Introduction
Microbes make up an unseen world, unseen at least by the naked eye. In the pages that follow, we will explore this world, the creatures that inhabit it, and the things they do. We will discover that processes carried out by microbes in the unseen world affect our visible world. These processes include virtually every chemical reaction in the biosphere that make up the elemental cycles of carbon, nitrogen, and all of the other elements necessary for life. The processes also involve interactions between organisms, both among microbes and between microbes and large organisms.
This chapter will introduce the types of microbes found in nature and some basic terms used throughout the book. It will also discuss why we should care about microbes in nature. The answers will give some flavor of what microbial ecology and this book are all about.
What is a microbe?
The microbial world is populated by a diverse collection of organisms, many of which having nothing in common except their small size. Microbes include by definition all organisms that can be observed only with a microscope and are smaller than about 100 µm. Microbes and its synonym “microorganisms” include bacteria, archaea, fungi, and many other types of eukaryotes (Figure 1.1). The microbial world also houses viruses, but arguably they are not alive and are not microbes, although some viral ecologists would argue otherwise. Whether dead or alive, viruses certainly are a big part of microbial ecology (Chapter 10). Bacteria and archaea are the simplest and the smallest microbes (excluding viruses) in nature, usually <10 µm in size, while microbial eukaryotes are
structurally more complex and bigger, ranging from 1 to 100 µm.
Microbes are found in all three domains of life: Bacteria, Archaea, and Eukarya (eukaryotes). Bacteria and archaea look quite similar under the microscope, and in fact archaea were once thought to be a type of bacteria. An old name for archaea is “archaebacteria,” while bacteria were once referred to as “eubacteria,” the “true” bacteria. Now we know that bacteria and archaea occupy separate domains, accounting for two of the three domains of life found on Earth. The third domain, Eukarya (sometimes spelled Eucarya), includes microbes with a nucleus and other organelles. Examples include fungi and algae (but not blue-green algae, more appropriately called cyanobacteria), as well as higher plants and animals. Prominent members of the third domain are protists, which are single-cell eukaryotes. Protists do not include the fungi even though some forms of fungi, the yeasts, are single cells. All of these microbes and viruses are discussed in this book.
