Contributors
LucaBaiamonte DepartmentofChemistry, ColoradoSchoolofMines,Golden,CO,United States
JosefBrandt DepartmentAnaylsis,LeibnizInstitutf € urPolymerforschungDresdene.V., Dresden,Germany
TaihyunChang DepartmentofChemistryand DivisionofAdvancedMaterialsScience, PohangUniversityofScienceandTechnology, Pohang,SouthKorea
MarkD.Dadmun DepartmentofChemistry, UniversityofTennessee,Knoxville;Chemical SciencesDivision,OakRidgeNational Laboratory,OakRidge,TN,UnitedStates
NadiaEdwin DepartmentofHealthand BiomedicalSciences,AdventHealthUniversity, Orlando,FL,UnitedStates
AnthonyP.Gies TheDowChemicalCompany, LakeJackson,TX,UnitedStates
DavidGillespie TosohBioscienceLLC,Kingof Prussia,PA,UnitedStates
AndrewGorman SchoolofMaterialsScience& Engineering,GeorgiaInstituteofTechnology, Atlanta,GA,UnitedStates
AlexanderS.Gubarev Departmentof MolecularBiophysicsandPhysicsofPolymers, Saint-PetersburgStateUniversity,SaintPetersburg,Russia
ShawLingHsu PolymerScienceand EngineeringDepartment,Universityof Massachusetts(Amherst),Amherst,MA, UnitedStates
WayneHuberty AdvancedComposites Institute,MississippiStateUniversity, Starkville,MS,UnitedStates
MuhammadImran H.E.J.ResearchInstituteof Chemistry,InternationalCenterforChemical
andBiologicalSciences(ICCBS),Universityof Karachi,Karachi,Pakistan
S.KimRatanathanwongsWilliams Department ofChemistry,ColoradoSchoolofMines, Golden,CO,UnitedStates
AlbenaLederer DepartmentAnaylsis,LeibnizInstitutfurPolymerforschungDresdene.V.; SchoolofScience,TechnischeUniversitat Dresden,Dresden,Germany;Departmentof ChemistryandPolymerScience,Stellenbosch University,Matieland,SouthAfrica
WeiLu TosohBioscienceLLC,KingofPrussia, PA,UnitedStates
MuhammadImranMalik H.E.J.Research InstituteofChemistry,InternationalCenterfor ChemicalandBiologicalSciences(ICCBS), UniversityofKarachi,Karachi,Pakistan
JimmyMays DepartmentofChemistry, UniversityofTennessee,Knoxville,TN,United States
ToshikazuMiyoshi DepartmentofPolymer Science,TheUniversityofAkron,Akron,OH, UnitedStates
HaraldPasch DepartmentofChemistryand PolymerScience,UniversityofStellenbosch, Stellenbosch,SouthAfrica
JigneshkumarPatel IntelCorporation (Chandler),Chandler,AZ,UnitedStates
GeorgesM.Pavlov InstituteofMacromolecular Compounds,RussianAcademyofSciences, Saint-Petersburg,Russia
IgorPerevyazko DepartmentofMolecular BiophysicsandPhysicsofPolymers,SaintPetersburgStateUniversity,Saint-Petersburg, Russia
JawadurRehman H.E.J.ResearchInstituteof Chemistry,InternationalCenterforChemical
andBiologicalSciences(ICCBS),Universityof Karachi,Karachi,Pakistan
SebastienRouzeau TosohBioscienceLLC,King ofPrussia,PA,UnitedStates
PaulS.Russo SchoolofMaterialsScience& Engineering;SchoolofChemistry& Biochemistry,GeorgiaInstituteofTechnology, Atlanta,GA,UnitedStates
SalimSaifullah H.E.J.ResearchInstituteof Chemistry,InternationalCenterforChemical andBiologicalSciences(ICCBS),Universityof Karachi,Karachi,Pakistan
MuhammadRazaShah H.E.J.Research InstituteofChemistry,InternationalCenterfor ChemicalandBiologicalSciences(ICCBS), UniversityofKarachi,Karachi,Pakistan
WilliamC.Smith DepartmentofChemistry, ColoradoSchoolofMines,Golden,CO,United States
AliSoleymannezhad TosohBioscienceLLC, KingofPrussia,PA,UnitedStates
KirilA.Streletzky DepartmentofPhysics, ClevelandStateUniversity,Cleveland,OH, UnitedStates
MichaelToney DepartmentofChemistry, ColoradoSchoolofMines,Golden,CO,United States
XujunZhang WyattTechnologyCorporation, SantaBarbara,CA,UnitedStates
WeiweiZhao NingboMaterialsInstitute, Ningbo,China
Foreword
Aswecontemplatethecentennialofmacromolecularchemistry,wemaybereasonably certainthatHermannStaudingerneversaid“Polymerscientiaestomnisdivisainpartestres,” butifhehad,hewouldhavebeenright!Thefirstpartofpolymerchemistryissynthesis: whatmolecularstructurescanwemake,howeasily,andhowreproducibly?Thethirdpart comprisesmaterialproperties:whatisthispolymergoodfor,howcanwemakeitbetter,and howcanwecombinemultipledesirablepropertiesinonematerial?Theintermediatedomain, andtheessentialcomponentinordertoclosethestructure-propertyloop,ispolymer characterization:whatdidwemake,exactly?
