https://ebookmass.com/product/practical-approaches-to-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Inorganic Chemistry 7th Edition Mark Weller
https://ebookmass.com/product/inorganic-chemistry-7th-edition-markweller/
ebookmass.com
Principles of General Organic & Biological Chemistry 2nd Edition, (Ebook PDF)
https://ebookmass.com/product/principles-of-general-organicbiological-chemistry-2nd-edition-ebook-pdf/
ebookmass.com
(Original PDF) Chemistry: An Introduction to General, Organic, and Biological Chemistry 13th Edition
https://ebookmass.com/product/original-pdf-chemistry-an-introductionto-general-organic-and-biological-chemistry-13th-edition/
ebookmass.com
The Concise Cengage Handbook 5th Edition Laurie G. Kirszner
https://ebookmass.com/product/the-concise-cengage-handbook-5thedition-laurie-g-kirszner/
ebookmass.com
Digital Electronics: Principles and Applications 9th Edition Roger L. Tokheim
https://ebookmass.com/product/digital-electronics-principles-andapplications-9th-edition-roger-l-tokheim/
ebookmass.com
Options, Futures, and Other Derivatives: Eleventh Edition [Global] John C. Hull
https://ebookmass.com/product/options-futures-and-other-derivativeseleventh-edition-global-john-c-hull/
ebookmass.com
Communication Works, 11th edition 11th Edition, (Ebook PDF)
https://ebookmass.com/product/communication-works-11th-edition-11thedition-ebook-pdf/
ebookmass.com
Spiritual Direction as a Medical Art in Early Christian Monasticism Jonathan L. Zecher
https://ebookmass.com/product/spiritual-direction-as-a-medical-art-inearly-christian-monasticism-jonathan-l-zecher/
ebookmass.com
Digital Supply Networks Amit Sinha
https://ebookmass.com/product/digital-supply-networks-amit-sinha/
ebookmass.com
Mission, Race, and Empire: The Episcopal Church in Global Context
Jennifer C. Snow
https://ebookmass.com/product/mission-race-and-empire-the-episcopalchurch-in-global-context-jennifer-c-snow/
ebookmass.com
PRACTICALAPPROACHESTO BIOLOGICALINORGANICCHEMISTRY PRACTICALAPPROACHESTO BIOLOGICALINORGANIC CHEMISTRY SECONDEDITION Editedby
RobertR.Crichton
CatholicUniversityofLouvain,Louvain-la-Neuve,Belgium
RicardoO.Louro
InstitutodeTecnologiaQuı´micaeBiolo ´ gicaAnto ´ nioXavierdaUniversidadeNovadeLisboa, Oeiras,Portugal
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierB.V.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical, includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthe publisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefound atourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmay benotedherein).
Notices Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbe mindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforany injuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseor operationofanymethods,products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-444-64225-7
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionEditor: EmilyMcCloskey
EditorialProjectManager: KelseyConnors
ProductionProjectManager: PremKumarKaliamoorthi
CoverDesigner: ChristianBilbow
TypesetbyMPSLimited,Chennai,India
ListofContributors MargaridaArcher InstitutodeTecnologia
Quı´micaeBiolo ´ gicaAnto ´ nioXavier(ITQB NOVA),UniversidadeNovadeLisboa, Oeiras,Portugal
EckhardBill Max-PlanckInstitutefor ChemicalEnergyConversion,Mulheiman derRuhr,Germany
Jose ´ A.Brito InstitutodeTecnologiaQuı´mica eBiolo ´ gicaAnto ´ nioXavier(ITQBNOVA), UniversidadeNovadeLisboa,Oeiras, Portugal
WesleyR.Browne MolecularInorganic Chemistry,StratinghInstituteforChemistry, FacultyofScienceandEngineering, UniversityofGroningen,Groningen,The Netherlands
RobertR.Crichton CatholicUniversityof Louvain,Louvain-la-Neuve,Belgium
MartinC.Feiters DepartmentofSynthetic OrganicChemistry,InstituteforMolecules andMaterials,FacultyofScience,Radboud University,AJNijmegen,TheNetherlands
VincentFourmond CNRS,AixMarseille University,BIP,Marseille,France
MajaGruden FacultyofChemistry,University ofBelgrade,Belgrade,RepublicofSerbia
W.R.Hagen DepartmentofBiotechnology, DelftUniversityofTechnology,Delft,The Netherlands
IrinaA.Kuhne SchoolofChemistry, UniversityCollegeDublin,Dublin,Ireland
ChristopheLe ´ ger CNRS,AixMarseille University,BIP,Marseille,France
RicardoO.Louro InstitutodeTecnologia
Quı´micaeBiolo ´ gicaAnto ´ nioXavierda UniversidadeNovadeLisboa,Oeiras, Portugal
WolframMeyer-Klaucke DeutschesElektronen SynchrotronDESY,Hamburg,Germany
GraceG.Morgan SchoolofChemistry, UniversityCollegeDublin,Dublin,Ireland
RobertL.Robson SchoolofBiological Sciences,UniversityofReading,Berkshire, UnitedKingdom
Ine ˆ sB.Trindade InstitutodeTecnologia
Quı´micaeBiolo ´ gicaAnto ´ nioXavierda UniversidadeNovadeLisboa,Oeiras, Portugal
MatijaZlatar DepartmentofChemistry, InstituteofChemistry,Technologyand Metallurgy,UniversityofBelgrade,Belgrade, RepublicofSerbia
Anoverviewoftheroleof metalsinbiology RobertR.Crichton
CatholicUniversityofLouvain,Louvain-la-Neuve,Belgium
OUTLINE Introduction
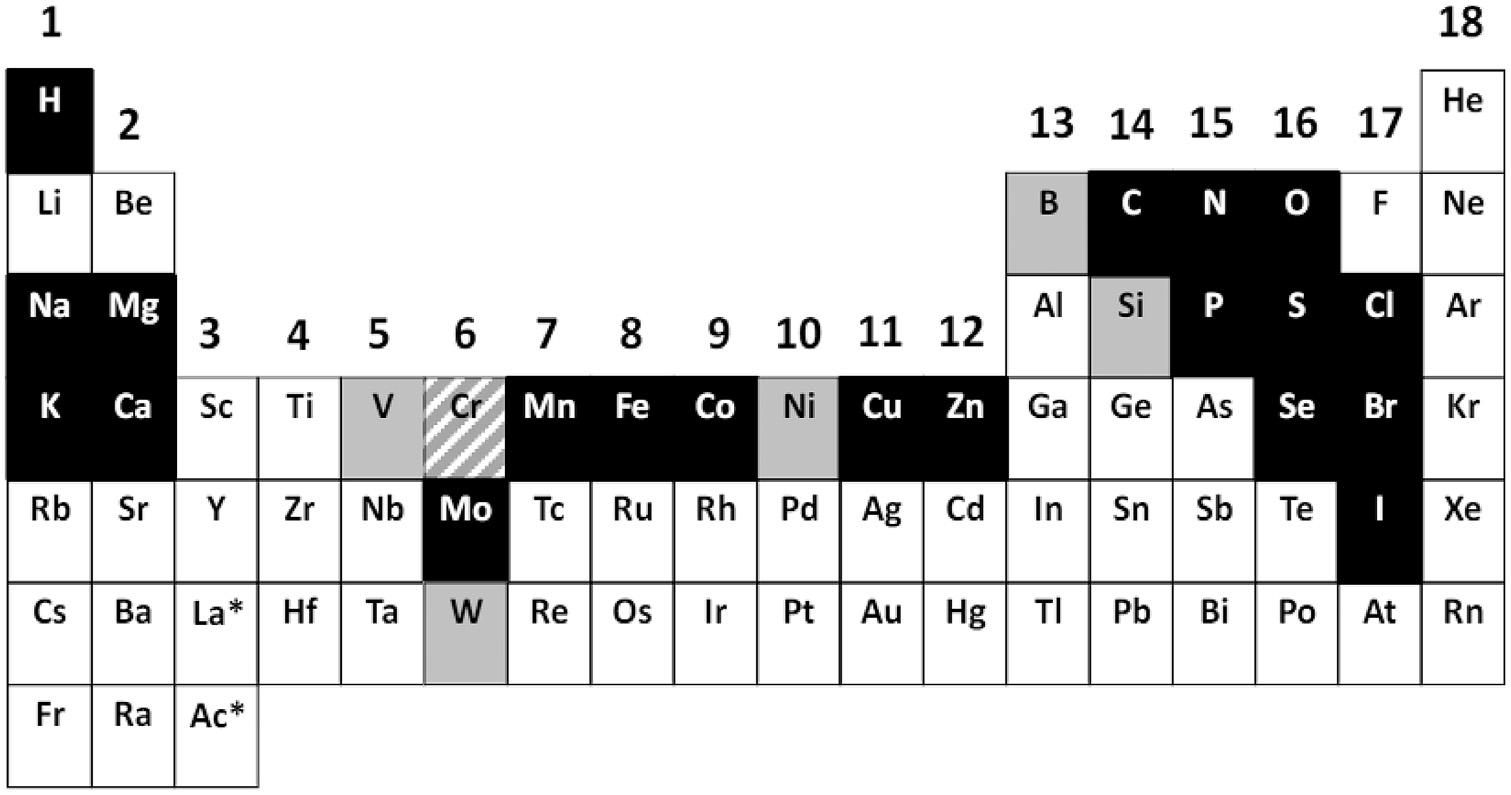
Metalsplaymanydifferentrolesinthebiologicalworld,whetherbytheirparticipation inessentialbiologicalprocesses,astoxicconstituentsofourenvironment,orasindispensablediagnosticandtherapeuticagentsinhumanmedicine.Onlyalimitednumberof metalionsareessentialformostlivingorganisms(Fig.1.1),andthisshortintroduction beginsbyillustratingthebiologicalimportanceofmetals,notonlyinvitalprocessessuch asintermediarymetabolism,electrontransfer,respiration,andphotosynthesis,butalsoin neurotransmission,cellsignaling,apoptosis,andfertilization.
Whilemanyessentialmetalscanbetoxic,particularlywhentheyareinexcess,inour modernenvironmentthereareanumberofnonessentialmetals,suchascadmium,lead, mercury,andaluminum,whicharethemselveshighlytoxic.
Finally,metalshaveassumedanextraordinarynumberofrolesinmedicine,not onlytherapeuticallyasdrugs,butalsoasnoninvasivecontrastagentsand radiopharmaceuticals.
Moredetailedaccountsoftheseaspectsofmetalionsarepresentedinthecompanion volumetothissecondedition(Crichton,2018).