The diversity in the types of microbes found in nature is more than matched by the diversity of processes they carry out. Microbes carry out some processes that are similar to the functions of plants and animals in the visible world. Some microbes are primary producers and carry out photosynthesis similarly to plants (“photoautotrophy”), some are herbivores that graze on microbial primary producers, and still others are carnivores that prey on herbivores; both herbivores and carnivores are “heterotrophic.” But microbes do many more things that have no counterparts among large organisms. These things, these processes, are essential for life on this planet.
Processes in Microbial Ecology. Second Edition. David L. Kirchman. Oxford University Press (2018). © David L. Kirchman 2018. DOI 10.1093/oso/9780198789406.001.0001
1
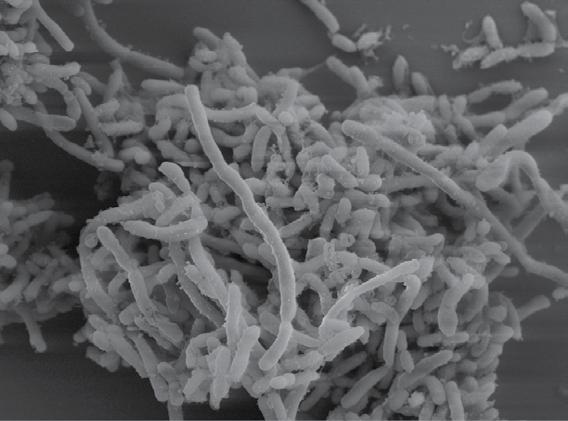
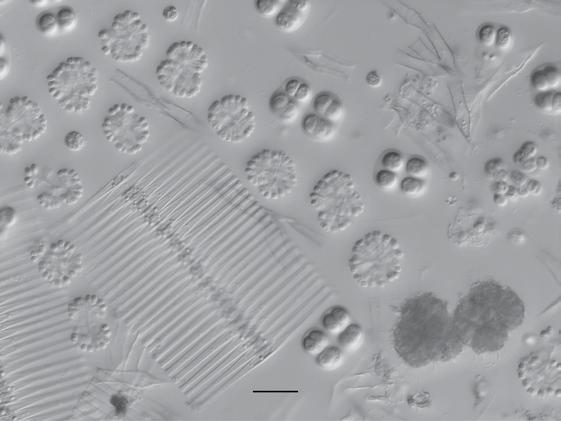
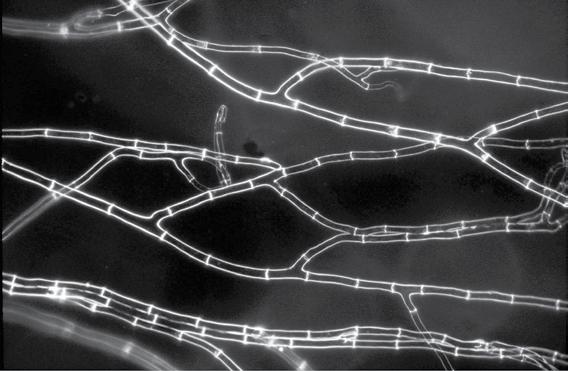
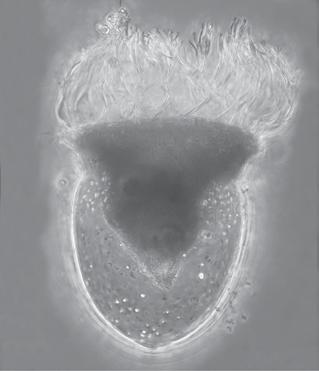
Figure 1.1 Examples of some microbes. Panel A: Soil bacteria belonging to the Gemmatimonadetes phylum, each about 1 µm wide. Image courtesy of Mark Radosevich. Panel B: Hyphae of the fungus Sordaria, commonly found in the feces of terrestrial herbivores. The width of each cell is about 10 µm. Image courtesy of George Barron, used with permission (http://hdl.handle. net/10214/7080). Panel C: Eukaryotic algae from a freshwater lake. The scale bar is 25 µm. Image courtesy of John Wehr. Panel D: A marine ciliate, Cyttarocylis encercryphalus, about 100 µm. Image courtesy of John Dolan. Color versions of panels B–D are available at http://www.oup.co.uk/companion/kirchman.
Why study microbial ecology?
The main reason has already been implied: microbes mediate many processes essential to the operation of the biosphere. But there are other reasons for studying microbial ecology. The following list of seven reasons starts with those that a nonscientist might give, if asked why we should learn about microbes. The list ends with the reasons that arguably are the most important, certainly in shaping the contents of this book.
Microbes cause diseases of macroscopic organisms, including humans
Most people probably think “germs” when asked how microbes affect their lives. Of course, some microbes do
cause diseases of humans and other macroscopic organisms. The role of infectious diseases in controlling population levels of macroscopic plants and animals in nature is recognized to be important (Ostfeld et al., 2008), but its impact probably is still underestimated. Sick animals in nature are likely to be killed off by predators, or simply die while hidden to avoid predation before being counted as being ill. We know less about the impact of diseases on smaller organisms, such as the zooplankton in aquatic systems (Figure 1.2) or invertebrates in soils. These small organisms are crucial for maintaining the health of natural ecosystems. There is some evidence that diseases in the ocean are becoming more common (Burge et al., 2014), and amphibians on land are now declining worldwide due to infections by chytrid fungi, perhaps linked to global warming (Raffel et al., 2015).