Thereisnodoubtthatpolymercharacterizationis,ingeneral,aformidableexperimental challenge.Attherootoftheproblemistheunavoidablefactthatallsyntheticpolymersareheterogeneousintheirmolecularstructure.Asimplecalculationrevealsthatevenatankcarfullof homopolymerwillnotcontaintwomoleculesthatarepreciselyidentical.Notonlyistherea distributionofmolarmass,butthereisalsoheterogeneityinmultiplevariables,including, forexample,regioisomerism,stereochemistry,long-andshort-chainbranching,andcopolymersequenceandcomposition.Ideally,wewouldliketocharacterizeallofthesedistributions, atleasttothelevelofameanvalueandavariance.Thisgoaliscurrentlybeyondthecapabilities ofmostlaboratories,althoughexperimentalsciencecontinuestoadvancesignificantly.
Itisnosurprisethatfullmolecularcharacterizationdemandsasuiteofexperimentaltools. Eachtechniquewillprovidevaluableinformation,butnonecanbesensitivetoallofthevariablesofinterest.Thiscreatesafurtherchallengeforthepolymerscientist:howtodecidewhich techniquestouse,andhowtousethemeffectively?Itistemptingtovieweachinstrumentasa blackbox:insertsomesamplematerial,andoutpopsananswer(typicallywitharidiculous numberofsignificantfigures).Asexperimentalscientists,weknowthisisdangerous;every techniquereliesonasetofassumptions,whichmayormaynotapplyforthesamplewecare about.Wecanconsultatextbook,or,morelikely,Wikipedia,andfindabriefdiscussionofthe technique,andastatementoftheworkingequations.Whilehelpful,thisisnotsufficient. Agoodpractitionerneedstounderstandtheassumptions,andalsotobeconversantwith thestrengthsandweaknesses,theopportunitiesandblindspots,ofeachcharacterizationtool.
Thisisthevoidthat MolecularCharacterizationofPolymers:AFundamentalGuide aimstofill. Theauthorsadoptatutorialstyle,sothatspecificpriorknowledgeisnotrequired.And,by takingthisapproach,thereaderisempoweredtoacquireadeeperunderstandingofthe theoryunderlyingeachapproach.Asnotedbefore,therehavebeensubstantialrecentadvancesininstrumentaltechniques,andthusthisup-to-datetreatmentwillbeveryvaluable forexperiencedpractitionersaswell.
TimothyP.Lodge
UniversityofMinnesota,Minneapolis,MN,UnitedStates
Preface
Itisveryappropriateaswecelebratein 2020the100-yearanniversaryofNobelLaureateHermannStaudinger’s“discovery”of polymers,thatweassemblethisnewbook on MolecularCharacterizationofPolymers:A FundamentalGuide.ThisisbecauseStaudinger,beginningin1920until1930,utilized aseriesofingenious molecularcharacterization studies toprovethelongchainnatureofpolymers,theirhighmolecularweights,andtheir distributionofchainlengths(molecular weightdistribution,MWD) [1–3].Thiswork earnedStaudingertheNobelPrizeinChemistryin1953.
InthedecadesafterStaudinger’sdiscoveryofpolymers,thesynthesisofpolymers ofever-increasingcomplexityledtothe “polymerage”ofmaterialswhichcontinues tothisday,andnewmethodshavebeen continuallydevelopedinordertobetter characterizepolymers.However,evenin 1975,theeminentpolymerscientistFred Billmeyerstated“…characterizationofpolymersisinherentlymoredifficultthanthatof othermaterials.Polymersareroughlyequivalentincomplexityto,ifnotmorecomplex than,othermaterials,ateveryphysicallevel oforganizationfrommicroscopictomacroscopic ”and“Wewouldwish,ideally,to characterizeallaspectsofpolymerstructure inenoughdetailtopredictitsperformance fromfirstprinciples.Iseriouslydoubtthat thiswilleverbepossible… ” [4].
Billmeyer’sremarksstemfromthefact thatsyntheticpolymersalwaysexhibita distributionofchainlengthsorMWD. Homopolymersarefurthercomplicatedby
tacticity,modeofmonomerinsertion(e.g., head-to-tailorhead-to-head),chainconformation(flexibility),endgroups,and branching(longchainand/orshortchain). Copolymersarefurthercomplicatedby monomersequencedistributionsandvariationsincomonomercontentacrossthe MWD.Allofthesefactorsaffectpolymer processingandthepropertiesofthepolymericmaterial,butpolymercharacterization methodsonlyyieldaveragevaluesanddistributionsofkeymolecularparameters.Even today,nearlyahalfcenturysinceBillmeyer’s statements,despitealltheadvancesincharacterizationtechniquesandcomputation,the thoroughandaccuratemolecularcharacterizationofpolymericmaterialsremainshighly challenging.
