NEWGENERATION GREENSOLVENTSFOR SEPARATIONAND PRECONCENTRATION
OFORGANICAND INORGANICSPECIES
Editedby
MustafaSoylak
DepartmentofAnalyticalChemistry,FacultyofPharmacy,NearEastUniversity, Nicosia,TRNC,Mersin,Turkey
ErkanYilmaz
DepartmentofAnalyticalChemistry,FacultyofPharmacy, ErciyesUniversity,Kayseri,Turkey
ERNAM—ErciyesUniversityNanotechnologyApplicationand ResearchCenter,Kayseri,Turkey
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhow toseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuchas theCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybenoted herein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding, changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation, methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirown safetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjury and/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationofany methods,products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-818569-8
ForInformationonallElsevierpublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: KathrynEryilmaz
EditorialProjectManager: BillieJeanFernandez
ProductionProjectManager: SojanP.Pazhayattil
CoverDesigner: MarkRogers
TypesetbyMPSLimited,Chennai,India
Contents
Listofcontributorsix
1.Historicalbackgrounds,milestonesinthe fieldofdevelopmentofseparationand preconcentrationmethods
ERKANYILMAZANDMUSTAFASOYLAK
Abbreviations1
1.1Introduction2
1.2Historicaldevelopmentofseparationand preconcentrationmethods3
1.3Conclusions36 References36
2.Historicalbackground:milestonesinthe fieldofdevelopmentofanalytical instrumentation
NASRULLAHSHAH,MUHAMMADBALALARAINAND MUSTAFASOYLAK
2.1Introduction45
2.2Developmentinthefieldofchromatography46
2.3Developmentinthefieldofspectroscopy49
2.4Developmentofelectroanalyticaltechniques62
2.5Hyphenatedtechniques67
2.6Advancementinsamplingsystemsforanalytical instruments70
2.7Conclusion71 References71
3.Typeofnewgenerationseparationand preconcentrationmethods
ERKANYILMAZANDMUSTAFASOYLAK
3.1Introduction75
3.2Liquidphasemicroextraction76
3.3New-generationsolidphaseextraction methods117 References138
4.Newmethodologiesandequipmentused innew-generationseparationand preconcentrationmethods
MOHAMMADHOSSEINAHMADIAZQHANDI, TAHEREKHEZELI,MEHRORANGGHAEDIAND ALIDANESHFAR
4.1Thehistoricaldevelopmentandoverviewofthese preconcentrationandseparation methodologies149
4.2Hyphenatedandnonhyphenatedchromatographic techniquesforextractionand/orseparationof targetcompounds150
4.3Ultrasound-assistedemulsification microextraction151
4.4Cloudpointextraction153
4.5High-diffusionliquids155
4.6Pressurizedliquidextraction156
4.7Microwave-assistedextraction157
4.8Vacuummicrowave-assistedextraction159
4.9Supercriticalfluidextraction159
4.10Dispersiveliquid liquidmicroextraction160
4.11Solidifiedfloatingorganicdrop microextraction164
4.12Moderntechniquesofisolationand/or preconcentration170
4.13Theapplicationofcarbonnanotubesand nanoparticlesinseparation195
4.14Conclusion198
References198
5.Typeofgreensolventsusedinseparation andpreconcentrationmethods
ERKANYILMAZANDMUSTAFASOYLAK
Abbreviations207
5.1Introduction208
5.2Greenanalyticalchemistry209
5.3Switchablehydrophilicitysolvents251
References258
6.Ionicliquidsinseparationand preconcentrationoforganicandinorganic species
TAHEREKHEZELI,MEHRORANGGHAEDI,
ALIDANESHFAR,SONIABAHRANI,ARASHASFARAMAND MUSTAFASOYLAK
6.1Introduction267
6.2Physicalpropertiesofionicliquids270
6.3Applicationofionicliquidsinextractionof organicandinorganiccompounds274
6.4Magneticionicliquidsandtheoryapplication300
6.5Conclusion304
References307
7.Supramolecularsolventsinseparation andpreconcentrationoforganicand inorganicspecies
MUHAMMADBALALARAINANDMUSTAFASOYLAK
Abbreviations319
7.1Introduction319
7.2Background322
7.3Solventextractionsystem325
7.4Preparationofsupramolecularsolvents327
7.5FormationmechanismofSUPRASphase329
7.6Componentsofsupramolecularsolvents331
7.7Supramolecularsolventsinseparationand preconcentrationmethods331
7.8Efficiency337
7.9Thermodynamics337
7.10Environment338
7.11TypesofSUPRASmolecularextraction338
7.12Supramolecularsolventsinliquid liquid microextraction338
7.13Integrateduseofsupramolecularsolventswith nanomaterials339
7.14Ultrasonic-assistedsupramolecularsolvent extraction339
7.15Microwave-assistedsupramolecularsolvent extraction340
7.16Vortexassistedsupramolecularsolvent extraction340
7.17Temperature-assistedsupramolecularsolvent extraction341
7.18Trends342
7.19Conclusion342
Acknowledgment343 References343
8.Switchablesolventsinseparationand preconcentrationoforganicandinorganic species
Abbreviations347
8.1Introduction347
8.2Switchable-hydrophilicitysolvents348
8.3Synthesisandchemistryofswitchable-hydrophilicity solvents349
8.4Applicationsofswitchable-hydrophilicity solvents353
8.5Switchable-hydrophilicitysolventsinlarge-scale extractions354
8.6Switchable-hydrophilicitysolventsin microextractions356
8.7Futureaspects377 References377
9.Deepeutecticsolventinseparationand preconcentrationoforganicandinorganic species
TAHEREKHEZELI,MEHRORANGGHAEDI,SONIA BAHRANI,ALIDANESHFARANDMUSTAFASOYLAK
Abbreviations381
9.1Introduction381
9.2Deepeutecticsolvent(definitionand preparation)383
9.3Physicochemicalpropertiesofdeepeutectic solvents385
9.4Applicationofdeepeutecticsolventsinextraction techniques402
9.5Conclusion416 References417
USAMAALSHANA,ERKANYILMAZANDMUSTAFA SOYLAK
10.Supercriticalfluidextractionin separationandpreconcentrationoforganic andinorganicspecies
TAHEREKHEZELI,MEHRORANGGHAEDI,SONIABAHRANI ANDALIDANESHFAR
Abbreviations425
10.1Introduction425
10.2Propertiesofsupercriticalfluid426
10.3Instrumentation427
10.4Mechanismandkineticofsupercriticalfluid429
10.5Applicationsofsupercriticalfluid430
10.6Conclusion440
References447
Index453
Listofcontributors
UsamaAlshana DepartmentofAnalytical Chemistry,FacultyofPharmacy,NearEast University,Nicosia,TRNC,Mersin,Turkey
MuhammadBalalArain Departmentof Chemistry,UniversityofKarachi,Karachi, Pakistan
ArashAsfaram MedicinalPlantsResearchCenter, YasujUniversityofMedicalSciences,Yasuj,Iran
MohammadHosseinAhmadiAzqhandi Applied ChemistryDepartment,FacultyofGasand Petroleum(Gachsaran),YasoujUniversity, Gachsaran,Iran
SoniaBahrani DepartmentofChemistry,Yasouj University,Yasouj,Iran
AliDaneshfar DepartmentofChemistry,Faculty ofSciences,IlamUniversity,Ilam,Iran
MehrorangGhaedi DepartmentofChemistry, YasoujUniversity,Yasouj,Iran
TahereKhezeli DepartmentofChemistry,Faculty ofSciences,IlamUniversity,Ilam,Iran
NasrullahShah DepartmentofChemistry,Abdul WaliKhanUniversityMardan,Mardan,Pakistan
MustafaSoylak DepartmentofChemistry,Faculty ofSciences,ErciyesUniversity,Kayseri,Turkey; TechnologyResearch&ApplicationCenter (TAUM),ErciyesUniversity,Kayseri,Turkey
ErkanYilmaz DepartmentofAnalytical Chemistry,FacultyofPharmacy,Erciyes University,Kayseri,Turkey;ERNAM—Erciyes UniversityNanotechnologyApplicationand ResearchCenter,Kayseri,Turkey;Technology Research&ApplicationCenter(TAUM),Erciyes University,Kayseri,Turkey
Historicalbackgrounds,milestones inthefieldofdevelopmentofseparation andpreconcentrationmethods
ErkanYilmaz1,2 andMustafaSoylak3
1DepartmentofAnalyticalChemistry,FacultyofPharmacy,ErciyesUniversity,Kayseri,Turkey
2ERNAM—ErciyesUniversityNanotechnologyApplicationandResearchCenter,Kayseri,Turkey
3DepartmentofChemistry,FacultyofSciences,ErciyesUniversity,Kayseri,Turkey
