MOVEMENT DISORDERSIN CHILDHOOD
THIRDEDITION
HARVEY S.SINGER
JohnsHopkinsUniversitySchoolofMedicine,DepartmentofNeurology,andtheKennedyKriegerInstitute, Baltimore,MD,UnitedStates
JONATHAN W.MINK
UniversityofRochesterMedicalCenter,DepartmentofNeurology,DivisionofChildNeurology,Rochester, NY,UnitedStates
DONALD L.GILBERT
DivisionofNeurology,CincinnatiChildren’sHospitalMedicalCenter;DepartmentofPediatrics, UniversityofCincinnati,Cincinnati,OH,UnitedStates
JOSEPH JANKOVIC
Parkinson’sDiseaseCenterandMovementDisordersClinic,DepartmentofNeurology,BaylorCollegeofMedicine, Houston,TX,UnitedStates
AcademicPressisanimprintofElsevier 125LondonWall,LondonEC2Y5AS,UnitedKingdom 525BStreet,Suite1650,SanDiego,CA92101,UnitedStates 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
Copyright © 2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperiencebroaden ourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationor methodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhom theyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceor otherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthe materialherein.
ISBN:978-0-12-820552-5
ForinformationonallAcademicPresspublicationsvisitour websiteat https://www.elsevier.com/books-and-journals
Publisher: NikkiLevy
AcquisitionsEditor: JoslynChaiprasert-Paguio
EditorialProjectManager: KristiAnderson
ProductionProjectManager: SelvarajRaviraj
CoverDesigner: MattLimbert
CoverImage: “BoysAfflictedwithChoreaKnown” , usedwithpermissionfromHistoria/Shutterstock.
TypesetbyTNQTechnologies
Prefaceix
SectionI
Overview
1.BasalGangliaAnatomy,Biochemistry, andPhysiology
Introduction4 BasalGangliaCircuits,CellTypes,and Compartments4
Neurotransmitters6
OtherBasalGangliaNuclei7
InhibitingandDisinhibitingMotorPatterns9 ImplicationsforDisease:FocalLesionsand AbnormalMovements10 References11
2.CerebellarAnatomy,Biochemistry, Physiology,andPlasticity
IntroductionandOverview16
OverviewofCerebellarStructure,Function,and SymptomLocalization16
CerebellarIntegrationwithBasalGanglia Circuits23
NeurotransmittersintheCerebellum26
NeuroplasticityintheCerebellum28 Conclusion30
References30
3.Classi ficationofMovementDisorders
Introduction33 Ataxia36 Athetosis36 Ballismus36 Chorea37
Dystonia37 Myoclonus38 Parkinsonism39 Startle39
Stereotypies39 Tics40 Tremor41 MixedMovementDisorders41
AtypicalMovementDisorders41 References42
4.DiagnosticEvaluationofChildrenWith MovementDisorders
Introduction44 Preclinic46 InClinic48
TheDiagnosis57 Summary64
References65
5.MotorAssessments
Introduction70
QuantitativeMeasurementinMovement Disorders70
RatingScalesforPediatricMovement Disorders72 References77
SectionII DevelopmentalMovementDisorders
6.TransientandDevelopmental MovementDisorders
Introduction85 References93
SectionIII
ParoxysmalMovementDisorders
7.TicsandTouretteSyndrome
Introduction100
DefinitionofTics101
ClinicalCharacteristics Phenomenologyand ClassificationofTics101
LocalizationandPathophysiology102
SpecificTicDisorderDiagnoses112
Treatment119
References126
8.MotorStereotypies
IntroductionandOverview142
Definitions142
ClinicalCharacteristics,Classi fications,and Differentiation142
Pathophysiology146
MajorDiseasesandDisorders151
StereotypyRatingScales157
Treatment157
References158
9.ParoxysmalDyskinesias
IntroductionandOverview165
ClinicalCharacteristics166 DiseasesandDisorders168
References176
SectionIV
HyperkineticandHypokinetic MovementDisorders
10.Chorea,Athetosis,andBallism
IntroductionandOverview184
DefinitionsofChorea,Athetosis,and Ballism184
ClinicalCharacteristics PhenomenologyofChorea, Athetosis,andBallisminChildren185
LocalizationandPathophysiology187
DiseasesandDisorders189
SummaryofDiagnosticandTherapeutic Approach217
References218
11.Dystonia
IntroductionandOverview230
ClassificationofDystonias231
LocalizationandPathophysiology238
DiseasesandDisorders239
DiagnosticApproachtoDystonia250
ManagementandTreatment251
PatientandFamilyResources253
References253
12.Myoclonus
IntroductionandOverview264
DefinitionofMyoclonus265
ClinicalCharacteristics Phenomenologyof MyoclonusinChildren265
LocalizationandNeurophysiology268
DiseasesandDisorders270
SummaryofDiagnosticandTherapeutic Approach295
References296
13.Tremor
IntroductionandOverview306
DefinitionofTremor306
ClinicalCharacteristics Phenomenologyand ClassificationofTremorinChildren307
LocalizationandPathophysiology310
DiseasesandDisorders314
ApproachtoDiagnosisandManagement324
References325
14.Ataxia
IntroductionandOverview334