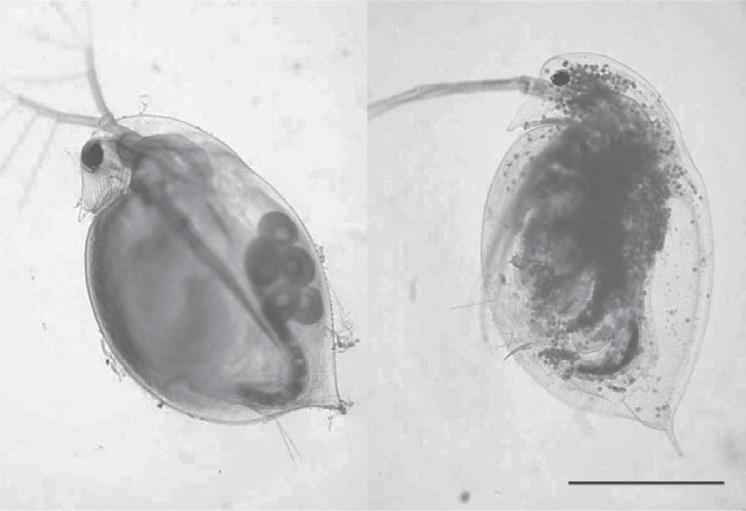

Figure 1.2 Two examples of animals infected by fungi. In panel A, the fungi are the small numerous dots visible in the transparent body cavity of a common freshwater zooplankton, Daphnia pulicaria (the common name is “water flea”). Taken from Johnson et al. (2006). Used with permission from the Ecological Society of America. In panel B, the arrows indicate the lesions affecting a salamander, a disease decimating these amphibians in Europe (Stegen et al., 2017). Picture provided by Frank Pasmans, Ghent University. A color version of panel B is available at http://www.oup.co.uk/companion/kirchman.
But pathogenic microbes are exceptions rather than the rule. The microbiologist John Ingraham pointed out that there are more murders among humans than pathogens among microbes (Ingraham, 2010). The vast majority of microbes in nature are not pathogenic, and that includes those living on and in us. The human body is host to abundant and diverse microbial communities. In fact, many of the cells in the human body are not human, but rather are bacteria. An average adult has about thirty trillion human cells but also thirty eight trillion bacteria (Sender et al., 2016), as well as numerous archaea and protists, not to mention viruses. Microbes inhabiting our skin and mucous membranes help to prevent invasion by pathogens, and the bacteria in the gastrointestinal tract do the same. Disruption of the microbial community in the colon, for example, allows the pathogen Clostridium difficile to flourish, causing severe diarrhea. One novel cure is “fecal transplantation” or “bacteriotherapy,” in which a normal microbial community is “transplanted” into the colon of a diarrhea-suffering patient.
Bacteria also aid digestion in humans and in many other mammals, while these microbes and others help invertebrates live on otherwise indigestible food such as wood (Chapter 14). There is even a connection between the microflora of our digestive tract and our brain (Collins
et al., 2012). A huge project is now examining the genomes of human-associated microbes, the “human microbiome” (http://www.hmpdacc.org/), using metagenomic approaches (Chapter 5) first designed for soils and oceans. The term “microbiome” is used by some investigators for any microbial community in a natural environment (Moran, 2015; Fierer, 2017), but here the term will be reserved for communities associated with larger organisms.
Microbes help to make our food and other useful products
Microbes produce several things that we eat and drink every day, including yogurt, cheese, kimchi, and wine. Some of these microbially produced comestibles were examined by early scientists who could be called microbial ecologists. Louis Pasteur (1822–95; see Box 1.1) was hired by the wine industry to figure out why some wines turned sour and became undrinkable. The problem, as Pasteur found out, was a classic one of competition between two types of microbes, one that produced alcohol (good wine) and the other, organic acids (sour, undrinkable wine). To this day, food microbiologists try to understand the complex microbial interactions and
Box 1.1 Two founders of microbiology
One of the founders of microbiology, Louis Pasteur, made many contributions to chemistry and biology, such as showing that life does not arise from spontaneous generation, a theory held in the mid-nineteenth century to explain organic matter degradation. Decomposition of organic material is still an important topic today in microbial ecology (Chapter 7). Pasteur also
explored the role of bacteria in causing diseases, but it was a contemporary of Pasteur, Robert Koch (1843–1910), another founder of microbiology, who defined the criteria, now known as Koch’s postulates, for showing that a particular microbe causes a specific disease. Koch developed the agar plate method for isolating bacteria, a method still used today in microbiology.
processes that affect our favorite things to eat and drink, an important job in applied microbial ecology. Microbes are also involved in meat and dairy products. Cows, goats, and sheep—ruminants—depend on complex microbial consortia to digest the polysaccharides in the grasses they eat (Chapter 14).
Microbes are also important in supporting life in lakes and the oceans, and eventually make possible the fish we eat. Microbes take over the role of macroscopic plants in aquatic environments and are the main primary producers, meaning they use light energy to convert carbon dioxide (CO2) to organic material (Chapter 6). These microbes, the “phytoplankton,” include cyanobacteria and eukaryotic algae. Phytoplankton are rarely eaten by fish, not even by their small, young stages, the fish larvae. Rather, the main herbivores in lakes and oceans are mostly microscopic animals (zooplankton) and protists.
