ListofContributors
DavidAbraham CentreforRheumatologyandConnective TissueDiseases,UniversityCollegeLondon,London, UnitedKingdom
MariaAlmeida DivisionofEndocrinologyandMetabolism,CenterforOsteoporosisandMetabolicBone Diseases,UniversityofArkansasforMedicalSciences, LittleRock,AR,UnitedStates;TheCentralArkansas VeteransHealthcareSystem,LittleRock,AR,United States
ElenaAmbrogini CenterforOsteoporosisandMetabolic BoneDiseases,UniversityofArkansasforMedical SciencesDivisionofEndocrinologyandMetabolism, LittleRock,AR,UnitedStates;CentralArkansasVeteransHealthcareSystem,LittleRock,AR,United States
AndrewArnold CenterforMolecularOncologyand DivisionofEndocrinologyandMetabolism,University ofConnecticutSchoolofMedicine,Farmington,CT, UnitedStates
BenceBakos 1stDepartmentofMedicine,Semmelweis UniversityMedicalSchool,Budapest,Hungary
ClemensBergwitz DepartmentsofPediatricsandInternal Medicine,YaleUniversitySchoolofMedicine,New Haven,CT,UnitedStates
DanielD.Bikle VAMedicalCenterandUniversityof CaliforniaSanFrancisco,SanFrancisco,California, UnitedStates
JohnP.Bilezikian DivisionofEndocrinology,Department ofMedicine,CollegeofPhysiciansandSurgeons, ColumbiaUniversity,NewYork,NY,UnitedStates
NeilBinkley UniversityofWisconsinSchoolofMedicine andPublicHealth,Madison,Wisconsin,UnitedStates
AlessandroBisello DepartmentofPharmacologyand ChemicalBiology,LaboratoryforGPCRBiology, UniversityofPittsburghSchoolofMedicine,Pittsburgh,PA,UnitedStates
L.F.Bonewald IndianaCenterforMusculoskeletalHealth, DepartmentsofAnatomyandCellBiologyandOrthopaedicSurgery,IndianaUniversity,Indianapolis,IN, USA
GeorgeBou-Gharios InstituteofAgeingandChronic Disease,UniversityofLiverpool,Liverpool,United Kingdom
RogerBouillon LaboratoryofClinicalandExperimental Endocrinology,DepartmentofChronicDiseases, MetabolismandAging,KULeuven,Belgium
MaryL.Bouxsein CenterforAdvancedOrthopaedic Studies,BethIsraelDeaconessMedicalCenter,Boston,MA,UnitedStates;DepartmentofOrthopaedic Surgery,HarvardMedicalSchool,Boston,MA,UnitedStates;EndocrineUnit, DepartmentofMedicine, MassachusettsGeneralHospital,Boston,MA,United States
BrendanF.Boyce DepartmentofPathologyandLaboratoryMedicine,UniversityofRochesterSchoolof MedicineandDentistry,Rochester,NY,UnitedStates
StevenBoyd McCaigInstituteforBoneandJointHealth, TheUniversityofCalgary,Calgary,AB,Canada
MariaLuisaBrandi DepartmentofExperimentaland ClinicalBiomedicalSciences,UniversityofFlorence, Florence,Italy
DavidB.Burr DepartmentofAnatomyandCellBiology, IndianaCenterforMusculoskeletalHealth,Indiana UniversitySchoolofMedicine,Indianapolis,IN,UnitedStates
LauraM.Calvi DepartmentofMedicineandWilmot CancerCenter,UniversityofRochesterMedicalCenter, Rochester,NY,UnitedStates
ErnestoCanalis DepartmentsofOrthopaedicSurgeryand Medicine,andtheUConnMusculoskeletalInstitute, UConnHealth,Farmington,CT,UnitedStates
XuCao DepartmentofOrthopedicSurgery,JohnsHopkins UniversitySchoolofMedicine,Baltimore,MD,United States
GeertCarmeliet LaboratoryofClinicalandExperimental Endocrinology,DepartmentofChronicDiseases, MetabolismandAgeing,KULeuven,Leuven,Belgium;Prometheus,DivisionofSkeletalTissueEngineering,KULeuven,Leuven,Belgium
ThomasO.Carpenter DepartmentsofPediatricsand InternalMedicine,YaleUniversitySchoolofMedicine, NewHaven,CT,UnitedStates
WenhanChang EndocrineResearchUnit,Departmentof VeteransAffairsMedicalCenter,DepartmentofMedicine,UniversityofCalifornia,SanFrancisco,CA, UnitedStates
ShekManChim RegeneronPharmaceuticals,Inc.Tarrytown,NY,UnitedStates
ShilpaChoudhary DepartmentofMedicineandMusculoskeletalInstitute,UConnHealth,Farmington,CT, UnitedStates
SylviaChristakos DepartmentofMicrobiology,BiochemistryandMolecularGenetics,Rutgers,NewJersey MedicalSchool,Newark,NJ,UnitedStates
Yong-HeePatriciaChun DepartmentofPeriodontics, UniversityofTexasHealthScienceCenteratSan Antonio,SanAntonio,TX,UnitedStates
CristianaCipriani DepartmentofInternalMedicineand MedicalDisciplines,SapienzaUniversityofRome, Italy
RobertoCivitelli WashingtonUniversityinSt.Louis, DepartmentofMedicine,DivisionofBoneandMineral Diseases,St.Louis,MO,UnitedStates
ThomasL.Clemens DepartmentofOrthopaedicSurgery, JohnsHopkinsUniversitySchoolofMedicine,Baltimore,MD,UnitedStates;BaltimoreVeteransAdministrationMedicalCenter,Baltimore,MD,UnitedStates
MichaelT.Collins SkeletalDisordersandMineral HomeostasisSection,NationalInstituteofDentaland CraniofacialResearch,NationalInstitutesofHealth, Bethesda,MD,UnitedStates
CaterinaConte Vita-SaluteSanRaffaeleUniversity, Milan,Italy;DivisionofImmunology,Transplantation andInfectiousDiseases,IRCCSSanRaffaeleScientific Institute,Milan,Italy
MarkS.Cooper TheUniversityofSydney,ANZAC ResearchInstituteandDepartmentofEndocrinology & Metabolism,ConcordHospital,Sydney,NSW,Australia
JillianCornish DepartmentofMedicine,Universityof Auckland,Auckland,NewZealand
SergeCremers DepartmentofPathology & CellBiology andDepartmentofMedicine,VagelosCollegeof PhysiciansandSurgeons,ColumbiaUniversityIrving MedicalCenter,UnitedStates
BessDawson-Hughes JeanMayerUSDAHumanNutritionResearchCenteronAgingatTuftsUniversity, Boston,Massachusetts,UnitedStates
BenoitdeCrombrugghe TheUniversityofTexasM.D. AndersonCancerCenter,Houston,TX,UnitedStates
HectorF.DeLuca DepartmentofBiochemistry,UniversityofWisconsin Madison,Madison,Wisconsin, UnitedStates
DavidW.Dempster RegionalBoneCenter,HelenHayes Hospital,WestHaverstraw,NY,UnitedStates; DepartmentofPathologyandCellBiology,Collegeof PhysiciansandSurgeons,ColumbiaUniversity,New York,NY,UnitedStates
MatthewT.Drake DepartmentofEndocrinologyand KogodCenterofAging,MayoClinicCollegeof Medicine,Rochester,MN,UnitedStates
PatriciaDucy DepartmentofPathology & CellBiology, ColumbiaUniversity,CollegeofPhysicians & Surgeons,NewYork,NY,UnitedStates
FrankH.Ebetino DepartmentofChemistry,Universityof Rochester,Rochester,NY,UnitedStates;Mellanby CentreforBoneResearch,MedicalSchool,University ofSheffield,UnitedKingdom
KlausEngelke DepartmentofMedicine,FAUUniversity Erlangen-NürnbergandUniversitätsklinikumErlangen, Erlangen,Germany;Bioclinica,Hamburg,Germany
ReinholdG.Erben DepartmentofBiomedicalResearch, UniversityofVeterinaryMedicineVienna,Vienna, Austria
DavidR.Eyre DepartmentofOrthopaedicsandSports Medicine,UniversityofWashington,Seattle,WA, UnitedStates
CharlesR.Farber CenterforPublicHealthGenomics, DepartmentsofPublicHealthSciencesandBiochemistryandMolecularGenetics,Universityof VirginiaSchoolofMedicine,Charlottesville,VA, UnitedStates
MarinaFeigenson DepartmentofDevelopmentalBiology, HarvardSchoolofDentalMedicine,Boston,MA, UnitedStates
MathieuFerron InstitutdeRecherchesCliniquesde Montréal,Montréal,QC,Canada
PabloFlorenzano EndocrineDepartment,Schoolof Medicine,Ponti ficiaUniversidadCatólicadeChile, Santiago,Chile
FrancescaFontana WashingtonUniversityinSt.Louis, DepartmentofMedicine,DivisionofBoneandMineral Diseases,St.Louis,MO,UnitedStates
BrianL.Foster BiosciencesDivisionatCollegeofDentistryatOhioStateUniversity,Columbus,OH,United States
PeterA.Friedman DepartmentofPharmacologyand ChemicalBiology,LaboratoryforGPCRBiology, UniversityofPittsburghSchoolofMedicine,Pittsburgh,PA,UnitedStates
SeijiFukumoto FujiiMemorialInstituteofMedicalSciences,InstituteofAdvancedMedicalSciences, TokushimaUniversity,Tokushima,Japan
LauraW.Gamer DepartmentofDevelopmentalBiology, HarvardSchoolofDentalMedicine,Boston,MA, UnitedStates
ThomasJ.Gardella EndocrineUnit,Departmentof MedicineandPediatricNephrology,MassGeneral HospitalforChildren,MassachusettsGeneralHospital andHarvardMedicalSchool,Boston,MA,United States
PatrickGarnero INSERMResearchUnit1033-Lyos, Lyon,France
HarryK.Genant DepartmentsofRadiologyandMedicine,UniversityofCalifornia,SanFrancisco,CA, UnitedStates
FrancescaGiusti DepartmentofExperimentalandClinical BiomedicalSciences,UniversityofFlorence,Florence, Italy
AndyGöbel DepartmentofMedicineIII,TechnischeUniversitätDresden,Dresden,Germany;GermanCancer Consortium(DKTK),PartnersiteDresdenandGerman CancerResearchCenter(DKFZ),Heidelberg,Germany
DavidGoltzman CalciumResearchLaboratoriesand DepartmentofMedicine,McGillUniversityandMcGill UniversityHealthCentre,Montreal,QC,Canada
JeffreyP.Gorski DepartmentofOralandCraniofacial Sciences,SchoolofDentistry,andCenterforExcellenceinMineralizedandDentalTissues,Universityof Missouri KansasCity,KansasCity,MO,UnitedStates
JamesGriffith DepartmentofImagingandInterventional Radiology,TheChineseUniversityofHongKong, HongKong,China
R.GrahamGRussell MellanbyCentreforBone Research,MedicalSchool,UniversityofSheffi eld, UnitedKingdom;Nuf fieldDepartmentofOrthopaedics, RheumatologyandMusculoskeletalSciences,The OxfordUniversityInstituteofMusculoskeletalSciences,TheBotnarResearchCentre,Nuf fieldOrthopaedic Centre,Oxford,UnitedKingdom
KurtD.Hankenson DepartmentofOrthopaedicSurgery, UniversityofMichiganMedicalSchool,AnnArbor, MI,UnitedStates
FadilM.Hannan DepartmentofMusculoskeletalBiology, InstituteofAgeingandChronicDisease,Facultyof Health & LifeSciences,UniversityofLiverpool,Liverpool,UnitedKingdom;AcademicEndocrineUnit, RadcliffeDepartmentofMedicine,Universityof Oxford,OxfordCentreforDiabetes,Endocrinologyand Metabolism(OCDEM),ChurchillHospital,Oxford, UnitedKingdom
StephenE.Harris DepartmentofPeriodontics,University ofTexasHealthScienceCenteratSanAntonio,San Antonio,TX,UnitedStates
IrisR.Hartley InterinstituteEndocrineTrainingProgram, EuniceKennedyShriverNationalInstituteofChild HealthandHumanDevelopment,NationalInstitutesof Health,Bethesda,MD,UnitedStates
ChristineHartmann InstituteofMusculoskeletalMedicine,DepartmentofBoneandSkeletalResearch, MedicalFacultyoftheUniversityofMünster,Münster, Germany
RobertP.Heaney CreightonUniversity,Omaha,NE, UnitedStates
GeoffreyN.Hendy MetabolicDisordersandComplications,McGillUniversityHealthCenterResearchInstitute,andDepartmentsofMedicine,Physiologyand HumanGenetics,McGillUniversity,Montreal,QC, Canada
MatthewJ.Hilton DepartmentofOrthopaedicSurgery, DepartmentofCellBiology,DukeUniversitySchoolof Medicine,Durham,NC,UnitedStates
LorenzC.Hofbauer CenterforRegenerativeTherapies Dresden,CenterforHealthyAgingandDivisionof Endocrinology,Diabetes,andBoneDiseases,DepartmentofMedicineIII,TechnischeUniversitätDresden, Dresden,Germany
GillHoldsworth BoneTherapeuticArea,UCBPharma, Slough,UnitedKingdom
Yi-HsiangHsu DepartmentofMedicine,BethIsrael DeaconessMedicalCenterandHarvardMedical School,HarvardSchoolofPublicHealth,Hindaand ArthurMarcusInstituteforAgingResearch,Hebrew SeniorLife,Boston,MA,UnitedStates
DavidM.Hudson DepartmentofOrthopaedicsandSports Medicine,UniversityofWashington,Seattle,WA, UnitedStates
