Dedication
Dedicated to my wife Sweta and daughter Aashwita.
Kuldeep Bauddh
Dedicated to my wonderful and blessed wife of 48 years, and our 4 beautiful daughters, 4 sons-in-law, and 10 amazing grandchildren. Proverbs 31:10-31 and Psalm 127:3-5.
John Korstad
Dedicated to my mother Mrs. Geeta Sharma and daughter Adya Jha.
Pallavi Sharma
Contributors
Joseph Acker Oral Roberts University, Tulsa, OK, United States
Muhammad Aqeel Ashraf School of Environmental Studies, China University of Geosciences (Wuhan), Wuhan, P. R. China
Sneha Bandyopadhyay Ecological Restoration Laboratory, Department of Environmental Science & Engineering, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
Kuldeep Bauddh Department of Environmental Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
Corne Beneke Oral Roberts University, Tulsa, OK, United States
Poulomi Chakravarty Department of Environmental Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
R.P. Choudhary Department of Mining Engineering, Faculty of Engineering, Jai Narain Vyas University, Jodhpur, India
Mary Claire Cooperrider Oral Roberts University, Tulsa, OK, United States
Bhupinder Dhir School of Sciences, Indira Gandhi National Open University, New Delhi, India
R.S. Dubey Department of Biochemistry, Faculty of Science, Banaras Hindu University, Varanasi; Central University of Gujarat, Gandhinagar, India
Elena-Luisa Iatan Department of Endogene Processes, Natural Hazards and Risk, Institute of Geodynamics “Sabba S. Stefanescu” of Romanian Academy, Bucharest, Romania
Sakinatu Issaka School of Environmental Studies, China University of Geosciences (Wuhan), Wuhan, P. R. China
A.B. Jha Department of Plant Sciences, Crop Development Centre, University of Saskatchewan, Saskatoon, SK, Canada
John Korstad Department of Biology and Global Environmental Sustainability, Oral Roberts University, Tulsa, OK, United States
Manoj Kumar Department of Environmental Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
Mukesh Kumar School of Environmental Sciences, Jawaharlal Nehru University, New Delhi, India
Alka Kumari Department of Botany, University of Lucknow, Lucknow, India
Khushbu Kumari Department of Environmental Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
Contributors
Elizabeth J. Lam Chemical Engineering Department, Universidad Católica del Norte, Antofagasta, Chile
Subodh Kumar Maiti Ecological Restoration Laboratory, Department of Environmental Science & Engineering, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
T. Mohan Manu Environmental Biotechnology and Genomics Division, CSIR-National Environmental Engineering Research Institute, Nagpur, India
Ítalo L. Montofré Mining Business School, ENM; Metallurgical and Mining Engineering Department, Universidad Católica del Norte, Antofagasta, Chile
Sangeeta Mukhopadhyay Environmental Management Division, CSIR—Central Institute of Mining and Fuel Research (CIMFR), Dhanbad, Jharkhand, India
Lakshmi Pathak Institute of Environment and Sustainable Development, Banaras Hindu University, Varanasi, India
Deepak Kumar Patra Department of Botany, Nimapara Autonomous College, Nimapara, Puri, India
Hemanta Kumar Patra Department of Botany, Utkal University, Bhubaneswar, India
Leandro Santos Peixouto Instituto Federal de Educação, Ciência e Tecnologia Baiano – IF Baiano, campus Guanambi, BA, Brazil
Yslai Silva Peixouto Instituto Federal de Educação, Ciência e Tecnologia Baiano – IF Baiano, campus Guanambi, BA, Brazil
Alanna Cibelle Fernandes Pereira Centro Universitário FG – UniFG, Guanambi, BA, Brazil
Chinmay Pradhan Department of Botany, Utkal University, Bhubaneswar, India
Nopi Stiyati Prihatini Department of Environmental Engineering, Lambung Mangkurat University, Banjarbaru, Indonesia
Sierra Pruitt Oral Roberts University, Tulsa, OK, United States
Yendery Ramírez Chemical Engineering Department, Universidad Católica del Norte, Antofagasta, Chile; School of Engineering Science, Lappeenranta-Lahti University of Technology, Lappeenranta, Finland
Shelby Reiser Oral Roberts University, Tulsa, OK, United States
Vaniele Souza Ribeiro Instituto Federal de Educação, Ciência e Tecnologia Baiano – IF Baiano, campus Guanambi, BA, Brazil
Madhumita Roy Department of Microbiology, Bose Institute, Kankurgachi, Kolkata, India
Lala Saha Department of Environmental Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
Kavita Shah Institute of Environment and Sustainable Development, Banaras Hindu University, Varanasi, India
Pallavi Sharma Department of Life Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
Tilak Raj Sharma
ICAR-Indian Institute of Agricultural Biotechnology, Ranchi, Jharkhand, India
V. Sheoran Department of Zoology, Faculty of Science, Jai Narain Vyas University, Jodhpur, India
Anil Kumar Singh ICAR-Indian Institute of Agricultural Biotechnology, Ranchi, Jharkhand, India
Lal Singh Environmental Biotechnology and Genomics Division, CSIR-National Environmental Engineering Research Institute, Nagpur, India
Shipra Singh School of Environmental Sciences, Jawaharlal Nehru University, New Delhi, India
Ragini Sinha ICAR-Indian Institute of Agricultural Biotechnology; Department of Life Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
Soemarno Department of Soil Sciences, University Brawijaya, Malang, Indonesia
Prafulla Soni Forest Research Institute, Dehradun, India
Sanjog T. Thul Environmental Biotechnology and Genomics Division, CSIR-National Environmental Engineering Research Institute, Nagpur, India
Jaya Tiwari Department of Environmental Studies, Zakir Husain Delhi College, University of Delhi, Delhi, India
Phytoremediation: A sustainable method for cleaning up the contaminated sites
Jaya Tiwaria, Poulomi Chakravartyb, Pallavi Sharmac, Ragini Sinhad, Manoj Kumarb, and Kuldeep Bauddhb
aDepartment of Environmental Studies, Zakir Husain Delhi College, University of Delhi, Delhi, India
bDepartment of Environmental Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India
cDepartment of Life Sciences, Central University of Jharkhand, Ranchi, Jharkhand, India dICAR-Indian
Institute of Agricultural Biotechnology, Ranchi, Jharkhand, India
1.1 Introduction
Sustainability is the only way for the survival of our planet Earth. The current human population of 7.8 billion as of January 2020, is exuding pressure on the limited area and natural resources of the Earth. Higher population results in higher consumption, as well as higher pollution. Pollution is spreading like an epidemic across the world because of recalcitrant nature of contaminants and their vast amount has been a cause of concern in this century (Glick, 2003, 2010). Furthermore, soil contamination with various organic and inorganic pollutants has become a critical global environmental problem with serious implications on public health (Salomons et al., 1995; El-Shahawi et al., 2010). Industrial activities, extraction of natural resources, burning of fossil fuels are some of the major sources of environmental pollution. Mining is another important sector that adds contributes a huge amount of heavy metals (HMs), metal leachates, acids, etc., to the soil, air, and water. The land near the mining areas becomes barren and lay unattended for several years. Similarly, oil drilling sites have various organic contaminants especially hydrocarbons which are considered as wasteland.