FIGURE1.1 Abiologicalperiodictableoftheelementsindicatingtheessentialelements.Theessentialelementsformostformsoflifeareshowninblackwiththeexceptionofchromium(Cr),whichisshownwithan upwarddiagonalpattern,andessentialelementsthataremorerestrictedforsomeformsoflifeshowningray. Source:ReproducedfromMaret,W.,2016.Themetalsinthebiologicalperiodicsystemoftheelements:conceptsandconjectures.Int.J.Mol.Sci.17,pii:E66. doi:10.3390/ijms17010066.ThisisanopenaccessarticledistributedundertheCreative CommonsAttributionLicense(CCBY)whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
Essentialmetalionsandtheirfunctions Mostlivingorganismsrequiresome25elements(Maret,2016;ChellanandSadler,2015) includingbetween10and14metalions(Fig.1.1).Inthecaseof Homosapiens,thereare10 essentialmetalions(sodium,potassium,calcium,magnesium,manganese,iron,cobalt, copper,zinc,andmolybdenum).Ofthese,thefirstfourareconsideredas“bulkelements” (Na1,K1,Ca21,andMg21),representing112g,160g,1.1kg,and25g,respectively,inan “average”personofbodyweight80kg(Thedataontheabundanceofelementsinthe 80kghumanbodyarethosegiveninWebElements: http://www.webelements.com/.). Together,theyconstitutesome99%ofthemetalioncontentofthehumanbody.The others,manganese,iron,cobalt,copper,zinc,andmolybdenum,designated“traceelements,”arepresentinmuchloweramountsthanthebulkelements(respectively,16mg, 4.8g,1.6mg,80mg,2.6g,and8mginan80-kgperson).
TheessentialalkalimetalionsNa1 andK1 onlyweaklybindorganicligands,rendering themextremelymobile,aswithH1 andCl .Thisenablesthemtogenerateionicgradients acrossbiologicalmembranes.ThedistributionofNa1 andK1 inmammalsisquitedifferent;Na1,togetherwithCl ,isthemajorelectrolyteintheextracellularfluid,whereasK1 isretainedwithinthecells.TheconcentrationofNa1 intheplasmaismaintainedwithin narrowlimitsatabout145mmol/L,anditsintracellularconcentrationisonlyabout 12mmol/L,whereastheintracellularconcentrationofK1 is150mmol/L,andtypically
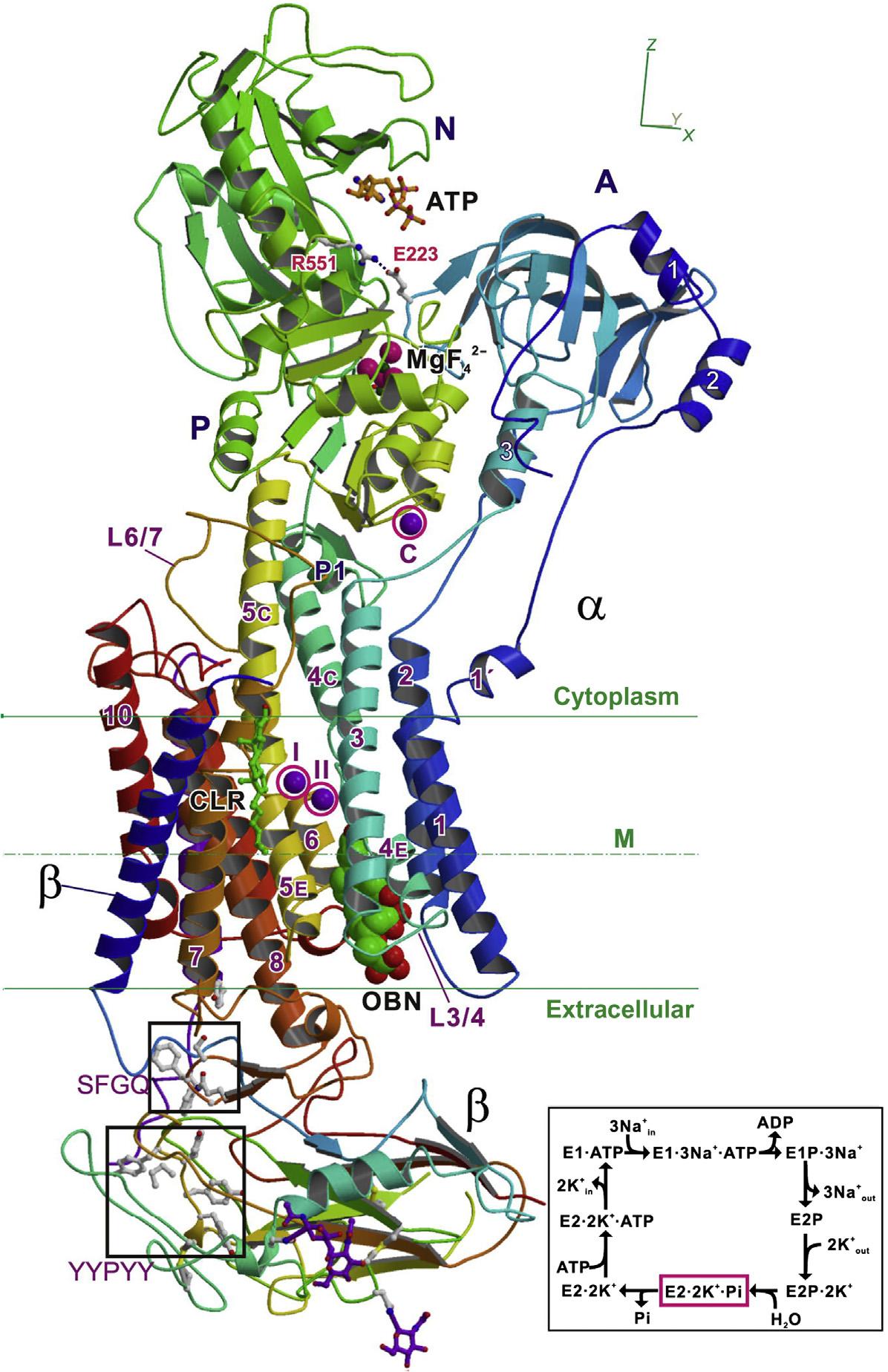
only4 5mmol/Lintheextracellularfluids.Thisconcentrationdifferential,maintainedby the(Na1 K1)-ATPaseoftheplasmamembrane,ensuresanumberofmajorbiological processes,suchascellularosmoticbalance,signaltransduction,andneurotransmission. (Na1 K1)-ATPasetransportsthreeNa1 ionstotheoutsideofthecellandtwoK1 ionsto theinside(Figs.1.2and1.3),contributingtotheactionpotentialinvolvedintransmission ofnerveimpulsesalongneuronalaxons.Actionpotentialscanbegeneratedbypresynapticneuronsattherateofabout250persecond,accountingforbetweenone-halfand two-thirdsoftheirtotalATPconsumption.TherepetitiveG-richsequencesfoundinthe telomeresattheendsofeukaryoticchromosomesarestabilizedbyK1 andNa1 ions.The retentionofNa1 (hypernatremia)whenNa1 intakeexceedsrenalclearanceisoneof themostcommonelectrolytedisordersinclinicalmedicine.Hyperkalemiahasbecome morecommonincardiovascularpracticeduetothegrowingpopulationofpatientswith chronickidneydiseaseandthebroadapplicationofdrugsthatmodulaterenalelimination ofpotassiumbyreducingtheproductionofangiotensinII.
Thealkalineearthmetalions,Mg21 andCa21,havegreaterbindingstrengthstoorganic ligandsthanNa1 andK1,andthereforearelessmobile.Bothplayimportantstructural andcatalyticroles,with99%ofthebody’sCa21 foundinboneandteeth.AlthoughMg21 istheleastabundantofthe“bulkelements,”theintracellularconcentrationoffreeMg21 is around0.5mM,makingitthemostabundantcation,andlessthan0.5%oftotalbody Mg21 isintheplasma.HalfofcytosolicMg21 isboundtoATPandmostoftherest,along withK1,isboundtoribosomes.Unliketheotherthreebulkcations,Mg21 hasamuch slowerwaterexchangerate,allowingittoplayastructuralrole,forexample,participating inATPbindinginmanyenzymesinvolvedinphosphoryltransferreactions—6ofthe10 reactionsofglycolysisarephosphoryltransfers.
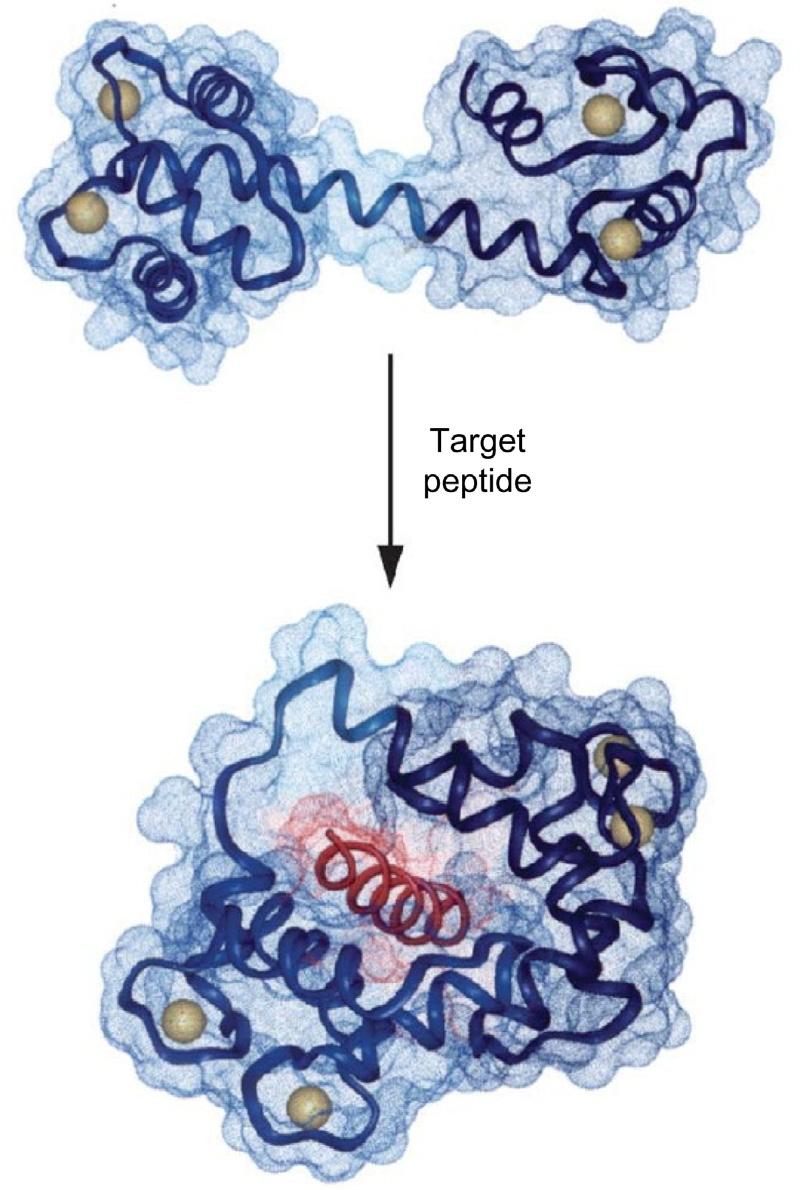
Ca21 servesasamessengerinvirtuallyalloftheimportantfunctionsofcells.Why Ca21 hasendedupinthispositionisprobablyduetoitsuniquecoordinationchemistry, whichenablesittobindtositesofirregulargeometryeveninthepresenceoflarge excessesofothercationssuchasMg21 (CarafoliandKrebs,2016).WhilethetotalCa21 concentrationinsidecellsismicromolar,inthecytosoltheconcentrationoffreeCa21 is about10,000timeslower.ThisnanometerconcentrationisachievedbyligationofCa21 by twobroadclassesofspecificproteins.(1)ThosewhichbufferCa21 inthenanometerrange, andinsomecases,alsoprocessitsinformation,byincreasing,orlessfrequentlydecreasing,theirbiologicalactivityuponCa21 bindingbyachangeinconformation,illustrated forcalmodulinin Fig.1.4—“Ca21 isnotanactivesitemetal,itistheallostericmetalpar excellence”(CarafoliandKrebs,2016).(2)Intrinsicmembraneproteinswhichtransport Ca21 inoroutofcells,orbetweenthecytosolandthelumenofcellularorganelles. Apoptosis(programmedcelldeath)playsamajorroleinthemaintenanceoftissuehomeostasis.Ca21,inadditiontoitsroleintheregulationofcellularprocesses,mayactasa proapoptoticagent,andbothintracellularCa21 depletionoroverloadmaytriggerapoptosis(Brinietal.,2013).Hypercalcemiaisacommonmetabolicperturbationandtheincrease inover-the-counterpurchaseofCa21 andvitaminDsupplements,notablytocombatosteoporosisintheagingpopulation,isacontributoryfactor.