MarjaHurley DepartmentofMedicine,Universityof ConnecticutSchoolofMedicine,UConnHealth, Farmington,CT,UnitedStates
KarlL.Insogna DepartmentsofPediatricsandInternal Medicine,YaleUniversitySchoolofMedicine,New Haven,CT,UnitedStates
RobertL.Jilka CenterforOsteoporosisandMetabolic BoneDiseases,UniversityofArkansasforMedical SciencesDivisionofEndocrinologyandMetabolism, LittleRock,AR,UnitedStates;CentralArkansasVeteransHealthcareSystem,LittleRock,AR,UnitedStates
MarkL.Johnson DepartmentofOralandCraniofacial Sciences,UMKCSchoolofDentistry,KansasCity, MO,UnitedStates
RachelleW.Johnson VanderbiltCenterforBoneBiology, DepartmentofMedicine,DivisionofClinicalPharmacology,Nashville,TN,UnitedStates
GlenvilleJones DepartmentofBiomedicalandMolecular Science,Queen’sUniversity,Kingston,ON,Canada
StefanJudex DepartmentofBiomedicalEngineering, BioengineeringBuilding,StateUniversityofNewYork atStonyBrook,StonyBrook,NY,UnitedStates
HaraldJüppner EndocrineUnit,DepartmentofMedicine andPediatricNephrology,MassGeneralHospitalfor Children,MassachusettsGeneralHospitalandHarvard MedicalSchool,Boston,MA,UnitedStates
IvoKalajzic DepartmentofReconstructiveSciences, UConnHealth,Farmington,CT,UnitedStates
GérardKarsenty DepartmentofGeneticsandDevelopment,ColumbiaUniversityMedicalCenter,NewYork, NY,UnitedStates
HuaZhuKe AngitiaBiopharmaceuticalsLimited, Guangzhou,China
SundeepKhosla DepartmentofMedicine,Divisionof Endocrinology,MayoClinicCollegeofMedicine, Rochester,MN,UnitedStates;TheRobertandArlene KogodCenteronAging,Rochester,MN,UnitedStates
DouglasP.Kiel DepartmentofMedicine,BethIsrael DeaconessMedicalCenterandHarvardMedical School,HarvardSchoolofPublicHealth,Hindaand ArthurMarcusInstituteforAgingResearch,Hebrew SeniorLife,Boston,MA,UnitedStates
J.Klein-Nulend DepartmentofOralCellBiology, AcademicCentreforDentistryAmsterdam(ACTA), UniversityofAmsterdamandVrijeUniversiteit Amsterdam,AmsterdamMovementSciences,Amsterdam, TheNetherlands
FrankC.Ko CenterforAdvancedOrthopaedicStudies, BethIsraelDeaconessMedicalCenter,Boston,MA, UnitedStates
YasuhiroKobayashi InstituteforOralScience,MatsumotoDentalUniversity,Nagano,Japan
MartinKonrad DepartmentofGeneralPediatrics,UniversityChildren’sHospitalMünster,Münster,Germany
PaulJ.Kostenuik PhylonPharmaServices,Newbury Park,CA,UnitedStates;SchoolofDentistry,UniversityofMichigan,AnnArbor,MI,UnitedStates
ChristopherS.Kovacs FacultyofMedicine,Memorial UniversityofNewfoundland,St.John’s,NL,Canada
RichardKremer CalciumResearchLaboratoriesand DepartmentofMedicine,McGillUniversityandMcGill UniversityHealthCentre,Montreal,QC,Canada
VenkateshKrishnan LillyResearchLaboratories,Eli Lilly & Company,LillyCorporateCenter,Indianapolis, UnitedStates
HenryM.Kronenberg EndocrineUnit,Massachusetts GeneralHospital,HarvardMedicalSchool,Boston, MA,UnitedStates
PeterA.Lakatos 1stDepartmentofMedicine,SemmelweisUniversityMedicalSchool,Budapest,Hungary
UriA.Liberman DepartmentofPhysiologyandPharmacology,SacklerFacultyofMedicine,TelAviv University,Tel-Aviv,Israel
JosephA.Lorenzo TheDepartmentsofMedicineand Orthopaedics,UConnHealth,Farmington,CT,United States
ConorC.Lynch DepartmentofTumorBiology,Mof fitt CancerCenter,Tampa,FL,UnitedStates
KarenM.Lyons DepartmentofOrthopaedicSurgery/ OrthopaedicHospital,UniversityofCalifornia,Los Angeles,CA,UnitedStates;DepartmentofMolecular, Cell, & DevelopmentalBiology,UniversityofCalifornia,LosAngeles,CA,UnitedStates
Y.LindaMa BiotechnologyandAutoimmunityResearch, EliLillyandCompany,Indianapolis,IN,UnitedStates
ChristaMaes LaboratoryofSkeletalCellBiologyand Physiology(SCEBP),SkeletalBiologyandEngineering ResearchCenter(SBE),KULeuven,Leuven,Belgium
MichaelMannstadt EndocrineUnit,MassachusettsGeneralHospital,HarvardMedicalSchool,Boston,MA, UnitedStates
StavrosManolagas DivisionofEndocrinologyand Metabolism,CenterforOsteoporosisandMetabolic BoneDiseases,UniversityofArkansasforMedical Sciences,LittleRock,AR,UnitedStates;TheCentral ArkansasVeteransHealthcareSystem,LittleRock,AR, UnitedStates
RobertMarcus StanfordUniversity,Stanford,CA,United States
DavidE.Maridas DepartmentofDevelopmentalBiology, HarvardSchoolofDentalMedicine,Boston,MA, UnitedStates
PierreJ.Marie UMR-1132Inserm(Institutnationaldela SantéetdelaRechercheMédicale)andUniversityParis Diderot,SorbonneParisCité,Paris,France
FrancescaMarini DepartmentofExperimentalandClinicalBiomedicalSciences,UniversityofFlorence, Florence,Italy
JasnaMarkovac CaliforniaInstituteofTechnology, Pasadena,CA,UnitedStates
T.JohnMartin St.Vincent’sInstituteofMedical Research,Melbourne,Australia;Departmentof MedicineatSt.Vincent’sHospital,TheUniversityof Melbourne,Melbourne,Australia
BryaG.Matthews DepartmentofMolecularMedicineand Pathology,UniversityofAuckland,Auckland,New Zealand
AntonioMaurizi DepartmentofBiotechnologicaland AppliedClinicalSciences,UniversityofL’Aquila, L’Aquila,Italy
SasanMirfakhraee TheUniversityofTexasSouthwestern MedicalCenter,DepartmentofInternalMedicine, EndocrineDivision,Dallas,TX,UnitedSates
SharonM.Moe DivisionofNephrology,IndianaUniversity SchoolofMedicine,RodebushVeteransAdministration MedicalCenter,Indianapolis,IN,UnitedStates
DavidG.Monroe DepartmentofMedicine,Divisionof Endocrinology,MayoClinicCollegeofMedicine, Rochester,MN,UnitedStates;TheRobertandArlene KogodCenteronAging,Rochester,MN,UnitedStates
CarolinaA.Moreira BoneUnitofEndocrineDivisionof FederalUniversityofParana,LaboratoryPRO,Section ofBoneHistomorphometry,ProRenalFoundation, Curitiba,Parana,Brazil
RalphMüller InstituteforBiomechanics,ETHZurich, Zurich,Switzerland
DavidS.Musson DepartmentofMedicine,Universityof Auckland,Auckland,NewZealand
TeruyoNakatani DepartmentofBasicScienceandCraniofacialBiology,NewYorkUniversityCollegeof Dentistry,NewYork,NY,UnitedStates
DoritNaot DepartmentofMedicine,UniversityofAuckland,Auckland,NewZealand
NicolaNapoli UnitofEndocrinologyandDiabetes,UniversityCampusBio-Medico,Rome,Italy;Divisionof BoneandMineralDiseases,WashingtonUniversityin StLouis,StLouis,MO,UnitedStates
TallyNaveh-Many MinervaCenterforCalciumandBone Metabolism,NephrologyServices,HadassahUniversity Hospital,HebrewUniversitySchoolofMedicine,Jerusalem,Israel
EdwardF.Nemeth MetisMedica,Toronto,ON,Canada
ThomasL.Nickolas DivisionofNephrology,Department ofMedicine,ColumbiaUniversityMedicalCenter, NewYork,NY,UnitedStates
MichaelS.Ominsky RadiusHealthInc.,Waltham,MA, UnitedStates
NoriakiOno DepartmentofOrthodonticsandPediatric Dentistry,UniversityofMichiganSchoolofDentistry, AnnArbor,MI,UnitedStates
DavidM.Ornitz DepartmentofDevelopmentalBiology, WashingtonUniversitySchoolofMedicine,St.Louis, MO,UnitedStates
NicolaC.Partridge DepartmentofBasicScienceand CraniofacialBiology,NewYorkUniversityCollegeof Dentistry,NewYork,NY,UnitedStates
VihitabenS.Patel DepartmentofBiomedicalEngineering, BioengineeringBuilding,StateUniversityofNewYork atStonyBrook,StonyBrook,NY,UnitedStates
J.WesleyPike DepartmentofBiochemistry,Universityof Wisconsin Madison,Madison,WI,UnitedStates
CarolPilbeam DepartmentofMedicineandMusculoskeletalInstitute,UConnHealth,Farmington,CT, UnitedStates
LoriPlum DepartmentofBiochemistry,Universityof Wisconsin Madison,Madison,Wisconsin,UnitedStates
JohnT.Potts,Jr. EndocrineUnit,Departmentof MedicineandPediatricNephrology,MassGeneral HospitalforChildren,MassachusettsGeneralHospital andHarvardMedicalSchool,Boston,MA,United States
J.EdwardPuzas DepartmentofOrthopaedicsandRehabilitation,UniversityofRochesterSchoolofMedicine andDentistry,Rochester,NY,UnitedStates
TilmanD.Rachner DepartmentofMedicineIII,TechnischeUniversitätDresden,Dresden,Germany;German CancerConsortium(DKTK),PartnersiteDresdenand GermanCancerResearchCenter(DKFZ),Heidelberg, Germany;CenterforHealthyAgingandDivisionof
Endocrinology,Diabetes,andBoneDiseases,TechnischeUniversitätDresden,Dresden,Germany
AudreyRakian DepartmentofAppliedOralSciences,The ForsythInstitute,Cambridge,MA,UnitedStates; DepartmentofOralMedicine,Infection,Immunity, HarvardSchoolofDentalMedicine,Boston,MA, UnitedStates
RubieRakian DepartmentofAppliedOralSciences,The ForsythInstitute,Cambridge,MA,UnitedStates; DepartmentofOralMedicine,Infection,Immunity, HarvardSchoolofDentalMedicine,Boston,MA, UnitedStates
NoraE.Renthal DivisionofEndocrinology,Children’s HospitalBoston,Boston,MA,UnitedStates
JulieA.Rhoades(Sterling) DepartmentofVeterans Affairs,Nashville,TN,UnitedStates;Vanderbilt CenterforBoneBiology,DepartmentofMedicine, DivisionofClinicalPharmacology,Nashville,TN, UnitedStates;DepartmentofBiomedicalEngineering, Nashville,TN,UnitedStates
MaraRiminucci DepartmentofMolecularMedicine, SapienzaUniversityofRome,Rome,Italy
ScottJ.Roberts BoneTherapeuticArea,UCBPharma, Slough,UnitedKingdom
PamelaGehronRobey NationalInstituteofDentaland CraniofacialResearch,NationalInstitutesofHealth, DepartmentofHealthandHumanServices,Bethesda, MD,UnitedStates
MichaelJ.Rogers GarvanInstituteofMedicalResearch andStVincent’sClinicalSchool;UniversityofNew SouthWales,Sydney,Australia
G.DavidRoodman DepartmentofMedicine,Divisionof HematologyandOncology,IndianaUniversitySchool ofMedicine,andRoudebushVAMedicalCenter, Indianapolis,IN,UnitedStates
CliffordJ.Rosen MaineMedicalCenterResearchInstitute,Scarborugh,ME,UnitedStates
VickiRosen DepartmentofDevelopmentalBiology,HarvardSchoolofDentalMedicine,Boston,MA,United States
DavidW.Rowe CenterforRegenerativeMedicineand SkeletalDevelopment,DepartmentofReconstructive Sciences,BiomaterialsandSkeletalDevelopment, SchoolofDentalMedicine,UniversityofConnecticut HealthCenter,Farmington,CT,UnitedStates
JanetRubin EndocrineDivision,DepartmentofMedicine, UniversityofNorthCarolina,ChapelHill,NC,United States
ClintonT.Rubin DepartmentofBiomedicalEngineering, BioengineeringBuilding,StateUniversityofNewYork atStonyBrook,StonyBrook,NY,UnitedStates
KarlP.Schlingmann DepartmentofGeneralPediatrics, UniversityChildren’sHospitalMünster,Münster, Germany
EgoSeeman DepartmentofEndocrinologyandMedicine, AustinHealth,UniversityofMelbourne,Melbourne, VIC,Australia;MaryMacKillopInstituteforHealth Research,AustralianCatholicUniversity,Melbourne, VIC,Australia
MarkusJ.Seibel TheUniversityofSydney,ANZAC ResearchInstituteandDepartmentofEndocrinology & Metabolism,ConcordHospital,Sydney,NSW,Australia
ChrisSempos VitaminDStandardizationProgram,Havre deGrace,MD,UnitedStates
DoloresM.Shoback EndocrineResearchUnit,DepartmentofVeteransAffairsMedicalCenter,Department ofMedicine,UniversityofCalifornia,SanFrancisco, CA,UnitedStates