Various techniques have been tried and employed over the years to curb the ever-increasing menace of contamination. The removal of contaminants from soil is a complex and expensive process. Most of the available physiochemical remediation technologies are either expensive or they demand a huge amount of chemicals and/or energy. There are some technologies that alter the actual characteristics of the soil like soil fertility, microbial diversity, etc. (Khalid et al., 2017; Shah and Daverey, 2020). Often these changes are irreversible for example, in vitrification method soil zone is treated with high temperature
(> 1500°C), at such that soil melts and once the soil cools down HMs are entrapped in a glassy matrix. Similarly, during the soil washing, various organic solvents (e.g., sulfuric acid, hydrochloric acid, phosphoric acid, etc.) are utilized to solubilize and mobilize HMs, which adversely affect the soil biology (Liu et al., 2018). Such treated soil is not fit to grow plants and cannot be used for agriculture purposes. One important aspect of soil remediation is that it is also location-specific. Results obtained from a technique in one site may not be transferred to other sites where the type of soil and contaminants are different. Furthermore, if the contaminated soil contains a mixture of organic and inorganic contaminants, the remediation becomes even more complex, owing to different properties of the contaminants and their possible interactions (Reddy and Cameselle, 2009). Phytoremediation was proposed as the most sustainable and benign tool that can deal with mixed contamination (US EPA, 2001). Phytoremediation is a process in which green plants are used for the accumulation, degradation, or stabilization of the contaminants. It is a novel technology that improves the biological quality of soil (Bauddh et al., 2017; Chakravarty et al., 2017; Pandey and Bauddh, 2018; Patra et al., 2020).
The remediation technology is used to decontaminate several toxic heavy metals, inorganic and organic pollutants present in the soil, sediments, wastewater, rivers, groundwater, or atmosphere by using wild or genetically modified plants are known as phytoremediation. The phytoremediation techniques have been in use since 1990s (Garbisu and Alkorta, 2001; Campos et al., 2008).
Phytoremediation technique has gained much importance in recent years, but despite several efforts in the last 20–30 years on removal of contamination from the soil a huge research gap was felt due to scattered information on phytoremediation. Cultivation of suitable plant species on the abandoned mine and oil drilling sites is found natural and cost-effective method for the reclamation of these sites. After the extensive efforts of several authors, the scattered knowledge on phytoremediation was amalgamated in order to bridge the research gap. Keeping this in view the present chapter focuses on the fundamental mechanism of various phytoremediation techniques for the removal of inorganic and organic contaminants along with its associated advantages and limitations.
1.2 Environmental contamination
Any substance that is present in the environment in excess to the original amount can be termed as pollutant. Pollutants can be completely new compounds produced directly from the source as a primary pollutant, as well as that are produced as byproducts of primary one ( Manisalidis et al., 2020 ). The diverse nature of contaminants is the reason for difficulty in categorization of pollutants and thus in effective management too. The wastes generated from industries, agricultural lands, towns, power plants are the general sources of pollution. Although there are various types of contaminants,













some contaminants are potentially more troublemaking than others, Fig. 1.1 . Several organic pollutants are persistent in the atmosphere and toxic in nature. The organic xenobiotics are also known as persistent organic pollutants (POPs) as they have a high residence time in the environment. The synthetic organic pollutants include wastes from pharmaceutical industries, pesticides, petroleum wastes, polychlorinated biphenyls (PCBs), and polyaromatic hydrocarbons (PAHs) ( Abhilash and Singh, 2009 ; Kumar et al., 2019 ). Algal bloom due to the presence of high amounts of fertilizer in agricultural runoff is the cause for the reduction in dissolved oxygen in water bodies which leads to the death of marine life. HMs are another type of pollutants which are naturally present and are also released in the environment by anthropogenic activities. The HM dust, sewage sludge, and leachates are hazardous when they travel far distances and pollute the land air or water ( Gaur and Adholeya, 2004 ). Arsenic, lead, mercury, and cadmium are few of the toxic metals that are harmful to floral and faunal species. Radioactive elements are also determinant to the environment and can cause hazardous effects to health of organism and pollute the surrounding land, water, and air. Radioactive substances are more harmful because of their long residence time in the environment.
1.3 Sources of HMs
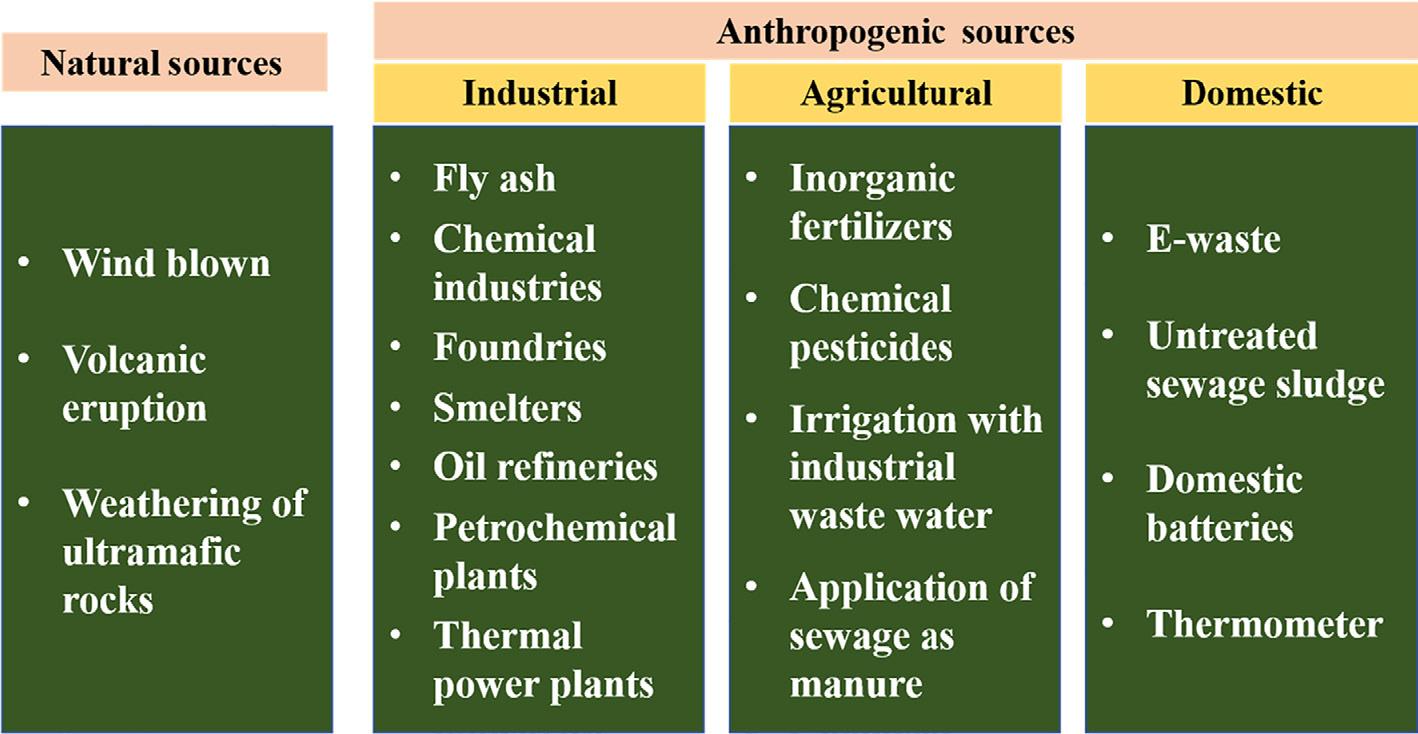
There are several inorganic and organic pollutants in various components of the environment. Widespread problem associated with toxicity of HMs has received a paramount attention from the researchers globally. As we can easily make out from Fig. 1.2, that we have myriad sources of HMs, which could be both natural and anthropogenic but finally end up in different components of the environment (e.g., lithosphere, hydrosphere, atmosphere, and biosphere). This section discusses various sources of HMs under the following categories.
Fig. 1.1
1.3.1 Natural sources of HMs
Many prior studies have extensively documented different natural sources of HMs. HMs s are found naturally under different and certain environmental conditions. These natural emissions are volcanic eruptions, sea-salt sprays, fires in forests, weathering of ultramafic rocks, biogenic sources, and air-borne soil particles. Natural weathering processes are responsible for the mobilization of HMs from their native spheres to different environmental compartments. The common HMs are nickel (Ni), chromium (Cr), mercury (Hg), lead (Pb), cadmium (Cd), zinc (Zn), arsenic (As), and copper (Cu). Although the aforementioned HMs are present in traces, but they still are harmful for the growth of plants and serious health implications in humans and other mammals (Herawati et al., 2000).
