Introductionandoverview
1.1Introduction
Thisisaseeminglysimpleandlogicalquestion.Atthemostbasiclevel,chromatographyisaseparationtechniqueorprocess.Itsvaluedependsontheabilitytoresolveorseparatethecomponentsof mixtureswithalargenumberofeithersimilar(molecularsize,polarity,etc.)and/ordissimilaranalytes.Theresult ispresentedinachromatogram,thenatureofwhichisdifferentdependingontheactualprocess.Inpaperchromatography,itisapieceofchromatographypaperwithaseriesofvisiblespots.However,themostcommonformof
chromatogramisnowacomputer-generatedprint-outcomprisingaseriesofpeaksrisingfromabaselinedrawnona timeaxis.Eachpeakrepresentsthedetectorresponseforadifferentcompound.Thetimefromthepointofinjectionof sampleintothechromatographtotheapexorpeakmaximumisreferredtoastheretentiontimeofthatparticular compound.
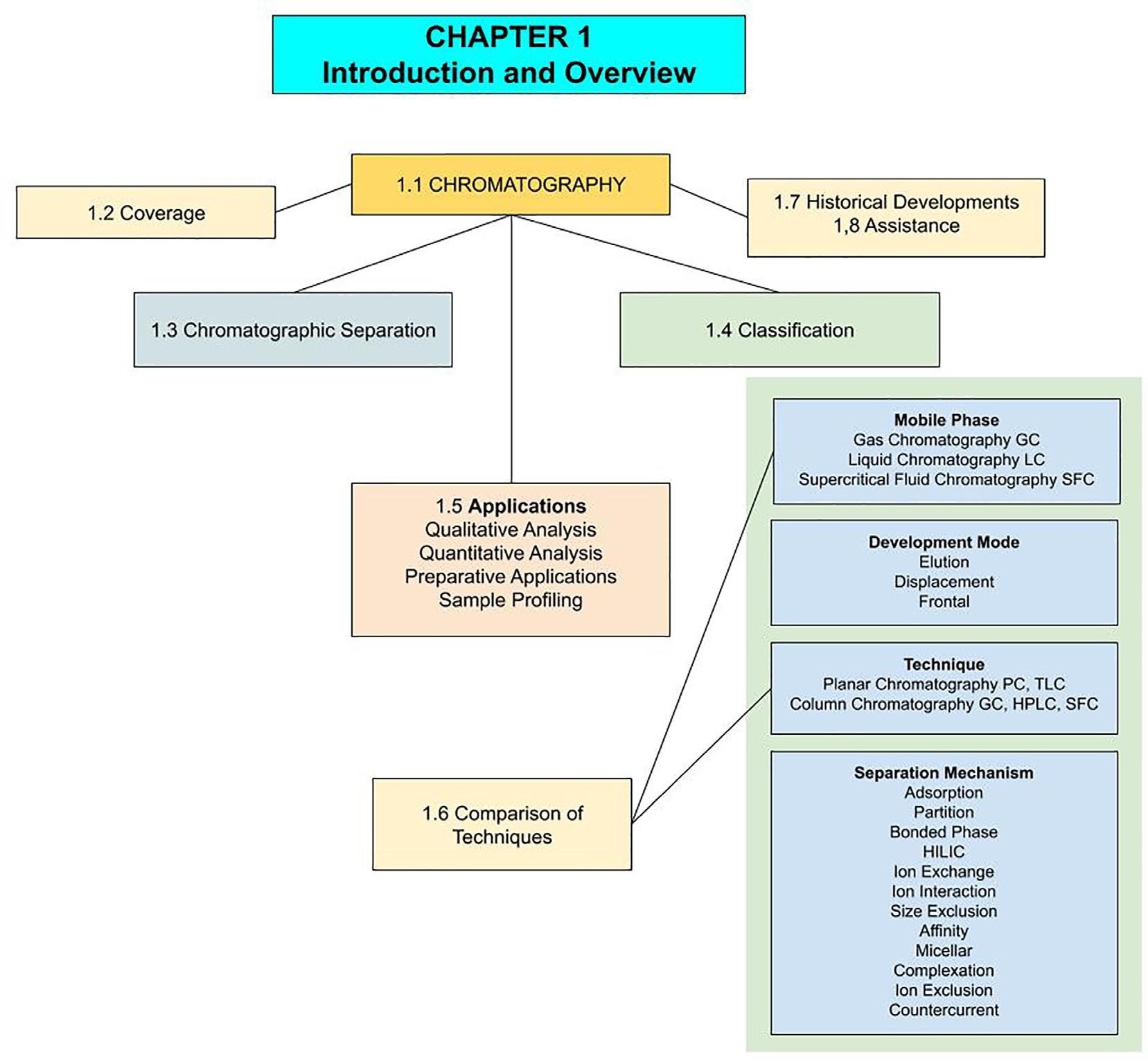
Anexampleofa2019state-of-the-artseparationbychromatographyisshowninthechromatogramof Fig.1.1 for 12analytesin6min.Thechromatogram(aplotofdetectorresponseversustime)providesdirectlybothqualitativeand quantitativeinformation.Eachcompoundinthemixturehasitsowncharacteristicelutionorretentiontime(thetime pointatwhichthesignalappearsinthechromatogram)underagivensetofconditions(qualitativeanalysis)andthe areaandheightofeachsignalareproportionaltotheamountofthecorrespondingsubstance(quantitativeanalysis). Thismakeschromatographyaverypowerfulandusefultechnique.
ChromatographywasoriginallydevelopedbytheRussianbotanistM.S.Tswett(1872–1919)(Fig.1.2)asatechniquefortheseparationofcolouredplantpigments.Acleardefinitionofchromatographyisclearlydesirableand shouldanswerourquestion.Tswettgaveaverypragmaticdefinition [1,2].However,thefirstdetaileddefinition
FIG.1.1 Anexampleofthemostcommonformofachromatogram inwhichthe x-axisrepresentstimeandthe y-axisisthedetector response.Thechromatogramshowstheseparationof12analytesin 6min.Thepeaksobservednear1minarecomponentsofthesample solventandsomeminorpeaksarealsoseenthroughoutthe chromatogram.
FIG.1.2 PhotographofMikhailTswett(1872–1919),thesonofaRussianforeignserviceofficialandanItalianmother.HestudiedattheUniversityofGenevabutthenreturnedtoRussia beforeworkingatWarsawUniversityandthenbeingevacuatedtoMoscowinWorldWarI. Credit:Elsevier.

appearstobeduetoZechmeister [3] andvarioussubsequentdefinitionshavesincebeenformulated.Ageneralized definitionwasprovidedin1974 [4] andessentiallyconfirmedwithminorrefinementsin1993 [5] byaspecialcommitteeoftheInternationalUnionofPureandAppliedChemistrywhichregardschromatographyas ‘ amethod,used primarilyforseparationofthecomponentsofasample,inwhichthecomponentsaredistributedbetweentwophases, oneofwhichisstationarywhiletheothermoves.Thestationaryphasemaybeasolid,liquidsupportedonasolid,ora gel.Thestationaryphasemaybepackedinacolumn,spreadasalayer,ordistributedasafilm … Themobilephase maybegaseousorliquid’.Thisdefinitionneglectedthepossibilityofusingasupercriticalfluidasthemobilephase whichhighlightsthedifficultiesassociatedwithprovidinganadequatedefinition.
WhiletheIUPACdefinitionregardschromatographyasa ‘method’,theScientificCouncilonChromatography, RussianAcademyofSciences [6] definedchromatographyasfollows:
• Scienceofintermolecularinteractionsandtransportofmoleculesorparticlesinasystemofmutuallyimmiscible phasesmovingrelativetoeachother;
• Processofmultipledifferentiatedrepeateddistributionofchemicalcompounds(orparticles),asaresultof molecularinteractions,betweenmutuallyimmisciblephases(oneofwhichisstationary)movingrelativetoeach otherleadingtoformationofconcentrationzonesofindividualcomponentsoforiginalmixturesofsuchsubstances orparticles;and
• Methodofseparationofmixturesofsubstancesorparticlesbasedondifferencesinvelocitiesoftheirmovementina systemofmutuallyimmisciblephasesmovingrelativetoeachother.
Thisdefinitionrecognizesthatchromatographyissimultaneouslyaprocess,amethod,andabranchofscience.Itis identifiedas ‘anewbranchofscience ’ [7] andas ‘abodyofknowledgethatisnowtoolargeformanyscientiststofully grasp?’ Thisreferenceisanexcellenttributetothepioneersandbuildersofchromatographyandtotheirachievements. Whilethedefinitionmightappearirrelevant,inactualfact,Socratesheldtheviewthatthe ‘preciselogicaldefinitionsof conceptsareafundamentalprerequisitetotrueknowledge’.Indeed,therehasbeenconsiderabledebateonthis topic [8].
Novákapproachesthisproblemfromadifferentperspectiveandprovidesaphenomenologicaldefinition,amolecularkineticdefinitionandvariousworkingdefinitions [9].Whatisofinterestisthatwitheachsuccessivedefinitionthe criteriaforaprocesstobecalledchromatographyhavegenerallybeenliberalized.Thisisnotsurprisingaschromatography,likemostscientificdisciplines,iscontinuouslyevolving.Thusweshouldnotallowourselvestobedistracted bytheneedforaclearandconcisedefinition,butratherregardchromatographyasagroupofseparationmethods whichareundergoingcontinuousdevelopmentandrefinement.Alternatively,itisalsoappropriatetoseechromatography [10] asaunifiedscientificdiscipline: ‘the “bridge”—asacentralscience akeyfoundationbuiltonthetwentieth centuryformajoradvancesanddiscoveriesyettocomeacrossmanysciencesofthetwenty-firstcentury’
Theoriginsoftheword ‘chromatography’ arenolessobscure [11,12].A1952paper [11] commentedontheuseofthe wordchromatographyforoveracenturyandahalfpriortoTswett’suseofthetermalthoughwithadifferentconnotation.InTswett’spapers,itwascoinedbycombiningtwoGreekwords, chroma, ‘colour’ and graphein, ‘towrite’ selectedtoindicatetheindividualcolouredbandsobservedbyTswettinhisseparations(Fig.1.3).Atthesametime Tswettemphasizedthatcolourlesssubstancescanbeseparatedinthesameway.However,itmaywellbethatTswett, whowasinvolvedinabittercontroversywithhispeers,gavereferencetotheGreekwordsonlyasanexcuseforas Purnell [13] states ‘ itwouldbenicetothinkthatTswett,whosename,inRussian,meanscolour,tookadvantageof theopportunitytoindulgehissenseofhumour’.TheGermanictranscriptionofhisCyrillicname,Tswettismostly usedbutitoccasionallyappearsastheEnglishtranscription,Tsvet.
