
Supportedbimetallicnanoparticlesasanode catalystsfordirectmethanolfuelcells:Areview AkaljotKaur&GagandeepKaur&PritPalSingh& SandeepKaushal
https://ebookmass.com/product/supported-bimetallicnanoparticles-as-anode-catalysts-for-direct-methanol-fuelcells-a-review-akaljot-kaur-gagandeep-kaur-prit-pal-singhsandeep-kaushal/

Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
A review of g-C3N4 based catalysts for direct methanol fuel cells Afdhal Yuda & Anand Kumar
https://ebookmass.com/product/a-review-of-g-c3n4-based-catalysts-fordirect-methanol-fuel-cells-afdhal-yuda-anand-kumar/ ebookmass.com
PEM Fuel Cells Gurbinder Kaur
https://ebookmass.com/product/pem-fuel-cells-gurbinder-kaur/
ebookmass.com
Elsevier Weekblad - Week 26 - 2022 Gebruiker
https://ebookmass.com/product/elsevier-weekbladweek-26-2022-gebruiker/ ebookmass.com
Dirty Plays (Florida Devils Book 2) (Florida Devils Series) Michelle A. Valentine
https://ebookmass.com/product/dirty-plays-florida-devilsbook-2-florida-devils-series-michelle-a-valentine/
ebookmass.com




Nutrition Préventive et Thérapeutique Jean-Michel Lecerf
https://ebookmass.com/product/nutrition-preventive-et-therapeutiquejean-michel-lecerf/
ebookmass.com
Taxation: Philosophical Perspectives Martin O’Neill
https://ebookmass.com/product/taxation-philosophical-perspectivesmartin-oneill/
ebookmass.com
Cambridge Latin Courses 1 to 4 all books complete Cambridge Press
https://ebookmass.com/product/cambridge-latin-courses-1-to-4-allbooks-complete-cambridge-press/
ebookmass.com
General Chemistry. Principles and Modern Applications 11th Edition Edition Carey Bissonnette
https://ebookmass.com/product/general-chemistry-principles-and-modernapplications-11th-edition-edition-carey-bissonnette/
ebookmass.com
Affirmative Aesthetics and Wilful Women: Gender, Space and Mobility in Contemporary Cinema 1st ed. 2020 Edition Maud Ceuterick
https://ebookmass.com/product/affirmative-aesthetics-and-wilful-womengender-space-and-mobility-in-contemporary-cinema-1st-ed-2020-editionmaud-ceuterick/
ebookmass.com




https://ebookmass.com/product/john-11-21-a-handbook-on-the-greek-textnovakovic/
ebookmass.com


ReviewArticle
Availableonlineat www.sciencedirect.com
journalhomepage: www.elsevier.com/locate/he internationaljournalofhydrogenenergy46(2021)15820 15849
Supportedbimetallicnanoparticlesasanode catalystsfordirectmethanolfuelcells:Areview
AkaljotKaur,GagandeepKaur,PritPalSingh**,SandeepKaushal*
DepartmentofChemistry,SriGuruGranthSahibWorldUniversity,FatehgarhSahib,Punjab,India highlights


Thesupportedbimetallicnanoparticlesasanodecatalystsfordirectmethanolfuelcellsarediscussed. Maincomponents,workingandchallengesinthedevelopmentofdirectmethanolfuelcellarehighlighted. Varioussupportssuchascarbonaceous,metaloxides,metalnitrides,conductingpolymersareexploredformethanolelectrooxidation. Criticalinsightsandfutureperspectivesaregiven.
articleinfo
Articlehistory:
Received21October2020
Receivedinrevisedform
2February2021
Accepted7February2021
Availableonline12March2021
Keywords:
Fuelcells
Directmethanolfuelcell
Methanolelectro-oxidation
Anodecatalysts
Supportedbimetallicnanoparticles
abstract
Directmethanolfuelcell(DMFC)isanenvironmentfriendlyenergysourcethattransforms chemicalenergyofmethanoloxidationintoelectricalenergy.ThePt-andnon-Ptbased bimetallicnanoparticles(BMNPs)withelectrocatalystsupportmaterialsareemployedas anodeelectrocatalystsformethanoloxidation.ThesesupportedBMNPshavedrawn prominentconsiderationduetotheirincrediblephysicalandchemicalproperties.This articlereviewstheadvancementsinthefieldofsupportedBMNPsofvariedstructures, compositionsandmorphologies,usinginnumerablecarbonaceoussupportmaterialssuch ascarbonblack,carbonnanotubes,carbonnanofibers,graphene,mesoporouscarbonas wellasnon-carbonaceoussupportslikeinorganicoxides,graphiticcarbonnitride,metal nitrides,conductingpolymersandhybridsupportmaterials.Theperformanceofelectrocatalystsonthebasisofsupportmaterial,structure,compositionandmorphologyof BMNPs,andprosandconsofvarioussupportmaterialshavebeendiscussed.
© 2021HydrogenEnergyPublicationsLLC.PublishedbyElsevierLtd.Allrightsreserved.
* Correspondingauthor
** Correspondingauthor E-mailaddresses: dhillonps2003@gmail.com (P.P.Singh), kaushalsandeep33@gmail.com (S.Kaushal).
https://doi.org/10.1016/j.ijhydene.2021.02.037 0360-3199/© 2021HydrogenEnergyPublicationsLLC.PublishedbyElsevierLtd.Allrightsreserved.
Introduction
Thedemandforinexhaustibleandcleanenergysourcesis escalatingday-by-dayduetoexpandingfinancialturnof eventsandpopulacedevelopment.Thedependencyonfossil fuelsasenergyresourceshasledtoglaringairpollutionand indiscriminateminingofworld’soilreserveswhichisprimarilyresponsibleforglobalwarming.Besideswellbeingand ecologicalconcerns,world’sfossilfuelreservesarediminishingrapidly.Thedevelopmentofproficient,inexhaustible andenvironmentfriendlyalternativeenergysourcesisa challengingandprofoundlyactivearea[1].Generally,fuel cellsprovideanexcellenttrackforconversionofchemical potentialenergyintoelectricalenergy.Themostdistinct qualityoffuelscellsisthattheyarepollutionfreepower sources[2,3].Theexplorationoffuelcellsstartedin1800’sbut thefuelcelltechnologieshaveflourishedduringpastthree decades.Currently,thefuelcellsarereplacinginternalcombustionengines,andareusedinstationaryandportable powerapplications[4,5].Afuelcellisagalvaniccellconsisting ofananode,acathodeandanelectrolytethatconverts chemicalenergyoffueloxidationintoelectricalenergy.The fuelissuppliedcontinuouslyintheanodechamberwhilean oxidantissuppliedcontinuouslyinthecathodechamber,and theelectriccurrentisproducedbythechemicalreactions occurringattheelectrodesoffuelcell.Thefuelcellshaveefficiencyintherangeof40 60%whichcanbeenhancedto about85%iftheremainingheatoffuelisalsoutilized[6].Fuel cellscanutilizeavarietyoffuelssuchashydrogen,methanol, methane,COandotherorganicandinorganicreducingagents (e.g.,H2S,hydrazine).Hydrogenisthemostcommonlyused fuelinfuelcellsbutanyhydrocarbonorotherhydrogen containingfuelthatisdecomposedthermallyorcatalytically, mayalsobeemployedasafuel.
Onthebasisofoperationaltemperature,fuelcellsare classifiedaslow-temperatureandhigh-temperaturefuelcells. Onthebasisofelectrolytematerial,theseareclassifiedas solidoxidefuelcell(SOFC),polymerelectrolytemembrane fuelcell(PEMFC),moltencarbonatefuelcell(MCFC),alkaline fuelcell(AFC)andphosphoricacidfuelcells(PAFC).The characteristicsofPEMFCssuchashighpowerdensity,small stacksize,lowcost,lowtemperature,fastresponseandhigh energyconversionefficiencymakethempromisingcandidatesforefficientenergyconversionamongvarioustypesof fuelcells.PEMFCsarefurtherclassifiedashydrogenfueled anddirectliquidfuelcell(DLFC)onthebasisoftypeoffuel.
DLFCsusedimethylether,formicacid,methanol,ethanol, ethyleneglycolandhydrazineasliquidfuels.Hydrogenisthe mostsuitablefuelbutthecleanproductionofhydrogenand thechallengesinitsstorageandtransportationrestrictitsuse asafuelfortheadvancementoffuelcelltechnologies.Theuse ofliquidfuelsoverhydrogenfuelisadvantageous,owingto theeaseoftheirstorage,transportationandhighenergy density[7 9].Directmethanolfuelcell(DMFC)isoneofthe mosteminentfuelcellsintermsofproficiencyandoperationalease,andisreckonedasapotentialreplacementof conventionalbatteriesinportabledevices.DMFCisalowtemperaturefuelcellbasedonpolymermembranetechnology.Thisfuelcelldoesnotrequirethereformingofalcohol intohydrogenbutmethanolisfeddirectlytothefuelcell. Anodedrawshydrogenbydissolvingmethanolinwaterduringchemicalreactionandtheoverallcostisreducedowingto thelackofreformer.Owingtoitshighefficiency,ultra-low pollution,lownoise,highreliabilityandeasymaintenance, DMFChasreceivedconsiderableattentionamongdifferent kindsoffuelcells[7,10 12].Thevolumetricenergydensityof methanol(17,900kJL 1)isapproximatelythreetimesthatof hydrogen[13].Methanolisconsideredtobeanattractivefuel, owingtoitslowcost,highenergydensity,easystorageand transportation,andproductionfromnaturalgasorrenewable biomassresources.ThismakesDMFCanexcellentpower sourceforelectronicvehicles,portableelectronicdevicesand stationaryapplications[14 17].
PtandPt-basedcatalysts,thefrequentlyutilizedanode electrocatalystsinDMFCs,arecostlyandsufferfromsurface poisoningwhichleadstosluggishkineticsofanodicreaction. Moreover,highcatalyticloadingofPtisrequiredtobreakthe strongcarbon-oxygenbondinmethanolwhichfurtherincreasesthecostofcatalyst[18].Thesearetheforemost limitationsinutilizationofDMFCs.ForthesuccessofDMFC technology,highlyactiveandstableanodeelectrocatalyst withlowPtloadingisrequired,toincreasetherateof methanoloxidationatanode.Inthepastdecades,theadvancementsinnanotechnologyhaveunrolledbetterapproachestocreateinnovativeelectrocatalystsforfuelcells. ThedesignandsynthesisofPt-andnon-PtBMNPanode catalystswithwell-definedstructure,compositionand morphology,withvariouscatalystsupportmaterialsto decreasethecostofproductionandincreasetheefficiencyof DMFChasemergedswiftlyinthelastfewyears.Thisreview articleisfocusedontherecentresearchanddevelopment activitiesinthefieldofsupportedbimetallicnanoparticlesas anodecatalysts.
Methanolelectrooxidationreaction
InDMFC,methanoloxidationtakesplaceatanode.Ptisthe mostwidelyemployedelectrocatalystinDMFCsformethanol electrooxidationreaction(MOR)[19 21].Themechanismof MORonPtelectrodeinacidicmediawaspresentedbyHamnett[22].Ingeneral,themechanismofmethanoloxidationin acidicmediacanbeexplainedinthefollowingsteps:(a) electrodepositionofmethanolonthePtelectrode,followedby formationofintermediatessuchas CHO, COOH, COetc. onPtsurfaceviaC Hbondactivation(b)formationof oxygenatedspecies(Pt OH)onthesurfaceofPtbydegradationofwater,and(c)removalofCO2 bytheadditionofoxygen tocarboncontainingintermediates[22,23].Amongvarious intermediatesformedduringMOR,COisadsorbedstronglyon thesurfaceofPt,leadingtoblockageofthesurfaceofPt catalystandloweringthedurabilityofcatalyst[20,21,24,25]. BymodifyingtheelectronicstructureofPt,itsMORactivity canbeameliorated.ThiscouldbeaccomplishedbymixingPt withadditionalmetalsthatpromotetheremovalofCO,by oxidationtoCO2.BimetallicPt-basednanocatalystsoften exhibitenhancedcatalyticpropertiesascomparedtotheir mono-metalliccounterparts,duetothecombinedsynergistic strainandligand/electroniceffect[26,27].Ruismostwidely studiedmetalandPtRuisreckonedtobethepotentialbimetalliccatalystforMOR.
AlloyingPtwithRumodifiestheelectronicstructureofPt. WatermoleculesundergodissociationonthesurfaceofRu intooxygenatedspecies(OHads)atloweroxidationpotential ascomparedtothatonPt,andhelpintheremovalofCOads on PtbyoxidizingittoCO2 throughabifunctionalmechanism [28 30].
materials,andincreasestheextentofmetalsthatcanbe exploitedaselectrocatalysts.PdandNi-basedcatalystsare moreactiveformethanoloxidationinalkalinemedium [38 40].However,themajordisadvantageofliquidalkaline electrolyteistheformationofcarbonateandbicarbonateon thesurfaceofelectrodewhichreducestheamountofOH adsorption,andultimatelydecreasesthereactionactivity. However,thisproblemcanbeovercomebyusingalkaline anionexchangemembraneaselectrolyte.
AnodecatalystsforDMFCs
Thepresentultra-modernelectrocatalystforDMFCin acidicmediaisPtRu/CwithPt:Ruatomicratioof1:1.ItpossessesimprovedactivitytowardsMORandgoodCOtolerance incontrasttounsupportedPtRunanoparticlesandPt/Ccatalyst[24,27,28].However,thePtRu/Ccatalystisnotstableovera periodoftimeduetoRudissolutionprocessesandcorrosion ofcarbonmaterials.UnderDMFCoperatingconditions,Ru dissolutiontakesplaceandcrossoverofdissolvedRuto cathodeinhibitsthekineticsofoxygenreductionreactionand alsoresultsinmembranedeterioration[31,32].
Ingeneral,theperformanceofcatalystisbetterinalkaline media.Severalgroupshaveinvestigatedmethanoloxidation onplatinumsinglecrystalandpolycrystallineelectrodesin alkalinemedium.Itwasobservedthattheelectrodedoesnot getpoisonedinalkali,owingtoweakbondinginintermediateschemisorbedonPtandlesseramountofpossible poisoningspeciesi.e.COads [33,34].Themechanismofmethanoloxidationinvolvestheformationofadsorbedmethanolic speciesonPtsurfacebysuccessiveelectrontransfer.These adsorbedmethanolicspeciesandOH ionsreacttoformCO2 [35 37].Therateofreactioninalkalinemediumisfasteras comparedtothatinacidicmedium.Alkalinemediumalso reducestheriskofcorrosionofcatalystsandcarbon
TwosignificantfactorsthatdecidethesuccessofDMFC technologyarethemembraneandelectrocatalyst,forpreventionofmethanolcrossoverandimprovementofmethanol oxidationkinetics,respectively.ThediscussionontheadvancementsofMEAtoincreasetheefficiencyofDMFCis beyondthescopeofthisreview.Theforemostquestionsin theevolutionofanodecatalystinclude:(i)catalystpresentationintermsofactivity,consistencyanddurability,and(ii) costreduction.Thedevelopmentofhighlyactivecatalystfor MORispropitiousastheefficiencyofDMFCsissustainedby MOR[41].Inthepastyears,manyresearcheszeroedinonthe advancementofanodecatalystswhilereducingitstariff.The metalsaregenerallyalloyedtoimprovetheirelectrocatalytic activity,andbinarynanoparticles(NPs)exhibithighercatalyticactivitythanmonometallicNPs.Theothertechniquefor enhancingtheefficacyofelectrocatalystswhilediminishing theamountofnoblemetalwithoutcompromisingitsperformanceinvolvesimmobilizationofNPsondifferentsupports. Pt,Pdandothernon-noblemetalbasedBMNPssupportedon variouscarbonaceousornon-carbonaceousmaterialsare highlybeneficialforMOR[42].Metalsarefinelydispersedon suitableporoussupportswhichallowahighloadingof dispersedmetalNPs.TheseNPsshowexcellentcatalytic propertieswhicharenotexhibitedbyNPsinlowdispersion. BMNPswithdissimilarstructuressuchasalloys,core-shell andintermetallicNPs,differentcompositionsandmorphologieshavebeensynthesizedtoenhancetheMOR.
VariousmethodsareusedtoassesstheactivityofelectrocatalyststowardsMOR.Theelectrocatalyticactivityofthe catalystisexpressedintermsofitsmassactivity/specificactivity,electrochemicallyactivesurfacearea(ECSA)andIf/Ib ratio.Massactivity/specificactivityisdeterminedbycurrent densityvalues.Higherthevalueofpeakcurrentdensity, greateristheactivityofcatalystforMOR.LargevalueofIf/Ib ratioindicateshigherstabilityofelectrocatalysttowardsCO poisoning.
Supportedbimetallicnanoparticlesasanodecatalysts
ThesupportedBMNPsareextensivelyutilizedasanodecatalysts.Variousorganicandinorganicsupportssuchascarbon, graphene,CNTs,CNFs,metaloxides,metalnitrides,conductingpolymersetc.areusedtosupporttheNPs.These supportsenhancetheirsurfacearea,offerhighdispersity, increasestabilityandreducecost,thusmakingthembetter candidatesformethanolelectrooxidation[43].Currently,the supportedNPsareemployedactivelyaselectrocatalystsfor
methanoloxidation[44 46].Thesupportmaterialmust possesshighporosity,highelectronicconductivity,large specificsurfacearea,goodsurfacemorphology,corrosion resistanceandgoodwaterhandlingcapabilityforeasyrecoveryandtoavoidflooding.Theperformanceofsupported BMNPsdependsmainlyonthenatureofsupportandmetal supportinteractions[47 49].Supportmaterialinfluencesthe sizedistributionanddispersionofNPsthatfurtheraffecttheir catalyticactivity.TheperformanceofNPsisimprovedbytheir interactionwithsupportmaterials.Supportcanassumea significantroleincatalyticprocessbyprovidingnewactive sitesandalsoaffectsthephysicalaswellaschemicalpropertiesofNPs.Severalcarbonaceousandnon-carbonaceous supportmaterialsareusedtoimmobilizeBMNPsfortheir useascatalystsinDMFCs,inboththeacidicaswellasbasic media.Theelectrocatalyticactivitiesofthesesupported BMNPsandtheeffectofvariousparameterssuchascomposition,morphology,structure,crystalplane,supportmaterial etc.ontheirelectrocatalyticactivityarediscussedbelow:
Carbonaceoussupports
CarbonblackssupportedBMNPs. Amongawiderangeofcarbonmaterials,carbonblacksarecommonlyusedassupport materialsforelectrocatalystsinDMFCsonaccountoftheir hugesurfaceareaandelevatedelectricalconductivity. Varioustypesofcarbonblacksareacetyleneblack,Ketjen black,BlackPearlandVulcanXC-72,havingdiverseproperties suchasporosity,specificsurfacearea,electricalconductivity andsurfacefunctionalitieswhicharedeterminedbytheir manufacturingprocess.Carbonblackshavinglowspecific surfaceareae.g.acetyleneblackusuallygivelowdispersionof NPs.HighlydispersedNPsarederivedfromcarbonblackswith elevatedsurfacearealikeKetjenBlackbutKetjenBlacksupportedmaterialsuffersfromtheproblemofhighohmic resistanceandmasstransfer.ThemostcommonlyusedcarbonsupportsforBMNPcatalystareVulcanXC-72and VulcanXC-72R(Cabot)whichhavedifferentmorphologies. VulcanXC-72isintheformofpelletsandVulcanXC-72Risin powderform,andthismorphologicaldifferencemightinfluencethefuelcellperformance[50].AnumberofBMNPssupportedoncarbonblackhavebeensynthesizedfortheiruseas anodecatalystsinDMFCs[51 58].Pt-basedNPsareusually employedasanodecatalystsforMOR.PtRu/Cisconsideredan excellentanodecatalystforMORinacidicmediabutitsstabilityinacidicconditionsstillneedstobeimprovedfurther. Varioussyntheticmethodsandreactionconditionsare employedtoboosttheelectrochemicalproperty,durability andreliabilityofthecatalyst.DiverseformsofPtRunanocatalystssuchasalloys,core-shellNPs,Ruelectrodeposits onPt,PtRuco-depositsetc.areusedformethanolelectrooxidationandallthesematerialsexhibitdifferentactivities towardsmethanoloxidation,dependingupontheirmethodof synthesis,surfacestructure,particlesize,morphology,crystal planestructureetc.Itiscompulsorytoaccuratelycontrolthe dispersionofRuonPtasthesurfacecompositionofcatalyst affectsitsactivity,andacatalystwithbetterelectrocatalytic propertiescanbeobtainedbyoptimizationofitssurface composition.Garricketal.usedelectrolessdeposition(ED) methodtocontrolthesurfacecompositionofPtRu/Ccatalyst. EDdoesnotallowthedevelopmentofconfinedcrystallitesof
optionalmetaloncatalystsupportbutleadstoregulated depositionofspecifiedamountofsecondarymetalonthe surfaceofmonometalliccatalyst.AseriesofRuPtbimetallic catalystswerepreparedbyEDusingVulcanXC-72asbase monometalliccatalyst.Thecatalystconsistingof20wt%Pt and1.1wt%Ruwithcoverageof0.51RuonPtexhibitedhigher activitythanothercatalystwithlowerorhighertheoretical coveragesofRuonPti.e.~3.5timeshigherthanitscommercialcounterpartand~7timeshigherthanacommercial monometallicPt/Ccatalyst[59].Wangetal.synthesizedthree differentPt/Rualloyswithvariationinratioofboththemetal ionsi.e.Pt1Ru0.5/C@NC(1),Pt1Ru2/C@NC(2)andPt1Ru1/C@NC (3)alloy.ItwasshownbyFTIRanalysis(Fig.1)thatnanocomposite(1)exhibitedmethanoloxidationreaction(MOR) pathwayoverHCOO intermediateandnanocomposite(2) exhibitedMORpathwayoverCOintermediate.Thenanocomposite(3)having1:1ratioofPt:RushowedbestMORactivityaswellasanti-COintoxicationcapabilitythroughthe collectivetrackofHCOO andCOintermediate[60].AhighPt utilizationandincreasedactivitycanbeachievedbysynthesizingbimetallicstructureswithislandsofPt,depositedinto lessnoblematerialwithfinesizes.Inthisregard,Zhengetal. synthesizedVulcanXC-72supportedRuPtbimetalliccatalyst withevendispersionandmeanparticlesizeof1.4nmforRu coreand1.9nmforbimetallicRuPt.Thecatalystpossessed ECSAof92.5m2 g 1 andmassactivityof483Ag 1 thatwas considerablymoreincomparisontothatofcommercialPtRu/ Candco-reducedPtRu/Ccatalysts[61].Acompositeofcobalt ferriteNPswithVulcanXC-72carbon(CoFe2O4-VC)wasused asasupportfordispersionofPd/NiNPs.TheresultingelectrocatalystwasusedforORRandborohydrideoxidationreactioninalkalinemedia[62].
ThecatalyticactivitytowardsMORcanbetunedbyregulatingthemorphologyandcrystalplanestructureofnanoparticles.Inthisrespect,Pt/Rubimetallic,icosahedraand cubesweredepositedonVulcanXC-72.Higherspecific (0.76mAcm 2)andmassactivity(74.43mAmgPt1)wasdisplayedbyboththeRudecoratedPtcatalystsascomparedto thatexhibitedbyPt7Ru/C(Fig.2).Itindicatedthatthecatalytic activityoficosahedraofPt/Runanocrystalsishigherthanthat ofcubicduetocombinedfaceteffectandtwininducedstrain effect[63].
