Solid-PhaseExtraction
Editedby ColinF.Poole
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandthe CopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby thePublisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professional practices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatter ofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-816906-3
ForinformationonallElsevierpublicationsvisitourwebsite at https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionEditor: KathrynEryilmaz
EditorialProjectManager: ReddingMorse
ProductionProjectManager: OmerMukthar
CoverDesigner: GregHarris
TypesetbyTNQTechnologies
Contributors
AbbiAbdel-Rehim FacultyofScienceandEngineering,UniversityofManchester, Manchester,UnitedKingdom
MohamedAbdel-Rehim DepartmentofClinicalNeuroscience,CenterforPsychiatryResearch,KarolinskaInstitutetandStockholmCountyCouncil,Stockholm, Sweden
MarthaB.Adaime LaboratoryofPesticideResidueAnalysis(LARP),Chemistry Department,FederalUniversityofSantaMaria,SantaMaria,RioGrandedoSul, Brazil
HossamAl-Suod DepartmentofEnvironmentalChemistryandBioanalytics, FacultyofChemistry,NicolausCopernicusUniversity,Torun,Poland;Centrefor ModernInterdisciplinaryTechnologies,NicolausCopernicusUniversity,Torun, Poland
BeatrizAlbero DepartamentodeMedioAmbienteyAgronomía,InstitutoNacional deInvestigacionyTecnologíaAgrariayAlimentaria(INIA),Madrid,Spain
TaherehGolzariAqda EnvironmentalandBio-AnalyticalLaboratories,DepartmentofChemistry,SharifUniversityofTechnology,Tehran,Iran
SergioArmenta DepartmentofAnalyticalChemistry,ResearchBuilding, UniversityofValencia,Valencia,Spain
MaríaAsensio-Ramos InstitutoVolcanologicodeCanarias(INVOLCAN),INtech LaLaguna,SanCristobaldeLaLaguna,Tenerife,IslasCanarias,Espana
SaraAsgari EnvironmentalandBio-AnalyticalLaboratories,Departmentof Chemistry,SharifUniversityofTechnology,Tehran,Iran
SankaN.Atapattu CanAmBioresearchInc.,Winnipeg,MB,Canada
HabibBagheri EnvironmentalandBio-AnalyticalLaboratories,Departmentof Chemistry,SharifUniversityofTechnology,Tehran,Iran
A.Ballester-Caudet MiniaturizationandTotalAnalysisMethods(MINTOTA) ResearchGroup,DepartamentdeQuímicaAnalítica,FacultatdeQuímica.Universitat ofValencia,Valencia,Spain
FrancescBorrull DepartmentofAnalyticalChemistryandOrganicChemistry, UniversitatRoviraiVirgili,Tarragona,Spain
Bogusł awBuszewski DepartmentofEnvironmentalChemistryandBioanalytics, FacultyofChemistry,NicolausCopernicusUniversity,Torun,Poland;Centrefor ModernInterdisciplinaryTechnologies,NicolausCopernicusUniversity,Torun, Poland
P.Campíns-Falco MiniaturizationandTotalAnalysisMethods(MINTOTA) ResearchGroup,DepartamentdeQuímicaAnalítica,FacultatdeQuímica.Universitat ofValencia,Valencia,Spain
S.Cardenas DepartamentodeQuímicaAnalítica,InstitutoUniversitariode InvestigacionenNanoquímica(IUNAN),EdificioMarieCurie(anexo),Campusde Rabanales,UniversidaddeCordoba,Cordoba,Spain
BeibeiChen DepartmentofChemistry,WuhanUniversity,Wuhan,Hubei,China
YanlongChen SchoolofChemistry,SunYat-senUniversity,Guangzhou, Guangdong,PRChina
MigueldelaGuardia DepartmentofAnalyticalChemistry,ResearchBuilding, UniversityofValencia,Valencia,Spain
JuliaA.deOliveira LaboratoryofPesticideResidueAnalysis(LARP),Chemistry Department,FederalUniversityofSantaMaria,SantaMaria,RioGrandedoSul, Brazil
M.C.Díaz-Li ~ nan DepartamentodeQuímicaAnalítica,InstitutoUniversitariode InvestigacionenNanoquímica(IUNAN),EdificioMarieCurie(anexo),Campusde Rabanales,UniversidaddeCordoba,Cordoba,Spain
JianweiDong SchoolofChemistry,SunYat-senUniversity,Guangzhou, Guangdong,PRChina
AntonioMartínEsteban DepartamentodeMedioAmbienteyAgronomía,INIA, Madrid,Spain
FrancescA.Esteve-Turrillas DepartmentofAnalyticalChemistry,Research Building,UniversityofValencia,Valencia,Spain
NestorEtxebarria DepartmentofAnalyticalChemistry,FacultyofScienceand Technology,UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain; ResearchCentreforExperimentalMarineBiologyandBiotechnology(PIE), UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain
NuriaFontanals DepartmentofAnalyticalChemistryandOrganicChemistry, UniversitatRoviraiVirgili,Tarragona,Spain
LeonFuks CentreofRadiochemistryandNuclearChemistry,InstituteofNuclear ChemistryandTechnology,Warszawa,Poland
KennethG.Furton InternationalForensicResearchInstitute,Departmentof ChemistryandBiochemistry,FloridaInternationalUniversity,Miami,FL,United States
BelénGonzalez-Gaya DepartmentofAnalyticalChemistry,FacultyofScienceand Technology,UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain; ResearchCentreforExperimentalMarineBiologyandBiotechnology(PIE), UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain
ManHe DepartmentofChemistry,WuhanUniversity,Wuhan,Hubei,China
IrenaHerdzik-Koniecko CentreofRadiochemistryandNuclearChemistry, InstituteofNuclearChemistryandTechnology,Warszawa,Poland
R.Herraez-Hern andez MiniaturizationandTotalAnalysisMethods(MINTOTA) ResearchGroup,DepartamentdeQuímicaAnalítica,FacultatdeQuímica.Universitat ofValencia,Valencia,Spain
BinHu DepartmentofChemistry,WuhanUniversity,Wuhan,Hubei,China
AbuzarKabir InternationalForensicResearchInstitute,DepartmentofChemistry andBiochemistry,FloridaInternationalUniversity,Miami,FL,UnitedStates
HianKeeLee NationalUniversityofSingaporeEnvironmentalResearchInstitute, NationalUniversityofSingapore,Singapore,Singapore;NUSGraduateSchoolfor IntegrativeSciencesandEngineering,NationalUniversityofSingapore,Singapore, Singapore;DepartmentofChemistry,NationalUniversityofSingapore,Singapore, Singapore;TropicalMarineScienceInstitute,NationalUniversityofSingapore, Singapore,Singapore
GongkeLi SchoolofChemistry,SunYat-senUniversity,Guangzhou,Guangdong, PRChina
YanxiaLi SchoolofChemistry,SunYat-senUniversity,Guangzhou,Guangdong, PRChina
A.I.Lopez-Lorente DepartamentodeQuímicaAnalítica,InstitutoUniversitariode InvestigacionenNanoquímica(IUNAN),EdificioMarieCurie(anexo),Campusde Rabanales,UniversidaddeCordoba,Cordoba,Spain
R.Lucena DepartamentodeQuímicaAnalítica,InstitutoUniversitariodeInvestigacionenNanoquímica(IUNAN),EdificioMarieCurie(anexo),CampusdeRabanales,UniversidaddeCordoba,Cordoba,Spain
FaranakManshaei EnvironmentalandBio-AnalyticalLaboratories,Departmentof Chemistry,SharifUniversityofTechnology,Tehran,Iran
RosaM.Marcé DepartmentofAnalyticalChemistryandOrganicChemistry, UniversitatRoviraiVirgili,Tarragona,Spain
MohammadMahdiMoein DepartmentofClinicalNeuroscience,Centerfor PsychiatryResearch,KarolinskaInstitutetandStockholmCountyCouncil, Stockholm,Sweden
Y.Moliner-Martinez MiniaturizationandTotalAnalysisMethods(MINTOTA) ResearchGroup,DepartamentdeQuímicaAnalítica,FacultatdeQuímica.Universitat ofValencia,Valencia,Spain
C.Molins-Legua MiniaturizationandTotalAnalysisMethods(MINTOTA) ResearchGroup,DepartamentdeQuímicaAnalítica,FacultatdeQuímica.Universitat ofValencia,Valencia,Spain
RosaMontes DepartmentofAnalyticalChemistry,NutritionandFoodScience, IIAA InstituteforFoodAnalysisandResearch,UniversidadedeSantiagode Compostela,SantiagodeCompostela,Spain
