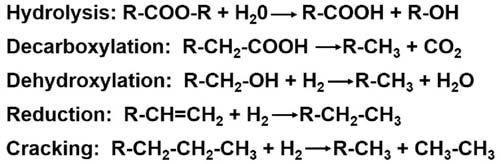https://ebookmass.com/product/practical-petroleumgeochemistry-for-exploration-and-production-2nd-edition-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Practical Petroleum Geochemistry for Exploration and Production 2nd Edition Harry Dembicki
https://ebookmass.com/product/practical-petroleum-geochemistry-forexploration-and-production-2nd-edition-harry-dembicki/
ebookmass.com
Practical Petroleum Geochemistry for Exploration and Production Dembicki
https://ebookmass.com/product/practical-petroleum-geochemistry-forexploration-and-production-dembicki/
ebookmass.com
Practical Petroleum Geochemistry for Exploration and Production 1st Edition Edition Harry Dembicki (Auth.)
https://ebookmass.com/product/practical-petroleum-geochemistry-forexploration-and-production-1st-edition-edition-harry-dembicki-auth/
ebookmass.com
Nanomaterials for Biocatalysis Guillermo R. Castro https://ebookmass.com/product/nanomaterials-for-biocatalysisguillermo-r-castro/
ebookmass.com
The End of Pax Britannica in the Persian Gulf, 1968-1971
1st ed. Edition Brandon Friedman
https://ebookmass.com/product/the-end-of-pax-britannica-in-thepersian-gulf-1968-1971-1st-ed-edition-brandon-friedman/
ebookmass.com
Physiologie humaine Bernard Lacour
https://ebookmass.com/product/physiologie-humaine-bernard-lacour/
ebookmass.com
¿Dónde estás, Emma Johnes? Vic Logan
https://ebookmass.com/product/donde-estas-emma-johnes-vic-logan/
ebookmass.com
Corporate Financial Accounting 16th Edition Carl S. Warren
https://ebookmass.com/product/corporate-financial-accounting-16thedition-carl-s-warren/
ebookmass.com
Total synthesis of bioactive natural products Brahmachari
https://ebookmass.com/product/total-synthesis-of-bioactive-naturalproducts-brahmachari/
ebookmass.com
Pucking Final: An MM Age Gap Hockey & Mafia Romance (Deadly Puck Daddies Book 7) Zack Wish
https://ebookmass.com/product/pucking-final-an-mm-age-gap-hockeymafia-romance-deadly-puck-daddies-book-7-zack-wish/
ebookmass.com
PRACTICAL PETROLEUM GEOCHEMISTRYFOR EXPLORATIONAND PRODUCTION Thispageintentionallyleftblank
PRACTICAL PETROLEUM GEOCHEMISTRYFOR EXPLORATIONAND PRODUCTION SECONDEDITION
HARRYDEMBICKI,JR. GeologicalTechnologyGroup,Anadarko,UnitedStates
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright © 2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions .
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
ISBN:978-0-323-95924-7
ForinformationonallElsevierpublicationsvisitourwebsite at https://www.elsevier.com/books-and-journals
Publisher: CandiceG.Janco
AcquisitionsEditor: AmyM.Shapiro
EditorialProjectManager: MariaElaineD.Desamero
ProductionProjectManager: StalinViswanathan
CoverDesigner: LimbertMatthew
TypesetbyTNQTechnologies
1.Introduction1
2.Theformationofpetroleumaccumulations21
Visualkerogentyping122
Thermalalterationindex124
Kerogen fluorescence126
Conodontalterationindex129
Wirelineloginterpretations130
Usingoutcropsamples137
Strategiesinsourcerockevaluation137
References 141
4.Interpretingcrudeoilandnaturalgasdata147
Introduction 147
Bulkpropertiesofcrudeoilandnaturalgas147
Phasebehavior 150
Crudeoilandnaturalgasalteration152
Oil-to-oilandoil-to-sourcerockcorrelations162
Crudeoilinversion177
Strategiesandobstaclesinoilcorrelationandoilinversionstudies189
Naturalgasdata 192
Thesourceofnaturalgas:biogenicversusthermogenic193
Thematurityofthermogenicnaturalgas198
Gas-to-gasandgas-to-sourcerockcorrelations200
Strategiesandobstaclesininterpretinggasdata205
References 206
5.Reservoirgeochemistry215
Introduction 215
Payzonedetection215
High-molecular-weightwaxes225
Asphaltenes 226
Reservoircontinuity229
Productionallocation234
Productionproblemsandperiodicsampling236
Monitoringenhancedoilrecovery237
Reservoirsouring238
Strategiesinreservoirgeochemistry240
References 241
6.Surfacegeochemistry245
Introduction 245
Microseepage 246
Directindicatorsofhydrocarbonmicroseepage247
Indirectindicatorsofhydrocarbonmicro-seepage250
Microseepagesurveydesignandinterpretation252 Onshoremacroseepage258 Offshoremacroseepage259
Locatingpotentialseafloorseepsites260
Samplingpotentialseafloorseepsites270
Analyzingseafloorsedimentsforthermogenichydrocarbons272
CHAPTER1 Introduction Introduction
Intherealmofpetroleumexplorationandproduction,thegeoscienceshavelongbeen referredtoasG&G,geology,andgeophysics.However,petroleumgeochemistryhas longbeenamajorcontributorto findingoilandgasanddeservestoberecognizedas thethird “G” alongwithgeologyandgeophysics.Itistheintentofthisvolumetodemonstratetheimportanceofpetroleumgeochemistrybyexplaininghowitcanbeappliedtoa varietyofexplorationandproductionproblems,inbothconventionalandunconventional plays,andtheroleofpetroleumgeochemistryinbasinmodelingandpetroleumsystem analysis.Bytheend,itishopedthatthereaderwillthinkaboutpetroleumgeosciences asG,G,&G.
Butbeforedelvingintothetheoreticalunderpinningsandapplicationsofpetroleum geochemistryinsubsequentchapters,Chapter1willbeginwithabriefhistoryofthesciencetoprovideaperspectiveonhowitcametobewhatitistoday.Thiswillbefollowed bysomefundamentaldefinitionssothediscussioncanbeginwithsomecommonlanguage.Thechapterwillthenconcludewithareviewofsomeimportantaspectsof organicchemistryandrelevantconceptsinstableisotopes.