Ofthesixessentialtracemetalions,Znhasligand-bindingconstantsintermediate betweenthoseofMg21 andCa21 andtheotherfive.Manganese,iron,cobalt,copper,and molybdenumallhavemuchstrongerbindingtoorganicligandsandarethereforeonly
FIGURE1.2 Architecture ofNa1,K1-ATPasefromshark rectalglandwithbound MgF42 andK1,astableanalogoftheE2 Pi 2K1 state.A ribbondiagramofNKAwith ouabain(showninspacefill) boundatlowaffinity(PDB ID:3A3Y).Colorchanges graduallyfromtheNterminal(blue)totheCterminal(red).ATPistaken fromtheE2(TG) ATPcrystal structureofCa21-ATPase (SERCA1a)(PDBID:3AR4) anddockedinthecorrespondingposition.BoundK1 ionsaremarked(I,II,andC) andcircled.Insetshowsa simplifieddiagramofthe post-Albersscheme. CLR, cholesterol; OBN,ouabain. Source:From Toyoshimaetal. (2011).Copyright2011.With permissionfromElsevier.
poorlymobile.Inaddition,theyhaveaccesstoatleasttwooxidationstates,andtherefore canparticipateinelectrontransferandredoxcatalysis,whereaszinchasaccessonlytothe Zn21 state.
Manganesecanoccurinbiologicalsystemsinthreeoxidationstates,Mn(II),Mn(III), andMn(IV).Inhumans,manganeseisessentialfordevelopment,metabolism,andthe antioxidantsystemthroughitsinvolvementinanumberofenzymes,includingarginase,
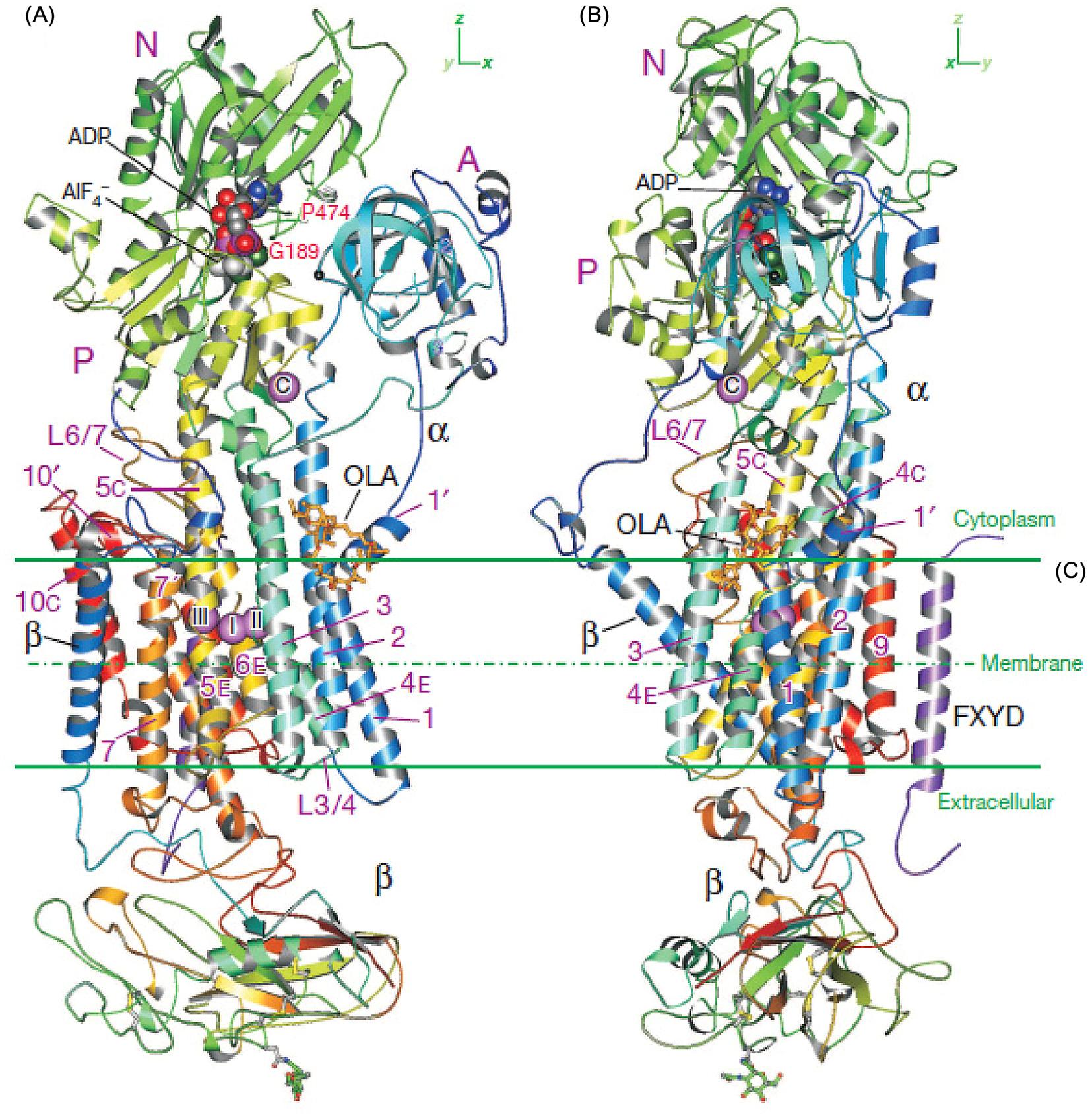
FIGURE1.3 CrystalstructureofNa1,K1-ATPaseinthetransitionstateanalogE1BP ADP 3Na1.(AandB) Ribbondiagramsviewedintwoorthogonaldirections.ColorchangesgraduallybetweentheNterminus(blue) andCterminus(red)forthe α-and β-subunits. Purple spheresshowboundNa1 ions[three(I III)inthetransmembraneregionandone(C)inthecytoplasmicregion].Sugarsattachedtothe β-subunitareshownasballand stick. OLA,oligomycinA. Source:FromKanai,R.,Ogawa,H.,Vilsen,B.,Cornelius,F.,Toyoshima,C., 2013.Crystal structureofaNa1-boundNa1,K1-ATPaseprecedingtheE1Pstate.Nature502,201 206.Copyright2013.WithpermissionfromElsevier.
theenzymeresponsibleforureaproduction,mitochondrialsuperoxidedismutase,and glutaminesynthetase,whichplaysanimportantroleinthebrain.Nevertheless,excessive exposureorintakemayleadtoaconditionknownasmanganism,aneurodegenerative
FIGURE1.4 Ribbonrepresentationshowinghowtargetbindinginduceschangesinthequaternarystructure ofcalmodulin.Theconformationofthetwodomainsof calmodulinisunaffectedbytargetbinding,buttheorientationofthedomainswithrespecttoeachother changesdrastically,bringingthetwopreviously independentdomainsintocontact.Calcium-loaded calmodulin(PDBcode1CLL)isshownatthetopand calcium-loadedcalmodulincomplexedwithapeptide derivedfromsmooth-musclemyosinlight-chainkinase (PDBcode1CDL)isshownatthebottom.TheN-terminaldomainofcalmodulinis mediumblue,theC-terminal domainis darkblue,andthelinkerloopbetweenthe domainsis lightblue.Thepeptideis red andthecalcium ionsarerepresentedas yellow balls.Thetintindicates theConnollysurfacesofthemolecules. Source:From Johnson,C.N.,Damo,S.M.,Chazin,W.J., 2014.EF-handcalcium-bindingproteins.In:EncyclopediaofLifeSciences.John Wiley&SonsLtd., https://doi.org/10.1002/9780470015902. a0003056.pub3.Copyright2014.WithpermissionfromJohn WileyandSons.
