https://ebookmass.com/product/polymer-nanocomposite-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Self-Healing Polymer-Based Systems 1st Edition Sabu Thomas
https://ebookmass.com/product/self-healing-polymer-based-systems-1stedition-sabu-thomas/
ebookmass.com
Wool Fiber Reinforced Polymer Composites Sabu Thomas
https://ebookmass.com/product/wool-fiber-reinforced-polymercomposites-sabu-thomas/
ebookmass.com
Nanocomposite Membranes for Water and Gas Separation
Mohtada Sadrzadeh (Editor)
https://ebookmass.com/product/nanocomposite-membranes-for-water-andgas-separation-mohtada-sadrzadeh-editor/
ebookmass.com
Earl's Well That Ends Well Jane Ashford
https://ebookmass.com/product/earls-well-that-ends-well-janeashford-2/
ebookmass.com
The Primacy of Metaphysics Christopher Peacocke https://ebookmass.com/product/the-primacy-of-metaphysics-christopherpeacocke/
ebookmass.com
Hanoverian to Windsor Consorts: Power, Influence, and Dynasty Aidan Norrie
https://ebookmass.com/product/hanoverian-to-windsor-consorts-powerinfluence-and-dynasty-aidan-norrie/
ebookmass.com
God of Ruin: A Dark College Romance (Legacy of Gods Book 4) Rina Kent
https://ebookmass.com/product/god-of-ruin-a-dark-college-romancelegacy-of-gods-book-4-rina-kent/
ebookmass.com
Handbook of Cancer Treatment-Related Toxicities (Mar 8, 2021)_(0323672418)_(Elsevier).pdf Vamsidhar Velcheti
https://ebookmass.com/product/handbook-of-cancer-treatment-relatedtoxicities-mar-8-2021_0323672418_elsevier-pdf-vamsidhar-velcheti/
ebookmass.com
Middleton's Allergy Essentials, 1e 1 Har/Psc Edition Robyn E O'Hehir Fracp Phd Frcpath
https://ebookmass.com/product/middletons-allergy-essentials-1e-1-harpsc-edition-robyn-e-ohehir-fracp-phd-frcpath/
ebookmass.com
Methods and Applications in Petroleum and Mineral
Exploration and Engineering Geology Said Gaci
https://ebookmass.com/product/methods-and-applications-in-petroleumand-mineral-exploration-and-engineering-geology-said-gaci/
ebookmass.com
POLYMER NANOCOMPOSITE MEMBRANESFOR PERVAPORATION POLYMER NANOCOMPOSITE MEMBRANESFOR PERVAPORATION Editedby
SABUTHOMAS InternationalandInterUniversityCentreforNanoscienceand Nanotechnology,MahatmaGandhiUniversity,Kottayam,India
SONEYC.GEORGE CentreforNanoScienceandTechnology,AmalJyothiCollegeof Engineering,Kanjirapally,India
THOMASUKUTTYJOSE DepartmentofBasicSciences,CentreforNanoScienceandTechnology, AmalJyothiCollegeofEngineering,Kanjirapally,India
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,including photocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher. Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswith organizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www. elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybe notedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding, changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation, methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheir ownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjury and/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationof anymethods,products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-816785-4
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: SimonHolt
EditorialProjectManager: MarianaC.Henriques
ProductionProjectManager: PrasannaKalyanaraman
CoverDesigner: GregHarris
TypesetbyMPSLimited,Chennai,India
1Polymernanocompositemembranesforpervaporation:an
ThomasukuttyJose,SoneyC.GeorgeandSabuThomas
JithinJoy,NeenuGeorge,CintilJoseChirayilandRuncyWilson
7.3Membranesforpervaporation........................................................158
7.4Nanodiamond...................................................................................160
7.5Pervaporationperformanceoffullerenes-basednanocomposite membranes.......................................................................................162
7.6Membranesmodifiedwithfullerenesandderivatives..................164
7.7Conclusions......................................................................................166 References...............................................................................................169
8Polymernanocompositemembranesforpervaporation desalinationprocess.......................................................................................175
DeepakRoyGeorge,ShalinTyni,AshaElizabethandAbhinavK.Nair
