https://ebookmass.com/product/practical-application-ofsupercritical-fluid-chromatography-for-pharmaceutical-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Bioinformatics Tools for Pharmaceutical Drug Product Development 1st Edition Edition Vivek Chavda
https://ebookmass.com/product/bioinformatics-tools-for-pharmaceuticaldrug-product-development-1st-edition-edition-vivek-chavda/
ebookmass.com
Evidence Based Practice for Nurses: Appraisal and Application of Research 4th Edition, (Ebook PDF)
https://ebookmass.com/product/evidence-based-practice-for-nursesappraisal-and-application-of-research-4th-edition-ebook-pdf/
ebookmass.com
Introduction to Forensic Psychology: Research and Application (NULL)
https://ebookmass.com/product/introduction-to-forensic-psychologyresearch-and-application-null/
ebookmass.com
John Locke and Agrarian Capitalism Neal Wood
https://ebookmass.com/product/john-locke-and-agrarian-capitalism-nealwood/
ebookmass.com
A Wolf in Duke's Clothing Susanna Allen https://ebookmass.com/product/a-wolf-in-dukes-clothing-susannaallen-3/
ebookmass.com
Multi-Scale Approaches in Drug Discovery. From Empirical Knowledge to In Silico Experiments and Back 1st Edition Edition Alejandro Speck-Planche (Eds.)
https://ebookmass.com/product/multi-scale-approaches-in-drugdiscovery-from-empirical-knowledge-to-in-silico-experiments-andback-1st-edition-edition-alejandro-speck-planche-eds/ ebookmass.com
To Honor (The Knight School Chronicles Book 1) Cecelia Mecca
https://ebookmass.com/product/to-honor-the-knight-school-chroniclesbook-1-cecelia-mecca/
ebookmass.com
The Danger Imperative: Violence, Death, and the Soul of Policing Sierra-Arévalo
https://ebookmass.com/product/the-danger-imperative-violence-deathand-the-soul-of-policing-sierra-arevalo/
ebookmass.com
Spinoza and Biblical Philology in the Dutch Republic, 1660-1710 Jetze Touber
https://ebookmass.com/product/spinoza-and-biblical-philology-in-thedutch-republic-1660-1710-jetze-touber/
ebookmass.com
England's Jews: Finance, Violence, and the Crown in the Thirteenth Century John Tolan
https://ebookmass.com/product/englands-jews-finance-violence-and-thecrown-in-the-thirteenth-century-john-tolan/
ebookmass.com
SEPARATIONSCIENCEANDTECHNOLOGY VOLUME14 Areferenceserieseditedby
SATINDERAHUJA PRACTICALAPPLICATIONOF SUPERCRITICALFLUID CHROMATOGRAPHYFOR PHARMACEUTICALRESEARCH ANDDEVELOPMENT VOLUME14 Editedby MICHAEL HICKS
AnalyticalResearch&Development,MerckResearchLabs,Rahway,NJ,UnitedStates
PAUL FERGUSON
NewModalities&ParenteralDevelopment,PharmaceuticalTechnology&Development,Operations, AstraZeneca,Macclesfield,UnitedKingdom
AcademicPressisanimprintofElsevier
125LondonWall,LondonEC2Y5AS,UnitedKingdom
525BStreet,Suite1650,SanDiego,CA92101,UnitedStates
50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(other thanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfrom anyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-88487-7
ISSN:1877-1718
ForinformationonallAcademicPresspublications
visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: CharlotteRowley
EditorialProjectManager: ZsereenaRoseMampusti
ProductionProjectManager: SruthiSatheesh
CoverDesigner: VickyPearsonEsser
TypesetbySTRAIVE,India
Dedication Forthetwoconstantsinmylife,IsobelandEmily,withoutwho’spatienceandsupportthisbook wouldnothavebeenpossible
PaulFerguson,November2022
Formyfamily,Thomas,Brendan,Georgina,andKathy,forinspiringmeandformycolleaguesfor sharingthisjourneywithme
MichaelHicks,November2022
Contents Contributorsxi
Prefacexiii
1.EvolutionofpackedcolumnSFC
asagreeneranalyticaltoolfor pharmaceuticalanalysis
SusanOlesikandRaffealBennett
Discoveryofsupercriticalfluidsanditsrelevanceto analyticalseparationscience1
Sustainableaspectsofsubcriticalandsupercritical chromatographicseparationmethods9
AnalyticalscalesubcriticalSFC10
Analyticalenhanced-fluidityliquid chromatography12
Preparativeandanalyticalscaleinstrument improvements13
Currentutilityandrecentadvancements20
Futuredirections22
References22
2.ApplicationspaceforSFCin pharmaceuticaldrugdiscovery anddevelopment
GioacchinoLucaLosacco,AmandineDispas,Jean-LucVeuthey, andDavyGuillarme
Introduction29
Discussion33
ConsiderationsonSFCasananalyticaltoolindrug discoveryanddevelopment38
Conclusionsandperspectives42 References43
3.SelectionofSFCstationary andmobilephases
CarolineWestandEricLesellier
Introduction49
CurrentstationaryphasechemistriesforSFC49
Flexiblemobilephasecomposition59
Otheroperatingparameters:Temperature,pressure andflowrate64
Predictingretentionandselectivity65
Summary66
References66
4.Measurementsofdrugsandmetabolites inbiologicalmatricesusingSFC andSFE-SFC-MS
BradyW.Drennan,A.PaigeWicker,BlairK.Berger, andKevinA.Schug
Introductiontodrugandmetaboliteanalysis inbiologicalmatricesusingSFCand SFE-SFC-MS73
On-lineSFE-SFC-MSmethoddevelopmentfor biologicalmatrices75
Drugmetabolismandpharmacokinetics(DMPK) monitoringindiscoveryanddevelopment88 Discoveryanddereplicationofnaturalproducts93
Conclusions94
References94
5.Syntheticchemistryscreeningforrobust analysisandpurificationfromdiscovery throughtodevelopment
TimUnderwood,SeanHindley,AndyKnaggs,andCraigWhite
Introduction101
Screeningstrategies103
Practicality119
Aspectsforconsideration123
Futurerefinements128
References129
6.ApplicationofpreparativeSFCinthe pharmaceuticalindustry
JenniferKingston,HannaLeek,AstridBuica,Kristina Ohlen, KatieProctor,JoannaRaubo,MatthewSanders,andLindaThunberg
IntroductiontotheuseofpreparativeSFC133
PreparativeSFCinstrumentationand infrastructure134
FeaturesandcontrolsduringpreparativeSFC138
Temperaturecontrol143
Detectionsystems143
Fractioncollection144
MethoddevelopmentinpreparativeSFC145
PreparativeSFCapplications:Casestudieswithin AstraZenecaresearchlaboratories148
SFCasasustainablechromatographictechnique159
Conclusions163 References163
7.Methoddevelopmentapproaches forsmall-moleculeanalytes
SyameKhater,PaulFerguson, andAlexandreGrand-Guillaume-Perrenoud
Introduction167
Methoddevelopment“prework”170
ScreeningtoolutilizationtoidentifyoptimalSFC parameters174
Methodvalidation205
Continuousmethodperformanceverification210
Summary211
Acknowledgments212 References212
8.ApplicationofSFCforthe characterizationofformulated drugproducts
PaulFerguson,RebeccaCross,andGesaSchad
Introduction221
Drugformulations222
Samplepreparationprocedures225
Waterandorganicsolventsassamplediluentsin SFCforAPIsandsolid-oraldosageforms230
AlternativeapproachestosolubilizeanalytesinSFC compatiblesolvents237
Characterizationofpolymerexcipients241
Conclusions246
Acknowledgments247
Appendix:Constituentsofformulateddrugsdiscussed inchapter247 References252
9.ExpandingtheboundariesofSFC: Analysisofbiomolecules
MartinBeres