Beforestarting,afewsentencesareneededtomanageexpectations.Thisisnotapetroleumgeochemistrybookintendedforpetroleumgeochemists.Itisalsonotanexhaustive reviewofalltheconceptsandtechniquesofthesubjectscoveredorallthesubtlenuisances ofdatainterpretations.Norisita “cookbook” with “recipes” thatgeologistsandgeophysicistscanusetodotheirowninterpretations.Althoughmanyreaderswillbecapableofdoingsomesimpleinterpretationsforthemselves,theopportunitytomakeseriouserrorswill stillexist.Itisinsteadareferencebookforthenonspecialistgeoscientisttogainabetterunderstandingofthevalueandpotentialapplicationsofpetroleumgeochemistrytotheir explorationandproductionprojects.Afterreadingthisbook,geologistsandgeophysicists willbebetterequippedtoreadandunderstandgeochemistryreports,askprobingquestions oftheirgeochemists,andapplythe findingsfromthegeochemistryreportstotheirexplorationand/orproductionprojects.Withthatinmind,letusbegin.
Abriefhistoryofpetroleumgeochemistry Petroleumgeochemistryisarelativelyyoungscience,tracingitsrootsbacktothe1934 discoveryofchlorophyll-likestructuresincrudeoilbyAlbertTreibs(Treibs,1934).
PracticalPetroleumGeochemistryforExplorationandProduction,SecondEdition ISBN978-0-323-95924-7, https://doi.org/10.1016/B978-0-323-95924-7.00008-9
Whilemanypetroleumgeologistsasearlyasthelate19thcenturybelievedoilwas derivedfromorganicmatterinsediments,Treibs’ findingswereundeniableproofof theorganicoriginofcrudeoil(Durand,2003).Bythe1950s,majoroilcompanies hadbegunresearchprogramstolearnmoreaboutoilandgas,especiallyhowitforms andmovesaboutinthesubsurface.Anindicationoftheimportancegiventopetroleum geochemistrybythepetroleumindustryisthe1958patentissuedforamethodforprospectingforpetroleumusingsourcerocksgrantedto HuntandMeinert(1958).
Bytheearly1960s,professionalsocietiesandresearchconferencesonorganic geochemistrywereestablishedandthe firstbookwaspublishedthatwasdevotedsolely tothisscience(Breger,1963).Itwasalsointhe1960sthatadvancesinanalyticaltools, suchasthedevelopmentofgaschromatographyandimprovementsinmassspectrometry, beganprovidingmoredetaileddataonthedistributionandstructureoftheorganiccompoundsinsedimentsandcrudeoils.Thesenewdataledtothedevelopmentofthe conceptofbiologicalmarkercompoundsorbiomarker(EglintonandCalvin,1967), chemicalfossilsthatwouldbecomeimportanttoolsforoil sourcerockcorrelations andoil oilcorrelations.
Inthelate1960sthroughearly1970s,majoradvancesinunderstandingthehydrocarbongenerationprocessweremadeleadingtotheoilwindowconcept.Theneed tounderstandthethermalmaturityofsedimentsandthecompositionofthekerogen wasalsorecognized.Andmigrationtheoriesweregivenseriousattention.Bythemid1970s,theRock-Evalinstrumentwasdevelopedandavailable(Espitalieetal.,1977). Thisstandardizedthepyrolysismethodforsource-rockcharacterizationandevaluation wouldbecomeanindustrystandardinpetroleumgeochemistrythatisstillusedtoday. Thelate1970salsosawthepublicationof PetroleumFormationandOccurrence by Tissot andWelte(1978) and PetroleumGeochemistryandGeology by Hunt(1979),the firsttextbooksonpetroleumgeochemistry.
The1980sbroughttheexpansionofpyrolysistechniques,theproliferationof biomarkerapplications,andabetterunderstandingofpetroleummigration.Itwasalso whenbasinmodelingbecameamainstreamapplication.Priorworkonbasinmodeling usedoversimplifiedtime temperaturerelationships,suchas Connan(1974),orrelied oncomplexcalculationsthatrequiredlargemainframecomputers.Then, Waples (1980) providedasimplemethodofestimatingmaturitybasedontheworkof Lopatin (1971).Itallowedgeologiststomakemodelsusinggraphpaperandahandheldcalculator.Withtheconcurrentintroductionofpersonalcomputers,basinmodelingwas adaptedtothisnewtoolandquicklybecameastandardmethodforpetroleumgeochemistryandexploration.
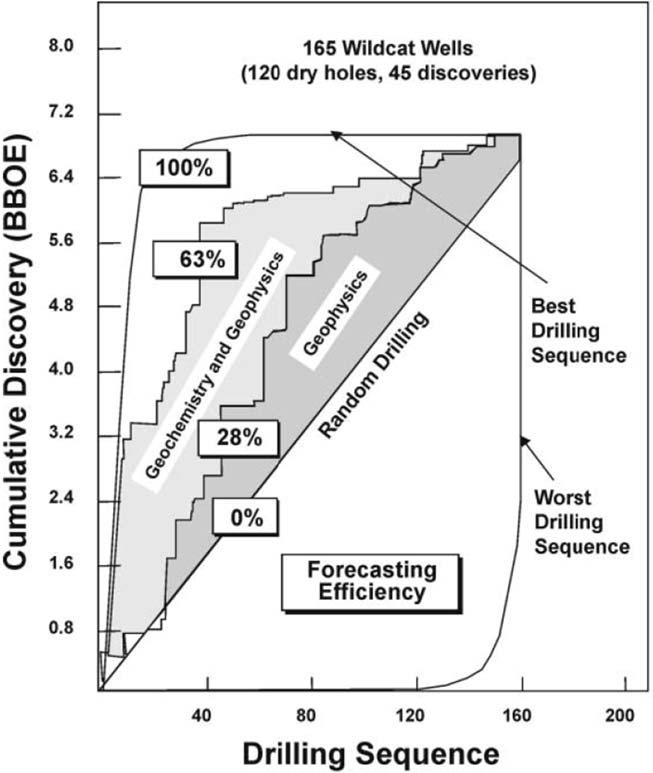
Anothersignificantdevelopmentinthe1980swasthepublicationofapaperby Sluijk andParker(1988) thataddressedthevalueofpetroleumgeochemistryinexploration.As shownin Fig.1.1,theyconsideredthreecases:randomdrilling,explorationbasedontrap sizeonlyfromgeophysicaldata,andexplorationusinggeophysicsinconjunctionwith
Figure1.1 Anassessmentofapproachestoexploration,by SluijkandParker(1988),comparing randomdrilling,explorationbasedontrapsizefromgeophysics,andtheuseofgeophysicsin conjunctionwithpetroleumgeochemistry.
petroleumgeochemistryforasetof165explorationprospects.Whileusingonlytrapsize, anexplorationefficiencyof28%formakingadiscoverycouldbeachieved.Butusingthe combinationofgeophysicsandgeochemistry,explorationefficiencywasincreasedto 63%.Thisclearlydemonstratedthatpetroleumgeochemistryisaneffectivetoolfor reducingexplorationrisk.