Anotherfactorcomplicatingthethorough characterizationofpolymersistheneedto employacombinationofdifferentcharacterizationtechniques,aswellassomeknowledgeonhowthepolymerwassynthesized, inordertorigorouslycharacterizeeventhe simplestofpolymers.Unfortunately,scientistsactivelyinvolvedincharacterizingpolymers,eitherinindustryorinacademia,are usuallyspecialistsinoneortwoanalytical techniquesandlackdetailedknowledgein awiderangeofcomplementarypolymer characterizationtechniques,aswellaspolymersynthesismethodsandtheirimpacton polymerstructure,thatmustbeemployed inordertounderstandthepolymer’smolecularstructureindetail.Asexamples,in industryanalyticalchemistsareoften confrontedforthefirsttimewith
characterizingpolymersandtheymayhave littleornoexperienceincharacterizingsuch complexmixtures.Also,fewpolymerscientistsarerigorouslytrainedinawiderangeof polymercharacterizationtechniques.
Thustheprimarypurposeofthisbookis toserveasatextbookforacourse(academic courseorshortcourse)onpolymercharacterizationinordertobettertrainthenext generationofpolymercharacterization experts.Thisbookisthuswritteninatutorial styletoserveasanintroductiontothevariouspolymercharacterizationtechniques. Weanticipatethatthisbook,writteninthis style,willalsobeusefultoscientistsinindustrialpolymeranalysislaboratorieswho areapplyingacharacterizationtechnique topolymersforthefirsttime.Inadditionto fundamentals,wehavealsoincludedineach chapterrecentadvancesinthetechnique, informationoninstrumentation,andrecent applicationstomakethisbookusefultoscientistswithexperienceinatechniquebut lookingforupdatesonrecentadvancesand applications.
Thisbookbeginswithseveralchapterson chromatographyofpolymers. Chapter1 introducesbasicprinciplesofchromatography ofpolymer,includingsizeexclusionchromatography(SEC),highperformanceliquid chromatography(HPLC),andliquidchromatographyatthecriticalcondition.Data reductionmethodsandcolumntechnologies arediscussed. Chapter2 discusses multidetectorSECofpolymers,usingdetectorssuchaslightscatteringandviscositydetectors,forcharacterizingsimpleand complex(copolymer,branched)polymers. SECremainstheworkhorseforcharacterizingpolymermolecularweightdistributions. Chapter3 discussestheuseoftemperature gradientinteractionchromatographyfor characterizationofbranchedpolymers andcopolymers,endfunctionalizedpolymers,andisotopicallylabeledpolymers.
Chapter4 describesbasicprinciplesandapplicationsoffieldflowfractionation(FFF)to polymers. Chapter5 focusesontheindustriallyimportantareaofcharacterizationof polyolefins,whichconstitutehalfoftheannualpolymerproductionworldwide.Becauseoftheirlimitedsolubility,polyolefins presentspecialchallengesintheircharacterization.MultidetectorSECofpolyolefinsis discussed,alongwithcrystallinity-based techniquessuchastemperaturerisingelutionfractionationandcrystallizationanalysis fractionation.
Chapter6 describestheuseofcombinationsoffundamentalhydrodynamicapproaches(analyticalultracentrifugation, intrinsicviscosity,translationaldiffusion, andSEC)tocharacterizemolecularweights, dimensions,andconformation.Thesecombinedtechniquesareespeciallyusefulwith complexpolymerssuchaspolyelectrolytes. Chapter7 describestheuseofviscometry tomeasurepolymersize,molecularweight, aswellasgatherinsightintoconformational characteristicsandbranching.Methodsfor detectingandquantifyinglongchain branching,includingviscometry,lightscattering,andmultidetectorSECaredescribed in Chapter8.
Chapter9 isfocusedonrecentadvancesin massspectrometryofpolymers,focusingon MALDI-TOF-MSandMS/MS. Chapters10 and11 describetheuseofvibrationalspectroscopyandNMRforstructuralcharacterizationofpolymers,includingendgroups, composition,tacticity,etc. Chapters12and 13 describetheuseofstaticanddynamic lightscatteringtocharacterizepolymermolecularweights,sizes,thermodynamicinteractionsandconformations. Chapter14 introducesLenS3,anewlightscatteringdetectorthatmeasurespolymermolecular weightsandallowsforradiusofgyration measurementsinthesub-10-nmrange.The useofX-raysandneutronsforprobing
polymerstructureandconformation,inbulk, thinfilm,andinsolution,isdescribedin Chapter15 alongwithselectedapplications. Chapter16 coversmicroscopyofpolymers, withabasicintroductiontoSEM,TEM,and AFMandrecentapplicationstopolymers. Wearegratefultoalltheauthorswho madethetimelyassemblyofthisbookpossibleevenunderthechallengesimposedby theCovid-19pandemic.