Irrespectiveofsuchconsiderationschromatographyisauniversalandversatiletechnique.Whilethemorelimited IUPACdefinitionjustifiesthoseinvolvedinapplicationsofchromatographyinfieldsasdiverseasmedicineandengineering,thebroaderconceptofascientificdisciplinelegitimizesbasicresearchinthefieldwhichisanessentialsupport fortheappliedaspects.Itisequallyapplicableinallareasofchemistryandbiochemistry,biology,qualitycontrol, research,analysis,preparativescaleseparations,andphysicochemicalmeasurements.Itcanbeappliedwithequal successonthemacroandmicroscale.Chromatographyisusedindustriallyinthepurificationofsuchdiversematerialsascanesugar,pharmaceuticals,andrareearths.Ontheotherhand,itiswidelyusedinthelaboratoryforthe separationofminutequantitiesofsubstance,asintheinitialchromatographicexperimentsleadingtothediscovery ofelementnumber100whichinvolvedonlyabout200atoms.Thissurelyrepresentsoneofthemostremarkable achievementsofmodernscience [14]
Theachievementofsuchseparationsdemonstratestheroleofchromatographynotonlyinchemistrybutalsoin scienceandmedicinewheretheimportanceofchromatographyisindisputable.TwoNobelPrizesinChemistry(to A.TiseliusofSwedenin1948andtoA.J.P.MartinandR.L.M.SyngeofGreatBritainin1952)havebeenawarded
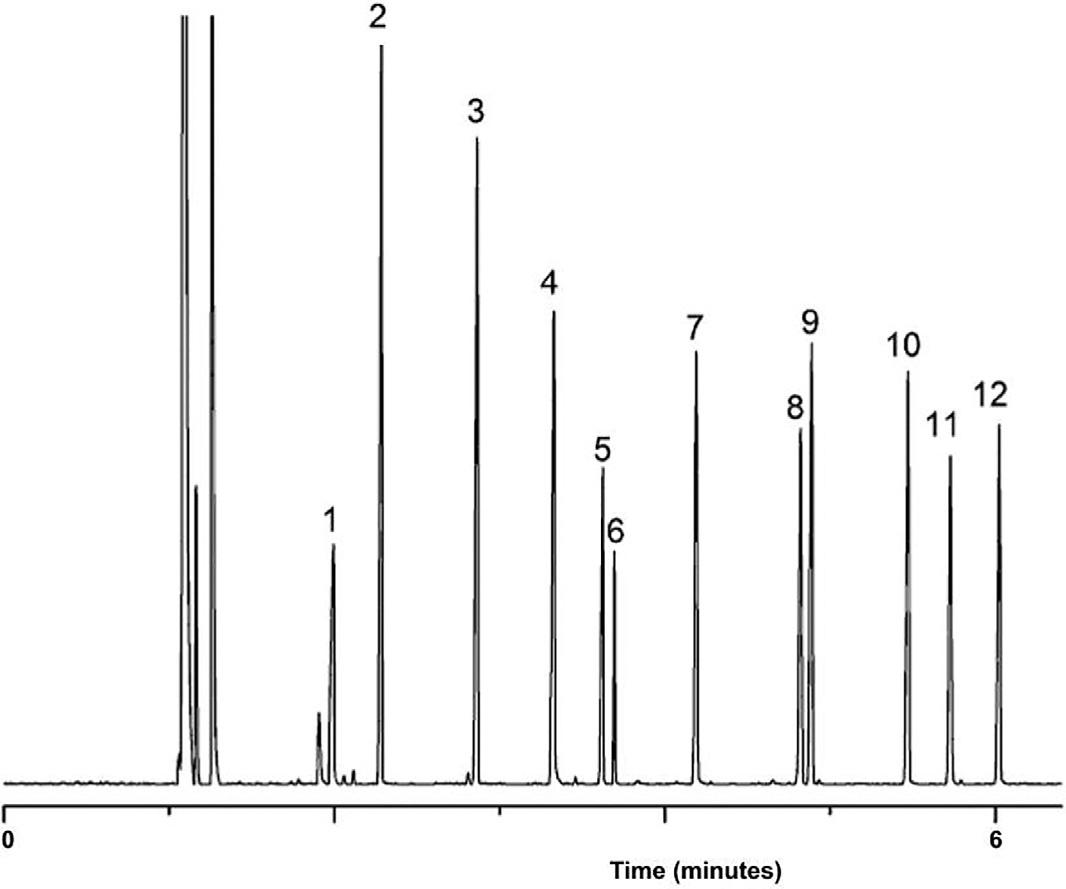
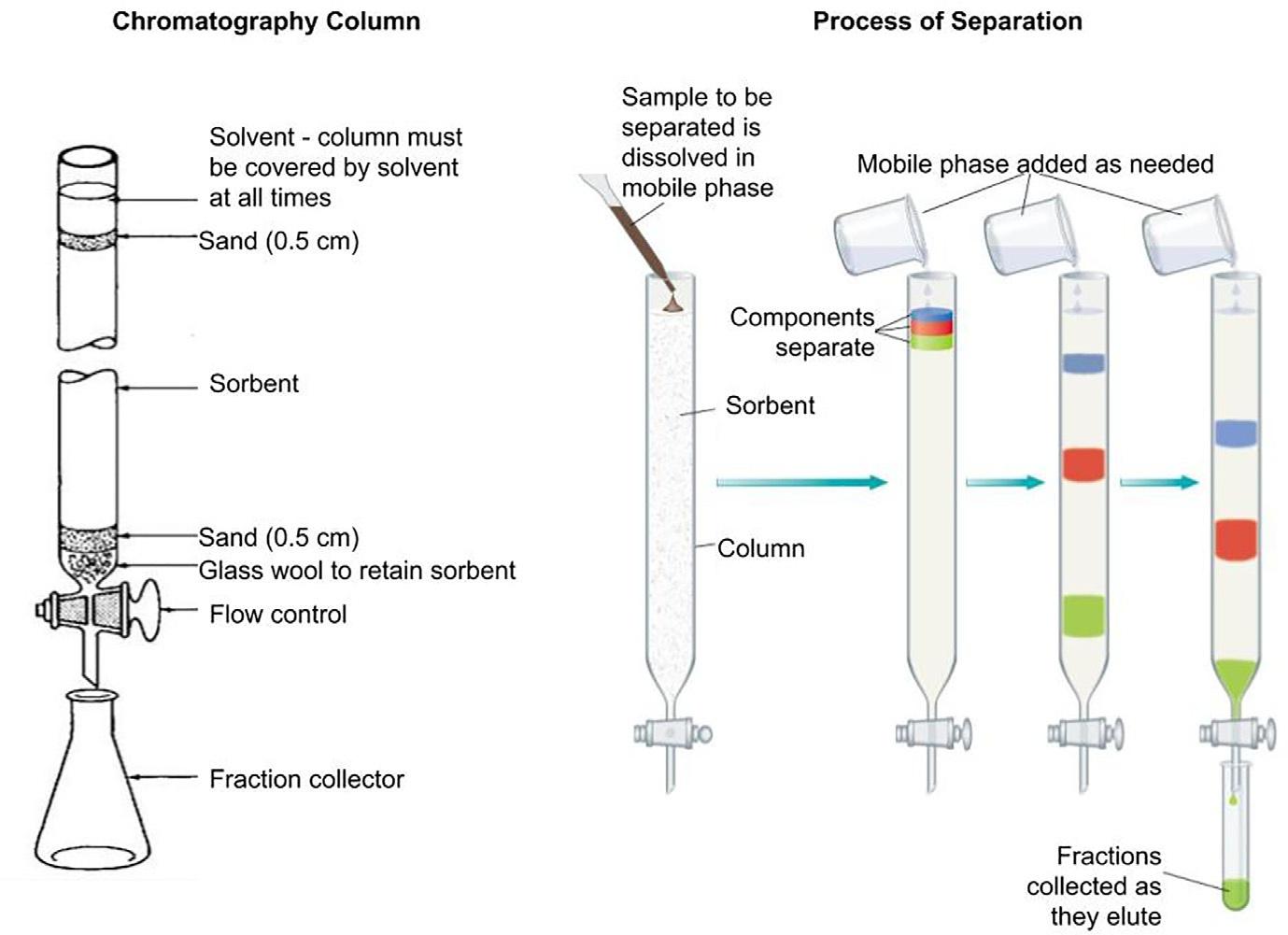
FIG.1.3 Illustration(idealized)ofclassical columnchromatographyshowingthecolumn priortoadditionofsampleandatfourdifferent stagesofdevelopmentillustratingtheseparationofthreeanalytesfromamixture.Sandis addedtothetopofthecolumntominimizedisturbancetothesorbentassampleandmobile phaseareadded.Thisisessentiallythesystem asusedbyTswettinhisoriginalexperiments. Priorto1935thecolumnpackingwasremoved fromthecolumnafteruseandtheseparated zoneswereextractedinordertorecoverthe ‘ pure ’ components.
forworkdirectlyinthefieldofchromatography.Inaddition,chromatographyplayedavitalroleinworkleadingto theawardofafurther25NobelPrizesinthe62yearsbetween1937and1999,and19between2000and2007 [10]
Theuseandimportanceofchromatographytosocietycanbeillustratedinmanyways.Thevalueofsalesofchromatographicequipmentdemonstratesadirecteconomicimportancetothecommunity.However,therearemany othereconomicimpactsthataredifficulttoassess.Forexample,theuseofchromatographyinensuringthehealth oftheenvironment,qualityofourfood,waterandairsupply,andclinicalmonitoring,amongothers,arelesstangible andmoredifficulttoassignadollarvalue.Equipmentsalesalsoprovideanindirectmeasureofhowwidelychromatographyisused.Anothermeasureistheuseofscientificpublicationsbutthisreflectsresearchoutputratherthan routinedailyuseoccurringingovernmentorganizationsanddepartments,foodandpharmaceuticalindustrylaboratories,hospitals,racingclubs,sportingorganizations,themobilesciencelabinFerrari’sF1garage(theovenwitha 30-mcoilinsideisagaschromatograph) [15],thePhilaelanderonComet67Plocatedover500millionkilometresfrom Earth [16],oranyoneofthemanylaboratoriesworldwideusingchromatography.
Theauthorsofapaperontheself-imageofchemistsbetweentheyears1950and2000 [17] identifiedthe1960sasthe periodof ‘chromatographictakeover.’ Theynotedtheexistenceatthattimeoftwoworlds, ‘thatoftraditional chemistry,basicallyunchangedfortwoorthreecenturies,andthatofmodernchemistry,withaplethoraofnew andpowerfulphysicalmethods’ withchemistsseeingthemselveswithafootineachofthetwoworlds.Hopefully, thereareatleastsomechemistsstillstraddlingbothoftheseworlds.
1.2Coverage
Inthisbookweexaminealltechniquesthatfitthedefinitionofchromatographyprovidedearlierinthischapter.The orderofpresentationoftopicshaschangedfromthefirsteditiontobetterreflectthedistinctionbetweenplanarand columntechniques.Newchaptershavebeenaddedonhyphenatedtechniquesandpreparativechromatographywhile thechapterontheoryhasbeenextendedandre-written.
Referenceshavebeenupdatedandextendedandpartlyforthisreasonbutalsoreflectingthegreatereaseof literaturesearching,thebibliographywitheachchapterhasbeendeleted.
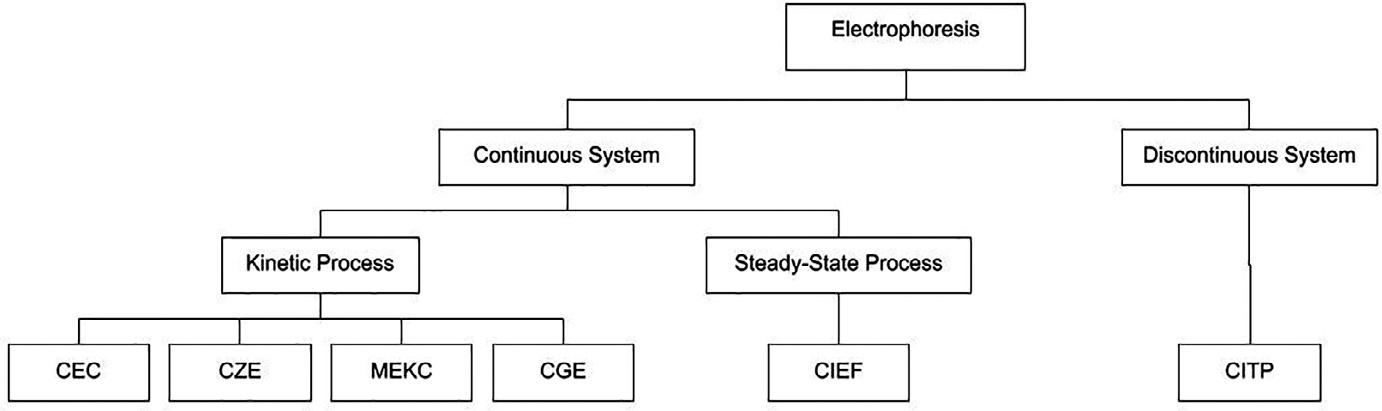
Electrophoretictechniquesbearsomesimilaritytochromatographyinthatamobileandstationaryphaseare involvedand,onthisbasis,itiseasytoarguefortheinclusionofmodernelectrophoretictechniquesinthecurrent book.Sixtypesofcapillaryelectroseparation(Fig.1.4)canbeidentifiedas:capillaryzoneelectrophoresis(CZE),capillarygelelectrophoresis(CGE),micellarelectrokineticcapillarychromatography(MEKC),capillaryelectrochromatography(CEC),capillaryisoelectricfocusing(CIEF),andcapillaryisotachophoresis(CITP) [18].
FIG.1.4 Flowchartshowingthecategorizationofelectrophoretictechniques.
Themeansofdrivingthemobilephaseinliquidchromatographicseparationshasevolvedfromgravityinclassical columnchromatographythroughcapillaryactioninpaperandthinlayerchromatographytohydrodynamicpressure inhigh-performanceliquidchromatography.However,inthecaseofelectrophoresis,solutemigrationisalsounder theinfluenceofanexternalforceintheformofanelectricfield.
Ofthesixtechniques,CECisatruehybridofelectrophoresisandchromatographyandastrongerargumentcould bemadeforitsinclusion.TheCECprocesstakesplaceinacapillarycolumn,containingaselectedstationaryphase, wherethemobilephaseisdeliveredbyanelectro-osmoticflowcontrolledbytheapplicationofarelativelyhighelectric field.CECpresentsanumberofadvantagesrelativetohigh-performanceliquidchromatography(HPLC) [19] butthe mostsignificantconsiderationistheimprovedchromatographicefficiency.TheflowrateinCECisindependentof particlediameterandcolumnlengthandthereisnopressuredependencyunlikeHPLC,wherethecolumnpressure isinverselyproportionaltotheparticlediametersquaredanddirectlyproportionaltocolumnlength,So,CEChasthe potentialtogeneratehigherplatecountsthanHPLC.Theplug-likeflowvelocityprofileintheelectro-drivensystem furtherreducesbanddispersionandthusachievesahigherefficiency [20] totheparabolicorGaussianprofile associatedwithhydraulic-drivenflowinHPLC.
