HANDBOOKOFCLINICAL NEUROLOGY
SeriesEditors
MICHAELJ.AMINOFF,FRANC¸OISBOLLER,ANDDICKF.SWAAB
VOLUME135
vi AVAILABLETITLES(Continued)
Vol.124,Clinicalneuroendocrinology,E.Fliers,M.KorbonitsandJ.A.Romijn,eds.ISBN9780444596024
Vol.125,Alcoholandthenervoussystem,E.V.SullivanandA.Pfefferbaum,eds.ISBN9780444626196
Vol.126,Diabetesandthenervoussystem,D.W.ZochodneandR.A.Malik,eds.ISBN9780444534804
Vol.127,TraumaticbraininjuryPartI,J.H.GrafmanandA.M.Salazar,eds.ISBN9780444528926
Vol.128,TraumaticbraininjuryPartII,J.H.GrafmanandA.M.Salazar,eds.ISBN9780444635211
Vol.129,Thehumanauditorysystem:Fundamentalorganizationandclinicaldisorders,G.G.Celesia andG.Hickok,eds.ISBN9780444626301
Vol.130,Neurologyofsexualandbladderdisorders,D.B.Vodus ˇ ekandF.Boller,eds.ISBN9780444632470
Vol.131,Occupationalneurology,M.LottiandM.L.Bleecker,eds.ISBN9780444626271
Vol.132,Neurocutaneoussyndromes,M.P.IslamandE.S.Roach,eds.ISBN9780444627025
Vol.133,Autoimmuneneurology,S.J.PittockandA.Vincent,eds.ISBN9780444634320
Vol.134,Gliomas,M.S.BergerandM.Weller,eds.ISBN9780128029978
Foreword
Weareproudtopresentthefirstvolumesdedicatedtoneuroimaginginthe HandbookofClinicalNeurology series. Neurologists,notjustthoseintraining,maywonderatthekindofmedicalworldthatexistedbeforemodernimaging. Indeed,theneurosciencecommunityhassinceitsbeginningattemptedtounderstandthehumanmindandbrain throughimaging.Asfarbackas1880,theItalianphysiologistAngeloMossointroducedthe“humancirculation balance,”whichcouldnoninvasivelymeasuretheredistributionofbloodduringemotionalandintellectualactivity. Morerecently,semi-invasivetechniquessuchaspneumoencephalography(introducedbyDandyin1918)andarteriography(pioneeredbyMonizin1927)allowedpartialvisualizationofthebrainanditssurroundingstructures.New methodsenablingeasier,safer,noninvasive,painless,andrepeatableimaginghaveonlybeendevelopedinthepast 50yearsorso,startingwithcomputedtomography,someofwhosedeveloperswonthe1979NobelPrizeformedicine orphysiology.Themanysubsequentdevelopmentsinneuroimagingaremasterfullypresentedinthesetwovolumes.
Thevolumesdealwithavarietyofneuroimaging-relatedtopics.Theyincludethoroughdescriptionsoftheinvolved methodsandtheirapplicationtospecificdiseasesofthebrain,spinalcord,andperipheralnervoussystem.Emphasisis giventothemostcommondisorders,suchastumors,strokes,multiplesclerosis,movementdisorders,infections, dementia,andtrauma,butlesscommonconditionssuchasneurocutaneoussyndromesarealsodiscussed.Theimportantquestionsofwhenandwheretoimage,aswellasthedifferentialdiagnosisofimagingfindings,arediscussedin thelightofspecificsyndromes.Aseparatesectioncoverspediatricneuroimaging.Thevolumesconcludewithsections dedicatedtointerventionalneuroimagingaswellastopostmortemimagingandneuropathologiccorrelations.
Wehavebeenfortunatetohaveasvolumeeditorstwodistinguishedscholars,Dr.JosephC.Masdeu,oftheDepartmentofNeurology,MethodistHospital,Houston,Texas,andDr.R.GilbertoGonza ´ lez,fromtheDepartmentofRadiology,MassachusettsGeneralHospitalinBoston.Bothhavebeenattheforefrontofneuroimagingresearchformany years.Theyhaveassembledatrulyinternationalgroupofauthorswithacknowledgedexpertisetocontributetothe textsandhaveproducedtwoauthoritative,comprehensive,andup-to-datevolumes.Theiravailabilityelectronicallyon Elsevier’sScienceDirectsiteaswellasinprintformatshouldensuretheirreadyaccessibilityandfacilitatesearchesfor specificinformation.
Wearegratefultothevolumeeditorsandtoallthecontributorsfortheireffortsincreatingsuchaninvaluable resource.Asserieseditorswereadandcommentedoneachofthechapterswithgreatinterest.Wearethereforeconfidentthatbothcliniciansandresearchersinmanydifferentmedicaldisciplineswillfindmuchinthesevolumesto appealtothem.
Andlast,butnotleast,itisalwaysapleasuretoacknowledgeandthankElsevier,ourpublisher – and,inparticular, MichaelParkinsoninScotland,andMaraConnerandKristiAndersoninSanDiego – fortheirunfailingandexpert assistanceinthedevelopmentandproductionofthesevolumes.
MichaelJ.Aminoff Franc ¸ oisBoller DickF.Swaab
C.Habas
NeuroimagingService,CentreNational d’OphtalmologiedesQuinze-Vingts,Paris,France
V.Haughton
SectionofNeuroradiology,DepartmentofRadiology, UniversityofWisconsin,Madison,WI,USA
J.A.Hirsch
NeurointerventionalService,MassachusettsGeneral Hospital,Boston,MA,USA
M.Ichise
MolecularNeuroimagingProgram,MolecularImaging Center,NationalInstituteofRadiologicalSciences, Anagawa,Inage,Chiba,Japan
S.Kamalian
DivisionofNeuroradiology,DepartmentofRadiology, MassachusettsGeneralHospitalandHarvardMedical School,Boston,MA,USA
H.R.Kelly
DepartmentofRadiology,HarvardMedicalSchooland MassachusettsEyeandEarInfirmary;andDivisionof Neuroradiology,MassachusettsGeneralHospital, Boston,MA,USA
A.J.M.Kiruluta
DepartmentofRadiology,MassachusettsGeneral Hospital,BostonandDepartmentofBiophysics, HarvardUniversity,Cambridge,MA,USA
N.Klar
DivisionofNeuroradiology,RussellH.Morgan DepartmentofRadiology,JohnsHopkinsUniversity SchoolofMedicine,Baltimore,MD,USA
K.Lameka
DepartmentofRadiology,TuftsUniversity,Bostonand DepartmentofRadiology,BaystateMedicalCenter, Springfield,MA,USA
M.Larvie
DivisionsofNeuroradiologyandNuclearMedicineand MolecularImaging,MassachusettsGeneralHospital, Boston,MA,USA
T.M.Leslie-Mazwi
NeurointerventionalService,MassachusettsGeneral Hospital,Boston,MA,USA
M.H.Lev
DivisionofEmergencyRadiologyandDivisionof Neuroradiology,DepartmentofRadiology, MassachusettsGeneralHospitalandHarvardMedical School,Boston,MA,USA
D.D.M.Lin
DivisionofNeuroradiology,RussellH.Morgan DepartmentofRadiology,JohnsHopkinsUniversity SchoolofMedicine,Baltimore,MD,USA
M.Manto DepartmentofNeurology,UniversiteLibredeBruxelles Erasme,Brussels,Belgium
K.-A.Mardal DepartmentofMathematics,UniversityofOslo,Oslo, Norway
J.C.Masdeu DepartmentofNeurology,HoustonMethodistHospital, Houston,TX,USA
M.Moghbel DepartmentofRadiology,HospitaloftheUniversityof Pennsylvania,Philadelphia,PA,USA
