Photophysics and Nanophysics in Therapeutics
Edited by
Nilesh M. Mahajan
Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Avneet Saini
Department of Biophysics, Panjab University, Chandigarh, India
Nishikant A. Raut
Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Sanjay J. Dhoble
Department of Physics, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Elsevier
Radarweg 29, PO Box 211, 1000 AE Amsterdam, Netherlands
The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States
Copyright © 2022 Elsevier Inc. All rights reserved.
No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions
This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein).
Notices
Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary.
Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility.
To the fullest extent of the law, neither the Publisher nor the authors, contributors, or editors, assume any liability for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions, or ideas contained in the material herein.
ISBN: 978-0-323-89839-3
For Information on all Elsevier publications visit our website at https://www.elsevier.com/books-and-journals
Publisher: Andre Gerhard Wolff
Acquisitions Editor: Michelle Fisher
Editorial Project Manager: Susan E. Ikeda
Production Project Manager: Omer Mukthar
Cover Designer: Miles Hitchen
Typeset by Aptara, New Delhi, India
2. Phototherapy for skin diseases
1. Phototherapy: A critical review
Nilesh Rarokar, Shailendra Gurav, Dadasaheb M. Kokare, Vijay Kale, Nishikant A. Raut
1.1
1.2.1
1.2.2
1.3
1.5
1.4.1
1.4.2
1.4.3
1.4.4
1.5.1
1.5.3
3. Phototherapy: The novel emerging treatment for cancer
Sagar Trivedi, Nishant Awandekar, Milind Umekar, Veena Belgamwar, Nishikant A. Raut 3.1
3.2
3.4
3.5
3.3.1
3.3.2
3.3.3
3.4.1
3.4.2
3.4.3
3.4.4
3.5.1
3.5.2
3.5.3
3.5.4
3.5.5
3.5.6
3.5.7
3.5.8
3.6
3.7
3.6.1
3.6.2
3.6.3
3.6.4
3.7.1
3.7.2
4. Fundamentals of photodynamic therapy
Mrunal M. Yawalkar, Samvit Menon, Hendrik C. Swart, Sanjay J. Dhoble
5.5.4 Urinary system tumors 103
5.5.5 Brain tumors 104
5.5.6 Nonsmall cell lung cancer and mesothelioma 105
5.6 Recent developments, future scope, and challenges 105
5.7 Conclusion 106 Acknowledgment 106 References 106
6. Photodiagnostic techniques
Anurag Luharia, Gaurav Mishra, Nilesh Haran, Sanjay J. Dhoble
6.1 Introduction 115
6.1.1 Ionizing radiations 115
6.2 Fundamentals of light used in diagnostic techniques 116
6.2.1 X-ray production 118
6.2.2 X-ray beam intensity 119
6.2.3 Target material 120
6.2.4 Voltage applied 120
6.2.5 X-ray tube current 120
6.3 Various photo diagnostic techniques 120
6.3.1 Plain radiography and digital radiography 120
6.3.2 Computed tomography 121
6.3.3 Fluoroscopy 123
6.3.4 Digital subtraction angiography 123
6.3.5 Digital radiography and picture archival and communication system 124
6.3.6 Dual energy X-ray absorptiometry 124
6.3.7 Dual energy computed tomography 124
6.3.8 Orthopantomography 124
6.4 Physics of photodiagnostic techniques 125
6.4.1 Interaction of radiation with matter 125
6.4.2 Importance of interaction in tissue 127
6.4.3 Picture archiving and communication system 130
6.5 Opportunities, challenges, and limitations of photodiagnostic techniques 134 References 135
7. The role of physics in modern radiotherapy: Current advances and developments
Anurag Luharia, Gaurav Mishra, D. Saroj, V. Sonwani, Sanjay J. Dhoble
7.1 Introduction 139
7.2 Role of radiotherapy in cancer treatment 140
7.2.1 What is radiotherapy and how it works? 140
7.2.2
of
8. Physics in treatment of cancer radiotherapy
Ravindra B. Shende, Sanjay J. Dhoble
8.1
8.1.16 Interaction of charged particle 171
8.1.17 Electron and electron interaction 172
8.1.18 Electron and nucleus interaction 172
8.1.19 Interaction of heavy charged particle 172
8.1.20 Biological effect of radiation 173
8.1.21 Linear energy transfer 174
8.1.22 Relative biological effectiveness 174
8.2 Principle of radiotherapy 175
8.2.1 Radiotherapy facility 175
8.3 Traditional facility in treatment of radiotherapy 175
8.3.1 Superficial therapy 175
8.3.2 Orthovoltage therapy or deep therapy 175
8.3.3 Supervoltage therapy machines 176
8.3.4 Cobalt-60 teletherapy unit 176
8.3.5 Betatron and microtron 176
8.3.6 Advance facility in treatment of radiotherapy 177
8.3.7 Linear accelerator (Linac) 177
8.3.8 Tomotherapy 178
8.3.9 CyberKnife 178
8.3.10 Proton and light ion therapy 178
8.3.11 Cyclotron 178
8.3.12 Synchrotron and synchrocyclotron 179
8.3.13 Add-on facility in treatment of radiotherapy 179
8.3.14 Conventional simulator 179
8.3.15 CT simulator 180
8.3.16 Commissioning of radiotherapy facility and quality assurance 180
8.3.17 Technique of radiotherapy 180
8.3.18 External beam radiation therapy 181
8.3.19 Conventional treatment techniques in EBRT 181
8.3.20 Three-dimensional conformal radiation therapy 181
8.3.21 Intensity modulated radiation therapy 182
8.3.22 Rotational therapy or volumetric modulated arc therapy (VMAT) 184
8.3.23 Stereotactic radiosurgery and stereotactic radiotherapy 184
8.3.24 Image-guided radiotherapy 184
8.3.25 Internal beam radiation therapy or brachytherapy 185
8.3.26 Process and treatment of radiotherapy 186
8.4 Patient preparation and simulation 187
8.5 Target delineation and treatment planning 187
8.5.1 Treatment verification and treatment delivery 187
8.5.2 Dosimetry in radiation therapy 188
8.5.3 Activity 188
8.5.4 Particle fluence 188
8.5.5 Energy fluence 188
8.5.6 Exposure 188
8.5.7 Kerma 188
8.5.8 Absorbed dose 189
8.5.9 Methods of radiation dosimetry and dosimeters in radiation therapy 189
8.5.10 Ionization chamber dosimetry 189
8.5.11 Film dosimetry 189
8.5.12 Luminescence dosimetry 190
8.5.13 Thermoluminescence 190
8.5.14 Optically stimulated luminescence 191
8.5.15 Semiconductor dosimetry 191
8.5.16 Physical and clinical dosimetry in radiotherapy 191
8.5.17 Physical dosimetry 191
8.5.18 Clinical dosimetry 192 References 192
9. Role of carbon ion beam radiotherapy for cancer treatment
Vibha Chopra, Nirupama S. Dhoble, Balkrishna Vengadaesvaran, Sanjay J. Dhoble
9.1 Introduction 193
9.2 Radiation therapy for the treatment of cancer 193
9.2.1 Gamma ray therapy 194
9.2.2 Proton therapy 194
9.2.3 Ion beam therapy 194
9.3 Role of carbon ion beam therapy 195
9.4 Development of TLD materials for carbon ion beam therapy 195
9.4.1 Lithium-based phosphors 195
9.4.2 Calcium-based phosphors 198
9.4.3 Some other phosphors 200
9.5 Conclusion 202 References 202
Part II
Nanotherapeutics
10. Nanomaterials physics: A critical review
Khushwant S. Yadav, Sheeba Jacob, Anil M. Pethe
10.1 Introduction 207
10.2 Fundamental concepts of nanomaterial physics 208