Abbreviations
AµE adsorptivemicroextraction
APDC ammonium pyrrolidinedithiocarbamate
ASE acceleratedsolventextraction
C60 fullerenes
CE capillaryelectrophoresis
CF-SD-LPME continuous-flowmicroextraction/ single-dropmicroextraction
CNTs carbonnanotubes
CP coprecipitation
CPE cloud-pointextraction
CPT cloud-pointtemperature
DDTC diethyldithiocarbamate
DESs deepeutecticsolvents
DI-SDME directimmersionsingle-drop microextraction
EPA environmentalprotectionagency
ETAAS electrothermalatomicabsorption spectrometry
FAAS flameatomicabsorptionspectrometer
FIA flowinjectionanalysis
FIP internationalpharmaceutical federation
G graphene
GC gaschromatography
GCB graphitizedcarbonblack
GC FID gaschromatography flame ionizationdetection
GC MS/MS gaschromatography tandemmass spectrometer
GFAAS graphitefurnaceatomicabsorption spectrometer
GO grapheneoxide
HGAAS hydridegenerationatomicabsorption spectrometry
HPLC high-performanceliquid chromatography
HR-CS-ETAAS highresolutioncontinuum,source electrothermalatomicabsorption spectrometer
HS-SDME headspace single-drop microextraction
ICP-AES inductivelycoupledplasmaatomic emissionspectrometry
ICP-OES inductivelycoupledplasma optical emissionspectrometer
ILs ionicliquids
ISO internationalorganizationfor standardization
LC-UV liquidchromatography ultraviolet spectrophotometer
LLE liquid liquidextraction
LLLME liquid liquid liquidmicroextraction
LPME liquidphasemicroextraction
LSA liquid solidadsorption
LSE liquid solidextraction
MAE microwave-assistedextraction
MALDI-MS matrix-assistedlaserdesorption/ ionizationmassspectrometer
MIPs molecularlyimprintedpolymers
MISPE molecularlyimprintedsolidphase extraction
MOFs metal organicframeworks
PAHs polycyclicaromatichydrocarbons
PGC porousgraphiticcarbon
PLE pressurizedliquidextraction
PS-DVB polystyrene-divinylbenzene
PTFE polytetrafluoroethylene
RAM restricted-accessmaterials
RDSE rotating-discsorbentextraction
SBSE stir-barsorptiveextraction
SCSE stir-cakesorptiveextraction
SDME single-dropmicroextraction
SFE supercriticalfluidextraction
SFODME solidifiedfloatingorganicdrop microextraction
SIA sequentialflowinjectionanalysis, solidphasemicroextraction
SPE solidphaseextraction
SPME solidphasemicroextraction
SRSE stir-rodsorptiveextraction
SSs switchablesolvents
SUPRAs supramolecularsolvents
TAN 1-2-thiazolylazo-2-naphthol
WCED WorldEnvironmentand DevelopmentCommission
TSP-MS-MS thermospraytandemmass spectrometer
UAE ultrasoundextraction
UNWFP UnitedNationsWorldFood Programme
WHO WorldHealthOrganization
WPC WorldPharmacyCouncil
1.1Introduction
Inparallelwiththedevelopmentoftechnology,therehasbeenasignificantincreaseinthe amountofharmfultraceorganic,inorganic, andbiologicalspeciesinlivingareas,living organisms,andecologicalenvironment [1,2]
Thescientificcommunityandthepublichave becomeawareofandconcernedaboutthe presenceoforganictracespeciessuchasactive ingredients,pesticides,azofoodadditives,and inorganictracespeciessuchasheavymetal ions,metalcompounds,anions,andnanoparticlesintheirworkingandlivingenvironments andaboutthehealthimpactofthesesubstances [3,4].
Thereisanincreasingrequirementtoestimatethepotentialhealthriskofthesetrace organicandinorganicspeciesundertheconditionsinwhichtheyareused.Thesespeciesare foundinbiological,food,environmentalsamples,andpharmaceuticalproductsasnaturally orsyntheticatdifferentconcentrations,and theyhavebeenmadeforusefulpurposes;however,theirprolongedusehasbecomeharmful. Evenifsomeofthesespeciesareusefulforlivingcellstoacertainextent,theyhaveadverse effectsonlivingcellsinprolongedexposures [5 7].
Whenhumanscomeintocontactwithor consumetheseorganicandinorganicspecies inliquid,solid,andgaseousformsatdifferent levels,thesespeciescanirritateanddamage theskin,eyes,andrespiratorytract;candamageinternalorganssuchasthenervoussystem,liver,andkidneys;andespeciallycan causespecificdiseasessuchascancer [4 8]. Worldwidefoundations,organizations,administrations,andenvironmentalprotectionagenciessuchastheWorldHealthOrganization (WHO),UnitedNationsWorldFood Programme(UNWFP),WorldPharmacy Council(WPC),InternationalPharmaceutical Federation(FIP),EnvironmentalProtection Agency(EPA)havesetuporganizationsfor thelong-termmonitoringandidentificationof organicandinorganicspeciesinvariousbiological,food,environmentalsamplesandpharmaceuticalproducts,includingtheeffectsat differentlevelsonlivingcellsandtheecology. Moreover,maximumpermissiblelevelsofthe organicandinorganicspeciesinrealsamples
arerecommendedbytheseorganizations. Hencetheanalysisoftraceamountsoforganic andinorganicspeciesinbiological,food,and environmentalsamplesandinpharmaceutical productshaveanimportantplaceinallenvironmentscontaininglivingcells(Fig.1.1).
1.2Historicaldevelopmentofseparation andpreconcentrationmethods
Intheendof19thcentury,WilhelmOstwald describedanalyticalchemistryastheartofseparating,recognizingdifferentsubstances,and determiningtheconstituentsofasample [9]. Takingthehumanhealthandenvironment safetyintoconsideration,thefast,accurate, andsensitivedeterminationoftheseorganic andinorganicspecies,especiallyattracelevels inbiological,food,andenvironmentalsamples andpharmaceuticalproducts,isalwaysasubjectofgreatinterest,especiallyinthefieldof analyticalchemistry.
FIGURE1.1 Subjectareasthatrequiretraceor ultratracedetectionoftheanalytes.
Intheageofclassicalanalysis,whilegravimetricandtitrimetricmethodswereusedfor thedeterminationofthemajorandminorcomponentsofmaterialssuchasrockandores,the proximityofthesumofcomponentsapproaching100%wasseenasameasureofthequality oftheanalysis.Althoughitisknownthattrace elementsarepresent,however,theyarenot consideredtohaveasignificantcontributionto clusteringinverysmallamounts.Anearly authoritywasHillebrand [10],whopublished hisclassicbook AnalysisofSilicateandCarbonate Rocks in1919andusedtheword“trace”to identifycomponentsbelow0.01%or0.02% belowthequantitativedetectionlimit.In1944 Sandellpublished ColorimetricDeterminationof TracesofMetals.Sandellclassifiedthemajor, minor,andtraceconstituentsasfollows:Major constituentsofthefractioncorrespondtohigherthan1%ofthetotalsampleamount;minor constituentsarethosepresentinamounts between0.01%and1%;andtraceconstituents arethosebelow0.01% [11].Inabook Trace
Analysis,publishedin1965,reportedthemoderndescriptionof“trace”asmoreflexible [12]. Thisbookcontainsbeautifulandimportant commentssuchas“Theconnotationofthe term‘trace’varieswiththebackgroundor interestsofthereader”and“anysharpdivisionis,ofcourse,superfluous,andwilldepend onthenatureofthesampletobeanalyzed, theanalyticaltechniqueemployed,andthe analyst.”Inthatbook,theupperlimitoftrace levelwasconsideredtobeabout100ppmby weight,andtheterm“ultratrace”wasconsideredforconstituentsbelow1ppm.
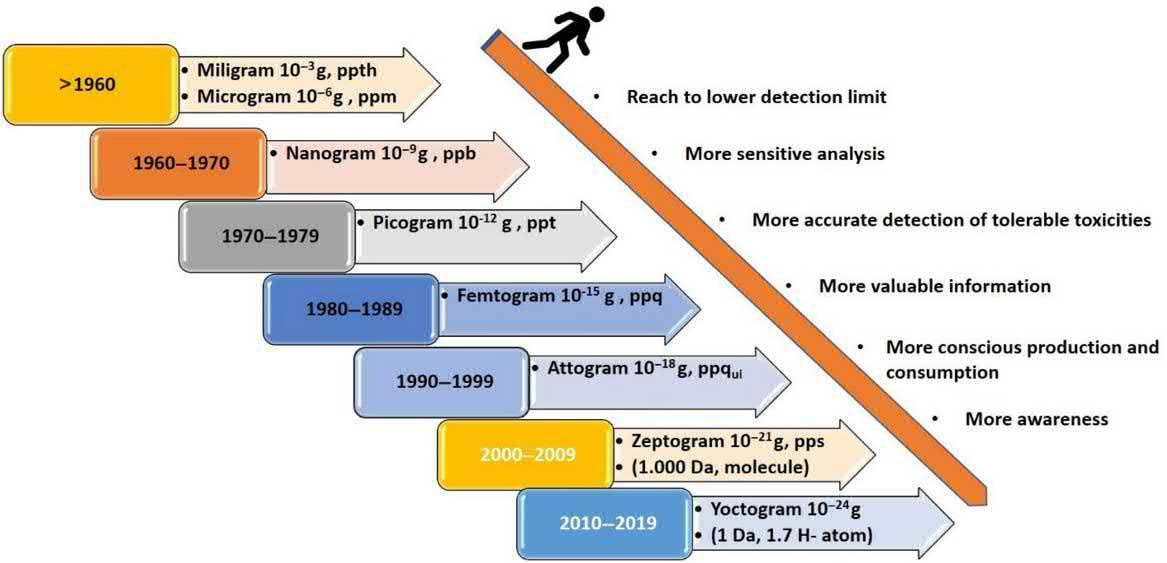
Thedevelopmentofnovelanalysismethods byanalyticalchemistsallowsforthedeterminationoflowerconcentrationsofanalyteor analytesindifferentsamplematrices.Abrief historyofanalyticalchemistryintermsofmilestonesindetectionlimitsisshownin Fig.1.2
Fortraceanalysistoemergeasanareaof expertiseinitself,twoconditionshadtobe met:specialneedsandapplicablemethods.In general,qualitativemethodsappearedmuch earlierthanquantitativeones.Therewerea
fewqualitativetestsandevensomequantitativesensitivitymethodsbeforethebeginning ofthecentury,buttheydidnotcausemuch curiosityuntilneeded.