DefinitionofAtaxia335
ClinicalCharacteristics PhenomenologyofAtaxia inChildren335
LocalizationandPathophysiology336
DiseasesandDisorders336
ApproachtoDiagnosisandManagement379
References382
15.Parkinsonism
IntroductionandOverview396
ClinicalFeaturesofParkinsonism396 PathophysiogyofParkinsonism397 DiseasesandDisorders399
TreatmentofParkinsonism407 References408
16.HereditarySpasticParaplegia
IntroductionandOverview416
DefinitionsofSpasticityand Hypertonia417
ClinicalCharacteristics PhenomenologyofSpastic ParaplegiainChildren417
LocalizationandPathophysiology419 DiseasesandDisorders420
ApproachtoDiagnosisand Management433 Summary435
References435
SectionV
SelectedSecondaryMovement Disorders
17.MetabolicDisordersWithAssociated MovementAbnormalities
PediatricNeurotransmitter Disorders445
StorageDisorders456 Leukodystrophies474 Aminoacidemias475 OrganicAcidemias479
Glycolysis,PyruvateMetabolism,andKrebsCycle Disorders484
MitochondrialDisorders488 PurineMetabolismDisorders493
CreatineMetabolismDisorders497
CongenitalDisordersofGlycosylation499
Cofactor,Mineral,andVitamin Disorders500
NeuroacanthocytosisSyndromes503
OtherMetabolicConditions506 References508
18.MovementDisordersinAutoimmune Diseases
Introduction536 ImmunologyOverview536 DiseasesandDisorders537
References552
19.MovementDisordersinSleep
Introduction562
OverviewofSleepPhysiology563
Sleep-RelatedMovementDisorders565 HyperkineticMovementDisordersthatArePresent DuringtheDaytimeandPersistDuring Sleep580
SeizuresinandAroundtheTimeofSleep580 References581
20.CerebralPalsy
Introduction592 Epidemiology593 Etiology593 Diagnosis595
HistoryandPhysicalExamination595 AssessmentScales596
CluesforDeterminingtheMotorCPType597 CerebralPalsySyndromes598 Management606 References610
21.MovementDisordersand NeuropsychiatricConditions
IntroductionandOverview620
AttentionDeficitHyperactivityDisorder621
ObsessiveCompulsiveDisorder623 AutismSpectrumDisorder625
Conclusions629 References629
22.Drug-InducedMovementDisorders inChildren
IntroductionandOverview638
DefinitionofDrug-InducedMovement Disorders639
ClinicalCharacteristics Phenomenologyof Drug-InducedMovementDisordersin Children639
Drug-InducedMovementDisorders640
Conclusion658
References658
23.FunctionalMovementDisorders
Introductions667 Epidemiology668
ClinicalFeaturesofFunctionalMovement Disorders670 Pathophysiology670 Diagnosis671
Conclusion676 References676
AppendixADrugAppendix681
AppendixBSearchStrategyforGenetic MovementDisorders705
Index713
BasalGangliaAnatomy, Biochemistry,andPhysiology
HarveyS.Singer1,JonathanW.Mink2, DonaldL.Gilbert3 andJosephJankovic4 1DepartmentofNeurology,JohnsHopkinsHospitalandtheKennedyKriegerInstitute, Baltimore,MD,UnitedStates; 2DivisionofChildNeurology,UniversityofRochesterMedical Center,Rochester,NY,UnitedStates; 3DivisionofNeurology,CincinnatiChildren’sHospital MedicalCenter,Cincinnati,OH,UnitedStates; 4DepartmentofNeurology,BaylorCollegeof Medicine,Houston,TX,UnitedStates
Introduction
Thebasalgangliaarelargesubcorticalstructurescomprisingseveralinterconnectednuclei intheforebrain,diencephalon,andmidbrain.Historically,thebasalgangliahavebeen viewedasacomponentofthemotorsystem.However,thereisnowsubstantialevidence thatthebasalgangliainteractwithalloffrontalcortexandwiththelimbicsystem.Thus, thebasalganglialikelyhavearoleincognitiveandemotionalfunctioninadditiontotheir roleinmotorcontrol.1 Indeed,mostdiseasesofthebasalgangliacauseacombinationof movement,affective,andcognitivedisorderswiththemovementdisorderbeingpredominant.Themotorcircuitsofthebasalgangliaarebetterunderstoodthantheothercircuits, butbecauseofsimilarorganizationofthecircuitry,conceptualunderstandingofbasal gangliamotorfunctioncanprovideausefulframeworkforunderstandingcognitiveandaffectivefunction,too.
BasalGangliaCircuits,CellTypes,andCompartments
Circuits
Thebasalgangliaincludethestriatum(caudate,putamen,nucleusaccumbens),thesubthalamicnucleus(STN),theglobuspallidus(internalsegment GPi,externalsegment GPe, ventralpallidum VP),andthesubstantianigra(parscompacta SNpcandparsreticulata SNpr)(Fig.1.1).ThestriatumandSTNreceivethemajorityofinputsfromoutsideofthebasal ganglia.Mostofthoseinputscomefromcerebralcortex,butthalamicnucleialsoprovide stronginputstostriatum.Thebulkoftheoutputsfromthebasalgangliaarisefromthe globuspallidusinternalsegment,VP,andsubstantianigraparsreticulata.Theseoutputs areinhibitorytothepedunculopontineareainthebrainstemandtothalamicnucleithatin turnprojecttofrontallobe.