10.2.1 Structure sensitive and structure insensitive properties 209
10.2.2 Phases and their distribution 209
10.2.3 Defects in body nanomaterials 209
10.3 Properties of materials 210
10.3.1 Factors affecting properties of a material 210
10.4 Rationale of nanoparticle physics with diverse functions involving nanomaterials 211
10.5 Self-assembly of nanostructures 212
10.6 Clinical applications of nanomaterials physics 212
10.6.1 Applications of nanomaterials physics in cancer 212
10.7 Conclusion: Nanotechnology, physics, and clinical outcome 213
Acknowledgments 214
References 214
11. Nanotherapeutic systems for drug delivery to brain tumors
Keshav S. Moharir, Vinita Kale, Mallesh Kurakula
11.1 Introduction 217
11.2 An overview of brain tumors 218
11.2.1 Malignant brain tumors 218
11.2.2 Benign brain tumors 218
11.3 Barriers and challenges in the treatment of brain cancer 219
11.3.1 BBB as a main hurdle 219
11.3.2 Chemoresistance and efflux 220
11.3.3 Tumor microenvironment (TME) dynamics and lack of brain tumor classification based on genetics 220
11.3.4 Resistance due to cancer stem cells (CSCs) of gliomas and GBM 220
11.3.5 Lack of proper brain cancer mimicking models 221
11.4 Conventional vs nanomedicines in drug delivery for brain cancers 221
11.5 Approaches and mechanisms of nanocarriers for chemotherapeutic drug delivery to brain tumors 222
11.5.1 Passive targeting 222
11.5.2 Active targeting 222
11.5.3 Stimuli responsive nanocarriers systems 224
11.6 Types of nanotherapeutic platforms for drug delivery to treat brain cancer 225
11.6.1 Inorganic (metallic) nanoparticles 225
11.6.2 Lipid-based and polymeric nanoparticles 228
11.7 Novel therapies to treat brain cancers 229
11.7.1 Artificial intelligence (AI)-enabled nanocarriers for oncotherapy 229
11.7.2 Gene-based nanotherapy 231
11.7.3 CRISPR/Cas 9-associated brain tumor therapy 232
11.7.4 Nose to brain drug delivery 232
11.8 Clinical translation of nanotherapeutic systems for brain cancers: From bench to bedside 232
11.9 Conclusion and future prospects 232 References 233
12. Progress in nanotechnology-based targeted cancer treatment
Shagufta Khan, Vaishali Kilor, Dilesh Singhavi, Kundan Patil
12.1 Introduction 239 12.2 Tumor microenvironment: Comparison with normal cells 239
12.2.1 Angiogenesis and endothelial permeability in cancer 240
12.2.2 Microenvironment pH 240
12.2.3 Microenvironment temperature 240
12.3 Nanotechnology-based diagnosis of cancer 240
12.4 Nanotechnology-based drug targeting strategies in cancer 241
12.4.1 Passive targeting 241
12.4.2 Active targeting
12.4.3 Physical targeting
12.5 Progress in nanotherapeutics for treating breast and lung cancer 245
12.5.1 Breast cancer 245
12.5.2 Lung cancer
12.6 Future of nanotechnology in cancer treatment
12.7 Conclusion 248 References
13. Nanotherapeutics for colon cancer
Nilesh M. Mahajan, Alap Chaudhari, Sachin More, Purushottam Gangane
13.1 Introduction 251
13.1.1 Anatomy 251
13.1.2 Pathogenesis and molecular pathways for CRC 252
13.1.3 Risk factors 253
13.1.4 Stages of CRC 254
13.1.5 Signs and symptoms
13.2 Diagnosis 254
13.2.1 Endoscopy
13.2.2 Imaging
13.2.3 Laboratory
13.2.4 Pathology
13.3 Current therapies 256
13.3.1 Conventional treatment strategies 256
13.3.2 Targeted therapy 259
13.3.3 Targeted therapies using nanocarriers 260
13.4 Nanodrug delivery in cancer therapy 261
13.4.1 Polymers used in formulations of NPs 261
13.5 Polymeric nanoparticles (PNPs) 262
13.5.1 Lipid-based nanoparticles 263
13.5.2 Superparamagnetic iron oxide nanoparticles (SPIONs) 263
13.5.3 Gold nanoparticles (AuNPs) 263
13.5.4 Enteric-coated nanoparticles 264
13.6 Conclusion 264
References 265
14. Nanoparticles for the targeted drug delivery in lung cancer
Veena Belgamwar, Vidyadevi Bhoyar, Sagar Trivedi, Miral Patel
14.1 Introduction 269
14.1.1 Stages of LC 269
14.1.2 Current treatment strategies on LC 270
14.1.3 Novel strategies for LC treatment by pulmonary route of administration 272
14.1.4 Pulmonary physiology and drug absorption 273
14.1.5 Role of nanoparticulate technology in the diagnosis and treatment of LC 273
14.1.6 Nanocarriers used for the diagnosis of lung diseases 274
14.2 Nanocarriers in LC treatment 275
14.2.1 Solid–lipid nanocarriers 275
14.2.2 Polymeric nanocarriers 276
14.2.3 Nanoemulsions as potential carrier in LC 276
14.2.4 Metal-based NPs 277
14.2.5 Dendrimers-based drug delivery 277
14.2.6 Target-mediated targeted therapy 279
14.2.7 Quantum dots (QDs) as a drug delivery system 279
14.2.8 Bio-NPs for LC 280
14.2.9 Hydrogel-based drug delivery for pulmonary cancer 281
14.2.10 Inhalation-based nanomedicine for pulmonary cancer 281
14.3 Marketed formulation 282
14.4 Toxicity issues of inhaled NPS 283
14.5 Conclusion 284 References 285
15. Role of nanocarriers for the effective delivery of anti-HIV drugs
Rohini Kharwade, Nilesh M. Mahajan
15.1 Introduction
291
15.1.1 HIV life cycle and pathogenesis 291
15.1.2 Pathophysiology 293
15.2 Conventional antiretroviral therapy 293
15.3 Types of nanocarriers for antiretroviral drugs delivery 295
15.3.1 Pure drug nanoparticles 296
15.3.2 Polymeric nanoparticles 297
15.3.3 Dendrimers 299
15.3.4 Polymeric micelles 301
15.3.5 Liposomes 302
15.3.6 Solid lipid nanoparticles 303
15.4 Nanaotechnological approaches for antiretroviral therapy 304
15.4.1 Immunotherapy for antiretroviral 304
15.4.2 Gene therapy 305
15.4.3 Vaccines 305
15.5 Nanotechnology for improving latency reservoir 306
16. Drug delivery systems for rheumatoid arthritis treatment
Mangesh Bhalekar, Sachin Dubey
16.1 Introduction 311
16.1.1 Stages of rheumatoid arthritis 311
16.1.2 Causes of RA 311
16.1.3 Symptoms of RA 312
16.1.4 Pathology of rheumatoid arthritis 312
16.2 Management of rheumatoid arthritis 314
16.3 Targeted delivery strategies to inflamed synovium 314
16.4 Passive targeting 315
16.4.1 Enhanced permeability and retention (EPR) effect 315
16.4.2 Hypoxia and acidosis 315
16.4.3 Stimuli responsive drug delivery 316
16.4.4 Angiogenesis 316
16.5 Active targeting 316
16.6 Factors for the selection of delivery system 316
16.6.1 Carrier type 316
16.6.2 Particle size 316
16.6.3 Shape 317
16.6.4 Surface modifications 317
16.6.5 Prolonged circulation time 317
16.6.6 Strategies for active targeting 317
16.7 Drug delivery vehicles for rheumatoid arthritis 318
16.7.1 Liposomes 318
16.7.2 Dendrimers 319
16.7.3 Nanoparticles 319
16.7.4 Polymeric micro- and nanoparticles 320
16.7.5 Macromolecules and the enhanced permeability and retention effect 320
16.7.6 Arthritis-specific antigens 321
16.7.7 The complement system 321