TherearedivergentviewsontheutilityofCEC.Accordingtoonesourcepublishedin2018 [21],interestinopentubularCEC(OT-CEC)continuestothrivewhereasa2009paperposedthequestion ‘whateverhappenedtocapillary electrochromatography?’ [22].Cantheseviewsbereconciledorhasthetechniqueexperiencedaresurgencebetween 2009and2017?Between1998and2009therewasanaverageofaround150publishedpapersperyear.Thetechnique doesappeartohavehadarenaissancesince2009basedonpublicationnumbersbutthesearestillrelativelyfewcomparedwithHPLCandCEChasfailedtobecomeamainstreamseparationtechnique [20].Lookingatthetitlesofpublishedpapersgivesafeelingthatitisatechniqueseekingapplicationareas.Thedevelopmentofnovelstationary phases [23] hasalwaysbeentheresearchfocus.ThetheoreticalbasisofCEChasbeenfirmlyestablishedsothataspect isnotanimpedance.Ratherthemajorissuesinthelackofwidespreadacceptancearetheabsenceofdedicatedcommercialinstruments [24] withthefeaturesneededtocompetewithHPLCandCE,andthelackofapplicationswhere establishedmethodsfail.However,scientistsarestillinvestigatingtheparametersforCECanddevelopingapplicationsdespitethelackofcommercialsupport.Indeed,thedevelopmentofrapid,effective,andselectivechiralseparationmethodsisgettingincreasinglyimportantfordrugqualitycontrol,pharmacodynamicandpharmacokinetic studies,andtoxicologicalinvestigations [25] andthisisoneareainwhichCECmaycompete.
WehaveexcludedtreatmentofCECinthisbookfortworeasons.Firstly,themeansofdrivingthemobilephasein electrophoresisisnotencapsulatedbythedefinitionwehavegivenofchromatography.Secondly,CEChasnotdevelopedintoaroutinetechniqueorestablishedparticularnichemarkets.TheauthorsacknowledgethepotentialCEC offersandrecognizethatitsinclusioninthenexteditionofthisbookmaybewarrantedtotheexclusionor,morelikely, reductionintreatmentofanothertechnique(s).
1.3Chromatographicseparationsimplyexplained
Thedefinitionofchromatographyimpliesthattheseparationofthecomponentsofasampleoccursbydistribution ofthecomponentsbetweentwophases,oneofwhichisstationary(asolidorliquid)andtheothermovingormobile(a liquid,gasorsupercriticalfluid).Consideratwo-componentmixturewhichisintroducedattime,to,intoamoving phasewhichisincontactwithasecondphase,thestationaryphase.Acontinuoussupplyoffreshmobilephaseisthen providedtotransportthesamplecomponentsthroughthestationaryphase.Astheanalytescomeintocontactwiththe stationaryphase,theydistributeorpartitionbetweenthetwophasesdependingontheirrelativeaffinitiesforthe phasesasdeterminedbymolecularstructuresandintermolecularforces.Thisprocessisdepictedin Fig.1.5 where
FIG.1.5 Schematicrepresentationofachromatographicdevelopmentonapackedcolumn showingtheseparationofamixtureoftwo dyesofequimolarconcentrationsunderideal conditionsandinthesituationpertainingin realsystems,wherebothseparationanddispersionofanalytebandsoccurduringtheseparationprocess.Thecolumnisdepictedatthe pointofinjection, t0,andattwosubsequent times, t1 and t2.Althoughnotdepicted,thesoluteconcentrationprofilealongthecolumn lengthisGaussianattimes, t1 and t2
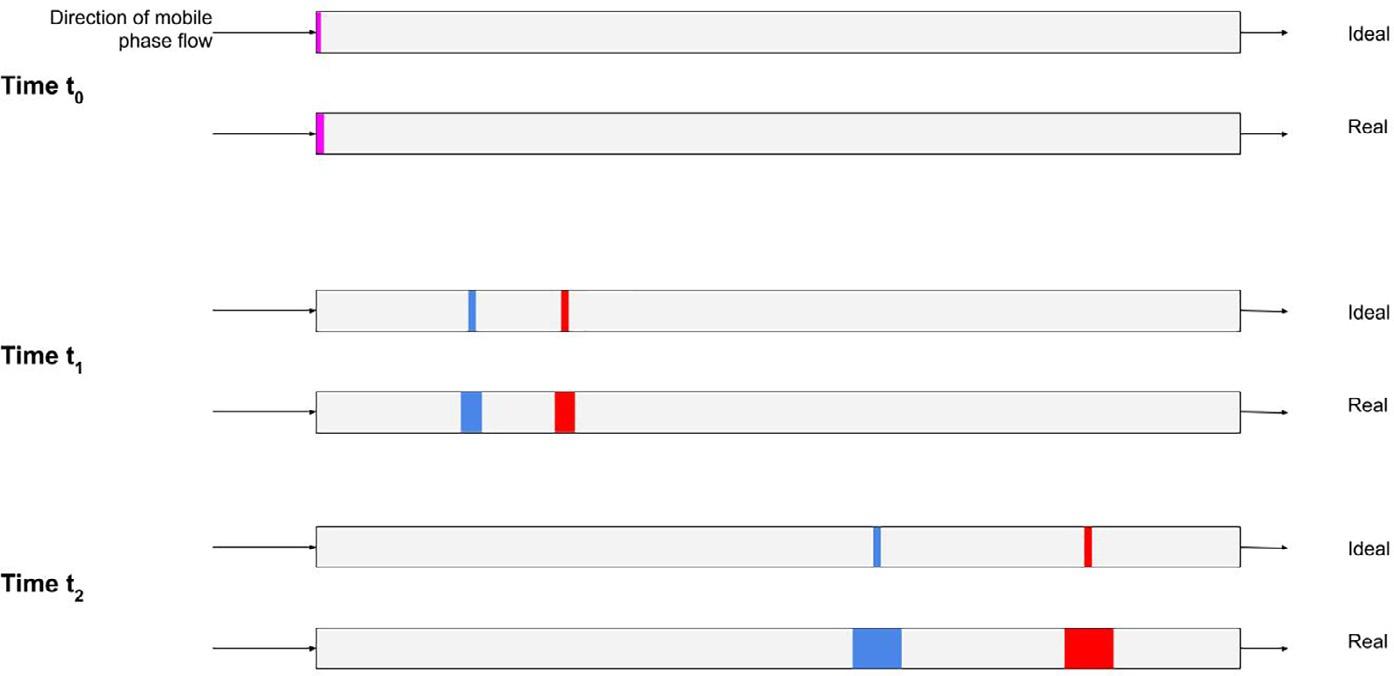
analyteAhasahigheraffinitythananalyteBforthestationaryphaseandthusspendsagreaterproportionofthe availabletimeinthestationaryphase.Whenananalyteispresentinthemobilephase,itwillpassthroughthesystem withthesamevelocityasthemobilephasebutwhenitisinthestationaryphase,itsvelocitywillbezero.Hence,analyteswithahighaffinityforthestationaryphasewillmovethroughthesystemveryslowlywhereasanalyteswitha loweraffinitywillmigratemorerapidly.Thisdifferentialmigrationrateofanalytesresultsinseparationofthecomponentsastheymovethroughthesystem,asshownin Fig.1.5 attimest0,t1,andt2,underidealandrealconditions.
Eventhoughthesystemisdynamic,itmustbeoperatedasclosetoequilibriumconditionsaspossiblebyoptimizing themobilephasevelocityanddesigningthestationaryphasetoallowrapidequilibrationtobeachieved;i.e.thetimescalefordistributionofsolutemoleculesbetweenphasesmustberapidcomparedtothevelocityofthemobilephase. Wecanwriteforanysolute, A:
where m and s representmobileandstationaryphase,respectively.Undertheseconditions,thesystemcanbecharacterizedbyathermodynamicdistributionconstant, K,whichisusuallyexpressedastheratioofanalyteconcentration inthestationaryphase, CA(s) tothatinthemobilephase, CA(
Thedescriptionof K asadistributionconstantisconsistentwithIUPACnomenclaturebutoldertermssuchaspartition coefficientanddistributioncoefficientarealsostillinuse.
Ananalogymightimproveunderstanding [26] ofEq. (1.1).Ifweassumethattherehasbeenrainwhichhaslefta puddleofwater.Itiswellknownthatthepuddledisappearsovertimeduetoevaporationeventhoughtheambient temperatureneverreachestheboilingpointofwater.Thiscanbedescribedbythefollowingequilibrium:
where KP isthepressure-basedequilibriumconstantfortheevaporationprocessand PH2O isthevapourpressureofthe water.Onacooldryday,theairabovethepuddlebecomessaturated(100%relativehumidity).Theliquidandgaseous watermoleculesareinorclosetoequilibriumandnofurtherevaporationoccurs.Onawindydaythepuddleevaporatesmorerapidlybecausethegaseouswatermoleculesarebeingcontinuouslyremovedsothatmoremolecules enterthegasphasetorestoretheequilibrium.
ThedistributionconstantofEq. (1.1) isacharacteristicphysicalpropertyofananalytewhichdependsonlyonthe structureoftheanalyte,thenatureofthetwophases,andthetemperature.Phenolicsolutes,forexample,wouldbe expectedtoformintermolecularattractionswithphenolicstationaryphasestoamuchhigherdegreethanwould hydrocarbonsolutesexposedtothesamestationaryphase.Thusthe K valueofaphenolishigherthanthatofahydrocarbonofcorrespondingchainlengthinaphenolicphase.Theseparationoftwocompoundsonaparticularchromatographicsystemrequiresthattheyhavedifferentdistributionconstants.Conversely,twocompoundswiththesame distributionconstantwillnotbeseparated.Inthiscase,theseparationcanbeimprovedbyvaryingthemobilephase,
thestationaryphase,orthetemperatureofthesystem.Inpractice,itisoftendifficulttopredicttheeffectsofchanging themobilephaseorstationaryphaseandtheonlymethodistomakethechangeexperimentally.Ingaschromatography,thepartitionpropertiesofthegasesusedasmobilephasearesimilarandthemobilephaseisdescribedas non-interactive,sothatonlythestationaryphaseandtemperaturecanbevariedtoimproveseparation.Thegreater versatilityofliquidandsupercriticalfluidchromatographyispossiblebecausethemobilephaseisinteractiveandall threevariablescanbealtered,althoughtemperaturechangesareveryrestricted.
Eq. (1.1) isanoversimplification,sinceK,likeanythermodynamicequilibriumconstant,isreallyaquotientofanalyteactivities.However,inchromatographicsystemswearenormallydealingwithsolutionswhichtendtowardsinfinitedilutionandthereforetheactivitycoefficientisone.Thisequationalsoassumesthattheanalyteispresentasonly onemolecularstructureorionandthattheanalytedoesnotinteractwithotheranalytemoleculesatinfinitedilution. Consideringthelowlevelsofanalytesinvolved,thisisareasonableassumption.Theconcentrationprofilesdepictedin Fig.1.5 asidealareneverachievedinpractice.Atthemolecularlevel,varioussolutediffusionaleffectsandrandom statisticalmotionofmoleculescausespreadingoftheanalytebands(asshownin Fig.1.5)whichassumethenormal Gaussiandistribution(providedadsorptiveeffectsareabsent discussedinlaterchapters)(notdepictedin Fig.1.5).
1.4Classificationofchromatography
Classificationisusedtosimplifyourunderstandingoftheuniversebydividingasubjectintosmaller,moremanageable,andmorespecificparts.Bookscanbeclassifiedaccordingtomanycriteriaincludingcolourofthecover, height,orsubjectmatter.Thelatteristhemostusefulsystemforalibrariantousebutheightismoreusefulforme becauseIcanthenfitbooksonmylimitedshelfspace.Similarly,anyoneofseveralfactors(stationaryormobilephase, separationprocess,oreventhetypeofsolute)canserveasabasisforclassificationofchromatography.Thuschromatographicseparationscanbeclassifiedinanumberofwaysdependingontheinterestsofthechromatographer. Whiletheultimategoalofanyclassificationsystemissimplification,unfortunatelythismultiplicityofoverlapping systems,togetherwiththediversityofchromatographyasnowpractised,hasledtoaproliferationofterminology whichcanbeconfusingforboththenoviceandspecialistchromatographeralike.
Thereareotheraspectsofnomenclaturethatarepotentiallyconfusingtothenovice.Forinstance,aswewillsee,a chromatographicsysteminwhichacolumnisconnectedtoapumpandspectrophotometeriscalledanHPLC,butif wetakethesamepumpandcolumnandconnectittoamassspectrometer,wehaveahyphenatedtechniqueor,more specifically,inthisinstance,anLC-MS.Onemightsaythatthisdistinctionisillogicalbutitisunderstandableinterms ofthehistoricaldevelopmentofthefieldofchromatography.Inotherexamples,thetermsrapidresolutionLC [27], ultra-high-performanceliquidchromatography(UHPLC) [28–31],andultra-highpressureLC [32] describeessentially thesameprocedure [33] butemphasizedifferentaspectsreflectingthepersonalbiasofthespeaker.Forexample, UHPLC [34,35] isdistinguishedfromHPLCbythesizeofstationaryphaseparticles:sub-2 μmforUHPLCversus 5 μmforHPLCwithtypicalpumppressuresa of1000bar(14,500psi)and4000bar(5800psi),respectively.
Agraspofthefundamentalspresentedhereandinwhatfollowswillhelptoreducesuchconfusion.