Historicalproblemsanalyzingpolarmoleculesvia SFC257
RoleofwaterinmodernSFC261
Enhanced-fluidityliquidchromatography269
ApplicationsofSFCtobiomolecules279
Concludingremarks290
References290
10.DifferentdetectorsusedwithSFC
G.JohnLangley,SergioCancho-Gonzalez, andJulieM.Herniman
Introductiontodetectorsusedwith modernSFC299
GenericdetectorsusedwithSFC300
CouplingSFCtomassspectrometric detectors313
Conclusions321
Acknowledgment321 References321
11.SFCinGMPtestingandquality controlofmedicinaldrugproducts
AdrianClarke,PaulFerguson,andMichaelHicks
Introduction325
CurrentuseofSFCinpharmaceutical development327
TransferofmethodstomanufacturingQC facilities332
Futurerequirementstowardregulatoryacceptanceof SFCmethods344
Conclusions348
Acknowledgments348 References348
12.Bestpracticesandinstrumental troubleshootingforsuccessful SFCmethods
FionaBell,PaulFerguson,RebeccaPoulten,EmilyRoddy, andWilliamFarrell
Introduction353
Systemconfiguration354
Bestpracticeforsystemsetup356
Systemperformancechecks357
Cylinderissues361
Instrumenttroubleshootinganderrors364
Eluenteffects369
Chromatographictroubleshooting371
Optimizationofdetectorsensitivity374
Conclusions374 References375
13.Thestate-of-the-artandfuture perspectivesforSFC
PaulFergusonandMichaelHicks
Introduction377
Reflectiononpreviouschapters378
TheoreticalperformanceofSFC382
Influenceofnewcolumnparticletechnologiesand instrumentdesignonSFCperformance383
Considerationsforfutureinstrumentapplicationand design384
Methoddevelopment,performanceandprediction aspects386
Methodscaling390
FuturedirectionsandapplicationsofSFC392
Sustainableinstrumentdesign394
Conclusions397
Acknowledgments398
References398
Listofabbreviations403
Index409
Contributors FionaBell GlobalChemicalDevelopment, PharmaceuticalTechnology&Development, Operations,AstraZeneca,Macclesfield, UnitedKingdom
RaffealBennett Merck&Co.,Inc.,MRL,AnalyticalResearch&Development,Boston,MA, UnitedStates
MartinBeres Separation&AnalysisTechnology Team,BristolMyersSquibb,Princeton,NJ, UnitedStates
BlairK.Berger DepartmentofChemistry&Biochemistry,TheUniversityofTexasatArlington,Arlington,TX,UnitedStates
AstridBuica EarlyChemicalDevelopment, PharmaceuticalSciences,BioPharmaceuticals Research&Development,AstraZeneca, Gothenburg,Sweden
SergioCancho-Gonzalez FacultyofEngineering&PhysicalSciences,SchoolofChemistry, UniversityofSouthampton,Southampton, UnitedKingdom
AdrianClarke Chemical&AnalyticalDevelopment,NovartisPharmaAG,TechnicalR&D, Basel,Switzerland
RebeccaCross GlobalChemicalDevelopment, PharmaceuticalTechnology&Development, Operations,AstraZeneca,Macclesfield, UnitedKingdom
AmandineDispas UniversityofLiege(ULiege), CIRM,Vibra-SanteHub,LaboratoryofPharmaceuticalAnalyticalChemistry;Universityof Liege(ULiege),CIRM,Mas-SanteHub,LaboratoryfortheAnalysisofMedicines,Liege, Belgium
BradyW.Drennan DepartmentofChemistry& Biochemistry,TheUniversityofTexasat Arlington,Arlington,TX,UnitedStates
WilliamFarrell OncologyMedicinalChemistry, Pfizer,Inc.,WorldwideMedicinalChemistry, LaJollaLaboratories,SanDiego,CA, UnitedStates
PaulFerguson NewModalities&Parenteral Development,PharmaceuticalTechnology& Development,Operations,AstraZeneca,Macclesfield,UnitedKingdom
AlexandreGrand-Guillaume-Perrenoud Chemical&AnalyticalDevelopment,Novartis PharmaAG,TechnicalR&D,Basel,Switzerland
DavyGuillarme SchoolofPharmaceuticalSciences;InstituteofPharmaceuticalSciencesof WesternSwitzerland,UniversityofGeneva, CMU,Geneva,Switzerland
JulieM.Herniman FacultyofEngineering& PhysicalSciences,SchoolofChemistry, UniversityofSouthampton,Southampton, UnitedKingdom
MichaelHicks AnalyticalResearch&Development,MerckResearchLabs,Rahway,NJ, UnitedStates
SeanHindley GlaxoSmithKlineMedicineResearchCentre,Stevenage,Hertfordshire, UnitedKingdom
SyameKhater TechnologieServier,Orleans, France
JenniferKingston OncologyChemistry,OncologyResearch&EarlyDevelopment, AstraZeneca,Cambridge,UnitedKingdom
AndyKnaggs GlaxoSmithKlineMedicineResearchCentre,Stevenage,Hertfordshire, UnitedKingdom
G.JohnLangley FacultyofEngineering& PhysicalSciences,SchoolofChemistry, UniversityofSouthampton,Southampton, UnitedKingdom
HannaLeek EarlyChemicalDevelopment, PharmaceuticalSciences,BioPharmaceuticals Research&Development,AstraZeneca, Gothenburg,Sweden
EricLesellier UniversityofOrleans,CNRS, InstituteofOrganic&AnalyticalChemistry (ICOA),UMR,Orleans,France
GioacchinoLucaLosacco SchoolofPharmaceuticalSciences;InstituteofPharmaceuticalSciencesofWesternSwitzerland,Universityof Geneva,CMU,Geneva,Switzerland;Analytical ResearchandDevelopment,MRL,Merck&Co, Inc.,Rahway,NJ,UnitedStates
Kristina € Ohlen EarlyChemicalDevelopment, PharmaceuticalSciences,BioPharmaceuticals Research&Development,AstraZeneca, Gothenburg,Sweden
SusanOlesik DepartmentofChemistry,The OhioStateUniversity,Columbus,OH, UnitedStates
RebeccaPoulten EarlyChemicalDevelopment, PharmaceuticalSciences,Research& Development,AstraZeneca,Macclesfield, UnitedKingdom
KatieProctor OncologyChemistry,Oncology Research&EarlyDevelopment,AstraZeneca, Cambridge,UnitedKingdom
JoannaRaubo OncologyChemistry,Oncology Research&EarlyDevelopment,AstraZeneca, Cambridge,UnitedKingdom
EmilyRoddy EarlyChemicalDevelopment, PharmaceuticalSciences,Research& Development,AstraZeneca,Macclesfield, UnitedKingdom
MatthewSanders OncologyChemistry,OncologyResearch&EarlyDevelopment, AstraZeneca,Cambridge,UnitedKingdom
GesaSchad ShimadzuEuropaGmbH, Duisburg,Germany
KevinA.Schug DepartmentofChemistry& Biochemistry,TheUniversityofTexasat Arlington,Arlington,TX,UnitedStates
LindaThunberg EarlyChemicalDevelopment, PharmaceuticalSciences,BioPharmaceuticals Research&Development,AstraZeneca, Gothenburg,Sweden
TimUnderwood GlaxoSmithKlineMedicine ResearchCentre,Stevenage,Hertfordshire, UnitedKingdom
Jean-LucVeuthey SchoolofPharmaceuticalSciences;InstituteofPharmaceuticalSciencesof WesternSwitzerland,UniversityofGeneva, CMU,Geneva,Switzerland
CarolineWest UniversityofOrleans,CNRS, InstituteofOrganic&AnalyticalChemistry (ICOA),UMR,Orleans,France
CraigWhite ExscientiaAILtd,Abingdon, Oxfordshire,UnitedKingdom
A.PaigeWicker DepartmentofChemistry& Biochemistry,TheUniversityofTexasat Arlington,Arlington,TX,UnitedStates
Preface Supercriticalfluids Theterm“supercritical”referstothestate ofasubstanceaboveacertaintemperature (termedits criticaltemperature, Tc)andpressure(criticalpressure, Pc).Thestatetransitions areoftendescribedthroughaphasediagram plottingstateboundaries(solid,liquid,gas, supercriticalregions)asafunctionoftemperatureandpressure.Inthesupercriticalregion,thefluidexhibitspropertiesthatare intermediatebetweenthatofaliquidanda gas.Inparticular,supercriticalfluidspossess liquid-likedensities,gas-likeviscosities,and diffusivitiesintermediatetothatofaliquid andagas.