Thecore-shellPtRu/Cnano-catalystsareexceedinglypropitiousforMORandpossesshigherCO-poisoningtolerance whencomparedwiththatofPtRu/Calloycatalysts[64,65].The stabilityandactivityofPtRuNPscanbeenhancedbyheteroatomdopingorbyfunctionalizationofcarbonblacksupport [66 69].ThecomparisonofactivitiesofdifferentPtRu/Ccatalystsisgivenin Table1
CarbonsupportedPtFe[55],PtSn[56],PtCo[71],PtCu[72], PtIr[73],PtAu[74,75]andPtNi[76]BMNPshavealsobeenreportedinliterature.One-potsolvothermalprocesswas employedtofabricateVulcanXC-72supportedPt CoNPswith tunablecomposition,withoutusingacappingagent.The synthesizedPtxCo/Ccatalystdisplayedhigherelectrocatalytic activitytowardsMOR.Outofthecatalystswithvarying composition,threetimeshighermassandspecificactivity wasobservedforMORincaseofPt1.2Co/C,with4.7 ± 0.8nm averageparticlesizeand64.30m2 g 1 activeexteriorarea whencomparedwithbusinessPt/Ccatalyst.Thisenhanced
internationaljournalofhydrogenenergy46(2021)15820 15849
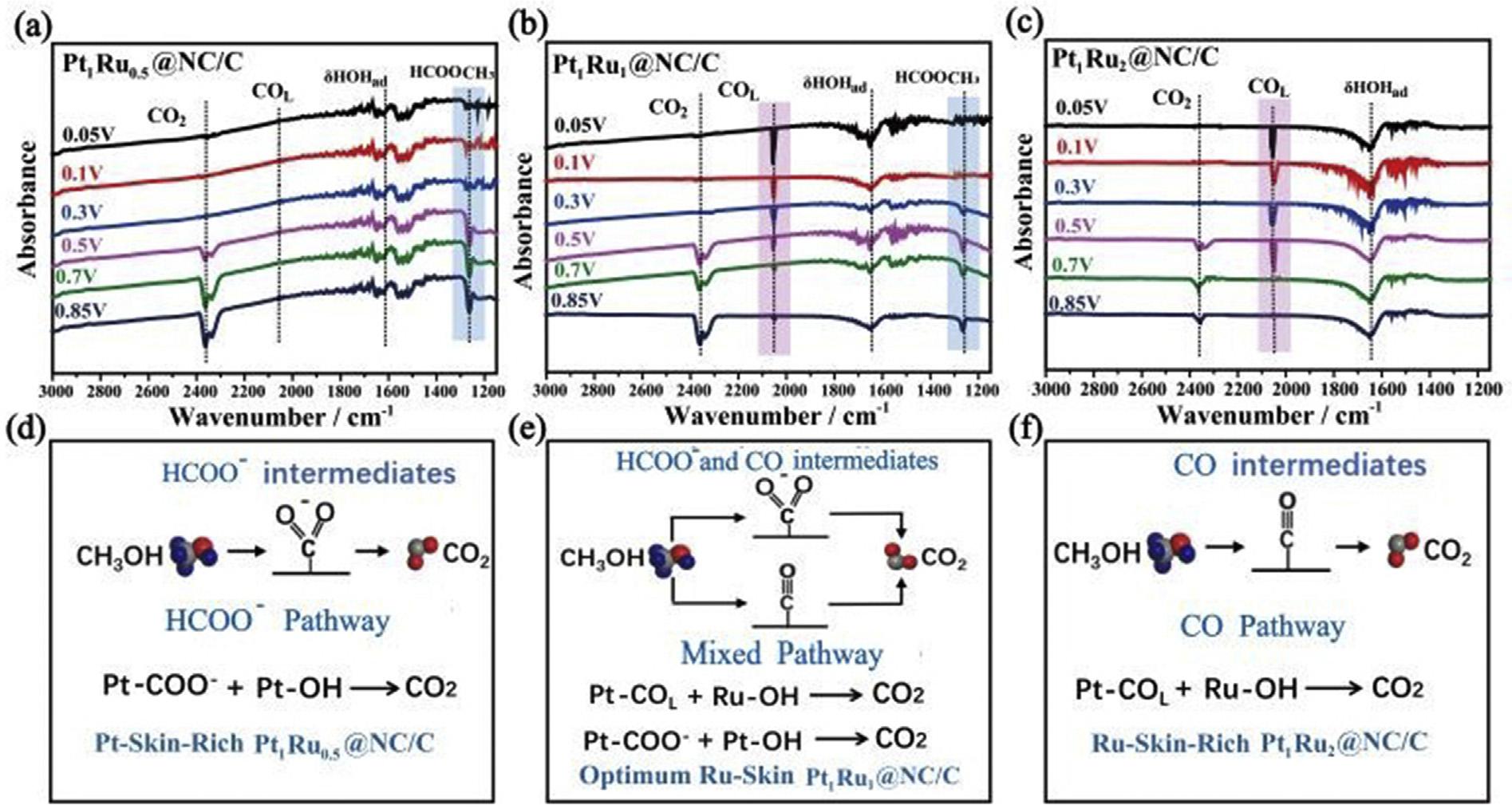
Fig.1 TheFTIRspectraofmethanoloxidationon(a)nanocomposite(1),(b)nanocomposite(2)and(c)nanocomposite(3),(d) graphicalrepresentationoftheMORon(d)nanocomposite(1),(e)nanocomposite(2)and(f)nanocomposite(3)[60] (Reproducedwithpermission,TheRoyalSocietyofChemistry,2020).
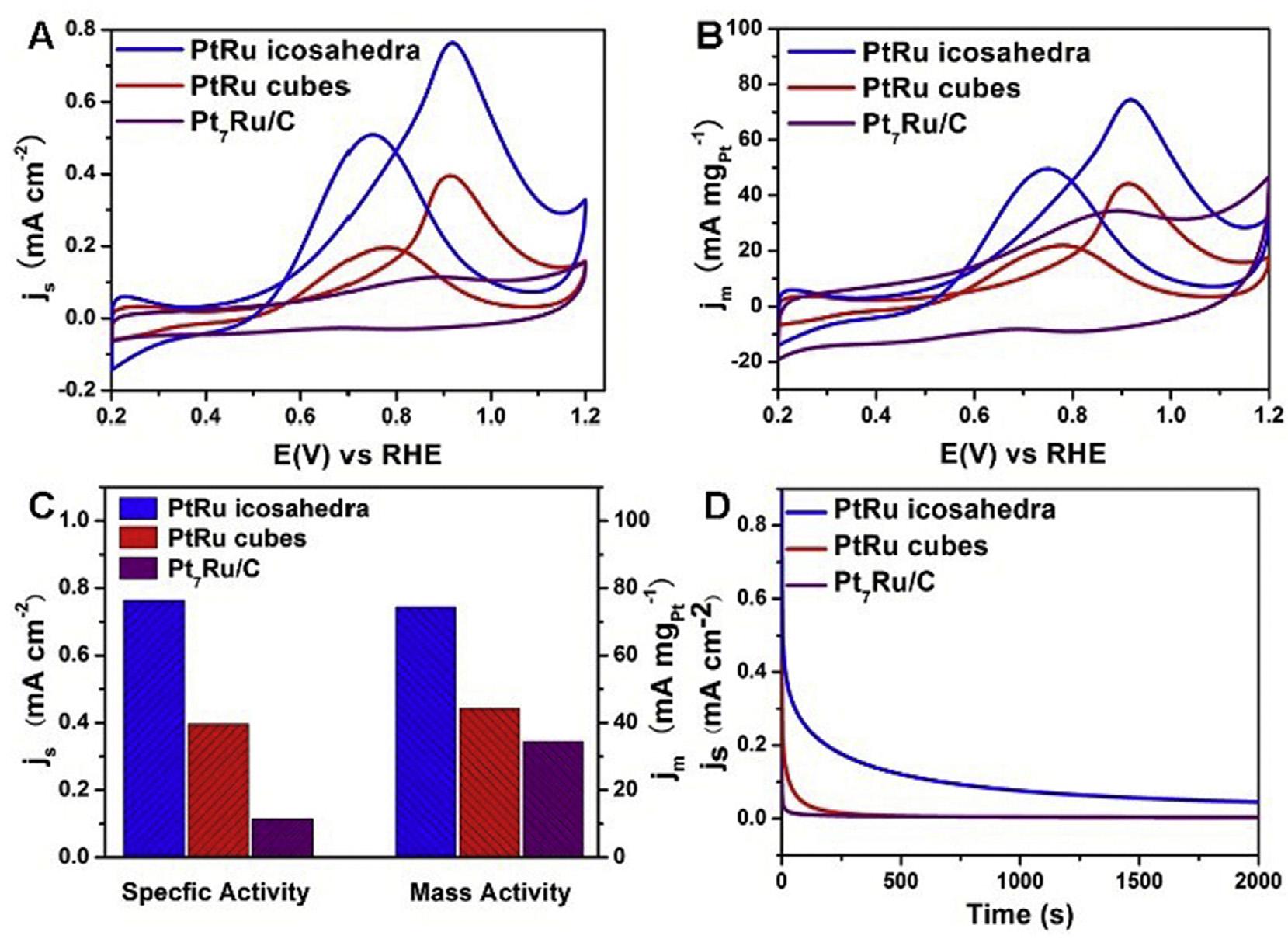
Fig.2 (A,B)Cyclicvoltammogramsofthreedifferentcatalysts,(C)Specificandmassactivity,(D)GraphofcurrentVstime forthreecatalysts[63](Reproducedwithpermission,TheRoyalSocietyofChemistry,2016).
/CMicrowaveassistedpolyolmethod1.53__0.5MH
catalyticactivityofthecatalystwascreditedtotheintroductionofCoandalloyingwithPtwhichdecreasedtheparticle size,leadingtomoreactivesitesonthecatalystandlowering ofelectronicbindingenergyinPtwhichpromotedthecleavageofC Hbondatsmallpotential[71].Yanetal.fabricated Pt/CandPt@Au/Ccatalystsemployingmicrowave-assisted polyolprocessandheteroepitaxialgrowthmethodwith differentconcentrationofAusaltsolution(0.24,0.48,0.72and 1.32mMforPt@Au-0.55/C).ItwasobservedfromelectrochemicalstudiesthatmassactivityofPt@Au/CcatalystdecreaseswithincreaseintheoreticalatomicratioofPtandAu (RAu/Pt)butmassactivitiesofPt@Au-0.1/C,Pt@Au-0.2/CandPt/ Ccatalystwerenearlythesame.ECSApossessedbyPt@Au0.1/CandPt@Au-0.2/CwashigherascomparedtothatofPt/ C.Pt/C-JM,andPt@Au-0.1/C,Pt@Au-0.2/CandPt@Au-0.3/C exhibitedimprovedperformanceforCOoxidation.TheresultsrevealedthatPt@Au/CcatalystswithRAu/Pt 0.2 exhibitedhigherMORactivity,superiorCOtoleranceandCO electrooxidationactivity[74].Thecore-shelldendriticAu/Pt NPssupportedonVulcanXC-72weresynthesizedusing ascorbicacidreductant.TheelectrocatalyticactivityofMOR wasaffectedbyascorbicacidreductiontime.AuPt/Cbased electrodeswerepreparedatascorbicacidreductiontimeof 1h,4h,8hand12h.Thehighestelectrocatalyticactivitywas achievedatreductiontimeof4h,withcurrentdensity2.3 timesmorethanthatofbusinessPt/Cbasedelectrode[75]. TheeffectofNionPtNPstowardsMORwasinvestigatedby synthesizingcarbonsupportedPt/NialloyNPs,withand withoutsurfaceNi.ItwasobservedthatthesurfaceNionPt/ NialloyNPsprovidedenhancedOHadsorptionsitesandwas mainlyresponsibleforpromotingtheMORactivityofPtbased catalysts[76].Theoilphasemethodwasemployedtofabricate highindexplanesbound,shape-controlledPt Cualloyhollow nanocubes.TEM,HAADF-STEMandEDStechniqueswere usedtosupporttheformationofcubicconcavestructure (Fig.3).Theas-synthesizedcubeswerefilledonacarbonblack support(VulcanXC-72).andtheirelectrocatalyticproperties forMORwerestudied.TheCVandchronoamperometric(CA) experiments(Fig.4)showedthatthecatalystexhibitedbetter durabilityandelectrocatalyticactivityforMORincontrast withnanostructureofPt-CuandbusinessPt/C,attributableto thecombinedeffectsofconfigurationaswellasplane[72].
PtRhalloysarealsopreferredMORcatalystsbecause alloyingofPtwithRhpromotesCOoxidationonneighboring Ptsites.Shietal.exploredtheevolutionincompositionand structureofPtRh/CalloyNPsduringtheelectrochemical methanoloxidationinacidicenvironment.Itwasobserved thattheinitialatomicratiobetweenPtandRhwas1:1,andRh atomswerepresentonthesurfaceofPt/RhNPswithan atomicratioof68.2%.Thepercentageandsurfacecontentof Rhreducedto34.4%and11.5%,respectively,after80cyclesof CVindicatingRhdissolutionwhichexposedthePtsitesofPt/ Rhalloy.After80cyclesofCV,aggregationandrecombination ofactivePtatomsonthesurfacepreventedRhdissolution, leadingtostablecatalyticperformanceascomparedtoPtRu/C whichexhibitedlowerstabilitytowardsMORinacidicmedia, duetodissolutionofRuduringthefirst39cyclesofCV[32]. IntermetallicNPsalsopossessgreaterstabilityandexcellent catalyticactivitytowardsmethanolelectrooxidation comparedtotheirrandomalloycounterpartsinsomecases.
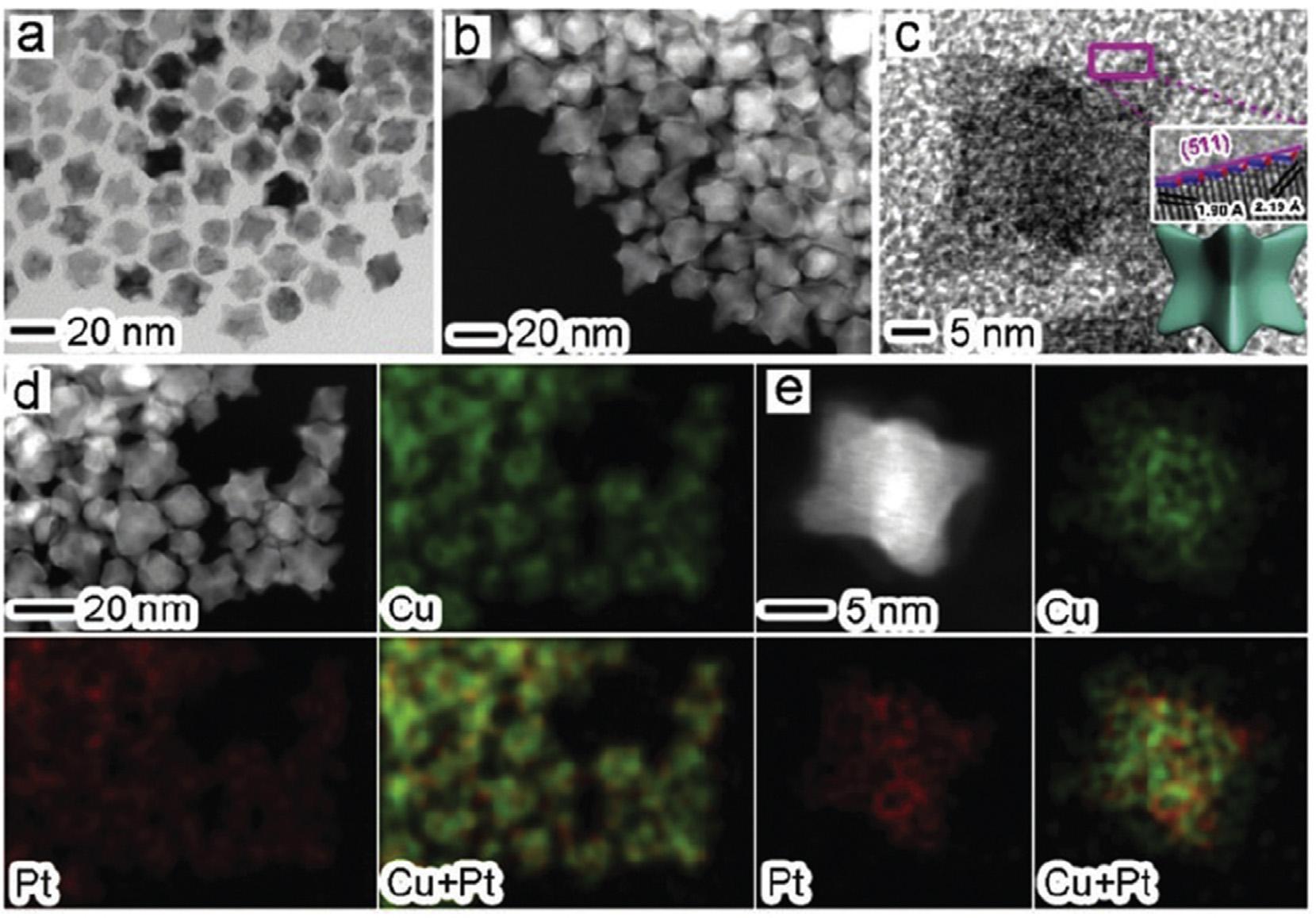
Fig.3 (a)TEM,(b)HAADF-STEM,(c)HRTEM(insethighresolutionimage),and(dande)HAADF-STEMEDXmappingpictures ofthePt Cuconcavenanocubes[72](Reproducedwithpermission,TheRoyalSocietyofChemistry,2014).
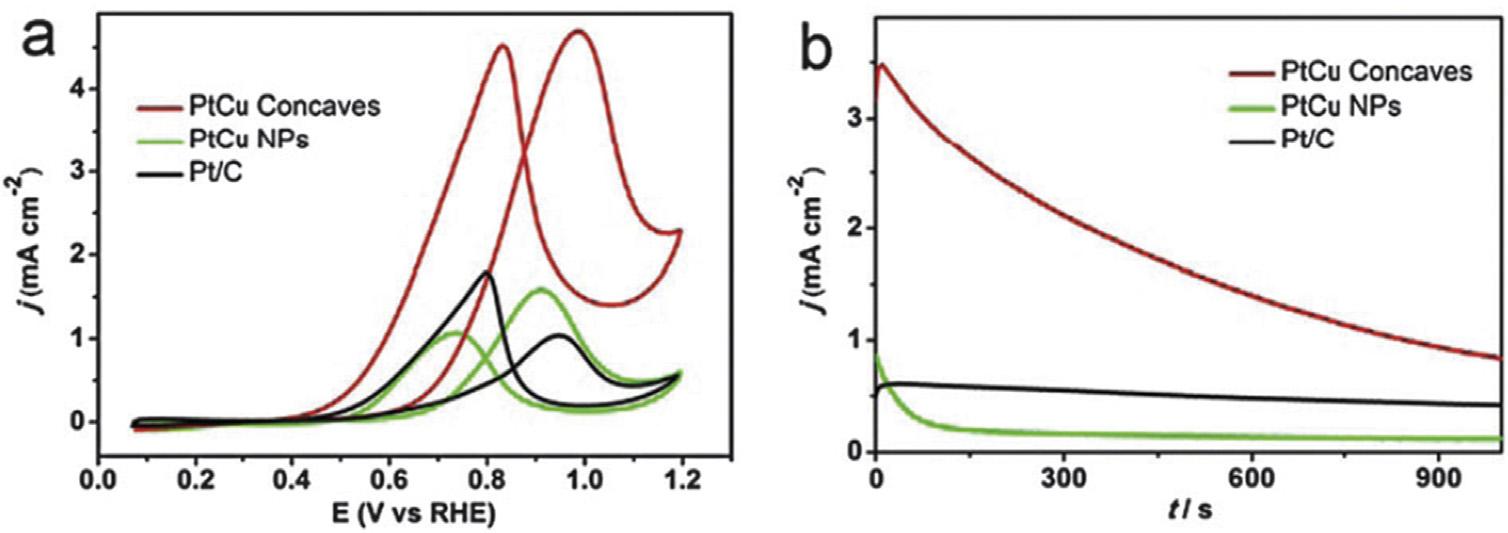
Fig.4 (a)CVsand(b)CAresultofmethanoloxidationonPt Cuconcavecubesin0.1MHClO4 1MCH3OHsolution[72] (Reproducedwithpermission,TheRoyalSocietyofChemistry,2014).
ThecarbonblacksupportedPt3TiandPt3VintermetallicNPs withultra-smallparticlesizeweresynthesized.Thepeak currentdensityofPt3Ti/C(380mAmg 1Pt)andPt3V/C(481mA mg 1Pt)wasgreaterthanthatofPt/C(161mAmg 1Pt)which indicatedtheirhigheractivityforMOR[77].
ThePd-basedcatalystsshowgoodactivityforMORin alkalineelectrolytes.However,theoxidationofPdtoPdO,at similarpotentialsastheMORandCOpoisoning,affectsthe reactionperformance.TheMORactivityofPd-basedelectrolytescanbeenhancedbyintroducingasecondmetalinto theirstructure[78 84].Kellyetal.investigatedAuPd/Ccore shellandalloynanocrystals,andobservedthatthecatalysts exhibitedbetteractivitythanPdnanocrystalsformethanol
electrooxidationduetopresenceofAu.Moreover,thecatalyst showednoCOpoisoningatPdsurfacebecauseofsurface coveragebyOHfromalkalinemedium.Itwasobservedthat thealloyNPsshowedbetterperformancethancore-shellNPs. AufacilitatedthereductionofPdObacktoPdandmadethe surfaceavailableatmorepositivepotentialforfurthermethanoloxidation[78].Fathiradetal.synthesizedVulcanXC-72R supportedPdandPd MoNPswithvaryingatomicratiosat pH9.ItwasobservedthatalloyingPdwithMoloweredthedbandpositionofnoblemetaloverlayer,resultinginmodified electrocatalyticactivitybyinducingstrainandelectron redistributionbetweensubstrateandoverlayer.Pd3Mo/C possessedhighactivityanddurabilityduetosmallsizeand
lowerMocontentthanPdMo/CandPdMo3/Ccatalysts[79].Pd/ NiNPswereimpregnatedonVulcanXC-72R,employingNaBH4 asareducingagent.Cyclicvoltammetryandchronoamperometrywereusedtoinvestigatetheelectrocatalytic activityofthecatalystforMORin0.5MKOH.Thecatalyst exhibitedhighercurrentdensity(1.92timeshigherthanthat ofPd/Cand1.68timeshigherthanthatofNi/C)andrate constantformethanoloxidation,andhigherstabilitythanPd/ CandNi/Ccatalysts[80].Recently,Kodiyathandco-workers synthesizedcarbonblacksupportedintermetallicPd3X (X ¼ TiandZr)BMNPs.Thespecificcurrentdensityexhibited byPd3Ti/CandPd3Zr/Cwasevaluatedtobe3and2.5times higher,respectively,relativetoPd/C,andthecurrentdensity remainedvirtuallysameevenafter300cycles[81].Thereare somelimitationsofusingcarbonblacksassupportmaterials. Carbonblacks(CBs)supportedBMNPssufferfromreduced catalyticactivityduetothepresenceoforgano-sulfphurimpuritiesintheCBsandtrappingofcatalystNPsindeepmicroporesofCBs,maketheNPsunapproachabletoreactants. ThethermodynamicinstabilityofCBsleadstocorrosionof carbonsupportthatultimatelycollapsesthecatalystlayer.So, thefocushasshiftedtoothernanostructuredsupportsasthey imparthighelectrocatalyticactivitytothecatalyst,dueto fasterelectrontransfer.
