Organogermanium Compounds
Theory, Experiment, and Applications
Edited by Vladimir Ya. Lee
Volume 1
This edition first published 2023 © 2023 John Wiley & Sons, Inc.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at http://www.wiley.com/go/permissions.
The right of Vladimir Ya. Lee to be identified as the author of editorial material in this work has been asserted in accordance with law.
Registered Office
John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA
For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com.
Wiley also publishes its books in a variety of electronic formats and by print-on-demand. Some content that appears in standard print versions of this book may not be available in other formats.
Trademarks: Wiley and the Wiley logo are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates in the United States and other countries and may not be used without written permission. All other trademarks are the property of their respective owners. John Wiley & Sons, Inc. is not associated with any product or vendor mentioned in this book.
Limit of Liability/Disclaimer of Warranty
In view of ongoing research, equipment modifications, changes in governmental regulations, and the constant flow of information relating to the use of experimental reagents, equipment, and devices, the reader is urged to review and evaluate the information provided in the package insert or instructions for each chemical, piece of equipment, reagent, or device for, among other things, any changes in the instructions or indication of usage and for added warnings and precautions. While the publisher and authors have used their best efforts in preparing this work, they make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives, written sales materials or promotional statements for this work. The fact that an organization, website, or product is referred to in this work as a citation and/or potential source of further information does not mean that the publisher and authors endorse the information or services the organization, website, or product may provide or recommendations it may make. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for your situation. You should consult with a specialist where appropriate. Further, readers should be aware that websites listed in this work may have changed or disappeared between when this work was written and when it is read. Neither the publisher nor authors shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages.
A catalogue record for this book is available from the Library of Congress
Hardback ISBN: 9781119613435; Set ISBN: 9781394177561 (Volume 1); ePub ISBN: 9781119613527; ePDF ISBN: 9781119613473; oBook ISBN: 9781119613466
Cover Images: © ALFRED PASIEKA/Getty Images; © Intothelight Photography/Shutterstock Cover design by Wiley
Set in 9.5/12.5pt STIXTwoText by Integra Software Services Pvt. Ltd, Pondicherry, India
Preface ix List of Contributors xiii
1 Computational and Theoretical Aspects of Structure and Bonding in Doubly Bonded Organogermanium Compounds 1
Miriam Karni and Yitzhak Apeloig
2 Organogermanium Compounds of the Main Group Elements 103
Kirill V. Zaitsev
3 Transition Metal Complexes of Germanium 195
Kohtaro Osakada
4 Germanium Cages and Clusters 225
Tanja Kunz and Andreas Schnepf
5 Arylgermanium Hydrides, ArnGeH4-n (n = 1–3) - Synthesis, Characterization, Reactivity 277
Ana Torvisco and Frank Uhlig
6 Germylium Ions and Germylium Ion-like Species 299
Thomas Müller
7 Germanium-Containing Radicals 339
Alexander Hinz and Frank Breher 8 Germanium-Centered Anions 361
Christoph Marschner 9 Germylenes 387
Norio Nakata
10 Multiple Bonds to Germanium 435
Vladimir Ya. Lee
Preface vii
List of Contributors xi
11 Germaaromatic Compounds 477
Yoshiyuki Mizuhata and Norihiro Tokitoh
12 Germanium-centered Ion Radicals 507
Mikhail P. Egorov, Viatcheslav V. Jouikov, Elena N. Nikolaevskaya, and Mikhail A. Syroeshkin
13 Donor-acceptor Stabilization of Species with Low-coordinate Germanium 561
Sakya S. Sen and Herbert W. Roesky
14 Synthesis of the Penta- and Hexacoordinate Germanium(IV) Complexes 597
Naokazu Kano
15 Dynamic Stereochemistry of Penta- and Hexacoordinate Germanium(IV) Complexes 629
Vadim V. Negrebetsky and Alexander A. Korlyukov
16 X-ray Crystallography of Organogermanium Compounds 667
Catherine Hemmert and Heinz Gornitzka
17 Organogermanium Photochemistry 745
William J. Leigh
18 Oligo- and Polygermanes 787
Charles S. Weinert
19 Bioorganic and Medicinal Organogermanium Chemistry 839
Takashi Nakamura, Yasuhiro Shimada, and Katsuyuki Sato
Index 867
Preface