Alongwiththeadvancementsinthe1980s,therewerenegativefactorsforpetroleum geochemistry.Mergersstartingintheearlytomid-1980sandadownturninthepetroleumindustrythatbeganin1986ledtoreducedresearchbudgetsandsignaledthe
beginningoftheendofmostindustrylabs.Whilepetroleumgeochemistsinindustrystill didresearch,itwaswithverylimitedfundsandanincreasingrelianceoncontractlabsfor analyticalservices.Asresearchatthecorporatelevelwasdwindling,theindustryphilosophywasthatacademia,governmentinstitutes,andcontractlabswouldmakeupthe shortfall.Tothisend,therewasanemphasisonjointindustryprojects,investigationcarriedoutbyacademiaandcontractlabsfundedbyagroupofindustrypartners.Butthe lackoflargevolumesofdata,diversesamplematerial,andexperiencefoundincompanies limitedthesuccessoftheseprojects.Muchoftheseshortcomingsarestillevidenttoday.
Withtheindustrydownturnextendingintothe1990s,moreemphasisinreservoir applicationsofpetroleumgeochemistrywasbeingseen.Explorationbudgetswerestill tightandcompanieslookedto “gettinganotherbarrelout” ofalreadydiscoveredreserves.Investigatingreservoircontinuity,makingproductionallocationsfromcommingledproduction,anddiagnosingproductionproblems(Kaufmanetal.,1990)were nowimportantpartsofthepetroleumgeochemist’stoolkit.Thisemphasisonreservoir applicationscontinuestoday.
Anothersignificantdevelopmentinthe1990swasthepublicationby Magoonand Dow(1990) ontheconceptofpetroleumsystems.Whiletheideaofpetroleumsystems hadbeenaroundsincethe1970s(e.g., Dow,1974),formalizingtheconceptbrought additionalfocusontherolepetroleumgeochemistryhasinunderstandingthepetroleum system’sworkings(moreonpetroleumsystemslaterinthischapterandin Chapter9).
From2000tothepresent,petroleumgeochemistrycontinuestoadvanceonallfronts. However,thelargesteffortsarebeingdirectedtowardtheso-calledunconventional plays.Inshalegasplays,thesourcerockisalsothereservoirrequiringadifferentapproach tounderstandthesourcerockandhowitfunctions(Passeyetal.,2010).Intightoilreservoirplays,moreattentionneedstobegivento fluidpropertiesandphasebehaviorfor successfulexploitation(Dembicki,2014).Whateverproblemsthefutureholdsinpetroleumexplorationandproduction,innovativeapplicationsoftheconceptandmethodsof petroleumgeochemistrywillcontinuetocontributetosolutions.
Formoredetailsofthehistoryofpetroleumgeochemistry,thereaderisreferredto Kvenvolden(2002), Huntetal.(2002), Durand(2003), Kvenvolden(2006),and Dow (2014).
Definitions Petroleum
Petroleum isanaturallyoccurringmaterialintheearthcomposedpredominantlyofchemicalcompoundsofcarbonandhydrogenwithandwithoutothernonmetallicelements suchassulfur,nitrogen,andoxygen.Itisformedbythediagenesisofsedimentaryorganic mattertransformingthebiologicalinputintosediments, firstintokerogen,thentheconstituentsofpetroleum,withthe finalproductbeinganinertcarbonresidue.Petroleum
mayexistasgas,liquid,orsoliddependingonthenatureofitschemicalconstituentsand temperatureandpressureconditionswhereitexists.Themainformsofpetroleumare: naturalgas,whichdoesnotcondenseintoaliquidatsurfaceconditions; condensate,which isgaseousatreservoirtemperatureandpressureandcondensesintoaliquidatthesurface; and crudeoil,theliquidpartofpetroleum.Oftenwithintheoilandgasindustry,theterm hydrocarbon issubstitutedforpetroleum,crudeoil,and/ornaturalgas.
Geochemistry Geochemistry isdefinedasthestudyoftheprocessesthatcontroltheabundance,composition,anddistributionofchemicalcompoundsandisotopesingeologicenvironments. Organicgeochemistry issimplythesubdisciplineofgeochemistrythatfocusesonorganic (carbonbearing)compoundsfoundingeologicenvironments. Petroleumgeochemistry is thepracticalapplicationoforganicgeochemistrytotheexplorationforandproduction ofpetroleum.
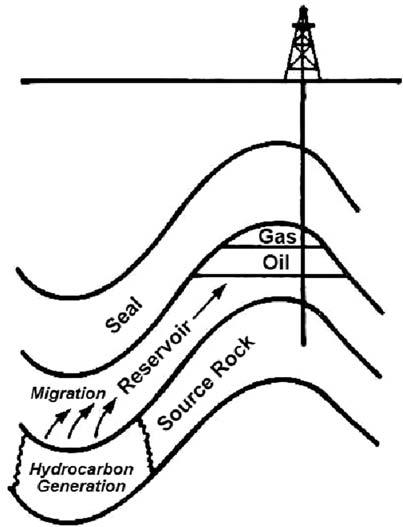
Petroleumsystem A petroleumsystem,shownschematicallyin Fig.1.2,isdefinedbyageneticrelationship linkingasourcerocktoalloilandgasithasgenerated,anditconsistsofallthegeologic elementsandprocessesthatareessentialfortheformationofapetroleumaccumulation (MagoonandDow,1990).Thegeologicelementsneededaresourcerock,reservoir,seal, andoverburden;whilethegeologicprocessesinvolvedaretrapformation,generation, migration,accumulation,andpreservation.Therearealsostratigraphic,temporal,and spatialcomponentstoa petroleumsystem,suchthattheelementsandprocessesmustoccur attherightplaceattherighttimetoproduceapetroleumaccumulation.Thekey elementfromapetroleumgeochemistrypointofviewisthe sourcerocks,rocksfrom whichpetroleumhasbeengeneratedoriscapableofbeinggenerated.Moreabout source rocks canbefoundinthenextchapter,whilemoreabout petroleumsystems canbefoundin Chapter9
Sedimentaryorganicmatter Organicmatterfoundinsedimentsandsedimentaryrocksistypicallysubdividedintotwo categories: bitumen,thatpartofthesedimentaryorganicmatterthatissolubleincommon organicsolvents;and kerogen,thatpartofthesedimentaryorganicmatterthatisinsoluble incommonorganicsolvents.Theterm bitumen hasdifferentconnotationsdependingon whattypeofsedimentaryrockitisfoundin.In fine-grainedsediments, bitumen isindigenoustotherockandmaybepreservedorganicmatterfromthedepositionalenvironmentortheproductofgeneration.Incoarse-grainedsediments(reservoirrocks), bitumen usuallyreferstoresidualcrudeoildispersedinthesediment. Bitumen isalsousedtorefer tosolidvein-fillingmaterial,pitch,tar,andasphalt.