The1940screatedanewneedforextraordinarilysensitiveanddifficultanalyticalprocedures.WorldWarIIhadaverystimulating influenceonnewneeds,butitalsosuppressed freepublicationforseveralyears.Thisresulted inthereleaseforpublicationofalargeamount ofpreviouslyclassifiedinformationshortly aftertheendofthewarin1945.Theinstrumentationandproceduresbuiltuptosolve specificwar-relatedquestionsbecameavailable forwiderapplications [12].
Analysisoftracelevelsoforganicandinorganicspeciesinbiological,food,andenvironmentalsamplesandinpharmaceutical matricesisadifficultprocess.Generally,a completeprocessofanalysisoforganicand inorganicspeciesinasampletakesfivesteps: (1)collectionofthesample,(2)conservationof thesample,(3)preconcentrationandseparationofanalyte/analytesfromsamplematrix,
FIGURE1.2 Briefhistoryofanalyticalchemistryintermsofmilestonesofdetectionlimits.
(4)instrumentalanalysis,and(5)dataprocessing.Asurveycarriedoutin1991declared thatsamplepreparationcanaccountfor aroundtwo-thirds(61%)oftheeffortofthe typicalanalyticalchemist,and92%ofthe respondentsconsideredsamplepreparationas veryimportantormoderatelyimportant [13].
Nodoubtitshouldbestatedthat,inthe last50years,analyticalchemistshavebeen abletopresentmanydevelopmentsintheir work,aswellascost-andtime-savingmethodology.Andhencetheclassicgravimetric andtitrimetricanalysismethods,whichallow theanalysisofasingleoratmosttwoanalytes,arenowinmostcasesreplacedby instrumentaldetectiontechniquesthatcan providetwo-andmultid imensionalinformationatquantitiesofafewnanograms,pictograms,orevenfemtograms [14]
Thoughmostmoderninstrumentaldetectionsystemsprovideexcellentdetectionlimits, combinedmethods,consistingofseveralstages suchassampling,decomposition,preconcentration,separation,anddetermination,bring importantimprovementsinrelativedetection limits,aswellasintheaccuracyandprecision oftheanalyticalresults [15].Theotherimportantadvantagesofsuchcombinationsareillustratedin Table1.1.
Thoughtheimportanceofsamplepreparationisoftenoverlooked,itmustberecognized thatthemostimportantandcriticalstagein thisprocessistheseparationandpreconcentrationstepduetothecomplexityofsample matricesandlowerlevelsofthesespeciesat tracelevelsandevenlowerthanthedetection limitsoftheavailablemeasurementsystem (fromngL 1 tomgL 1 orfromngkg 1 to mgkg 1).Inthisway,analyteconcentrationis increasedabovethedetectionlimitofthemeasurementsystem,interferenceeffectsofthe matrixspeciesisdecreasedoreliminated,and theanalyteinsamplematrixisconvertedinto amoresuitableformfordetection,whichcan
promotethelossofanalytesorcontamination ofsample.
Awiderangeofseparationandpreconcentrationtechniqueshavebeendevelopedover thelast100years.Whiledevelopmentwas slowuntilthe1990s,thedevelopmentsinthis fieldhavegainedmomentuminthelasttwo decadesbecausescientistscontinuetoexplore accurate,sensitive,simple,cheap,andwidely availablesamplepreparationprocedureswith reasonabledetectionlimits.
Solidphaseextraction(SPE),liquid liquid extraction(LLE),cloud-pointextraction(CPE) andcoprecipitation(CP)applicationswerethe conventionalandmoreusedsampletreatment techniquesuntil2000s.However,theproductionofthenewextractionmediums,suchas nanosizedsorbentsandnew-generationsolvents,andtheadventofnewextractionequipment,suchasminisizedextractioncolumns, minisizedextractiontubes,microinjectionsystems,ultrasonicandmicrowavevibrationshakers,vortexmixerequipment,andsoon,have ledtoanimportanttransitionfrommacroscale separationandpreconcentrationmethodsto microscaleones.Microscaleseparationand preconcentrationmethodsmaybecomemore simpleandrapidwithoutcausingdeterioration intheanalyticalaccuracyandprecision.The microscalenew-generationseparationandpreconcentrationmethodshavecometobeknown assolidphasemicroextraction(SPME)andliquidphasemicroextractionmethods(LPME)for 30years.
Whentheliteraturestudiesareexamined,it isseenthatwhileseparationandpreconcentrationprocedures,especiallycoprecipitation, liquid liquidextractionandsolidphase extractionfortheextractionofthemajorcomponentsinrealsamples,havebeenusedover thelast100years,theyhavebeenfrequently usedforextractionandpreconcentrationof traceorganic,inorganic,andbioactiveanalytes for70years [16 84].
1.Historicalbackgrounds,milestonesinthefieldofdevelopmentofseparationandpreconcentrationmethods
TABLE1.1 Comparisonofthedirectinstrumentalanalysisandcombinedmethodswithinstrumentaltechniquesfor traceorganicandinorganicanalyteanalysis.
ItemsDirectanalysiswithinstrumentaltechniquesCombinedmethodswithinstrumentaltechniques
InterferencesStrongmatrixeffectsinterferencesmayoccurMatrixinterferencescanbeeliminatedbyseparating traceanalytesfromthesamplematrix
DetectabilityDetectionlimitsdependonthesensitivityofthe detectorandthesignalgenerationareaofthe instrumentaltechnique
Traceanalytesinthesamplearecompletely concentratedintoasmallvolume,whichleadtoa strongincreaseinthedetectionlimit
CalibrationReferencematerialsareusedCalibrationcanbedonereadilybyapplyingthe standardsolutions
Systematic errors
Lossofanalytes,contamination,andblankproblems areminimallevel
Contamination,volatilizationhazardsandblank problemsaremorecommon
However,thesedrawbackscanbeeliminatedby carefuloperations
Economical aspects
Simultaneousanalysisofanalytesaresimpleand rapid
Thecoprecipitationmethodisoneofthe firstseparationandpreconcentrationapplicationsfortraceanalytes,followedby liquid liquidextractionandsolidphase extractionmethods.
1.2.1Historicaldevelopmentof coprecipitationmethods
Inthecoprecipitationmethod,aprecipitatingagentisusedforthereliableisolationof theanalyteprecipitatesinoptimumconditions. Theprecipitatesareisolatedfromthesample solutionwithcentrifugationorfiltrationwith membranefilters.Isolatedanalyteprecipitates aredissolvedinasuitablesolventsuchasdifferentconcentrationsofHNO3,H2SO4,HCl, andthelike.Thenanalyteconcentrationinthe lastphaseisanalyzedbymeansofa suitabledetectiontechnique.
Themechanismofthecoprecipitation methodwasexplainedforfirsttimebyShapiro andKolthoffin1896 [16].Theyexplainedthe
Expertperson,capability,time,andmoreapparatus aregenerallyrequired,butthemethodscanalsobe carriedoutrapidlyandsimplybyusingwell designedtechniquesandapparatus
precipitationofsilverbromidewiththecoprecipitationmechanism.Afterthisreport,many studieswerecarriedouttoexplainthecoprecipitationmechanismsfordifferentspecies.For example,in1924McCandlessandBurtonproposedthecoprecipitationmechanismforthe determinationofphosphoricacidbythe molybdate-magnesiamethod [17].Inadifferentstudy,in1934abenzoatemethodwas developedbyKolthoffandcoworkerstoseparatetheiron,aluminum,andchromiumfrom otherionsofthethirdgroupandalkalineearth ions [18].Thisstudymaybethefirstworkto reportontheseparationandanalysisofthe metalswiththecoprecipitationmethod. Currently,thesearchfornewprecipitating agentsforthequantitativeprecipitationand determinationofmetalionsisongoing.Mover andRemingtonprovidedmagnesiumand8hydroxyquinolineprecipitationasacoprecipitationagentforzincin1938.Moreover,they explainedtheimportanceofthepHofthesamplesolutionphaseoncoprecipitationefficiency andreportedthattheseparationofferriciron
andaluminumispossiblewithininsameconditionswithzincions [19] .In1939areport wasdeclaredbyKolthoffandOverholseron thecoprecipitationofdivalentzinc,nickel, cobalt,magnesium,calcium,andmanganese ions.Theyusedferrichydroxideasthecoprecipitationagentandcheckedtheinfluenceof theconcentrationofammoniaandammoniumchlorideonthecoprecipitationefficiencyofthemetalions.Theyfoundthat coprecipitationefficienciesofcobalt,nickel, andzincionsdecreasewithincreasingconcentrationsofammoniaandammoniumchloride,whilethoseofcalcium,manganese,and magnesiumdecreasewithincreasingconcentrationsofammoniumchloridebutincrease withincreasingconcentrationsofammonia [20] .In1941LouisWaldbauerandcoworkers usedbariumsulfateasacoprecipitationagent forthecoprecipitationofnickel,chromium, cobalt,iron,andmanganeseionsandused leadsulfateasthecoprecipitationagentfor thecoprecipitationofcopperandzincions. Theyfoundthatchromium,manganese,and ironcouldbecoprecipitatedwithbariumsulfate,whilenickelandcobaltdidnotcoprecipitatewithbariumsulfate.Zincandcopper werecoprecipitatedwithleadsulfate [21] . Witteprovidedacoprecipitationmethodfor thedeterminationoftungstenasBaWO 4 in 1943 [22] . Thefirstknownstudyfortheseparation andanalysisofsmallamountsofarsenic,antimony,andtininleadandleadalloysbycoprecipitationmethodwasperformedbyLukein 1943.Inthisstudy,Lukeusedmanganesedioxideasthecoprecipitationagent [23].Theturningpointinthisareatookplacein1948with thefirststudyontheanalysisofthetracelevel ofmetalionsreportedbyKolthoffandCarr. Theyusedmagnesiumammoniumphosphate asthecoprecipitationagentfortheaccumulationoftracesofarsenate.Inthismethod, 150 μgL 1 ofarseniccanbeanalyzedwithan accuracyof2%.Theyusedanexcessoftartrate
topreventtheprecipitationofothermetalions intheammoniamedium.Theysuggestedthat thedevelopedcoprecipitationmethodcanbe successfullyusedforthedeterminationofarsenicinsteelsamplesthatcontainsmorethan 0.01%ofarsenic [24].