16.7.8 Specific surface receptors 321
16.7.9 Monoclonal antibodies 322
16.7.10 mAbs targeted against B cells 322
16.7.11 mAbs directed against IL-6function 322
16.7.12 mAb directed against NFKB ligand 323
16.8 Conclusion 323 References 323
17. Peptide functionalized nanomaterials as microbial sensors
Shubhi Joshi, Sheetal Sharma, Gaurav Verma, Avneet Saini
17.1 Introduction 327
17.2 Conventional techniques for microorganism detection 328
17.2.1 Pure culture-based protocols 328
17.2.2 Immunological techniques 328
17.2.3 Nucleic acid-based assays 329
17.3 Principle behind using biosensors for microorganism detection 330
17.4 Commonly used biosensing recognition elements 331
17.4.1 Antibodies as biosensing recognition elements 331
17.4.2 Aptamers as biosensing recognition elements 332
17.4.3 Bacteriophages as biosensing recognition elements 332
17.4.4 Carbohydrates as biosensing recognition elements 332
17.4.5 Peptides as biosensing recognition elements 333
17.5 Advantages and challenges of using peptide-based detection of microorganisms 335
17.6 Properties of nanomaterials making them suitable for construction of microbial sensors 335
17.6.1 Carbon-based nanoparticles 335
17.6.2 Metallic nanoparticles 336
17.6.3 Magnetic nanoparticles 336
17.6.4 Quantum dots 337
17.7 Techniques enabling microorganism detection 337
17.7.1 Colorimetric detection 337
17.7.2 Fluorescence-based detection 338
17.7.3 Microscopic techniques 338
17.7.4 Spectroscopic detection 338
17.8 Recent advances in on-site detection of microorganisms using peptide functionalized nanosensors 339
17.8.1 Bacteria detection 339
17.8.2 Detection of fungal spores 339
17.8.3 Virus detection 340
17.9 Conclusion and future perspectives 341 References 341
18. Theranostic nanoagents: Future of personalized nanomedicine
Vidya Sabale, Shraddha Dubey, Prafulla Sabale
18.1 Introduction 349
18.1.1 Theranostics 349
18.1.2 Nanoagents 349
18.1.3 Nanotheranostics 349
18.2 Recent approaches versus theranostic nanoagents
350
18.2.1 Contemporary treatment methods and their drawbacks 350
18.3 Nanotheranostics and neurological disorders
350
18.3.1 Blood–brain barrier 350
18.3.2 Theranostic nanoparticles employed in neurology 351
18.3.3 Theranostic applications of nanosystems in neurological disorders 355
18.4 Nanotheranostics and rheumatoid arthritis 360
18.4.1 Rheumatoid arthritis (RA) 360
18.4.2 Current treatments and their drawbacks 360
18.4.3 Nanotheranostic approach for rheumatoid arthritis 361
18.5 Nanoparticle-based theranostic agents 363
18.5.1 Iron oxide nanoparticle-based theranostic agents 363
18.5.2 Quantum dot-based theranostic agents 365
18.5.3 Gold nanoparticle-based theranostic agents 366
18.5.4 Carbon nanotube-based theranostic agents 367
18.5.5 Silica nanoparticle-based theranostic agents 368
18.6 Theranostic nanoagents: future of nanomedicine 369
18.7 Conclusion 369 References 370
19. Improving the functionality of a nanomaterial by biological probes
Panchali Barman, Shweta Sharma, Avneet Saini
19.1 Introduction to nanomaterials 379
19.2 Classifications of nanoparticles 380
19.2.1 Metallic nanoparticles 380
19.2.2 Semiconductor quantum dots 383
19.2.3 Metal oxide nanoparticles 384
19.2.4 Organic nanoparticles 385
19.2.5 Upconversion nanoparticles 387
19.3 Common conjugation approaches for biomolecule functionalized nanomaterials 389
19.3.1 Conjugation approaches 389
19.3.2 Functionalization of nanoparticles 391
19.4 Basic chemistries behind conjugation approaches 397
19.4.1 Functional groups and conjugation reactions 397
19.4.2 Polyhistidine–nitrilotriacetic acid chelation 398
19.4.3 Biotin–avidin chemistry 399
19.5 Applications 400
19.5.1 Detection of DNA, protein, and metal ions 400
19.5.2 Detection of human pathogens 401
19.5.3 Enhancement of antibacterial and anti-inflammatory activity 402
19.5.4 Theranostics 403
19.6 Conclusion and future perspective 404 References 405
20. Nanostructures for the efficient oral delivery of chemotherapeutic agents
Ravindra Satpute, Nilesh Rarokar, Sunil Menghani, Anjali Ganjare, Vivek S. Dave, Nishikant A. Raut, Pramod B. Khedekar
20.1 Introduction 419
20.1.1 Limitations of conventional chemotherapy 420
20.1.2 Edges of nanoparticles over the other delivery system 420
20.1.3 Components of nanoparticles as a targeting system 420
20.1.4 Characteristics features of ideal targeting moieties 421
20.1.5 The potential of nanocarriers as drug delivery systems 421
20.1.6 Nanoparticle properties 421
20.1.7 Cancer therapy: Selective targeting of tissues by nanotechnology 421
20.2 Nanodrug carriers 422
20.2.1 Classification of nanoparticles as drug carriers 422
20.2.2 Micelles 423
20.2.3 Solid-lipid nanoparticles (SLNs) 423
20.2.4 Cubosomes 423
20.2.5 Drug-polymer conjugates 424
20.2.6 Antibody-drug conjugates 424
20.2.7 Inorganic nanoparticles 425
20.2.8 Carbon nanotubes (CNTs) 425
20.2.9 Gold nanoparticles (GNPs) 426
20.2.10 Porous silicon particles (PSiPs) 426
20.2.11 Quantum dots (QDs) 426
20.2.12 Iron oxide nanoparticles (IONPs) 427
20.2.13 IONPs 427 References 428
21. Photo-triggered theranostics nanomaterials: Development and challenges in cancer treatment
Neha S. Raut, Divya Zambre, Milind J. Umekar, Sanjay J. Dhoble
21.1 Introduction of nanomaterials in phototherapeutics 431
21.2 Types of nanomaterials 432
21.2.1 Magnetic nanoparticles 432
21.2.2 Properties and materials for preparation of photo-based nanomaterials 433
21.2.3 Gold-based nanoparticles 433
21.2.4 Carbon nanotubes 433
21.3 Polymeric nanocarriers for photosensitizer/ dye encapsulation 434
21.4 Nanoconstructs for photodynamic therapy 434
21.5 Photo-triggered theranostic nanocarriers 435
21.6 Approaches to measure drug release through theranostic nanomedicine 436
21.6.1 Silicon photonic crystals with pores 436
21.6.2 Fluorescent nanoparticles 437
21.6.3 Upconversion nanoparticles 437
21.6.4 Radioluminescent nanoparticles 437
21.7 Magnetic resonance imaging for monitoring release of drug 437
21.8 Photo-triggered theranostics nanomaterials: Principle and applications 438
21.8.1 Applications of photo-triggered theranostics nanomaterials in cancer treatments 438
21.8.2 Therapeutic applications of photobased theranostic nanoparticles 438
21.9 Opportunities and limitations of nanomaterials 439
21.10 Preclinical challenges 439
21.11 Future aspects of nanomaterials in the therapeutics 439
References 440
22. Nanocrystals in the drug delivery system
Raju Ramesh Thenge, Amar Patel, Gautam Mehetre
22.1 Introduction to nanocrystals and nanosuspension 443
22.1.1 Properties of nanocrystals 443
22.1.2 Nanocrystals and bioavailability 444