Kerogen,theinsolubleorganicmatterpreservedinsedimentaryrocks,isacomplexmaterialderivedfromthebreakdownanddiagenesisofthecomponentsofplants,animals,and bacteriadepositedinthesediment.Thechemicalcompositionof kerogen isvariable anddependsontheorganicmaterialincorporatedintothesedimentandthechemicalprocessesinvolvedinitsdiageneticalterationandpolymerization.Foradditionalinformation ontheformationofkerogen,seethediscussionofKerogenFormationin Chapter2.
Anotherformofinsolubleorganicmatteris pyrobitumen.Itissolidifiedbitumenthatis theinsolubleresidueremainingafterresidualbitumeninsourcerocksoroilinreservoir rocksiscrackedinsitutogas.
Othersedimentaryorganicdeposits Inadditiontooilandgasdeposits,thereareseveralformsofsedimentaryrockveryrichin organicmatter. Coal isacombustiblesedimentaryrockcontainingatleast w75%by weightorganicmatter(w60%byweighttotalorganiccarbon).Most,butnotall,coals arefromtheaccumulationandpreservationofhigherplantmaterials,usuallyinaswamp environment.An oilshale isanimmatureorganic-rich,oil-pronesourcerock,whichcan beheatedtoyieldoil.Andtarsandsaresandstonereservoirscontainingviscousheavyoil usuallyatorneartheearth’ssurface.
Figure1.2 Schematicofapetroleumsystem.
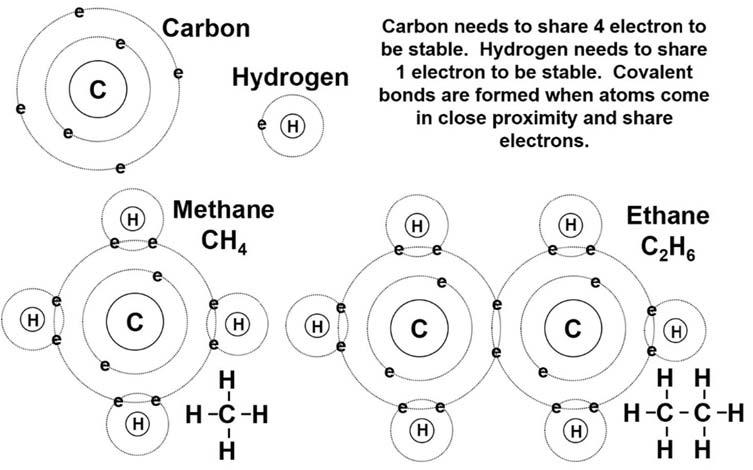
Organicchemistryreview Covalentbonds Asstatedearlier,petroleumiscomposedoforganic(carbon-bearing)chemicalcompoundsmadeofpredominantlycarbonandhydrogenwithandwithoutothernonmetallicelementssuchassulfur,nitrogen,andoxygen.Organiccompoundsrangeinsize andcomplexityfromthesimpleone-carbongasmethanetothecomplexgeopolymer kerogen,withamolecularweightof50,000ormore.Allofthesecompoundsarebased onbuildingmolecularstructureswithcovalentbonds.Covalentbondsinvolvethe sharingofapairofelectronsbytwoatoms.Bysharingelectrons,bothatomscan filltheir outerelectronshellsleadingtomorestabilityforboththeatomsandtheresulting molecule.
Lookingattheexamplesofcarbon hydrogenandcarbon carboncovalentbonding in Fig.1.3,thesharingofelectronscanbedemonstrated.Carbonhastwoelectronshells, aninnershellwithtwoelectronsandanoutershellwithfourelectrons.Whiletheinner electronshellisstable,theouterelectronshellwouldliketohavefourmoreelectronsto reachastablestatewitheight.Hydrogenhasonlyoneelectronshellcontainingasingle electron.Astableconfigurationforhydrogenwouldbetohavetwoelectrons,likethe innershellofthecarbonatom.Methaneisformedbybringingfourhydrogenatoms intocloseproximitywithacarbonatom.Thesharingofapairofelectronsconsistsof oneelectronfromthecarbonpairingwiththeoneelectronfromoneofthehydrogens.
Figure1.3 Carbon hydrogenandcarbon carboncovalentbondsinmethaneandethane.
The finalresultfromtheelectronsharingiseightelectronsintheouterelectronshellof thecarbonandtwoelectronsintheelectronshellofeachhydrogen,witheachpairof sharedelectronsconstitutingacovalentbond.
Sharingofelectronscanalsooccurbetweentwocarbonatomsasshownintheethane moleculein Fig.1.3.Inthiscasetwocarbonatomscomeintocloseproximitytosharea pairofelectronsalongwithsixhydrogenatoms.Thisisessentiallytakingtwomethane molecules,removingonehydrogenfromeach,andformingthecarbon carbonbond betweentheremainsofthetwomolecules.Thisprocesscouldbecontinuedtoform largermolecules.
Becauseitisnotefficienttoputinalltheelectronsoftheatomswhiledrawingacompound,organicchemistsdevisedashorthandfordepictingacovalentbond.Thesharing oftwoelectronsisusuallyshownasabarconnectingtheatoms.In Fig.1.3,methaneand ethaneareshowninthisstylejustbelowandtotherightofthefullelectron representations.
