NANOMATERIALSFOR CLINICALAPPLICATIONS
CaseStudiesinNanomedicines
Editedby
NATASSAPIPPA
SectionofPharmaceuticalTechnology,DepartmentofPharmacy, SchoolofHealthSciences,NationalandKapodistrianUniversityofAthens, Athens,Greece
TheoreticalandPhysicalChemistryInstitute,NationalHellenicResearchFoundation, Athens,Greece
COSTASDEMETZOS
SectionofPharmaceuticalTechnology,DepartmentofPharmacy, SchoolofHealthSciences,NationalandKapodistrianUniversityofAthens, Athens,Greece
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearanceCenter andtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodsthey shouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessional responsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,or fromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-816705-2
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: SimonHolt
EditorialProjectManager: EmmaHayes
ProductionProjectManager: JoyChristelNeumarinHonestThangiah
CoverDesigner: GregHarris
TypesetbyMPSLimited,Chennai,India
Listofcontributors ix
1.Solidlipidnanoparticlesindermaceuticals1
InduPalKaur,GarimaSharma,MandeepSingh,MohhammadRamzan,JogaSingh, SimarjotKaurSandhuandJaspreetSinghGulati
1.1Generalintroduction1
1.2Whysolidlipidnanoparticles?2
1.3Evolutionoflipidicnanoparticlesfromsolidlipidnanoparticlestonanostructured lipidcarriers3
1.4Cosmeticandtopicalapplicationsofsolidlipidnanoparticles5
1.5Skinpenetrationwithsolidlipidnanoparticles11
1.6Mechanismofdrugpenetrationwithsolidlipidnanoparticles12
1.7Incorporationintosemisolidvehicle13
1.8Casestudiesofsuccessfultopicaldeliverywithlipidicnanoparticles13
1.9Deliveryofagentsforotherskindiseases15
1.10Conclusions22
References 22
2.Cyclodextrin-baseddrugdeliverysystems29
MarioJug
2.1Cyclodextrins structure,physiochemicalproperties,andtoxicologicalprofile29
2.2Cyclodextrininclusioncomplexes formation,stability,andapplicationindrugdelivery31
2.3Cyclodextrin-basedproductsinclinicalpractice33
References 60
3.Lipidvesiclesfor(trans)dermaladministration71
SilviaFranzè,UmbertoM.MusazziandFrancescoCilurzo
3.1(Trans)dermaldrug-deliverysystems71
3.2Lipidvesiclesforbreachingtheskinbarrier74
3.3Liposomalformulationinclinics88
3.4Finalremarks92
References 92
4.Stimuli-responsivenanocarriersfordrugdelivery99
MariaChountoulesi,NikolaosNaziris,NatassaPippa,StergiosPispasandCostasDemetzos
4.1Introduction99
4.2Typesofstimuli100
4.3Developmentofchimericstimuli-responsiveliposomeswithincorporated stimuli-responsivepolymers103
4.4Thermotropicbehaviorofstimuli-responsiveliposomes106
4.5Physicochemicalpropertiesofstimuli-responsiveliposomes110
4.6Developmentofstimuli-responsivelyotropicliquidcrystallinenanosystems112
4.7Conclusionandfuturedirections116 References 117
5.Biodegradablenanomaterials123 KaterinaAnagnostou,MinasStylianakis,SotirisMichaleasandAthanasiosSkouras 5.1Introduction123
5.2Naturalpolymers125
5.3Syntheticpolymers130
5.4Polymericnanoparticles136
5.5Clinicalapplicationsofbiodegradablenanoparticles145
5.6Futureperspectives151 Acknowledgments152 References 153 Furtherreading 157
6.Modulatingtheimmuneresponsewithliposomaldelivery159 DavidNardo,DavidHenson,JoeE.SpringerandVincentJ.Venditto
6.1Introduction159
6.2Liposomalimmunemodulationwithsmall-moleculetherapeutics164
6.3Liposomalimmunemodulationwithliposomalgenevectors174
6.4Immunestimulationwithliposomalvaccines184
6.5Conclusionsandfuturedirections193 Listofabbreviations194 Acknowledgments195 References 195
7.Recentadvancesinsolidlipidnanoparticlesformulationandclinical applications213
HelenaRouco,PatriciaDiaz-Rodriguez,CarmenRemuñán-LópezandMarianaLandin
7.1Lipidnanoparticles213
7.2Formulationcomponents214
7.3Preformulationstudies220
7.4Formulationprocedures221
7.5Characterizationtechniques225
7.6Drugincorporationmodels231
7.7Administrationroutes233
7.8Solidlipidnanoparticlesandnanostructuredlipidcarrierscasestudiesinhumans formedicalapplications239 Self-assessmentquestions241 References 242
8.Biopolymers,liposomes,andnanofibersasmodifiedperoraldrugrelease formulants249
MarilenaVlachouandAngelikiSiamidi
8.1Introduction249
8.2Mathematicalmodelsfordrugrelease252
8.3Releaseprofilescomparison260
8.4Biopolymersinmodifiedperoraldrugdelivery261
8.5Nanofibersinmodifiedperoraldrugdelivery263
8.6Examplesofliposomal-modifiedreleaseformulationsinclinicaluse264
8.7Conclusion265
Self-assessmentquestions266 References 267
9.Graftedpolymethacrylatenanocarriersindrugdelivery271
DorotaNeugebauer
9.1Graftpoly(meth)acrylates,includingmolecularbrushes271
9.2Carriersbasedonpoly(ethyleneglycol)poly(meth)acrylatebrushes273
9.3Carriersbasedonpoly(ethyleneglycol)graftedpoly(meth)acrylates275
9.4Poly(ethyleneglycol)andbiodegradablepolyesternonlinearamphiphilics281
9.5Otherthermoresponsivegraftpolymethacrylatenanocarriers283
9.6HeterograftedJanus-typecarriers284
9.7Core shellgraftcopolymers287
9.8Graftpolymerscontainingdisulfidelinkers289 9.9Summary
LISTOFCONTRIBUTORS
KaterinaAnagnostou
DepartmentofElectrical&ComputerEngineering,HellenicMediterraneanUniversityHeraklion,Crete, Greece