Thestriatumreceivesthebulkofextrinsicinputtothebasalganglia.Thestriatum receivesexcitatoryinputfromvirtuallyallofcerebralcortex.2 Inaddition,theventralstriatum(nucleusaccumbensandrostroventralextensionsofcaudateandputamen)receivesinputsfromhippocampusandamygdala.3 Thecorticalinputusesglutamateasits neurotransmitterandterminateslargelyontheheadsofthedendriticspinesofmedium spinyneurons.4 Theprojectionfromthecerebralcortextostriatumhasaroughlytopographicorganizationthatprovidesthebasisforanorganizationoffunctionallydifferent circuitsinthebasalganglia. 5, 6 Althoughthetopographyimpliesacertaindegreeofparallel organization,thereisalsoevidenceforconvergenceanddivergencei nthecorticostriatal projection.Thelargedendritic fi eldsofmediumspinyneurons7 allowthemtoreceiveinput fromadjacentprojections,whicharisefromdifferentareasofcortex.Inputstostriatumfrom severalfunctionallyrelatedcorticalareasoverlapandasinglecorticalareaprojectsdivergentlytomultiplestriatalzones.8 ,9 Thus,thereisamultiplyconvergentanddivergentorganizationwithinabroaderframeworkoffun ctionallydifferentparallelcircuits.This organizationprovidesananatomicalframewor kfortheintegrationandtransformationof corticalinformationinthestriatum. 1.BasalGangliaAnatomy,Biochemistry,andPhysiology

FIGURE1.1 Simplifiedschematicdiagramofbasalganglia thalamo-corticalcircuitry.Excitatoryconnections areindicatedby openarrows,inhibitoryconnectionsby filledarrows.Themodulatorydopamineprojectionisindicated bya three-headedarrow.Abbreviations: dyn,dynorphin, enk,enkephalin, GABA,gamma-amino-butyricacid, glu, glutamate, GPe,globuspallidusparsexterna, GPi,globuspallidusparsinterna, IL,intralaminarthalamicnuclei, MD, mediodorsalnucleus, PPA,pedunculopontinearea, SC,superiorcolliculus, SNpc,substantianigraparscompacta, SNpr,substantianigraparsreticulata, SP,substanceP, STN,subthalamicnucleus, VA,ventralanteriornucleus, VL, ventrallateralnucleus.
CellTypes
Mediumspinystriatalneuronsmakeup90% 95%ofthestriatalneuronpopulation.They projectoutsideofthestriatumandreceiveanumberofinputsinadditiontotheimportant
corticalinput,including(1)excitatoryglutam atergicinputsfromthalamus;(2)cholinergic inputfromstriatalinterneurons;(3)gamma -amino-butyricacid(GABA),substanceP, andenkephalininputfromadjacentmediumspinystriatalneurons;(4)GABAinput fromfast-spikinginterneurons;(5)alarge inputfromdopamine-containingneuronsin theSNpc;(6)amoresparseinputfromtheser otonin-containingneuronsinthedorsal andmedianraphenuclei.
Thefast-spikingGABAergicstriatalinterneuronsmakeuponly2% 4%ofthestriatal neuronpopulation,buttheyexertpowerfulinhibitiononmediumspinyneurons.Likemediumspinyneurons,thestriatalinterneuronsreceiveexcitatoryinputfromcerebralcortex. Theyappeartoplayanimportantroleinlimitingtheactivityofmediumspinyneurons andinfocusingthespatialpatternoftheiractivation. 10 Abnormalitiesinthenumberorfunctionoftheseneuronshavebeenlinkedtothepathobiologyofinvoluntarymovements. 11 13
Compartments
Althoughtherearenoapparentregionaldifferencesinthestriatumbasedoncelltype,an intricateinternalorganizationhasbeenreve aledwithspecialstains.Whenthestriatumis stainedforacetylcholinesterase(AChE),there isapatchydistributionoflightlystainingregionswithinmoreheavilystainedregions. 14 TheAChE-poorpatcheshavebeencalled striosomes andtheAChE-richareashavebeencalledtheextrastriosomal matrix .Thematrix formsthebulkofthestriatalvolumeandreceivesinputfrommostareasofcerebralcortex. Withinthematrixareclustersofneuronswithsimilarinputsthathavebeentermed matrisomes.ThebulkoftheoutputfromcellsinthematrixistobothsegmentsoftheGP,VP,and toSNpr.Thestriosomesreceiveinputfrom prefrontalcortexan dsendoutputtoSNpc. 15 Immunohistochemicaltechniqueshavedemon stratedthatmanysubstancessuchassubstanceP,dynorphin,andenkephalinhaveapatchydistributionthatmaybepartlyor whollyinregisterwiththestriosomes.Thestri osome-matrixorganizationsuggestsalevel offunctionalsegregationwithinthestriatumthatmaybemaintainedbydifferential in fl uencesofdopamine.16 Whilepreferentialinvolvemento fthestriosomeormatrixcompartmentshasbeensuggestedinsomedisorders,17 theclinicalsigni fi canceofthisorganizationisstillnotwellunderstood.
Neurotransmitters
Dopamine
Thedopamineinputtothestriatumterminateslargelyontheshaftsofthedendritic spinesofmediumspinyneuronswhereitisinapositiontomodulatetransmissionfrom thecerebralcortextothestriatum.18 Theactionofdopamineonstriatalneuronsdepends onthetypeofdopaminereceptorinvolved.FivetypesofGprotein-coupleddopaminereceptorshavebeendescribed(D1.D5).19 Thesehavebeengroupedintotwofamiliesbasedon theirlinkagetoadenylcyclaseactivityandresponsetoagonists.TheD1familyincludes D1andD5receptorsandtheD2familyincludesD2,D3,andD4receptors.Theconventional viewhasbeenthatdopamineactsatD1receptorstofacilitatetheactivityofpostsynapticneuronsandatD2receptorstoinhibitpostsynapticneurons.20 Indeed,thisisafundamental 1.BasalGangliaAnatomy,Biochemistry,andPhysiology
conceptforsomemodelsofbasalgangliapathophysiology.21,22 However,thephysiologiceffectofdopamineonstriatalneuronsismorecomplex.WhileactivationofdopamineD1receptorspotentiatestheeffectofcorticalinputtostriatalneuronsinsomestates,itreducesthe efficacyofcorticalinputinothers.23 ActivationofD2receptorsmoreconsistentlydecreases theeffectofcorticalinputtostriatalneuron.24 Dopaminecontributestofocusingthespatial andtemporalpatternsofstriatalactivity.
