https://ebookmass.com/product/spectroscopy-of-lanthanide-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Mössbauer Spectroscopy: Applications in Chemistry and Materials Science Yann Garcia
https://ebookmass.com/product/mossbauer-spectroscopy-applications-inchemistry-and-materials-science-yann-garcia/ ebookmass.com
Winterberries-like 3D network of N-doped porous carbon anchoring on N-doped carbon nanotubes for highly efficient platinum-based catalyst in methanol electrooxidation Tong Wang https://ebookmass.com/product/winterberries-like-3d-network-of-ndoped-porous-carbon-anchoring-on-n-doped-carbon-nanotubes-for-highlyefficient-platinum-based-catalyst-in-methanol-electrooxidation-tongwang/ ebookmass.com
Chemometrics in Spectroscopy (Second Edition) Howard Mark
https://ebookmass.com/product/chemometrics-in-spectroscopy-secondedition-howard-mark/ ebookmass.com
Hitler: A Biography 1st Edition Peter Longerich
https://ebookmass.com/product/hitler-a-biography-1st-edition-peterlongerich/
ebookmass.com
Hebrew For Dummies 2nd Edition Jill Suzanne Jacobs
https://ebookmass.com/product/hebrew-for-dummies-2nd-edition-jillsuzanne-jacobs/
ebookmass.com
Being Feared: The Micro-Dynamics of Fear and Insecurity
Ben Ellis
https://ebookmass.com/product/being-feared-the-micro-dynamics-of-fearand-insecurity-ben-ellis/
ebookmass.com
Early Modern Debts: 1550–1700 Laura Kolb
https://ebookmass.com/product/early-modern-debts-1550-1700-laura-kolb/
ebookmass.com
Human: A History Karolina Hubner (Editor)
https://ebookmass.com/product/human-a-history-karolina-hubner-editor/
ebookmass.com
Thicker than water W. C. Tuttle
https://ebookmass.com/product/thicker-than-water-w-c-tuttle/
ebookmass.com
https://ebookmass.com/product/optical-fiber-sensors-fundamentals-fordevelopment-of-optimized-devices-ignacio-del-villar/
ebookmass.com
Editedby
S.J.Dhoble
VijayB.Pawade
HendrikC.Swart
VibhaChopra
WoodheadPublishingisanimprintofElsevier
TheOfficers’ MessBusinessCentre,RoystonRoad,Duxford,CB224QH,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OX51GB,UnitedKingdom ©2020ElsevierLtd.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizations suchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatour website: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatment maybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligence orotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontained inthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-08-102935-0(print)
ISBN:978-0-08-102936-7(online)
ForinformationonallWoodheadpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionEditor: KaylaDosSantos
EditorialProjectManager: PeterAdamson
ProductionProjectManager: SojanP.Pazhayattil
CoverDesigner: GregHarris
TypesetbySPiGlobal,India
VijayB.Pawade,VibhaChopra,S.J.Dhoble
2Developmentintheinnovationofleadhalide-basedperovskite quantumdotsfromrareearth-dopedgarnet-basedphosphorsfor light-emittingdiodes21 RajanKumarSingh,NehaJain,PushpendraSingh,RanveerKumar, JaiSingh,SomritaDutta,Teng-MingChen,SudiptaSom, HendrikC.Swart
3Luminescentdynamicsofrareearth–dopedCaTiO3 phosphors57
LuyandaL.Noto,SefakoJ.Mofokeng,FokotsaV.Molefe, HendrikC.Swart,AngelinaS.Tebele,MokhotjwaS.Dhlamini
3.1Introduction
3.2CrystalstructureofCaTiO3
4Ln2+,3+-dopedaluminosilicate–basedphosphors87
D.A.Hakeem,AndrzejSuchocki
4.1Introduction
5Lanthanide-dopedorthometallatephosphors113 GoutamSinghNingombam,NongmaithemRajmuhonSingh
6Lanthanide-dopedorthovanadatephosphors:Syntheses, structures,andphotoluminescenceproperties235
DraganaJ.Jovanovic 6.1Introduction
7StudyofREion–dopedoxideglassmaterialsforphotonic applications293
B.NaveenKumarReddy,S.Sailaja,K.Thyagarajan, B.SudhakarReddy
8Advancedresearchonoxynitridephosphors305 AbhilashaJain,S.J.Dhoble,D.R.Peshwe 8.1Introduction
9HighlyluminescentZnObasedupconversionthinfilmsgrown bysol-gelspincoating327 TrilokK.Pathak,R.E.Kroon,L.P.Purohit,HendrikC.Swart
10Luminescencepropertiesofrare-earthdopedoxidematerials345 VinodKumar,S.P.Tiwari,O.M.Ntwaeaborwa,HendrikC.Swart
11Roleoflanthanidesubstitutiononsuitablesitesinenhancing thepropertiesofvariouselectroceramics365
PareshH.Salame,JayantT.Kolte 11.1Introduction
11.2Effectoflanthanide(Ln3+)substitutiononBaTiO3 ceramics
12Rare-earthdopinginafterglowoxidephosphors:Materials, persistencemechanisms,anddarkvisiondisplayapplications393
G.Swati,VishnuV.Jaiswal,D.Haranath
12.1Introductiontoafterglowphosphors
Contributors
Teng-MingChen NationalChiaoTungUniversity,Hsinchu,Taiwan
VibhaChopra P.G.DepartmentofPhysics&Electronics,DAVCollege,Amritsar, India
MokhotjwaS.Dhlamini DepartmentofPhysics,CollegeofEngineeringScience andTechnology,UniversityofSouthAfrica,Johannesburg,SouthAfrica
S.J.Dhoble DepartmentofPhysics,RashtrasantTukadojiMaharajNagpur University,Nagpur,India
SomritaDutta NationalChiaoTungUniversity,Hsinchu,Taiwan
D.A.Hakeem InstituteofPhysics,PolishAcademyofSciences,Warsaw,Poland
D.Haranath DepartmentofPhysics,NationalInstituteofTechnology(NIT), Warangal,Telangana,India
AbhilashaJain VisvesvarayaNationalInstituteofTechnology,Nagpur,India
NehaJain Dr.HariSinghGourCentralUniversity,Sagar,India
VishnuV.Jaiswal DepartmentofPhysics,NationalInstituteofTechnology(NIT), Warangal,Telangana,India
DraganaJ.Jovanovic VincaInstituteofNuclearSciences,UniversityofBelgrade, Belgrade,Serbia
JayantT.Kolte SchoolofPhysicsandMaterialsScience,ThaparInstituteof EngineeringandTechnology,Patiala,India
R.E.Kroon DepartmentofPhysics,UniversityoftheFreeState,Bloemfontein, SouthAfrica
RanveerKumar Dr.HariSinghGourCentralUniversity,Sagar,India
VinodKumar CentreforEnergyStudies,IndianInstituteofTechnologyDelhi, NewDelhi,India;DepartmentofPhysics,UniversityoftheFreeState, Bloemfontein,SouthAfrica
