CROP IMPROVEMENT THROUGH MICROBIAL BIOTECHNOLOGY
Edited by Ram Prasad
Amity University, Noida, India
Sarvajeet S. Gill
MD University, Rohtak, India
Narendra Tuteja
Amity University, Noida, India ICGEB, New Delhi
3 Conclusion 98 References 99 Further Reading 106
6 Innate Immunity Engaged or Disengaged in Plant-Microbe Interactions
SRIDHAR RANGANATHAN
1 Beginning of Molecular Basis of Plant-Microbe Interactions 107
2 Plant-Pathogen Interactions 108
3 Innate Immunity 108
4 Mutualistic Interactions 120
5 Epilogue 130 References 131 Further Reading 144
7 Novel Strategies for Engineering Resistance to Plant Viral Diseases
MEENAKSHI DANGWAL, SHIVARAJ M. MATHAD, BASAVAPRABHU L. PATIL
1 Introduction 145
2 Natural and Engineered Resistance Against Plant Viruses 147
3 Mechanisms of Resistance to Plant Viruses 151
4 RNA Silencing Pathways 155
5 Genome Editing Tools to Combat Plant Viruses 162
6 Prospects 164 Acknowledgments 164 References 164 Further Reading 174
8 Molecular Characterization of Sugarcane Viruses and Their Diagnostics
RASAPPA VISWANATHAN, BALASUBRAMANIAN PARAMESWARI, KATHIRVEL NITHYA
1 Introduction 175
2 Mosaic 176
3 Leaf Fleck 181
4 Yellow Leaf Disease 184
5 Detection and Diagnosis of Mixed Infections 186
6 Conclusion 187 Acknowledgments 188 References 188
9 Cyanobacterial Biodiversity and Biotechnology: A Promising Approach for Crop Improvement
SHIVAM YADAV, RUCHI RAI, ALOK K. SHRIVASTAVA, PRASHANT K. SINGH, SONIA SEN, ANTRA CHATTERJEE, SHWETA RAI, SHILPI SINGH, LAL C. RAI
1 Introduction 195
2 Crop Yield Constraint, Population Increase and Food Security 196
3 Application of Cyanobacteria in Crop Improvement and Sustainable Agriculture 200
4 Conclusion 211 Acknowledgments 211 References 211 Further Reading 219
10 Pseudomonas fluorescens: A PlantGrowth-Promoting Rhizobacterium (PGPR) With Potential Role in Biocontrol of Pests of Crops
BALIAH V. DAVID, GOVINDAN CHANDRASEHAR, PAMILA N. SELVAM
1 Introduction 221
2 General Characteristics of Pseudomonas fluorescens 222
3 Plant Growth Promoting Properties of Pseudomonas 222
4 Mechanisms of Plant Growth Promotion by Pseudomonas 223
5 Induction of Systemic Resistance by PGPR Against Diseases, Insect and Nematode Pests 225
6 Synergistic Effect of PGPR Strain Mixtures 227
7 PGPR as Endophytes 227
8 Mode of Action of Pseudomonas Against Fungal Pathogens 228
9 Plant Diseases Control by P. fluorescens 229
10 Interaction of P. fluorescens With Chemical Pesticides 232
11 Formulation Characteristics of Biopesticides 233
12 Approved Uses of P. fluorescens Formulations in India 233
13 Regulation for Biopesticides 236
14 Data Requirements for Biopesticides
Registration 236
15 Regulatory Mechanisms for Biopesticides 237
16 Factors Affecting Growth of Biopesticides 238
17 Future Issues and Research Needs in Biopesticides 238 References 239 Further Reading 243
11 Crop Improvement Through Microbial Technology: A Step Toward Sustainable Agriculture
PANKAJ BHATT, TAPAN K. NAILWAL
1 Introduction 245
2 Crop Production Scenario in World 246
3 Crop Production in India 247
4 Microbial Technology for Crop Production 247
5 Microbial Biotechnology for Crop Production 249
6 Conclusion 250 Acknowledgment 251 References 251 Further Reading 253
12 Microbial Technologies for Sustainable Crop Production
CHITTRANJAN BHATIA, PRASUN K. MUKHERJEE
1 Introduction 255
2 Origin of Farming 256
3 Urgent Need to Increase Sustainable Crop Productivity 256
4 Undesired Effects of Increased Inputs of Chemical Fertilizers and Pesticides 257
5 Rhizosphere Microbial Diversity 257
6 Crop Production as an Energy Harvesting Process 257
7 Root Exudates Support Microbial Populations in the Rhizosphere 258
8 New Techniques 258
