MicroandNanoTechnologies NANOSTRUCTURED CARBONNITRIDES FORSUSTAINABLE ENERGYAND ENVIRONMENTAL APPLICATIONS
Editedby SHAMIKCHOWDHURY
AssistantProfessorintheSchoolofEnvironmentalScience andEngineering,IndianInstituteofTechnologyKharagpur, WestBengal,India
MU.NAUSHAD
FullProfessorintheDepartmentofChemistry,CollegeofScience, KingSaudUniversity,Riyadh,SaudiArabia
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermission inwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’ s permissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearanceCenterandthe CopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthan asmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodsthey shouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessional responsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityfor anyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromany useoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN978-0-12-823961-2
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: EdwardPayne
EditorialProjectManager: ClodaghHolland-Borosh
ProductionProjectManager: NirmalaArumugam
CoverDesigner: MilesHitchen
TypesetbySTRAIVE,India
1.Synthesisandpropertiesofcarbonnitridematerials1
RajangamVinodh,RajiAtchudan,MoonsukYi,andHee-JeKim
SectionISustainableenergyapplications
2.Exploringsmartgraphiticcarbonnitridematerialtowardflexible energystoragesupercapacitors21
MeenakshiTalukdarandPritamDeb
3.Carbonnitridesascatalystsupportinfuelcells:Currentscenario andfuturerecommendation39
ChanchalGupta,AmanBhardwaj,RamaKant,andSatyabrataPatnaik
1.Introduction
3.Graphiticcarbonnitridesasfuelcellelectrocatalystsupportmaterial45
4.Enhancingmicrobialfuelcellperformancebycarbonnitride-based nanocomposites63
M.M.Ghangrekar,AnilDhanda,S.M.Sathe,andIndrajitChakraborty
1.Introduction 63
2.DesirablepropertiesofacathodecatalystinMFC66
3.ApplicationofgraphiticcarbonnitrideascathodecatalystinMFC67
4.Futurescope 74
5.Solarenergyharvestingwithcarbonnitrides81
ArabindaBaruah,NirupamjitSarmah,SantoshKumar,PriyaGhosh, RituMalik,andVijayK.Tomer
1.Introduction 81
2.Magneticallyseparableg-C3N4-Fe3O4 asvisible-light-drivenphotocatalyst83
3.Solarenergyharvestingusingg-C3N4-Ag3PO4 hybridnanocomposite91
4.Conclusionandfutureoutlook101 References 102
SectionIIEnvironmentalremediationapplications
6.Superioradsorptionofenvironmentalcontaminantsontocarbon nitridematerials111
AliKhadir,MehrdadNegarestani,EbrahimPakzad, andAfsanehMollahosseini
1.Introduction 111
2.Pollutantremovaltechniques112
3.Fundamentalsofadsorption114
4.Carbonnitride-basedadsorbentsfortheremovaloftoxicmetals/heavymetals115
5.Carbonnitride-basedadsorbentsfortheremovalofdyes123
6.Conclusion 130 References 131
7.Carbonnitridephotocatalystsforwatertreatmentandpurification137
MehdiAlKausorandDhrubaChakrabortty
1.Introduction 137
2.Spectroscopicmethodsforcharacterizationofg-C3N4 andg-C3N4-basedmaterials143
3.Photocatalyticdegradationoforganicpollutantsbyg-C3N4 152
4.Mechanisticpathwayofphotodegradationbyg-C3N4-basedmaterials162
5.Conclusionandfuturescope166 References 167
8.Graphiticcarbonnitride-basedcompositesforphotocatalyticabatementof emergingpollutants175
ShabnamTaghipour,BehzadAtaie-Ashtiani,SeiyedMossaHosseini,andKingLunYeung
1.Introduction175
2.Emergingpollutants177
3.Photocatalyticreactionsasadvancedoxidationprocesses177
4.Graphitic-C3N4 179
5.Differentmorphologiesofg-C3N4 181
6.Synthesismethodsofg-C3N4 forwaterpurification186
7.Defectsofg-C3N4 188
8.Methodstominimizedefects188
9.Photocatalyticapplicationsofg-C3N4 190
10.Conclusionandfutureperspectives200
References 202
9.Artificialphotosynthesisbycarbonnitride-basedcomposite photocatalysts215
KonstantinosC.Christoforidis
1.Elementarystepsinphotocatalyticprocesses218
2.FundamentalsofphotocatalyticwatersplittingandCO2 reduction220
3.Compositephotoactivematerials Generalremarks221
4.CNsynthesis 222
5.CN-basedcompositematerialsforartificialphotosynthesis227
6.Concludingremarksanddirections238 References 239
10.Carbonnitride-basedopticalsensorsformetaliondetection245
EktaSharma,AshishGuleria,KulvinderSingh,RituMalik,andVijayK.Tomer
1.Introduction 245
2.GraphiticcarbonnitridequantumdotsasanopticalsensorforHg2+ ions247
3.GraphiticcarbonnitridequantumdotsasanopticalsensorforFe2+ andFe3+ ions251
4.Conclusion 255 References 256 Index 261
Contributors
BehzadAtaie-Ashtiani
DepartmentofCivilEngineering,SharifUniversityofTechnology,Tehran,Iran
RajiAtchudan
DepartmentofChemicalEngineering,YeungnamUniversity,Gyeongsan,RepublicofKorea
ArabindaBaruah DepartmentofChemistry,GauhatiUniversity,Guwahati,Assam,India
AmanBhardwaj
SchoolofPhysicalSciences,JawaharlalNehruUniversity,NewDelhi,India
DhrubaChakrabortty DepartmentofChemistry,B.N.College,Dhubri,Assam,India
IndrajitChakraborty
DepartmentofCivilEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,India
KonstantinosC.Christoforidis
DepartmentofEnvironmentalEngineering,DemocritusUniversityofThrace,Xanthi,Greece; InstitutdeChimieetProcedesPourl’Energie,l’EnvironnementetlaSante(ICPEES),ECPM UniversityofStrasbourg,Strasbourg,France
PritamDeb
AdvancedFunctionalMaterialLaboratory(AFML),DepartmentofPhysics,TezpurUniversity (CentralUniversity),Tezpur,India
AnilDhanda
DepartmentofCivilEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,India
M.M.Ghangrekar
DepartmentofCivilEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,India
PriyaGhosh
DepartmentofAppliedOrganicChemistry,CSIR-NEIST,Jorhat,Assam,India
AshishGuleria
DepartmentofAppliedSciences,WITDehradun,Dehradun,Uttarakhand,India
ChanchalGupta
DepartmentofChemistry,UniversityofDelhi,NewDelhi,India
Contributors
SeiyedMossaHosseini
PhysicalGeographyDepartment,UniversityofTehran,Tehran,Iran
RamaKant
DepartmentofChemistry,UniversityofDelhi,NewDelhi,India
MehdiAlKausor
DepartmentofChemistry,ScienceCollege,Kokrajhar,Assam,India