SefakoJ.Mofokeng DepartmentofPhysics,CollegeofEngineeringScienceand Technology,UniversityofSouthAfrica,Johannesburg,SouthAfrica
FokotsaV.Molefe DepartmentofPhysics,CollegeofEngineeringScienceand Technology,UniversityofSouthAfrica,Johannesburg,SouthAfrica
B.NaveenKumarReddy DepartmentofPhysics,SVDegreeCollege;SKR&SKR GovernmentDegreeCollegeforWomen(Autonomous),Kadapa,India
GoutamSinghNingombam DepartmentofChemistry,ManipurUniversity, Canchipur,India
LuyandaL.Noto DepartmentofPhysics,CollegeofEngineeringScienceand Technology,UniversityofSouthAfrica,Johannesburg,SouthAfrica
O.M.Ntwaeaborwa SchoolofPhysics,UniversityoftheWitwatersrand, Johannesburg,SouthAfrica
TrilokK.Pathak DepartmentofPhysics,UniversityoftheFreeState,Bloemfontein, SouthAfrica;TKCOE,TeerthankerMahaveerUniversity,Moradabad;Gurukul KangriUniversity,Haridwar,India
VijayB.Pawade LaxminarayanInstituteofTechnology,R.T.M.NagpurUniversity, Nagpur,India
D.R.Peshwe VisvesvarayaNationalInstituteofTechnology,Nagpur,India
L.P.Purohit DepartmentofPhysics,GurukulKangriUniversity,Haridwar,India
S.Sailaja DepartmentofHumanitiesandBasicSciences,G.PullaReddy EngineeringCollege(Autonomous),Kurnool,India
PareshH.Salame DepartmentofPhysics,InstituteofChemicalTechnology Mumbai,Mumbai,India
JaiSingh Dr.HariSinghGourCentralUniversity,Sagar,India
NongmaithemRajmuhonSingh DepartmentofChemistry,ManipurUniversity, Canchipur,India
PushpendraSingh Dr.HariSinghGourCentralUniversity,Sagar,India
RajanKumarSingh Dr.HariSinghGourCentralUniversity,Sagar,India
SudiptaSom NationalTaiwanUniversity,Taipei,Taiwan
AndrzejSuchocki InstituteofPhysics,PolishAcademyofSciences,Warsaw,Poland
B.SudhakarReddy DepartmentofPhysics,SVDegreeCollege;SKR&SKR GovernmentDegreeCollegeforWomen(Autonomous),Kadapa,India
HendrikC.Swart DepartmentofPhysics,UniversityoftheFreeState, Bloemfontein,SouthAfrica
G.Swati CentreforNanotechnologyResearch,VelloreInstituteofTechnology University,Vellore,TamilNadu,India
AngelinaS.Tebele DepartmentofPhysics,CollegeofEngineeringScienceand Technology,UniversityofSouthAfrica,Johannesburg,SouthAfrica
K.Thyagarajan DepartmentofPhysics,JNTUACollegeofEngineering, Pulivendula,India
S.P.Tiwari DepartmentofPhysics,UniversityoftheFreeState,Bloemfontein, SouthAfrica
1
VijayB.Pawadea,VibhaChoprab,S.J.Dhoblec aLaxminarayanInstituteofTechnology,R.T.M.NagpurUniversity,Nagpur,India, bP.G.DepartmentofPhysics&Electronics,DAVCollege,Amritsar,India, cDepartmentof Physics,RashtrasantTukadojiMaharajNagpurUniversity,Nagpur,India
1.1Introduction
Theelementshowninthebottomoftheperiodictableisclassifiedaslanthanideseries element.Therearetotal15elementoccurredinthisseriesincludingLa3+,Ce3+,Pr3+, Nd3+,Pm3+,Sm3+,Eu3+,Gd3+,Tb3+,Dy3+,HO3+,Er3+,Tm3+,Yb3+,andLu3+.These elementsarearrangedonthebasisofatomicnumber.Fromthelongyears,backlanthanideelementsareusedasdopantionsinmanyinorganichostlatticessuchasaluminates,borates,silicates,tungstate,fluoride,phosphate,andvanadates.Inpastfew years,alotofstudyhadbeencarriedoutonthelocationoflanthanidelevelintoband structureofseveralinorganichostcompounds [1–4].Thistypeofstudyisverymuch usefultopredictingtheexactlocationoflanthanideleveland4f-electronbindingenergiesbasedonsometheoreticalmodeltocalculateredshift,centroidshift,charge transfer,andchemicalshift.Thesearestudiedonthebasisoftheobservedspectroscopicdatatodrawthehostreferredbindingenergylevelschemeinwhichthe4fn and4fn 1 5dlevelsforalldivalentandtrivalentlanthanidesionsareplacedrelative tothetopoftheV.B;however,incaseofvacuumreferredbindingenergyscheme,all theenergylevelsarerelativetothatofanelectronatrestinvacuum [5].Therefore, suchenergylevelschemeprovidedmethodsforthevisualizationoftherelevantlocationoflanthanidelevelsinrelativeinthebandstructureoftheinorganichostlattice. Also,weknowthattheapplicationoflanthanide-dopedinorganichostdependsupon theobservedphotophysicalproperties.Amongthe15lanthanideelement,Ce3+,Pr3+, Sm3+,Eu3+,Tb3+,andDy3+ arepotentiallyusedinLEDsanddisplaydevices.However,theotherelementssuchasNd3+,HO3+,Er3+,Tm3+,andYb3+ shownearvisibleNIRemissionspectraandexhibittheemissionbandsinvisibletoNIRrange [6–9]. Recently,upconversionluminescentnanoparticleisproposedforwastewatertreatment.Inpastfewyears,researcherreportedmanytypesoflanthanide-dopedluminescenthostmaterialsforlightingdevices.Butonlyfewofthemarecommerciallyused. Currently,QD-basedLEDsattracttheattentionofmanyresearchersworldwidedueto theirhighbrightnessandhighquantumyield.Onlyfewmaterialsarereportedforsuch typesofLEDapplications.Hence,thesearchfornewluminescentmaterialsandQDs
2SpectroscopyofLanthanide-DopedOxideMaterials
isnotendinnearfutureduetocontinuousinnovationanddevelopmentinexiting indoorandoutdoorlightingtechnology.Recently,lanthanide-doped(Ce3+,Pr3+, Sm3+,Yb2+,Nd3+,etc.)oxidematerialsshowexcellentluminescencepropertiesin UVvisibletoNIRrangeandalsoapplicableasanactivelayeronthefrontsurface toimproveconversionefficiencyofSisolarcell.Amongthedifferenttypesofrenewableandsustainablesourceofenergy,solarenergyisaprominentandacleansourcefor energygeneration.Thus,PVsolarcellsarethenonpollutingenergysourceofthe21st century [10].AnditiswellknownthatSisolarcelliseverywhereacceptedforthePV applicationthantheotherPVdeviceslikeorganicsolarcell,perovskitesolarcell,Tandemsolarcell,andCdTecell [11].Itismostcommerciallyusedfromfewyears.Now, researchersarefocusedonfindingotheralternativesforSicellandalsoaretakingan effortonhowtoincreasetheefficiencyofexistingSisolarcell.Therefore,theluminescentmaterialswithUC/DCphosphormechanismweretheoreticallyandpractically proposedbymanyresearchgroupstoenhancethePVresponseofSisolarcell [12]. Hence,thelanthanideionsplayanimportantroleinmodifyingtheoptical,electronic, electrical,andcatalyticactivitywhenitdopedwithsomehostlattices.Inthefollowing section,wehavediscussedthesomeimportantpropertiesoflanthanidesions.