Zooplankton and protists in turn are eaten by still larger zooplankton and fish larvae as part of a food chain leading eventually to adult fish (Figure 1.3). There can be more
direct connections between microbes and fish. In some aquaculture farms, shrimp feed on “bioflocs” formed from bacteria growing on added wheat flour. The simple, linear food chain shown in Figure 1.3 is accurate in only some aquatic systems. But even in waters with more complex food webs, microbes are the base upon which the fisheries of the world depend. Consequently, there is a general relationship between microbial production and fishery yields.
Phytoplankton
Figure 1.3 A simple food chain, from phytoplankton to fish, found in aquatic habitats. Microbes (phytoplankton) are at the base of this food chain, and other microbes (small grazers) make up the first few transfers. Still other microbes (mainly bacteria), not shown, contribute to the release of nutrients used by phytoplankton.
Another important connection with our food is the role of microbes in producing the inorganic nutrients that are essential for growth and biomass production by higher plants in terrestrial environments (Figure 1.4) and by phytoplankton in aquatic environments. Essential inorganic nutrients, such as ammonium and phosphate, come from microbes as they degrade organic material (Chapter 7). Other microbes “fix” nitrogen gas, which cannot be used by plants as a nitrogen source, into ammonium, which can (Chapter 12). The fertility of soils depends on microbes in other ways. Organic material from higher plants, partially degraded by microbes, and other organic compounds directly from microbes, make up soil organic material. This organic component of soil contains essential plant nutrients and affects water flow, fluxes of oxygen and other gases, pH, and many other physical–chemical properties of soils that directly contribute to the growth of crop plants. So, our food indirectly and directly depends on microbes and what they do.
In addition to things we can eat or drink, microbes produce a vast array of other compounds that enrich our lives. These include antibiotics, chemicals such as butanol and citric acid, and enzymes used in the food,
Figure 1.4 Some of the roles of microbes in supplying key nutrients for crops and plants in terrestrial habitats. The three N-cycle reactions depicted here include mineralization of organic nitrogen to ammonia (NH3), the fixation of N2 gas to ammonia, and the reduction of nitrate (NO3 ) to N2 gas or ammonia. The complete N cycle is discussed in Chapter 12.
pharmaceutical, and textile industries. Other microbially produced enzymes, such as the one used for the polymerase chain reaction (PCR; see Chapter 4), are essential in biotechnology. Most of “industrial microbiology” is far from the environmental sciences, but some aspects of the field touch on the basics of microbial ecology covered in this book.
Microbes degrade and detoxify pollutants
The modern environmental movement is often said to have started with the publication in 1962 of Silent Spring by Rachel Carson (1907–64). The book chronicled the damage to wildlife and ecosystems caused by the pesticide dichloro-diphenyl-trichloroethane, better known as DDT. Fortunately, the concentrations of DDT have decreased over time, in part due to regulations banning it in most developed countries, following the publication of Silent Spring. In addition, microbes, mostly bacteria, degrade DDT and other organic pollutants to innocuous compounds and eventually to CO2, despite the pollutants being recalcitrant and difficult to degrade. With very few exceptions, bacteria and fungi are quite adept at degrading organic compounds, even those quite toxic to macroscopic organisms.
Inorganic pollutants cannot be removed by microbial activity, but by changing the chemical state of these pollutants, microbes affect their toxicity or movement through the environment or both. Microbes produce a
toxic form of mercury, methylmercury, that is more easily transferred to larger organisms than inorganic mercury. Another example is uranium (Williams et al., 2013). The most oxidized form of uranium, U(VI), moves easily through subsurface environments. When U(VI) is reduced by Geobacter and probably other bacteria, the resulting U(IV) is less mobile and less likely to cause environmental problems.
The study of pollutant–microbe interactions is a part of “environmental microbiology,” but that term is also used more generally to describe the study of microbes in the environment regardless of whether a pollutant is involved or if the microbes are mediating a process of practical interest (Madsen, 2016). “Environmental microbiology” and “microbial ecology” are essentially synonymous.