Carbonnanotubes(CNTs)supportedBMNPs. CNTsareallotropicformofcarbonrelatedtofullerenefamilyandconsistof rolled-upsheetsofgraphenei.e.singlelayercarbonatoms. PureCNTscanbeviewedtobeformedfromtherollingupofa graphenesheet(SWCNTs)orfromaseriesofconcentric rolled-upgraphenesheets(MWCNTs).Thediameterof SWCNTsrangesfrom0.4to3nm,withmostcommonvalue around1.4nmwhileMWCNTshavediametervaryingfrom0.4 to100nm.SWCNTshavelargersurfaceareawhileMWCNTs possesshighconductivity.CNTsconstituteafavorablesupportmaterialforheterogeneouscatalystsowingtotheir excellentpropertiessuchaschemicalinertness,highoxidationstability,outstandingelectronconductivityandlarge specificsurfacearea.Severalresearchershavereportedthe synthesisofCNTssupportedBMNPsfortheiruseasanode catalystinDMFCs[85 88].Solutionplasmasputtering,followedbythermalannealingwasemployedtofabricate MWCNTssupportedPt/Co PtNPswhichexhibitedexcellent improvementinelectrooxidationofmethanolinacidicmedia withmassactivity1719mAmg 1 Pt [89].Pt/RuNPsandPt/Mo NPswithtypicalsizeof10nmweresynthesizedbychemical reductionwithcarbonylcompoundsandsupportedon SWCNTs.TheresultsindicatedthatPt50%Ru50%/SWCNTs exhibitedbetterelectrocatalyticactivityasanodecatalystin hydrogenandmethanolfuelcellwhencomparedwith Pt50%Mo50%/SWCNTsandPt100%/SWCNTs[90].Ahighfuelcell performancewasachievedusingPt Pd/CNT-Scathodeelectrocatalystsynthesizedviasurfactantassistedmethodusing anionicsodiumdodecylsulfate[91].
ItisdifficulttoattachmetalNPsonpristineCNTsduetotheir chemicalinertness.Thefunctionalization(covalentandnoncovalent)ofCNTsprovidesbetterdistributionofmetalNPs andsizecontrol,toimprovetheactivityofcatalystinDMFCs. Thecovalentfunctionalizationismostcommonlyperformedby oxidizingCNTsusingH2SO4,HNO3,HClO4,O2,O3,KMnO4 and
H2O2 whichgeneratesurfaceoxygengroupsincludinghydroxyl, carboxyl,carbonyl,esteretc.Thismakesthesurfacemorehydrophilicandimprovesthemetal-supportinteractions.The dispersionanddistributionofNPscanalsobeimprovedbynoncovalentfunctionalizationofCNTswithbiomolecules,polyelectrolytes,polymers,surfactantsandaromaticcompounds whichprovidelargenumberofanchoringsiteswhilekeeping theintrinsicpropertiesofCNTsintact.ThecovalentfunctionalizedCNTshavebeenusedtosupportBMNPs[92 96].The chemicalco-reductionmethodwasusedtodisperseCo@Pt core-shellNPsonfunctionalizedMWCNTssupportinthe presenceofsurfactant(Fig.5).ThecatalystpossessedhighMOR (433.4mAmg 1)activityinbasicmediawithmassactivity1.61 and3.36timesmoreincontrasttothatofPt Co/MWCNTsand Pt/MWCNTs,respectively[92].ThecatalyticactivityoffunctionalizedMWCNTssupportedNi@Ptcore-shellNPsforMOR wasinvestigatedinacidicaswellasbasicmedium.Cyclicvoltammetryresultsrevealedthatthiscatalystdisplayedhigh valuesofpeakcurrents(10.40,13.05mA),massactivities(216.7, 271.9mAmg 1Pt)andexchangecurrents(182.5,284.2mA mg 1Pt)inacidicandbasicmedia,respectively[93].
ThiolfunctionalizedSH-CNTssupportedPt RuNPswere foundtoexhibitgreaterCOtolerancethanPtRu/COOH-CNTs. TheadsorptionofCOonPtinvolvesbondingbetween2p orbitalsofCOand5sPtorbitals.Thesynergisticback-donation ofelectronsfromPtto2p* orbitalofCOmolecule strengthensthe s p bond.Byrestrictingtheelectrontransfer fromPtdp toCO2p* orbital,the s p bondisweakenedand COcouldberemovedfromthesurfaceofPt.InPtRu/SH-CNTs catalyst,stronginteractionsbetweenPtatomsand SH groupsweakenthe s-p bondbetweenCOandPt,leadingto highMORactivity(Fig.6)[94].
Satyanarayanetal.investigatedthesynergisticeffectofAg substitutedPdNPsanchoredonCOOH-MWCNTsmatrixfor MORinalkalinemedia.ThesynthesisofcatalystPd20-xAgx/ COOH-MWCNTs(xwt% ¼ 0,5,10and15)wascarriedout throughmicrowaveassistedpolyolmethod.TheelectrocatalyticactivitywasimprovedbyinductionofAgintoPdNPs. Theorderofelectrochemicalactivityofdifferentcatalysts was:Pd10Ag10/COOH-MWCNTs > Pd5Ag15/COOHMWCNTs > Pd15Ag5/COOH-MWCNTs > Pd20/COOH-MWCNTs. ThePd10Ag10/COOH-MWCNTsexhibitedhigherpeakcurrent, loweronsetpotential,higherstabilityandbetterpoison tolerancetowardsCOandothercarbonintermediates[97].
ThemethanoloxidationmechanismonPt/Pd(111)andPt/ Au(111)wasinvestigatedbyjointcomputational/experimentalstudybyYouandco-workers.Forthispurpose,hydroxylfunctionalizedSWCNTssupportedPt/Pd(111)andPt/ Au(111)NPsweresynthesizedbyelectrodeposition.Cyclic voltammetrywasperformedforaspecifiednumberofcycles ina1MsolutionofeachofMeOHandNaOHatambient temperature,todeterminetheelectrochemicalactivityand stabilityofNPsforMOR.ItwasobservedthatmethanoldecomposesonPt/Au(111)surface,preferablybyanon-CO pathwayincontrasttothatonpurePt(111)surfaceasthe energybarrierofrate-determiningstepis0.29eVlowerin non-COpathwaythanthatinCOpathway.Thedecompositionofmethanolfollowsthepathway:CH3OH / CH3O / CH2O / CHO / HCOOH / HCOOHC/ COOHT/ CO2.The changeinreactionpathwayfromCOtonon-COisthemain
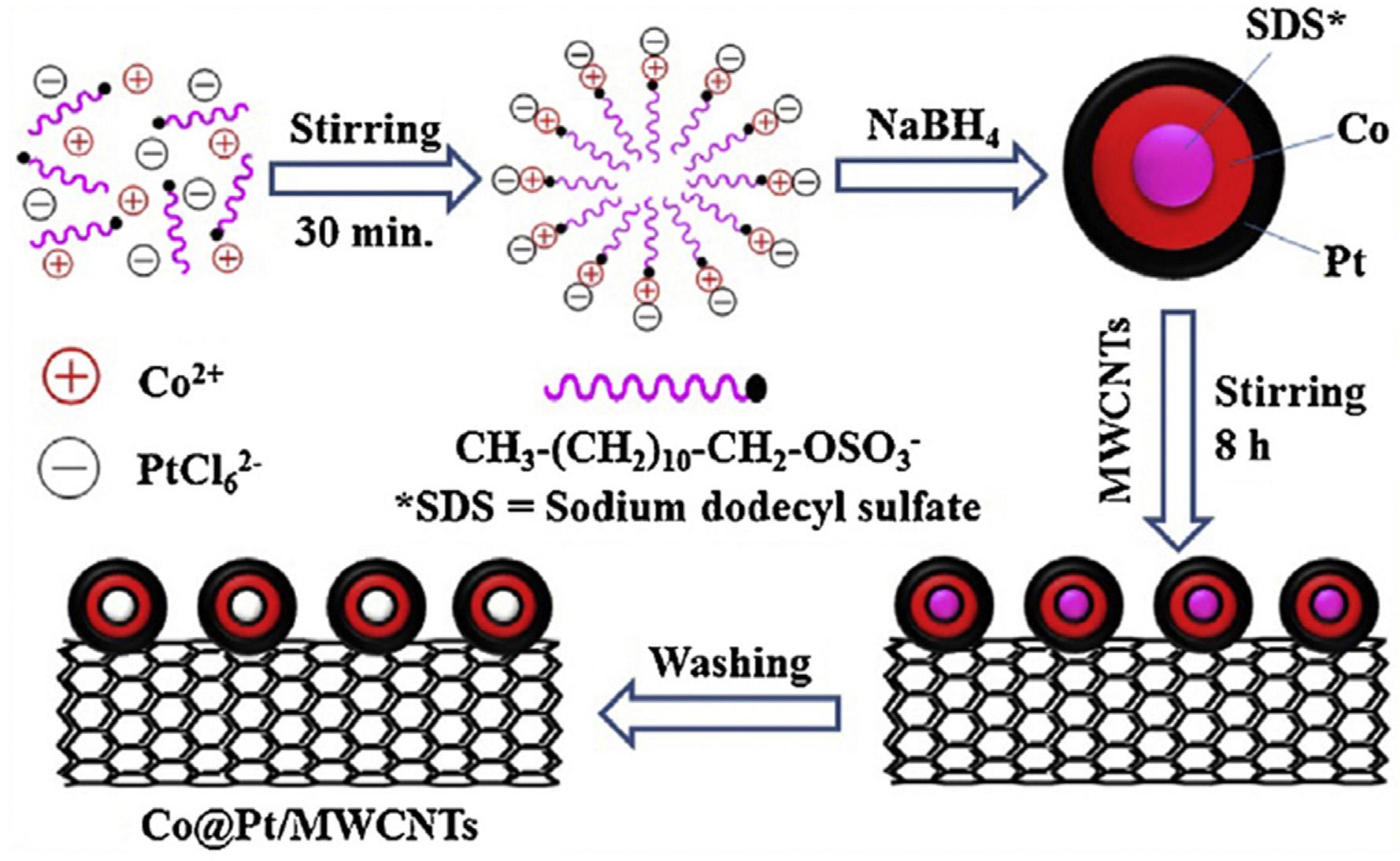
Fig.5 ProposedmechanismofCo@Pt/MWCNTscore shellnanoparticlesformation[92](Reproducedwithpermission, Elsevier,2015).
reasonforbetterelectrocatalyticactivityandhighertolerance towardsCO-poisoningofPt/Aucatalyst.IncaseofPt/Pd(111) catalyst,methanoloxidationoccursthroughenergeticallyand kineticallyfavoredCOpathway;CH3OH / CH2OH / CHOH / CHO / CO / COOH / CO2.Theelectrochemicalactivity ofPt/Pd(111)wasfoundtobegreaterthanthatofPt/Au(111) catalystwhichwasattributedtolowerenergybarrierforrate
determiningsteponPt/Pd(111)catalystandhigheraccessibilityofPtactivesiteswhichenhancesmethanoladsorption andoxidationviaOH-assistedCOremovalusingPt/Pdcatalyst.ButincaseofPt/Au(111)catalyst,theremovalofCO adsorbedonthesurfaceisdifficult[98].HighlydispersedPt/Ru NPswith20and40%metalloadingweresupportedoncationic poly(ethyleneimine)functionalizedMWCNTs(PtRu/PEI-
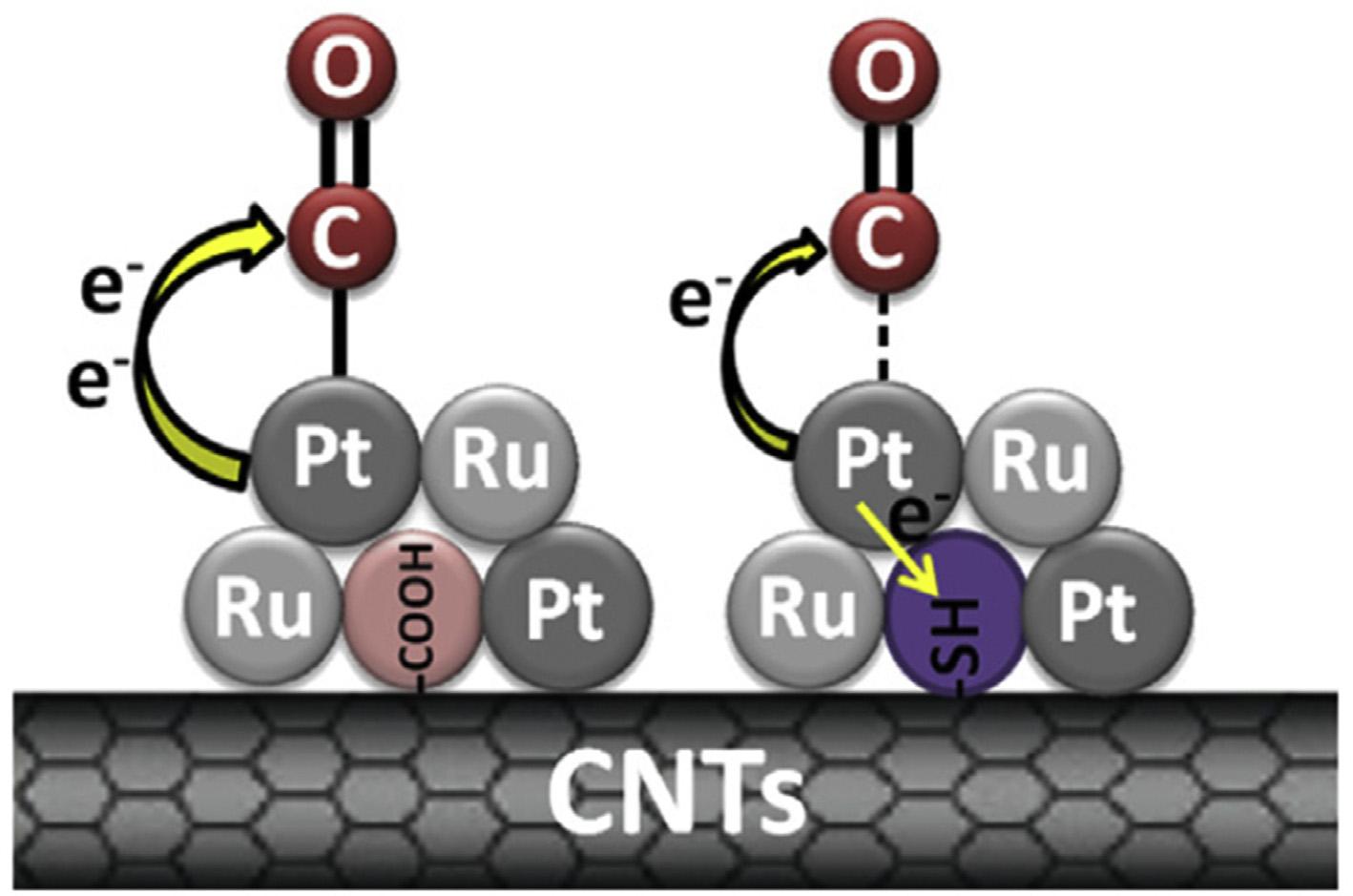
Fig.6 The s p bondofCO Ptisweakenedbyinhibitionofelectrontransferwiththeintroductionof SHtoCNTs[94] (Reproducedwithpermission,Elsevier,2015).
MWCNTS)foruseasanodecatalystinDMFC.ItselectrocatalyticactivitywascomparedwiththatofPt/RuNPssupportedonacidtreatedCNTs(PtRu/AO-MWCNTs).The observedstabilityorderofdifferentcatalystswas:40%PtRu/ PEI-MWCNTs > 20%PtRu/PEI-MWCNTs » 20%PtRu/AOMWCNTs40%PtRu/AO-MWCNTs.ThePtRuNPssupportedon PEI-MWCNTsavoidedaggregationinhighcatalystloadingand providedbettermorphologyanduniformdistributionwith enhancedinterconnectivity,leadingtobetterelectrocatalytic activityandstability[99].Similarly,Chenetal.synthesized highlyefficientPt/RuNPssupportedonpolydopaminefunctionalizedCNTs(PtRu/CNT@Pdop)byfacile,one-potmethod withoutusinganystabilizer,withhighelectrochemicalactivityforMORandsuperiortolerancetoCOpoisoningin comparisontoPtRu/CNTscatalyst.Thehighcatalyticactivity ofPtRu/CNT@PdopmaybeattributedtothepresenceofpolydopaminewhichimpartsenhancedstabilitytoPt/RuNPs andfavorsCOoxidationthanthatonnon-functionalized CNTs[100].Chengetal.investigatedtheeffectofvarying functionalizationofCNTsonthemorphology,dispersionand electrocatalyticactivityofPt/RuNPssupportedonthese differentlyfunctionalizedCNTs.ThePt/RuNPsweresupportedonpoly(diallyldimethylammoniumchloride)(PDDA), polyethylenimine(PEI),1-aminopyrene(1-AP),andtetrahydrofuran(THF)functionalizedMWCNTsandwererepresented asPtRu/PDDA-CNTs,PtRu/PEI-CNTs,PtRu/AP-CNTsandPtRu/ THF-CNTs.Forcomparison,CNTsfunctionalizedbyaconventionalacidoxidationtreatmentwerealsousedassupport (PtRu/AO-CNTs).Itwasobservedthattheparticlesizeand morphologywereaffectedbyfunctionalization(Fig.7).TEM imagesrevealedthatPt/RuNPs(averagesize~4.2nm)form aggregatesandtheirdispersionwasnotuniformonconventionallyfunctionalizedAO-CNTs.ButabetterdispersionofPt/ RuNPswasachievedincaseofPDDA-CNTs,PEI-CNTs,APCNTsandTHF-CNTs.Thecyclicvoltammetry(Fig.8)was achievedinamixturehavingconcentrationof0.5MH2SO4 and1.0MCH3OHforMOR.Theparticlesize,ECSA,current density(jp)andcurrentdensityoffunctionalizedCNTssupportedPt/RuNPsmeasuredatdifferentpotentialsforMORis shownin Table2 [101]:
ThepeakcurrentdensityexhibitedbyPt/RuNPssupported onCNTsfunctionalizedbydifferentpolyelectrolytesi.e. PDDA,PEI,APandTHFwasconsiderablyhigherascompared tothatofconventionallyfunctionalizedPtRu/AO-CNTs.A muchbetterstabilityat0.6and0.4Vafterpolarizationfor5000 and2000s,respectivelywasobservedforPt/RuNPsonPDDA, PEIandAPfunctionalizedCNTs(Fig.9).Fromalltheseresults, theactivityorderofvariouscatalystswasfoundtobe:PtRu/ PEI-CNTs(1)>PtRu/AP-CNTs(2)>PtRu/PDDA-CNTs(3)»PtRu/ THF-CNTs(4)~PtRu/AO-CNTs(5)[101].
NitrogendopingisalsoemployedtomakeCNTschemically activebyamelioratingtheirelectricalconductivity,NPs dispersion,stabilityandactivity.Johanssonetal.usedatomic layerdeposition(ALD)techniqueforthesynthesisofRu decoratedPtNPssupportedonN-dopedCNTs.PtNPssupportedonN-dopedCNTswith1.5 6nmsizewereobtainedby varyingthenumberofALDcyclesandthendecoratedwith variousRuALDcycles(5,10and20).Thecatalystdecorated with5ALDcyclesexhibitedhighestactivitytowardselectrooxidationofmethanolandCO,followedbycatalystwith10
and20ALDRucycles,respectively[102].Recently,Fardetal. carriedoutthesynthesisofN-dopedCNTssupportedPt/Ru NPs.TheconcentrationofNandmetalcatalystinthesamples wasfoundtobe5%and20%,respectively.TheNPspossessed smallsizeandlargersurfaceareaduetomoreactivesitesand stronginteractionsbetweenmetalandsupport[103].Chandranetal.carriedoutthesynthesisofPt/SnNPssupportedon ahybridcarbonhetero-structureof1Dcarbonnanotubesand 2-Dgraphene.Thecatalystdemonstratedenhancedelectrocatalyticactivitytowardsethanoloxidationreactionfordirect ethanolfuelcellandORRforpolymerelectrolytemembrane fuelcellduetohomogenousdispersionofNPsonpartially exfoliatedcarbonnanotubes[104].
CarbonnanofiberssupportedBMNPs. Carbonnanofibers (CNFs)havebeenreportedasexcellentsupportmaterials becauseofgoodcompromisebetweentexturalandstructural properties.CNFshavesignificantlyhigherspecificsurface areaandelectronicconductivity(103 104 Scm 1)comparedto VulcanXC-72Rwhichmakesthembettersupportmaterials [105].VariousBMNPssupportedonCNFshavebeenreported asanodecatalystsforDMFCs[106 111].PdxCoy/CNFscatalyst wasfabricatedbyelectrospinningandthermaltreatment techniques.ThesizeandcompositionofPdxCoy NPswas controlledbyadjustingfeedratioofthemetalprecursors.A linearincreaseinthesizeofPdxCoy alloyNPswasobserved from19.5nmforPd1Co2 to36.2nmforPd2Co1 whichwas attributedtothelargeratomicvolumeofPd(8.9cm3 mol 1) comparedtoCO(6.7cm3 mol 1),andfastcrystallizationof PdxCoy alloywithhighPdcontent.ThecatalystPd1Co1/CNFs exhibited2.9and2.4timeshighermassactivitythancommercialPd/CandPd/CNFs,respectively,andbettertolerance towardspoisoningspeciesforMORinalkalinemedia.The catalystalsoexhibitedhigherstabilityandelectrocatalytic activityforformicacidoxidation[106].Thedirectgrowthof CNFsoncarbon-basedsubstratesreducesthecostofelectrode productionbyeliminatingthediffusiveandcatalyticink conventionalsprayingprocess.Inthisregard,Giorgietal. synthesizedPtAu/p-CNFscatalystbydirectlygrowingp-CNFs ongraphitepaperbyplasmaenhancedchemicalvapour depositionandPt/AuNPsweredepositedonp-CNFsbyelectrodeposition.TheactivityofPtAu/P-CNFswithdifferentAu/ PtatomicratioswasinvestigatedforMOR.Thegreateractivity wasexhibitedbyPtAu/p-CNFscomparedwithPtRu/ VulcanXC-72RwhichmaybeduetohigherutilizationofPt atoms.Moreover,theelectrodepositionlocalizedthecatalyst NPsonlyontheexposedsurfaceofelectrodewithgreat catalystloadreductionwhichledtoincreasedPttolerance towardsCOpoisoningandalsoincreasedthestabilityof catalyst[107].CoxCuy/CNFswereutilizedforoxidationof methanolinalkalinemedia.Theelectrocatalyticactivityof thiscatalystforMORwasinfluencedbyCocontentanditwas foundthatCu5%Co95% alloyNPsshoweddistinctlyhighperformance[108].Inanotherexperiment,acost-effectivecatalystwassynthesizedbydecoratingPd/CealloyNPsonCNFs usingelectrospinningtechniqueforMORinalkalinemedium [109].Chenetal.fabricatedahighlyactivePt/Nibimetallic catalystsupportedonporousCNFs.Inatypicalmethod, polyacrylonitrilewasaddedintoDMFandafterstirringat 80 Cfor1h,nickel(II)acetylacetonate(NiAA)wasadded.A
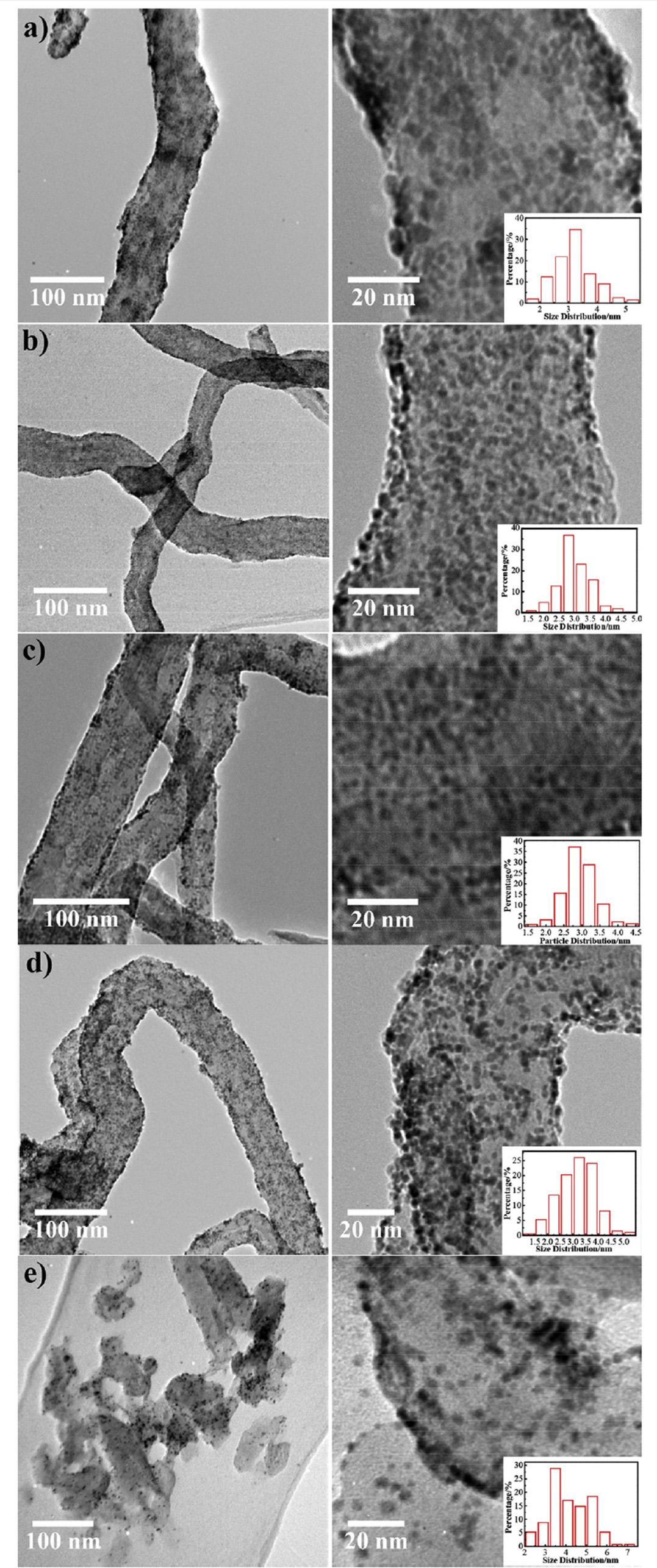
Fig.7 Left(lowmagnification)andright(highmagnification)TEMimagesofnanostructure(a)1,(b)2,(c)3,(d)4and(e)5, respectively(insetisthehistogramsofparticlesizedistribution)[101](Reproducedwithpermission,Elsevier,2015).