MuhammadImranMalika,JimmyMaysb,and MuhammadRazaShaha aUniversityofKarachi,Karachi,Pakistan bUniversityofTennessee,Knoxville,TN, UnitedStates
References
[1] H.Staudinger, Uberpolymerisation,Ber.Deut. Chem.Ges.53(1920)1073–1085.
[2] H.Staudinger, UberdieKonstitutionder Hochpolymeren,Ber.Deut.Chem.Ges.61(1928) 2427–2431.
[3] H.Staudinger,W.Heuer, Uberhochpolymere Verbindungen,33.Mitteilung:Beziehungen zwischenViscosit€ atundMolekulargewichtbei Poly-styrolen,Ber.Deut.Chem.Ges.63(1930)222–234.
[4] F.W.Billmeyer,Trendsinpolymercharacterization, J.Polym.Sci.Symp.55(1975)1–10.
Basicprinciplesofsizeexclusionand liquidinteractionchromatography ofpolymers
MuhammadImranMalika andHaraldPaschb
aH.E.J.ResearchInstituteofChemistry,InternationalCenterforChemicalandBiological Sciences(ICCBS),UniversityofKarachi,Karachi,Pakistan bDepartmentofChemistryand PolymerScience,UniversityofStellenbosch,Stellenbosch,SouthAfrica
Polymersareinherentlycomplexmulticomponentmaterialshavingseveralsimpleand distributedproperties.Simplepropertiesincludetotalweightofthepolymer,residualmonomer/oligomer,gelcontent,etc.Distributedpropertiesarethoseinwhichdifferentmolecules ofthesamesamplehavedissimilarvalues.Theimportantdistributedpropertiesofpolymers includemolarmass,chemicalcomposition,sequencelength,endgroupfunctionality,moleculartopology,etc.Theperformancepropertiesofpolymersarehighlydependentuponthese distributedproperties.Theperformanceofpolymersforanyparticularapplicationcanbeimprovedsignificantlybycarefullymonitoring,adjusting,andunderstandingtheirmolecular distributions.Animportanttoolforthedeterminationofdistributedmolecularpropertiesof polymersisseparationscience.
Thesize,chemicalcomposition,sequenceofrepeatunits,andarchitecturearesomeimportantparametersthatneedtobeconsideredwhenanalyzinganypolymer.Theconstitution, configuration,andconformationofmacromoleculesarealsocriticalforregulatinganypotentialapplication.Polymershavingsimilarmolarmassesandchemicalcompositionscanhave completelydifferentpropertiesdependinguponthesequence,constitution,configuration, andconformationoftheirrepeatunits.Polymershavinganydistributionbeyondonlymolar massaretermedascomplexpolymers.
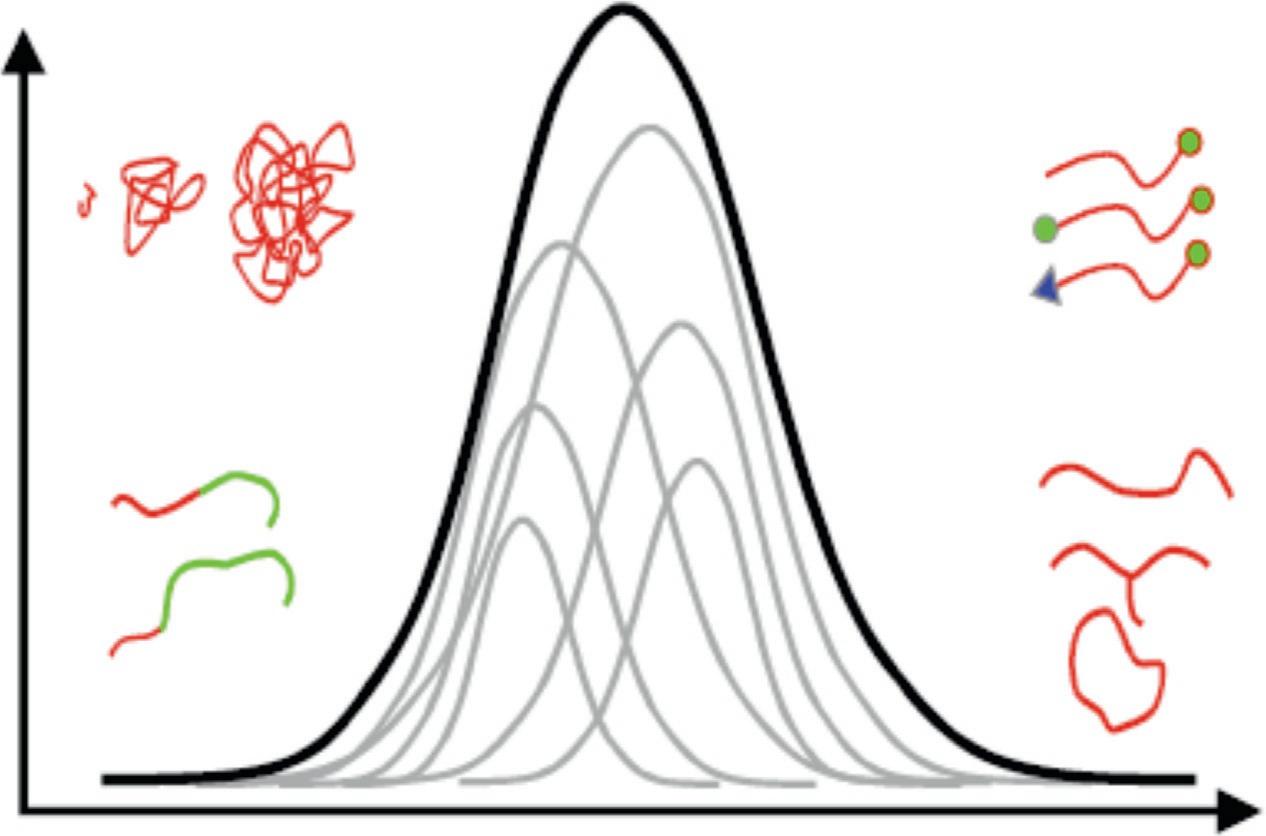
Theconceptofmolecularheterogeneitycanbeutilizedtodescribethestructuralcomplexityofsyntheticpolymers,see Fig.1.1.Differenttypesofheterogeneitiesofpolymerchains mightsuperimposeeachotherandagivenpolymersamplemayexhibitamolarmassdistribution,achemicalcompositiondistribution,individualblocklengthdistributions,
2 1.Basicprinciplesofsizeexclusionandliquidinteractionchromatographyofpolymers
FIG.1.1 Molecularheterogeneityofcomplexpolymers. ReproducedfromH.Pasch,Hyphenatedtechniquesinliquid chromatographyofpolymers,Adv.Polym.Sci.150(2000)1–66,withpermissionfromSpringerNature.Copyright2000.