N.Nagornaya DepartmentofRadiology,UniversityofMiamiMiller SchoolofMedicine,Miami,FL,USA
A.Newberg
MyrnaBrindCenterofIntegrativeMedicine,Thomas JeffersonUniversityandHospital,Philadelphia,PA, USA
B.Pascual DepartmentofNeurology,HoustonMethodistHospital, Houston,TX,USA
N.Pavese
DivisionofBrainSciences,ImperialCollegeLondon, UKandAarhusUniversity,Denmark
R.Pizzolato
DepartmentofNeuroradiology,MassachusettsGeneral Hospital,HarvardMedicalSchool,Boston,MA,USA
S.R.Pomerantz
DepartmentofNeuroradiology,MassachusettsGeneral Hospital,Boston,MA,USA
M.J.D.Post
DepartmentofRadiology,UniversityofMiamiMiller SchoolofMedicine,Miami,FL,USA
P.Preziosa
NeuroimagingResearchUnit,InstituteofExperimental Neurology,DivisionofNeuroscience,SanRaffaele ScientificInstitute,Vita-SaluteSanRaffaeleUniversity, Milan,Italy
T.Ptak
DivisionofNeuroradiologyandDivisionofEmergency Radiology,MassachusettsGeneralHospital,Boston, MA,USA
J.D.Rabinov
NeurointerventionalService,MassachusettsGeneral Hospital,Boston,MA,USA
O.Rapalino
DivisionofNeuroradiology,DepartmentofRadiology, MassachusettsGeneralHospitalandHarvardMedical School,Boston,MA,USA
E.-M.Ratai
DivisionofNeuroradiology,DepartmentofRadiology, MassachusettsGeneralHospitalandHarvardMedical School,andAthinoulaA.MartinosCenterfor BiomedicalImaging,Boston,MA,USA
S.Rincon
DivisionofNeuroradiology,MassachusettsGeneral Hospital,Boston,MA,USA
M.A.Rocca
NeuroimagingResearchUnit,InstituteofExperimental Neurology,DivisionofNeuroscience,SanRaffaele ScientificInstitute,Vita-SaluteSanRaffaeleUniversity, Milan,Italy
J.M.Romero
DepartmentofNeuroradiology,MassachusettsGeneral Hospital,HarvardMedicalSchool,Boston,MA,USA
J.Rosand NeuroscienceIntensiveCareUnit,Departmentof Neurology,MassachusettsGeneralHospital,Boston, MA,USA
A ´ .Rovira MRUnit,DepartmentofRadiology,Hospital UniversitariValld’Hebron,AutonomousUniversityof Barcelona,Barcelona,Spain
A.Rovira Corporacio ´ SanitàriaParcTaulı´,CD-UDIAT,Sabadell, Spain
G.Saigal DepartmentofRadiology,UniversityofMiamiMiller SchoolofMedicine,Miami,FL,USA
P.W.Schaefer DepartmentofRadiology,MassachusettsGeneral Hospital,Boston,MA,USA
L.H.Schwamm DepartmentofNeurology,MassachusettsGeneral HospitalandHarvardMedicalSchool,Boston,MA, USA
A.B.Singhal DepartmentofNeurology,MassachusettsGeneral Hospital,Boston,MA,USA
J.G.Smirniotopoulos DepartmentofRadiologyandRadiologicalSciences, UniformedServicesUniversityoftheHealthSciences, Bethesda,MD,USA
Y.F.Tai DivisionofBrainSciences,ImperialCollegeLondon, UK
K.VanLaere DivisionofNuclearMedicine,UniversityHospital LeuvenandKULeuven,Leuven,Belgium
screening(Napeletal.,1992;Schwartzetal.,1992). Thinnerslices(0.6mm,512–1024 512–1024matrix) andincreasedscanningspeedhavefacilitatedmore widespreadadoptionofcoronalandsagittalimage reformatting – improvingdetectionofsubtlecontusion andsubarachnoidhemorrhage(SAH)intheanteriorfrontalandtemporallobesandcorticalsulci(Baker,1981;Wei etal.,2010).Moreover,CTperfusion(CTP)imaginghas increasinglybeenutilizedatmanycentersforqualitative assessmenttoimprovedifferentialdiagnosis,determine strokesubtype,guidehypertensivemanagement,andsort treatmentoptionsforvasospasmfollowinganeurysmal SAH(Koenigetal.,1998;Eastwoodetal.,2002).
AdvancesinCTneuroimaginghaveparalleled advancesincomputerprocessingspeedandinefficiency ofimagereconstructionalgorithms.Mostrecently,iterativereconstructiontechniques – thefirstgenuinely novelCTimage-processingdevelopmentsinceHounsfield’sfilteredbackprojectionmethodology(forwhich hewasawardedtheNobelPrizein1979) – havemade possiblehighspatialresolution,reducednoise,very lowradiationdoseCTscanning(Rapalinoetal.,2012). Theimprovedscanningspeed, z-directioncoverage,spatialresolution,contrastresolution,andlowradiation dosecapabilityofpresent-dayCTscannershavealso facilitateddual-energyimaging,which – forthefirst time,likeMRI – hasallowedtissue-specificcharacterizationofintracranialpathology,includingdedicatedCT imagingthatcanreliablydistinguishcalcium,iodine,fat, water,andhemorrhage(Guptaetal.,2010).Virtual monochromaticdual-energyCTimagesalsohavethe potentialtohelpreducetheposteriorfossabeamhardeningartifactcausedbydenseboneattheskullbase (Pomerantzetal.,2013).
PRINCIPLES
Noncontrastcomputed tomography(NCCT)
ThephysicalbasisofCTscanningisthattheattenuation ofanX-raybeamthroughlivingtissueisproportional totheelectrondensityofthattissue,generallycorrespondingtothephysicaldensityofthetissue,andthat agray-scaleimage – reflectingtherelativedensitiesof differentvoxelsofsuchtissue – canbereconstructed fromtheattenuationvaluesobtainedwhenrotatingan X-raysourcearoundapatient,usingamathematical techniqueknownasfilteredbackprojection(FBP).The gray-scalevaluesareassignedanarbitrarylinearvalue, theHounsfieldunit(HU),namedafterSirGodfrey Hounsfield,thephysicistwhowasawardedthe1979 NobelPrizeinMedicine(aswellasearningaknighthood) forhisinventionofCTscanninginthelate1960s–early
1970s,whilehewasanemployeeoftheBritishmusic companyEMI(thefirstrecordlabelfortheBeatles).
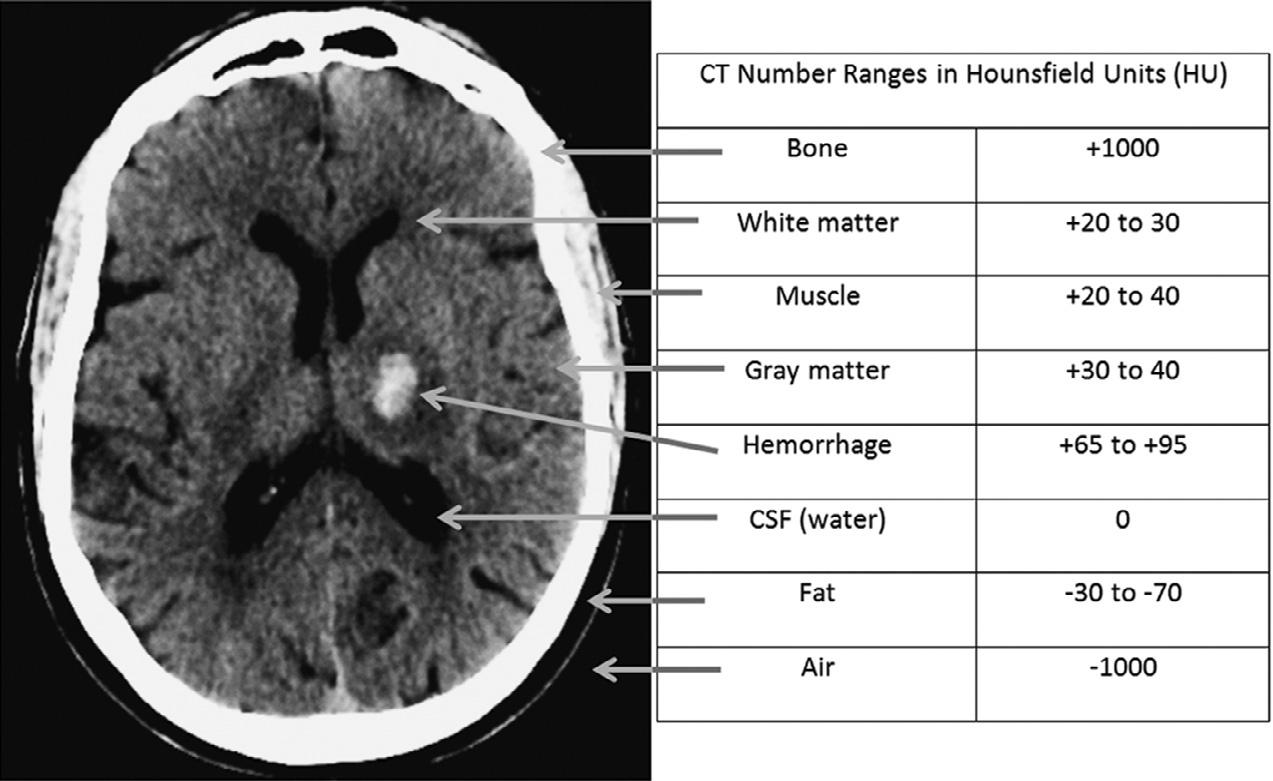
TheHounsfieldscaleisdefinedastheattenuation valueoftheX-raybeaminagivenvoxel,minusthe attenuationofwater,dividedbytheattenuationof water,multipliedby1000.Hence,waterisarbitrarily assignedanHUvalueofzero,withmaterialsmoredense thanwaterhavingpositivevaluesandmaterialsless densethanwaterhavingnegativevalues.Although roughlylinearlyproportionaltophysicaldensity(based onso-calledComptonscatter),theHounsfieldscaleis relative,ratherthanabsolute,inthatdifferent-energy X-raybeamswillresultindifferentattenuationvalues andhencedifferentHUvalues.Moreover,becausesome elements – suchasiodine – preferentiallyabsorbphotons ofcertainspecificenergiesbasedonthephotoelectric effect(so-called k-edgeorcharacteristicradiation),they willappeartohavedisproportionatelylargeattenuation valuesrelativetotheiractualphysicaldensity.Indeed, thisiswhyiodine-basedintravascularcontrastagents areideallysuitedtoCTimaging.
