PolymerElectrolytes
CharacterizationTechniquesandEnergyApplications
Editedby
TanWinie,AbdulK.Arof,andSabuThomas
Editors
Dr.TanWinie SchoolofPhysicsandMaterialScience FacultyofAppliedSciences UniversitiTeknologiMARA 40450ShahAlam,Selangor Malaysia
Dr.AbdulK.Arof CentreforIonicsUniversityofMalaya DepartmentofPhysics UniversityofMalaya 50603KualaLumpur Malaysia
Dr.SabuThomas SchoolofChemicalSciences MahatmaGandhiUniversity PriyadarsiniHills Kottayam686560 Kerala,India
Allbookspublishedby Wiley-VCH arecarefullyproduced.Nevertheless, authors,editors,andpublisherdonot warranttheinformationcontainedin thesebooks,includingthisbook,to befreeoferrors.Readersareadvised tokeepinmindthatstatements,data, illustrations,proceduraldetailsorother itemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>.
©2020Wiley-VCHVerlagGmbH& Co.KGaA,Boschstr.12,69469 Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc.used inthisbook,evenwhennotspecifically markedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34200-6
ePDFISBN: 978-3-527-80543-3
ePubISBN: 978-3-527-80546-4
oBookISBN: 978-3-527-80545-7
Typesetting SPiGlobal,Chennai,India PrintingandBinding
Printedonacid-freepaper 10987654321
Contents
Preface xiii
1PolymerElectrolytes:StateoftheArt 1
MasashiKotobuki
1.1Introduction 1
1.2SolidPolymerElectrolyte 4
1.3GelPolymerElectrolyte 8
1.4CompositePolymerElectrolyte 12
1.5Summary 17 References 17
2ImpedanceSpectroscopyinPolymerElectrolyte Characterization 23
MohamedAbdulCareem,IkhwanSyafiqMohdNoor,andAbdulK.Arof
2.1Introduction 23
2.2IS:PrincipalOperationandExperimentalSetup 23
2.2.1BasicPrinciplesofImpedanceSpectroscopy 23
2.2.2ImpedanceSpectroscopy(IS)Technique 25
2.2.3ElectricalConductivityofaSample 26
2.2.4ConditionsNecessaryforISMeasurements 26
2.2.5ImpedancePlotsofSimpleCircuits 28
2.2.5.1APureResistance, R28
2.2.5.2APureCapacitance, C28
2.2.5.3 R and C ConnectedinSeries 29
2.2.5.4 R and C ConnectedinParallel 30
2.2.5.5CombinedSeriesandParallelCircuits 31
2.2.5.6ImpedanceSpectraofModelElectrolyteSystems 32
2.2.6PossibleConductionProcessesinaSolidElectrolyte 35
2.2.7ImpedanceSpectraofRealSystems 36
2.2.7.1TheConstantPhaseElement(CPE) 37
2.2.7.2EquivalentCircuitsforRealSystems 37
2.2.7.3Electrolyte/Electrode(E/E)Interface 39
2.2.7.4DiffusionImpedanceorMassTransportImpedance 39
2.2.7.5WarburgImpedance 40
2.2.7.6EquivalentCircuitRepresentationofanE/ESystem 41
2.2.8Impedance-RelatedFunctions 42
2.2.8.1ImmittanceFunctions 43
2.2.8.2RelationshipsBetweenImmittanceFunctions 43
2.2.8.3ImmittancePlots 43
2.2.8.4ChoiceBetweenImmittanceFunctions 46
2.2.9ExperimentalSetup 46
2.2.9.1SampleandCellArrangement 47
2.2.9.2OtherPracticalDetailsandPrecautions 48
2.3IS:ExperimentalDataInterpretationandAnalysis 49
2.3.1DeterminationofBulkResistancefromtheImpedancePlots 49
2.3.2ImpedanceDataInterpretationandAnalysis 50
2.3.2.1InterpretationofImpedanceData 51
2.3.2.2ChoiceofEquivalentCircuits 51
2.3.3DeterminationofTransportParametersfromImpedanceData 53
2.3.3.1Bandara–Mellander(B–M)Method 53
2.3.3.2NyquistPlotFittingMethod 57
2.3.4SomeExperimentalResultsandAnalysis 59
2.3.4.1ConductivityCalculationofImpedancePlots 59
2.3.4.2ConductivityDeterminationfromFittingEquivalentCircuit 60
2.3.4.3EvaluationofTransportPropertiesusingNyquistPlotFitting Method 60
2.4Conclusions 63 References 64
3ThermalCharacterizationofPolymerElectrolytes 65
AparnaThankappan,ManuelStephan,andSabuThomas
3.1Introduction 65
3.2TGA:ExperimentalDataInterpretationandAnalysis 67
3.3DSC:ExperimentalDataInterpretationandAnalysis 75
3.4DSC:ExperimentalErrorsandSuggestionforImprovement 82
3.4.1Transition(s)at0 ∘ C 83
3.4.2ApparentMeltingat T g 83
3.4.3ExothermicPeaksBelowDecompositionTemperatureWhile Heating 84
3.4.4BaselineShiftafterEndothermicorExothermicPeaks 86
3.4.5SharpEndothermicPeaksDuringExothermicReactions 86
3.5DMA:ExperimentalDataInterpretationandAnalysis 87 References 91
4EnergyinaPortableWorld 93
NoorSyuhadaZakuan,WooHawJiunn,andTanWinie
4.1Introduction 93
4.2HistoryDevelopmentofMobilePower 94
4.3CaringforMobilePowerfromBirthtoRetirement 102
4.3.1GettingtheMostOutofthePrimaryBatteries 103