8.1Introduction......................................................................................175
8.2Synthesismethodsofpolymernanocompositepervaporation membranes.......................................................................................177
8.3Factorsaffectingtheperformanceofpervaporation desalinationmembranes.................................................................178
8.4Polymermembranesforpervaporationdesalination...................179
8.5Polymernanocompositemembranesforpervaporation desalination......................................................................................184
8.6Conclusionandfutureaspects........................................................195
9Polymer/polyhedraloligomericsilsesquioxanenanocomposite membranesforpervaporation.......................................................................201
ValiyaParambathSwapna,VakkoottilSivadasanAbhishaandRanimolStephen
9.1Introduction......................................................................................201
9.2Polyhedraloligomericsilsesquioxane............................................205
9.3Pervaporationperformanceofpolymer/POSSmembranes.........210
9.4Factorsaffectingthepervaporationthroughpolymer membrane.........................................................................................214
9.5Applicationsofpolyhedraloligomericsilsesquioxaneembeddedpolymericsystems........................................................218
9.6Challengesandfutureaspects........................................................220 References...............................................................................................221
10Nanometalandmetaloxide-basedpolymernanocomposite
SamitKumarRay,AmritanshuBanerjee,SwastikaChoudhuryandDebapriyaPyne
10.1Introduction...................................................................................231 10.2Synthesisofpolymer
10.4Nanometalandmetaloxide-basedpolymer
I.G.Wenten,K.Khoiruddin,G.T.M.Kadja,RinoR.MuktiandPutuD.Sutrisna
13Polymer/metal-organicframeworksmembranesandpervaporation....329 PeiyongQin,ZhihaoSi,HouchaoShanandDiCai
Listofcontributors VakkoottilSivadasanAbhisha DepartmentofChemistry,St.Joseph’sCollege (Autonomous),Devagiri,Calicut,India
AmritanshuBanerjee DepartmentofPolymerScience&Technology,Universityof Calcutta,Kolkata,India
DiCai NationalEnergyR&DCenterforBiorefinery,BeijingUniversityofChemical Technology,Beijing,P.R.China
CintilJoseChirayil NewmanCollege,Thodupuzha,India
SwastikaChoudhury DepartmentofPolymerScience&Technology,UniversityofCalcutta, Kolkata,India
AshaElizabeth DepartmentofChemicalEngineering,AmalJyothiCollegeofEngineering, Kottayam,India
BincyFrancis PGDepartmentofChemistry,St.ThomasCollege,Ranny,India
DeepakRoyGeorge DepartmentofChemicalEngineering,AmalJyothiCollegeof Engineering,Kottayam,India
GejoGeorge SchoolofPure&AppliedPhysics,MahatmaGandhiUniversity,Kottayam, India
NeenuGeorge St.Joseph’sCollege,Moolamattom,India
SoneyC.George CentreForNanoscienceandTechnology,AmalJyothiCollegeof Engineering,Kanjirapally,India
NeethaJohn CentralInstituteofPlasticsEngineering&Technology(CIPET),Instituteof PlasticsTechnology(IPT),KochiJNMCampus,Udyogamandal,Kochi,India
ThomasukuttyJose DepartmentofBasicSciences,CentreForNanoscienceand Technology,AmalJyothiCollegeofEngineering,Kanjirapally,India
JithinJoy NewmanCollege,Thodupuzha,India
G.T.M.Kadja ResearchCenterforNanosciencesandNanotechnology,InstitutTeknologi Bandung,Bandung,Indonesia;DivisionofInorganicandPhysicalChemistry,Institut TeknologiBandung,Bandung,Indonesia;CenterforCatalysisandReactionEngineering, InstitutTeknologiBandung,Bandung,Indonesia
GeethaKathiresan NanotechnologyDivision,DepartmentofElectronicsand CommunicationEngineering,PeriyarManiammaiInstituteofScienceandTechnology, Vallam,Thanjavur,India
K.Khoiruddin DepartmentofChemicalEngineering,InstitutTeknologiBandung, Bandung,Indonesia
TorajMohammadi DepartmentofChemical,PetroleumandGasEngineering,Centerof ExcellenceforMembraneResearchandTechnology,IranUniversityofScienceand Technology(IUST),Narmak,Tehran,Iran
MuhammadMujiburohman DepartmentofChemicalEngineering,Muhammadiyah UniversityofSurakarta,Surakarta,Indonesia
RinoR.Mukti ResearchCenterforNanosciencesandNanotechnology,InstitutTeknologi Bandung,Bandung,Indonesia;DivisionofInorganicandPhysicalChemistry,Institut TeknologiBandung,Bandung,Indonesia;CenterforCatalysisandReactionEngineering, InstitutTeknologiBandung,Bandung,Indonesia