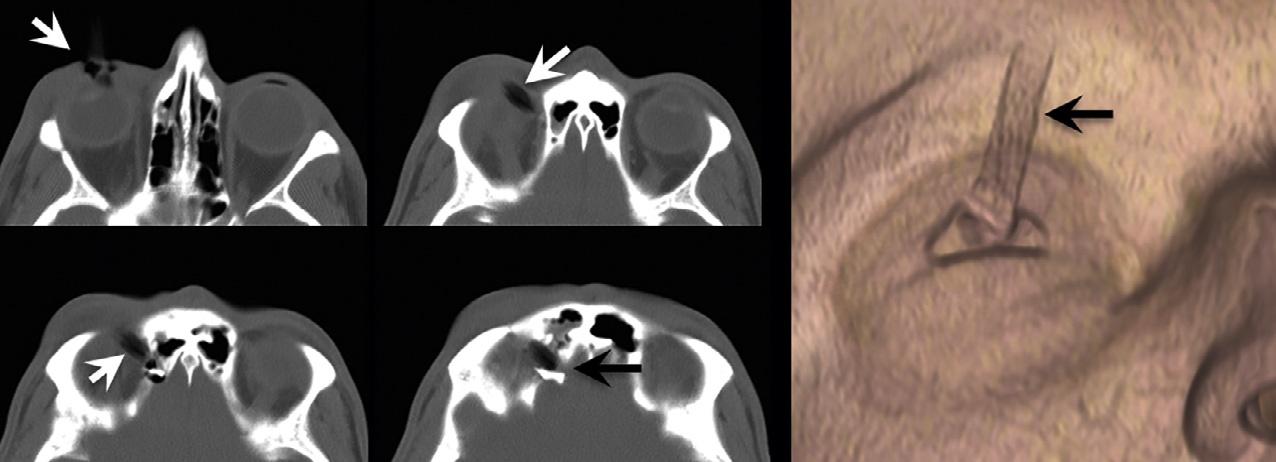
Forexample,atroutineCTX-raybeamenergiesof 120–140kV,theHUvalueofairisapproximately –1000andtheHUvalueofdensecorticalboneisapproximately+1000.Fat,whichfloatsonwater(i.e.,isless dense)istypicallyinthe –30to –70HUrange.Whitematterisabout25HU,graymatterabout35HU,andsoft tissueabout20–30HU.ThestandarddeviationofHU valuesisusuallyinthe 10–20%range.TheHUvalue of invivo bloodis(notsurprisingly)proportionalto thehematocritlevel,andtypicallyabout30.Extravascular,intracranialblood,however,clotsrapidly,andas plasmaisextrudedandresorbedfromtheclot,theconcentrationofthehemoglobinproteincandoubleandtriple,sothatintracranialhemorrhagetypicallymeasures 60–90HU(butrarely >100)(Fig.1.1).Animportant caveatwithregardtoevaluatingtraumapatientsisthat notallpotentialforeignbodiesarehigh-density,highHUstructures.TheCTnumberofadry,woodenforeign body,forexample,istypicallyinthe –100to –170HU range,duetodrywood’sair-filledporousmicrostructure(Yamashitaetal.,2007)(Fig.1.2).
InCTimagedisplay,higherHUvaluesappear brighterandlowerHUvaluesappeardarker.Because thehumaneyecanonlydistinguishapproximately128 shadesofgray,thedynamicrangeoftheCTimagedisplaymustbeadjustedsoastobeappropriatetothetissue beingevaluated.Themid-valueofthisgrayscaleis termedthecenterlevel,andthefulldynamicrangeis knownasthewindowwidth.Forexample,withstandard headCTimagedisplayparametersofcenterlevel30HU andwindowwidth100HU,eachpixelgreaterthan80HU (¼30+50)wouldbeequallybright,whereaseachpixel lessthan –20HU(¼30 – 50)wouldbeequallydark.With
Fig.1.1. Axialcomputedtomography(CT)imagewithsampletypicalCTnumbersinHU.CSF,cerebrospinalfluid.
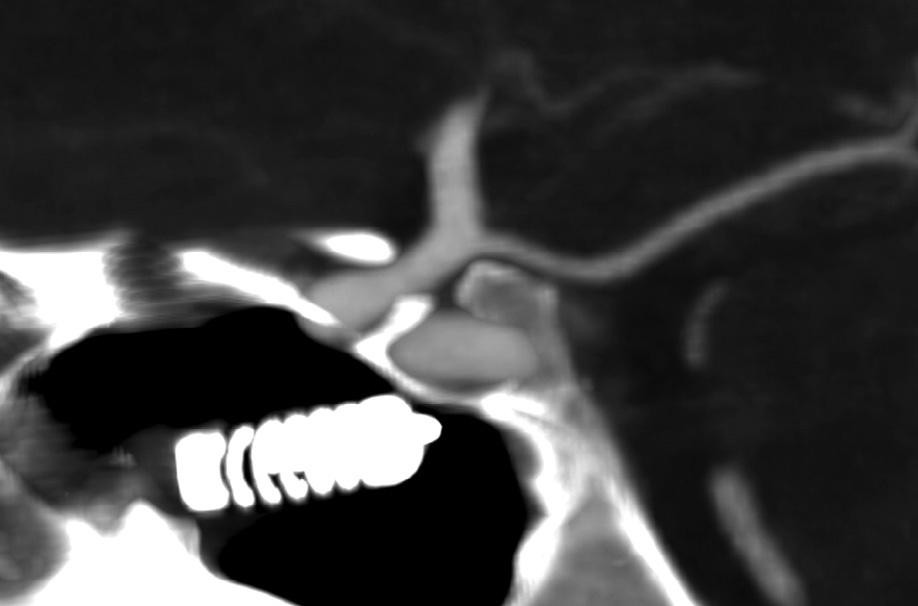
Fig.1.2. Hypodenseforeignbody – awoodenpencil – penetratingthesuperiormedialorbit,withperforationintotheanterior cranialfossa.Notethatthewoodhasasimilarcomputedtomographyappearancetoair.