AliKhadir
YoungResearcherandEliteClub,Yadegar-e-ImamKhomeini(RAH)ShahreReyBranch, IslamicAzadUniversity,Tehran,Iran
Hee-JeKim
SchoolofElectricalandComputerEngineering,PusanNationalUniversity,Busan,Republicof Korea
SantoshKumar
DepartmentofChemicalEngineering,ImperialCollegeLondon,London,UnitedKingdom
RituMalik
DepartmentofMechanicalEngineering,UniversityofCalifornia,Berkeley,CA,UnitedStates
AfsanehMollahosseini
ResearchLaboratoryofSpectroscopy&MicroandNanoExtraction,DepartmentofChemistry, IranUniversityofScienceandTechnology,Tehran,Iran
MehrdadNegarestani
DepartmentofCivilandEnvironmentalEngineering,IranUniversityofScienceand Technology,Tehran,Iran
EbrahimPakzad
DepartmentofCivilandEnvironmentalEngineering,IranUniversityofScienceand Technology,Tehran,Iran
SatyabrataPatnaik
SchoolofPhysicalSciences,JawaharlalNehruUniversity,NewDelhi,India
NirupamjitSarmah
DepartmentofChemistry,GauhatiUniversity,Guwahati,Assam,India
S.M.Sathe
DepartmentofCivilEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,India
EktaSharma
DepartmentofChemistry,SchoolofBasicandAppliedSciences,MaharajaAgrasenUniversity, Baddi,HimachalPradesh,India
KulvinderSingh DepartmentofChemistry,DAVCollege,Chandigarh,India
ShabnamTaghipour
DepartmentofCivilEngineering,SharifUniversityofTechnology,Tehran,Iran;Departmentof ChemicalandBiologicalEngineering,TheHongKongUniversityofScienceandTechnology, HongKong
MeenakshiTalukdar AdvancedFunctionalMaterialLaboratory(AFML),DepartmentofPhysics,TezpurUniversity (CentralUniversity),Tezpur,India
VijayK.Tomer
DepartmentofMechanicalEngineering,UniversityofCalifornia,Berkeley,CA,UnitedStates
RajangamVinodh
DepartmentofElectronicsEngineering,PusanNationalUniversity,Busan,RepublicofKorea
KingLunYeung DepartmentofChemicalandBiologicalEngineering;DivisionofEnvironmentand Sustainability,TheHongKongUniversityofScienceandTechnology,HongKong
MoonsukYi
DepartmentofElectronicsEngineering,PusanNationalUniversity,Busan,RepublicofKorea
Preface
Withescalatingworldpopulation,unsustainableconsumptionoffossilfuels,insatiable energydemand,rapidenvironmentaldegradation,andglobalclimatechange,energy andenvironmentalissuesarereceivingconsiderableattentionworldwideinthecontext ofsustainabledevelopment.Inordertoaddresstheseinterconnectedchallenges,theuse ofinexpensive,robust,andhighlyefficientenergyconversion/storagedevicesandenvironmentalremediationtechnologiesbasedonadvancedmaterialshasintensifiedinrecent years.Inparticular,carbonnitride,anewtypeoftwo-dimensional(2D)material,has stimulatedgreatinterestforfundamentalscientificinvestigationsandpotentialpractical applicationsinamultitudeofcleanenergytechnologies(e.g.,lithium-ionbatteries, sodium-ionbatteries,supercapacitors,fuelcells,microbialfuelcells,solarcells,photoelectrochemicalwatersplittingdevices,andhydrogenstorage)andenvironmentalremediationtechniques(suchaswastewatertreatment,waterpurification,airpollution control,andclimatechangemitigation).Thiscanbelargelyattributedtoitsexcellent optoelectronicandphysicochemicalproperties,includingmoderatebandgapenergy, adjustableenergybandconfiguration,tailor-madesurfacefunctionalities,lowcost, metal-freenature,remarkablethermochemicalstability,andenvironmentallybenign manufacturingprotocol.Additionally,duetotheirpolymericstructure,thesurface chemistryofcarbonnitridescanbeeasilytailoredbymeansofsurfaceengineeringat themolecularlevel,leadingtonewmaterialsystemswithnovelfunctionalities.Indeed, inthelast5years,over1000researcharticleshavebeenpublishedwithaparticularfocus onfabricatinghigh-performancenanostructuredcarbonnitridesforenergyconversion andstorageaswellasenvironmentalremediationapplications.Assuch,acomprehensive andup-to-dateaccountofcarbonnitride-based2Dmaterials,exploredforsustainable energyandenvironmentalapplications,ishighlydesirableasitwouldpromotefurther advancesinthisrapidlyevolvingcross-disciplinaryresearchfieldofcurrentglobalinterest.Tothisend,webelievethatthisbookwillhelptheglobalscientificcommunityto gaindeepinsightsintovariousaspectsofcarbonnitridematerialsfrommultidisciplinary perspectivesandinapplyingthesematerialstotackleglobalenergyandenvironmental challengesinasustainablemanner.Specifically,thebookwillhaveagreatappealto chemists,electrochemists,physicalchemists,solidstatephysicists,chemicalengineers, materialscientists,environmentalscientistsandengineers,andenergyspecialists.Needlesstosay,thisinturnwillstimulatefurtheradvancesinthedevelopmentofmultifunctionalmaterialsforcleanenergy-relatedapplicationsandenvironmentalremediation.
Thebookissystematicallyorganizedinto10chapters. Chapter1 narratestheunique setofoptical,electronic,andchemicalpropertiespossessedbycarbonnitrides,which
makethemparticularlyattractiveforenergy-andenvironment-relatedapplications.It thencollatesthecurrentstate-of-the-artsynthesisstrategiesavailabletorealizecarbon nitridenanostructureswithsuperiorphysiochemicalproperties.Thenextninechapters aredividedintotwosections.SectionIfocusesonsustainableenergyapplications (Chapters2–5),whileSectionIIdealswithenvironmentalremediationapplications (Chapters6–10).
Chapter2 summarizestherecentadvancesinthedesignanddevelopmentofexotic carbonnitride-basedelectrodesforthecreationofsupercapacitorswithunprecedented performance. Chapter3 highlightstherecentprogressincarbonnitride-basednanocatalystswithcontrollablesizeandshapeforfuelcellapplications. Chapter4 presentsasystematic,updatedsummaryofthecurrentstatusoftheapplicationofcarbonnitride-based materialswithhighconductivityandbiocompatibilityinmicrobialfuelcells(MFCs)and discussesthekeyscientificandtechnologicalchallengesinusingthemtoimprovethe performanceofMFCs. Chapter5 providesabroadoverviewofthelatestdevelopments incarbonnitride-mediatedsolarenergyharvestingforpotentialapplicationsinsterilizationofwasteandseawaterdesalination.