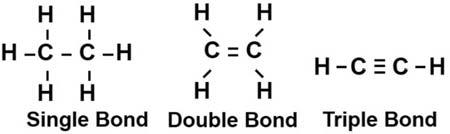
Hydrocarbons The firstclassoforganiccompoundstodiscussarethehydrocarbons.Theyarebyfarthe mostabundantclassofcompoundsinpetroleum.Hydrocarbonsaremadeexclusivelyof carbonandhydrogen.Saturatedhydrocarbons,alsocalledalkanesorparaffins,arehydrocarbonsthatcontainonlycarbon-to-carbonsinglebonds.Unsaturatedhydrocarbonsare hydrocarbonsthatcontainatleastonecarbon-to-carbondoubleortriplebond.While carbonandhydrogencanonlyshareonepairofelectrons,carbonandcarboncanshare uptothreeinasinglebond.Thesharingoftwopairsofelectronsbetweencarbonatoms resultsinadoublebond,shownbytwobarsbetweenthecarbons,andthesharingof threepairsofelectronsmakesatriplebond,shownbythreebarsbetweenthecarbons, asshownin Fig.1.4.Asthenumberofpairsofsharedelectronsincreases,thenuclei ofthetwocarbonatomscomeclosertogether.Asaresult,thenucleibegintorepel eachothermakingdoubleandtriplebondslessstablethanthecarbon carbonsingle bond.Thisisreflectedinthatveryfewcarbon carbontriplebondsarefoundinnaturally occurringsubstances.Carbon carbondoublebondsarecommoninsomebiologicalmaterials,butnotverycommoningeologicalmaterials,theexceptionbeingaromatic hydrocarbons,whichwillbediscussedshortly.
Figure1.4 Carbon carbonsingle,doubleandtriplebonds.
Hydrocarbonscanexistaslinearchainsofcarbonsatoms,asbranchedchainsofcarbon atoms,asoneormoreringsofcarbonatoms,orasacombinationofringedandchain structures.Manyofthecommonhydrocarbonshaveso-calledtrivialnames.Thisis typicalformanyofthesmallerlesscomplexhydrocarbons.Butasthestructural complexityofhydrocarbonsincreases,trivialnamesareoftenreplacedbymoreformal scientificnamesthatarearrivedatbyasetofstrictrulesbasedonthenumberand typeofatomsinvolved,thestructureofthecompound,andthetypeofbondspresent. Thepurposeofthescientificnamesisthatascompoundsizeandcomplexityincrease,the nameshouldprovideneededinformationaboutthecompound’sstructuresothatitcan bedrawnbasedsolelyontheformalname.
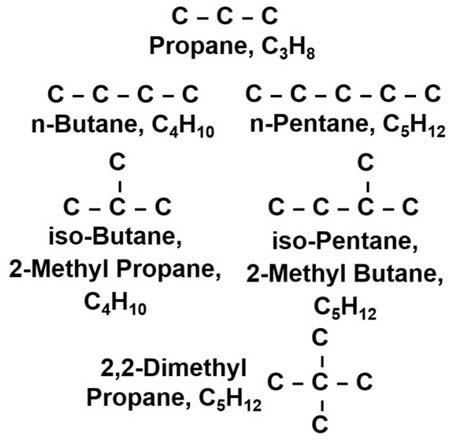
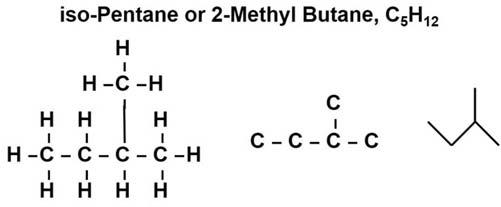
Somesimpleexamplesofhydrocarbonsareshownin Fig.1.5.Propaneisasimple three-carbonsaturatedhydrocarbon.Thestructurecannotbeanymorecomplexfor thisthree-carbonchain.However,ifonecarbonisaddedtoformbutane,thenumber ofpossiblestructuresincreases.Itisnowpossibletohavethebutaneexistasastraightchained(normal),n-butane,andiso-butane(thetrivialname)or2-methylpropane, whichisthethree-carbonpropanechainwithamethylgroup(methanelessone hydrogenatom),locatedatthemiddleornumber2carbon.Thesetwocompounds arecalledisomers,whichmeanstheyhavethesamenumberofcarbonandhydrogen atoms,butarearrangedindifferentstructures.Startingwithpentaneontherightside of Fig.1.5,asimilarpatterncanbeobserved.Itstartswithn-pentaneandtheisomer iso-pentane(thetrivialname),or2-methylbutane,afour-carbonchain,butane,with
Figure1.5 Someexamplesofsmallhydrocarbonmoleculestoillustratetheconceptofisomers.
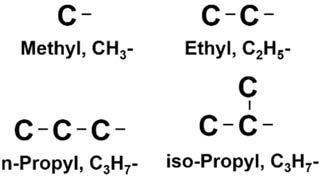
amethylgrouplocatedatthenumber2carbon.Inaddition,anotherisomer2,2dimethylpropaneisalsoapossiblestructuralconfiguration.Withtheincreasingnumber ofcarbons,thenumberofpossibleisomersalsoincreases.Inadditiontomethylgroupsas sidechainstothemainstraight-chainedstructure,therecanbeethylgroups(ethaneless onehydrogenatom),n-propylgroups(propanelessonehydrogenatom),andgreater,as wellasbranchedsidechainssuchasaniso-propylgroup,asshownin Fig.1.6.
Forthecompoundsshownin Figs.1.5and1.6,adifferentnotationisusedtodepict thecompounds’ structures.Ascompoundsbecomelargerandtheirstructuresbecome morecomplex,itisnotefficienttoputinallthehydrogenswhiledrawingacompound. Inthisnotation,organicchemistssimplydonotshowthem,theassumptionbeingthere aresuf ficienthydrogenatomsinplacetomatchupwithanyunpairedcarbonelectrons notalreadyinvolvedincarbon carbonbonds.Thesharingofapairofelectronsisstill shownasasinglebarconnectingtheatoms.Butasorganiccompoundsbecomelarger, evenshowingthecarbonbecomescumbersomeandinefficient,givingrisetothe “stick” notation.Theexamplein Fig.1.7 foriso-pentane(2-methylbutane,C5H12)showthree singlesticksconnectedinazig-zagpatternwithafourthstickconnectedtooneofthe intersections.Thisnotationindicatesthatthereisacarbonattheendofeach “stick” andthatthesesingle “sticks” representcarbon carbonbonds.Thethreesingle “sticks” connectedinazig-zagpatternrepresentfourcarbonsinthebutanechain,andthefourth “stick” connectedtooneoftheintersectionsrepresentsthemethylgroupsidechain.Asin thepreviousnotationscheme,thereisanassumptionthattherearesuf ficienthydrogen atomsinplacetomatchupwithanyunpairedcarbonelectronsnotalreadyinvolvedin carbon carbonbonds.Twoparallel “sticks” wouldindicateadoublebondbetweenthe carbons.Thisnotationsystemisusedextensivelywiththelargestraight-chained, branched-chained,andcyclichydrocarbons.