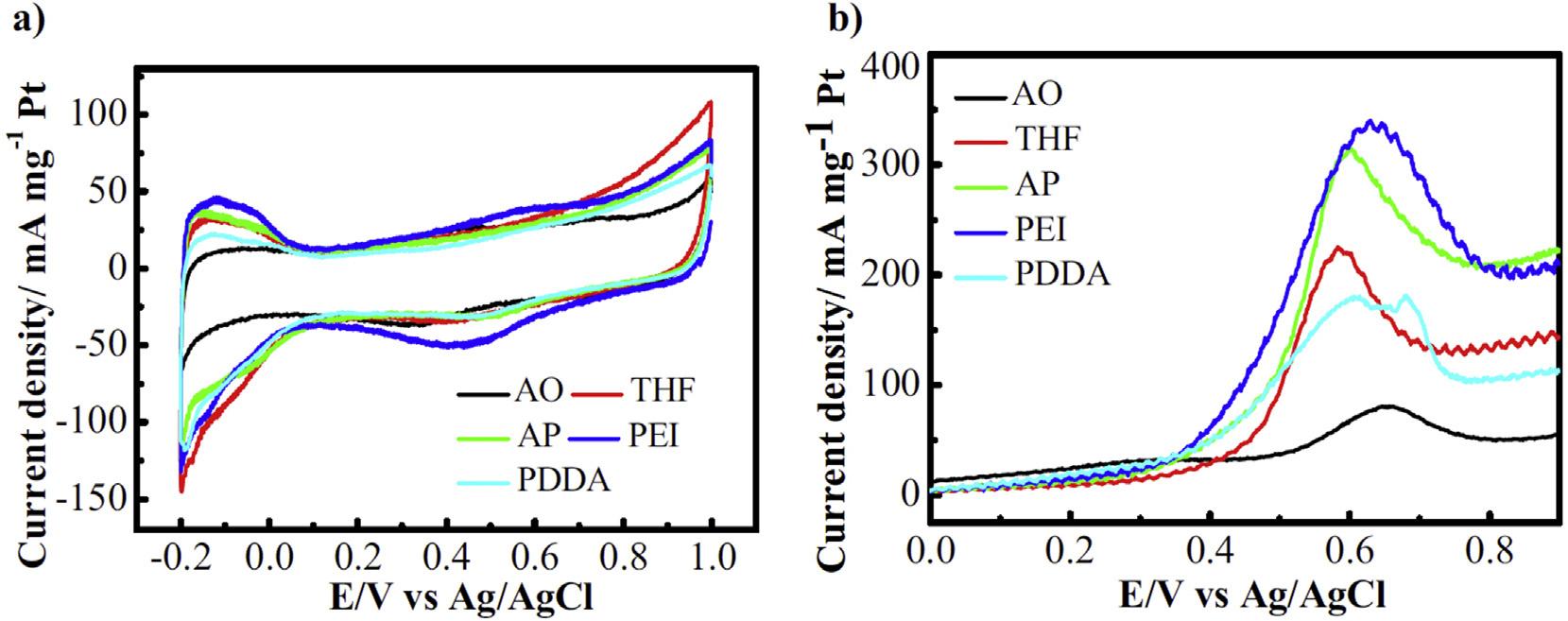
Fig.8 Cyclicvoltammogramsofnanostructure1,2,3,4and5inaN2-saturated0.5MH2SO4þ 1.0MCH3OHsolution, measured(a)from 0.2to1.0Vand(b)from 0.2to0.6V[101](Reproducedwithpermission,Elsevier,2015).
PDDA3.13123.3250440115182214 PEI2.98141.4200635152291375 AP2.94132.5200585121202276 THF3.28126.2250425105158195 AO4.262.35001105275100
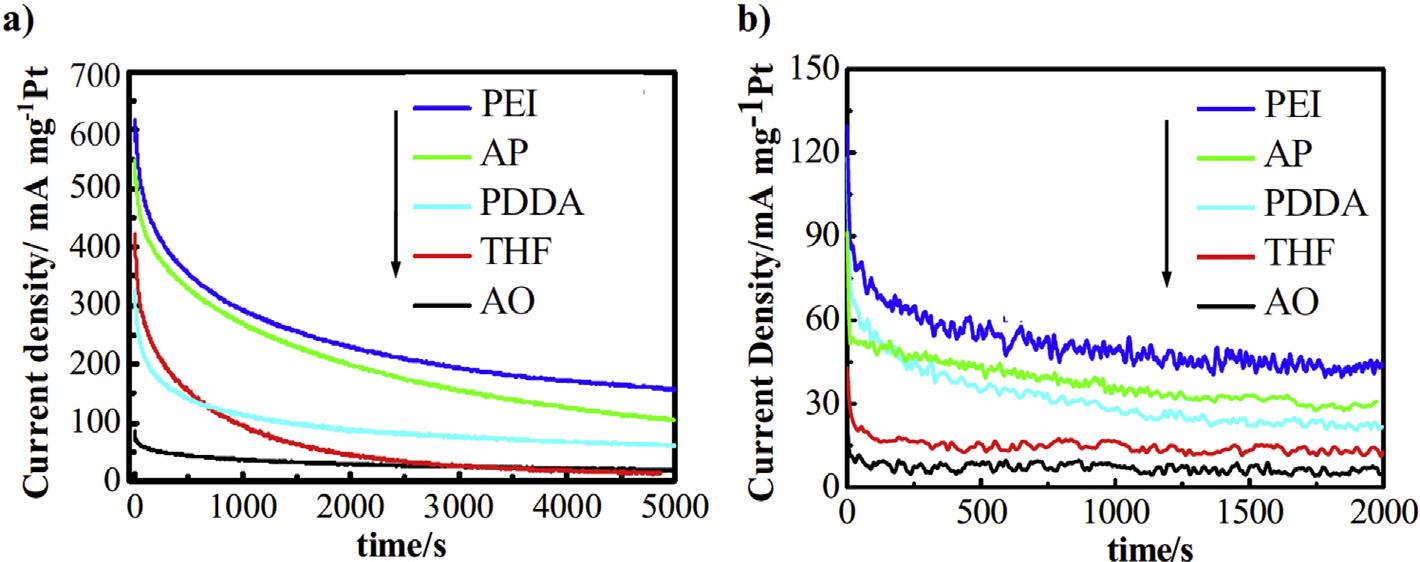
Fig.9 Chronoamperometrycurvesofnanostructure1,2,3,4and5measuredina0.5MH2SO4þ 1.0MCH3OHsolution underapotentialof(a)0.6Vand(b)0.4V[101](Reproducedwithpermission,Elsevier,2015).
homogeneoussolutionwasobtainedafterstirringtheabove mixtureovernight.Theelectrospinningofsolutionformed NiAA/PANwhichwasdriedandstabilizedinair.The carbonizationofprecursornanofibersformedgraphiticCNFs withNiNPsunderN2 atmosphere.Then,PtNPsweredepositedonporousNi/CNFsusingchemicalreductionmethod.The resultsrevealedthatPtandNiNPshavingtypicaldiametersof 3.8 & 17.8nm,correspondingly,werecompletelyreducedand evenlydistributedontheCNFsintheexperimentalprocess.
HigherelectrocatalyticactivitytowardsmethanolandCO oxidationwasshownbyNi50/Pt/CNFs[110].
GraphenesupportedBMNPs. Grapheneisahexagonalnetwork ofsp2 hybridizedcarbonatoms.Ithasahighsurfacearea (~2630m2 g 1)whichisgreaterthanthatofSWCNTs (1315m2 g 1)andgraphite(2 10m2 g 1).Comparedwithother allotropesofcarbon,grapheneprovidesgreaterintrinsiccarriermobility,highchemical,mechanicalandthermalstability
whichmakesitappropriateforelectrochemicalapplications [112 115].Graphene-basedmaterialsareusuallypreparedby reductionofgraphitepowderthatresultsintheformationof grapheneoxide(GO)whichisthenreducedtorGOby employingvariousreductionmethods.Graphene-basedmaterialsarehighlyefficientsupportmaterialsforelectrocatalysts [116 120].Theoxygen-containingfunctionalgroups presentonthesurfaceoredgesofGOmaterialsincreasethe rateofelectrontransfer,providereactivesitesandexcellent dispersioninsolution,andhelpinoxidationofadsorbedCO species.Graphene-basedmaterialssupportedBMNPsareused aselectrocatalystsinDMFCs[121 125].rGOsupportedPd/Au NPswithcore-shellmorphologywereusedfortheelectrocatalyticORRatroomtemperatureinalkalinemedia.The catalystexhibitedsuperioractivityandhighermethanol toleranceORRactivitycomparedwithPd/rGO,Au/rGOand commercialPt/Ccatalysts[126].Ni/CoBMNPssupportedon silanemodifiedgrapheneoxidewereusedascathodecatalyst forORRinmicrobialfuelcell.Fuelcellexhibitedpowerdensity of1003.18mWm 2 withNi Co/MGOcatalyst[127].Ahigh electrocatalyticactivityof1738mAmg 1 Pt thatwas3.7times higherthanthatofcommercialPd/Ccatalyst,wasachieved withPd75Cu25/N-rGOcatalystforelectrooxidationofformic acid[128].PtCu/NrGOcatalystwasusedfortheelectrooxidationofmethanol,ethyleneglycolandglycerolindirect alcoholfuelcellinacidicmedia.Thisultrafinenanostructured catalystwithPtrichsurfaceexhibitedexcellentperformance towardsalcoholoxidationreaction[129].
Awasthietal.usedmicrowave-assistedpolyolreduction methodforthesynthesisofPd/RuBMNPswithaveragesizeof 6nm,supportedongraphenenanosheets(GNS).Theelectrocatalyticactivityofcatalystwasdeterminedin1MKOH þ 1M MeOHsolutionat25 C.ItwasobservedthattheelectrocatalyticactivityexpandedwithincrementinquantityofRu inPt/Rualloy,andthecatalystwith5wt%Ruexhibitedhigher activity.Theelectrocatalyticactivityof40wt%Pd-5wt%Ru/ GNSwas2.6timeshigherthanthatof40wt%Pd/GNSandit alsopossessed72%and625%moretolerancetowardsCO poisoningwhencomparedwith40wt%Pd/GNSand40wt%Pd/ MWCNTs,respectively[130].Reddyandco-workersfabricated PtRu/rGO(1)catalystusingdifferentreducingagentsi.e.PtRu/ rGO-AB(2)andPtRu/rGO-AAbyusingmethylammonia borane(MeAB)andascorbicacid(AA),respectively,as reducingagents.MeABwasalsoutilizedasareductantinthe fabricationofPt/rGO.TheNPspossessednarrowsizedistributionwithaveragediameterof2.8 ± 1.1nmand52.5 ± 3.4nm for(PtRu/rGO-AB)and(PtRu/rGO-AA),respectively(Fig.10). TheactivitiesofsynthesizedPtandPtRu/rGOcatalysts,and commercialPt/CandPt Ru/CcatalystswereobservedbyCV (Fig.11)inamixtureof0.5Msulphuricacidand0.5MMeOH. TheobservedECSAvaluesofcatalystsfollowtheorder:PtRu/ rGO-AB(130.46m2 g 1) >PtRu/C(80.76m2 g 1) > Pt/rGo (63.23m2 g 1) > Pt/C(59.05m2 g 1) >PtRu/rGO-AA(58.0m2 g 1). ThefavorableECSAofPtRu/rGO-ABcatalystwasduetouniformdistributionofextremelysmallsizedPt/RuNPsonthe rGOlayer.PtRu/rGO-ABcatalystexhibitedhighestspecific currentat0.65VandIf/Ib ratiowhichindicatedhigherelectrochemicalactivityofPtRu/rGO-ABtowardsMORandCO oxidation[131].Inordertopreventtheagglomerationof BMNPsduetothermodynamicinstability,varioussurfactants
orcappingagentsareusedfortheirfabrication.Pt@Pd/rGO catalyst,synthesizedbyanoversimplifiedmethodusing caffeineasacappingandstructuredirectingagent,exhibited specificactivityof42.63Am 2 andmassactivity4.6times higherthanthatofPt/rGOcatalyst.ThehighercatalyticactivityisascribedtothesynergeticeffectofPtandPd,unique nanostructureofcatalyst,andhighloadingandgooddispersionofNPsonrGO[132].ItisnecessarytoremovethesurfactantsafterfabricatingNPsastheirimproperremovalmay leadtoreducedcatalyticactivityofNPs.Sincetherearemany problemsassociatedwithsurfactant-removaltechniques,the researchhasbeenfocusedonthesurfactant-freemethodsto fabricateBMNPs.ThegrowthrateofNPscanbecontrolledby thehalideligandreplacementstrategytopreventtheir agglomeration.Reddyetal.utilizedthisstrategytofabricate Pt/PdBMNPsonrGOsupportbyusingascorbicacidasa reducingagentforreducinggrapheneoxideandmetalprecursorssimultaneously.Halideionswereaddedtocontrolthe reductionofmetalprecursors.UniformlydispersedPt/PdNPs ofabout6nmwereformedusing5mgKI,andtheparticlesize wasfurtherreducedtoabout3 4nmbyincreasingthe amountofKIto15mgcomparedtotheagglomeratedNPsof about50nmformedintheabsenceofhalideions.Pt Pd/rGO15KIcatalystexhibitedIf/Ib ratioof1.709andtwotimeshigher massactivitycomparedtocommercialPt/Cduetoitshigher ECSA(81.2m2 g 1),uniformdispersionofNPs,andimproved electrontransportbygraphene[133].
ThecatalyticactivityofNPsisalsoinfluencedbytheir shapes.Toachieveefficientelectrocatalyticactivity,BMNPsof variousshapessuchasnanoflowers[134,135],cube-shaped [136],star-shaped[137]etc.havebeensynthesized.Chen etal.synthesized3-DPd@Ptcore-shellnanoflowershaving typicalsizeof16.4nmwhichwereinterconnectedintheform ofnanorodsandcreateda3-Dnano-porousstructuresupportedongraphenenanosheets.Theelectrochemicalstudies ofthecatalystwereperformedinasolutioncontaining1M KOHand1MMeOH.Themassactivityofdifferentcatalysts suchasPt/GandPt/Cwascomparedbynormalizingtothe stackingamountofPt.Themaximummassactivitywas exhibitedbyPd@Pt/Gwithapeakcurrentof9.23Amg 1 Pt that was8.9and14.2timeshigherthanthatofPt/G(1.04Amg 1Pt) andcommercialPt/C(0.6Amg 1Pt)catalysts,respectively.The maximumelectrocatalyticactivityofPd@Ptnanoflowers catalystwasaccreditedtotheoutstandingmixingandhomogeneousness,supportedongraphenesheetshavinglarge surfacearea[135].One-potandsurfactantfreemethodwas usedtodispersePd/Ptnanocubeswithaveragesize8nmon graphenenanosheetssupport.DMFwasusedasabifunctional solventforreducinggrapheneoxideaswellasthemetal precursors.ThecatalystexhibitedhigherECSA(45.1m2 g 1), currentdensity(0.48Amg 1)andIf/Ib ratiothanthatofPdPt/C [136].Star-likePt/CuNPsonrGOsupportweresynthesized whichshowed2.3timesmoredurabilitythanPt/rGOcatalyst [137].Aporousdandelion-likeAu@Pdcore-shellnanostructuresupportedonrGOwasfabricatedviaone-potwet chemicalreductionmethod.Cetyltrimethylammoniumchloride(CTAC)andiodideionswereusedasshapedirecting agents.TheCTACandI selectivelybindtotheAu(111)facets tofacilitatethecrystalgrowthalong(111)directions.I actsasa retardertoslowdownthereductionkinetics,resultinginthe
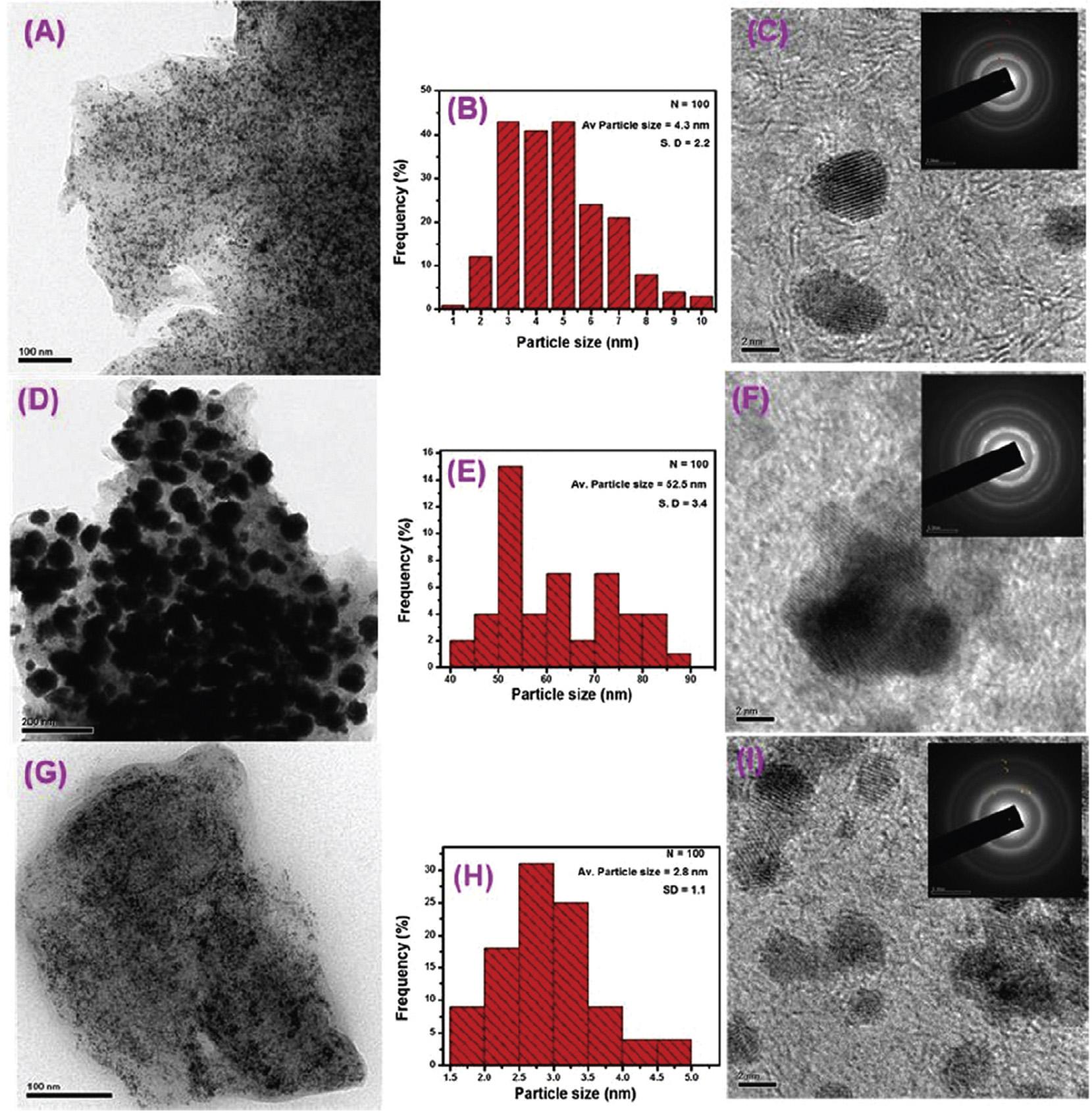
Fig.10 TEMimages(A,DandG),Particlesizehistograms(B,EandH)andHR-TEMimagesofPt/RGO,Pt Ru/RGO-AA,and Pt Ru/RGO-AB,(insetcorrespondingSAEDpattern)respectively.[131](Reproducedwithpermission,TheRoyalSocietyof Chemistry,2016).
formationofdandelion-likecore-shellAu@Pdnanostructure welldispersedonrGO.ThespecificactivityofAu@Pd/rGOwas 1.65timeshigherthanthatofPd/Cand2.46timeshigherthan thatofPd/rGO.Similarly,themassactivityandstabilityofthe catalystwasalsohighincomparisontoPd/CandPd/rGO catalysts[138].Pd@Pt/GNss(PDDA)electrocatalystexhibited highermassactivity(870.8mAmg 1),higherspecificsurface area,andabetteranti-poisoningabilityformethanolelectrooxidationcomparedtocommercialPt/CandalloyedPdPt/ GNs(PDDA)catalysts[139].Someofthesyntheticmethods utilizehazardousreducingagents,templateorsurfactant, cappingagentsetc.whichrequiremultistepreactionsand complicatethesynthesis.Thus,itisimperativetoemploya facileandlesstime-consumingmethodtosimplifythesynthesis.Inthisregard,afacileandrapidhydrothermal-assisted chemicalreductionwithformicacidwasemployedtofabricatePt/PdalloyNPssupportedonrGO,avoidingtheuseofany surfactant,cappingagentorhazardousreducingagent.The
synthesizedcatalystwashighlyactivetowardsmethanol oxidation[140].Therestackingofgraphenelayersdueto p-p interactionsandVanderWaalsforcesresultsintoaggregation andlimitsitsapplications.Verticallyorientedgraphene(VG)is abettercatalyticsupportbecauseofitsinimitableorientation, non-stackingstructureandhighsurfacetovolumeratio whichprovidebetternucleationanddispersionofNPs.A repeatedpulsepotentialapproachwasusedfortheelectrodepositionofPt/RuNPsonverticallyorientedgraphenecoated carbonpaper(VG-CP).Forthispurpose,VGwasgrowndirectly ontheCP(9mm 8mm,TorayInc)usingahome-made microwave-PECVDdevice,followedbyco-electrodeposition ofPt/RuNPswithdifferentcompositions.Highercatalyst loading,smallercatalystsizeandincreasedNPsdispersion wasobtainedusingVGcoatedCPwhichresultedintobetter electrocatalyticactivityforMORascomparedtoPtRu/CP, PtRu/CandwasalsomoreactivethanPtRu/CandPtRu/CNTs withsmallerNPsize[141].