AbhinavK.Nair DepartmentofChemicalEngineering,AmalJyothiCollegeofEngineering, Kottayam,India
NaveenRoobaDossM. NanotechnologyDivision,DepartmentofElectronicsand CommunicationEngineering,PeriyarManiammaiInstituteofScienceandTechnology, Vallam,Thanjavur,India
DebapriyaPyne DepartmentofPolymerScience&Technology,UniversityofCalcutta, Kolkata,India
PeiyongQin NationalEnergyR&DCenterforBiorefinery,BeijingUniversityofChemical Technology,Beijing,P.R.China
SamitKumarRay DepartmentofPolymerScience&Technology,UniversityofCalcutta, Kolkata,India
InciSalt DepartmentofChemicalEngineering,YildizTechnicalUniversity,Esenler, Istanbul,Turkey
YavuzSalt DepartmentofChemicalEngineering,YildizTechnicalUniversity,Esenler, Istanbul,Turkey
M.B.SethuLakshmi ResearchandPGDepartmentofChemistry,N.S.S.HinduCollege, Changanacherry,India
HouchaoShan NationalEnergyR&DCenterforBiorefinery,BeijingUniversityofChemical Technology,Beijing,P.R.China
ZhihaoSi NationalEnergyR&DCenterforBiorefinery,BeijingUniversityofChemical Technology,Beijing,P.R.China
RanimolStephen DepartmentofChemistry,St.Joseph’sCollege(Autonomous),Devagiri, Calicut,India
PutuD.Sutrisna DepartmentofChemicalEngineering,UniversityofSurabaya(UBAYA), Surabaya,Indonesia
ValiyaParambathSwapna DepartmentofChemistry,St.Joseph’sCollege(Autonomous), Devagiri,Calicut,India
SabuThomas InternationalandInterUniversityCentreforNanoscienceand Nanotechnology,MahatmaGandhiUniversity,Kottayam,India
BerkTirnakci DepartmentofChemicalEngineering,YildizTechnicalUniversity,Esenler, Istanbul,Turkey
MaryamAhmadzadehTofighy DepartmentofChemical,PetroleumandGasEngineering, CenterofExcellenceforMembraneResearchandTechnology,IranUniversityofScience andTechnology(IUST),Narmak,Tehran,Iran
ShalinTyni DepartmentofChemicalEngineering,AmalJyothiCollegeofEngineering, Kottayam,India
I.G.Wenten DepartmentofChemicalEngineering,InstitutTeknologiBandung,Bandung, Indonesia;ResearchCenterforNanosciencesandNanotechnology,InstitutTeknologi Bandung,Bandung,Indonesia
RuncyWilson DepartmentofChemistry,St.CyrilsCollege,Adoor,India
Preface Theneweraofmembranetechnologyiskeentodevelop efficientwaystoreduceindustrialpollutionandenergyconsumption.Pervaporationisoneofthemostenergy-efficient methodstodevelopsustainableseparationandpurification systems.Permeationandevaporationcombinetogetacosteffectiveandpollution-freealternativetoconventionalseparationprocessessuchasdistillation.Polymernanocomposite membranesgivemoreviabilitytothesemembrane-basedseparationtechnologies.Thepolymernanocompositemembranes andtheirapplicationinpervaporationaretheprimeareaof researchtodevelopnewenergy-efficientandecofriendlyseparationandpurificationstrategies.Therecentadvancementin polymernanocompositemembranesandthepervaporation processpromptedustosummarizetheresultsinacollective way.Thebookgivesdetailedinsightintodifferentpolymer nanocompositemembranesandtheirroleinpervaporation separationprocesses.Itconsistsof15chaptersincludingabrief introductionaboutthepervaporationprocess.Thefirstfour chaptersexclusivelydealwiththe21stcenturynanomaterials suchasnanocellulose,nanochitin,andnanoclay-basednanocompositemembranesanditspervaporationapplications.The pervaporationperformanceofnanocompositemembraneswith differentnanoscaleallotropesofcarbon(graphene,carbon nanotubes,fullerene,andnanodiamond)iswellexplainedin fifthtoseventhchapters.Desalinationisanothersignificant areaofresearch,andonechapterisforpervaporation-based desalinationprocesses.NanocompositemembraneswithdifferentnanomaterialssuchasPOSS,nanometalandmetaloxides, andmodifiedzeolitesandtheirpervaporationperformanceare explainedinsubsequentchapters.Thechaptersbasedon computationalmodelingofthepervaporationandhybridpervaporationprocessesaddattractiontothereaders.Thechapters providedetailedinsightstoyoungresearchersandindustrialist toknowmoreaboutdifferentnanocompositemembranesand theirpervaporationapplications.
Theenormoussupportandhelpofallthecontributorsto thebookarewellappreciated.Wegratefullyacknowledgethe greateffortsofallthereviewerswhoreviewedthechaptersin
theagreedtime.Averyspecialwordofthankstotheeditorial teammembersofElsevierfortheirguidanceandcontinuous supportinthisventure.Weindebtedtothesupport,guidance, andmotivationofourmanagementandcolleagues.Wehope thatthebookgivesawonderfulexperiencetothereaders whofocusedontheoreticalandexperimentalaspectsof pervaporation.
