https://ebookmass.com/product/nanostructured-functional-and-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Biomass-Derived Carbon Materials: Production and Applications Alagarsamy Pandikumar
https://ebookmass.com/product/biomass-derived-carbon-materialsproduction-and-applications-alagarsamy-pandikumar/
ebookmass.com
Graphene-Based Electrochemical Sensors for Biomolecules Alagarsamy Pandikumar
https://ebookmass.com/product/graphene-based-electrochemical-sensorsfor-biomolecules-alagarsamy-pandikumar/
ebookmass.com
Energy Storage Systems: System Design and Storage Technologies Armin U. Schmiegel
https://ebookmass.com/product/energy-storage-systems-system-designand-storage-technologies-armin-u-schmiegel/
ebookmass.com
Human Communication: University of Central Florida 7th Edition Judy C. Pearson
https://ebookmass.com/product/human-communication-university-ofcentral-florida-7th-edition-judy-c-pearson/
ebookmass.com
Narratives of African American Women's Literary Pragmatism and Creative Democracy 1st ed. Edition Gregory Phipps
https://ebookmass.com/product/narratives-of-african-american-womensliterary-pragmatism-and-creative-democracy-1st-ed-edition-gregoryphipps/
ebookmass.com
Introduction to global studies Second Edition John Mccormick
https://ebookmass.com/product/introduction-to-global-studies-secondedition-john-mccormick/
ebookmass.com
DiPiro's Pharmacotherapy: A Pathophysiologic Approach, 12th Edition Dipiro
https://ebookmass.com/product/dipiros-pharmacotherapy-apathophysiologic-approach-12th-edition-dipiro/
ebookmass.com
The Greek Life of Adam and Eve 1st Edition John R. Levison
https://ebookmass.com/product/the-greek-life-of-adam-and-eve-1stedition-john-r-levison/
ebookmass.com
Panther's Catch (Animal Rescue Shifters Book 3) Zoe Chant
https://ebookmass.com/product/panthers-catch-animal-rescue-shiftersbook-3-zoe-chant/
ebookmass.com
Weinstein
https://ebookmass.com/product/venomous-bites-from-non-venomous-snakesscott-a-weinstein/
ebookmass.com
NANOSTRUCTURED, FUNCTIONAL,AND FLEXIBLEMATERIALS FORENERGY CONVERSIONAND STORAGESYSTEMS Editedby
ALAGARSAMYPANDIKUMAR
PERUMALRAMESHKUMAR
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyright LicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby thePublisher(otherthanasmaybenotedherein).
Notices Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professional practices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasa matterofproductsliability,negligenceorotherwise,orfromanyuseoroperationofany methods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-819552-9
ForinformationonallElsevierpublicationsvisitourwebsite at https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: ChristinaGifford
EditorialProjectManager: GabrielaD.Capille
ProductionProjectManager: NirmalaArumugam
CoverDesigner: MarkRogers
TypesetbyTNQTechnologies
Contributors
RafaelAbargues
UMDO,InstitutodeCienciadelosMateriales,UniversidaddeValencia,Valencia,Spain
SouravAcharya
DepartmentofChemistry,IIT(ISM)Dhanbad,Dhanbad,Jharkhand,India
SomasundaramAnbuAnjugamVandarkuzhali
NationalCentreforCatalysisResearch,IndianInstituteofTechnologyMadras,Chennai, TamilNadu,India
A.Arulraj
GrapheneandAdvanced2DMaterialsResearchGroup(GAMRG),SchoolofScienceand Technology,SunwayUniversity,Selangor,Malaysia
SaravanaVadivuArunachalam
DepartmentofChemistry,SchoolofAdvancedSciences,KalasalingamAcademyof ResearchandEducation,Virudhunagar,TamilNadu,India
NorfatehahBasiron
SchoolofMaterials&MineralResourcesEngineering,EngineeringCampus,Universiti SainsMalaysia,NibongTebal,PulauPinang,Malaysia
R.JosephBensingh
AdvancedResearchSchoolforTechnologyandProductSimulation(ARSTPS),Schoolfor AdvancedResearchinPolymers(SARP),CentralInstituteofPlasticsEngineeringand Technology(CIPET),Chennai,TamilNadu,India
GaneshChandraNayak
DepartmentofChemistry,IIT(ISM)Dhanbad,Dhanbad,Jharkhand,India
MyongYongChoi
DepartmentofChemistryandResearchInstituteofNaturalScience,GyeongsangNational University,Jinju,Gyeongnam,RepublicofKorea
ShrabaniDe
DepartmentofChemistry,IIT(ISM)Dhanbad,Dhanbad,Jharkhand,India
DuraisamiDhamodharan
CASKeyLaboratoryofDesignandAssemblyofFunctionalNanostructure,FujianKey LaboratoryofNanomaterials,FujianInstituteofResearchontheStructureofMatter, ChineseAcademyofSciences,Fuzhou,Fujian,China
NidhinDivakaran
CASKeyLaboratoryofDesignandAssemblyofFunctionalNanostructure,FujianKey LaboratoryofNanomaterials,FujianInstituteofResearchontheStructureofMatter, ChineseAcademyofSciences,Fuzhou,Fujian,China
KingshukDutta
AdvancedResearchSchoolforTechnologyandProductSimulation(ARSTPS),Schoolfor AdvancedResearchinPolymers(SARP),CentralInstituteofPlasticsEngineeringand Technology(CIPET),Chennai,TamilNadu,India
ChandraSekharEspenti
DepartmentofChemistry,RajeevGandhiMemorialCollegeofEngineeringand Technology,Kurnool,AndhraPradesh,India
A.Gowrisankar
DepartmentofChemistry,BharathiarUniversity,Coimbatore,TamilNadu,India
VasanthRajendiranJothi
DepartmentofChemicalEngineering,HanyangUniversity,Seongdong-gu,Seoul, SouthKorea
Ho-YoungJung
DepartmentofEnvironment&EnergyEngineering,ChonnamNationalUniversity, Gwangju,RepublicofKorea;CenterforEnergyStorageSystem,ChonnamNational University,Gwangju,RepublicofKorea
M.AbdulKader
AdvancedResearchSchoolforTechnologyandProductSimulation(ARSTPS),Schoolfor AdvancedResearchinPolymers(SARP),CentralInstituteofPlasticsEngineeringand Technology(CIPET),Chennai,TamilNadu,India
ManojB.Kale
CASKeyLaboratoryofDesignandAssemblyofFunctionalNanostructure,FujianKey LaboratoryofNanomaterials,FujianInstituteofResearchontheStructureofMatter, ChineseAcademyofSciences,Fuzhou,Fujian,China
K.Karthick
AcademyofScientificandInnovativeResearch(AcSIR),CSIR-Campus,NewDelhi, India;MaterialsElectrochemistry(ME)Division,CSIR-CentralElectrochemicalResearch Institute(CECRI),Karaikudi,TamilNadu,India
K.Karuppasamy
DivisionofElectronicsandElectricalEngineering,DonggukUniversity-Seoul,Seoul, SouthKorea
Hyun-SeokKim
DivisionofElectronicsandElectricalEngineering,DonggukUniversity-Seoul,Seoul, SouthKorea
VijayS.Kumbhar
DepartmentofEnergyChemicalEngineering,SchoolofNano&MaterialsScienceand Engineering,KyungpookNationalUniversity,Sangju,Gyeonsang-daero,Republicof Korea
SubrataKundu
MaterialsElectrochemistry(ME)Division,CSIR-CentralElectrochemicalResearch Institute(CECRI),Karaikudi,TamilNadu,India
N.LakshmanaReddy