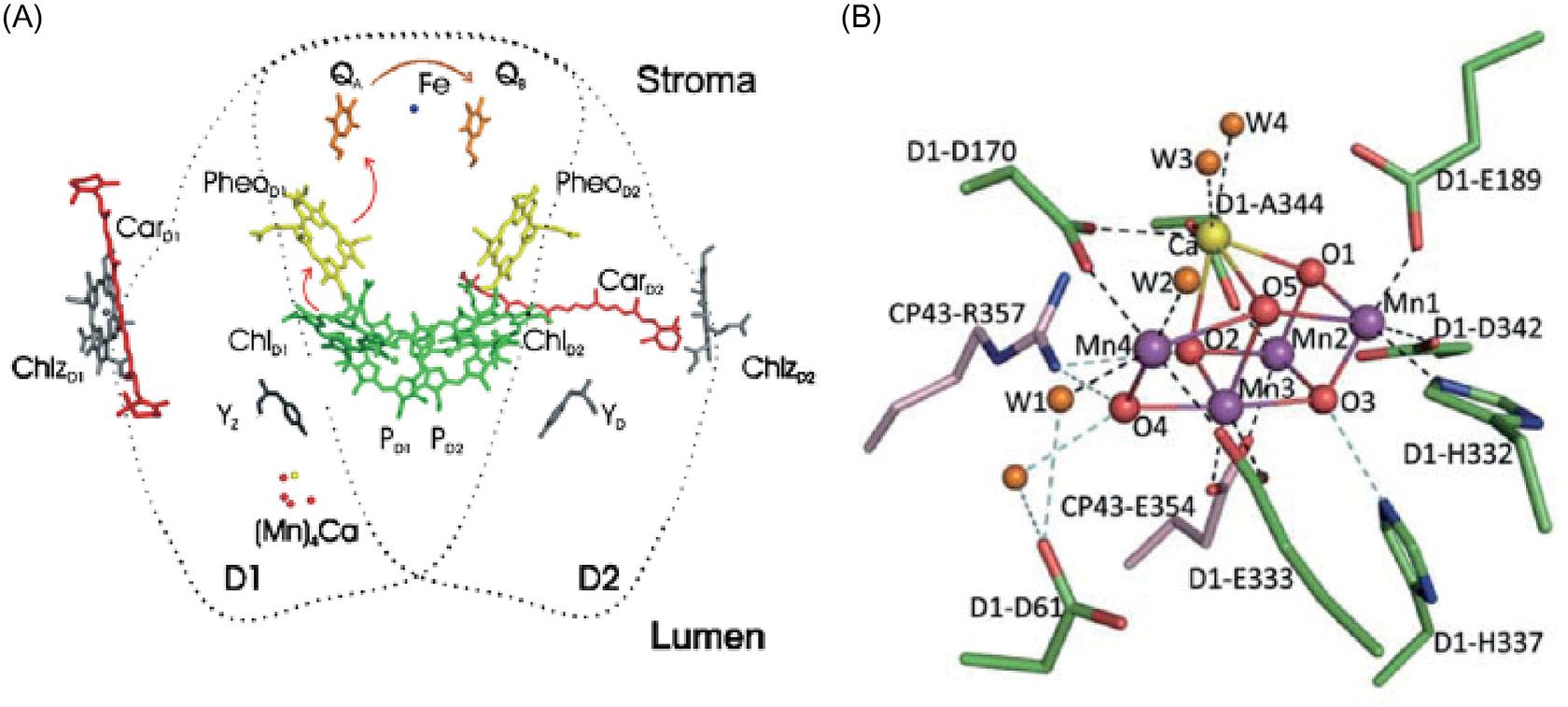
disorderthatcausesdopaminergicneuronaldeathandparkinsonian-likesymptoms(Avila etal.,2013).Clearlythemostimportantroleofmanganeseinbiologyisitsinvolvementin theoxygenevolvingcomplexofphotosystemIIincyanobacteria,algae,andgreenplants, whichoxidizeswaterintodioxygen,protons,andelectrons(Eq. 1.1).
ThedeterminationofthestructureoftheMn4CaO5 cluster(Fig.1.5)atthecenterof PSII(Sugaetal.,2015)hasprovokedanintensiveflurryofbiomimeticchemistry,with theaimofgenerating“greenenergy”usingourunlimitedaccesstosolarpower (Najafpouretal.,2015).
Ironisthemostabundantofthetransitionmetalionsinhumans,withthebulkpresent intheoxygen-bindinghemeproteins,hemoglobinandmyoglobin.Thesebothcontainiron withintheprotoporphyrinIXnucleus,requiringanumberofgenesforbiosynthesisofthe porphyrin,insertionofiron,andsubsequenthemetransport(Crichton,2016;Andreini etal.,2009).Theremainingmuchsmallerproportionofbodyironispresentinotherironcontainingproteins(hemeproteins,Fe Sproteins,andnonheme,non-Fe Sproteins)with awidevarietyoffunctions,encodedbythehumangenome(Crichton,2016).Arecentbioinformaticsapproachindicatesthatabout2%ofhumangenesencodeanironprotein(48% heme-bindingproteins,17%Fe Sproteins,and35%whichbindindividualironions).
FIGURE1.5 (A)Schematicrepresentationofthecofactorarrangementinthecoreofthereactioncenter(the viewisalongthemembraneplane).Organiccofactors(forthesakeofsimplicity,thehemegroupofcytochrome b559 isomitted)arecolored green (Chl), yellow (Pheo), magenta (plastoquinonesQA andQB),and red (carotenoids). Ca(yellow),Fe(blue),andMn(red)areshownasspheres;thefigurewasgeneratedusingPyMOL(http://www. pymol.org).ThecoordinatingproteinsubunitsD1andD2areindicatedbydottedlines.(B)StructuralarrangementoftheMn4CaOx clusterandMn O,Ca O,Mn water,andCa waterdistancesintheoxygenevolving complex(OEC)(inA ˚ )Mn1,Mn2,Mn3,andMn4denotethedifferentMnionsoftheOEC. Source:(A)Reprinted withpermissionfromReger,G., 2012.MechanismoflightinducedwatersplittinginphotosystemIIofoxygenevolvingphotosyntheticorganisms.Biochim.Biophys.Acta1817,1164 1176.Copyright2012Elsevier.(B)Reprintedwithpermission fromSuga,M.,Akita,F.,Hirata,K.,Ueno,G.,Murakami,H.,etal.,2015.NativestructureofphotosystemIIat1.95A ˚ resolutionviewedbyfemtosecondX-raypulses.Nature517,99 103.Copyright2015.NaturePublications.
Morethanhalfofthehumanironproteinshaveacatalyticfunction,andtheauthorsestimatethat6.5%ofallhumanenzymesareiron-dependent(Andreinietal.,2018).
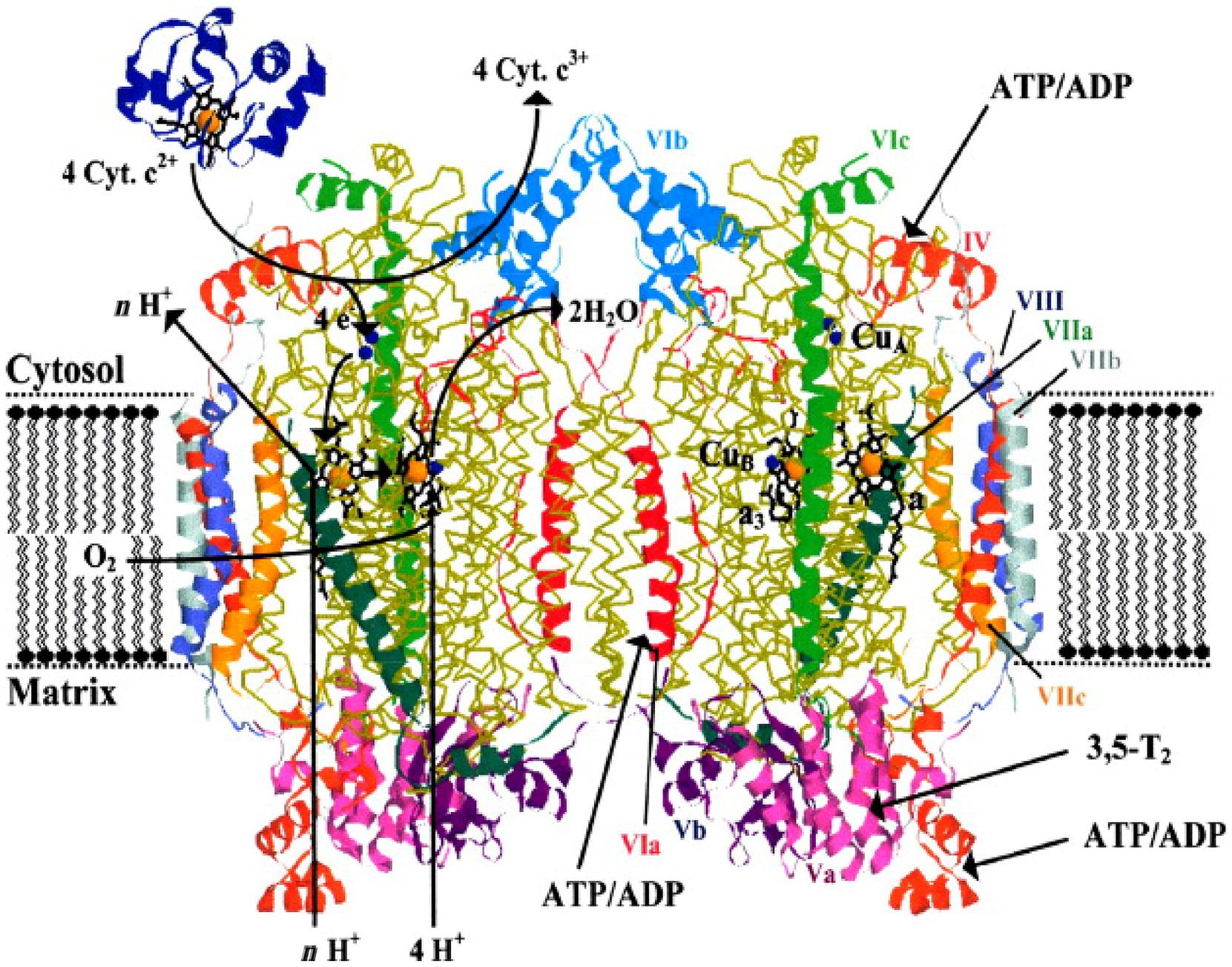
Feisaconstituentofalargenumberofproteinsinvolvedinelectrontransferchainsin humans,notablytherespiratorychainintheinnermembraneofthemitochondria,involvingcytochromes,Fe Sproteins,andquinines,channelingelectronstotheterminalcomponent,theCu Fe-dependentcytochrome c oxidase(COX)whichmediatesthereductionof O2 (Eq.1.2).
4H1 1 4e 1 O2 -2H2 O ð1 2Þ
MammalianCOXiscomposedof13subunits,threecatalyticsubunitsI IIIencodedby mitochondrialDNA,and10nuclear-codedsubunitsencodedbynuclearDNA(Fig.1.6).
Electronsfromcytochrome c aretransferredtothedimetallicCuA site,whichrapidly reducestheheme a,some19A ˚ away.Heme a thentransferselectronstotheactivesite heme a3 andCuB,whereO2 binds.
Copperisthethirdmostabundantessentialtransitionmetalioninthehumanbody, involved,forexample,inrespiration,angiogenesis,andneuromodulation,yetCu proteinsrepresentlessthan1%ofthetotalproteomeinbotheukaryotesandprokaryotes
FIGURE1.6 Crystalstructureofdimericcytochrome c oxidasefrombovineheart(Tsukiharaetal.,1996).The nuclear-codedsubunitsareincolor,themitochondrial-codedsubunitsI,II,andIIIarein yellow.Indicatedschematicallyontheleftmonomeraretheelectrontransportpathwaysfromcytochrome c (Cyt.c)tooxygenaccompaniedbyuptakeofprotonsfromthematrixforwaterformationandpumpedprotons(nH1).Ontheright monomerbindingsitesfor3,5-diiodothyronine(T2)andATPorADPareindicated. Source:FromKadenbach,B., Hu¨ttemann,M., 2015.Thesubunitcompositionandfunctionofmammaliancytochrome c oxidase.Mitochondrion24, 64 76.Copyright2015.WithpermissionfromElsevier.