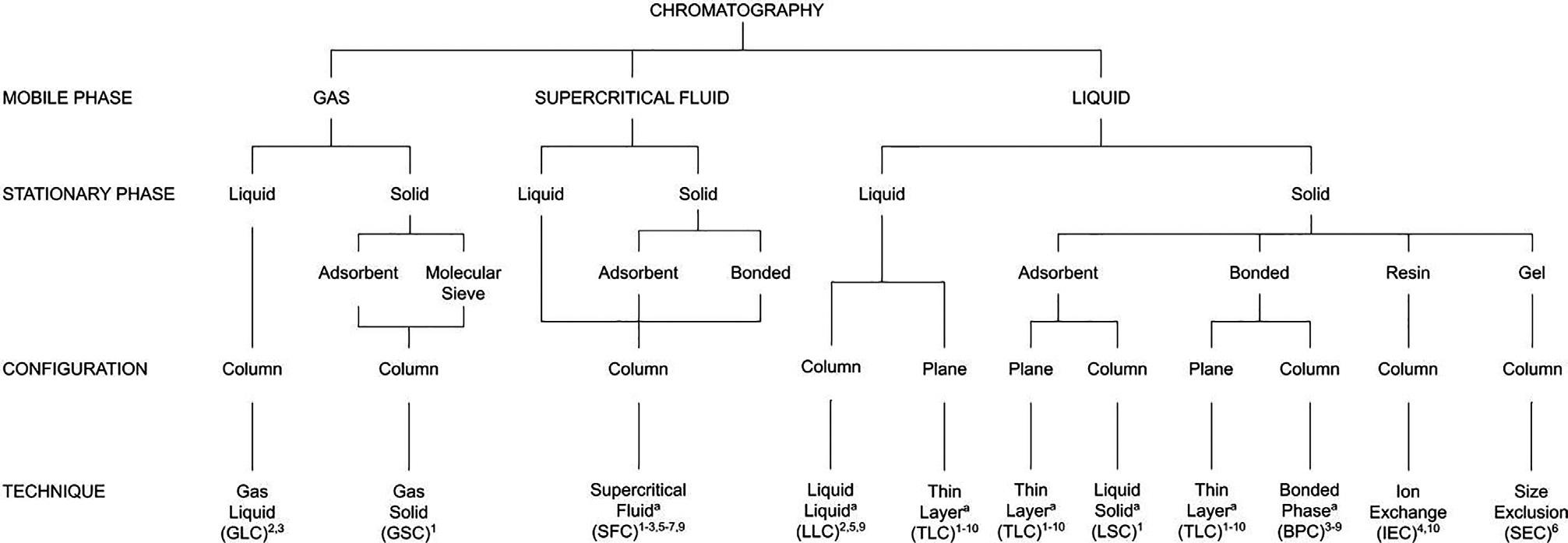
1.4.1Mobilephase
Onesystemofclassificationrecognizestheimportanceofthemobilephaseanddivideschromatographyintothree broadareasofliquidchromatography(LC),gaschromatography(GC),andsupercriticalfluidchromatography(SFC) (Fig.1.6),dependingonwhetherthemobilephaseisaliquid,gas,orsupercriticalfluid,respectively.Furtherclassificationispossiblebyspecifyingboththemobileandstationaryphasesleading,forexample,togassolidchromatography(GSC)andgasliquidchromatography(GLC)inwhichthemobilephaseisagasbutthestationaryphaseisa solid(GSC)oraliquid(GLC).AsGSCislittleusedexceptinveryspecificapplicationsonenormallyreferstogaschromatographyorGCunlessasolidstationaryphaseisusedinwhichcaseitisspecificallynotedbyspeakingofGSC.
Morerecently,supercriticalfluidshavebeenemployedasmobilephasesandthesetechniquesareatpresenttermed supercriticalfluidchromatography(SFC)irrespectiveofthestateofthestationaryphase.Thesituationwithliquid chromatographyisintermediateinthatdistinctionbetweenliquid-solidchromatography(LSC)andliquid-liquid
a Variouspressureunitsareusedinchromatography.Thosecommonlyencounteredandrelevantconversionsare
FIG.1.6 Flowchartshowingtheclassificationofchromatographicsystemsbasedonmobilephase,stationaryphase,configuration,and technique.
chromatography(LLC)issometimesmadebut,inmostcases,itisjustreferredtoasliquidchromatography(LC) regardlessofthestateofthestationaryphase.
1.4.1.1Reversed-phaseandnormal-phasechromatography
Inliquidandsupercriticalfluidchromatography,systemsinvolvingapolarstationaryphaseandanon-polar mobilephasearetermednormal-phasesystems.Withthiscombinationofphases,soluteretentiongenerallyincreases withsolutepolarity.Ontheotherhand,ifthestationaryphaseislesspolarthanthemobilephase,thesystemis describedasreversedphaseandpolarmoleculeshavealoweraffinityforthestationaryphaseandelutefaster (Table1.1).
Thechoiceofthesetermsispurelyhistoricalandnospecialsignificance(beyondthatindicated)isattachedtothe useoftheterm ‘normal’.TheoriginalseparationsofplantextractsperformedbyTswettusedapolarstationaryphase (chalkinaglasscolumn)withamuchlesspolar(indeed,non-polar)mobilephaseandthiscombinationbecameknown asnormalphase.However,reversed-phasesystemsbecauseoftheirbroadapplicabilityandbetterreproducibilityare nowfarmorecommonthannormal-phasesystemsinliquidchromatography.
Withnormal-phasesystems,increasingthemobilephasepolaritymakesitmorelikethestationaryphasesothatthe mobilephasecompetesmoreeffectivelywiththestationaryphaseforsolutemolecules.Thesolutemoleculestherefore spendlesstimeinthestationaryphaseandelutefaster.Usingasimilarargument,wepredictslowerelutionasmobile phasepolarityisincreasedinreversed-phasechromatography [36].
TABLE1.1 Normal-phaseandreversed-phasechromatography.
ClassificationStationaryphaseMobilephaseOrderofsoluteelution
NormalphasePolarNon-polarMosthydrophobicsolutesfirstandfinallymosthydrophilicsolutes
ReversedphaseNon-polarPolarMosthydrophilicsolutesfirstandfinallymosthydrophobicsolutes
1.4.2Developmentmode
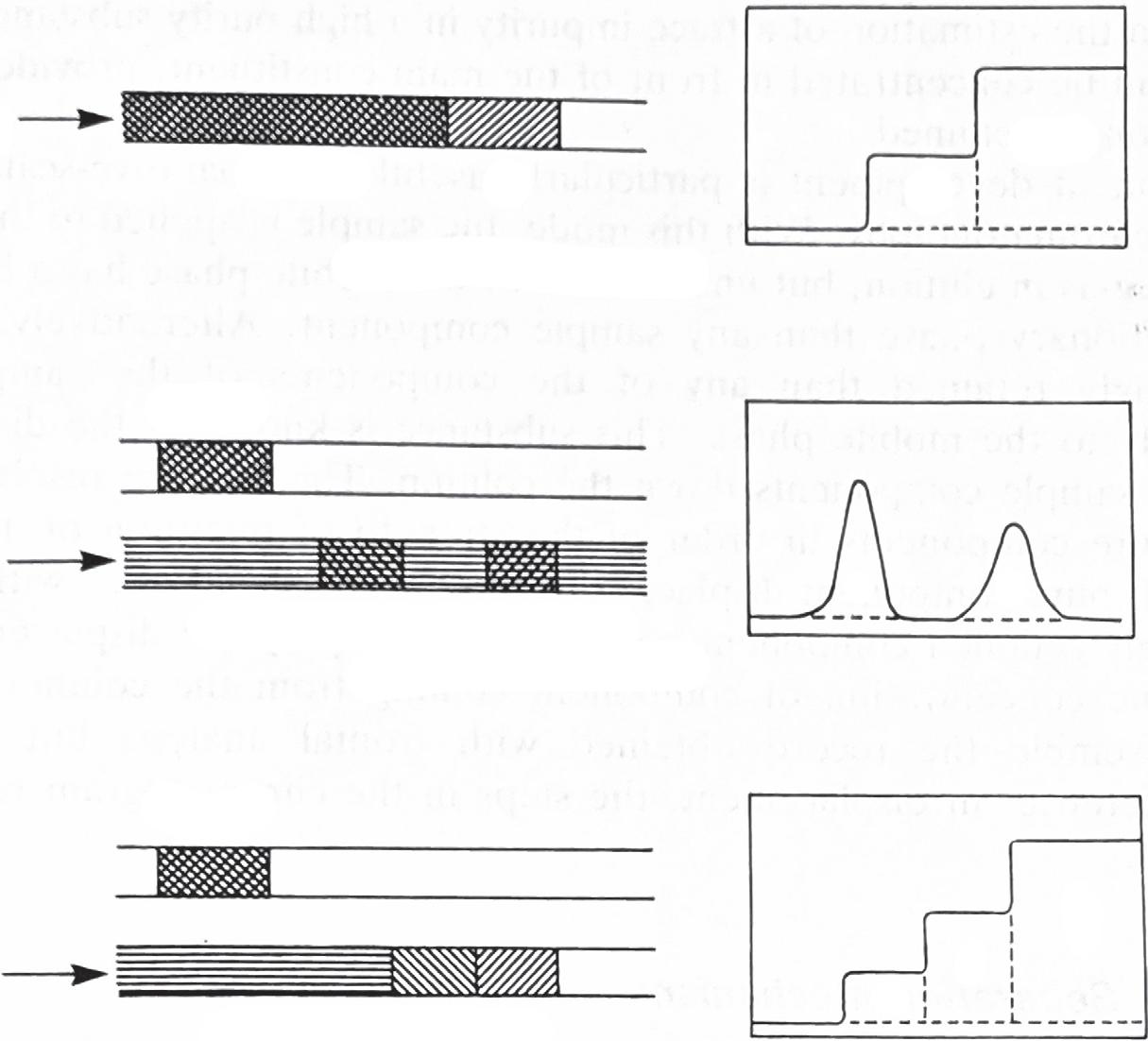
Thechromatographicbeddescribestheconfigurationofthestationaryphase.Inthesimplestterms,thebediseither planaroracolumn.Theprocesswherebyasamplecomprisingamixtureofsolutesprogressesthroughthechromatographicbed,whileinthemobilephase,iscalledchromatographicdevelopment.Threemodesofchromatographic developmentwereidentifiedin1943 [37] bythe1948ChemistryNobelLaureate [38],ArneTiselius,as elution development, displacementdevelopment, andfrontalanalysis.Thedevelopmentmodereferstothemannerinwhichthe sampleandmobilephaseareappliedtothestationaryphasebed(columnorplane)andasshownin Fig.1.7,thenature oftheresultingpeakprofile,termedthechromatogram,differsbetweenthethreemodes.IUPACdistinguishesthe threemodesasfollows:
FIG.1.7 Schematicrepresentationofthedifferent chromatographicdevelopmentmodesshowingthe effectonmigrationofsamplecomponentsandthe resultingzoneprofile.AandBrepresentsamplecomponentsandCthedisplacer.
• FrontalChromatography.Aprocedureinwhichthesample(solid,liquid,orgas)dissolvedinmobilephaseisfed continuouslyintothechromatographicbed.Infrontalchromatographynoadditionalmobilephaseisused.
• DisplacementChromatography.Aprocedureinwhichthemobilephasecontainsacompound(theDisplacer)more stronglyretainedthanthecomponentsofthesampleunderexamination.Thesampleisfedintothesystemasa finiteslug.
• ElutionChromatography.Aprocedureinwhichthemobilephaseiscontinuouslypassedthroughoralongthe chromatographicbedandthesampleisfedintothesystemasafiniteslug.Elutiondevelopmentisnowvirtuallythe onlydevelopmenttechniqueemployedforanalyticalseparations.
Infrontalchromatographythesampleissweptcontinuouslyontothecolumnbythemobilephaseduringtheentire courseoftheprocess.Whenthecolumnbecomessaturatedwithrespecttoaparticularcomponent,thatcomponentis thenelutedfromthecolumn.Whenthezoneofpurecomponenthascompletelyeluted,itisfollowedbyamixturewith thenextcomponent,andsoon.Acompleteseparationcannotbeachievedandthemethodhaslimitedapplicationfor quantitativemeasurements.Atypicalapplicationwouldbetheestimationofatraceimpurityinahighpuritysubstancewheretheimpuritycanbeconcentratedinfrontofthemainconstituent,providedthatitwasthelessstrongly retained.Frontalanalysisisnowprobablyofacademicinterestonlyalthoughitisusefulforobtainingthermodynamic datafromchromatographicmeasurements [39,40].Itisquiteinappropriateformostpracticalanalyticalapplications andhasbeencompletelysupersededbyelutiondevelopment.
Theadventofdisplacementchromatographycanbeattributed [37] toTiselius.Withthismode,thesampleis appliedtothesystemasadiscreteplugasinelution,butunlikeelution,themobilephasehasahigheraffinityfor thestationaryphasethananysamplecomponent.Alternatively,asubstancemorestronglyretainedthananyof thecomponentsofthesampleisaddedcontinuouslytothemobilephase.Thissubstanceisknownasthedisplacer andit ‘pushes’ thesamplecomponentsdownthecolumn.Themixtureresolvesitselfintozonesofpurecomponents inorderofthestrengthofretentiononthestationaryphase.Eachpurecomponentdisplacesthecomponentaheadofit withthelastandmoststronglyretainedcomponentbeingforcedalongbythedisplacer.Therecorddepictingtheconcentrationofcomponentcomingfromthecolumn(Fig.1.7)isseentoresembletherecordobtainedwithfrontalanalysisbutwithanimportantdifference.Indisplacement,thestepsinthechromatogramrepresentpurecomponents.