Germanium is one of the few chemical elements in the Periodic Table, for which the theoretical prediction of its very existence has preceded its actual experimental discovery. This prediction was made by the Russian chemist Dmitri Mendeleev based on the general trends of valence and atomic weights within his Periodic Table of the chemical elements (1869) [D. Mendelejeff “Ueber die Beziehungen der Eigenschaften zu den Atomgewichten der Elemente”, Z. Chem 1869, 12, 405–406]. In an updated version of this Periodic Table (1871-1872) [D. Mendelejeff “Die Periodische Gesetzmässigkeit der Chemischen Elemente”, Ann. Chem. Pharm 1872, Suppl. 8, 133–229; D. Mendelejeff “Zur Frage über das System der Elemente”, Ber. Dtsch. Chem. Ges. 1871, 4, 348–352], Mendeleev proposed that there was a missing element in the carbon family with the atomic weight 72 that should be placed in the fourth row, just below silicon and just above tin within the carbon group. He named this non-existing (at that time) element as “eka-silicium”. Following this seminal Mendeleev prediction, German chemist Clemens Winkler finally succeeded in 1886 in the isolation of “eka-silicium” from the mineral argyrodite (Ag8GeS6) and named this new element as germanium (Ge) [C. Winkler “Germanium, Ge, Ein Neues, Nichtmetallisches Element”, Ber. Dtsch. Chem. Ges. 1886, 19, 210–211; C. Winkler “Mittheilungen über das Germanium”, J. Prakt. Chem. 1886, 34, 177–229]. Winkler also pioneered the preparation of the first organic derivative of germanium, namely, tetraethylgermane Et4Ge, in 1887 [C. Winkler “Mittheilungen über das Germanium”, J. Prakt. Chem. 1887, 36, 177–209]. Since then and up the present date, the chemistry of organogermanium compounds (that is, compounds featuring Ge–C bonds) has experienced an explosive growth, especially after the recognition of the key role of metallic germanium in semiconductor electronics in the midtwentieth century, followed by the extensive use of germanium and its organic derivatives in optical fibers, polymerization catalysts, microchip manufacturing, and biomedical applications. Given the undoubted importance of organogermanium compounds, it comes as no surprise that the field of organogermanium chemistry is continuously growing, thus requiring regular reviewing and updates on its latest advances. Among the most important previously published books on organogermanium chemistry, one should first of all mention excellent monograph by Satgé and coworkers [J. Satgé, M. Lesbre, P. Mazerolles, “The Organic Compounds of Germanium, Wiley, 1971] and two comprehensive volumes of the Patai’s series of books [(a) The Chemistry of Organic Germanium, Tin, and Lead Compounds (Eds. S. Patai, Z. Rappoport), Wiley, 1995; (b) The Chemistry of Organic Germanium, Tin, and Lead Compounds, Volume 2 (Ed. Z. Rappoport), Volume 2, Parts 1–2, Wiley, 2002]. Patai’s latest book was published 20 years ago, and since then, critical progress has been made in organogermanium chemistry with the majority of milestone developments achieved since 2000. That is why we have attempted in this book to survey, analyze and summarize the current state of affairs in the field of organogermanium chemistry, focusing on the latest (published mostly after 2000) groundbreaking advances with comprehensive and up-to-date literature coverage up to the end of 2021.
Our “Organogermanium Compounds” book is organized into the three major parts, Theory, Experiment, and Applications, made up of a total of 19 chapters, each written by leading experts in their respective fields comprehensively covering the relevant literature, first published within the last two decades.
1) The first part, Theory (one chapter) includes a contribution “Computational Aspects of Structure and Bonding in Doubly Bonded Organogermanium Compounds” from Miri Karni and Yitzhak Apeloig (Technion – Israel Institute of Technology, Haifa, Israel). In their chapter, the authors present state-of-the-art computational approaches to the flourishing field of doubly bonded organogermanium compounds and thoroughly discuss their structural and bonding aspects depending on the nature of the double bond and its substituents.
The Experiment part is completed by the Physico-Chemical Studies of Organogermanium Compounds section which comprises two contributions each dealing with state-of-the-art instrumental techniques for assessing the structures of organogermanium compounds. In the first one, “X-Ray Crystallography of Organogermanium Compounds”, Catherine Hemmert and Heinz Gornitzka (University of Toulouse, Toulouse, France) comprehensively overview the vast literature on the structurally characterized organogermanium compounds discussing their particular X-ray crystallographic features. The following chapter “Organogermanium Photochemistry”, by William J. Leigh (McMaster University, Hamilton, Canada) deals with developments in the photochemistry of organogermanium compounds, particularly emphasizing systems involving low-coordinate organogermanium compounds as either photoproducts or photoreactants.