1.2Electronicstructure
Table1.1 showstheelectronicstructureoflanthanideions.Theseared-blockelementsthatstartfromceriumtolutetium;theyareformedbytheadditionof1,2, 3…14elementsinto4flevel.Mostofthe5delectronisenergeticallymoveinto4f level.ButitisnotpossibleincaseofCe,Gd,andLa.Itiswellknownthatthelanthanidehas+3stableoxidationstate(L2+.4+ ionsarelessstable).Therefore,the
Table1.1 ElectronicstructureandoxidationstateofLn3+ ions
ElementElectronicstructureofatomsOxidationstate
Lanthanum(La)[Xe]5d1 6s2
Cerium(Ce)[Xe]4f1 5d1 6s2
Praseodymium(Pr)[Xe]4f3 6s2
Neodymium(Nd)[Xe]4f4 6s2
Promethium(Pm)[Xe]4f5 6s2
Samarium(Sm)[Xe]4f6 6s2
Europium(Eu)[Xe]4f7 6s2
Gadolinium(Gd)[Xe]4f1 5d1 6s2
Terbium(Tb)[Xe]4f9 6s2
Dysprosium(Dy)[Xe]4f10 6s2
Holmium(Ho)[Xe]4f11 6s2
Erbium[Xe]4f12 6s2
Thulium(Tm)[Xe]4f13 6s2
Ytterbium(Yb)[Xe]4f14 6s2
Lutetium(Lu)[Xe]4f14 5d1 6s2
electronicstructureoflanthanideionsisrepresentedasCe3+ f1,Pr3+ f2,Nd3+ f3+, Lu3+ f14.Here,the4foutermostelectroniswellshieldedby5sand5pelectrons.Consequently,itdoesnotparticipateinchemicalbonding.Hence,itcannotparticipatein chemicalbondingandcrystalfieldstabilizationofcomplexes,whichisimportantfor d-blockelement.Therefore,theoctahedralsplittingofforbitalisapproximately1KJ/ mol.However,theoutermostorbitalisfilledorempty,andithaslittleeffectonthe chemicalproperties,butitdoesnotaffectthemagneticpropertiesandtheirspectra. Thehigheroxidationstategenerallyoccursinfluoridesandoxides,andloweroxidationstateoccursinhalides(bromidesandiodides).Oxidationstateof+2and+4occurs whentheLn3+ ionsleadto.
1. Anoblegasconfiguration(ex-Ce4+(f 0)),
2. Ahalf-filledfshell(ex-Eu2+ andTb3+ (f7)),
3. Completelyfilledflevel(ex-Yb2+ (f14)),
Thus,the+2and+4statesexistforelementsthatareclosertothesestates.Hence, +3stateisthemoststable,while+4and+2statehaveaqueouschemistry(¼Ce4+, Sm2+,Yb2+ etc.) [13].
1.3TransitionsinLn3+ ions
1.3.1Allowedtransition
TheL-absorptionspectraoflanthanideionshavingthe4fN electronicconfiguration occurbecauseof4f–5d(f–d)transitions.Usually,thistypeoftransitionexhibitsa strongbroadband(4fn 1 5dª4fn).Thisabsorptionandemissionspectracanbewell understoodwiththehelpofFranck-Condonprinciple;4fn 1 5delectronicstatecorrespondstothehighdensityofstates.Thistransitioncorrespondstothelargenumber ofenergylevels.Thenatureoff-dtransitionisdifferentfordifferenthostlatticedueto thepresenceofcrystalfieldsplittingdifference.Amongthedifferentlanthanideelements,Ce3+ andEu2+ ionsexhibitlong-wavelengthf–dspectralband.Thistypeof emissionbandunderNUVexcitationwavelengthisquitefavorableforthefabrication ofopticaldevices(LEDs,PDP,etc.) [14]
1.3.2Forbiddentransition
Here,wehavediscussedtheforbiddentransitioninlanthanideelementbytakinga suitableexampleofEu3+ ions.WeknowthatEu3+ ionsshowthecharacteristics orangeanddeepredbandunderUVandNUVexcitation.Suchas 5D0-7FJ(J¼0,1,2…), here, 5D0 ! 7F0 isreferredasspinandelectric-dipole-forbiddentransition.Here, J ¼ 0indicatedthegroundstate(7F0)andtheexcitedstate(5D0).Inthiscase,stark splittingcannotbeobserved,andtheselectionrule(spin)isrelaxedbythemixing of 7F0 into 5D0 duetospin-orbitinteraction.This0 $ 0transitionisnondegenerate andnotasccociatedwithcrystalfieldofthetrivalentlanthanideions.Therefore, thepositionandlinewidthoff–f(0 $ 0)transitionprovideaninformationregarding thelocalbondingenvironmentandthecoordinationoftrivalentlanthanideions Introductiontoelectronicspectroscopyoflanthanide3
4SpectroscopyofLanthanide-DopedOxideMaterials
(ex-Eu3+)inhostlattice.Suchtypeof0 $ 0transitionisverymuchsuitedtostudying thelinebroadeninginmanyglassmaterialsduetoitssinglejumpbetween nondegenerateenergylevels.Therefore,manyfactorslikeelectron-phononcoupling, localcrystalfields,andion-ioninteractionmaycontributeinsuchkindoflinebroadeningmechanism.However,theenergygapbetweenhigherandlowerstate isapproximately10timeshighest,andhence,theprobabilityofnonradiative deexcitationfromtheexcitedstateisverysmall.Thejumpfrom 5D0 ! 7F0 is asymmetricinwhich J mixingisdominant,whennearlysymmetric,andthenother mechanismsaredominant.Duetoinhomogeneousbroadeninginthelienspectra, wegetasharpspectralbandthatreflectsthattransitionenergyisstatistically distributed [14].