MariaChountoulesi
SectionofPharmaceuticalTechnology,DepartmentofPharmacy,SchoolofHealthSciences,National andKapodistrianUniversityofAthens,Athens,Greece
FrancescoCilurzo
DepartmentofPharmaceuticalSciences,UniversityofMilan,Milan,Italy
CostasDemetzos
SectionofPharmaceuticalTechnology,DepartmentofPharmacy,SchoolofHealthSciences,National andKapodistrianUniversityofAthens,Athens,Greece
PatriciaDiaz-Rodriguez
R+DPharmaGroup(GI-1645),DepartmentofPharmacology,PharmacyandPharmaceutical Technology,FacultyofPharmacy,UniversityofSantiagodeCompostela,SantiagodeCompostela,Spain
SilviaFranzè
DepartmentofPharmaceuticalSciences,UniversityofMilan,Milan,Italy
JaspreetSinghGulati
HitechFormulationsPvtLtd,IndustrialArea1,Chandigarh,India
DavidHenson
DepartmentofPharmaceuticalSciences,UniversityofKentucky,Lexington,KY,UnitedStates
MarioJug
FacultyofPharmacyandBiochemistry,UniversityofZagreb,Zagreb,Croatia
InduPalKaur
UniversityInstituteofPharmaceuticalSciences,PanjabUniversity,Chandigarh,India
MarianaLandin
R+DPharmaGroup(GI-1645),DepartmentofPharmacology,PharmacyandPharmaceutical Technology,FacultyofPharmacy,UniversityofSantiagodeCompostela,SantiagodeCompostela,Spain
SotirisMichaleas
DepartmentofLifeSciences,SchoolofSciences,EuropeanUniversityCyprus,Nicosia,Cyprus
UmbertoM.Musazzi
DepartmentofPharmaceuticalSciences,UniversityofMilan,Milan,Italy
DavidNardo
DepartmentofPharmaceuticalSciences,UniversityofKentucky,Lexington,KY,UnitedStates
NikolaosNaziris
SectionofPharmaceuticalTechnology,DepartmentofPharmacy,SchoolofHealthSciences,National andKapodistrianUniversityofAthens,Athens,Greece
DorotaNeugebauer
SilesianUniversityofTechnology,FacultyofChemistry,DepartmentofPhysicalChemistryand TechnologyofPolymers,Gliwice,Poland
NatassaPippa
SectionofPharmaceuticalTechnology,DepartmentofPharmacy,SchoolofHealthSciences,National andKapodistrianUniversityofAthens,Athens,Greece;TheoreticalandPhysicalChemistryInstitute, NationalHellenicResearchFoundation,Athens,Greece
StergiosPispas
TheoreticalandPhysicalChemistryInstitute,NationalHellenicResearchFoundation,Athens,Greece
MohhammadRamzan
UniversityInstituteofPharmaceuticalSciences,PanjabUniversity,Chandigarh,India
CarmenRemuñán-López
NanoBiofarGroup(GI-1643),DepartmentofPharmacology,PharmacyandPharmaceuticalTechnology, FacultyofPharmacy,UniversityofSantiagodeCompostela,SantiagodeCompostela,Spain
HelenaRouco
R+DPharmaGroup(GI-1645),DepartmentofPharmacology,PharmacyandPharmaceutical Technology,FacultyofPharmacy,UniversityofSantiagodeCompostela,SantiagodeCompostela,Spain
SimarjotKaurSandhu
UniversityInstituteofPharmaceuticalSciences,PanjabUniversity,Chandigarh,India
GarimaSharma
UniversityInstituteofPharmaceuticalSciences,PanjabUniversity,Chandigarh,India
AngelikiSiamidi
SectionofPharmaceuticalTechnology,DepartmentofPharmacy,SchoolofHealthSciences,National andKapodistrianUniversityofAthens,Athens,Greece
JogaSingh
UniversityInstituteofPharmaceuticalSciences,PanjabUniversity,Chandigarh,India
MandeepSingh
UniversityInstituteofPharmaceuticalSciences,PanjabUniversity,Chandigarh,India
AthanasiosSkouras
DepartmentofElectrical&ComputerEngineering,HellenicMediterraneanUniversityHeraklion,Crete, Greece;DepartmentofLifeSciences,SchoolofSciences,EuropeanUniversityCyprus,Nicosia,Cyprus
JoeE.Springer
SpinalCordandBrainInjuryResearchCenter,UniversityofKentucky,Lexington,KY,UnitedStates
MinasStylianakis
DepartmentofElectrical&ComputerEngineering,HellenicMediterraneanUniversityHeraklion,Crete, Greece
VincentJ.Venditto
DepartmentofPharmaceuticalSciences,UniversityofKentucky,Lexington,KY,UnitedStates
MarilenaVlachou
SectionofPharmaceuticalTechnology,DepartmentofPharmacy,SchoolofHealthSciences,National andKapodistrianUniversityofAthens,Athens,Greece
Solidlipidnanoparticlesin dermaceuticals
InduPalKaur1,GarimaSharma1,MandeepSingh1,MohhammadRamzan1, JogaSingh1,SimarjotKaurSandhu1 andJaspreetSinghGulati2
1UniversityInstituteofPharmaceuticalSciences,PanjabUniversity,Chandigarh,India
2HitechFormulationsPvtLtd,IndustrialArea1,Chandigarh,India
1.1Generalintroduction
WorldHealthOrganizationhasincludedskindiseasesasthemostcommon noncommunicablediseasesinhotandhumidcountries,includingIndia;prevalenceof thesediseasesisontherise,worldover.Skinbeingthelargestorganthatinterfaces withtheenvironmentisexposedtoavarietyofphysical[ultraviolet(UV)radiations], chemical,andmicrobialinsultsthataffectitsstructureandfunction.Sincesignificant partofskinisvisibletoothers,anydisfigurementofskinisoftenassociatedwithsocial andpsychologicalimplicationsmuchbeyondtheactualdiseasesymptoms.Global BurdenofDiseasesurveyreportedskinandsubcutaneousdiseasesas18thleadingcause ofglobaldisability-adjustedlifeyearsand4thleadingcauseofnonfatalburden (Karimkhanietal.,2017).Yearslivedwithdisabilityfromthesediseases(36.4million) aremorethanthosecausedbydiabetesmellitus(29.5million)andmigraines(28.9 million)(Karimkhanietal.,2017).Theglobaldermaceuticalmarket(overthecounter andprescription)ishugeandevolvingquicklyindicatingaglobalmarketofUSD 91.40billionin2028fromUSD49.22billionin2018.