afunctionalitytypedistribution(endgroupdistribution),andamoleculararchitecture distribution.Importantinformationrequiredforthecomprehensivecharacterizationof complexpolymersarethemolarmassdistributionswithineachtypeofheterogeneity.
Syntheticpolymersalwayshaveadispersitywithregardtomolarmassthatoriginates fromthepresenceofpolymerchainshavingdifferentlengths.Molarmassesofpolymers maybeexpressedasdifferentaverages,e.g.,number-averagemolarmass(Mn),weightaveragemolarmass(Mw),etc.Thebroadnessofthemolarmassdistributioncanbeexpressed bythemolarmassdispersity(Ð)thatistheratioofweight-averageandnumber-averagemolarmasses(Mw/Mn).Sizeexclusionchromatography(SEC)isoneofthemostimportant methodsfortheanalysisofmolarmassdistributionsofpolymers [1].
Differentfunctionalgroupsatthepolymerchainendoralongthepolymerchainintroduce anotherheterogeneitythatisparticularlyimportantforoligomersandtelechelicpolymers. Thepropertiesandreactivitiesofoligomersandtelechelicpolymersdifferwithregardto thenumberandnatureofthefunctionalgroups.Spectroscopictechniquescanonlyprovide averagenumbers,whereaswell-designedchromatographicseparationmethodscanreveal thefunctionalitydistributionasafunctionofotherheterogeneities,e.g.,themolarmass distribution [2]
Theinvolvementofmorethanonemonomerinapolymerizationreactioncomplicatesthe situationandtheresultingpolymermayhaveachemicalcompositiondistribution superimposingthemolarmassdistribution.SpectroscopictechniquessuchasNMRandFTIR canprovideanaveragechemicalcompositionofthesample.However,noinformationonthe distributionofchemicalcompositionasafunctionofmolarmasscanbeobtained.Moreover, spectroscopictechniquescantypicallynotdifferentiatebetweencopolymersandmixturesof theirrespectivehomopolymers.Interactionchromatographictechniquescanprovidemore
insightintothecomplexcompositionofcopolymers.Therecanbeseveraladditionalheterogeneitiesincopolymerssuchasthebulkmolarmassdistribution,thedistributionofrepeat unitsinthecopolymer,thelengthsofdifferentsegmentsincaseofsegmentedcopolymers,the presenceofanytypeofhomopolymerinthebulksample,etc.Theabove-mentionedfactors makethecomprehensivecharacterizationofpolymersamultifacetedtask.Fortheanalysisof thechemicalcompositionasafunctionofmolarmass,multidetectorapproachesmayberequired(see Chapter2 fordetaileddiscussion).Theindependentanalysisofeachtypeofheterogeneityrequiresindependentseparationtechniquesthatareselectiveregardingspecific typesofdistributions.Inordertogetaccesstoonetypeofheterogeneityasafunctionofother heterogeneities,couplingofindependentchromatographictechniquesorhyphenationof chromatographicseparationwithspectroscopictechniquesisimperative.