(Andreinietal.,2009).Coppersitesinproteinscanbeclassifiedasbelongingtooneof threeclasses.Type1(blueCuproteins)functioninsingleelectrontransfer,typeIIarecatalyticsiteswhichbinddirectlytosubstrates,whiletypeIIIsitesaredinuclearandare involvedintheactivationandtransportofoxygen.Thecopperchaperonesareaspecific classofproteinswhichensurethesafeandspecificdeliveryofpotentiallyharmfulcopper ionstoavarietyofessentialcopperproteins(Palumaa,2013).Cuisalsoinvolvedasthe catalyticcomponentindetoxification(Cu/Znsuperoxidedismutase).
Bothironandcopperarecharacterizedbygeneticdisordersassociatedwiththeaccumulationofthesemetalsinparticulartissues,withtoxicconsequences.Wilson’sdiseaseis achronicdiseaseofthebrainandliverduetoadisturbanceofcoppermetabolism,
FIGURE1.7 StructureofvitaminB12 shownastheCo31 corrincomplex,whereR 5 50 deoxyadenosyl,Me,OH ,or CN (cyanocobalamin). Source:Withpermissionfrom Wikipedia(releasedintothepublicdomainbyitsauthor Ymwang42attheWikipediaproject).
accompaniedbyprogressiveneurologicaldysfunction,withprogressiveaccumulationof copperinthebrain,liver,kidneys,andthecorneaoftheeye.Ironoverloadcanresult fromgeneticdefectsinironabsorptionfromthegastrointestinaltract(hereditaryhemochromatosis),butcanalsoresultfromgeneticdysfunctionoferythropoiesis,asinthalassemia,necessitatingregularbloodtransfusions(secondaryhemochromatosis).
Althoughonly1.6mgispresentinthehumanbody,cobaltremainsanessentialtrace element,andisrequiredinthehumandietintheformofcobalamin(vitaminB12),aproductofmicrobialbiosynthesis,wheretheCoistightlyboundinacorrinring(Fig.1.7). VitaminB12 uptakefromthegutrequiresaspecificprotein,intrinsicfactor,whichis secretedbythegastricmucosaandisessentialforefficientabsorptionofthevitamin.Lack ofintrinsicfactorcausesperniciousanemia,andvitaminB12 wasidentifiedin1925asthe antiperniciousanemiafactor.ThisisduetoB12 beinganessentialcofactorforanumberof B12-dependentisomerasesandmethyltransferases(Banerjeeetal.,2009)involvedinDNA synthesis,aminoacidandfattyacidmetabolism,inthesynthesisofmyelinbyoligodendrocyteswrappedaroundtheaxonsofmotorneurons,andinthematurationofdevelopingredbloodcells.Cobaltisacutelytoxicinlargedosesandthiswasdramatically observedinthe1960samongheavybeerdrinkers(15 30pints/day),whenCo21 salts wereaddedasfoamstabilizers,resultinginsevereandoftenlethalcardiomyopathy (Kestelootetal.,1968).
Bioinformaticsanalysisofthehumangenomeindicatesthatoneproteinin10(about 3000intotal)isazincmetalloprotein(Andreinietal.,2006,2009).Zn21 isrepresentedin allsixclassesofenzymes(asdefinedbytheInternationalUnionofBiochemistry),whereit canplaybothastructuralaswellasacatalyticrole,oftenfunctioninglikeMg21 asa Lewisacid.Itcanalsofulfillaveryimportantregulatoryfunctioninthestructuralmotifs
FIGURE1.8 Thestructureofthepyranopterincofactorcommontoallofthese enzymesisgivenatthetop.Activesite structuresfortwofamiliesofmononuclear molybdenumenzymes(xanthineoxidase andsulfiteoxidase). Source:Reprintedwith permissionfromHille,R.,Hall,J.,Basu,P., 2014.Themononuclearmolybdenumenzymes. Chem.Rev.114,3963 4038.Copyright2014. AmericanChemicalSociety.
knownas“zincfingers”involvedintheregulationoftranscriptionandtranslationbyits bindingtoDNAandRNA.Zn21 isthesecondmostabundantofthetracemetals(after iron),andisextensivelyinvolvedinbrainfunction,withmMconcentrationsinsynaptic vesiclesinwhichZnisstoredandfromwhichitisreleasedinacontrolledmanner (BitanihirweandCunningham,2009).Inthecourseofmeioticmaturation,oocytestakeup over2 3 109 zincatoms,andwhenaspermcellentersandfertilizestheoocyte,thistriggersthecoordinatedreleaseofzincintotheextracellularspaceinaprominent“zinc spark,”detectablebyfluorescence(Duncanetal.,2016).Thislossofzincisnecessaryto mediatetheegg-to-embryotransition.
AlthoughMoisrelativelyrareintheEarth’scrust,itisthemostabundanttransition metalinseawater,andsincetheoceansareascloseaswegettotheprimordialsoupin whichlifefirstevolved,itisnosurprisethatMohasbeenwidelyusedinbiology.While MoiswellknownasanimportantcomponentoftheFeMocofactorinnitrogenase,thekey enzymeofnitrogen-fixingorganisms,thereareanumberofMo-dependentenzymesin humans,whichallcontainMointheformofamolybdenumpyranopteridindithiolate cofactor(Fig.1.8).Theseincludexanthineoxidase,involvedinthecatabolismofpurine bases,sulfiteoxidaseinvolvedinsulfurmetabolism,andaldehydeoxidase,involvedin themetabolismofmanydrugs(Hilleetal.,2014).
Ni,V,andCrappeartobebeneficial,andhavebeenproposedtobeessentialforman. Althoughthehumanbodycontainsaround8mgofnickel,noNi-dependentenzymesare known,however,itmaybethatNiisessentialformicroorganismsthatcolonizethe humangut(Zambellietal.,2016).
Toxicmetals Essentialmetalscanbetoxicifexcessiveconcentrationsofthemetalionsaccumulate, ofteninspecifictissuesororgans—anumberofexamplesofwhicharegivenabove. However,asaconsequenceofenvironmentalexposure,anumberofnonessentialmetal
ionscanaccumulatewithinthebodywithtoxicconsequences.Inwhatfollows,webriefly describesomeofthemorecommontoxicmetals.
WhilelittlecredenceisgiventodaytothetheorythatRomancivilizationcollapsedasa resultofchronicleadpoisoning(saturnism),arecentstudyhasshownthat“tapwater” fromancientRomecontained100timesmorePbthanlocalspringwaters(Delileetal., 2014).Wearenowacutelyawarethatsaturnismisamajorenvironmentalconcern,andPb exposureremainsawidespreadproblem,particularlyinthedevelopingworld.Pbtoxicity affectsseveralorgansystemsincludingthenervous,hematopoietic,renal,endocrine,and skeletalsystems.Italsocausesbehavioralandcognitivedeficitsduringbraindevelopment ininfantsandyoungchildren.Pbappearstotargetproteinsthatnaturallybindcalcium andzinc,andexamplesincludesynaptotagmin,whichactsasacalciumsensorinneurotransmission,and δ-aminolevulinatesynthase(ALAD),thesecondenzymeintheheme biosyntheticpathway.HumanALADisactivatedbyZn21 witha Km of1.6pMandinhibitedbyPb21 witha Ki of0.07pM.Pb21 andZn21 appeartocompeteforasinglemetalbindingsite(Simons,1995).
ThetoxicityofcadmiummanifesteditselfamongtheinhabitantsoftheJinzuriverbasin inJapaninthe1950sduetoenvironmentalCdpollutionoriginatingineffluentfromazinc minelocatedintheupperreachesoftheriver.IntheCd-pollutedareas,50% 70%ofthe Cdingestedorallywasderivedfromrice.Itremainsthemostsevereexampleofchronic CdpoisoningcausedbyprolongedoralCdingestion.Cd21 isasoftLewisacidwitha preferenceforeasilyoxidizedsoftligands,particularlysulfur.ItcandisplaceZn21 from proteinswheretheZncoordinationenvironmentissulfurdominated,andgiventhesimilarityofitsionicradiuswiththatofCa21 itcanexchangewithCa21 incalcium-binding proteins.Cadmiumoccursintheenvironmentnaturallyandasapollutantemanating fromindustrialandagriculturalsources.Exposuretocadmiuminthenonsmokingpopulation(thereisahighconcentrationofCdincigarettes)occursprimarilythroughfood,and chronicexposureresultsinrespiratorydisease,emphysema,renalfailure,bonedisorders, andimmunosuppression.
Thebrutalrealityofmercurytoxicitywashighlightedin1956byanenvironmental disasterwhichstruckthepopulationofMinamata,Japan,anditssurroundings. Methylmercurywasreleasedintheindustrialwastewaterfromachemicalfactoryand bioaccumulatedinaquaticfoodchains,reachingitshighestconcentrationsinshellfishand fishinMinamataBayandtheShiranuiSea,whichwheneatenbythelocalpopulation resultedinmercurypoisoning.Ofthe2265victimsofficiallyrecognized,1784died.The symptomsincludeataxia,numbnessinthehandsandfeet,generalmuscleweakness,narrowingofthefieldofvision,anddamagetohearingandspeech.Thebrainistheprincipal targettissueofMeHganditsmajortoxiceffectsareonthecentralnervoussystem,accumulatingparticularlyinastrocytes.ThebiochemicaltargetofHgistheselenocysteineresiduesinselenoenzymes,asHghasanaffinityforSe B1milliontimesgreaterthanits affinityforsulfur(RalstonandRaymond,2010,2018).Theselenoenzymesglutathioneperoxidase,thioredoxinreductase,andthioredoxinglutathionereductasearerequiredtopreventandreverseoxidativedamagetothebrainandneuroendocrinesystem,andthey undergoirreversibleinhibitionbymethylmercury(MeHg).Selenoenzymeinhibition appearslikelytocausemostifnotallofthepathologicaleffectsofmercurytoxicity,asoutlinedin Fig.1.9.
FIGURE1.9 SesequestrationmechanismofHgtoxicity.Asimplifiedportrayalofthenormalcycleofselenoproteinsynthesisisdepictedontheleft.DisruptionofthiscyclebyexposuretotoxicquantitiesofHg(MeHg)is depictedontheright.SelenidefreedduringselenoproteinbreakdownbecomesboundtoHg,formingHgSethat accumulatesincellularlysosomes.IfHgispresentinstoichiometricexcess,formationofinsolubleHgselenides abolishesthebioavailabilityofSeforproteinsynthesis(indicatedbygraytext)andresultsinlossofnormalphysiologicalfunctionsthatrequireselenoenzymeactivities. Source:FromRalston,N.V.,Raymond,L.J.,2018.Mercury’s neurotoxicityischaracterizedbyitsdisruptionofseleniumbiochemistry.Biochim.Biophys.ActaGen.Subj.1862, 2405 2416.