Almostatthesametime,investigationvariousorganicandinorganicprecipitatingagents forcoprecipitationmethodshasbeenstepped up.In1950WestandConradreportedinan extensiveworktitled“AComparisonStudyof theCoprecipitationofCationsbyOrganicand InorganicPrecipitants.”Theycarriedoutthe experimentsonthetypicalclassesofinorganic organicprecipitationreactionsinrelation totheirtendencytocoprecipitateforeign cations.Theyfoundthatorganiccoprecipitationagentstendtocoprecipitatecationstoa greaterextentthaninorganicones.Thepossiblemechanismsinthissortofprecipitation includetheformationofcoagulatedcolloidal particlesundertheactionoftheelectrolytes presentatthetimeofthereaction.Trivalent cationshaveverysuitableflocculationvalues, andasaresulttheprecipitationwillbeatthe highestlevel [25].
Inthe1950s,itwaspossibletomeasurethe analytesinlowerconcentrationsinparallel withthedevelopmentsininstrumentalmeasurementtechniques,andthestudiesinthis fieldgainedspeed.In1951GarrisonandGile developedamethodfortheradiochemical analysisofAt211 infat,bone,skin,andmuscle ofratsusingacoprecipitationmethod. Accordingtoourbestknowledge,thedevelopedmethodmaybethefirstreportonthe analysisinbiologicalsamples.Theyusedthe metallictelluriumascoprecipitationagentand analyzedAt211 withanalpha-particle-X-ray countingmethod [26].Fr223 hasahalf-lifeof 21min;henceitisimperativetouseaveryfast methodforanalysisofFr223.Basedonthis requirement,Hyde,whowasaresearcherat theUniversityofCalifornia,usedsilicotungstic acidascoprecipitationagentforthe
precipitationandisolationoffrancium-87from bombardedthoriumtargets [27].
Theimportantadvancementsonthecoprecipitationmethodhasbeenlivedafter1950s withtheapplicationofnewanalyticalinstrumentsandcombinedseparationmethods,such asionexchange,adsorption,andliquid liquid extractionfortraceanalysis.
Someofimportantcoprecipitationapplicationstodatearedescribedhere.
In1963GirardiandPietrafoundthatthe separationoftracespeciesfromthematrix environmentpriortoisotopeanalysiswasa veryimportantstage.Theyusedionexchange, isotopicexchange,andcoprecipitationmethodsfortheseparationof13traceimpuritiesin aluminumpriortoneutron-activationanalysis. Thecarrierfreecoprecipitationstepprovided thesimplerandfasterfinalseparationofindividualrareearths [28].Thoughthephotometric determinationoftinwiththephenylfluoron methodprovidesanimprovedsensitivity, matrixionsshowadverseeffectsonaccurate determination.ShimizuandOgatadevelopeda coprecipitationmethodbasedontheformation offerrichydroxideatpH6B7,whichledto thecollectionoftininthesamplesolution. Afterthisstep,theprecipitatewasdissolved andadsorbedonananion-exchangecolumn. Zirconium,selenium,germanium,titanium, andantimonywereremovedbyelutionwith 0.5Nhydrochloricacid.Mercuryandbismuth wereremovedinahydrochloricacid sulfuric acidsolutionbyusingisobutylketoneasaliquidphaseextractionmedium.Theobtained resultsshowedthataslittleas1 μgkg 1 oftin incommonsalt,seawater,andbitterncouldbe analyzedwithsatisfactoryresults [19]
In1966Abdel-Rassoulandcoworkerspreferredacombineduseofseparationmethods consistingofadsorptiononsilicagel, liquid liquidextraction,andcoprecipitation forthesimultaneousdeterminationofiron, chromiumzinc,andcobaltinmetallicuranium,uraniumoxidessamples,anduranium
concentratespriortoneutron-activationanalysis.Theyusedaluminumchlorideasacoprecipitationagent.Inlaststep,theyusedionexchangechromatographyforthefractional separationoftheelements.Themethoddevelopedallowedthedeterminationofiron,chromiumzinc,andcobaltatpartspermillion levelswithareproducibilityof10% 15% [30].
In1967acombinedmethoddesignedas coprecipitationwithalkalineearthsalts,followedbysolventextractionoftransitionmetal dithiocarbamates,wasdevelopedbyJoyner andothersfortheseparationandpreconcentrationofnickel,manganese,iron,cobalt,zinc, lead,andcopperattracelevelsinseawater samples.Theywereabletorecoverymorethan 90%fortheseelementspriortoatomicabsorptionorflameemissionanalysis [31]
Fujinagaetal.developedapreconcentration methodbasedonorganiccoprecipitationfor tracelevelsofzinc,copper,vanadium,molybdenum,aluminum,anduraniuminnatural watersamplespriortoneutron-activationanalysis.Thesolubleionswereconvertedtooxine chelatesatpH5.2,followedbyextractionwith o-phenylphenolabove56 Candsolidification atroomtemperature.Thesolidparticleswere accumulatedonafilter,dried,andwrapped upinapolyethylenesheet.Inlaststep, amountsofanalytesweremeasuredby neutron-activationanalysisatthepartsper billionlevel [32].In1975acombined coprecipitation X-rayfluorescencemethod wasdevelopedbyBruninxandVanMeylfor thepreconcentrationanddeterminationofzinc andleadbetweenthe10and100 μgL 1 levels insurfacewaters.Zincandleadinsurface waterswereprecipitatedbyformationofiron hydroxideprecipitants [33].Inthesameyear, RollierandRyansearchedtheapplicabilityof solid-statefluorescenceanalysisfortrace elementsbyusingaluminumastheexample element.Theyprecipitatedthealuminumas aluminumoxinatewithahighamountofoxine andmeasuredtheluminescenceofthe
aluminumoxinateprecipitate(λex,385nm: λex, 524nm).Thedevelopedmethodallowsthe analysisofthepartsperbillionlevelofaluminum.Inthe1970s,theimportanceofseparation andpreconcentrationmethodsindetermining traceelementswasunderstoodbyscientists, andinterestinthisfieldincreaseddaybyday. Animportantresearchonthecoprecipitation, ionexchange,andsolventextractionwascarriedoutbyOkochiin1975.Okochideveloped eightkindsofcoprecipitation,threekindsof ionexchange,andthreekindsofsolvent extractionmethodsfortheseparationandpreconcentrationoftraceelementsinmetals. Okochiinvestigatedthepossibilityoftheseparationandpreconcentrationofsmaller amountsofelementsthanintheconventional methods,whichledtothecreationofnew ideas [34].Fromtheliterature,itisseenthat ironhydroxideisoneofthemostusedof coprecipitationagents.In1975Bruninxused ferrichydroxideasacoprecipitationagentfor theprecipitationoftraceamountsofZn,Cd, andHg [35].Oneofthemostcomprehensive coprecipitationmethodsfortraceelementswas carriedoutbyNagatsukaandTanizakiin1976. Theydevelopedacoprecipitationmethod basedontheformationofFe(OH)3 andPbS precipitantsfortheseparationandpreconcentrationofCa,Al,Mg,Dy,V,Ti,As,Ag,Cr,Co, Cu,Cs,Fe,Eu,Lu,Na,Rb,La,Sc,Se,Sb,W, Zn,andSmpriortoneutron-activationanalysis.Theyappliedthisdevelopedmethodfor analysisofthesetraceelementsinriverwaters [36].
UedaandYamazakidevelopedacoprecipitationprocedurefortraceamountsofcadmium priortoflamelessatomicabsorptionspectrometricandthedifferential-pulsepolarographic determinationsin1986.Cadmiumchanging from0.01 μgtoatleast1000 μgamountsin 50 400cm3 ofasamplesolutionwasprecipitatedquantitativelybyusingahafnium hydroxidecoprecipitationagent.Theysuccessfullyusedthesemethodsforthedetermination oftraceamountsofcadmiuminriverwater samples [38]
Acombinedsequentialseparation/preconcentrationmethod,consistingofcoprecipitationwithironhydroxideandbismuth phosphate,ionexchange,electrodeposition, andcountingbyalphaspectrometry,wasprovidedbyPuandAmattracelevelsinenvironmentalsamples.TodetermineThinachicken bonesample,acombinedsequentialseparation/preconcentrationmethodwasusedasfollows:oxalateprecipitation,ionexchange, electrodeposition,andalphaspectrometry [39].