Historicallyitwasusedforpreparativeseparationsofaminoacidsandrareearthelements.Morerecently,ithasfound manyapplicationsintherealmofbiologicalmacromoleculepurifications [41].
Inthisbookwearereferringtoelutiondevelopmentinallcasesunlessspecificallymentionedotherwise.
1.4.3Technique
ChromatographycanbeperformedusingverysimpleapparatusasinTswett’soriginalexperimentsor,ascommonlypractisednowadays,withmuchmoresophisticatedequipment(Fig.1.8)costingintothemillion-dollarmark forthemostsophisticatedsystem.However,allofthesediversetechniquescanbeclassifiedintooneoftwobroad categories;namely,columnandplanarchromatography,buttheseencompassanumberofvariants.
1.4.3.1Planarandcolumnchromatography
Therearetwotechniquesinwhichthestationaryphaseissupportedonaplanarsurface:paperchromatography (PC)andthinlayerchromatography(TLC)whichcollectivelyaretermedplanarchromatography.WithPC,asheetof paperorsubstrate-impregnatedpapercomprisestheplane(sorbedwateristhestationaryphase)whereasinTLC,the planeisaflatsheetofglass,plasticoraluminiumcoateduniformlywithathinlayerofsolidcomprisingthestationary phase.Alternatively,thestationaryphasemaybepackedinaclosedcolumnandthetechniqueisreferredtoascolumn chromatography.
Ifthestationaryphaseisaliquid,itmustbeimmobilizedonthethinlayerorinthecolumnandthisisconveniently achievedbycoatingorchemicallybondingtheliquidstationaryphasetoaninertsolidsupport(e.g.silica)whichis thenpackedinthecolumnorspreadinathinlayeroveraflatplate.Planarprocedureshavebeenrestrictedtoliquid chromatographybecauseofthetechnicaldifficultiesassociatedwithconfiningagasorsupercriticalfluidtoaplanar surface.Incontrasttoplanarprocedures,columnchromatographyisusedinLC,GC,andSFC.Inthecaseofliquid chromatography,therearetwovariantswhichbecauseoftheirchronologicaldevelopmentmaybetermedclassical columnchromatographyandmoderncolumnchromatography.Thelatterisreferredtoashigh-performanceliquid chromatography,HPLC.Thetermliquidchromatographyisoftenusedtodenoteliquidchromatographyincolumns andparticularly(ultra)high-performanceliquidchromatography,(U)HPLCbecauseofitsextensiveuse.
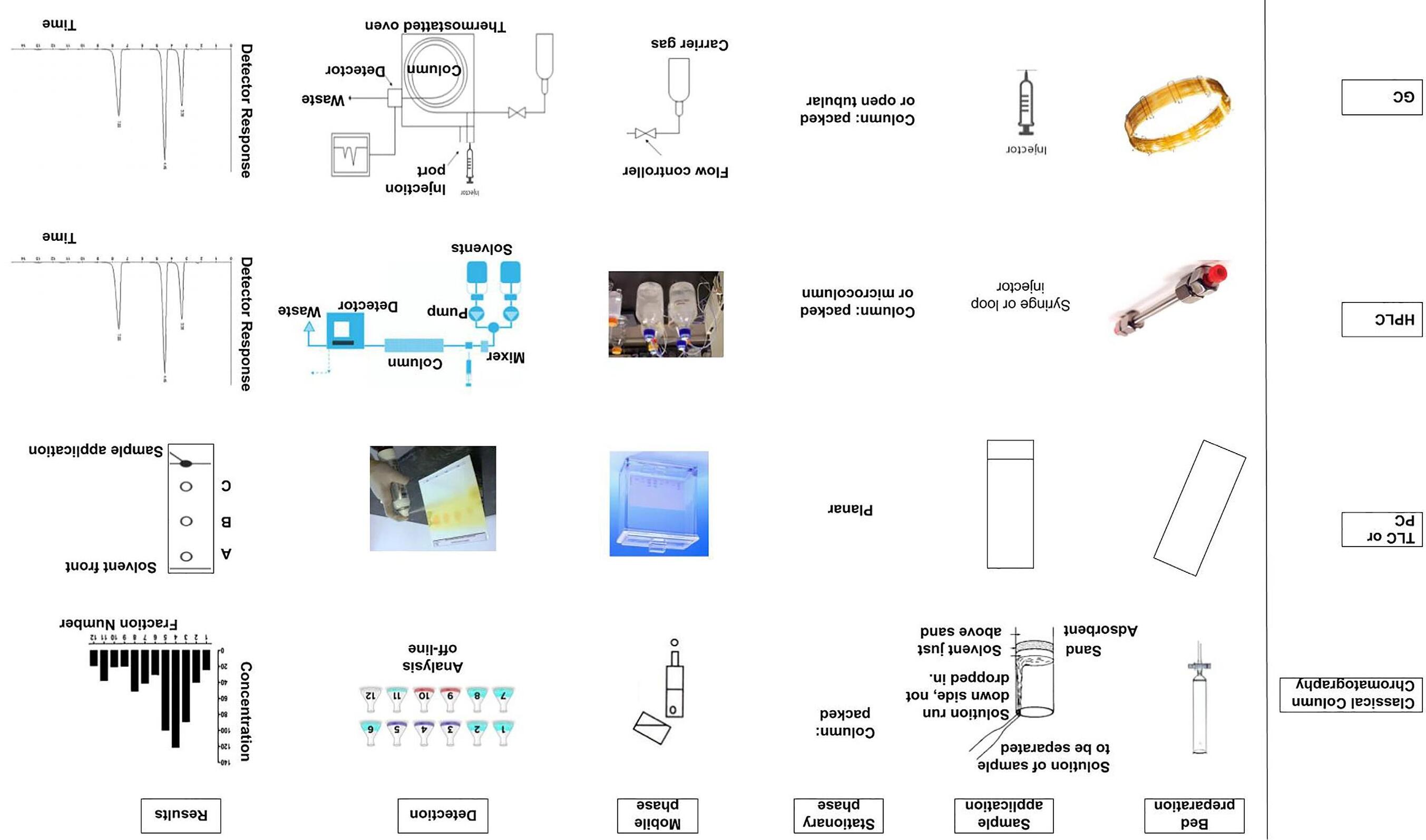
Methodsinvolvinggaseous,liquid,andsupercriticalfluidmobilephaseswillbetreatedindividuallyinlater chapters.Althoughtherearefewfundamentalreasonsforseparatetreatment,itisnonethelesswarrantedbydifferencesinequipmentandoperationalprocedures.Fornow,itissufficienttomakeacomparisonofthedifferent techniquesonthebasisofoperationalproceduresandresultsasshownin Fig.1.9
FIG.1.8 Photographofalaboratoryset-upforliquid chromatography-massspectrometry. Credit:DanielleRyan.
FIG.1.9 Pictorialcomparisonofchromatographictechniquesshowingthevariousstagesoftheprocess:bedpreparation,sampleapplication,natureofthestationaryphase,methodof mobilephaseaddition,methodofdetection,andformatforpresentationofresults.
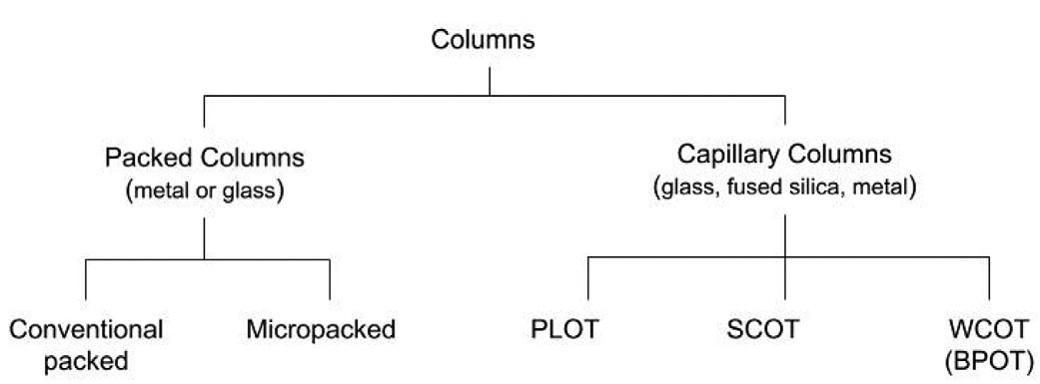
Columntypes
Itisoftenstatedthatthecolumnistheheartofthechromatographemphasizingitsimportancetotheseparation. Columnproceduresmaybefurtherclassifiedaccordingtothenatureanddimensionsofthecolumn.Conventional columnproceduresbothingasandliquidchromatographyand,morerecently,supercriticalfluidchromatography, exploit ‘wide’ borepackedcolumnswithinternaldiameters(i.d.)exceeding1.0mm.InGC,theinternaldiametersof suchcolumnsaretypicallyof2–4mmandinHPLCof4.6mm.Thesepackedcolumnsgenerallycontainastationary phaseconsistingofeitherasolidoraliquidcoatedorbondedtoaninertsolidsupport.Specificdetailsrelatingto stationaryphaseswillbepresentedintherelevantchapter.Thissectionisconcernedwiththephysicalconstruction oftheactualcolumn.
Therearemanybenefitsassociatedwithcolumnminiaturizationandthefirststepinthisdirectionwasmadein1957 withthedevelopmentofcapillarycolumnsforGC [42].Itisnowrealizedthatminiaturizedseparationcolumns, whetherusedinvariousformsofgas,liquid,orsupercriticalfluidchromatography,sharesimilartechnologiesand instrumentalrequirements [43].In1981Novotny [44] identifiedadvantagesofmicrocolumnsashighercolumnefficiencies,improveddetectionperformance,variousbenefitsofdrasticallyreducedflowrates,andtheabilitytowork withsmallersamples.Prioritieshavechangedovertheyearsasdifferentapplicationshavevariedtheemphasisof theseuniquecapabilitiesofminiaturizedsystems.Miniaturizationisnotwithoutproblemshowever,andthechief disadvantagesofcapillarycolumnsarethattheyaremoredemandingofinstrumentperformance,lessforgivingof pooroperatortechnique,andpossessalowersamplecapacitythanpackedcolumns.
In gaschromatography,columnscanbeclassified [45] asfollows:
Conventionalpackedcolumnswereoriginallyconstructedofstainlesssteelorothermetalsuchascopperofabout 1–2mlong,2–4mmi.d.,andformedintoacoil.Astheperformanceofdetectorsimproved,lesssamplewasappliedto thesystemandthesemetalcolumnsweretooreactiveforthesmallerquantitiesofanalytes.Forexample,withasample injectioncorrespondingto1mganalyte,decompositioncatalysedbythemetaltubingof1–10 μgoftheanalytewas relativelyinsignificant.However,withanimproveddetectorandinjectionequivalentto1–10 μgofanalyte,catalytic decompositionofthissameamountofanalyterepresentedaverysignificantloss.Thuscolumnsconstructedofglass whichwasmoreinertwereintroducedbutwithsimilardimensionstothepreviouslyusedmetalcolumns.
Miniaturizationofthecolumns(diameter)proceededintwodirections.Micropackedorpackedcapillarycolumns [46,47],characterizedbysmallinternaldiameters,usuallylessthan1.0mm,areminiaturizedversionsofconventional packedcolumns.Theyaretypicallyconstructedofstainlesssteelandare1–2mlong.Theirusehasbeenlimitedby practicalproblems,particularlywithinjectionathighbackpressuresbuttheyarecommerciallyavailableandareusefulforseparationofgasmixturesincludingsulphurcompoundsorlighthydrocarbons.Incontrast,thedevelopmentof opentubularcolumns [48] withaninternaldiameterlessthan1.0mmhasbeenimmenselysuccessful.Opentubular columnsarealsoreferredtoascapillarycolumns.However,thecharacteristicfeatureofthesecolumnsistheiropenness,whichprovidesanunrestrictedgaspaththroughthecolumn.Hence,opentubularcolumnisamoreaptdescriptionalthoughbothtermswillundoubtedlycontinuetobeusedandcanbeconsideredinterchangeable(butwitha cautiontocheckthecontext).Thecolumnswereoriginallyconstructedofglassbutthecommercialavailabilityin the1980soffusedsilicaopentubularcolumnscoatedontheoutsidewithaprotectivepolyimidelayerrevolutionized theindustry.Theyrapidlybecamewidelyacceptedandusedasthestandardagainstwhichothercolumnswere judged.Highlyinertmetalopentubularcolumnswereamorerecentaddition.
Ifthestationaryphaseisapplieddirectlyasathinlayertotheinternalwalloftheopentubularcolumn,thenitis knownasawallcoatedopentubular(WCOT)column.Whilenon-bondedphasesaresimplycoatedontheinternal wallofthecolumn,thestationaryphasecanalsobechemicallybondedtothewall.Inmostcases,thepolymerchainsof thestationaryphasearealsocross-linked.Generally,abondedandcross-linkedphaseispreferablebecauseitcanbe usedtohighertemperaturesandhaslesscolumnbleed(Chapter4)duringuse.Moreover,thesephasescanberinsed
withsolventstoremoveanyaccumulatednon-volatilematerials.Lowpolaritystationaryphasesaretypicallybonded andcross-linkedbut,insomeinstances,suchashighlypolarphases,optionsaremorerestricted [49].