Tosummarize,aminimumoftwoindependentmethodsarerequiredfortheanalysisof complexpolymerseachwithacertainselectivityforonetypeofheterogeneity.Inthiscontext, differentmodesofHPLCofpolymerssuchasSEC,liquidchromatographyatcriticalconditions(LCCC),andinteractionchromatography(IC)orliquidadsorptionchromatography (LAC)canbecoupledtoeachother.Couplingofachromatographicseparationwithspectroscopictechniquescanalsobeafascinatingapproachinordertoobtainthedistributionofone propertyasafunctionofanotherdistribution.
1.1Liquidchromatographyofpolymers
Thebasicprincipleofanychromatographicprocessisbasedontheselectivedistributionof theanalytemoleculesbetweenthemobileandthestationaryphase.Theseparationprocess ofchromatographycanbedescribedby
where Ve, Vi, Vp,and Kd representtheelution(retention)volumeoftheanalyte,theinterstitial volumeofthecolumn,theporevolumeofthepacking,andthedistributioncoefficient,respectively.Thedistributioncoefficientistheratiooftheanalyteconcentrationinthemobile andthestationaryphase. Kd isrelatedtothevariationsinGibbsfreeenergy Δ G thatdepends onanalytepartitioningbetweeninterstitialandporevolume [3].
Thelogarithmicplotofthedistributioncoefficientallowsthedeterminationoftheentropic (ΔS)andenthalpic(ΔH)contributions(vant’Hoffplot):
DifferenteffectsthatcontributetothechangeinGibbsfreeenergyare(1)thedecrease inconformationalentropythatoriginatesfromlimiteddimensionsinsidetheporesofthe stationaryphase,and(2)changesinenthalpythatoriginatefromthe(adsorptive)interaction ofmacromoleculeswiththestationaryphase.
SECseparatesmacromoleculeswithregardtotheirhydrodynamicvolumeindilutesolution.ThestationaryphaseinSECisaswollengelhavingacharacteristicporesize
distribution.Themacromoleculesmayhavelessormoreaccesstotheporesdependingon theirhydrodynamicsizes.Verylargemoleculescannotentertheporesandareexcluded,elutingattheinterstitialvolume Vi.Verysmallmoleculeshavefullaccesstotheporesofthestationaryphaseandeluteatthevoidvolumeofthecolumnwhichisthesumofinterstitialand porevolume(Vo ¼ Vi + Vp).Hence,theseparationrangeofSECis0 < KSEC < 1.
InidealSEC,thedistributioncoefficientdependsonlyonentropychangeswithoutany involvementofenthalpicinteractions;however,inrealSECthisisdifficulttoachieve.On theotherhand,thedistributioncoefficientinthecaseofICtotallydependsontheinteraction strengthoftheanalytemoleculeswiththestationaryphase.Thisisperfectlytrueonlyfor smallmolecules.Longerchainsofpolymersmaynothaveaccesstothewholeporevolume, hence,entropicfactorsmustbeassumedtocontributeinadditiontoenthalpiccontributions.
Incaseofpolymers,oftenmixedmodesofchromatographyareoperativeandmethodsare definedbythepredominanceofentropicorenthalpicinteractions.Entropicinteractionsare predominantincaseofthesizeexclusionmode,i.e. T Δ S > Δ H correspondstonegativevalue of Δ G,whileseparationintheinteractionmodeisdominatedbyenthalpicinteractions,i.e. Δ H > T Δ S correspondstopositivevalueof Δ G.Interactionforcesexactlycompensate entropylossesatthetransitionpointoftheexclusionandinteractionmodes,i.e. Δ H ¼ T Δ S correspondstozerovalueof Δ G.Thismodeofliquidchromatographyofpolymersisoften termedasliquidchromatographyatcriticalconditions.
Hence,Gibbsfreeenergyatthechromatographiccriticalpointisconstant(Δ G ¼ 0)andthe valueofthedistributioncoefficientequals1, Kd ¼ 1,independentofthemolarmassofthe polymerandtheporesizeofthestationaryphase.Anarrowrangebetweensizeexclusion andinteractionmodesofLCthatissensitivetochangesintemperatureandmobilephase compositionisrelatedtothechromatographiccriticalpoint.Thistransitionfromonemode ofseparationtotheotherwasreportedforthefirsttimebyBelenkiietal. [4] andTennikov etal. [5].Theydemonstratedsuddenchangesintheelutionbehaviorbyslightvariationsin thecompositionofthemobilephase.Hence,thetransitionpointbetweentheSECandIC modescanberealizedbycarefullyadjustingthemobilephasecompositionandthetemperature.Thisspecifictransitionpointislabeledasthechromatographiccriticalpoint(CCP)and thecorrespondingmodeofliquidchromatographyistermedasliquidchromatographyat criticalconditions(LCCC).