Amodifiedphasediagramforcarbon dioxide(CO2)isshownin Fig.P1.Above modestvaluesof31°Cand74bar(7.3MPa), thefluidexistsinasupercriticalstate.Near thecriticalpoint,smallchangesinpressure ortemperaturecanresultinlargechanges indensity.Thisfactcanbeconveniently exploitedinSFC,tomodifypropertiesof supercriticalfluidstoeluteanalytesfroma chromatographiccolumnbymodification ofthetemperature,pressure,orboth.The propertieslikedensity,viscosity,anddiffusivityofsupercriticalfluidsareintermediate betweenthegasandaliquid.Asthereisno distinctgasorliquidphaseandsupercritical fluidshaveverylowornosurfacetension, thisallowstheuseofhighflowratesforfast separations,orlongercolumnsforhigher efficiency/resolutionseparationsandshort reequilibrationtimes.
Althoughthephysicochemicalproperties ofCO2 areimportanttoitsselectionasa supercriticalfluidchromatography(SFC) mobilephase,theelutionofmanyanalytes willonlyoccurwiththeinclusionofa cosolventmodifier,typicallymethanol. AlthoughthephasediagramofCO2 iscommonlyshown(see Chapter1,Fig.1.1), Fig.P1 showstheimpactoncriticalpoint withtheadditionofmethanolasamodifier. Eventheadditionofsmallvolumesofmethanolcanshiftthepropertiesawayfromsupercriticalconditionsintoa“subcritical” regionbelowthecriticaltemperatureand pressureofthemixture.However,forall practicalchromatographicpurposes,itisjust amatterofsemanticsiftheCO2-solventmodifierissub-orsupercriticalasitmaintainsits monophasicbehaviorandadvantageous chromatographicproperties,e.g.,lowviscosityandhighdiffusivity.
Historicalperspectives Supercriticalfluidbehaviorwasfirst reportedin1822byaFrenchengineerand physicistCharlesCagniarddeLaTour throughhis“cannonbarrel”experiments [1] attheAcademiedesSciencesinParis.He listenedtodiscontinuitiesinthesoundofa rollingflintballinasealedgunbarrelfilled withavarietyofsolvents(water,ethanol, diethylether,andpetroleumspirit)atdifferenttemperaturesandhenotedthatthe splashingsoundattheliquid–airinterface
FIG.P1 Phasediagramforcarbondioxide(bluearea)orcarbondioxide/methanolmixtures(greenarea).Thered linesdenoteaconstantdensity(pertinenttotheelutioncharacteristicsofthesystem)atdifferentpercentagesofmethanolinthemobilephase. ReproducedwithpermissionofElsevierfromE.Lesellier,C.West,Themanyfacesofpackedcolumn supercriticalfluidchromatography—acriticalreview,J.Chromatogr.A1382(2015)2–46.
disappearedattemperatureswellabovethe liquid’sboilingpoints.Hehadunknowingly discoveredtheirtransitionpointstoa supercriticalphase.Inarelatedexperiment, heheatedasealedglassvialofethanolnoting itexpandedtotwiceitsoriginalvolume,and theliquidappearedtovanish.Oncooling,a cloudofmistappearedthatcorrespondedto thesolvent’scriticaltemperature [2].Inasubsequentpaper,hereportedthecriticaltemperatureofaseriesofliquidswhentheinterface tensionvanished—visualizedthroughthe disappearanceoftheliquid’smeniscus [3]
Morethan45yearslater,Andrewsauthored thefirstsystematicstudyofgas–liquidcriticalpointofcarbondioxide(generatedfrom carbonicacid)andnitrousoxide [4].The firstapplicationof“supercriticality”was reportedin1879byHannayandHogarth
whodescribedthesolubilizationofcobalt chlorideinsupercriticalethanol [5].These fundamentalexperimentssetinplacethe cornerstonesforfurtherpivotalworksin the20thcentury(see Chapter1).
In1957,JamesLovelock,oneofthepioneersofgaschromatography(GC),firstproposedtheuseofsupercriticalfluidsfor chromatographicmobilephasesfortheanalysisofnonvolatilecompounds(“criticalstate chromatography”—[6,7]).In1962,Ernst Klesperandcoworkerspresentedthefirst studyontheuseofaninorganicgasasachromatographicmobilephase [8].Theyused chlorofluorocarbons(CHClF2 andCCl2F2) abovetheircriticalpoints(pressuresof 800–2300psiand115°C)witha30-in.packed columncontainingCarbowax20M(polyethyleneglycol)ona180–250 μmChromosorbW
diatomaceousearthsupporttoseparate colorednickel–porphyrincomplexes.He termedthis“highpressuregaschromatography.”Theelutionstrengthwasfoundto beproportionaltothesystempressure, whichwascontrolledthroughrestriction capillariesandmobilephaseflowrate [6]. Astheworkwasundertakenonmodified gas-chromatographicinstrumentation(GC), thetechniquewascoined“highpressure GC”.Thisisconsideredthefirstpractical demonstrationofwhatwaslatertobecome knownasSFC.
ThetermSFCwasfirstproposedbySie andcoworkersatShellResearchLaboratories (Amsterdam,TheNetherlands)inaseriesof papersinthelate1960s.Intheseexperiments, theyinvestigatedtheeffectsofpressureand temperatureinopen-tubular(squaleneand glycerolcoated,andlaterpackedcolumn) gaschromatographywithcarbondioxideas thecarriergas [9–11].Theydescribedhowincreasingthecarbondioxidepressurealtered thedensityofthegasandreducedanalyte retention.Atasimilartimepointca.1968, J.CalvinGiddingsgroupproposedtheterm “densegaschromatography” [12] andsoon afterreportedtheinfluenceofhighpressures onanalyteretentioninGCwithcarbondioxideandammoniaasmobilephase/carrier gas [13].
Asisoftenthecaseinearlyresearch,thelate 1960sthroughtothemid-1980ssawtheriseof certainclaimsthatultimatelyhinderedthe paceofdevelopmentofthetechnique,notably theassertionthatthesolventstrengthofcarbondioxidewassimilartoisopropylalcohol [14],publicationshighlightingthedeficiencies ofpackedcolumnsonchromatographicperformance [15],andthebeliefthatmodifiers didnotincreasemobilephasesolventstrength [16].Similarly,therewerelimitationsinthe availableinstrumentation,whichmeantthat thefullbenefitsofsupercriticalmobilephases couldnotbeexploited [17].