9 Using Microbial Diversity for Enhanced Crop Production 258
10 Registration and Commercial Issues 259
11 Challenges of Microbial Products 260
12 Conclusions and Outlook for the Future 260 References 261
13 Trichoderma: Its Multifarious Utility in Crop Improvement
MUJEEBUR R. KHAN, FAYAZ A. MOHIDDIN
1 Introduction 263
2 Taxonomy of Trichoderma 265
3 Factors Influencing Activity of Trichoderma
Species 271
4 Mechanism of Action of Trichoderma
Species 272
5 Plant Growth Promotion by Trichoderma
Species 276
6 Conclusion and Future Prospects 282 References 282 Further Reading 291
14 Microbe-Mediated Enhancement of Nitrogen and Phosphorus Content for Crop Improvement
MANOJ NATH, DEEPESH BHATT, MEGHA D. BHATT, RAM PRASAD, NARENDRA TUTEJA
1 Introduction 293
2 Plant Growth Promoting Rhizobacteria (PGPR) Mediated N and P Enhancement During Plant Microbe Interaction 294
3 AMF and Enhancement of N and P in Plants 298
4 Conclusions 301 References 301
15 Microbiome in Crops: Diversity, Distribution, and Potential Role in Crop Improvement
AJAR N. YADAV, VINOD KUMAR, HARCHARAN S. DHALIWAL, RAM PRASAD, ANIL K. SAXENA
1 Introduction 305
2 Isolation and Characterization of Crop Microbiomes 307
3 Diversity and Distribution of Crop Microbiomes 310
4 Beneficial Role of Microbes in Crop Improvement 316
5 Conclusion and Future Scope 322 References 323
16 Plant Growth-Promoting Rhizobacteria (PGPR): Perspective in Agriculture Under Biotic and Abiotic Stress
AJAY KUMAR, VIPIN K. SINGH, VIJAY TRIPATHI, PREM P. SINGH, AMIT K. SINGH
1 Introduction 333
2 Stress Conditions Affecting Plant Growth 335
3 Role of PGPR Against Biotic Stress 335
4 Role of PGPR in Mitigation of Draught and Salinity Stress 337
5 Role of PGPR in Phytoremediation of Metal Contaminated Sites 338
6 Conclusions 339 References 340 Further Reading 342
17 Rhizosphere Metabolite Profiling: An Opportunity to Understand Plant-Microbe Interactions for Crop Improvement
AMIT VERMA, SATENDRA KUMAR, HEMANSI, GOVIND KUMAR, JITENDRA K. SAINI, RUCHI AGRAWAL, ALOK SATLEWAL, MOHAMMAD W. ANSARI
1 Introduction 343
2 Plant Microbial Environment and Root Exudates 344
3 Rhizosphere Metabolites 347
4 Transcriptomics in Rhizosphere Study 348
5 Metabolomics in Rhizosphere Study 351
6 Future Prospects and Conclusion 356 References 356 Further Reading 361
18 Phosphate-Solubilizing Pseudomonads for Improving Crop Plant Nutrition and Agricultural Productivity
BALA RATHINASABAPATHI, XUE LIU, YUE CAO, LENA Q. MA
1 Phosphorus Nutrition for Crop Production 363
2 Phosphate Solubilization by Rhizosphere Microorganisms 364
3 Mechanisms of Phosphorus-Solubilizing Bacteria 366
4 Use of Pseudomonads in Agriculture Products 369 Acknowledgments 370
References 370 Further Reading 372
19 Targeted Genome Editing for Crop Improvement in Post Genome-Sequencing Era
CHANDRA P. SINGH, NAVNEET S. CHAUDHARY, BASKARAN KANNAN, RATNA KARAN
1 Introduction 373
2 Basic Mechanism of Genome Editing 374
3 Double Strand Breaks (DSBs) and Repairing Pathways 374
4 Sequence Specific Nucleases 376
5 Application of Sequence Specific Nucleases in Plants 382 References 385 Further Reading 389
20 Endophytic Microorganisms: Their Role in Plant Growth and Crop Improvement
MANJU SHARMA, REKHA KANSAL, DINESH SINGH
1 Introduction 391
2 Mode of Transmission 392
3 Colonization 393
4 Types of Endophytes 395
5 Role of Endophytes in Crop Improvement 399
6 Conclusion 405 References 405
21 Microbes in Crop Improvement: Future Challenges and Perspective
KASHYAP K. DUBEY, PUNIT KUMAR
1 Introduction 415
2 Microbes to as Biocontrol Control Plant
Disease Control 416
3 Antagonism 417
4 Competition 417
5 Induced Resistance 419