In1989Elleretal.usedSeasacoprecipitationagentforgold,platinum,palladium,and rhodiumatthenanogramandpicogramlevels innaturalwater,geologicalandbiologicalstandards,andmanganesecrust.Theyachieved thequantitativeisolationfortheseelements priortoZeemangraphitefurnaceatomic absorptionspectrometryandtotalreflectionXrayspectrometryanalysis [40].Inthesame year,Kurataetal.suggestedaneasyandrapid procedureconsistingofcoprecipitationandXrayfluorescencefortheanalysisofAs,Sb,Bi, Sn,Fe,andPbattracelevelsincopper.Inthe procedure,theysolvedacoppersamplerangingfrom0.2to2.0gin20mLof8MHNO3 by heating.Aftercooling,1mLfrom1mgmL 1
Takemotoandcoworkerssuggesteda coprecipitation X-rayfluorescenceanalysis procedureformicrogramlevelsofPb(II),Fe (III),Cu(II),Zn(II),Mn(II),Cd(II),Cr(III),Sb(III), andAs(III)inindustrialwastewaterandriver watersamples.Theyusedmodelsolutions includingdiethyldithiocarbamate(DDTC)or ammoniumpyrrolidinedithiocarbamate (APDC)ascomplexingagentsatpH5.0 5.5. Themetalchelatecomplexformedwas precipitatedbytheadditionofdibenzylideneD-sorbitol.Theprecipitateswerecollectedona filterpaper,dried,andanalyzedbyX-rayfluorescencespectrometer.Theobtainedanalytical resultswereingoodagreementwiththose obtainedbytheatomicabsorptionmethod [37].
1.Historicalbackgrounds,milestonesinthefieldofdevelopmentofseparationandpreconcentrationmethods
ofZrsolutionwasputinsamplesolution,and thepHwasadjustedto9.4 9.5.Thevolumeof thesamplesolutionwasincreasedto70mL withpurewaterandlefttositfor1htocompletetheprecipitation,andprecipitateswere collectedonthe1.0 μmmembranefilter.The precipitateonthefilterwasdriedpriortoXrayanalysis.Threepercentofrelativestandard deviation(RSD)wasobtainedforanalysisof 20 μgofallelements.Theyappliedtheir methodforanalysisofcertifiedreferencematerials(CRMs).Theobtainedresultswerein goodagreementwiththecertifiedvalues [41].
In1990McLarenetal.usedtheisotopedilutionICP-MSformultielementtracedeterminationinseawaterandthenondefattedlobster hepatopancreastissuecertifiedreferencematerialsandHPLC-ICP-MSfordeterminationof tributyltinanddibutyltinintheharborsedimentreferencematerial.Whiletheelementsin seawaterwereseparatedfromthematrix mediumbyusingeitheradsorptiononimmobilized8-hydroxyquinolineorbyreductive coprecipitationwithironandpalladium,butyltincompoundsintheharborsedimentreferencematerialwereseparatedfromthematrix mediumbycation-exchangehighperformance liquidchromatography(HPLC).Detectionlimitsfortributyltinanddibutyltinwerefoundas 5and12ngSng 1,respectively [42].AdifferentusageofcoprecipitationandICP-MSprocedurewasreportedbyNakamuraandFukuda todetermineSb,As,Bi,Pb,andSntracesin high-puritycopper.Theydissolved1.0gof coppersamplein8mLof7MHNO3 and added10mgofLatothesolution.As,Sb,Bi, Sn,andPbionswerecoprecipitatedwithlanthanumionsastheirhydroxidesbyadjusting thepHofthesamplesolutionbetween9and 10.Theprecipitatewascollectedonafilterand dissolvedwithacidicsolution,andconcentrationsofSb,As,Bi,Pb,andSnweredetermined byICP-MS.Therespectivedetectionlimits werefoundbetween0.01and0.08ngmL 1 withtheRSDlessthan2.5%for100ngofanalytes [43].
NiskavaaraandKontassuggestedareductivecoprecipitationmethodtoseparatePd,Au, Rh,Pt,Se,Ag,andTetracesfromgeological samples.Inthismethod,whilemercurywas usedasacollector,tin(II)wasusedasareductant.Afterthecoprecipitationstep,graphite furnaceatomicabsorptionspectrometer (GFAAS)wasusedtomeasuretheconcentrationofanalytes.Theaccuracyofthedeveloped methodwascheckedontheanalysisofgeochemicalreferencesamples [44].Inadifferent application,lanthanumhydroxideasacoprecipitationagentwasusedfortheseparationof hydride-formingBi(III),As(V),Sb(V),Te(IV), andSe(IV)traceelementsinaMomatrixprior toanalysiswithcontinuoushydridegeneration andinductivelycoupledplasmaatomicemissionspectrometry.DetectionlimitsforAs,Bi, Sb,Se,andTewerefoundas0.2,0.7,2,0.5, and2 μgg 1,respectively [45].
Thetransitionfrommacroscalecoprecipitationmethodsneedinglargesampleamounts andreagentstothemicrosizedminimumsampleamountsandreagentshasgainedimportanceinrecentyears.Themostimportantstep inthedevelopmentofmicrosizedcoprecipitationtechniquesisthesolvingofprecipitatesin microliterlevelsoffinalsolventsbeforethe detectionstep.Theimprovementshaveledto highpreconcentrationfactorsandreachtolowereddetectionlimits.Anexampleapplication wascarriedoutbyAgakiandHaraguchiin 1990.Theyusedcoprecipitationandinductivelycoupledplasmaatomicemissionspectrometry(ICP-AES)forpreconcentrationand determinationofCr,TI,Al,Fe,Mn,Ni,Co,Zn, Cu,Pb,andYtracesinseawater.Theyprecipitatedtraceelementswithgalliumhydroxide anddissolvedtheprecipitatewith50 μLof nitricacid.Across-flownebulizerwasusedto introducethe50 μLofsolutionintotheplasma. Thedetectionlimitsoftheseelementswere foundtobe10 500ngL 1 withabout10%
precision.Inthisanalyticalmethod,about50 samplesof10mLvolumecouldbeenriched andanalyzedwithin1h.Thelowsamplevolumeneededandthespeedofthemethodwere themostimportantadvantages [46].
Takedaetal.usedthecoprecipitation methodforthepreconcentrationoftrace amountsoftinbeforeelectrothermalatomic absorptionspectrometry(ETAAS).Theyused yttriumhydroxidefortheprecipitationoftinat pH9.5 11.2.Alinearcalibrationgraph between0.004and0.12 μgmL 1 wasobtained. Theyusedthisdevelopedcoprecipitationelectrothermalatomicabsorptionspectrometry (ETAAS)procedureforthedeterminationof tininzincmetal [47].Hiraideetal.developed acoprecipitationmethodbasedontheformationofindiumhydroxideatpH9.5forparts perbillionlevelsofCu(II),Cr(III),andMn(II). Afterthepreconcentrationstep,theprecipitate wasdissolvedin0.5Mhydrobromicacidand determinedwithGFAAS.Theyappliedthis developedmethodtowatersamples [48].
Thetransitionmetalshavedifferentoxidationstepsbecausetheoxidationstepsforeach elementhavedifferenthealtheffectsonliving cells.Thereforeinasample,theanalysisofthe specieswiththecorrectoxidationstepsismore importantthanthetotalquantificationofthe relevantelement.In1991Wengietal.provided achelatecoprecipitation graphitefurnace AASproceduretodeterminepartsperbillion levelsofCr(III)and(VI)innaturalwaterand humanurinesamples.Theycheckedseveral metalion chelatingagentcoprecipitationcarriers:Co(II)-l-nitroso-2-naphthol,Co(II)-dithizone,Ni-dimethylglyoxime,Ni(II)-dithizone, Co(II)-pyrrolidinedithiocarbamate,Fe(II)-dithizone,Fe(III)-diethyldithiocarbamate(DDTC), Zn(II)-APDC(APDC),Mn(II)-DDTC,andCu (II)-DDTC.TheyfoundthatMn(II)-DDTCwas themostsuitablechelatecarriersfortheseparationofCr(III)andCr(VI)atsuitablepH. WhiletherecoveryvaluesforCr(III)were changingfrom87.4%to105.3%,therecoveries
forCr(VI)werechangingfrom85.4%to108.2% [49].