Thetreatmentsavailableatpresentareunabletocompletelycurevariousdiseases oftheskinandmeettheexpectationsofpatients.Thisisattributedtoeitheralackof suitabletreatment/agentsorpoordeliveryofdrugagenttotheappropriatelayerof theskin.Theoutermostpart(15 20 μm)oftheepidermis,namelystratumcorneum (SC),isthemajorbarriertodrugabsorptionintotheskin.Theresistantenvelopesof SCcorneocytesandkeratinmicrofibrilsareconsideredasbricks,andthelipidiclayers foundbetweenthesecellsarecalledasmortar.Thisuniquearrangementisresponsible forbasicskinpermeationresistanceandreducesthepassageofmolecules(largerthan 500Da)throughskin(Erdogan,2009).Althoughdrugsmaydiffuseintotheskinvia hairfollicles,sebaceousglands,orsweatglands,permeationthroughthemultiplelipid
bilayersofSCremainsthemainpathway(Tingetal.,2004)becausetheformer compriseonlyasmallareaoftheskin.Theinabilityofdrugmoleculestopenetrate theSCandreachthedeeperdermislayeroftheskininsufficientconcentrationcan usuallyresultinrecurrenceofseveralskindiseasesincludinginfectionsthatareoften notlimitedtotheSC.
Small-sizedcarriersincludingliposomes,niosomes,aquasomes,transfersomes,elastosomes,microemulsions,nanoemulsions,selfmicroemulsifyingdrugdeliverysystem,self nanoemulsifyingdrugdeliverysystem,andsolidlipidnanoparticles(SLNs)arecurrently beingexploredextensivelyfortheirabilitytopermeatetheSCandreachthelowerskin layersincludingdermisandattimesthesubcutaneoustissuetoo.Bothmicrometer-and nanometer-rangecarrierswerefoundeffectiveinimprovingthedeliverytoskin.Since thedrugisreleasedgraduallyandoveraprolongedperiodoftime,irritancyorother sideeffectsassociatedwiththeactiveingredients,whenappliedinconventionalformulations,aresignificantlyreducedwhenincorporatedintothesesystems,without compromisingtheefficacy(CastroandFerreira,2008).Thesesystemsnotonlymaskthe irritationandsideeffectsoftheselectedagenttobedeliveredbutalsoinvariably improveitssolubilityandpermeability.
1.2Whysolidlipidnanoparticles?
In1990thelipidicnanoparticleswereinventedasanalternativetotraditional drugcarrierssuchaspolymericnanoparticlesandliposomes.Manyquestionsofability toproduceatindustriallevel,regulatorystatusofexcipients,andnanotoxicityrose regardingtheuseoftheseconventionalnanocarriers.Suchquestionswereaddressed withthedevelopmentofSLNsasanalternative.VariousadvantagesofSLNsover polymericnanoparticlesandliposomesareelaboratedlater.
1.2.1Formulationaspects
• SLNscanbepreparedwithoutemployingorganicsolvents.Residuesofthesesolventshavetoxiceffects(Kauretal.,2014).
• Highdrugloading(B10%ormore)canbeachievedwithSLNsversusalowdrug loadingof , 5%incaseofpolymericnanoparticles(Singhetal.,2010).
• SLNsarereportedtobestableforupto3years(Kakkaretal.,2011).Thisisan importantadvantageoverothercolloidalcarriersystems.
• Remarkablescalabilityandreproducibilityofimportantpropertiesnamely,particle sizeandencapsulationefficiencyinlarge(Liuetal.,2007)batchesusinga
cost-effectivehigh-pressurehomogenizationtechniqueasthepreparationprocedureisagainanexclusiveadvantagewithSLNs(Albaneseetal.,2012).
• SLNscanbesterilizedbyautoclaving.Othernanocolloidalsystemsaresterilized bygammairradiation,whichisnotonlycostlyandaspecializedtechniquebutmay possiblyleadtotheformationoffreeradicalsandsubsequentlytoxicreactionproducts(Kauretal.,2014).
• QuantificationofSLNincreamsissimplifiedascomparedtootherparticles.Many creambasesdonotexhibitameltingpeakbelow100 C,whichmeansthecontent ofSLNinacreamcanbequantifiedbytheirmeltingpeakdeterminedbydifferentialscanningcalorimetry.
1.2.2Physiologicalaspects
• SLNsactasdrugreservoirsinvariousskinlayers(Vyasetal.,2014)byvarietyof uptakemechanismslikeenteringintoashuntsuchashairfollicle,accumulating betweencorneocytes,andinterminglingwithskinlipids,orbydisintegratingand mergingwithlipidiclayers(Tolletal.,2004;Bseisoetal.,2015).
• DependingontheproducedSLNtype,controlledreleaseoftheactiveingredients ispossible.SLNswithadrug-enrichedshellshowburstreleasecharacteristics whereasSLNswithadrug-enrichedcoreleadtosustainedrelease(Wissingand Müller,2003b).
• SLNsactasocclusives,thatis,theycanincreasethewatercontentoftheskinmakingitmorehydratedandthusmorepermeable(Wissingetal.,2001).
• SLNsshowaUV-blockingpotential,thatis,theyactasphysicalsunscreenson theirownandcanbecombinedwithmolecularsunscreenstoachieveimproved photoprotection(WissingandMüller,2003b).