1.3.2 Anthropogenic processes
The release of pollutants in various components of the environment are mainly observed through various anthropogenic processes such as industrial, agricultural, domestic, wastewater, mining, and metallurgical. Furthermore, anthropogenic processes for the release of HMs have been observed to go beyond the natural fluxes for some metals. Metals emitted in wind-blown dusts are mostly from industrial areas. Some important anthropogenic sources which significantly contribute to the HM contamination in the environment include automobile exhaust which is responsible for the emission of lead; smelting which emits arsenic, copper, and zinc; insecticides which release arsenic and burning of fossil fuels emits nickel, vanadium, mercury, selenium, and tin.
Fig. 1.2
Potential sources of HMs.
1.4 Toxicity of HMs
1.4.1 Toxicity to plants
Adverse effects of HM toxicity on plants are visible through many symptoms, such as low biomass production, chlorosis, growth and photosynthesis inhibition, senescence, perturbed nutrient assimilation, and water balance, which result in plant injury, reduced growth, and even death. HMs influence plant physiology by promoting or inhibiting their growth. Some metals that are required in high concentration have a substantial role in the structural or osmotic activity, while those required at low concentration may indicate their role as a cofactor for specific enzymes. Certain HMs like Fe, Co, Cu, Mo, Mn, and Ni are elements essential for basic metabolisms of plants. However, beyond a certain concentration, these metals inhibit the biochemical processes of plants (as reviewed by Fernandes and Henriques, 1991; Sarma and Sarma, 2007; Sarma et al., 2009). To begin with, many metals affect photosynthetic pigments which, eventually affect the plant’s tolerance ability (Vajpayee et al., 2001). In general, metals cause a reduction in fluorescence associated with chlorophyll (Atal et al., 1991; El-Sheekh, 1992). Atriplex halimus subsp. schweinfurthii, hyperaccumulator of Cd, shows decreased chlorophyll pigments and stomatal transpiration rate and lower root hydraulic conductivity due to high Cd concentration (Nedjimi and Daoud, 2009). Also, the uptake of Cr affects pigmentation and amino acid content in aquatic plants. Vajpayee et al. (2000) showed inhibition of δ-amino laevulinic acid dehydratase by Cr (VI). Cr led carotenoid degradation has been observed in some plants which are metal and plant-specific (Barcelo et al., 1986).
In other examples, Hg toxicity has been reported where mercuric cation shows a higher affinity for sulphydryl (-SH) groups, that bind to two sites of protein molecule causing subsequent precipitation of protein (Clarkson, 1972). Therefore, Hg influences light as well as dark reactions of photosynthesis, inhibiting electron transport activity, chlorophyll fluorescence quenching in photosystem II and oxygen evolution. Maximum damage is caused due to the replacement of Mg by Hg that prevents light reaction and ultimately collapse of photosynthesis (Krupa and Baszynski, 1995). Similarly, specific ligands are present on cell membranes that preferentially bind with Hg. Inside the cell, Hg blocks functional groups of enzymes, utilizes transport systems for nutrient ions, denatures enzymes, and disrupts the integrity of cell and organelle membrane (Ochiai, 1987). Thus, Hg toxicity happens due to change in membrane permeability, reaction with sulfhydryl groups and phosphate groups (ADP or ATP), and cation replacements (Kabata-Pendias and Pendias, 1989). In excess amounts, metals like Cu and Fe can be dangerous because they participate in redox cycles and generate hydroxyl radicals which are very harmful to living cells (Stohs and Bagchi, 1995). Cd which is a non-redox metal is strongly phytotoxic and leads to growth inhibition and plant death. It induces alterations in lipid profile (Ouariti et al., 1997) and affects the activities of enzymes such as H+-ATPase which are associated with membranes (Fodor et al., 1995).
It also damages the photosynthetic apparatus (Siedlecka and Baszynsky, 1993), reduces chlorophyll content, and impedes the stomatal regulations (Barcelo and Poschenrieder, 1990).
1.4.2 Toxicity to animals
Inappropriate disposal of e-waste, uncontrolled industrial activities, urban runoff from municipal solid and liquid waste, agricultural runoff, burning of fossil fuels, e.g., coal, etc., end up into the aquatic ecosystem either through direct input or precipitation, or weathering or erosion, etc. Thus, there are all the possibilities for aquatic animals of getting exposed to a higher concentration of HMs and causing toxicity (Idrees et al., 2020).
Due to the long persistent nature of HMs, they can easily contaminate the food chains and thereafter, the entire ecosystem. As the concentration of HMs gets magnified at each trophic level, the top consumers of a food chain (e.g., human beings) are considered as most vulnerable to exhibit the severe adverse effects. Chronic exposure of HMs may decrease the activities of the central nervous system. It may damage the functioning of various organ systems like pulmonary, hepatic, renal, haematic etc. which may cause muscular dystrophy, Alzheimer’s disease, different types of cancers, and multiple sclerosis (El-Kady and Abdel-Wahhab, 2018).
However, the adverse effects caused due to exposure of different HMs, it largely depends on its route of exposure. For example, oral ingestion, inhalation, and dermal contact are perceived as three noteworthy routes of exposure, which also depends upon the type and concentration of HMs (Vardhan et al., 2020). Therefore, in the light of above-discussed problem associated with the toxicity of HMs in plants, as well as animals it is imperative to develop some environmentally sustainable treatment strategies to decrease the HMs load from various components of the environment.
1.5 Remedial measures (traditional measures)
As contamination of polluted sites is a common problem throughout the world, several measures for remedy and removal of contaminants are applied. Some of the techniques that are most commonly used to clean the contaminated sites are listed below.
1.5.1 Soil washing
Soil washing is an onsite method of contamination removal where two processes are applied; (a) contaminated soil is washed by chemical solvents to remove contamination and (b) concentrating the contaminants by attrition scrubbing, gravity separation, and particle size separation to reduce the size of the contaminants. Radionuclides, HMs, and organic pollutants can be removed with this technique although the process is not cost-effective and a high amount of contaminant laden residue is left which requires further treatment (Tangahu et al., 2011).
1.5.2 Soil excavation
In this process, the contaminated soil is excavated and dumped in another location, which is not a feasible or effective method as it merely dislocates the contaminants to another site (Tangahu et al., 2011).
1.5.3 Electrokinetic treatment
This is also an onsite treatment technique where electrodes are used to apply electric potential to contaminated soil and through this process the contaminants migrate toward electrodes by two processes, electromigration and electroosmosis (Cameselle et al., 2013).
1.5.4 Stabilization/solidification
In this process a stabilizing agent is utilized to bind the contaminants in the soil (Gomes, 2012). In another method heat is applied to the contaminants to melt them and then solidify them. This process is termed as vitrification.