6 Microbes as Biofertilization (Improved Plant Nutrient Availability) 419
7 Nitrogen Fixation 419
8 Phosphate Solubilization 420
Contributors
Chetana Aggarwal Indian Council of Agricultural Research—Indian Institute of Maize Research, New Delhi, India
Ruchi Agrawal Govind Ballabh Pant University of Agriculture and Technology (GBPUAT), Pantnagar, India
Mohammad W. Ansari Zakir Hussain College, Delhi University, New Delhi, India
Mehmet C. Baloglu Kastamonu University, Kastamonu, Turkey
Chittranjan Bhatia Mumbai, India
Pankaj Bhatt Dolphin Institute of Biomedical and Natural Sciences, Dehradun, Uttarakhand, India
Deepesh Bhatt Shree Ramkrishna Institute of Computer Education and Applied Sciences, Affiliated to Veer Narmad South Gujarat University, Surat, India
Megha D. Bhatt GSFC AgroTech Ltd., Gujarat State Fertilizers & Chemicals Ltd., Vadodara, India
Subrata N. Bhowmik ICAR Research Complex for NEH Region, Lembucherra, India
Yue Cao Nanjing University, Nanjing, China
Govindan Chandrasehar International Institute of Biotechnology and Toxicology (IIBAT), Padappai, India
Antra Chatterjee Banaras Hindu University, Varanasi, India
Navneet S. Chaudhary University of Rajasthan, Jaipur, Rajasthan, India
Meenakshi Dangwal ICAR-National Research Centre on Plant Biotechnology, IARI, New Delhi, India
Baliah V. David International Institute of Biotechnology and Toxicology (IIBAT), Padappai, India
Harcharan S. Dhaliwal Eternal University, Sirmour, India
Kashyap K. Dubey Central University of Haryana, Mahendergarh; Maharshi Dayanand University, Rohtak, Haryana, India
Sarvajeet S. Gill Maharshi Dayanand University, Rohtak, India
Songül Gürel Sugar Institute, Ankara, Turkey
Ekrem Gürel Abant Izzet Baysal University, Bolu, Turkey
Hemansi Department of Microbiology, Central University of Haryana, Mahendergarh, Haryana, India
Baskaran Kannan University of Florida, Gainesville, FL, United States
Rekha Kansal Indian Agricultural Research Institute, New Delhi, India
Ratna Karan University of Florida, Gainesville, FL, United States
Musa Kavas Ondokuz Mayıs University, Samsun, Turkey
Mujeebur R. Khan Aligarh Muslim University, Aligarh, India
Krishan Kumar Indian Council of Agricultural Research—Indian Institute of Maize Research, New Delhi, India
Vinod Kumar Eternal University, Sirmour, India
Ajay Kumar Banaras Hindu University, Varanasi, India
Satendra Kumar Govind Ballabh Pant University of Agriculture and Technology (GBPUAT), Pantnagar, India
Govind Kumar Govind Ballabh Pant University of Agriculture and Technology (GBPUAT), Pantnagar, India
Punit Kumar Maharshi Dayanand University, Rohtak, Haryana, India
Xue Liu Nanjing University, Nanjing, China
Lena Q. Ma University of Florida, Gainesville, FL, United States; Nanjing University, Nanjing, China
Shivaraj M. Mathad ICAR-National Research Centre on Plant Biotechnology, IARI, New Delhi, India
Fayaz A. Mohiddin SKUAST-K, Srinagar, India
Prasun K. Mukherjee Bhabha Atomic Research Centre, Mumbai, India
Tapan K. Nailwal Kumaun University, Nainital, Uttarakhand, India
Manoj Nath Amity Institute of Microbial Technology, Amity University, Noida, India
Kathirvel Nithya ICAR-Sugarcane Breeding Institute, Coimbatore, India
Keishi Osakabe Tokushima University, Tokushima, Japan
Yuriko Osakabe Tokushima University, Tokushima, Japan
Balasubramanian Parameswari ICARSugarcane Breeding Institute Regional Centre, Karnal, India
Hemant J. Patil Institute of Soil, Water and Environmental Sciences, Volcani Center, Agricultural Research Organization, Bet Dagan, Israel
Ramabhau T. Patil Benevole Welfare Society for Post Harvest Technology, Bhopal, India
Basavaprabhu L. Patil ICAR-National Research Centre on Plant Biotechnology, IARI, New Delhi, India