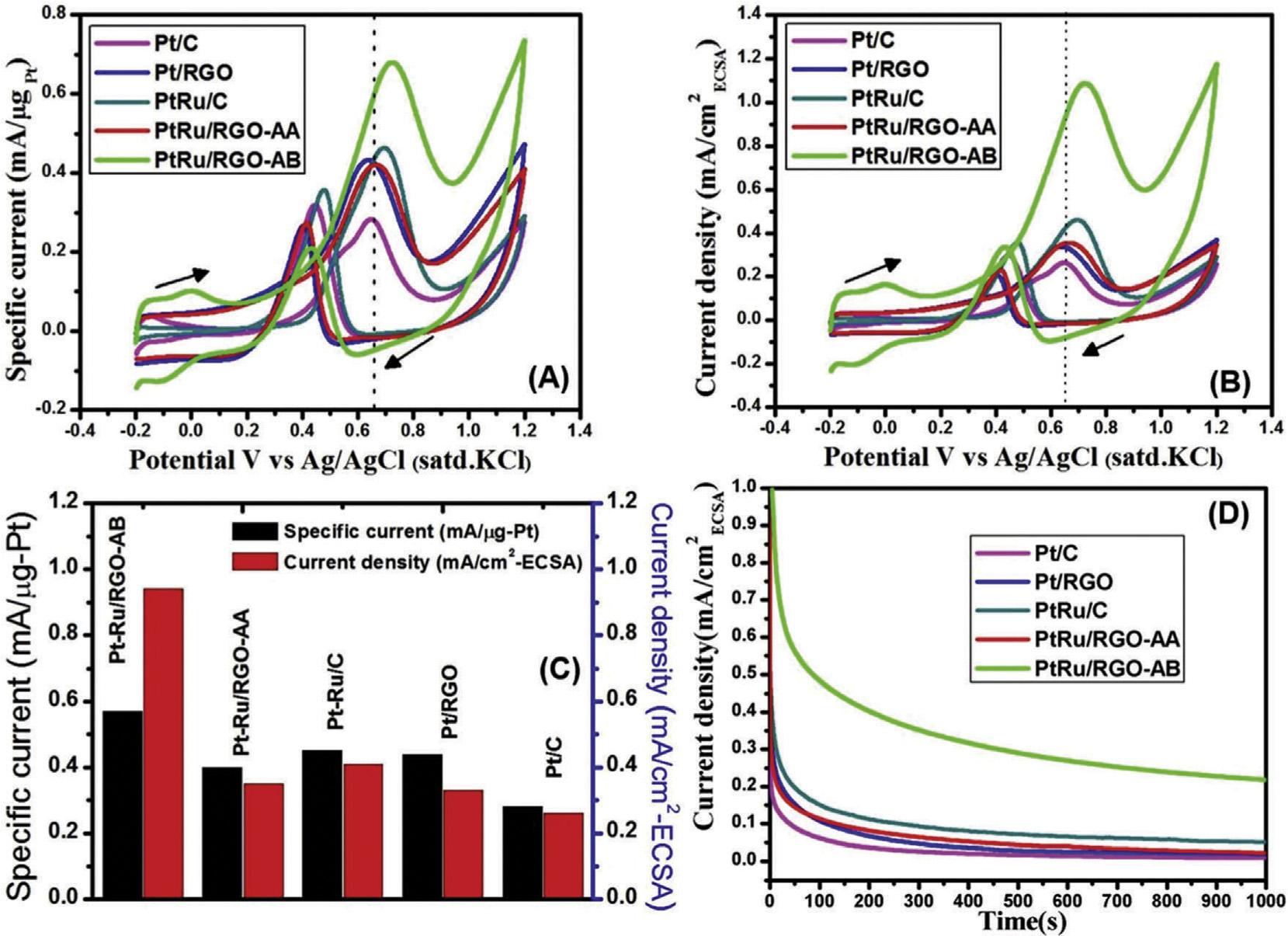
Fig.11 Cyclicvoltammogramsformethanoloxidation(A)Pt-massnormalizedcurrent,(B)ESCAnormalizedcurrentonPt/ RGO,PtRu/RGO,(C)comparisonofthespecificcurrentandESCA-normalizedcurrentand(D)Chronoamperometriccurveson Pt/RGO,PtRu/RGO,andcommercialPt/CandPtRu/Celectrocatalystsataconstantpotentialof0.65Vfor1000s[131] (Reproducedwithpermission,TheRoyalSocietyofChemistry,2016).
Thefunctionalizationofgrapheneviacovalentandnoncovalentmethodscandevelopvariousfunctionalgroups whichimproveitschemicalstabilityanddispersibility,and inducesdesirableinteractionswithothermolecules.The dopingofgraphenewithheteroatomssuchasN,BandSalso improvesitsproperties[142 145].Functionalizedanddoped grapheneandgraphene-basedmaterialsareefficientsupportmaterialsforelectrocatalysts.Pt/PdNPsweresupported oncarboxylicsodiumfunctionalizedgraphenebyin-situ electrochemicalmethod.ThefunctionalizedgrapheneprovidedmorebindingsitesforPt/PdNPs.CVwascarriedoutin 1MH2SO4 þ 1MMeOHatascanrateof50mVs 1.The catalystcoatedelectrodeexhibitedhigherpeakcurrent density(15.7mAcm 2)thannon-functionalizedgraphene supportedPt/PdNPs(3.91mAcm 2).Theresultsshowedthat carboxylicsodiumgroupwaspresentuniformlyandwell orderlyonthesurfaceofgraphene.Thesegroupsprovided bindingsitestoPt/PdNPsandincreasedtheelectrocatalytic activityandstabilityofthesynthesizedcatalyst[146]. LCysteinefunctionalizedrGOwasusedasasupportfor Ag@AuNPs.ECSAvalue(243m2 g 1)showedthatAg@Au/cis/ rGOelectrocatalysthasagreaternumberofavailableelectrochemicalsitesthanAg/cis/rGO(182m2 g 1)andAu/cis/ rGO(195m2 g 1)catalysts,leadingtoitshighelectrocatalytic activity[147].Kuyuldaretal.fabricatedPt/RuNPsonthiourea functionalizedGOhavingaverageparticlesizeof 3.86 ± 0.59nm.Thefunctionalizationbythioureaimproves theproperties,solubilityandprocessingofGOinwateras
wellasinorganicsolvents.Thesynthesizedcatalystshowed excellentMORactivityinbasicmediawhichwasmaintained upto97.3%evenafter1000cycles[148].
N-dopingimprovestheperformanceofgraphenefor methanoloxidationowingtoconjugationbetweenelectron pairofnitrogenandgraphenethatcreatesdisruptioningraphenestructure.AbetterdispersionofNPsandmoreactive surfaceareaisachievedwithN-dopedgrapheneasasupport material.Byusingsolvothermalmethod,KiyaniandcoworkerssynthesizedN-dopedgraphenesupportedPd/Co BMNPsforDMFC.TheparticlesizeofmetalNPswas10nm. ThecatalystPdCo/N-Gexhibitedhigherpeakcurrentdensity (21.33mAcm 2)andimprovedECSA(44.2m2 g 1)contrasted withPd/N-GwhichindicatedbettercatalyticactivityofPdCo/ N-G[149].rGOwasfunctionalizedwith2-thiobenzimidazole (TBI)andusedasasupportforPt/PdNPs.ThecatalystdisplayedECSAvaluewhichwas2.06and1.78timesgreaterthan thoseofPd/TRI-rGOandPt/TBI-rGO,respectively,andhigh valueofcurrentpeakdensity.Thecatalystpossessedhigh catalyticactivityforMORandimprovedCOtolerancedueto thepresenceofsmallsizedandhighlydispersedPt/PdNPson modifiedrGOsurface[150].Ahighlyefficientelectrocatalyst withhighcurrentdensity(35.13mAcm 2)andECSAvalue (447.72m2 g 1)wasfabricatedbydepositingPt/PdNPson sulfonatednitrogensulfurco-dopedgraphenenanocomposite (PtPd/S-NS-GN)byelectrochemicalmethod[151].B,Ncodopedgraphene(BNG)wasusedassupportforanchoring Ni/PtBMNPs.Boricacidandureawereannealedat800 Cinan
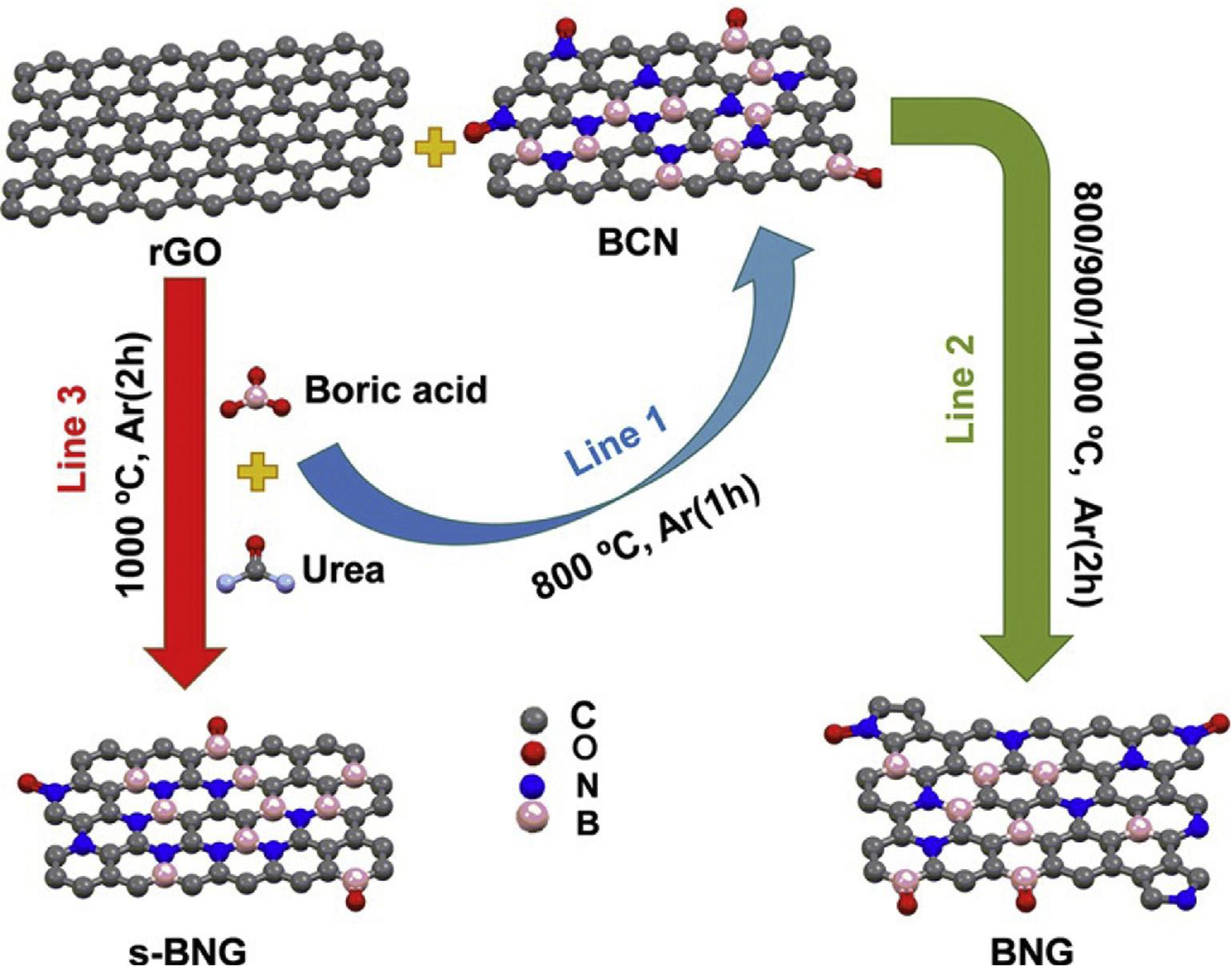
Fig.12 SimplifieddiagramforsynthesisofBCN,BNG,ands-BNG [152] (Reproducedwithpermission,JohnWileyandSons, 2016).
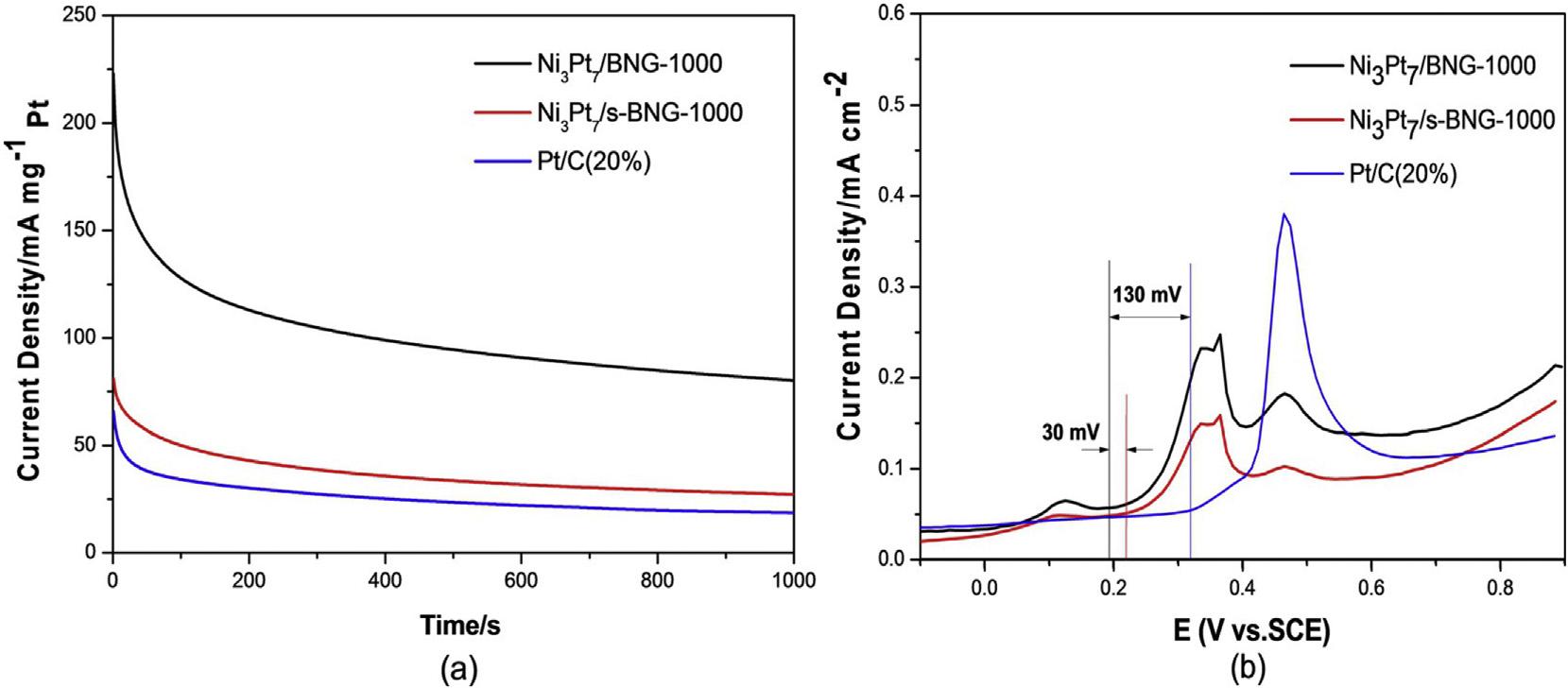
Fig.13 a)Cyclicvoltammetrycurvesin(A)0.5MH2SO4 (B)0.5MH2SO4þ0.5MCH3OHforNi3Pt7/BNG-1000,Ni3Pt7/s-BNG1000,andcommercialPt/C[152](Reproducedwithpermission,JohnWileyandSons,2016).
AratmosphereforthesynthesisofBCN.BNGwaspreparedby annealingboron-carbon-nitride(BCN)andrGOattemperatures800,900and1000 CinanAratmospheretogiveBNG800,BNG-900andBNG-1000,respectively.Thesynthesis schemeofcatalystisshownbelow(Fig.12).
BNG-1000exhibitedmaximumboronandpyridinicN content,andlowestcovalentBNratioandthus,itwasusedfor anchoringofNi/PtNPswithdifferentcompositions.Ni3Pt7/
BNG-1000exhibitedECSAvalueandcurrentdensityof 176.8m2 g 1 and359.8mAcm 2,respectivelywhichwere higherthanthoseofPt/CandNi3Pt7/s-BNG-1000,indicatingits higheractivityforMORinacidicmedia(Fig.13).Thecurrent densityofcatalystsdecreasedwithtimeduetoadsorptionof COandotherintermediatespeciesformed(Fig.14 a).However,Ni3Pt7/BNG-1000catalystshowedhigheractivitythan Ni3Pt7/s-BNG-1000andcommercialPt/Ccatalysts.Anegative
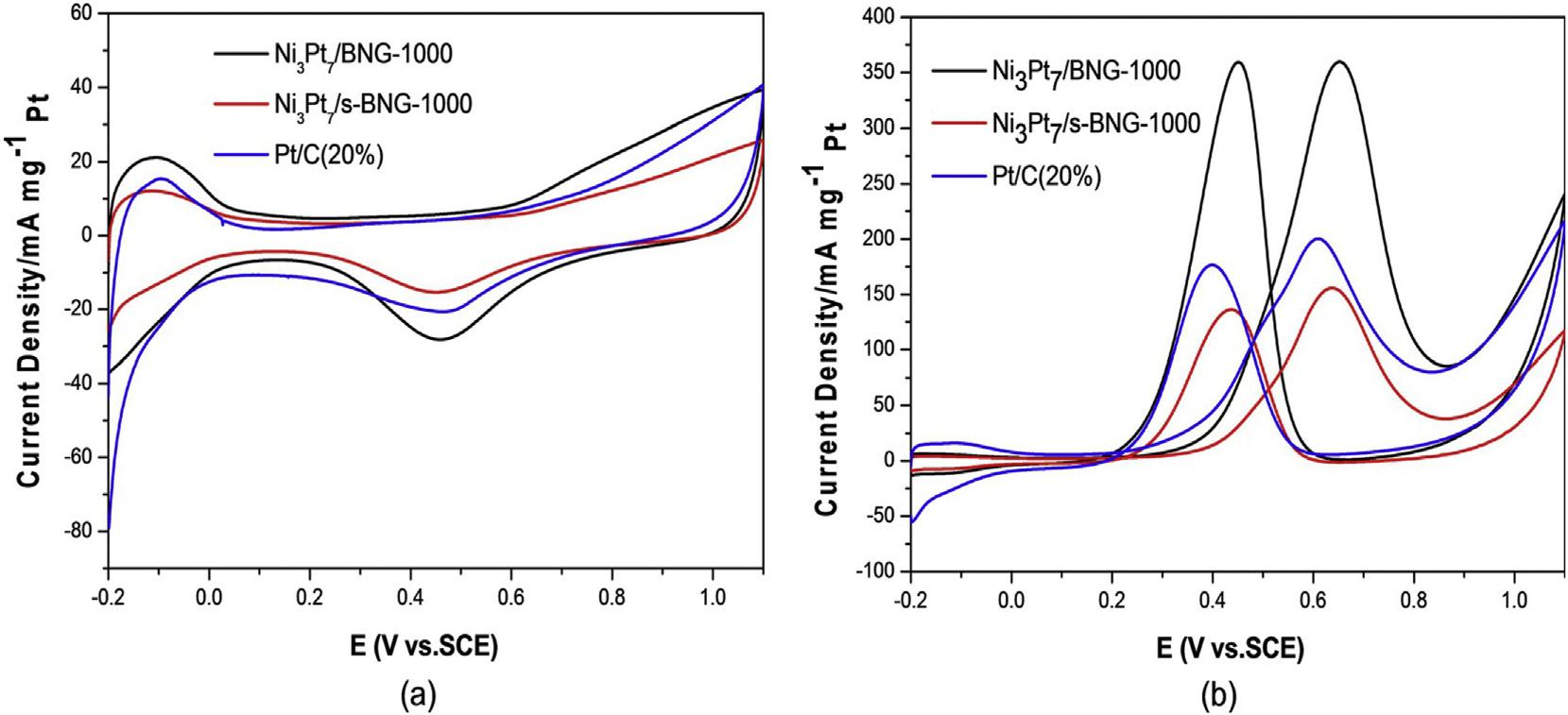
Fig.14 (A)Chronoamperometriccurvesin0.5MH2SO4þ0.5MCH3OHsolutionand(B)COstrippingcurvesin0.5MHClO4 solutionforNi3Pt7/BNG-1000,Ni3Pt7/s-BNG-1000,andcommercialPt/Ccatalysts[152](Reproducedwithpermission,John WileyandSons,2016).
shiftinonsetpotentialforCOoxidationwasnoticedforthe catalystinCOstrippingvoltammetrystudyaccomplishedin 0.5MHClO4 whichindicatedthefacileoxidationofCO adsorbedonNi3Pt7/BNG-1000catalyst(Fig.14 b)[152].
Inordertopreventtherestackingofgraphenesheetsand toextenditsuniquepropertiesintothree-dimensions(3D), greateffortshavebeendevotedtothepreparationof3Dgrapheneskeletons[153,154].Inthisregard,Kungandco-workers reportedthedevelopmentofPt/RuNPsanchoredonto3D graphenefoam(GF).Physicochemicalcharacterizationofthe catalystrevealedamacroporousstructureof3DGFwithpore diameterof50 200 mmandPt/Ruparticlesizeof3.51nm indicatinghighsurfaceareaperunitvolume.Thecatalyst exhibitedimprovedMORactivityandanti-poisoningability owingtoenhancedactivesurfacearea,uniformdistributionof NPs,andincreasedactivetransportofreactantsprovidedby 3Dporousgraphene[154].TheexamplesofsomeanodecatalystsforDMFCsaregivenbelowin Table3
Othercarbonaceoussupportmaterials. Mesoporouscarbon,3Dporouscarbonfoam,carbonxerogels,carbonnanosheets, fullerene-C60 etc.havealsobeenemployedassupportfor BMNPs.Hungetal.synthesizedPtbasedbifunctionalmetal (PtM;M Ru,FeandMo)alloycatalystsdispersedonordered mesoporouscarbon(OMC).Thecatalystsweresynthesized viaadirectreplicationmethodbymixingknownamountsof metalprecursorsinfurfurylalcoholandtrimethylbenzene carbonsourcesundersonication.Polymerizationofcarbon precursorswasinitiatedbyaddingoxalicacidintothesolution.SBA-15wasimpregnatedbythismixedsolution,followedbypolymerization.TheetchingofresultingsiliconcarboncompositebyaqueoussolutionofHFremovedsilicontemplate,andthenwashinganddryingofthematerials producedPtM-OMCcatalysts.Thehighcatalyticactivityand exceptionaltolerancetoCOpoisoningduringMORas comparedtoPtRu/VulcanXC-72catalystwasobserveddueto highlydispersedNPswithaveragesize2 3nm,withinthe
porechannelsofOMC[187].Defectivemesoporouscarbons arealsoeffectivesupportmaterialsastheelectronicstructureofcatalystsisregulatedbydefectswhichleadto improvedcatalyticperformance.Veryrecently,Liandcoworkersuseddefectivemesoporouscarbon(CMK-3-D)supportforPd/NiNPs.Theresultsindicatedthatthedefects createseveralactivesitesforPd/NialloyunderPdloadingof 10wt%,resultingintoenhancedelectrocatalyticperformanceofPdNi/CMK-3-Dcatalyst[19].Pt/RuNPssupported onN-dopedcarbonnanohornswithNPsize1.9nmexhibited 2.5timeshighermassactivitythancommercialPtRu/C-JM catalyst,and1.8timeshigherMORspecificactivitythan PtRu/Vulcancatalyst[188].Khanetal.usedhighlyporous carbonobtainedbyheattreatmentofMOF-5asasupport materialfor30wt%Pt/CuBMNPsand20wt%PtNPs.The highlydispersedPt/CuNPsexhibitedhighcatalyticactivity towardsMORandORR[189].HeteroatomdopedporouscarboncanbesynthesizedbyusingMOFandMOFbasedcomposites.Lvandco-workersrevealedthefabricationofPt/Cu alloyNPsonN-dopedcarbonnanosheetsbypyrolysisof grapheneoxide/ZIF-67/H2PtCl6 atdifferenttemperatures. ThespectroscopicstudiesrevealedthatNoriginatedfrom theorganiclinkerofZIF-67andwasdopeduniformlyintothe carbonnanosheetsprovidingdenselydistributedPt/CoNPs. Thestructure,morphologyandperformanceofNPswasalso affectedbypyrolysistemperature.ThesmallersizedNPs wereproducedat800 Candthecatalystexhibitedspecific activity(1.25mAcm 2)2.6timeshigherthanthatofPt/C commercialcatalystinacidicmedia[190].Porouscarbons (PCs)withhoneycomb-likestructuresweresynthesizedby facileself-assemblyrouteusingtetraethylorthosilicateand phenolicresinsassourceofsiliconandcarbon,respectively andusedtosupportPt/PdbimetallicalloyNPswhichwere employedasanodecatalystsforMORinalkalinemedia. Pt3Pd1/PCswithhigherpeakcurrentdensityandloweronset potentialwasmoreactivethanotherPt Pd/PCs,Pt/C,Pd/PCs andPt/PCscatalystsbecauseofitshighelectrochemically
Table3 ComparisonofmethanoloxidationactivityofsomeBMNPsonvarioussupportmaterials.