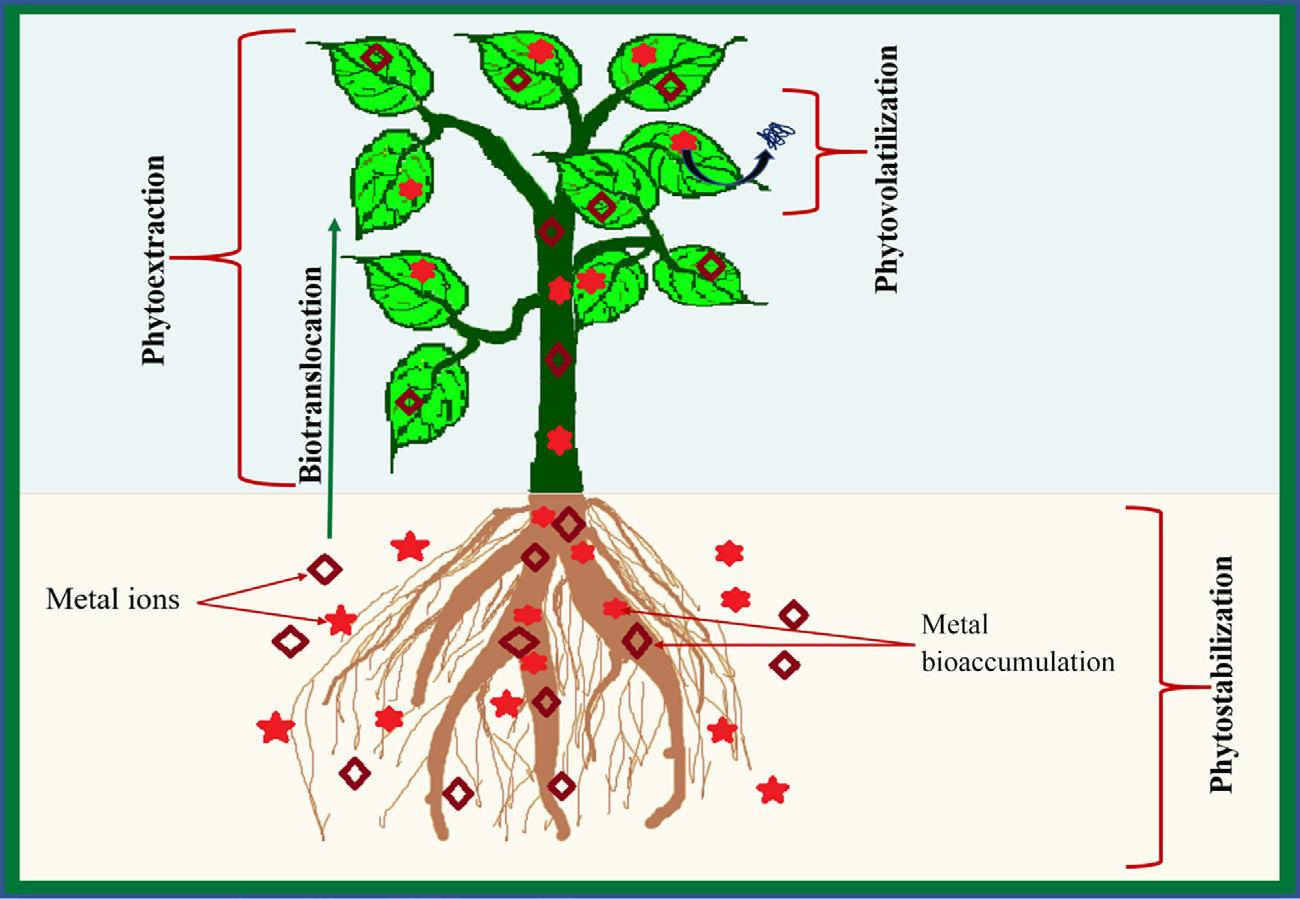
1.6 Phytoremediation
Phytoremediation is the application of plant or plant-based technologies to remediate the contaminated sites (Fig. 1.3). Methods such as chemical reduction/oxidation and incineration are also applied to contaminated sites. The traditional methods have feasibility issue due to expenses, disposal of residues and destruction of soil microflora and fauna. Therefore, researchers are applying innovative techniques to control the contaminants along with maintaining the physical, chemical and biological equilibrium of the sites. Several plants have been investigated to have feasibility to grow in the contaminated sites and bear substantial efficiency to accumulate the toxic substances. These plants can be utilized to remediate the contaminated sites. Different phytoremediator plants have different metabolism and, thereby, the process of contaminant removal may be varied from plant to plant. Some plants can accumulate toxic substance in their roots but restrict the translocation in to their aerial parts. However, other plants have significant efficiency to transfer the toxicants in the shoots. Some common mechanisms of phytoremediation have been discussed as follows.
1.6.1
Phytoextraction
In areas of low to medium contamination where the contaminants are present at a shallow depth phytoextraction is a very efficient and cost-effective process (Kumar et al., 1995a, 1995b; Blaylock and Huang, 2000). The roots of the plant extract the contaminants from the medium and then they are translocated to other parts of the plant. The process is beneficial
Fig. 1.3 Mechanism and different aspects of phytoremediation.
as the contaminated metals can be extracted from the ash of the aerial parts of the plants after they are burnt as fuel (Prasad and De Freitas, 2003; Erakhrumen and Agbontalor, 2007; Moreno et al., 2008).
1.6.2 Phytostabilization
Some plants have capability to retain contaminants from soil or water medium and keep them stabilized in their tissues, this process is known as phytostabilization. The contaminants are contained by adsorption on surface or precipitation to other parts within the root zone (Prasad and De Freitas, 2003; Erakhrumen and Agbontalor, 2007; Moreno et al., 2008). For the process of phytostabilization to be successful, the plants have to be fast growing with well-developed roots and aerial parts, also need to be tolerant toward external stresses (Ismail, 2012).
1.6.3 Phytovolatilization
In the phytovolatilization process, the plants absorb contaminants along with moisture, then accumulate and transfer them into aerial parts, from where they are released into the atmosphere by transpiration ( Prasad and De Freitas, 2003 ; Erakhrumen and Agbontalor, 2007 ; Moreno et al., 2008 ). The disadvantage of this process is the return of the toxic volatile contaminants with rainfall and wider spread of the contaminants ( Henry, 2000 ).
1.6.4 Rhizofiltration
Domestic greywater and effluents can be treated applying rhizofiltration method where plants are grown in constructed wetlands and they absorb and accumulate or adsorb and precipitate contaminants in their roots (Prasad and De Freitas, 2003; Erakhrumen and Agbontalor, 2007; Moreno et al., 2008). The plants efficient in rhizofiltration require extensive fast-growing root systems and capacity to accumulate contaminants over a long period of time (Flathman and Hannza, 1998).
1.6.5 Phytodegradation
Several studies have confirmed that the organic contaminants can be removed with the help of plants and this process is commonly popularized as phytodegradation (Newman and Reynolds, 2004; Peng et al., 2006; Wang et al., 2008; Vafaei et al., 2012; Zazouli et al., 2014). The plant can remove organic compounds from water, air or soil. Phytodegradation can be done as the degradation of compounds in the rhizospheric region of plants or within the plant body, volatilization after accumulation of compounds (Newman and Reynolds, 2004). Organic contaminants which can be degraded by the plants include explosives, synthetic pesticides, petroleum hydrocarbons (especially poly-aromatic hydrocarbons), solvents, dyes etc. Several plants species like Erythrina crista-galli, Azolla filiculoides, Brassica, Phaseolus vulgaris, Sorghum bicolor, Phragmites australis etc. have been identified to bear potential to degrade the above-mentioned contaminants present in the soil and water ecosystems (Fig. 1.4).