CarolineSilve HôpitalBicêtre,Paris,France;Centrede RéférencedesMaladiesraresduCalciumetduPhosphoreandFilièredeSantéMaladiesRaresOSCAR, AP-HP,Paris,France;ServicedeBiochimieetGénétiqueMoléculaires,HôpitalCochin,AP-HP,Paris, France
JustinSilver MinervaCenterforCalciumandBone Metabolism,NephrologyServices,HadassahUniversity Hospital,HebrewUniversitySchoolofMedicine,Jerusalem,Israel
NatalieA.Sims St.Vincent’sInstituteofMedical Research,Melbourne,Australia;DepartmentofMedicineatSt.Vincent’sHospital,TheUniversityofMelbourne,Melbourne,Australia
FrederickR.Singer JohnWayneCancerInstitute,Saint John’sHealthCenter,SantaMonica,CA,UnitedStates
JosephP.Stains DepartmentofOrthopaedics,University ofMarylandSchoolofMedicine,Baltimore,MD, UnitedStates
SteveStegen LaboratoryofClinicalandExperimental Endocrinology,DepartmentofChronicDiseases, MetabolismandAgeing,KULeuven,Leuven,Belgium;Prometheus,DivisionofSkeletalTissueEngineering,KULeuven,Leuven,Belgium
PaulaH.Stern DepartmentofPharmacology,NorthwesternUniversityFeinbergSchoolofMedicine,Chicago, IL,UnitedStates
GaiaTabacco DivisionofEndocrinology,Departmentof Medicine,CollegeofPhysiciansandSurgeons, ColumbiaUniversity,NewYork,NY,UnitedStates; EndocrinologyandDiabetesUnit,Departmentof Medicine,CampusBio-MedicoUniversityofRome, Rome,Italy
IstvanTakacs 1stDepartmentofMedicine,Semmelweis UniversityMedicalSchool,Budapest,Hungary
NaoyukiTakahashi InstituteforOralScience,Matsumoto DentalUniversity,Nagano,Japan
DonovanTay DivisionofEndocrinology,Departmentof Medicine,CollegeofPhysiciansandSurgeons, ColumbiaUniversity,NewYork,NY,UnitedStates; DepartmentofMedicine,SengkangGeneralHospital, Singhealth,Singapore
AnnaTeti DepartmentofBiotechnologicalandApplied ClinicalSciences,UniversityofL’Aquila,L’Aquila, Italy
RajeshV.Thakker AcademicEndocrineUnit,Radcliffe DepartmentofMedicine,UniversityofOxford,Oxford CentreforDiabetes,EndocrinologyandMetabolism (OCDEM),ChurchillHospital,Oxford,United Kingdom
RyanE.Tomlinson DepartmentofOrthopaedicSurgery, ThomasJeffersonUniversity,Philadelphia,PA,United States
FrancescoTonelli DepartmentofExperimentalandClinicalBiomedicalSciences,UniversityofFlorence, Florence,Italy
DwightA.Towler TheUniversityofTexasSouthwestern MedicalCenter,DepartmentofInternalMedicine, EndocrineDivision,Dallas,TX,UnitedSates
ElenaTsourdi DepartmentofMedicineIII,Technische UniversitätDresden,Dresden,Germany;Centerfor HealthyAging,TechnischeUniversitätDresden, Dresden,Germany
Chia-LingTu EndocrineResearchUnit,Departmentof VeteransAffairsMedicalCenter,DepartmentofMedicine,UniversityofCalifornia,SanFrancisco,CA, UnitedStates
NobuyukiUdagawa DepartmentofBiochemistry,MatsumotoDentalUniversity,Nagano,Japan
ConnieM.Weaver PurdueUniversity,WestLafayette, IN,UnitedStates
MarcN.Wein EndocrineUnit,MassachusettsGeneral Hospital,HarvardMedicalSchool,Boston,MA,United States
LeeS.Weinstein MetabolicDiseasesBranch,National InstituteofDiabetes,Digestive,andKidneyDiseases, Bethesda,MD,UnitedStates
MaryAnnWeis DepartmentofOrthopaedicsandSports Medicine,UniversityofWashington,Seattle,WA, UnitedStates
MichaelP.Whyte CenterforMetabolicBoneDiseaseand MolecularResearch,ShrinersHospitalsforChildrenSt.Louis,St.Louis,MO,UnitedStates;Divisionof BoneandMineralDiseases,DepartmentofInternal Medicine,WashingtonUniversitySchoolofMedicine atBarnes-JewishHospital,St.Louis,MO,UnitedStates
BartO.Williams ProgramforSkeletalDiseaseandCenter forCancerandCellBiology,VanAndelResearch Institute,GrandRapids,MI,UnitedStates
XinXu StateKeyLaboratoryofOralDiseases & National ClinicalResearchCenterforOralDiseases,Department ofCariologyandEndodontics,WestChinaHospitalof Stomatology,SichuanUniversity,Chengdu,PRChina
ShoshanaYakar DavidB.KriserDentalCenter,DepartmentofBasicScienceandCraniofacialBiology,New YorkUniversityCollegeofDentistryNewYork,New York,NY,UnitedStates
YingziYang DepartmentofDevelopmentalBiology, HarvardSchoolofDentalMedicine,Boston,MA, UnitedStates
StefanoZanotti DepartmentsofOrthopaedicSurgeryand Medicine,andtheUConnMusculoskeletalInstitute, UConnHealth,Farmington,CT,UnitedStates
HongZhou TheUniversityofSydney,ANZACResearch Institute,Sydney,NSW,Australia
Molecular and cellular regulation of intramembranous and endochondral bone formation during embryogenesis
Christine
Hartmann 1 and Yingzi Yang 2
1Institute of Musculoskeletal Medicine, Department of Bone and Skeletal Research, Medical Faculty of the University of Munster, Munster, Germany; 2 Department of Developmental Biology, Harvard School of Dental Medicine, Boston, MA, United States
Chapter outline
Introduction
lntramembranous ossification
The axial skeleton
Somitogenesis
Sclerotome differentiation
The limb skeleton Overview of limb development Proximal-distal axis
Anterior-posterior axis
patterning of
The skeletal system performs vital functions: support, movement, protection, blood cell production, calcium storage, and endocrine regulation. Skeletal formation is also a hallmark that distinguishes vertebrate animals from invertebrates. In higher vertebrates (i.e., birds and mammals), the skeletal system contains mainly bones and cartilage, as well as a network of tendons and ligaments that connects them. During embryonic development, bones and cartilage are formed by osteoblasts and chondrocytes, respectively, both of which are derived from common mesenchymal progenitor cells called osteochondral progenitors. Skeletal development starts from mesenchymal condensation, during which mesenchymal progenitor cells aggregate at future skeletal locations. As mesenchymal cells in different parts of the embryo are derived from different cell lineages, the locations of initial skeletal formation determine which of the three mesenchymal cell lineages contribute to the future skeleton. Neural crest cells from the branchial arches contribute to the craniofacial bone, the sclerotome compartment of the somites gives rise to most of the axial skeleton, and lateral plate mesoderm forms the limb mesenchyme, from which limb skeletons are derived.
Howosteoblastcellsareinducedduringbonedevelopmentisacentralquestionforunderstandingtheorganizational principlesunderpinningafunctionalskeletalsystem.Abnormalosteoblastdifferentiationleadstoabroadrangeof devastatingskeletaldiseases.Therefore,itisimperativetounderstandthecellularandmolecularmechanismsunderlying temporalandspatialcontrolsofboneformation.Boneformationoccursbytwoessentialprocesses:intramembranous ossifi cationandendochondralossi ficationduringembryonicdevelopment.Osteochondralprogenitorsdifferentiateinto osteoblastsdirectlytoformthemembranousboneduringintramembranousossifi cation,whereasduringendochondral ossifi cation,theydifferentiateintochondrocytesinsteadtoformacartilagetemplateofthefuturebone.Bothossification processesareessentialduringthenaturalhealingofbonefractures.Inthischapter,wefocusoncurrentunderstandingof themolecularregulationofendochondralandintramembranousboneformationanditsimplicationindiseases.
Intramembranousossification
Intramembranousossificationmainlyoccursduringformationofthe fl atbonesoftheskull,mandible,maxilla,and clavicles.Themammaliancranium,orneurocranium,istheupperandbackpartoftheskull.Itprotectsthebrainand supportsthesensoryorgans,suchastheear,andtheviscerocranium,whichsupportstheface.Theneurocraniumcanbe dividedintocalvariumandchondrocranium,whichgrowtobethecranialvaultthatsurroundsthebrainandtheskullbase, respectively.Thecalvariumiscomposedof flatbones:frontalbones,parietalbones,theinterparietalpartoftheoccipital bone,andthesquamouspartsofthetemporalbone(Jinetal.,2016).Inmice,thecalvariumconsistsoffrontalbones, parietalbones,interparietalbone,andsquamouspartsofthetemporalbone,allgoingthroughintramembranousossification(Ishiietal.,2015).Bylineageanalysisinmousemodels,frontalbonesshowamajorcontributionfromneuralcrest andasmallcontributionfromheadmesoderm,whileparietalbonesentirelyoriginatefromheadmesoderm(Jiangetal., 2002;Yoshidaetal.,2008;Deckelbaumetal.,2012).Neuralcrest derivedandheadmesoderm derivedcellscoalesceto formcalvarialboneprimordia(Jiangetal.,2002;Yoshidaetal.,2008).Themandibleandmaxillaarederivedfromthe neuralcrestcellsoriginatinginthemid-andhindbrainregionsoftheneuralfoldsthatmigrateventrally,whiletheclavicles areformedfrommesoderm.
Theprocessstartsfrommesenchymalcondensationandprogressesthroughformationoftheossi ficationcenter, ossifi cationexpansion,trabeculaformation,andcompactboneformationandthedevelopmentoftheperiosteum(Fig.1.1).
FIGURE1.1 Schematicsofintramembranouscranialboneformation. Seetextfordetails.
Condensationofmesenchymalprogenitorcellsisthe firststepforbothintramembranousandendochondralossifi cation. Duringintramembranousossification,mesenchymalprogenitorcellsdifferentiateintoosteoblastsinsteadofchondrocytes asoccursduringendochondralossifi cation.Theosteoblaststhatappear firstinthecondensationsecretebonematrixand formtheossi ficationcenter.Theearlyosteoblastssecreteosteoid,uncalcifiedmatrix,whichcalcifiessoonafter,whilethe osteoblastsmatureandterminallydifferentiateintoosteocytesthatareentrappedintheosteoid.Asosteoblastsdifferentiate intoosteocytes,moremesenchymalprogenitorssurroundingtheosteoiddifferentiateintonewosteoblastcellsatthe osteoidsurfacetoexpandthecalci ficationcenter.Osteoidexpansionaroundthecapillariesresultsinatrabecularmatrixof thespongybone,whileosteoblastsonthesuperfi ciallayerbecometheperiosteum.Theperiosteumisalayerthatalso containsmesenchymalprogenitorcells,osteoblastdifferentiationofwhichcontributestotheformationofaprotectivelayer ofcompactbone.Thebloodvesselsalongwithothercellsbetweenthetrabecularboneeventuallyformtheredmarrow. Intramembranousossificationbeginsinuteroduringfetaldevelopmentandcontinuesonintoadolescence.Atbirth,the skullandclaviclesarenotfullyossified.Suturesandfontanellesareunossifi edcranialregionsthatallowtheskullto deformduringpassagethroughthebirthcanal.Suturesarejointsbetweencraniofacialbones,whicharecomposedoftwo osteogenicfrontswithsuturemesenchymebetweenthem(Fig.1.2).Fontanellesarethespacebetweentheskullbones wherethesuturesintersectandarecoveredbytoughmembranesthatprotecttheunderlyingsofttissuesandbrain.In humans,cranialsuturesnormallyfusebetween20and30yearsofageandfacialsuturesfuseafter50yearsofage(Badve etal.,2013;Senarath-Yapaetal.,2012).Mostsuturesinmiceremainpatentthroughouttheanimal’slifetime.Suturesand fontanellesallowthecraniofacialbonestoexpandevenlyasthebraingrows,resultinginasymmetricallyshapedhead. However,ifanyofthesuturesclosetooearly(fuseprematurely),intheconditioncalledcraniosynostosis,theremaybeno growthinthatarea.Thismayforcegrowthtooccurinanotherareaordirection,resultinginanabnormalheadshape.