Chapter6 criticallyexaminestherecentprogressinthedevelopmentofnovelcarbon nitride-basednanostructuresforfastandefficientremovalofavarietyofcontaminants fromwater,withaspecialfocusoninteractionmechanismswithcontaminantmolecules. Chapters7and8 collatetherecentadvancesintherationaldesignofcarbonnitride-based photocatalysts,withaspecialemphasisongraphiticcarbonnitride,andhighlighttheir applicationsinphotocatalyticdegradationofenvironmentalcontaminants. Chapter9 providesasystematicoverviewofthelatestprogressinthedevelopmentandapplication ofcarbonnitride-basedphotocatalystsforCO2 reductiontosolarfuels. Chapter10 introducesthebasicprinciplesofsensordesignandexplorestheapplicationofcarbonnitridebasedsensorsfortheon-sitedetectionofvariousheavymetalions.
Wearethankfultoalltheleadandcontributingauthorsforsharingtheirvaluable expertiseinvariousaspectsofcarbonnitrideswithoutwhichthisbookwouldnothave beenpossible.WearealsogratefultotheElsevierEditorialProjectManager,Cloe Holland-Borosh,forherconstructivefeedback,logisticalsupport,andconstant encouragement.
Synthesisandpropertiesofcarbon nitridematerials
RajangamVinodha,RajiAtchudanb,MoonsukYia,andHee-JeKimc aDepartmentofElectronicsEngineering,PusanNationalUniversity,Busan,RepublicofKorea bDepartmentofChemicalEngineering,YeungnamUniversity,Gyeongsan,RepublicofKorea cSchoolofElectricalandComputerEngineering,PusanNationalUniversity,Busan,RepublicofKorea
Carbonnitrides(C3N4),oftenreferredtoasg-C3N4,isapolymericmaterialcontaining carbon(C),nitrogen(N),andfewcontaminantssuchashydrogen(H),whicharebonded throughtri-s-triazine-basedpatterns.Theg-C3N4,oneoftheancientpolymersexisting intheliterature,hasthemolecularformulaof(C3N4H)n.Thedevelopmenthistoryof g-C3N4 canbetracedfrom1834 [1].Inthe1990s,researchworkwasinspiredbythe hypotheticalprophecythatdiamond-likeC3N4 maypossessthehighesthardness [2] Theg-C3N4 wasconsideredtobeamorestableallotropeatroomtemperature.The g-C3N4 hasalayeredstructuresimilartographitematerial,containsVanderWaalsforce layers,andeverylayerismadeupoftri-s-triazineunitslinkedtoplanar –NH2 groups [3]. Theringstructureoftri-s-triazinegivesthepolymerhighthermalandchemicalstability inalkalineandacidicconditions [4]
Whencomparedtoothermaterials,g-C3N4 possessesmanyadvantages,suchas (i)lowcost,(ii)excellentCO2 activationcharacteristicsbecauseofitsnitrogen-rich
configuration [5],(iii)photocatalyticCO2 reduction,(iv)itcantransferelectronstosurfacechemicaladsorptionsitesduetoitstwo-dimensional(2D)layerstructure,and(v)the structure/shapeofg-C3N4 structurecanbeadjustedbyoptimizingtheparameterslike precursorsofmonomers,time,andtemperatureofthepolymerizationreaction;thus, aminorbandoffcouldbeattained [6].Furthermore,co-monomerscanbemadebyaddingcombinationsofmonomermoleculespreferredtoprecursors [7].Theycanappreciablyalterthecapabilityoflightabsorptionandelectrontransfercapabilitiesofg-C3N4 in organiccompounddegradation,andhydrogenmanufactureandoxidationofNOx (nitrogenoxides)canbeimprovedbymetalstimulants [8]
Thesemiconductingpropertiesofg-C3N4 differsignificantlyfromthatofgraphene sheetswithabandgapof2.7eVforbulkg-C3N4;thismakesitanormalbandgapsemiconductor.Theg-C3N4 hasanopticalabsorptionpeakofabout460nmbecauseofits yellowcolor.Theg-C3N4 isoneoftheexcellentmaterialsforproducingsolarenergy duetoitsremarkablechemicalandthermalstability [9].Furthermore,g-C3N4,also knownas“melon,”isastableallotrope.Moreover,g-C3N4 isextractedfromtheEarth’s crust,soitiscost-effectiveandreasonableforcommercialutilizationsinenergyconversionandwatersplitting [10].However,itselectricalresistancehaslimiteditselectrocatalyticactivity [11].Untilnow,g-C3N4 ismostlyusedinitspowderform,which preventsitfrombeingreusedforsomeapplications.Therefore,thetransformationof 2Dconstructionmodulesintomacroscopic3Dstructuraldesignsiscriticalformany applications [12].
Inthischapter,wegiveanoverviewofthebasicconceptsandstructureofg-C3N4,synthesisprotocols,aswellasthepropertiesofg-C3N4.Wefirmlyhopethiswillincreaseofthe growthofg-C3N4.Novelphysicochemicalpropertiesbasedong-C3N4 nanostructureshave notyetbeendiscovered.Weareatacrucialjuncturetoemphasizethedevelopmentandoffer high-qualityevidenceforthisemergingresearchtopic.
2.Structureofg-C3N4
Usually,g-C3N4 ispreparedbythepolycondensationofamelamine(MA)precursor, whichisalow-costnitrogen-containingmonomer.Sincethediscoveryofcarbonnitride materials,manyeffortshavebeencarriedouttoconcludehowdifferentsyntheticprocessesinfluencethematerialproducedbasedonitsmorphologyandreactivity.