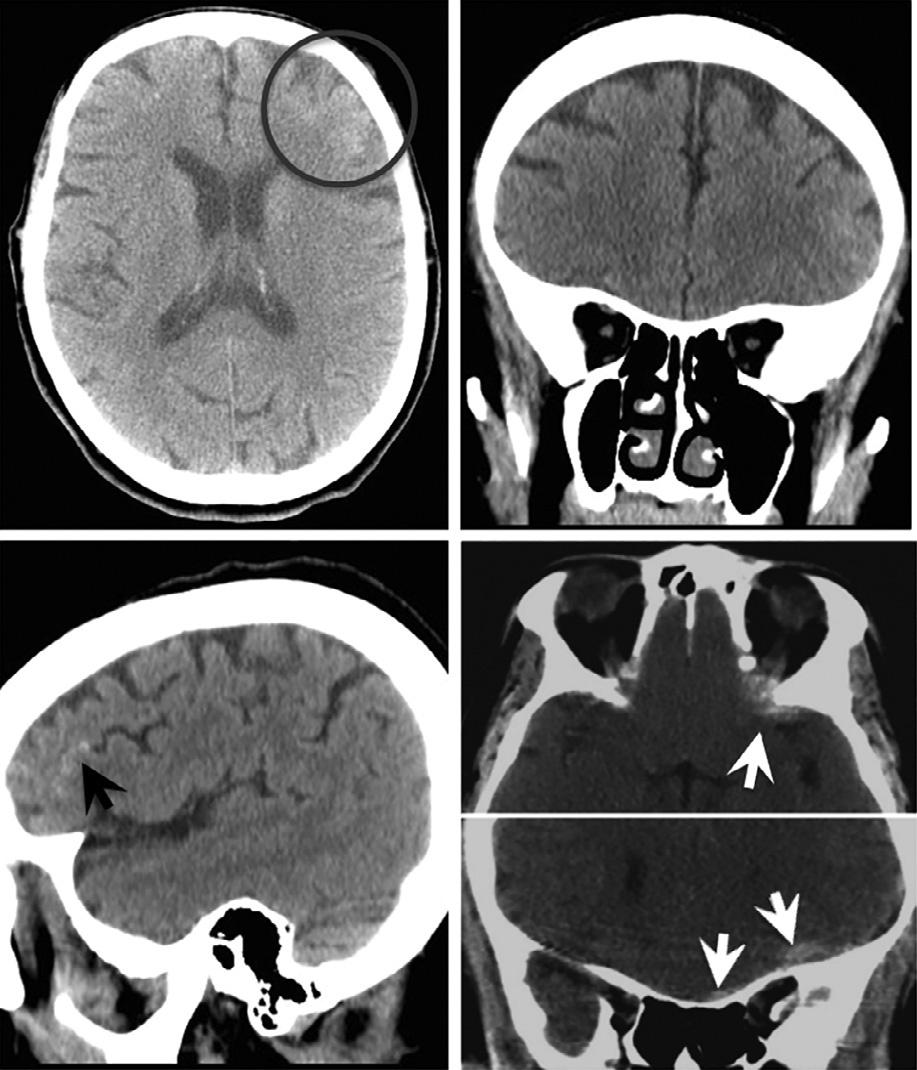
suchsettings,asubtlecrescenticsubduralhematoma ( 90HU)wouldappearequallyasbrightastheadjacent skull( 1000HU),andhencebeundetectable.Conversely,fat( –30HU)andair( –1000HU)would appearequallydark,andhence,airwithinintraorbital fatresultingfromaparanasalsinusfracturewouldalso beundetectable(Fig.1.3).Byexpandingthewindow widthdisplayandcenteringthegrayscaleatahigher HUlevel,thedifferenceindensitybetweenthesame subduralhematomaandadjacentbonecanbemade visuallyapparent.Similarly,ithasbeensuggestedthat subtlevasogenicedemainacutestrokecanbemoresensitivelydetectedbysoftcopyimagereviewusingnarrowedwindowwidthdisplaysettingsthatexaggerate theHUdifferencesbetweengrayandwhitematter (Levetal.,1999).
Givenitsspeed,convenience,lowcostrelativeto MRI,andwidespreadavailability,headCThasbecome afirst-linemethodforassessmentoffocalneurologic symptoms,andhaslargelybecomeanextensionofthe routinephysicalexam.CT,unlikeMRI,isidealforevaluatingfracturesandcalcifications.Amajorstrengthof
Fig.1.3. Computedtomographyimageatlefthascenter-level displaysettingof30HUandwindowwidthof100HU.The imageatrighthascenter-level80HUandwindowwidth 200HU.Withthedisplaysettingsontheright,thesubtlecrescenticsubduralhematomaadjacenttobonebecomesvisually apparent.
CTistherapidandaccuratediagnosisofintracranial hemorrhage,whichappearshyperdense(i.e.,bright)relativetonormalbraintissue.Indeed,currentguidelines forthrombolytictherapywithin4.5hoursofacutestroke
Table1.1
Pearlsandpitfallsofunenhancedheadcomputed tomography
Pearls Pitfalls
Widelyavailable
Accuratemethodfor:
● Intracranialhemorrhage
● Fracture
● Temporalbone evaluation
● Spinalstenosis
● Sinusitis
● Calcificationincentral nervoussystemlesions
● Pre-andpostoperation evaluation
Soft-tissueevaluationwhen magneticresonance imagingis contraindicatedornot available
Radiationexposure
Limitedsensitivityforsofttissueresolution(early ischemicchanges,multiple sclerosislesions,early neoplasticchanges)
Anatomyandpathologymay beobscuredduetopartial volumeaveraging,patient motion,beamhardening, andmetallicstreak artifacts
suchasdemyelinativewhite-matterplaques,orearly strokeedema.Low-contrastresolutionconspicuityis proportionaltoimagenoise;therefore,techniquesthat reducenoisecanimprovesensitivityforlesiondetection. Suchtechniquesincludeusingsoft-tissuekernelimage reconstructionalgorithms,thick-sliceaxialimages (2.5–5mm)forgreatersignalrelativetonoise,optimizedcenterlevelandwindowwidthdisplaysettings, andtheuseofneweriterativereconstructionalgorithms (Fig.1.5)(discussedinfurtherdetailbelow).
Additionaluser-definedspecificscanparameters, suchasX-raybeamenergyinmilliamperes(mA)and kilovoltage(kV),aswellastablespeedandhelicalpitch, canalsobeoptimizedformaximalimagequalityatminimalradiationdose.Detaileddiscussionoftheseimportantparameters,however,isbeyondthescopeofthis chapter.
Computedtomographyangiography(CTA)
onsetrequireanunenhancedheadCTtoruleout hemorrhageastheonlyabsolutecontraindicationto intravenoustissueplasminogenactivator(IV-tPA) administration.Withcurrent-generationheadCTscanners,whole-brainimagescanbeobtainedinseconds, sothatimagereviewcantakeplaceinrealtimeatthe scannerconsole,expeditingclinicalmanagement.Some strengthsandweaknessesofCTareoutlinedin Table1.1
WeaknessesofCTincluderadiationexposure,reconstructionartifacts,limitedsensitivityfordetectingsubtledifferencesinsoft-tissuedensity(e.g.,theearly edemaassociatedwithhyperacutestroke),andpoor interobserverreliability.Indeed,objectivemeasuresof CTimagequalitytypicallydistinguishbetweenhighversuslow-contrastresolutioncapability(Table1.2). Lesionconspicuityisafunctionofsizeanddensityrelativetothatofsurroundingnormaltissues,aswellasthe degreeofimagenoise(so-calledquantummottle).Highcontrast(spatial)resolutionreferstotheabilityto resolvesmallobjectsbutofwidelydifferentdensities thatareveryclosetogetherasdistinctstructures,such asthebonytrabeculaeofthemastoidaircells,nondisplacedskullfractures,orpunctateintracranialhemorrhage.High-contrastresolutionstructurescantypically bemoresensitivelydetectedwiththinnerslices(lessvolumeaveraging)andsharperimagereconstructionalgorithms(e.g.,bonekernel)(Fig.1.4).Low-contrast resolutionreferstotheabilitytoresolveadjacentobjects ofsimilardensitiesthatdifferonlyminimallyinHU,
Neurovascularimagingiscriticalforlocatingarterial occlusions,determiningdegreeofstenosis,andidentifyingdissections,aneurysms,venoussinusthrombosis, andothervascularlesionssuchasarteriovenousmalformations(AVMs).CTA,withCTvenography(CTV),is themostaccuratewidelyavailableminimallyinvasive imagingmethodtoevaluatethevesselsofthehead andneck,and,withgreaterthan95%sensitivityand specificityfordiagnosingproximalarteryocclusion, haslargelyreplaceddirectcatheterarteriographyas thediagnosticmethodofchoiceforemergencyvascular assessmentofstrokeandothercerebrovasculardisorders.CTArequiresintravenousadministrationofiodinatedcontrastsolutionviaapowerinjector.TheCT scannerisprogrammedtodetectthearrivaloftheradiopaquecontrastwithintheaorticarch,andthentriggers scanningforoptimalvascularopacification.CTAcanbe tailoredtooptimallydelineateeitherthearterialor venousphaseofcontrastenhancement,orboth.With modernmultidetectorrowscanners,imagesofthehead andneckarteriescanbeobtainedinunder15seconds, minimizingmotionartifact.