Ratna Prabha ICAR—National Bureau of Agriculturally Important Microorganisms, Indian Council of Agricultural Research, Maunath Bhanjan, India
Siddegowda R. Prasad ICAR—Indian Institute of Seed Science, Indian Council of Agricultural Research, Maunath Bhanjan, India
Ram Prasad Amity Institute of Microbial Technology, Amity University, Noida, India
Ruchi Rai Banaras Hindu University, Varanasi, India
Shweta Rai Banaras Hindu University, Varanasi, India
Lal C. Rai Banaras Hindu University, Varanasi, India
Sridhar Ranganathan Independent Researcher, Chennai, Tamil Nadu, India
Vavilala R. Rao Central Rice Research Institute, Cuttack, India
Bala Rathinasabapathi University of Florida, Gainesville, FL, United States
Jitendra K. Saini Department of Microbiology, Central University of Haryana, Mahendergarh, Haryana, India
Sapna Indian Council of Agricultural Research—Indian Institute of Maize Research, New Delhi, India
Alok Satlewal Govind Ballabh Pant University of Agriculture and Technology (GBPUAT), Pantnagar, India
Anil K. Saxena ICAR-National Bureau of Agriculturally Important Microorganisms, Mau, India
Pamila N. Selvam International Institute of Biotechnology and Toxicology (IIBAT), Padappai, India
Sonia Sen Banaras Hindu University, Varanasi, India
Manju Sharma Amity University Haryana, Manesar, India
Krishna K. Sharma Maharshi Dayanand University, Rohtak, India
Alok K. Shrivastava Banaras Hindu University, Varanasi, India
Bhuvnesh Shrivastava Panacea Biotech Limited, New Delhi, India
Dhananjaya P. Singh ICAR—National Bureau of Agriculturally Important Microorganisms, Indian Council of Agricultural Research, Maunath Bhanjan, India
Ishwar Singh Indian Council of Agricultural Research—Indian Institute of Maize Research, New Delhi, India
Prashant K. Singh Banaras Hindu University, Varanasi, India
Shilpi Singh Banaras Hindu University, Varanasi, India
Vipin K. Singh Banaras Hindu University, Varanasi, India
Prem P. Singh Banaras Hindu University, Varanasi, India
Amit K. Singh Banaras Hindu University, Varanasi, India; Agricultural Research Organization, Volcani Center, Bet-Dagan, Israel
Chandra P. Singh University of Rajasthan, Jaipur, Rajasthan, India
Dinesh Singh Indian Agricultural Research Institute, New Delhi, India
Deepti Singh Maharshi Dayanand University, Rohtak, India
Bijender Singh Maharshi Dayanand University, Rohtak, India
Amarjeet Singh University of Delhi, New Delhi, India
Shigeo S. Sugano Ritsumeikan University, Kyoto, Japan
Vijay Tripathi Sam Higginbottom University of Agriculture Technology and Sciences, Allahabad, India
Narendra Tuteja Amity Institute of Microbial Technology, Amity University, Noida; ICGEB, New Delhi, India
Amit Verma Gujarat Agricultural University, SK Nagar, India
Rasappa Viswanathan ICAR-Sugarcane Breeding Institute, Coimbatore, India
Shivam Yadav Banaras Hindu University, Varanasi, India
Ajar N. Yadav Eternal University, Sirmour, India
Pranjal Yadava Indian Council of Agricultural Research—Indian Institute of Maize Research, New Delhi, India; Stanford University, Stanford, CA, United States
Mahesh S. Yandigeri ICAR—National Bureau of Agricultural Insect Resources (NBAIR), Hebbal, India
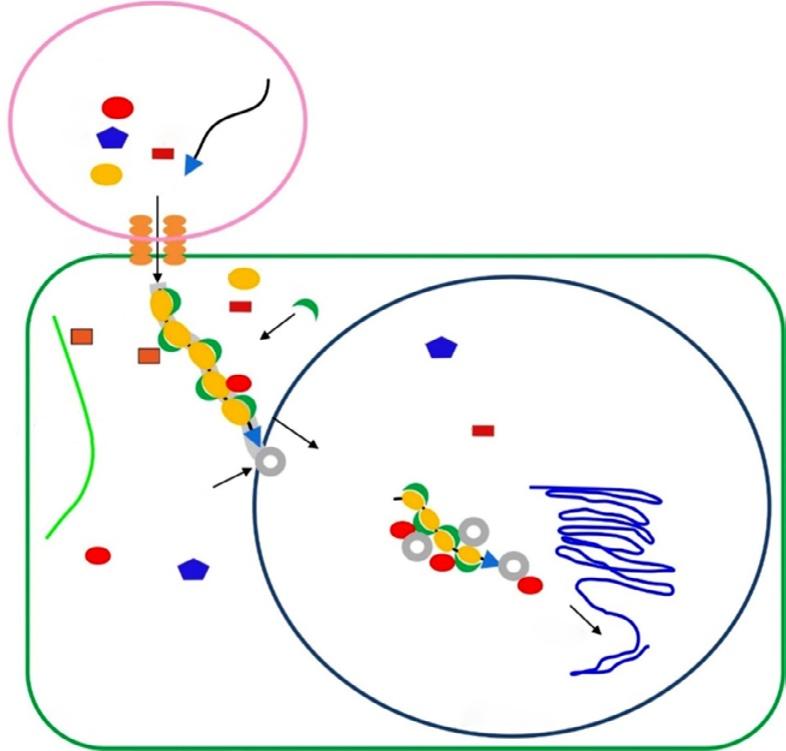