Alltheaforementionedexperiments, amongothers,generatedacuriositythat pavedthewayfornumerousadvancesin thefield.Attheheartofthesedevelopmentswereimprovementsinunderstanding theimpactoforganicsolventonthemobile phaseelutionstrength,theroleofmobile phaseadditives,andthesignificantrole stationaryphaseinteractionsplayonanalyte retentionandselectivity.Alongsidethese theoreticaldevelopments,improvementsin instrumentdesignsawthetransitionfrom earlymodificationofgaschromatographic instruments,tomodifiedHPLCinstrumentationandthentheconcerteddevelopment ofinstrumentationdedicatedtothetask. Ateachjuncture,improvedunderstanding ofCO2 fluidmeteringallowedformore accuratedeliveryofvolumesofthecompressiblegasmobilephaseandcosolvent modifiers.Theseadvancesweresupported bydevelopmentsinthermostatedhighpressureUVflowcellsforimproveddetectionsensitivity,improvedpumpcheck valvesmaterials,andadvancesinback pressuresystemregulation.
SFCinthepharmaceuticalindustry Havingbeenavailableformanydecades, SFCisachromatographictechniquethat manyanalyticalchemistsareawareof,but mayhavenotyetused.Itisoftenconsidered anichetechniquewithonlylimitedapplicationforpreparativeandchiralchromatography.SFChasaperceivedcomplexityover moreestablishedtechniquessuchasGC andliquidchromatography(LC),andthese perceptionshavepreventeditsapplication asastandardlabtechnique.However,ifan analysthasexperienceinliquidchromatography,thenmuchoftheknowledgeandinstrumentalunderstandingisrelevantand transferabletoSFC,andapplicationtoSFC
isnotashighabarriertoovercomeasone mightbelieve.
SFChasseveralinterestingaspectsasa separationtechniquethatarepertinentto theanalysisofbothsmallandlargepharmaceuticalmoleculesaswellasrelated materialssuchascertainformulationexcipients.Inmanycases,SFCcanprovidea bettersolutionthanLCforthecharacterizationofpharmaceuticallyrelevantmolecules. Fig.P2 showsthenumberofpublications reportingtheuseofSFCforpharmaceutical analysisyearonyearfrom1985.Thereisa clearexponentialupwardtrendreflecting thegrowinginterestandimportanceofthe techniqueintheindustry.Infact,application ofthetechniqueisexpandingsorapidlyin pharmaceuticalsciencethatwefounditchallengingtoproposepossiblefutureapplicationsareas(see Chapter13).
SomeoftheaspectsthatmakeSFCattractiveforpharmaceuticalanalysisincludethe following:
• Typicallyfasteranalysistimesand gradientreequilibrationthan(U)HPLC
duetothelowviscosityofthemobile phaseallowinghighflowratestobeused;
• Effectivewithawiderangeoforganic solventscommonlyusedinsynthetic chemistryreactions,andisparticularly usefulforanalysisofwaterlabile molecules;
• Virtuallynostationaryphaselimitations intermsofselectionchoice,usingcarbon dioxide–basedmobilephases(see Chapter3);
• Compatiblewithmanysamplediluents rangingfromfullyorganictofully aqueoussamples(dependingonmobile phasecomposition,see Chapter8);
• Asaresultofthelowmobilephase viscosity,highlyefficientcolumnswith sub-2 μmstationaryphasescanbe successfullyemployedtoachievehigher efficiencyandresolutionthananalogous LCseparations(see Chapter7);
• Isbecomingtheindustrytechnique-ofchoiceforchiralandpreparativeseparations duetothetechniqueusinglesssolvent andinstrumentalenergythantheanalogous LCseparations(see Chapters5 and 6);
FIG.P2 CASScifindersearchusingkeywords“SFC”+“pharmaceutical”fortheperiod1985–2021.
• ProduceslesssolventwastethanLCand usesa“recyclable”gasastheprimary mobilephasecomponent.Inaddition, alcoholsareoftenusedasacosolventthat furtherimprovesits“green”credentials.
Scopeandrelevanceforthistext TheseareexcitingtimesforSFC.Therehas beenaproliferationinnewunderstanding, workflows,andinstrumentaldevelopments inrecentyears,andthepaceisnotslowing. Asanalyticalchemistsworkinginthepharmaceuticalindustry,wewantedtooutline theopportunitiestoutilizeSFCthroughout thecompletedevelopmentcycleofadrug molecule,fromdrugdiscoverythroughto developmentandcommercialreleaseofthe formulatedmedicine,inamanufacturingenvironment.Webelievethistextfillsacrucial gapinthemarket,onethatfocusesonfully utilizingthetechniqueinapharmaceutical setting.Weenvisagethisbookwillappeal notonlytoanalyticalscientistsinthepharmaceuticalindustrybutalsotoanalyticalscientistsinotherindustries.Wealsohopethat thebookwillserveasausefulreferencefor academicsofferingin-depthandpractical insightandalsoprovideaplatformtohelp teachthenextgenerationofseparationscientistsaboutthisenvironmentallysustainable technique.
Inourfinalcomments,wewouldliketo acknowledgethecontributionsofourcolleaguesandcollaborators.Indevelopingthis book,wewantedtopartnerwithscientists whohaveextensivetheoreticalknowledge andpracticalexperiencewithSFC.Thistext wasthereforecompiledbypractitionersof thetechniqueandincludesunderstanding gatheredovermanyyears(oftendecades) ofexperience.Weareindebtedtothemfor theircontribution,insight,anddedication inhelpinguscompilethistext.Wehope
youenjoythebookandfindithelpfulin expandingyourknowledgeofSFC.
MichaelHicks Rahway,NJ,UnitedStates PaulFerguson Macclesfield,UnitedKingdom November2022
References [1] C.CagniarddeLatour,Exposedequelques resultatsobtenuparl’actioncombineedelachaleur etdelacompressionsurcertainsliquides,telsque l’eau,l’alcool,l’ethersulfuriqueetl’essencede petrolerectifiee,Ann.Chim.Phys.21(1822) 127–132.
[2] B.Berche,M.Henkel,R.Kenna,Criticalphenomena:150yearssinceCagniarddeLatour,J.Phys. Stud.13(2009)3001–3005.
[3] C.CagniarddeLatour,Nouvellenotede M.CagniarddeLatour,surleseffetsqu’onobtient parl’applicationsimultaneedelachaleuretdela compressiona ` certainsliquids,Ann.Chim.Phys. 22(1823)410–415.
[4] T.Andrews,Onthecontinuityofthegaseousand liquidstatesofmatter,Philos.Trans.R.Soc.Lond. 159(1869)575–590.
[5] J.B.Hannay,J.Hogarth,VI.Onthesolubilityof solidsingases,Proc.R.Soc.Lond.29(1879) 324–326.
[6] T.A.Berger,Thepast,present,andfuture(?)ofanalyticalsupercriticalfluidchromatography—a2018 perspective,Chromatogr.Today(2018)4–8.
[7]L.T.Taylor,Past,current,andfuturedirectionsinsupercriticalfluidchromatography,LCGC 31(2013). https://www.chromatographyonline. com/view/past-current-and-future-directionssupercritical-flui d-chromatography.(Accessed onApril3,2022).