Apartfromcraniofacialbonedevelopment,intramembranousossificationalsocontrolsboneformationintheperichondralandperiostealregionsofthelongbone,whereosteoblastsdirectlydifferentiatefrommesenchymalprogenitor cells.Yet,thisrequiresasignalfromthecartilaginouselement.Furthermore,intramembranousossi ficationisanessential mechanismunderlyingbonerepairandregenerationinthefollowingprocesses:fracturehealingwithrigid fixation; distractionosteogenesis,abone-regenerativeprocessinwhichosteotomyfollowedbygradualdistractionyieldstwo vascularizedbonesurfacesfromwhichnewboneisformed(Ai-Aqletal.,2008);andblastemicbonecreation,which occursinchildrenwithamputations(Fernandoetal.,2011).
Intramembranousossi ficationistightlyregulatedatbothmolecularandcellularlevels.Cranialmalformationsareoften progressiveandirreversible,andsomeofthemneedaggressivesurgicalmanagementtopreventormitigatesevere impairmentsuchasmisshapenheadorabnormalbraingrowth(Bronfin,2001).Forinstance,craniosynostosisisacommon congenitaldisorderthataffects1in2500livebirths.Itischaracterizedbyprematurecranialsuturefusion,whichmay resultinsevereconditionssuchasincreasedintracranialpressure,craniofacialdysmorphism,disruptedbraindevelopment, andmentalretardation.Craniosynostosisisgenerallyconsideredadevelopmentaldisorderresultingfromadisrupted balanceofcellularproliferation,differentiation,andapoptosiswithinthesuture(Senarath-Yapaetal.,2012;Levietal., 2012;Slateretal.,2008;Lattanzietal.,2012;CiureaandToader,2009).Surgicalcorrectionfollowedbyreshapingofthe calvarialbonesremainstheonlytreatmentavailableforcraniosynostosispatients(MartouandAntonyshyn,2011;Posnick etal.,2010;Hankinsonetal.,2010).Incontrasttocraniosynostosis,cleidocranialdysplasia(CCD)iscausedbyreduced intramembranousboneformation,underdevelopedorabsentclavicles(collarbones)aswellasdelayedmaturationofthe skull,manifestedbydelayedsutureclosureandlargerthannormalfontanellesthatarenoticeableas “softspots” onthe headsofinfants(Farrowetal.,2018).SeverecasesofCCDrequiresurgicalintervention.Identifyingmolecularpathways thatcontrolintramembranousossifi cationiscriticallyimportantinthemechanisticunderstandingofcraniofacialbone diseasesandtheirtargetedtherapeuticdevelopment.
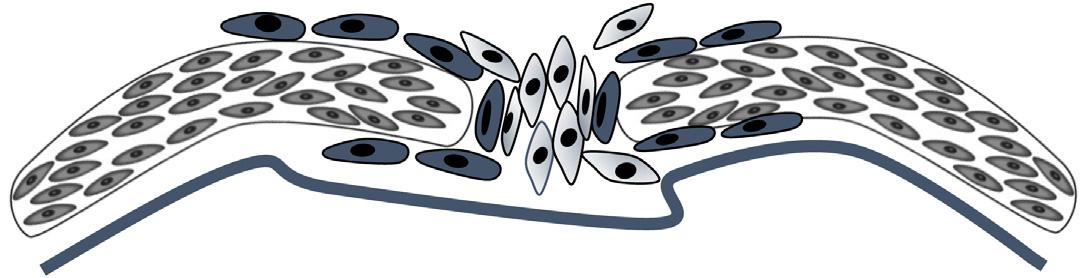
FIGURE1.2 Schematicsofcellularcompositionofthesuture. Inthesuture,mesenchymalstemcells(MSCs)arelocatedinthemiddle.Theymay first becomecommittedpreosteoblastsandthen finallymatureosteoblasts.
Studiesofbothdevelopmentalbiologyandraregeneticdiseaseshaveledtotheidenti ficationofcriticalregulatorsof intramembranousossifi cation.TranscriptionalregulationoftheosteoblastlineageisconsideredindetailinChapter7.The runt-relatedtranscriptionfactor2,RUNX2(alsoknownasCBFA1),andazinc fingertranscriptionfactor,Osterix(OSX), areosteoblastlineage determiningfactorsrequiredforbothintramembranousandendochondralossi fications. Runx2 is expressedinosteogenicprogenitorcellsandrequiredforosteoblastcellfatedeterminationbydrivingosteoblast-specifi c geneexpression(Ducyetal.,1997;Ottoetal.,1997). Runx2 loss-of-functionmutationsarefoundinbothmiceandhumans andcauseCCD(Ottoetal.,1997;Mundlosetal.,1997;Leeetal.,1997).RUNX2inducestheexpressionof Osx,whichis requiredforosteoblastcellfatecommitment,aslossof Osx leadstoconversionfromosteoblaststochondrocytes (Nakashimaetal.,2002).UnderthecontrolofRUNX2andOSX,osteoblastcellsproduceosteoblast-specificcollagenI togetherwithavarietyofnoncollagenous,extracellularmatrix(ECM)proteinsthataredepositedalongwithaninorganic mineralphase.Themineralisintheformofhydroxyapatite,acrystallinelatticecomposedprimarilyofcalciumand phosphateions.
Cell cellcommunicationthatcoordinatescellproliferationanddifferentiationalsoplaysacriticalroleinintramembranousossification.TheWNTandHedgehog(HH)signalingactivitiesarerequiredforcellfatedeterminationof osteoblastsbycontrollingtheexpressionof Runx2.ActiveWNT/b-cateninsignalingisdetectedinthedeveloping calvariumandperichondrium,whereosteoblastsdifferentiatethroughintramembranousossi fication.Indeed,enhanced WNT/ b-cateninsignalingenhancesboneformationand Runx2 expression,butinhibitschondrocytedifferentiationand Sox9 expression(HartmannandTabin,2000;Guoetal.,2004;Dayetal.,2005). Sox9 isamastertranscriptionfactorthat determineschondrocytecellfate(Bietal.,1999;Akiyamaetal.,2002).Conversely,removalof b-catenin inosteochondral progenitorcellsresultedinectopicchondrocytedifferentiationattheexpenseofosteoblastsduringbothintramembranous andendochondralossi fication(Hilletal.,2005;Huetal.,2005;Dayetal.,2005).Therefore,duringintramembranous ossifi cation,WNT/ b-cateninsignalinglevelsinthemesenchymalcondensationarehigher,whichpromotesosteoblast differentiationwhileinhibitingchondrocytedifferentiation.Inaddition,upregulatedWNT/b-cateninsignalinginthe perichondriumalsopromotedosteoblastdifferentiation.IncontrasttotheWNT/b-cateninsignaling,Indianhedgehog(IHH) signalingisnotrequiredforosteoblastdifferentiationofintramembranousbonesintheskull(St-Jacquesetal.,1999).Itisstill notclearwhatcontrols Ihh-independent Runx2 expressionduringintramembranousossificationanditisimportantto understandfurtherthedifferentialregulationofintramembranousversusendochondralossificationbycellsignaling.As removingSmoothened,whichmediatesallHHligand-dependentsignaling,doesnotabolishintramembranousossification either(Jeongetal.,2004),HHsignalingislikelytobeactivatedinaligand-independentmannerinthedevelopingcalvarium. Indeed,ithasbeenfoundthatintherarehumangeneticdiseaseprogressiveosseousheteroplasia,whichiscausedbynull mutationsin Gnas,whichencodesGas,HHsignalingisupregulated.SuchactivationofHHsignalingisindependentofHH ligandsandisbothnecessaryandsufficienttoinduceectopicosteoblastcelldifferentiationinsofttissues(Regardetal., 2013).Importantly, Gnas gain-of-functionmutationsupregulateWNT/b-cateninsignalinginosteoblastprogenitorcells, resultingintheirdefectivedifferentiationandin fibrousdysplasiathatalsoaffectsintramembranousossification(Regard etal.,2011).Therefore,Gas isakeyregulatorofproperosteoblastdifferentiationthroughitsmaintenanceofabalance betweentheWNT/b-cateninandtheHHpathways.ThecriticalroleofWNTandHHsignalinginintramembranous ossificationisalsoshowninthesuture.Mesenchymalstemcellsthatgiverisetothecranialboneandregulatecranial bonerepairinadultmicehavebeenidentifiedinthesuture.ThesecellsareeitherGLI1þ orAXIN2þ (Zhaoetal.,2015; Maruyamaetal.,2016),whichmarkscellsthatreceiveHHorWNTsignaling,respectively(Baietal.,2002;Leungetal., 2002;Jhoetal.,2002).
Othersignalingpathways,includingthosemediatedbytransforminggrowthfactor(TGF)superfamilymembers, Notch,and fibroblastgrowthfactors(FGFs),arealsoimportantinintramembranousossi fication.MutationsintheFGF receptorsFGFR1,FGFR2,andFGFR3causecraniosynostosis.ThecraniosynostosissyndromesinvolvingFGFR1, FGFR2,andFGFR3mutationsincludeApertsyndrome(OMIM101200),Beare Stevensoncutisgyrata(OMIM123790), Crouzonsyndrome(OMIM123500),Pfeiffersyndrome(OMIM101600),Jackson Weisssyndrome(OMIM123150), Muenkesyndrome(OMIM602849),crouzonodermoskeletalsyndrome(OMIM134934),andosteoglophonicdysplasia (OMIM166250),adiseasecharacterizedbycraniosynostosis,prominentsupraorbitalridge,anddepressednasalbridge,as wellasrhizomelicdwar fismandnonossifyingbonelesions.Allthesemutationsareautosomaldominantandmanyofthem areactivatingmutationsofFGFreceptors.FGFsignalingcanpromoteorinhibitosteoblastproliferationanddifferentiation dependingonthecellcontext.ItdoessoeitherdirectlyorthroughinteractionswiththeWNTandbonemorphogenetic protein(BMP)signalingpathways.
ApartfromRUNX2andOSX,othertranscriptionfactorsarealsoimportant,asmutationsinthemcausehumandiseases withdefectsinintramembranousossification.Mutationsinthehuman TWIST1 genecauseSaethre Chotzensyndrome (OMIM101400),oneofthemostcommonlyinheritedcraniosynostosisconditions.Inaddition,mutationsinthehomeobox
genes MSX1, MSX2,and DLX arealsoassociatedwithhumancraniofacialdisorders(Cohen,2000;KrausandLufkin,2006). MSX2 haploinsufficiencydecreasesproliferationandacceleratesthedifferentiationofcalvarialpreosteoblasts,resulting indelayedsutureclosure,whereasits “overexpression” resultsinenhancedproliferation,favoringearlysutureclosure (DodigandRaos,1999).Itislikelythat MSX2 normallypreventsdifferentiationandstimulatesproliferationof preosteoblasticcellsattheosteogenicfrontsofthecalvariae,facilitatingexpansionoftheskullandclosureofthesuture.It wouldbecriticaltounderstandfurtherhowthesetranscriptionfactorsinteractwithoneanotherandthesignalingpathways toregulateintramembranousboneformation,maintenance,andrepair.
Theaxialskeleton
Theaxialskeletonconsistsoftheoccipitalskullbones,theelementsofthevertebralcolumn,andtheribcage(ribsand sternum).Withtheexceptionofthesternum,theaxialskeletonisderivedfromtheparaxialmesoderm,whichissegmented intosomitesduringearlyembryonicdevelopment.Theoccipitalskullbonesaregeneratedfromthefusedsclerotomesof thecranial-most4.5somites(Goodrich,1930).Thebilateralanlagenofthesternumoriginatefromthelateralplate mesodermandfuseattheventralmidlineinthecourseoftheformationoftheribcage(Chen,1952).