4.3.2GettingtheMostOutoftheLead-AcidBatteries 103
4.3.3GettingtheMostOutoftheNickel-BasedBatteries 104
4.3.4GettingtheMostOutoftheLithiumIonBatteries 105
4.4MobilePowerRecycling 106
4.4.1RecyclingPrimaryBatteries 106
4.4.2RecyclingRechargeableBatteries 109 Acknowledgments 111 References 111
5InsightonPolymerElectrolytesforElectrochemicalDevices Applications 113 MariaManuelaSilva,VerónicadeZeaBermudez,andAgnieszkaPawlicka
5.1Introduction 113
5.2Theory:IonicConductivity 117
5.3Applications 120
5.3.1ConventionalBatteriesandTransientBatteries 120
5.3.2FuelCells 123
5.3.3Supercapacitors 124
5.3.4ElectrochromicDevices 125
5.3.5Dye-SensitizedSolarCells 127
5.3.6Sensors 128
5.3.7Light-EmittingElectrochemicalCells 128 References 129
6PolymerElectrolyteApplicationinElectrochemical Devices 137
SitiNorFarhanaYusufandAbdulK.Arof
6.1Introduction 137
6.2PropertiesofPolymerElectrolytes(PEs) 137
6.3ReviewofPolymerElectrolytes 138
6.3.1DrySolidPolymerElectrolytes(SPEs) 138
6.3.2GelPolymerElectrolytes(GPEs) 141
6.3.2.1IonicLiquidGelPolymerElectrolytes(ILGPEs) 144
6.3.2.2GelPolymerElectrolyteswithNanomaterials 146
6.4ApplicationofPEsinElectrochemicalDevices 148
6.4.1Dye-SensitizedSolarCells(DSSCs) 148
6.4.2LithiumIonBatteries 150
6.4.3ElectricalDoubleLayerCapacitors(EDLCs) 152
6.4.4PolymerElectrolyteFuelCells 156
6.4.5ElectrochromicWindows 163
6.4.6ElectrochromicMaterials 164
6.4.6.1TransitionMetalOxides 164
6.5ChallengesandImprovements 167
6.5.1InElectrolytes 167
6.5.2InDevices 169
6.5.2.1DSSCs 169
6.5.2.2FuelCell 170
6.5.2.3Batteries 171
6.5.2.4EDLCs 172
6.5.2.5ElectrochromicWindows(ECWs) 172
6.6FutureAspects 173
6.6.1ElectrochromicWindows 173
6.6.2Batteries 173
6.6.3DSSCs 173
6.6.4FuelCells 174 References 175
7PolymerElectrolytesforLithiumIonBatteriesandChallenges: PartI 187
ShishuoLiang,WenqiYan,MinxiaLi,YusongZhu,LijunFu,andYupingWu
7.1Introduction 187
7.2ClassificationofPolymerElectrolytes 188
7.2.1SolidPolymerElectrolytes(SPEs) 188
7.2.2GelPolymerElectrolytes(GPEs) 190
7.3PerformanceandImprovements 190
7.4ApplicationandPerformanceofPolymerLithiumIonBatteries 194
7.5FutureTrends 195 Acknowledgments 196 References 197
8PolymerElectrolytesforLithiumIonBatteriesandChallenges: PartII 201
SitiNorFarhanaYusufandAbdulK.Arof
8.1Introduction 201
8.2StructureandOperationofLithiumIonBatteries 202
8.2.1AnodeMaterials 204
8.2.2CathodeMaterials 205
8.2.3Electrolytes 206
8.2.4Li+ IonTransportinPolymerElectrolytes 206
8.3PolymerElectrolyteforLithiumIonBatteries 207
8.4PerformanceCharacteristicsofLithiumIonBatteries 216
8.5ChallengesandImprovement 218
8.6FutureTrends 219 References 221
9PolymerElectrolytesforSupercapacitorandChallenges 231 SafirAhmadHashmi,NitishYadav,andManojKumarSingh
9.1Introduction 231
9.2PrincipleandWorkingProcessofSupercapacitors 232
9.2.1ChargeStorageMechanismsinEDLCs 233
9.2.2ChargeStorageMechanismsinPseudocapacitors 236
9.2.2.1UnderpotentialDeposition 237
9.2.2.2RedoxPseudocapacitance 237
9.2.2.3IntercalationPseudocapacitance 238
9.3ElectrolytesforSupercapacitors 239
9.3.1LiquidElectrolytes 239
9.3.2Polymer-BasedElectrolytes 241
9.3.2.1Solvent-FreeSolidPolymerElectrolytes(SPEs) 242
9.3.2.2GelPolymerElectrolytes(GPEs) 242
9.3.2.3PorousPolymerElectrolytes 252
9.4PerformanceCharacteristics 255
9.4.1ElectrodeCharacterization 255
9.4.2CharacterizationofSupercapacitors 258
9.4.2.1ElectrochemicalCharacterizationTechniquesandImportant Parameters 258
9.4.2.2PerformanceofPolymerElectrolyte-BasedSupercapacitors:Some CaseStudies 262
9.5ChallengestoSolid-StateSupercapacitorsandFutureScopeof Improvement 284 References 285
10PolymerElectrolytesforQuantumDot-SensitizedSolarCells (QDSSCs)andChallenges 299
T.M.W.J.BandaraandJ.L.Ratnasekera
10.1DemandandSupplyofEnergy 299
10.2TheSunasaPotentialEnergyResource 300
10.3AdvantagesofSolarCells 301
10.4Photo-ElectrochemicalSolarCells 301
10.4.1GeneralMechanismofaPhoto-ElectrochemicalSolarCell 303
10.4.2MechanismofaPhoto-ElectrochemicalSolarCell 304
10.4.3Semiconductor/PolymerElectrolyteJunction 308
10.4.4Photo-sensitizationofWideBandgapSemiconductors 308
10.5QuantumDot-SensitizedSolarCells(QDSSCs) 310
10.5.1QuantumDots 310
10.5.2MechanismofaQDSSC 313