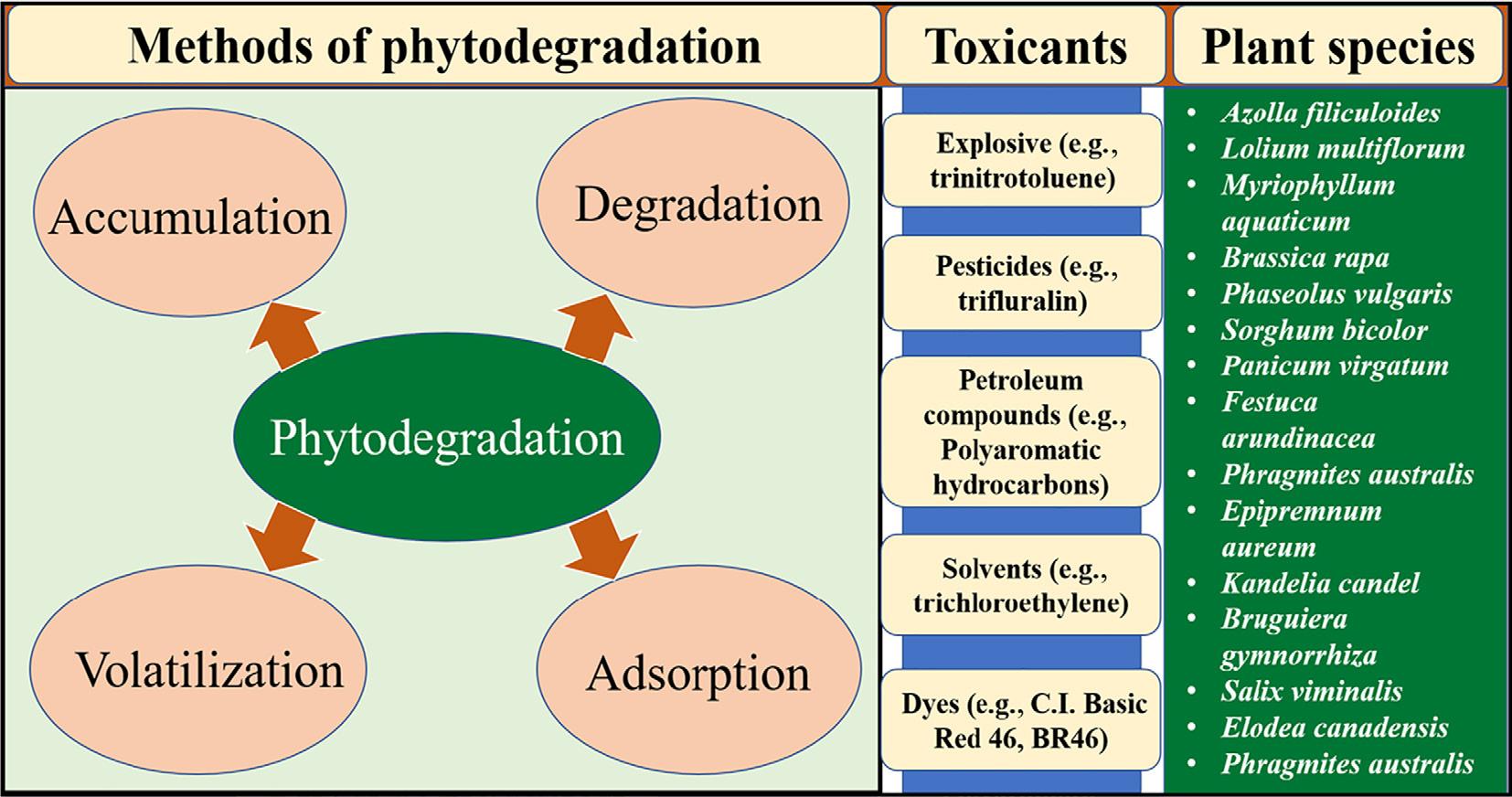
Fig. 1.4
Phytodegradation of organic contaminants (Garrison et al., 2000; Newman and Reynolds, 2004; de Farias et al., 2009; Zazouli et al., 2014; He et al., 2017).
1.7 Mechanisms of phytoremediation
Plants accumulate essential elements along with other components from their growing medium. Non-essential substances especially contaminants are also be absorbed through the same process and channels. The researchers have also reported that several plants behave like selective in nature and on that basis, these plants are categorized in to metal accumulators and metal excluders (Sinha et al., 2004). The mechanisms behind this have several aspects which make a plant metal accumulator or metal excluder. The basic mechanism which makes a plant phytoremediator is the production of root exudates which enhance the mobility of HMs and nutrients, production of metal chelating agents like phytochelatins (PCs) and metallothionines (MTs), antioxidant compounds like catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (ASX), proline, amino acids etc. The soil fertility parameters like pH, EC, presence of nutrients, microbial diversity etc. also affect the contaminant removal ability of the plants.
Presence of microbes in the plant growing medium significantly enhances phytoremediation of the contaminants in various ways like by increasing plant biomass, metal bioavailability, metal bioaccumulation in roots (bioaccumulation) and transfer of the accumulated metal in to aerial tissues of the plants (Ma et al., 2011, 2013; Rajkumar et al., 2012; Singh et al., 2014; Baghaie and Aghilizefreei, 2019; Shi et al., 2020).
1.7.1 Plant soil interaction and bioactivation of contaminants
For the bioaccumulation of any substance, bioactivation is the first and necessary process. Several toxic substances remain in the soil for longer period without causing any direct adverse effect to the plant. The root exudates released from the roots of the plants are reported not only to enhance the growth and productivity of pants, but also the bioavailability of toxic metals present in soil (Seshadri et al., 2015). The mechanisms of functioning of root exudates in removal of contaminants is done by several ways like altering the rhizospheric soil pH, solubilizing the minerals, chelating, enhancing the microbial number and activities in soil etc. (Yuan-Wen et al., 2003).
Kim et al. (2010) observed that the root exudates enhanced the soil pH and dissolved organic carbon which resulted in increased bioavailability of Cd and Zn. They also suggested that among the different factors which govern the metal uptake, root-induced changes in the soil make the phytoremediation process more effective. Root exudates are also reported to be produced during metal stressed conditions (Rengel, 2002). The release of oxalic and malic acids from the root exudates of three plant species Poa annua, Medicago polymorpha and Malva sylvestris in the presence of Cd, Cu and Zn were reported which found to be a vital mechanism to defend the HMs (Montiel-Rozas et al., 2016). Recently, Wu et al. (2019) reported that six organic acids were found to be produced by the plant Leersia hexandra
Swartz in root exudates in the presence of Cr. It was also observed that the release of these organic exudates significantly enhanced the Cr mobilization.
In a study conducted by Lu et al. (2013), it was found that the effect of exogenous application of two organic acids viz. citric acid and tartaric acid on Cd uptake and its translocation in Sedum alfredii was performed. It was observed that the application of both the acids significantly enhanced the metal uptake in the plant.
1.7.2 Contaminant accumulation and translocation into aerial parts
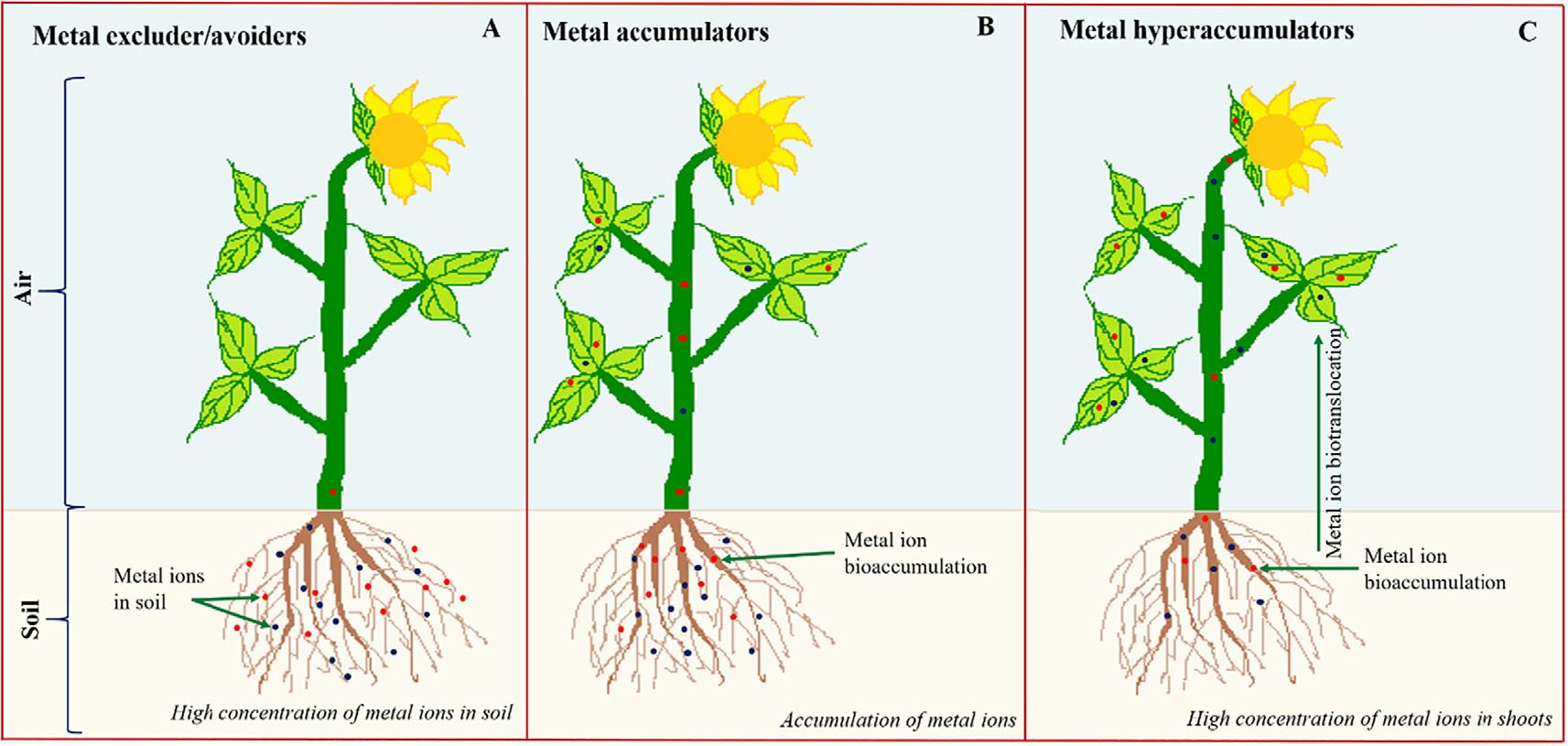
The accumulation of contaminants in the plants is the first and most important feature of a phytoremediator plant. The accumulation of contaminants depends on several factors like plant species, level of contamination, soil fertility, microbial diversity in the soil, etc. Baker (1981) and Baker and Walker (1990) classified plants into three categories on the basis of metal accumulation: metal excluder, metal accumulator, and hyperaccumulator plants (Fig. 1.5).