Somitogenesis
Thebasicbodyplanofvertebratesisdefinedbythemetamericsegmentationofthemusculoskeletalandneuromuscular systems,whichoriginatesduringembryogenesisfromthesegmentationoftheparaxialmesoderm(forreviewssee Winslowetal.,2007;Pourquie,2000).Theparaxialmesodermislaiddownduringgastrulation,appearingasbilateral stripsofunsegmentedtissue(referredtoassegmentalplateintheavianembryoandpresomiticmesoderminthemouse).It flanksthecentrallylocatedneuraltubeandnotochordandgivesrisetotheaxialskeleton(headandtrunkskeleton)andall trunkandlimbskeletalmuscles,aswellasthedermis,connectivetissue,andvasculatureofthetrunk.Duringdevelopment, theparaxialmesodermissegmentedthroughaseriesofmolecularandcellulareventsinananteriortoposterior (craniocaudal)sequencealongthebodyaxis,theanterior-mostsomitesbeingthemorematureones.Theposterior, unsegmentedpartoftheparaxialmesodermisalsoreferredtoasthepresomiticmesoderm(PSM),andthesequentially arising,pairedtissueblocksarecalledsomites.ThePSMisaloosemesenchymaltissue.Thecellsreachingtheanterior borderofthePSMprogressivelyundergoamesenchymal-to-epithelialtransition(Christetal.,2007).Newlyformed somitesareepithelialballswithamesenchymalcore.Asthesomitesmature,accompaniedbythecommitmentofthecells tothedifferentlineages,thisorganizationchanges.Inresponsetosignalsfromthenotochordandtheventral floorplateof theneuraltube(SonicHedgehog[SHH]andtheBMPantagonistNoggin),cellsontheventralmarginundergoan epithelial mesenchymaltransition,scatter,andmovetowardthenotochord(Christetal.,2004;Cairnsetal.,2008;Yusuf andBrand-Saberi,2006).ThesecellswillexpressthetranscriptionfactorsPAX1,NKX3.1,andNKX3.2andformthe sclerotome,givingrisetothevertebraeandribs.Thedermomyotomeisspeci fiedbyWNTligandssecretedfromthedorsal neuraltubeandtheectodermcoveringthedorsalsomite.LowlevelsofSHHsignalingare,incombinationwithWNT signaling,requiredtomaintaintheexpressionofdermomyotomalandmyotomalmarkers(Cairnsetal.,2008).The dermomyotomeremainsepithelialandeventuallygivesrisetotheepaxialmusclesofthebackandvertebrae,thehypaxial musclesofthebodywallandlimb,thedermisunderneaththeskinofthetrunk,andthebrownadiposetissue(Scaaland Christ,2004;Atitetal.,2006).Tendonsandligamentsofthetrunkarisefromthefourthsomiticcompartment,the syndetome,whichisinducedbythenewlyformedsclerotomeanddermomyotome(Brentetal.,2003;Dubrulleand Pourquie,2003).
ThemolecularmechanismdrivingsomitogenesisattheanteriorendofthePSMisintrinsictothePSM,whilenewcells arecontinuouslyaddedtothePSMfromaposteriorlylocatedprogenitorpool(Martin,2016).Theso-calledsegmentation clock,amolecularoscillatorcoordinatingtherhythmicactivationofseveralsignalingpathwaysandtheoscillatory expressionofasubsetofgenesinthePSM,isthoughttobeatthemolecularheartofsomiteformation(Hubaudand Pourquie,2014).OneofthemainsignalingpathwayswithoscillatorygeneexpressionistheNotch/Delta/DELTA pathway.Thispathwayalsosynchronizestheoscillationsbetweentheindividualcells(HubaudandPourquie,2014).Also, membersoftheWNT/b-cateninandtheFGFsignalingpathwaydisplaycyclicgeneexpression(AulehlaandPourquie, 2008).Theoscillatoryexpressionofthesegenesappearstogolikeawavefromthecaudalend,sweepinganteriorly throughthePSM(Fig.1.3A).Anothermolecularsysteminvolvedinsomiteformationisthewavefront,whichisdefined byopposingsignalinggradientsinthePSM(Fig.1.3B).Here,aposterior anteriorgradientofFGF8andnuclear b-catenin isopposedbyananterior posteriorgradientofretinoicacid(RA)activity(Mallo,2016).Despitethefactthattheexistence ofanRAgradientisdebated,thereiscleargeneticevidencethatagradientofWNTsignalingactivityinteractswiththe
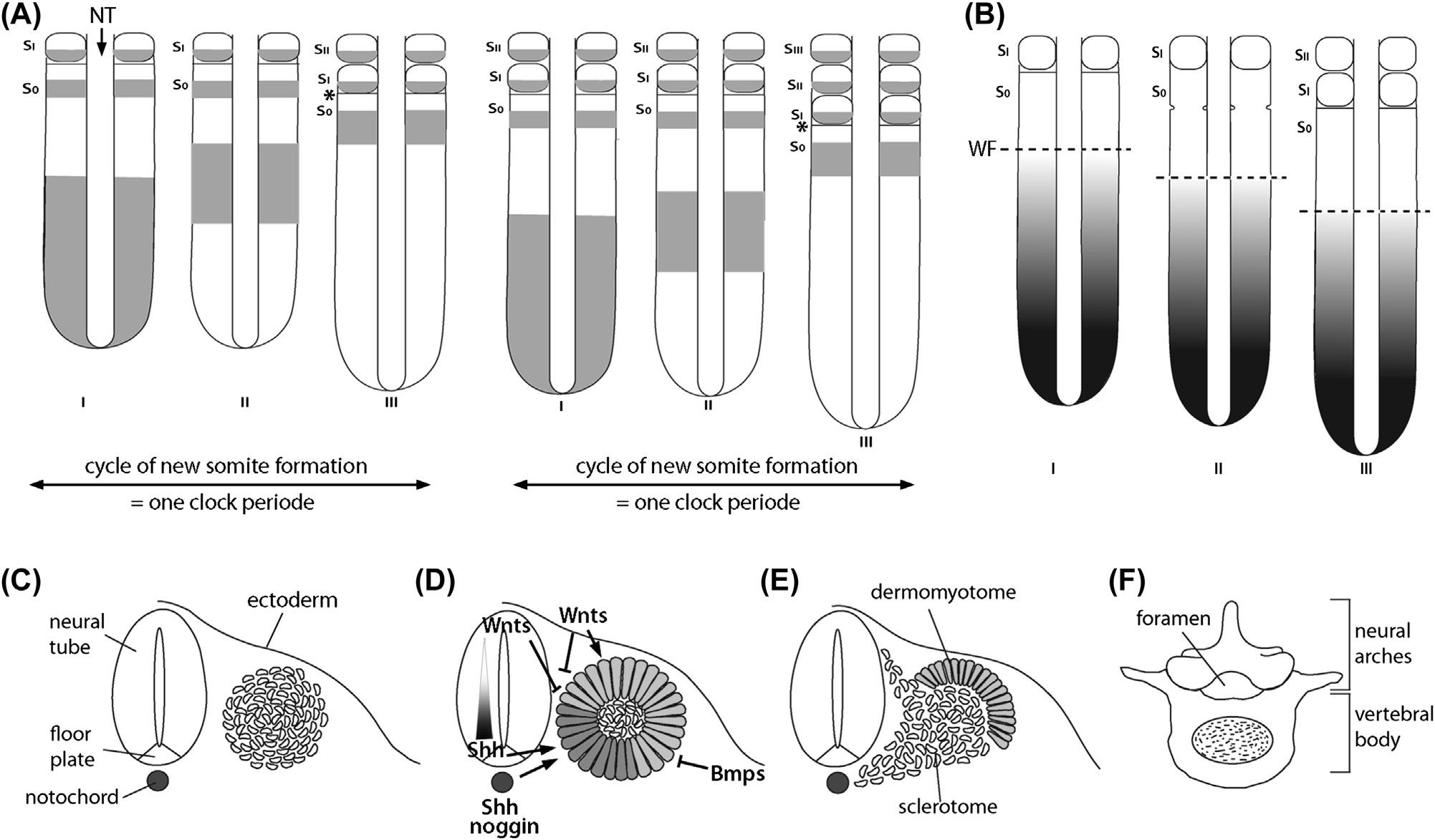
FIGURE1.3 Somiteformationanddifferentiation.(A)Cyclicgeneexpressionduringsomiteformation.The asterisk marksthepositionofnew boundaryformation. NT,neuraltube; S0,somitestage0; SI,somitestageI; SII,somitestageII; SIII,somitestageIII.(B)Signalgradientwithinthe presomiticmesoderm(PSM),withthe dashedline markingthepositionofthewavefront(WF).(C E)Schematicrepresentationsofthedifferentsomite stages.(C)LoosemesenchymalPSM,(D)epithelialballstage(theventraldarkercoloredregionmarksthePAX1-positivesclerotomalregion)andfactors involvedinthesomitecompartmentalization,(E)sclerotomedifferentiation.(F)Superiorviewofavertebralelementderivedfromtheposteriorand anteriorsclerotomalcompartmentsoftwoadjacentsomites.
segmentationclocktodeterminetheposteriorborderofanewlyformingsomite(Mallo,2016).Themorphologicalchanges thateventuallyleadtotheformationofanewsomiteattheanteriorendofthePSMaretriggeredbyNotchactivityin combinationwiththeT-boxtranscriptionfactor,TBX6,andstartwiththeexpressionofthebasichelix loop helix transcriptionfactormesodermposterior2(MESP2)(Saga,2007;Sasakietal.,2011).Incellsposteriortothedetermination front, Mesp2 isrepressedbyFGFsignaling(Sasakietal.,2011).Inaddition, Mesp2 expressionbecomesrestrictedtothe anteriorhalfofthenewlyformedsomite,asTBX6-mediatedtranscriptionof Mesp2 issuppressedbytheRIPPLY1/2 proteinsexpressedintheposteriorpartofthesomite(Morimotoetal.,2007;Takahashietal.,2007).MESP2activityis essentialforestablishingsomitepolarity,whichisinturnvitalforthelaterformationofthevertebralbodiesfromthe caudal/posteriorpartofonesomiteandtherostral/anteriorpartoftheneighboringsomite(Christetal.,2007).
Thepositionalidentityofasomitedefinesthetypeofvertebralelement(occipital,cervical,thoracic,lumbar,orsacral) itwilleventuallycontributeto,andthisiscontrolled,inpart,bytheregionalcodeof Hox genesalongtherostral caudal bodyaxis(forreviewsee Wellik,2007).Humansandallotherbilateralanimalshavemultiple Hox genes,encoding transcriptionfactorswithahomeoboxDNA-bindingdomain,whichareclusteredtogether(Krumlauf,1992).Through duplicationevents,theancestralclusteroforiginallyeight Hox geneshasbeenmultipliedtofourgeneclusters(HoxA, HoxB, HoxC,and HoxD)of13paralogous Hox genesinvertebrates.Aparticularfeatureof Hox geneexpressionfromone clusteristhattheyareexpressedinatemporalandspatialorderthatreflectstheirorderonthechromosome,withthemost 30 Hox genebeingexpressed firstandinthemostanteriorregion.Itisthoughtthatthe Hox genesprovideasortof positionalcodethroughtheiroverlappingexpressiondomains,whicharecharacterizedbyarelativelysharpanteriorborder. Forexample,theexpressionofthe Hox5 paralogs(HoxA5, HoxB5,and HoxC5)correlatesindifferentspeciessuchas mouseandchicken,alwayswiththepositionofthelastcervicalvertebra,whiletheanteriordomainsofthe Hox6 paralogs lieclosetotheboundarybetweencervicalandthoracicvertebrae(Burkeetal.,1995;Burke,2000).Yet,thiscorrelationis notmaintainedatthelevelsofthesomites,asmouseandchickendifferintheirnumbersofcervicalelements.Changesin theHOXcodecanleadtohomeotictransformation,whichreflectsashiftintheregionalbordersandaxialidentities.
Membersofthepolycombfamily( Bmi and Eed)andtheTALEclassofhomeodomaintranscriptionfactorsareinvolved infurtherre fi ningthepositionalid entityprovidedbythe Hox code.BMIandEEDaretranscriptionalrepressorslimiting therostral(anterior)transcriptionboundaryofindividual Hox genes(Kimetal.,2006).TheTALEproteins,encodedby the Pbx and Meis genes,furthermodifythetranscriptionalactivity oftheHoxproteinsthroughheterodimerization (MoensandSelleri,2006).
