EditionChandraP.Sharma
https://ebookmass.com/product/regenerated-organs-futureperspectives-1st-edition-chandra-p-sharma/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Challenges in Science
Education: Global
Perspectives for the Future Gregory P. Thomas
https://ebookmass.com/product/challenges-in-science-education-globalperspectives-for-the-future-gregory-p-thomas/
ebookmass.com
Unravelling Plant-Microbe Synergy 1st Edition Dinesh Chandra
https://ebookmass.com/product/unravelling-plant-microbe-synergy-1stedition-dinesh-chandra/
ebookmass.com
Phytoremediation Potential of Perennial Grasses 1st
Edition Vimal Chandra Pandey
https://ebookmass.com/product/phytoremediation-potential-of-perennialgrasses-1st-edition-vimal-chandra-pandey/
ebookmass.com
An Extraordinary Lord Anna Harrington https://ebookmass.com/product/an-extraordinary-lord-anna-harrington-3/
ebookmass.com
Chapman & Nakielny’s Guide to Radiological Procedures 7th Edition Nick Watson
https://ebookmass.com/product/chapman-nakielnys-guide-to-radiologicalprocedures-7th-edition-nick-watson/
ebookmass.com
Atlas of Laparoscopic and Robotic Urologic Surgery 3rd Edition Jay T. Bishoff
https://ebookmass.com/product/atlas-of-laparoscopic-and-roboticurologic-surgery-3rd-edition-jay-t-bishoff/
ebookmass.com
The Year-Long Adventures of the Blue Shoes & Their Friends: A Pedagogical Experiment in Visual Blogging and Tutoring University Athletes at the University of Nebraska-Lincoln Michael R. Hill
https://ebookmass.com/product/the-year-long-adventures-of-the-blueshoes-their-friends-a-pedagogical-experiment-in-visual-blogging-andtutoring-university-athletes-at-the-university-of-nebraska-lincolnmichael-r-hill/ ebookmass.com
A Michaelmas Truce (The Mercer's House Book 4) Mary Kingswood
https://ebookmass.com/product/a-michaelmas-truce-the-mercers-housebook-4-mary-kingswood/
ebookmass.com
Mathematics for Elementary School Teachers 1st Edition, (Ebook PDF)
https://ebookmass.com/product/mathematics-for-elementary-schoolteachers-1st-edition-ebook-pdf/
ebookmass.com
of Interventional Cancer Pain Management 1st ed. 2019 Edition, (Ebook PDF)
https://ebookmass.com/product/essentials-of-interventional-cancerpain-management-1st-ed-2019-edition-ebook-pdf/
ebookmass.com
REGENERATEDORGANS REGENERATED ORGANS FuturePerspectives Editedby CHANDRA P.SHARMAFBSE
DepartmentofPharmaceuticalBiotechnology,ManipalCollegeofPharmaceuticalSciences, ManipalUniversity,Manipal,Karnataka CollegeofBiomedicalEngineering&AppliedSciences,PurbanchalUniversity,Kathmandu,Nepal BiomedicalTechnologyWing,SreeChitraTirunalInstituteforMedicalSciences&Technology(SCTIMST), Thiruvananthapuram,India
AcademicPressisanimprintofElsevier
125LondonWall,LondonEC2Y5AS,UnitedKingdom
525BStreet,Suite1650,SanDiego,CA92101,UnitedStates
50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
Copyright©2021ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical, includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthe publisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefound atourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmay benotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbe mindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforany injuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseor operationofanymethods,products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-821085-7
ForInformationonallAcademicPresspublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher:StacyMasucci
AcquisitionsEditor:ElizabethBrown
EditorialProjectManager:BillieJeanFernandez
ProductionProjectManager:SelvarajRaviraj
CoverDesigner:MarkRogers
TypesetbyMPSLimited,Chennai,India
Listofcontributorsix
Prefacexiii 1
EngineeringApproaches:From ScaffoldingtoBioprinting Applications
1.Tissueandorganregeneration:An introduction
WilliPaulandChandraP.Sharma
1.1Introduction3
1.2Guidedtissueregeneration4
1.3Stemcellsintissueregeneration5
1.4Conclusionandfutureperspective8 References8
2.Tissuerepairwithnaturalextracellular matrix(ECM)scaffolds
ThomasChandy
2.1Summary11
2.2Background12
2.3Smallintestinalsubmucosa15
2.4Acellulardermis18
2.5Bladderacellularmatrix18
2.6Amnioticmembrane19
2.7Pericardiumandfascialata24
2.8ECMforrepairofdamagedmuscle26
2.9Stemcellsforskeletalmuscle regeneration27
2.103Dbioprintingfororganregeneration27
2.11Nanosystemdeliveryofcellularmediators29
2.12Perspective31 References32
3.Engineeredsurfaces:Aplausible alternativeinoverviewingcriticalbarriers forreconstructingmoderntherapeuticsor biomimeticscaffolds
PreetamGuhaRay,RagaviRajasekaran,TrinaRoy,AbirDutta, BaisakheeSaha,HemaBora,SubrataK.DasandSantanuDhara
3.1Introduction39
3.2Currentstatusofmedicaldevicesandrelative complicationsinvolved40
3.3Substratesdeployedinbiomedical applications41
3.4Typesofsurfacemodificationtechniques towardsligandspecificactivation44
3.5Surfaceengineeringofpolymeric substrates45
3.6Surfaceactivationofmetallicsubstrates50
3.7Engineeringorganoids54
3.8Theconceptofengineered invivo system (organ-on-chipdevices)57
3.9Engineeredbactericidalsystems amodern paradigmtoregenerativemicrodevices63
3.10Futureperspectivesandchallenges71 References71
4.Strategiesof3Dbioprintingand parametersthatdeterminecellinteraction withthescaffold-Areview
GreeshmaRatheesh,CedryckVaquette,PrashantSonarand YinXiao
4.1Introduction81
4.2Typesofbioprinting82
4.3Hydrogelsfor3Dbioprinting85
4.4Propertiesofabioink87
4.5Parametersthatdeterminesthecellresponses ontissueengineeredscaffold87
4.6Conclusion90 References91
5.Multipotentnatureofdentalpulpstem cellsfortheregenerationofvaried tissues Apersonalizedmedicine approach
V.P.Sivadas,D.P.RahulandPrabhaD.Nair
5.1Introduction97
5.2ImportanceofDPSCsinpersonalized regenerativemedicine98
5.3UsefulnessofDPSCsinosteogenicregeneration therapy99
5.4DPSCsfortheregenerationofneuronaltissues andcentralnervoussystem101
5.5ApplicabilityofDPSCsasastemcellsourcefor theregenerationofmyocardialandvascular tissues102
5.6Dentalpulpstemcellsasmediatorsofoptic systemregeneration104
5.7RegenerativetherapeuticpotentialofDPSCsin diabetes105
5.8DPSCsasatherapeutictoolforthe regenerationofcartilageandtendon106
5.9Futureperspectivesandconclusions108
References114
2 CardiovascularSystem 6.Regeneratingtheheart:Thepast, present,&future
AdityaSenguptaandRaghavA.Murthy
6.1Introduction121
6.2Cardiomyocyteregenerative potential122
6.3Cell-basedstrategies122
6.4Cardiacstemcells123
6.5Pluripotentstemcells123
6.6Cell-freestrategies124
6.7Growthfactors124
6.8ExosomesµRNAtechnology124
6.9Directreprogramming125
6.10Endogenousrepair®eneration126
6.11Thefuture127
6.12Conclusion128 References128
7.Engineeredcardiactissue:Conceptsand future
SoumyaK.Chandrasekhar,FinoshG.Thankam,JoshiC.Ouseph andDevendraK.Agrawal
7.1Introduction133
7.2CardiacECM134
7.3Post-MIscarring135
7.4Cardiactissueengineering136
7.5BiomaterialsforCTE139
7.6DecellularizedECM140
7.7Tissue biomaterialinteraction140
7.8FunctionalmodificationsforCTE scaffolds143
7.9Bottlenecksandfuture145 References146
8.Vascularregenerationandtissue engineering:Progress,clinicalimpact,and futurechallenges
SantanuHati,SwatiAgrawalandVikrantRai
8.1Introduction153
8.2Regenerativetherapies155
8.3Conclusionandfuturedirections162 References163
3 MusculoskeletalRegenerationof Tissues 9.Oraltissueregeneration:Currentstatus andfutureperspectives
MajiJose,VrindaRajagopalandFinoshG.Thankam
9.1Introduction169
9.2Histologyoforaltissues169
9.3Oralmicrobiologyinhealthanddiseases170
9.4Oraldiseases,prevalenceand management171
9.5Oralmucosallesions173
9.6Oralcancer174
9.7Oro-dentaltissueengineering:amodern epochintoothmanagement175
9.8Strategiesadaptedforperiodontaltissue regeneration177
9.9ECMregenerationwithECM-based scaffolds177
9.10Biomaterialscaffoldsfororo-dentaltissue regeneration178
9.11Stemcellbiologyupdatesfororo-dentaltissue regeneration180
9.12Summary182 References182
Furtherreading187
10.Regenerativetechnologiesfororal structures
PrachiHanwatkarandAjayKashi
10.1Introduction189
10.2Embryologyoforalstructures190
10.3Regenerationofteeth190
10.4Regenerationofmuscles/tongue194
10.5Regenerationofbone195
10.6RegenerationofTMJ196
10.7Regenerationofsalivaryglands196
10.8Microgravity197
10.9Ethicalconsiderations198 References198
11.State-of-the-artstrategiesand futureinterventionsinboneand cartilagerepairforpersonalized regenerativetherapy
YogendraPratapSingh,JosephChristakiranMoses,Ashutosh Bandyopadhyay,BibritaBhar,BhaskarBirru,NandanaBhardwaj andBimanB.Mandal
11.1Introduction203
11.2State-of-the-artstrategiesfor regeneration205
11.3Diseasemodels223
11.4Graftsubstitutes229
11.5Clinicalstatus232
11.6Conclusionandfutureperspectives237 Acknowledgment239 References239
12.Muscletissueengineering A materialsperspective
JohnP.Bradford,GerardoHernandez-MorenoandVinoyThomas
12.1Introduction249
12.2Bio-interfacingmaterials254
12.3Engineeringapproaches(scaffolds)263
12.4Summary265
12.5Futureperspective268 References269
RegenerativeNeuroscience 13.Recentdevelopmentsandnew potentialsforneuroregeneration
SreekanthSreekumaran,AnithaRadhakrishnanandSanjuP.Joy
13.1Nervoussystem,neurodegenerationand regeneration anutshell278
13.2Strategiesforneuralregenerationand repair281
13.3Futureperspectives285 References285 5
RespiratoryResearch 14.Lungdiseaseandrepair Is regenerationtheanswer?