10.5.3QuantumDot-SensitizedSolarCells(QDSSCs) 314
10.5.4PolymerElectrolytesforQDSSCs 317
10.6PerformancesofDifferentQDSSCsAssembliesBasedonPolymer Electrolytes 318
10.6.1Quasi-Solid-StateQDSSCsBasedonPolyacrylamideHydrogel Electrolytes 318
10.6.1.1HydrogelElectrolytewithPolyacrylamide 318
10.6.2CdS-SensitizedCellwithPANandPVDFElectrolytes 319
10.6.3ZnO-BasedQuasi-SolidQDSSCsSensitizedwithCdSandCdSe 323
10.6.3.1Quasi-Solid-StateElectrolytePreparation 324
10.6.4NaturalPolysaccharideThinFilm-BasedElectrolyteforQuasi-Solid StateQDSSCs 324
10.6.5Dextran-BasedHydrogelPolysulfideElectrolyteforQuasi-Solid-State QDSSCs 325
10.6.6CarbonDotsEnhanceLightHarvestinginaSolid-StateQDSSC 326
x Contents
10.6.7QuantumDot-SensitizedSolarCellsBasedonOligomerGel Electrolytes 326
10.6.8QDSSCswithThiolate/DisulfideRedoxCoupleand Succinonitrile-BasedElectrolyte 327
10.6.9Graphene-ImplantedPolyacrylamideGelElectrolytesfor QDSSCs 328
10.6.10PEOandPVDF-BasedElectrolyteforSolid-StateElectrolytesfor QDSSCs 329
10.6.11HydroxystearicAcid-BasedPolysulfideHydrogelElectrolytefor CdS/CdSeQDSSCs 329
10.6.12QDSSCsBasedonaSodiumPolyacrylatePolyelectrolyte 330 10.7Summary 331 References 334
11PolymerElectrolytesforPerovskiteSolarCelland Challenges 339 RahulSingh,Hee-WooRhee,BhaskarBhattacharya,andPramodK.Singh
11.1Introduction 339
11.2PrincipleandWorkingProcessofPerovskiteSolarCell 341
11.2.1PerovskiteMaterials 342
11.2.2PerovskiteStructure 344
11.2.3SynthesisofPerovskite 349
11.2.3.1Solution-ProcessedMethod 349
11.2.3.2HotCastingTechnique 352
11.2.3.3VaporDepositionMethod 352
11.2.3.4ThermalEvaporationTechnique 352
11.3PolymerElectrolyteforPerovskiteSolarCell 354
11.3.1DeviceFabrication 354
11.3.2HoleTransportLayer 355
11.4PerformanceCharacteristics 355
11.5ChallengesandImprovement 356
11.6FutureTrends 357
11.7Conclusion 358
CompetingInterests 358 Acknowledgments 358 References 358
12PolymerElectrolytesforElectrochromicWindows 365 LiNaSimandAgnieszkaPawlicka
12.1Introduction 365
12.2PrinciplesandWorkingProcessofElectrochromicWindow 366
12.3TypesofElectrochromicElectrodes 367
12.4MechanismofECW 368
12.5PolymerElectrolytesforElectrochromicWindows 369
12.5.1Background 369
12.5.2CriteriaofPolymerElectrolytesandElectrochromicDevice 369
12.5.3TypesofPolymerElectrolytesUsedinECWs 370
12.5.3.1SolidPolymerElectrolytes(SPEs) 370
12.5.3.2GelPolymerElectrolytes(GPEs) 374
12.5.3.3CompositePolymerElectrolyte 383
12.6PresentECDsUses/Applications 385
References 385
Index 391
Preface
Polymerelectrolytesresultingfromthecomplexationofpolymerwithorganic orinorganicsaltswerefirstintroducedbyP.V.Wrightin1975andproposedas potentialmaterialforelectrochemicaldevicesbyM.Armandin1978.Thepolymericpropertyofpolymerelectrolytesgivesitadvantagesoverliquidelectrolytes intermsofleak-proof,sizeandshapeflexibility.Polymerelectrolytesaresubstitutedfortheliquidelectrolyteinnewgenerationelectrochemicaldevicessuchas batteries,supercapacitorsandsolarcells.
Chapter1ofthisbookprovidesanimportantreviewofthedevelopment ofpolymerelectrolytes.Chapters2and3presenttheelectricalandthermal propertiesofpolymerelectrolytescharacterizedbyimpedancespectroscopy, TGAandDSC,respectively.Thesechaptersincludetheoreticalconsiderations, theknow-howneededtosetuptheexperimentsandhowtoanalyzetheir results.Therearealsodiscussionsonsourcesoferrorsalongwithsuggestions forimprovement.Chapters4to12focusonapplicationsofpolymerelectrolytes, whichcoverthebatteries,supercapacitors,solarcellsandelectrochromic windows.Challengesinfabricationandperformanceimprovementofthese deviceswereindentifiedalongwithsuggestionsforimprovement.
Thisbookbringstogetherprestigiousinternationalauthors.Novicereaderwill findanoutlineofbasictheory,experimentalsetupandadiscussionofexperimentalmethodsanddataanalysis,withexamplesandappropriatereferences. Leadingresearchersandfacultymemberswillfindthisbookveryvaluableasan excellentreviewandacomprehensivesummaryoftheliteratureonthesubject withadiscussionofcurrenttheoreticalandexperimentalissues.
Finally,wewishtoexpressourgratitudeandappreciationtothechapter contributors.Commentsfromreviewersaregratefullyappreciated.