Sclerotomedifferentiation
Theearliestsclerotomalmarkersarethetranscriptionfactors Pax1, Nkx3.1,and Nkx3.2/Bapx1,whichbecomeexpressed undertheinfl uenceofSHHandNogginsignalingintheventralsomiteregion(Kosetal.,1998;Ebenspergeretal.,1995; Murtaughetal.,2001). Pax9 expressionappearsslightlylaterinthesclerotomeandoverlapsinpartwith Pax1 (Muller etal.,1996).Bothgenesactredundantlyintheventromedialregionofthesclerotome,asinthe Pax1/Pax9 double-mutant micethedevelopmentoftheventralvertebraisstronglyaffected(Petersetal.,1999).NKX3.2appearstoactdownstream of Pax1/Pax9 andcanbeectopicallyinducedbyPAX1(TribioliandLufkin,1999;Rodrigoetal.,2003).Althoughthe initial Pax1 expressionisnotaffectedbythelossof Nkx3.2,thevertebraldifferentiationalsodependsonthefunctionof NKX3.2(TribioliandLufkin,1999). Nkx3.1 mutantmice,ontheotherhand,donotdisplayanyskeletaldefects(Schneider etal.,2000).AsPAX1isabletoactivatetheexpressionofearlychondroblastmarkersinvitro,ithasbeensuggestedthat theactivationofPAX1isthekeyeventthattriggerssclerotomeformation(Monsoro-Burq,2005).
Aftertheirinduction,thesclerotomalcellsundergoepithelial mesenchymaltransitionandmigratetowardthe notochord,aroundtheneuraltube,andinthethoracicsegmentsalsolaterally,andthencondensetoformthevertebral bodiesandtheintervertebraldiscs,neuralarches,andproximalpartoftheribs,respectively(Fig.1.3C F).Some notochordalcellssurroundedbysclerotomalcellsdie,whileothersbecomepartoftheintervertebraldiscandformthe nucleuspulposus(McCannandSeguin,2016).Theneuralarchesandspinousprocessesarederivedfromthemediolateral regionsofthesclerotomesandfromsclerotomalcellsthatmigrateddorsally.TheactivityofPAX1/PAX9isnotrequired forthesetwocompartments(Petersetal.,1999).Thedorsallymigratingsclerotomalcellscontributingtothedorsalpartof theneuralarchesandspinousprocessesdonotexpress Pax1 butanothersetoftranscriptionfactors, Msx1 andMsx2 (reviewedin Monsoro-Burq,2005;RawlsandFischer,2010).Othertranscriptionfactors,suchasthewinged-helixfactor, MFH1(FOXC2),arepossiblyrequiredfortheclonalexpansionofcellstakingplacewithintheindividualsclerotome-derived populations,astheymigrateventrally,laterally,andmediallyandthencondense(Winnieretal.,1997).Inaddition,the homeodomaintranscriptionfactors Meox1 and Meox2 havebeenimplicatedinvertebraldevelopmentandmayevenact upstreamofPAX1/PAX9(Mankooetal.,2003;Skuntzetal.,2009).Withintheindividualsclerotomalcondensationsthe chondrogenicandosteogenicprogramsaretheninitiatedtoeventuallyformthevertebralelements.
Thelimbskeleton
Overviewoflimbdevelopment
Themesenchymalcellscontributingtotheskeletonoftheappendages(limbs)originatefromthebilaterallylocatedlateral platemesoderm.Thelateralplatemesodermisseparatedfromthesomiticmesodermbytheintermediatemesoderm,which givesrisetothekidneyandgenitalducts.Ourknowledgeaboutlimbdevelopmentduringembryogenesisisprimarily basedontwoexperimentalmodelsystems,chickandmouse.Inalltetrapods,forelimbdevelopmentprecedeshindlimb development.Theaxialpositionoftheprospectivelimb fieldisinregisterwiththeexpressionofaspeci ficsetof Hox geneswithinthesomites(Burkeetal.,1995).Thelimb fieldsaredemarcatedbytheexpressionoftwoT-boxtranscription factors, Tbx5 intheforelimband Tbx4 inthehindlimb field(Petitetal.,2017;DubocandLogan,2011).Yet,theidentityof thelimbisconveyedbytheactivityofanothertranscriptionfactor,PITX1,whichisexpressedspecifi callyinthehindlimb regionandspeci fieshindlimbidentity(LoganandTabin,1999;Minguillonetal.,2005).Inmouse,theforelimbbudstarts todeveloparoundembryonicday(E)9andthehindlimbaroundE10.Inchick,forelimbdevelopmentstartsonday2½ (HamburgerHamiltonstage16)withathickenedbulge(HamburgerandHamilton,1992).Inhumans,theforelimbis visibleatday24ofgestation.ExperimentalevidencefromthechicksuggeststhatWNTsignalinginducesFGF10 expressionandtheFGF-dependentinitiationofthelimboutgrowth(Kawakamietal.,2001).Forcontinuouslimb outgrowththeexpressionof Fgfs inthemesenchymeandinanepithelialridgecalledtheapicalectodermalridge(AER)is essential(BenazetandZeller,2009;Martin,2001)(Fig.1.4A).Patterningoftheoutgrowinglimboccursalongallthree axes,theproximal distal,theanterior posterior,andthedorsal ventral(Niswander,2003).Forexample,inthehuman arm,theproximal distalaxisrunsfromtheshouldertothe fingertipsandcanbesubdividedintothestylopod(humerus),
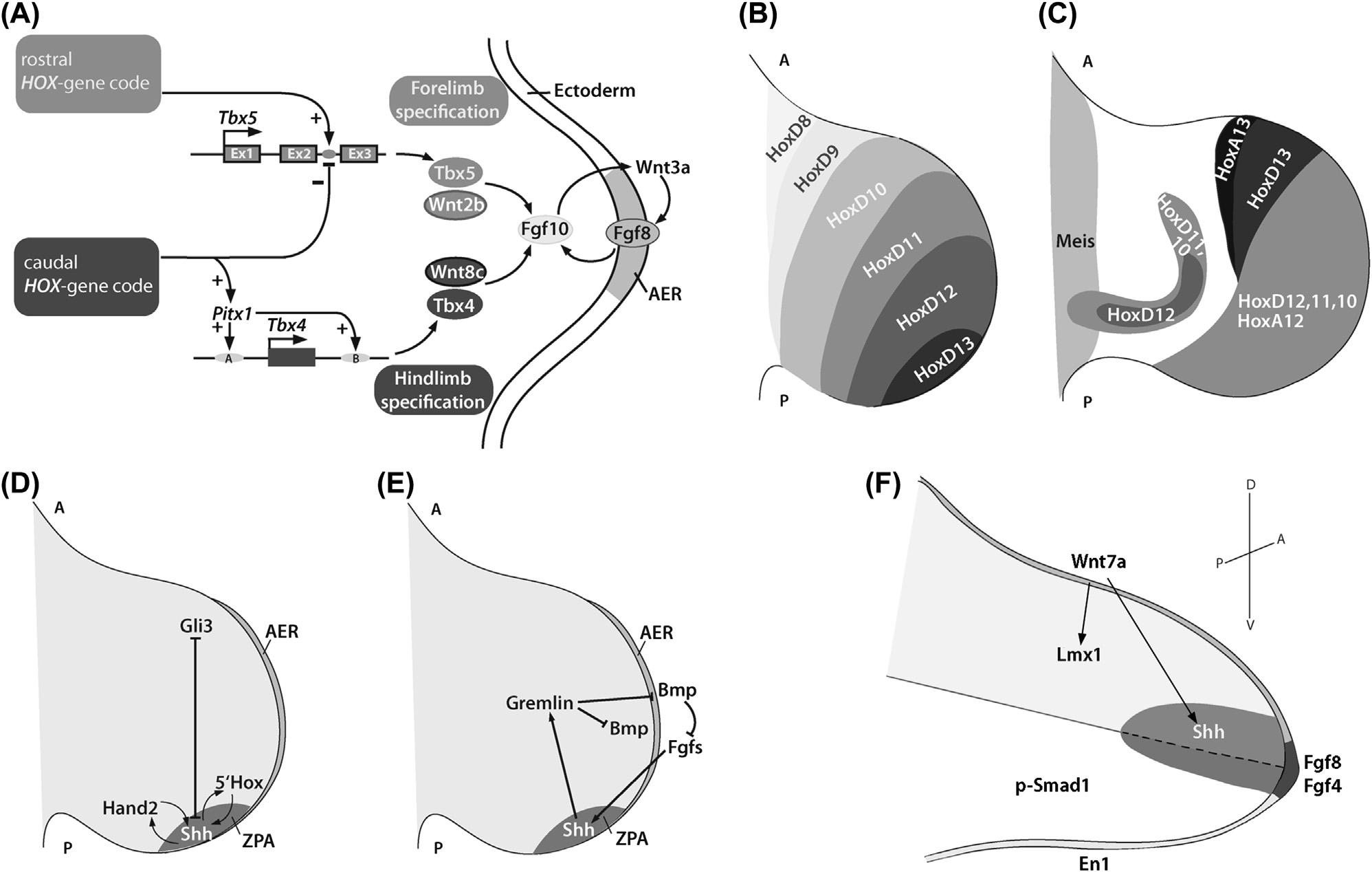
FIGURE1.4 Limbdevelopmentoverview.(A)Earlyeventsinlimbbuddevelopment:factorsinvolvedintheestablishmentofthelimbidentityand signalsrequiredfortheinitiationoflimboutgrowth. Hox genesinthelateralplatemesodermdefinethepositionswherethelimbswilldevelopandactivate orrepressviaspecificenhancerstheexpressionof Pitx1 andthe Tbx4/5 genes.Togetherwiththeactivityoflimb field specificWNTsanFGF10/WNT3a/ FGF8loopisestablished,whichdrivesproximal distallimboutgrowth. AER,apicalectodermalridge.(B)EarlynestedexpressionoftheHOXDcluster inthelimb. A,anterior; P,posterior.(C)Lateexpressionofthe HoxA and HoxD genesintheautopodstageandexpressionoftheproximaldeterminant Meis1.(D)Factorsinvolvedinanterior posteriorpatterningofthelimb,with Shh expressedinthezoneofpolarizingactivity(ZPA)underthepositive controlofthetranscriptionfactorsHAND2andthe50 HOXproteins,whileitsactivityintheanteriorisopposedbytherepressorGLI3.(E)Molecules involvedintheinterregulationoftheanterior posteriorandproximal distalaxes.(F).Moleculesinvolvedinthespecificationofthedorsal ventralaxis: Wnt7a expressedinthedorsalectodermactivates Lmx1 expressioninthedorsalmesenchymespecifyingdorsalfate,whileEN1intheventralectoderm andphospho-SMAD1intheventralmesenchymespecifyventralfate.WNT7aalsopositivelyenforcestheexpressionof Shh (A)Adaptedfrom Fig.1.2, Petit,F.,Sears,K.E.,Ahituv,N.,2017.Limbdevelopment:aparadigmofgeneregulation.Nat.Rev.Genet.18,245 258.
zeugopod(radiusandulna),andautopodregions(wristanddigitsofthehand)(Fig.1.5A).Theanterior posterioraxis runsfromthethumbtothelittle fingerandthedorsal ventralaxisextendsfromthebackofthearm/handtotheunderside ofthearm/palm.Thesethreeaxesareestablishedveryearlyindevelopment,andspeci ficsignalingcenters,whichwillbe brie flydiscussedinthefollowing,coordinatetheoutgrowthandpatterningofthelimb.
Proximal distalaxis
Asalreadymentioned,duringtheinitiationstage,apositiveFGFfeedbackloopisestablishedbetweenthe Fgfs expressed inthemesenchyme(Fgf10)andthe Fgfs intheAER(Fgf8,Fgf4,Fgf9,Fgf17).MesenchymalFGF10activityisessential fortheformationoftheAER(Sekineetal.,1999).Inthepositivefeedbackloop,FGF10induces Fgf8 expressioninthe AER,whichisprobablymediatedbya Wnt gene’sexpression(Wnt3a inchickand Wnt3 inmouse)(Kawakamietal., 2001;Kengakuetal.,1998;Barrowetal.,2003).TheAERplaysacriticalroleinthelimboutgrowth.Removalofthe AERatdifferenttimepointsofdevelopmentleadstosuccessivetruncationofthelimb(Saunders,1948;Summerbell, 1974;RoweandFallon,1982).The Fgf genesexpressedintheAERconferproliferativeandantiapoptoticactivityon thedistalmesenchymeandmaintainthecellsinanundifferentiatedstate(Niswanderetal.,1994;Niswanderetal.,1993; Fallonetal.,1994;TenBergeetal.,2008).ThisisfurthersupportedbygeneticstudiesshowingthatFGF4and FGF8arebothrequiredforthemaintenanceoftheAER(Bouletetal.,2004;Sunetal.,2002).Themostproximalpart
Molecularandcellularregulationofintramembranousandendochondralboneformation
FIGURE1.5 Patterningoftheappendicularskeleton.(A)Schematicoverviewoftheskeletalelementsinahumanarm.(B)Insituhybridizationson adjacentsectionsofamouseforelimb(embryonicstagesE11.5,E12.5,andE13.5),showingthebranchedstructureofanearlycartilaginoustemplate (Col2a1 expressing)consistingofthehumerus(h),radius(r),andulna(u).NotethatatE11.5markersofthejointinterzone(Gdf5 and Wnt4)areexpressed incellsthatalsoexpressthechondrogenicmarker Col2a1.AtE12.5,duringinterzoneformation, Col2a1 becomesdownregulatedintheshoulder(sh)and elbow(e)region,whiletheexpressionpatternsof Gdf5 and Wnt4 undergorefinement.AtE13.5, Col2a1 isnolongerexpressedinthejointareasandthe expressiondomainsof Gdf5 and Wnt4 becomedistinct.(C)Schematicrepresentationofthemajorstepsduringsynovialjointformation.
ofthelimbexpressestheTALEhomeoboxtranscriptionfactorMEIS1underthecontrolofopposingRAandFGF signaling(Mercaderetal.,2000).MEIS1aloneissuf ficienttoproximalizethelimbinthechickandmousesystems (Mercaderetal.,1999,2009).Alongtheproximal distalaxis,the50 Hox genes,whichareexpressedearlyinanested pattern(see Fig.1.4B),arethoughttoprovidepositionalcuesforgrowth.Assuch,membersofthegroup11paralogs (HOXA11andD11intheforelimbandHOXA11,C11,andD11inthehindlimb)arerequiredforthegrowthofthe zeugopod,whiletheautopodestablishmentdependsonthefunctionofgroup13paralogs(ZakanyandDuboule,2007). Hox genesarealsoinvolvedinconnectivetissuepatterninginthelimb(PineaultandWellik,2014).Inadditiontotheirrole withregardtotheproximal distalaxis, Hox genesalsoplayanimportantroleinestablishingthesignalingcenterwithin thelimbbudregulatingtheanterior posterioraxis.