1.7.2.1
Metal excluder plants
Metal excluder plants avoid translocating the metals into the aerial parts from the roots, irrespective of the soil metal contamination is higher. The common examples of excluder species are Commelina communis, Oenothera biennis, Agrostis stolonifera, Silene maritime, Populus, Salix, and Pinus radiata (Wei et al., 2005; Maestri et al., 2010). Wei et al.
(A) Metal excluder, (B) metal accumulator, and (C) hyperaccumulator plants.
Fig. 1.5
(2005) found species Oenothera biennis and Commelina communis as Cd-excluders and Taraxacummongolicum as a Zn-excluder among 54 weed species cultivated in the pots.
1.7.2.2
Metal indicator plants
Metal indicator plants accumulate metals into the roots and transfer them in their aerial tissues. Although, the level of metal accumulated in tissues of these plants is low but is sufficient to reflect the level of contamination in the environment (Baker and Walker, 1990). These plants are not able to survive in the soil having high level of metal contamination, as they do not bear a strong defense mechanism to overcome the toxic effects exerted by the metals. Metal indicator plants are considered as pollution indicators therefore, are also ecologically significant (Mganga et al., 2011).
1.7.2.3
Metal accumulator plants
The plants which actively uptake metals are known as metal accumulator plants. These plants have the efficiency to accumulate the metals up to the levels higher from the soil. The roots of metal accumulator plats absorb the HMs and also transfer them into their shoots. Majority of these plants have tolerance toward the contaminants to survive without exhibiting any adverse effect. For instance, Ricinus communis a species of Euphorbiaceae family has been extensively studied for it metal accumulating potential (Bauddh and Singh, 2012a, b; Bauddh and Singh, 2015a, b; Bauddh et al., 2015, 2016, 2017; He et al., 2020; Palanivel et al., 2020). Among the category of metal accumulator plants, several plant species have been identified for their application in phytoremediation and popularized as hyperaccumulator plants due to their potential to accumulate comparatively higher amount of contaminants in their roots and also transfer them into the aerial parts. These plants have a special characteristic of transfer of metal in to their aerial parts up to a higher level than accumulated in to the roots (Chaney et al., 1997, 2005; Reeves and Baker, 2000; Ellis and Salt, 2003; Reeves, 2003, 2006; PilonSmits, 2005; Sors et al., 2005; Milner and Kochian, 2008). More than 450 plant species have been recognized as metal and metalloids hyperaccumulators especially for Ni, Cu, Zn, Co, Mn, Cd, and As (Reeves and Baker, 2000; Ellis and Salt, 2003; Reeves, 2003, 2006; Sors et al., 2005; Milner and Kochian, 2008).
1.7.2.4
Hyperaccumulator plants
The hyperaccumulator plants have specifically designed mechanisms at physiological and biochemical levels to accumulate HM and sustain normal growth without producing severe adverse effects. The secretion of organic acids, protons, enzymes, amino acids in the rhizosphere increases metal bioavailability. Due to the secretion of protons by the roots, rhizosphere is acidified resulting in enhanced dissolution of metal. In solution culture pHdependent proton release and plant growth were observed in Ni-hyperaccumulator plant (Alyssum murale) (Bernal et al., 1994). Some examples of common hyperaccumulators include Sedum plumbizincicola, Pteris vittata, Alyssum serpyllifolium, Thlaspi caerulescens,
Phytolacca Americana, and Solanum nigrum. Over 500 hyperaccumulator plants have been investigated belonging to the family Asteraceae, Caryophyllaceae, Cunouniceae, Brassicaceae, Cyperaceae, Fabaceae, Flaconrtiaceae, Laminaceae, Poaceae, Euphorbiaceae, etc. (Prasad and De Freitas, 2003; Krämer, 2010). Among these, Brassicaceae family is considered one of the important family having several hyperaccumulator plants.
1.8 Mechanisms behind metal accumulation and their translocation
The accumulation of toxic substances from the contaminated medium is the key feature of a phytoremediator plant. Initially HMs get accumulated in the root tissues of the plants. Three roots exudates citric acid, glycine, and maltose were used to assess their impacts on Cd accumulation (Chen et al., 2020). It was found that all three biochemicals enhanced the growth of the plants along with enhanced Cd accumulation in the roots and its translocation into the shoots. Organic acid secretion can mobilize HMs and enhance root absorption. To tolerate Al, plants show either apoplastic or symplastic detoxification mechanism (Ma et al., 2001; Pilon-Smits and Leduc, 2009). Cd organic complex formation was associated with the uptake of 40% of total Cd in the soil and phyto-availability of Cd (Krishnamurti et al., 1997). The metals are complexed with carboxylic acid in the form of citrate for Ni and tartrate or malate for Co (Van der Ent et al., 2018a, 2018b). In T. caerulescens stable Ni-nicotianamine complex is formed in xylem sap (Mari et al., 2006).
Co and Ni, bind strongly to roots and are passively absorbed from soil solutions. They enter inside the cell through plasma membrane carriers and may be transported by IRT1 (Iron regulated transporter 1) (Pilon-Smits and Leduc, 2009). The metal Cd is adsorbed by root apoplast and competes with symplastic absorption for accumulation in roots (Redjala et al., 2009). Similarly, plant sp., viz. Stanleya sp. and Astragalus sp. have specialized transporter systems that adapt them to accumulate 1000–1500 ppm (0.1%–1.5%) of Se, even from the low level of soil concentrations. In A. bisulcatus and B. oleracea, specific selenocysteine methyl transferases have been identified that lead to the accumulation of Se (Tamaoki et al., 2008). The transport of Cd in the leaf mesophyll layer is maintained by transporters present in the plasma membrane and tonoplast. It generally affects the Vmax of root without affecting Km (Lasat et al., 1996; Lombi et al., 2001). Mode of Ni uptake in leaf tissue has been explained in A. lesbiacum, where it activates vacuolar H+-ATPase in the presence of Mg/ATP (Ingle and Fricker, 2008).