3) The last, third, part of the book, Applications (two chapters), reports on the synthetic approaches towards materials based on organogermanium compounds and their practical use. In the first contribution, “Oligo- and Polygermanes”, Charles S. Weinert (Oklahoma State University, Stillwater, USA) reviewed and updated the recent advances in the field of the oligo- and polygermanes, discussing general synthetic methods for their preparation and also their peculiar physical properties (luminescence, electrochemistry). In the very final chapter of the Applications part (and the whole book) “Bioorganic and Medicinal Organogermanium Chemistry”, Takashi Nakamura, Yasuhiro Shimada, and Katsuyuki Sato (Asai Germanium Research Institute, Hakodate, Japan) give an account on the production and use of bioactive organogermanium compounds (such as Ge-132) in medicinal chemistry.
As an editor of the volume, I highly appreciate the works of all above-mentioned authors whose excellent contributions will hopefully turn this book into a guide to contemporary organogermanium chemistry and a useful reference source, to further inspire those already working in the field and to attract newcomers to join the fascinating world of organogermanium compounds. Although the book is primarily and foremost addressed to specialists in the field of organogermanium and organometallic chemistry, it could also be of interest to both Main Group and transition metal chemistry experts, as well as to computational and material science chemists, and to the rest of the chemical community, including undergraduate, graduate, and post-graduate students of the advanced levels.
Vladimir Ya. Lee (Editor) University of Tsukuba, Tsukuba, Japan April 2022
List of Contributors
Yitzhak Apeloig
Schulich Faculty of Chemistry
Technion-Israel Institute of Technology Haifa, Israel
Frank Breher
Institute of Inorganic Chemistry Karlsruhe Institute of Technology (KIT) Karlsruhe, Germany
Mikhail P. Egorov
N. D. Zelinsky Institute of Organic Chemistry Moscow, Russia
Heinz Gornitzka
Laboratoire de Chimie de Coordination du CNRS
Université Toulouse Toulouse, France
Catherine Hemmert
Laboratoire de Chimie de Coordination du CNRS
Université Toulouse Toulouse, France
Alexander Hinz
Institute of Inorganic Chemistry
Karlsruhe Institute of Technology (KIT) Karlsruhe, Germany
Viatcheslav V. Jouikov
N. D. Zelinsky Institute of Organic Chemistry Moscow, Russia
Naokazu Kano
Department of Chemistry
Gakushuin University Tokyo, Japan
Miriam Karni
Schulich Faculty of Chemistry
Technion-Israel Institute of Technology Haifa, Israel
Tanja Kunz Institut für Anorganische Chemie Universität Tübingen Tübingen, Germany
Alexander A. Korlyukov Pirogov Russian National Research Medical University Moscow, Russia
Vladimir Ya. Lee Department of Chemistry University of Tsukuba Tsukuba, Japan
William J. Leigh Department of Chemistry & Chemical Biology McMaster University Hamilton, Ontario, Canada
Christoph Marschner Institut für Anorganische Chemie Technische Universität Graz Graz, Austria
Yoshiyuki Mizuhata Institute for Chemical Research Kyoto University Kyoto, Japan
Thomas Müller
Institute of Chemistry
Carl von Ossietzky University Oldenburg Oldenburg, Germany
Takashi Nakamura
Asai Germanium Research Institute Co., Ltd. Hokkaido, Japan
Norio Nakata Department of Chemistry
Saitama University Saitama, Japan
Vadim V. Negrebetsky Pirogov Russian National Research Medical University Moscow, Russia
Elena N. Nikolaevskaya
N. D. Zelinsky Institute of Organic Chemistry Moscow, Russia
Kohtaro Osakada Chemical Resources Laboratory Tokyo Institute of Technology Yokohama, Japan
Herbert W. Roesky Institute of Inorganic Chemistry
Georg-August-University Göttingen Göttingen, Germany
Katsuyuki Sato
Asai Germanium Research Institute Co., Ltd. Hokkaido, Japan
Andreas Schnepf Institut für Anorganische Chemie Universität Tübingen Tübingen, Germany
Sakya S. Sen
Institute of Inorganic Chemistry
Georg-August-University Göttingen Göttingen, Germany
Yasuhiro Shimada
Asai Germanium Research Institute Co., Ltd. Hokkaido, Japan
Mikhail A. Syroeshkina
N. D. Zelinsky Institute of Organic Chemistry
Moscow, Russia
Norihiro Tokitoh Institute for Chemical Research
Kyoto University
Kyoto, Japan
Ana Torvisco Institute for Inorganic Chemistry
Graz University of Technology
Graz, Austria
Frank Uhlig Institute for Inorganic Chemistry
Graz University of Technology
Graz, Austria
Charles S. Weinert Department of Chemistry
Oklahoma State University Stillwater, Oklahoma
Kirill V. Zaitsev Department of Chemistry
Moscow State University Moscow, Russia
RRKM Rice-Ramsberger-Kassel-Marcus theory
SDD Sttutgart-Dresden Double zeta Electron Core Potential (ECP)