S.S.PradeepKumarandA.MayaNandkumar
14.1Embryonicdevelopment293
14.2Stemcellsinthelung294
14.3Epithelial mesenchymalinteractions295
14.4Roleofmechanicalforcesinlung architecture296
14.5Lungregenerationindisease296
14.6Invivo Animalmodels297
14.7Invitromodels297
14.8Lung-on-achip(LOC)model298
14.9Threedimensionalprintingofthe lung299
14.10Futureperspectiveslatest4D printing299
Acknowledgments299 Conflictofinterest300 References300 Furtherreading301
KeyEnablingTechnologiesfor RegenerativeMedicineFuture OutlookandConclusions
15.3Dprintinginregenerativemedicine AynurUnalandNidhiArora
15.1Introduction305
15.2Conclusions326 References327
16.Roleofumbilicalcordstemcellsin tissueengineering MerlinRajeshLalandOormilaKovilam
16.1UmbilicalcordandMSCs332
16.2TransplantationbiologyofUCMSCs: biomaterials,differentiationand regeneration332
16.3Concernsandperspective335 References336
Index339
Listofcontributors DevendraK.Agrawal Departmentof TranslationalResearch,WesternUniversityof HealthSciences,Pomona,CA,UnitedStates
SwatiAgrawal DepartmentofSurgery, CreightonUniversitySchoolofMedicine, Omaha,NE,UnitedStates
NidhiArora SSInfotech,Indore,MP,India
AshutoshBandyopadhyay Biomaterialand TissueEngineeringLaboratory,Departmentof BiosciencesandBioengineering,IndianInstitute ofTechnologyGuwahati,Guwahati,India
BibritaBhar BiomaterialandTissueEngineering Laboratory,DepartmentofBiosciencesand Bioengineering,IndianInstituteofTechnology Guwahati,Guwahati,India
NandanaBhardwaj Departmentof Biotechnology,NationalInstituteof PharmaceuticalEducationandResearch Guwahati,Guwahati,India
BhaskarBirru BiomaterialandTissue EngineeringLaboratory,Departmentof BiosciencesandBioengineering,IndianInstitute ofTechnologyGuwahati,Guwahati,India
HemaBora BiomaterialsandTissueEngineering Laboratory,SchoolofMedicalScienceand Technology(SMST),IndianInstituteof Technology Kharagpur,Kharagpur,India
JohnP.Bradford Polymers&Healthcare Materials/Devices,DepartmentofMaterials Science&EngineeringUniversityofAlabama atBirmingham(UAB),Birmingham,AL, UnitedStates
SoumyaK.Chandrasekhar Departmentof Zoology,KKTMGovernmentCollege,Pullut, CalicutUniversity,Kerala,India;Department ofZoology,ChristCollege,Irinjalakuda, CalicutUniversity,Kerala,India
ThomasChandy PhillipsMedisizeLLC, Hudson,WI,UnitedStates
SubrataK.Das BiomaterialsandTissue EngineeringLaboratory,SchoolofMedical ScienceandTechnology(SMST),IndianInstitute ofTechnology Kharagpur,Kharagpur,India
SantanuDhara BiomaterialsandTissue EngineeringLaboratory,SchoolofMedical ScienceandTechnology(SMST),Indian InstituteofTechnology Kharagpur, Kharagpur,India
AbirDutta BiomaterialsandTissueEngineering Laboratory,SchoolofMedicalScienceand Technology(SMST),IndianInstituteof Technology Kharagpur,Kharagpur,India
PrachiHanwatkar RochesterGeneralHospital, Rochester,NY,UnitedStates
SantanuHati DepartmentofBiomedical Science,CreightonUniversitySchoolof Medicine,Omaha,NE,UnitedStates
GerardoHernandez-Moreno Polymers& HealthcareMaterials/Devices,Departmentof MaterialsScience&EngineeringUniversity ofAlabamaatBirmingham(UAB), Birmingham,AL,UnitedStates
MajiJose DepartmentofOralPathology& Microbiology,YenepoyaDentalCollege, Yenepoya(DeemedtobeUniversity), Mangalore,India
SanjuP.Joy Neurology,NationalInstituteof MentalHealthandNeuro-Sciences,Bangalore, India
AjayKashi PrivatePractice,Rochester,NY, UnitedStates
OormilaKovilam StJoseph’sHospital, Indiana,UnitedStates
MerlinRajeshLal LifeCellInternational(Pvt) Ltd,Chennai,India
BimanB.Mandal BiomaterialandTissue EngineeringLaboratory,Departmentof BiosciencesandBioengineering,Indian InstituteofTechnologyGuwahati,Guwahati, India;CentreforNanotechnology,Indian InstituteofTechnologyGuwahati,Guwahati, India
A.MayaNandkumar DivisionofMicrobial Technology,SreeChitraTirunalInstitutefor MedicalSciences&Technology Thiruvananthapuram,India
JosephChristakiranMoses Biomaterialand TissueEngineeringLaboratory,Department ofBiosciencesandBioengineering,Indian InstituteofTechnologyGuwahati,Guwahati, India
RaghavA.Murthy Departmentof CardiovascularSurgery,IcahnSchoolof MedicineatMountSinai,NewYork,NY, UnitedStates
PrabhaD.Nair DivisionofTissueEngineering andRegenerationTechnologies,Biomedical TechnologyWing,SreeChitraTirunal InstituteforMedicalSciencesand Technology(SCTIMST),Poojapura, Thiruvananthapuram,India
JoshiC.Ouseph DepartmentofZoology, ChristCollege,Irinjalakuda,Calicut University,Kerala,India
WilliPaul BiomedicalTechnologyWing,Sree ChitraTirunalInstituteforMedicalSciences &Technology,Thiruvananthapuram,India
S.S.PradeepKumar DivisionofMicrobial Technology,SreeChitraTirunalInstitutefor MedicalSciences&Technology Thiruvananthapuram,India
AnithaRadhakrishnan Researchand DevelopmentDepartment,Pharmaceutical Corporation(IndianMedicine),Thrissur, India
D.P.Rahul DepartmentofOrthodonticsand DentofacialOrthopedics,SchoolofDentistry, AmritaVishwaVidyapeetham,Amrita InstituteofMedicalSciences,Kochi,India
VikrantRai DepartmentofBiomedical Science,CreightonUniversitySchoolof Medicine,Omaha,NE,UnitedStates
VrindaRajagopal Departmentof Biochemistry,UniversityofKerala, Karyavattom,India
RagaviRajasekaran BiomaterialsandTissue EngineeringLaboratory,SchoolofMedical ScienceandTechnology(SMST),Indian InstituteofTechnology Kharagpur, Kharagpur,India
GreeshmaRatheesh InstituteofHealthand BiomedicalInnovation,QueenslandUniversity ofTechnology,Brisbane,QLD,Australia
PreetamGuhaRay BiomaterialsandTissue EngineeringLaboratory,SchoolofMedical ScienceandTechnology(SMST),Indian InstituteofTechnology Kharagpur, Kharagpur,India
TrinaRoy BiomaterialsandTissue EngineeringLaboratory,SchoolofMedical ScienceandTechnology(SMST),Indian InstituteofTechnology Kharagpur, Kharagpur,India