Inadditiontoshort-termfacilitationorinhibitionofstriatalactivity,thereisevidencethat dopaminecanmodulatecorticostriataltransmissionbymechanismsoflong-termdepression (LTD)andlong-termpotentiation(LTP).Throughthesemechanisms,dopaminestrengthens orweakenstheefficacyofcorticostriatalsynapsesandcanthusmediatereinforcementofspecificdischargepatterns.LTPandLTDarethoughttobefundamentaltomanyneuralmechanismsoflearningandmayunderliethehypothesizedroleofthebasalgangliainhabit learning.25 SNpcdopamineneurons fireinrelationtobehaviorallysignificanteventsand reward.26 Thesesignalsarelikelytomodifytheresponsesofstriatalneuronstoinputsthat occurinconjunctionwiththedopaminesignalresultinginthereinforcementofmotorand otherbehaviorpatterns.Striatallesionsorfocalstriataldopaminedepletionimpairsthe learningofnewmovementsequences,27 supportingaroleforthebasalgangliaincertaintypes ofprocedurallearning.Dopaminemayalsoplayaroleinotheraspectsofmotorlearning.28
GABA
MediumspinystriatalneuronscontaintheinhibitoryneurotransmitterGABAandcolocalizedpeptideneurotransmitters.29,30 Basedonthetypeofneurotransmittersandthepredominanttypeofdopaminereceptortheycontain,themediumspinyneuronscanbedividedinto twopopulations.OnepopulationcontainsGABA,dynorphin,andsubstancePandprimarily expressesD1dopaminereceptors.Theseneuronsprojecttothebasalgangliaoutputnuclei, GPi,andSNpr.ThesecondpopulationcontainsGABAandenkephalinandprimarilyexpressesD2dopaminereceptors.Theseneuronsprojecttotheexternalsegmentoftheglobus pallidus(GPe).21
Acetylcholine
Cholinergicinterneuronsdenselyinnervatethestriatum 31 andmodulatedopamine release.32 Additionalcholinergicinputintostriatumcomesfromthepedunculopontinenucleusandthelaterodorsaltegmentalnucleiinthebrainstem.33 Viamuscarinicacetylcholine receptors,cholinergicinterneuronsin fluencebothdopamineD1andD2receptorexpressingmediumspinyneurons.Akeypropertyofcholinergicinterneuronsistheirtonic spikingactivity,andthustheyarealsoreferredtoastonicallyactiveneurons.34
OtherBasalGangliaNuclei
SubthalamicNucleus
TheSTNreceivesanexcitatory,glutamatergicinputfrommanyareasoffrontallobeswith especiallylargeinputsfrommotorareasofcortex.35 TheSTNalsoreceivesinhibitory
GABAergicinputfromGPe.TheoutputfromtheSTNisglutamatergicandexcitatorytothe basalgangliaoutputnuclei,GPi,VP,andSNpr.STNalsosendsanexcitatoryprojectionback toGPe.ThereisasomatotopicorganizationinSTN36 andarelativetopographicseparationof “motor” and “cognitive” inputstoSTN.
OutputNuclei:GlobusPallidusInternaandSubstantiaNigraParsReticulata
TheprimarybasalgangliaoutputarisesfromGPi,aGPi-likecomponentofVP,andSNpr. Asdescribedabove,GPiandSNprreceiveexcitatoryinputfromSTNandinhibitoryinput fromstriatum.TheyalsoreceiveaninhibitoryinputfromGPe.Thedendritic fieldsofGPi, VP,andSNprneuronsspanupto1mmdiameterandthushavethepotentialtointegrate alargenumberofconverginginputs.37 TheoutputfromGPi,VP,andSNprisinhibitory andusesGABAasitsneurotransmitter.Theprimaryoutputisdirectedtothalamicnuclei thatprojecttothefrontallobes:theventrolateral,ventroanterior,andmediodorsalnuclei. ThethalamictargetsofGPi,VP,andSNprproject,inturn,tofrontallobe,withthestrongest outputgoingtomotorareas.Collateralsoftheaxonsprojectingtothalamusprojecttoanarea atthejunctionofthemidbrainandponsintheareaofthepedunculopontinenucleus.38 Other outputneurons(20%)projecttointralaminarnucleiofthethalamus,tothelateralhabenula, ortothesuperiorcolliculus.39
Thebasalgangliamotoroutputhasasomatotopicorganizationsuchthatthebodybelow theneckislargelyrepresentedinGPi,andtheheadandeyesarelargelyrepresentedinSNpr. Theseparaterepresentationofdifferentbodypartsismaintainedthroughoutthebasal ganglia.Withintherepresentationofanindividualbodypart,italsoappearsthatthereis segregationofoutputstodifferentmotorareasofcortexandthatanindividualGPineuron sendsoutputviathalamustojustoneareaofcortex.40 Thus,GPineuronsthatprojectviathalamustomotorcortexareadjacentto,butseparatefrom,thosethatprojecttopremotorcortex orsupplementarymotorarea.GPineuronsthatprojectviathalamustoprefrontalcortexare alsoseparatefromthoseprojectingtomotorareasandfromVPneuronsprojectingviathalamustoorbitofrontalcortex.Theanatomicsegregationofbasalganglia-thalamocorticaloutputssuggestsfunctionalsegregationattheoutputlevel,butotheranatomicevidence suggestsinteractionsbetweencircuitswithinthebasalganglia(seeabove).5,41
GlobusPallidusExterna
TheGPeandtheGPe-likepartofVPmaybeviewedasintrinsicnucleiofthebasalganglia. LikeGPiandSNpr,GPereceivesaninhibitoryprojectionfromthestriatumandanexcitatory onefromSTN.UnlikeGPi,thestriatalprojectiontoGPecontainsGABAandenkephalinbut notsubstanceP.21 TheoutputofGPeisquitedifferentfromtheoutputofGPi.Theoutput fromGPeisGABAergicandinhibitory,andthemajorityoftheoutputprojectstoSTN. TheconnectionsfromstriatumtoGPe,fromGPetoSTN,andfromSTNtoGPiformthe “indirect” striatopallidalpathwaytoGPi42 (Fig.1.1).Inaddition,thereisamonosynaptic GABAergicinhibitoryoutputfromGPedirectlytoGPiandtoSNprandaGABAergicprojectionbacktostriatum.43 Thus,GPeneuronsareinapositiontoprovidefeedbackinhibition
toneuronsinstriatumandSTNandfeedforwardinhibitiontoneuronsinGPiandSNpr.This circuitrysuggeststhatGPemayacttooppose,limit,orfocustheeffectofthestriatalandSTN projectionstoGPiandSNpraswellasfocusactivityintheseoutputnuclei.