22.1.3 Various methods of characterization of nanocrystals formulations 444
22.2 Production methods and technology of nanocrystals 445
22.2.1 Top down technology 445
22.2.2 Bottom up technology 446
22.2.3 Top down and bottom up technology 446
22.2.4 Spray drying 447
22.3 Advantages and Disadvantages of nanocrystals 448
22.3.1 Potential advantages and disadvantages of nanocrystals 448
22.3.2 Disadvantages of nanocrystals 448
22.4 Pharmaceutical Nanocrystals of API 448
22.4.1 Case studies of drug loaded in the nanocrystals 448
22.4.2 Application of nanocrystalsloaded carrier 449
22.5 Conclusion 452
Contributors
Nishant Awandekar Smt. Kishoritai Bhoyar College of Pharmacy, Kamptee, Nagpur, India
Panchali Barman Institute of Forensic Science and Criminology (UIEAST), Panjab University, Chandigarh, India
Veena Belgamwar Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Mangesh Bhalekar Department of Pharmaceutics, AISSMS College of Pharmacy, Pune, India
Vidyadevi Bhoyar University Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Alap Chaudhari Formulation R&D, Teva Pharmaceuticals, Weston, FL, USA
Vibha Chopra P.G. Department of Physics & Electronics, DAV College, Amritsar, Punjab, India
Vivek S. Dave Department of Pharmaceutical Sciences, St. John Fisher College, Wegmans School of Pharmacy, Rochester, NY, USA
Nirupama S. Dhoble Deaprtment of Chemistry, Sevadal Mahila Mahavidhyalaya, Nagpur, Maharashtra, India
Sanjay J. Dhoble Department of Physics, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Sachin Dubey Drug Product and Analytical Development, Ichnos Sciences SA, La Chaux-de-Fonds, Switzerland
Shraddha Dubey Inselpital University of Bern, Bern, Switzerland
Purushottam Gangane Dept of Pharmaceutics, Dadasaheb Balpande College of Pharmacy, Besa, Nagpur, MS, India
Anjali Ganjare Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Shailendra Gurav Department of Pharmacognosy, Goa College of Pharmacy, Goa University, Panaji, Goa, India
Nilesh Haran Department of Radiology HCG-NCHRI Cancer Centre, Near Automotive Square, Nagpur, Maharashtra, India
Prakash R. Itankar Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Sheeba Jacob Virginia Commonwealth University, Richmond, VA, USA
Shubhi Joshi Energy Research Centre, Panjab University, Chandigarh, India
Vijay Kale College of Pharmacy, Roseman University of Health Sciences, South Jordan, UT, United States
Vinita Kale Pharmaceutics Deptt., Gurunanak College of Pharmacy, Nagpur, Maharashtra, India
Shagufta Khan Department of Pharmaceutics, Institute of Pharmaceutical Education and Research, Borgaon (Meghe) Wardha, Maharashtra, India
Rohini Kharwade Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Pramod B. Khedekar Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Vaishali Kilor Department of Pharmaceutics, Gurunanak College of Pharmacy, Nagpur, Maharashtra, India
Dadasaheb M. Kokare Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Mallesh Kurakula Product Development, CURE Pharmaceutical, Oxnard, CA, USA
Anurag Luharia Department of Radiology, Datta Meghe Institute of Medical Science (Deemed to be University), Sawangi, Wardha, Maharashtra, India
Renuka K. Mahajan Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Nilesh M. Mahajan Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Gautam Mehetre Dr. Rajendra Gode College of Pharmacy, Malkapur, Buldana, MS, India
Sunil Menghani Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Samvit Menon Department of Physics, University of the Free State, Bloemfontein, South Africa
Gaurav Mishra Department of Radiology, Datta Meghe Institute of Medical Science (Deemed to be University), Sawangi, Wardha, Maharashtra, India
Keshav S. Moharir Pharmaceutics Deptt., Gurunanak College of Pharmacy, Nagpur, Maharashtra, India
Sachin More Dept of Pharmacology, Dadasaheb Balpande College of Pharmacy, Besa, Nagpur, MS, India
Amar Patel Bristol Myers Squibb, New Jersey, USA
Miral Patel Department of Pharmaceutical Sciences, Arnold and Marie Schwartz College of Pharmacy and Health Science, Long Island University, Brooklyn Campus, NY; Office of Pharmaceutical Quality, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD, USA
Kundan Patil Department of Pharmaceutics, Government College of Pharmacy, Amrawati, Maharashtra, India
Anita Paunikar Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Anil M. Pethe Datta Meghe College of Pharmacy, Datta Meghe Institute of Medical sciences, (Deemed-to-be) University, Sawangi (Meghe), Wardha, India
Nilesh Rarokar Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Nishikant A. Raut Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Neha S. Raut Department of Pharmaceutical Chemistry, Smt. Kishoritai Bhoyar College of Pharmacy, Kamptee, India
Vidya Sabale Dadasaheb Balpande College of Pharmacy, Besa, Nagpur, Maharashtra, India
Prafulla Sabale Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Avneet Saini Department of Biophysics, Panjab University, Chandigarh, India
D. Saroj Department of Radiotherapy, Allexis Hospital, Mankapur, Nagpur, India
Ravindra Satpute Toxicology Laboratory, Defense R & D Establishment, Nagpur, Maharashtra, India
Sheetal Sharma Department of Biophysics, Panjab University, Chandigarh, India
Shweta Sharma Institute of Forensic Science and Criminology (UIEAST), Panjab University, Chandigarh, India
Ravindra B. Shende Department of Radiation oncology, Balco Medical Centre, New Raipur, Chhattisgarh, India
Dilesh Singhavi Department of Pharmaceutics, Institute of Pharmaceutical Education and Research, Borgaon (Meghe) Wardha, Maharashtra, India
V. Sonwani HCG NCHRI Cancer Center, Nagpur, India
Hendrik C. Swart Department of Physics, University of the Free State, Bloemfontein, South Africa
Raju Ramesh Thenge Dr. Rajendra Gode College of Pharmacy, Malkapur, Buldana, MS, India
Sagar Trivedi Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India
Milind J. Umekar Department of Pharmaceutics, Smt. Kishoritai Bhoyar College of Pharmacy, Kamptee, India
Balkrishna Vengadaesvaran Higher Institution Centre of Excellence (HICoE), UM Power Energy Dedicated Advanced Centre (UMPEDAC), Level 4, Wisma R&D University of Malaya, Kuala Lumpur, Malaysia
Gaurav Verma Dr. S.S. Bhatnagar University Institute of Chemical Engineering & Technology (Dr. SSBUICET), Panjab University, Chandigarh, India; Centre for Nanoscience and Nanotechnology (UIEAST), Panjab University, Chandigarh, India
Khushwant S. Yadav Shobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM’s NMIMS, Mumbai, India
Mrunal M. Yawalkar Department of Physics, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, India