October8,2019
TanWinie AbdulK.Arof SabuThomas
PolymerElectrolytes:StateoftheArt
MasashiKotobuki
NationalUniversityofSingapore,DepartmentofMechanicalEngineering,21LowerKentRidgeRd., Singapore119077,Singapore
Polymersaredefinedaslargemoleculesormacromolecules,whichconsistof repeatedsubunits.Thepolymersmaybesyntheticplasticsornaturalbiopolymerssuchasprotein,DNA,andsoon.Inthepast20years,polymershavebeen tailoredaselectronorionconductors.Whenappropriatesaltisaddedintosome polymers,theirionicconductivitycanbeimprovedtothevaluethatcanbeused aselectrolyte.Inthepastthreedecades,manyresearchershaveendeavoredto developnewpolymerelectrolytes(PEs)duetotheirpotentialapplicationinelectrochemical/electricalpowergeneration,storage,andconversionsystems.Asa result,alotofnewPEshavebeenfound,characterized,andtriedtobeappliedin electrochemical/electricaldevices.Particularly,Liion-conductivePEshavebeen ofinterestforapplicationinLibatteriesduetotheirhighenergydensity.Inthis chapter,thestate-of-the-artdevelopmentofLiion-conductivePEsisdescribed.
1.1Introduction
PEwasfirstintroducedin1973[1].Sincethen,theresearchonPEhasbeen eagerlyperformedbymanyresearchers,especiallyintheearly1980s,duetothe recognitionofPEsinindustrialapplications.
PEisamembranecomposedofsaltsdissolvedinapolymer[2].Somepolymer matrixessuchaspolyethyleneoxide(PEO)andpoly(methylmethacrylate) (PMMA)candissolvesaltsandformsalt–polymercomplexesduetotheinteractionbetweenoxygenatominthepolymerchainandcationinthesalt.This solvent-freeandion-conductivesystemhasbeenexpectedtobewidelyapplied inelectrochemicaldeviceslikerechargeablesolid-statebatteries,especially rechargeableLiionbatteries.Inrecentyears,PEshaveotherprospectiveapplicationsinadvancedelectrochemical,electrochromic,andelectronicdevicessuch asfuelcells,supercapacitors,electrochemicalsensors,analogmemorydevices, andelectrochromicwindows[3–7].Figure1.1showsthestructureofcommercial LiionbatteriesusinggraphiteandLiCoO2 asananodeandcathode,respectively. Liionsonlyexistinthecathodesidewhenthebatteriesareconstructed.TheLi PolymerElectrolytes:CharacterizationTechniquesandEnergyApplications, FirstEdition.EditedbyTanWinie,AbdulK.Arof,andSabuThomas. ©2020Wiley-VCHVerlagGmbH&Co.KGaA.Published2020byWiley-VCHVerlagGmbH&Co.KGaA.
Libattery.
ionsmovefromcathodetoanodeinachargeprocess.Inadischargeprocess,Li ionsmigratetotheoppositedirection.Theelectrolytedoesnotgetinvolvedin batteryreactionsintheLibatteriesandjustactsasLiion-conductivemedia.In general,theelectrodesofLibatteriesarepreparedbymixingthreecomponents, i.e.activematerial,binder,andconductivematerial.Poly(vinylidenefluoride) (PVdF)hasbeenusedasabinderthusfar.Asaconductivematerial,acetylene blackandKetjenblackhavebeennormallyemployed.Thesethreecomponents aremixedandaddedintoasolvent N -methyl-2-pyrrolidone(NMP)tomakea slurry.TheslurryispaintedontoCu(foranode)orAl(forcathode)foil,which isusedasacurrentcollector.Asfortheelectrolytes,nonaqueouselectrolytes havebeenusedsofarduetonarrowelectrochemicalwindowofaqueous electrolytes.ThenonaqueouselectrolytesarecomposedofLisalt(usuallyLiPF6 ) dissolvedintoorganicsolvents,whichareamixtureofacyclicsolventwithlow viscositylikedimethylcarbonate(DMC)andethylmethylcarbonate(EMC) andcyclicsolventwithhighdielectricconstantlikeethylenecarbonate(EC). However,flammabilityofthesenonaqueousorganicsolventssometimeshas causedserioussafetyissuessuchasfirehazardandleakageofelectrolyte[8–10]. Contrarytothenonaqueouselectrolytes,solidpolymerelectrolytes(SPEs)can solvetheissueofleakage.Additionally,PEspossessmuchlowerflammability thantheorganicelectrolytesduetolowvaporpressure.ThePEsshouldhavethe followingphysical,chemical,andelectrochemicalproperties[11]:
(1)Highionicconductivityatoperatingtemperature(normallyroomtemperature),whileelectronicconductivitycanbenegligible.
(2)Sufficientmechanicalstrengthattheoperatingtemperatureforselfsupportedcell.
(3)Highelectrochemicaldecompositionvoltage(wideelectrochemical window).
(4)Highcationicoranionictransferencenumber.
Figure1.1
(5)Environmentalbenign,non-hygroscopic,lowcost,andeaseofpreparation.
(6)Stabilityagainstchemicalandelectrochemicalreactionswithbothelectrodes (cathodeandanode)duringpreparationandoperationofthebattery.
(7)Thermalexpansioncoefficientmatcheswiththatoftheelectrodestoensure goodcontactsbetweenPEandelectrodes.
Table1.1summarizesacomparisonofpropertiesofconventionalnonaqueous electrolyteandPE.Ceramicelectrolyte,whichisanothertypeofsolidelectrolyte, isalsocompared.Theceramicelectrolytespossessexcellentsafetyduetotheir nonflammablenaturealthoughstructuralflexibilityisverylowandconductivityislowbecauseofhighgrainboundaryresistance.PEsnormallypossesshigh conductivityandstructuralflexibilityaswellasrelativelywideelectrochemical window.
PEscanbecategorizedintothreegroupsbasedontheirphysicalstateand composition:(i)Solidpolymerelectrolyte(SPE),(ii)gelpolymerelectrolyte (GPE),and(iii)compositepolymerelectrolyte(CPE)[12,13].Also,PEscanbe dividedintotwogroupsbypolymersource,i.e.syntheticpolymerandnatural polymer(Figure1.2).SyntheticpolymerssuchasPEOandpolycarbonatehave beenusedaspolymermatrix.Naturalpolymerslikechitosanareusuallylow cost,eco-friendly,biodegradable,andabundant.Therefore,researchesonnatural polymerforPEshavebeeneagerlyperformedinrecentyears.