SOJT second-order Jahn Teller
Tbb
2,6-[CH(SiMe3)2]2-4-tBu-C6H2
Tip 2,4,6-triisopropylphenyl
VB valence bond
WBI Wiberg bond index
Xylyl (CH3)2C6H3
1.1 Introduction
Unsaturated hydrocarbons having C=C double bonds; heteroelement- substituted doubly bonded carbon compounds, e.g., ketones; alkynes with C≡C triple bonds; hydrocarbons with an extended number of double bonds, such as dienes and allenes; all are of fundamental importance to chemistry and the chemistry of life. In contrast stable heavier group 14 analogues, with Si, Ge, Sn and Pb atoms, were believed to be nonexistent until several decades ago (see below the historical overview). The last four decades have witnessed an enormous progress in the synthesis and in the theoretical understanding of the properties of these groups of compounds. The experimental progress was accompanied, and in many cases even preceded, by theoretical and computational studies. These advances were enabled by two important developments: (a) the substantial advances in quantum mechanical computational methodology [1–10] that now enables reliable, accurate and efficient calculations of the properties of relatively large molecules possessing heavy elements. These developments make quantum chemical calculations a reliable tool for the quantitative analysis of chemical phenomena and a reliable guide to experiment; and (b) the dramatic developments in computer technology that have accelerated calculations dramatically [11, 12].
The synthesis, molecular structure determination, physical and chemical properties, and the development of bonding models of multiply bonded compounds of heavier group 14 elements, Si, Ge, Sn and Pb, have been reviewed extensively in the past three decades [13–26]. The numerous experimental and theoretical studies carried out in this field have revealed fundamental differences in the structures, physical properties and chemical behavior between carbon multiply bonded compounds and analogous group 14 heavier congener compounds. The heavier group 14 compounds were considered “unusual”. In contrast, Kutzelnigg suggested that the heavier main group element compounds have “regular” behavior, while carbon compounds are the exception [27]. This revolutionary view is now widely accepted by main group researchers (not only for group 14 elements). The trends in the physical properties of group 14 elements, E, are largely responsible for the unprecedented structures and chemical behavior of the heavier group 14 compounds compared to those of the analogous carbon compounds. Selected properties of group 14 elements are given in Table 1.1. A comparison of the important physical properties of group 14 elements was also provided by Basch and Hoz [28]. The effects of the trends in the properties of the E atoms shown in Table 1.1, on the structure and nature of bonding of group 14 multiply bonded compounds, are discussed in section 1.4.1.
The unusual structures and bonding motifs of multiply bonded heavier group 14 molecules attracted the attention of many theoreticians, who studied their molecular structures, nature of bonding, kinetic and thermodynamic stability, potential energy surfaces for a variety of their reactions, etc. Many of these theoretical studies were carried out before stable compounds of these classes were synthesized, and were therefore truly predictive, and in some cases directed experiment. Two comprehensive reviews on the theoretical studies of the chemistry of organic Si, Ge, Sn and Pb compounds were published: one by Apeloig, Karni and Schleyer in 2001 [44] and one in 2002 by Frenking and coworkers [45].
In this chapter we review the theoretical studies of doubly bonded organogermanium compounds, mainly those published in the years 2000–2020 but occasionally we added important studies published in 2021. We have highlighted the insights that these theoretical studies provide on molecular structures, nature of bonding, stability vs. isomeric forms, and the physical properties, of these intriguing compounds. We do not include in this review their reactions and reaction mechanisms. Where available, we relate and compare the computational results to reported experimental studies. In our view, it is important to examine organogermanium compounds in comparison to their closest neighbors in the Periodic Table, Si and Sn. Thus, when available, we discuss comparisons with analogous organosilicon compounds (the element most similar to germanium) and other group 14 elements, mainly Sn. For pre-year 2000 theoretical studies, the readers are referred to the above-mentioned reviews [44, 45] and to specific papers cited in this chapter.