2.1Geometricstructure
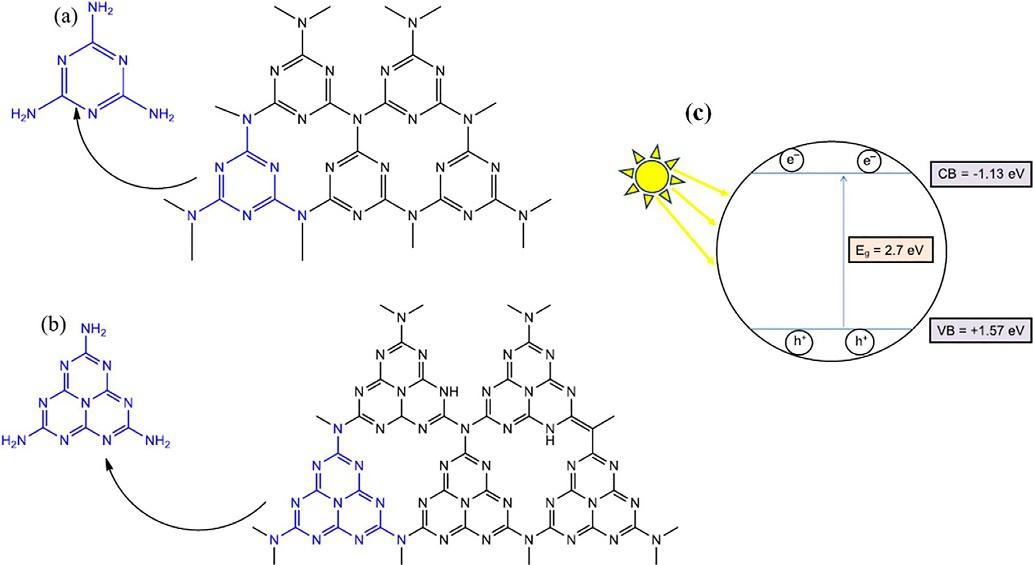
Theexactbuildingblockofg-C3N4 containstwoimportantunits,tri-s-triazine(C6N7) and s-triazine(C3N4)rings,asshownin Fig.1.1AandB [13].Tri-s-triazineisrecognized tobehighlystableatroomtemperature [14].Thetwomajorunitsofg-C3N4 alsoshow thattri-s-triazineisthermodynamicallymorestablebasedondensityfunctionaltheory
Fig.1.1 g-C3N4 structuresof(A) s-triazineand(B)tri-s-triazine.(C)Acharge-transfermechanism fortypicalg-C3N4 ((A)and(B)ReproducedwiththepermissionfromS.Zhang,P.Gu,R.Ma,C.Luo, T.Wen,G.Zhao,W.Cheng,X.Wang,Recentdevelopmentsinfabricationandstructureregulationof visible-light-driveng-C3N4-basedphotocatalyststowardswaterpurification:acriticalreview,Catal. Today335(2019)65–77, https://doi.org/10.1016/j.cattod.2018.09.013 .(C)Reproducedwiththe permissionfromN.Rono,J.K.Kibet,B.S.Martincigh,V.O.Nyamori,Areviewofthecurrentstatusof graphiticcarbonnitride,Crit.Rev.SolidStateMater.Sci., https://doi.org/10.1080/10408436.2019.1709414 .)
(DFT)calculations [15].Theoretically,ithasbeenmentionedthattheoptimalsurface areaofamonolayersheetcanbeenhancedtoapproximately2500m2 g 1 [16,17].Itusuallyconsistsofa2Dsheetofsp2 carbons [18],whereasg-C3N4 hasp-conjugatedgraphitic planesproducedbyasp2 hybridofCandNatoms [19].Finaetal. [20] elucidatedthe3D structureofg-C3N4 viapowderX-raydiffraction(PXRD)andneutrondiffractiontechniques.Theyclearlyrevealedthattheas-synthesizedg-C3N4 showsa3Darrangement withmisalignmentoftri-s-triazine-basedlayers.Thelayersweremisalignedtoevade therepulsiveforcesofp-electronsinadjacentlayers.
2.2Electronicstructure
Theg-C3N4 hasbecomeacenterofdebateduetoitsextraordinaryelectronicproperties andprospectiveutilizations [21].ItconsistsofCandN,whichisasp2 hybridthatforms thep-conjugateddelocalizedsystem.ThelonepairofelectronsfromNcausesalonepair ofvalancebandstoform,andtherefore,creatingthebandstructure [22].Itisimportantto notethatthelonepairofnitrogenisimportantintheelectronicconfigurationofg-C3N4 [23].TheoreticalapproachesconcerningDFTmeasurementspredictthatthevalance
bandwillhavenitrogenPz orbitals,whereastheconductionbandwillhavecarbonPz orbitals;therefore,Catomsactaspointswherereductionoccurs,andNatomsactas pointswhereoxidationoccurs [24].Asaphotocatalyst,g-C3N4 tendstosplittheholes andelectrons.The2.7eVofbandgapallowsittoabsorbsunlight,whichisusedtopurify water,producehydrogen,andusedforsolarcellapplications [25]. Fig.1.1Crepresentsa chargetransfering-C3N4.
3.Preparationofg-C3N4
Theg-C3N4 canbepreparedbythermalpolycondensationofnitrogen-containingprecursors(triazineandheptazinederivatives),suchasurea [26],MA(C3H6N6) [27], dicyandiamide(C2N3 ) [28],cyanamide(CH2N2) [29],thiourea(CH4N2S) [30],guanidiniumchloride(CH5N3 HCl) [31],guanidinethiocyanate(C2H6N4S) [32],andthioureaoxide(CH4N2O2S) [33].ThecondensationroutesfromtheseC-Nprecursorsare easyandprominentwaystobuildag-C3N4-interpenetratingarchitecture [34].Among them,MAisacommonandstraightmonomersupplytosynthesizeC-N,whereasthe highbondingenergyofthechemicalbondbetweenC3H6N6 unitsand –NH2 groupsof MAisinactivatedatlowtemperatures,withoutthecatalyst,andwithnootherreactionto formCN.
Ithasbeenarguedthatthephysicochemicalcharacteristicsoftheresultingg-C3N4 canbeseverelyaffectedbyavarietyofprecursorsandtreatments,includingporevolume, surfacearea,photoluminescence(PL),CandNratio,absorption,andnanostructures. Differentfunctionalitiesandsurfacemodificationshavebeenusedtogetpreferredmorphology/structuressuchas2Dnanosheets(NSs),3Dbulks,1Dnanorods,2Dfilms,1D nanowires,1Dnanotubes,and0Dquantumdots.