Microbes are models for exploring principles in ecology and evolution
Microbes have served as models for exploring many questions in biochemistry, physiology, and molecular biology. They are good models because they grow rapidly and can be manipulated easily in laboratory experiments. For similar reasons, microbes are also used as models to explore general questions in ecology, population genetics, and evolution (Barberán et al., 2014). Virus–bacteria interactions, for example, have served as models of predator–prey interactions, and experiments with protozoa and bacteria were crucial for establishing Gause’s “competitive exclusion principle” (Figure 1.5). The principle is that only one species can occupy a particular niche at a time because it excludes other species due to competition for a limiting resource. Experiments with microbes have demonstrated basic principles about natural selection and adaptation in varying environments. Richard Lenski and colleagues have explored the evolution of the bacterium Escherichia coli over 50,000 generations by growing it in new, fresh media every day since 1988, including weekends and holidays (Lenski et al., 2015). Genome sequencing (Chapter 5) has revealed how this bacterium has changed over time, providing insights into evolution not possible with large organisms.
Just as we can learn about large organisms from microbes, the flow of ideas can go the other way. General
Figure 1.5 Experimental evidence for the competitive exclusion principle, which states that no two species can occupy the same niche at the same time. Here, two species of the protozoan Paramecium are forced to compete for the same food source, a bacterial prey (Bacillus pyocyaneus). Only one Paramecium species wins. Data from Gause (1964).
theories developed for exploring the ecology of plants and animals are often useful for exploring questions in microbial ecology. For example, microbial ecologists have used island biogeography theory, which was first conceived for large animals by R. H. MacArthur and E. O. Wilson in 1967, to examine the dispersal of microbes and relationships between microbial diversity and habitat size. Likewise, models about stability and diversity developed for large organism communities are now being applied to microbial communities and processes. Microbial ecologists look at microbial diversity for patterns that have been seen for plant and animal diversity, such as how diversity varies with latitude (Chapter 4). Not all large organism-based theories are applicable to thinking about microbes, but many are.
Microbes living today are models for early life on Earth and perhaps life on other planets
Life first appeared on Earth about 3.7 billion years ago and perhaps even earlier than that, not long after the planet was formed 4.5 billion years ago (Fig 1.6). The first cell on Earth was a microbe, perhaps a hydrogen-gaspowered acetogen or methanogen, types of bacteria and archaea that produce acetate or methane, respectively (Chapter 11), which may have evolved at hydrothermal vents (Martin and Sousa, 2016). Viruses have probably been around since cellular life began; some investigators
even hypothesize that life started in viral form. Cyanobacteria first appeared probably about 2.8 billion years ago, leading eventually to high oxygen concentrations in the Earth’s atmosphere (Chapter 11).
The date when eukaryotes evolved is controversial, and estimates depend on the data being considered. Fossil structures perhaps from the first eukaryote have been found in 3.2 billion-year-old estuarine sediments, while steranes, derived from steroids or sterols produced only by eukaryotes, have turned up in shales estimated to be about 2.7 billion years old (Arndt and Nisbet, 2012). But the most convincing fossils, backed up by DNA sequence data, indicate that eukaryotes appeared on Earth about two billion years ago. Regardless of the exact dates, microbes were the only life forms on the planet for the first three billion years of Earth’s history. Multicellular animals and plants did not appear until about a billion years ago, two to three billion years after microbes had invented most of the various metabolisms now known to operate on Earth. As Harvard University paleontologist Andrew Knoll once said, “Animals might be evolution’s icing, but bacteria are really the cake.”
We can gain additional insights into the evolution of early life by looking at microbes and environments around today that may mimic those on early Earth. These include bacteria and archaea living today with gene sequences that put them near the root of the Tree of Life and are perhaps closely related to the first cell that
P. caudatum
No life
Millions of years before the present
Bacteria, archaea and viruses
Cyanobacteria and oxygen production
Only microbes on earth
Microbial eukaryotes
Metazoans
Simple animals
Land plants
Dinosaurs
Figure 1.6 A few key dates in the history of life. For much of this history, only microbes were present, as multicellular eukaryotes appeared only one billion years ago, or 3.5 billion years after Earth had formed. Based on Humphreys et al. (2010), Payne et al. (2009), and Arndt and Nisbet (2012).
appeared on the planet. These microbes may have metabolisms similar to those of the first cell or two. Some of the first fossils of microbes have been found in stromatolites, which are large, laminated mats of different microbes and minerals abundant in the Precambrian when life was just starting on Earth (Chapter 13). These complex microbial mats can also be found today in some saline lakes and marine lagoons. Our planet still has many “warm little ponds” where Darwin speculated that life first evolved. Studying the Earth’s first organisms is part of a branch of microbial ecology: geomicrobiology (Chapter 13).