S. No. ElectrocatalystElectrolytePeakcurrentfromCVcurvesECSA(m2g 1)Scanrate(mVs 1)Ref.
1.PtNi/ERPGO0.5MH2SO4 þ 0.5MCH3OH4.65mAcm 2 vsPt/ERPGO(2.1mAcm 2)263.22vsPt/ERPGO(151.8)50[155]
2.Pt2Ag1/rGO0.1MHClO4 þ 1.0MCH3OH2.82mAcm 2 vsPt/C(0.61mAcm 2)74.97vsPt/C(20.30)50[156]
3.H PtPd(3:1)/G0.5MH2SO4 þ 1.0MCH3OH198.0mAmg 1 Pt vsH PtPd(2:1)/G(76.7mAmg 1Pt)andPt/C (125.0mAmg 1Pt) 82.81vsH PtPd(2:1)/G(69.99) andPt/C(20.17)
4.PtPd(1:1)/GN0.1MH2SO4 þ 0.5MCH3OH265mAmg 1 Pt vsPtPd(2:1)/GN(75mAmg 1Pt)46.11vsPtPd(2:1)/GN(69.99) andPt/C(20.17)
50[157]
100[158]
5.PtRu@CS0.5MH2SO4 þ 1.0MCH3OH56.3mAmg 1 Pt vsPtRu/VC(39.6mAmg 1Pt)86.0vsPtRu/VC(67.0)10[159]
6.Pd80Ag20/C1.0MKOH þ 1.0MCH3OH691.6mAmg 1 Pt vsPd65Ag35/C(629.6mAmg 1Pt)andPt/C (689.3mAmg 1Pt)
50[160]
7.AuPt/TC0.5MH2SO4 þ 0.5MCH3OH0.68mAcm 2,2.5timeshigherthanPt/C13.7vsPt/C(61.1)100[161]
8.PtCu/C0.5MH2SO4 þ0.1MCH3OH1338.2mAmg 1 Pt vsPtCu(1137.12mAmg 1Pt)14.8vsPtCu(7.042)50[162]
9.PtRu/C-MWCNTs0.5MH2SO4 þ 0.5MCH3OH1236.0mAmg 1 Pt vsPt/C(214.2mAmg 1Pt)133.2vsPt/C(55.6)50[163]
10.PtNi(1:1)/MWCNTs0.5MH2SO4 þ 1.0MCH3OH390.0mAmg 1 Pt vsPt/C(198.0mAmg 1Pt)36.65vsPt/C(18.71)50[164]
11.PtNi(3:1)/FCNTs0.5MH2SO4 þ 1.0MCH3OH730.3mAmg 1 Pt vsPt/FCNTs(292.9mAmg 1Pt)70.7vsPt/FCNTs(61.3)100[165]
12.PtNi/CNTs0.5MH2SO4 þ 1.0MCH3OH84.18mAmg 1 Pt vsPtNi/C(64.3mAmg 1Pt)29.1,higherthanPtNi/C100[166]
13.PtNi(0.1:5.7)/C TiO2 1.0MNaOH þ 0.5MCH3OH123.0mAcm 2 50[167]
14.PdRu-4/CNTs1.0MNaOH þ 1.0MCH3OH3.12mAcm 2 vsPd/C(1.22mAcm 2)48.1vsPd/C(51.7)50[168]
15.Pd7Rh2/rGO1.0MNaOH þ 1.0MCH3OH32.1mAcm 2 vsPd/GO(23.2mAcm 2)
16.PdCu(1:1)/rGO1.0MKOH þ 1.0MCH3OH1153.4mAmg 1 Pt vsPd/rGO(385.5mAmg 1Pt)
30[169]
50[170]
17.Pd2Cu2/rGo1.0MKOH þ 1.0MCH3OH916mAmg 1 Pt vsPd1Cu3/rGO(560mAmg 1Pt)2.49timeshigherthanPd/C50[171]
18.Pt1Pd3/G1.0MNaOH þ 0.5MCH3OH0.464mAcm 2 vsPtNPs/G(o.196mAcm 2)23.34vsPtNPs/G(21.93)100[172]
19.PtCo(1:9)/rGO1.0MH2SO4 þ 2.0MCH3OH38.02mAmg 1 Pt vsPt/rGO(3.81mAmg 1Pt)320.3vsPt/rGO(9.436)20[173]
20.Pt3Ni C/rGO0.1MHClO4 þ 1.0MCH3OH360.4mAmg 1 Pt vsPt3Ni/rGO(210.7mAmg 1Pt)52.7vsPt3Ni/rGO(42.8)50[174]
21.AuPd@PdNPs/N-rGOH0.5MKOH þ 1.0MCH3OH11.5mAcm 2 vsPd/C(1.9mAcm 2)53.8vsPd/C(26.3)50[175]
22.Fe@Au/GO0.1MHClO4 þ 0.5MCH3OH8.75 ± 2mAcm 2 vsAu/GO(1.12 ± 0.5mAcm 2) 100[176]
23.PtAu/poly(CP-co-Bz)-f-GO0.5MKOH þ 0.5MCH3OH22.05mAcm 2 vsPt/poly(CP-co-Bz)-f-GO(14.18mAcm 2)223.63vsPt/poly(CP-co-Bz)-f-GO (210.47) 50[177]
24.PtRu/TiO2 CNF0.5MH2SO4 þ 2.0MCH3OH603.06mAmg 1 vsPtRu/C(75.8mAmg 1) 20[178]
25.PtRu/TCCNF0.5MH2SO4 þ 0.5MCH3OH72.2mAmg 1 vsPtRu/CNF(27.9mAmg 1)60.1vsPtRu/CNF(25.9)20[179]
26.PtRu/TECNF0.5MH2SO4 þ 0.5MCH3OH143.0mAmg 1 PtRu vsPtRu/C(75.8mAmg 1)105.5vsPtRu/C(90.7)20[180]
27.Pt1Cu2.81/CeO2/CNTs0.5MH2SO4 þ 1.0MCH3OH2.03mAcm 2,1.7timeshigherthanPt/C66.38vsPt/C(30.95)50[181]
28.PtRu/CNC0.5MH2SO4 þ 1.0MCH3OH79.79mAcm 2 vsPtRu/Vulcan(14.66mAcm 2) 50[182]
29.PtCu3/C0.5MH2SO4 þ 1.0MCH3OH2.44mAcm 2 vsPt/C(0.56mAcm 2)28.1vsPt/C(61.5)10[183]
30.Pt2Ir/MWCNT0.1MHClO4 þ 0.5MCH3OH9.72mAcm 2 vsPt/MWCNT(6.97mAcm 2)85.3vsPt/MWCNT(60.4)100[184]
31.Cu@Pt/C0.5MH2SO4 þ 0.5MCH3OH5.86mAcm 2 vsPt/C(3.32mAcm 2)74.3vsPt/C(50.4)50[185]
32.PtCo/C0.1MHClO4 þ 1.0MCH3OH2110.0mAmg 1 Pt vsPt/C(382.0mAmg 1Pt)54.5vsPt/C(74.3)50[186]
activesurfacearea[191].3-Dporouscarbonfoamshaving extremelyinterconnectedopen-cellstructuresaremost preferredascatalystsupportsinDMFCsbecausethelarger surfaceareaoffoamsprovidesabetterdispersionofNPsand theporesassistindiffusionofelectrolyteandtransferof electron.Butthepenetrationofelectrolytesintoinnerlayer ofcarbonfoamsisdifficultduetotheirinertsurfaces.Heteroatomsareintroducedinthesurfaceofcarbonfoamsfor increasingthewettabilityandutilizationefficiencyofthe surface.Yietal.fabricatedPt/RualloyNPsdispersedonnitrogenandoxygenco-dopedporouscarbonaceousfoam.Pt/ RualloyNPsweresupportedoncarbohighinternalphase emulsion(carboHIPE-c)andthecatalystexhibitedhigher currentdensity,goodstabilityandresistancetoCO poisoningduetosynergisticeffectbetweencarboHIPE-cand metalNPs[192].Bhavanietal.reportedthesynthesisofPd/W NPsonfullerene-C60 thinfilm.ThecatalystexhibitedECSA 96.56m2g 1,highIf/Ib ratioandmaximumcurrentdensityin comparisontoWNPs@fullereneC-60,Pt/CandPdNPs@fullereneC-60 owingtoexcellentmechanical/thermalproperties,extended-conjugationbetweencarbonatomsand higheractivesurfaceareaoffullerene-C60 whichenhanced theelectrochemicalperformance[193].MetalNPscanalsobe loadedorencapsulatedonhollowcarbonspheres(HCS)that possessporousshellandlargecavity,highlyexposedcatalyticactivesites,largesurfaceareaandhighelectronic conductivity,whichmakethempropitioussupportforfuel cellcatalysts.Pt/NinanocrystalssupportedonHCS(PtNi NCs-PVP@HCS)werefabricatedbyone-stepsolvothermal synthesis.Thesurfacecleaningofcatalystwasdoneby annealingat300 C,followedbyelectrochemicalCOstrippingmethodtoproducePt/NiNCs@HCS(COS)which improvedtheelectrocatalyticactivityofthecatalystby removingPVPcappedonthesurfaceofPt/Ninanocrystals. ECSAofPt/NiNCs@HCS(COS)wasfoundtobe5.7and5.9 timeshigherthanthatofPt/NiNCs-PVP@HCSandPt/C, respectivelyandpeakcurrentdensity(13.89Amgpt1)9times higherthanthatofcommercialPt/C(0.43Amgpt1)[194].
Non-carbonaceoussupports
Thecarbonaceoussupportssufferfromcorrosioninacidic mediawhichlimitstheirapplicability.Hence,noncarbonaceousmaterialssuchasTiN[195],TiO2 [196],ZnO [197],conductingoxides[198],g-C3N4 [199]etc.areusedas supportsforelectrocatalysts.Theuseofchromiumnitride (CrN)andtitaniumnitride(TiN)asacatalystandcatalyst supportmaterialhasbeenexploredduetotheiroutstanding electricalconductivity,thermalandelectrochemicalstability, andpeculiarhardness[200].ButthereplacementofcarbonaceoussupportswithTiNdeterioratestheactivityofcatalyst duetocorrosionofTiNinhighlyacidicenvironment.Ternary catalystshavebeensynthesizedtoovercomethisproblem. Cuietal.synthesizedTi0.5Cr0.5Nsupportwithexcellentelectronicconductivity,largesurfaceareaandhighchemical stabilityinacidicaswellasbasicmediabysolid-solidphase separationmethod.Pd/AgNPsweresupportedonTi0.5Cr0.5N andusedforMORinalkalinemediaandformicacidoxidation inacidicmedia.Thecatalystpossessedhighactivityand stabilityforelectrochemicalreactions[201].Ti-supported NimCon (m þ n ¼ 4)alloyNPswithdifferentNi/Coatomic
ratiosweresynthesizedusinghydrogenevolutionassisted electrodepositionmethodforMORinalkalinemedia.In comparisontomonometallicNi,Ni/CoalloyNPsdisplayed improvedpropertiesforMOR.ItwasobservedthattheintroductionofCoenhancessurfaceexposureoftheredoxspecies forNimCon catalystsandweakenedtheadsorptionofCO, leadingtodecreasedCOpoisoning.Ni2Co2/Tiexhibitedhighestcatalyticactivityanddurability[202].Phametal.synthesizedanataseTi0.7W0.3O2-supportedPt3RuBMNPsofdiameter ~3nmusingsurfactantfreemicrowave-assistedpolyol method.ThecatalystdemonstratedoutstandingelectrochemicalstabilityandCOanti-poisoningabilityduring methanolandethanolelectrooxidationduetobifunctional effectofPt3Runanoalloyandstronginteractionsbetween Pt3Ruandnon-corrosiveTi0.7W0.3O2 nanosupport[203].Xu etal.investigatedtheelectrocatalyticactivityofrutileTiO2 supportedPt1Pd3 NPstowardsmethanolelectrooxidationin alkalinemedia.Anenhancedcatalyticactivityaswellashigh toleranceforCOpoisoningwasobservedwhichisattributed tostrongsynergicinteractionsbetweenPt/PdNPsandbetweenPt1Pd3 NPsandTiO2 support[204].
Thepropertiesoftransitionmetaloxidessuchasstability andstronginteractionswithNPsmakethemhighlyefficient electrocatalystsupportmaterials.Anon-Ptelectrocatalyst, PdSn/TiO2 wasfabricatedusingsphericalTiO2 NPsassupport, usingsurfacereduction-depositionmethod.Pt/SnNPsof about1 2nmweredistributedontheexteriorofTiO2. Amore uniformdispersionofNPswasobservedatPd:Snmolarratio of1:1and3:1comparedto2:1.ThePdSn/TiO2 catalysts showedgoodanti-poisoningabilityforMORasnooxidation peakofintermediatessuchasCOandCO-likespecieswas foundinthecyclicvoltagramof1MH2SO4 and2MMeOHata scanrateof100mVs 1.ThecatalystwithPd:Snratioof1:1 wasmostactivethancatalystwithPd:Snratioof3:1andthe catalystwithPd:Snratioof2:1wasleastactive.TheelectrocatalyticactivityofcatalystwithPd:Snratioof1:1was investigatedatdifferentmolarconcentrationsofmethanol (1M,2Mand4M)in1MH2SO4 atascanrateof10mVs 1.A highercatalyticactivityforMORandtolerancetowardsintermediateswasobservedforthecatalystascomparedto commercialPtRu/C[205].ThesynthesisofTiO2 supportedPt/ CuNPswascarriedoutbyphoto-depositionofCuonTiO2 powdersupport,andsubsequentpartialgalvanicdisplacementofdepositedCuwithPt.TheMORexperimentswere regulatedindeaeratedmixtureof0.1MHClO4 and0.5M methanol.Ahighintrinsicactivityandsimilarmassactivity wasshownbyPtCu/TiO2 whencomparedwithcommercialPt/ Ccatalyst.Theenhancedcatalyticactivitymaybeduetothe interactionsofCuandPtNPswithTiO2,andthesynergistic effectofTiO2 onoxidativeremovalofCO.TheDensityfunctionaltheory(DFT)studieswerecarriedouttoevaluatethe interatomicinteractionsofPt,CuandPt/CuNPsdispersedon TiO2 surface.Thecatalystwasmodeledbythreetypesof alloyedPt38-xCux (x ¼ 0and6)structures,dispersedonsurface ofTiO2
Itwasrevealedwithcomputedadhesiveenergies(Fig.15) thatCuandPtpresentincoreandshell,respectively,incoreshell(CS(Pt32Cu6))structure,hashigheststability,trailedby (RA((Pt32Cu6))with6Cuatomsinthecentersofhexagonal facets.Thealloy(PhSep(Pt32Cu6)containingCuatomsatthe
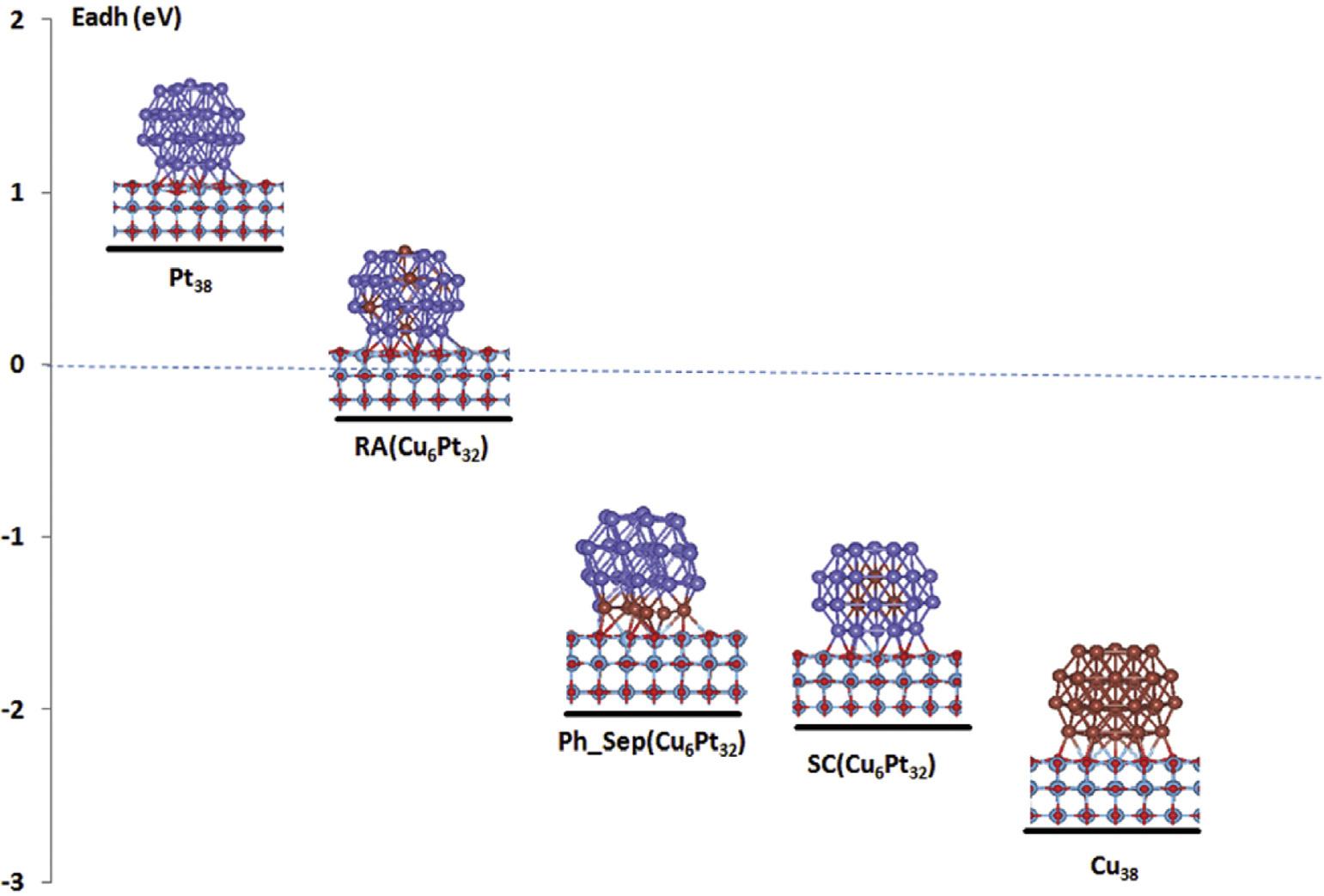
Fig.15 ComputedadhesionenergiesofsolePtandbimetallicPtCustructuresonTiO2 support[196](Reproducedwith permission,TheRoyalSocietyofChemistry,2019).
surfaceofclusterofhexagonalfacet,wasleaststable.These resultsconcludedtheformationofCSand/orPhSepNPsin PtCu/TiO2 catalyst[196].Themajordrawbackofusingtransitionmetaloxidesupportsistheirlowelectricalconductivity whichimpedestheirpracticalapplications.Theirelectrical conductivitycanbeimprovedbyfunctionalizationoftheoxides.Forthispurpose,Liandco-workerssynthesizedMo dopedMoxTi1-xO2-d NPs(IF-MTNPs)withacrystallinecoreand interfacialfunctionalizedshell,andPt/RuNPsweredeposited onthissupport.TheinterfacialfunctionalizationofTiO2 with Moincreaseditselectronicconductivitybymodulatingits bandstructureaswellasfacilitatedtheelectrontransfertoPt/ RuNPs.Thecatalystpossessed3.3timeshighermassactivity and10.3%improveddurabilityoverundopedPtRu/P-TNPs catalyst[206].
Tianetal.utilizednano-porousgold(NPG)filmsasasupportandPt/RualloyNPswithchangingPt/Rumolarratios weredispersedonit,toinvestigatetheircatalyticactivitytowardsMORusing0.5MH2SO4 and0.5MMeOHsolution saturatedwithN2,employinglinearscanvoltammetryat 10mVs 1 between0and0.75VandCVat50mVs 1 between 0and1.2V.ThecatalyticactivitywashighlydependentonRu content.WithincreaseinRucontent,thecatalystexhibited improvedCOtolerancebutadecreaseinMORactivity.NPGPt2Ru1 catalystshowedoptimalpropertywith3-foldcatalytic activityandsimilarCOtolerancewhencomparedwithcommercialPtRu/Ccatalyst.Apowerdensityof96mWcm 2 and about6timesincreasedPtefficiencywasobtainedbythe membraneelectrodeassemblywithanodeofNPG-Pt2Ru1 [207].AnITO-glasssubstratewasemployedasasupportfor Au/PtalloyNPsandthecatalystdisplayedhighcatalyticactivitytowardsMORinalkalinesolution[200].Recently,
MXeneshavedrawngreaterattentioninthefieldofelectrocatalysisowingto2-Dlayeredstructurecomprisingoftransitionmetalcarbides,nitridesorcarbonnitrides.These exhibithighmetallicityandhydrophilicityandexcellentmechanicalproperties,makingthempotentialcandidateasa supportmaterial,todisperseandstabilizecatalysts[208,209]. PtRu/MXenecatalyst(M ¼ Ti3C2)wassynthesizedbydepositionofPtandRuontoMXeneusingchemicalreduction method.ResponsesurfacemethodologywasusedforoptimizationofelectrocatalystperformanceofPtRu/MXene.With MXenecomposition78.90wt%,Nafioncontent19.71wt%and methanolconcentrationof2.82Masoptimumfactors,the optimumresponsewaspredictedtobe186.59mAmgPtRu 1 whichwasveryclosetotheactualvalue,andthisvalueof currentdensitywas2.34timeshigherthanthatofPtRu/C catalyst[210].