Apresentationofthetransitionbetweenthethreemodesofliquidchromatographyof polymersisshownin Fig.1.2.InSEC,retentiondecreaseswithincreasingmolarmass whereasretentionincreaseswithmolarmassinICorLAC.AtLCCC,theexclusionandinteractioneffectsarecompensatedrenderingamolarmassindependentelutionofaparticular polymerataconstantelutionvolume.Theseseparationmodescanbecombinedinvarious waystorealizeseparationsofpolymerswithregardtodifferentdistributionssuchasmolar mass,chemicalcomposition,andfunctionality.SEC,themostfrequentlyusedmethodfor polymeranalysis,separatespolymerswithregardtotheirhydrodynamicsizeindilutesolution,andseveralapproachesareinplacetoobtainchemicalcompositioninformationasa functionofmolarmassthatincludemultipleconcentrationdetectorsystems,anduniversal calibrationwithviscometricandlightscatteringdetection(see Chapter2 fordetaileddiscussion).Onemustkeepinmind,however,thatSECseparationisbasedonsizeandthechemical compositionsobtainedbydifferentapproachesareonlyaveragevaluesrelatedtoagivenSEC fraction.

Dependenceofelutionvolumeonmolarmassindifferentmodesofliquidchromatographyofpolymers.
1.2Theoryofpolymerchromatography
RetentionofananalyteonthestationaryphaseinHPLCisexpressedintermsofthe distributioncoefficientasfollows:
Thedistributioncoefficientmayassumedifferentvaluesindifferentmodesofliquid chromatography.Inthiscontext,deGennesintroducedafunctionthatistermedtheinteractionparameter, c [6,7].Thevalueofinteractionparameterdependsonthemobilephase composition(foragivenpolymer-stationaryphasesystem)andtemperature.Theunitof theinteractionparameterisinverselength(nm 1).
1.2.1Sizeexclusionchromatography
InSEC,thevalueoftheinteractionparameter c isnegativewhilethedistributioncoefficient assumesvaluesbetweenzeroandone(0 < K < 1).SECseparationiscontrolledbyentropy changesinducedbydifferentlysizedspeciesmovingthroughthechromatographiccolumn. TheelutionvolumeofSECinidealconditionsisgivenbyEq. (1.5)[8]
FIG.1.2
where R istheradiusofgyrationoftheanalytewhile D istheporediameterofthestationary phase.Theradiusofgyrationexpressedintermsoflengthandnumberofrepeatunitsis givenas
where a isthelengthand n isthenumberoftherepeatunits.
SeparationinSECisrealizedwithregardtomoleculardimensionsregardlessofcompositionandfunctionality.
1.2.2Interactionchromatographyorliquidadsorptionchromatography
ICisbasedonthestrengthofinteractionoftheanalytemoleculeswiththestationaryphase. Thevalueof c ispositivewhereasthedistributioncoefficienthasvalueslargerthanone(k > 1) thatincreasesexponentiallywiththenumberofrepeatunits.
RetentioninICisoftendescribedintermsofMartin’srule [9,10]
Thedimensionlessfactor k isdeducedfromtheelutionvolumeofthesoluteandholdup volume(differentfromvoidvolume)ofthecolumn.Anexcellentreviewwithregardto holdupvolumeofthecolumnispresentedbyRimmeretal. [11].Inthiscontext,Gorbunov andSkvortsovdevelopedatheoryofchromatographyforflexiblehomopolymersthatcan interactwiththestationaryphase [12,13].Accordingtothistheory,theelutionvolumeof anonfunctionalpolymerchaininICisgivenby
wherein D istheporediameterofthestationaryphase, R istheradiusofgyrationofthemacromolecule, c istheinteractionparameter,and erf(cR)istheerrorfunction.Itispertinentto mentionherethatthevalueof c isindependentof D, Vp, Vi,or R (i.e.,thedegreeofpolymerization n).
Theterm erf(cR)approachesunityatsufficientlystronginteraction(typicalforIC)and, therefore,Eq. (1.8) canberewrittenas
where Vo∗ istheaccessiblevolumeforthepolymerchain.
Obviously,theaccessiblevolumeissmallerthanthevoidvolume(Vo ¼ Vi + Vp).TheaccessiblevolumeinICcanbeobtainedbyplottingtheelutionvolumesofoligomers Vn with n repeatunitsversusthedifferenceinelutionvolumeofconsecutivepeaks(Δ Vn ¼ Vn Vn 1)
Theaccessiblevolumeisrepresentedbytheinterceptoftheplot,andslope γ ¼ e B/(e B 1) canbeusedtocalculatetheinteractionparameter c,wherein B ¼ (c 2 a2)/6istheslopein Martin’srule(seeEq. 1.7)
Itcanbenoticedthatthenumber n oftheindividualpeaksisnotrequiredforthe determinationoftheaccessiblevolumeandtheinteractionparameter,providedsufficiently stronginteractionispresent.Moreover,Gorbunovetal.developedasoftwareforthedeterminationofinteractionparametersinallmodesofLC [14].Theapproachisbasedonasetof measurementsofnonfunctionalpolymerstandardsofknownmolarmass.Eq. (1.10) canalso berewrittenintermsoftheretentionfactor k
ThelogarithmicformoftheequationcorrespondstoMartin’srule.