In addition to helping us look at life millions of years ago, today’s microbes may potentially provide insights into life on planets or moons of other planets millions of kilometers from Earth. Studying microbes in extraterrestrial-like environments on Earth is the main focus of “astrobiology.” These environments are extreme, where only microbes, and often only bacteria and archaea, the “extremophiles,” survive. Microbes live in hot springs and deserts, polar ice, permafrost of the tundra, and within rocks—unworldly habitats where it is hard to imagine life existing. Perhaps these earthly extremophiles are similar to life on other planets, and perhaps insights gained from astrobiological studies on this planet will help in the
search for life elsewhere in the universe. But the work would be worthwhile even if there were no extraterrestrial microbes. Extreme environments and extremophiles are often bizarre and always fascinating.
Microbes mediate biogeochemical processes that affect global climate
This reason shapes many topics appearing in this book. The role of microbes in degrading pollutants has already been mentioned, but microbes are involved in an even more serious “pollution” problem.
Humans have been polluting the Earth’s atmosphere with various gases that affect our climate. These “greenhouse gases” trap long-wave radiation, better known as heat, from the sun. Most of these gases also have natural sources, and the Earth always has had greenhouse gases, fortunately. Because of these gases, the average global temperature is 16 °C, much warmer than the chilly 18 °C Earth would be without them (Schlesinger and Bernhardt, 2013). Mars does not have any greenhouse gases and is even colder ( 53 °C). High concentrations of greenhouse gases, mainly carbon dioxide, in Venus’s atmosphere, along with its proximity to the sun, explain why that planet has an average surface temperature of
474 °C. However, although some greenhouse gases are beneficial for the Earth’s climate, their excessive levels are leading to global warming and many other related global problems.
Greenhouse gases have been increasing in Earth’s atmosphere since the start of the industrial revolution in the early 1800s (Figure 1.7). Although water vapor is the dominant greenhouse gas, human society has a bigger, more direct impact on other gases, most notably carbon dioxide. Other important greenhouse gases include methane (CH4) and nitrous oxide (N2O). The concentrations of these gases in the atmosphere are much lower, but they trap more heat per molecule than does CO2 Because of higher levels of greenhouse gases, the average temperature for the planet is about 1 °C warmer now
than it was in the nineteenth century (Figure 1.7), and is projected to increase even more in the near future. A degree or two warmer may not seem like much, but it has already caused many changes in the biosphere, to say the least. Another problem caused by raising atmospheric CO2 is the acidification of the oceans, which threatens corals and other calcifying organisms, including some microbes (Chapters 13 and 14).
Microbial ecology has an essential role in understanding the impact of greenhouse gases on our climate and the response of ecosystems to climate change, one reason being that nearly all of these gases are either used or produced, or both, by microbes (Table 1.1). Carbon dioxide, for example, is used by higher plants on land and by phytoplankton in aquatic ecosystems. This gas is
Figure 1.7 Atmospheric CO2 concentrations (A) and global temperature anomaly (B) since the nineteenth century. Concentrations were estimated from ice cores (Etheridge et al., 1996) or measured directly at the Mauna Loa Observatory, Hawaii, provided by Pieter Tans (NOAA Earth System Research Laboratory and Ralph Keeling (Scripps Institution of Oceanography), used with permission (http://www.esrl.noaa.gov/gmd/ccgg/trends/#mlo_data). The global temperature is expressed as the difference between the average temperature for a year minus the average over 1951–80 (Hansen et al., 2010). Updated temperature data from http://data.giss.nasa.gov/gistemp/, accessed on November 20, 2016.