BaisakheeSaha BiomaterialsandTissue EngineeringLaboratory,SchoolofMedical ScienceandTechnology(SMST),Indian InstituteofTechnology Kharagpur, Kharagpur,India
AdityaSengupta DepartmentofCardiovascular Surgery,IcahnSchoolofMedicineatMount Sinai,NewYork,NY,UnitedStates
ChandraP.Sharma BiomedicalTechnology Wing,SreeChitraTirunalInstitutefor MedicalSciences&Technology, Thiruvananthapuram,India
YogendraPratapSingh BiomaterialandTissue EngineeringLaboratory,Departmentof BiosciencesandBioengineering,IndianInstitute ofTechnologyGuwahati,Guwahati,India
V.P.Sivadas DivisionofTissueEngineering andRegenerationTechnologies,Biomedical TechnologyWing,SreeChitraTirunal InstituteforMedicalSciencesand Technology(SCTIMST),Poojapura, Thiruvananthapuram,India
PrashantSonar SchoolofChemistryand Physics,QueenslandUniversityof Technology,Brisbane,QLD,Australia
SreekanthSreekumaran Departmentof Biochemistry,UniversityofKerala, Thiruvananthapuram,India
FinoshG.Thankam Departmentof TranslationalResearch,WesternUniversityof HealthSciences,Pomona,CA,UnitedStates
VinoyThomas Polymers&Healthcare Materials/Devices,DepartmentofMaterials Science&EngineeringUniversityofAlabama atBirmingham(UAB),Birmingham,AL, UnitedStates
AynurUnal DigitalMonzoukuri,PaloAlto, CA,UnitedStates
CedryckVaquette SchoolofDentistry,The UniversityofQueensland,Brisbane,QLD, Australia
YinXiao InstituteofHealthandBiomedical Innovation,QueenslandUniversityof Technology,Brisbane,QLD,Australia; Australia-ChinaCentreforTissue EngineeringandRegenerativeMedicine, CentreforBiomedicalTechnologies, QueenslandUniversityofTechnology, Brisbane,QLD,Australia
Preface Regeneratedorgansisanemergingarea toencouragethepatientspecificorgan developmentarealityinfuture.Therefore, theobjectiveofthisbookistobringallthe relatedinterdisciplinaryconceptstogether anddiscussthecomprehensivedevelopmentspossibleofthisfieldcurrentlywith futuredirections.Thebookcontainssixsections Section1:Engineeringapproaches: fromscaffoldingtobioprintingapplications, Section2:Cardiovascularsystem,Section3: Musculoskeletalregenerationoftissues, Section4:RegenerativeNeuroscience, Section5:Respiratoryresearch,Section6: Keyenablingtechnologiesforregenerative medicinefutureoutlookandconclusions. Eachchapterhasbeenwrittenbyexperts intheirspecializedarea.
Thisbookisexpectedtobeanessential referenceresourceforyounggraduatestudents,academicfacultyandcollaborating industrialpartnerswhoareinterestedin advancingtheknowledgeandtranslational researchintheareaofRegeneratedOrgans.
Ithankalltheauthorsfortheireffortsof preparingexcellentcontributionsandMs. BillieJeanFernandezforhereffectivecoordinationofthisproject.
Ialsoappreciateverymuchandthank mywifeArunaSharmaforhersustained supportduringthecourseofthisproject.
ChandraP.Sharma
1 Tissueandorganregeneration:An introduction WilliPaulandChandraP.Sharma
BiomedicalTechnologyWing,SreeChitraTirunalInstituteforMedicalSciences&Technology, Thiruvananthapuram,India
1.1Introduction Tissuerepairorhealingisanatural,complicatedandcontinuousprocessinanyliving organism,i.e.restorationoftissuefunctionandarchitectureaftertheinjury.Itcomprisesof twoessentialcomponents;RegenerationandRepair.Repairafterinjurycanoccurbyregenerationofcellsortissuesthatrestoresnormaltissuestructure,orbyhealing,whichleadstothe formationofascar.Incaseofregeneration,thedamagedorlosttissueisreplacedbytheproliferationofsurroundingundamagedcellsandtissue.Thenormalstructureofthetissueis restoredorcompleteregenerationoccursinepidermis,GItractepitheliumandhematopoietic systemwherethecellshavehighproliferativecapacity.Inthecaseofstabletissueslikeliver andkidney,compensatorygrowthoccursratherthantrueregeneration.However,repairpredominantlyisthedepositionofcollagentoformascar.Butwillcertainlydependuponthe abilityofthetissuetoregenerateandtheextentoftheinjury.Woundsonsuperficialskin healthroughregenerationofsurfaceepithelium(regeneration);however,restorationoforiginalECMdamagedbysevereinjuryinvolvescollagendepositionandscarformation(repair) therebythenormalstructureofthetissueispermanentlyaltered.Chronicinflammationmay causemassivefibrosis.
Differentcelltypeshavedifferentcapacityforregeneration.Labilecellswhichactsasphysicalbarriershaveunlimitedregenerativecapacity.Theyarecellsfoundonskin,GItract,respiratorytractandurinarytractthatarecharacterizedbycontinuousregeneration.Quiescent celltypesfoundinmostoftheinternalorgans likeliver,kidney,endocrineandmesenchymal cells(fibroblasts,smoothmuscle,vascular) havelimitedregenerativecapacityandarein responsetostimuli.Itrequiresanintactbasementmembranefororganizedregeneration.
PermanentcelltypeslikeCNSneurons,skeletalandcardiacmusclecellshaveverylittle regenerativecapacityanditsrepairformsscar.
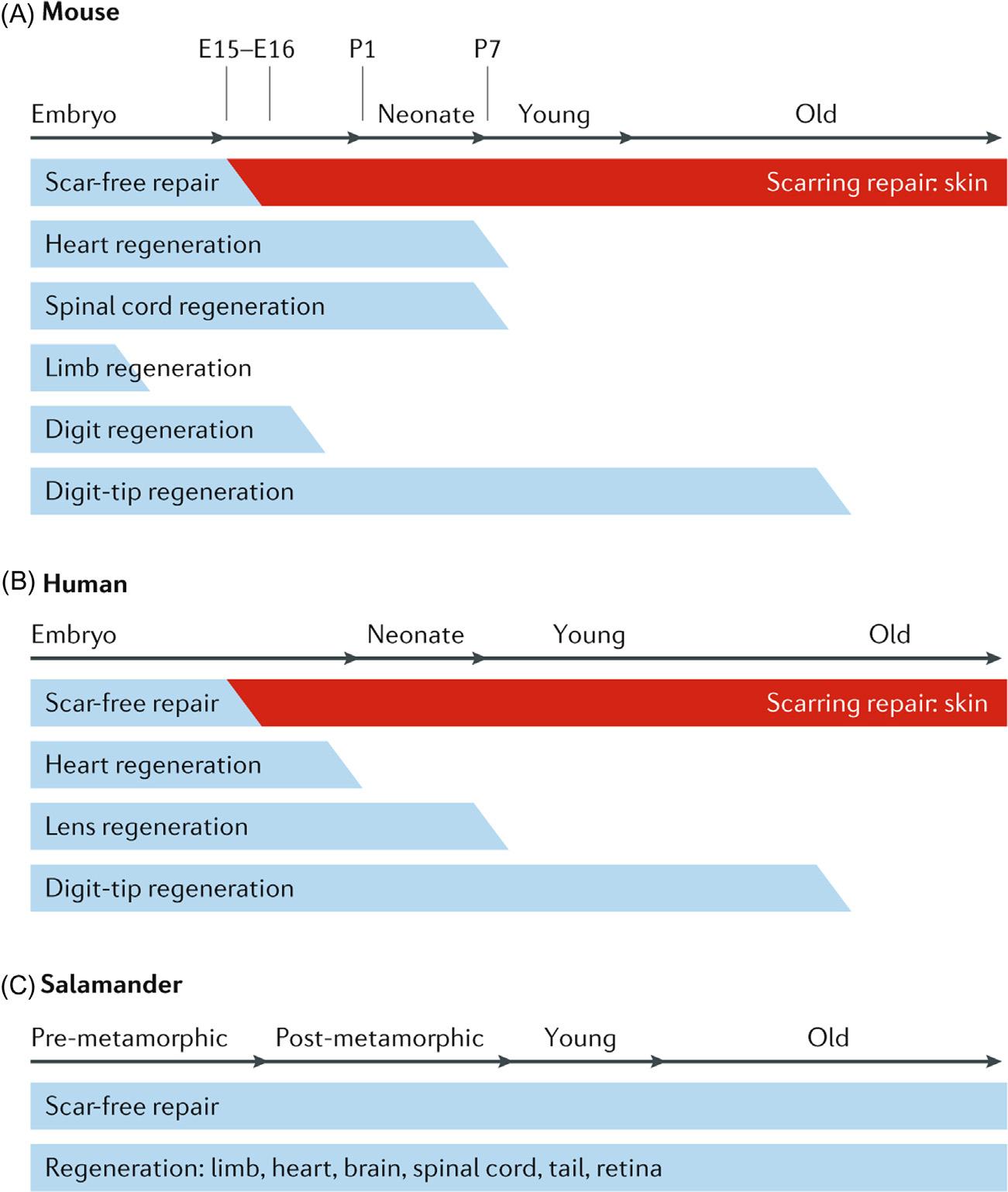
Mammalsandhumansaregenerallyconsideredasapoorexampleforregeneration whencomparedwithmostvertebratespeciesduetothedifferencesingenetics,development,immunesystemsandtissuecomplexity [1].Incaseofmammals,scar-freehealing andregenerationnormallyoccursduringtheearlystagesoflife.Theabilitytoregenerate islostduringadulthood,butmanynon-mammalianvertebratesretainthecapacityto regenerateorgansandlimbsafterinjuryasdepictedin Fig.1.1[1].Physiologicalregenerationinmammalsislimitedtotissueswithhighproliferativecapacity.Theepitheliaofthe skinandgastrointestinaltract,thehematopoieticsystem(redbloodcellreplacement),hair cyclingandantlerregenerationareexamples.Thisformsthebasisofguidedtissueregenerationwhichmaybenecessaryforefficientrestorationofdamagedtissues.Thustheoriginalfunctionandformseemstobemimickedascloselyaspossiblebytheregenerated tissues.Regenerationthusrequiresanintactconnectivetissuescaffold.