Divya Zambre Department of Pharmaceutics, Smt. Kishoritai Bhoyar College of Pharmacy, Kamptee, India
1.2 Background
1.2.1 Historical perspective of phototherapy
Modern scientific discoveries and technological inventions created the basis for applying artificial and modified light sources in phototherapy. Undoubtedly, these achievements included Isaac Newton’s (1642–1727) splitting of a light beam into seven basic colors, using a prism and his discovery of the color wheel, Friedrich Wilhelm Herschel’s (1738–1822) discovery of the infrared spectrum of the sun in 1800, and the discovery of ultraviolet radiation (Roelandts, 2002) in 1801 independently by Johann Wilhelm Ritter (1776–1810) and William Hyde Wollaston (1766–1828). Michel Eugène Chevreul (1786–1889) expanded upon Newton’s theory of seven colors by formulating the concept of simultaneous contrast in 1830. He described the phenomenon of an interaction of two colors, side by side, changing human perception. This contrasting effect is more distinct during the interaction between complementary colors (e.g., blue and yellow) (Chevreul, 1839).
The advancement of research on electricity and artificial light sources preceded the application of phototherapy in clinical therapy. Thereafter, Hans Christian Oerstedt (1777–1851) discovered that electric current creates a magnetic field (1777–1851). Michael Faraday (1791–1867) described electromagnetic induction as a source of electric power and built the first electric generator and the motor. Subsequently, Thomas Alva Edison (1847–1931) invented the electric light bulb and the battery as a source of light and electric power, respectively.
In the same historical period, scientific attempts were performed to explain the positive influence of light on humans. The first modern scientific data on the effects of light and colors on human health was published in the early nineteenth century by the German poet and writer Johann Wolfgang von Goethe (1749–1832). In 1810, he published a work on the perception of color vision and the influence of light and colors on the human emotional state (Goethe, 1810). It is considered as the very first report on the psychological effects of colors. However, it is far from perfection as it includes several erroneous assertions, like the thesis on light’s homogeneity inherent in the polemics of Newtonian optics. For example, he contradicted Newton with reference to the view that colors arise from the decomposition of light emerging from a prism into tiny particles called corpuscles but suggested that colors derive from the interaction of light and dark, and light is indivisible into any particles.
In the second half of the 19th century, scientific reports pointed to the healing properties of sunlight and reported the bactericidal properties of sunlight along with its therapeutic application in the treatment of rickets (Downes et al., 1877; Palm, 1890).
Activities of sanatoria, using natural solar radiation, were an important element in the historical process of creating contemporary phototherapy. The end of the nineteenth century saw the development of these “sun sanatoria.” They became the centers for heliotherapy and hydrotherapy. In addition, attempts were made to combat the tuberculosis epidemic by associating phototherapy with climatic treatment (i.e., therapy by bathing in cold or warm water and walking in the fresh air) (Roelandts, 2002). Pioneers in this therapeutic trend included the “Sunapostle” Arnold Rikli (1823–1906) (Levental, 1977), Oskar Bernhard (1861–1939), and August Rollier (1874–1954). Although from 1855, balneotherapy might include light treatment, as found in the Alpine Bed in Slovenia (Zupanic-Slavec and Toplak, 1998), Rikli applied the principle “Water is good, the air is better, and most of all the sunlight.” Bernhard promoted heliotherapy at the beginning of 1899 at a private clinic in St. Moritz, Switzerland. Finally, Rollier applied climatic treatment in combination with phototherapy to treat tuberculosis of the bone, beginning in 1903 at a sanatorium in Leysin, Switzerland (Rollier and Rosselet, 1923).
The discoveries (e.g., ultraviolet radiation) and inventions (e.g., the electric generator or the electric-light bulb), as well as balneological experiences of the treatment with the sunlight, contributed to the development of modern phototherapy and the transition from heliotherapy to artificial light phototherapy at the end of the nineteenth century. Nils Ryberg Finsen’s (1860–1904) studies on phototherapy led to its rise as a new field in physiotherapy (Grzybowski and Pietrzak, 2012). Finsen is also famous for creating the Medical Light Institute in Copenhagen, Denmark in 1896 (Finsen, 1896) and use of an electric carbon arc torch to treat lupus vulgaris patients with ultraviolet radiation (Finsen and Forchhammer, 1904).
1.2.1.1 Progress in the twentieth century
Phototherapy was developed for neonatal jaundice in the late 1950s. Sister Jean Ward at Rochford General Hospital in Essex, England noted in 1956 that sunshine decreased neonatal jaundice. Concurrently, hospital biochemists observed significantly low bilirubin levels in samples exposed to sunlight before processing (Dobbs and Cremer, 1975). Thus, it was the first evidence for effective light therapy for infantile hyperbilirubinemia (Cremer, Perryman and Richards, 1958). Jerold Lucey, the editor of the journal Pediatrics, published the “1968 landmark randomized controlled trial” results showing the efficacy of phototherapy (Lucey et al., 1968), which continued an important method for treating newborn jaundice (Weiss and Zimmerman, 2013).
Goethe’s idea on the impact of light on emotional states was resurrected when phototherapy was employed to treat depression. In 1946, in Scandinavia, the first descriptions of depression treatment using light were published (Marx, 1946); however, the fundamental development of phototherapy in the treatment of depression did not occur for four more decades (Rosenthal et al., 1984).
Phototherapy was also explored in ophthalmology. Gerhard Meyer-Schwickerath (1920–1992) investigated the use of natural sunlight for treating retinal disorders. Gerhard Meyer-Schwickerath performed a successful surgical operation with a photocoagulator in 1949 (Meyer-Schwickerath, 1960). It was a device designed and placed on the clinic’s roof to aggregate light onto a mirror in the operating room, paving the way for the application of laser therapy in retinal diseases (Grzybowski et al., 2016).