NanocatalysisandSolarFuelsResearchLaboratory,DepartmentofMaterialsScience& Nanotechnology,YogiVemanaUniversity,Kadapa,AndhraPradesh,India;Departmentof EnergyChemicalEngineering,SchoolofNano&MaterialsScienceandEngineering, KyungpookNationalUniversity,Sangju,Gyeonsang-daero,RepublicofKorea
SeungJunLee
DepartmentofChemistryandResearchInstituteofNaturalScience,GyeongsangNational University,Jinju,Gyeongnam,RepublicofKorea
KiyoungLee
DepartmentofEnergyChemicalEngineering,SchoolofNano&MaterialsScienceand Engineering,KyungpookNationalUniversity,Sangju,Gyeonsang-daero,Republicof Korea
N.Malarvizhi
DepartmentofChemistry,GuruNanakCollege,Chennai,TamilNadu,India
JuanP.Martínez-Pastor
UMDO,InstitutodeCienciadelosMateriales,UniversidaddeValencia,Valencia,Spain
SuhailMubarak
CASKeyLaboratoryofDesignandAssemblyofFunctionalNanostructure,FujianKey LaboratoryofNanomaterials,FujianInstituteofResearchontheStructureofMatter, ChineseAcademyofSciences,Fuzhou,Fujian,China
A.Murali
SchoolforAdvancedResearchinPolymers(SARP)-ARSTPS,CentralInstituteofPlastics Engineering&Technology(CIPET),Chennai,TamilNadu,India
A.Nichelson
DepartmentofPhysics,NationalEngineeringCollege,K.R.Nagar,Kovilpatti,Tamil Nadu,India
S.T.Nishanthi
ElectrochemicalPowerSourcesDivision,CSIR-CentralElectrochemicalResearch Institute(CECRI),Karaikudi,TamilNadu,India
AlagarsamyPandikumar
FunctionalMaterialsDivision,CSIR-CentralElectrochemicalResearchInstitute, Karaikudi,TamilNadu,India
S.Priya
DepartmentofPlantBiology&Biotechnology,LoyolaCollege,Chennai,TamilNadu, India
AlagarRamar
GraduateInstituteofAppliedScienceandTechnology,NationalTaiwanUniversityof ScienceandTechnology,Taipei,Taiwan,RepublicofChina
PedroJ.Rodríguez-Canto
UMDO,InstitutodeCienciadelosMateriales,UniversidaddeValencia,Valencia,Spain
T.Sadhasivam
DepartmentofEnvironment&EnergyEngineering,ChonnamNationalUniversity, Gwangju,RepublicofKorea;CenterforEnergyStorageSystem,ChonnamNational University,Gwangju,RepublicofKorea
KhairulArifahSaharudin
SchoolofMaterials&MineralResourcesEngineering,EngineeringCampus,Universiti SainsMalaysia,NibongTebal,PulauPinang,Malaysia
SumantaSahoo
DepartmentofChemistry,IIT(ISM)Dhanbad,Dhanbad,Jharkhand,India
M.Sakar
CentreforNanoandMaterialSciences,JainUniversity,Bangalore,Karnataka,India
S.SamSankar
AcademyofScientificandInnovativeResearch(AcSIR),CSIR-Campus,NewDelhi, India;MaterialsElectrochemistry(ME)Division,CSIR-CentralElectrochemicalResearch Institute(CECRI),Karaikudi,TamilNadu,India
T.Saravanakumar
DepartmentofNanoscienceandTechnology,AnnaUniversityRegionalCampus, Coimbatore,TamilNadu,India
M.Selvaraj
DepartmentofChemistry,GuruNanakCollege,Chennai,TamilNadu,India
T.Selvaraju
DepartmentofChemistry,BharathiarUniversity,Coimbatore,TamilNadu,India
T.Senthil
AdvancedResearchSchoolforTechnologyandProductSimulation(ARSTPS),Schoolfor AdvancedResearchinPolymers(SARP),CentralInstituteofPlasticsEngineeringand Technology(CIPET),Chennai,TamilNadu,India
M.V.Shankar
NanocatalysisandSolarFuelsResearchLaboratory,DepartmentofMaterialsScience& Nanotechnology,YogiVemanaUniversity,Kadapa,AndhraPradesh,India
ParamasivamShanmugam
DepartmentofChemistry,St.JosephUniversity,Dimapur,Nagaland,India
SubramanianSingaravadivel
DepartmentofChemistry,SSMInstituteofEngineeringandTechnology,Dindigul,Tamil Nadu,India
GandhiSivaraman
DepartmentofChemistry,GandhigramRuralInstitute DeemedtobeUniversity, Gandhigram,TamilNadu,India
AnanthakumarSoosaimanickam
UMDO,InstitutodeCienciadelosMateriales,UniversidaddeValencia,Valencia,Spain
SrimalaSreekantan
SchoolofMaterials&MineralResourcesEngineering,EngineeringCampus,Universiti SainsMalaysia,NibongTebal,PulauPinang,Malaysia
TammineniVenkataSurendra
DepartmentofChemistry,RajeevGandhiMemorialCollegeofEngineeringand Technology,Kurnool,AndhraPradesh,India
WaqasHassanTanveer
ResearchCentreforCarbonSolutions,Heriot-WattUniversity,Edinburgh,United Kingdom;DepartmentofMechanicalEngineering,SchoolofMechanicaland ManufacturingEngineering,NationalUniversityofSciencesandTechnology(NUST), Islamabad,Pakistan
JayaramanTheerthagiri
DepartmentofChemistryandResearchInstituteofNaturalScience,GyeongsangNational University,Jinju,Gyeongnam,RepublicofKorea;CentreofExcellenceforEnergy Research,CentreforNanoscienceandNanotechnology,SathyabamaInstituteofScience andTechnology(DeemedtobeUniversity),Chennai,TamilNadu,India
DhanasekaranVikraman
DivisionofElectronicsandElectricalEngineering,DonggukUniversity-Seoul,Seoul, SouthKorea
Fu-MingWang
GraduateInstituteofAppliedScienceandTechnology,NationalTaiwanUniversityof ScienceandTechnology,Taipei,Taiwan,RepublicofChina
LaiChinWei
Nanotechnology&CatalysisResearchCentre,InstituteforAdvancedStudies,University ofMalaya,KualaLumpur,Malaysia
LixinWu
CASKeyLaboratoryofDesignandAssemblyofFunctionalNanostructure,FujianKey LaboratoryofNanomaterials,FujianInstituteofResearchontheStructureofMatter, ChineseAcademyofSciences,Fuzhou,Fujian,China
Sung-ChulYi
DepartmentofChemicalEngineering,HanyangUniversity,Seongdong-gu,Seoul,South Korea;DepartmentofHydrogenandFuelCellTechnology,HanyangUniversity,Seoul, SouthKorea
CHAPTER1 Basicprinciplesinenergy conversionandstorage JayaramanTheerthagiri1, 2, a,SeungJunLee1, a , ParamasivamShanmugam3,MyongYongChoi1
1DepartmentofChemistryandResearchInstituteofNaturalScience,GyeongsangNationalUniversity, Jinju,Gyeongnam,RepublicofKorea; 2CentreofExcellenceforEnergyResearch,Centrefor NanoscienceandNanotechnology,SathyabamaInstituteofScienceandTechnology(Deemedtobe University),Chennai,TamilNadu,India; 3DepartmentofChemistry,St.JosephUniversity,Dimapur, Nagaland,India
1.Introduction Energyisindisputablyoneoftheforemostissuesofmodernsocietyand playsanimportantroleineconomicgrowth.Inthecurrentenergyscenario,researchershaveparticularlyfocusedontheproductionandconsumptionofenergy.However,energyconsumptionincreaseisinevitable becauseofthegrowingpopulationandeconomicgrowthindeveloping countries.Nevertheless,thetremendousexploitationoffossilfuelsas nonrenewablesourceshasraisedconcernsaboutthelackofenergyresourcesandCO2 emissionsthatdeterioratetheenvironment[1 3].To overcometheseissues,anaffordable,clean,andrenewableenergyresource, whichcanbeanalternativetofossilfuels,isurgentlyrequired.Asan importantsteptowardtheutilizationofrenewableenergyresources,energy storageandconversiondeviceshaveattractedglobalattention.Highly efficientelectrochemicalenergystorageandconversiondeviceswith minimaltoxicity,lowcost,and flexibilityinenergyutilizationare consideredtomeettheever-expandingenergydemandinelectricvehicles (EV),consumerelectronics,andminiaturizeddevices.