SubstantiaNigraParsCompacta
DopamineinputtothestriatumarisesfromSNpcandtheventraltegmentalarea(VTA). SNpcprojectstomostofthestriatum;VTAprojectstotheventralstriatum.TheSNpcand VTAaremadeupoflargedopamine-containingcells.SNpcreceivesinputfromthestriatum, speci ficallyfromthestriosomes.ThisinputisGABAergicandinhibitory.TheSNpcandVTA dopamineneuronsprojecttocaudateandputameninatopographicmanner,41 butwithoverlap.Thenigraldopamineneuronsreceiveinputsfromonestriatalcircuitandprojectbackto thesameandtoadjacentcircuits.Thus,theyappeartobeinapositiontomodulateactivity acrossfunctionallydifferentcircuits.
InhibitingandDisinhibitingMotorPatterns
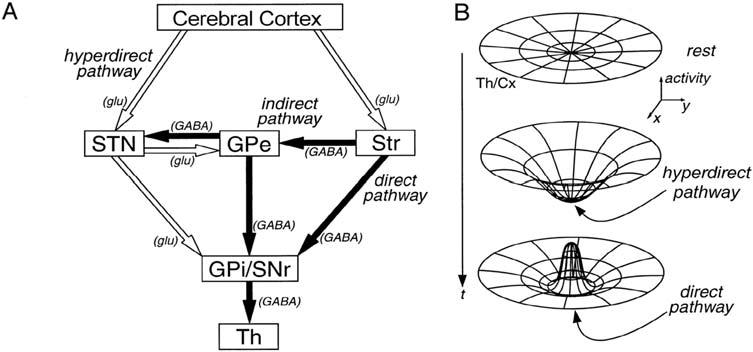
Althoughthebasalgangliaintrinsiccircuitryiscomplex,theoverallpictureisoftwoprimarypathwaysthroughthebasalgangliafromcerebralcortexwiththeoutputdirectedvia thalamusatthefrontallobes.Thesepathwaysconsistoftwodisynapticpathwaysfromcortex tothebasalgangliaoutput(Fig.1.2).Inaddition,thereareseveralmultisynapticpathways involvingGPe.Thetwodisynapticpathwaysarefromcortexthrough(1)striatum(the direct pathway) and(2)STN(the hyperdirectpathway) tothebasalgangliaoutputs.Thesepathways haveimportantanatomicalandfunctionaldifferences.First,thecorticalinputtoSTNcomes onlyfromfrontallobe,whereastheinputtostriatumarisesfromvirtuallyallareasofcerebral cortex.Second,theoutputfromSTNisexcitatory,whereastheoutputfromstriatumisinhibitory.Third,theexcitatoryroutethroughSTNisfasterthantheinhibitoryroutethroughstriatum.44 Finally,theSTNprojectiontoGPiisdivergentandthestriatalprojectionismore focused.45 Thus,thetwodisynapticpathwaysfromcerebralcortextothebasalgangliaoutput nuclei,GPiandSNpr,providefast,widespread,divergentexcitationthroughSTNand slower,focused,inhibitionthroughstriatum.17 ThisorganizationprovidesananatomicalbasisforfocusedinhibitionandsurroundexcitationofneuronsinGPiandSNpr(Fig.1.3). BecausetheoutputofGPiandSNprisinhibitory,thisresultsinfocusedfacilitationandsurroundinhibitionofbasalgangliathalamocorticaltargets.47 Thetonicallyactiveinhibitory outputofthebasalgangliaactsasa “brake” onmotorpatterngenerators(MPGs)inthecerebralcortex(viathalamus)andbrainstem.Whenamovementisinitiatedbyaparticular MPG,basalgangliaoutputneuronsprojectingtocompetingMPGsincreasetheir firing rate,therebyincreasinginhibitionandapplyinga “brake” onthosegenerators.Otherbasal gangliaoutputneuronsprojectingtothegeneratorsinvolvedinthedesiredmovement decreasetheirdischarge,therebyremovingtonicinhibitionandreleasingthe “brake” from thedesiredmotorpatterns.Thus,theintendedmovementisenabledandcompetingmovementsarepreventedfrominterferingwiththedesiredone.35,48
1.BasalGangliaAnatomy,Biochemistry,andPhysiology
FIGURE1.2 Schematicdiagramofthehyperdirectcortico-subthalamo-pallidal,directcortico-striato-pallidal,and indirectcortico-striato-GPe-subthalamo-GPipathways. Whiteandblackarrows representexcitatoryglutamatergic(glu) andinhibitoryGABAergic(GABA)projections,respectively. GPe,externalsegmentoftheglobuspallidus; GPi,internalsegmentoftheglobuspallidus; SNr,substantianigraparsreticulata; STN,subthalamicnucleus; Str,striatum; Th,thalamus. B:aschematicdiagramexplainingtheactivitychangeovertime(t)inthethalamocorticalprojection (Th/Cx)followingthesequentialinputsthroughthehyperdiectcortico-subthalamo-pallidal(middle)anddirect cortico-striato-pallidal(bottom)pathways. ModifiedfromRef.[44].