3.1Chemicalvapordepositionmethod
Urakamietal. [35] reportedthatg-C3N4 filmsweregrownuniformlyonthesurfaceof differentsubstratesbythermalchemicalvapordeposition(CVD)method.Thestoichiometricamountandnatureofatomicbondswerecharacterized,anditwasfoundtobe equivalenttothoseofidealg-C3N4.ForaPLstudy,althoughelectronexcitationisto thesp3 C-Nconductionband,thesp2 C-NconductionbandwasidentifiedasthepreferredelectroninjectionfromthePLintensityandtheexcitation-energydependenceof thePLpeakshift.Also,Urakamietal. [36] discussedthegrowthofborane(B)atoms attachedtotheg-C3N4 filmsinc-planesapphiresubstratesbythermalCVDatdifferent augmentationtemperatures(Fig.1.2).AmmoniumboraneandMAwereusedasprecursors.TheincorporationofBisaccomplishedat618°Cofgrowthtemperature.Itwas higherthantheg-C3N4 thinfilm’sidealgrowthtemperaturetosomeextent.Thesignal peakfortheB1scorepositionduetotheB NbondswasnoticedbyXPS,whichindicatestheperceptionofBpenetrationintog-C3N4 films.Whenthegrowthtemperature
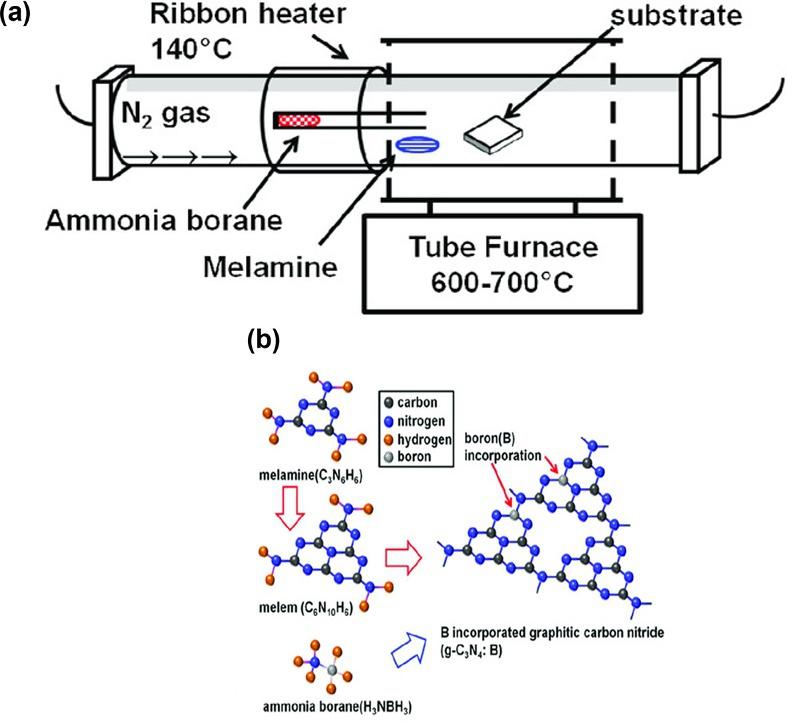
Fig.1.2 (A)Sketchofin-househow-wallCVDequipmentforB/g-C3N4 films;(B)pictorialrepresentation oftheB/g-C3N4 growthprocessusingmelamineasprecursorandammoniaboraneasborane molecularspecies. (ReproducedwiththepermissionfromN.Urakami,M.Kosaka,Y.Hashimoto, Chemicalvapordepositionofboron-incorporatedgraphiticcarbonnitridefilmforcarbon-basedwide bandgapsemiconductormaterials,Phys.StatusSolidiB27(2)(2019)1900375, https://doi.org/10.1002/ pssb.201900375.)
wasincreasedto650°C,auniformlyenhancingBcompositionandadeclininggraphite compositionwerenoticed,indicatingthatBatomsareintegratedintog-C3N4 asanalternativetothegraphitesites.Wangetal. [37] preparedorderedcubicmesoporous(OCM) g-C3N4 byasimpleCVDmethodusingMAastheprecursorand3DOCMsilicaKIT-6 asthetemplate.ThesynthesizedOCMg-C3N4 couldbeseentohavea3Dcubicsymmetrywithahighsurfacearea(129.8m2 g 1)andregularporesize(3.5nm).Duetothese excellentproperties,theOCMg-C3N4 showedimprovedphotocatalyticactivityto reduceCO2 withwatercomparedtoflak-likeg-C3N4.Yadavetal. [38] produced free-standingfilmswithg-C3N4 nanolayersbytheannealingofdicyandiamide (DCN)usingaCVDmethod.Thepyrolysiswascarriedoutunderlow-pressure(approximately3Torr)at600°C.Furthermore,excitation-dependentPLspectraoftheprepared g-C3N4 filmexhibitedastable,strong,andbroademissionpeakof459nminthevisible region.Thefree-standingg-C3N4 filmsshowedablueshiftandbandsharpeningofthe emissionspectra(ES)comparedtotheg-C3N4 powder.Cuietal. [39] reportedaneasy
andair-conditionedCVDprocessthatwouldproduceonionring-likeg-C3N4 (OR-gC3N4)microstructuresinafacile,ecofriendly,andconsistentway.Thistechniqueuses approximatelypacked350nmSiO2 microspheresasarigidtemplateandMAasaCVD precursortoformathinlayerofg-C3N4 inthenarrowspacebetweentheSiO2 microspheres.AfterdissolutionoftheSiO2 microspherehardtemplate,theresultingg-C3N4 uniformlyexhibitsOR-likemicrostructures.Ashortdescriptionofthesynthesisprocedureisasfollows:Typically,afewgramsofMAprecursorweretakeninanaluminaboat, andthefewgramsofSiO2 microspheresweredistributeduniformlyontheMApowder surface.Thealuminaboatwithalidwasannealedat320°Cfor2hinamufflefurnace witharampingrateof10°Cmin 1.Then,themufflefurnacetemperaturewasfurther increasedto550°Candsubjectedintocalcinationfor3htoformg-C3N4 phase.Theresultantyellow-coloredg-C3N4/SiO2 solidsediment(toplayer)wascarefullyseparatedand furthersubjectedwithammoniumbifluoride(4M)for12htoisolatetheSiO2 microspheres.Afterthoroughwashingwithwater,centrifugation,andultrasonication,ayellow powderofOR-g-C3N4 wasprocured.ThedeterminedbandgapforOR-g-C3N4 was 2.58eV,whichwasconsiderablyshorterthanthatof2.70eVofbulkg-C3N4.Furthermore,thepreparedOR-g-C3N4 facilitateschargeseparation,extendsthelifespanofphotoinducedcarriers,andexhibitsfivetimesmorephotocatalytichydrogenevolutionthan thatofbulkg-C3N4
3.2Hydrothermalmethod