Theperformanceoftheelectrochemicalenergystorageandconversion devicesiscloselyassociatedwithphysicochemicalpropertiesofmaterials utilized.Forexample,materialswithlimitedelectrochemicalactivesurface sitesandbulkmaterialswithslowdiffusioncannotbeutilizedinenergy devicessuchasbatteriesandsupercapacitors.Also,thelowelectrocatalytic behaviorofenergymaterialsisnotsuitableformakinghighlyefficient
a Theseauthorscontributedequallytothiswork.
Nanostructured,Functional,andFlexibleMaterialsforEnergy ConversionandStorageSystems
ISBN978-0-12-819552-9 https://doi.org/10.1016/B978-0-12-819552-9.00001-4
2 Nanostructured,Functional,andFlexibleMaterialsforEnergyConversionandStorageSystems
dye-sensitizedsolarcells(DSSCs)andfuelcells[4 6].Ontheotherhand, nanomaterialswithexceptionalsurfacestructureshavegainedimmense interestbecauseoftheiruniqueelectricalandmechanicalproperties,and theirsurfacepropertiesalsoplayavitalroleintheelectrochemicalbehavior. Theadventofnanotechnologyoffersanewplatformforthedesignand fabricationofenergymaterialswithnanosizedstructures.Thus,theperformanceofelectrochemicalenergystorageandconversiondeviceshas highlybenefittedfromthe fieldofnanotechnology.
Thischapteroutlinesthespecificfeatures,basiclandscape,general components,andperformanceevaluationofvariouselectrochemicalenergy storageandconversiondevices,suchasbatteries,supercapacitors,DSSCs, photocatalytichydrogenproductionviawatersplitting,andfuelcells.Also, nanostructuredmaterialsinenergystorageandconversiontechnologiesare emphasized.
2.Lithiumbatteries Lithium-ionbatteries(LIBs)arethemostpromisingcandidatesforportable electronicsandEVapplications.Itwas firstdevelopedinJapanbyAsahi KaseiCompanyin1991.The firstrechargeableLIBmadeupofLiCoO2 cathodeandgraphiteanodewascommercializedbySony[7].Currently, availableLIBsinthemarketpossesshighenergydensityandgoodperformance,aslithiumisthelightestmetalandmostelectropositivemetallic element( 3.04Vvs.standardhydrogenelectrode)andthereforeenables anelectrochemicalstoragedevicewithhighenergydensities[8].Moreover, LIBscanundergomorethan1000charge/dischargecyclesandcanbe manufacturedinvarioussizesanddimensions.ThemaintenanceofLIBsis quitesimplecomparedwiththeotherbatterytechnologies,suchas lead acid,Na NiCl2,andNi MHbatteries.TheLIBtechnologyhas beenwidelyusedinelectronicdevicesandhasrecentlybeenintroducedto thehybridEVmarketasasuitablecandidatetopowerelectriccars[9].Still, researchershavebeenfocusingonelectrodes,electrolytematerials,and designsofthistechnologytodecreasethecost,improvethecyclinglife,and increasethesafety.
2.1Batteryprincipleandbasics ALIBisatypeofrechargeableenergystoragedevicethatconvertsstored chemicalenergyintoelectricalenergybymeansofchemicalreactionsof lithium.ThesimplestunitofLIBscalledelectrochemicalcellconsistsof
threekeycomponents:cathode,anode,andelectrolyte.Faradaicredox reactionstakeplaceatalowerelectrodepotentialcalledtheanode(negative electrode)andamorepositiveelectrodecalledthecathode.The first workingrechargeableLIBsconsistedofLiCoO2 asthepositiveelectrode andgraphiteasthenegativeelectrode[10]. Fig.1.1 showstheschematic diagramoftheLIBdesign.IntheLIBs,Liþ ionsaretransferredbetweenthe cathodeandanodeduringchargeanddischargeprocesses.TheLIBdesign shownin Fig.1.1 isanexampleof “rockingchair” battery[11],whereLiþ ionsaredeintercalatedfromthelayeredlithiumcobaltoxideduringthe chargingprocess(oxidationofLiCoO2).ThedeintercalatedLiþ ionsfrom LiCoO2 migratetothegraphitetoformLiC6.Duringthedischargeprocess,thereversereactionofLiþ ionsisintercalatedtoLixCoO2.The electrochemicalreactionsatthepositiveandnegativeelectrodeduringthe charge/dischargeprocessaregivenbelow[12]:
LiMO 2 #Li1 x MO 2 þ xLiþ þ xe
Figure1.1 SchematicrepresentationofLi-ionbatterydesignusingaLiCoO2 cathode andgraphiteanode [13].Copyright(2020)AmericanChemicalSociety.
Positive:
OneofthethreemaincomponentsinLIBsisthecathode/positive electrode.ThecapacityandvoltageoftheLIBsdependonthecathode material,whichisthelimitingfactorofthedevice.Forexample,the theoreticalcapacityofLiFePO4 islimitedto170mAhg 1 basedontheone Liþ transfer,althoughthetheoreticalcapacityoftheanodematerial, graphite,is372mAhg 1.ElectrolytesareusedtotransportLiþ ionsfrom oneelectrodetoanotherelectrode.Theycanbedividedintotwotypes:(1) liquidelectrolyteand(2)solidelectrolyte.Theliquidelectrolytes,suchas NaPF6 inethylenecarbonate/propylenecarbonate,requireseparatorto preventshortcircuits.However,solidelectrolytesdonotneedanyadditionalseparatorastheycanfunctionasbothaseparatorandanelectrolyte. Inaddition,electrolytesshouldhavehighionicconductivityandbroad operatingvoltagerangewithoutanydecomposition[13].
3.Supercapacitors Asupercapacitorisanelectrochemicalenergystoragedevice,whichcanbe usedtostoreanddeliverchargebyreversibleadsorptionanddesorptionof ionsattheinterfacebetweentheelectrodematerialandelectrolyte. Supercapacitorsarealsocalledultracapacitorsorelectrochemicalcapacitors. Theycanwithstandmanychargeanddischargecyclescomparedto rechargeablebatteries.Supercapacitorshavemanyadvantages,suchashigh powerdensityandspecificcapacitance(SC),longlifecycle,ecofriendliness, and flexibilityofworkingtemperature[14,15].Inaddition,theycanrapidly chargewithquickpowerconveyanceandarecompetenttoreplaceconventionalcapacitors.Also,supercapacitorscanactlikebridgesanddecrease thegapamongcapacitors,batteries,orfuelcells.Theoperatingvoltage rangeofastandardcapacitorisveryhigh,butforsupercapacitors,itis between2.5and2.7V.