Anterior posterioraxis
Classicalembryologictransplantationexperimentsuncoveredtheexistenceofaregionpresentintheposteriorlimbbud conveyingpatterninginformationalongtheanterior posterioraxis(SaunderandGasseling,1968).Transplantationstudies alsorevealedthatthisregion,whichwasreferredtoasthezoneofpolarizingactivity(ZPA),mustcontainsomekindof positionalinformationintheformofasecretedmorphogenthatspeci fiesdigitidentityalongtheanterior posterioraxis (Tickle,1981;Tickleetal.,1975;Wolpert,1969).Themolecularidentityofthismorphogenwasuncoveredonlyin1993 withthecloningofavertebratehomologofthe Drosophilahh gene,called Shh Shh expressionoverlapswiththeZPA,and Shh-producingcellstransplantedintotheanteriormesodermofthelimbbudcouldreproducemirror-imageduplicationsof ZPAgrafts(Riddleetal.,1993).Geneticexperimentsconfirmedthat Shh isrequiredtoestablishposteriorstructuresofthe limb(Chiangetal.,1996).The Shh expressiondomainisestablishedbytheactivityofpositiveandnegativeregulators. ThetranscriptionfactorHAND2(dHAND)isexpressedinaposteriordomainprecedingandencompassingthe Shh domainandactsasapositiveregulatorofSHH,whichfeedsbackpositivelyontheexpressionofHAND2(Chariteetal., 2000;Fernandez-Teranetal.,2000).Earlyinlimbdevelopment, Hand2 isexpressedcomplementarytothetranscription factor Gli3 andGLI3represses Hand2 intheanterior(Wangetal.,2000).HAND2,ontheotherhand,represses Gli3 inthe posterior(TeWelscheretal.,2002).SHHsignalingintheposteriorpreventsthecleavageofthefull-lengthactivatorGLI3 intotheGLI3repressor(GLI3R)form.Hence,theGLI3Rformisrestrictedtotheanteriorofthelimbbud.The 50 Hox genesandSHHsignalingarealsoconnectedbyapositivefeed-forwardregulatoryloop(Tarchinietal.,2006;Rosetal., 2003),whichmayalsoinvolveFGFsignaling(Rodriguesetal.,2017)(Fig.1.4D).Thereisalsoaninterconnectionbetweentheanterior posteriorandtheproximal distalaxis:SHHsignalingupregulatestheBMPantagonistGremlininthe posteriorhalfofthelimb.GremlinantagonismofBMPsignalingisrequiredtomaintaintheexpressionof Fgf4, Fgf9,and Fgf17 intheAER,andFGFsignalingfeedspositivelyonto Shh (Khokhaetal.,2003;Lauferetal.,1994)(Fig.1.4E).
Dorsal ventralaxis
Thethirdaxisthatneedstobeestablishedisthedorsal ventralaxis.Here,theWNTligandWNT7aisexpressedinthe dorsalectodermandregulatestheexpressionoftheLIMhomeoboxtranscriptionfactorLMX1(LMX1Binthemouse)in thedorsalmesenchyme(Riddleetal.,1995;Vogeletal.,1995).LMX1Bisrequiredtomaintainthedorsalidentityof structuressuchastendonsandmusclesinthelimb(Chenetal.,1998).Theventralcounterplayeristhetranscriptionfactor Engrailed1(EN1),whichisexpressedintheventralectodermandtheventralhalfoftheAER,andisessentialforthe formationofventralstructures(Davisetal.,1991;GardnerandBarald,1992;Cyganetal.,1997;Loomisetal.,1996).
BMPsignalingappearsalsotoberequiredforestablishmentofthedorsal ventralaxis,astheactivateddownstream component,phospho-SMAD1,isdetectedthroughouttheventralectodermandmesenchyme(Ahnetal.,2001)(Fig.1.4F). DeletionofaBMPreceptorgene, Bmpr1a,fromthelimbbudectodermresultsinanexpansionof Wnt7a and Lmx1b into ventralterritories,analmostcompletelossof En1,andseveremalformationofthelimbsmissingtheventral flexortendons (Ahnetal.,2001).
Mesenchymalcondensationandpatterningoftheskeleton
Patterningofthesomitictissueandthelimbsalongthedifferentaxesisaprerequisiteforthemesenchymalcondensationsto takeplace.Inthecraniofacialskeleton,epithelial mesenchymalinteractionsoccurduringtheprecondensationphase(Hall andMiyake,1995).Mesenchymalcondensationsarepivotalforintramembranousandendochondralboneformation.They definethepositionsandthebasicshapesofthefutureskeletalelements.Theycanbevisualizedinthesclerotome,developing skull,andlimbsinvivoandinmicromasscellculturesinvitrobythepresenceofcellsurfacemoleculesthatbindpeanut agglutinin(StringaandTuan,1996;Milaire,1991;HallandMiyake,1992).Duringtheprechondrogenicandpreosteogenic condensationphaseECMmolecules,suchastheglycoproteinsFibronectin,Versican,andTenascin;cell celladhesion molecules,suchasN-CAMandN-cadherin;thegap-junctionmoleculeConnexin43(CX43);andSyndecans(typeI transmembraneheparansulfateproteoglycan)becomeupregulated,buttheirexpressionoftenchangesdynamicallyduring thesubsequentdifferentiationprocess(forreviewsee HallandMiyake,2000;DeLiseetal.,2000).Celladhesionand ECMproteinspromotetheformationofthecondensationsbyestablishingcell cellcontactsandcell matrixinteractions. Yet,throughgeneticstudies,theirfunctionalrequirementforthecondensationprocesshasnotbeendemonstratedsofar. Forthecell matrixinteractions,integrinsalsoplayanimportantroleastheyactasreceptorsforFibronectin(a5b1; aVb3), typesIIandVIcollagen(a1b1, a2b1, a10b1),Laminin(a6b1),Tenascin(a9b1, aVb3, a8b1, aVb6),andOsteopontin (OPN)(aVb1; aVb3; aVb5; a8ßb1)(Loeser,2000,2002;TuckerandChiquet-Ehrismann,2015;Dochevaetal.,2014).
Variousgrowthfactors,suchasmembersoftheTGF b superfamily,regulatethecondensationprocess(reviewedin MosesandSerra,1996 ).Thishasalsobeenelegantlydemonstratedinv itroforasubclassofthissuperfamilyofgrowth factors,theBMPfamily( BarnaandNiswander,2007 ).Fortheproximalelements(femur,tibia,and fi bula)inthe hindlimb,geneticsrevealedadualrequirementforthezinc fi ngertranscriptionfactorsGLI3andPLZFtoestablishthe correcttemporalandspatialdistributionofchondrocyteprogenitors(Barnaetal.,2005 ).
Mesenchymalcellswithinthecondensationscandifferentiateintoeitherosteoblasts(intramembranousossification)or chondrocytes(endochondralossification).WNT/ b-cateninsignalingisessentialforthedifferentiationofosteoblasts,asno osteoblastsdevelopinconditionalmousemutantsinwhichthe b-catenin-encodinggene Ctnnb1 wasdeletedinmesenchymalprecursorcellsofthelimband/orskull(Huetal.,2005;Hilletal.,2005;Dayetal.,2005).Instead,theprecursor cellsdifferentiateintochondrocytes(Dayetal.,2005;Hilletal.,2005).Hence, b-cateninactivityisnotessentialfor chondrogenesis.WNT/ b-cateninsignalingismostlikelyactingasapermissivepathwayatthisearlystepofdifferentiation, astoohighlevelsofWNT/ b-cateninsignalingblockosteoblastaswellaschondrocytedifferentiation(Hilletal.,2005). WNT/b-cateninsignalinginperichondrialcellsisampli fiedbySOXCproteinfamilymemberstofurthersecurethe nonchondrogenicfateofthesecells(Bhattarametal.,2014).Forosteoblastdifferentiationtooccur,thetranscriptionfactor RUNX2needstobeupregulatedwithinthepreosteogeniccondensations,whiletheHMG-boxtranscriptionfactorSOX9is requiredforthefurtherdifferentiationofcellswithinthecondensationsalongthechondrocytelineageandprobably alsoforthecondensationprocessitself(Bietal.,1999;Akiyamaetal.,2002;Karsenty,2001;LianandStein,2003). Thelatteraspecthasbeenchallengedbytheresultsofinvitroexperimentsby BarnaandNiswander(2007) showingthat Sox9-deficientmesenchymalcellscompactandinitiallyformcondensations,yetthecellswithinthecondensationsdonot differentiateintochondroblasts(BarnaandNiswander,2007).
Theskeletalelementsinthelimbs,whichareformedbytheprocessofendochondralossifi cation,developinpartas continuous,sometimesbifurcated(pre)chondrogenicstructures,suchas,e.g.,thehumerusbranchingintotheradiusand ulnaintheforelimb(Fig.1.5B),beingsubsequentlysegmentedbytheprocessofjointformation(ShubinandAlberch, 1986;HinchliffeandJohnson,1980;Osteretal.,1988).Furthermore,studieshaveshownthatthecartilagemorphogenesis ofthedevelopinglongbonesalsooccursinamodularway,withtwodistinctpoolsofprogenitorcellscontributingtothe primarystructuresandtheboneeminences(Blitzetal.,2013;Sugimotoetal.,2013).Cellswithinthebifurcated,SOX9 þ primarystructuresexpressthegene Col2a1,characteristicofchondroblasts/chondrocytes.Althoughtheyappearduring earlylimbdevelopment(E11.5)tobemorphologicallyuninterrupted,theregionwhereajoint(heretheshoulderjoint)will beformedcanbevisualizedusingmolecularjointmarkers,suchas Gdf5 (growthdifferentiationfactor5)or Wnt4 (see Fig.1.5B).Interestingly,thecartilagematrixproteinMatrilin-1isneverexpressedintheinterzoneregion,norinthe adjacentchondrogenicregion,whichpossiblygivesrisetothearticularcartilage(Hydeetal.,2007).Howthepositionof jointinitiationwithinthelimbisdeterminedisnotcompletelyunderstoodasofthiswriting.Alimbmolecularclock operatinginthedistalregionmaybeinvolvedinthisprocess.Ithasbeenproposedthattwooscillationcyclesofthegene Hairy2 (Hes2)arerequiredtomakeoneskeletalelementinthezeugopodandstylopodregionofthelimb(Sheebaetal., 2016).Asthejointsdevelopsequentiallyalongtheproximal distalaxisatacertaindistancefromeachother,secreted factorsproducedbythejointitselfmayprovidesomekindofself-organizingmechanism(HartmannandTabin,2001; Hiscocketal.,2017).WNT/b-cateninsignalingisalsorequiredforjointformation(HartmannandTabin,2001;Guoetal., 2004;Spateretal.,2006a,2006b).Yet,again,itmayactinthisprocessalsoasapermissivepathway,repressingthe chondrogenicpotentialofthejointinterzonecells.However,asWNT/b-cateninsignalingalsoinducestheexpressionof Gdf5,itmayalsoplayanactiveroleinjointinductionbyinducingcellularandmolecularchangesrequiredforjoint formation.TheAP1-transcriptionfactorfamilymemberc-JUNactsupstreamofWNTsignalinginjointdevelopment regulatingtheexpressionof Wnt9a and Wnt16,whicharebothexpressedintheearlyjointinterzone(KanandTabin, 2013).Numerousothergenes,including Noggin, Hif1a, Gdf5, Gdf6, Gli3, Ihh, PTH/PTHrPR1, Tgfb, Mcp5,and Crux1, havebeenimplicatedinavarietyofcellularprocessesduringjointformationbasedongeneticormisexpression experiments(Brunetetal.,1998;Amanoetal.,2016;Spagnolietal.,2007;Longobardietal.,2012),forreviewsee (Archeretal.,2003;Paci ficietal.,2006).