Despitecomprising8%oftheEarth’scrust,themostabundantmetalandthethird mostabundantelementafteroxygenandsilicon,aluminumisnotusedinbiology;however,severalfactorshaveincreaseditsaccesstothebiosphere.First,anincreaseinanthropogenicacidificationofsoils,duetoacidraingeneratedbyemissionsofsulfurdioxide andnitrogenoxidesintheatmosphere,hasresultedinelevatedconcentrationsofAl31 in groundwaters.Onaccountofitslightnessandcorrosionresistance,aluminumiswidely usedforindustrialpurposes,fromtheaerospaceindustrytoconstruction,fromfoodpackagingtopharmaceuticals.Aluminumsaltsareextensivelyutilizedasaflocculentinwater treatment.Thisenhancedbioavailabilityhasresultedintheaccumulationofthismetalin livingorganismsincludinghumans,particularlyintheskeletalsystem,theliver,andthe brain.ThetoxicityofAl31 isassociatedwithanemia,osteomalacia,hepaticdisorders,and certainneurologicaldisorders.ThemoleculartargetsofAltoxicityinvolvedisruptionto thehomeostasisofessentialmetalions,notablyFe,Ca,andMg.AlcanreplaceCainbone andinterferewithCa-basedsignalingevents,andcancompetewithMg21 bindingto phosphategroupsoncellmembranes,ATP,andDNA.However,itislikelythatthemain targetsofAltoxicityareFe-dependentbiologicalprocesses.Al31 hascoordinationgeometrysimilartoFe31,whichshouldenableAl31 tosubverttheplasmairontransportpathway.InthecytosolAl31 isunlikelytobeincorporatedintoferritin,whichrequiresredox cyclingbetweenFe21 andFe31,anditseemslikelythatmostaluminumaccumulatesin mitochondria,whereitcaninterferewithCa21 homeostasis.
Metalsindiagnosisandtherapeutics Inadditiontotheessentialmetalions,alargenumberofothermetals,includingsome thataretoxic,areroutinelyusedinclinicalmedicinebothasdiagnosticandtherapeutic agents,includinggadoliniumandtechnetiumcomplexesusedintheirmillionseveryyear fordiagnosis.Themostwidelyuseddrugsforcancerchemotherapyareplatinumcomplexes(ChellanandSadler,2015).
Webeginbyreviewingsomeofthegrowingnumberofmetallo-organiccomplexes usedforthenoninvasiveimagingtechniqueswhichhaverevolutionizedmodernmedicine, allowingbetterdiagnosisandgreaterpatientcomfort.
Webeginwithoneoftheoldestnoninvasivediagnosticapplications,theuseoftherelativelyinsolublebariumsulfate,BaSO4,asaradiopaquecontrastagentforX-rayimagingof thegastrointestinaltract.
Incontrast,themetastableisotopeoftechnetium, 99mTc,wasfirstcreatedin1937by cyclotronbombardmentofmolybdenum,Itisa γ-emitterwithahalf-lifeof6hours,andis usedintensofmillionsofnuclearmedicineimagingprocedureseveryyear.The3Dscanningtechnique,single-photonemissioncomputedtomography,usesa γ-camerawhich usuallyperformsafull360degreerotationaroundthepatienttoobtainanoptimalreconstruction. 99mTcispredominantlyusedforboneandbrainscans.Fortheformer,pertechnetateionsareuseddirectly,astheyaretakenupbyosteoblasts,whileforbrainscans 99mTcischelatedtohexamethylpropyleneamineoxime,whichlocalizesinthebrain accordingtoregionalbloodflow. 99mTcscintigraphycanalsobecombinedwithcomputed tomographyformorerefinedresolution.
MillionsofdosesofgadoliniumareadministeredeveryyearasMRIcontrastagents.Gd (III)hashighparamagnetism(sevenunpairedelectrons)andafavorableslowelectronic relaxationtime,whichmakesiteffectiveinrelaxingwaterprotonswhichcangenerate contrastinMRimages.Gdasthefreeionishighlytoxicevenatlowdoses(10 20 μmol/ kg),andforthisreasonitisnecessarytouseligandsthatformstablecomplexeswiththe lanthanideion.ThehighaffinityofGdtowardpolyaminocarboxylicacidshasbeen exploitedtoformverystablecomplexes(uptolog KML . 20).Thefirstcontrastagenttobe approvedforclinicalusewas[(Gd-diethylenetriaminepenta-aceticacid(DTPA))(H2O)]2 (Fig.1.10)in1988,whichwasadministeredtomorethan20millionpatientsinthefirst10 yearsofclinicalexperimentation.
Turningnexttometalsasdrugs,prideofplacemustgotoplatinum.Currentlyaround 50%ofallcancertreatmentsinvolvePtcomplexes,essentiallytheoriginaldrugcisplatin cis-[PtCl2(NH3)2],discoveredinthe1960sandapprovedforclinicaluseinthelate1970s, andsubsequentlyjoinedbyitsanalogscarboplatin cis-[Pt(1,1-dicarboxycyclobutane) (NH3)2]andoxaliplatin[Pt(1R,2R-1,2-diaminocyclohexane)(oxalate)](Fig.1.11).Ruthenium complexeshavealsoshownpromiseasanticancerdrugs(Bergamoetal.,2012).
Lithiumisthesimplesttherapeuticagentforthetreatmentofdepressionandhasbeen usedformorethan100years.Lithiumcarbonatecanreversethesymptomsofpatients withbipolardisorder(manic-depression),achronicdisorderwhichaffectsbetween1% and2%ofthepopulation.Thisdiseaseischaracterizedbyepisodicperiodsofelevatedor depressedmood,severelyreducesthepatient’squalityoflife,anddramaticallyincreases
FIGURE1.10 ThestructureoftheMRIcontrast agentGd-DTPA(diethylenetriaminepenta-aceticacid). Source:FromWikipedia www.LookForDiagnosis.
FIGURE1.11 Platinumanticancerdrugsapprovedfor clinicaluse:(A)cisplatin;(B)carboplatin;and(C)oxaliplatin. Source:FromWikipedia.
FIGURE1.12 TheorallyactiveantirheumatoidarthritisdrugAuranofin. Source:FromWikipedia,byBenMills—ownwork,publicdomain, https://commons. wikimedia.org/w/index.php?curid 5 5934319.
theirlikelihoodofcommittingsuicide.Today,itisthestandardtreatment,oftencombined withotherdrugs,forbipolardisorderandisprescribedtoover50%ofbipolardisorder patients.Themolecularbasisofmooddisorderdiseasesandtheirrelationshiptothe effectsoflithiumremainunknown.
Silveriswellknownforitspotentantibacterialproperties,andacombinationofsilver nitrateandasulfonamideantibioticasatopicalantibacterialagentforburnmanagement isstillinusetoday,asaresilver-containingwounddressingsinlieuofantibiotics.
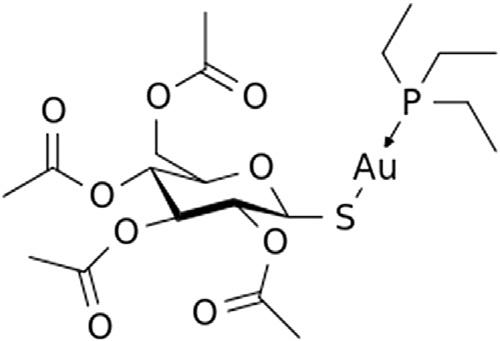
Goldhasalsobeenusedasadrugsinceantiquity(RaubenheimerandSchmidbauer, 2014),withtheAu(I)complexauranofin(Fig.1.12)beingdevelopedforthetreatmentof rheumatoidarthritisasasubstitutefortheinjectablegoldcompoundsaurothiomalateand aurothioglucose.Despiteefficacyinthetreatmentofbothrheumatoidarthritisand
psoriasis,currentlyauranofinisseldomusedasatreatmentforpatientswithrheumatoid arthritisasmorenovelantirheumaticmedicationshavebecomeavailable.However,potentialnewapplicationsofauranofin,notablyforcancertherapyandtreatinginfections includingHIV,basedonitsdualinhibitionofinflammatorypathwaysandthiolredox enzymeslikemitochondrialthioredoxinreductase,haveemerged(Madeiraetal.,2012). Goldnanoparticleshavegreatpotentialforcontrolleddrugdelivery,cancertreatment,biomedicalimaginganddiagnosis,andphotothermaltherapy(Elahietal.,2018).
Whiletherearemanymoreexamplesofmetalsusedasdiagnosticsordrugs,itseems appropriatetoendthissectionbybrieflymentioningtheexcitingdevelopmentoftheranosticagents(Terrenoetal.,2012).Theseintegratediagnosisandtherapy,allowing imaging-guideddrugdelivery,imagingofdrugrelease,monitoringtherapybyimaging, usingtheranosticagentsforradiation-basedtherapies,andtheuseofimagingprobesin imaging-guidedsurgery.
References
Andreini,C.,Banci,L.,Bertini,I.,Rosato,A.,2006.Countingthezinc-proteinsencodedinthehumangenome.J. ProteomeRes.5,196 201.
Andreini,C.,Bertini,I.,Rosato,A.,2009.Metalloproteomes:abioinformaticapproach.Acc.Chem.Res.42, 1471 1479.
Andreini,C.,Putignano,V.,Rosato,A.,Banci,L.,2018.Thehumaniron-proteome.Metallomics10,1223 1231. Avila,D.S.,Puntel,R.L.,Aschner,M.,2013.Manganeseinhealthanddisease.Met.IonsLifeSci.13,199 227. Banerjee,R.,Gherasim,C.,Padovani,D.,2009.Thetinker,tailor,soldierinintracellularB12trafficking.Curr. Opin.Chem.Biol.13,484 491.
Bergamo,A.,Gaiddon,C.,Schellens,J.H.,Beijnen,J.H.,Sava,G.,2012.Approachingtumourtherapybeyondplatinumdrugs:statusoftheartandperspectivesofrutheniumdrugcandidates.J.Inorg.Biochem.106,90 99. Bitanihirwe,B.K.,Cunningham,M.G.,2009.Zinc:thebrain’sdarkhorse.Synapse63,1029 1049.
Brini,M.,Calı`,T.,Ottolini,D.,Carafoli,E.,2013.Intracellularcalciumhomeostasisandsignaling.In:Banci,L. (Ed.),MetallomicsandtheCell,MetalIonsinLifeSciences,12.Springer,Dordrecht,pp.119 168.
Carafoli,E.,Krebs,J.,2016.Whycalcium?howcalciumbecamethebestcommunicator.J.Biol.Chem.291, 20849 20857.
Chellan,P.,Sadler,P.J.,2015.Theelementsoflifeandmedicines.Philos.Trans.AMath.Phys.Eng.Sci.373, Availablefrom: https://doi.org/10.1098/rsta.2014.0182,pii:20140182.
Crichton,R.R.,2016.IronMetabolism:FromMolecularMechanismstoClinicalConsequences,fourthed.John WileyandSons,Chichester,UK,556pp.
Crichton,R.R.,2018.BiologicalInorganicChemistry.ANewIntroductiontoMolecularStructureandFunction, thirded.AcademicPress,London,669pp.
Delile,H.,Blichert-Toft,J.,Goiran,J.P.,Keay,S.,Albare ` de,F.,2014.LeadinancientRome’scitywaters.Proc. Natl.Acad.Sci.U.S.A.111,6594 6599.