DisadvantagesofCTAincluderadiationexposure andutilizationofiodinatedcontrast,whichmayresult inallergicreactionsorrenalinjury(thelatterespecially inpatientswithdiabetesorpreexistingkidneyimpairment)(Table1.3).AdvantagesofCTAincludehighresolutionimagesfromtheaorticarchtothevertex. Indeed,CTAisoftenusedastheconfirmatorytestor tiebreakerwhenthereisdiscordancebetweencarotid duplexultrasoundimagingandmagneticresonance angiographyforevaluatingthedegreeofcarotidstenosis.Unlikeultrasoundandmagneticresonanceangiography,CTAimagesarenotflow-weighted,andhence,
Table1.3
Advantagesversusdisadvantagesofcomputedtomography (CT)angiography
Advantages Disadvantages
Widelyavailable
Excellentnoninvasive methodtoevaluate vascularanatomyand pathologies:
● Arterialocclusionand stenosis
● Aneurysm
● Arterial-venous malformations
● Venousocclusivedisease
● Vasospasm
● Blood–brainbarrier disruption(neoplasm, infection)
Moresensitivethan noncontrastCTto evaluateparenchymal ischemicchanges
Fastandcritical reconstructionscanbe performedatthescanner consolewithoutneedfor complexpostprocessing
Additionalradiation exposure
Requiresiodinatedcontrast administration(limitation inpatientswithallergyor renalimpairment)
Noflowinformation; providesastaticsnapshot ofvascularanatomy(not reliabletoassessbrain tissueviability)
Vesselpatencymaybe obscuredduetoheavy calcification,beam hardening,andmetallic streakartifacts
Inadditiontoinformationregardingvesselpatency, CTAsourceimages(CTA-SI)canprovideasensitive evaluationofischemicchangeswithinthebrainparenchyma.ParenchymalhypoattenuationonCTA-SIrepresentsdecreasedcontrastopacificationwithinthe capillarybed,andismorereadilydetectablethanan unenhancedCThypodensity(Camargoetal.,2007). Onecaveatisthatthetypeanddegreeoftheperfusion weightingoftheseCTAsourceimagesarehighlyvariable,dependingoncirculationtimeandanumberof otherfactorsrelatedtocollateralflow,sothattheycannotbeusedtoreliablydistinguishtissuelikelytoinfarct (core)fromhighlyischemicbutstillsalvageabletissue (penumbra)(Schrammetal.,2002;Couttsetal.,2004).
Giventhesepotentiallyverylargeaxialimagingdatasets,imagepostprocessingisrequiredtoefficientlyvisualizevesselabnormalitiesandfacilitatediagnoses (Fig.1.6).Inparticular,maximum-intensityprojection (MIP)imagesoftheintracranialcirculationprovidean easywaytodetectproximalarterialocclusionsinstroke patients,forexample,thatmaybeamenabletocatheterbasedtreatments.TheseMIPimagesdepictthehighest densityalongaparticularimagingray.Forevaluation oftheintracranialarteries,MIPimagesreformattedto 20–30mmthicknesswith3–5mmoverlapcanbecreated inaxial,coronal,andsagittalplanesquicklyatthe scannerconsolebytheCTtechnologist.Morecomplex postprocessingtechniquesincludecurvedreformats, multiplanarvolumereformats,andvolume-rendered images.Curvedreformatsdepicttheentirecourseofa particularvesselinasingletwo-dimensionalimage, andprovideagoodevaluationofarterialsteno-occlusive diseaseintheneck,suchasatthecarotidbifurcation. The3Dvolume-renderedandothersurfacetechniques arelesshelpfulforischemicstrokeevaluation,butare routinelyusedinaneurysmdetectionandtreatment planning(Fig.1.7).
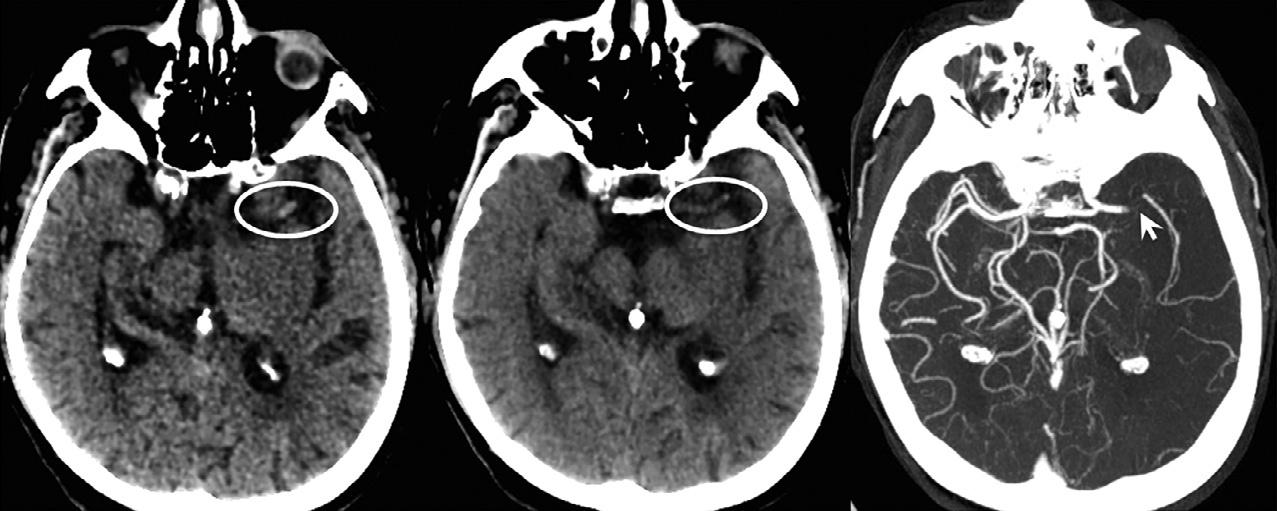
Indeed,therehavebeennumerousstudiesregarding theutilityofCTAforgradingtherobustnessofpialcollateralflowinpatientswithacuteembolicstroke.These gradingschemeshavenotbeengenerallyusefulformakingmanagementdecisionsinindividualpatients,astheir accuracyforpredictingtissueandclinicaloutcome – in theabsenceofearly,robustrecanalization – istypically poor.Anexceptiontothis,however,isthemalignant CTAcollateralprofile,which – again,intheabsence ofearly,robustreperfusion – correlatesstronglywith theconcurrentMRdiffusion-weightedimagingfindings ofirreversibleinfarction(Souzaetal.,2012).ThismalignantCTAcollateralpatternisdefinedasthecomplete absenceofvascularenhancementwithinalargecortical area(typically >33–50%ofamiddlecerebralarterydivision).Asnotedabove,time-resolvedCTAwith4DvolumedynamicCTAscanningshouldproveusefulfor moreaccuratecharacterizationofsuchdelayedcollateralflowpatterns.Specifically,ifintracranialcollateral flowisimagedtooearlyinarterialphase,arrivaltime delayscausedbyslowflow(inthesettingofproximal extracranialorcircle-of-Willismajorarteryocclusions/ stenoses)canresultinafalse-positivemalignantcollateralpattern(Fig.1.8).
CT/CTAselectedtechnical-clinicalpearls andpitfalls
Detaileddiscussionregardingtheimagingevaluationof specificneurologicdisordersisprovidedinsubsequent chapters.Inwhatfollows,selectedtechnicalpearlsand pitfallsforcommonclinicalsituationswillbehighlighted.
AllheadCTinterpretationshouldfollowaconsistent searchpattern,toinsurethatincidentalfindingsarenot overlooked.Thesymmetryoftheventricles,sulci,andcisternsshouldbeassessed;midlineshift,sulcaleffacement, herniation,masslesions,andbleedsintheepidural,subdural,subarachnoid,andparenchymalcompartmentsof thesupratentorialandinfratentorialspacesshouldbe excluded.Extracranially,theglobes,orbits,paranasal
Fig.1.9. Coronalandsagittalreformattedimagescanmitigate volumeaveragingandstreakartifactsfromadjacentbony structures,improvingtheconspicuityofsubtlehemorrhage comparedtothatofthick-sliceaxialimagesalone.