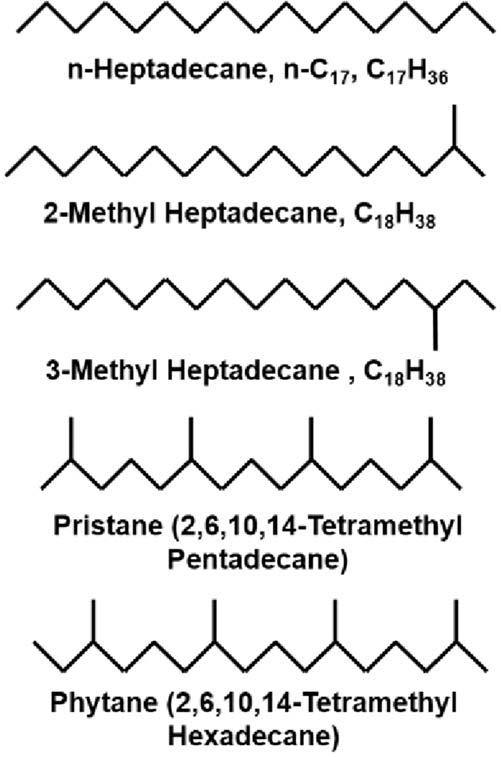
Thestraightandbranched-chainedsaturatedhydrocarboncangetquitelarge, commonlyupto60carbonsorgreater.However,mostofthecompoundsusedin petroleumgeochemistryareintheC1 C35 range,primarilyduetoanalyticalconsiderations.Branchingcanbeverysimpleorverycomplex,asshownintheexamplesin Fig.1.8.Startingwiththestraight-chainedn-heptadecane(n-C17),commonlyencounteredhydrocarbonssuchas2-methylheptadecaneand3-methylheptadecane,areformed
Figure1.6 Commonsmallsidechainsforhydrocarbonmolecules.
Figure1.7 Differentformsofstructuralnotationsforhydrocarbonmolecules.
Figure1.8 Someexamplesofstraight-chainedandbranchedsaturatedhydrocarbons.
simplybyaddingamethylgrouptothenumber2ornumber3carboninthebasechain. However,morecomplexbranched-chainedcompounds,suchaspristane(2,6,10,14tetramethylpentadecane)andphytane(2,6,10,14-tetramethylhexadecane),arealso abundant.Thestructuralsimplicityorcomplexityofmoleculessuchasthesecontribute tothegeochemicalinformationtheycarry,aswillbediscussedinsubsequentchapters.
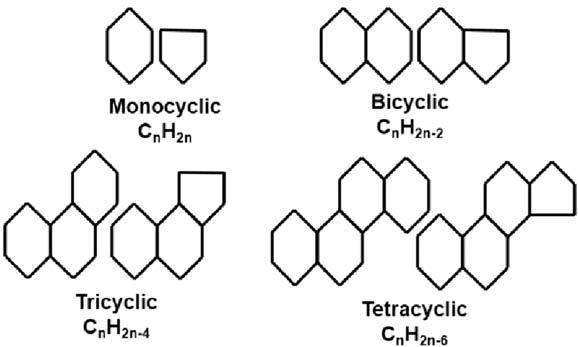
Saturatedhydrocarbonscontainingoneormoreringsofcarbonatomsintheirstructuresarecalledcycloalkanesornaphthenes.Theseringsusuallyconsistof fiveorsixcarbons,withsixcarbonringsbeingthemoststableand,therefore,themostcommon.
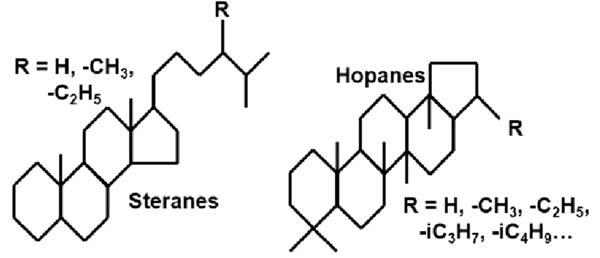
Geochemicallysignificantnaphthenescanconsistof1 6rings.Someexamplesof 1 4ringsstructuresareshownin Fig.1.9.Inadditiontotherings,oneormoreside chainscanbeaddedtostructureatanyofthecarbons.Twoexamplesofthisareshown bythesteraneandhopanestructuresin Fig.1.10.Thesegeochemicallysignificantnaphthenegroupsalsodemonstratehowaseriesofrelatedcompoundsareformedbysimply varyingthesidechainatonelocation,R,onthebasemolecule.
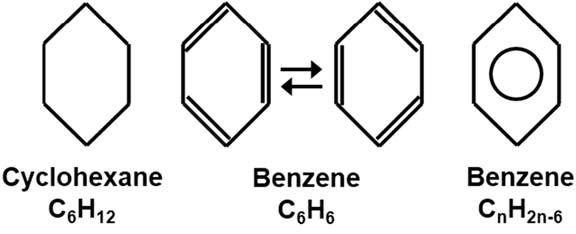
Aromatichydrocarbons Aromatichydrocarbonsareaspecialclassofunsaturatedhydrocarbonbasedonasix-carbon ringmoietycalledbenzene.Thesaturatedhydrocarboncyclohexaneistransformedinto thearomatichydrocarbonbenzenebyaddingthreealternatingcarbon carbondouble bonds,asshownin Fig.1.11.Thebenzenestructurecanhavetwoarrangementsofthese doublebonds,shownbythepairofbenzenemoleculesinthemiddleof Fig.1.11.Innature,thesearrangementsofbondsrapidlyalternateinthebenzenestructure.Becauseofthis rapidchangingorresonatingofthepositionofthethreecarbon carbondoublebonds, benzeneisusuallyrepresentedasahexagonwithacircleinthecenter,asshownonthe
Figure1.9 Someexamplesof five-andsix-memberedringcyclicsaturatedhydrocarbons,alsocalled naphthenesorcycloalkanes.