1.2.2 Overview on various types of phototherapies
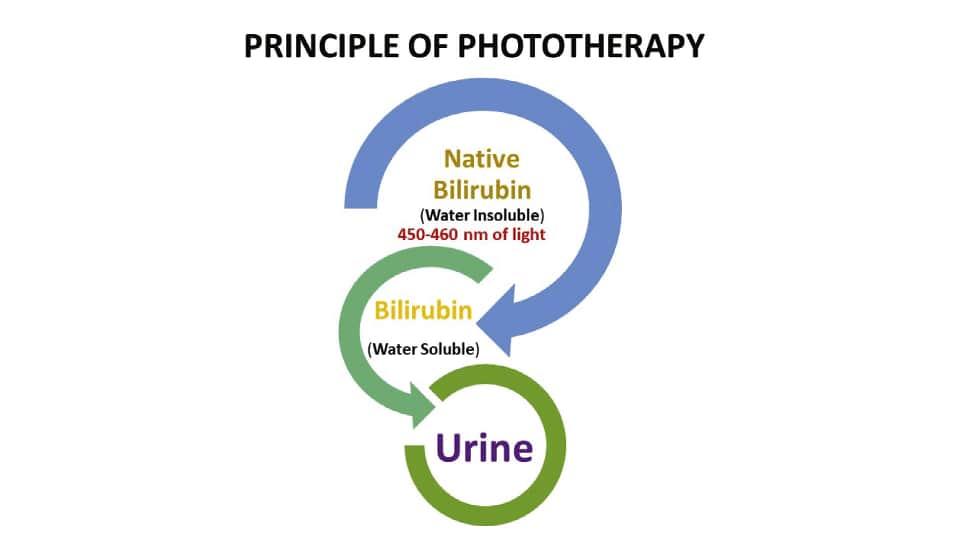
The traditional phototherapy units using fluorescent tubes contain standard blue (Westinghouse F20T 12B), daylight (F20 T12D), and cool white (F20 T12CW) lamps. The most effective lights are those with a high energy output near the maximum absorption peak of bilirubin (450 to 460 nm) as shown in Fig. 1.1 (Weiss and Zimmerman, 2013). Special blue lamps (Phillips TL 52/20W, Westinghouse 20 watt F20 T12BB) are the most efficient for neonatal phototherapy because it has more than twice the energy output at 450 nm than the standard blue bulb (Ennever et al., 1983). Investigators reported the significance of blue light in phototherapy with rapid reduction of serum bilirubin than with daylight or standard blue bulbs. On the other hand, these special blue bulbs cause nausea and dizziness among the neonatal care staff. A combination of four special blue lamps placed in the center of the phototherapy unit with two days’ light lamps on either side has been found to provide excellent irradiance without producing significant discomfort to staff. Non-fluorescent halogen lamps make a more intense light over a smaller surface. If lamps are placed closer than 50 cm, halogen lamps, unlike fluorescent bulbs, incur a risk of burns to an infant.
Light-Emitting Diode (LED) lights are now commercially available for use in the United States (Maisels and McDonagh, 2008). The Neo Blue LED systems incorporate optimal blue LED technology and are manufactured by Natus Medical Inc., San Carlos, CA, USA. Neo Blue LED’s emit blue light in the 450–470 nm spectrum. It is one of the safest phototherapy devices available as they do not emit light in the harmful ultraviolet and infrared radiation range. Further, the absence of heat when delivering overhead neo blue phototherapy is less likely to cause physiological water loss (Seidman et al., 2000). Fiber-optic phototherapy systems first appeared in the market around 1989. They are widely considered to be equally effective and more convenient than overhead lights. Light is delivered from a halogen bulb through a fiber optic cable and is
FIG. 1.1 Principle of phototherapy showing conversion of water-insoluble bilirubin to the soluble form upon exposer to a light wavelength of 450–460 nm.
emitted from the sides and ends of fibers inside a plastic blanket protected by a disposable cover. Infants lie on the blanket or are held with the blanket wrapped around them, and the need for eye patches, otherwise required in neonatal phototherapy, is eliminated.
Technology currently provides the clinician with three different modes of phototherapy delivery: fiber optic, low intensity, and high-intensity phototherapy. For low-intensity phototherapy, overhead lamps are typically set at a distance of 50 cm from the patient. The American Academy of Pediatrics has defined High-intensity phototherapy as a spectral irradiance of at least 30 MW per square meter per nanometer. High-intensity phototherapy is achieved by using a unit with eight special blue lamps, or Neo Blue LED systems 25 cm above the unclothed infant on a fiber optic phototherapy blanket in a bassinet while wearing a tie-on surgeon’s mask as a diaper. This method allows maximum skin exposure and achieves irradiance as high as 50 uw/cm2/nm.
However, as overhead lamps to infant distance are decreased, there is an increase in the heterogeneity of irradiation, with a much greater expansion at the center than at the periphery. Lining the bassinet with a white cloth produces greater homogeneity or irradiance and an increase in indirectly reflected irradiance (Maisels et al., 2007; Zauk, 2015).
1.2.2.1 UVB therapy
The UVB therapy includes NB-UVB and BB-UVB. The UVB therapy exerts effect by damaging nuclear DNA within epidermal-dermal junctional cells leading to apoptosis and cellular death of keratinocytes, immune cells, and fibroblasts (Bulat et al., 2011; Bolognia and Schaffer, 2012; Vangipuram and Feldman, 2016). NB-UVB (wavelength 311–312 nm) has largely replaced the use of BB-UVB (290–320 nm) due to its greater efficacy and emission duration at lower cumulative doses resulting in a reduction of associated long-term complications. NB-UVB is indicated as a first-line treatment of moderate-to-severe psoriasis and is also efficacious in treating numerous other dermatologic conditions (Ighani et al., 2018). The excimer laser is a targeted form of UVB treatment. It uses an active medium composed of excited dimers (noble gas argon/krypton/xenon) and reactive halogen gas (fluoride or chloride), delivering 308 nm light and facilitating the delivery of high doses of UVB to localized areas of skin (Mehraban and Feily, 2014). This focal treatment is beneficial in areas, which are difficult to treat such as the scalp, palms, and soles. It allows for a higher initial dose, less treatments, and thus, low long-term side effects. The excimer laser is currently approved in the USA for the treatment of conditions including psoriasis, and vitiligo, in addition to other localized inflammatory dermatoses (Alshiyab et al., 2015).
1.2.2.2 UVA therapy
The UVA is absorbed in the dermis and exerts its apoptotic effect on dermal blood vessel components, dendritic cells, fibroblasts, endothelial cells, and mast cells. UVA is further categorized into UVA1 (340–400 nm) and UVA2 (320–340 nm). UVA1 utilizes non-erythemogenic wavelengths and is indicated in the treatment of atopic dermatitis, localized scleroderma (morphea), systemic sclerosis, urticaria pigmentosa, cutaneous T cell lymphoma, dyshidrotic eczema, as well as other dermatoses (York and Jacobe, 2010).