• ThecomponentsusedtoformulateSLNsaresafeascomparedtopolymericnanoandmicroparticleswhichmaycausesystemictoxicitybyimpairmentofthereticuloendothelialsystemduetoslowdegradationofitscomponentsupto4weeks (Cavallietal.,2000).
1.3Evolutionoflipidicnanoparticlesfromsolidlipid nanoparticlestonanostructuredlipidcarriers
Themostimportantparametersforevaluationoflipidnanoparticlesareparticle sizeandsizedistribution,zetapotential,polymorphism,degreeofcrystallinity,drug loading,entrapmentefficiency,anddrugrelease.
Thefirstgenerationoflipidicnanoparticles,thatis,SLNs,necessarilycomprise high-meltingpointlipid(s)whichareheatedatleastoncetomeltandconsecutively cooled.Latterresultsintherecrystallizationofthelipidmatrixleadingtohighpossibilityofpolymorphismoccurrence.Lipidparticlescrystallizeinahigherenergymodification(α or β0 )whichduringstoragetransformtothelow-energy,more-ordered modification(β).
DrugmoleculesinSLNs,orientedbetweenthefattyacidchainsorglycerides,can bepotentiallyexpelledduringtransformationofthelipidfrom α to β formonstorage. Thishappensduetotheformationofmore-orderedstructureorreducednumberof imperfectionsinthecrystallattice(GuimarãesandRé,2011).Moreoverbecauseof theirperfectcrystallinestructure,SLNsexhibitalowdrug loadingefficiency (GhasemiyehandMohammadi,2018).
ToovercomethesepotentialchallengesfacedbySLNs,thesecond-generation lipidicnanoparticlescallednanostructuredlipidcarriers(NLCs)wereintroducedin 1999.EvolutionoflipidicnanoparticlesfromemulsiontoNLCsisshownin Fig.1.1 (GuimarãesandRé,2011).
NLCsarecomposedofblendsofsolidandliquidlipidsresultinginimperfections inthelatticewhichcanaccommodateagreateramountoftheactiveingredient.The less-orderedstructureisduetoinhibitionofcrystallizationbyliquidlipids.This enablesasignificantincreaseinloadingcapacityandalsominimizesprematureactive ingredientexpulsion.ThestructuralcomparisonofSLNandNLCisdepictedin Fig.1.2
Figure1.1 Evolutionoflipidnanoparticleconceptincomparisonwiththeconventionaltechnologiestillthebeginningofthe1990s. NLC,Nanostructuredlipidcarrier; SLN,solidlipidnanoparticle. ObtainedwithpermissionfromGuimarães,K.L.,Ré,M.I.,2011.Lipidnanoparticlesascarriersforcosmetic ingredients:thefirst(SLN)andthesecondgeneration(NLC).In:Beck,R.,Guterres,S.,Pohlmann,A.(Eds.), NanocosmeticsandNanomedicines,Springer,Berlin,Heidelberg,pp.101 122.
“Brick wall” structureUnstructure matrix
Low drug load
Long-term drug stability Drug expulsion during storage
High drug load
Figure1.2 “Symmetricbrickwall” and “Welshnaturalstonewall” modeldepictingdifference betweenparticlematrixstructureofSLNsandNLCs,respectively. NLCs,Nanostructuredlipidcarriers; SLNs,solidlipidnanoparticles. ObtainedwithpermissionfromBeloqui,A.,Solinís,M.A.,RodríguezGascón,A.,Almeida,A.J.,Préat,V.,2016.Nanostructuredlipidcarriers:promisingdrugdeliverysystems forfutureclinics.Nanomed.Nanotechnol.Biol.Med.,12,143 161.
(Beloquietal.,2016).DifferentpatentedandmarketedproductsusingSLN/NLCtechnologyaregivenin Tables1.1and1.2,respectively(Mülleretal.,2007;Kauletal., 2018),respectively.
1.4Cosmeticandtopicalapplicationsofsolid lipidnanoparticles
Occlusion :Epidermallayerofskinhas20%watercontentandplayprincipal roleasthebarrieroftopicalabsorptionofforeignparticles.Occlusionprocesscan enhancehydrationofSClayerwhichinfluencetopicalabsorption.SLNshavethe abilitytoformahydrophobicmonolayeredfilm,whichhasattractionfortheepidermallayerofskin.Theocclusivenatureofthisfilmretardsthewaterlossdueto
Table1.1 Importantpatentsconcerningtheuseoflipidicnanoparticlesfortopicaldermaladministration.
PatentnumberTitleMedicalconditionReference
US8715736B2Nanoparticleformulationsforskin delivery
Skininflammation SinghandPatlolla(2009)
ES2384060B1CapsuleslipidnanoparticlesHormonereplacement therapy ViladotPetitetal.(2010)
RU2602171C2Compositioncontaininglipid nanoparticlesandcorticosteroid orvitaminDderivative
EP2919756B1Solidlipidnanoparticlesof roxithromycinforhairlossor acne
CN102670484BMannose-modifiedsolidlipid nanoparticlepluralgeland preparationmethodthereof
KR101860555B1Solidlipidnanoparticles compositionforskin-whitening effectcomprisingMHY498and preparationmethodthereof
CN102342914ACalcipotriolsolidlipidnanoparticle andpreparationmethodofsame
RU2491911C1Moisturizingcreamwithsolidlipid nanoparticles
KR101810695B1Peptidesusedinthetreatmentand/ orcareofskin,mucous membranes,and/orscalpand theiruseincosmeticor pharmaceuticalcompositions
WO2010112749A1Solidlipidnanoparticles encapsulatingminoxidiland aqueoussuspensioncontaining same
Atopicdermatitis
BastholmandPeterson (2012)
Hairlossoracne CalandFrackowiak (2012)
Immunoboosterand antiinflammatory
JianqingChenandPing (2012)
Skinwhitening Jinetal.(2016)
Psoriasisand ichthyosis
MinminandHaijun (2011)
Moisturizingcream Omelyanchukand Vilinskaya(2012)
Skincare Sanzetal.(2009)
Hairloss Padoisetal.(2009)
WO2017143421A1Nanoscalesystemforthesustained releaseofactivecosmeticand/or repellentsubstances
WO2008041116A2Formulationsofactiveprinciples incorporatedinSLNssuitablefor transdermaladministration
US6875438B2Preparationsfortopical administrationofsubstances havingantiandrogenicactivity
US20180296493A1Lipidnanoparticlecompositionsand methodsascarriersof cannabinoidsinstandardized precision-metereddosageforms
JP2018521052ALipidandlipidnanoparticles formulationsfordeliveryof nucleicacids
Insectrepellent DePaulaetal.(2017)
Transdermaldelivery ofdrugswithshort halflife Gasco(2006)
Androgenicalopecia Kraemeretal.(2003)
Antiinflammatory Kaufman(2015)
Diseasesrelatedto adeficiencyof aproteinand enzymes DuandAnsell(2016)
Table1.2 Marketedproductscontaininglipidicnanoparticles(Mülleretal.,2007;Kauletal.,2018).