Table 1.1 Selected physical properties of group 14 elements.
Pauling
Allen
Hybridization of E in EH4e
Hybridization of E in H2E=EH2 (C2h) f
a Metallic distance;
b Difference of orbital radii between the valence p and s orbitals;
c For the transition: ns2np2(3P) → ns1np2(4P);
d For the transition: ns2np2(3P) → ns2np1(2P);
e Calculated using NBO analysis;
f Calculated using NBO analysis at B3LYP/6-31++G(d,p).
The theoretical literature of organogermanium compounds is vast. When we collected the literature for this review, we were amazed how vast is this field, and because of space limitations we had to limit the scope of this review, which even so includes more than 540 references! In this chapter, we concentrate on doubly bonded organogermanium compounds, which in their bonding and other fundamental properties are significantly different from analogous carbon compounds. We discuss homonuclear and heteronuclear organogermanium compounds, R2Ge=ER2, E=C, Si, Ge, and extended doubly bonded organogermanium compounds such as germadienes, germaallenes, and germaaromatic derivatives, i.e., germabenezene analogues. For lack of space, we do not discuss doubly bonded compounds between group 14 elements and groups 13, 15 and 16 elements or triply bonded compounds.
For completeness, we present a brief historic overview of the field, since the isolation of the first stable doubly bonded heavier group 14 element compounds in 1976.
1.2 Computational Methods
For the benefit of readers unfamiliar with the theoretical methods and terms, we bring a brief overview of the theoretical methods that are currently frequently used in theoretical calculations of organogermanium compounds. Details of the specific computational methods or of the basis sets mentioned in this review can be found in the cited papers.
The rapid development of computational packages for electronic structure calculations in the last three decades has been recently reviewed [10] (for a comprehensive list of available software packages see Ref. [46]). These computational software packages apply state-of-the-art theoretical methods for electronic
modelling of molecules in the gas phase,
solution, and the solid-state. Currently, these software packages apply mainly two theoretical approaches, ab initio wavefunction based molecular orbital (MO) methods [1–5], and electron density based Density Functional Theory (DFT) methods [6–8]. These software packages enable highly reliable calculations of molecular structures, potential energy surfaces, electron density and population analysis, vibrational frequencies, UV-Vis, NMR, EPR, IR and Raman spectroscopic properties, electronic circular dichroism (ECD), excitation energies, and other properties [1–9, 47].
Below we briefly summarize the theoretical approaches most commonly used for the calculation of the above-mentioned molecular properties. A detailed description of the field is given in the monographs cited above. Specific definitions and description of the computational methods used in the studies reviewed in this chapter are provided in the cited papers and references therein.
1.2.1 Ab initio Methods
Ab initio MO methods are based on Hartree-Fock (HF) self-consistent field (SCF) calculations, in which the total multielectron wave function Ψ is composed from single electron spin orbital wave functions ϕl (MO) via a single anti-symmetrized determinant (the Slater determinant). The single electron wave functions are expressed by a sum of basis functions (basis sets). A very large choice of basis sets exists; they differ in size and in their adaptation for a specific calculated property [48, 49]. In addition to choosing the appropriate computational method, a correct choice of a basis set determines the computational accuracy and efficiency. However, even with the largest basis set and reaching the HF limit, the HF method recovers only 95–99% of the total energy. The missing fraction is relatively small in absolute terms, but most chemistry happens in this small remaining fraction. The missing fraction is corrected by including electron correlation energy.
The most frequently used methods for including electron correlation are by perturbation theory, i.e., Møller-Plesset [50] in the nth order, (MPn) [51], configuration interaction (CI) [52], and coupled-cluster (CC) [53] methods [1–5, 46]. Today, coupled-cluster methods provide the most accurate results among the practical ab initio electronic structure theories and often serve as a benchmark for evaluating, in the absence of experimental results, the reliability of new computational methods, e.g., density functionals. Ab initio methods can be improved systematically by increasing the size of the basis sets and the amount of correlation energy that is included. It is assumed that with a complete basis set and full CI the exact solution to the Schrödinger equation can be obtained. However, the major drawback of these methods is the large computer resources required, and they are therefore applicable only to moderately-sized molecules.