ability to transfer its segment of plasmid (Ti-Ri plasmid) surrounded by repeated nucleotides into plant genome naturally. The typical Ti plasmid, with a crucial role in crown gall disease, is about 200 kb. Naturally grown Agrobacterium cells carry two types of gene on T-DNA region. The first one known as oncogenic genes includes auxin and cytokinin genes. The others are responsible for opine and agropine synthesis in infected plant tissues (Gustavo et al., 1998). The proteins coded by vir (virulence) genes carried out the transfer of T-DNA region into the plant cells. Phenolic substances released from wounded plant tissues induce the activation of vir genes located in tumor-inducing (Ti) plasmid of A. tumefaciens. There are about 30 genes in A. tumefaciens vir regulon, and about 20 of them are required for tumor formation in plant tissues (Gelvin, 2003). This regulon consists of at least six operon (VirA, VirB, VirC, VirD, VirG, and VirE) required for single-stranded T-DNA generation and transfer into the host plant cell genome (Fig. 1) (Gustavo et al., 1998; Zupan and Zambryski, 1997).
The improvement of plants through the gene transfer mainly relies on the tissue-culture response of genotypes or species. In order to generate transgenic plants, suitable transformation methods and a robust regeneration protocol are required. By this context, some of the species and explants may not be suitable for Agrobacterium-mediated gene transfer. Especially, monocotyledon plant species are recalcitrant to Agrobacterium-mediated transformation. These groups of plants are not naturally infected by A. tumefaciens due to the lack of phenolic substances required for induction of vir genes. By using artificial phenolic substances and hypervirulent strain, monocot plants such as cereal can be transformed by A. tumefaciens. Another
FIG. 1 Single-stranded T-DNA generation and transfer into the host plant cell genome.
group of plants also cannot be genetically engineered because of their low regeneration potential. As previously mentioned, the production of transgenic plants requires tissue- culture steps. Recently, tissue-culture-independent methods have been demonstrated to work in a limited number of plant species. One of the most promising tissue-culture-independent methods is called floral-dip transformation. Arabidopsis thaliana plant can efficiently be transformed by using this technique (Feldmann and Marks, 1987). This technique, which removes the need for tissue culture, has been successfully applied to other plants such as soybean, radish, tomato, brinjal, and snake gourd (Hu and Wang, 1999; Curtis and Nam, 2001; Park et al., 2005; Yasmeen et al., 2009; Subramanyam et al., 2015).
1.2 Gene Transfer Through Agrobacterium Rhizogenes
The other important phytopathogens have gene transfer ability, and the cause to hairy root disease is A. rhizogenes (Gelvin, 2009). The A. rhizogenes-mediated transformation characterized by hairy root formation takes place by transferring T-DNAs from the Ri plasmid into plant cell (Tepfer and Cassedelbart, 1987). The most prominent characteristic of hairy roots induced by A. rhizogenes is that they can able to grow rapidly in the absence of exogenous plant growth regulators (Collier et al., 2005). Testing of gene functions in a short period by using stable transgenic tissues is the most important advantage of A. rhizogenes-mediated gene transfer (Kim et al., 2002). Because of this valuable property, A. rhizogenes-mediated gene transfer has turned into a powerful tool for gene functional and root biology studies (Cao et al., 2009). There are many plant species; some of them are recalcitrant to A. tumefaciens-mediated gene transfer that has been transformed by A. rhizogenes (Georgiev et al., 2007). In this context, soybean and tobacco plants were transformed to analyze gene function (Wang et al., 2016; Hao et al., 2011). In addition to the gene function analyses, A. rhizogenes-mediated gene transfer is generally used for the excess production of plant chemicals and therapeutic agents (Yao et al., 2016; Kiani et al., 2016).