Table1.1 Comparisonofvariouspropertiesamongnonaqueous,polymer, andceramicselectrolytes.
ConductivityHighMiddleLow
SafetyLowHighHigh
StructuralflexibilityMiddleHighLow ElectrochemicalwindowMiddleMiddleWide
Figure1.2 Classificationofpolymerelectrolytes.
Nonaqueous electrolyte Polymer electrolyte Ceramics electrolyte
1.2SolidPolymerElectrolyte
SPEiscomposedofhostpolymermatrix(es)andLisalts.Thesaltsaredissolved intothepolymermatrix(es)andprovideionicconduction.Theresearchon SPEscommencedthreedecadesago[14].ThePEO-basedSPEwasinvestigated firstandhasbeenmostwidelyresearchedsofar[1].Theetheroxygenatoms inthePEOmatrixcomplexwithLiion[15]anddissolvetheLisalts.Itis widelybelievedthatthecationtransportisrelatedtothecomplexingsegmental motionofPEOchain[16,17](Figure1.3).OtherpolymerhostssuchasPVdF, poly(vinylidenefluoride–hexafluoropropylene)(PVdF–HFP),PMMA,poly(vinyl chloride)(PVC),poly(acrylonitrile)(PAN),poly(acrylicacid)(PAA),poly(ethyl methacrylate)(PEMA),andsoonalsocontainoxygen,nitrogen,chlorine,or fluorineatom,whichcanformacomplexwithLiion,andthesegmentalmotion wouldcauseionconduction.Table1.2summarizesrepeatunitofpolymer
Polyethylene oxide (PEO)
Table1.2 Polymerhostgenerallyusedinpolymerelectrolytes.
Figure1.3 Segmental motion-assistedLiion conductioninPEO-based polymer.
PolymerhostRepeatunit
Exampleof polymerelectrolyte
Ionic conductivity atroom temperature (Scm 1 )References
PEO–(CH2 CH2 O)n –(PEO-HBP)–LiTFSI–BaTiO3 2.6 × 10 4 [18]
PVdF–(CH2 CF2 )n –PVdF–PEO–LiTFSI5.4 × 10 4 [19]
PVdF-HFP–[(CH2 CF2 )–(CF2 CFCF3 )]n –P(VdF-HFP–SiO2 –LiTFSI 4.3 × 10 3 [20]
PMMA–[(CH2 C(–CH3 )–COOCH3 )]n –PMMA–LiClO4 –DMP–CeO2 7.3 × 10 6 [21]
PVC–(CH2 CHCl)n –PVC–Li2 B4 O7 –DBP2.83 × 10 6 [22]
PAN–[CH2 CH(–CN)]n –PAN–LiClO4 –Al2 O3 5.7 × 10 4 [23]
PAA–[CH2 CH(–COOH)]n –PAA–LiClO4 –Sb2 O3 2.15 × 10 4 [24]
PEMA–[(CH2 C(–CH3 )–COOCH2 CH3 )]n –PEMA–LiTf-IL1.17 × 10 4 [25]
Figure1.4 SchematicArrheniusplotof PEO–LiClO4 polymerelectrolyte.

hostsandexamplesofPEs.Thestructuralflexibilitydirectlyrelatestothe ionicconductivity.Inotherwords,theamorphousphaseintheSPEssupports ionicconduction.Thecontinuoussegmentalmotionoftheamorphouschain occursabovetheglasstransitiontemperature(T g )[26]. T g ofthePEsshould belowerthanroomtemperature.Moreover,increaseintheamorphousregions withraisedtemperatureimprovestheionicconductivity.Figure1.4depicts aschematicArrheniusplotofPEO–polystyrene(PSt)-LiClO4 PE[27].An inflectionpointaround40 ∘ Ccanbeobservedinthisplot.Thiscorrespondsto aphasetransitionfromcrystallinetoamorphousphaseofthepolymerhost. Duetothephasetransition,theactivationenergyofionicconductionislowered considerably.However,mechanicalstrengthoftheSPEsystemisrelatedto themovementofpolymerchain.Increaseintheamorphousregionsimplies thatthepolymerchainsmoveactively,whichenhancestheionicconductivity, butdecreasesthemechanicalstrength.Thisadverseeffectleadstodifficultyin constructionofself-supportedpolymerbatteries.
Inordertosolvethisproblem,manyeffortshavebeendevotedtodevelop novelpolymermatrixescontainingamorphousstate,includingblendingofpolymers,cross-linking,copolymerization,andsoon.Theseapproachescanlower crystallinityor T g oftheSPEsystemsandincreasetheionicconductivityand mechanicalstrength[28–30].
BlendPEsarepreparedbymixingtwoormorekindsofmolecularchains (Figure1.5).Thechainsaremixedwith/withoutanychemicalbondingbetween them.Thisblendedpolymerchaindestroystheregularityofonepolymer chainandpreventsitsrearrangement,resultinginformationofamorphous structure.Tanakaetal.preparedapolymerblendcomprisingofPEOand polyethyleneimine(PEI).Theionicconductivityof[(8:2)PEO/PEI]–LiClO4 was ∼10 4 Scm 1 at30 ∘ C[31].Thishighionicconductivityisconsideredto
Figure1.5 Schematicimageofblendpolymer.
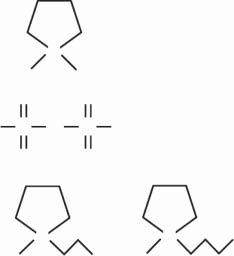
Polymer APolymer BBlend polymer
1PolymerElectrolytes:StateoftheArt
beduetomixingofPEOwithPEIthathinderedtheircrystallizationandledto moreusefulmatrices.