Table 1.1 Some greenhouse gases and how they are affected by microbes. Concentrations are expressed as parts per million (ppm), per billion (ppb), or per trillion (ppt). Data from the Mauna Loa Observatory (http://www.esrl.noaa.gov/gmd/) and Myhre et al. (2014).
GasConcentrations in 2005 Concentrations in 2016 Greenhouse effect* Microbial process
Carbon dioxide (CO2) 379 ppm 401 ppm 1Primary production and organic carbon mineralization
Methane (CH4)1774 ppb1834 ppm21Methanogenesis and methanotrophy
Nitrous oxide (N2O) 319 ppb 329 ppb270 denitrification and nitrification CFC-11 (CCl3F)** 250 ppt 230 ppt4660 degradation by heterotrophs?
* Relative to CO2
** One of many hydrochlorofluorocarbons. Some of these other gases have even greater greenhouse effects than that of CFC-11.
released by heterotrophic microbes in all ecosystems. The impact of biological activity can be seen in the yearly oscillations of carbon dioxide in Figure 1.7; it goes down in the summer when plant growth is high and it increases in winter when carbon dioxide produced by respiration exceeds carbon dioxide use by plant growth. Methane, another gas that has been increasing in the atmosphere, is also produced and consumed by microbes (Chapter 11). It and another powerful greenhouse gas, nitrous oxide, are produced in anoxic environments which have expanded over the years, mainly due to the growth in agriculture.
What complicates our understanding of these greenhouse gases is that nearly all are produced or consumed by natural processes mediated by microbes, in addition to the anthropogenic inputs. Although the input of CO2 to the atmosphere by human activity is substantial, it is still small compared to the input and uptake by natural processes. In contrast, production of the important plant nutrient, ammonium, directly by humans (fertilizer synthesis) or aided by humans (microbial production in agriculture) rivals the natural production of ammonium by microbes (Chapter 12). To complicate things further, greenhouse gases vary with the seasons, as already seen for CO2, and have varied greatly over geological time, independent of human intervention. So, the challenge is to separate the natural changes from those effected by humans and to understand the consequences of these changes.
Microbial ecologists cannot solve the greenhouse problem. But many of the topics discussed in this book can help us understand the problem and predict future changes. One job of microbial ecologists and other scientists studying the Earth system is to figure out the impact of increasing greenhouse gases and other global
changes on the biosphere. How will an increase in global temperature affect the balance between photosynthesis and respiration? How much CO2 and CH4 will be released if the permafrost of the tundra in Alaska and Siberia melts? Will a decrease in diversity caused by climate change affect the C cycle? Answering these and other questions depends on the work of microbial ecologists.
Microbes are everywhere, doing nearly everything Most of the reasons discussed so far for studying microbial ecology have focused on practical problems facing human society. But microbial ecology would be an exciting field even if all of these problems were solved tomorrow (Box 1.2). One goal of this book is to show the importance of microbial ecology in explaining basic processes in the biosphere, even if they may appear to be far from any problem facing us today. Regardless of what microbes do, we should want to know about them
Box 1.2 Praise of microbial ecology from an ant ecologist
E. O. Wilson did most of his scientific work on ants and went on to write several award-winning books, including Sociobiology and On Human Nature. Near the end of his autobiography, Naturalist, published in 1994 (Island Press), Wilson mentioned that if he had to do it all over again, he would be a microbial ecologist. He ends with a paean to the microbial world: “The jaguars, ants, and orchids would still occupy distant forests in all their splendor, but now they would be joined by an even stranger and vastly more complex living world virtually without end.”
because they are the most numerous and diverse organisms on the planet.
As a general rule, the smaller the organism, the more numerous it is (Figure 1.8). Viruses are the smallest and also the most abundant biological entity in both aquatic habitats and soils, whereas large organisms, such as zooplankton and earthworms, are rare, being 1010 less abundant than viruses. A typical milliliter of water from the surface of a lake or the oceans contains about 107 viruses, 106 bacteria, 104 protists, and 103 or fewer phytoplankton cells, depending on the environment. A typical gram of
soil or sediment contains about 1010 viruses, 109 bacteria, and so on for larger organisms. Even deep environments, kilometers below the Earth’s surface, have thousands of microbes. Even seemingly impenetrable rocks can harbor dense microbial communities. Overall, the biomass of bacteria and archaea rivals that of all plants in the biosphere, and is certainly greater than the biomass of animals.