1.9 Mechanisms of contaminant tolerance in plants
Mining and oil drilling sites are key sources of organic and inorganic contaminants such as PAHs and HMs in nearby areas (Sarma et al., 2016). Plants respond to these pollutions quickly and their responses can be categorized as (i) signaling, (ii) detoxification, and (iii) degradation. Various hormones and enzymes including ethylene, brassinosteroids, and
diphosphate kinase have been shown to be involved in response to polycyclic aromatic hydrocarbon exposure and signaling in plants (Weisman et al., 2010; Ahammed et al., 2012; Liu et al., 2015). In plants, the detoxification pathway for PAHs can be categorized as transformation, conjugation, and neutralization/sequestration/export outside the cell. Plants possess various enzymes such as hydrolases, oxidoreductases, carboxylesterases, dioxygenases, cytochromes P450, and peroxidases (POD) for PAH transformation (Campos et al., 2008; Fu et al., 2016; Hernández-Vega et al., 2017). Transformation makes PAHs more reactive which can then conjugate to various endogenous molecules including glutathione, sugars, malonic acid, and amino-acids (Coleman et al., 1997). Conjugation of PAHs with endogenous molecules is catalyzed by various transferases such as glutathione-S-transferases, glycosyltransferases, and malonyl transferases. Conjugation process makes PAHs soluble hydrophilic polar compounds. PAH conjugates are neutralized by the cell wall polymer such as lignin, sequestered via ATP-binding cassette transporters in the vacuoles or exported outside cells (Ishikawa, 1992; Lu et al., 1997).
Organic contaminants also induce oxidative stress, through excessive production reactive oxygen species (ROS) accumulation in plant cells, which in turn reduce plant growth and development. Antioxidant enzymes and molecules reduce oxidative stress damages conferred by PAH stress (Shen et al., 2018; Houshani et al., 2019). Carotenoid and SOD were reported as two most active antioxidants for ROS scavenging among 9 main antioxidants SOD, CAT, APX, and glutathione-S-transferase (GST), GSH, ascorbate (AsA), α-tocopherol, polyamines, carotenoid in wheat leaves under phenanthrene stress. Ascorbate-glutathione cycle turns active under higher phenanthrene treatments (Shen et al., 2018). However, Sobhani et al. (2020) reported decreased activity of SOD and POD but increased CAT activity with increase in the concentration of pyrene and phenanthrene compounds in wheat leaves. In comparison to glycophytic Arabidopsis thaliana, halophytic Thellungiell asalsuginea demonstrated enhanced phenanthrene tolerance and recovery from stress. Accumulation of phenanthrene in stomata of T. salsuginea suggested possible volatilization (Shiri et al., 2015).
For protection against HMs, plants have developed mechanisms to exclude or detoxify them (Jha et al., 2019; Singh et al., 2019). The first living structure of the cell that encounters HMs is the cell wall. It restricts the movement of HMs into the cytoplasm. Cell wall sequestration of HMs and their limited translocation are considered as HM tolerance mechanisms in plants (Torasa et al., 2019). Plants also secrete a variety of metabolites including low-molecularweight organic-compound in the rhizosphere for detoxification of HM present in soils (Chen et al., 2017; Osmolovskaya et al., 2018). Once metals enter plant cells, they generate signaling molecules such as nitric oxide, ethylene, salicylic acid, jasmonic acid, etc. which contribute to HM tolerance (Popova et al., 2012; Jan and Parray, 2016). Components that cause HM detoxification significantly influence the plant metal tolerance. These components include chelation of metals with different ligands such as PCs, MTs, GSH, chaperons, organic acid, and amino acid and its transportation to above-ground parts and accumulation
in vacuoles. Thiol groups play a key role in conjugation and detoxification of HMs which need to be sequestered within the vacuole. PCs, MTs, and GSH have a high affinity for HMs due to their thiol groups. PCs are peptides synthesized by enzyme PC synthase. Some of the HMs strongly produce PCs, a sulfur-rich molecule with the capability to bind metals strongly. MTs are low molecular-weight (5–10 kDa) cysteine-rich proteins implicated in homeostasis and tolerance of HM in plants (Cobbett and Goldsbrough, 2002). As MT-metal complexes have high thermodynamic and low kinetic stability, it can tightly bind metals and promptly exchange them with other proteins (Domènech et al., 2007). Organic acids such as oxalate, citrate, malate, etc., bind strongly with HM with their carboxyl groups. They participate in both intracellular and extracellular HM chelation. Secretion of organic acids by plant roots forms an extracellular complex with HM and reduces their bioavailability whereas intracellular chelation by organic acids improves metal tolerance in plants (Osmolovskaya et al., 2018). Sequestration of HMs in vacuoles imparts tolerance to plants. Various families of transporters including HMs ATPases (HMAs), natural resistance-associated macrophage proteins (NRAMPs) Ca2 + exchangers (CAXs), and ATP-binding cassette subfamily C proteins (ABCCs), are associated with sequestration of HMs in vacuoles (Korenkov et al., 2007; Park et al., 2012). Like PAHs, HMs also induce oxidative stress in plants. Enzymatic as well as non-enzymatic antioxidants contribute to HM tolerance by scavenging ROS. Depending on the plant and HM content responses of antioxidant differs. Various studies have reported enhanced ROS formation and considerable enhancement in the activities of SOD, CAT, and APX due to exposure of HMs (Mishra and Sharma, 2019; Sharma et al., 2019). Non-enzymatic components such as ascorbate and GSH also provide tolerance against oxidative stress by protecting plants against HM induced oxidative stress (Asgher et al., 2017; Sharma et al., 2019).
1.10 Molecular mechanisms of phytoremediation of toxicants
Various molecular mechanisms enhance the potential of plants to phytoremediate organic and inorganic contaminants, such as PAHs, RDX, TNT, and HMs present in oil drilling and mining sites. Phytoremediation of organic contaminants includes (i) phytoremediation ex planta and (ii) direct phytoremediation (Reichenauer and Germida, 2008). Root exudates containing organic acid, phenolics, proteins, alcohol which are released by plants stimulate the growth and metabolic capabilities of microbes that have the capability of degrading organic contaminants in the rhizosphere (Gao et al., 2010; Sun et al., 2010). Some of these microbes enhance remediation process by breaking down organic pollutants to generate humic substances or volatilizing contaminants such as PAHs (Salt et al., 1998). In plants, there is no known natural transporter for organic contaminants, therefore these contaminants are taken up passively. Uptake of organic contaminants in plants is limited by physicochemical properties, availability, and uptake mechanisms. Physicochemical properties such as hydrophobicity/hydrophilicity, octanol-water partition coefficient, log
Kow, acidity constant. pKa, concentration, and others (Namiki et al., 2018). Compounds that are moderately hydrophobic and have octanol-water partition coefficient ranging from 0.5 to 3 are the most likely contaminants taken up by plants (Ryan et al., 1988). Also, plants differ significantly in their capability to uptake specific contaminants.
Evaporation rate is a key player in the uptake of organic compounds and that might be the reason of differential uptake. Natural (rhamnolipids) and artificially produced (SDS, Triton X-100) biosurfactants have been reported to enhance water solubility and bacterial degradation of organic contaminants. Mixed surfactants (sodium dodecylbenzene sulfonate and Tween 80 in 1:1, 1:2, and 2:1 ratio) could also enhance phytoremediation of PAHs in soil. It enhanced the quantity of PAHs degrading bacteria and degradation related genes (Lu et al., 2019). Cyclodextrins also solubilize organic contaminants (Shirin and Buncel, 2005).
Partitioning of various contaminants vary significantly between root and aboveground parts.