Polymernanocomposite membranesforpervaporation: anintroduction ThomasukuttyJose1,SoneyC.George2 andSabuThomas3
1DepartmentofBasicSciences,CentreForNanoscienceandTechnology, AmalJyothiCollegeofEngineering,Kanjirapally,India 2CentreFor NanoscienceandTechnology,AmalJyothiCollegeofEngineering, Kanjirapally,India 3InternationalandInterUniversityCentreforNanoscience andNanotechnology,MahatmaGandhiUniversity,Kottayam,India
1.1Introduction Oneofthemostsignificantmembraneseparationprocesses ispervaporation(PV).Itistheonlymembraneprocessinwhich aliquid-to-vaporphasetransitionoccursthroughadenseor microporousmembraneduringthetransportofsolvents.The partialpressuredifferenceexistbetweenthemembraneis therealdrivingforcebehindthephasetransitionanditcanbe duetothevacuumatthepermeateside.Theefficiencyofseparationisobtainedbyselectivediffusionofcomponentsfroma liquidmixturethroughthemembrane.PVisanenergyefficient separationmethod,especiallyfortheseparationofazeotropic, isomeric,andcloseboilingpointliquids.Theazeotropesor closeboilingpointliquidscanbeseparatedbyaclassicaldistillationprocesswhereseparationisbasedonthedifferencesin theirrelativevolatility.Theseparationofdifferentmixtures occursonthebasisoftheiraffinitywiththemembranematerialsinPV.AnotherprimeconditionforthePVseparationof azeotropsandothercloseboilingliquidsisthattheyhaveclear differenceintheirtransportcharacteristicsthroughmembrane material.So,PVisausefultechniquefortheseparationofazeotropicmixtures [1],closeboilingpointmixtures [2],andstructuralisomers [3].PVismainlyappliedfortheseparationand concentrationofmixturesthataredifficulttoseparatebydistillation [4].Thetransportpropertiesofwaterthroughpolymer membranesaremuchmoredifferentthanthatoforganic
solvents.Thustheseparationofwater organicmixturesbyPVis gettingmoreattentionduetotheirdifferenceintransport characteristics.Selectionofproperpolymersandpreparationofa suitablemembranewithhighperformancearethemainfactorsin PV.Manyresearchersdevelopeddifferentpolymericmembranes forthePVseparationofdifferentorganic watermixtures [5,6].
TheuseofPVfortheseparationofazeotropicmixturesis firstintroducedbyApteletal. [7] in1976andafterthatPVis potentiallyappliedfortheseparationofvariousliquidmixtures. However,theconceptofPVisfirstintroducedbyKober [8] in 1917fortheselectivepermeationofwaterfromaqueoussolutionsofalbuminandtoluenethroughcellulosenitratefilms.He observedthe“permselectiveevaporation”ofcomponents throughthemembraneandwasabbreviatedas“pervaporation.” ButthesystematicandpotentialapproachtowardPVwasfirst carriedoutbyBinningetal.fortheseparationofhydrocarbon mixturesthroughdensepolythenefilmsandobservedthatthe linearhydrocarbonspermeatefasterthanbranchedisomers [9,10].Thenalargenumberofhydrophilicandorganophilic membranesweredevelopedandextensivelyappliedforthe dehydrationoforganicsolventsandseparationoforganicsolvents,respectively.Polymeric-basedhydrophilicmembranesare widelyusedforthePVdehydrationoforganicsolventssuchas alcohols,ketones,acids,andethers [11].Polydimethylsiloxane (PDMS)-basedhydrophobicmembranesweredevelopedand usedfortheextractionoforganiccompoundsfromwatermixtures [12,13].GesellschaftfurTrenntechnikintroducedfirst commercializedPVmembranesbycoatingpoly(vinylalcohol) (PVA)onaporoussupportofpoly(acrylonitrile) [14]
1.2Basicprinciplesofpervaporation PVofmembranesisdescribedbasicallybysolution-diffusion mechanism,andmasstransportinPVisexplainedbytwomodels:(1)solution-diffusionmodeland(2)poreflowmodel.
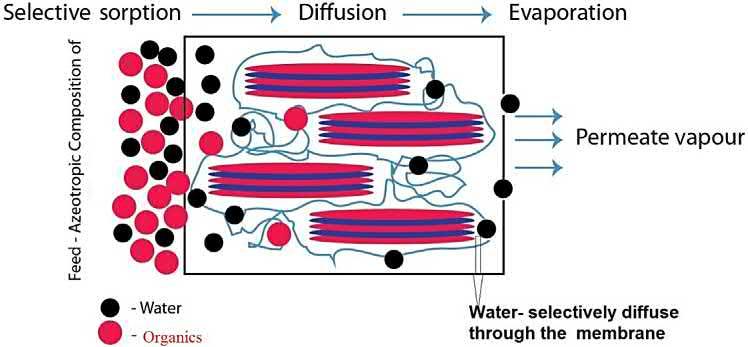
1.2.1Solution-diffusionmodel Thesolution-diffusionmodelwasfirstproposedbyThomas Grahamtoexplaingastransportthroughthediaphragms.In thismodel,therearethreeconsecutivestepsinPV:(1)permeateselectivelysorbedfromthefeedliquidtothemembrane, (2)diffusionofthepermeatethroughthemembranes,and (3)desorptionofthepermeatecomponenttothevaporphase
(Fig.1.1) [15].Thephysicochemicalinteractionsofthemembranematerialsandthepermeatingmoleculesplayavitalrole inPVseparation.Sorptionselectivityand/ordiffusionselectivity ofthepermeatingmoleculesarethekeyfactorsthataffectsPV performances.Thepermeatingmoleculeswhichhavemore interactionwiththemembranefavorssorptionselectivityandis basicallyidentifiedbythesolubilityparameterofthemembrane andthepenetrants.