Microbes are found even where macroscopic organisms are not, in environments with extremes in temperatures, pH, or pressure: “extreme” for humans, but quite
Figure 1.8 Size distribution and abundance of some microbes and other organisms in a typical aquatic habitat (A) and in soils (B). “Bacteria” here refers only to heterotrophic ones, while “other phytoplankton” are phytoplankton other than cyanobacteria or photoautotrophic protists. The size given for fungi is the diameter of the hyphae. Some fungi can be several meters long.
normal for many microbes. Some hyperthermophilic bacteria and archaea thrive in near boiling water (>80 °C), which kills all other organisms, including eukaryotic microbes. The hot springs of Yellowstone are famous for harboring dense and exotic microbes that not only thrive at high temperatures but also at low pH; these microbes live in boiling acid baths. At the other extreme, microbes live in the brine channels of sea ice where water is still liquid but very salty (20% versus 3.5% for seawater) and cold ( 20 °C). Viable microbes have been recovered encased in salt millions of years old (Jaakkola et al., 2016). The deep ocean may be extreme to us with its high hydrostatic pressure, one hundred-fold higher at 1000 m than at sea level, and perennially cold temperatures (about 3 °C). Many microbes thrive and grow, albeit slowly, in these deep waters, one of the largest ecosystems on the planet. Over 70% of the globe is covered by the oceans of which 75% (by volume) is deeper than 1000 m.
In addition to being numerous, there are many different types of microbes, some with strange and weird (from our biased perspective) metabolisms on which the biosphere depends. In addition to plant- or animal-like metabolism, some microbes can live without oxygen and “breathe” with nitrate (NO3 ) or sulfate (SO42−) (Chapter 11). Compounds like hydrogen sulfide (H2S) are deadly to macroscopic organisms, but are essential comestibles for some microbes. Several metabolic reactions, such as methane production and the synthesis of ammonium from nitrogen gas, are carried out only by archaea and bacteria. Other microbes are capable of producing chemicals, like acetone and butane, which would kill off eukaryotic cells and seem incompatible with life. Microbes are truly capable of doing nearly everything.
How do we study microbes in nature?
The facts about microbes in nature discussed so far have come from many studies using many approaches and methods. It is a great intellectual puzzle to figure out the actions and creatures in the unseen world and how they affect our visible world. This book will introduce some of the methods used in microbial ecology so that you can gain deeper insights and appreciation of the boundaries between the known and unknown. By learning a little about the methodology, you will also understand better
why some seemingly simple questions are difficult to answer.
Here we start with one of the most basic questions: how many bacteria are in an environment? We will focus on just two ways to answer this question, in order to introduce a fundamental divide between approaches for all microbes: “cultivation-dependent” versus “cultivationindependent.” The number of bacteria in an environment was first estimated by the “plate count method,” which consists of growing or cultivating organisms on solid agar media (Figure 1.9; Box 1.3). The assumption behind the plate count method is that each microbe in
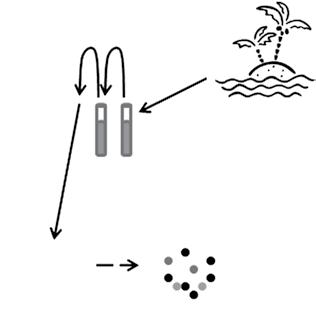
Figure 1.9 The plate count method. A sample from the environment is usually diluted first by adding 1.0 ml or 0.1 g of soil to 9.0 ml of an appropriate buffer, then sometimes diluted again by adding 1.0 ml of the first dilution to a new tube containing 9.0 ml of the buffer. Several more dilutions may be necessary. After a few days, if ten colonies grew up after two dilutions, for example, we could deduce that there were 104 culturable bacteria per ml or gram of sample.
Box 1.3 Able assistance with agar plates
Agar plates are made by pouring molten agar amended with various compounds into Petri dishes. Once cool, the agar solidifies and becomes a porous support on which microbes grow to form colonies— many, many cells piled together into a mound big enough to see. Although the approach is usually attributed to Robert Koch, two assistants of Koch came up with the key parts. Petri dishes were thought up by Julius Richard Petri, while Koch’s wife, Fannie Hesse, suggested agar, which was used at the time to make jam.