1.4Selectionrulesforlanthanide
1.4.1Laporteselectionrule
Theorbitalquantumnumber(‘)forelectric-dipoletransitionschangesto Δ‘ ¼ 1. Therefore,initialandfinalstateshaveoppositeparitystate.Duetolargeinteraction withelectronicwavefunctionoftheion,thisselectionrulecanberelaxeddueto electron-phononinteractionandinteractionwithhigherorbitals.Therefore,forbidden transitionsareusuallymuchweakeronlatticesiteswithinversionsymmetry [14].
1.4.2Spinselectionrule
Duringthejumpbetweentwoenergylevels,thespinofelectronsisconserved (ΔS ¼ 0).Therefore,thespin-forbiddentransitionisveryweakfortransitionmetal complexes,andintensityofthespectrallineisincreasingbecauseofstrongspin-orbit coupling,andhence,spinselectionruleisrelaxedduetothepresenceofthiscoupling.
1.4.3Selectionrulefor J
Thenotation J representsorbitalangularmomentum(i.e.,spectroscopicnotation 7Fj). Theselectionruleforoutermost4forbitaloflanthanides j ΔJ j 6isexplainedonthe basisofJuddandOfelttheory [15,16].Theelectric-dipoletransitions(J ! J transitions)arestrictlyforbidden.Hence,theparityselectioncannotberelaxedbecause Ln3+ ionispresentatthecenterofsymmetryinthehostlattice.Forthemagneticdipoletransition,selectionruleisrepresentedby ΔJ ¼ 0, 1.Itisnotaffectedmore bythesitesymmetryduetoitsparity-allowednature.Oscillatorstrengthsforallowed electric-dipoletransitionscorrespondto10 5 –10 6, whereas10 8 correspondsto magnetic-dipoletransitions [14].
Thetotalangularmomentum(J )oftheelectroncanberepresentedasfollows:
J ¼ L S(no.of4felectronsislessthan7
J ¼ L+S(no.of4felectronsislargerthan7
1.5Propertiesoflanthanides
Itiswellknownthatlanthanideelementswith+2and+3oxidationstateshavethe characteristicfeature,andtheyaresimilarinproperties.Theyarealsonamedasrare earth(RE)ions,butnow,theyaremostlycalledasinnertransitionserieselements. Hence,alternatively,theyaretermedas“rareearth.”Andweknowthattheword earth,whichisusedbychemiststorepresentthechemicalcompounds,iscalledas oxides.Acompoundiscomposedofatomsofdifferentelementsthatgetclosely bondedtoeachotherduringthechemicalreaction.TherareearthionsareknownbetterfortheoxidesofLn3+ ionstodifferentiatethemfrombetterknownearths.Usually, thed-blockandf-blocktransitionserieselementswiththeirtrivalentoxidationstate alsoexhibitcolorsintheircrystallineandinsolutionforms.Therangeanddepthof colorincreasewiththeincreaseinthenumberofunpairedelectronsintheirforbital state.Amongthedifferentlanthanideelement,theionswith+3oxidationstatearethe moststablestate.Certainelementswith+2and+4oxidationstatesarealsoformed, buttheyarelessstable.Becausesumvalueofthefirstthreeionizationenergyislower, however,thesecondandfourthionizationenergiesarehigher.Thebromideandiodide compoundshavetheloweroxidationstates,whereastheoxidesandfluorideshavethe higheroxidationstate.Theoxidationstatessuchas+2and+4arepossibleonlyifthis formsanoblegasconfigurationwithfullyorhalf-filledorbital.Alongwiththis,there aresomesimilaritiesofthelanthanideelementsinrelationtotheirionizationenergies, electrodepotentials,ionicradii,etc.Thereductioninatomicandionicradiuswiththe increaseinatomicnumberisaspecialcharacteristicsfeatureoftheLn3+ ions.Therefore,thepropertiesofLn3+ ionsaredeterminedonthebasisoftheirsizeandshape. TheseLn3+ ionshaveidenticalsizeandshowthesimilarchemicalproperties.
1.5.1Physicalproperties
Thelanthanideionshavedifferentelectronicconfigurations(asshownin Table1.1), crystalstructures,andlatticeconstants.Generally,lanthanideionsexistinoneoffive crystalstructures.Atroomtemperature,thesedifferentlanthanideelementshave 9-hcp(hexagonalclose-packed)structure,4-dhcp(doublec-axishcp)structure, 2-fcc(face-centeredcubic),and1-bccandrhombicstructures.Therefore,thisdepends ontemperatureandpressure,becausemanyelementsshowastructuralphasetransitionsatelevatedtemperature.ItiswellknowthatthephysicalpropertiesofLn3+ ions arebasedontheirsolubility,color,magneticproperties,oxidationstates,complexformation,etc.MostoftheLn3+ ionsshowsuncharacteristicfeature,andtheyarealmost similarinproperties.Theyreferasrareearthmetalsoralsocalledasinnertransition serieselements.Thetermrareearthmeanstheelementfoundinearthcrustinoxide form.Itisformedbythebondingofdifferentelementsduringachemicalreaction. Thus,rareearthisusedfortheoxidesofLn3+ ions.MostofLn3+ insaltformaresolubleinwaterandformcrystallization.Thiscanbeassociatedwiththepresenceof voidsindandforbital.Transitionelementcorrespondstod-andf-blocklanthanides in+3oxidationstatethatproducecolorsincrystallineaswellasinsolutionforms. Introductiontoelectronicspectroscopyoflanthanide5
Thedepthofcolordependsonthenumberofunpairedelectronsinforbitalandit increaseswithincreaseinelectrons.Butthelanthanideswithotheroxidationstatecannotformcolorinacompound.From Table1.1,itisseenthatLa3+ andCe4+ haveno4f electrons,whereasLu3+ has14electronsintheirfshell;itmeansthatthereareno unpairedelectrons.Thus,itisdiamagneticinnature.However,theotherLn3+ ionswill haveunpairedelectrons,andtheyexhibittheparamagneticquality.Therefore,the magneticeffectsoccurduetothemobilizationofparticles,whichhavebothmass andelectriccharges(electrons,holes,protons,andpositiveandnegativeions).We knowthatspinningelectric-chargedparticlecreatesamagneticdipole,soitiscalled asmagneton.Therefore,paramagnetismincreaseswithincreaseinthenumberof unpairedelectrons.Amagneticdomainisreferredasvolumeofferromagneticmaterial inwhichallmagnetonsarealignedinthesamepathbytheexchangeforces.Fromthis domain,wecandistinguishtheferromagnetismandparamagnetismmaterials.The domainstructureofferromagneticmaterialindicatessizedependenceofitsmagnetic behavior.However,whenthesizeofthisferromagneticmaterialreducedtoacritical value,thenitexhibitsasingledomain.Thus,fineparticlemagnetismoccursfromthe sizeeffectsofthematerials,whicharebasedonthemagneticdomainstructureof ferromagneticmaterials.Hence,thecriticalsizeofthesingledomainisaffectedby severalfactorslikethevalueofthemagneticsaturation,strengthofthecrystalanisotropy,shapeoftheparticles,andsurfaceordomain-wallenergyexchangeforces.