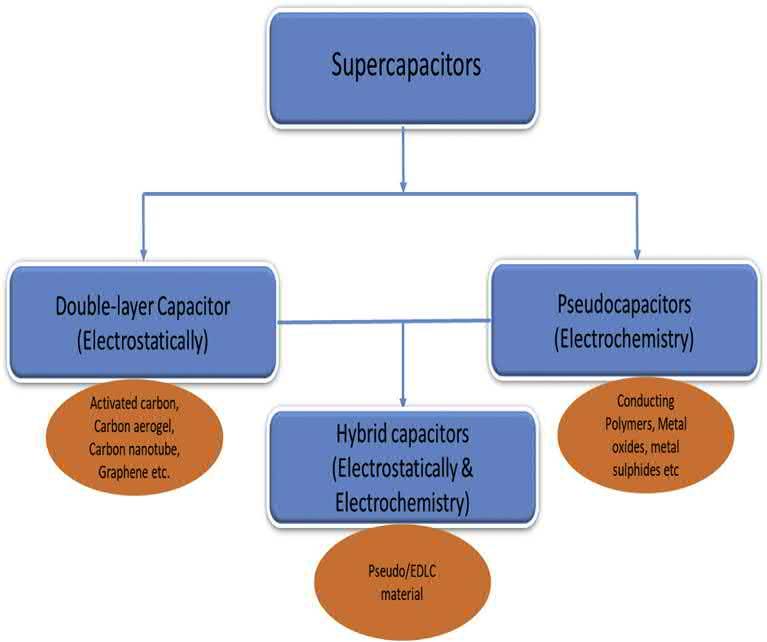
Theelectrochemicalsupercapacitorsareclassifiedintothreecategories basedonthechargestoragemechanism:(1)electrochemicaldouble-layer capacitors(EDLCs),(2)pseudocapacitors,and(3)hybridcapacitors. EDLCsconsistoftwoelectrodesandanelectrolyte.Thetwoelectrodesare separatedbyaseparator,andtheelectrolyteisthecombinationofnegative andpositiveionsdissolvedinsuitablesolvents.Inpseudocapacitors,energy isstoredfaradaicallybymeansofchargetransferbetweentheelectrolyteand
electrode.Thechargetransfermayoccurviaredoxreactionsandelectrosorption.Thus,theFaradaicprocesscanachievehighSCandenergy densitycomparedwithnon-FaradaicprocessofEDLCs.Hybridcapacitors areacombinationofEDLCs(non-Faradaic)andpseudocapacitors(Faradaic).Typesofsupercapacitorsandthecorrespondingmaterialsareshown in Fig.1.2.
3.1Workingprincipleofasupercapacitor Acurrentcollectorattractsoppositelychargedionswhenvoltageisapplied, andtheionsfromtheelectrolytearecollectedonboththecurrentcollector surfacesandthenthechargeisbuilt.Supercapacitorscomprise currentcollectors(conductingmetalplates),electrodes,anelectrolyte, andaseparator.Thestructuresofsupercapacitorsvaryfromstandard capacitorstobatteries.Theutilizationofactivatedcarbonincreasesthe surfacearea,therebyincreasingthecapacitancevalue.Moreover,electrolyte withalowinternalresistanceincreasespowerdensity.Thesetwo featuresleadtotheabilitytoquicklystoreandreleaseenergyin supercapacitors.
Figure1.2 Typesofsupercapacitors.
4.Dye-sensitizedsolarcells Therenewableenergydeviceofsolarcellsconvertssolarenergy(sunlight) intoelectricalenergyandpotentiallycansolvethegrowingenergydemand. Currently,solarlight basedtechnologiesaregainingrecognitionbecause ofitsvariousadvantages,suchaslowtoxicityandnoise.Amongvarious solarcells,DSSCshavebeenreceivingconsiderableinterestbecauseoftheir lowmanufacturingcost,simplefabrication,ecofriendliness,excellentstability,andrelativelyhighpowerconversionefficiency.Sincethemid1980s,Gratzel’sgroupatEPFL(Switzerland)hasbeenthemaindriving forceforthedevelopmentofDSSCs.In1991,theyinventedaDSSCwith aconversionefficiencyof7.1%basedonlow-costnanoporousTiO2 particlestogetherwithnewlydevelopedrutheniumdyes,whichopenedanew wayintheareaofDSSCs(akaGratzelcells)byprovidingbetterstabilityand enhancedefficiency[16].
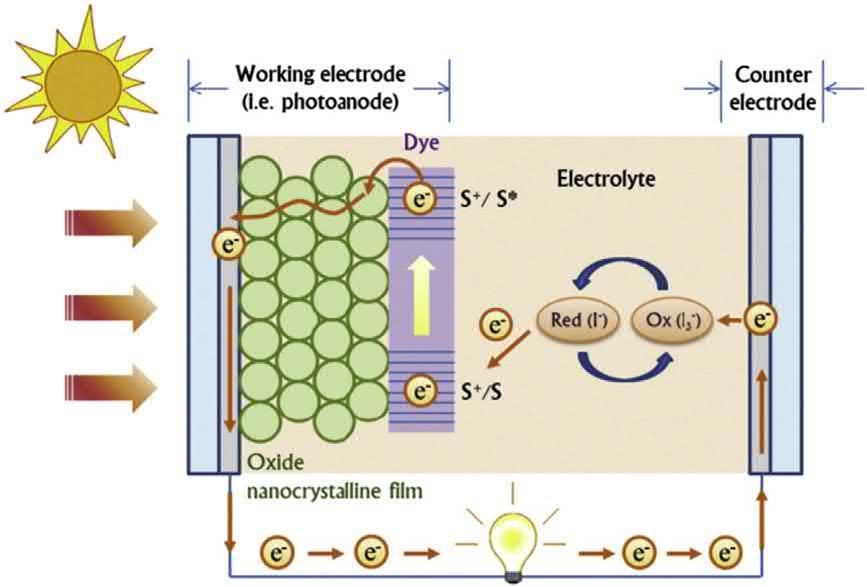
4.1Majorcomponentsofdye-sensitizedsolarcells ADSSCcontainsfourmaincomponents:photoanode(photoelectrode), counterelectrode,dyesensitizer,andelectrolyte.Aschematicofatypical DSSCisshownin Fig.1.3.Specificfunctionsofallfourcomponentsare
Figure1.3 Schematicofadye-sensitizedsolarcell[23].Copyright(2020)Elsevier.
crucialtoaccomplishamaximumpowerconversionefficiencyandlifetime ofthedevice.
ThephotoanodeisanimportantpartofaDSSC.Ingeneral,photoanodespreparedbyanonporouslayerofasemiconductormetaloxide,such asTiO2,ZnO,SiO2,andNb2O5,arecoatedonatransparentconducting (indium-or fluorine-dopedtinoxide)glasssubstrate,whichlargelyenhancesthesurfaceareaofphotoanodeandallowsmoredyemoleculestobe adsorbed,therebyimprovingthelightabsorptionefficiency.Thedyesensitizedsemiconductoroxidecollectsthephotoinducedelectronfrom thelowestunoccupiedmolecularorbitalofthedyesensitizer,providing electronmobilitytotheconductingsubstrate.Thedyemolecules’ (sensitizer)functioninatypicalDSSCis,withtheabsorptionofmaximumsolar light,toproduceelectronsthatareconsequentlyinjectedintotheconductionband(CB)ofthenonporousoxideelectrodeonwhichthedye moleculesareattached.Generally,ruthenium-basedpolypyridylcomplexes (N719andN3dye)areusedashigh-performancedyemoleculesinthe fabricationofDSSCs.AnotherimportantcomponentofDSSCsisredox electrolytes.Thefunctionoftheelectrolyteistoregeneratetheoxidized formofdyemoleculesandcompletionofanelectriccircuitbytransporting positivechargestothecounterelectrode.Also,thestabilityofthefabricatedDSSCdeviceiscloselyassoci atedwiththenatureoftheredox electrolytes.Torecoveroxidizeddyemolecules,thepotentialofthe redoxelectrolyteshouldbemargina llysituatedatamorenegativelevel thanthehigherunoccupiedmolecularorbitalofthedyemolecules. Numerousredoxmediatorsareutili zedintheDSSCelectrolyte,for example,I /I3 ,SCN /SCN3 ,Br /Br3 ,andCo(II)/Co(III).I /I3 isthe mostcommonlyutilizedamongredoxmediatorsinelectrolytes.In DSSCs,thecounterelectrodecomprisesanelectrocatalystcoatedonthe conductingsubstrate.Thefunctiono fthecounterelectrodeistoinject electronsintotheredoxelectrolytestoelectrocatalyzethetriiodideinto iodideions.Agoodcounterelectrodeshouldpossesshighelectrocatalytic behaviortowardthereductionofI 3 intoI ions,lowresistancetoobtain ahigh fi llfactor,andbeavailableatlowcost.Generally,platinumisused asaconventionalelectrocatalyticmaterialbecauseofitshighcatalytic reductionperformance[ 6,16].However,itslarge-scaleapplicationsare limitedbecauseofitshighcostandscarcity;thus,theselimitationsfueled variousstudiestodevelopasubstitu tematerialfortheplatinum-based counterelectrodesanddecreasetheircostwhilemaintainingtheef ficiencyofDSSCs.