SomeothersupportmaterialsforBMNPs
Besidesthesecarbonaceousandnon-carbonaceoussupports, conductingpolymersandhybridsupportsarealsousedfor BMNPsimmobilization.Conductingpolymerssuchaspolypyrrole(Ppy),polyaniline(PANI),polythiophene(PTh), poly(3,4-ethylenedioxythiophene)(PEDOT)andpolyvinylpyrrolidone(PVP)areusedassubstratesfordepositionof NPsinMORapplicationsowingtotheirhighconductivity. TheyprovideuniformlydistributedNPsofnarrowsizerange withgoodstabilityandhighutilization[211,212].However,the synthesisofthesepolymerfilmsisdifficultbecauseofinsolublenatureofmonomers.Duetosimplicityoffabricationas conductingfilm,highchemicalandelectrochemicalstability andwatersolubilityofbrilliantcresylblue(BCB)monomer, Soltanietal.utilizeditasamonomerandsynthesizedpoly
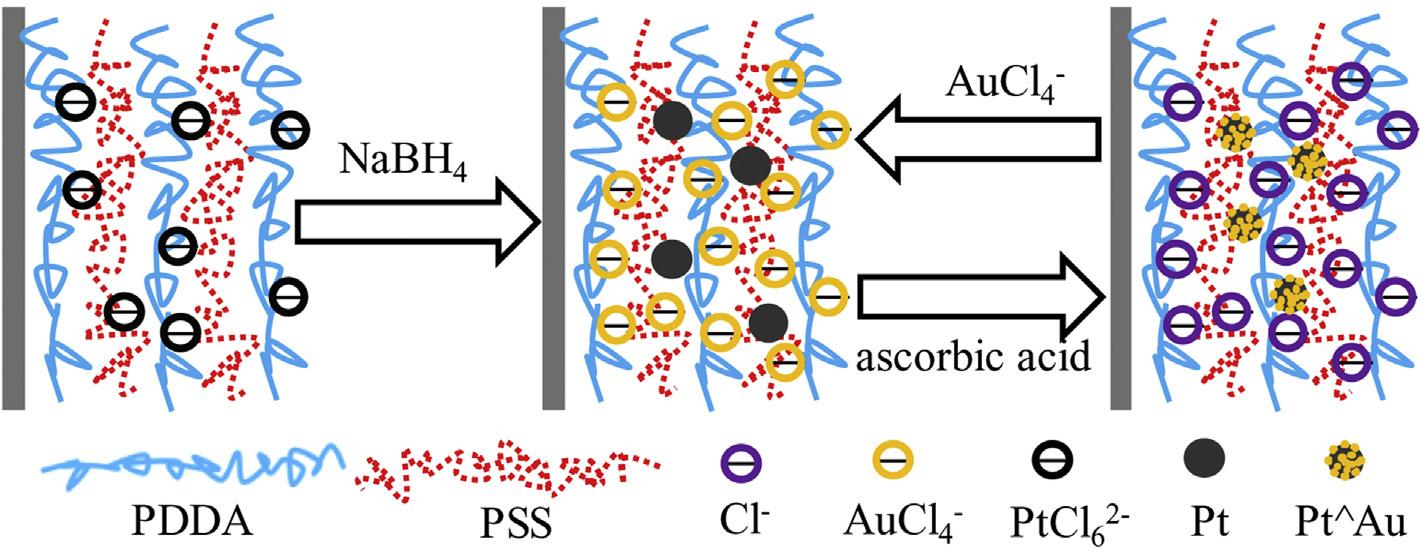
Fig.16 SimplifiedrepresentationofthefabricationofPtAuNPs[214](Reproducedwithpermission,Elsevier,2017).
(brilliantcresylblue)(PBCB)polymer.Theglassycarbonelectrode(GCE)wasmodifiedbyPBCBandthenPt/RuNPswere depositedonitbyelectrodeposition.Thesynthesizedcatalyst exhibitedhighmassactivityandlowestonsetpotential comparedwithGCE/NPsindicatingitshighMORactivityand tolerancetowardsCOpoisoning[213].Layer-by-layerfilms havebeenemployedasprotonexchangemembranesdueto easeoffabrication,precisecontrolandtunabilityofthefilm properties,andassupportmaterialsforfuelcellcatalyst owingtotheirintimatecontactwithcatalystparticles,resistancetofuelpermeationandstabilizationofthecatalyst particles.Chuetal.synthesizedpolymersupportedAudecoratedPtNPsbyion-exchangein-situreductionandastepwise seed-mediatedgrowthinpolyelectrolytemultilayers(PEMUs) atroomtemperature.
Thesynthesiswascarriedoutbydippinga(PDDA/PSS)4.5 filmintoH2PtCl4 solutionandtreatingwithNaBH4.Thisionexchange/reductionprocesswasrepeated m timestoyield Ptm NPsembeddedPEMU.ThePtm NPsembeddedPEMUwas thenimmersedintoHAuCl4,ascorbicacidsolutionrepeatedly n timestogetAudecoratedPtembeddedPEMU(Fig.16).The PEMUsupportedPt/AuNPswerehighlyactiveforMORand alsopossessedbettertolerancetopoisoningandlong-term stabilityincertaincompositionrangeascomparedtoPtNPs. Thebettercatalyticactivitymaybeattributedtothesynergic interactionsbetweenPtandAu.Audecorationprovided anotherdimensionfortuningthecatalyticproperties[214]. SomehybridsupportsconsistingofcarbonaceousandnoncarbonaceousmaterialsarealsousedinBMNPssynthesis andtheyalsoprovideadvantagesassupportmaterials.Optimizedperformanceisachievedbyfabricationofprimaryand secondarysupportsindifferentratios[215].Thehybridsupportmaterialconsistingofmetaloxideandcarbonblack providesabettercorrosionresistance,improvedhydrophilicityaswellashigherelectricalconductivity.Yousafetal. synthesizedanumberofnanoelectrocatalystsbyimplanting Pt/PdNPsoncarbonsupportedCeO2 nanospheresbyafacile surfactant-freemethod.TheCVwasperformedbetween0.05 and1.2VvsRHEat50mVs 1 scanrate.Thepeakduringthe forwardandreversescancorrespondstomethanoloxidation andeliminationofintermediatespeciessuchasCO,respectively(Fig.17).Pt3Pd1 CeO2/Ccatalystexhibitedloweronset potentialofmethanoloxidation.ThemechanismofMORfor
Pt3Pd1 CeO2/Ccatalystisproposedin Fig.17(C).Thecatalyst possessedhigherspecificandmassactivitiesincomparisonto othercatalystsandcommercial20wt%Pt/Ccatalyst(Fig.17 D) [216].
Twotimeshighermassactivitythanthatofcommercial catalystwasobtainedbyutilizingCeO2 embeddedcarbon nanofibers(CECNFs)supportedPt/RuNPsatCe/Ccontent0.4 [217].TiO2 supportedPt-basedNPsareextensivelyemployed ascatalystsinfuelcellsduetometal-supportinteractions.But thelowerelectricalconductivityofTiO2 decreasesitsapplicabilityforfuelcell.TheelectricalconductivityofTiO2 canbe improvedbydopingorbypreparingthecompositewithan electricallyconductingmaterial.Inthisregard,Itoetal.synthesizedTiO2 embeddedCNFs(TECNFs)andPt/RuNPswere supportedonit.AsignificantlyhigherMORactivitywas observedforPtRu/TECNFcomparedtothatofPtRu/C,PtRu/ TiO2,PtRu/(C þ TiO2)andPtRu/TiO2 catalysts[218].Hanetal. fabricatedgrapheneandTiO2 nanotubearrays(TNTs)modifiedTiwire(RGO/TNTs).Pt/RuNPsweredepositedonthe surfaceof(RGO/TNTs)supportthroughasquarepulsedelectrodepositionmethod.Pt/Runanoalloyswerewelldispersed ontheinnerendwallsofTNTsaswellasrGOsurfacewhich facilitatedthemasstransportandenhancedtheelectrochemicalactivity[219].Anelectrocatalyst,Ru@Pt/C TiO2 with peakcurrentdensity1.08mAcm 2,ECSA69.6m2 gPt1,high durabilityandresistancetoCOwasdesignedbysupporting Ru@PtNPsontoC TiO2 byatwo-stepethanolreduction method[220].AnultrafinecrystallinesizedMomodifiedTiO2 nanocrystallinecompositewithcarbonblackwasemployed asahybridsupporttoimmobilizePt/RuNPsandthesynthesizedPtRu/iMTNCs-CanodecatalystwashighlyactivetowardsMOR[221].TheeffectofAl2O3 incorporationonthe electrocatalyticpropertiesofPtRu/Ccatalystwasexplored anditwasobservedthattheadditionofAl2O3 incarbon supportdecreasedtheparticlesizeofNPs.WithAl2O3:Cratio of2:1(w/w),lowestparticlesizewasobtainedatwhichthe catalystexhibitedhighMORactivitywithcurrentdensityof 148.92mAcm 2 [222].Acompositeofpolyaniline(PANI)and ricehuskash(RHA)wassynthesizedbychemicaloxidation method(PANI-RHA)whichwasusedasasupportforPtandPt/ SnNPs.ThePtSn/PANI-RHAexhibitedenhancedactivityfor MORthanPt/PANI-RHA[223].Restackingofreducedgraphene oxidelayerswhichinhibitsthemovementofreactants
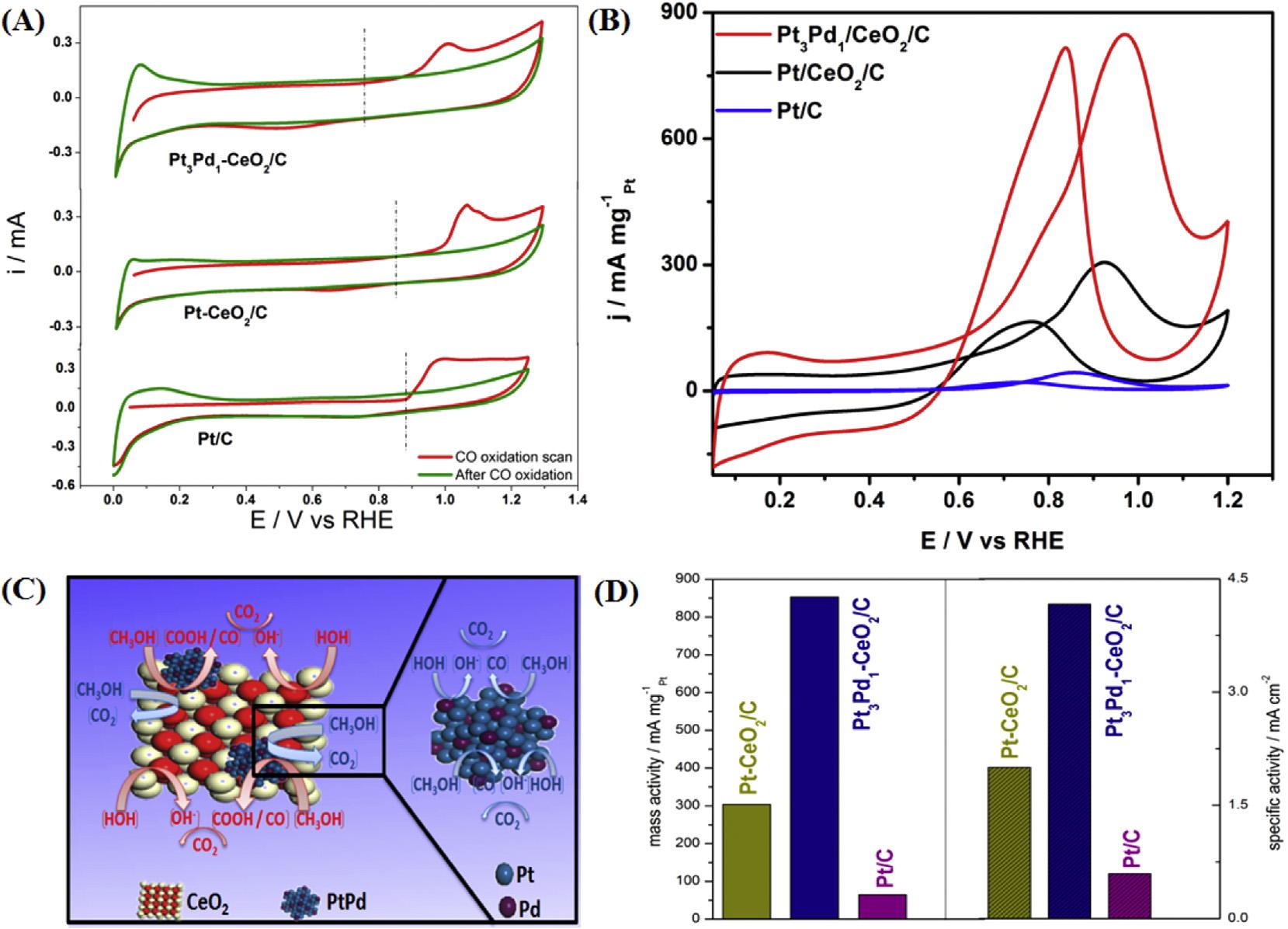
Fig.17 (A)ElectrochemicalCOoxidationand(B)MORperformanceofPt3Pd1 CeO2/C,Pt CeO2/Ccatalystsincontrastto 20%Pt/Ccatalyst,(C)SimplifiedillustrationofMORontheexteriorofPt3Pd1 CeO2/Ccatalysts(D)Evaluationofspecificand massactivitiesforallcatalystscomparedwith20%Pt/Ccatalyst[216](Reproducedwithpermission,AmericanChemical Society,2017).
towardscatalyticallyactivesitesandlimitsitsapplicationasa supportmaterial,canbepreventedbyincorporatingconductingpolymersbetweenitslayers.Forthispurpose,Arukulaandco-workerssynthesizedrGO/PANI/Pt Pdcatalystby incorporatingpolyaniline(PANI)betweenthelayersofrGO.Pt/ PdNPswithaveragesizeof3.5nmwereuniformlydistributed ontherGO/PANIhybridsupportandthecatalystdisplayed enhancedcatalyticactivity,operationalstabilityandconcrete celldurability(70h)comparedtoPt/C[224].
In-depthcriticalanalysis
DMFCisoneofthemosteminentfuelcells,basedonpolymer electrolytemembranetechnologyandusesmethanolasa fuel.Itisanexcellentpowersourceforelectronicvehicles, portableelectronicdevicesandstationaryapplications.Ptand Pt-basedcatalystsarewidelyusedfortheelectrooxidationof methanolinDMFC.ThecommercializationofDMFCis impededbythelowactivity,stabilityandhighcostofthe electrocatalyst.Alotofresearchisinprogressforthedevelopmentofactive,robustandstableelectrocatalystsforMOR, usingPt-andnon-PtbasedBMNPsandvarioussupportmaterials.TheactivityandstabilityofPtcanbeenhanced significantlybymodifyingitsstructurethroughbimetallizationwhichalsoreducesthecostofcatalyst.Theuseofsupport materialresultsingooddispersionofNPs,increasingtheir catalyticactivitybyincreasingtheelectrochemicallyactive
surfacearea.Manystudieshavebeenconductedinrecent yearstosynthesizesupportedBMNPs,toincreasetheefficiencyofDMFCs.ThesupportplaysanimportantroleinstabilizingtheNPsandamelioratingtheircatalyticactivity. Carbonblackisthemostcommonlyusedsupportmaterialbut thepresenceofimpurities,thermodynamicinstabilityand trappingofNPsinitsmicroporeslimitsitsuseandhas resultedintheuseofothernanostructuredcarbonmaterials suchasCNTs,CNFs,graphene,mesoporouscarbonetc.The propertiesoftheelectrocatalystmainlydependuponthe electrical,mechanical,surfaceandtexturalpropertiesofthe supportmaterial.Variousstudieshavebeenconductedto modifythepropertiesofcarbonaceoussupportmaterialsby covalentornon-covalentfunctionalizationand/ordopingof thesematerials.Thefunctionalizedcarbonaceoussupports exhibitbettercatalyticpropertiesascomparedtononfunctionalizedsupportmaterialsbutcovalentfunctionalizationdestroysthestructureofsupport.Researchhasbeen carriedoutforthesynthesisofBMNPsusingnoncarbonaceoussupportmaterialssuchasmetaloxides, MXenesetc.,hybridsupportmaterialsandconductingpolymers.Theuseofnon-carbonaceoussupportsincreasesthe stabilityofcatalystbypreventingthecorrosionofcatalyst underworkingDMFCconditions.Thestudiesindicatethatthe electrochemicalactivityofthecatalystisdependentuponthe synthesisprocess,experimentalconditions,structure,size, compositionandshapeofNPs,andnatureofsupportmaterial.Therehasbeensignificantprogressincontrollingthe
size,structure,shapeandcompositionofBMNPstoimprove theircatalyticperformance.TheNPswithhigh-indexfacets exhibitimprovedelectrochemicalactivity.
Conclusionandfutureperspectives
Theever-increasingdependenceonconventionalenergy sourcesduetoincreasingpopulation,andeconomicdevelopmenthasresultedinenvironmentaldegradationwhichhas triggeredsearchforefficientandenvironmentfriendlyenergy sources.DMFCsarepromisingcandidatesasalternativeenergysourcesforelectronicvehiclesandportableelectronic devices.Themajorchallengesinthecommercializationof DMFCsincludetheslowkineticsofmethanolelectrooxidation andhighloadingofPtcatalystwhichdecreasestheefficiency offuelcells.Toimprovethekineticsandincreasetheefficiencyanddurabilityofthecell,variousnanostructuredmaterialsandnewcatalyticsupportshavebeenemployedas catalysts.Theadventofextremelyactive,stableandinexpensivecatalystsforDMFCsisanemergingareaofresearch. Numerousinvestigationshavebeencarriedouttostudythe applicationofnanoparticlesinDMFCs.BimetallicnanoparticlesarehighlyactiveanodecatalystsforDMFCs.The activityofthecatalystscanbeimprovedbyusingasupport materialwhichprovidesbetterdispersion.Newmesoporous carbons,graphene,fullereneetc.havebeenusedtosupport bimetallicnanoparticlestoincreasetheiractivity,stability anddurability,andtoachievedesirablepropertiesatlowcost. Thefunctionalizationanddopingofcarbonaceoussupports improvetheelectronicpropertieswhichinturnincreasetheir efficiencyforfuelcellapplications.However,carbonaceous supportssufferfromcorrosionwhichlimitstheirapplicability forfuelcellapplications.
Eventhoughthecarbonaceoussupportssuchascarbon black,CNTs,CNFsandgrapheneoffernewdirectionsinthe synthesisofcatalysts,yetvariousdegreesofcarboncorrosionstillpersistsforoxidizedand/orfunctionalizedcarbonaceoussupports.Thecovalentfunctionalizationinvolves theintroductionofnewgroupsintothecarbonnanomaterial structure,favorableforthesynthesisofcatalystbutitalso leadstothedestructionofcarbonnanomaterialsbyweakeningorlosingsomeofitsspecialproperties.Theoriginal structureofthecarbonnanomaterialscanbemaintainedby non-covalentfunctionalizationbyusingVanderWaals forcesorhydrogenbondsbutitisunstable.Thestructureof carbonnanomaterialisalsodestroyedbydopingmethod. Effortsshouldbemadetoovercomethesechallengessothat thesefunctionalizedcarbonaceousmaterialsbecomepracticallyviable.Thesurfacemodificationtechniquesrequire furtherimprovement,andnewmethodsshouldbeexplored forthefunctionalizationofcarbonnanomaterial,without affectingitsoriginalstructure.Measuresshouldalsobetaken toincreasethestabilityofcarbonaceoussupportmaterialsto preventtheirdegradationundertheworkingDMFC conditions.
Severalinorganicmaterialssuchasmetaloxides,metal nitrides,MXenesetc.andsomeconductingpolymersand hybridsupportshavealsobeenemployedtoimmobilize metalNPs.Forinorganicsupportssuchasmetaloxides
andmetalnitrides,themainconcernsincludeelectronic conductivityandcorrosion resistanceinacidicmedia whichrequiresfurtherimprovements.Theuseofconductingpolymerseffectivelyincreasestheutilizationof metalNPsbecauseoftheirhighprotonandelectronic conductivitiesandtheypossessthecharacteristicsofan idealsupport.Butowingtodifficultiesinthesynthesisof conductingpolymerfilmsbecauseoflowsolubilityof monomersinwater,theirapplicationisstilllimited.The newconductingpolymerswithcomparablewatersolubilityshouldbesynthesized.Thehybridsupportsprovidethe bestactivityduetocombinedeffectsandpropertiesof bothcarbonaceousandnon-carbonaceoussupportswhich demandsthesynthesisofnewhybridsupportmaterialsin future.
Forhighperformancecatalysts,narrowsizedistributionof nanoparticleswithhighdispersionandfavoredcrystalplanes isthekeyrequirement.So,thenanoparticleswithcontrollable size,shapeandhigh-indexfacetsshouldbedesignedtoachieveoptimalelectrochemicallyactivesitesforimprovedcatalyticactivity.Themorphologicalcontrolalongwith synergisticeffectbetweenmetalscanenhancetheactivityof catalystandcanalsoreduceitscost.Thenewsynthetic methodsshouldbedevelopedtopreciselymanagethestructure,shapeandcompositionofNPs.Thesemethodscanbe developedfurtherbystudyingtheaccuraterelationbetween crystalparameters,surfacemorphology,electronicstateof thecatalystsandtheircatalyticactivity.
Theutilizationofnoblemetalsmustbeoptimizedand attemptshouldbemadetoattainthehighestpossibleactivity withlowestcontentofnoblemetal,andthebimetallic formulationshouldbetuned.Mostcommonly,Pt-basedNPs arebeingsynthesizedwhichareexpensive.Pureplatinum catalysthasmajorlimitationofbeingdeactivatedbycarbon monoxide(CO)producedinelectro-oxidationofmethanol. WhenPtcatalystisutilizedinfuelcells,theadsorbedCOintermediatesdecreaseitslifetime.Thedopingofadditional metalscanavertthissituationandupgradetheanti-CO poisoningcapabilityofthecatalystbyalteringtheCO adsorptionsites.ThesynthesisofPt-basednano-catalysts havinglittlequantityofhigh-costPtandbetteranti-CO poisoningactivityisextremelydesired,butthisisaserious challenge.Themostprominentwaytoresolvethisissueisby alloyingofPtwithlow-costmetalstoformbimetallicelectrocatalysts.TheelectronicstructureofPtwouldbeinfluencedbyadjoiningmetalatomspresentinthebimetallic catalyst,whichmaydebilitatetheconnectionbetweenthePt sitesandCO-likeintermediates.Forreducingthecostofproduction,furtherresearchontailoringofnon-preciousmetal electrocatalystswithimprovedmetalloadingsisrequiredin future.Thesimplifiedsynthesisprocessesshouldbedevelopedforthepreparationofefficientandinexpensivecatalysts fortheirmassproduction.
Declarationofcompetinginterest
Theauthorsdeclarethattheyhavenoknowncompeting financialinterestsorpersonalrelationshipsthatcouldhave appearedtoinfluencetheworkreportedinthispaper.
[1] ChuS,MajumdarA.Opportunitiesandchallengesfora sustainableenergyfuture.Nature2012;488:294 303
[2] DuanC,KeeR,ZhuH,SullivanN,ZhuL,BianL,JenningsD, O0 HayreR.Highlyefficientreversibleprotonic electrochemicalcellsforpowergenerationandfuel production.NatEnergy2019;4:230 40
[3] ShengT,LinX,ChenZY,HuP,SunSG,ChuYQ,MaCA, LinWF.Methanolelectro-oxidationinplatinummodified tungstencarbidesindirectmethanolfuelcells:aDFTstudy. PhysChemChemPhys2015;17:25235 43
[4] DanielL,BonakdarpourA,WilkinsonsDP.Benefitsof Platinumdepositedinthepolymermembranesubsurface ontheoperationalflexibilityofhydrogencells.JPower Sources2020;471:228418 29.