MarketedproductActiveingredientsIntendeduse
CutanovaCreamNanoRepair Q10
IntensiveSerumNanoRepair Q10
CutanovaCreamNanoVital Q10
SURMERCrèmeLegère
Nano-Protection
SURMERCrèmeRiche
Nano-Restructurante
SURMERElixirduBeauté
Nano-Vitalisant
SURMERMasqueCrème
Nano-Hydratant
Q10,polypeptide,hibiscus extract,gingerextract, ketosugar
Q10,polypeptide,mafane extract
Q10,TiO2,polypeptide, ursolicacid,oleanolicacid, sunflowerseedextract
Kukuinutoil,MonoiTiare Tahiti,pseudopeptide,milk, extractfromcoconut,wild indigo,noniextract
Kukuinutoil,MonoiTiare Tahiti,pseudopeptide,milk extractfromcoconut,wild indigo,noniextract
Kukuinutoil,MonoiTiare Tahiti,pseudopeptide,milk extractfromcoconut,wild indigo,noniextract
Kukuinutoil,MonoiTiare Tahiti,pseudopeptide,milk extractfromcoconut,wild indigo,noniextract
NanoLipidRestoreCLRBlackcurrantseedoil containingomega3and6 unsaturatedfattyacids
NanolipidQ10CLRCoenzymeQ10andblack currantseedoil
IOPESuperVitalcream, serum,eyecream,extra moistsoftener,extramoist emulsion
CoenzymeQ10,omega3and unsaturatedfattyacids
NLCDeepEffectEyeSerumCoenzymeQ10,highlyactive oligosaccharides
NLCDeepEffectRepair Cream Q10,TiO2,highlyactive oligosaccharides
NLCDeepEffect ReconstructionCream Q10,acetylhexapeptide-3, micronizedplantcollagen, high,activeoligosaccharides inpolysaccharidematrix
NanoLipidRepairCLRBlackcurrantseedoiland manukaoil
Antiaging,smoothesfine lines,promotes restructuring
Antiaging,antiwrinkle
Antiaging
Skin-protectingserum
Intenselyhydrating
Antiagingandmoisturizing
Skinhydration
Skinhydration
Antiaging
Antiaging,moisturizerunder eyewrinkles, facelift
Undereyewrinkles
Antiaging
Antiaging
Skindamagerepair (continued )
Table1.2 (Continued)
MarketedproductActiveingredientsIntendeduse
NLCDeepEffect Reconstruction
SerumRegenerationscreme Intensiv
SwissCellularWhite IlluminatingEyeEssence
SwissCellularWhiteIntensive Ampoules
Macadamiaternifolia seedoil, avocadooil,urea,black currantseedoil
Glycoproteins, Panaxginseng rootextract, Equisetum arvense extract, Camellia sinensis leafextract, Viola tricolor extract
Glycoproteins, Panaxginseng rootextract, Equisetum arvense extract, Camellia sinensis leafextract, Viola tricolor extract
Skinrejuvenation
SURMERCremeContour
DesYeuxNanoRemodelante
OlivenölAntiFalten Pflegekonzentrat
OlivenölAugenpflegebalsam
Kukuinutoil,MonoiTiare Tahiti,pseudopeptide, protein
Oleaeuropaea oil,panthenol, Acaciasenegal,tocopheryl acetate
Oleaeuropaea oil, Prunus amygdalusdulcis oil, hydrolizedmilkprotein, tocopherylacetate, Rhodiola rosea rootextract,caffeine
Undereyedarkcircle lightening
CelazomeMAXSun ProtectionFactor(SPF)29
Aloebarbadensis leafjuice, artemiaextract,tocopheryl acetate,benzophenone-3, butyl methoxydibenzoylmethane (Parsol,1789),ethylhexyl methoxycinnamate, ethylhexylsalicylate, homosalate
AllureBodyCreamEthylhexylglycerin,benzyl salicylate,citronellol, tocopherol,geraniol,alphaisomethylionone,coumarin, citral,ascorbylpalmitate, benzylbenzoate,ascorbic acid,citricacid,farnesol
AllureParfumBottleSparklingnotesofmandarin, rose,andvanilla
AllureEauParfumSpraySparklingmandarin,mayrose, sensualvanilla,and intoxicatingpassionfruit notesmixedwithpeony
Skinlightening
Antiwrinkle
Antiaging
Antiaging
Protectfromsun,remove finelines
Bodymoisturizer
Perfume
Perfume
evaporation.Experimentalverificati onofmoisturebarrierpropertieshas demonstratedthedifferentdegreeofo cclusion,dependingonthesizeofthe appliedparticles.Itwasfurtherobservedthatmaximumocclusivitywasreached withSLNshavinglow-meltinglipids,highcrystallinity,andlow-particlesize ( Mülleretal.,2002 ).Inastudy,nanosizedSLNandNLCsystemsweredeveloped andshowedasimilarocclusionfactorof36% 39%withareductionintransepidermalwaterlossof34.3% 6 14.8%and26.2% 6 6.5%,respectively.Themarker (nilered)howevershowedthatNLCspenetratedeeperintotheSCascompared toSLNs( López-GarcíaandGanem-Rondero,2015 ).