AlineL.H.Muller LaboratoryofPesticideResidueAnalysis(LARP),Chemistry Department,FederalUniversityofSantaMaria,SantaMaria,RioGrandedoSul, Brazil
NyiNyiNaing NationalUniversityofSingaporeEnvironmentalResearchInstitute, NationalUniversityofSingapore,Singapore,Singapore
OscarNu ~ nez DepartmentofChemicalEngineeringandAnalyticalChemistry,UniversityofBarcelona,Barcelona,Spain;SerraHunterFellow,GeneralitatdeCatalunya, Barcelona,Spain
MaitaneOlivares DepartmentofAnalyticalChemistry,FacultyofScienceand Technology,UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain; ResearchCentreforExperimentalMarineBiologyandBiotechnology(PIE),UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain
RosaAnaPérez DepartamentodeMedioAmbienteyAgronomía,InstitutoNacionaldeInvestigacionyTecnologíaAgrariayAlimentaria(INIA),Madrid,Spain
ValériePichon DepartmentofAnalytical,BioanalyticalSciencesandMiniaturization,UMR,CBI,ESPCIParis,PSLResearchUniversity,Paris,France;Sorbonne University,Paris,France
ColinF.Poole DepartmentofChemistry,WayneStateUniversity,Detroit,MI, UnitedStates
OsmarD.Prestes LaboratoryofPesticideResidueAnalysis(LARP),Chemistry Department,FederalUniversityofSantaMaria,SantaMaria,RioGrandedoSul, Brazil
AilettePrieto DepartmentofAnalyticalChemistry,FacultyofScienceandTechnology,UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain;Research CentreforExperimentalMarineBiologyandBiotechnology(PIE),Universityofthe BasqueCountry(UPV/EHU),BasqueCountry,Spain
MariaEugêniaC.Queiroz DepartamentodeQuímica,FaculdadedeFilosofi a CiênciaseLetrasdeRibeiraoPreto,UniversidadedeSaoPaulo,RibeiraoPreto,SP, Brasil
JoséBenitoQuintana DepartmentofAnalyticalChemistry,NutritionandFood Science,IIAA InstituteforFoodAnalysisandResearch,UniversidadedeSantiago deCompostela,SantiagodeCompostela,Spain
MaríaRamil DepartmentofAnalyticalChemistry,NutritionandFoodScience, IIAA InstituteforFoodAnalysisandResearch,UniversidadedeSantiagode Compostela,SantiagodeCompostela,Spain
OmidRezvani EnvironmentalandBio-AnalyticalLaboratories,Departmentof Chemistry,SharifUniversityofTechnology,Tehran,Iran
RosarioRodil DepartmentofAnalyticalChemistry,NutritionandFoodScience, IIAA InstituteforFoodAnalysisandResearch,UniversidadedeSantiagode Compostela,SantiagodeCompostela,Spain
Miguel AngelRodríguez-Delgado DepartamentodeQuímica,UnidadDepartamentaldeQuímicaAnalítica,FacultaddeCiencias,UniversidaddeLaLaguna (ULL).AvenidaAstrofísicoFranciscoSanchez,SanCristobaldeLaLaguna,Tenerife, Espa~ na
RuthRodríguez-Ramos DepartamentodeQuímica,UnidadDepartamentalde QuímicaAnalítica,FacultaddeCiencias,UniversidaddeLaLaguna(ULL).Avenida AstrofísicoFranciscoSanchez,SanCristobaldeLaLaguna,Tenerife,Espana
JackM.Rosenfeld DepartmentofPathologyandMolecularMedicine,McMaster University,Hamilton,ON,Canada
YoshihiroSaito DepartmentofAppliedChemistryandLifeScience,Toyohashi UniversityofTechnology,Toyohashi,Aichi,Japan
AlvaroSantana-Mayor DepartamentodeQuímica,UnidadDepartamentalde QuímicaAnalítica,FacultaddeCiencias,UniversidaddeLaLaguna(ULL).Avenida AstrofísicoFranciscoSanchez,SanCristobaldeLaLaguna,Tenerife,Espana
JavierSaurina DepartmentofChemicalEngineeringandAnalyticalChemistry, UniversityofBarcelona,Barcelona,Spain
SoniaSentellas DepartmentofChemicalEngineeringandAnalyticalChemistry, UniversityofBarcelona,Barcelona,Spain
BarbaraSocas-Rodríguez DepartamentodeQuímica,UnidadDepartamentalde QuímicaAnalítica,FacultaddeCiencias,UniversidaddeLaLaguna(ULL).Avenida AstrofísicoFranciscoSanchez,SanCristobaldeLaLaguna,Tenerife,Espana
IsraelD.Souza DepartamentodeQuímica,FaculdadedeFilosofiaCiênciaseLetras deRibeir aoPreto,UniversidadedeSaoPaulo,Ribeir aoPreto,SP,Brasil
Mał gorzataSzultka-Mł ynska DepartmentofEnvironmentalChemistryand Bioanalytics,FacultyofChemistry,NicolausCopernicusUniversity,Torun,Poland
JoséL.Tadeo DepartamentodeMedioAmbienteyAgronomía,InstitutoNacional deInvestigacionyTecnologíaAgrariayAlimentaria(INIA),Madrid,Spain
SzeChiehTan NUSGraduateSchoolforIntegrativeSciencesandEngineering, NationalUniversityofSingapore,Singapore,Singapore;DepartmentofChemistry, NationalUniversityofSingapore,Singapore,Singapore
EstherTuriel DepartamentodeMedioAmbienteyAgronomía,INIA,Madrid, Spain
IkuoUeta DepartmentofAppliedChemistry,UniversityofYamanashi,Kofu, Yamanashi,Japan
AresatzUsobiaga DepartmentofAnalyticalChemistry,FacultyofScienceand Technology,UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain; ResearchCentreforExperimentalMarineBiologyandBiotechnology(PIE), UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain
J.Verdu-Andrés MiniaturizationandTotalAnalysisMethods(MINTOTA) ResearchGroup,DepartamentdeQuímicaAnalítica,FacultatdeQuímica.Universitat ofValencia,Valencia,Spain
LingXia SchoolofChemistry,SunYat-senUniversity,Guangzhou,Guangdong, PRChina
RenatoZanella LaboratoryofPesticideResidueAnalysis(LARP),Chemistry Department,FederalUniversityofSantaMaria,SantaMaria,RioGrandedoSul, Brazil
ShakibaZeinali EnvironmentalandBio-AnalyticalLaboratories,Departmentof Chemistry,SharifUniversityofTechnology,Tehran,Iran
NaiyuZheng DepartmentofBioanalyticalSciences,Bristol-MyersSquibb Company,Princeton,NJ,UnitedStates
OlatzZuloaga DepartmentofAnalyticalChemistry,FacultyofScienceand Technology,UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain; ResearchCentreforExperimentalMarineBiologyandBiotechnology(PIE), UniversityoftheBasqueCountry(UPV/EHU),BasqueCountry,Spain
Coreconceptsandmilestonesin thedevelopmentofsolid-phase extraction
ColinF.Poole DepartmentofChemistry,WayneStateUniversity,Detroit,MI,UnitedStates
1.1Introduction
Theoriginsofsolid-phaseextractionareasoldaschromatography,whichinitsearly dayswasexploitedfortheisolationofcompoundsfrommixturesbytheirselective interactionwithasolidstationaryphaseandsubsequentrecoverybyelutionina mobilephase.Chromatographyandextractionhavesincedivergedintheirgeneral functioninchemicalanalysisandareregardedascomplementarytechniquestoday. Extractionistypicallyemployedforisolation,preconcentration,matrixsimplification, orsolventexchangeaheadoftheseparationandidentifi cationofcompoundsby chromatographic-based(andother)techniques.Thekeytounderstandingtherelationshipbetweenthesecommonlaboratorytechniquesistoconsiderextractionasan enablingtechniquethatmodi fiessamplepropertiestofacilitateasuccessfulseparation anddetectionoftargetcompoundsbythemostappropriatetechnique.Intheabsence ofanextractionstepthesamplewouldappeartobetoocomplex,toodiluteorincompatiblewithsustaininginstrumentperformancerenderingtheanalysisunsuccessful. Overtimethescale,speed,materialcosts,andlevelofautomationfortheextraction stephaveadaptedtochanginglaboratoryneeds.Thus,solid-phaseextraction,one variantofextractionmethods,isadynamic fi eld,andwhileold,itisstillheavily researchedwiththe fluxofadvancesfarfromconcluded.Atthetimeofwriting,it isreasonabletoidentifyminiaturization,advancesinmaterialscience,easeofautomation,andcompatibilitywiththegoalsofgreenanalyticalchemistryastheprimary drivingforcesmaintainingthegeneralinterestinadvancingthetechniquesofsolidphaseextraction [1 3].