Duncan,F.E.,Que,E.L.,Zhang,N.,etal.,2016.Thezincsparkisaninorganicsignatureofhumaneggactivation. Sci.Rep.6,24737.Availablefrom: https://doi.org/10.1038/srep24737. Elahi,N.,Kamali,M.,Baghersad,M.H.,2018.Recentbiomedicalapplicationsofgoldnanoparticles:areview. Talanta184,537 556.
Hille,R.,Hall,J.,Basu,P.,2014.Themononuclearmolybdenumenzymes.Chem.Rev.114,3963 4038.
Johnson,C.N.,Damo,S.M.,Chazin,W.J.,2014.EF-handcalcium-bindingproteins.EncyclopediaofLifeSciences. JohnWiley&Sons,Ltd.Availablefrom: https://doi.org/10.1002/9780470015902.a0003056.pub3 Kadenbach,B.,Hu ¨ ttemann,M.,2015.Thesubunitcompositionandfunctionofmammaliancytochromecoxidase. Mitochondrion24,64 76.
Kanai,R.,Ogawa,H.,Vilsen,B.,Cornelius,F.,Toyoshima,C.,2013.CrystalstructureofaNa 1 -boundNa 1 , K 1 -ATPaseprecedingtheE1Pstate.Nature502,201 206.
Kesteloot,H.,Roelandt,J.,Willems,J.,Claes,J.H.,Joossens,J.V.,1968.Anenquiryintotheroleofcobaltinthe heartdiseaseofchronicbeerdrinkers.Circulation37,854 864.
Maret,W.,2016.Themetalsinthebiologicalperiodicsystemoftheelements:conceptsandconjectures.Int.J. Mol.Sci.17,Availablefrom: https://doi.org/10.3390/ijms17010066,pii:E66.
Madeira,J.M.,Gibson,D.L.,Kean,W.F.,Klegeris,A.,2012.Thebiologicalactivityofauranofin:implicationsfor noveltreatmentofdiseases.Inflammopharmacology20,297 306.
Najafpour,M.M.,Renger,G.,Hoły ´ nska,M.,Moghaddam,A.N.,Aro,E.M.,etal.,2015.Manganesecompoundsas water-oxidizingcatalysts:fromthenaturalwater-oxidizingcomplextonanosizedmanganeseoxidestructures. Chem.Rev.116,2886 2936.
Palumaa,P.,2013.Copperchaperones.Theconceptofconformationalcontrolinthemetabolismofcopper.FEBS Lett587,1902 1910.
Ralston,N.V.C.,Raymond,L.J.,2010.Dietaryselenium’sprotectiveeffectsagainstmethylmercurytoxicity. Toxicology278,112 123.
Ralston,N.V.,Raymond,L.J.,2018.Mercury’sneurotoxicityischaracterizedbyitsdisruptionofseleniumbiochemistry.Biochim.Biophys.ActaGen.Subj.1862,2405 2416.
Raubenheimer,H.G.,Schmidbauer,H.,2014.Thelatestartandamazingupswingingoldchemistry.J.Chem. Educ.91,2024 2036.
Reger,G.,2012.MechanismoflightinducedwatersplittinginphotosystemIIofoxygenevolvingphotosynthetic organisms.Biochim.Biophys.Acta1817,1164 1176.
Simons,T.J.,1995.TheaffinityofhumanerythrocyteporphobilinogensynthaseforZn2 1 andPb2 1 .Eur.J. Biochem.234,178 183.
Suga,M.,Akita,F.,Hirata,K.,Ueno,G.,Murakami,H.,etal.,2015.NativestructureofphotosystemIIat1.95A
resolutionviewedbyfemtosecondX-raypulses.Nature517,99 103.
Terreno,E.,Uggeri,F.,Aime,S.,2012.Imageguidedtherapy:theadventoftheranosticagents.J.Control.Release 161,328 337.
Toyoshima,C.,Kanai,R.,Cornelius,F.,2011.FirstcrystalstructuresofNa 1 ,K 1 -ATPase:newlightontheoldestionpump.Structure.19,1732 1738.
Tsukihara,T.,Aoyama,H.,Yamashita,E.,Tomizaki,T.,Yamaguchi,H.,etal.,1996.Thewholestructureofthe13subunitoxidizedcytochromecoxidaseat2.8A.Science272,1136 1144.
Zambelli,B.,Uversky,V.N.,Ciurli,S.,2016.Nickelimpactonhumanhealth:anintrinsicdisorderperspective. Biochim.Biophys.Acta1864,1714 1731.
Furtherreading Miller,A.,Korem,M.,Almog,R.,Galboiz,Y.,2005.VitaminB12,demyelination,remyelinationandrepairinmultiplesclerosis.J.Neurol.Sci.233(1 2),93 97.
Orrenius,S.,Zhivotovsky,B.,Nicotera,P.,2003.Regulationofcelldeath:thecalcium-apoptosislink.Nat.Rev. Mol.CellBiol.4,552 565.
MatijaZlatar1 andMajaGruden2 1DepartmentofChemistry,InstituteofChemistry,TechnologyandMetallurgy,University ofBelgrade,Belgrade,RepublicofSerbia 2FacultyofChemistry,UniversityofBelgrade, Belgrade,RepublicofSerbia
Introduction18
Introductiontoquantumchemistry18 Approximationsinquantumchemistry21
Somequantitativeconsiderations48
Introductiontocomputational chemistry53 Thewavefunction basedmethods55 Densityfunctionaltheory58 Computationalmethodsforexcited states62
Computationalmethodsforbiological systemscontainingtransitionmetal63 Concludingremarks65
Introduction Chemists,biochemists,andbiologistsusuallydonotlikemathematics.However,to understandbasicconceptsofchemicalbondingintransitionmetal(TM)compounds, theirspectral,magnetic,andotherproperties,ligandfieldtheory(LFT),computational chemistry,andquantummechanics(QM)areabsolutelynecessary.Thischapterstarts withanintroductiontoquantumchemistry,followedbyadescriptionofanelectronic structureofatoms,andbriefsymmetryconsiderations.ThisgivesthebasisfortheLFT whichisthendescribed.Inthesecondpartofthechapter,anoverviewofthetechniques ofcomputationalchemistryisgiven,withanemphasisonthepracticalcomputationofthe electronicstructureandpropertiesofTMcompounds.Theauthorshavetriedtoreduce thenumberofmathematicalequationstotheminimum,andstronglyencouragereaders nottobedemoralizedreadingthesetopics,becauseallbasicconceptsnecessarytounderstandLFT,thebeautyofcoordination,andcomputationalchemistryarepresented.
Introductiontoquantumchemistry Understandingthepropertiesofmatteratthemolecularlevelreliesonthelawsof QM.Thisbranchofphysicswasdevelopedinthefirsthalfofthe20thcenturytoexplain experimentalobservationsunaccountableby classicalphysics.Theclarificationofblackbodyradiation,photoelectriceffect,theComptoneffect,andthelinespectrumofthe hydrogenatomledtothekeyconceptsinQM:quantizationofenergyandmomentum, wave particleduality,andtheuncertaintyprinciple.Quantizationofphysicalquantities impliesthattheycanhaveonlydiscretevaluesandarenotcontinuousvariables. Wave particledualityindicatestherelationbetweenmomentum(propertyofparticles) andwavelength(waveproperty).Forexample,electronswhichare“normally”consideredasnegativelychargedparticleshavewave-likepropertiesand,ontheotherhand, lightwaveshaveparticle-likeproperties.Asparticlesbecomesmaller,itislessvalidto considerthemashardspheresbecausethey aremorewave-like.Anelectrondoesnot movealongthedefinitepath,itisawavedistributedthroughspace.Consequently,QM dictatesthatthepositionandmomentumofaparticlecannotbemeasuredatthesame time.Thisisnothingtodowiththeprecisionorqualityofthemeasurements,butis intrinsictotheQMdescriptionofphenomena.ItisimportanttoemphasizethatQMis madetoexplaintheobservedfactseventhoughourcommonsensemaybepuzzledwith it.Ourexperienceisbuiltuponmacroscopic,everydayphysicsthatmaynotworkinthe worldofatomsandmolecules.WewillnotdelvehereintothedetailsofQM,butwill mainlyfocusonconceptsessentialforunderstandingcoordinationcompoundsandthe basicsofcomputationalchemistry.Theinterestedreaderisreferredtomorespecialized textbooksonthetopic(seeReferencessectionattheendofthischapter; Szaboand Ostlund,1996; AtkinsandFriedman,2005; Levine,2017; Atkinsetal.,2018).
AcentralquantityinQMisthewavefunction, Ψ.Itisafunction,oftencomplicated,in generalcomplex,ofthecoordinatesofalltheparticlesinthesystemunderconsideration, aswellasoftime.Itcontainsalltheinformationthatcanbedeterminedexperimentally. However,wavefunctionitselfcannotbeobserved.ThisisoneoftheQMquirks—a
quantitythatdescribeseverythinginthesystemisjustamathematicalconstruction.But thesquareofthewavefunction, Ψ2 5 Ψ Ψ (Ψ isthecomplexconjugateof Ψ)isareal quantity,interpretedasaprobabilitydistributionofaparticle.Inotherwords,the probabilityoffindingaparticleinadifferentialvolumeelement, dV isproportionalto Ψ2 dV .Themeasureoftheprobabilityoffindinganelectronataspecificlocationisthe electrondensity, ρ.Electrondensityisdetermined,fromitsdefinition,by Ψ,butalso,the otherwayaround,electrondensitydeterminesthewavefunction(apartfromitsphase). Thisistheformalfoundationof densityfunctionaltheory (DFT)(seebelow).Electron density,unliketheelectron’swavefunction,isobservable—forexample,X-raycrystallographyandscanningtunnelingmicroscopyareexperimentaltechniquesthatcanmeasure theelectrondensity.
Thewavefunctionsaresolutionsofthe Schrodinger’sequation (SE).Itisafundamental equationofQMandappliestoallkindsofsystems,atoms,molecules,macromolecules, materials.Initsnonrelativistic,time-independentformSEreads
where E istheenergyand ^ H isthe Hamiltonian Eq.(2.1) hidesallthecomplexityinits elegance.Wemustnotethattherightsideoftheequationisnotasimplemultiplication, thuswecannoteliminate Ψ frombothsidestosimplygetthat ^ H and E areequal.Thisis because ^ H isnotasimplecoefficientmultiplying Ψ,itisan operator,andthisisindicated bythefunny“hat”symbolontopofthesymbol H.Anoperatorrepresentsasetof mathematicalrulesthat“act”onthewavefunction.Anoperatorcanbeasimple multiplicationbyanumber,orbyafunction,o radifferentiation.InQM,everyphysical quantityisdescribedbyanoperator.Wehaveoperatorsforthemomentum,position, dipolemoment,etc.Energy, E ,isdescribedbytheHamiltonian,the operatorofenergy . Thereforealthoughwecannotmaketheequivalenceof ^ H and E inastrictsense,SEtells usthatonceweactonawavefunctionwithasetofrulesdescribedbytheHamiltonian, wewillgettheenergyofasystem(multiplyingtheoriginalwavefunction).The Hamiltoniancanbeconstructedforanysystem,andboththeenergyandthewave functionareobtainedasasolutionofSEsimultaneously.TheSEisaneigenvalueequation,andtherearetwounknowns(Ψ and E ),unliketypicalequationsweareusedto. ThewavefunctionsatisfyingSEforasystemistheeigenfunctionoftheHamiltonianfor suchasystem,andenergyisitseigenvalue.SEdoesnothaveasingle,specificsolution. Therewillbeadifferentsolutionforeverydifferentdistributionofparticles,thatis,for differentvaluesofenergy.Although,therewillbeaninfinitenumberofsolutions, energycanhaveonlydiscretevalues.Thisquantizationofenergyisadirectconsequence oftheSE.Thewavefunctionwiththelowestenergyiscalledthe groundstatewave function,orsimplythe groundstate.Alltheothersare excitedstates.Iftwo(ormore)wave functionshavethesame E, thesewavefunctionsare degenerate .Degeneracyofthe solutionsofSEisaconsequenceofthesymmetryofasystem.SolvingSEisnottheonly wayofgettingenergies,differenceinenerg iesbetweenthestatescanbedetermined,for example,byspectroscopicmeasurements.Thereforethereisalwaysaone-to-one correspondencebetweentheQMdescriptionofthesystemandexperiment.