Thehydrothermal(HT)processisessentiallytheleastexpensiveandmostcommon methodofproducingg-C3N4-basedNSs.Thisistheeasiestandmostreliablemethod ofproducingg-C3N4-basedternaryheterostructure,whichcanobtainhighpurityheterostructureNSs [40,41].Zhangetal. [42] highlightedaHTmethodatlowtemperature toproducecarbon-richg-C3N4 NSs,whichshowsenhancedphotocurrentandphotocatalyticactivity,becauseofitssuperiorabilityofelectrontransportandimprovedlifespanofphotoexcitedchargecarriers.Inatypicalprocedure,MA(5.5mmol)andglucose powder(16.5mmol)wereplacedinTeflon-linedautoclave(100mL)andthenfilledwith Milliporewaterupto60%oftheautoclavetotalvolume.Thereactionmixtureinthe autoclavewasstirredfor12hwiththemagneticstirrer,thentheautoclavewassealedwith thenickelfoamsubstrateandmaintainedat180°C.Afterheatingtothespecifiedtemperature,theautoclavewasnaturallycooledtoambientcondition.Thefinalmaterialwas thoroughlycleanedwithMilliporeH2Oand92.1%ethanoltoeliminateresidualcontaminants,andtheresultingyellowpowderwasdriedfor12hat60°Ctogettheultimate product.Thedetailedschematicviewisdepictedin Fig.1.3.Guoetal. [43] successfully synthesizedanovelg-C3N4 andBiVO4 (bismuthvanadate)composite(g-C3N4/BiVO4) byasimpleHTmethodforphotocatalyticdegradationreaction.Thegeneralreaction
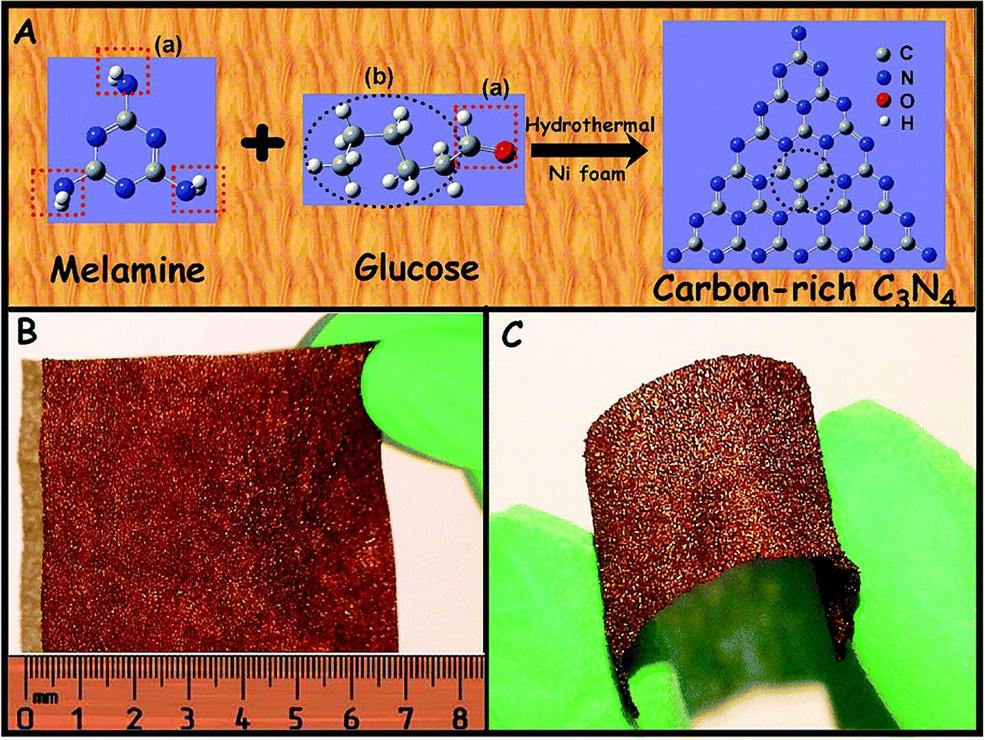
Fig.1.3 (A)Thermalpolycondensationofmelamineprecursorandglucoseinwatersolution;(B)and (C)exhibitionoftheg-C3N4 onnickelfoam. (ReproducedwiththepermissionfromP.Zhang,X.Li,C. Shao,Y.Liu,Hydrothermalsynthesisofcarbon-richgraphiticcarbonnitridenanosheetsforphotoredox catalysis,J.Mater.Chem.A3(2015)3281–3284, https://doi.org/10.1039/C5TA00202H .)
protocolisasfollows:Atfirst,theg-C3N4 wassynthesizedfromMAmonomer.Briefly, DCN(2g)reactantwasplacedintoanaluminajarwithalid,thenannealedtoreacha temperatureof550°Catarateof2.3 °Cmin 1 andthencontinuedat550°Cforanother 2h.Theyellowproductwascomposedandgroundedintofineparticlesforfurtheruse. Second,g-C3N4/BiVO4 heterojunctionswerepreparedbyaHTroute.Inatypicalprocedure,g-C3N4 (0.3g)andNH4VO4 (0.1083g)wereplacedindistilledwater(30mL) andstirredvigorouslyfor3hfollowedbyahomogeneoussedimentation.Simultaneously,Bi(NO3)3 5H2O(0.449g)wasmixedin3mLofHNO3 (1molL 1)toattain aplainsolution.Theclearsolutionwasquicklytransferredtothesedimentationand immediatelymixedwellatroomtemperatureforanextra3h.AfteralteringthepHvalue (pH ¼ 8)usingsodiumhydroxidesolution,thereactioncontentwascarefullymovedinto anautoclave,whichwasannealedinanovenfor20hat160°C.Atlast,theresulting g-C3N4/BiVO4 wasgatheredandcleanednumeroustimesinethanolanddeionized wateranddesiccatedfor2hat100°C.Tianetal. [44] coupledg-C3N4 byBi2WO6 (g-C3N4/Bi2WO6)viaaHTmethod.Aninterfacewasformedbetweeng-C3N4 and
Bi2WO6 heterojunctions,whichwasconfirmedbyhigh-resolutiontransmissionelectron microscopy(HR-TEM).Further,moreintensiveabsorptionwithinthevisiblelight regionoccursinthecompositethanpristineBi2WO6,whichwasattributedby UV-visiblediffusereflection(DRSUV-visible)spectraresults.Theseoutstandingstructuralandspectralcharacteristicsprovidedtheg-C3N4/Bi2WO6 heterojunctionswith improvedphotocatalyticactivities.Furthermore,itshowedagreaterstrengthandlifetime duringsixconsecutivecycles.Zhuangetal. [45] developedaneasyandecofriendlyHT methodologyfortheone-steppreparationofg-C3N4 NSusingsodiumcitrate (Na3C6H5O7)andMA(C3H6N6)astheprecursors.Inbrief,Na3C6H5O7 (0.075g) wastakenalongwithwater(20mL)andC3H6N6 (0.22g)ina100-mLbeaker.After 5minofsonication,thecontentwasmovedintoaTeflon-linedautoclave(50mL) andannealedfor4hat200°C.Aftercompletionofthereaction,thebrown-yellowcoloredmaterialwascentrifugedat12,000rpmfor30min.Theattainedmaterialwas thendialyzedbesidedeionizedwatertoeliminatecontaminantsviacellulosedialysis membrane.Thesynthesizedg-C3N4 NSreleasedpowerfulfluorescencewithahighpercentagequantumyield(48.3%).Shietal. [46] fabricated n-typeg-C3N4 andmodified with p-typeInVO4 (indiumvanadate)toformanovelInVO4/g-C3N4 p-n heterojunctionphotocatalystforthecompetentphotocatalyticdegradationofRhB(rhodamineB). Inaclassicpreparationmethod,g-C3N4 wassynthesizedbypolycondensationofanMA monomer.Briefly,afewgramsofDCNpowderwaskeptinaporcelaincruciblewitha stopperandthenannealedto550°Cwitharampingrateof2.3 °Cmin 1,andthencontinuedfor2h.Theobtainedyellowproductwasgatheredandgroundintofineparticles forfutureuse.InVO4/g-C3N4 heterojunctionswereproducedbyHTmethodology.In aclassicprotocol,g-C3N4 (0.54g)andNaVO3 2H2O(1.58g)weretakenin30mLof deionizedwaterandthenstirredfor3hforsedimentation.Simultaneously,0.381gof indiumnitratepentahydrate(In(NO3)3 5H2O)wasmixedindilutenitricacid(3mL) toattainaplainsedimentation.Thereactantwastransferredquicklytothesedimentation andimmediatelywellmixedatambienttemperatureforextra3h.AftertuningthepH value(pH ¼ 4)withthehelpof1Msodiumhydroxidesolution,thecombinedsolution wasmovedintoanautoclaveandpyrolyzedinatemperature-controlledovenfor24hat 150°C.Finally,thesynthesizedInVO4/g-C3N4 compositewasgatheredandcleaned numeroustimeswithabsoluteethanolanddeionizedwateranddesiccatedfor2hat 100°C.