After uptake, organic contaminants may (i) get translocated to other plant part and get volatilized (ii) get partially and fully degraded (iii) get transformed to less toxic compounds (iv) bind to plant tissue such as insoluble lignin and remain non-available (v) get sequestered in vacuoles. Lipid bilayer of the plasma membrane and hemicellulose of cell wall bind hydrophobic organic contaminants (Pilon-Smits, 2005). Various chemical reactions including oxidation, reduction, and hydrolysis can modify organic contaminants which can then conjugate with organic acids, sugars, and GSH. Conjugation enhances solubility and movement of organic contaminants into vacuoles, where they can be further metabolized to CO2 and water (Campos et al., 2008). GST enzyme catalyzes the transfer of GSH to organic contaminants which results in detoxification of organic contaminants (Schröder et al., 2008). ATP-dependent membrane pumps actively transport glutathione S-conjugates to the vacuole/ apoplast. In hyperaccumulators, cytochrome P450 participates in degradation and hence phytoremediation of organic pollutants (Rostami and Azhdarpoor, 2019). Plants can detoxify RDX and TNT up to a limited extent.
Direct conjugation of the TNT molecule with glutathione by GSTs has been shown (Gunning et al., 2014). Expression of GST (DmGSTE6) from Drosophila melanogaster in Arabidopsis improved its TNT phytoremediation potential (Tzafestas et al., 2017). The TNT transformation is also done by the enzyme oxophytodienoate reductases which is then subsequently conjugated by the enzyme uridine diphosphate glycosyltransferases (GandiaHerrero et al., 2008; Beynon et al., 2009). Transgenic Agrostis stolonifera and Panicum virgatum expressing xplB (flavodoxin reductase) and xplA gene (cytochrome P450 activity) also showed better removal of RDX (Zhang et al., 2017). Arabidopsis plants expressing XplA and partnering XplB from Rhodococcusrhodochrous strain 11Y and nfsI from Enterobacter cloacae which encodes a nitroreductase showed enhanced TNT detoxification and RDX degradation (Rylott et al., 2011). Recently, a putative flavonol synthase has been shown to be involved in detoxification of PAHs in Arabidopsis thaliana (Hernández-Vega et al.,
2017). Different plant growth regulators such as cytokinins, gibberellins, salicylic acid, and auxins also increase the phytoremediation efficiency of organic contaminants (Rostami and Azhdarpoor, 2019). They enhance the plant biomass and reduces the harmful effects of organic contaminants in the plant.
Like organic contaminants, phytoremediation of HMs also includes several steps, such as: (a) secretion of various compounds in rhizosphere to augment the mobility of contaminants, (b) transport of contaminants through root cell plasma membrane, (c) translocation through xylem, (d) detoxification of contaminant, and (e) sequestration of contaminants. Among HMs, some like Cd and Zn are more available and mobile for uptake in plants compared to others which are comparatively immobile like Pb (Lasat, 1999). Soil pH and presence of chelating compounds like EDTA can enhance metal mobility and hence uptake of metals.
Concentration of metals, metal interaction, temperature, organic matter, nutrients, microbial consortium, etc., also play a role to influence the mobility of metal ions in soil (Rieuwerts et al., 1998; Luo et al., 2016; Cui et al., 2019). The enzyme secretion by microorganisms present in the plant’s rhizosphere makes metal ions available for absorption by roots (Burns and Dick, 2002). Several processes are used by plants to increase the availability of metal ions e.g. acidification of the rhizosphere, secretion of organic acids such as malate, citrate, phytosiderophores which enable the solubilization and chelation of soil-bound metals (Kinnersley, 1993).
The changes in composition and content of root exudates that facilitate phytoremediation of HM contaminated soils take place upon exposure to metals (Javed et al., 2017; Ping et al., 2017). Hou et al. (2015) observed more secretion of organic acids, including citric, succinic, and glutaric acid under lead stress. These organic acids have the capability to increase uranium accumulation in plants by desorbing uranium in soil colloid and enhancing the content of free-moving HMs. Metallic contaminants enter roots through apoplastic/symplastic pathway. Cell walls have a comparatively high exchange capacity for cations (Raskin et al., 1997). Thus, as many metals are insoluble and incapable of moving in the vascular system themselves, they are immobilized in apoplastic and symplastic compartments after forming carbonate, sulfate, or phosphate precipitates (Raskin et al., 1997; Garbisu and Alkorta, 2001).
Metal chelates can follow apoplastic movement. Metal hyperaccumulator plants translocate a very high concentration of metal ions into the shoot via symplastic movement through the xylem. Through root symplasm, HMs enter xylem stream (Tester and Leigh, 2001). After uptake, metallic contaminants translocation takes place through xylem vessel by xylem loading. In shoot, unloading of metallic contaminants takes place in sap of the xylem.
Metal entry from root tissue to xylem is primarily regulated by three activities: metal ion sequestration in root cells, symplastic transport in stele, and discharge in xylem (Ghosh and Singh, 2005; Saxena and Misra, 2010; Cui et al., 2019; Hu et al., 2019). The metallic contaminants sequestration begins when it is taken up in root cells where they bind to cell
wall components such as pectin and suberin (Baxter et al., 2009; Parrotta et al., 2015). Many low-molecular-weight organic compounds, e.g., GSH, non-proteinogenic amino acids, and organic acids having metal chelating properties are used by plants to detoxify, sequester, or transport metals (Krämer, 2003; Osmolovskaya et al., 2018; Dubey et al., 2018). They can be manipulated to improve phytoremediation process. Nicotianamine plays an important role in detoxification of metals and has been proposed as an important tool for utilization in phytoremediation. Overexpression of HvNAS1 gene from Hordeum vulgare in Arabidopsis plants led to increased accumulation of Ni. Metals also form complex with thiol chelators, e.g., PCs, GSH, MTs. These metal chlelators play a key role in phytoremediation of HMs such as Zn, As, Cu, and Cd (Srivastava, 2016). Tobacco and Arabidopsis plants overexpressing MnPCS1 and MnPCS2 from Morusnotabilis exhibited increased Cd and Zn phytoremediation (Fan et al., 2018). Cu exists as thiol-S bound Cu(I) complex in root xylem (Cui et al., 2019). Hence, the reduction of Cu (II) to Cu (I) in rice roots is considered as an adaptive Cu stress tolerance mechanism with phytoremediation implications (Cui et al., 2019).
Metal chelator complexes are transferred from cytosol to vacuoles (Song et al., 2014).
Vacuolar sequestration of HM contaminants is an important molecular mechanism for the purpose of phytoremediation (Zhang et al., 2018; Rai et al., 2020; Tan et al., 2019). Metal sequestration in roots restrict its translocation to aerial parts. Vacuoles contain many transport proteins such as ATP-binding cassette (ABC) family (AtSuc4); vacuolar sugar transporters; vacuole iron transporter (VIT) H+-ATPase; Na+/H+ antiporter; Ca2 +/H+ antiporters; multidrug and toxic compound extrusion (MATE) for the sequestration of HM contaminants (Zhang et al., 2018; Tan et al., 2019). In transgenic studies, ABC genes were found localized in tonoplast and improved metal sequestration in lumen of vacuoles (Yazaki et al., 2006; Song et al., 2014). Metal tolerance/transport protein (MTP) which is encoded by cation diffusion facilitator (CDF) family participates in excluding excessive ions from the cytoplasm. Overexpression of CDF genes enhanced thiol concentration and metal chelation and led to efficient vacuolar sequestration and hyperaccumulation of metals in plants. Tobacco plant overexpressing OsMTP1 from indica rice caused Cd hyperaccumulation and increased arsenic accumulation and tolerance upon exogenous Cd and As treatments suggesting wide substrate specificity of this transporter (Das et al., 2016). The HMW compounds get dissociated in vacuoles owing to acidic pH and then chelated with organic acids (e.g., amino acids, oxalate, citrate, and malate) (Choppala et al., 2014; Luo et al., 2016; Rai et al., 2020). In addition to vacuoles, Golgi apparatus has been associated with exocytosis of metals, facilitating phytoremediation (Shi et al., 2019; Rai et al., 2020). Metal sequestration inside compartments of cells can be a mechanism to detoxify and hence protect the important enzymes and metabolites actively associated with metabolic machinery. Furthermore, the role of antioxidants in scavenging excess ROS due to HMs is a key strategy for metal tolerance and hence important for successful HM phytoremediation (Ali et al., 2003; Goswami and Das, 2016).