1.3 Non-Agrobacterium-Based Technologies
Over 10 years ago, Broothaerts et al. (2005) have reported that there is non-Agrobacterium plant-associated bacterial species such as Sinorhizobium meliloti, Mesorhizobium loti, and Rhizobium NGR 234 that could transform A. thaliana. Although there are a limited number of studies reporting the successful transformation event by using these species, Wendt et al. (2011) and Rathore et al. (2016) have demonstrated that Ensifer adhaerens strain OV14 has the ability to transform potato and oilseed rape.
2 CROP IMPROVEMENT THROUGH TRANSGENIC TECHNOLOGY
2.1 Herbicide Resistant Transgenic Plants
The use of genetic engineering techniques to develop glyphosate-resistant (GR) crops was a scientific discovery that leads to revolutionizing the weed management strategies (Green, 2012).
Glyphosate (N-(27)-glycine) is a powerful and most widely used broad-spectrum herbicide targeting the shikimate pathway enzyme 5-enolpyruvylshikimate 3-phosphate (EPSP) synthase (Steinrücken and Amrhein, 1980). Up till now, many genes have been shown to provide a glyphosate resistance effect to different cells (Stalker et al., 1985; Comai et al., 1985; Yu et al., 2015; Ye et al., 2001; Zhou et al., 1995; Padgette et al., 1991). By using these genes, various herbicidetolerant transgenic plants have been generated (Table 1). Among these genes, the CP4-EPSPS gene, isolated from naturally glyphosate-resistant Agrobacterium strain CP4, has been widely used to produce commercially available herbicide-resistant transgenic plants (Dill et al., 2008). The CP4-EPSPS gene codes glyphosate-insensitive form of EPSP synthase enzyme. Since weed control with classical tools was time-consuming and costly, the usage of GR crops made weed management easy, efficient, economical, and environmentally compatible. The first herbicideresistant transgenic plant was produced in 1986 by introducing EPSPS gene into soybean cells (Shah et al., 1986). By this context, natural or codon-optimized EPSPS gene has been introduced into several plant species including rice, maize, bent grass, cotton, sugar beet, lettuce, and cotton (Mannerlof et al., 1997; Nida et al., 1996; Nagata et al., 2000; Cerny et al., 2010; Lee et al., 2011; Sun et al., 2015; Chhapekar et al., 2015).
TABLE 1 Important Transgenes/Transgene Products Being Used for Engineering Crop Plants Possessing Herbicide Resistance
Transgene(s) Source Plant Species Target Herbicide Reference
Bar Streptomyces hygroscopicus Tobacco, potato, tomato Phosphinothricin and bialaphos
De Block et al. (1987)
Bar Streptomyces hygroscopicus Lotus japonicus Phosphinothricin Lohar et al. (2001)
Bxn Klebsiella ozaenae Tobacco Bromoxynil (3,5-dibromo4-hydroxybenzonitrile) Stalker et al. (1988)
Bxn Klebsiella ozaenae Trifolium subterraneum L. Bromoxynil (3,5-dibromo4-hydroxybenzonitrile) Dear et al. (2003)
PgrA Ochrobactrum anthropi Tobacco Paraquat Jo et al. (2004)
Bar Streptomyces hygroscopicus Sweet potato Glufosinate Choi et al. (2007)
MxPPO Myxococcus xanthus Tall fescue Oxyfluorfen, acifluorfen Lee et al. (2008)
Bar Streptomyces hygroscopicus Ipomoea batatas Phosphinothricin and bialaphos Zang et al. (2009)
AtDHAR1 Arabidopsis thaliana Potato Methylviologen Eltayeb et al. (2011)
G6/EPSSPS Pseudomonas putida Rice Glyphosate Te et al. (2011)
G2/EPSSPS and GAT Pseudomonas fluorescens Bacillus licheniformis Soybean Glyphosate Guo et al. (2015)
DAAO Bradyrhizobium japonicum Arabidopsis Glyphosate Han et al. (2015)
Bar Streptomyces hygroscopicus Salvia miltiorrhiza Phosphinothricin Liu et al. (2015a)
Dehd Rhizobium sp. RC1 Nicotiana benthamiana Mohamed et al. (2016)
These plants have more cultivating area each year because they are making the management with weeds both easier and cheaper. This is especially the case for soybean, which is the most widely grown herbicide-tolerant plant in the world. Glyphosate-resistant soybeans represented 50% of all herbicide-resistant crops and about 80% of all globally cultivated soybeans in 2014 (James, 2015).