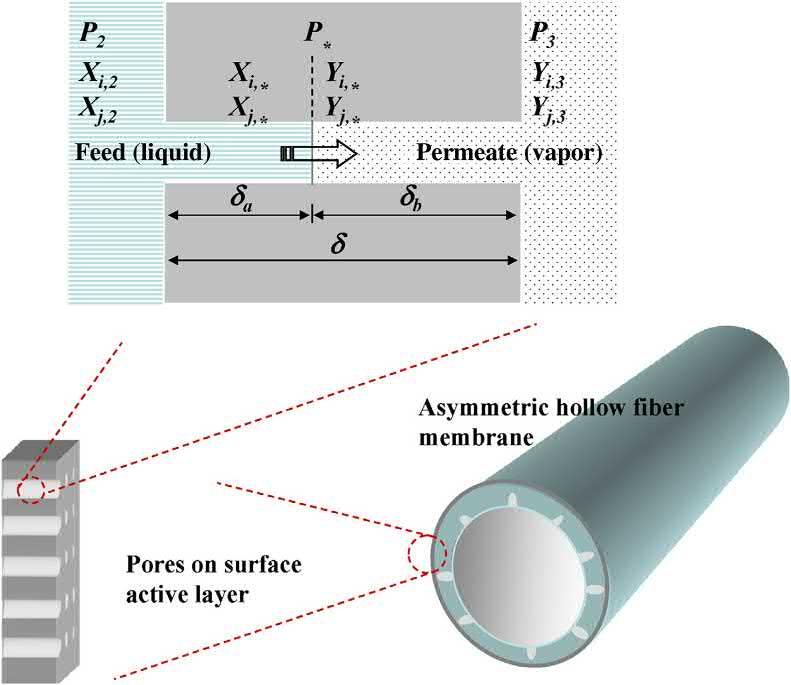
1.2.2Poreflowmodel OkadaandMatsuura [15] proposedtheporeflowmodel whichisanalternativetoinvestigatethemasstransportinPV. Accordingtothismodel,thePVtakingplacethroughthecylindricalporesexistsinthemembranesandmicroporesfavors betterPVseparation.Themainstepsinpore-flowmodelare (1)thepermeanttransportsthroughtheliquid/vapor-filledportionoftheporeand(2)thephasechange(liquidtovapor) occurinsidethepore.
Fig.1.2 describesthePVtransportthroughtheasymmetric hollowfiber [16].Thepenetratinglength,liquid-filledsectionof theporeforliquid-phasetransportofthepermeants,and vapor-filledsectionforvapor-phasetransportarewellexplained inthefigure.ThePVperformanceisacompliedeffectoftransportthroughtheliquidandvaporphases,andtheevaporation isoccurringattheboundaryregionofliquidandvapor sections.
ThebasictheoryofPViswellexplainedbymanyresearchers.DrioliandGiornoexplainedthemasstransportthrough membranesinthebook“Encyclopediaofmembranes” [17]. Bakeretal. [18],Neeletal. [19],FengandHuang [20],etc.are primescientistswhostudiedandexplainedthedifferent theoreticalaspectsofPVseparationtechnology.
Figure1.1 Schematic representationofsolutiondiffusionmodelfor pervaporation. Source:Adapted fromJoseetal.,Ind.Eng.Chem. Res.2014,53,43,16820 16831. Copyright r 2014,American ChemicalSociety.
Figure1.2 Schematic representationofthepore-flow modelinpervaporation transportwithinasymmetric hollowfibermembranes.
Source:AdaptedfromP. Sukitpaneenit,T.S.Chung,L.Y. Jiang,JournalofMembrane Science362(2010)393 406, Copyright r 2010ElsevierB.V.All rightsreserved.
Recentlyin2010Bakeretal. [18] introducednewterms calledpermeability,permeance,andselectivitytounderstand therealmembraneintrinsicproperties.Thebasictermssuchas fluxandseparationfactorlagtheintrinsic,drivingforcenormalizedpropertiesbehindtheseparation.Inthischapter,we correlatedthefluxandseparationfactorwithpermeabilityand intrinsicselectivity,respectively,togetmoreideaonrealmembraneintrinsicproperties.
1.2.3Permeability:anormalizedflux Theefficiencyofthemembranetoseparateaparticular componentfromthemixtureisdeterminedbyflux( J ),permeabilityorpermeance,andthesefactorsareassociatedwiththe weightofthepermeate(Q)ingramsorkilograms.