4.2Workingprinciplesofdye-sensitizedsolarcells UpontheilluminationofsolarlightontothefabricatedDSSCdevice,dye moleculesareexcitedfromthegroundstatetotheexcitedstate.The exciteddyemoleculesinjectitselectrontotheCBofthesemiconductor metaloxide basedphotoanode.Then,theoxidizedformofdyemolecules isregeneratedbyI oftheelectrolyte,andI isconvertedtoI3 ions. Simultaneously,I3 isreducedtoI becauseofelectrongainfromthe counterelectrode.Thecycleiscompletedbythe flowofinjectedelectrons fromthephotoelectrodetothecounterelectrodeviaanexternalcircuit.
TheperformanceofaDSSCdevicecanbeassessedbythe fillfactor(FF) andapowerconversionefficiency(h)usingthefollowingequations[6]:
where Vmax isthemaximumvoltage, Jmax isthemaximumcurrentdensity, Voc istheopencircuitvoltage, Jsc istheshortcircuitcurrent,and Pin and Pout arethepowerinputandoutputfromsolarlight,respectively.
5.Hydrogenproductionbyphotocatalyticwatersplitting Hydrogenisakindofenergysourcewithzerogreenhousegasemissionand thushasattractedmuchattentionasanalternativetofossilfuels,providing ecofriendliness,sustainability,andcleanenergy[17,18].Hydrogenismainly obtainedfromthedecompositionoffossilfuels(naturalgas,oil,andcoal). However,theconsumptionoffossilfuelshasgeneratedasubstantial quantityofCO2 emissionandisthemainreasonforglobalwarming.Itis criticallyneededtosupplantfossilfuelswithadvancedalternateenergy sources.Hence,hydrogenproductionviawatersplittinghasgainedinterest becausewaterisnaturallyabundant,carbon-free,andenvironmentally friendly[19].
Watersplittingviaphotocatalyticreactionisinitiatedbytheabsorption ofphotonsbyasemiconductorphotocatalyst,whichcanbephotoexcited bytheabsorptionofphotonswithequalorhigherenergythanitsbandgap energy.Then,thephotoinducedholesandelectronsaredirectlyinvolved inthesplittingofwater.Theproductionofhydrogenbywatersplitting usingTiO2 photocatalystsunderUVlightilluminationwas firstreportedby
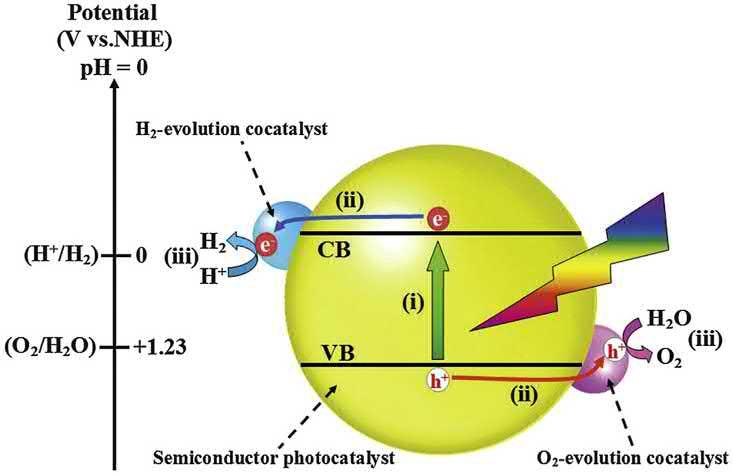
FujishimaandHondainthe1970s,wheretheyutilizedaphotoelectrochemicalcellcomprisingTiO2 andPtelectrodesunderanelectric potential[20].Thechemicalprocessinvolvedinphotocatalyticwater splittingisdemonstratedin Fig.1.4
Mostly,thewatersplittingprocessviaphotocatalysistakesplacein severalreactions:
(1) Intrinsicionizationofphotocatalystsbytheabsorptionofphotonsover thebandgap,generatingholesinvalenceband(VB)andelectronsin CBofcatalysts;
Photocatalyst þ 2hv / 2hþ þ 2e
(2) Watermoleculesoxidizedbyphotogeneratedholes;
2H2O þ 4hþ / 4Hþ þ O2
(3) Hydrogenionreductionbyphotogeneratedelectrons;
2Hþ þ 2e / H2
Theoverallreactionofwatersplittingis
2H2O / 2H2 þ O2
Duringthewatersplittingreaction,thephotoinducedelectronscan reduceHþ intoH2 onlywhentheCBpotentialismorenegativethanthe
Figure1.4 Chemicalprocessinvolvedinthephotocatalyticwatersplitting[24]. Copyright(2020)RoyalSocietyofChemistry.
0Vofhydrogenevolution,whereasholescanoxidizewatermoleculesinto O2 onlywhentheVBismorepositivethan þ1.23Vofoxygenevolution. Toachievehighlyefficienthydrogenproduction,itiscriticaltodevelop semiconductormaterialswithappropriatebandenergygapsasacatalystfor watersplittingtoproducehydrogenundervisiblelightillumination. Furthereffortwasputintodevelopinghigh-performancephotocatalystsfor hydrogenproduction,buttheyarestillstrugglingwithlowactivitybecause oftherecombinationofchargecarriers.Thephotocatalyticmaterialsare oftentunedwithothercocatalyst,whichencouragestheseparationof chargebydevelopinganewsemiconductorphotocatalyst/cocatalystinterfaces,subsequentlyimprovingthephotoinducedchargecarrierlifetime andphotocatalyticactivity.
6.Fuelcell Fuelcellisanelectrochemicaldevicethatconvertschemicalenergy (hydrogen,methanol,ornaturalgas)andpureoxygenorairintoelectricity, water,andheat.Insomecases,by-productsofcarbondioxideandlow molecularweighthydrocarbonsmaybeproduced[21].Fuelcellscan continuouslyproduceelectricityaslongasfuelandoxidantaresupplied. Fuelcellsaresuitablechoicesforapplicationsinbuildingsbecauseoftheir highelectricalefficiency,environmentalcompatibility,flexibility,andquiet operation.Currently,fuelcellsarerecognizedintransportationandvarious markets(boomingpowersector,fuelcell basedvehicles,portability,and stationaryapplications,etc.)asabetterchoicecomparedtocombustionbasedconventionalgenerators[22].