1.2Firstgenerationformats
Solid-phaseextractionisbasedonthetransferoftargetcompoundsinagas,liquid,or supercritical fluidmatrixtoasolidsorbent [4].Typically,thesamplecontainingthe targetcompounds fl owsoverthesolidsorbentwhichretainsthecompoundsbytheir favorableinteractionswiththesorbent.Thesorbentissubsequentlyseparatedfromthe sampleandthetargetcompoundsrecoveredbysolventdisplacementorthermal desorptionintothegasphase.Anearlyapplicationofsolid-phaseextractioninthe
1950swastheuseofactivatedcarbon- filledcolumnstoisolateorganiccontaminants fromsurfacewatersfortoxicityevaluation [5].Thelowconcentrationofcontaminants andthepoorcapabilityofinstrumentalmethodstoidentifycompoundsandassesstheir toxicityatthattimeresultedinlarge-scaleoperationsinwhichthousandsoflitersof waterweresampledoverseveraldays.Theintroductionofmacroreticularporous polymersintheearly1970swasresponsibleforredirectinginterestinsolid-phase extractionforboth fieldandlaboratoryapplicationsaswellasextendingitsscopeto airsamplingandtheisolationofdrugsfrombiological fl uids.Thesesorbentshad reasonablemechanicalstrength,alargesurfacearea,alargesamplecapacity,low waterretention,andprovidedhighrecoveryoftargetcompoundsbysolventorthermal desorption.Comparedwithactivatedcarbontheoverallrecoveryoftargetcompounds wasgenerallybetterandirreversibleadsorptionandcatalyticactivitygreatlydiminished.Thesepropertiestogetherwithfurtherimprovementsininstrumentalmethods facilitatedageneraldownsizingofsorbentbeds,areductioninsamplesize,and increasinguseofsolid-phaseextractionasagenerallaboratorytechniqueforawider rangeofapplicationsthanwaspreviouslythecase [6,7].Porouspolymersofhighthermalstabilityandlowwaterretentionwereresponsibleforrevolutionizingtheanalysis ofvolatileorganiccompoundsinairandpurgegassamplesfromdynamicstrippingof volatileorganiccompoundsfromaqueoussolution.Compoundstrappedonthe sorbentbedwerethermallydesorbeddirectlyintoagaschromatographforanalysis eventuallyleadingtofullyautomatedsamplingandanalysissystemsforroutineuse [8,9].Thegeneralacceptanceofsolid-phaseextractionforsamplingliquids,however, occurredlaterintheearly1980swiththeintroductionofdisposablecartridgedevices containingsilica-basedchemicallybondedsorbentsofasuitableparticlesizeforsampleprocessingbygentlysuction [10 14].Withinafewyearscartridge-basedsolidphaseextractionwasconsideredasuitablealternativetoliquid-liquidextractionfor manyapplicationsandenteredaperiodofevolutionarychange.Typicalcartridge devicesconsistofshortcolumns(generallyanopensyringebarrel)containingsorbent withanominalparticlesizebetween20and60 mm,preferablywithanarrowparticle sizerange,packedbetweenporousplasticormetalfrits, Fig.1.1.Awiderangeof sorbentchemistries(silica-basedchemicallybonded,mixedmode,porouspolymer, restrictedaccessmedia,molecularlyimprintedpolymers,immunosorbent,bonded cryptands,etc.)areavailabletodayprovidingforthediverseapplicationbaseof moderncartridge-basedsolid-phaseextraction [12 14].Low-volumecartridgesor precolumndevicessoonappearedasthebasisofonlineintegratedsystemsforautomationofthesamplingandseparationprocesses,inforexample,solid-phaseextraction (SPE)-liquidchromatography(LC),SPE-gaschromatography(GC),SPE-capillary electrophoresis(CE),SPEinductivelycoupledplasmaspectroscopy(ICP)and LC-SPE-nuclearmagneticresonancespectroscopy(NMR).Bythemid-1990sthese systemshadmaturedintorobustpracticalsystemsinuseinmanylaboratorieswith ahighsampleworkloadandlittlevariationinsamplematrix,forexample,drugsin biological fluids,contaminantsinsurfacewaters,targetcompoundsinfoodextracts, etc. [15 18].Standardsolid-phaseextractionprocedureslendthemselvestoautomationusingroboticplatformsorspecialpurposeprocessingunitsthatsimultaneously extractandpreparesamplesforseparation [12,19].Multiwellplateswithasorbent
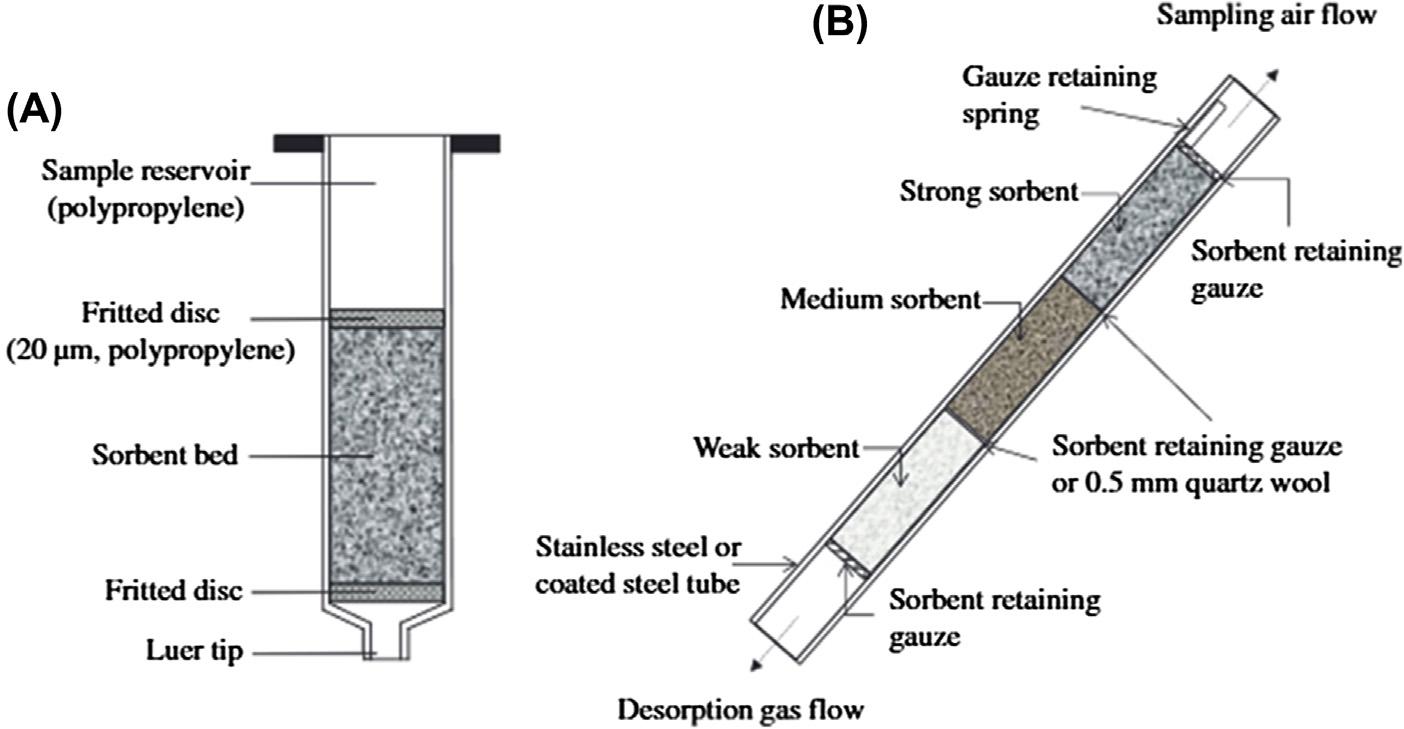
Figure1.1 Solid-phaseextractionusingacartridgedeviceforliquidsamples(A)andgas-phase samples(B).
bedatthebottomofthewellcombinedwithliquidhandlingrobotsaresuitablefor high-throughputparallelsampleprocessing [20].
Cartridge-basedsolid-phaseextractionwasinitiallymarketedasareplacementfor traditionalliquid-liquidextraction.Traditionalliquid-liquidextractionmethodswere viewedaslaborintensive,dif ficulttoautomate,andfrequentlyplaguedbypractical problems,suchasemulsionformation.Inaddition,theytendtoconsumelarge volumesofhighpuritysolvents,someofwhichareconsideredsignifi canthealth hazardswithhighdisposalcosts [5,12,21].Cartridge-basedsolid-phaseextractionprocedures,ontheotherhand,weremoreeconomical,affordedshortersampleprocessing times,consumedlesssolvent,andallowedforsimplersamplehandlingprocedures. Theyarealsomoreconvenientfor fieldsamplingbecausetheyminimizethetransport andstorageproblemsassociatedwithbulksamples,whichhavetobereturnedtothe laboratoryforprocessing.Thesearguments,althoughpersuasiveatthetime,arenotas truetoday.Modernliquid-liquidextractionproceduresintheirseveralforms,known collectivelyasliquidphasemicroextraction,addressmanyoftheproblemsassociated withtraditionalliquid-liquidextractionandcompeteeffectivelyandcomplement solid-phaseextractionprocedures [22 24].
Itshouldnotbeoverlookedthatcartridge-basedsolid-phaseextractionhasitsown, albeitdifferent,problemstothoseofliquid-liquidextraction.Theextractionproperties ofsolid-phasesorbentsarenotasreproducibleasthoseforsolvents.Typicalsorbent surfacescontainmorethanonefunctionalgroupinamountsnoteasilyreplicatedby synthesis.Inaddition,theirsorptionpropertiesaretypicallymoreadverselyaffected bycontaminants.Sorbentsalsotendtohaveahigherlevelofextractablecontaminants originatingfromthemanufacturingprocessandfrompackagingmaterials.This chemicalbackgroundmayinterfereinthesubsequentsampleanalysis.Rinsingof thesorbentbedpriortouseandusingblankstoestablishbackgroundcontamination levelsdiminishessamplethroughputandaddssignificantlytosolventconsumption
andsampleprocessingcosts.Sorbentsarealsoaffectedbysampleprocessingconditions,suchasoverloading,displacementoftargetcompoundsbyexcessmatrix,and blockingofsorbentpores.Theseproblemseasilygounnoticedresultinginunforeseen changesintherecoveryoftargetcompounds.