Inanotherstudy,Zhangetal.suggesteda coprecipitationmethodforthedetermination ofarsenic(III)andarsenic(V)tracesinwater samples.Inthisstudy,As(III)wascoprecipitatedquantitativelywithaNi-ammonium APDCcomplexatthepHbetween2and3, but,inthesameexperimentconditions,arsenic (V)wasnotcoprecipitatedwiththeNi-PDC complex.Sothedevelopedmethodwasbased ontheprecipitationofAs(III).Theamountof As(III)intheprecipitatewasmeasuredby solidsamplingETAAS.As(V)wasconverted toAs(III)byusingsodiumthiosulfateand potassiumiodide,andthetotalAsconcentrationwasanalyzed.Thedetectionlimitwas foundas0.02ngmL 1 for500mLportionsof watersamples [50]
Currently,theprospectofdevelopingonline preconcentrationandanalysismethods,which enablesautomaticanalysisinashorttime,has createdexcitementinthescientificcommunity. In1992FandandDongsuggestedanonline flowinjectioncoprecipitationsystemcoupled toanelectrothermalatomicabsorption spectrometerforthedeterminationofheavy metaltracesinwholeblooddigests.They usedtheiron(II)-hexamethylenedithiocarbamate (HMDTC)complextocollectnickelandcadmiumonthewallsofaknottedreactor.The precipitatewasdissolvedin60 μLofisobutyl methylketoneandintroducedontoagraphite furnaceplatformofatomicabsorptionspectrometer.Theprecipitatecollectiontimeswere 20 40s,andsampleflowratesof2mLmin 1 forNiand3mLmin 1 forCdwereapplied. Thedetectionlimitsforcadmiumandnickel werefoundas0.003and0.02 μgL 1 ,respectively [51].Inanotherstudy,DongandFang designedaflowinjectiontechniqueincluding anonlinecoprecipitationandflameatomic absorptionspectrometerdeterminationfor traceamountsofcadmium,nickel,andcobalt. MetalionswerecoprecipitatedwithAPDC-Fe
complexonaknottedreactor(KR).TheprecipitatewasdissolvedinMIBKanddirectly injectedintoflameatomicabsorptionspectrometer(FAAS).Theenhancementfactorsfor Ni,Cd,andCowere30,33,and42,respectively.Theyappliedthedevelopedmethodfor theanalysisofbovineliver(NBS1577a)and urine(GBWO9103)standardreferencematerialswithgoodagreement [52].Adifferentflow injectiononlinepreconcentrationprocedure wasdevelopedforsilverattracelevelingeologicalsamples.Theseparationandpreconcentrationofsilverionswereaccomplishedby usinganonlinecoprecipitationwithiron(II)diethyldithiocarbamatecomplexinthepresenceof1,10-phenanthrolineinaflowinjection system.Aknottedreactorwasusedtocollect theprecipitate.Theprecipitate,dissolvedinisobutylmethylketone,wasinjectedintothenebulizerofanatomicabsorptionspectrometer. Highconcentrationsofiron(II)weremasked with1,10-phenanthroline.Enhancementfactor, detectionlimit(3σ),concentrationefficiency, coprecipitationtime,andsamplingfrequency werefoundas26,0.5 μgL 1,28min 1,45s, and62h 1,respectively [53].
Nielsenetal.combinedhydridegeneration atomicabsorptionspectrometry(HGAAS)with onlinecoprecipitation.Thedevelopedflow injectionprocedurewasusedforanalysisof ultratraceamountsofselenium(IV).Thesuggestedapplicationprovidedanonlineaddition ofthecoprecipitanttothetime-basedaspirated sample.Inthissystem,thesamplesolutionand lanthanumnitrateasthecoprecipitatingagent weremixedonlineandmergedwithanammoniumbuffersolutionofpH9.1,whichledto precipitationandquantitativecollectiononthe wallsofthemicrolinereactor.Afterward,the precipitatewasdissolvedinhydrochloricacid andanalyzedwithhydridegenerationatomic absorptionspectrometry(HGAAS) [54].In 1998Maoetal.designedaflowinjectiononline coprecipitationpreconcentration,combined withFAASfortheaccurateanalysisoftrace
silver.Copper-diethyldithiocarbamate(DDTC) chelatewasusedasthecoprecipitationagent. Thedetectionlimitforsilverwas0.6 μgL 1 for aloadingtimeof30s.Thescientistssuccessfullyanalyzedtraceamountsofsilverin geologicalsamples [55].Liuetal.usedNi(II)diethyldithiocarbamate(DDTC)coprecipitation agentintheflowinjectiononlinecoprecipitationsystemforpreconcentrationsoftracelead, copper,iron,andcadmiuminenvironmental andbiologicalsamples.Theyalsousedaknottedreactortocollecttheprecipitatepriorto FAASdetermination.Whileenhancementfactorsforiron,lead,cadmium,andcopperwere 59,58,65,and60,thedetectionlimitsforiron, lead,cadmium,andcopperwere2.5,2.7,0.2, and0.5 μgL 1,respectively [56]
DivrikliandElciprovidedacerium(IV) hydroxidecoprecipitationmethodtoseparate andpreconcentrateCo,Cu,Pb,Ni,andCd tracespriortoFAASdetermination.Theyused thismethodforaccuratedeterminationofthe tracemetalsinaqueoussolutions,water,and sedimentsamples [57].
Usageofcoprecipitationmethods,together withtheelectroanalyticaltechniques,hasan importantplaceintheliterature.Sekharan etal.developedacoprecipitationmethodfor theseparationoftheantimonyinimpure zincsulfateelectrolyte,followedbyits voltammetricdeterminationin1996 [58] . Hydrousmanganesedioxidewasusedasthe coprecipitationagent [58] .Inadifferent method,Kirgo zetal.designedavoltammetriccellallowingdirectmeasurementof precipitateswithoutanyneedfordissolution orfiltrationsteps.Theysuccessfullyusedthis systemonthepreconcentrationofPb(II) tracesinaqueoussolutionbyusingAl(OH)3 asthecoprecipitationagent.Theobtained peakcurrentsfromvoltammetricdeterminationwerefoundtobemuchhigherthanthose withoutapplyingcentrifugation.Thedetectionlimitofthedevelopedmethodwas 2.2 3 10 9 M [59] .
SoylakandBalgunesprovidedagadolinium hydroxidecoprecipitation-atomicabsorption spectrometrysystemtodeterminetrace amountsofcobalt(II),lead(II),copper(II),and manganese(II).ThepHofthesamplesolution wasadjustedto11toobtaingadolinium(III) hydroxidefromgadolinium(III)salt.Atthis stage,cobalt(II),lead(II),copper(II),andmanganese(II)ionswereprecipitated.Theprecipitatewasdissolvedin1mLof1molL 1 HNO3 andanalyzedbyFAAS.Thedetectionlimitsof theanalyteswerefoundbetween0.52and 12.0 μgL 1 [60].
Aimportantmicroprecipitationmethod basedontheformationofCd(II) 1-(2-thiazolylazo)-2-naphthol(TAN)microprecipitatewas providedbyALOthmanetal.forthepreconcentrationofcadmiumtraces.Inthissystem, microvolumeofthelastphasewasinjectedinto aflameatomicabsorptionspectrometerusinga microinjectionsystem.TheoptimumprecipitationmediumwasobtainedatpH8.Thelimitof detection,limitofquantification,andrelative standarddeviationwerefound0.25 μgL 1 , 0.83 μgL 1,and5.5%,respectively.They appliedthedevelopedmicroprecipitationmicrosamplingFAASproceduretotheanalysis ofcadmiuminfoodsamples [61].
1.2.2Historicaldevelopmentofliquid phaseextraction/microextractionmethods
Liquid liquidextraction(solventextraction)hasbeenoneofthebestprocessesknown fortwocenturiesforseparatingthecomponentsofaliquid(thefeed)bycontactwitha secondliquidphase(thesolvent).Thebasisof theprocessisbasedonthedispersionratesof thecomponentsbetweentwoessentially immiscibleliquids.Whentherelevantanalytes/analytesinthesamplesolutionaretransferredtotheotherphase,thematrix componentsremaininthesamplesolutionor viceversa.Inthisway,extractionofthe
analyte/analytesfromthematrixenvironment iscarriedoutsuccessfully.Inotherwords,the transferofthecomponentsfromonephase (thefeed)totheotherisguidedbyadeviation fromthethermodynamicequilibrium,andthe equilibriumstatedependsontheinteractions betweenthetwophases [31,62 95].Thefoundationsofsolventextractionareprobably basedonthestudiesconductedbyBuchozin 1805 [62,63].Hediscoveredthaturanylnitrate isfreelysolubleindiethylether.Thefirst knownapplicationofliquid liquidextraction (LLE)wasbyE.Peligot [64].In1842uranyl nitratewasextractedfromnitricacidsolution totheetherphaseinthisstudy.Nearly100 yearslater,nuclearenergy,ofgreatinterest duringWorldWarII,wasusedintheextractionandenrichmentoflargeamountsofuraniumfromthematrixenvironment.In1867 Skeysuggestedtheextractionofvariousmetal ionsasthiocyanateformsintotheetherphase, amethodthatisstillpopular,butdidnotuse thismethodhimself.Heprovidedether-based thiocyanateextractionfortheseparationof goldfromplatinum,ironfromthealkaline earthsaluminum,chromium,manganese,uranium,platinumandnickel,andcobaltfrom nickel [65].Etherextractionwasbecoming increasinglypopular,andin1892Rothesucceededinextractingferricchloridepresentin concentratednitricacidintotheetherphase. Theseapplicationscontinuedrapidlyuptothe etherextractionofgalliumtrichloridein1924 [66].Oneofthemostimportantmilestonesin thehistoryofsolventextractionisthesynthesisanduseoforganicchelatingagentsthat formorganicsolublecomplexeswithvarious metalions.Averyimportantapplicationin thisfieldwascarriedoutbyFischer. Dithizone,aversatilechelatingagent,was usedintheextractionandquantitativedeterminationofvariousmetalionsinhisstudy [66,67].Inordertoperformthedesiredextractionandanalysis,theeffectsofpHandtheuse ofmaskingagentvariablesthatareimportant
inextractionstudieswereinvestigated.Then differentorganicchelatingreagentssuchas β-diketones,dimethylglyoxime,and8hydroxyquinolineweresuccessfullyusedin LLEprocedures.Inthe19thcentury,LLEwas apopularproceduretoextractandobtain valuableingredients,suchasperfumeand paint,fromdifferentplantsources.