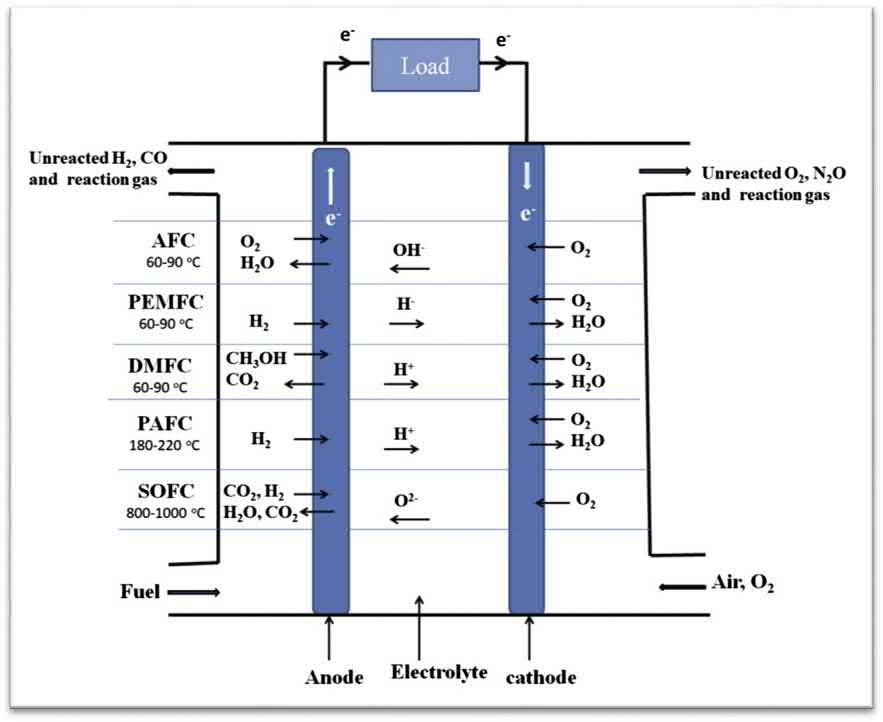
Atypicalfuelcellcomprisesacathode,ananode,andanelectrolyte [1,21].Thechoiceofelectrocatalystsutilizedinelectrodematerialsrelieson theoperatingtemperature.Thetypesofelectrochemicalreactionsthattake placeinthefuelcelldeviceareassociatedwithelectrolytetype.Basedon thefuelandelectrolyteutilized,thefuelcelliscategorizedintodifferent types,asshownin Fig.1.5.Alkalinefuelcell,polymericelectrolyte membranefuelcell,directmethanolfuelcell,andphosphoricacidfuelcell havealowoperatingtemperaturerangefrom60to220 C,whereassolid oxidefuelcellscanoperateathightemperaturesfrom800to1000 C (Fig.1.5).Uponconstantsupplyoffuelattheanodesideandoxidantatthe cathodesideofthedevice,thefuel(hydrogen)attheanodesideis decomposedintohydrogenionsandelectrons(H2 / 2Hþ þ 2e ).Thus, theproducedhydrogenionsmovetothecathodepartthroughan
Figure1.5 Differenttypesoffuelcellsoperatingatvarioustemperatures[25]. Copyright(2020)Elsevier.
electrolyte,andtheelectrons flowthroughtheexternalcircuittothecathode.Subsequently,thereachedionsandelectronsatthecathodearecombinedwithanoxidanttoproducewater(1/2O2 þ 2Hþ þ 2e / H2O).In contrasttothewaterelectrolysis,theanodeisnegativeandthe cathodeispositiveinfuelcells.Besides,theproducedelectricitybyafuel cellmostlydependsontheelectrocatalystsutilizedinthefabricationof electrodes.
Nevertheless,somedrawbacksoffuelcellsincludehighfabrication cost,lowstability,andlimitationoffuelsinthemarket.Toovercome thesedisadvantages,researchersareworkingtoadvancefuelcellsby usingalternativematerialswithlowcostandexcellentstability,in additiontofocusingonthechallengesrelatedtotheproductionoffuel (hydrogen)andconsumption.Thechoiceofelectrocatalystsforelectrode fabricationmayinvolveuniquesurfacestructuresatthenanoscale,high electrocatalyticactivity,andimprovedmasstransportattheelectrode surface.
7.Conclusions Inthischapter,fundamentalconsiderationsofenergyconversionand storagedevicesaresummarizedtosolvechallengesrelatedtotheutilization ofnonrenewablefossilfuelenergysources(coal,gas,andoil),suchas increasingCO2 emissionbecauseofhumanactivitiesandglobalwarming. Energyconversionandstoragedevicesthatcanconvertorstoreenergyin variousformsarebeingimprovedbyvariousadvancednanomaterials. Currently,the fieldofnanotechnologyhasopenednewavenuesfornovel energyconversionandstoragedevices.Wediscussedbasicworkingprinciples,components,andanalysismethodsofthesetechnologicaldevices, includingbatteries,supercapacitors,DSSCs,hydrogenproductionviawater splitting,andfuelcells.
Energyproductionfromrenewableenergysourcesrequiresstoring energyinthedeviceforutilizationonanas-neededbasis.Designingnew integratedtechnologiesforbothenergyconversionandstorageneedsmuch considerationforthemanagementandcontrolofelectricalgrids.
Acknowledgments ThisworkwassupportedbytheKoreaBasicScienceInstitute(KBSI)NationalResearch FacilitiesandEquipmentCenter(NFEC)grantfundedbytheKoreagovernment(Ministry ofEducation)(No.2019R1A6C1010042).TheauthorsProf.M.Y.ChoiandDr.J. Theerthagiriacknowledgethe financialsupportfromNationalResearchFoundationof Korea(NRF),(NRF-2019H1D3A1A01071209,NRF-2017M2B2A9A02049940).Also, Dr.J.Theerthagirithankfullyacknowledgesthe financialsupportfromIndianSpace ResearchOrganization(RespondprogramgrantNo.ISRO/RES/3/792/18-19),India.
References [1]J.Theerthagiri,J.Madhavan,S.J.Lee,M.Ashokkumar,B.G.Pollet,Sonoelectrochemistryforenergyandenvironmentalapplications,UltrasonicsSonochemistry63 (2020)104960.
[2]J.Theerthagiri,S.Sunitha,R.A.Senthil,P.Nithyadharseni,A.Madankumar, A.Prabhakarn,T.Maiyalagan,H.S.Kim,AreviewonZnOnanostructuredmaterials:energy,environmentalandbiologicalapplications,Nanotechnology30(2019) 39200.
[3]J.Theerthagiri,A.P.Murthy,V.Elakkiya,S.Chandrasekaran,P.Nithyadharseni, Z.Khan,R.A.Senthil,R.Shanker,M.Raghavender,P.Kuppusami,J.Madhavan, M.Ashokkumar,Recentdevelopmentoncarbonbasedheterostructuresfortheirapplicationsinenergyandenvironment:areview,JournalofIndustrialandEngineering Chemistry64(2018)16 59.
[4]J.Liu,J.Wang,C.Xu,H.Jiang,C.Li,L.Zhang,J.Lin,Z.X.Shen,Advancedenergy storagedevices:basicprinciples,analyticalmethods,andrationalmaterialsdesign, AdvancementofScience5(2017)1700322.
[5]J.Theerthagiri,K.Karuppasamy,G.Durai,A.H.SarwarRana,P.Arunachalam, K.Sangeetha,P.Kuppusami,H.S.Kim,Recentadvancesinmetalchalcogenides(MX; X ¼ S,Se)nanostructuresforelectrochemicalsupercapacitorapplications:abriefreview,Nanomaterials8(2018)256.
[6]J.Theerthagiri,R.A.Senthil,J.Madhavan,T.Maiyalagan,Reviewonrecentprogress innonplatinumcounterelectrodematerialsfordye-sensitizedsolarcells,ChemElectroChem2(2015)928 945.
[7]M.S.Whittingham,R.F.Savinelli,T.Zawodzinski,Introdeuction:batteriesandfuel cells,ChemicalReviews104(2004)4243 4886.