1.2Guidedtissueregeneration Guidedtissueregenerationisaprocedurewhereabiodegradableconduitprovidescontactguidanceforenhancingtheopportunityforonecelltypetopopulateanareafor regeneratingtissue.Theconduitorabiomaterialconstructshouldbebiocompatibleand shouldnotmakeanydamageorberejectedbythehosttissue.Regenerationisclassified intoguidedtissueregeneration(GTR)whichreferstotheregenerationofperiodontal attachmentandguidedboneregeneration(GBR)thatreferstoridgeaugmentationand focusedondevelopmentofhardtissuesinadditiontothesofttissueregeneration.
Ridgeaugmentationtechniqueisrequiredforsuccessfulimplantplacementintheright prosthodonticpositions.Guidedtissueregenerationisonetechniqueusedforridgeaugmentationinrehabilitationofatrophicjawswithdentalimplants [2].Itusesbarriermembraneswithorwithoutbonegraftsorsubstitutesforosseousregenerationforexclusionof cellsimpedingboneformation.Epitheliumandconnectivetissueareexcludedfromthe rootsurfaceinthebeliefthattheyinterferewithregeneration.Thiswasbasedonthe assumptionthatonlyperiodontalligamentcellshavethepotentialforregeneration. TheoreticallyguidedtissueregenerationwasdevelopedbyMelcherin1976 [3].Primarily, therearefourstagesforasuccessfulboneortissueregeneration,whicharegenerally abbreviatedasPASS.(1)Primaryclosureofthewoundtopromoteundisturbedanduninterruptedhealing;(2)angiogenesistoprovidenecessarybloodsupplyandundifferentiated mesenchymalcells;(3)spacecreationandmaintenancetofacilitatespaceforboneingrowth,and(4)stabilityofthewoundtoinducebloodclotformationandallowuneventfulhealing.
AdvantagesofGTRmembranesarethatothertissuesthatinterferewiththeosteogenesisandboneformationcanbepreventedbyusingabarrier.Thisbarrieralsoactsasa dressingforthewoundcoverageandanchorageforthebloodclot.Preventbacterialinvasionandinflammationandprovidesuitablemicroenvironmentforregeneration.There areseveralGTRmembranesusedclinicallywhichrangesfromacellulardermalallograft topolymericmembranesbothresorbableaswellasnonresorbable.
FIGURE1.1 Tissueregenerationindifferentspecies. Thecapacityoftissueandorganregenerationvariesin differentanimalspecies.(A)Inmice,thecapacityforscar-freerepairdecreasesbetweenembryonicday15(E15) andE16.Thecapacityforheartandspinalcordregenerationislostinearlypostnatallifebetweenpostnatalday1 (P1)andP7.Limbregenerationislostearlyindevelopment(beforeE15).Wholedigitscanberegenerateduntil E16,anddigittipregenerationismaintainedthroughoutthedevelopmentstages.(B)Inhumans,theregenerative potentialislimitedtoearlydevelopmentalstages,similartomice.(C)Insalamanders,theabilityforscar-free repairandtheregenerationoflimbs,heart,brain,spinalcord,tailandretinaaremaintainedthroughouttheirlife. AdaptedfromXiaHM,etal.Tissuerepairandregenerationwithendogenousstemcells.NatRevMater,2018;3(7):174 93, underlicensefromSpringerNature.
1.3Stemcellsintissueregeneration Stemcellsarethecoreofthemodernregenerativemedicine.Stemcellshaveprolonged self-renewalcapacityandabilitytoasymmetricreplicationandarefoundinspecialized
nicheswithineachtissue.Duringnormalhomeostasisthedeadcellswillbereplenishedby thestemcells,andalsorepairdamagedtissue.Theextrinsicsignalsinteractwiththeproteins expressedbythestemcellsinadynamicmannerinthenichemicroenvironmentthatinfluencetheabilityofstemcellstoself-renew.Inasymmetricreplication,ineverycelldivision, onecellwillbeidenticaltotheoriginalstemcellwhereastheotheroneterminallydifferentiates.Instochasticdifferentiation,onestemcelldevelopsintotwodifferentiateddaughter cells.Whereasasecondoneproducestwostemcellsidenticaltotheoriginal.
Therearedifferentsourcesforstemcells.Somecomefromembryosthatare3 5days oldcalledembryonicstemcells.Theyarepluripotentcells,candevideintomanystemcells andcandifferentiateintoanytypeofcellinabody.Thusthesecellsareversatileandcan beusedtoregenerateandrepairofanydiseasedtissueororgan.Adultstemcellsarefound insmallnumbersinadulttissuesuchasbonemarroworfatandhavelimiteddifferentiation potential.Bygeneticreprogramming,adultcellscanbetransformedintoembryonicstem cells.Stemcellsarealsofoundinamnioticfluidaswellasumbilicalcordblood,andare calledperinatalstemcells,whichalsohavetheabilitytochangeintospecializedcells.
Ithasbeenreportedthatstemcellsexistintwodistinctstatesdependingupontheirrelativeactivityandwound-inducedregeneration.Theamountoftissuegeneratedisaffected bythetimingandlengthofstemcellactivity.Arecentstudyonhairfolliclehasshown thatsignalsemanatingfrombothheterologousnichecellsandfromlineageprogenyinfluencethetimingandlengthofstemcellactivity [4].Thecapacityofbonetoregenerateand repairitselfdependsonthesizeofthewoundandthepresenceofcertaindiseases.Large bonedefectsmayrequiresurgicalintervention.Implantationofthebonestemandprogenitorcellswithtissueengineeredscaffoldshasimmensepotentialinfracturebonehealing [5].Mesenchymalstemcellsdifferentiatesintoosteoblasts,chondrocytes,andadipocytes andarecriticallyimportantformusculoskeletaltissueregenerationandrepair [6].Stem cellshavebeenexploredforitsregenerativeabilitywidelyinboneregenerationstudies. Bothadiposederivedmesenchymalstemcells(ASC)andbonemarrowstemcells(BMSC) haveshowedalmostsimilarpotentialinboneregeneration,althoughBMSChasshown betterresultsinvitro.Anewmethodfortherepairofinjuredboneorperiodontaldisease usingbonemarrowstemcells(BMSC)hasbeenreported [7].Proliferationandosteogenic differentiationtoosteoblastcellshasbeenachievedusingred-lightabsorbingcarbon nitridesheetsusedalongwithBMSC.Ithasbeenshownthatthematerialabsorbsredlightandemitsfluorescencethatspeedsupboneregeneration.BMSCtherapyhasbeen shownasapromisingchoiceinboneregenerationandrepairparticularlyforcritical-sized defects.However,studyonthecellularandlocalinteractionintheprocessofboneregenerationisrequiredfortheapprovalofFoodandDrugAdministration.