1.2.2 Density Functional Theory ( DFT) Methods
DFT methods [6–8], which include electron correlation indirectly, have become the most popular electronic structure methods in computational chemistry over the last 30 years. Recent advances in the development of density functionals (DF) allow one to reliably describe numerous properties of large systems at an affordable computational expense. A major difficuly in developping reliable DFs is that in contrast to the rigor of ab intio methods, DFT methods do not allow systematic improvement of the theoretical method. The strategy for improving DFs is by adding new ingredients and parametrizing them by fitting to a set of properties, e.g., atomization energy, thermochemistry, barrier heights, etc. For example, addition of a small percentage of exact HF exchange (i.e., hybrid DFs) improves significantly the performance of local DFs as shown in Figure 1.1. For example, the performance of the PBE (Perdew–Burke-Ernzerhof) functional is improved by a factor of two in PBE0, which mixes the PBE exchange energy with 25% exact Hartree–Fock exchange energy [8, 54]. Figure 1.2 shows the importance of including London dispersion corrections (D2 [55] and D3(BJ) [56]) that capture noncovalent interactions more effectively. Based on an assessment of 200 DFs tested on 84 data sets including thermodynamic data, isomerization energies, barrier heights, etc., it was concluded that addition of dispersion corrections should be applied with caution [8]. Recommendations for effective use of DFs that are suited for specific applications are given in Refs. [8] and [57], and the reader is advised to consult these references before applying a specific method to his own research or when evaluating the research of others. As can be seen in Figures 1.1 and 1.2, calculated errors can be very large, e.g., with BLYP, B3LYP, and BP86.
The common notations of the theoretical methods used in the literature are the following: each computational level (ab initio and DFT) is defined by the type of method and the basis sets which are used. By convention, the computational level is designated as follows: [method 1/basis set 1]//[method 2/basis set 2]. Method 2 and basis set 2 are those used for geometry optimizations while method 1 and basis set 1 are those used for obtaining the final energy or other properties that are calculated.
Figure 1.1 Stacked root-mean-square deviations (RMSD, in kcal/mol) for a set of six popular local/hybrid pairs (e.g., BLYP/B3LYP, BP86/B3P86, etc.) derived from 155 isomerization energies (ID), 206 barrier heights (BH), and 91 noncovalent (NCD) interactions. Reproduced with permission from Ref. [8]. Copyright (2017) Taylor and Francis, Ltd.
Figure 1.2 Stacked root-mean-square deviations (RMSD, in kcal/mol) for a set of four DFs and their dispersion corrected (D2 and D3(BJ)) counterparts. Based on 1744 non-covalent dimer interactions (NCED) and on 755 isomerization energies (IE). Reproduced with permission from Ref. [8]. Copyright (2017) Taylor and Francis, Ltd.
1.2.3 Methods for Analysis of Electronic Structure
Important information and deeper insight into the chemical and physical properties of the studied molecules can be gained by analyzing their wave function, the electron distribution, hybridization at various atoms, the nature of the chemical bond, etc. Several analysis methods are mentioned throughout this chapter. The most popular is the Natural Bond Orbital method (NBO) [58–63] that calculates the Natural Population Analysis charges (NPA). The NPA charges are believed to be more accurate [64, 65] than the Mulliken atomic charges [66, 67], which are still often used. Several other methods for calculating atomic charges are available and a comparison of their accuracy for specific purposes was discussed, e.g., in References [64] and [65]. NBO analysis, based on localized orbitals, provides a Lewis structure of the molecule, bond orders, hybridization at the various atoms, and suggests possible resonance structures that contribute to the molecular electronic structure and their relative weights (NRT)[68], thus providing important information for analyzing the structures and bonding of the studied molecules. Other frequently used computational procedures are: (a) Atoms in Molecules (AIM), developed by Bader [69], which is based on a topological analysis of the electron density in atomic basins, and calculates also atomic AIM charges; and (b) Energy Decomposition Analysis and Natural Orbitals for Chemical Valence (EDA-NOCV), which proved to be a powerful tool for bonding analysis. For a detailed description of the above-mentioned methods and their performance, the reader is referred to the recent review by Frenking, Schwerdtfeger, and coworkers [70].
1.2.4
How Important are Relativistic Effects for Ge?