[8]J.M.Tarascon,M.Armand,Issuesandchallengesfacingrechargeablelithiumbatteries, Nature414(2001)359 367.
[9]A.Yoshino,K.Sanechika,T.Nakajima,SecondaryBattery,UnitedStatesPatent 4668595,1987.
[10]K.Mizushima,P.Jones,P.Wiseman,J.Goodenough,LixCoO2 (0 < x 1):anew cathodematerialforbatteriesofhighenergydensity,SolidStateIonics3 4(1981) 171 174.
[11]A.J.Salkind,J.J.Kelly,A.G.Cennone,in:D.Linden(Ed.),HandBookofBatteries, McGrawHill,NewYork,1995.
[12]K.Ozawa,Lithium-ionrechargeablebatterieswithLiCoO2 andcarbonelectrodes:the LiCoO2/Csystem,SolidStateIonics69(1994)212 221.
[13]K.Xu,Nonaqueousliquidelectrolytesforlithium-basedrechargeablebatteries, ChemicalReviews104(2004)4303 4418.
[14]J.Theerthagiri,G.Durai,K.Karuppasamy,P.Arunachalam,V.Elakkiya, P.Kuppusami,T.Maiyalagan,H.S.Kim,RecentAdvancesin2-Dnanostructured metalnitrides,carbidesandphosphideselectrodesforelectrochemicalsupercapacitors abriefreview,JournalofIndustrialandEngineeringChemistry67(2018)12 27.
[15]J.Li,J.Qiao,K.Lian,Hydroxideionconductingpolymerelectrolytesandtheirapplicationsinsolidsupercapacitors:areview,EnergyStorageMaterials24(2020)6 217.
[16]B.O.Regan,M.Gratzel,Alow-cost,high-efficiencysolarcellbasedondye-sensitized colloidalTiO2 films,Nature353(1991)737 740.
[17]J.Theerthagiri,E.Cardoso,G.Fortunato,G.Casagrande,B.Senthilkumar, J.Madhavan,G.Maia,HighlyelectroactiveNipyrophosphate/Ptcatalysttowards hydrogenevolutionreaction,ACSAppliedMaterialsandInterfaces11(2019) 4969 4982.
[18]N.Fajrina,M.Tahir,Acriticalreviewinstrategiestoimprovephotocatalyticwater splittingtowardshydrogenproduction,InternationalJournalofHydrogenEnergy44 (2019)540 577.
[19]A.P.Murthy,D.Govindarajan,J.Theerthagiri,J.Madhavan,K.Parasuraman,Metaldopedmolybdenumnitride filmsforenhancedhydrogenevolutioninnear-neutral stronglybufferedaerobicmedia,ElectrochimicaActa283(2018)1525 1533.
[20]A.Fujishima,K.Honda,Electrochemicalphotolysisofwateratasemiconductor electrode,Nature238(1972)37 38.
[21]P.E.Dodds,I.Staffell,A.D.Hawkes,F.Li,P.Grunewald,W.McDowall,P.Ekins, Hydrogenandfuelcelltechnologiesforheating:areview,InternationalJournalof HydrogenEnergy40(2015)2065 2083.
14 Nanostructured,Functional,andFlexibleMaterialsforEnergyConversionandStorageSystems
[22]K.AmarsinghBhabu,J.Theerthagiri,J.Madhavan,T.Balu,G.Muralidharan, T.R.Rajasekaran,Superioroxideionconductivityofnovelacceptordopedcerium oxideelectrolytesforIT-SOFCapplications,JournalofPhysicalChemistryC120 (2016)18452 18461.
[23]A.Pandikumar,S.P.Lim,S.Jayabal,N.M.Huang,H.N.Lim,R.Ramaraj, Titania@goldplasmonicnanoarchitectures:anidealphotoanodefordye-sensitizedsolar cells,RenewableandSustainableEnergyReviews60(2016)408 420.
[24]J.Ran,J.Zhang,J.Yu,M.Jaroniec,S.Z.Qiao,Earth-abundantcocatalystsfor semiconductor-basedphotocatalyticwatersplitting,ChemicalSocietyReviews43 (2014)7787 7812.
[25]N.Seselj,C.Engelbrekt,J.Zhang,Graphene-supportedplatinumcatalystsforfuel cells,ScienceBulletin60(2015)864 876.
CHAPTER2 Low-dimensionalcarbon-based nanomaterialsforenergy conversionandstorage applications T.Senthil1,NidhinDivakaran2,ManojB.Kale2,SuhailMubarak2, DuraisamiDhamodharan2,LixinWu2,R.JosephBensingh1, M.AbdulKader1,KingshukDutta1
1AdvancedResearchSchoolforTechnologyandProductSimulation(ARSTPS),SchoolforAdvanced ResearchinPolymers(SARP),CentralInstituteofPlasticsEngineeringandTechnology(CIPET), Chennai,TamilNadu,India; 2CASKeyLaboratoryofDesignandAssemblyofFunctional Nanostructure,FujianKeyLaboratoryofNanomaterials,FujianInstituteofResearchontheStructureof Matter,ChineseAcademyofSciences,Fuzhou,Fujian,China
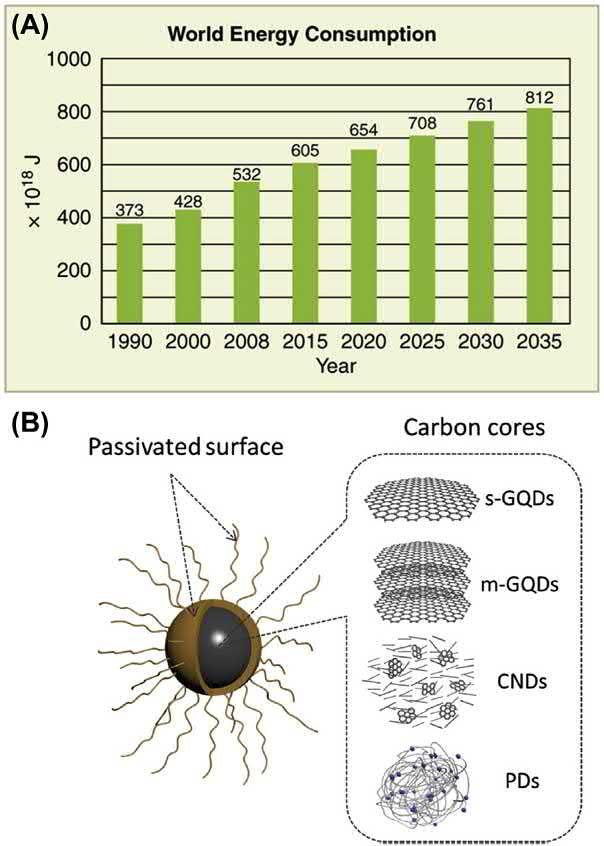
1.Introduction TheUSdepartmentofenergyestimatesthatin15yearsfromnowthe globalenergydemandwillincreasebyabout20%(Fig.2.1A).Theuseof fossilfuelshascreatedaconcernoveritseffectonclimatechange,whichled tothesearchforrenewableandsustainableenergyresources.Itisimportant todeveloprenewableenergygenerationandstoragesystemstocounterthe problemofclimatechangeandmeettherequirementofincreasingenergy consumption[1].Theworldpopulationisexpectedtobearound9billion by2050,andasaresulttheenergydemandisgoingtoincreaseto13trillion wattsby2050.Thenaturaloilresourcesmaybeabletofulfilltheneedof energyfor60years,naturalgasfor60years,andcoalfor200years[2].