Theeffectiveareaofthemembranes(inm2)andtime(t inh) usedforseparationarealsoinfluencedthepermeationflux. Componentfluxistheanotherfactorwhichdependsonthe permeatecompositionofoneofthecomponentsandtotalflux.
Ji and Jj arethecomponentfluxes, J istheflux, Xi and X are thepermeatecompositionofthemixtures.
Permeabilityofthemembraneiscomponentfluxesnormalizedformembranethicknessandaregivenfromthefollowing equation:
Permeanceisathicknessnormalizedcomponentandis expressedas
Itismostcommonlyreportedasgaspermeationunit(gpu) (1gpu 5 3.349 3 10 10 molm 2 s 1 Pa 1).
1.2.4Selectivity:anintrinsicmembraneproperties Separationfactor(α),ofthePVisdefinedas
XA and XB arethecompositionofmixture“ X”inthefeed andpermeate,respectively. YA and YB arethecompositionof component“Y”inthefeedandpermeate,respectively.The preferentialsorptionofselectivecomponentincreasestheseparationefficiencyofthePVprocess.
Theenrichmentfactorisalsousedtodescribetheselective separationofpreferentiallysorbedcomponentandisexpressed as
Theintrinsicmembraneperformanceisanalyzedbythe parameter,selectivity,anditistheratioofpermeabilityofcomponent i bycomponent j.
Themembraneintrinsicpropertiesobtainedinmolarterms andareconcentrationdependent,evenifthedrivingforce contributionisremoved.Thisisthemainadvantageofthe selectivity,permeance,andpermeability.
1.3Membranesforpervaporation ThemembranesusedinPVprocessareusuallydenseor microporouspolymericmembraneswhichallowthepassageof selectivecomponentsthroughit.Themembranematerial wouldbechemicallyandthermallystableinnatureandableto transportselectivecomponentsatelevatedtemperature.Inthis regard,thestiffandrigidpolymerchainshavebeenconsidered aspotentialcandidateforPVapplication [21].Theselectionof polymericmaterialforPVmainlydependsonthedesired application.Thedifferentapplicationareasandselectionof suitablemembranetypearesummarizedin Fig.1.3.
PVA,chitosan,andsodiumalginatearemainlyusedPVmembranesfortheseparationofwaterfromaqueous organicmixtures [22,23].Polyimides(PIs),polybenzimidazole(PBI),and PDMS-basedmembranesareusedforhydrophobicororganophilicPVseparation [24,25].Kungetal. [26] reportedPBI/PIblend membranesfortheseparationoftoluene/isooctanemixtures.The hydrophobicPVisveryusefulinmanyfieldsandthenumbersof polymerichydrophobicmembranesareusedfortherecoveryof organics,butitlooksunattractiveduetotheirpoorperformance.
Figure1.3 Pervaporationmembranetypesanditsapplicationareas.
ThehydrophilicmembranesarewidelyusedforthePVseparation ofdifferentmixtures.ThePVmembranesarebroadlycategorized into(1)inorganicmembranes,(2)mixedmatrixmembranes (MMMs),and(3)polymericmembranes,
1.3.1Inorganicmembranes Zeolites,silica,titania,andzirconiaarethemaininorganic membranesusedforthePVseparationofwaterfromorganic mixtures.Theinorganicmembranesoffersuperiorthermaland chemicalstabilitycomparedtopolymers.Veenetal. [27] reportedthedewateringofsolventssuchasethanol,methylethylketone,DMF,THF,andethylacetateusingsilicamembranes.Silicamembranesexhibitedapermeationfluxof 1485gm 2 h 1 withprocessselectivityof350forthedewateringofethanol.Micorporoussilicaanddopedsilicamembranes showedexcellentalcoholdehydration [28].The10%zirconia dopedsilicamembranesshowedafluxof0.86kgm 2 h 1 with separationfactor300forthedehydrationofisopropanol/water (90/10wt.%)mixtures.ZeolitemembranesareoneoftheextensivelystudiedinorganicmembranesforPVprocess.Boththe hydrophilicandhydrophobiczeolitesexhibitedbetterPVperformance.Sodiumalginatehydrophiliczeolitemembranes effectivelyusedforthedehydrationofalcoholswithhigherseparationfactor [29,30].Thesilicate-1hydrophobiczeoliteswere usedfortheremovaloforganicsolventsfromwater [31 33]. Matsufujietal. [34] observedaseparationfactorof270forthe separationof n-hexane/2,3-DMBusingZSM-5zeolitemembranes,buttheseparationfluxdecreaseswithmembraneconcentrationandisduetothepresenceofAl2O3 support.The uniform,molecular-sizedporescausesignificantdifferencein thetransportofvarioussolvents,butthehighcostofzeolite limitedtheirapplications [35].