In addition to EPSPS gene, bar, dehd, daao, dhar1, pat, ppo, cryp1a, pgra, bxn, gat, and gst27 genes were successfully transferred into plant cell, and various herbicide-tolerant transgenic plants were obtained (Table 1). In this context, another important gene was called bar that was isolated from Streptomyces hygroscopicus. Phosphinothricin acetyltransferase (PAT) enzyme coding by bar gene has an ability to convert phosphinothricin into a nontoxic acetylated form (Gordonkamm et al., 1990). Transgenic plants have been obtained by transferring this gene to many plants including sorghum, cowpea, cotton, soybean, salvia, grape, sugar beet, apricot, and sweet potato during the last 30 years (Liu et al., 2015a, 2014; Ilori and Pellegrineschi, 2011; Petri et al., 2015; Metwali et al., 2016; Do et al., 2016; Li et al., 2009; Zang et al., 2009; Mishutkina et al., 2010).
2.2 Insect Resistant Transgenic Plants
One of the most important abiotic stress factors reducing agricultural productivity is pests. So, the second important trait introduced by Agrobacterium-mediated gene transfer into plant cell was insect resistance. There are two main approaches for the production of genetically engineered insect-resistant plants. In the context of the first approach, insect-resistant transgenic plants are generally obtained through the transferring of genes encoding crystal toxin proteins (Cry proteins) from Bacillus thuringiensis. These proteins inactivate their targets through the affecting guts. Cry genes code resistance in plants against a variety of insects belonging to Lepidoptera (Zhao et al., 2014), Coleoptera (Tohidfar et al., 2013), Hemiptera (Rausch et al., 2016), and Diptera (Andrews et al., 1987). The first example of insect-resistant transgenic plant was transgenic tobacco plant produced through the introduction of bt genes by using A. tumefaciens (Hilder et al., 1987), although the first commercially available bt transgenic plant, a transgenic maize generated for controlling corn borer (Ostrinia nubilalis), was produced using biolistic method. The number of bt transgenic species produced via Agrobacterium-mediated gene transfer has dramatically increased (Narva et al., 2013). Important plant species including maize, rice, potato, cotton, tomato, alfalfa, and chickpea have been transformed with bt genes by using A. tumefaciens (Table 2). GM crops with bt genes were globally planted over 35 million hectares in 13 different countries in 2014, and they constitute 15% of all GM crops (James, 2015).
In another strategy, other insecticidal genes from a variety of organism including plant and bacteria were transferred into plant cell. These are proteinase inhibitors, lectins, amylase inhibitors, etc. (Tran et al., 1997; Ishimoto et al., 1995; Tamayo et al., 2000; Rao et al., 1998; Christeller et al., 2002).
2.3 Nutritional Improvement
After the successful use of recombinant DNA techniques in the development of plants, improving the nutritional quality of food crops has become an important target. Genes from
2.5 Engineering for Molecular Farming/Pharming
Plants can be used to produce several chemicals and recombinant proteins that have strong effects on human health. In the light of this information, plant-manufactured chemicals are one of the most remarkable applications of transgenic plants. The cell's intrinsic metabolic processes are not designed for industrial production of these chemicals. Genetic engineering can manipulate metabolic pathways of cell, and resulted transgenic plants can synthesize more biopharmaceutical products and small chemicals suitable for industrial applications (Karuppusamy, 2009). There are many studies showing the successful production of specialty chemicals, biopharmaceuticals, and edible vaccines in seeds, leaves, or fruits (Hesselink et al., 2014; Niemer et al., 2014; Piller et al., 2005; Hudson et al., 2014; Lombardi et al., 2012; Kashani et al., 2012; Pniewski et al., 2011). In this context, especially the production of plant-based vaccines for human and animal was accepted as an attractive approach (Hudson et al., 2014). Up till now, several vaccine candidates with proved potential at the preclinical level were produced (Hernandez et al., 2014) Additionally, some of them are close to get license for implementation, such as a vaccine against influenza (Grabowski et al., 2014; Ward et al., 2014). In addition to the vaccines, other plant-based biopharmaceuticals are also approved for human usage. A good example for this application is Taliglucerase alfa, which is an enzyme produced in carrot cells after A. tumefaciens-mediated gene transfer. This plant-based chemical has already been approved and commercially available for the treatment of Gaucher's disease (Pastores et al., 2014). Although a variety of plant-based chemicals and vaccines have been produced, there are many challenges to overcome such as the timing of application, the vaccine, the dosage, and the capacity of the protein to induce immunity on oral administration (Yusibov et al., 2015).