ImplicationsforDisease:FocalLesionsandAbnormalMovements
Thisschemeprovidesaframeworkforunderstandingboththepathophysiologyofparkinsonism35,49 andinvoluntarymovement.35,48 Differentinvoluntarymovementssuchasparkinsonism,chorea,dystonia,orticsresultfromdifferentabnormalitiesinthebasalganglia circuits.LossofdopamineinputtothestriatumresultsinalossofnormalpausesofGPi dischargeduringvoluntarymovement.Hence,thereisexcessiveinhibitionofMPGsandultimatelybradykinesia.49 Furthermore,lossofdopamineresultsinabnormalsynchronyofGPi neuronaldischargeandlossofthenormalspatialandtemporalfocusofGPiactivity.49 51 BroadlesionsofGPiorSNprdisinhibitbothdesiredandunwantedmotorpatternsleading toinappropriateactivationofcompetingmotorpatterns,butnormalgenerationofthe wantedmovement.Thus,lesionsofGPicausecocontractionofmultiplemusclegroups anddifficultyturningoffunwantedmotorpatterns,similartowhatisseenindystonia, butdonotaffectmovementinitiation.52 LesionsofSNprcauseunwantedsaccadiceyemovementsthatinterferewiththeabilitytomaintainvisual fixationbutdonotimpairtheinitiation ofvoluntarysaccades.53 Lesionsofputamenmaycausedystoniaduetothelossoffocused inhibitioninGPi.48 LesionsofSTNproducecontinuousinvoluntarymovementsofthecontralaterallimbs(hemiballismorhemichorea).48 Despitetheinvoluntarymovements,voluntary movementscanstillbeperformed.Althoughstructurallesionsofputamen,GPi,SNpr,or STNproducecertaintypesofunwantedmovementsorbehaviors,theydonotproduce tics.Ticsaremorelikelytoarisefromabnormalactivitypatternsinthestriatum.12,48

FIGURE1.3 Schematicofnormalfunctionalorganizationofthebasalgangliaoutput.Excitatoryprojectionsare indicatedwith openarrows;inhibitoryprojectionsareindicatedwith filledarrows.Relativemagnitudeofactivityis representedbylinethickness. ModifiedfromRef.[46].
Althoughthefocusofthisdiscussionofbasalgangliacircuitshasbeenonmotorcontrol andmovementdisorders,itislikelythatthefundamentalprinciplesoffunctioninthesomatomotor,oculomotor,limbic,andcognitivebasalgangliacircuitsaresimilar.Ifthebasic schemeoffacilitationandinhibitionofcompetingmovementsisextendedtoencompass morecomplexbehaviorsandthoughts,manyfeaturesofbasalgangliadisorderscanbe explainedasafailuretofacilitatewantedbehaviorsandsimultaneouslyinhibitunwantedbehaviorsduetoabnormalbasalgangliaoutputpatterns.Indeed,manymovementdisorders areaccompaniedbycognitiveandaffectivesymptoms.54 56
References
1.Baez-MendozaR,SchultzW.Theroleofthestriatuminsocialbehavior. FrontNeurosci.2013;7:233.
2.KempJM,PowellTPS.Thecorticostriateprojectioninthemonkey. Brain.1970;93:525 546.
3.FudgeJ,KunishioK,WalshC,RichardD,HaberS.Amygdaloidprojectionstoventromedialstriatalsubterritoriesintheprimate. Neuroscience.2002;110:257 275.
4.CherubiniE,HerrlingPL,LanfumeyL,StanzioneP.Excitatoryaminoacidsinsynapticexcitationofratstriatal neuronesinvitro. JPhysiol(Lond).1988;400:677 690.
1.BasalGangliaAnatomy,Biochemistry,andPhysiology
5.KellyR,StrickPL.Macro-architectureofbasalganglialoopswiththecerebralcortex:useofrabiesvirustoreveal multisynapticcircuits. ProgBrainRes.2004;143:449 459.
6.AlexanderGE,DeLongMR,StrickPL.Parallelorganizationoffunctionallysegregatedcircuitslinkingbasal gangliaandcortex. AnnuRevNeurosci.1986;9:357 381.
7.WilsonCJ,GrovesPM.Finestructureandsynapticconnectionsofthecommonspinyneuronoftheratneostriatum:astudyemployingintracellularinjectionofhorseradishperoxidase. JCompNeurol.1980;194:599 614.
8.SelemonLD,Goldman-RakicPS.Longitudinaltopographyandinterdigitationofcorticostriatalprojectionsinthe rhesusmonkey. JNeurosci.1985;5:776 794.
9.FlahertyAW,GraybielAM.Corticostriataltransformationsintheprimatesomatosensorysystem.Projections fromphysiologicallymappedbody-partrepresentations. JNeurophysiol.1991;66:1249 1263.