Initially,wesaidthat Ψ hasalltheinformationaboutanyexperimentalobservation.So far,wedidnotspecifywhereisthisinformation.Foreveryphysicalpropertyofthe
system O,describedbyamatchingoperator ^ O ,theaveragevalueofthatpropertyisgiven bythe expectationvalue oftheoperator: hOi 5 Ð Ψ ^ OΨ dt 5 hΨ ^ O Ψ i.Thewavefunction doesnotneedtobeaneigenfunctionoftheoperator,butthewavefunctionmustbe normalized,thatis, Ð Ψ Ψ dt 5 hΨΨ i 5 1 j .Thelastexpressionisjuststatingthatthe probabilityoffindingaparticleanywhereinthespaceisequalto1,thatis,aparticleexists inthesystem.Typically,whentalkingabout Ψ,itisconsideredthattheyarenormalized. Onemayalways normalize awavefunction,simplymultiplyingitwithaconstant, normalizationconstant,suchthatthenormalizationconditionissatisfied.Normalizationis, ingeneral,aseparateproblemformtheSE,anditremovesanyambiguityrelatedtothe magnitudeof Ψ.Theexpressionswith“ ,. ”areintegralswritteninDirac’snotation, whichiscommonlyusedinQM.
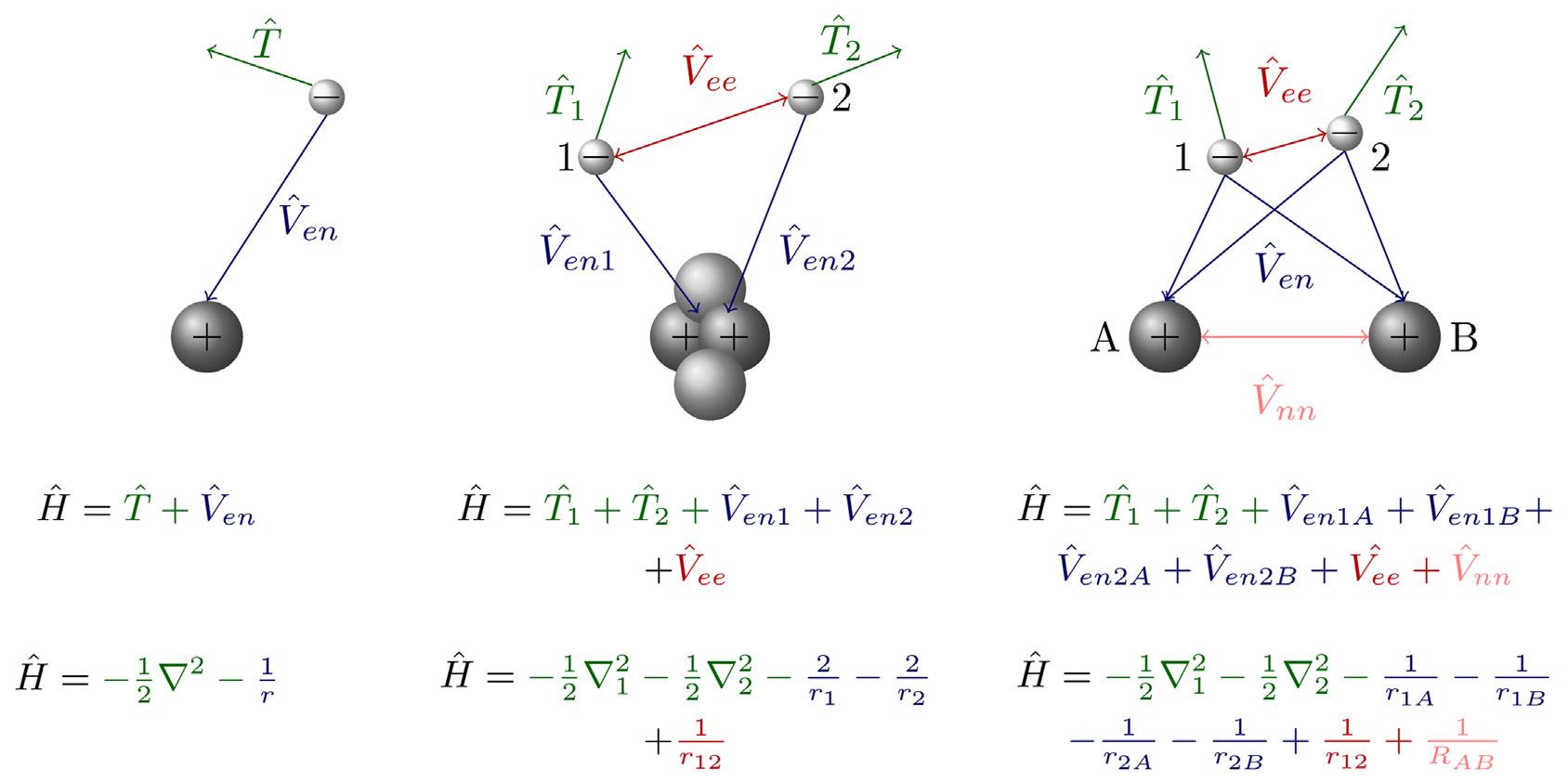
TheHamiltonianportraysourknowledgeaboutthesystem.Constructingthe Hamiltonianfromafewfundamentalformulasi sstraightforward.Inclassicalphysics totalenergyisasumofpotentialandkineticenergies.AnalogouslytheHamiltonian,the totalenergyoperator,isasumofthekineticenergyoperatorandthepotentialenergy operator: ^ H 5 ^ T 1 ^ V .Thepotentialenergyoperatoristhesameasitscorresponding classicalquantity.Moreprecisely,itisapositionoperatorthatisthesame.Considering atomsandmolecules,potentialenergyisjusttheCoulombicinteractionbetweencharged particleswithcharges qi onadistance r.Kineticenergyrequiresdealingwiththe momentumoperator,whichhasaspecialform,forthemomentumalong x -coordinate: ^ p x 52 i hd=dx ,where i2 52 1,andh¯istheconstant(thePlanck’sconstantdividedby2 π, calledthereducedPlanck’sconstantandequalto1:054571800 3 1034 Js).Thisformofthe momentumoperatorensuresthatposition/momentumuncertaintyissatisfied. Operatorscorrespondingtootherphysicalobservablescanbeconstructedinasimilar manner.Expressionsforthekineticenergyoperatorandthepotentialenergyoperatorin atomicandmolecularproblemsaregivenin Eq.(2.2).Examplesfortheformof Hamiltonianexpressedasthesumofkinetic andpotentialenergytermsforH-atom, He-atom,andH2 moleculearegivenin Fig.2.1.In Fig.2.1 expressionsaregivenin atomicunits(thenumericalvaluesofelectro nmass,elementarycharge,reducedPlanck’s constant,andCoulomb’sconstantarealltakentobeunity),andsymbol r 2 (Laplacian)is usedforasumofthesecondderivativesalongallthreeCartesiancoordinates.This somewhatsimplifiestheexpressions.
Weseethatonlytwofundamentalinteractionsarenecessarytodescribeatomsand molecules.Notethatoneneedstoconsiderallpossibleinteractionsbetweenelectrons andnuclei,andamongeachother,aswellasthekineticenergyofallelectrons. ThereforetheHamiltonianquicklybecomesquitecomplicatedasthenumberofparticles increases.Here,andintherestofthechapter,weconsidertheelectronicSE—fromthe pointofviewofelectrons,nucleiarenotmoving,thereforethereisnokineticenergyof nucleiintheexpressions,and ^ V nn isaconstantforagivenmolecularstructure.Inother words,muchheaviernucleimoveslowerthanelectrons,andSEcanbeseparatedinto theelectronicandnuclearpart.Forato msandions,thecenterofmassisinagood
FIGURE2.1 Schematicrepresentationoftheinteractionsin(fromlefttoright)hydrogenatom,heliumatom, andhydrogenmolecule.
approximationatthenucleusandthemotio nsrelativetothecenterofmassarethe motionsoftheelectron.Formolecules,separationofelectronandnuclearmotionis thebasisofthe Born Oppenheimer(BO)approximation.IntheBOapproximation,wesolve theSEforelectronsonly.Still,theresultingmolecularelectronicenergydependsonthe nuclearcoordinates,leadingtothemolecularpotentialenergycurvefordiatomics,and potentialenergysurfacesforageneralpolyatomicmolecule.
Approximationsinquantumchemistry ThecomplexityoftheSEpreventstheexactanalyticalsolvabilityofthissecond-order differentialeigenvalueequation,apartfromsomesimpleproblems.Thedifficultiesthat arisewhenweattempttosolvetheSEforthesystemthathasmorethanoneelectronis notsomeexoticQMcomplexitybutoriginatesfromthethree-bodyproblemthatdoesnot haveanalyticalsolutionsinclassicalmechanicseither.Even“simple”problems,likethe H-atom,arenotwhatmostchemistswouldliketosolveonpaper,orevenreadinabook chapter.Inaddition, Ψ isveryhardtovisualize,afactthatgoesagainstachemist’s intuition.Thuswemustturntoapproximations.Theroleofapproximationsistwofold. First,simplifiedsolutionsallowustobuildupmodelsofhierarchicalcomplexitythatwill letusimproveourknowledgeofthesystem.Second,approximationsaretheonlywayto solvetheSEequation.Evenwithapproximations,solvingSEwillrelyonnumerical mathematicsandonthepowerofcomputers.Resultsofsuchcomputationsneedtobe comparedwiththechemist’squalitativeunderstandingoftheproblem.Todothatwe needmodels.Anydiscrepancybetweenourqualitativemodelandcomputationalresults willeithermakeuslearnsomethingnewbymakingourmodelsbetterormakeusincrease theaccuracyofthecomputations.