1.5.2Chemicalproperties
Generally,allmetalsarewhitesliverincolorandhavesoftnature;theyareelectropositiveinnatureandreactwithothermetalinwhichheaviermetalarelessreactive thanlighter.TheionizationenergiesoftheelementvarieswithminimaatLa3+,Gd3+, andLu3+,whichhasempty,half,andfullshellorbital,whereasthemaximaoccursat Eu3+ andYb3+,whichrelatedtobreakingofhalforfullshell.Thechemicalreactivity ofLn3+ ionismuchmorethanthatofAlandslightlyreactivethanMg.Thebasicityof Ln(OH)3 decreasesfromCetoLuastheionicradiusdecreases.Thus,Ce(OH)3 is mostbasicthanLn(OH)3,anditintermediatesbetweenscandiumandyttrium. Ln3+ elementtarnishesreadilyinopenatmosphereandformoxides.Alsowhen heatingwithoxygen,theycanformoxideM2O3.However,Ceisanexceptioncase inwhichitformsCeO2.Athightemperature,Ln3+ elementreactswithnP,As,and Sb.Lanthanideelementsformlargevarietyofoxosaltswhentheyreactwithnitrates, sulfates,phosphates,etc. [13].
1.5.3Opticalproperties
Inperiodictable,manytypesofcompoundofthesquarateanionwithdivalentortrivalentcationshavebeenstudiedandindexedinthedatabase,whichexhibitaninterestingstructuresandpropertieslikemechanical,electrical,physical,chemical, optical,etc. [17–21].Amongthem,opticalpropertiesoflanthanideionsareverymuch usefulinengineering,medical,andenvironmentalfieldsduetoitscurrentneedsin emergingtechnologicaldevelopment.TheLn3+ ionsareregardedasafascinating 6SpectroscopyofLanthanide-DopedOxideMaterials
groupofelements;theseelementshowsthecharacteristicsofopticalspectra,which arisefromtheinnerfelectrons.Ceriumionsconsistoneelectron,whileytterbiumhas 13electrons.AccordingtoLaporterule,thetransitionprobabilitiesoftheforbitalare strictlyforbidden,andtheybecomepartiallyallowedbythemixingoffanddorbital [22,23].Thebasicfundamentalandtheoryofelectronicspectrainlanthanideionshas beendiscussedwellinprovidedreferences [24,25].Inthe20thcentury,withfurther improvementinmanytypesoflightinganddisplaydevices,thelanthanideelements receivedmuchmoreacademicandindustrialattention.LikeLn3+-dopedinorganic compound,currentlylanthanideactivatedwithorganiccompoundshasbeenwidely applicablebecauseitexhibitsdesirableopticalpropertiessuchasintensesharpband emissionviaefficientintramolecularenergytransfer(ET)processfromtheligand excitedstatetothecentralmetalionswhenexcitedbyUVlight.However, lanthanide-dopedorganicligandshavepoorthermalstabilityandmechanicalproperties,buttheyshowbetteropticalproperties.Sothattheyhavelimitedpracticaluses [26].Therefore,thelanthanideionsshowsthecharacteristicabsorptionandemission bandinvisible,nearultraviolet(NUV),andinfrared(IR),whichareattributeddueto thetransitionsbetween4flevelsthatoccursinsharplinewithhighoscillatorstrengths (10 6).Thesetransitionscorrespondtoelectricdipoleforbidden,buttheybecame allowedduetoforcedelectric-dipoletransitions [15,16,27–29],whereas magnetic-dipolef–ftransitionsareallowedandmuchweaker.Ln3+ ionsdopedinto inorganicnanocrystals(NCs)areaninterestingtopicofresearch,whichopenedan opportunityindiversefieldssuchaslight-emittingdisplaysandbiologicallabeling andimaging [30–32].Thus,researchertakingconsiderableeffortsonthesynthesis andtuningtheopticalpropertiesofLn3+ ionsdopedwithsemiconductorNCs.In, Section1.5,wehavediscussedindetailspectroscopicpropertiesofdifferent lanthanideions.
1.5.4Mechanicalproperties
Currently,Ln3+-basedcompoundshavebeenextensivelystudiedduetoitsemerging applicationsinthefieldsofscienceandengineering [33–35].Amongthedifferentlanthanidecompounds,LnOisextensivelystudiedbecauseitshowsbettermagnetic, electronic,andthermochemicalandthermophysicalproperties.Thus,amongthedifferentpropertiesoflanthanides,mechanicalpropertiesofthesetypesofcompounds havegainmoreimportanceinthescientificcommunities.Inthissection,weare mainlyfocusedontheLnOs(Ln ¼ La,Ce,Pr,Nd,Sm,Eu,Tb,Ho,Er,andYb)to introducethescopeofthesecompoundstomodifytheirmechanicalproperties.The parametersuchasbulkmodulus,shearmodulus,Young’smodulus Y,Poisson’sratio m,andanisotropicratio A,calledasmechanicalparameter,hasmoreindustrialimportance.Theseparametersareverymuchusefultostudyingthemechanicalcharacteristicsofamaterial.Itiswellknownthattheaverageshearmodulusofacompoundcan bemeasuredwiththehelpofresistancetoreversibledeformationsuponshearstress [36].Therefore,fromthisobtainedvalueofshearmodulus,thehardnessofamaterial canbeobtainedcorrectly.Thus,fromthedifferentLn3+ oxideelements,YbOshows thehighvalueofshearmodulus,whereasTbOshowslowvalueofshearmodulus. Introductiontoelectronicspectroscopyoflanthanide7
8SpectroscopyofLanthanide-DopedOxideMaterials
Young’smodulus(Y )ofthecompoundcanbecalculatedwiththehelpofstressto strainratio.Hence,itisusedforthemeasurementofmaterialstiffness(i.e.,ifthevalue of Y islarger,thenmaterialwillbestiffer).Thus,fromtheliterature,itisseenthat LaO,CeO,NdO,SmO,EuO,HoO,ErO,andYbOhavehighervaluesofYoung’s modulusascomparedwithBulkmodulus,whichindicatesthatthesematerialsare stifferthanTbO.Theductile/brittlecharacteristicsofmaterialscanbestudiedwith thehelpofratiobetweentheirbulkmodulusandshearmodulus [37,38].Ifthisratio isfoundtobe >1.75,thenthematerialisductile,otherwiseitisbrittleinnature.