3.3Thermalexfoliationmethod
Generally,thermalexfoliation(TEXF)isachievedbysubjectingbulkg-C3N4 toheat, whichbreakstheweakVanderWaalsforcesofattractionbetweenthelayersensuing inexfoliation [47].Supremely,whileheating,H2 anchoredtothetri-s-triazineor s-triazineunitsreactswithO2,andwhenthisgasreleases,itproducesporesinthe
aggregateandformssheets [48].Asaresult,theg-C3N4 NSsarerelatedwithahigher surfaceareaandporesizes,thusincreasingtheporosityofthematerial.Xuetal. [49] studiedg-C3N4 NSswithsingleatomiclayerstructurebyafacilechemicalexfoliation (CEXF)method.Theas-synthesizedg-C3N4 NSsexhibited0.4nmofsingleatomic thicknessandmicrometersoflateralsize.Thesynthesisprocedureinvolvestwosteps: First,g-C3N4 wassynthesizedbyheatingDCNfor4hat550°Cinthemufflefurnace andaeratedatthesametemperatureforanother4h.Secondly,single-layerg-C3N4 (SLg-C3N4)NSswerepreparedbyCEXFmethod.Onegramofas-synthesized g-C3N4 wasmixedwith98%of10mLsulfuricacidinaglassbeakerandwell-mixed atambienttemperaturefor8h.Subsequently,thereactioncontentwasgentlytransferred intodeionizedwater(100mL)andultrasonicatedforexfoliation.Thesuspensiontemperaturewasincreasedquickly,andthecolorchangedfromyellowtopaleyellow. Theattainedsedimentwasthencentrifugedfor10minat3000rpmtoeliminate unreacted/unexfoliatedg-C3N4 afterthoroughwashingwithethanolanddeionized water,andatlastdriedinairovernightat80°C.Toremovestructuraldefects,thepreparedpale-yellowpowders(0.3g)wereplacedintoaglassround-bottomflaskcontaining 150mLofmethanolandrefluxedinaheatingmantlefor6hat65°C.Theg-C3N4 NSs wereobtainedaftercentrifugationanddrying.Whencomparedwithbulkg-C3N4,SL g-C3N4 NSsexhibitedsuperiorenhancementinphotogeneratedchargecarrier’stransfer andseparation.Consequently,thephotocatalyst’shydrogenproduction,contaminant decompositionactivities,andphotocurrentgenerationofSLg-C3N4 NSsarefarbetter thanthatofthebulkg-C3N4,illustratingthattheSLg-C3N4 NSswasapowerfulcandidateforphotosynthesisandphotocatalysis.
Thetransformationandtransportationofphotogeneratedcarriersduringthephotocatalyticprocessofg-C3N4 werecontrolledbytheefficacyoflowerchargeseparation andinadequatesurface-activesite.Asatop-downstrategy,theexfoliationoflayerstackedbulkg-C3N4 intoNSswasextensivelyacknowledgedasacompatiblepathway butisstillchallengingintermsofscalabilityandcleansetofsynthesis.ThisissuewasovercomebyCuietal. [50] usingafacileHTmethodinanNaClOsolution,whichcombined theeffectofalkalinemetalionintercalationandtheoxidativeexfoliationofbulkg-C3N4 (Fig.1.4).Highlyactiveg-C3N4 NSswereproducedinthelaboratorybyasimplemanner,anditcouldbeimmediatelyfar-extendedtoapilotscale(large-scaleproduction). TheHTmethodproducedaverticallyorientedpathwayfordirectelectrontransfer andresultantultrathing-C3N4 NSswithsignificantporosity(meso-,macro-,andmicropores)andexcellenthydrophilicity.Theg-C3N4 NSsexhibitedexcellentsurfaceareaof 170.7m2 g 1,narrowbandgapof2.55eV,highnumberofexposededges,andoutstandingelectrontransportcapability.Theseg-C3N4 NSshaveanaverageH2 evolutionrate ninetimeshigherthanthatofbulkg-C3N4.Moreover,theyconcludedthatthisgreen, simple,andscalablemethodtopreparelayeredg-C3N4 NSsprovidesanewapproachfor designingandfabricatingotherfunctional2Dobjects.Inthiswork,Lietal. [51]
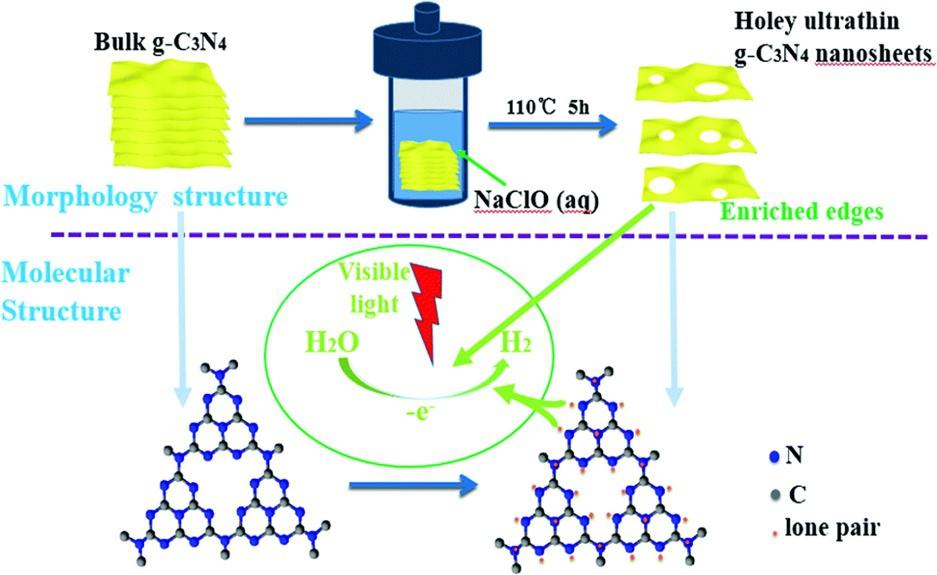
Fig.1.4 SketchoftheaqueousNaClOHTexfoliationofbulkg-C3N4 intoaSL,molecularstructure modelsofbulkg-C3N4 (left)andexfoliatedg-C3N4 (right),andanticipatedmechanismforthe photocatalytichydrogenevolution. ReproducedwiththepermissionfromL.Cui,Y.Liu,X.Fang,C. Yin,S.Li,D.Sun,S.Kang,ScalableandCleanExfoliationofGraphiticCarbonNitrideinNaClOSolution: EnrichedSurfaceActiveSitesforEnhancedPhotocatalyticH2Evolution,GreenChem.20(2018)13541361. https://doi.10.1039/C7GC03704J