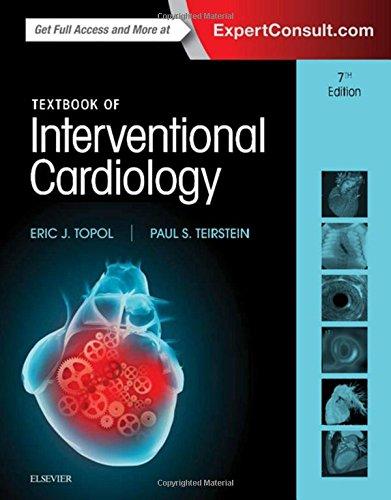
Solid-phasemicroextraction(SPME)wasintroducedin1990asanalternative approachtocartridge-basedsolid-phaseextraction,whichwaswellestablishedby thistime [3,25].Itissometimesthoughtofasaminiaturizedversionofsolidphaseextractionasimpliedbyitsname,but thisisnotthecase.Thedownscaling ofsolid-phaseextractiontoaccommodatesmallsamplevolumes,butotherwise incorporatingthesameextractionprinciples,iscorrectlyknownasporousmembrane protectedmicro-solid-phaseextraction,orsimplymicro-solid-phaseextraction ( mSPE)and fi rstappearedin2006 [26,27].Itemploysasmallsorbentbedenclosed inaporousmembraneallowingsolventandlow-masscompoundsaccesstothesorbentbedwhileexcludingmacromolecule s.Theratioofsample volumetosorbent surfacearea(orvolume)islowintherelativesensebutlargecomparedwiththe fi ber formatusedinsolid-phasemicroextraction, andexhaustiveextractionoftargetcompoundsremainsthegeneralgoal.The fi berformatemploysathinlayerofimmobilizedextractionphasecoatedontheoutsideofafused-silicaormetal-wiresupport. Theextraction fi berisattachedtotheplungerofamodifi edmicrosyringewhichboth protectsandfacilitatesmanipulationofthe fi berduringsampling, Fig.1.2[28] .The maindifferencebetweensolid-phasemicroextractionandsolid-phaseextractionis theextremeratioofthesampletosorbentvolumeorsurfacearea.Anextremelysmall amountofsorbentisutilizedincomparisontothesamplevolumeinsolid-phase microextractionandexhaustiveextractionoftargetcompoundsisnotfavored.Typicallyonlyasmallportionofthetargetcompoundsisextractedfromthesample (negligibledepletionunlessthedistributi onconstantisunusuallylarge).Thisamount increaseswiththeextractiontimeuntile quilibriumconditionsarereached,asillustratedby Fig.1.3[29] .Forcalibrationeitherthelinearportionofthepreequilibrium oratornearequilibriumconditionsareselected [3,30,31].Thetimetoreachequilibriumcanbelong,inwhichcasethelinearportionofthepreequilibriumcurvebecomesthepreferredchoice.Extractionin thepreequilibriumregioniskinetically controlledandmasstransferisdominated bydiffusion(stagnantsolution)orby themethodofagitation(agitatedsolution) .Theselectivityoftheextractionprocess isusuallypoorinthepreequilibriumregioncomparedwithequilibriumsampling. Thelatteriscontrolledbythedifferencesin theextractionphase-samplesolutiondistributionconstants.Thesmallvolumeofextractionphasefavorsapplicationsin fi eld sampling(spotandtimeweightedaverage), asitisunnecessarytodeterminethesamplevolumeprocessed.Theamountofextractedtargetcompoundsisindependentof thesamplevolumesolongastheproductofthedistributionconstantandextraction phasevolume(orsurfacearea)issmallcomparedwiththesamplevolume [29,32].
Solid-phasemicroextractioncanbeusedforsamplingbydirectimmersion, headspaceextraction,orextractionwithmembraneprotection [28].Itintegratessampling,extraction,concentration,andsampleintroductionintoasinglesolvent-freestep forgaschromatographyandmassspectrometryandwithminimalsolventusefor liquidchromatographyandcapillaryelectrophoresis.Sampleprocessingrequires
Coreconceptsandmilestonesinthedevelopmentofsolid-phaseextraction5
Figure1.2 Deviceforsolid-phasemicroextractionutilizinga fiberformat.
twosteps:thedistributionoftargetcompoundsbetweentheextractionphaseandthe samplematrixanddesorptionoftheextractedcompoundsbythermaldesorption (solventlessextraction)orsolventdesorption.Onlyasmallvolumeofsolventis requiredforsolventdesorptionduetothesmallvolumeofextractionphase.Thus, theconcentrationoftargetcompoundsinthedesorptionsolventisrelativelyhigh eventhoughtheamountextractedisconsiderablylessthanforexhaustiveextraction. Theminimaldilution,orcompletetransferinthecaseofgasphasedesorption, compensatesforthelowabsoluteamountofextractedcompounds.Theoftencited advantagesofsolid-phasemicroextractionareitseaseofminiaturization,easeofautomation,andstraightforwardcouplingwithdifferentmeasurementsystems [2,3,33,34] Themainfactorsaffectingsamplingefficiencyinclude:theextractionphasechemistry, extractionmode,agitationmethod,samplemodification(pH,ionicstrength,presence oforganicsolvent,etc.),extractiontime,anddesorptionconditions.Limitations includethesmallnumberofcommerciallyavailableextractionphases,thefragile natureoffused-silica fibers,limited fiberreusabilityduetocarryoverormatrix contamination,andtheshortlifetimeofphysicallycoated fibers.Problemsaremore

Figure1.3 Extractiontimeprofileforsamplingwithacoated fiber(solid-phase microextraction).Amountofanalyteextracted ¼ n(ne atequilibrium),Kfs ¼ analyte distributionconstantbetweentheextractionphaseandsample,Vf ¼ volumeofextractionphase, Vs ¼ samplevolume,andCs ¼ analyteconcentrationinthesamplematrix. ReproducedfromSouza-silvaEA,JiangR,Rodriguez-LafuenteA,GionfriddoE,PawliszynJ. Acriticalreviewofthestateoftheartofsolid-phasemicroextractionofcomplexmatricesI. Environmentalanalysis.TrendsAnalChem2015;71:224 235withpermission.
commonwithdirectimmersioninsamplesofhighcomplexity,suchasbiologicaland foodsamples,forwhichthedevelopmentofbiocompatible fibercoatingsisanactive researcharea [35 37].
1.3Secondgenerationformats
Thelowpackingdensitytypicalofcartridgedevicesresultsintheuseoflongersorbent bedsthannecessarytocompensateforreducedretentionduetochanneling [38].The largerbedmass,inturn,increasesnonspecificmatrixadsorptionresultinginan increaseincontaminationofextracts.Thediskformatutilizessmallerparticlesina morestablepackingconfigurationtominimizechanneling,affordingacleanerchemical backgroundthroughreducedmatrixadsorption.Solid-phaseextractiondisksareavailableinatleastthreedifferentformats.Particle-loadedmembranes,introducedin1990, consistofawebofpolytetrafluoroethylene(PTFE)microfibrils,suspendedinwhichare sorbentparticlesofabout8 12 mmindiameter [39,40].Thesemembranesare flexible withahomogeneousstructurecontainingupwardsof90%(w/w)sorbentparticlesinthe formofcirculardisksabout0.5mmthickwithdiametersfrom4to96mm.Forgeneral usetheyaresupportedonasinteredglassdisk(orothersupport)inastandard filtration apparatususingsuctiontogeneratethedesired flowthroughthemembrane. Particle-loadedmembranesarealsoavailableinasyringebarrel(cartridge)format.In thiscase,thesorbentbedcontainsparticlesofalargerdiameter,about50 mm,inthicker disks,about1.0mm,sealedintothebaseofanopensyringebarrel.Particle-embedded
glass fiberdiskscontain10 30 mmdiametersorbentparticleswovenintoaglass fiber matrix [41].Thesmall-diameterdisksarerigidandself-supporting,whilethelarger diameterdisksrequireasupportingstructuresimilartoparticle-loadedmembranes. Laminardisksconsistofasandwichof10 mmsorbentparticlesinaconsolidated 0.5 1.0mmbedlocatedbetweentwoglass-fiber filters,withascreentoholdthe filters inplace [10].The50mmdiameterlaminardisksaretypicallymountedinanopen syringebarrelsuperficiallysimilartoconventionalcartridgedevices [10].