1.3.2Mixedmatrixmembranes Tocombinethestrengthsofinorganicfillersandpolymeric membranes,MMMswereusedforthePVapplications.The porousmagnesiumoxide(MgO)particle-incorporatedMatrimid matrixmembraneswereusedforthePVdehydrationofIPAand themembranesshowedhigherselectivitywith15wt.%MgO loading [36].TheSiO2-reinforcedpolyelectrolytecomplexes (PECs)membranesshowedgoodperformancefortheIPAdehydration [37].The5wt.%SiO2-loadedPECsmembranesexhibited aseparationfluxof2.3kgm 2 h 1 andseparationfactorof
1721at75 Cwithafeedcompositionof10wt.%waterinIPA. Numerousinorganicfillerssuchaszeolite [38,39],silica [40], MgO,morerecentlypolyhedraloligosilsesquioxane(POSS) [41,42],etc.wereincorporatedintothepolymermatrixand theresultantmembranesshowedpromisingPVseparationperformance.Metal-organicframeworks(MOFs)areothermembranecomponentswithcontrollableporesizesandhigh porosity,andtheygetconsiderableattractioninPVapplication duetoitsexceptionalthermalandchemicalstability [43,44]
1.3.3Polymermembranes PolymericmembranesarewidelyusedforthePVseparation ofvariousmixturesbecauseofitsexcellentchemicalstability, easeofprocessability,bettermechanicalproperties,thermal stability,etc.Thepolymericmembranesareusedforthedehydrationoforganicsolvents [45],theremovaloforganiccomponentsfromwater [46,47],biofuelsfromfermentationbroth [48,49],theseparationoforganicliquidmixtures [50 52],and thedesulfurizationofgasoline [53].ThePVseparationefficiency largelydependsonthenature,size,andmolecularstructureof thepolymers.Thenaturalandsyntheticpolymersexhibited excellentPVperformance.Chitosanandsodiumalginateare twonaturallyoccurringpolymersthatarewidelyusedinPV process.Chitosan-basedmembranesarewidelyusedforthe dehydrationoforganicsandremovalofalcoholsfromorganics duetoitsexcellentfilmformingandhydrophilicnature [54 56].TheUVcross-linkedchitosan/polyvinylpyrrolidone blendmembranesshowedaPSIof167fortheseparationof water ethanolmixtures [57].Sodiumalginate(NaAlg)isanaturallyoccurringhydrophilicpolymerwhichiswidelyusedin separationofwater organicmixtures [58,59].Butthemain drawbackofNaAlgisitshighswellingwhichreducestheselectivityandmechanicalstrength,andhenceitisnotfavorablefor commercialapplications.
SyntheticpolymerssuchasPIs [60,61],poly(etheramides) [62],polyurethanes [63],poly(methylmethacrylates)(PMMA) [64],PDMS [65],polyacrylates [66],polyesters,polyethylenimines [67],etc.areusedforthePVseparationofvariousmixtures.Ribeiroetal. [47] synthesizedpoly(siloxane-co-imide) membranesforthePVseparationoftoluene/n-heptane mixturesandfoundthattheincorporationofsiloxaneinthe polymergreatlyimprovedthePVperformance.Thepolypropylene-,polyvinylidenefluoride-,andPMMA-basedmembranes wereusedforthePVseparationofbenzeneandcyclohexane
mixtures [68].ThePVdehydrationof90%ethanolwascarried outusingPVA-carboxylatedchitosanandachievedaseparation factorof1231at30 C [69].
1.4Factorsaffectingthepervaporation Themainfactorsthataffecttheefficiencyofseparationare asfollows.
1.4.1Pressure Feedandpermeatepressurearethemaindrivingforcefor PV.Theactivityofthecomponentsatthedownstreamsideof themembraneandthepermeatepressureisindirectrelation whichstronglyinfluencethePVperformances. Eqs.(1.3)and (1.4) showtherelationshipbetweenthepartialpressureandthe permeationflux.Themaximumgradientcanbeobtainedat zeropermeatepressure.Mouliketal. [70] studiedtheeffectof pressureonthePVprocessandobservedthatthedrivingforce formethanoltransportationdecreasesatlowvacuumwhich slowsdownthedesorptionrateofmolecules.HenceSeparation factorandpermeationdecreasewithlowvacuumconditions.