[8] E.Klesper,A.H.Corwin,D.A.Turner,High pressuregaschromatographyabovecritical temperatures,J.Organomet.Chem.27(1962) 700–706.
[9] S.T.Sie,G.W.A.Rijnders,High-pressuregaschromatographyandchromatographywithsupercriticalfluids.III.Fluid-liquidchromatography,Sep. Sci.2(1967)729–753.
[10] S.T.Sie,G.W.A.Rijnders,High-pressuregas chromatographyandchromatographywithsupercriticalfluids.II.Permeabilityandefficiencyof packedcolumnswithhigh-pressuregasesasmobile
fluidsunderconditionsofincipientturbulence,Sep. Sci.2(1967)699–727.
[11] S.T.Sie,G.W.A.Rijnders,High-pressuregaschromatographyandchromatographywithsupercriticalfluids.IV.Fluid-solidchromatography,Sep. Sci.2(1967)755–777.
[12] L.McLaren,M.N.Myers,J.C.Giddings,Densegaschromatographyofnonvolatilesubstances ofhighmolecularweight,Science159(1968) 197–199.
[13] J.C.Giddings,M.N.Myers,L.M.McLaren,R.A. Keller,Highpressuregaschromatographyofnonvolatilespecies,Science162(1968)67–73.
[14] J.C.Giddings,M.N.Myers,J.W.King,Densegas chromatographyofpressuresto2000atmospheres, J.Chromatogr.Sci.7(1969)276–283.
[15] M.Novotny,W.Bertsch,A.Zlatkis,Temperature andpressureeffectsinsupercritical-fluidchromatography,J.Chromatogr.61(1971)17–28.
[16] B.W.Wright,R.D.Smith,Investigationofpolar modifiersincarbondioxidemobilephasesfor capillarysupercriticalfluidchromatography, J.Chromatogr.355(1986)367–373.
[17] T.A.Berger,Separationofpolarsolutesbypacked columnsupercriticalfluidchromatography, J.Chromatogr.A785(1997)3–33.
EvolutionofpackedcolumnSFC asagreeneranalyticaltool forpharmaceuticalanalysis SusanOlesika,∗ andRaffealBennettb
aDepartmentofChemistry,TheOhioStateUniversity,Columbus,OH,UnitedStates bMerck& Co.,Inc.,MRL,AnalyticalResearch&Development,Boston,MA,UnitedStates
∗Correspondingauthor:E-mail:olesik@chemistry.ohio-state.edu
Discoveryofsupercriticalfluidsanditsrelevanceto analyticalseparationscience
TheInternationalUniononPureandAppliedChemistry [1] andtheAmericanSocietyof TestingandMaterials [2] bothdescribetheregiononaphasediagram(Fig.1)abovethecriticaltemperatureandpressureasa“supercriticalfluid.”Thecriticalpointconditions(temperatureandpressure)arespecifictoeachsubstance.At,orabove,thecriticalpoint,theinterface betweenthegasandliquidphasedisappearsandahomogenousfluidresults.Supercritical fluidswerediscoveredbyCagniarddeLatourwherehenotedthedisappearanceofthemeniscusatthecriticalpointformethylalcoholandether [3].Later,HannayandHogarthillustratedthationiccompounds,suchasCoCl2,KBr,KI,andFeCl3,dissolveinsupercritical fluids,suchasCS2 andmethylalcohol [4].Theystatedthe“theliquidconditionofthefluids hasverylittletodowiththeirsolventpower,butonlyindicatesmolecularcloseness.” AnotherimportantpointdescribedbyHannayandHogarthisthatmovingfromthefluid conditionabovethecriticalpressureandtemperaturetoaliquid(downortothelefton thephasediagramrelativetothesupercriticalregion)doesnotcauseaphasetransitionor ameniscustoform,illustratingacontinuumofhomogenousconditions.Theresultanthigh fluidityconditionsbetweenasupercriticalfluidandconventionalliquidconditionsare increasinglyusedinSFCpharmaceuticalanalyses.
Theprogressionofanalyticalsupercriticalfluidchromatography Thephysicalattributesofsub-/supercriticalfluidledtothefirstsupercriticalchromatographicseparationthatinvolvedtheresolutionofthermallylabileporphyrinsthatcould notbeseparatedbygaschromatography [5].Chlorofluorocarbonswereusedasthemobile phasewithadiatomaceousearthpackingmaterial.Shortlythereafter,Giddings,illustrated thepossibilityofdissolving,separating,anddetectingcompoundswithmolecularweights ashighas400,000amu,includingcarotenoids,cortisolsteroids,sterols,nucleosides,amino acids,carbohydrates,witheithersupercriticalNH3 orCO2 asthemobilephaseattemperaturesof140°Cand40°C,respectively,whichwasabovetheircriticaltemperatures,and PorosilB,Chromosorb-W,orUconpackingmaterialinshort1–1.5mlongcolumnswitha flameionizationdetector [6,7]
Inthe1970s,instrumentationforhigh-performanceliquidchromatography(HPLC) becameavailableandquicklyresultedinaflurryofapplicationsinmanyfieldsofscience. WiththeadvancementofHPLC,furtherdevelopmentofpackedcolumnsupercriticalfluid chromatography(SFC)didnotkeeppacewithHPLCuntilthe1980s.Intheearly1980s,a renewedinterestinpackedcolumnSFCwasshown.ThefirstcommercialSFCwasdeveloped byHewlett-PackardthroughtheleadershipofDennisGere.ThefirstSFCinstrumenthad independentcontrolofflow,mobilephasecomposition,andoutletpressureandusedacombinationofgasandliquidchromatographycomponents.Usinga10cm 4.6mmcolumn packedwith3 μmODS(octadecylsilicaorC18)particles,oligomericubiquinonesextracted from Legionellapneumophilia cellwallswereseparatedandidentifiedusingultraviolet
FIG.1 Phasediagramforcarbondioxide.
absorptionspectra [8].Thesecompoundscontainabenzoquinoneandalongisoprenoidtail group.Therepeatunitoftheseoligomersistheisoprenoidtail.Theubiquinonesinthisextract contained8–13repeatingunitswithbaselineresolutionachievedinlessthan8min.
Aroundthesametimeperiod,LeeandNovotny’sresearchgroupstogetherillustratedthe firstuseofopentubularSFCusingsilicacapillarycolumnsof50–100 μminternaldiameter [9,10].Thistechnologyprovidedconsiderablechromatographicefficiency,buttheflowrate andpressurecouldnotbeseparatelycontrolledduetotheuseoffixedrestrictors(typically smalleri.d.capillaries)tocontrolpressureandflowwithinthecolumn.Theserestrictorswere placedafterthedetectorifaspectroscopydetectorwasusedandbeforethedetectorifflame ionizationormassspectrometric(MS)detectorswereused.TheearlyadvancesofSFCinthe 1980swereclosertoopen-tubularcapillaryGCwithCO2 asthecarriergas.Accordingly,Lee ScientificprovidedopentubularSFCinstrumentation [11] thatwascommerciallyreleasedin 1985.AsopentubularSFCdidnothaveseparatecontroloftheflowrateandcolumnpressure, itwasnotwidelyused,especiallyforpharmaceuticalcompoundswhereaccuracyandprecisionofmeasurementsiscritical.