As the nuclei become heavier the strong attraction of the electrons by the heavier nuclear charge causes the electrons to move faster and behave relativistically [71, 72], i.e., their relative mass increases and the effective Bohr average radius for
double bond in both 1 and 2 is 0°. Based on these geometries, the authors suggested that 1 and 2 result from a double donor-acceptor interaction of two singlet 1A1 ER2 monomers, in which the filled lone-pair orbital on one E(II) atom donates electrons into the vacant p orbital of the second E(II) monomer (Scheme 1.1, for E=Sn). However, 1 and 2 were stable only in the solid state, while in solution they dissociate to their corresponding monomers [87]. (tBu2MeS i)2Sn=Sn(SiMetBu2)2 synthesized in 2004 by Sekiguchi et al. [88] was the first, of only few isolated distannenes, that are stable also in solution [15, 89].
Lone-pair donation Sn
Scheme 1.1 Mode of interactions of two stannylenes producing a trans-bent distannene.
In 1981, in two milestone papers, Brook and West and their coworkers reported independently the syntheses, isolation, and characterization, including by X-ray crystallography, of the first stable silene, (Me3Si)2Si=C(OSiMe3)1-Ad (3) [90–92] and disilene, Mes2Si=SiMes2 (4), [93–96], respectively. Silene 3 has a planar skeleton with a Si=C bond length of 1.764 Å, (r(Si-C) = 1.869 Å in H3CSiH3 [97]). In disilene 4, r(Si=Si) = 2.143 Å (r(Si-Si) = 2.327 Å in H3SiSiH3 [97]), and the Si centers are slightly pyramidal with Σ∠(Si) = 358° and a trans-bent angle of 12°.
Since these pioneering landmark achievements, this field has witnessed an enormous progress. The key point to success is the use of large bulky substituents that protect the highly reactive E=E’ double bonds from further reactions. Stable compounds with a variety of E=E and E=E’ bonds (E, E’=Si, Ge, Sn, Pb; E’=group 13–16 elements), as well as compounds having an extended number of conjugated double bonds, i.e., heavier allenes and butadiene analogues, and heavier benzene analogues, were synthesized, isolated, and characterized and their chemistry has been reviewed extensively [16, 18, 24, 98–100].
Heavy alkyne analogues RE≡ER (E=Si, Ge, Sn) have also been isolated, but they are much less abundant than heavy doubly bonded compounds [17, 18, 24]. Unlike the linear alkynes, all known heavy alkynes have a highly trans-bent geometry [17, 18, 24]. The first digermyne ArGe≡GeAr (5) and distannyne ArSn≡SnAr (6) [Ar = 2,6-(2,6-iPr2-C6H3)2C6H3], were reported by Power and coworkers in 2002 [101]. Several digermynes and distannynes with other substituents on the aryl groups were reported by these authors in 2010 [102]. In 2006, Tokitoh et al. reported the synthesis of BbtGe≡GeBbt (7) (Bbt = 2,6-[CH(SiMe3)2]-4-[C(SiMe3)3]-C6H2) [103]. The synthesis and characterization by X-ray crystallography of the first stable disilyne RSi≡SiR, R=[SiiPr{CH(SiMe3)2}2] (8) was reported by Sekiguchi’s group in 2004 [104, 105]. Wiberg reported earlier the detection of a relatively stable disilyne, RSi≡SiR, R=SiMe(Sit-Bu3)2 (9), but its crystal structure could not be obtained [106]. A second known stable disilyne with R = Bbt (10) was reported in 2008 by Tokitoh’s group [107, 108]. In 1999, Schwarz and Apeloig reported the identification of two silynes HC≡SiX (X=F, Cl) in neutralization–reionization mass-spectrometric experiments, and demonstrated their microsecond existence under high-vacuum conditions [109]. More recently, Kato and Baceiredo reported a base stabilized silyne that was stable up to −30°C. Its X-ray structure revealed a short Si–C bond length of 1.667 Å, indicating the possible existence of a Si–C triple bond [110, 111]. Germa- [112] and stanna [113]-acetylenes were identified only as intermediates in a low-temperature matrix or by their trapping reactions [113]. To date, efforts to synthesize isolable E≡C (E=Si, Ge, Sn) triply bonded molecules were not successful.
It should be emphasized that all known stable multiply bonded heavier alkene and alkyne analogues are substituted with very bulky substituents that stabilize them kinetically, preventing their dimerization and significantly slowing their other reactions. Theoretical studies in the last decade pointed to the importance of attractive noncovalent London Dispersion Forces (LDF) [114] between the bulky substituents, in stabilizing these weakly bonded multiply bonded molecules against dissociation to their tetrylene monomers [115–118].