1.5.5Electricalproperties
EffectofLn3+ additiononelectricalconductivityofdifferenthostmaterials(e.g., glasses)hasbeenreportedinmanyliteratures.Itdependsonthecompositionofmaterials andatomicsizeofLn3+ ions.However,conductivitydecreaseswithincreaseinatomic weightofLn3+ ions,whichisobservedinmanyLn3+ dopedwithtelluriteglasses.This correspondstotheslowmobilityofLn3+ ions,becauseoftheirheavymasses.Whenthe telluriteglassesdopedwithHo3+ ions,itshowsdecreaseinconductivitywithincreasein dopantconcentration.Thisisattributedduetotheformationofquasimolecularcomplex oflanthanideionswithglassmaterials.WhenLn3+ iondopedwithphosphateglasses, thentheconductivityincreasedbyanorderofmagnitude,andtheactivationenergy remainedconstantordecreasedinverysmallamountwithincreaseofLa2Oconcentration.Presently,manyreportsareavailableonthestudyofelectricalconductivityina seriesofLn3+ (Sm,Gd,Dy,Ho,Y,Er,andYb)iondopedwithsodiumborateglasses. OthermaterialslikeRCrO3 (R Yorlanthanide)andRFeO3 orthoferritescorrespondto thefamilyofDzyaloshinskyinteractionantiferromagnets,whichexhibittheunusual varietyofmagneticandstructuralchanges [39–41].TheLn3+ dopedLaFeO3 exhibits thenumberofcharacteristicssuchashighelectricalconductivity,dielectricconstant, thermalstability,lowdielectricloss,moderatepermittivity,susceptibility,polarizability, ferroelectricity,andpiezoelectricity,whichactsasbettermaterialforsolidoxidefuel cell [42–44].Also,itisusedashotelectrodeformagnetohydrodynamic[MHD]power generation [45].AndLn3+-dopedLaFeO3 materialsarealsobeingusedassensors [46–48].Thedecreaseinresistivityisobservedwithfurtherincreaseintemperature, whichreflectsthesemiconductorbehaviorofthematerials [49].
1.5.6Magneticproperties
Lanthanideionsshowtheparamagneticpropertiesduetothepresenceofunpaired4f electrons:theyareeffectivelyshieldedwellbytheoutermostfilled5sand5porbitals. AlsothebehavioroftheLn3+ ionsislessaffectedbythecoordinationenvironmentof theionascomparedwith3dtransitionserieselement.La3+ andCe4+ ionshaveno4f electronsintheiroutermostshell,whereaslutetium(Lu3+)has14electronsinfshell; itmeansthatunpairedelectronsdonotexist,andtherefore,theyarediamagnetic, whereasalltheotherLn3+ ionshaveunpairedelectronsandshowparamagnetic quality.Hence,paramagnetismincreaseswithincreaseinthenumberofunpaired electrons.
Introductiontoelectronicspectroscopyoflanthanide9
1.6SpectroscopicpropertiesofsomeLn3+ ions
Luminescentmaterialscanbeofthreetypes:hostluminophores,“host+activator” type,and“host+sensitizer+activator”type.Ifthehostmaterialshaveluminescent centersthatcanabsorbtheradiationduringexcitationandcanemitradiationdirectly andcomebacktogroundstate,thematerialsaredefinedashostluminophoressuchas vanadateandtungstate.Iftheluminescentmaterialsneedactivatorsasluminescent centersintheinactivehost,thensuchtypeofmaterialsisknownas“host+activator” type.Forexample,Y2O3 asahostmaterialcannotluminescencedirectly.ButifEu3+ ionsaredopedintoY2O3,itcanemitredlight.Further,toenhancetheluminescent properties,sensitizersareusedtoenhancethepropertiesofmaterials;whenadded tohostmaterials,theycanabsorbtheexcitationenergyandtransferittotheactivators. Asaresult,theluminescentpropertiesareenhancedtoagreaterextent.Such luminescentmaterialsaredefinedas“host+sensitizer+activator”type.Forexample, intheLaPO4/Ce3+,Tb3+ phosphor,theCe3+ ionactasagoodsensitizerthatcantransfer itsexcitationenergytoTb3+ andemitgreenlight.So,itisconcludedthatLn3+ ionsplay animportantroleindecidingthecolorofemissiondependingupontheirspectroscopy. So,atthisstage,itisofutmostimportancetostudythespectroscopyoftheseLn3+ ions thatcanbeexplainedbyusingtheirenergyleveldiagrams.
1.6.1EnergylevelsofEu3+
TheemissionsinCdPbBAlF/Eu3+ glassesareshownbyenergyleveldiagramin Fig.1.1.Theabsorptionbandoccursat395nmwithtransitionfrom 7F0 ! 5L6 [1,2]. ItwasobservedthatEu3+ emissionoccursfrom 5D0 ! 7Fj transitions.Themain transitionsare 5D0 ! 7F0 (around580nm), 5D0 ! 7F1 (around595nm),and 5D0 ! 7F2 (around610nm).Thefirsttransitionisstronglyforbiddenbutstill observedinfewhosts.Thesecondoneistheelectricdipoleforbiddenbutis magnetic-dipoleallowed.Forthistransitiontooccur,Eu3+ shouldoccupyacenter ofsymmetrysite.However,ifEu3+ ionissituatedatasitethatlackstheinversion symmetry,thetransitionsareelectric-dipoleallowedcorrespondingtoevenvalues of j (2–4,except0) [3–6].Further,basedonthelocalsymmetry,allthelinesofthese transitionssplitintwocomponents [7],andspectraarealsosensitivetocationsize [8] andchemicalbonding [9]
1.6.2EnergylevelsofDy3+
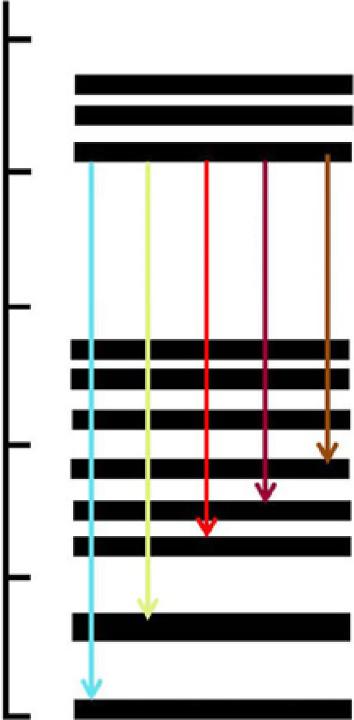
DifferenttransitioninvolvedinDy3+ ionsisshowninenergyleveldiagramof Dy3+ [10] shownin Fig.1.2.