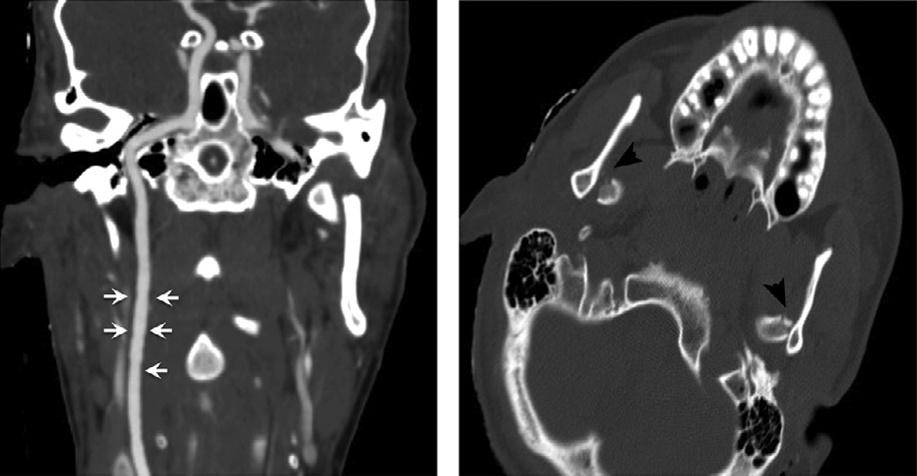
WheninterpretingCTAimages,caremustalsobe takentonotconfusecertainimagingartifactscommonly seeninassociationwithdirectcatheterarteriography withCTAartifacts.Forexample,thedifferential diagnosisofcircumferentialirregularityofthecarotid arterywallinthesettingofbluntcarotidtraumaincludes carotidintimalinjury,fibromusculardysplasia,atheromatousdisease,orpotentiallyapoor-contrastbolus (Fig.1.10).Standingwavesthatarecausedbyvibrations fromhigh-pressurepowerinjectionofcontrastduring selectivecatheterarteriographyarenotincludedinthis differentialdiagnosis.
Fig.1.10. Subtleinternalcarotidintimalirregularity(arrows) causedbyblunttrauma,withbilateraldisplacedmandibular condylefractures(arrowheads).
Similarly,anintraluminalfillingdefectintheproximalandmidbasilararteryonCTAislikelytorepresent afree-floatingthrombus,orpotentiallybeam-hardening artifact,butshouldneverbemistakenformixingartifact,whichagainisaphenomenonexclusivelyassociatedwithdirectcatheterarteriography(Fig.1.11). Mixingartifactoccursduringselectiveinjectionofcontrastintoonevertebralartery,whenunopacifiedblood fromthecontralateralvertebralarterymixeswiththe contrastcolumninthebasilarlumen.WithCTA,for whichcontrastisadministeredintravenously,mixing occursintheheartandlungs,withuniformlyopacified bloodexitingtheaorta.
Stroke – technicalpearlsandpitfalls
Imagingindicationsaredrivenbytheavailablemanagementoptions.Hence,itwasnotuntilFoodandDrug Administrationapprovalofthrombolytictreatmentfor strokeusingIV-tPAin1996thattheuseofCTforstroke assessmentbecamethestandardofcare(NINDS,1995). Intracranialhemorrhageisanabsolutecontraindication toIV-tPAadministration(vonKummeretal.,1997). Alarge(>30%)middlecerebralarteryterritorylowdensitylesionsuggestingestablishedinfarctisarelative contraindication,owingtoincreasedhemorrhagicrisk (vonKummeretal.,2001).Giventhelowsensitivity andspecificityoftheearlyCTsignsofstroke,compared tothatofMRdiffusion-weightedimaging,obtaininga correlativeclinicalhistoryofabruptonsetoffocalneurologicsymptoms – priortoCTimageinterpretation – is essential(Mullinsetal.,2002).
AnearlyCTsignofembolicstrokethatmighthelp guidepatientselectionforintra-arterialclotretrieval therapyisthehyperdensevesselsign(Fig.1.12).More distalclotsinthird-orderbranchesmaybeidentified asdotsigns.Arecentretrospectivestudyshowedthat patientswithCThyperdenseclotlengths > 8mm,as measuredonthin-sectionCT,haveanear-zeroprobabilityofrespondingtoIV-tPAalone;hence,suchpatients mightbenefitfromintra-arterialclotretrieval (Somfordetal.,2002;Riedeletal.,2011;Kamalian etal.,2013).
AclassicearlyischemicCTsignisfocalcorticalswelling.OtherearlyCTimagingsignsofstrokeinclude obscurationofthelentiformnucleusandinsularribbon (Fig.1.13)sign,bothattributabletolossofgray/whitematterdifferentiationwithhypodensityrelatedtovasogenicedema(Tomuraetal.,1988;Truwitetal.,1990). Thedetectionoftheseearlystrokesignsvariesbetween observers,buttheyaretypicallyseeninlessthantwothirdsofpatientsimagedat3hourspoststrokeonset. Parenchymalhypoattenuationisrelatedtoincreased watercontentfromvasogenicedemaandappearsto
Fig.1.11. Fillingdefectwithinbasilararteryduetointraluminalfree-floatingthrombus,withassociatedleftsuperiorcerebellar infarctseenondiffusion-weightedmagneticresonanceimaging.Thefillingdefectshouldnotbeattributedtomixingartifactinthe absenceofdirectcatheterarteriography.
Fig.1.12. Hyperdenseleftmiddlecerebralarteryclotisvisibleontheaxialthin,1.25-mmcomputedtomography(CT)slice,but onlyasadotsignonthethick,5-mmCTsliceduetopartialvolumeaveraging;confirmedontheaxialCTangiographymaximumintensityprojectionimage.
Fig.1.13. Hypoattenuationoftheleftinsularribbon,anearly ischemiccomputedtomographysignofstroke,bettervisualized withnarrowwindowwidthdisplaysettings(35HU,right),than atstandardwindowwidthdisplaysettings(70HU,left).
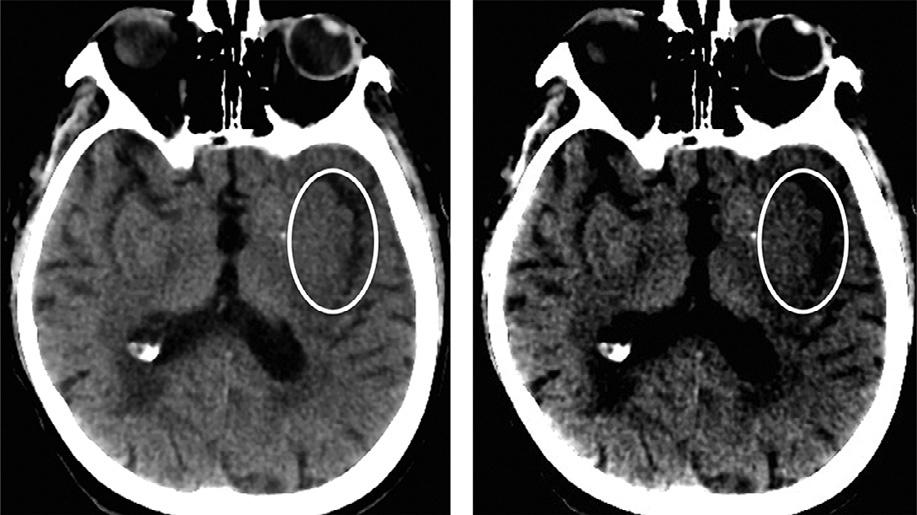
beasignofirreversibletissueinjury,whilerecentstudies suggestthatfocalswellingalonemaybereversible. A10%increaseintissuewatercorrespondstoa20–30 HUdecreaseintissuedensity.