Blockcopolymerelectrolytes(BCEs)havebeenproposedasanovelSPE tosatisfyboththeionicconductivityandthemechanicalstrength[32].The mostcommonstructuresresearchedarethediblockandtriblockcopolymers.Alithiumsalt-solvatingpolymerisusedtoensurecontinuousionic conductionpathways,andanotherpolymerhost,whichformsapolymer frameworkoftheSPEs,ischosentoprovidethemechanicalstrengthtothe SPEs[33].Polymerfilmswithgoodmechanicalstrengthcanbeobtained withoutsacrificingtheionicconductivity.Thus,abalanceofthesalt-solvating polymerandtheframeworkpolymerisimportant.Niitanietal.reportednovel PSt–poly(ethyleneglycol)(PEG)methylethermethacrylate(PME)–PSt–LiClO4 triblockcopolymers(Figure1.6)[34,35].ThetriblockSPEexhibitedhigh ionicconductivityof2.0 × 10 4 Scm 1 withoutplasticizer.ThePStblockwas usedtoimprovemechanicalproperties,whilethePEOmoietyincreasedthe ionicconductivity.Anall-solid-statebatteryofLiCoO2 /SPE/Licelldemonstratedexcellentcharge–dischargepropertyatroomtemperature.Thisconcept canalsoincreaseLiiontransferencenumber.Bouchetetal.reporteda single-ionPEbasedonpoly[lithium4-styrenesulfonyl(trifluoromethylsulfonyl) imide][P(STFSILi)–PEO–P(STFSILi)]polyanionictriblockcopolymers[36]. ThismaterialdemonstratedhighLiiontransferencenumber(>0.85),excellentmechanicalstrength,andgoodionicconductivity(1.3 × 10 5 Scm 1 at60 ∘ C).Thebatterytestsexhibitedgoodpowerandcyclingperformances at60 ∘ C.
Cross-linkingPEsshowgoodionicconductivityatambienttemperatureand fullyamorphousfeature(Figure1.7)[37].However,thecross-linkingpolymer generallyexhibitslowelasticityandbrittlenessaswellaslowprocessability[38]. Across-linkedhighmolecularweightpoly(oxyethylene)hasbeenreportedasa
Figure1.6 Schematicimageofnovelnanostructure-controlledSPEanditssyntheticscheme.
Polymer APolymer BCross-linked polymer
Figure1.7 Schematicimageofthecross-linkedpolymer.
newSPE,whichdemonstratedfavorableionicconductivityandgoodmechanical strength[39].
Copolymerisapolymerpreparedfromatleasttwodifferenttypesof monomers.PVdF-HFPisthemostcommoncopolymerresearchedasPE. ThePVdF-HFPcopolymerispreparedbycopolymerizationofPVdFand HFP(Figure1.8).Thecopolymerexhibitsbetterfeaturescomparedwiththe mono-polymersalone,whichcouldbeattributedtothesynergisticeffectsin thecombinedstructure.Also,PVdFhasreceivedmuchattentionduetogood electrochemicalstabilityandhighdielectricconstant[40,41].Thepresenceof strongelectron-withdrawingfluorineatoms(C–F)promotesdissociationof saltsandincreasestheconcentrationofchargecarriers,leadingtohighionic conductivity.Jiangetal.reportedionicconductivityofPVdF-basedSPEsof above10 4 Scm 1 atroomtemperature[41].
Otherpolymerhostsalsohavebeenresearched.ThepotentialofPMMAasa polymerhostwasreportedbyIijimaetal[42].ThePMMA-basedSPEsshowed lowmechanicalintegrityandhighbrittleness[43].BlendingofPMMAwithPVC hasalsobeenresearched[44,45].PVCisalsoanattractivepolymerhostdue toitslowcostandeasyprocessing.GPEsbasedonPVCwithplasticizershave beenwidelyresearched.Poly(vinylalcohol)(PVA)isnontoxicandcosteffectiveandpossessgoodtensilestrength,goodmechanicalstrength,goodoptical properties,hightemperatureresistance,highabrasionresistance,goodflexibility,biocompatibilities,highhydrophilicity,andexcellentchemicalandthermal stabilities[46–50].PVAcontainsalargeamountofpolarhydroxylgroup,leadingtohighhydrophilicity.Thisprovidesotheradvantageslikeeaseinpreparation andhighdielectricconstant.Duetothesesuperiorproperties,PVAhasreceived considerableresearchinterestaselectrolytesforfuelcellsandelectricaldouble layercapacitors[51].APAN-basedPEhassomeoutstandingcharacteristicssuch ashighthermalstabilityandhighionicconductivity[52].PANissuperiorover PVdFwithrespecttomechanicalstability[53].The–CNgroupsinPANcaninteractwithcations.StructuresofthesepolymerhostsareshowninFigure1.9.
Additionally,naturalpolymersuchaschitosan[54],ricestarch[55],andcorn starch[56]hasalsobeenstudied.Thesehaveanadvantageasnovelpolymerhosts duetobeinglowcost,biodegradable,eco-friendly,andabundant.
1.3GelPolymerElectrolyte
GPEisalsoknownasplasticizedPE,whichwasfirstintroducedbyFeuilladeand Perchein1975[57].GPEcontainsaplasticizerorgelledpolymermatrix,whichis swollenbyadditionoftheplasticizers[58],andcanbepreparedbysimplyheatingamixtureofpolymerandLisaltwithsolvent.Byintroducingaplasticizer and/orsolvent,theiontransportisnotdominatedbythesegmentalmotionof polymerchainsbutoccursintheswollengelledphaseorliquidphase.Ingeneral, whenthepolymeriscomposedofinterconnectedmicropores,theionicconductivityofGPEsmainlydependsonthepropertiesoftrappedliquidelectrolyte.On thecontrary,iontransportmainlyoccursintheswollengelledphaseifthepolymerdoesnotcontainmanyinterconnectedpores.TheGPEsshouldpossessgood mechanicalstrength,capabilityofholdingaliquidelectrolyte,highionicconductivity,andelectrochemicalstabilitytowardbothcathodeandanode.Manykinds ofpolymermatrixsuchasPEO[59],PMMA[60],PAN[61],PVC[62],PVdF [63],andP(VdF-HFP)copolymer[64]havebeenwidelystudiedasaframework forGPEs.