ThesamplecapacityofWCOTcolumnscanbeadjustedbyvaryingthecolumndiameterandthefilmthicknessof thestationaryphase.AlternativestotheWCOTcolumnaretheporouslayeropentubular(PLOT)columnandsurface coatedopentubular(SCOT)column.TheinnerwallofthecolumnisextendedinPLOTcolumnsbyadditionofa porouslayersuchasfusedsilica.InSCOTcolumns,thestationaryphaseisappliedtoasolidsupportwhichiscoated ontheinternalwallofthecolumn.SCOTcolumnswerepopularbecauseofhighersamplecapacityalthoughwidebore ormegabore(0.53–1.00mmi.d.)WCOTcolumnsarecompetitiveinthisrespectandareeasiertouseandmorestable. SCOTcolumntechnologyallowsaccesstomanystationaryphasesthatarenotcompatiblewithconventionalWCOT columnmanufacturingtechnology.PLOTcolumnsareidealforseparatingcompoundsthataregasesatroomtemperatures,lowmolecularmasshydrocarbonisomers,andreactiveanalytessuchashydrides,amines,andsulphur gases.ThevariouscolumntypeshavebeencomparedbyDuffy [50]
Itisdifficulttoestablishaccuratelytheproportionofroutineseparationsthatuseopentubularversuspackedcolumns.Thevastmajorityofpublishedpapers(probablywellinexcessof90%)involvetheformer.However,papers representresearchactivityratherthanroutineapplications.Ontheotherhand,therelativeamountofspacedevotedto thedifferentcolumntypesincolumnmanufacturers ’ literatureandthevolumeofsalesprobablygiveagoodindicationoftherelativeimportanceacrossalluses.Onthisbasis,wecanstatethatseparationsonopentubularcolumns dominateGCexceptinaveryfewspecialtyareas.
In liquidchromatography,conventionalpackedcolumnsforclassicalcolumnchromatographycomprisedacylindricalglasstubeabout200–800mmlongand20–60mmwidealthoughlargercolumnshavealsobeenusedinthelaboratory(Fig.1.10).Inalmostallinstances,suchcolumnsweredesignedforasingleuse.
ThedesignofacolumnforHPLCisverysimpleintheory.Allthatisneededisaninerttubethatwillretainthe stationaryphaseandallowmovementofthemobilephase.However,thepracticalrequirementsareverydemanding asnotedbyMajors [51,52]: ‘Thetubeitselfmustbeabletowithstandthepressuregeneratedbythepackedbedand shouldnotexpandorchangeitsdimensionsifthepressureortemperatureishighorcontractifthetubeiscooled.Next,
FIG.1.10 PhotographofachemistemployedbytheUSFoodand DrugAdministrationinthemid-1950susingacolumnchromatographicapparatustoseparatetheconstituentsinacoaltarcolouranalysis. Credit: https://en.wikipedia.org/wiki/Column_chromatography#/ media/File:FDA_History_-_Column_Chromatography.jpg
oneneedsadevicetocontainthepackingandnotpermitanyminuteparticles(fromadistributionofparticles)toexit thepackedcolumn.Evenwiththisrestriction,thisdevicemustbeabletoprovideadequateflowandbeinert’.IncolumnsusedathighpressuresforHPLCasinteredmetalfritisusedforthispurposeandameansofsealingthecolumn isrequiredsothatitholdsthispressurewithoutleakingfluid. ‘Sealingisaccomplishedbycompressionendfittingsor specialmodifiedendfittingsthatcanconsistentlywithstandthesehighpressures.Thesefittingsmustallowflowto enterandexitthecolumnatthesehighpressureswithoutleaking.Thefittingsalsoshouldprovidenarrow,well-swept flowchannelssothatflowingsolventcanpassthroughthemathighandlowlinearvelocitieswithoutthecreationof flowdisturbances,floweddys,orunevenflow.Formodernhighefficiencycolumns,theflowdesignshouldallowthe injectedsampletopassthroughthesechannelsinanarrowplugwithoutspreadingthisband.Othercolumndesign issuesthatareimportantarethesmoothnessoftheinsidewall,whichisincontactwiththepacking,thechemical resistance,andinertnessofthecolumncomponentsincontactwiththemobilephaseandthesample,thecostof thematerialsusedtoconstructthehardware,andthesturdinessoftheassembledcolumnhardware’
ColumntechnologyinHPLC [51–54] advancedrapidlyinthelate1960sandearly1970s.Between1975and2000,the conventionalcolumnforHPLCwasastainlesssteeltubeof4.6mmi.d.packedwithsphericalparticlesofbetween 10and5 μmindiameter.Overthisperiodcolumnlengthgraduallydecreasedfrom300to150mm.Interestingly, theoriginalcolumnswereeither1.0mmor2.1mmi.d.Theestablishmentofthe4.6mmi.d.columnasstandard resultedfrompurelypracticalandpragmaticconsiderationsratherthanarigoroustheoreticalstudy.Stainlesssteel tubeof4.6mmwasreadilyavailableatareasonableprice,wascompatiblewithcompressionfittingsborrowedfrom packedcolumngaschromatographs,andwasofsufficientwallthicknesstowithstandoperatingpressures.Thusit wasadoptedasthestandardmaterialforcolumnconstruction.Morerecently,glass-linedorTeflon-linedstainlesssteel hasbeenintroducedforcolumnconstructiontoenhanceinertnessandreducesurfaceinteractions.Manymanufacturerssupplycolumnsforbiochromatographywithpolyetheretherketone(PEEK)construction.
ThenextmajordevelopmentwasthecommercializationofmonolithicsilicacolumnsbyMerckin1999.Thesenew columnsweremadeofasingleblockofsilica,withabimodalporesizedistribution,encapsulatedinaPEEKtubewith dimensionsof100mm 4.6mm.Thesecolumnsofferedfasteranalysesthantheconventionalpackedcolumnsat equivalentresolutionandlookedaverypromisingalternativeintheearly2000s.Polymericmonolithcolumnswere alsodevelopedconsistingofacontinuouscross-linked,porousmonolithicpolymerusuallypolymethacrylatesor methacrylatecopolymerizates.Interestinmonolithiccolumnsfadedandtheyseemedtobeontheirwaytooblivion [55] buttheyexperiencedarenaissance [56] andareenjoyingrenewedpopularity.
Manufacturersofconventionalpackingsrespondedtothepotentialthreatofmonolithiccolumnsbycommercializingcolumnspackedwithfullyporousparticlesofdecreasingsizes,5,then3.5,2.5,andeventually1.7and1.5 μm.At thesametime,columnlengthsgraduallydecreasedfrom150to50mm.Thesedevelopmentswitnessedthebirthof veryhigh-pressureliquidchromatographyin2004involvingnarrowbore(2.1mmi.d.)short(50mmlong)columns packedwithsub-2 μmparticles [57].Thesecolumnsofferedsuperiorperformancetothemonolithiccolumnsduetothe radialheterogeneityofthesilicarodsinthelatterandthisprobablyexplainedthesuddendeclineininterestinthe monolithiccolumns.
Asecondandveryunexpecteddevelopmentincolumntechnologyoccurredafewyearslaterwithare-visittopellicularparticles.Theoriginalmaterials,alsoreferredtoassuperficiallyporouspackingsorporouslayeredbeads,were of40–50 μmdiameterandexhibitedgoodefficiency(ameasureofseparation)relativetothelarge(100 μm)porous particlestheninvoguebuttheyhadpoorsamplecapacity.Thustheywererejectedintheearly1970sinfavourof packingsbasedonsmallerporousparticles.Thepellicularpackingsthathaveemergedinthelastfewyearshavemuch smallerparticlesizesthantheir1960sforerunnersandpotentiallycandelivercomparableseparationstotheveryfine fullyporousparticles.
Therearethreetypesofmicrocolumnforliquidchromatographyalthoughtheiruseisnotwidespread.Microbore columnsaresimilarinconstructiontoconventionalpackedcolumnsexceptthatthecolumndiameterisreducedto 1mm.Packedcapillarieshaveacolumndiameterof70 μmorlessandarelooselypackedwithparticleshavingdiametersoffrom5to30micrometres.Nanooropenmicrotubularcolumnsaretheequivalentofthecapillaryoropen tubularcolumninGC.Ideally,theyhavediametersof10–30micrometresandcontainastationaryphaseoranadsorbenteithercoatedon,orchemicallybondedto,thecolumnwall [58] Table1.2 presentsanomenclatureappliedtoLC columnsbyMajors [51].Nanocolumnsaremostcommonlyconstructedfromfusedsilicawhilestainlesssteel,lined stainlesssteel,andPEEK-cladfusedsilicatubinghavebeenusedtomakecapillaryLCcolumns.
Nanocolumnscanbereplacedpotentiallybychip-basedLCsystems [52] inwhichthechromatographycolumnis fabricatedonglassorplasticchips.Thenarrowchannelscanbeetchedorlaserablatedwithdimensionsinthe50 μm diameterrangebylengthsaslongastensofcentimetres.Althoughtheflowofmobilephasecanbeviaconventional
TABLE1.2 ClassificationandcolumndimensionsforHPLC.
ColumnInternaldiameter(mm)Length(mm)Particlesize(
Nano0.1,0.07550,1503.55,5.0
Capillary0.3,0.535–2503.55,5.0
Microbore1.030–1503.55,5.0
Narrowbore2.115–
Solventsaver3.0150,250sub-2,3.0
Analytical4.615
Semi-preparative9.450–2505.0
Preparative21.2,30.0,50.050–2505.0,7.0,10.0
hydraulicmeansusingpumpingsystemsandnano-valvesintegratedontothechip,electroosmoticflowisalso possible.
Althoughthesituationislessclear-cutthaninGC,conventionalpackedcolumnsstilldominateroutineseparations byHPLC.Anumberofpublications [53,55,59–64] coverthedevelopmentofcolumntechnologyinLC.Conventional analyticalcolumnsintheyear2020fallintoparticle-packedcolumnsandmonolithiccolumnsofbetween1mmand 4.6mminternaldiameter.Theformercanbesubdividedintofullyporous,core-shell,andnon-porousparticleswhich arefurtherdiscriminatedonthebasisoftheirchemicalcompositionintoeithersilica-basedorpolymer-based (cross-linkedorganicpolymers).
Thedevelopmentof supercriticalfluidchromatography occurredinthedecadefrom1980atatimewhencolumn technologyinbothGCandHPLChadbeenwelldeveloped.CommercialcolumnmanufacturerstendedtoignoreSFC untilthemarketgrewlargeenoughtojustifyaninvestment [65].ThustherewerefewSFC-specificcolumnsandcolumntechnologyforSFCwaslargelyborrowedfromHPLCforthepackedcolumnformatorfromGCfortheopen tubularformat.Intheearly1980s,foraverybriefperiod,theusualcolumnwasastandardnormal-phasecolumn borrowedfromHPLC,typically250 4.6mmwith5 μmtotallyporoussilicapackings.Capillaryoropentubular SFCwasoriginallyreportedbyMiltonLeeandothers [44,66] in1981anditwascommercializedby1986.Itwasin thisperiodthatSFCresearchwasdominatedbygaschromatographerswhotransferredtheirknowledgeofopentubularcolumnstotheirnewinterestandopentubularcolumnSFCrapidlybecamethestandardapproachalthoughthere remainedasignificantcoreemployingpackedcolumnSFC [67,68].Generally,thediameterofopentubularcolumns forSFCwassmallerthan100 μmtomaintainbothreasonableanalysistimesandhighresolution [69].By2000,itwas acknowledged [70] thatcapillarySFChadbeenoversoldespeciallyfortheelutionofpolarsolutes.Aboutthissame timeSFC-specificcolumnsofbothpackedandopentubularvarietiesbecameprogressivelyavailablefrommanufacturers.Nevertheless,SFCalthoughconsideredamaturetechniquebyage,inotherways,itisstillinitsinfancyand doesnotreceivethesameattentionbycolumnmanufacturersasdoesHPLCorGC [71].
1.4.4Separationmechanism
Molecularinteractionsplayafundamentalroleinthebehaviourofthechemicalandphysicalpropertiesofany physicochemicalsystemincludingchromatography.Thenatureofthisinteractionbetweensamplecomponents andthetwophasesformsafurtherbasisforclassificationofchromatography.Sincethesemolecularinteractions (Table1.3)determinetheretentionbehaviourofthesolute,thisisthemostfundamentalofallclassificationsofchromatography.However,inmanywaysitisthemostdifficultsince,inanumberofinstances,itisnotclearexactlywhat mechanismisinvolved.Nevertheless,aknowledgeofthemechanismiscrucialtoourunderstandingofthechromatographicprocess,toenablepredictionsabouttheexpectedbehaviourofasystemandinchoosingastationaryphase/ mobilephasecombinationtoobtainadesiredseparation.Inmostinstancesitispossibletospecifythepredominant mechanismoperatinginaparticularsituationeventhoughthenominatedmechanismisrarely,ifever,thesole mechanism.