Theslowsampleprocessingratesforlargesamplevolumestypicalofcartridgesand theirlowtolerancetoblockagebyparticlesandsorbedmatrixcomponentsprovided theinitialinterestindisktechnologyforenvironmentalapplications,suchasthe analysisofsurfacewatersfortracecontaminants [40].Onaccountoftheirlarger cross-sectionalareaanddecreasedpressuredropdisksallowtheuseofhighersample flowratesresultinginshortersampleprocessingtimes [38].Anintegralprefilter attachedtothetopsurfaceofthediskreducespluggingbysuspendedparticles. Small-diameterdisksaresuitableforhandlingsamplesofrestrictedvolume.Smalldiameterdisksfacilitateintegratedsampleprocessingtechniques,suchasin-vial desorptionandon-diskderivatization [42].Thelargesurfaceareaperunitbedmass ofdisksfacilitatestheiruseforpassivesamplinginwhichthediskissuspendedin thesampleasopposedtotheconventionalapproachofpassingthesamplethrough thediskinamannersimilarto filtration.Theslowequilibriumoftheextractionprocess,evenforagitatedsolutions,however,isalimitationforlaboratoryapplications butislessofaconcernfor fieldstudies [43].Particle-loadedmembraneshavebeen utilizedforbiomimeticextractionassurrogatemodelsforbioconcentrationandfor toxicityriskassessment [44].Disktechnologycontributeddirectlytotheautomation ofsolid-phaseextractionmethodsthroughthedevelopmentofmultiwellextraction plates,usedforthecleanupofsamplesinhigh-throughputscreeningtechniquesin drugdevelopment [20].Thecharacteristicphysicalpropertiesofdiskshavesupported theirapplicationforintegratedsampling/detectiontechniquessuchas insitu radiochemical,phosphorescence,andX-ray fluorescencedetectionandasasubstratefor MALDImassspectrometry [45 47]
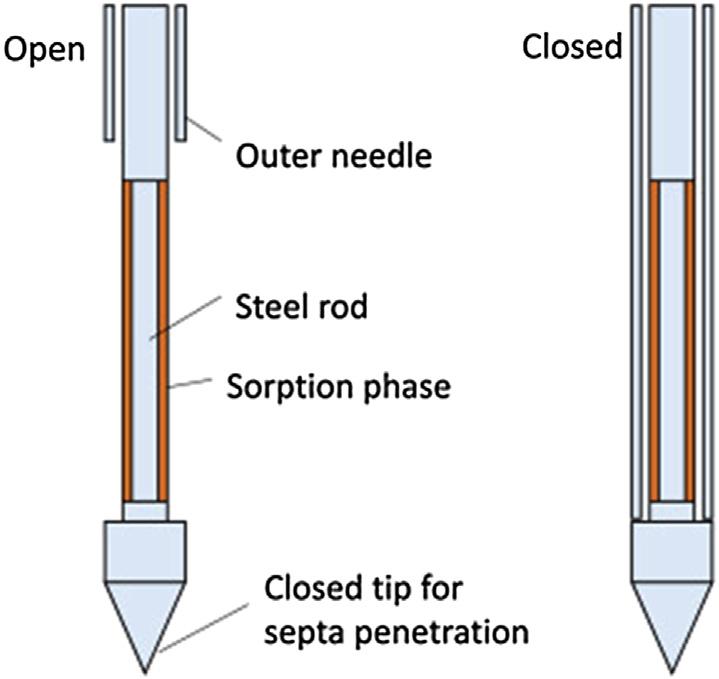
Microextractionbypackedsorbentbed(MEPS)wasintroducedin2004asaminiaturizedversionofcartridge-basedsolid-phaseextractiondesignedforhandlingsmallsamplevolumeswithaviewtoeasyautomationusingalaboratoryliquidhandler [48,49].Theextractiondeviceistypicallya100 250 mLsyringehousingashort bedcontaining1 2mgsorbentlocatedeitherbetweentheplungerandtheneedle orbuiltintotheneedle, Fig.1.4.TheMEPSsyringefacilitateslow-deadvolume sampleprocessingbyverticalmovementoftheplungerwithsampleandsolvent flowpossibleinbothdirectionsthroughthesorbentbedinacyclicfashion.Thisis incontrasttoconventionalsolid-phaseextractiontechniqueswhichutilizeaunidirectional flowandasinglecontactbetweenthesampleandsolventsandthesorbentbed. Needle-basedextractionformatsnowincludeinternallycoatedneedles [50] andneedle trapdeviceswithsorbent-packedneedles [50,51].Internallycoatedneedlestypically employastainlesssteelneedleinternallycoatedwitha50 mmthick filmofimmobilizedstationaryphaseresultinginanopenstructuresimilartoopentubularcolumnsin gaschromatography.Typicalapplicationsincludeautomatedheadspacesamplingof Coreconceptsandmilestonesinthedevelopmentofsolid-phaseextraction7

Figure1.4 Modifiedsyringeformicroextractionbypackedsorbentwithanexpandedviewof thesorbentbed(canister).Thecanisterhasadeadvolumeofabout7 mL. ReproducedfromMoeinMM,Abdel-RehimA,Abdel-RehimM.Microextractionbypacked sorbent(MEPS).TrendsAnalChem2015;67:34 44withpermission.
volatileorganiccompoundsreferredtoassolid-phasedynamicextraction(SPDE). Needletrapsconsistofaspeciallydesignedstainlesssteelneedlepackedwithadsorbent.Forextractiona fixedvolumeofagasorliquidsampleispassedthroughthe extractionneedlewithrecoveryoftargetcompoundsbythermaldesorption,orless commonly,bysolventdesorption.Needletrapshavealsobeenusedaspassive samplingdevicesforgasphasesamples.Needletrapsaremoredurablethansolidphasemicroextraction fibersandhaveahigherextractioncapacitywiththepossibility ofexhaustiveextraction.
Theextractionrateandsensitivityof fi ber-basedsolid-phasemicroextraction methodscanbeenhancedbyincreasingthevolumeand/orsurfaceareaofthe extractionphase.Simplyincreasingthethicknessoftheextractionphaseinthe fiber formatresultsinlongextractiontimesandalternativeformatshavebeenexploredto tacklethisissueresultinginthedevelopmentofin-tube,stirbar,rotatingdisk, thin-film,anddispersedparticleapproaches [2,3,52,53].Animportantperformance parameterforsolid-phasemicroextractionistheequilibriumtime(thetimerequired toreach95%equilibriumwherestatisticallynodifferenceintheamountextracted isobservedbyextendingthesamplingtime).Shorterequilibrationtimescanbe obtainedbyutilizingageometryfortheextractionphasewithahighersurface area-to-volumeratiothanthecylindricalgeometryof fiber-basedsolid-phasemicroextractiondevices.Thesolid-phasemicroextractionarrow, Fig.1.5,consistsofasteel
Coreconceptsandmilestonesinthedevelopmentofsolid-phaseextraction9
Figure1.5 Arrowsolid-phasemicroextractiondevicewithexposedsorbent(samplingmode) leftandinjection(covered)moderight.
ReproducedfromHelinA,RonnkoT,ParshintserK,HartonenK,SchillinB,LaubliT,Riekkola ML.Solid-phasemicroextractionarrowforthesamplingofvolatileaminesinwastewaterand atmosphere.JChromatogrA2015;1426:56 63withpermission.
rodoflargerdiameterthanaconventional fibercoatedwithalargeramountofextractionphase,whilestillbeingcompatiblewiththermaldesorptioninastandardinjection linerofagaschromatographonaccountofitsdimensionsandsharp,closedtip [50,54].Alternativelythesurfacearea-to-volumeratiooftheextractionphasecan beincreasedusingathin-filmformatinwhichtheextractionphaseisimmobilized ontheoutersurfaceofasupportofsuitablegeometry, Fig.1.6[29,55,56].The thin-filmgeometryisthebasisforfullyautomatedparallelsampleprocessingina
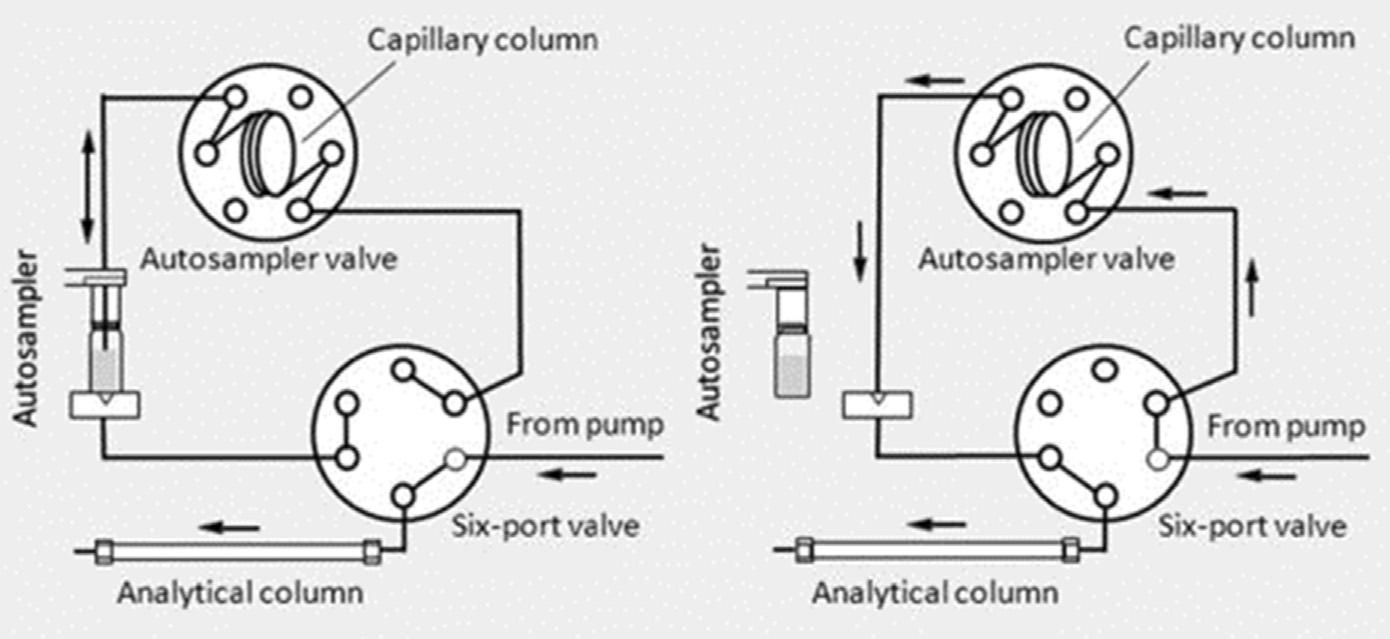
Figure1.6 In-tubesolid-phasemicroextractionwiththeextractioncolumnutilizedasthe sampleloopoftheinjectionvalveofaliquidchromatograph.Thevalveisshowninthe extractionposition(load)ontheleftandextractinjectionposition(inject)ontheright.