Traumaticmuscleinjuriesarechallengingtotreat.Cellbasedapproachhaveshown promisingresultsinmanypre-clinicalstudies.Myogenicstemcellsaswellasnonmyogeniccellsarestudiedinmuscleregeneration.Satellitecells(SC)giverisetolargenumber ofprogenywhichformsmyofibersandrepopulatetheSCnicheinhostmuscles.Mesenchymal stemcells(MSC)canmodulatethefunctionofmyoblastssuchastheirfusionintomyotubes,and theirmigrationandproliferationkinetics.BonemarrowderivedMSChasbeenshownto improvecontractilemusclefunctionafterintramuscularimplantation [8].Aclinicalstudy reportstheimplantationofanacellularbiologicalscaffoldatthemuscleinjurysiteandprovidingthepatientwithaggressivephysicaltherapyhasshownsignificantfunctional
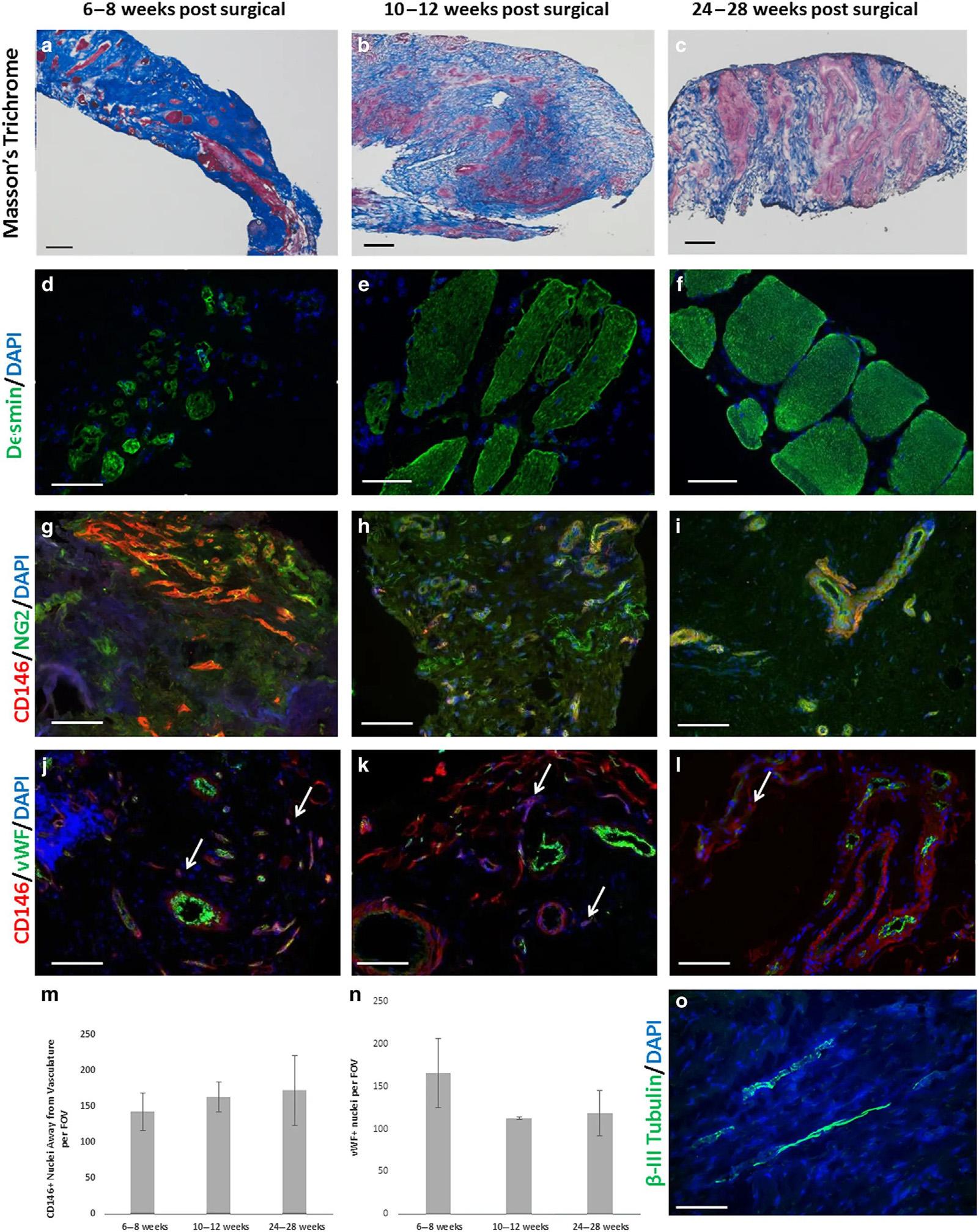
FIGURE1.2 Site-appropriatetissueremodelingbyECMbioscaffolds. (A C)Massonstrichromestainingof humanmusclebiopsiesshowsislandsofskeletalmusclepresentat6 8weeks,10 12weeksand24 28weekspostsurgery,respectively.(D F)Humanmusclebiopsiesarecharacterizedbydesminexpressionatalltimepoints,indicating newmuscleformationwithinthesiteofimplantation.(G I)ECMbioscaffoldimplantationisassociatedwiththepresence ofCD146 1 NG2 1 perivascularstemcells.(J L)PVSCswereshowntomigrateawayfromtheirnormalvesselassociatedanatomiclocationatalltimepoints.ArrowsindicateCD146 1 PVSCsmigratingawayfromvessels.(M,N) MigratingPVSCsandvascularitywasquantifiedusingCellProfilerimageanalysissoftware.(O)At24 28weekspost surgery,ECMbioscaffoldimplantationwasassociatedwiththepresenceof β-IIItubulin 1 cells,implicatinginnervated skeletalmuscle.(Scalebars 5 50 μm). AdaptedfromDzikiJ,etal.Anacellularbiologicscaffoldtreatmentforvolumetricmuscleloss:resultsofa13-patientcohortstudy NpjRegenerativeMed2016;1,underCreativeCommonsLicense.
improvementinthirteenpatientswithvolumetricmuscleloss.Asthescaffoldsstarted degradingthestemcellsmigratetotheareaandgetdifferentiatedintomusclecells [9]. Newmuscleformationandpresenceofneurogeniccellsattheremodelingsiteisevident in Fig.1.2.Althoughvariousstudieshaveprovidedapositiveoutlook,aninnovativecellbasedtherapyisyettobestandardizedfortraumaticmuscleinjuries.
1.4Conclusionandfutureperspective Tissueengineeringconceptshavebeenwidelyexperimentedforcartilage,skin,bone,vascularandnervetissueregeneration.The3Dstructureanditsphysicalpropertiesareequally importantlikeitscombinationofmaterials,thecell-cellandthecell-matrixinteractions. Traditionalscaffoldfabricationtechniquehasitslimitationthatthecomplexstructureofthe realorganscannotbeduplicated.3Dbioprintingtechniquehasbeenstudiednowadayasa strategytoimproveregenerationoforgans.Theinventionofstereolithographyin1983laterled tothedevelopmentof3Dbioprintingmethodforprintingartificialhumanorgans.It’saversatile3Dprintingmethodutilizingbio-inkforprintingartificialorganslikebloodvessel,skeleton andskin.3Dbioprintingtechnologyishighlypreciseandfast,andhasthebenefitofindividualizedmedicaltreatment.Ithasbeendemonstratedwithtricalciumphosphatethat3Dprinted scaffoldscanhavepreciseandcontrollableporestructurewithoptimalmechanicalstrength comparablewithhumancancellousbone [10].Thisscaffoldwasbiocompatible,andhadadherenceandrapidproliferationofbonemesenchymalstemcells(hMSC)foritsapplicationinloadbearingbone.Anewbio-inkwithprecisecontroloverprintability,mechanicalanddegradation propertieshasdemonstratedendochondraldifferentiationofencapsulatedhMSCs [11].This could3Dprintpatientspecificbonetissueforregenerationofdiseasedbone.Similarly3Dprintingcouldalsobeusedinbioprintingofheartvalvesandheartmusclesforthetreatmentofcardiacpatients [12].AcriticalreviewbyDeoetal. [13] discussesvariousdesigncriteriaand processingparametersofbioinktohelpfabricationofcomplexstructuresforbioartificialorgan manufacturing.Thereisashortageofdonororgansworldwidewhichprojectstheurgencyof developmentofbiocompatible3Dprintedartificialorgans.Thestrategiesandprocessparametersforbioprintingoforganslikeskin,cardiactissue,bone,cartilage,liver,lung,neuraltissues,pancreasetc.arereviewedindetailbyMataietal. [14].Theprogressmadeinorganbio printinginregenerationhasmadeconsiderableprogress;however,stillvariouschallengeslike structuralstabilityinvivoanddegradation,biocompatibility,maintenanceofsterilityetc.need tobeoptimizedbeforeclinicaltranslation.Theversatilityofthebioprintingcouldimprove withthelatestinnovationlike5Dprintingofadditivemanufacturing (wheretheprintingcan achievecurvedpathsmakingtheartificialorgansmorerealistic).Theadventof4Dprinting wherethereisafourthdimensionaddedseemsmoredynamicwhichmakesasmartmaterial thatrespondstoastimulus.Theseseemtobemoresuitableforbioartificialorganregeneration.
References
[1] XiaHM,etal.Tissuerepairandregenerationwithendogenousstemcells.NatRevMater2018;3(7):174 93.
[2] LiuJ,KernsDG.Mechanismsofguidedboneregeneration:areview.OpenDentJ2014;8:56 65.
[3] MelcherAH.Ontherepairpotentialofperiodontaltissues.JPeriodontol1976;47(5):256 60.