[5] AndujarJM,SeguraF.Fuelcell:historyandupdating.Awalk alongtwocenturies.RenwSustEnergRev2009;13:2309 22
[6] LindorferJ,RosenfeldDC,BohmH.Chapter23-fuelcells: energyconversiontechnology.3rded.FutureEnergy;2020. p.495 517
[7] CarretteL,FriedrichKA,StimmingU.Fuelcells -fundamentalsandapplications.FuelCell2001;1:5 39.
[8] WangC,BaiS,XiongY.Recentadvancesinsurfaceand interfaceengineeringforelectrocatalysis.ChinJCatal 2015;36:1476 93
[9] SamimiF,RahimpourMR.Chapter14-directmethanolfuel cell.MethSciEng2018:381 97
[10] YangZ,BerberMR,NakashimaN.Polymer-coatedcarbon black-basedfuelcellelectrocatalystwithhighCO-tolerance anddurabilityindirectmethanoloxidation.JMaterChemA 2014;2:18875 80
[11] LeiW,LiM,HeL,MengX,MuZ,YuY,RossFM,YangW.A generalstrategyforbimetallicPt-basednano-branched structuresashighlyactiveandstableoxygenreductionand methanoloxidationbifunctionalcatalysts.NanoRes 2020;13:638 45
[12] JiaJ,ZhaoL,ChangY,JiaM,WenZ.Understandingthe growthofNiSenanoparticlesonreducedgrapheneoxideas efficientelectrocatalystformethanoloxidationreaction. CeramInt2020;46:10023 8
[13] GhoshA,RamaprabhuS.Anefficientanddurablenovel catalystsupportwithsuperiorelectrondonationproperty andfueldiffusivityfordirectmethanolfuelcell.CatalSci Technol2017;7:5079 91
[14] HigaM,MehdizadehS,FengS,EndoN,KakihanaY.Cell performanceofdirectmethanolalkalinefuelcell(DMAFC) usinganionexchangemembranespreparedfromPVAbasedblockcopolymer.JMembrSci2020;597:117618
[15] ChengQ,WangY,JiangJ,ZouZ,ZhouY,FangJ,YangH. Shape-controlledporousheterogeneousPtRu/C/Nafion microspheresenablinghighperformancedirectmethanol fuelcells.JMaterChemA2015;(3):15177 83
[16] KamarudinSK,AchmadaF,DaudWRW.Overviewonthe applicationofdirectmethanolfuelcell(DMFC)forportable electronicdevices.IntJHydrogenEnergy2009;34:6902 16
[17] GoorM,MenkinS,PeledE.Highpowerdirectmethanolfuel cellformobilityandportableapplications.IntJHydrogen Energy2019;44:3138 43
[18] GlusenA,DionigiF,PaciokP,HeggenM,MullerM,GanL, StrasserP,Dunin-BorkowskiRE,StoltenD.DealloyedPtNicore-shellnanocatalystsenablesignificantloweringofPt electrodecontentindirectmethanolfuelcells.ACSCatal 2019;9:3764 72
[19] LiG,ShiB,GongY,ZhangY,WangX,GuoM,LyuXi.PdNi nanoparticlesdecoratedondefectivemesoporous carbon:anefficientbifunctionalelectrocatalystsin
alkalinedirectmethanolfuelcells.MaterChemPhys 2020;243:122570 80
[20] ChangJ,FengL,LiuC,XingW,HuX.Ni2Penhancesthe activityanddurabilityofthePtanodecatalystindirect methanolfuelcells.EnergyEnvironSci2014;7:1628 32
[21] ShiW,ParkHU,ParkAH,KwonYU.Effectofcompositionof Pd10 xCux (x¼2,3,4,and5)alloynanoparticlesontheir electrocatalysisformethanoloxidation.JElectroanalChem 2020;865:114144 50
[22] HamnettA.Mechanismandelectrocatalysisinthedirect methanolfuelcell.CatalToday1997;38:445 57
[23] HamnettA.Handbookoffuelcells fundamentals, technologyandapplications,directmethanolfuelcells (DMFC).2010
[24] SanetuntikulJ,KetpangK,ShanmuganS.Hierarchical nanostructuredPt8Ti-TiO2/Casanefficientanddurable anodecatalystfordirectmethanolfuelcells.ACSCatal 2015;5:7321 7
[25] LiZ,JiangX,WangX,HuJ,LiuY,FuG,TangY.Concave PtConanocrossesformethanoloxidationreaction.Appl CatalB:Environ2020;277:119135
[26] ChettyR,XiaW,KunduS,BronM,ReineckeT, SchuhmannW,MuhlerM.Effectofreductiontemperature onthepreparationandcharacterizationofPt-Ru nanoparticlesonmultiwalledcarbonnanotubes.Langmuir 2009;25:3853 60
[27] ZhangJ,QuX,HanY,ShenL,YinS,LiG,JiangY,SunS. EngineeringPtRubimetallicnanoparticleswithadjustable alloyingdegreeformethanolelectrooxidation:enhanced catalyticperformance.ApplCatalB:Environ 2020;263:118345
[28] ZhaoF,YeJY,YuanQ,YangX,ZhouZ.RealizingCO-free pathwaysandboostingdurabilityonhighlydispersedCu dopedPtBinanoalloytowardsmethanolfull electrooxidation.JMaterChemA2020;8:11564 72
[29] HuangL,ZhangX,WangQ,HanY,FangY,DongS.Shape controlofPtRunanocrystals:tuningsurfacestructurefor enhancedelectrocatalyticmethanoloxidation.JAmChem Soc2018;140:1142 7
[30] LiL,XingY.Pt-Runanoparticlessupportedoncarbon nanotubesasmethanolfuelcellcatalysts.JPhysChemC 2007;111:2805 8
[31] SawyEE,BirssV.Nano-engineeredIrcore@Ptshell nanoparticleswithcontrolledPtshellcoveragesfordirect methanolelectro-oxidation.ACSApplMaterInterfaces 2018;10:3459 69.
[32] ShiZ,LiX,LiT,ChenY,TangY.Evolutionofcomposition andstructureofPtRh/Cintheacidicmethanoloxidation process.ElectrochemCommun2020;113:106690
[33] MorallόnE,VazquezJL,AldazA.Electrochemicalbehavior ofbasalsinglecrystalPtelectrodesinalkalinemedium.J ElectroanalChem1990;288:217 28
[34] TripkovicAV,PopovicKD,MomcilovicJD,DraẑicDM. Kineticandmechanisticstudyofmethanoloxidationona Pd(111)surfaceinalkalinemedia.JElectroanalChem 1996;418:9 20
[35] YuEH,ScottK,ReeveRW.Astudyoftheanodicoxidationof methanolonPtinalkalinesolutions.JElectroanalChem 2003;547:17 24
[36] BedenB,KadirganF,LamyC,LegerJM.Oxidationof methanolonaplatinumelectrodeinalkalinemedia,Effect ofmetaladd atomsontheelectrocatalyticactivity.J ElectroanalChem1982;14:171 90
[37] BursteinGT,BarnettCJ,KucemakAR,WilliamsKR.Aspects oftheanodicoxidationofmethanol.CatalToday 1997;38:425 37.
[38] TheresGS,VelayuthamG,SureshC,KrishnanPS, ShanthiK.PromotionaleffectofNi-Co/ordered
mesoporouscarbonsasnon-noblehybridelectricatalyst formethanolelectrooxidation.JApplElectrochem 2020;50:639 53
[39] HuaD,ZhangLY,MengK,JiaZ,WangY,QiT. ElectrocatalysisofPd-Erbimetalliccatalystsformethanol oxidationinalkalinemedia.Ionics2020;26:3456 64
[40] JohnJ,HugarKM,MalendezJR,KostalikHA,RusED, WangH,CoatesGW,ArunaHD.Anelectrochemicalquartz crystalmicrobalancestudyofprospectivealkalineanion exchangemembranematerialsforfuelcells:Anion exchangedynamicsandmembraneswelling 2014;136:5309 22
[41] TengX,ShanA,ZhuY,WangR,LauWM.Promoting methanol-oxidationreactionbyloadingPtNinano-catalysts onnaturalgraphitic-nano-carbon.ElectrochimActa 2020;353:136542
[42] ChenZ,HeYC,ChenJH,FuXZ,SunR,ChenY,WongCP. PdCualloyflowerlikenanocageswithhighelectrocatalytic performanceformethanoloxidation.JPhysChemC 2018;122:8976 83
[43] ElsherifSA,SawyEN,GhanyNAA.Polyolsynthesized graphene/PtxNi100-x nanoparticlesalloyforimproves electrocatalyticoxidationofmethanolinacidicandbasic media.JElectroanalChem2020;856:113601 11
[44] KakatiN,MaitiJ,LeeSH,JeeSH,ViswanathanB,YoonYS. AnodecatalystsforDirectmethanolfuelcellsinacidic media:dowehaveanyalternativeforPtorPt Ru?Chem Rev2014;114:12397 429.
[45] ChenA,OstromC.Palladium-basednanomaterials: synthesisandelectrochemicalapplications.ChemRev 2015;115:11999 2044
[46] AntoliniE.Formationofcarbon-supportedPtMalloysfor lowtemperaturefuelcells:areview.MaterChemPhys 2003;78:563 73
[47] GerberIC,SerpP.Atheory/Experiencedescriptionof supporteffectsincarbonsupportedcatalysts.ChemRev 2020;120:1250 349.
[48] SankarM,HeQ,EngelRV,SainnaMA,LogsdailAJ,RoldanA, WillockDJ,AgarwalN,KielyCJ,HutchingsGJ.Roleofthe supportingold-containingnanoparticlesasheterogeneous catalysts.ChemRev2020;120:3890 938
[49] DestroP,KokumaiTM,ScarpelliniA,PasqualiL,MannaL, ColomboM,ZanchetD.Thecrucialroleofthesupportin thetransformationofbimetallicnanoparticlesandcatalytic performance.ACSCatal2018;8:1031 7
[50] CarmoM,SantosARD,PocoJGR,LinardiM.Physicaland electrochemicalevaluationofcommercialcarbonblackas electrocatalystssupportsforDMFCapplications.JPower Sources2007;173:860 6
[51] CalderonJC,Nieto-MongeMJ,Perez-RodriduezS,PardoJI, MolinerR,LazaroMJ.Palladium-nickelcatalystssupported ondifferentchemicallytreatedcarbonblacksformethanol oxidationinalkalinemedia.IntJHydrogenEnergy 2016;43:19556 69
[52] HuangM,GuanL.FacilesynthesisofcarbonsupportedPtCunanomaterialswithsurfaceenrichedPtashighlyactive anodecatalystformethanoloxidation.IntJHydrogen Energy2015;40:6546 51
[53] LuL,NieY,WangY,WuG,LiL,LiJ,QiX,WeiZ.Preparation ofhighlydispersedcarbonsupportedAuPtnanoparticles viaacappingagent-freerouteforefficientmethanol oxidation.JMaterChemA2018;(6):104 9
[54] JurzinskyT,BarR,CremersC,TubkeJ,ElsnerP.Highly activecarbonsupportedpalladium-rhodiumPdXRh/C catalystsformethanolelectrooxidationinalkalinemedia andtheirperformanceinanionexchangedirectmethanol fuelcells(AEM-DMFCs).ElectrochimActa 2015;176:1191 201
[55] LvQ,XiaoY,YinMin,GedJ,XingW,LiuC.Reconstructed PtFealloynanoparticleswithbulk-surfacedifferential structureforMethanolOxidation.ElectrochimActa 2014;139:61 8
[56] AmaniM,KazemeiniM,HamedanianM,PahlavanzadehH, GharibiH.Investigationofmethanoloxidationonahighly activeandstablePt-Snelectrocatalystsupportedon carbon polyanilinecompositeforapplicationinapassive directmethanolfuelcell.MaterResBull2015;68:166 78
[57] Cruz-CruzJJDL,CrespoMAD,MenesesER,HuertaAMT, SibajaSBB,CastroNC,RosalesHJD.Efficientstabilizationof in-situfabricationofPtxPd1-x nanostructuresforelectrooxidationofmethanolinalkalinemedium.IntJHydrogen Energy2020;45:4570 86
[58] NjokiPN,RootsMED,MayeMM.Thesurfacecompositionof Au/Agalloynanoparticlesinfluencesthemethanol oxidationreaction.ACSApplNanoMater2018;10:5640 5. [59] GarrickTR,DiaoW,TengcoM,StachEA,SenanayakeSD, ChenDA,MonnierJR,WeidnerJW.Theeffectofthesurface compositionofRu-Ptbimetalliccatalystsformethanol oxidation.ElectrochimActa2016;195:106 11
[60] WangQ,ChenS,JiangJ,LiuJ,DengJ,PingX,WeiZ. ManipulatingthesurfacecompositionofPt-Rubimetallic nanoparticlestocontrolthemethanoloxidationreaction pathway.ChemCommun2020;(56):2419 22
[61] ZhengY,ZhanH,FangY,ZengJ,LiuH,YangJ,LiaoS. Uniformlydispersedcarbon-supportedbimetallic ruthenium platinumelectrocatalystsforthemethanol oxidationreaction.JMaterSci2017;52:3457 66
[62] MartinsM,SantosD.PdNialloynanoparticlesassembledon cobaltferrite-carbonblackcompositeasfuelcellcatalyst. IntJHydrogenEnergy2019;44:14193 200
[63] LinZ,ChenW,JiangY,BianT,ZhangH,WuJ,WangY, YangD.FacilesynthesisofRu-decoratedPtcubesand icosahedraashighlyactiveelectrocatalystsformethanol oxidation.Nanoscale2016;(8):12812 8
[64] HuY,ZhuA,ZhangQ,LiuQ.PreparationofPtRu/Ccoreshellcatalystwithpolyolmethodforalcoholoxidation.IntJ HydrogenEnergy2016;41:11359 68
[65] XieJ,ZhangQ,GuL,XuS,WangP,LiuJ,DingY,YaoYF, NanC,ZhaoM,YouY,ZhouZ.Ruthenium-Platinumcoreshellnanocatalystswithsubstantiallyenhancedactivity anddurabilitytowardsmethanoloxidation.NanoEnergy 2016;20:247 57
[66] CorpuzAR,WoodKN,PylypenkoS,DameronAA,JogheeP, OlsonTS,BenderG,DinhHN,GennettT,RichardsRM, O’HayreR.Effectofnitrogenpost-dopingonacommercial platinum-ruthenium/carbonanodecatalyst.JPower Sources2014;248:296 306
[67] PereiraVS,daSilvaJCM,NetoAO,SpinaceEV.PtRu nanoparticlessupportedonPhosphorus-dopedcarbonas electrocatalystsformethanolelectro-oxidation. Electrocatalysis2017;8:245 51
[68] WangR,DaH,WangH,JiS,TianZ.Seleniumfunctionalizedcarbonforhighdispersionofplatinumrutheniumnanoparticlesanditseffectonthe electrocatalyticoxidationofmethanol.JPowerSources 2013;233:326 30
[69] LingX,YuX,ZhangQ,CaiW,LuF,YangZ.Highstability andperformanceofPtRuelectrocatalystderivedfrom doublepolymercoatings.IntJHydrogenEnergy 2017;42:11803 12
[70] LeeH,KimTJ,LiC,ChoiID,KimYT,CokerZ,ChoiTY,LeeD. Flameaerosolsynthesisofcarbon-supportedPt-Ru catalystsforafuelcellelectrode.IntJHydrogenEnergy 2014;39:14416 20.
[71] WangX,YangC,CaoL,LiangHP.Facilesolvothermal synthesisofPt1.2Co/Cbimetallicnanocrystalsasefficient
electrocatalystsformethanoloxidationandhydrogen evolutionreaction.NewJChem2020;(44):5792 9
[72] QiY,BianT,ChoiS,JiangY,JinC,FuM,etal.Kinetically controlledsynthesisofPt Cualloyconcavenanocubes withhigh-indexfacetsformethanolelectro-oxidation. Chem.Commun.2014;50:560 2
[73] MintsouliI,GeorgievaJ,ArmyanovS,ValovaE,AvdeevG, HubinA,etal.Pt-Cuelectrocatalystsformethanoloxidation preparedbypartialgalvanicreplacementofCu/carbon powderprecursors.Appl.Catal.B:Environ.2013;136137:160 7
[74] KwonS,HamDJ,KimT,KwonY,LeeSG,ChoM.Active methanoloxidationreactionbyenhancedcotoleranceon bimetallicPt-Irelectrocatalystsusingelectronicand bifunctionaleffects.ACSApplMaterInterfaces 2018;46:39581 9
[75] YanS,ZhangS,ZhangW,LiJ,GaoL,YangY,GaoY. ApplicationofcarbonsupportedPtcore Aushell nanoparticlesinmethanolelectrooxidation.JPhysChemC 2014;118:29845 53
[76] CaoR,XiaT,ZhuR,LiuZ,GuoJ,ChangG,ZhangZ,LiuX, HeY.Novelsynthesisofcore-shellAu-Ptdendritic nanoparticlessupportedoncarbonblackforenhanced methanolelectro-oxidation.ApplSurfSci2018;433:840 6
[77] LuS,LiH,SunJ,ZhuangZ.Promotingthemethanol oxidationcatalyticactivitybyintroducingsurfacenickelon platinumnanoparticles.NanoRes2018;11:2058 68
[78] CuiZ,ChenH,ZhaoM,MarshallD,YuY,AbrunaH,J DiSalvoF.SynthesisofstructurallyorderedPt3TiandPt3V nanoparticlesas,methanoloxidationcatalysts.JAmChem Soc2014;136:10206 9
[79] KellyCHW,BenedettiTM,AlinezhadA,SchuhmannW, GoodingJJ,TilleyRD.UnderstandingtheeffectofAuin Au Pdbimetallicnanocrystalsontheelectrocatalysisof themethanoloxidationreaction.JPhysChemC 2018;122:21718 23
[80] FathiradF,MostafaviA,AfzaliD.BimetallicPd-Mo nanoalloyssupportedonVulcanXC-72Rcarbonasanode catalystsfordirectalcoholfuelcell.IntJHydrogenEnergy 2017;42:3215 21
[81] AminRS,HameedRMA,KhatibKME,YoussefME. ElectrocatalyticactivityofnanostructuredNiandPd-Nion VulcanXC-72Rcarbonblackformethanoloxidationin alkalinemedium.IntJHydrogenEnergy2014;39:2026 41
[82] KodiyathR,RameshGV,ManikandanM,UedaS,FujitaT, AbeH.IntermetallicPd3X(X¼ TiandZr)nanocrystalsfor electro-oxidationofalcoholsandformicacidinalkaline andacidicmedia.SciTechnolAdvMater2020;21:573 83.
[83] RobledoAM,CostaNJS,PhilippotK,RossiLM,MenesesER, Guerrero-OrtegaLPA,QuirogaSE.Electro-oxidationof methanolinalkalineconditionsusingPd-Ninanoparticles preparedfromorganometallicprecursorsandsupportedon carbonVulcan.JNanoparticleRes2015;17:474 82
[84] MatinMA,KumarA,BhosaleRR,SaadMAHS,AlmomniFA, Al-MarriMJ.PdZnnanoparticleselectrocatalysts synthesizedbysolutioncombustionformethanol oxidationreactioninanalkalinemedium.RSCAdv 2017;7:42709 17
[85] ChowdhurySR,GhoshS,BhattachryaSK.Enhancedand synergisticcatalysisofone-potsynthesizedPalladiumNickelalloynanoparticlesforanodicoxidationofmethanol inalkali.ElectrochimActa2017;250:124 34
[86] YangG,ZhouY,PanHB,ZhuC,FuS,WaiCM,DuD,JhuJJ, LinY.UltrasonicassistedsynthesisofPd-Pt/carbon nanotubesnanocompositesforenhancedelectro-oxidation ofethanolandmethanolinalkalinemedium.Ultrason Sonochem2016;28:192 8
[87] RashidM,JunTS,JungY,KimYS.Bimetalliccore-shellAu@ Ptnanoparticle-decoratedMWNTelectrodesfor amperometricH2 sensorsanddirectmethanolfuelcells. SensorActuatorBChem2015;208:7 13
[88] RenX,LvQ,LiuL,LiuA,LiuB,WangY.Facilesynthesisof alloyedPtNi/CNTselectrocatalystwithenhancedcatalytic activityandstabilityformethanoloxidation.InorgChem Commun2020;120:108130.
[89] AdamAAM,DengM,ZhuA,ZhangQ,LiuQ.Facileone-step roomtemperaturesynthesisofPdAgnanocatalysts supportedonmulti-walledcarbonnanotubetowards electro-oxidationofmethanolandethanol.Electrochim Acta2020;339:135929
[90] HuangH,HuX,ZhangJ,SuN,ChengJX.Facilefabricationof platinum-cobaltalloynanoparticleswithenhanced electrocatalyticactivityforamethanoloxidationreaction, methanoloxidationreaction.SciRep2017;7:45555.
[91] CornejoJM,BernabeAG,CompanV.BimetallicPt-M electrocatalystssupportedonsingle-wallcarbonnanotubes forhydrogenandmethanolelectrooxidationinfuelcells applications.IntJHydrogenEnergy2018;43:872 84
[92] BhartiA,CheruvallyG.SurfactantassistedsynthesisofPtPd/MWCNTandevaluationascathodecatalystforproton exchangemembranefuelcell.IntJHydrogenEnergy 2018;43:14729 41
[93] AliS,AhmedR,SohailM,KhanSA,AnsariMS.Co@Pt core shellnanoparticlessupportedoncarbonnanotubesas promisingcatalystformethanolelectro-oxidation.JIndEng Chem2015;28:344 50
[94] AliS,KhanI,KhanSA,SohailM,AhmedR,urRehmanA, AnsariMS,MorsyMA.Electrocatalyticperformanceof Ni@Ptcore shellnanoparticlessupportedoncarbon nanotubesformethanoloxidationreaction.JElectroanal Chem2017;795:17 25
[95] GuoL,ChenS,LiL,WeiZ.ACO-tolerantPtRucatalyst supportedonthiol-functionalizedcarbonnanotubesforthe methanoloxidationreaction.JPowerSources 2014;247:360 4
[96] RahseparM,KimH.Microwave-assistedsynthesisand characterizationofbimetallicPtRualloynanoparticles supportedoncarbonnanotubes.JAlloysCompd 2015;649:1323 8
[97] ChenW,ZhangY,WeiX.CatalyticperformancesofPdNi/ MWCNTforelectrooxidationsofmethanolandethanolin alkalinemedia.IntJHydrogenEnergy2015;40:1154 62
[98] SatyanarayanaM,RajeshkhannaG,SahooMK,RaoGR. ElectrocatalyticactivityofPd20-xAgx nanoparticles embeddedincarbonnanotubesformethanoloxidationin alkalinemedia.ACSApplEnergyMater2018;8:3763 70
[99] YouG,JiangJ,LiM,LiL,TangD,ZhangJ,ZengXC,HeR. PtPd(111)surfaceversusPtAu(111)surface:whichoneis moreactiveformethanoloxidation?ACSCatal 2018;8:132 43
[100] ChengY,JiangSP.HighlyeffectiveandCO-tolerantPtRu electrocatalystssupportedonpoly(ethyleneimine) functionalizedcarbonnanotubesfordirectmethanolfuel cells.ElectrochimActa2013;99:124 32.
[101] ChenF,RenJ,HeQ,LiuJ,SongRui.Facileandone-pot synthesisofuniformPtRunanoparticlesonpolydopaminemodifiedmultiwalledcarbonnanotubesfordirect methanolfuelcellapplication.JColloidInterfaceSci 2017;497:276 83
[102] ChengY,XuC,ShenPK,JiangSP.Effectofnitrogencontainingfunctionalizationontheelectrocatalyticactivity ofPtRunanoparticlessupportedoncarbonnanotubesfor directmethanolfuelcells.ApplCatalB:Environ 2014;158 159:140 9