Endochondralboneformation
Overview
Theaxialandappendicularskeletalelementsareformedbytheprocessofendochondralboneformationstartingwitha cartilaginoustemplate(Fig.1.6A E).Thisprocessstartswiththecondensationofmesenchymalcellsatthesiteofthe futureskeleton.Asmentionedalready,thisinvolvesalterationsincell celladhesionpropertiesandchangesintheECM
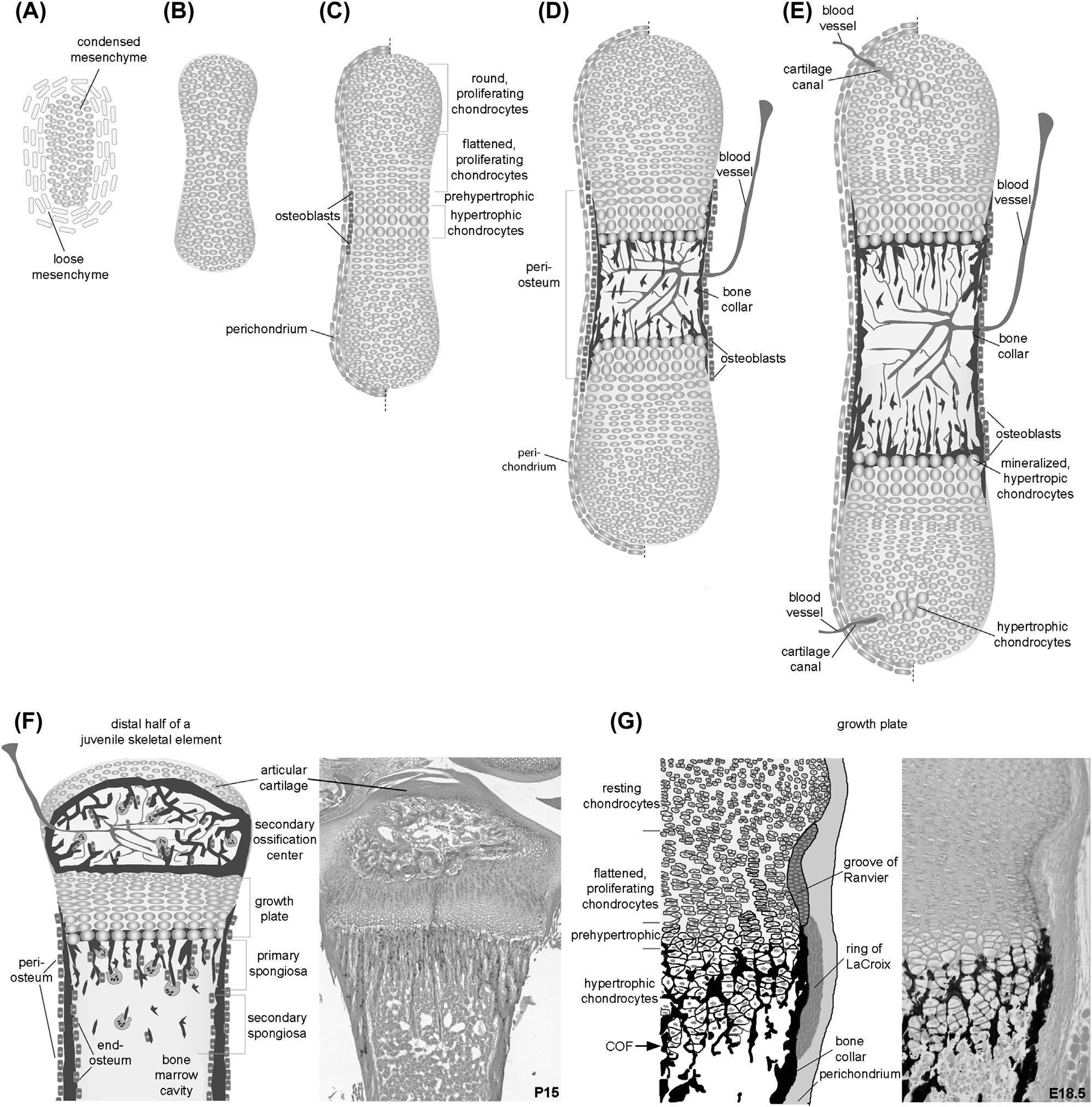
FIGURE1.6 Schematicrepresentationoftheformationandgrowthoflongbonesbyendochondralossification.(A)Mesenchymalcondensationwith surroundingloosemesenchymalcells.(B)Cartilaginoustemplateprefiguringthefutureskeletalelement.(C)Chondrocytedifferentiationwithinthe cartilaginoustemplateanddifferentiationofosteoblastswithinaregionoftheperichondrium,whichisthenreferredtoastheperiosteum.(D)Bloodvessel invasionandonsetofbonemarrowcavityformation.(E)Onsetoftheformationofthesecondaryossificationcenterwithdifferentiationofhypertrophic chondrocytesinthecentralregionoftheepiphysisandbloodvesselinvasionfromtheperichondriumthroughthecartilagecanals.(F)Schematic representationontheleftandcorrespondingAlcianblue/eosin stainedimageoftheproximalendofapostnatalday15(P15)mousetibiaontheright. (G)SchematicrepresentationofthedifferentfeaturesofamousegrowthplatebasedonthevonKossa/Alcianblue stainedproximalendofamouse humerusatembryonicday18.5(E18.5). COF,chondro-osseousfront.
(DeLiseandTuan,2002a;DeliseandTuan,2002b;HallandMiyake,1995;Bhatetal.,2011).Mesenchymalcellswithin thecondensationsstarttoexpresschondro-osteogenicmarkers,suchasthetranscriptionfactors Sox9 and Runx2 (Hilletal., 2005;Akiyamaetal.,2005;Wrightetal.,1995).Next,theprechondrogenicprecursorpopulationofchondroblasts differentiatesintochondrocytes,whichproduceanECMrichintheproteoglycanaggrecanand fibrillarcollagenoftypeII.
Cartilaginoustemplateformationprefiguresthefutureskeletalelementandissurroundedbytheso-calledperichondrium, alayerofmesenchymalcells.Ascartilageisavascular,limbvasculatureregressionneedstooccurwherecartilaginous structuresform(Hallmannetal.,1987).Yet,interestingly,thechondrogeniccondensationdoesexpressvascular endothelialgrowthfactor(VEGF)(Eshkar-Orenetal.,2009).Theoutgrowthofvertebratelimbsoccursprogressively alongtheproximal distalaxis(Newmanetal.,2018;Zelleretal.,2009).Concomitantly,theskeletalelementsdevelopin aproximodistalsequence,withtheanlagenoftheproximalelements(humerusintheforelimbandfemurinthehindlimb) forming first,branchingintomoredistalelements,andthenbeingsegmentedintoindividualelementsasthelimbgrows (Hinchliffe,1994).Thecartilaginoustemplateincreasesinsizebyappositionalandinterstitialgrowth(Johnson,1986). Interstitialgrowthbydividingchondrocytesallowsthecartilagetogrowrapidlyalongthelongitudinalaxis.Thewidthof thecartilageelementiscontrolledbyappositionalgrowth,wherebytheperichondriumsurroundingthecartilagetemplate servesastheprimarysourceofchondroblasts.Earlyon,allchondrocytesarestillproliferating.Asdevelopmentprogresses, thechondrocytesdistanttothearticulationsinthecentraldiaphysiswillstarttoundergoadifferentiationprogram.First, they fl attenandrearrangeintoproliferativestacksofchondrocytesformingthezoneofcolumnarproliferating chondrocytes.Theelongationofthesecolumnsoccursinternallythroughorientedcelldivisionfollowedbyintercalation movementsofthedaughters(Ahrensetal.,2009;LiandDudley,2009).A2014studyshowedthatthedaughtercells maintainintimatecontactaftercelldivision,preservingcadherin-mediatedcell cellinteractionuntiltheendofthe rotationalmovement(Romereimetal.,2014).Interferingwithcadherin-mediatedcell celladhesionstallstherotation processinvitro(Romereimetal.,2014).Asimilarrotationdefectwasobservedinmicelackingintegrin b1(Aszodietal., 2003).Chondrocytesatthelowerendofthecolumnswillthenexitthecellcycleandbecomeprehypertrophic;astagethat isnotmorphologicallydistinctbutcanbevisualizedusingmolecularmarkerssuchastheexpressionofthegenes Ihh and parathyroidhormone/parathyroidhormone-likepeptidereceptor1(Pthr1).Next,theprehypertrophicchondrocytes increasedramaticallyinvolumeandbecomehypertrophic(Cooperetal.,2013;Hunzikeretal.,1987).Thealmost10-fold increaseinvolumeoccursinpartsbytruecellularhypertrophyandswellingandsignifi cantlycontributestothelongitudinalexpansionoftheskeletalelementsasthecellsarelaterallyrestrictedbymatrixchannels(Cooperetal.,2013). Hypertrophicchondrocytes(HCCs)aredistinctintheirECMproducingtypeXinsteadoftypeIIcollagen.Furthermore, theyproduceVEGF,whichinthiscontextattractsbloodvesselstothediaphysisregion(Gerberetal.,1999).TheECMof matureHCCsmineralizesandthecellsproducematrixmetalloproteinase13(MMP13)aswellasOPN/SSP1.MMP13 (collagenase3)breaksupthematrixofHCCsforthesubsequentremovalbyosteoclasts(Inadaetal.,2004;Stickensetal., 2004),whileSSP1hasmultiplefunctions;itregulatesmineralization,servesasachemoattractantforosteoclasts,andis functionallyrequiredfortheiractivity(Franzenetal.,2008;Rittlingetal.,1998;Boskeyetal.,2002;Chellaiahetal., 2003).The finalfateofHCCshaslongbeenbelievedtobeapoptoticcelldeath(Shapiroetal.,2005).Yet,exvivoand invitroexperimentsalreadyhintedatanalternativefate,withHCCstransdifferentiatingintoosteoblasts(Shapiroetal., 2005).Lineagetracingexperimentshaveconfi rmedthisalternativefate,proposingamodelofdualosteoblastorigin(Zhou etal.,2014;Yangetal.,2014a,2014b;Parketal.,2015).Atleastduringembryonicdevelopment,about20%ofosteoblastsarechondrocytederivedandabout80%arederivedfromtheperichondrium/periosteum.Thelatterpopulation migratesintothebonemarrowcavityalongtheinvadingbloodvessels(Maesetal.,2010).Thisinvasionoriginatesfrom theperiostealcollar,theareaoftheperichondriuminwhichosteoblastsdifferentiateandthebonecollarisbeingformed (Colnotetal.,2004).Inaddition,monocyticosteoclastprecursorsaswellasmacrophages,bothofwhichareof hematopoieticorigin,entertheremodelingzoneviathevascularsystem,whichisattractedbyVEGF(Henriksenetal., 2003;Engsigetal.,2000).Bloodvesselshaveadditionalrolesduringtrabecularboneformationintheprimaryspongiosa, whichwillbefurtherdiscussedinthefollowing.Endothelialcells,chondroclasts,andosteoclastsacttogethertoerodethe bonemarrowcavitybyremovingHCCremnants.Interestingly,abonemarrowcavitycanforminmousemutantslacking osteoclastsorevenmacrophagesandosteoclasts(Ortegaetal.,2010).Inthesemutants,MMP9-positivecellsarestill presentatthechondro-osseousjunctionandmaybeinpartresponsibleforbonemarrowcavityformation(Ortegaetal., 2010).Withtheformationofthemarrowcavityinthediaphysis,thetwogrowthplatesbecomeseparatedfromeachother. Thegrowthplatesserveasacontinualsourceofcartilagebeingconvertedintoboneatthechondro-osseousfrontduring thelatestagesofdevelopmentandpostnatally.Inmostspecies,asecondossi ficationcenterappearsduringpostnatal developmentwithintheepiphysealcartilage.Theonsetdiffersbetweenspeciesfortheindividualbonesandevenwithin oneboneforthetwoepiphyses(AdairandScammin,1921;Shapiro,2001;Zoetisetal.,2003).Here,cartilagecanals containingmesenchymalcellsandbloodvesselsenterfromthesurroundingperichondrium,reachingeventuallythe hypertrophiccenteroftheepiphysis(Blumeretal.,2008;Alvarezetal.,2005).Aftertheformationofthesecondary ossificationcenter,theepiphysealarticularcartilagebecomesdistinctandthemetaphysealgrowthplateissandwiched betweentheepiphysealsecondaryossifi cationcenterandtheprimaryossifi cationcenterinthediaphysis(Fig.1.6F). Molecularandcellularregulationofintramembranousandendochondralboneformation