1.EngineeringApproaches:FromScaffoldingtoBioprintingApplications
[4] BlanpainC,FuchsE.Stemcellplasticity,plasticityofepithelialstemcellsintissueregeneration.Science 2014;344(6189):1243- 1
[5] WalmsleyGG,etal.Stemcellsinboneregeneration.StemCellRevRep2016;12(5):524 9.
[6] ChenY,etal.Mesenchymalstemcells:apromisingcandidateinregenerativemedicine.IntJBiochemCell Biol2008;40(5):815 20.
[7] TiwariJN,etal.Acceleratedboneregenerationbytwo-photonphotoactivatedcarbonnitridenanosheets.Acs Nano2017;11(1):742 51.
[8] QaziTH,etal.Celltherapytoimproveregenerationofskeletalmuscleinjuries.JCachexiaSarcopenia Muscle2019;10(3):501 16.
[9] DzikiJ,etal.Anacellularbiologicscaffoldtreatmentforvolumetricmuscleloss:resultsofa13-patient cohortstudy.NpjRegenerativeMed2016;1.
[10] ManX,etal.Researchonsinteringprocessoftricalciumphosphatebonetissueengineeringscaffoldbased onthree-dimensionalprinting.ShengWuYiXueGongChengXueZaZhi2020;37(1):112 18.
[11] ChimeneD,etal.Nanoengineeredosteoinductivebioinkfor3Dbioprintingbonetissue.ACSApplMater Interfaces2020.
[12] BirlaRK,WilliamsSK.3Dbioprintinganditspotentialimpactoncardiacfailuretreatment:anindustry perspective.APLBioeng2020;4(1):010903.
[13] DeoK,etal.Bioprinting101:design,fabricationandevaluationofcell-laden3Dbioprintedscaffolds.Tissue EngPartA2020.
[14] MataiI,etal.Progressin3Dbioprintingtechnologyfortissue/organregenerativeengineering.Biomaterials 2020;226:119536.
Tissuerepairwithnatural extracellularmatrix(ECM)scaffolds ThomasChandy
PhillipsMedisizeLLC,Hudson,WI,UnitedStates
2.1Summary Extracellularmatrix(ECM)scaffoldsthatprovideaconduciveenvironmentfornormal cellulargrowth,differentiationandangiogenesisareimportantcomponentsoftissueengineeredgraftsforlongtermviability.ECMhasshowntobeaneffectivescaffoldfortherepair andreconstitutionofseveraltissues,includingbloodvessels,skingraft,duralrepair,soft tissuegrafts,herniarepair,myocardialrepair,urinarytractstructures,ophthalmicreconstructionandnervetissueregeneration.TheseECMscaffoldsarecompletelydegraded invivoandinduceahostcellularresponsethatsupportsconstructiveremodelingrather thanscartissueformation.Severalnaturallyoccurringscaffoldmaterialshavebeeninvestigated,includingsmallintestinalsubmucosa(SIS),acellulardermis(AlloDerm)bladder acellularmatrixgraft(UBM),amnioticmembranetissue(anthromatrix,ambiodry,amniograft),cadavericfascia(Tutoplast)andporcinepericardium(IOpatch).Commonfeaturesof ECM-associatedtissueremodelingincludeextensiveangiogenesis,recruitmentofcirculating progenitorcells,rapidscaffolddegradationandconstructiveremodelingofdamagedor missingtissues.Thesources,themethodsofprocurementandprocessing,andtheeffectsof thesenaturallyoccurring,materialsonangiogenesisandtissuedepositionarereviewed.SIS hasfounditsapplicationinavarietyoftissueinterfacesfortissuerepairandreconstruction. ThebladderacellularmatrixisverysimilartoSISstructurally.However,extensiveprocessingmethodsareneededtoseparatetheattachedmuscularbladderwallfromthesubmucosalmembrane.Theseharshchemicalandenzymatictreatmentsonbladdermatrixcauses deleteriousresultsonlongtermimplantsontissuereconstructionandrepair.Acellular humanamnioticmembraneshowspromisingintissuerepairandneovascularizationdueto theirimprovedstrength,flexibility,suturability, antibacterialeffectsandlowimmunogenicity. Itseemshumanamnion,whichisprocessedtoyieldauniform,acellularbiofabric,isa superiormaterialforavarietyofproductapplications.
https://doi.org/10.1016/B978-0-12-821085-7.00002-6
Crosslinkingisaneffectivemeansofcont rollingthebiodegradationrateofcollagenbasedbiomaterials.Crosslinkedcollagenorcollagenbasedmaterialshasagreatermodulusofelasticity(Young’smodulus),greaterresistancetoproteases,andalowerdegreeof swellingthanuncrosslinkedcollagen.Glutaraldehydefixationofbio-prosthetictissue hasbeenusedsuccessfullyforalmost40years.However,itisgenerallyrecognizedthat glutaraldehydefixationofbio-prosthesesis associatedwiththeoccurrenceofcalcification.Accordingly,manyeffortshavebeenun dertakentodeveloptechniquesforthe fixationofbio-prostheses,whichwillnotleadt ocalcification.Severalalternativecrosslinkingtechniqueshavebeenexploredindifferentapplications,includingphysicalmethodssuchasUVirradiation,dehydrothermal, freezedrying,etc.andtheuseofchemical reagents,suchasdiepoxides,diisocyanates,carbodiimides,diisothiocyanates,and glycidylethers.
Thecrosslinkingoftissuesreducesimmunogenicityofthematerialandincreaseresistancetodegradationbyhostandbacterialen zymes.Itissuggestedthatthefunctionality oftheamnioticmembranemaybeimprovedviaselectingasuitablecrosslinkingtechniquetosuitaspecificapplication.However,thesuccessfulutilizationofmammalian ECMasatherapeuticdevicewilldependinlargepartuponourabilitytounderstand andtakeadvantageofthenativestructure/fun ctionrelationshipsofthebiologicalscaffoldmaterial.
Thisreviewalsopresentstherecentadvancesinthe3Dbioprintingandtheirrelative components,includingthebioinks,thecells,andapplicationsfororganregeneration. Althoughchallengesstillremaininthisresearchfield,furthermultidisciplinaryresearch toadvanceprintingtechniques,printablebioinkmaterialsandengineeringdesignscan addressthecurrentchallengesandrealizetheemergingpotentialof3Dorganbioprinting. Weconcludethischapterbyhighlightingongoingchallengesandopportunitiesassociated withgrowthfactor(GF)deliveryandaddressthebiomaterialsselectioncriteriaforthefabricationoftraditionalandmodernnanodeliverysystemsthataccomplishthespatiotemporalreleaseofsingle/multipleGFsforfunctionalregenerationofcomplextissues.
2.2Background Effectiverepairandregenerationofinjuredtissuesandorgansdependsonearlyreestablishmentofthebloodflowneededforcellularinfiltrationandmetabolicsupport. Implantablebiomaterialsdesignedtoreplacedamagedordiseasedtissuesmustactassupports(i.e.,scaffolds)intowhichcellscanmigrateandestablishthisneededbloodsupply [1 4].Oneapproachtotreatingdamagedordiseasedtissuesreliesuponsynthetically derivedbiocompatiblepolymerscaffoldstoserveasbackbonesfortissuerepairandregeneration.Althoughmanysyntheticbiopolymershavebeenusedtoreplacedamagedvascularstructures,andwhilelong-termpatencyrateshaverisenovertheyears,theideal vasculargraftscaffoldremainselusive.Forexample,nosyntheticbiopolymercurrently availableforclinicalusecanrestorenormalstructureandfunctiontoinjuredvasculartissueswhileavoidingseverecomplicationssuchasthrombosis,neointimalhyperplasia, acceleratedatherosclerosis,and/orapproachtorepairandregenerationofdamaged
tissuesusesintactextracellularmatrixobtainedfromanimaltissuesasthegrowthsupport forhostcells.Theextracellularmatrix(ECM)isacomplexmixtureofstructuralfunctional proteins,proteoglycansandglycoproteinsarrangedinaunique,tissuespecificthreedimensionalultrastructure.Theseproteinsprovidestructuralsupportandtensilestrength fortheorgansandtheydeliverdiversehostprocessesasangiogenesisandvasculogenesis, cellmigration,cellproliferationandorientation,inflammation,immuneresponsiveness andwoundhealing.Implantablebiomaterialsdesignedtoreplacedamagedordiseased tissuesmustactassupports(i.e.,scaffolds)intowhichcellscanmigrateandestablishthis neededbloodsupply [1,2].Similarly,thisECMmustbestrongenoughtowithstandthe physiologicdemandsplaceduponthemwhenimplantedintoasite-specificorgansystem andmustretaintheirmechanicalpropertiesovertime.