10.MalletN,LeMoineC,CharpierS,GononF.Feedforwardinhibitionofprojectionneuronsbyfast-spikingGABA interneuronsintheratstriatuminvivo. JNeurosci.2005;25:3857 3869.
11.KataokaY,KalanithiPS,GrantzH,etal.Decreasednumberofparvalbuminandcholinergicinterneuronsinthe striatumofindividualswithTourettesyndrome. JCompNeurol.2010;518:277 291.
12.McCairnKW,BronfeldM,BelelovskyK,Bar-GadI.Theneurophysiologicalcorrelatesofmotorticsfollowing focalstriataldisinhibition. Brain.2009;132:2125 2138.
13.GittisAH,LeventhalDK,FensterheimBA,PettiboneJR,BerkeJD,KreitzerAC.Selectiveinhibitionofstriatal fast-spikinginterneuronscausesdyskinesias. JNeurosci.2011;31:15727 15731.
14.GraybielAM,AosakiT,FlahertyAW,KimuraM.Thebasalgangliaandadaptivemotorcontrol. Science. 1994;265:1826 1831.
15.GerfenCR.Theneostriatalmosaic:multiplelevelsofcompartmentalorganizationinthebasalganglia. AnnuRev Neurosci.1992;15:285 320.
16.PragerEM,DormanDB,HobelZB,MalgadyJM,BlackwellKT,PlotkinJL.Dopamineoppositelymodulatesstate transitionsinstriosomeandmatrixdirectpathwaystriatalspinyneurons. Neuron.2020;108:1091 1102.e5.
17.CrittendenJR,GraybielAM.Basalgangliadisordersassociatedwithimbalancesinthestriatalstriosomeandmatrixcompartments. FrontNeuroanat.2011;5:59.
18.BouyerJJ,ParkDH,JohTH,PickelVM.Chemicalandstructuralanalysisoftherelationbetweencorticalinputs andtyrosinehydroxylase-containingterminalsinratneostriatum. BrainRes.1984;302:267 275.
19.SibleyDR,MonsmaFJ.Molecularbiologyofdopaminereceptors. TrendsPharmacolSci.1992;13:61 69.
20.GerfenCR,EngberTM,MahanLC,etal.D1 andD2 dopaminereceptor-regulatedgeneexpressionofstriatonigral andstriatopallidalneurons. Science.1990;250:1429 1432.
21.AlbinRL,YoungAB,PenneyJB.Thefunctionalanatomyofbasalgangliadisorders. TrendsNeurosci 1989;12:366 375.
22.DeLongMR.Primatemodelsofmovementdisordersofbasalgangliaorigin. TrendsNeurosci.1990;13:281 285.
23.Hernandez-LopezS,BargasJ,SurmeierDJ,ReyesA,GalarragaE.D1receptoractivationenhancesevoked dischargeinneostriatalmediumspinyneuronsbymodulatinganL-typeCa2þ conductance. JNeurosci. 1997;17:3334 3342.
24.NicolaS,SurmeierJ,MalenkaR.Dopaminergicmodulationofneuronalexcitabilityinthestriatumandnucleus accumbens. AnnuRevNeurosci.2000;23:185 215.
25.JogM,KubotaY,ConnollyC,HillegaartV,GraybielA.Buildingneuralrepresentationsofhabits. Science. 1999;286:1745 1749.
26.SchultzW,RomoR,LjungbergT,MirenowiczJ,HollermanJR,DickinsonA.Reward-relatedsignalscarriedby dopamineneurons.In:HoukJC,DavisJL,BeiserDG,eds. ModelsofInformationProcessingintheBasalGanglia. Cambridge:MITPress;1995:233 249.
27.MatsumotoN,HanakawaT,MakiS,GraybielAM,KimuraM.Roleofnigrostriataldopaminesysteminlearning toperformsequentialmotortasksinapredictivemanner. JNeurophysiol.1999;82:978 998.
28.WoodAN.Newrolesfordopamineinmotorskillacquisition:lessonsfromprimates,rodents,andsongbirds. LID.2020. https://doi.org/10.1152/jn.00648
29.KreitzerAC.Physiologyandpharmacologyofstriatalneurons. AnnuRevNeurosci.2009;32:127 147.
30.PennyGR,AfsharpourS,KitaiST.Theglutamatedecarboxylase-,leucineenkephalin-,methionineenkephalinandsubstanceP-immunoreactiveneuronsintheneostriatumoftheratandcat:evidenceforpartialpopulation overlap. Neuroscience.1986;17:1011 1045.
31.KawaguchiY,WilsonCJ,AugoodSJ,EmsonPC.Striatalinterneurones:chemical,physiologicalandmorphologicalcharacterization. TrendsNeurosci.1995;18:527 535.
32.ThrelfellS,LalicT,PlattNJ,JenningsKA,DeisserothK,CraggSJ.Striataldopaminereleaseistriggeredbysynchronizedactivityincholinergicinterneurons. Neuron.2012;75:58 64.
33.DautanD,Huerta-OcampoI,WittenIB,etal.Amajorexternalsourceofcholinergicinnervationofthestriatum andnucleusaccumbensoriginatesinthebrainstem. JNeurosci.2014;34:4509 4518.
34.BennettBD,CallawayJC,WilsonCJ.Intrinsicmembranepropertiesunderlyingspontaneoustonic firinginneostriatalcholinergicinterneurons. JNeurosci.2000;20:8493 8503.
35.MinkJW.Thebasalganglia:focusedselectionandinhibitionofcompetingmotorprograms. ProgrNeurobiol 1996;50:381 425.