Plasticizers,whichareusuallylowmolar-massorganics,organicsolvents,or ionicliquids(ILs),largelyaffectthepropertiesofGPEs.Aplasticizercanincrease thecontentoftheamorphousphaseinaPEandpromotesegmentalmotion [65].Inaddition,itcanalsopromotedissociationofionpairs.Asaresult,the numberofchargecarriersisincreased,leadingtoenhancedionicconductivity [59].PEGhasbeenwidelyusedasalowmolar-massplasticizer.Itwasreported thattheionicconductivityofPEO–LiCF3 SO3 complexincreasedwiththe decreaseofmolecularweightofPEGandwiththeincreaseofPEGcontent[66]. However,thehydroxylendgroupsinPEGreactwithelectrodematerialssuch aslithiummetal.Therefore,variousmodifiedformsofPEGweresynthesizedby replacingactiveoxygenatomsinPEGwithmonomethoxy(MMPEG),dimethoxy (DMPEG)groups,orlithium(LPEG)ions[67].TheLPEGplasticizercanimprove thecompatibilityofthePEwithlithiummetalanode[13].Onthecontrary,in somepolymersystemssuchasPEO-PMMA,nosignificantimprovementof ionicconductivitybyadditionofPEGwasreported.Theplasticizermustbe chosencarefullydependingonthepolymerhostused.Otherlowmolar-mass
plasticizerssuchaspolyethyleneglycoldimethylether(PEGDME)[68],borate estersuchasPEGborateester[69],tris(2-(2-methoxyethoxy)ethyl)borate (B2 ),andtris(2-(2-(2-methoxyethoxy)ethylborate(B3 )[70],phthalatessuchas dibutylphthalate(DBP)[71],dimethylphthalate(DMP)[65],dioctylphthalate (DOP)[72],succinonitrile(SN)[73],andsoonhavealsobeenstudied.Theionic conductivityofthePEscontainingtheseplasticizersissummarizedinTable1.3.
TheorganicsolventsusuallyusedasplasticizerarepolarandnonvolatilesolventssuchasEC,propylenecarbonate(PC),diethylcarbonate(DEC),andDMC. Thesolventshelptosolvateionsandfacilitatetheirtransportation.Therefore, highdielectricconstantandlowviscosityarerequiredforthesolvents.Individualsolventisdifficulttomeetalltherequirements;thus,amixtureofthesolvents usuallyhasbeenemployed.Themixtureofsolventsismoreefficienttoenhance theionicconductivitycomparedwithasinglesolvent,whichisduetothecombinedactionofdielectricconstantandviscosity[75].Theionicconductivityof someGPEsusingtheplasticizersistabulatedinTable1.4.Theionicconductivitycanbeincreasedto ∼10 3 Scm 1 bytheadditionofsuitablesolvents.Choi etal.studiedtheionicconductivityofPANpolymerswollenby1MLiPF4 in EC/DMC(1:2wt%),EC/DMC(1:1),EC/EMC(1:1),EC/DEC(1:1),and EC/DMC/DEC(1:1:1)[61].TheorderofionicconductivitywasEC/DMC/DEC (1:1:1) > EC/DMC(1:1) > EC/EMC(1:1) > EC/DEC(1:1) > EC/DMC(2:1). ILshaveattractedconsiderableinterestasanovelplasticizer.Theyareroom temperaturemoltensalts,whicharecomposedofabulkyorganiccationanda largedelocalizedinorganicanion.TheILspossesssomeuniquepropertiessuch ashighchemicalandthermalstabilities,nonflammability,negligiblevolatility, andhighelectrochemicalstability[83–85].Duetotheseuniqueproperties,the incorporationofroomtemperatureionicliquids(RTILs)intoPEscanovercome inherentlimitationsoftheionicconductivityinSPEsasproposedbyPasserini
Table1.3 Ionicconductivityofvariouspolymerelectrolyteswithplasticizers. Polymer
(PEO)15 /LiTFSI/10wt%PEGDME >10 3 50[59] (PEO)20 /LiCF3 SO3 /50wt%PEGDME1.2 × 10 4 30[68] 30wt%PVA/10wt%LiClO4 /60wt%DMP1.5 × 10 4 29[65]
17.5wt%PVA+7.5wt%PMMA/8wt% LiClO4 /67wt%DMP 6 × 10 5 30[74]
PEO/15wt%LiCF3 SO3 /15wt%PEG1.7 × 10 5 RT[72]
PEO/15wt%LiCF3 SO3 /20wt%DOP7.6 × 10 4 RT[72] (PEO)20 /LiBOB/24mol%SN >6 × 10 4 60[73]
PEO/13wt%LiCF3 SO3 /10wt%DBP1.6 × 10 4 27[71] (PEO)20 /LiTFSI/100wt%PEGborateester1.36 × 10 5 30[69]
PSi/15wt%LiCF3 SO3 /40wt%borateesterB2 3.70 × 10 5 RT[70]
PSi/15wt%LiCF3 SO3 /40wt%borateesterB3 1.60 × 10 4 RT[70]
1PolymerElectrolytes:StateoftheArt
Table1.4 Ionicconductivityofvariouspolymerelectrolyteswithorganicsolvents.