ThemostcommonconstituentoftheECMisthestructuralprotein,collagen.Whenharvestedfromthetissuesourceandfabricatedintoagraftprosthesis,theseECMmaterials maybereferredtoasnaturallyoccurringpolymericscaffolds,bio-scaffolds,biomatrices, ECMScaffolds,ornaturallyoccurringbiopolymers [3,5 7].Thesematerialsareharvested fromseveraldifferentbodysystems,buttheysharesimilaritieswhenprocessedintoa graftmaterial.Specifically,sincetheyaresubjectedtominimalprocessingaftertheyare removedfromthesourceanimal,theyretainastructureandcompositionnearlyidentical totheirnativestate.Thehostcellsareremovedandthescaffoldsareimplantedacellularly toreplacediseasedordamagedtissues(Table2.1).
Naturallyoccurringbiopolymersincludesmallintestinalsubmucosa,acellular dermis,cadavericfascia,porcinepericardia,thebladderacellularmatrixgraftand
TABLE2.1 ECMScaffoldsandselectedinvestigationaluses.
Scaffoldmaterial/ commercialnameTissuesourcePrimaryuses(Ref.)
Smallintestinalsubmucosa (Surgisiss softtissuegraft, Cook)
Acellulardermis (AlloDerms )
Bladderacellularmatrix (AcellvetV1000-LY)
Amnioticmembrane (Ambiodry,Amniograft, Anthromatrix)
PorcinesmallintestineVascularconduits [8],Skingraft [9] Duralrepair, Softtissuegraft [10] Herniarepair [11],Ligament reconstruction [12] Myocardialrepair [5],Urinary reconstruction [13]
PigskinSkingraft [14],Duralrepair [15],Urinary reconstructionPlasticandcosmeticsurgery [16]
PorcinebladderVascularconduits [17],Bladderreconstruction [18] Esophagusreconstruction,Cardiactissuerepair f13J,
HumanamnionSkingraft [19],Urinarytractreconstruction, Ophthalmology [20],Abdominalherniarepair, Closureofpericardium [21],Vascularrepair [22] Nervetissueregeneration [23]
FasciaLata(Tutoplastt)HumancadaverFascialataLigamentreconstruction,Duralrepair [24], Craniofacialreconstruction,Heartvalves [25]
Pericardium(IOpatcht)MammalianPericardium (porcine,calf)
Heartvalves [26],Cornealrepair,Skingraft
amnioticmembrane [5,27].Thesenaturallyoccurringmaterialsofferpromisingalternatives tosyntheticallyengineeredpolymericscaffoldsfortissuerepairandregeneration [28 30]. Thesenaturallyoccurringscaffoldscanbeprocessedinsuchawayastoretaingrowthfactors,suchasbasicfibroblastgrowthfactor(FGF-2),transforminggrowthfactor-β,vascular endothelialcellgrowthfactor(VEGF),andepidermalgrowthfactor(EGF) [31 33],glycosaminoglycans,suchasheparin,hyaluronicacid,dermatansulfate,chondroitinsulfateAand C [15,34],andstructuralelementssuchasfibronectin,elastinandcollagen [27,34].AllECMs sharethecommonfeaturesofprovidingstructuralsupportandservingasareservoirof growthfactorsandcytokines [1,5].Thesematerialspreventmanyofthecomplicationsassociatedwithforeignmaterialimplantsbecausetheyprovideanaturalenvironmentonto whichcellscanattachandmigrate,withinwhichtheycanproliferateanddifferentiate. Thesenaturallyoccurringbiopolymershavebeenshowntointeractquicklywiththehost’s tissues,inducethedepositionofcellsandadditionalECM,andpromoterapidangiogenesisfunctionsthatareessentialtotherestorationoffunctionalsofttissue.Inthismanner,the ECMaffectslocalconcentrationsandbiologicactivityofgrowthfactorsandcytokinesand makestheECManidealscaffoldfortissuerepairandreconstruction.
Theidealbiomaterialmustallowtissueincorporationandresultinremodeled,functionaltissue(Fig.2.1)withoutleadingtoencapsulation,breakdownofthematerial,tissueerosion,or adhesionformation.Thepurposeofthisliteratureanalysisistopresentanoverviewdetailing theuseofnaturallyoccurringpolymersasacellularbio-scaffoldsandreviewthecurrentknowledgeaboutthebiochemicalcompositionofthesematerialsthatcontributetotheirabilitytoelicit anappropriateangiogenicresponse.ItisassumedthattheECMscaffoldsthatretainessentially unchangedfromnativeECMelicitahostresponsethatpromotecellinfiltrationandrapidscaffolddegradation,depositionofhostderivedneo-matrixandeventuallyconstructivetissue remodelingwithminimumofscartissue [1,35].Severalofthesematerialsandtheirprimary usesarelistedinTable2.1theirknownbiochemicalcompositionissummarizedin Table2.2.
FIGURE2.1 Remodeledtissue.
TABLE2.2 BiochemicalcompositionofECMscaffolds.
ScaffoldmaterialComponentsidentified
Smallintestinal Submucosa
(30 μm)
Size:2 3 3cm upto7 3 10cm
CollagenTypes:I,III,IV,V,VI
OtherProteins:fibronectin
Proteoglycan,laminin. Glycosaminoglycans:hyaluronicacid,Heparin,heparinsulfate,chondroitin SulfateA,dermatansulfate,ChondroitinsulfateC
Growthfactors:FGF 2TGF 8,VEGF
Acellulardermis(40 μm)CollagenTypes:I,IV,VII
OtherProteins:elastin
Glycosaminoglycans:Notdetected(ND)
Growthfactors:ND
Bladderacellularmatrix (60 μm)
Size:10 3 7cm
Amnioticmembrane (20 30 μm)
Sizes:1 3 2cm,2 3 3cm 4 3 4cm
Fascialata (400 650pm)
Size:0.3 3 15cm
Pericardium
(400 1000 μm)
Size:1 3 3cm 1.5 3 1.5cm
CollagenTypes:I,III,IV
OtherProteins:elastin,fibronectin. Glycosaminoglycans:hyaluronicacid,Heparin,heparinsulfate,chondroitin SulfateA,dermatansulfate, Growthfactors:FGF-2.TGF-0.VEGF
CollagenTypes:I,III,IV,V,VII
OtherProteins:laminin,fibronectin,decorin. Glycosaminoglycans:hyaluronicacid,heparinsulfate, Growthfactors:EGF.FGF-2.TGF-8.TGF-o,KGF
CollagenType:I
OtherProteins:ND. Glycosaminoglycans:ND Growthfactors:ND
Collagen:Type:I
OtherProteins:ND.
Glycosaminoglycans:ND Growthfactors:ND
2.3Smallintestinalsubmucosa
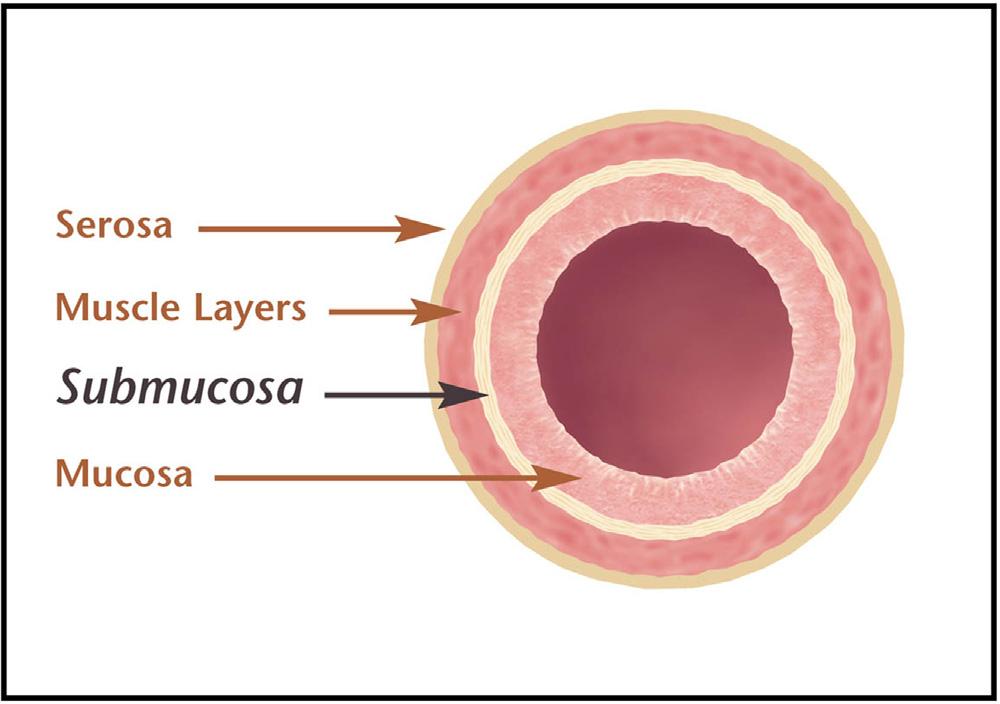
Smallintestinalsubmucosa(SIS)isaresorbable,acellularbio-scaffoldcomposedofextracellularmatrix(ECM)proteinsderivedfromthe jejunumofpigs.SIShascharacteristicofan idealtissueengineeredbiomaterialandcanactasabioscaffoldforremodelingofmanybody tissuesincludingskin,bodywall,musculoskeletalstructure,urinarybladder,bloodvessels,and supportsnewbloodvesselgrowth [8,11 13].SISconsistsofthreedistinctlayersofthemammaliansmallintestine:thelaminapropriaandmuscularismucosaeoftheintestinalmucosa,and thetunicasubmucosa(Fig.2.2) [1].Thetunicasubmucosaisthelayerofconnectivetissue arrangedimmediatelyunderthemucosalayeroftheintestineandisa100 200 μmthickinterstitialECM:itmakesupthebulkoftheSISbiopolymerscaffold.SISinducessite-specificremodelingofbothorgansandthetissuedependingonthesiteofimplantation [27].SISstimulates hostcellstoproliferateanddifferentiateintosite-specificconnectivetissuestructures,andthis replacestheSISmaterialwithin90days [36].SIS’sabilitytoinducetissueremodelingisassociatedwithangiogenesis,cellmigrationanddifferentiationanddepositionofECM [36].