Animportantinnovationtoallowthedevelopment,andapplicationofpackedcolumnSFC inthepharmaceuticalindustrywasthebackpressureregulator.Thefirstbackpressureregulatorthatprovidedpressurecontrolindependentofmobilephasecontrolwasdevelopedin 1988,andthisdeviceconceptcontinuestobeappliedinallcommercialSFCinstrumentation today [12].TerryBergerledthedevelopmentofHewlett-Packard’s(nowAgilent)firstsupercriticalfluidchromatograph [13] thatfocusedontheuseofpackedcolumnSFC. AtabulatedchronologyreflectingtheapplicationofSFCtodifferentmoleculetypesand milestonesisshownin Table1.Forfurtherdetailonthehistoryoftheuseofsupercritical fluidsseereferences[37–40].
TABLE1 NotableapplicationsandmilestonechronologyinthedevelopmentofSFC.
YearApplicationMilestoneAuthor/vendor
1957Lovelockproposes“criticalstate chromatography”
1962Metalporphyrins“HighpressureGC.”Firstapplicationof open-tubularchromatographicseparation withfluidsabovetheircriticaltemperature andpressures
1967Term“supercriticalfluidchromatography” proposed
1968Carotenoids,steroids,sterols, nucleosides,aminoacids, carbohydrates,polymers
1969Purines,nucleosides, nucleotides,steroids,sugars, aminoacids,proteins, terpenes
Firstreportofanalysisofsmalldrug-like molecules “Densegaschromatography”terminology coined
ProposedsolventstrengthofCO2 was similartoIPA
Klesperetal. [5]
SieandRijnders [14]
McLarenetal. [7]
Giddingsetal. [15] Continued
1.EvolutionofpackedcolumnSFCasagreeneranalyticaltool
TABLE1 NotableapplicationsandmilestonechronologyinthedevelopmentofSFC.—Cont’d
YearApplicationMilestoneAuthor/vendor
1970Polyaromatichydrocarbons (PAHs)
1972PAHs Styreneoligomers
1972–1977PAHs Styreneoligomers
Firstuseofpressureprogrammingto controlmobilephasedensity
JentoftandGouw [16]
FirstautomatedpreparativeSFCJentoftandGouw [17]
Automated/preparativeSFC withmobilephasemodifiers(methanolin n-pentane)
Hartmannand Klesper [18]
1978CouplingofSFCwithMSdetectionRandalland Wahrhaftig [19]
1982KitallowingconversionofModel1084 HPLCintoSFC.Includedfirstmechanical backpressureregulator
1982PAHsUseof3 μmsphericalsilicastationary phasesinSFCillustratedthatdecreasing particlesizeincreasedchromatographic efficiency
1985CaffeineincoffeeFirstreportofhyphenatedSFE-SFCand utilizationofDADdetection
1985PhosphineoxidesFirstchiralseparationusingSFC.First mentionofadditionofwatertomobile phase
1986 D,L-AminoacidsChiralseparationofpharmaceutically relevantcompoundswithmethanolas modifier
1986OpentubularSFCinstrument commercialized
HewlettPackard
Gereetal. [20]
Sugiyamaetal. [21]
Mourieretal. [22]
Haraetal. [23] Jasco
LeeScientific
1988DevelopmentofelectronicAPBRSaitoetal. [12] Jasco
1990CouplingofSFCwithMSwithatmospheric pressureionization
1990Carbondioxideshowntobesimilarin polaritytohexane
1991Firstpublicationofenhanced-fluidity liquidchromatography
1992Releaseof“secondgeneration” instrumentationallowingindependent flowcontrolunderpressuregradient condition.HPinstrumentincludedPeltier pumpheadcooling
1995HewlettPackardSFCproductlinesoldto Bergerinstruments
Huangetal. [24]
Deyeetal. [25]
CuiandOlesik [26]
HewlettPackard Gilson
TABLE1 NotableapplicationsandmilestonechronologyinthedevelopmentofSFC.—Cont’d
YearApplicationMilestoneAuthor/vendor
2000Developmentofsemi-prepSFCsystem with“nextgeneration”samplecollection Bergeretal. [27] Berger
2001Developmentof2-ethylpridine(2-EP) phaseallowinganalysisofbasicanalytes withoutmobilephaseadditives Princeton Technologies
2001DrugmoleculesMassdirectedsemi-prepSFCWangetal. [28]
2005EstrogenmetabolitesinurineSFC/MS/MSXuetal. [29]
2006PolypeptidesSFC/MSwithmethanol/TFAmodifierZhengetal. [30]
2009Releaseof1260InfinitySFCsystemAurora/Agilent
2010Steroids,profens,xanthenes, sulfadrugs,nucleicacids
2011Steroids,profens,sulfadrugs, nucleicacids
Assessmentofsub-2 μmparticlestationary phasesinSFC Berger [31]
Assessmentofsub-2 μmsuperficially porousstationaryphasesinSFC Berger [32]
2012Releaseof1260InfinitySFC/HPLCsystemAgilent Aurigemmaetal. [33]
2012ReleaseofUPC2 systemWaters
2014ReleaseofNexerra(SFE-)SFCsystemShimadzu
2015Proprietarypharmaceutical compounds
FilingofGMPSFCmethodsfor measurementofchiralpurity Hicksetal. [34]
2018SalbutamolFirstcross-instituteSFCmethodtransfer (identicalinstrumentplatform) Dispasetal. [35]
2018–2021SalbutamolFirstcross-instituteSFCmethodtransfer (differentinstrumentplatforms) Dispasetal. [36]
Fundamentalsofmobilephases
CarbondioxideremainsthemostcommonlyusedcomponentinSFCmobilephases.The carbondioxidemoleculehasazero-dipolemoment.Carbondioxidehasadielectricconstant lowerthanthoseofhydrocarbons,butmanyhydrocarbonsarenotmisciblewithCO2.Carbon dioxideisachargeseparatedmolecule,meaningithastwopolarbondswithnonzero bond dipolemomentsthatresultsinasignificantquadrupolemoment [41].Thechargeseparation andelectronicstructureallowCO2 toactasbothaweakLewisbase(electron-pairdonor)and Lewisacid(electron-pairacceptor).Stronginteractionswithcarbonyloxygengroupshave beennotedwithCO2 actingasaLewisbase.CO2 isalsocapableofformingconventionalhydrogenbondswithproticdonorsthatassistinthesolvationofhydrogenbondingmolecules. Eventhoughforspecificcompoundssuchasketoneandaldehydessignificantintermolecular interactionsoccur,undermostconditions,CO2 isgenerallyconsiderednonpolar [25].Other morepolarsubstancesthatmayformsupercriticalfluidshavebeenconsidered,buttheir criticaltemperaturesandpressuresaretoohightobeusedforpracticalapplication.
Amajoradvancementintheunderstandingofsolvationatthemolecularlevelinsupercriticalfluidswasdensityvariationnearasolute [42,43].Fromnumerousexperimental andtheoreticalstudies,theconclusionsarethatdensityenhancementsnearasoluteoccur outtolongmoleculardistances(10–30A ˚ )awayfromthesolute [43].Accordingly,themolecularlevelviewofsolvationinsupercriticalfluidsishigherdensityclusteringaroundthe solutewithsubstantialfreevolumeinthefluidatgreaterdistances.However,specific intermolecularinteractionsbetweenthebondsofCO2 arenotusuallythecauseofthisclusteringandthereforenonpolarinteractionsarecontrollingthissolvation.Conversely,polar solutessuchassugaracetatesarehighlymisciblewithsupercriticalandliquidCO2 based onspecificintermolecularinteractions [41],whichillustratesthevariedsolvatingcapabilities ofCO2.