36.NambuA,TakadaM,InaseM,TokunoH.Dualsomatotopicalrepresentationsintheprimatesubthalamicnucleus:evidencefororderedbutreversedbody-maptransformationsfromtheprimarymotorcortexandthesupplementarymotorarea. JNeurosci.1996;16:2671 2683.
37.PercheronG,YelnikJ,FrancoisC.Agolgianalysisoftheprimateglobuspallidus.III.Spatialorganizationofthe striato-pallidalcomplex. JCompNeurol.1984;227:214 227.
38.ParentA.Extrinsicconnectionsofthebasalganglia. TrendsNeurosci.1990;13:254 258.
39.FrancoisC,PercheronG,YelnikJ,TandeD.Atopographicstudyofthecourseofnigralaxonsandofthedistributionofpallidalaxonalendingsinthecentremedian-parafascicularcomplexofmacaques. BrainRes. 1988;473:181 186.
40.HooverJE,StrickPL.Multipleoutputchannelsinthebasalganglia. Science.1993;259:819 821.
41.HaberSN,FudgeJL,McFarlandNR.Striatonigrostriatalpathwaysinprimatesformanascendingspiralfromthe shelltothedorsolateralstriatum. JNeurosci.2000;20:2369 2382.
42.AlexanderGE,CrutcherMD.Functionalarchitectureofbasalgangliacircuits:neuralsubstratesofparallelprocessing. TrendsNeurosci.1990;13:266 271.
43.BolamJP,HanleyJJ,BoothPA,BevanMD.Synapticorganisationofthebasalganglia. JAnat.2000;196:527 542.
44.NambuA,TokunoH,HamadaI,etal.Excitatorycorticalinputstopallidalneuronsviathesubthalamicnucleus inthemonkey. JNeurophysiol.2000;84:289 300.
45.ParentA,HazratiL-N.Anatomicalaspectsofinformationprocessinginprimatebasalganglia. TrendsNeurosci 1993;16:111 116.
46.MinkJW.BasalgangliadysfunctioninTourette’ssyndrome:anewhypothesis. PediatrNeurol.2001;25:190 198.
47.OzakiM,SanoH,SatoS,etal.Optogeneticactivationofthesensorimotorcortexreveals “localinhibitoryand globalexcitatory” inputstothebasalganglia. CerebCortex.2017;27:5716 5726.
48.MinkJ.Thebasalgangliaandinvoluntarymovements:impairedinhibitionofcompetingmotorpatterns. Arch Neurol.2003;60:1365 1368.
49.BoraudT,BezardE,BioulacB,GrossCE.Fromsingleextracellularunitrecordinginexperimentalandhuman Parkinsonismtothedevelopmentofafunctionalconceptoftheroleplayedbythebasalgangliainmotorcontrol. ProgrNeurobiol.2002;66:265 283.
50.RazA,VaadiaE,BergmanH.Firingpatternsandcorrelationsofspontaneousdischargeofpallidalneuronsin thenormalandthetremulous1-methyl-4-phenyl-1,2,3,6-tetrahydropyridineVervetmodelofparkinsonism. JNeurosci.2000;20:8559 8571.
51.TremblayL,FilionM,BedardPJ.ResponsesofpallidalneuronstostriatalstimulationinmonkeyswithMPTPinduceparkinsonism. BrainRes.1989;498:17 33.
52.MinkJW,ThachWT.Basalgangliamotorcontrol.III.Pallidalablation:normalreactiontime,musclecocontraction,andslowmovement. JNeurophysiol.1991;65:330 351.
53.HikosakaO,WurtzRH.ModificationofsaccadiceyemovementsbyGABA-relatedsubstances.II.Effectsofmuscimolinmonkeysubstantianigraparsreticulata. JNeurophysiol.1985;53:292 308.
54.AsmusF,GasserT.Dystonia-plussyndromes. EurJNeurol.2010;17(Suppl1):37 45.
55.PolettiM,DeRosaA,BonuccelliU.AffectivesymptomsandcognitivefunctionsinParkinson’sdisease. JNeurol Sci.2012;317:97 102.
56.RossCA,AylwardEH,WildEJ,etal.Huntingtondisease:naturalhistory,biomarkersandprospectsfortherapeutics. NatRevNeurol.2014;10:204 216.
Thispageintentionallyleftblank
CerebellarAnatomy,Biochemistry, Physiology,andPlasticity
HarveyS.Singer1,JonathanW.Mink2, DonaldL.Gilbert3 andJosephJankovic4
1DepartmentofNeurology,JohnsHopkinsHospital,Baltimore,MD,UnitedStates; 2Division ofChildNeurology,UniversityofRochesterMedicalCenter,Rochester,NY,UnitedStates; 3DivisionofNeurology,CincinnatiChildren’sHospitalMedicalCenter,Cincinnati,OH,United States; 4DepartmentofNeurology,BaylorCollegeofMedicine,Houston,TX,UnitedStates
IntroductionandOverview16
OverviewofCerebellarStructure, Function,andSymptomLocalization16
MacroscopictoMicroscopicCerebellar Structure17
CerebellarStructural “Threes” 17
TheThreeAnatomic Regions StructuresandAfferent Connections18
TheThreeCerebellarFunctionalRegions ConnecttoThreeDeepCerebellar Nuclei18
TheThreePairedCerebellarPeduncles21 TypesofAfferentFibers23
TheThreeLayersofCerebellarCortex andTheirCellTypes23
CerebellarIntegrationwithBasal GangliaCircuits23
NeurotransmittersintheCerebellum26 Glutamate26 Gamma-AminobutyricAcid27 Acetylcholine,Dopamine, Norepinephrine,andSerotonin27 Endocannabinoids28
NeuroplasticityintheCerebellum28 CerebellarStimulation28 Conclusion30 References30