UV-blockingeffect:ThecapabilityofSLNstoscatterandreflecttheUVradiations makesthemsuccessfulUVblockers.ThematrixofSLNsmeasuredhigherUVabsorptionascomparedtosunscreenofoilinwater(o/w)nanoemulsion.Titaniumdioxide isacommonlyusedUVblockeratmolecularlevel.However,itexhibitssignificant sideeffectslikephotoallergiesandphototoxicity(WissingandMüller,2003b).AsynergisticeffectwasobtainedwhenSLNwerecombinedwithsunscreenformulations. TheamountofsunscreenActivePharmaceuticalIngredients(API)couldbedecreased ifcombinedwithSLNs,thusminimizingtheadversereactionsassociatedwiththese sunscreenmolecules.Followingaresomeexamplesofsuchcombinations:
1. SLNswerefoundtoactasexcellentdrugtransportsystemsforoxybenzone,and theadverseeffectslikeskinrashes,redness,andirritationwerereducedsignificantly (Maneaetal.,2014).
2. StabilityofUVblockeragentswasincreasedbyincorporationintoSLNsascarriers.Photodegradationofbis-ethylhexylphenolmethoxy-phenyltriazinewas decreasedconsiderablybyincorporationintoSLNs(Leeetal.,2007).SLNsencapsulatingUVprotectormoleculesalsoshowedabetterSPFfactorandphotostability (Lacatusuetal.,2011).
3. TheSLNformulationwithgreenteapreparedbyhigh-pressurehomogenization techniqueexhibitedahigherphotoprotectiveeffect(Boseetal.,2013),goodantioxidantactivity,andbetterstabilityatroomtemperature.
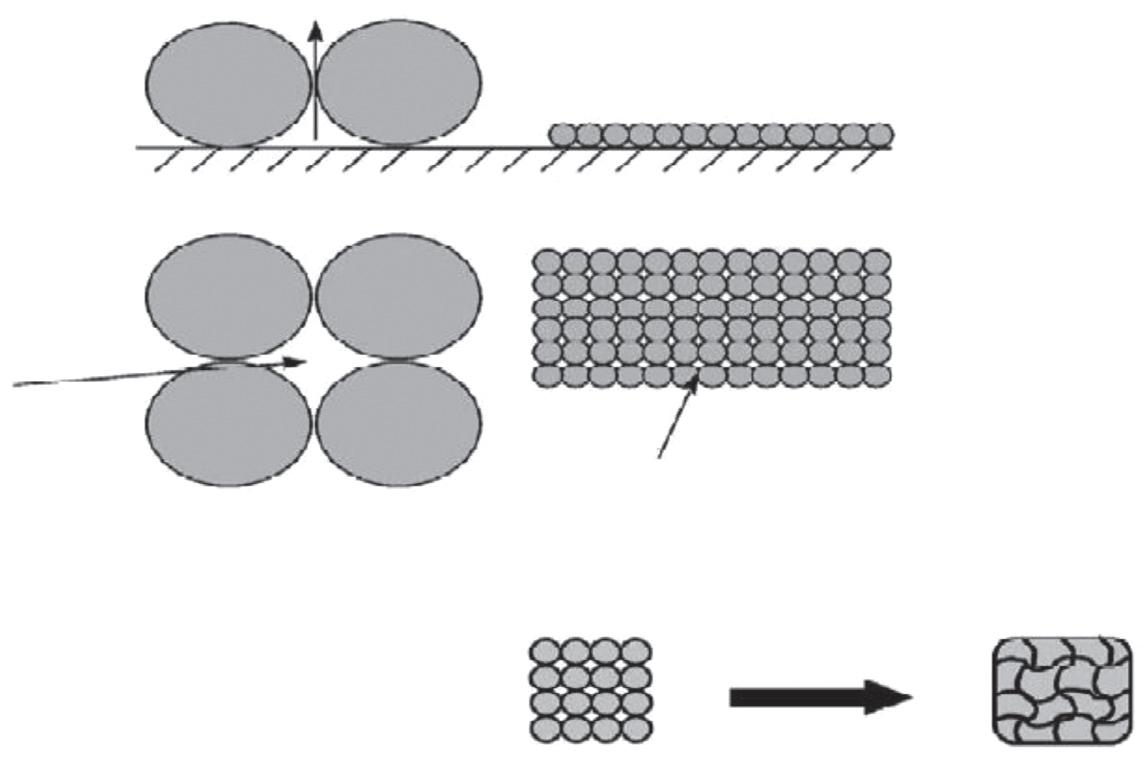
Adhesiveness:Theapplicationofformulationcontainingsubmicron-sizedSLN (B200nm)ondryhornylayershowsgoodadhesiveness.SLNsformafilmoftightly packedroundparticles,whichundertheappliedforceduringapplicationformedan intelligiblefilm(Fig.1.3).Suchtypeoflipidfilmcanhelprestoredamagedskinora brokenlipidfilmontheskinsurface.Inadditiontothis,itcanalsohaveanocclusive effect(WissingandMuller,2003a).
pHcontrolandosmoticeffect:SkinsurfaceusuallyexhibitsslightlyacidicpH(pH 5.0 7.0).ItisobservedthatasignificantchangeinpHbyapplicationofanyformulationcanleadtoirritationandrednessoftheskin.Stronglyacidicandalkalineapplication willprimarilyactasdeterioratingagents.SLNdispersionscanbeformulatedorbuffered attheskinoptimumpHthusmakingthemoptimalfordermalapplication.Some
Figure1.3 Modeloffilmformationontheskinforlipid2-mmparticlesandlipid200-nmparticles shownassection(upper)andfromthetop(middle),andanewmodeloffusionofthenanoparticlestoaporelessfilm(lower). ObtainedwithpermissionfromMüller,R.H.,Radtke,M.,Wissing,S.A., 2002.Solidlipidnanoparticles(SLN)andnanostructuredlipidcarriers(NLC)incosmeticanddermatologicalpreparations.Adv.Drug.DeliveryRev.54,S131 S155.