Nontraumaticintracranialhemorrhage –technicalpearlsandpitfalls
Thisgroupincludesintraparenchymalhemorrhage (IPH)andSAH.AcuteSAHisdetectedonCTas
hyperdensity(bloodclotdensity)inthecerebrospinal fluidspacessurroundingthebrain.AlthoughtheprimarycauseofSAHisrupturedaneurysm,itcanalso beduetointracranialdissection,trauma,vasculitis, duralAVM,orcervicalfistulas.CTAishighlyaccurate todetectaneurysms,withaccuraciesapproachingthat ofdigitalsubtractioncatheterarteriography(thedetectionrateforaneurysms 3mmiscloseto100%).Of note,aneurysmruptureneednotnecessarilypresent asSAH;forexample,ifthedomeofatop-of-internal carotidarteryaneurysmispointingsuperiorlyintothe brainparenchyma,aneurysmrupturecandissectinto theadjacentbraintissuecausingtheappearanceof anIPH,whichcanmimicahypertensivehemorrhage (Fig.1.14).
ThevastmajorityofIPHsareprimary;these areoftenrelatedtohypertensionand/oranticoagulation.Hypertensivebleedstypicallyaffectthebasal ganglia,pons,anddeepcerebellarnuclei.Imagingis criticalforidentifyingpotentialcausesofsecondary IPH,whichincludeAVM,aneurysm,venoussinus thrombosis,tumor,andvasculitis.OnCTA,cluesto thediagnosisofAVMincludenumerousenlargedvesselscorrespondingtofeedingarteries,thevascular nidusordrainingveins,aswellasassociated phleboliths.
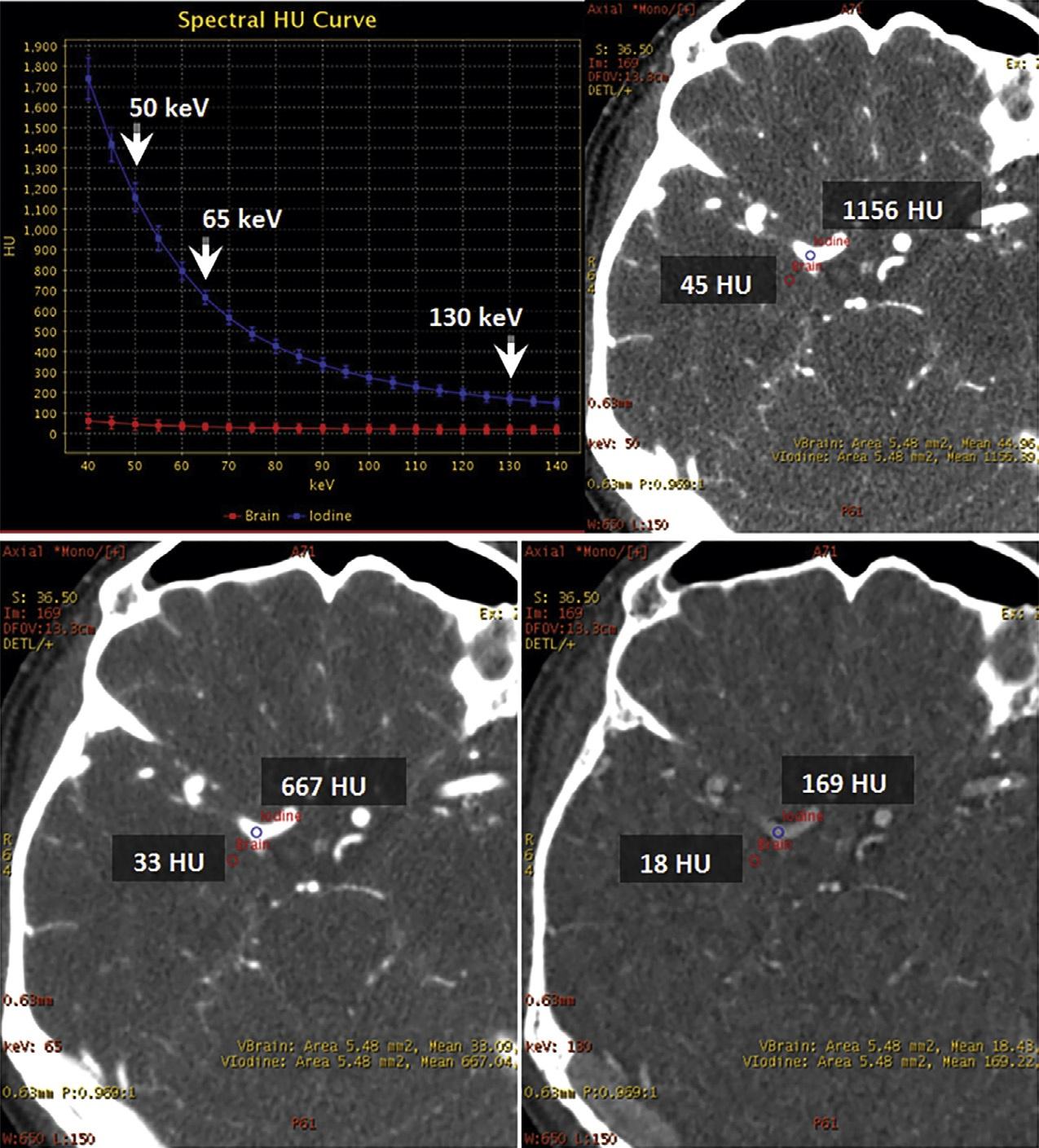
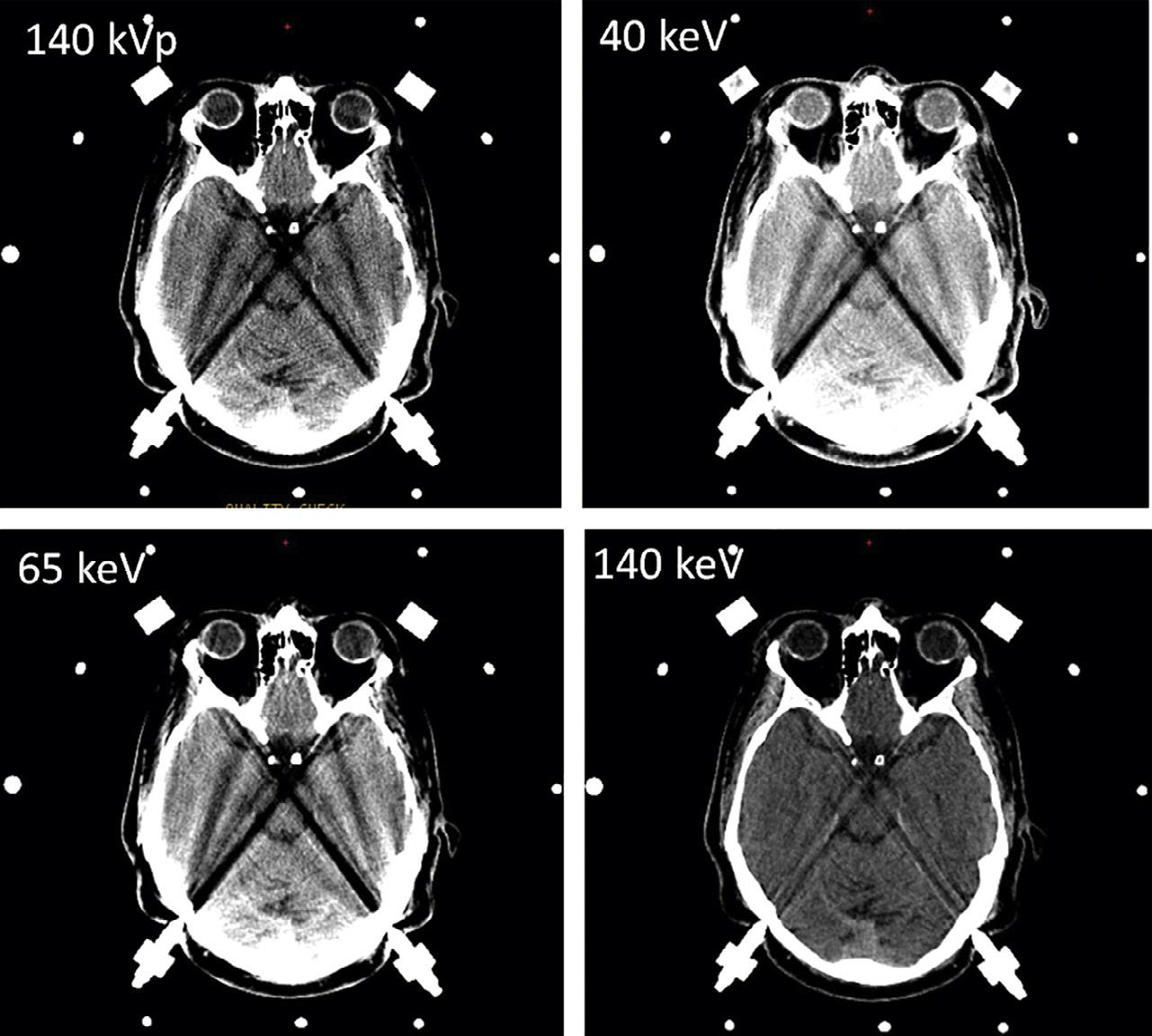
Fig.1.17. Dual-energyvirtualmonochromaticimagesofacontrast-enhancedbrainatthreedifferentenergylevels:50keV(top right),65keV(bottomleft),and130keV(bottomright).Withdual-energycomputedtomography,virtualmonochromaticreconstructionatlowerkeVlevelsimprovesintravascularenhancementandcontrast-to-noiseratioastheX-rayphotonenergymoves closertothe k-edgeofiodine(33.2keV).Ascanbeseen,theattenuationwiththevesseljumpsfrom169HUto1156HUwhenwe movefrom130keVto50keV.Withthischange,thebrainparenchymaonlygoesfrom18HUto45HU.Thisfactcanbeusedto drasticallycutdowntheamountofcontrastthatisadministered,withobviousbenefitsintermsofrenalhealth.