InmostofthematerialswhereDyactsasanactivator,fourmainpeakshavebeen observedthatarelocatedatabout480,575,670,and757nm [10]. 4F9/2 ! 6H15/2 transitioncorrespondstoabluecoloremissionatabout480nmandisofmagnetic-dipole origin;greenyellowlightemissionat575isdueto 4F9/2 ! 6H13/2 transitionandisof electricdipoleorigin [11].Further,theredcolorpeakatabout670nmoccursdueof
Fig.1.1 EnergylevelschemeforemissionprocessinEu3+/CdPbBAlFglasses. ReproducedwithpermissionfromS.Novais,A.Dobrowolska,A.Bose,P.Dorenbos,Z. Macedo,J.Lumin.148(2014)353.Copyright2019,Elsevier.
4F9/2 ! 6H11/2 transition,andfinallyred-brownishcolortransitionat757nmoccurs dueto 4F9/2 ! 6H9/2 transition.
1.6.3EnergylevelsofCe3+
TheschematicenergyleveldiagramofCe3+ ionisexplainedbyDanieletal. [12]. Ce3+ exhibitsabroademissionband,butinsomehostmaterials,itcanshowthe characteristicsofdoubletband(2F5/2,2F7/2 levels)undernear-UVexcitationwavelength.Excitationat280nmreferstothetransitionsfromgroundlevel4ftoexcited level5dofCe3+ ions [12].Theemissionbandsfromabout330–420nmhave beenobservedandareduetothetransitionfrom5dlevelto 2F5/2 and 2F7/2 levels ofCe3+.Theseareallowedelectric-dipoletransitions [13,14]
1.6.4EnergylevelsofTb3+
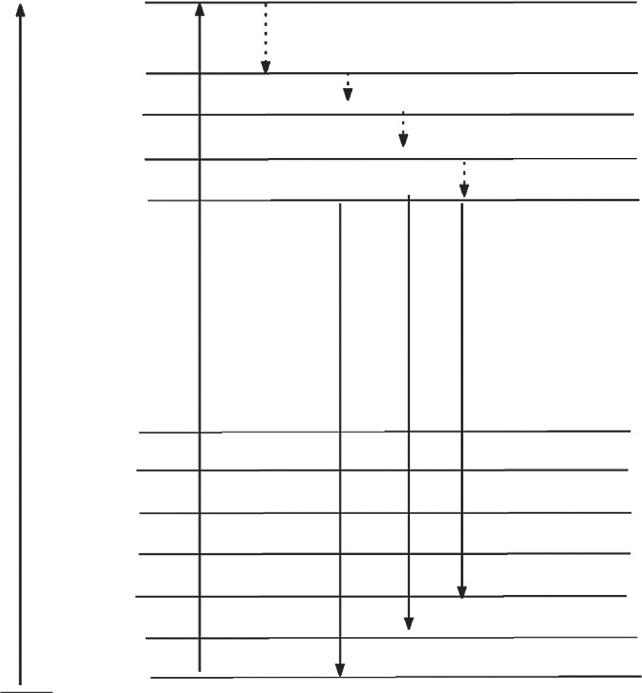
TheenergyleveldiagramofTb3+ ions [2] withmechanismispresentedin Fig.1.3. Forexcitationat377nm,theelectronswereexcitedfromthegroundstate 7F6 tothe excitedstate 5G6.Afterthat,thenonradiativetransitionoccurs,resultinginthe
4G11/2 4I15/2
4F9/7
Fig.1.2 Energyleveldiagramof Dy3+ion. Reproducedwithpermissionfrom J.Zhao,C.F.Guo,T.Li,D. Song,X.Y.Su,Phys.Chem.Chem. Phys.17(2015)26330.Copyright 2015,Springernature.
6F3/2+6F1/2
6F5/2
6H5/2+6F7/2
6H7/2+6F9/2
6H9/2+6F11/2
6H11/2
6H13/2
6H15/2
Fig.1.3 EnergyleveldiagramofTb3+ ionsalongwiththeproposedPLprocesses. ReproducedwithpermissionfromS.Novais,A.Dobrowolska,A.Bose,P.Dorenbos,Z. Macedo,J.Lumin.148(2014)353.Copyright2019,Elsevier.
populationofmetastable 5D3 level.Further,theelectronsweredecayedfrom 5D3 level tothe 5D4 levelcausingsecondnonradiativetransition.Finally,theradiativetransitions, 5D4 ! 7F6 at491nm, 5D4 ! 7F5 at545nm, 5D4 ! 7F4 at585nm,and 5D4 ! 7F3 at622nm,occur,givingrisetothecharacteristicemissionsofTb3+ ions [17].
1.6.5EnergylevelsofSm3+
TheenergylevelschemeofSm3+ ionsinborateglasswasexplainedbyNishaetal. [18].TheemissionspectraofSm3+ ionsintheborateglassesfor402-nmexcitation showsthreepeaks;563nmcorrespondingto 4G5/2 ! 6H5/2transition,600nmthat showstransition 4G5/2 ! 6H7/2,and645nmcorrespondingto 4G5/2 ! 6H9/2 transition [18].Themostintensepeakamongthethreetransitionsisat600nmthatgives reddish-orangeemission.Thetransition 4G5/2 ! 6H7/2 with ΔJ ¼ 1isa magnetic-dipole(MD)allowedandelectric-dipole(ED)dominated,whiletheother transition 4G5/2 ! 6H9/2 ispurelyanED [19,20].