Structuresofsteranesandhopanesasexamplesofsomeofthevariationsinrelated moleculesthatcanachievedfromdifferentsidechains.
rightsideof Fig.1.11.Asdiscussedearlier,carbon carbondoublebondsareusually consideredlessstablethancarbon carbonsinglebonds.However,theresonatingalternatingdoublebondsinthebenzenedistributetheelectronsharingoverallsixcarbons andimpartmorestabilitytothemolecule.
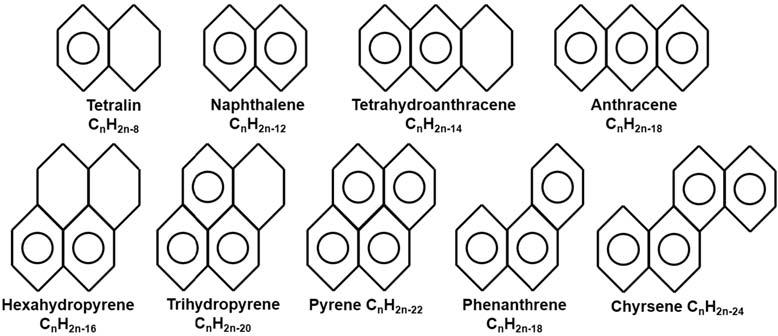
Thebasicbenzeneringstructurecanbeusedtobuildmuchlargemoleculesby attachingsaturatedhydrocarbonchains(eitherstraightorbranched)orbybuildingmultipleringstructures,similartothenaphthenes.Theseringstructures,asillustratedin Fig.1.12,mayconsistofallaromaticrings,purearomaticcompounds,oramix ofaromaticandsaturatedrings,naphthenoaromaticcompounds.Inthepurearomatic structures,theresonatingstabilizationofthebenzeneunitisextendedtotheentirestructure.Andjustlikeinthenaphthenes,oneormoresidechainscanbeaddedtoanyofthe carbonsinanyofthearomaticringstructures.
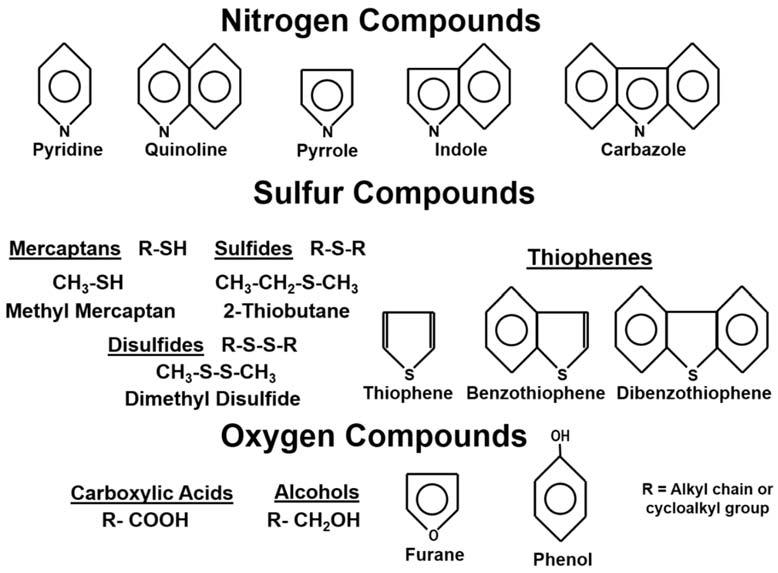
N S Ocompounds N S Ocompounds,orresins,areorganiccompoundsthatcontainnitrogen,sulfur,or oxygeninadditiontothecarbonandhydrogen.Inmanycrudeoils,theN S Os
Figure1.10
Figure1.11 Thebenzenestructure.
representonlyasmallportionoftheoilascomparedtothesaturatedandaromatichydrocarbons;however,thereareinstanceswheretheN S Osareinhigherconcentration.SomeexamplesofN S Ocompoundscommonlyfoundinbitumenandcrudeoil aregivenin Fig.1.13.Thesmaller,lesscomplexcompoundsaremoretypicallyfound duringsedimentdiagenesisandearlygeneration.Theseincludethealcohols,acids,mercaptans,sul fides,anddisulfides.Theremainderofthecompounds,mainlyringedstructures,aremoreabundantlaterinthegenerationhistoryofthesediment.Becauseof analyticaldifficulties,mostoftheN S Ocompoundsarenotfrequentlyusedinmaking geochemicalinterpretations.Theexceptiontothisisthethiophenegroup,whichisused indecipheringcrudeoilorigins,asdiscussedin Chapter4.