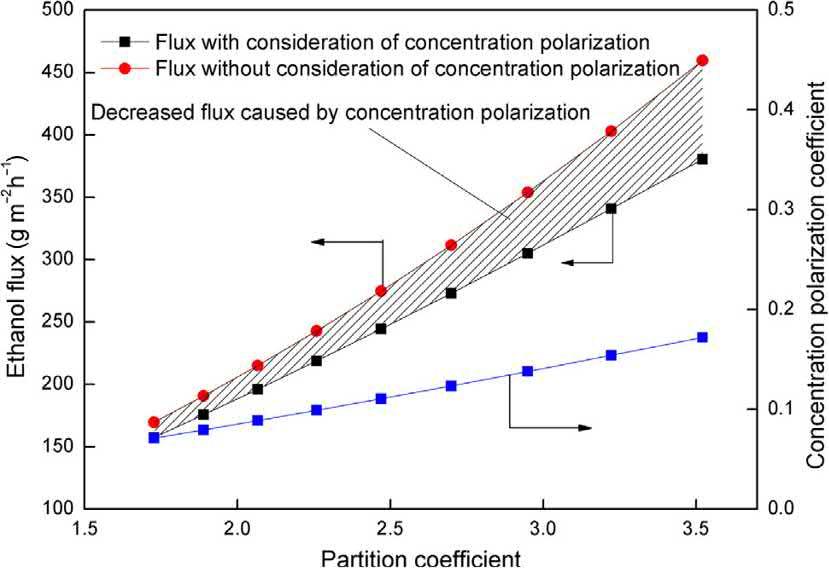
1.4.2Concentrationpolarizationandpartition coefficient PVperformancesarealsoaffectedbythepartitioncoefficient (Fig.1.4).Accordingtophysicalsciences,partitioncoefficientis theratioofconcentrationsofacompoundinamixtureoftwo immisciblesolventsatequilibrium.But,inPV,itrepresentsan equilibriumstateofonesolute(permeate)attheinterphaseof membranephaseandsolutionphase.Qiuetal. [71] studiedthe effectofpartitioncoefficientonthePVseparationofethanol andobservedthattheethanolfluxwasincreasedwithpartition coefficient.Theincreaseinpartitioncoefficientincreasedthe masstransferduringPVwhichismainlyduetothestrongpolymerandsolventinteraction.From Fig.1.5,itisobservedthat theconcentrationpolarizationcoefficienthasmucheffecton flux.Higherconcentrationpolarizationcoefficienthasbad effectonincreasedflux.Thiscouldbeovercomebychoosea membranewithmoresolventinteraction.
10 Chapter1 Polymernanocompositemembranesforpervaporation:anintroduction
Figure1.4 Effectofpartitioncoefficientonethanolpervaporation,ethanolconcentration:5wt.%;temperature:45˚ C. Source:AdaptedfromB.Qiu,Y.Wang,S.Fan,J.Liu,S.Jian,Y.Qin,etal.Sep.Purif.Technol.220(2019)276 282.Copyright r 2019ElsevierB.V.Allrightsreserved.
1.4.3Temperature Thepermeationperformancecanbedescribedasafunction oftemperaturethatobeysfollowingArrhenius-type relationship:
where J isthepermeateflux(kgm 2 h 1), Ea istheactivation energy(J/mol)associatedwiththepermeateprocess, Rg isthe gasconstant(Jmol 1 K 1),and T istheabsolutefeedtemperature(K).
Asthefeedtemperatureincreases,thethermalmotionsof thepolymerchainsarestimulatedandpermeatemolecules becomemoreenergetic,andhencepermeationrateincreases. Thusthepermeationfluxincreaseswithriseintemperature. Theactivationenergyoftheprocesscanbeobtainedfromthe semilogarithmicplotsofthepermeatefluxesagainstthereciprocaloftheabsolutetemperature(1/T ).From Table1.1,itis observedthatthepermeationfluxincreaseswithincreasein feedtemperature.But,theseparationfactordecreasessignificantlywithincreaseintemperature.Thisisduetothefactthat
therandomthermalmotionincreasedthefreevolumeavailable forpermeationofthemolecules.Theincreaseinfreevolumein themembraneprovidesmorespaceforthemoleculetodiffuse whichinturndecreasetheselectivepermeationofoneofthe componentthroughthemembrane.Hence,thepermeationflux increases,butselectivitydecreasessignificantly.
Figure1.5 Totalfluxagainst PVAlayerthicknessfor10% and40%feedethanolmixtures at25˚C. PVA,Poly(vinyl alcohol). Reproducedwith permissionfromM.N.Hyder,R.Y.M. Huang,P.Chen,J.Membr.Sci.318 (2008)387 396,Copyright r 2008 ElsevierB.V.Allrightsreserved.
Table1.1Effectonfeedcomposition,temperature,andthicknessofthemembraneonthePV characteristics.
Notes:PDMS,Polydimethylsiloxane; PVA,poly(vinylalcohol).
1.4.4Membranethickness Membranethicknessisanotherimportantparameterwhich affectsthePVperformances.Thepermeationrateofacomponentisinverselyproportionaltothemembranethickness becauseasthemembranethicknessincreases,thepermeation resistanceincreases.Thinmembranesgivehigherpermeation fluxesandisduetothefactthatthepermeatemoleculeshave tocrosslessdiffusivepathinsidethemembraneandmass transferbecomeeasierthroughathinnermembrane [72]. Fig.1.5 showsthatthetotalfluxdecreasedwithincreasein membranethickness.