considerationsarealsogiventoosmoticeffectofthetopicalformulation.Changeinisotonicitycanleadtoirritation.NLCsandSLNsshowremarkableisotonicityandgood osmoticeffectfollowingtheirapplicationonskin(SoutoandMüller,2008)
Improvedchemicalstability:SolidmatrixofSLNsisstableatroomtemperatureas wellasunderphysiologicalconditions.ThesolidcoreofSLNsbetteraccommodates APIswhicharepronetohydrolysisandoxidation,protectingthemagainstchemical degradationfromwaterandoxygen,forexample,SLNdispersionsoftocopherol,retinol,andcoenzymeQ10arechemicallymorestableascomparedtothecorresponding aqueousdispersionsoffreeorunencapsulatedagents(Dingleretal.,1999).Itmaybe notedthattretinoinincorporatedintoliposomeswasfoundpronetophotodegradation (Brisaertetal.,2001),whileretinolencapsulatedwithinSLNswaschemically stable(VolkhardandGohla,2001).SLNspersealsoexhibitedhighphysicalstability duringlong-termstorage(Mülleretal.,2007).
1.5Skinpenetrationwithsolidlipidnanoparticles
Forlocalaswellassystemiceffects,skinisconsideredtobetheimportantsite fordrugapplicationwhereSCisthemainpenetrationbarrier.Moderntechniques
Fusion:
Large pores
Skin
Small Application and capillary forces ''capillary pores''
evaporation
Top view: Section:
usuallyaimatdisruptingorbypassingthecomplexskinstructurefordrugdelivery. Intercellularrouteisthemostcommonroutefollowedbythemoleculestopenetrate throughtheskin.
SCiscomposedofmorphologicallydifferentcellswhichcarrydiversefunctionsofthis secondlargestorganofthebody.Itis6 10 μminthicknesswithcellandlipidlayeralternatingwitheachother.Itismadeupof B14 18cellularlayersandthebarriernatureis attributedtothepresenceof75% 80%proteins.Inaddition,lipids(5% 20%)and unidentifiedelements(5% 8%)alsocontributetowardhindranceforthedrugmolecules topassthroughtheskin(Wertz,2018).Corneocytesaretheuppermostcellsoftheepidermisandareobservedtobestackedintheformofpillarswhenseen microscopically.
1.6Mechanismofdrugpenetrationwithsolidlipid nanoparticles
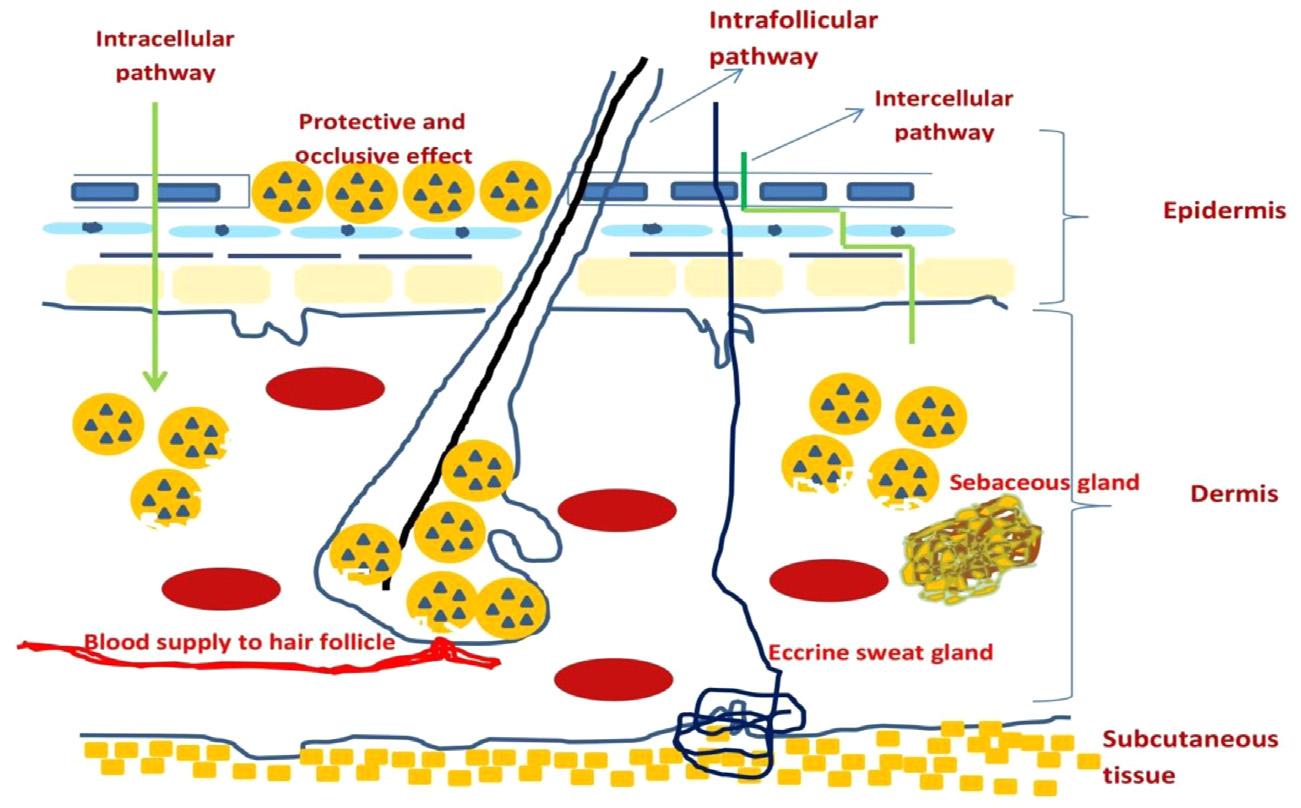
Ashighlightedearlierintercellularpathway,thatis,movementofdrugmolecules betweenthecorneocytesoftheSCisthemostpreferredroute.Anotherimportant pathwayfortransportistheintrafollicularpathwayalsocalledshuntorappendageal pathwaywherepenetrationintotheskinoccursthroughthesweatglandsorthehair follicularrouteasseenin Fig.1.4 (PalmerandDeLouise,2016).