Forthechromatographicseparationofmanypolaranalytes,polarmodifiersarenecessary toprovidehighefficiencythroughincreaseddesorptionkineticsfromthestationaryphase. Alargerangeofpolarmodifiershasbecomepopular,especiallytheadditionoflowmolecular-weightalcohols,suchasmethanol,ethanol,andisopropylalcohol,whicharefavoredduetotheirsignificantpolarityandhydrogen-bondingability.Also,thepolarityof thesolventshellaroundasoluteallowspredictionofthesolubilityandchromatographicretention.Reportsonthepolarityofmixturesofsupercritical/liquidCO2 combinedwithpolar modifiersbeganinthe1980susingsolvatochromicstudies,whichinvolvesmonitoringshifts inthewavelengthmaximaintheopticalspectraofindicatordyesandpredictionofthepolarityofthemixturesbasedontheseshifts [44–46]
In1990,thesolventstrengthofpuresupercriticalCO2 andCO2 withpolarmodifierswas comparedtothesolventstrengthofcommonliquids. Fig.2 comparesthetransitionenergyof theabsorptionmaximumforNileRed,asolvatochromicdyeintroducedbyDeyeetal. [25],to thatofacommonlyusedsetofsolvatochromicdyes,andtheReichardt’sdyesthatareacompoundfamilyofpyridiniumbetainedyes.ReichardtdyeswerenothighlysolubleinCO2 and thatwasthereasonforintroducinganewsolvatochromicdye.NileRedissolubleinCO2,and itisabsorbancemaximashiftssubstantiallywithincreasedpolarityasshownin Fig.2.Nile Redisalsostableinallstudiedsolvents. Fig.2 comparestheshiftinthetransitionenergyfor NileRedandthespecificReichardtdye(E30)forcommondyesandforCO2.Thetransition energyforbothdyeswascalculatedusingthisequation, E ¼ 28,591.44/(wavelengthmaximuminnanometers) ¼ transitionenergyinkcal/mol [47].Usingthisanalysis,CO2 was documentedtohavealowsolventstrengthsimilartothatoflow-molecular-weighthydrocarbons(Fig.2) [25].Inthissamestudy,thesolventstrengthof10v/v%methanol/CO2 wasobservedtobeclosertothesolventstrengthexpectedforanequalmixture(i.e.,50:50)of methanol/CO2 assumingidealsolutionconditionsandindicatessignificantclusteringof methanolaroundthepolarsolvatochromicdyes.
Fromanearlystage,manyresearchersobservedthattheadditionofasmallproportionof modifierinthemobilephaseabsorbedontobothpolarandnonpolarstationaryphasesoradsorbents.Forslightlyhigherdensityconditionsthanatthecriticalcondition(i.e.,increased pressureabovethecriticalpressure),adisproportionateamountofthemodifieradsorbsto thestationaryphase,evenforanonpolarstationaryphasesuchasODS.Forexample,with 2%methanol/CO2 mixedmobilephase,theadsorbedlayeronODSwasnearly25wt% methanol [48].Therefore,theadditionofmodifiersimpactstheretentionofpolaranalytes throughchangesinthepolarity,density,andcriticalconditionofthemobilephase,and
FIG.2 ComparisonoftransitionenergiesforNileRedandE(30) solvatochromicdyes foranumberofcommonliquids andCO2. E(NR) transitionenergyfortheabsorptionmaximumofNileRed. Et(30) isthetransitionenergyforabsorption maximumofaReichardt’sdyedesignatedas30. ReprintedwithpermissionfromJ.F.Deye,T.A.Berger,A.G.Anderson, Nileredasasolvatochromicdyeformeasuringsolventstrengthinnormalliquidsandmixturesofnormalliquidswith supercriticalandnearcriticalfluids,Anal.Chem.62(1990)615–622.Copyright2022AmericanChemicalSociety.
modificationofthestationaryphase(withtheadsorptiveinteractionswithsurfacesilanols typicallyhavingthemostsignificantimpact [48–50]).
AnotheradvancementinSFCthatallowedtheseparationofionicpharmaceuticalcompoundswastheadditionofionpairingreagentsintothemobilephasemodifiers [51]. However,therearelimitationstothismethodbecausemanyofthedesiredionpairing reagentswerenotsolubleinconventionalSFCmobilephases—evenwhenmodifierswere added [51].
Asmodifierswereincreasinglyemployed,theneedtoremainatsupercriticalconditions becamelessrelevant.Forexample,Sandra’sresearchgroupin1994showedsub-andsupercriticalpackedcapillaryconditionsworkedwellfortheseparationofbothbasicandacidic drugsandillustratedtheimportanceofpolarmodifiers [52]
AlfredFranciswasthefirsttostudythesolubilityofalargerangeofcompoundsinliquid carbondioxideandtostudyternarysystemsofliquidCO2 mixedwithorganicsolvents [53] Inthiswork,hedescribed464ternarymixturesystemscontainingliquidCO2.In1991,the Olesikresearchgrouppublishedthefirststudyofenhanced-fluidityliquidchromatography, EFLC,whichmixesconventionalliquidswithsmallamountsofliquidcarbondioxide [26]. Soonthereafter,thecapacityforenhanced-fluidityliquidmixturestoformsolventclustering
orenhancedsolventdensityaroundanalyteswasreported.Thiswassimilartothatobserved insupercriticalfluidsexceptthatanintermediateclustersize,betweenthatobserved insupercriticalfluidsandconventionalliquids,wasnoted [54].Also,formanypolarliquids, asmuchas40–50molw/w%CO2 canbeaddedtothemixturebeforethesolventstrengthis markedlydecreased(i.e.,thesemixtureshaveasolventstrengthsimilartotheoriginalorganic solventorsolventmixture [26,55,56]).Thisattributewasnotedforalltheenhanced-fluidity liquidmixturesstudiedtodate.Enhanced-fluidityliquidmixturesprovidediffusionrates approachingthoseofsupercriticalfluids(andloweredviscosities),butwithhighsolvent strength.Chemicalengineersbeganstudyingsimilarliquidmixturesaroundthesametime forseparationsinprocessingconditionsandcoinedtheterm“gas-expandedliquids”or “GXLs” [57].Asnumerousscientistshavenoted,SFCorchromatographycontainingliquefiedgasesprovidesacontinuumconditioninpropertiesandapplicationsbetweenthatofGC andHPLC.Termssuchas“unifiedchromatography”and“convergentchromatography” emergedtodescribetheseconditions [6,58–60].
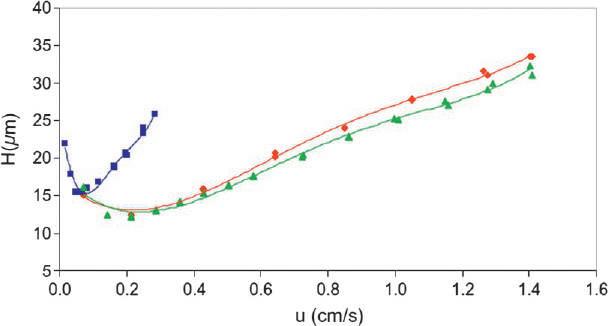
Insummary,supercriticalfluids,subcriticalliquids,orenhanced-fluidityliquidshave physicochemicalpropertiesthatareintermediatebetweenthoseofgasesandliquids.These fluidsaretypicallycompatiblewithdetectorsthatarecommonlyusedbygaschromatographyandliquidchromatography.Inthemostcommonpractice,whichistousecarbon dioxideinthesemixtures,thefluidshavelowtoxicity.Furthermore,theretentionfactors inthesechromatographicsystemsareprimarilycontrolledbythesolventstrengthofthe CO2-solventmixture.However,finetuningofthesolventstrengthofagivensolvent-CO2 mixtureiscontrolledbysystemdensity(i.e.,pressure).Finally,mostchromatographicexperimentsareaccomplishedatflowratesabovetheoptimalflowvelocitythatmeanstheband dispersionisinverselyrelatedtothediffusioncoefficient;andchromatographicefficiencies aredirectlyproportionaltothediffusioncoefficient,whichexplainswhytheefficienciesof gaschromatographyarehigherthanthoseforSFC,whichareinturnhigherthanEFLC andconventionalreversedandnormal-phaseliquidchromatography. Table2 providesa comparisonofthediffusioncoefficientsandviscositiesofgases,supercriticalfluids, enhanced-fluidityliquids,andconventionalliquids. Fig.3 showsacomparisonoftheband dispersionforthesamecompoundwiththesamechromatographiccolumnusingreversedphaseHPLCconditionscomparedtoreversed-phaseSFCconditions.SFCshowsmarked efficiencygainsinlinewiththetheorydiscussedearlier.