Currently,theworld’selectricityneedisful filledbyfossilfuel-andnuclearpoweredplants,whicharecausingenvironmentalpollutionbecauseof greengaseffects.Therefore,newenergygenerationtechniqueshavebeen developed,whicharenonpollutingandeasytostore,transfer,anduse wheneverneeded.Theconventionalenergyresourcescanbereplacedand/ oraccompaniedbysolarpower,windenergy,andwaterenergy,butthese energyresourcesneedefficientconversionandstoragedevices.Thestorage devices,suchasbatteriesandsupercapacitors,canbeusefultostorethe excessenergyanduseitwheneverneeded.
Nanostructured,Functional,andFlexibleMaterialsforEnergy ConversionandStorageSystems ISBN978-0-12-819552-9 https://doi.org/10.1016/B978-0-12-819552-9.00002-6
Figure2.1 (A)Thepastandprojectedenergyconsumptiononaglobalscale; (B)schematicsetupofthecarbonnanodots(CNDs)possessingvariousstructures. (A) BasedondatafromtheUSEnergyInformationAdministration,2011(https://voer.edu.vn/ m/world-energy-use/99605200#import-auto-id1764068 ,accessedonJuly10,2019).(B) ReproducedfromC.Hu,M.Li,J.Qiu,Y.-P.Sun,Designandfabricationofcarbondotsfor energyconversionandstorage,ChemicalSocietyReviews48(2019)2315 2337, https:// doi.org/10.1039/c8cs00750k withpermissionfromTheRoyalSocietyofChemistry.)
Carbonhasplayedaveryimportantroleinthedevelopmentofmodern scienceandtechnology.Itoriginatedfromthediscoveryoffullereneand carbonnanotubes(CNTs)tothehighlyefficientgrapheneinthemid2000s. Theconstanturgeofinclusivepropertiesofnanofillerspropelledthesearch ofthecarbon-basedmaterialshavingluminescentproperty.Itwasuntilthe discoveryofthecarbonnanodots(CNDs)intheyear2006.CNTshavebeen discoveredin1991byIijima[3].Theyhavehighsurfacearea,better electricalconductivity,highmechanicalstrength,andcorrosionresistance [4 6].CNTsaremadeupofcovalentlybondedcarbonatomsinhexagonal
shape,justlikeingraphene.TheCNTsareclassifiedintosingle-walled carbonnanotube(SWCNT)andmulti-walledcarbonnanotube (MWCNT).TheSWCNTsarejustlikesingle-foldedsheetsofgraphene, havinginnerdiameterofabout0.4 3nmandfewmicrometerslong.The MWCNTsareconcentricfoldedsheetswithvaryingdiametersofdifferent sheetseachrangingfrom1.4to25nm,andtheintersheetsdistanceisabout 0.4nm[7 9].TheCNTscanbepreparedbyvariousmethods,suchas arc-dischargemethod[10],spraypyrolysis[11],chemicalvapordeposition (CVD)[12],electrocatalyticmethod,andlaserablation.CNTsareas conductiveascopperandhavethecapacitytotransfermorecurrent.The highelectricalconductivityistheresultoftransferofelectronsthroughthe sidewalls.TheCNTsaremostlylimitedtothelaboratoryresearchforenergy storageapplicationbecauseoftheirlowdispersion,poorelectrochemical performance,andlowspecificcapacitance[13,14].Butthesurface functionalizationofCNTscanenhancethedispersion,electrochemical performance,andspecificcapacitance.TheCNTshavebeenresearchedfor variousapplications,suchashydrogenstorage[15],fuelcells[16],anode materialforLi-ionbatteries[17],drugdeliverysystem[18],andenergy conversionandstorage[19].TheCNTshavehighmechanicalandcharge transferproperties,whicharefavorableintheapplicationofenergy conversion.Also,thesurfaceofCNTscanbefunctionalizedusingsurface functionalgroupstoenhancetheirelectricalanddispersionproperties[20]. TheprecursorcarbonmaterialtosynthesizeCNTsischeapandabundant,so theuseofCNTsinstoragedevicesmakestheproducteconomicallycheap.
CNDsarealsoknownbythenameofcarbonquantumdots(QDs)or carbondots(CDs)[21].Thediscoveryaddedalaurelintheever-blooming applicationsofthecarboniferousmaterialswithCNDs,beingoneofthe proponents.Itcameunderthecategoryofthenanoparticleswiththe dimensionlessthan10nm.ThediscoveryofCNDstransformedthearch typeofcarbonbeingthematerialthatisunabletoemitlightasitisblackin color[22].TherevelationoftheCNDscouldbetracedbacktotheresearch on fluorescentmaterialsderivedfromCNTs(SWCNTs)intheyear2004. These fluorescentmaterialswerecoinedasCNDsbySunetal.,whoalso devisedthesynthesisroutetodevelopCNDswithaugmented fluorescent propertiesbysurfacepassivation[23].TheinventionoftheCNDscreated ripplesofresonanceinthe fieldofscienceandtechnologywithprosinthe formofchemicalstability,watersolubility,lowtoxicity,highluminescence,andlessphotobleaching[24 27].CNDsareoftenanalogoustothe semiconductorQDsandtheirfascinatingpropertiesmakethempompous
tothatofconventionalluminousQDsandluminousorganicdyes[28,29]. ThesubsequentsurveyintheapplicationofCNDsintheapplicationpoint ofviewhasopenedthegatesfortheircommercialuseinvarious fields,such asoptoelectronics,photovoltaicdevices,photocatalysis,biosensing,and bioimagingdevices[30 38].
ThestructuralperspectiveoftheCNDscanbeelaboratedintermsofa carbogeniccorecomprisingofcrystallineandamorphousfragmentwith functionalgroupsattachedonthesurface.Thereseemstobeasimilarityto thatofthegraphenestructurewiththeresearcherssuggestingthepresence ofcrystallinesp2 carbonsegments.ThecoreCNDsseemtohavepossessed theamorphouscarbonstructure,graphene/graphiticstructure,orthe diamond-likestructure.ThepresenceofdefectswithintheCNDsstructure appearstobemorethanthatofthegraphenequantumdots(GQDs).Italso holdspoorercrystallinitythanGQDs.Nevertheless,GQDscouldbe consideredasoneoftheCNDsastheybothhavesimilararrangementof oxygen-terminatedfunctionalgroupsontotheirsurface[39]. Fig.2.1B displaystheschematicsetupofthestructureoftheCNDswithvarious configurations.Themolecularorbitaltheoryencapsulatestheelectronic structureoftheCNDs.TheCNDsdisplayn / p and p / ntransition becauseofthepresenceofdifferenceintheenergylevels.The p states indicatethesp2 hybridizationinthecore,whilethenstatesspecifythe oxygenfunctionalgroupattachedtothesp2-hybridizedcarbon.The functionalgroupcouldbecarbonyl,amine,amides,andthiol[40].CNDs canactashydrophobicorhydrophilic,dependingonthestructureofthe surface.The fluorescencecharacteristicsoftheCNDscouldbedepictedon thebasisofthesynthesisproceduretodevelopit.Itdependsonthe synthesismethodswhethertheywillbecapableofemitting fluorescenceat differentwavelength.The fluorescenceiscommonlytunablewithit, havingtheabilityofemittingblue,green,orredlight,independentofthe excitationwavelength.
Thearrangementofhoneycombcrystallatticestructureofsp2-bonded carbonatomsiscalledgraphene.Ithasnumberofapplicationsinvarious sectorsbecauseofitshighsurfacearea,uniqueelectronicquality,andhigh mechanicalandthermalproperties[41,42].Theadjacentbondingbetween thecarbonatomsandthedelocalizationofbondingelectronsthroughout thelatticestructuremaybethekeyreasonfortheattractivepropertiesof graphene[43].Grapheneisacombinationofmanypolycyclicaromatic hydrocarbons,suchasanthracene,pyrene,naphthalene,etc.[44].The first successfulgrapheneproductionwasdonebyAndreGeimandhisfellow