phenomenonmanifestsitselfaslinearstreaksinthe images,asshowninthecaseofasurgicalfixationframe in Figure1.18.Asthisfigureillustrates,onecansubstantiallyreducetheseartifactsbyincreasingthesimulated monochromaticenergylevel.Anotherexampleofthis useofDECTisshowninatraumaCTA,performed forassessingpotentialinvolvementofthecarotidartery byaforeignbodywithsubstantialmetalinit(Fig.1.19). Thesingle-energyimagesinthiscasewereunrevealing becauseofexcessivesprayartifactfromthemetal. High-keVvirtualmonochromaticimages,however, clearlydemonstratedthatthecavernouscarotidwas notinvolved.
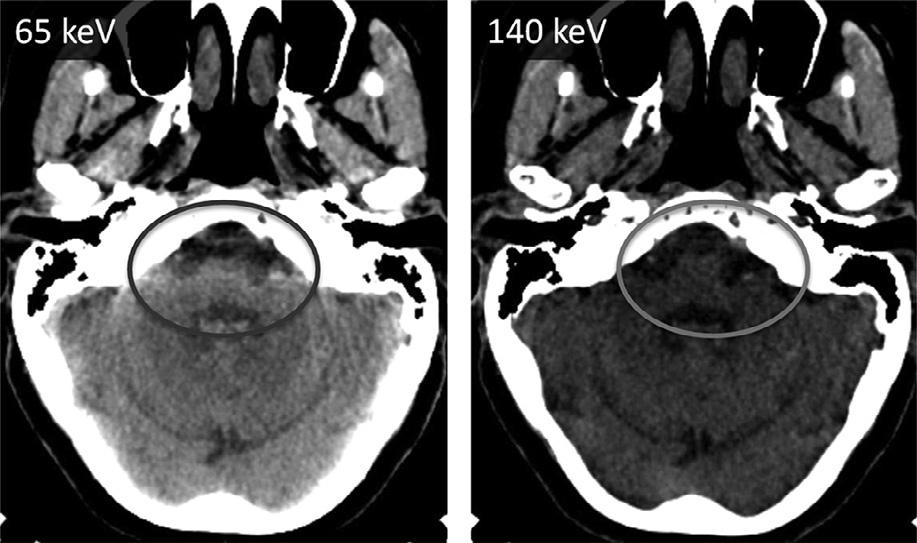
Thesametrick,infact,canbeusedwithanysubstancewithhighdensitythatcausesbeamhardening. Forexample,thevisualizationoftheposteriorfossacontents,especiallythebrainstem,isseverelydegradedby beam-hardeningartifactarisingfromthepetrousridges
laterallyandtheclivusanteriorly.Asshownin Figure1.20,onecanconsiderablyreducethisartifact usingthevirtualmonochromaticimages.
Iterativereconstructionalgorithms
AnX-rayimageprovidesasuperpositionofallthestructuresinthepathoftheX-raybeam.InCT,closeto1000 suchprojectionimagesareacquiredandconverted intotomographicslicedatausingaspecializedreconstructionalgorithm.JohannRadon,anAustrianmathematician,providedthemathematicbasisforthis conversionprocessalmostacenturyago.Radonproved thata2-Dfunction(e.g.,animageofatomographicslice throughthebody)ismathematicallyequivalenttoitsprojections.Itwasnotuntiltheearly1970sthatSirGodfrey HounsfieldrecognizedthatX-raysprovidedanexperimentalmethodforobtainingasetofprojectionimages
Fig.1.18. Dual-energycomputedtomographyofstereotacticframeimagingforpre-opmeasurements;reductionofbeamhardeningduetometallicframe.
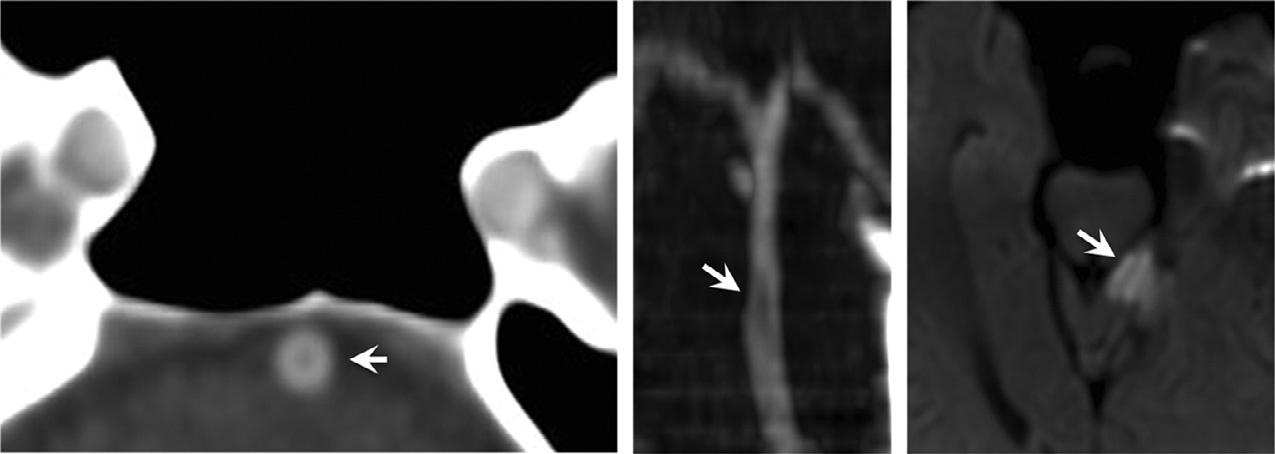
Fig.1.19. Anexampleofvirtualmonochromaticimagingat 180keVformetalartifactreductioninapatientwithaforeign body(aballpointpen)inthesphenoidsinus.Despitethepresenceofmetal,onecanclearlydiscernthatthecarotidarteryis notinvolvedbythetrauma.
ofanyobject,thatare,inafundamentalmathematic sense,equivalenttothetomographicimagesofthat object.
Themostcommonmethodforconvertingasetof projectionimagesintothecorrespondingsetoftomographicslicesisviaanalgorithmcalledfilteredbackprojectionorFBP(RamachandranandLakshminarayanan, 1971).Inthisalgorithm,thesetofprojectionsareconvolvedwithafunctioncalledakernel,andthenprojected
Fig.1.20. Anexampleofvirtualmonochromaticimagingfor posteriorfossabeam-hardeningartifactreduction.
intothetomographicplane.FBPprovidesananalytic methodforimagereconstruction;noattemptismade tominimizetheoverallerrorbetweenthereconstructed tomographicimageanditscorrespondingsetofprojectionimages.Suchanalyticreconstructionalgorithms provideasingle-passsolutiontothereconstructiontask: eachprojectionisconvolvedwiththekernelandthen backprojectedwithnoattemptaterrorminimization. Asaresult,FBPisveryfastandnearlyuniversallyavailableonallCTscanners.However,thisalgorithmisprone tonoiseandartifacts,especiallyinthepresenceofbeam hardeningandmetallicobjectsinthefieldofview.FBP