BovinetypeIcollagen(i.e.,reconstitutedcollagen)isperhapsthemostwidelyusedbiologicalscaffoldfortherapeuticapplicationsduetoitsabundantsourceanditshistoryof
successfuluse.Scaffoldsfortissuereconstructionandreplacementmusthavebothappropriatestructuralandfunctionalproperties.CollagentypesotherthantypeIexistnaturally occurringECMlikeSIS [8 10].Thesealternativecollagentypeseachprovidedistinct mechanicalandphysicalpropertiestotheECMandcontributetotheutilityoftheintact ECM(asopposedtotheisolatedcomponentsofECM)asascaffoldfortissuerepair. Structurally,SISconsistsoftypeI,III,IV,VandVIcollagen [9 11] inadditiontoother componentsasshownin Table2.2.ThisdiversityofcollagensandtheirstructuralarrangementwithinasinglescaffoldmaterialisparticularlyresponsibleforthedistinctivebiologicalactivityofSISscaffoldwhencomparedtosinglereconstitutedcollagenmatrix.
SISispreparedfromporcinejejunum [10] immediatelyafterharvestingtheintestine. Thesuperficiallayersofthetunicamucosaareremovedbymechanicaldelamination.The tissueisthenturnedtotheoppositesideandthetunicamuscularisexternaandtunica serosalayersaremechanicallyremoved.TheremainingtissuerepresentedtheSISand consistedofthetunicasubmucosaandbasilarlayersofthetunicamucosa.Thebiopolymer isthoroughlyrinsedinwater,treatedwithanaqueoussolutionof0.1%peraceticacid,and rinsedinsequentialexchangesofwaterandphosphatebufferedsaline.Itisthenstoredin antibioticsolutioncontaining0.05%Gentamycinsulfate [10,34].
SURGISISESsheetshaveathicknessandmechanicalstrengththatisseveraltimesthat ofasingle-layerSURGISISsheet [1].NominalpropertiesforSURGISISESandsingle-layer SURGISISsheetsarelistedin Table2.3.
ThemechanicalpropertiesandcomplementactivationofSISisindicatedin Tables2.3 and2.4.respectively.Thematerialhasgoodmechanicalandsutureretentionstrengthand hasnocomplementactivation.SIShasfounditsapplicationinavarietyoftissueinterfaces fortissuerepairandreconstruction.SIShassignificantpotentialasavasculargraftmaterial andwasexperimentallyevaluatedtorepairlargediameter( 10mmID)vasculargraft [37], smalldiameterarteriesandveins,venacava,carotidarteriesandheartvalves [37,38].In additiontovascularapplications,theSISbiomaterialhasbeenusedextensivelyinthegenitourinarysystemtorepaircongenitalabnormalitiesofthebladderpatch [13] hasshown rapidandaggressiveregenerationofbladdertissuewithin2 4weeks.SIShasbeenusedto
FIGURE2.2 Cross-sectiondiagramof smallintestine.
treatabdominalherniasandrepairbodywall,totreatchronicdermalwounds,torepair duramater,andtoreplacetendonandligamentinorthopedicapplications [1,5,9,27].Inall ofthesecases,SISsupportedangiogenesisandcausedreplacementofdamagedstructures leadingtotherestorationoffunctionaltissues.However,mildinflammationandanti-SIS antibodyproductionhavebeenreportedfollowingimplantation,theimmuneresponseelicitedbySIShavenotleadtoarejectionimmuneresponse [39].
Table2.5 providestheinvivo(Dogimplantationbodywallrepairmodel)degradation ofSISandtissuerepairprofile.Thereisarapiddecreaseinstrengthoftherepairdeviceat
TABLE2.3 MechanicalpropertiesofSIS.
PropertySURGISISa singlelayerSURGISISb enhancedstrength
NominalThickness(mm)0.200.42
asingle-layerSURGISISsheetsaredesignedtotoleratethemechanicalstressesassociatedwithlow-stressbodysystems. bSURGISISESsheets(2sheets)aredesignedtotoleratethemechanicalstressesassociatedwithhigher-stressbodysystems. *5-0suturewith2mmbitedepth. **9.5mmdiametersphere.
TABLE2.4 ComplementactivationwithSIS.
MaterialC3complementactivation(ng/mL)
Negativecontrol148 6 42 SISmaterial115 6 24
Positivecontrol2449 6 930
TABLE2.5 MechanicalpropertiesofexplantedSISHRDusingtheballbursttesta
Survivaltime(days)
Burstload(lb)
aImplantreadySISdevices(n 5 40)weretestedforpreimplantstrengthvalues.Themeanburstloadwas 73.37 6 11.45lb.*P , 0.05.
thesurgicalsiteduringthefirst10dayspostsurgerytoavalueof40.0pounds.Allsubsequenttimepointsofevaluationrangingfrom1monthto2yearsshowaprogressive increaseinstrengthofthesurgicalsite [37,39].ItappearsthatthenaturallyoccurringSIS showrapiddegradationwithassociatedandsubsequentremodelingtoatissuewith strengththatexceedsthatofthenativetissuewhenusedasabodywallrepairdevice. Thus,theSISconsistsofacomplexmixtureofstructuralandfunctionalproteinsand servesanimportantroleintissueandorganmorphogenesis,maintenanceofcellandtissuestructureandfunction,andinthehostresponsetoinjury.
2.4Acellulardermis Skiniscomprisedoftwoprimarylayersthatdifferinfunction,thicknessandstrength: theepidermisandthedermis.Thedermisunderliestheepidermisandisthethickerlayer oftheskin.Thedermisiscomposedoffibroblasts,whichproduceacollagen-containing ECMthatprovideselasticityandsupporttoskin.Acellulardermisismainlythenormal dermaltissuestructuresthatremainafterthecellsareremoved.Likeothernaturally occurringbiopolymers,acellulardermisisrichincollagenType1.CollagentypeIVand typeVIIarealsoretainedduringprocessinginthedermalskin [15] alongwithelastin.
Acellulardermisisharvestedfromeitherpigskinorhumancadaverskin.Theepidermisisremovedbysoakingtheskinin1MNaCIfor8h.Dermalfibroblastsandepithelial cellsareremovedbyincubationofthematerialin2%deoxycholicacidcontaining10mM ethylenediaminetetraacetate(EDTA).Whenimplantedasanacellulartissuegraft,acellulardermissupportedreendothelializationofrepairedvascularstructures,inhibitedexcessivewoundcontractionandsupportedhostcellincorporationandcapillaryingrowthinto thegraftedsite [15,40,41].
Allogenicacellulardermisisusedclinicallytomanagefull-thicknessburns.Theskin healedwithminimalscarformationincomparisontoanuntreatedsiteonpatient’s [14]. Thisstudydemonstratedthatcellulardermissupportedfibroblastinfiltration,neovascularization,andepithelializationintheabsenceofaninflammatoryresponse [14,16]. Acellulardermishasbeenusedinseveralothertissuerepairapplications,includingdermalreplacement,internalsoft-tissuerepair,andsmall-diametervascularreplacement [16,42].However,theallograftuseforlong-termperformanceofthetissuesremainsquestionsregardingtheirsourcetissue,intermsofviralandotherdiseasetransfer.
2.5Bladderacellularmatrix Bladderacellularmatrixgraft(BAMG)wasfirstdescribedin1975 [43] andwasderived fromalayeroftheurinarybladderthatisanalogoustothesubmucosaltissuecomprising thebulkoftheSISbiomaterial.Structurally,theBAMGisdeterminedtobecomposedof typeI,typeIIIandtypeIVcollagenandelastin,butalsocontainsotherECMcomponents, includingfibronectin,Glycosamino-glycans(hyaluronicacid,heparinsulfate,chondroitin sulfateA,dermatansulfate)andseveralgrowthfactors(FGF-2,TGF-β,VEGF)(Fig.2.3).
1.EngineeringApproaches:FromScaffoldingtoBioprintingApplications