1.2.2.3 PUVA therapy
PUVA involves administering either oral, topical, or bath psoralen followed by subsequent exposure to UVA, either via phototherapy or direct sunlight (PUVAsol). Psoralens (commonly methoxsalen or 8-methoxypsoralen) intercalate between DNA base pairs and, upon photon absorption, chemically activate to crosslink DNA. It results in several antiproliferative, antiangiogenic, apoptotic, and immunosuppressive effects. Melanogenesis is also stimulated via unknown mechanism. PUVA is effective in the treatment of several dermatologic conditions. PUVA treatments can result in severe blistering photoreactions/burns. PUVA is associated with a higher risk of skin cancer compared to other forms of phototherapy (Youssef et al., 2016).
1.2.2.4
Home phototherapy
Home phototherapy is another type that is limited to otherwise healthy term infants older than 48 hours with bilirubin levels 15-20 mg/dl with no hemolysis. Parents are required to be able to monitor the temperature and hydration status of infant. Home visits by a skilled nurse in evaluating newborns are performed, and bilirubin levels are assessed periodically. UVB therapy is equally effective when performed at home versus in the outpatient office setting and could be cost effective and more convenient to patients. Home phototherapy was associated with higher rates of dose reduction and/or discontinuation of biologics and apremilast, especially in patients with multiple comorbidities. There are several advantages assessing the efficacy of home phototherapy in treating other dermatologic conditions (Click et al., 2017; Howell et al., 2018). Tanning beds are an efficacious method of UV exposure and are recommended for patients who benefit from a more accessible,
less costly alternative to office and/or home phototherapy. The effect of tanning beds may be from UVA exposure, though common tanning bulbs emit varying degrees of UVB light. Of concern, use of tanning bed require limited supervision of control settings and duration of treatments, leading to the potential for increased adverse events (AEs) (Radack et al., 2015), however the amount of UV radiation received is likely to be better controlled with tanning beds than with sun exposure (Krenitsky et al., 2020).
1.3 Various light sources and methods of phototherapy
There is a considerable selection of several custom-made and commercial phototherapy devices, which have been produced for investigative and clinical applications. The phototherapy devices are categorized by their light source as follows: (1) fluorescent tube (TL12, 60 cm, 20W) devices with different colors of light (cool white [CW], blue, special blue [BB, 52, and 03], turquoise, or green) of straight or U-shaped (18 cm, 18W tubes), (2) metal halide bulbs used in spotlights and incubator lights, (3) metal halide bulb and fiberoptic light guide combinations as used in pads, blankets or spotlights, and (4) high-intensity LEDs used presently as canopies.
1.3.1 Fluorescent tubes
The most commonly used light source in the U.S. is the special blue tube, such as F20 T12/BB or TL52/20W (Philips, The Netherlands). CW light has also been used together with special blue tubes to ameliorate caregivers’ complaints regarding the blue hue of the light (Sisson and Kendall, 1973). Still, this combination of tubes dramatically decreases efficacy by 50% depending on the proportion of CW to special blue tubes. At a standard distance of 40 cm, the devices with a 1:1 ratio of tubes can deliver up to 11 W/cm2/nm, while a unit containing only special blue tubes can deliver up to 24 W/cm2/nm. However, the use of CW light typically provides only homeopathic doses of phototherapy. In addition, it may be inadequate in sufficiently decreasing total bilirubin levels in a jaundiced infant unless the lights are positioned in close proximity, such as directly above the infant (De Carvalho et al., 1999).
1.3.2 Halogen spotlights
Halogen spotlight systems utilize single or multiple metal halide lamps as the light source and provide fairly high irradiance, often exceeding 20 W/cm2/nm. However, these units can generate considerable heat, which can, in turn, cause thermal injury to the infant and staff if applied too closely and can emit ultraviolet (UV) radiation if not appropriately shielded. The use of spotlights is sometimes preferred in the neonatal intensive care unit because with premature or critically ill neonates on radiant warmers, its design allows for ad hoc positioning of these devices for the convenience of caregivers. However, their variable positioning with respect to the distance from the infant and angle of application and their irradiance heterogeneity can lead to unreliable dosing and unpredictable clinical response (Vreman et al., 2004).
1.3.3 Fiberoptic blankets
Fiberoptic devices contain a tungsten-halogen bulb that delivers light via a cable into a plastic pad containing fiberoptic fibers. The pad remains cool and can be placed directly under an infant to increase the skin surface area that is exposed. The pad can also be wrapped around the infant’s midsection to provide phototherapy (Vreman et al., 2004). The spectral power of the pad alone is low, therefore it is commonly used in conjunction with overhead lights to provide double phototherapy.
1.3.4
Light-emitting diodes
The gallium nitride LED is one of the most recent innovations in phototherapy. These devices provide high irradiance in the blue to blue-green spectrum without excessive heat generation (Maisels, 2005) Light-emitting-diode units are efficient, long-lasting, and cost-effective. The latest models incorporate amber LEDs to counteract the “blue hue” effect that can irritate caregivers (Stokowski, 2006).
1.3.5
Filtered sunlight
The lack of devices and/or reliable electric power limits the use of phototherapy in underdeveloped countries. Further, modern phototherapy devices are not affordable, often break down because of electrical power surges, and are difficult to maintain due to the unavailability of replacement parts. Even where phototherapy devices are available, most hospitals lack
the resources necessary to replace fluorescent lamps. Thus, it is not uncommon, especially in areas without access to phototherapy, for the parents/guardians of jaundiced infants to place their babies in direct sunlight unaware of the potential harm. Using direct sunlight for phototherapy has several clinical and practical drawbacks that could make its use undesirable. Sunlight contains altitude-, seasonal-, and time-of-day-dependent levels of harmful ultraviolet A, B, and C radiation, which can cause serious and permanent damage to human skin. It also contains significant levels of warming infrared radiation, which could raise core body temperature to unsafe levels in the absence of sufficient cooling. It must be underlined that the use of sunlight, when filtered to exclude the harmful spectral radiation, is a novel, practical, and inexpensive method of phototherapy. It potentially offers a safe and efficacious treatment strategy for management of neonatal jaundice in economically disadvantaged countries where conventional phototherapy treatment is not available (Slusher et al., 2014). The most practical and low-cost sunlight filters are the commercially available window-tinting films, widely used in vehicles and residential and commercial structures in sunny climates. Window tinting films can effectively reduce ultraviolet and infrared radiation and offer a range of significant attenuations of therapeutic blue light. Although window-tinting films are traditionally affixed to a glass surface, these films can also be applied over a support frame, under which an infant basket, bassinet, or crib can be placed (Vreman et al., 2013; Yurdakök, 2015).
1.4 Applications and limitations of phototherapy
1.4.1 Application in neonatal
jaundice
There is the use of visible light for the phototherapeutic treatment of hyperbilirubinemia in newborns. This relatively common therapy lowers the serum bilirubin level by transforming bilirubin into water-soluble isomers that can be eliminated without conjugation in the liver. The dose of phototherapy largely determines how quickly it works; the dose, in turn, is determined by the light’s wavelength, the light’s intensity (irradiance), the distance between the light source and the infant, and the body surface area exposed to the light. Commercially available phototherapy systems include delivery of light via fluorescent bulbs, halogen quartz lamps, light-emitting diodes, and fiberoptic mattresses. Proper neonatal and infant care enhances the effectiveness of phototherapy and minimizes complications. Caregiver responsibilities include ensuring effective irradiance delivery, maximizing skin exposure, providing eye protection and carefully monitoring thermoregulation, maintaining adequate hydration, promoting elimination, and supporting parent-infant interaction (Stokowski, 2006).