TABLE2 Masstransportpropertiesofmobilephasesobservedwithdifferentseparationapproaches.
FIG.3 Variationplateheight, H,vsmobilephaselinearspeed(u)forHPLC(squares)andSFCat20°C(diamonds are for95/5v/v%CO2/methanoland triangles areforpureCO2)usinga5 μmRP-C18,250mm 4.6mm.i.d.column. ReproducedwithpermissionofElsevierfromE.Lesellier,L.Fougere,D.P.Poe,Kineticbehaviorinsupercriticalfluidchromatographywithmodifiedmobilephasefor5 μmparticlesizeandvariedflowrates,J.Chromatogr.A1218(2011)2058–2064.
Sustainableaspectsofsubcriticalandsupercriticalchromatographic separationmethods
Worldwide,annualsolventuseanditsdisposalacrossallapplicationsisapproximately 30millionmetrictons [61].IntheUnitedStates,theaveragehumanweighs137lbs(62kg). Thirtymillionmetrictonsistheweightof482,763,504humans,whichiswelloverthetotal population(327.2million)oftheUnitedStates.Inaddition,solventusagecontinuesto increaseworldwide—mainlyduetoexpandingeconomies.Theapplicationsofsolventsin separationsciencecontributesignificantlytoorganicwaste.Estimatesofsolventuseinliquid chromatography(LC)areapproximately150,000tonsannually [62].
Thepharmaceuticalindustryisaheavyuserofliquidchromatographyrangingfrom applicationsindrugdiscoverytolarge-scaledrugproduction.Theindustryisthereforea significantcontributortoglobalsolventuseanddisposalchallenges.Accordingly,most pharmaceuticalmanufacturershavemadetheuseofgreensolventsahighpriority.Many pharmaceuticalcompanies,includingPfizer [63],AstraZeneca [64],andGlaxoSmithKline [65],havedevelopedsolventselectionguidestofacilitatetheuseoflesshazardoussolvents bytheiremployees.Theseguidesareuseful;however,thecharacterizationofasolvent’s propertiesisnotuniform.TheAmericanChemicalSocietyGreenChemistryInstitutePharmaceuticalRoundtablewasestablishedin2007 [66].TheRoundtablehasdevelopedtools [67,68] toprovideastandardizedassessmentofsolventgreenness.Recently,theRoundtable publishedtheAnalyticalMethodGreennessScore(AMGS)calculator [69] toquantifythe “greenness”ofcommonlyusedseparationtechniques.TheAMGScalculatorincludes cumulativeenergydemand,instrumentalenergydemand,themassofsolventconsumed, andenvironmental,healthandsafety(EHS)scores.Also,theAMGStoolisthefirstselection guidethatincludestheinformationonthegreennessofsupercritical/subcriticalfluid chromatography.
1.EvolutionofpackedcolumnSFCasagreeneranalyticaltool
TheAMGScalculatorallowsfordetailedcomparisonsofseparationsmethodsbasedon solventwaste,instrument,andsolventenergy.Fast,efficientseparationsprovidethegreenest conditions.UsingtheAMGScalculator,scientistsfromeightpharmaceuticalcompanies comparedtypicalinternalmethodsusingHPLC,ultrahighpressureHPLC(UHPLC),and SFCforanalyticalandpreparativeseparations.ThegeneraltrendwasthatSFCmethods andUHPLCmethodsweregreenerthanHPLCmethodsforanalyticalseparations.Forpreparativeseparations,preparativeSFC,andaqueous-basedHPLChadsimilargreenness scores.However,whentheenergyneededtoremovethewateraftertheseparationwas included,SFCwassubstantiallygreener [69].TheAMGScalculatorisexpectedtoprovide greatvalueinmethodandsolventselectionatboththeanalyticalandpreparativescalebeing aonestopshopforsolventsafety,solventenergy,instrumentenergy,andwaste.EnhancementstotheAMGScalculatorforsupercriticalandsubcriticalfluidchromatographyusing CO2 wererecentlydevelopedthatincludecorrectionstotheEHSparameter,acorrection forthedensityofCO2 valuesusedintypicalSFCconditions,andanewparametertoadd totheAMGSthatallowsforthecalculationoftheflammabilityhazardofmobilephasesif aleakoccurs [70].Thisisparticularlyimportantasthescaleoftheseparationincreasesfrom analyticaltosemipreparativeandpreparativescale.
UsingtheupdatedAMGScalculatorandalif ecycleanalysis(LCA),thegreennessof HPLCandSFCreversed-phaseseparationsofp harmaceuticalcompoundswerecompared. BoththeAMGSandtheLCAshowedsimilartrend s.Acorrectholisticanalysismustinclude theinstrumentenergyconsumptionforboth.WhencomparingHPLCseparationsusing alcohol/watermobilephasetoSFCseparationswithalcohol/H 2 O/CO2 ,theresultsshow thattheadditionalenergyconsumptionofc urrentSFCinstrumentsmustbecounterbalancedbytakingadvantageofhigherflowrat es.HPLCmethodsusingacetonitrile-water, whichareconsideredthegoldstandardforHPLC separations,weresignificantlylessgreen thantheSFCmethodsexaminedonbothananalyticalandpreparativescale [71].Duetothe lowvolumesused,commonlyusedacidandbaseadditivestothemobilephasessuchas formicacidorammoniumhydroxidedidnotsignificantlyimpactthegreennessofthe separations.
AnalyticalscalesubcriticalSFC Asnotedinthephasediagram(Fig.1),thereisnobarriertotransitioningfromsupercritical tosubcriticalconditionsorviceversa.Asearlyastheearly2000s,mostofthereported SFCfunctionedundersubcriticalconditions.ConsiderableprogressintheSFCfieldwas drivenbytheadventofnewSFCinstrumentationthatprovidedreproducibleretentiontimes foranalytes,reproduciblecontrolofcolumnbackpressure,andseparatecontrolofflow rate.Furthermore,effectiveinterfacestomassspectrometersandcommonlyusedHPLCdetectorsbecamecommonplace.Theseadvanceswerequicklyacceptedbythepharmaceutical, foodcharacterization,andenvironmentalresearchers—greatlyexpandingtheapplication spaceofSFC.
ImprovementsinSFCinstrumentation,includingpumps,mixers,autosamplers,back pressureregulators,andreducedextracolumndispersionvolumes,enabledhigh-performance andhigh-speedseparationsofcompoundsinsecondsthatcomparefavorablytofastHPLC