1.6.6EnergylevelsofHo3+
Theenergylevelsforthebariummagnesiumaluminatenanopowdersdopedwithholmium(Ba0.5Mg0.5Al2O4: x%Ho3+ [0 < x < 2])havebeenexplainedbyMelatoetal. [21].Themechanismshowsthattherearetwoabsorptionbands,around240and 352nmforthehostmaterialandtheemissionsataround422and733nm.
TheemissionfromHo3+ occursindependentlytothehostemissionasshownin diagram.ForHo3+ ion,theabsorptionbandsarecenteredat418nm(5I8 ! (5K5; 5G5)),454nm(5I8 ! (5F1;5G6)),474nm(5I8 ! (5F2,3K8)),and486nm (5I8 ! 5F3).Theelectronsareexcitedat454nmfromthegroundstate 5I8 tothe energylevel(3K5, 5G5)aftergettingenoughenergy.Finally,theemissionsoccurred at491nm(5F3 ! 5I8),550nm(5F4 ! 5S2 ! 5I8),662nm(5F5 ! 5I8),and758nm (5S2 ! 5I7)forHo3+ ion [22–24]
1.7EnergytransferinLn3+ ions
Energytransferforionsplaysanimportantroleinlight-emittingdiodes.Energytransferfromdonortoacceptormoleculeoccurswhentheyareclosetoeachother,orin otherwords,theemissionspectrumofthesensitizerionandtheabsorptionspectrum oftheactivatorionhavetoshowspectraloverlap.Everyenergytransfercanhavetwo typesoforigin:electrostaticorexchangeinteractions.Inelectrostaticenergytransfer, theinteractionofsensitizerandactivatoriondoesnotrequireanyphysicalcontact, whereasincaseofexchangeenergytransferinteraction,physicalcontactisessential. Further,thedistancebetweensensitizerandactivatorplaysanimportantroleinenergy transferprocessastransferispossibleoverthedistancesupto10A ˚ .Further,thereare twotypesofenergytransfersthatareradiativeandnonradiative.Theradiativeenergy processisbasedonvirtualphotonexchangemechanism,andnonradiativeenergy 12SpectroscopyofLanthanide-DopedOxideMaterials
transferisradiationlesstransferattributedtochargemigration.Theenergytransfer betweendifferentionsisexplainedinsubsequenttopics:
1.7.1EnergytransferofCe3+ toTb3 ions
TheenergytransfermechanismforCe3+ ! Tb3+ [25] isshownin Fig.1.4.
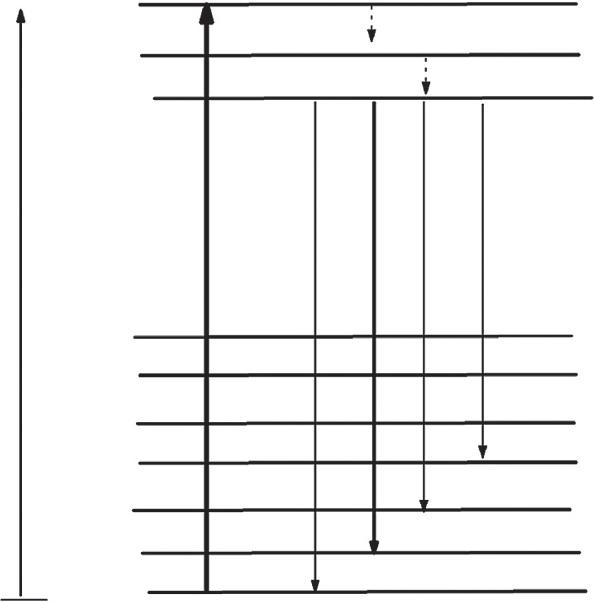
Theelectronsfromthegroundstate 2F5/2 ofCe3+ getexcitedtothe5dexcitedstate withirradiation.Fewoftheelectronsreturntothegroundstates(2F7/2 and 2F5/2)of Ce3+ ionsby5d ! 4ftransition,showingviolet-blueemissions.Atthesametime, aportionofexcitedelectronsmaybetransferredtoexcitedstate 5D4 ofTb3+ ions, andthen,theelectronscometothegroundstate 7FJ ofTb3+ ions,givinggreen emissionscorrespondingto 5D4 ! 7FJ (J ¼ 0–6)transitions. [26].
1.7.2EnergytransferofTb3+ toEu3+ ions
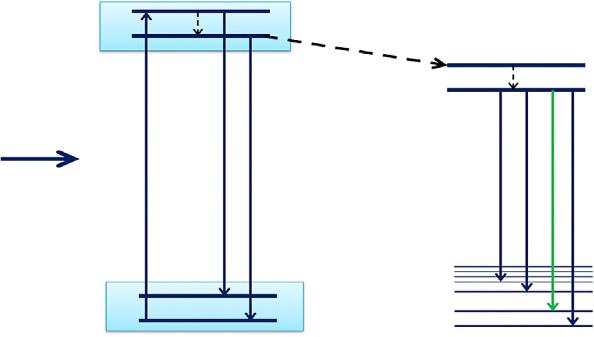
TheenergylevelsofTb3+ andEu3+ ionsalongwiththeirpossibletransfermechanism fromTb3+ ! Eu3+ [27] isshownin Fig.1.5.
Inthiscase,thenonradiativeenergytransferoccursastheenergylevelofTb3+ (5D4)isalittlehigherthanthatofEu3+ (5D1 and 5D0).TheelectronsofTb3+ ions areexcitedfromgroundstate(7F6)tohigherexcitedstate(5D3)byexcitationof 260nmandrelaxto 5D4 stateviamultiphononrelaxation.Then,theseelectrons caneitherreturntogroundstates(5D4 ! 7Fj (j ¼ 4–6)resultingintogreenemission ofTb3+ ortransfertheirenergyfrom 5D4 (Tb3+)leveltotheexcitedlevelsofEu3+ (7F0 ! 5D1,0)throughcrossrelaxationandthenrelaxesnonradiativelytothe 5D0 level from 5D1 and 5D2 states.Finally,radiativetransitiontakesplacesfrom 5D0 ! 7FJ (J ¼ 0–4),causingredemissionsofEu3+ [15].
Fig.1.4 EnergytransfermechanismofCe3+ ! Tb3+ ions. ReproducedwithpermissionfromG.K.Liu,X.Y.Chen,in:K.A.GschneidnerJr.,J.C.G. Bunzli,V.K.Pecharsky(Eds.),HandbookonthePhysicsandChemistryofRareEarths,Elsevier ScienceB.V.,Amsterdam,vol.37,2007,99Copyright2018,Elsevier. Introductiontoelectronicspectroscopyoflanthanide13