Inthe1800sthreeresearchstudiesconductedbyBerthelotandJungfleisch,Nernst, andGibbswereintroducedtoexplainthetheoryofphaseequilibrium,thedissolutionrates ofsolutesdissolvingbetweentwounmixed liquids,andpresenteddatadescribingthescientificfactsofliquid liquidextraction [68,69]. Theseandotheradvanceshavemadesignificantcontributionstothedevelopmentofthe chemicalindustry.
In1883Goeringdevelopedacountercurrent extractionprocessusingethylacetatetorecover aceticacidfrom“pyrolyticacid”producedby thepyrolysisofwood [70].Inthelate1890s,the emergenceofthechemicalengineeringprofessionpavedthewayforfurtherconsiderationof processdesignsandquantitativefoundationsin processdevelopment.Mostofthepathway recordedindistillationandabsorptionstudies wasreadilyadaptedtoliquid liquidextraction duetoitssimilaritywithanotherdiffusionbasedoperation.Theuseofliquid liquid extractionstudiesinthechemicalindustry increaseddaybydayandbecameoneofthe mostpopularmethodsinthe1930s.Manyof theliquid liquidextractionmethodsstillused todayinthechemicalindustryaretheresultof innovationsanddevelopmentsmadebetween 1920and1970.
Forexample,inthe1940s,theDow ChemicalandUniversalOilProducts Companiesintroducedawell-knowncommercialUdexprocedure.Inthisprocedure, aromaticcompoundswereseparatedfrom hydrocarbonmixturesbyusingdiethylene glycol.
The1920s 1940sareconsideredimportant milestonesintheprogressofliquid liquid extractionmethodssincetheproductionof newsolventsandinnovativeapparatus,such ascentrifugalextruders,mixer-settlingequipment,andmechanicallyagitatedextraction columns,werestartin gtobeusedinextractionprocedures.Attheendofthe1920s,oil refineriesandchemicalcompanieswereable toproducedifferentalcohol,ketone,esterand chlorinatedhydrocarbonderivativesinlarge volumesbyusingpetro leum-refiningprocessesornaturalgasproducts.Afterthese developments,manyspecialtysolventssuch assulfolane(tetrahydrothiophene-1,1-dioxane)andNMP( N -methyl-2-pyrrolidinone) wereprovidedtoextractaromaticsfrom hydrocarbonsinthesameyears.Moreover, specialorganophosphorousextractionsolventswerealsoutilizedforextractionand recoveryofmetalsdissolvedinaqueous solutions.
Afterthe1950s,liquid liquidextraction methodsbegantogainpopularityforseparationandpreconcentrationoftraceamountsof organicandinorganicanalytesduetothe accessibilityofnewextractionsolventsand detectionsystems.AlthoughtheLLEmethods werefrequentlyusedintheseparationand enrichmentoftheorganicandinorganicspeciesatthetracelevelbetween1950sand 1990s,inthis40-yearperiod,themainimportantdevelopmentscameaboutintheanalysis step,combinedwiththeLLEextractionmethods,andnosignificantfurtherimprovement wasmadeinLLEextraction.Majordevelopmentsandinnovationswereexperienced withtheintroductionofgreenchemistry, new-generationextractionsolvents,and microextractionmethodsinthe1990s. ThereforeLLEcanbeexplainedintwoparts, beforeandafter1990.Developmentsuptothe 1990sareexplainedinthesomeliterature studies.
1.2.2.1Classificationofliquid liquid extractionsystems
Extractioncanbeclassifiedonthebasisofthe
• natureofextractedspeciesand
• processofextraction.
Onthebasisof natureofextractedspecies,there aretwotypes
1. chelateextractionand 2. ionassociation
Classificationbasedonthebasisof processof extraction,therearefourtypes
1. extractionbychelationorchelateformation, 2. extractionbyionpairformation, 3. extractionbysalvation,and 4. synergisticextraction.
Theorganicextractionsolventscommonly useduntilthe1990sfortheextractionofanalytesfromaqueousphasetoorganicphasein theLLEareasfollows:
Carbon-basedsolvents:Carbontetrachloride (CCl4),chloroform(CHCl3),dichlromethane (CH2Cl2),carbondisulfide(CS2), N-hexane.
Acid-basedsolvents:Carboxylicacids (naphthenicacids,Versaticacids,decanoicacid, pentanoicacid,hexanoicacid,heptanoicacid,octanoicacid),alkylphosphoricacids[Di2ethylhexylphosphoricacid(DEHPA)].
Amine-basedsolvents:Primaryamines,secondaryamines,tertiaryamines,quaternary amines(N,N-dimethylcyclohexylamine,N,Ndimethyloctylamine,triethylamine,primeneJMT, Adogen283,Alamine336,Adogen381).
Alcohol-basedsolvents:Decanol,undecanol, heptanol.
Cyclicstructuresolvents:Cyclohexane, cyclohexanone.
In1951AndrewsandLloyddevelopedan LLEmethodfortheseparationofzinctraces priortoitscolorimetricdetermination.Zncomplexedwithdi-beta-naphthylthiocarbazone givesacherry-redsolublecomplexincarbon tetrachloride.Othermetals,whichshow interferenceeffectupondetermination,were
eliminatedbyapplyingapreliminaryextractionasdiethyl-dithiocarbamates.Themethod wasdevelopedandusedforanalysisof0.01 ppmzincinliquidor0.05ppminsolidsamples [71].In1953GarnerandHaleresearched theeffectofsurface-activematerialsonLLE procedures.Asamodelapplication,they researchedtheeffectofTeepoladdedinthe waterphaseontherateofextractionofdiethylaminefromtoluenedropsbywater.Results showedthatextractionefficiencywasreduced to45%withtheadditionofonly0.015%of surface-activematerial(Teepol) [72].
Pijcketal.developedadifferentLLE methodfortheseparationofradioactivetrace analytes.Theyusedisoamylacetateforextractionofchromiumtracesfromironmatrixwith highamounts.Cuproineinisoamylalcoholas theextractionsolventphasewasusedtoseparatecopperattracelevelsfromzincmatrix [73].In1962Attawayetal.developedaprocedureforLLEandthegas-liquidchromatographyfractionoftraceamountsofcarbonyl componentsinorangeessence.Carbonylcomponentswereconvertedtotheirdinitrophenylhydrazonesbyreactionsofcarbonylwith2,4dinitrophenylhydrazinesulfateinethanoland analyzedasdinitrophenylhydrazones.Thecarbonylcomponentsidentifiedincludehexanal, hexenal(twoisomers),acetaldehyde,octenal, octanal,neral,furfural,carvoneandgeranial [74].
In1965Brookscheckedtheusabilityof extractionsolventslighterthanwater.He developedanewmethodtoseparateandanalyzetraceelementsinsilicaterocks.The methodwasbasedontheextractionofelementsaschlorocomplexesintomethylisobutyl ketonephaseandsubsequentspectrographic analysis.Theresultsshowedthattraceelementsinsilicaterockcouldbeextractedfrom themainmatrixmediumandcouldbe fractionatedfromeachother.Themethod developedprovidedfast,flexible,relatively freeextractionandsimpleanalysiswith
quantitativeresults.Authorsclaimedthatthe methoddevelopedwasapplicableforseparationradioactivespeciesanddifferentoxidation statesofthesameelement,forexample,antimony(III)fromantimony(V) [75].
In1967Joyneretal.introducedasimple separationandpreconcentrationprocedurefor metaltraces.Theprocedureconsistsofcoprecipitationwithalkalineearthsalts,followedby liquid liquidextractionoftransitionmetal dithiocarbamatespriortoflameemissionor atomicabsorptiondeterminations.Themethod developedwasappliedtothequantitative analysisofiron,manganese,cobalt,nickel,lead copperandzincattracelevels [31].
In1968Campbelldevelopedaliquid liquid extractionfortheseparationofrhodiumand palladiumfromwastesolutions.Theyusedtricaprylmonomethylammoniumchlorideas extractionsolvent.Theyusedflamephotometrytodeterminerhodiumandpalladiuminthe lastphase.Themethoddevelopedprovided 0.05 μgmL 1 ofdetectionlimit [76].MirzasuggestedanLLEfor 115Cdand 89,90Srasfission products.Theysucceedtheextractionofthese elementswith1-phenyl-3-methyl-4-caprylpyrazolone-5.TheLLEmethodprovidedextraction efficiencyofmorethan80%for 89,90Srand90% for 115Cd [77].
Inadifferentstudy,Farraretal.used2thenoyltrifluoroacetone-xyleneastheextractionsolventphaseforliquid liquidextraction of 249Bk.Theyusedthebetacountingsystem foranalysisof 249Bk [78].
In1971BonsackdevelopedanLLEmethod fortheseparationoftracelevelsofniobium fromindustrialtitaniumsulfatesolutions. Cyclohexanoneanddiisobutylcarbinolwere usedasextractionsolventsinacidicextraction medium.Niobiumwasseparatedfromthe matrixmediumwithahighextractionefficiency(92% 98%) [79].In1972Barrattetal. introducedanLLEmethodforseparationand preconcentrationoftraceamountsofnickel priortogas-liquidchromatographyanalysis.