Themechanismscanbeclassified b intoanumberoftypesasfollows:
b Thetermssorptionandsorbareusedinthistexttodenoteasolute–stationaryphaseinteractionofunspecifiednature.Itisusedintwo circumstances;wherethenatureoftheinteractionisunknownorasagenerictermtocoverseveraltypesofinteraction.
TABLE1.3 Intermolecularinteractionsinvolvedindifferentliquidchromatographicmethods.
Intermolecularinteractions(interactionenergykJmol 1)
Mechanism
Vander Waals(0.4–4)Repulsion
dispersion (2–4)
(4)
(4–17)
Important; ♦Mostimportant; ♣ Importancevariabledependentoncolumnpacking.
• adsorption
• partition(includingcountercurrentchromatography)
• bondedphase
• hydrophilicinteractionliquidchromatography(HILIC)
• ionexchange
• ioninteraction
• sizeexclusion
• affinity
• micellar
• complexation
• ionexclusion
• chiral
Separationsexploitingeachofthesemechanismshavebeendevelopedinliquidchromatographywhereasgas chromatographyisrestrictedtoseparationsinvolvingoneormoreofthefirstthreenamedmechanismspluschiral separations.TheflexibilityofSFCintermsofthemechanismsexploitedapproachesthatofliquidchromatography.
Chromatographicretentionisaverycomplexprocessinvolvingvariousspecificandnon-specificphysicochemical interactions,reflectingtherelativeattractionandrepulsionthattheparticlesofthecompetingphasesshowforthe soluteandforthemselvesthroughmultiplemolecularinteractions.Thethreebasictypesofmolecularinteraction orforce,allofwhichareelectricalinnature,aredispersionforces(duetochargefluctuationsthroughoutamolecule resultingfromrandomelectron/nucleivibrations),polarforces(arisefromelectricalforcesbetweenlocalizedcharges suchaspermanentorinduceddipoles),andionicforces.Manydifferenttermsareusedtodescribethemolecularinteractions(e.g.hydrogenbonding, π-π interactions,coulombicorelectrostaticinteractions,hydrophobicforceswhich refertodispersiveinteractions,etc.)butallareofoneofthethreebasictypes.Thedifferentmolecularinteractionsthat determinesoluteretentioncomprise:
• ArepulsivecomponentresultingfromthePauliexclusionprinciplethatpreventsthecollapseofmolecules.
• Attractiveorrepulsiveelectrostaticorcoulombicorientationforcesbetweenpermanentcharges(inthecaseof molecularions),dipoles(inthecaseofmoleculeswithoutinversioncentre),andquadrupoles(sometimescalledthe Keesominteraction).Hydrogenbondingisanexampleofanextremedipole-dipoleinteraction.
• Attractiveinductionorpolarizationforcesbetweenapermanentdipoleononemoleculewithaninduceddipoleon another(sometimescalledDebyeforce).
• Attractive(London)dispersionforcesbetweenanypairofmolecules,includingnon-polaratoms,arisingfromthe interactionsofinstantaneousdipolescausedbyrandomdistortionoftheelectroniccloudofamolecule,causinga slightelectrostaticpolarization.Thisspontaneouspolarizationtheninducesanoppositepolarizationin neighbouringmolecules.
Theterm ‘vanderWaalsforces’ issometimesusedasacollectivetermforthetotalityofforces(includingrepulsion) but,moreoften,itisrestrictedtotheattractiveforces(Keesom,Debye,anddispersionforces).
Thecomplexinteractionbetweensolute,stationaryphase,andmobilephaseduetothemolecularinteractionsleads todistributionofthesolutebetweenthetwophases.Chromatographicbehaviourisalsoimpactedbyotherfactors suchassterichindranceofsubstituentgroupswithinthesolutemolecule.ThemolecularforcesinvolvedintheseinteractionsareusuallyweakervanderWaalsforcesorhydrogenbondingbut,insomeinstances,strongerionicinteractionsasinion-exchangechromatographyareexploited [72] and,inrarecases,specificinteractionssuchascharge transferforces [73,74]
Bywayofillustration,dispersionforcesareobservedbetweenallmolecules,buttheyaretheonlyforcesexerted betweennon-polarmoleculessuchashydrocarbons.Thustheseparationofn-alkanesonasqualane(abranchedparaffin)stationaryphasebyGCinvolvesdispersionforcesalone.Forthesenon-polarspecies,polarizabilityofthesolute moleculesincreasesasthemolecularsizeincreasesandthedispersionforcesalsoincreaseallowingustopredictthat thechromatographicretentionwillincreaseasthecarbonnumberofthen-alkaneincreases.Asafurtherexample, aromatichydrocarbonsarepolarizableduetothe π-electronsandexhibitinduceddipoleinteractionsaswellasdispersiveinteractions.RetentionandseparationofthesecompoundscanbeachievedbyGConasqualanestationary phaseexploitingdispersiveforcesalone.Alternatively,theycanberetainedandseparatedbyexploitinginduced dipoleanddispersiveinteractionsinHPLConsilicagelasastationaryphaseandadispersivemobilephasesuch asn-hexane.
Inthischaptersufficientdetailisgiventoprovideanunderstandingofthegeneralprinciplesinvolvedineachmechanism.Thisissupplementedintherelevantsectionsongaschromatography,liquidchromatography [75–77] (planar andcolumn),andsupercriticalfluidchromatography.Itishopedthatreaderswillexaminethisandsubsequent materialcloselyfor,asPoole [71] hasnoted,thereisadeclineinunderstandingofseparationmechanismsbythose whoperformthemajorityofroutinemethodsandmethoddevelopment.Theoutlookforthefuturedevelopment ofchromatographywillbebleakifthissituationisallowedtopersist.
1.4.4.1Adsorptionchromatography
AdsorptionwasexploitedbyTswettintheformofliquid-solidchromatographyincolumnsandthusrepresentsthe oldestofthechromatographictechniques.Inseparationsinvolvingadsorption [78–81],soluteandmobilephasemoleculescompeteforactivesitesonthesurfaceofthesolidstationaryphasewhichiscalledtheadsorbent.Adsorption ontothesurfaceoftheadsorbentisdistinguishedfrompartitionprocessesinwhichthesolutealsodiffusesintothe interiorofthestationaryphase.Amoreappropriatetermforthelatterwouldprobablybeabsorptioninwhichcasethe generalprocesscouldbetermedsorption.Nevertheless,theterminologyusedinthismonographconformstousual practiceandreferstoadsorptionandpartition.
Separationinadsorptionchromatographyisduetointeractionofpolarfunctionalgroupsonthesolutewithdiscrete adsorptionsitesontheadsorbentsurface.Theselectivityoftheseparationisdependentontherelativestrengthofthese polarinteractions.Theextenttowhichasolutecanbeaccommodatedonanadsorbentsurfacedependsonitsspatial configurationanditsabilitytohydrogenbondwiththeadsorbentsurface.Adsorptionprocessesarethereforesensitive tospatialdifferencesinsolutesandareideallysuitedtoseparationsofmoleculeshavingslightdifferencesinshape(i.e. geometricisomers).Adsorbentsalsodemonstrateauniqueabilitytodifferentiatesolutespossessingdifferentnumbers ofelectronegativeatomssuchasoxygenornitrogen,orformoleculeswithdifferentfunctionalgroups.Thisleadstothe useofadsorptionforclassseparations.Ontheotherhand,partitionprocessesdependonacompetitivesolubility betweentwoliquidphasesandarequitesensitivetosmalldifferencesinmolecularmass.Forthisreason,members ofanhomologousseriesaregenerallybestseparatedbyapartitionsystem.Moreover,partitionisusuallymoresuitablethanadsorptionforhighlypolarsubstancessuchasaminoacidsandcarbohydrates.Inthiscase,theneedtouse highlypolarmobilephasesinadsorptionwouldnegateanysmalldifferencesbetweenadsorptivepropertiesofthe solutesandproducenoseparation.
Adsorptionstillfindsuseinliquidcolumnchromatography(bothclassicalandhighperformance)andiswidely exploitedinthinlayerchromatography,whereasapplicationsofadsorptioninGCarelimitedmainlytoseparations wheretheanalytesarepermanentgases.Becausethestationaryphaseinsuchseparationsisasolid,thesystemsare referredtoasliquid-solidchromatographyandgassolidchromatography(GSC).ThepracticalapplicationofGSC (basedonadsorption)antedatedthenowmorepopularformofGLC(basedonpartition).Oneofthefirstimportant accountsofGSCwaspublishedin1946byClaesson [82] whouseddisplacementdevelopmentfortheseparationof hydrocarbonsoncolumnspackedwithactivatedcarbon.Phillipsandco-workersusedthesamemethod [83] butthis approachwasabandonedafter1952infavourofpartitionsystemsasdevelopedbyMartinandJames [84].Nevertheless,forcertainseparations,namelythatofpermanentgases,GSChascomebackintofavour.Typicaladsorbentsfor GSCarezeolites(aluminiumsilicates),Porapaks(cross-linkedpolystyrene),andmolecularsievesinadditiontothe morecommonadsorbentssuchassilicagel,charcoal,andaluminaencounteredinliquidchromatography.
Theparticlesizeisanimportantcharacteristicofanadsorbent.Toafirstapproximationsampleretentionisproportionaltosurfaceareawhich,inturn,dependsontheparticlesizeandontheinternalstructureoftheadsorbent particles.Thesmallertheaverageparticlesize,thegreaterthesurfaceareaoftheadsorbentandhencethenumber ofactivesitesavailableforadsorption.Mostadsorbentsareavailableinarangeofparticlesizestosuittheneeds ofthevariouschromatographictechniques.Forthinlayerchromatography,particlesizesof20to40 μmhavebeen mostcommon,whereasforclassicalliquidchromatographyincolumnstheparticlesarelarger(100to30 μm).For themoderncounterpart(i.e.HPLC)ofthisparticulartechniquetheyaresmaller(10,5or3 μm)andforhighperformancethinlayerchromatography,particlesizesof5 μmareused.AdsorbentsforGCaretypicallyinthesizerangeof 125–150 μmupto177–250 μm.
1.4.4.2Partitionchromatography
PartitionchromatographyoriginatedwiththeNobelPrizewinningworkofMartinandSyngein1941andhasasits basisthepartitioningofasolutebetweentwoimmiscibleliquids,asinsolventextraction,exceptthatoneoftheliquids isheldstationaryonasolidsupportsuchassilicagel,diatomaceousearth,cellulose,polytetrafluoroethylene(PTFE),or polystyrene.Thesolidsupportis,inprinciple,inertandsolelyprovidesalargesurfaceareaonwhichthestationary phaseisretained.Partitionchromatographyexploitsthefactthatasoluteincontactwithtwoimmiscibleliquids(or phases)willdistributeitselfbetweenthemaccordingtoitsdistributionconstant, K (Eq. 1.1).Theprincipal
intermolecularforcesinvolvedaredispersion,induction,orientation,anddonor-acceptorinteractionsincluding hydrogenbonding.Theseforcesprovidetheframeworkforaqualitativeunderstandingoftheseparationprocess.
Theimportanceofpartitionsystemshasdeclinedinallareasofchromatographywiththedevelopmentofbonded phases.Nevertheless,inGCwithpackedcolumns,partitionsystemsarestillusedonrareoccasions.Ontheotherhand, useofpartitionsystemsinliquidandsupercriticalfluidchromatographyisrestrictedbyinstabilityofcoatedliquid stationaryphases.Thisiscausedbythesmallbutfinitesolubilityoftheliquidstationaryphaseinsolventsusedas mobilephasesleadingtostrippingofthestationaryphasefromthecolumn.Oneapplicationareainliquidchromatographywheretheseparationmechanismispredominantlypartitionispaperchromatography.Here,thewater sorbedoncellulosefunctionsasthestationaryphase.
Countercurrentchromatography [85–87] issimilartoconventionalliquid-liquidpartitionchromatographywiththe distinctionthatthestationaryliquidphaseisretainedintheapparatuswithoutuseofanadsorptiveorporoussupport. Inonevariant,calleddropletcountercurrentchromatography,thestationaryphaseisretainedbygravitationalforcein anarrowverticaltube(ca.2mmi.d.)whiledropletsofanimmisciblemobilephasearepassedthroughit.Themobile phaseiseitheraddedatthetoporbaseofthetubedependingonwhetherithasahigherorlowerdensitythanthe stationaryphase.Typically,300tubesareconnectedinseriesusingcapillary-borepolytetrafluoroethylene(PTFE)tubingtoprovideacolumnofhighefficiency.Withthemorecommonsystems,thestationaryphaseisretainedinmoreor lesssegmentedcompartmentswithinacoiledtubingwhilethemobilephaseispassedthroughit.Coils(2–3mmi.d.) areusuallymadeofPTFEandrangeinlengthfromafewmetrestomorethan100m.Incontrasttoconventionalliquidliquidchromatographywherethevolumeofstationaryphaseinthecolumnisrelativelysmall,thestationaryphasein countercurrentchromatographyoccupiesfrom40%to90%ofthetotalcolumnvolume.Oneoftheproblemsofcountercurrentchromatographyistherelativelylonganalysistimes.Forexample,arelativelysimpleseparationof10compoundsmayrequireanythingfromafewtoseveralhoursusingcentrifugallyoperatedunitsupto1–3daysforunits operatedinaunitgravitationalfield.Themainroleforcountercurrentchromatographyisforpreparativescaleseparationsinthemilligramtogramrange.Newerdevicesalsofunctionasefficientextractorstoconcentratetracecomponentsfromenvironmentalsamples,suchasriverwaterandbiologicalfluids,suchasurinebyreplacingthe relativelylongseparationcolumnwithashortcolumn.