successfullypreparedultrathingraphene-likeg-C3N4 NSswithrichnanoporousenrichedandsuperiorhydrophilicpropertiesbyafacileandprominentTEXFofbulk g-C3N4.TounderstandtheeffectofTEXFconditionsonthetexture,surfacestate, andphotocatalyticactivityoftheresultingg-C3N4,aseriesofexfoliatedg-C3N4 NSs weresynthesizedbyoptimizingtheTEXFparameters,suchastimeandtemperature. Theextensivephysicochemicalcharacterizationresultsclearlyrevealedthattheexfoliationtemperatureledtoagreaternumberofnitrogenvacancies;thespecificsurfacearea alsoincreased,aswellasprolongedexfoliationtime,increaseddegreeofTEXF,higher carbonvacancies,andtheexpandedporevolumeintheendmaterials.Furthermore,the degreeofexfoliationandphotocatalyticefficiencyoftheresultantproductswere improvedbyincreasingTEXFtimeandtemperature.Sunreported [52] g-C3N4 NSs withhighphotoactivityproducedwiththehelpofisopropanol(IPA)inthesynthesis process.Theg-C3N4 NSsweresynthesizedbythefollowingtwosteps.First,bulk g-C3N4 waspreparedfromthepolycondensationofprecursor,MA.Inbrief,MA (3g)wascompletelyspreadintoIPA(30mL)ina50-mLporcelaincruciblewitha lid,andthenthereactantwascalcinedat550°Cfor3hinthemufflefurnaceataheating rateof5 °Cmin 1.Thematerialinthesilicacruciblewasgatheredafterbeingnaturally
cooleddowntoambienttemperatureandthenwasgroundtoapowderinamortarand pestle.Thepreparedmaterialwasbulkg-C3N4.Second,g-C3N4 NSswerepreparedbya TEXFprocessofbulkg-C3N4 insemiclosedsurroundings.Bulkg-C3N4 (0.1g)was takenwithIPL(10mL),andthenthesuspensionwasmovedintoa30-mLcruciblewith alidforTEXFprocess.Theoxidationtreatmentwascontinuedfor2hafterthefurnace temperaturewasraisedto550°Cwitharampingrateof5 °Cmin 1.Theproductfinally collectedwasg-C3N4 NSs.TheintroductionofIPAcausesverypowerfuloxidationin theexfoliationprocess,andthederivedg-C3N4 NSshasitsdistinctivepropertiesofa visiblelightwithawideabsorptionrange,highersurfacearea,andunevensurface.As aresult,theg-C3N4 NSshavegoodphotocatalyticactivityinthedegradationoforganic contaminants.Furthermore,thephotocatalyticH2 evolutionrateofg-C3N4 NSswas threetimesthatofg-C3N4 NSs,whichispreparedwithoutIPAusinganidentical method.Pattnaik [53] reportedexfoliatedg-C3N4 nanoparticles(NPs)byagreenpath. Degradationofanaqueoussolutionofciprofloxacin(CPN)byexposuretosolarradiation inthepresenceofg-C3N4 NPswasstudiedtoevaluatethephotocatalyticactivitiesofa semiconductorphotocatalyst.Thephotocatalyticactivitiesofg-C3N4 NPsenhanced afteritsexfoliation.Theimprovedbehaviorofphotocatalysisofexfoliatedg-C3N4 is theresultofitsefficientseparation,lowrearrangementofphotogeneratedchargecarriers, andhighspecificsurfacearea.Asawhole,theexfoliationmethoddeliversmoreadvantagessuchassimplechemicalsandequipment(lowcost),nosolvents,rapidandtimely performance,goodproductyield,andproducesvaluablestructuraldefectsintheresultant NSs.However,thisexfoliationmethodproducesvaluablestructuraldefectsintheresultantNSs.However,thisexfoliationmethodproducesaproductwithlowcrystallinity andcomparativelysmallersurfacearea.
3.4Solvothermalmethod
Solvothermal(ST)techniqueisdevelopedbasedontheHTmethod;themostimportant differenceofSTprocessfromthelatteristhatthepreparativeconditionisorganicsolvent ratherthanwater.Tianetal. [54] in-situsynthesizedanewg-C3N4/Bi2MoO6 heterojunctionswithvaryingcontentofBi2MoO6 NSsbyafacileSTmethod.Atypicalsynthesisprocedureofg-C3N4/Bi2MoO6 heterojunctionsisasfollows:Arequiredquantity ofsodiummolybdatedihydratewasdissolvedinethyleneglycol(10mL)andethanol (60mL)mixturetoformaclearsolution.Subsequently,fewgramsofg-C3N4 powder wasuniformlydispersedinthisclearsolutionviaultrasonicationfor5min,andthena solutionofethyleneglycol(10mL)withacertainamountofbismuthnitratepentahydratewasrapidlyadded.Aftervigorousstirring(30min),thesedimentwasgentlymoved toanautoclaveandkeptfor24hat160°C.Finally,thesuspensionwasgathered,washed numeroustimeswithH2Oandethanol,driedat60°Cinanoven,andgrindedforfuture use.Theobtainedg-C3N4/Bi2MoO6 heterojunctionshaveimprovedabsorptionwithin