Lipidicnanoparticlesgettingattachedtotheskinsurfacehavetheabilitytoconduct theexchangeoflipidbetweenSC(composedofhighconcentrationoflipids)and
Figure1.4 Diagrammaticrepresentationofskinpenetrationwithsolidlipidnanoparticles.
nanocarriers.RichnessofSCinepidermallipidsandthenanosizeofSLNstriggera preciseinteractionbetweenthetwo,thusprovidinganincreasedpenetratingpower, occlusivity,andconcentrationofencapsulateddruginthedermalregionoftheskin. SkinocclusivityprovidedbytheSLNsalsoincreasesskinhydrationandthustheskin penetration(Kortingetal.,2007).Nanoparticlesofsizegreaterthan100nmdonot perfusetheSC,majorlyduetotherigidityanddimensionsofthebarrierlayer. However,particlesof B200nmsizeprovideanocclusiveprotectivelayerthatinturn enhancespenetrationoftheskin.
Sebaceousglands,designatedtosecretesebum,areassociatedwiththehairfollicles insidetheskin.Sebaceoussecretionsarerichinlipidswhichprovideaperfectenvironmentforlipidnanoparticlestodissolveandreleasetheencapsulatedactive.Sebumisa mixtureofwaxesandtriglycerideswhicharealsousedinpreparingSLNs/NLCs(ones preparedwithbiocompatiblelipids).Formeracceleratetheabsorptionofthedrug throughtheseglands.Thusthisrouteisparticularlyfavorableforlipidnanoparticles andisexploitedinantiacnetherapy(Ranpiseetal.,2014).
1.7Incorporationintosemisolidvehicle
SLNdispersionsareusuallyfreeflowingandcanbeeasilyincorporatedintodermal carriersystemslikegelsandcreamstoformulateadosageformofdesiredconsistencyto beappliedtopically.Controlrelease,targetingtheviableepidermis,andtissuecompatibilityarethecharacteristicsparticularlyachievablewiththegelsystems.Gelsarealsopreferredduetoeaseinmanipulationforswellinglevelrequiredinthefinalformulation (Housinyetal.,2018).Carbopolismainlyusedasgellingagent,polymersofwhichcrosslinktogethertoformamicrogelstructurethatmakeitidealfordermatologicalpurposes. Thesestructuresgetadheredtotheskin,increasingthecontacttime(Deshkaretal.,2018)
1.8Casestudiesofsuccessfultopicaldeliverywithlipidic nanoparticles
1.8.1Deliveryofantimicrobials
1. Gideetal.preparedSLNswithacyclovir(ACV-SLNs)intheirmatrixandfurther incorporatedACV-SLNsintosemisolidgel.StudyshowedthatamountofacyclovirfromACV-SLNsinthelowerepidermallayerwastwotimesofthatachieved withcommercialacyclovirgel(Gideetal.,2013).
2. Aqueousdispersionsofketoconazole-loadedSLNsandNLCswithCompritol888 ATOasthelipidweredeveloped(SoutoandMüller,2005).Stabilitystudy revealedthatSLNdispersionwasphysicallystableandnosignificantchangeinparticlesizewasobserved;howeverNLCsalsoprotectedthedrugfromdegradation butincreaseinsizewasobserveduponstorage.
3. Sannaetal.preparedSLNsloadedwitheconazolenitrate(ECN)foradministration acrosstheskin.Theyconcludedfromskinpermeationstudythatcontrolledrelease ofdrugacrossSCwasgovernedbylipidportioninSLNs.Invivoresultsshowed thatSLNsenhancedthepenetrationofECNtodeeperskinlayersafter3hoursof administration(Sannaetal.,2007).
4. Miconazole(MN)-loadedSLNs(MN-SLN)whenincorporatedintoahydrogel showedacontrolledreleaseofMNovera24-hourtimeperiod.About10-foldincrease inretentionwasnotedwithMN-SLNascomparedtofreeMNdrugsuspensionand MNgel.InvivostudiesindicatedthatthehydrogelofMN-SLNprovidedsustained topicaleffectandtreatedthefungalinfectionsatafasterrate( Jainetal.,2010).
5. Topicalgeloffluconazole(FLZ)-loadedSLNsweredevelopedandevaluatedclinicallyforpityriasisvesicolor.Resultsshowedasignificantimprovement(P , .05)in therapeuticresponsewithFLZ-loadedSLNs,as90%patientsshowedcomplete eradicationand10%showedsignificantimprovementincomparisontomarketed cream(Housinyetal.,2018).
6. Stabilitystudywithclotrimazole-loadedSLNandNLCshowedthatthesesystems retainedtheircolloidalphaseafter3monthsofstorageattemperatureof4 C, 20 C,and40 C.Releasestudyshowedcontrolledreleaseofclotrimazolefrom bothSLNsandNLCsoveraperiodof10hours(Soutoetal.,2004).
7. Voriconazole(VCZ)lipid-basednanoparticles(LNP)developedforthetreatment ofaspergillosiscouldimprovethesolubilityofVCZ.Theantifungalstudyrevealed thatoptimizedformulationofVCZ-LNPstoppedfungusreproduction(Füredi etal.,2017).
8. Griseofulvin-loadedSLNs(GF-SLNs) enhancedtherateofdissolutionofGF duetothedecreaseinmeanparticlesize(165nm).ReleaserateofGFshowed sustainedrelease(cumulative63 .53%)overaperiodof12hours( Anuraketal., 2011 ).
9. AmphotericinB loadedSLNsfortopicalapplicationwithsmallsize (111.1 6 2.2nm)andhigherentrapmentefficiency(93.8% 6 1.8%)are reported.Freeze-driedSLNsshowedtw otimeshigherpermeationandhigher zoneofinhibitionagainst Trichophytonrubrum ascomparedtofreedrugdispersion.Theformulationwasstableatrefrigeratorandroomtemperatureoverthe periodof3months( Butanietal.,2016 ).