ReproducedfromQueirozMEC,MeloLP.Selectivecapillarycoatingmaterialforin-tubesolidphasemicroextractioncoupledtoliquidchromatographytodeterminedrugsandbiomarkersin biologicalsamples.Areview.AnalChimActa2014;826:1 11withpermission.
96-wellplateformatutilizinganextractionunitfashionedintoa96-bladedevicein whichtheextractionphaseiscoatedoverthe fl atendofeachblade [33,52,55].
Anearlyapproachforautomatedsolid-phasemicroextractionfrom1997wasthe couplingofaninternallycoatedcapillarycolumn,theextractiondevice,toaliquid chromatographforseparationanddetectionknownasin-tubesolid-phasemicroextraction [57 61].Twoapproachesaretypicallyusedtoday [59].Inthe firstapproacha shortcapillarycolumnisplacedbetweentheinjectionloopandinjectionneedleof anautosampler.Theinjectionsyringerepeatedlydrawsandejectssamplesfroma seriesofvialsundercomputercontrolcyclingthesamplethroughthecapillarycolumn intheforwardandreversedirectionforeachsampleuntilequilibriumisreachedor extractionissufficient.Theextractedcompoundsaresubsequentlydesorbedby solventfromtheextractioncolumnandtransportedtotheanalyticalcolumnforseparation.Alternatively,thecapillarycolumnisusedasasampleloopforaninjection valveandtargetcompoundsareextractedfromthesamplewiththevalveintheload positionandthentransferredtothecolumnbymobilephaseafterswitchingthevalve totheinjectposition, Fig.1.6[59].Thisarrangementisfavoredforhandlinglargersamplevolumestoincreasesensitivity.Wire-in-tubeor fiber-in-tubeconfigurationscanbe usedtoenhancetheextractionrateandefficiencybyreducingthephaseratioofthe extractioncolumn.Capillarycolumnsofthetypeusedforgaschromatographyare oftenusedastheextractiondevice,aswellasawiderrangeoflaboratory-madeextractionphasestoenhanceselectivity,forexample,poly(pyrrole),restrictedaccessmedia, immunosorbents,molecularlyimprintedpolymers,monolithicsorbents,etc. [59].Samplesareprocessedsequentiallythroughin-tubesolid-phasemicroextraction,which cannotbeconsideredhigh-throughputwhencomparedwithparallelsampleprocessing usingthe96-wellplateformat.Othergenerallimitationsincludealowextractionefficiencyforsomecompounds,alowsorbentloadingcapacity,instabilityofsomeextractionphases,andlongextractiontimesresultingfrompoormasstransferkinetics.
Stirbarsorptiveextractionwasintroducedin1999andquicklygainedpopularity [62 65].Theextractiondeviceconsistsofamagneticstirbarinaglasssleeveexternallycoatedwitha0.3 1.0mmlayerofextractionphase.Liquidsamplesareanalyzed bydirectimmersionofthestirbarwithvigorousstirringandgasphasesamplesbysuspendingthestirbarintheheadspaceabovethesample.Extractedcompoundsare recoveredbyeitherthermaldesorptionorsolventdesorption.Thelimitednumberof commerciallyavailableextractionphases[poly(dimethylsiloxane),poly(acrylate), andanethyleneglycol/siliconecopolymer]limitgeneralapplicationstotheextraction ofcompoundsoflow-andintermediate-polarityfromwater [63,65].Thelargervolumeofextractionphase,typicallytwoordersofmagnitudeormorecomparedwith fi ber-baseddevices,isresponsiblefortheenhancedextractionefficiencyaswellas thelongerextractiontimesrequiredtoreacheitherequilibriumorsuf ficientextraction. Therelativelylow-selectivityofcommerciallyavailableextractionphasesresultin signi ficantmatrixsorptionwithpossibleinterferenceintheseparationprocess [65] Thesamplingprocessisnoteasytoautomateandaspecialpurpose-designedthermal desorptionunitisrequiredforthesequentialdesorptionofextractedcompoundsfrom stirbarsforgaschromatography [62].Directcontactbetweenthestirbarandthe extractionvesselandthehighstirringratesemployedfordirectimmersionextraction
resultsinalossofcoatingduetofriction,thusreducingthelifetimeofthestirbar. Rotatingdisksorbentextractionattemptstoaddressthisproblemwithanovelstir bardesign [66 68].APTFEdiskwithanembeddedmagneticbaratitsbaseand extractionphasecoatedonlyonthetopsurfaceisused.Analternativedesignhasa cavityonthetopsurfaceloadedwithsorbentandcoveredbyaglass fiber fi lterorsemipermeablemembrane.Thereisnocontactbetweentheextractionphaseandtheextractionvesselduringstirringprolongingthelifetimeofthediskandtheprotectedcavity onthetopsurfaceallowsawiderchoiceofsorbentchemistriestobeexploited.Further developmentsbasedonstirrodandstirmembranedeviceshavebeenproposed [67].
Nanoparticleshavealargesurfacearea-to-volumeratioandmagneticnanoparticles arepromisingextractionmaterialsfordispersivesolid-phaseextraction [69 72].The sorbentmaterialdoesnotneedtobepackedinaparticulartypeofsamplingdevice, unlikefortraditionalsolid-phaseextractionmethods.Thesmallparticlesizeallows theirdispersionthroughoutthesamplevolumetominimizemasstransferproblems andtheirmagneticcorefacilitateseasycollectionwithapermanentmagnet.Theefficientdispersionofthesorbentintothematrixyieldsahighrecoveryoftarget compoundslimitedonlybytheirdistributionconstantsandcleanextractsthatdepends ontheabilityofthesorbenttorejectmatrixcomponents.Thesequenceofstepsinthe extractionprocessisillustratedin Fig.1.7[70].Particlesaretypically1 100nmin diameterandmadeupofseverallayers,typicallywithamagneticcoreofiron,nickel, cobalt,oranyoftheiroxides.Magnetite(Fe3O4)isthemostcommonmagneticcore.
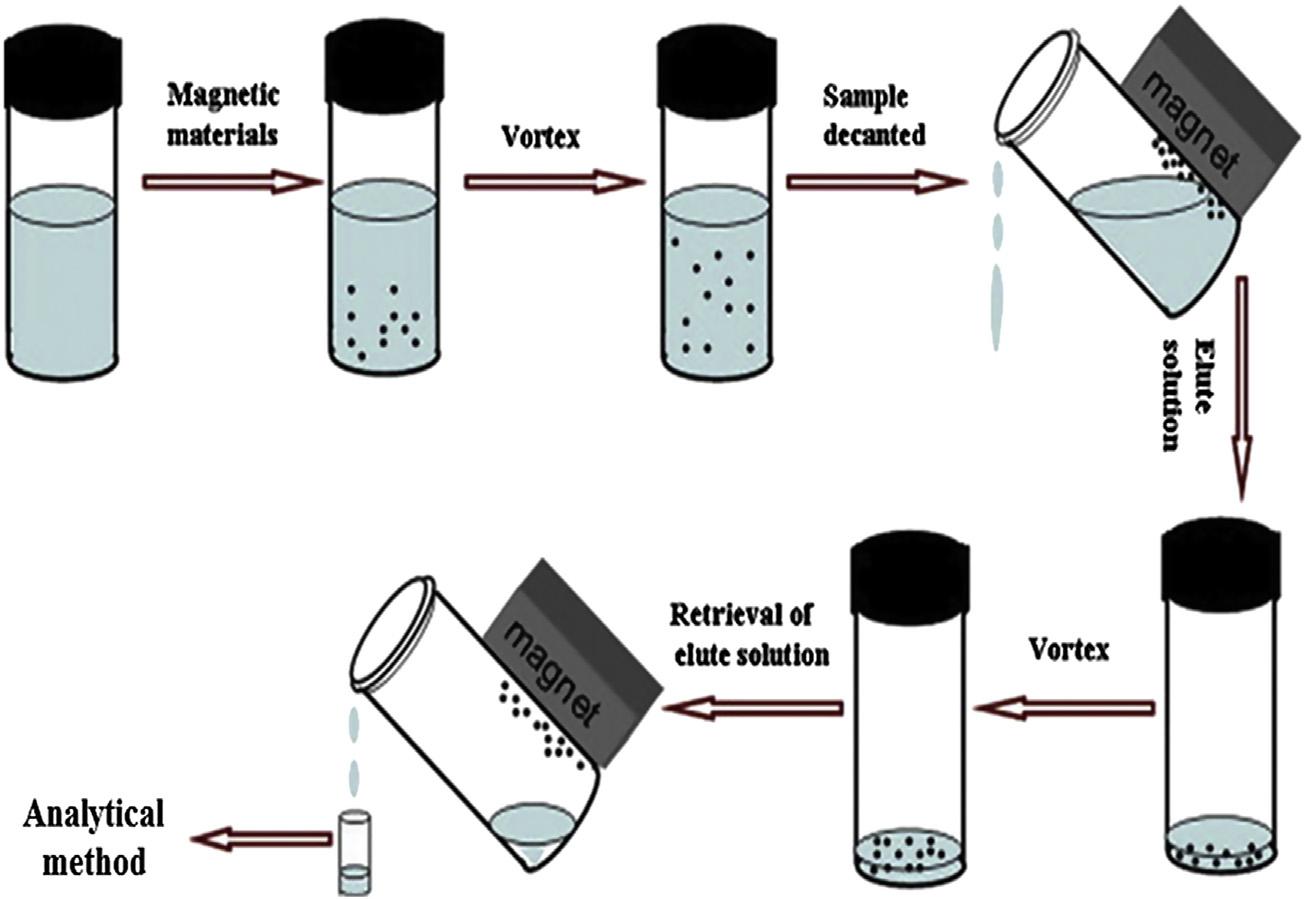
Figure1.7 Proceduralstepsfordispersivesolid-phaseextractionusingmagneticnanoparticles. ReproducedfromWleruckaM,BiziukM.Applicationofmagneticnanoparticlesformagnetic solid-phaseextractioninpreparingbiological,environmentalandfoodsamples.TrendsAnal Chem2014;59:50 58withpermission. Coreconceptsandmilestonesinthedevelopmentofsolid-phaseextraction11
Themagneticcoreistypicallyencasedinashellofinorganicoxide(silica,alumina, manganesedioxide,etc.)orcarbon.Thiscanbecoatedwithpolymerorthesurface modi fiedbychemicalreactiontomodifyselectivity.Alargenumberofsurfacemodifi ednanoparticleshavebeensynthesizedfordispersivesolid-phaseextractionbutonly afewarecommerciallyavailable [71,72]