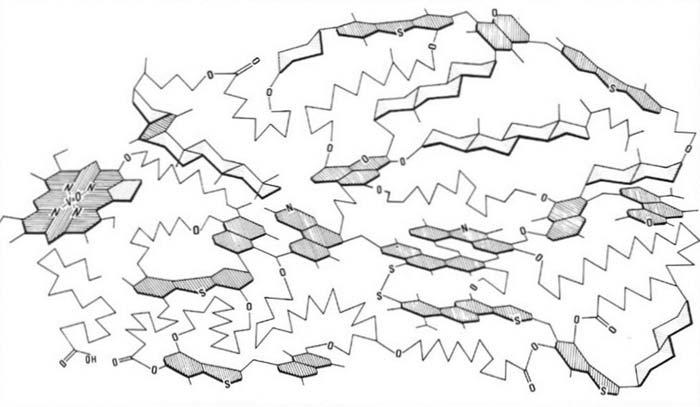
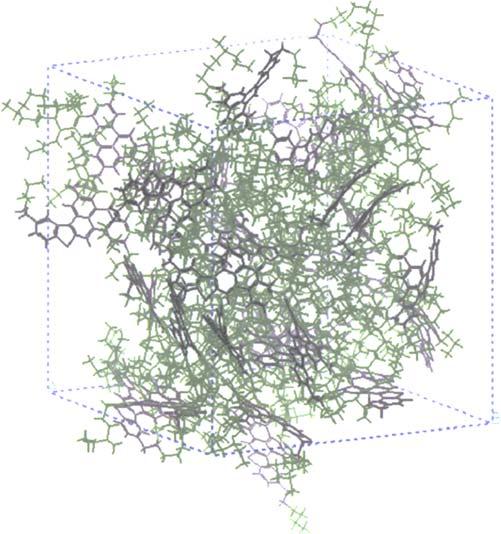
Asphaltenes Asphaltenesareahigh-molecular-weightcomponentofcrudeoilsandbitumen,whichis insolubleinn-heptane.Chemically,theyarepolyaromaticnucleilinkedbyaliphatic chainsorringsandfunctionalgroupswithmolecularweightintherangeof 1000 10,000(Peletetal.,1986).Arepresentationofatypicalasphaltenestructureis shownin Fig.1.14.Itshouldberememberedthatwhiletheexactcompositionwill varyfromoneasphaltenemoleculetoanother,themolecularcompositionoftheasphaltenesofaparticularcrudeoilorbitumenexhibitsahighdegreeofsimilarity(Beharand Pelet,1985).Asphalteneswereoriginallythoughttobesmallfragmentsofkerogenafter somethermaldegradation(LouisandTissot,1967).However, Peletetal.(1986) showed thatasphaltenescouldalsobederivedfromthecondensationprocessessimilartothose thatfromkerogen(seediscussionofKerogenFormationin Chapter2).Whileasphaltenesexistasfreemoleculesincolloidalsolutionwithincrudeoilsandbitumens,these solutionsareoftenunstableandcanbeperturbedcausingasphaltenemoleculesto comeoutofsolutionandclustertogetherinaggregates.Amodelofanasphalteneaggregateisshownin Fig.1.15.Theseaggregatescanhaveamolecularweightofafewtensof
Figure1.12 Someexamplesofbasicpurearomaticandnaphthenoaromaticstructures.
Figure1.13 Someexamplesofnitrogen,oxygen,andsulfurcontainingorganiccompounds commonlyfoundincrudeoilsandbitumen.
Figure1.14 Arepresentationofthestructureofatypicalasphaltenemolecule.Chainaliphaticstructuresareshownas “saw-toothed” lines,naphthenicstructuresareshownasunshadedpolygons,and aromaticstructuresaretheshadedpolygons.OxygenisshownasO,hydrogenasH,andnitrogenasN. (From Peletetal.(1986).)
Figure1.15 Athree-dimensionalrepresentationofanasphalteneaggregatefrom Mullins(2003).The differentcolorsandshadesofgrayrepresentindividualasphaltenemoleculesthathavecome togethertoformtheaggregate.
thousandstonearamillion.Clustersofasphalteneaggregatescanreduceporosityand clogpermeabilityinreservoirrocks(moreonthisin Chapter4).
Reactions Organiccompoundsaresubjecttoavarietyofreactionsthatcanaltertheircomposition andstructure.Someofthereactionsthatoccuringeologicalenvironmentsareshownin
Fig.1.16.Hydrolysisistheaddingofwateratacarbonylgroup,suchasanesterlinkage,
Figure1.16 Someofthereactionsthatsedimentaryorganicmattermayundergoduringdiagenesis andhydrocarbongeneration.
tocreateacarboxylicacidandahydroxylgroup.Decarboxylationisthelossofacarboxylicacidgroupcreatingcarbondioxideandanalkylgroup.Thecarbondioxidemaythen bedissolvedinthesedimentporewatersorgototheformationofcarbonatecements. Dehydroxylationistheadditionofhydrogentoahydroxyl(alcohol)groupcreatingwaterandanalkylgroup.Reductionistheconversionofacarbon carbondoublebondtoa carbon carbonsinglebondbytheadditionofhydrogen.Andcrackingisthebreakingof acarbon carbonbondcreatingtwosmallercompounds.Ifsufficientextrahydrogenis present,thetwosmallercompoundswillbesaturated,asshown.Ifhydrogenisnotpresent,anunsaturatedoraromaticcompoundmayform.Whilehydrolysis,decarboxylation,dehydroxylation,andreductionarereactionsobservedmainlyintheearly diagenesisoforganicmatterinsediments,crackingisanimportantreactioninthehydrocarbongenerationprocess.
Stableisotopereview Elementsaretheprimaryconstituentsofallmatterandassuchcannotbechemically brokendownintosimplerconstituents.Theyarecomposedoftheprotons,electrons, andneutronswitheachelementbeingdistinguishedfromtheothersbythenumberof protonsinitsnucleus.Protonsarepositivelycharged.Tobalancethischarge,thenumber ofelectronsinanelementequalsthenumberofprotons.Theneutronshavenocharge andcanvaryinnumber.Isotopesaresimplyformsofanelementthathavedifferent numbersofneutronsinthenucleioftheiratoms.
Arelevantexampleiscarbon,whichhasthreenaturalisotopes:carbon12, 12C,six protons,andsixneutrons;carbon13, 13C,sixprotons,andsevenneutrons;andcarbon 14, 14C,sixprotons,andeightneutrons.Someisotopes,suchas 14C,areradioactiveand decaytoformadifferentelementorisotopeplusahighenergyparticle.Stableisotopes, suchas 12Cand 13C,donotdecaybutaresubjecttochangesintheirrelativeconcentrationduetochemical,physical,andbiologicalprocesses.Forexample,biologicalprocesses,suchasphotosynthesis,tendtofavorutilizationof 12Cover 13Canddifferent typesofplantshaveastrongerpreferencefor 12Cthanothers.Thiscanresultincarbon isotoperatiosdistinctiveforspecificplantsgroups.Nonbiologicalprocessesalsoshowan isotopicpreference.Thereisakineticisotopeeffectobservedwhencleavingmethane (methylgroups)fromlargerorganicmoleculesduringcracking.Itisenergetically morefavorabletoremovea 12Cmethylgroupover 13Cmethylgroup.Asaresult, methaneformedearlyintheprocessofcleavingofmethylgroupswillhavehigher amountofthe 12Cisotopethanmethaneformedlater.
Thestableisotopesofhydrogen,nitrogen,sulfur,andoxygenbehavesimilarlytocarbon.Theseisotopesalongwithcarbonareshownin Fig.1.17 withtheirnaturalabundanceandthebaseratiosoftheisotopesusedingeochemicalstudies.Whileallthese