Ni(II)inaqueousphaseatpH4.5 5.0was extractedtotheextractionphaseconsistingof monothiotrifluoroacetylacetoneandn-hexane. TheLLEmethodappliedfordeterminationof traceamountsofnickelinfatandteasamples [80].
YatirajamandRamintroducedanLLE methodfortheseparationofmolybdenum fromahardmatrixmediumincludingaluminum,uraniummanganese,chromium,zirconium,iron,nickel,cobalt,andtitanium. Molybdenumwasturnedtophosphomolybdenumbluecomplexinacidicmediumand extractedtoisobutylketonephase. Concentrationofmolybdenuminthelast phasewasdeterminedbycerimetryorother standardmethods [81].TinsleyandIddonused aliquidionexchangerAmberliteLA2inthiocyanateformastheextractionphaseforthe separationandextractionofcopperpriorto atomicabsorptiondetermination [82]
GenerallyLLEmethodsareusedinthe extractionofanalytesintheaqueousphase intotheorganicphase.However,insomestudies,anoppositeapplicationhasbeenmade.For example,LameyandMaloyusedsulfuricacid asaselectiveextractionsolventforextraction ofanthracenetracesfromcyclohexanesolutionsofphenanthrene.Inthismethod,anthracenewasremoved,andhighamountsof ultrapurephenanthrenewereeasilyobtained [83].
In1970sinordertofindtheoptimumand bestseparation-preconcentrationprocedure, thescientistsstartedtomakecomparisonstudiesbetweenextractionmethodsfortraceanalytes.Mieureetal.developedthreedifferent extractionmethods,whichwereliquid liquid extraction,preconcentrationofanalytesina porouspolymerresincolumn,andpreconcentrationofanalytesinporouspolymerresincolumn.Theyselectedorganiccomponentsas traceanalytes.Itwasreportedthatdetection limitsof0.01 0.1ppmwereobtainedwith thesemethods [84].
Asaresultofthefactthatthemethodsof traceanalysishavebecomeimportantanda lotofstudieshavebeenmadeinthisfield,a literaturedatasource wasformed.Scientists havebeguntowritereviewstudiesinthis areabyusingtheseresources.Oneofthemost importantdevelopmentsinextractionmethodswastocollectallinformationabout extractioninreviews.In1975Eisenbrand wroteareviewtitled“RecentDevelopments inTraceAnalysisofVolatileNitrosamines:A BriefReview.”Inthisreview,manyextraction anddetectiontechniquesuptothe1970swere discussed [85].Asinothersamplepreparation methods,onlineextrac tionapplicationsin LLEstartedtoattractattentionatthistime.In 1976WuandSuffetdesignednewcontinuous LLEdevices,whichpavedthewaytothe designandconstructionofnewonlineextractionapparatus.Themainpartofthisdevice wasahelicalTeflonmixingcoil.Thecontinuousdeliveryofthesamplesolutionandthe extractionsolventtotheextractionmedium wasprovidedbyadual-channelmicropump. Separationoftheextractionphasefromthe aqueoussamplephasewasaccomplishedbya glasscolumnphaseseparatorandacontinuoussolventevaporatorconcentrator.Inthis way,theextractionsolventseparatedwaspreparedforrecycling.Thedevelopedapparatus hadasimpleandcheapmodularconceptthat providegreateranalyticalflexibilityandwas capableofrecyclingtheextractionsolvent. Thedevelopedapparatuswasusedforanalysisoforganophosphatepesticidesinwater samples.Moreover,theauthorsclaimedthat thiscontinuousLLEapparatusisapplicable fortheextractionandanalysisofanytrace organicmolecules [86] .NordandKarlberg alsointroducedanautomatedliquid liquid extractionsystemfortheseparationandpreconcentrationoftraceamountsofmetalions priortoflameatomicabsorptionspectrometer determination [87] .Coelloetal.introduceda continuous-flowLLEcombinedwithflame
atomicabsorptionspectrometerforextraction andanalysisofindium.Inthisprocedure, indiumtracesindilutednitricacidsolution wereextractedintobis(2-ethylhexyl)phosphoricacidphasedissolvedin4-methylpentane2-one.Thecontinuous-flowLLEsystemwas connectedtoonlineflamespectrometrytouse afullymechanizedsystem.Thedetection limit,relativestandarddeviation,andsample throughputofthedevelopedmethodwere 0.03mgL 1,1.5%,and60h 1,respectively [88] . OliverandBothencombinedtheLLE methodwithacapillarygaschromatography electroncapturedetectionsystemforthe separation,preconcentrationandanalysisof traceamountsof12chlorobenzenesinwater samples.Theyusedpentaneastheextraction solventintheLLEmethod.Theyachieveda preconcentrationfactorof1000 2000and detectionlimitsof0.01 500ngL 1 (ppt)for chlorobenzenes [89].Inadifferentapplication, tracelevelsofherbicidesandnitroderivatives wereextractedwithCH2Cl2 andanalyzedby gaschromatographyandcaptureelectron detector [90].In1983Vahtilacomparedthe applicabilityandanalyticalperformancevalues ofdifferentpreconcentrationmethods,suchas liquid liquidextraction,solidphaseextraction,ionexchange,andthelike,foranalysisof tracemetalsinseawater,biologicalmaterials, andmarinesediments [91].Agostianoetal. appliedadifferentLLEmethodforseparation andpreconcentrationofsometracepesticides (carbamates,chlorinated,carbonatesphosphorated)inwatersamples.GC(gaschromatography)andHPTLCwereusedinthe measurementstage [92]
In1987PetrovandZhivopistsevusedantipyrineanddiantipyrylmethanesaltsinsteadof organicsolventsforextractionoftraceelementspriortospectrochemicalanalysis [93]. HamannandKettrupcombinedLLEandSPE methodsfortheseparationandpreconcentrationofphenoxyacidherbicidesinwater
samples.AnalyseswereconductedbyHPLCUV [94].Inadifferentapplication,LLEand SPEmethodswasusedtogetherfortheseparationandpreconcentrationoftracemetals [95].
Astheworldpopulationincreases,the needsofpeopleandproductionrequiredto meettheseneedshaveincreasedrapidly.In parallel,therehasbeenalinearincreasein theamountofchemicalactivitiesinboth researchlaboratoriesandindustryinorderto meetproductionneeds.Chemicalactivitiesin thelaboratoryandindustrycannegatively affectlivingcellsandthenaturalenvironment andmayleadtoadecreaseinthequalityof life.
Perhapsenvironmentalawarenessformost ofusstartedwiththebook SilentSpring publishedbyRachelCarsonin1962 [96].Oneof Carson’salarmingobservationswastheenvironmentalpollutioncausedbypesticidessuch asDDT,leadingtothedeathofmillionsof birds.Duringthistime,theindustrywasstrivingtoprotectthenearbyenvironmentalzones bybuildinglongchimneys.Butitcouldnot accountforseriousenvironmentalproblemsin thelongrun.After20years,theUN ConferenceonEnvironmentandDevelopment (theEarthSummit)wasorganized,andmany reportswerepublished.In1987TheWorld EnvironmentandDevelopmentCommission (WCED)publishedareportcalled“Our CommonFuture.”Inthisreport,forthefirst time,theconceptofsustainabledevelopment wasmentioned [97].In1993atechnicalcommittee,codedas207,wasfoundedbythe InternationalOrganizationforStandardization (ISO)forenvironmentalmanagement.ISO/TC 207wasestablishedasanumbrellacommittee underISO14,000environmentalmanagement standards.Environmentalmanagementsystems,lifecycleassessment,environmental labeling,greenhousegasmanagement,and similaractivitiesareevaluatedbythiscommittee.Thesestudies,publishedreports,and books,aswellasorganizedmeetings,arethe
mostimportantmilestonesincreatingamore sustainablefuture.
Awarenessoflifeandoftheneedforenvironmentalprotectionhasledchemiststoreconsiderthetechniques,processes,chemicals,and thereforeallchemicaleventsandtodevelop environmentallyfriendlyprocesses.In1991the term“greenchemistry”wasintroducedwith the12principlesagreeduponbydifferentkey individuals,scientists,andinstitutions [98 101].
Themainrulesofgreenchemistryare relatedtothedevelopmentsofthe“greeningof methodologies”byapplyinglowerconsumptionsofenergy,atomeconomy,eliminating hazardouschemicalprocessesandtheuseof toxicchemicalsandmaterialsandreducing waste.
Sevenprinciplesthatcanbeappliedtogreen analyticalchemistry,takenfromthe12principlesofgreenchemistryacceptedbyall branchesofchemistryandindustry,mustbe considered [99 101]:
1. Preventionofwastesthatmayoccurafter theprocessorminimizingthemasmuchas possible,forexample,byusingsmaller extractionsystemsinsolidphaseorliquid phaseextractionsystemsorsmallerinner diametercolumnsinchromatography.
2. Useofnontoxicenvironmentallyfriendlyor aslittletoxicchemicalsandsolventsas possible.
3. Designanduseofminimalenergyconsuminganalyticalsystemsandmethods toensureenergyefficiency.
4. Avoidingtheuseofchemicalcatalysts, derivatizationreagents,andotherauxiliaries avoidedasmuchaspossible.
5. Useofcatalystsinsteadofstoichiometric reactions.
6. Useofinsituanalysisinsteadofoffline analysis.
7. Useofsaferchemistrytopreventaccidents suchasexplosionsandfires.