1.4.2 Application for morphea, scleroderma, and other sclerosing
skin conditions
As discussed above, UVA1 phototherapy potentially exerts its therapeutic effect through modulation of the three predominant pathologic mechanisms in sclerosis: immune dysregulation, imbalance of collagen deposition, and endothelial dysfunction. This beneficial effect is predominantly reported in morphea (localized scleroderma), systemic sclerosis (scleroderma), lichen sclerosis, and chronic graft versus host disease (GVHD). Morphea has been the subject of several studies utilizing UVA1 therapy. These studies have shown that patients with morphea respond to low, medium, and highdose UVA1 therapy. A comparative study of low dose versus high dose therapy revealed that high-dose UVA1 treatment is more effective. In a second comparative study, low and medium doses were compared with narrowband UVB therapy. This study revealed that medium-dose UVA1 therapy was superior to both low-dose UVA1 therapy and narrowband UVB therapy. A study showed no significant difference in improvement with low-dose UVA1 and narrowband UVB (NBUVB). The existing evidence indicates that medium to high dose UVA1 therapy delivered over treatment significantly benefits patients with morphea. These studies specifically included patients with linear and plaque subtypes of morphea. In all these studies, no adverse effects were reported, with the worst adverse effect being transient headaches. UVA1 is unlikely effective in burned-out atrophic lesions, deep morphea Parry Romberg/facial hemiatrophy, and eosinophilic fasciitis. Depth of the pathogenicity decides whether systemic treatment should be considered as first-line therapy in these cases (York and Jacobe, 2010). Hither to, phototherapy has evolved through more complex treatment, and is widely used to treat various diseases, such as atopic dermatitis (Patrizi et al., 2015), psoriasis (Diffey, 1980), vitiligo (Adauwiyah and Suraiya, 2010), acne vulgaris (Hession et al., 2015; Pei et al., 2021), and cancer (Morton et al., 2002).
1.4.3 Application for cancer
Conventional use of chemotherapy and radiation has some drawbacks in clinical practice, which are minimized by remodulating the current therapy using photosensitizers (PS). Combining PS with light generates reactive oxygen species (ROS) to kill cancerous cells is a novel noninvasive approach known as photodynamic therapy (PDT). It is a selective and
targeted approach that enhances specificity towards cancer cells. However, success of PDT depends on the selection of PS, as the administered PS must be deep entered into the tumor cells and then activated by irradiation of light. The cell death that occurs during PDT is the apoptotic response of the sub-cellular localization of PS. There are two approaches generally used in PDT: electron transfer (eT) happens due to the generation of radical species at the excited state. Another is energy transfer (ET) created by electronic excitation and energy transfer from an excited triplet of PS to a triplet oxygen molecule. Hence, the clinical use of PDT in combination with chemotherapy would be the better option as it works at the electronic level inside the cell.
Phototherapy involves the irradiation of tissues with light and is commonly implemented in PDT and photothermal therapy (PTT). Photosensitizers (PSs) are often needed to improve the efficacy and selectivity of phototherapy via enhanced singlet oxygen generation in PDT and photothermal responses in PTT. In both cases, efficient and selective delivery of PSs to the diseased tissue is of paramount importance. PTT monotherapy typically cannot completely eradicate tumors due to non-homogeneous heat distribution in tumor tissues. Several strategies have been adopted to increase the anticancer efficacy of PTT and PTT-based combination therapies. First, better PTT agents with high light absorptivity in the near-infrared spectrum, high photothermal conversion efficiency, long blood circulation times, and enhanced tumor uptake are being sought to improve photothermal therapy. Second, synergistic effects of PTT and other therapeutic modalities are being explored to enhance anticancer efficacy. The combination of PTT with reactive oxygen species, small interference RNAs, or chemotherapeutics can drastically increase treatment efficacy. Third, image-guided PTT with theranostic agents (which are used for treatment and diagnosis both) based on multifunctional nanomaterials can also increase treatment efficacy of PTT via selective delivery of PTT agents to tumors ( Morton et al., 2002 ).
1.4.4 Limitations of home phototherapy and sunlight
The irradiance and surface area exposure produced by the home phototherapy unit is lower than typical units used in the hospital, making them less efficient at lowering the serum bilirubin level. Although phototherapy is indicated as treatment, current guidelines state that clinically high bilirubin warrants treatment should be managed in the hospital. Lack of control treatment guidelines, duration of therapy, and variable sunlight prevented its use to treat hyperbilirubinemia and/or poor treatment, leading to adverse outcomes. Although in the past, parents had been told to expose jaundiced infants to the sunlight at courtyard of an English hospital, this practice is not considered a safe or reliable way to treat jaundice. There are reports in the literature of infants developing kernicterus after their parents were instructed to treat their jaundice at home by exposing them to sunlight, in some cases for 15 min per day. Sunlight is ineffective and contributes to delays in recognizing the severity of the hyperbilirubinemia (Stokowski, 2006; Lan et al., 2019).
1.5 Recent developments and future scopes
1.5.1 The immunoregulatory effects of phototherapy: Possible pathways
Psoriasis is caused by abnormal interactions among innate immune cells, T cells, and keratinocytes, leading to activation of the T helper cell type 1/T helper cell type 17 (Th1/Th17) immune axes and related cytokines. It contributes to the hyperproliferation, and inflammation seen in psoriasis. There are various mechanisms by which phototherapy may be effective for psoriasis. First, UV light induces apoptosis of keratinocytes and T cells in the epidermis and dermis. Second, UV light promotes immunosuppression by promoting migration of Langerhans cells of the epidermis and decreasing mast cell degranulation and histamine release. Lastly, UV light induces alteration in the cytokine profile of psoriasis ( Bulat et al., 2011 ). The schematic representation for mechanism of phototherapy has been depicted in Fig. 1.2
Research carried out recently has led to a better understanding of the specific pathways and alteration of cytokines by phototherapy. This therapy shifts the immune response away from the Th1/Th17 pathway toward the counter regulatory Th2 axis. The Th1/Th17 pathway is suppressed by NB-UVB, leading to decreased interleukin-12 (IL-12), IL-17, IL-20, IL-22, and IL-23. These effects on cytokines appear to be systemic and not just localized to psoriatic lesions. PUVA and NB-UVB lower plasma levels of tumor necrosis factor-alpha, IL-17, IL-22, and IL-23 at the end of 6 weeks of treatment. Furthermore, regulatory T (Treg) cells in patients with severe psoriasis display an enhanced propensity to convert into IL-17A-producing cells, linked to the loss of forkhead box P3 (Foxp3). UVB increases Foxp3-positive Treg cells in psoriatic skin lesions. This increase in Foxp3 expression improves Treg cell stability and reduces pro-inflammatory Th1/Th17 cytokines in psoriatic skin lesions (Wong et al., 2013).