In Table 1.2 we provide a list of characterized doubly bonded germanium compounds and some of their important geometric parameters (both homo- and heteronuclear) that were synthesized since 2010 and not covered in the reviews of Power [18] and Lee [24]. Table 1.2 also includes, where available, the results of theoretical calculations of these compounds’ structures.
Table 1.2 Doubly bonded organogermanium compounds, R 2 Ge=E’R’ 2 , reported in 2010–2020. Compound
[119,120]
[124] 2018
[124] 2018
[124] 2018
[125] 2016
(Ge 2 )
R 2 Ge=GeRSiMe 2 Ph R = Tip = 2,4,6i Pr 3 C 6 H 2 –
R 2 Ge=GeRSiMe 3 R = Tip = 2,4,6i Pr 3 C 6 H 2 –
R 2 Ge 2 =Ge 1 RLi 2dme R = Tip = 2,4,6i Pr 3 C 6 H 2 , dme = 1,2-dimethoxyethane 2.284
K 2 2+ [(boryl)Ge=Ge(boryl)] 2–Boryl = (HCDippN) 2 B, Dipp = 2,6i Pr 2 C 6 H 3 h 2.392
Compound Ge=E’ (Å) Σ ∠ (Ge); Σ ∠ (E’) (°)
476 (Ge=Ge) and 386 (C=O) i [123] 2019
[127] 2019
R 2 Ge 2 =Ge 1 R(C(R’) = O) R = Tip = 2,4,6i Pr 3 C 6 H 2; R’ = (a) t Bu, (b) 2-methylbutan-2-yl and (c) 1-adamantyl –
21
22 ( E )-(L)HGe=GeH(L) L = N(Si i Pr 3 )R R = 2,6-[CHPh 2 ]-4i PrC 6 H 2 2.486
460 [126] 2013 23 ( E )-(L)HGe=GeH(L) N B N Dip Dip N SiMe 3 Dip=Diisopropylphenyl L= toluene/18-crown-6
[128] 2018 25 ArBrGe=GeBrAr a) Ar = Bbt = 2,6-[CH(SiMe 3 ) 2 ] 2 -4[C(SiMe 3 ) 3 ]-C 6 H 2 b) Ar = Tbb = 4t Bu-2,6-[CH(SiMe 3 ) 2 ] 2 -C 6 H 2
494 [131] 2015 (Continued)
24 ( E )-(Eind)XGe=GeX(Eind) X= (a) Br and (b) Cl j Et Et Et Et Et Et Et Et Eind
Ge Ge B b t B b t Bbt = 2,6-[CH(SiMe 3 ) 2 ]-4-[C(SiMe 3 ) 3 ]C 6 H 2 r(Ge=Ge) = 2.413 r(C-C) = 1.532 ∠ C(Bbt)-Ge-Ge- C(Bbt) = 133.6 39.5 b
Table 1.2 (Continued)
413 [119] 2010
[141] 2021
Compound Ge=E’ (Å)
ii) Ge=Si bonds 36 ( t BuMe 2 Si) 2 Ge=Si(SiMe 2 t Bu) 2 2.221 0.6 b
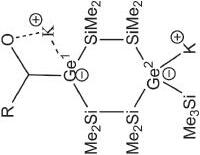
C Si Ge Si K 18-crown-6 Tip Tip Tip N Xyl • r(Ge=Si) = 2.246 r(Ge-K) = 3.407
iii) Ge=E’ (E’=group 13, 15 and 16 elements)
[143] 2016
[142] 2020 39 N Ge 1 Ge 2 N Di pp Di pp P 1 P 2 N N Di pp Dipp Dipp=2,6i Pr C 6 H 3 r(Ge 1 -P 1 ) = 2.266, r(Ge 1 -P 2 ) = 2.272 –
38 Ge B Ph 2 P X X= Cl, Br Ar * =2,6-Tip 2 -C 6 H 3 (Tip=2,4,6-iPr 3 C 6 H 2 ) 1.886 X=Cl: 359.6 (Ge), 360.0 (B)
q , 450 r [144] 2018 41 Eind 2 Ge=O
297 (IR (Ge=O) 916 cm −1 ) [145,146] 2012
40 ( t Bu 2 MeSi) 2 Ge=As-Mes* Mes* = 2,4,6t Bu 3 -C 6 H 2 2.273