Although there are many transgenic plants serving as biofactory for small chemicals and recombinant proteins obtained through A. tumefaciens-mediated gene transfer, A. rhizogenesinduced hairy root cultures are also good candidates to produce these substances. Different plant species especially medicinal ones have been transformed such as Callerya speciosa, Dracocephalum forrestii, and Artemisia tilesii (Yao et al., 2016; Weremczuk-Jeżyna et al., 2016; Matvieieva et al., 2016). Likewise, increased resveratrol content was obtained via A. rhizogenes-induced hairy root cultures of soybean (Kim et al., 2008).
3 VIRUS-INDUCED TRANSIENT GENE EXPRESSION IN PLANTS
Virus-induced gene silencing (VIGS) is mainly used for identification of gene function. This technique utilizes viral vectors that carry gene fragment of the target gene. As a result of virus induction, dsRNA molecule is produced, and this leads to starting of RNA-mediated gene silencing. VIGS is considered as a reverse genetic tool that provides an alternative way for characterization of gene functions in a transient way. In this part, mechanisms, development, and improvement of this method have been examined.
3.1 Basic Mechanism of VIGS
VIGS technique actually uses antiviral defense mechanism of plants in which posttranscriptional gene silencing (PTGS) occurs (Baulcombe, 1999a). In normal conditions, when
The first VIGS application was performed with TMV that caused knockdown of pds gene in N. benthamiana (Kumagai et al., 1995). It was shown that the minimum sequence length of RNA for gene silencing was detected in different studies. They indicated that 23-nucleotide RNA was the minimum for 100% homology to the target gene. However, longer similar sequences were required for efficient PTGS (Thomas et al., 2001). Modified TRV is another VIGS vector that has been used for more than 15 years for gene silencing in plants. The main benefits of TRV vector are easy transfer into plants, especially Solanaceae family members and higher spreading capability throughout whole plant parts (Unver and Budak, 2009). Using this vector, gene silencing was succeeded in N. benthamiana (Liu et al., 2002a) and tomato (Liu et al., 2002b). Traditionally, VIGS vector is located between right and left borders of TDNA (Liu et al., 2002b; Feldman and Levy, 2012; Ratcliff et al., 2001). Strong promoters such as 35S or duplicate 35S promoters and terminator such as a ribozyme were added to cassette and inserted into A. tumefaciens. These regulators provide more effective and faster spreading of TRV vectors. pYL156 and pYL279 were TRV vectors with double 35S promoters that caused infection of different plant species (Liu et al., 2002a,b; Ratcliff et al., 2001). PVX is an RNA virus that has a limited host range when compared with TMV-based vectors. However, modified PVX vectors provide more stability than TMV vectors (Burch-Smith et al., 2004). So, we understand from the literature (Senthil-Kumar and Mysore, 2011a) that although VIGS technique has been performed for more than 20 years, there are several limitations and drawbacks for this gene silencing method. These problems have actually caused the development of some new methodologies to find out solutions. In this part, we summarized and listed some of the problems and their solutions:
(i) The main problem is to find right and suitable VIGS vectors for plant species. This can be solved by two ways. One is that, to silence gene in VIGS-recalcitrant species, heterologous gene sequences can be used from close relative of VIGS-compatible species (Senthil-Kumar et al., 2007; Zheng et al., 2011). The second is the development of new VIGS vectors with broad host range. So, an appropriate virus and vectors can infect several plant species (Senthil-Kumar et al., 2007).
(ii) Another drawback of VIGS method is the transfer of virus vector to plants. Generally, delivery of VIGS vectors to dicot plants is achieved by Agrobacterium , which is not suitable for monocots. Therefore, new strategies such as virus sap inoculation method ( Lu et al., 2003 ) or RNA transcript inoculation ( Ding et al., 2007 ) or DNA bombardment (Krenz et al., 2010) have been developed by different research groups.
(iii) There is an another problem arisen from movement of virus in plants that results in the lack of silencing in certain tissues ( Senthil-Kumar and Mysore, 2011a ). This can be achieved by keeping stable environmental conditions for proper virus movement ( Senthil-Kumar et al., 2008, 2007 ). In addition, suitable VIGS vectors should be selected for spreading of insert into plant tissues and should not possess a strong silencing suppressor. They should also carry a reporter gene whose expression provides visualization and discrimination of silenced tissues for interpretation of effect of silencing ( Senthil-Kumar and Mysore, 2011a ; Burch-Smith et al., 2004 ).
(iv) Both symptoms arisen from VIGS vector and virus cause a prevention of interpretation of data. VIGS vectors that manufacture serious signs in host plants should be evaded