Polymer Ionic conductivity (Scm 1 ) Temperature (∘ C)References
P(VdF-HFP)/1.0MLiPF6 /EC + DEC1.0 × 10 3 RT[76]
P(VdF-HFP)/1.0MLiPF6 /EC + DMC + DEC1.43 × 10 3 RT[64]
PVdF/1.0MLiPF6 /EC + DMC + DEC1.0 × 10 3 RT[77]
30wt%PVC/8wt%LiClO4 /62wt%PC6.70 × 10 6 30[62]
PEO/15wt%LiCF3 SO3 /20wt%EC8.12 × 10 5 RT[78]
(PEO)16 /LiClO4 /40wt%EC2.67 × 10 4 RT[79]
4.5wt%PMMA/46.5wt%LiClO4 /30wt%
PC + 19wt%EC 5.0 × 10 4 RT[80]
PAN/1MLiPF6 /EC + DMC + DEC >1.0 × 10 3 RT[61]
PAN/1MLiBF4 /EC + DEC2.80 × 10 3 RT[81]
PVdF/1.0MLiPF6/EC + DMC1.00 × 10 3 RT[63]
7.5wt%PVC/5wt%LiBF4 /42wt%
EC + 28wt%PC 8.60 × 10 5 RT[82]
etal.in2003[86].Sincethen,manygroupshavedevotedmuchefforttostudy GPEscontainingILs.ManytypesofILcomprisingcationsbasedonpyridium, imidazolium,piperidinium,quaternaryammonium,andsoonandanions basedon[BF4 ] ,[PF6 ] ,[N(CF3 SO2 )2 ] ,[CF3 SO3 ] ,[C4 F9 SO3 ] ,[N(CN)2 ] , [CF3 CO2 ] ,[CF3 CONCF3 SO2 ] ,andsoonhavebeeninvestigated.Inmost cases,theILscontainthesameanionassaltssuchasILcontaining[PF6 ] anioninLiPF6 .ThisisbecausethesolubilityofthesaltintotheILincorporatingthesameanionismuchhigherthaninasystemofdifferentanions. Passerinietal.studiedaseriesofILscontainingpyrrolidinium-basedcations andTFSIanions[87–94].Thestructureof N -alkyl-N -methyl-pyrrolidium bis(trifluoromethanesulfonyl)imide(PYR1A TFSI,A = Cn H2n+1 ,1 < n < 10) isdepictedinFigure1.10.ThecommonlyusedILsforPEsarePYR13 TFSI (1-propyl-1-methylpyrrolidiniumbis(fluorosulfonyl)imide)andPYR14 TFSI (1-butyl-1-methylpyrrolidiniumbis(trifluorosulfonyl)imide).TheionicconductivityofPEO/LiTFSI/PYR13 TFSIPEis ∼10 4 Scm 1 at20 ∘ C,whichisabouttwo ordersofmagnitudehigherthanthatwithouttheIL[86].Itwasreportedthatthe PYR13 TFSIalsoimprovedtheionicconductivityofPVdF-basedpolymer.The GPEcomposedofP(VdF-HFP)/LiTFSI/PYR13 TFSIshowedhighionicconductivityof2.7 × 10 4 Scm 1 [95].Also,incorporationofPYR14 TFSIplasticizerinto P(VdF-HFP)/LiTFSIPEshowedagoodionicconductivityof4.0 × 10 4 Scm 1 andhighthermalstability[96].AninterestingstudywasperformedbyWinter etal.[83,84].TheyconductedinsituUVphotoradiationofacomplexof PRO/LiTFSI/PYR14 TFSIusingbenzophenoneasacross-linkingagenttoobtain highconductivePEswithhighmechanicalproperties. ILscontaining1-alkyl-3-methylimidazoliumcationhavealsobeenused asaplasticizerinGPEs.Thestructureof1-alkyl-3-methylimidazolium
Figure1.10 Structureof N-alkyl-N-methyl-pyrrolidium bis(trifluoromethanesulfonyl)imide.
n-methyl A= iso-propyl A= n-propyl (PYR13)
A= n-butyl (PYR14) A= n-ethyl
A= iso-butyl
A= sec-butyl
A= tert-butyl
Figure1.11 Structureof 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide.
bis(trifluoromethylsulfonyl)imideILisdepictedinFigure1.11.Thealkylgroup inthecationaffectstheionicconductivityofGPEs.EMImTFSI,PMImTFSI,and BMImTFSIwereemployedasplasticizerstoformGPEsbasedonP(VdF-HFP) matrix[97].AlltheseGPEsrevealedahighionicconductivityintherange of2.4 × 10 3 to4.5 × 10 3 Scm 1 .TheGPEcontainingEMImTFSIshowed thehighestconductivity.Also,effectsofaniononconductivityofPEO-based GPEwerestudiedusingILscontainingBMImcation[98].Inthestudy,three differentanions(TFSI ,BF4 ,andCF3 SO3 )andBMImcationwereemployed. ThePEO/LiTFSI/BMImTFSIelectrolyteshowedthehighestionicconductivity andgoodelectrochemicalstability[99].
Asexpected,compatibilitybetweenILsandpolymer iscriticaltodeterminethepropertiesofGPEs.Itwas reportedthatquaternaryammonium-basedILsweremore compatiblethanimidazolium-basedILswithPEO-PMMA copolymer[100].Thisisthoughttobeduetopreferable interactionbetweenILandpolymermatrix.
TheGPEscomprisingpolymericlithiumsaltsand ILshavebeenproposedasnovelGPEs.Lithium poly(2-acrylamido-2-methylpropanesulfonate)(PAMPSLi,Figure1.12)isusedasthepolymericlithiumsalt.The PAMPSLi-PVdFcopolymerwascombinedwith1-ethyl-3-methylimidazolium tricyanomethanide(EMImTCM).TheionicconductivityofPAMPSLi-PVdF
Structure ofPAMPSLi.
Figure1.12