Nanofibersrepresentanalternativetonanoparticlesforsomeapplicationsinsolidphaseextraction [73].Nanofibersarecontinuouslyspunwireswithtunablediameters inthenanometertomicrometerrange,variableporosity,andlargesurfacearea;they aresynthesizedinthelaboratorybyapplyingahighvoltagebetweenaviscoussolution ofapolymerandacollectorelectrode(intheformofawireforcoatedroddevicesor sheetofconductivematerialformembranedevices).Amatofelectrospun fiberssealed inaspecialholderaffordsasuitabledeviceforconventionalsolid-phaseextractioncharacterizedbyahighretentioncapacityandalowbackpressure.Depositingamatof fibers onthesurfaceofasuitablesubstrateprovidesmaterialsforthin-filmmicroextraction. Positioningananofibersheetintheloopofaninjectionvalveaffordsasuitableinterface forin-tubesolid-phasemicroextractioncoupledtoliquidchromatography.Fiber-coated wiresaresuitableforsolid-phasemicroextraction.Nanofiberscanalsobepackedinto thelowerportionofpipettetips,tofashionextractiondevicesforsamplingsmall volumes.Awiderangeofsinglepolymers,copolymers,composites,andhybridnanoparticleloaded fibershavebeendescribedsofar,butonlyafewhavebeenusedin solid-phaseextraction.Limitedcommercialavailabilityofnanofibersanddevices containingnanofibershasrestrictedtheirapplicationstoexperimentalstudies.
Monolithsprovideanalternativebedstructuretoparticle-packedbedsforsolidphaseextraction [74 77].Amonolithisasinglerodofbiporousorganicorinorganic polymerwithauniformstructureconsistingof flow-throughpores(1 2 mmindiameter)providinghighpermeability(lowbackpressure)andmesoporesprovidingahigh surfaceareaforretentionandsamplecapacity.Organicmonolithsareeasilyprepared in(usually)aone-stepprocessinasuitablemoldcontainingmonomers(styrene,acrylate,methacrylate,etc.),cross-linkingagent(dimethacrylate,divinylbenzene,etc.), porogen,andafreeradicalinitiator.Inorganicmonolithsaremostlybasedonsilica buttheirpreparationismoredifficult.Theyaretypicallypreparedfrommixturesof atetraalkyloxysilane,poly(ethyleneglycol),andanacidcatalystforminganinitialsilicasolthatistransformedtoagelbyaging.Silicamonolithsaremoremechanically stable,lesspronetochangingdimensions(swelling)indifferentsolvents,havea highersurfacearea,andareeasilymodifiedbyreactionofsurfacesilanolgroups.Silica monolithsarecommonlyusedfortheextractionofsmallmoleculesincartridgeand diskformats.Organicmonolithsareabetterchoicefortheextractionofbiopolymers duetotheirrelativelylowsurfaceareas,greaterbiocompatibility,andhighertolerance ofextremepH.Hybridorganicsilicamonolithscombinethepositivefeaturesof organicandsilicapolymermonoliths,butarenotcommerciallyavailable [77].Monolithsincorporatingnanoparticles,molecular-organicframeworks,immunosorbents, molecularlyimprintedpolymers,aptamers,etc.,havebeendevelopedtoenhance selectivityorsamplecapacity,butonlyasexperimentalmaterials [76 79].Wide diameter( 4mm)rodstructuresbecameavailableonlyrecently.Themainapplicationsofmainlynarrowdiametersilicamonolithsorthin-filmcoatingshavebeen
describedforin-tubesolid-phaseextraction,onlineprecolumnliquidchromatography, fiberandstirbarcoatings,andmicro fluidicdevices [75,77].Inthediskformatmonolithsaretypicallyusedinpipettetips,spincolumn(disksolid-phaseextractionutilizingcentrifugalforcesforsampleprocessing),andinmultiwellextractionplates [80].
1.4Sorbentchemistriesandproperties
Commonsorbentsforsolid-phaseextractioncanbeclassi fiedasinorganicoxides,lowspeci ficitysorbents(silica-basedchemicallybondedphases,porouspolymers,and carbon)andcompoundandgroup-selectiveorhigh-speci ficitysorbents(ionexchange, mixedmode,macrocyclic,restrictedaccess,affinity-based,andmolecularimprinted polymers) [13].Alternativeclassi ficationschemesarepossibleaswellasawiderrange ofsubcategories [1,3,52,81 84].Thereisalsoaconsiderabledisconnectbetweenthe enormousnumberofexperimentalsorbentsdescribedinthecontemporaryliterature andthemuchsmallernumberofcommerciallyavailablesorbents.Outsideofresearch centersanduniversitylaboratoriestheskills,interest,andexperiencenecessarytosynthesizeexperimentalsorbentsarenotusuallyavailable.Furtherdetailsofexperimental sorbentscanbefoundinthechaptersthatfollowthisgeneraloverview.Industrialand regulatoryapplicationsaredominatedbycommerciallyavailablesorbents,whichare thefocusofthissection.
1.4.1Inorganicoxides
Themostimportantinorganicoxideadsorbentforsolid-phaseextractionissilicagel andtoalesserextentalumina,titania,zirconia, florisil,anddiatomaceousearth.Inorganicoxideshaveahighconcentrationofactivefunctionalgroupsontheirsurface responsiblefortheadsorptionofanalytesbypolar,ion-exchange,andLewisacid/ baseinteractions [85].Foraqueoussamplesion-exchangeandLewisacid/baseinteractionsdominatewhiledipole-typeandhydrogen-bondinginteractionsareusuallyasor moreimportantfornonaqueoussamples.Silicagelisinmanywaysanearidealadsorbentfortheextractionofsmallpolarorganiccompoundsandisavailableinawide rangeofparticlesizesforuseindifferentextractionformats,averageporesizes,in therange4 30nm,andspecificsurfaceareasfrom300to800m2/g [5,12].Silica gelisalsoacommonsubstrateformonolithicsorbents [77].Thesol-gelprocessallows silicageltobeutilizedasathinporouscoatingwithchemicalattachmenttothesupportingsubstratetoincreasedurability [25,52].Silicaissolubleinaqueoussolutionsat pH > 7.4anditsuseislimitedtoacidicandneutralsolutions.Organic-inorganichybrid particlescanbeusedtoextendthepHoperatingrangeofsilica-basedsorbents [86].
Aluminaisavailableasneutral,acidic,orbasicformsdeterminedasanapparent surfacepHdependingonprocessingconditions.Typicaladsorbentsforsolid-phase extractionhaveanaverageporesizeof6nmandaspecificsurfaceareaof150m2/g. Thesurfacechemistryofaluminaismorecomplexthansilicaandisdominatedby ion-exchangeandLewisacid/baseinteractions.StabilitytoextremepHfavorsitsuse Coreconceptsandmilestonesinthedevelopmentofsolid-phaseextraction13
forextractionbasedonion-exchange.Italsohasahighadsorptioncapacityformetal ions.TitaniaandzirconiahavestrongLewisacid/basesitesandareusedfortheselectiveisolationofpolyoxyanions(phosphates,phosphonates,borates,carboxylates,sulfates,andinparticular,phospholipids) [85].Florisilisasyntheticmagnesiumsilicate withasurfaceareaofabout250 300m2/gandanapparentsurfacepHofabout8.5. Itiscommonlyusedforsamplecleanupintheisolationofpesticideresiduesfrom fatsandoils.Diatomaceousearthisa flux-calcinedformofsilicagelwithalowsurface area.Itismainlyusedasa filteraidinsamplepreparationandasadispersantforsolvent extraction(matrixsolid-phasedispersion) [87].