1.4.4.3Bondedphasechromatography
Bondedphases,inwhichthestationaryphaseiscross-linkedandbondedtotheinternalwallofthecolumnorchemicallybondedtoasolidsupport,arepopularinallformsofchromatography.Thegroup(e.g.anoctadecylhydrocarbon)thatischemicallybondedtothesupportisreferredtoasaligand.Thepopularityofbondedphasesistestimonyto theirmanyadvantages.TheywereoriginallydevelopedforopentubularcolumnsinGCinanattempttostabilizethe stationaryphaseatelevatedtemperatures.Bondedphasesarealsoavailableinliquidandsupercriticalfluidchromatographywheretheyovercometheproblemsofcolumnbleedassociatedwithphysicallybondedphasesinwhichthe stationaryphaseissimplycoatedonasupportmaterial.Comparedtoadsorptionsystemsinliquidchromatography, theyequilibratefaster,donotexhibitirreversiblesorption,andareavailablewithawiderangeoffunctionalities.
GCisuniqueinthatthemobilephasetransportssolutesthroughthechromatographicbedbutitisotherwisenoninteractive.ThissimplifiestheretentionmechanisminGCasmolecularinteractionsareessentiallylimitedtothesolute andstationaryphase.Themechanismwastreatedasapartitionthatcanbeapproximatedbyaphysicalprocessof solutevaporizationandsolutioninthemobileandstationaryphase.Thusretentionisdeterminedmostlybysolute vapourpressureandvolatility.Indeed,partitionisthedominantprocessformanysolutes;however,amixedmechanismofadsorptionandpartitionisnowregardedasamoreaccuratedescriptionalthoughtheextentofcross-linking ofbondedphasesisanimportantfactorforGC(Chapter4).
InLCandSFC,chemicallybondedphasesarenowcommoninallareasincludingionexchange,hydrophilicinteractionliquidchromatography(HILIC),etc.butthemechanismintheseprocessesissufficientlydistinctthattheretentionmechanismisnotregardedasbondedphasechromatography.Thebondedphasemechanismisrestrictedto normal-phase(liquid)chromatography(NPLC)andreversed-phase(liquid)chromatography(RPLC).Withnon-polar ligandssuchasanoctadecylhydrocarbon,thestationaryphaseisalwayslesspolarthantheassociatedmobilephase andthisconstitutesRPLC.Withmorepolarligands,thebondedphasescanbeusedwitheithermoreorlesspolar mobilephases,andincasesinvolvinglesspolarmobilephases,thenitconstitutesNPLC.
Themechanismofbondedphasechromatographyiscomplex [76] butappearstoinvolveacombinationofpartition andadsorption.Inanumberofinstances,bondedphasechromatographyhasbeenreferredtoaspartitionchromatographybecausetheorganicsurfacelayerisregardedasa ‘boundliquidfilm’.However,Locke [88] concluded,asearlyas 1974,thatbondedphasesactedmorelikemodifiedsolidsthanthinliquidfilms.Nevertheless,themechanisminvolved withbondedphases [75] issufficientlydifferentfrombothadsorptionandpartitiontowarrantseparatetreatment.
Innormal-phasesystems,thedominantinteractionsbetweenthesoluteandthestationaryphasethatcauseretentionandselectivityarepolarinnaturewhiledispersiveinteractionsdominateinthenon-polarmobilephase.Retention innormal-phasebondedLCwasoriginallyregardedassimilartothatinadsorptionchromatographyinvolvingcompetitiveadsorptionbetweensoluteandmobilephasemoleculesforactivesitesonthestationaryphasesurface.However,subtlenuancesinretentionbehaviourrequiredmodificationstothissimplisticretentionmechanism.
Inreversed-phaseLC,themostextensivelyusedofallformsofchromatography,theretentionmechanismhasbeen studiedoverseveraldecades [89] basedonclassicalandstatisticalthermodynamicstodescribethefundamentalprinciplesgoverningseparationbutaunifiedviewhasnotbeenachieved.Thiscanbeattributedtothecomplexityofthe retentionprocessandtheinterplayofmyriadmolecularinteractionsbetweentheanalyte,mobilephase,andstationary phase.Thesolvophobictheorywasoneofthefirstattemptstodescribechromatographicretentionusingclassicalthermodynamics.Thepartitionmodelwasdevelopedasananswertothecriticismsofthesolvophobictheorywiththe adsorptionmodelpresentingasanalternativeapproach.Anadsorption-partitionmodelbasedonsolutiontheory andsolubilityparametermodels,forexample,hasalsobeenpresented(seeRef. [90]).Eachoftheseandthemanyother models,withafocusonthestationaryphaseand/ormobilephase,representsadifferentviewoftheretentionmechanisminreversed-phasechromatography.Theygenerallyinvokeeitherthepartitionoradsorptionprocesswhichhas causeddebateovermanyyearsabouttherelativeroleofsolutepartitionbetweenthephasesandadsorptionofthe solutetothestationaryphase [91].Thesetworetentionprocessesrepresenttheextremesofaspectrumofpossible retentionmodels.Inthepartitionmechanism,thesolutetransfersfromthebulkmobilephasetothestationaryphase whereitisfullyembedded.Intheadsorptionmechanism,thesolutetransfersfromthebulkmobilephasetotheinterfacebetweenthestationaryphaseandmobilephasebutisnotfullyembeddedinthestationaryphase [91].Thesolvophobictheorywasthefirstrigorousattempttoexplainreversed-phaseretentionandattributedtheretentionprocess tothemobilephase,ignoringcontributionsofthebondedstationaryphase [75].Thetheoryidentifiedthemajorcontributiontotheretentionprocessasthehydrophobicbindinginteractionbetweenthesolutemoleculeinthemobile phaseandthestationaryphase.However,theactualnatureofthehydrophobicinteractionremainsamatterofheated debate.Indeed,itismoreappropriatelytermedasolvophobicinteractioninreversed-phasesystemsasthemobile phaseisrarelywaterbutahydroorganicmixtureorapolarorganicsolvent.Itisnowclearthatthestationaryphase alsoinfluencesretentionandthatthepropertiesofthestationaryphaseareinfluencedbythecompositionofthemobile phase.
1.4.4.4Hydrophilicinteractionliquidchromatography(HILIC)
HILICemploystraditionalpolarstationaryphasessuchassilicaandaminoorcyanobondedphasesbutwith mobilephasesmorecloselyassociatedwithreversed-phasesystems.Itprovidesanalternativeapproachtoseparate smallpolarcompounds.Theseparationmechanismisbasedonbothspecificandnon-specificinteractionsandhas beenmodelledaspartition,adsorption,ionexchange,andsizeexclusion [92].Ithasbeenreportedasavariantof normal-phaseLC(NPLC)buttheseparationmechanisminvolvedinHILICismorecomplicatedthanthatinNPLC [93].ThemechanismofHILICisnotcompletelyelucidated [94] butisthoughttoinvolvevariouscombinationsof hydrophilicinteractions,ionexchange,andreversed-phaseretentionbythesiloxaneonthesilicasurfaceofthestationaryphase,whichcontributetovariousdegreesdependingontheparticularconditionsemployed [93,95,96].
1.4.4.5Ion-exchangechromatography
Ionexchangeentailsareversible,stoichiometricexchangebetweensampleionsinthemobilephaseandionsoflike chargeassociatedwiththeion-exchangesurface.Thestationaryphaseisarigidmatrix,thesurfaceofwhichcarries fixedpositivelyornegativelychargedfunctionalgroups(A).Counter-ions(Y)ofoppositechargeareassociatedwith eachsiteinthematrixandthesecanexchangewithsimilarlychargedionsinthemobilephase.Ifthematrixcontains negativelychargedacidicfunctionalgroupsthenitiscapableofexchangingcationsandiscalledacationexchanger;if itbearspositivelychargedbasicgroupsitisananionexchangercapableofexchanginganions.Ifthesampleionsare depictedasM+ orX theprocesscanberepresentedasfollows:
Inordertoachieveaseparation,thematrixmustexhibitsomeaffinityforthesampleions.Thecounter-ionalreadyon theresinmustalsonotbetoostronglyheldthatitcannotbedisplacedbysampleions.Secondaryeffectscanarisedue toadsorptionorhydrophobicinteractionbythematrixitself.Duetotheionicnatureoftheinteractions,ionexchangeis restrictedtoaqueousliquidchromatography.
1.4.4.6Ion-interactionchromatography
Ionpairextractionisavaluableliquid-liquidseparationtechniqueforisolatingwater-solubleioniccompoundsby partitioningthembetweenwaterandanimmiscibleliquid.Theionicsolutespartitionformallyasionpairsaccording totheequilibrium:
inwhich Am+ representsthesoluteand Bn thepairingionorviceversa.ThisprinciplewasextendedbyEksborgand Schill [97] tochromatographyduringthe1970sandquicklygainedacceptanceasaversatiletechniquefortheseparationofionizedandweaklyionizedsolutes.Theearlysuccessespromotedgeneralinterestinion-interactionchromatographywhichusesconventionalhighefficiencymicroparticulate,normal-phaseorreversed-phasepackings. However,theforteofion-interactionchromatographyliesinitsabilitytosimultaneouslyseparateionicandmolecular species.Inreversed-phasechromatography,ionicspeciesgenerallyshowlittle,ifany,retentionandareelutedasan unresolvedmixture.Ion-interactionchromatographydoeshavesomedisadvantages.Theionicsolutionscanresultin shortcolumnlifeoraffectmetalcomponentsofthesystem.
ThetechniqueasdescribedbySchillandhisgroupbecameknownasextractionchromatographybutwasdubbed soapchromatographybyKnoxandco-workers [98] becauseoftheiruseofthedetergentcetyltrimethylammonium bromideasthepairingioninthemobilephase.Subsequenttermsusedtodescribetheprocedurehaveincluded ionpair,pairedion,andmobilephaseionchromatography(proposedbytheDionexCorporation),solventgenerated ionexchange,ionassociationchromatography,anddynamicionexchange,althoughtheauthorsprefertheuseofioninteractionchromatography.Itisunfortunatethatthediversityoftermsanddebateoverthemechanismofretention havecausedconsiderableconfusion.
1.4.4.7Size-exclusionchromatography
Separationsinsize-exclusionchromatographyarebasedonaphysicalsievingprocessandthusdifferfromallother mechanismsintherespectthatneitherspecificnornon-specificinteractionsbetweenanalytemoleculesandthestationaryphaseareinvolved.Infact,everyeffortismadetoeliminatesuchinteractionsbecausetheyimpaircolumn efficiency.Variousnameshavebeenusedtodescribethisformofchromatographyincludinggelpermeation,gelfiltration,andstericexclusion.Historically,gelfiltrationreferredtoseparationsofbiopolymers,suchasproteins,ondextranoragarosegelsusingaqueousmobilephases,whereasseparationsoforganicpolymersinorganicmobilephases onapolystyrenephaseweretermedgelpermeation.
Internalsurfacereversed-phasesupports,orPinkertoncolumns,becameavailableinthemid-1980s [99] andinvolve adualmechanism sizeexclusionandreversed-phasebondedsupport.Thesematerialscontainstationaryphaseon theinternalsurfaceoftheporesofthesupportwithanexternalsurfacewhichisnon-adsorptive.Thuslargebiomoleculesareelutedunretainedwhereassmallermoleculespenetratetheporesandareseparatedbyaconventional reversed-phasemechanism.
1.4.4.8Affinitychromatography
Affinitychromatographyisattheoppositeextremetosizeexclusioninthatveryspecificanalyte-stationaryphase interactionsareexploitedtoachieveseparation.Thestationaryphaseconsistsofabioactiveligandbondedtoasolid support(e.g.cross-linkedagaroseorpolyacrylamide).Sincethelattermaystericallyhindertheligand’saccessibility, theconceptofaspacerarmwasintroduced.Thisconsistsofashortalkylchaininsertedbetweentheligandandsolid supporttoreduceoreliminatethestericinfluenceofthematrix.Separationreliesonbiospecificinteractionssuchas antibody-antigeninteractions,chemicalinteractionssuchasthebindingofcis-diolgroupstoboronateorotherinteractions,whosenatureisnotfullyunderstood,suchastheattractionofalbumintoCibacronBlueF3G-Adye.Thespecificityoftheligandsetsthesebondedphasesapartfromallothers.Ligandsmayshowabsolutespecificityforasingle substanceormaybegroupspecific [100].Theinteractionbetweenligandandanalytemustbespecificbutreversible. Onaddingthesampleinasuitablemobilephase,the ‘active’ componentswithanaffinityfortheligandarebound andretainedwhiletheunboundmaterialiselutedinthemobilephase.ThecompositionorpHofthemobilephaseis thenalteredtoweakenthespecificinteractionofligandandactiveanalyte,whichisreleasedandeluted.