1.4.2Low-specificitysorbents
Low-speci ficitysorbentsforaqueoussamplesincludesilica-basedchemicallybonded sorbentsandcoatings,porouspolymersandpolymercoatings,andvariousforms ofcarbon [1,10 13].Forgasphasesamplesporouspolymersandvariousformsof carbon,andoccasionally,inorganicoxidesorinorganicoxidesphysicallycoated withaliquidphaseorreactivematerialaretypicallyused [8,9,88 90]. Low-speci ficitysorbentsarecharacterizedbyretentiongovernedbydispersiveinteractionsaccompaniedbyweakormodestpolarintermolecularinteractions.
Silica-basedporousparticle,monoliths,andthin filmswithchemicallybondedsurfacesarepreparedbyreactingsurfacesilanolgroupswithorganosilanesformingstable siloxanebonds.Byvaryingthesilicasubstrateandorganosilanereagentchemically bondedsurfaceswithawiderangeofproperties(poresize,boundligandtype,bonding density,etc.)canbepreparedbychemistrydevelopedfororganosiloxane-bonded silicastationaryphasesforliquidchromatography [91,92].Someexamplesareindicatedin Table1.1.Chemicallybondedsorbentsaregenerallysynthesizedbyreaction ofmonofunctionalortrifunctionalsilaneswithsilica,followedbyendcapping(reactionofstericallycrowdedsilanolgroupswithasmall-sizeorganosilane)insomecases. Monofunctionalreagentsresultinmonomericsurfacecoveragewhiletrifunctional reagentscanreactbothwiththesilicasurfaceandhydrolyzedreagentforming extendedpolymericlayersofhighercarbonloadingandgreaterpHstability.Chemicallybondedsorbentswithhighsurfaceareas(500 600m2/g),longalkylchains,and highphaseloadingaregenerallyusedfortheisolationoflow-masscompoundsfrom aqueoussolution,whilewideporematerialswithshortalkylchainsareusedformacromolecules.Forsilica-based,alkylsiloxane-bondedsorbentsretentionincreaseswith solutesize,andforcompoundsofasimilarsize,isreducedbypolarinteractionswith water(particularlyhydrogenbonding)andbyionizationforaqueoussamples.
Siloxane-bondedsorbentscontainingpolarfunctionalgroups(e.g.,3-cyanopropyl, 3-aminopropyl,andspacer-bondedpropanediol)areusedmainlytoisolatetargetcompoundsfromsamplesdissolvedinorganicsolventsbasedontheirselectiveinteractionswithpolarfunctionalgroupsofthetargetcompounds.Theyaregenerallyless retentivethantypicalalkylsiloxane-bondedsorbentsforaqueoussamples.
Silica-basedorganosiloxane-bondedsorbentshavetwogenerallimitationsinadditiontosmall,andpossiblyinadequate,breakthroughvolumesforlow-mass,highly polarorganiccompounds.Thesilica-basedsorbentscontainalowconcentrationof
Coreconceptsandmilestonesinthedevelopmentofsolid-phaseextraction15
Table1.1 Structuresofporoussilica-based,monomeric,organosiloxane-bondedsorbents (hSi-R).
TypeSymbolFunctionalgroupStructure(R)
AlkylC30Tricontane-C30H61
C18Octadecyl-C18H37
C8Octyl-C8H17
C4Butyl-C4H9
CyclohexylCHCyclohexyl-C6H11
AromaticPHPhenyl-C6H5
BHBiphenyl-C12H9
CyanoCN3-Cyanopropyl-CH2CH2CH2CN
AminoNH23-Aminopropyl-CH2CH2CH2NH2
HydroxylDIOLSpaced-bonded propanediol -CH2CH2CH2OCH2CH(OH) CH2OH
Quaternary amine
SAXTrimethylaminopropyl-CH2CH2CH2N(CH3)þ Cl
CarboxylicacidCBACarboxylpropyl-CH2CH2CH2CO2H
SulfonicacidPRSPropanesulfonicacid-CH2CH2CH2SO3 Hþ
SCXBenzenesulfonicacid-C6H4SO3 Hþ
ionizedsilanolgroupscapableofion-exchangeinteractionswithbasiccompoundsas wellaspoorhydrolyticstabilityatextremepH(2 > pH > 8) [93,94].Lowrecoveryof basiccompoundscanresultfromtheinabilityoftheelutionsolventstodisplacethese compoundsfromtheion-exchangesites(additionofacompetingbasetotheelution solventmightsolvethisproblem).
Macroreticularporouspolymersarebiporouscopolymersofstyrenedivinylbenzeneoracrylicesters,typically,consistingofagglomeratesofrandomly packedmicrospherespermeatedbyanetworkofholesandchannels [91,95].Thehighlystrainedporousstructureisswollentodifferentextentsbyorganicsolventsand aqueous-organicsolventmixtures,andispartiallyresponsibleforthefavorableretentionpropertiesofthesematerials.Theadditionofsmallvolumesofwater-miscible organicsolvents,usedasaprocessingaidtominimizedewettingofthepolymerduring sampling,canhavearemarkableeffectonthebreakthroughvolumeforthesesorbents [96].Macroreticularporouspolymerswithsurfaceareastypicallylessthan600m2/g, exhibitedinadequateretentionforlow-masspolarcompounds,whichledtothedevelopmentofhyper-cross-linkedpolymerswithspeci ficsurfaceareasof800 2000m2/g andcommensurateincreasedretention [97,98].Hydrophiliccopolymersorbentswere developedtoreduceinterfacialtensionbetweenthepolymersurfaceandwater,
increasingtheretentionofpolarcompoundsbyenhancingtheircontactwiththesorbent.Theyhavetheaddedadvantageofbeingfullywaterwettablesimplifyingsample processing.Thesearetypicallycopolymersof N-vinylpyrrolidoneanddivinylbenzene, methacrylateanddivinylbenzene,orsurface-modifiedconventionalstyrenedivinylbenzenepolymers [97]
Themainformsofcarbonusedforsolid-phaseextractionareactivatedcarbon, graphitizedcarbonblacks,carbonnanotubes,andporousgraphiticcarbon [99 104]. Granularactivatedcarbonispreparedbythelowtemperatureoxidationofvegetable charcoalsandhasalargesurfacearea(300 2000m2/g),awideporedistribution andaheterogeneoussurfacecontainingactivefunctionalgroups.Graphitizedcarbon blacksare(largely)nonporouswithmoderatesurfaceareasof5 100m2/g.Their surfacesarecontaminatedbyoxygenspeciesandotherfunctionalgroupsdepending ontheoriginofthematerialusedforpreparationofthecarbonsorbentbypyrolysis. Theretentionmechanismforactivatedandgraphiticcarbonisquitecomplexinwhich surfacefunctionalgroupscontributetoretention,forexample,byionexchange.Porous graphiticcarbonpreparedbythesilica-templateprocesshasalowlevelofsurface contaminationbutisusedlessfrequentlythanotherformsofcarbonbecauseofitsrelativelyhighcost.Foraqueoussamplesretentionisinfluencedbyhydrophobicforces (increaseswithsolutesize),byadsorptionontheelectronicallypolarizablesurface (dipolarinteractionsandelectronlonepairinteractionsincreaseretention),andbycontributionsrelatedtothemicroscopically flatsurfaceofgraphiticcarboninwhichplanar compoundsareretainedpreferentiallyoverbentandangularcompounds [105].
Carbonnanotubes(alsocones,disks,horns, fibers)arerelativelynewmaterials characterizedbyalargesurfaceareatovolumeratio,highthermalstability,and withasurfacethatiseasilymodi fiedtoadjusttheirproperties [83,101 104].Carbon nano fibershavesignificantlylargerdimensionsthancarbonnanotubesandare preferredforcartridge-basedsolid-phaseextraction [106].Single-walledcarbonnanotubesconsistofasinglesheetofgraphenerolledupintheshapeofacylinderwith typicaldiametersfrom0.4to3.0nmandwithendsnormallycappedbyfullerenelike structures.Multiwalledcarbonnanotubesconsistofseveralrolledupgraphenesheets concentricallyarrangedaroundacommonaxiswithtypicaldiametersfrom1.4to greaterthan100nmwith(usually)cappedends.Carbonnanotubescanbeprepared invariouslengthsuptoafewmicrometers.Graphenehasahighadsorptioncapacity forcompoundsofalltypesincludinginorganicions [107].Surfacemodificationnot onlyenlargestheirpotentialrangeofapplicationsbutequallyimportant,enhances theirdispersioninsolvents,whichotherwisebarelyoccursduetostrongintertube interactions.Experimentalmaterialswithawiderangeofphysicallycoatedandchemicallybondedsurfacefunctionalgroupshavebeendescribedbutarenotcommercially available [103,104].Grapheneisquitereactiveandsurfacemodifi cationbyhalogenation,hydrogenation,oxidation,andradicalandnucleophilicadditionmakeitsuitable forthepreparationofmoreselectivesorbents.
Cross-linkedpoly(siloxanes)analogoustothoseusedasstationaryphasesforgas chromatographyarecommonlyusedascoatingsfor film-basedformatssuchas fiber, in-tubeandthin-filmsolid-phasemicroextractionandstirbarsorptiveextraction [108,109].Morepolarexperimentalcoatingsbasedoncopolymerswithpolarand