Martin’srulecanalsoberewrittenfornonfunctionalchainsas
TheporesurfacecanalsobedeterminedfromtheinterceptofMartin’splotoncethe interactionparameterisdeterminedusingtheearlierequations.However,theidentification ofthepeaksisrequiredforthedeterminationoftheporesurface.Formono-functionalchains, anadditionalparameter q isrequired [15].Thespecificendgroupparameter q measuresthe differenceoffreeenergyofadsorptionofendgroupandrepeatunit [16].Afacilemethod forthedeterminationof q hasbeenelaboratedbyNguyenandTrathnigg [17].
InIC,retentionofmono-functionalchainswithanadsorbingendgroupcanbewrittenas
V0,m ∗ istheaccessiblevolumeformono-functionalchainsthatissmallerthantheaccessible volumefornonfunctionalchains.
1.Basicprinciplesofsizeexclusionandliquidinteractionchromatographyofpolymers
Thevalueof k increasesexponentiallywiththenumberofrepeatunits.Straightlines withthesameslopesareobtainedinaplotof lnk vs n inbothcases,havingratherdifferent intercepts.
ICallowsforseparationofoligomersofnonfunctionalpolymersaswellasmonofunctionalpolymerswitharatherweaklyadsorbingendgroup.Strongerinteractionofthe endgroupresultsinpoorresolutionofindividualoligomers.
1.2.3Liquidchromatographyatcriticalconditions
Entropicandenthalpictermscompensateeachotherinliquidchromatographyatcritical conditions.Atthisso-calledchromatographiccriticalpoint(CCP),thevaluesofthedistributioncoefficientandtheinteractionparameterequaltooneandzero,respectively.Thecompensationofenthalpicinteractionandentropicexclusionrendersmolarmass-independent elutionofnonfunctionalchainsatthevoidvolumeofthecolumn.Anadditionalterm qa is requiredtodescribetheinfluenceofaninteractingendgroup a onretention.Theelutionvolumeofchainswithanadsorbingendgroupattheirchromatographiccriticalpointislarger thanthevoidvolume,however,independentofmolarmass [16,18].
Thecontributionoftheendgrouponretentionatthecriticalpointallowsforseparationof mono-functionalchainswithregardtotheinteractionstrengthoftheendgroupindependent ofmolarmass.However,averydifferentbehaviorisobservedwithdi-functionalchains(with adsorbinggroupsatbothends) [16,19].Theelutionvolumeofchainswithsymmetrical groupsatbothendsisgivenby
Theequationindicatesthatdi-functionalchainsareseparatedwithregardtotheradiusof gyration R ofthecriticalpolymerchain.Asymmetricaldi-functionalpolymerchains(bearing differentgroupsatthechainends)holdthesamerelation,
where a and b areendgroups.
Di-functionalchainselutelaterthanmono-functionalpolymericchains;however,their elutionvolumeisnotonlydependentoncontributionsoftheendgroups a and b butalso containanadditionaltermthatistheratiooftheporediameter D ofthestationaryphase andtheradiusofgyration R ofthepolymermolecules [16,19].Consequently,elutionof di-functionalpolymerchainswithadsorbingendgroupsfollowsSECorder.
Thereisanotherspecialsituationwheretheendgroupadsorbswhiletherepeatunitsarein SECmode.Thisregimeofliquidchromatographyofpolymersistermedasliquidexclusion adsorptionchromatography(LEAC) [20].Undertheseconditions,theinteractionparameter oftherepeatunit c isnegativewhiletheinteractionparameteroftheendgroup cB ispositive. Thisconditionrenderselutionofmono-functionalchainsinSECorderbeyondthevoid
Vaa Vi + Vp 1+2qa + q
Vab
volumeofthecolumn.Themathematicalrelationfortheelutionvolume VAB ofshort mono-functionalpolymerchainsunderthedescribedconditionsisgivenby
where A istherepeatunitwhile B istheendgroup.
Theelutionvolumedecreaseswithincreaseinthenumberofrepeatunits(andhence radiusofgyration).Themethodcanbeusedforoligomerseparationsofmono-functional polymerchainscontaining10–15repeatunitsinSECorderbeyondthevoidvolumeofthe column.ThisisessentiallyanisocraticseparationthatallowsaRIdetectortobeemployed, resultinginaccuratequantification [21].
1.3Thermodynamicsofpolymerchromatography
ThedistributioncoefficientdependsonchangesinGibbsfreeenergythatcorrespondto variationsinentropyandenthalpy,seeEqs. (1.2)and(1.3).Separationinthesizeexclusionregimeisgovernedbytheentropictermwhereasinteractionisanenthalpicprocess.However, entropicorenthalpiccontributionsarenoteasytoavoidcompletelyespeciallyinthecase ofmacromolecules.Bothenthalpicandentropictermscompensateeachotheratthecritical modeofliquidchromatographyofpolymerswhichmeans Δ G ¼ 0,as Δ H ¼ T Δ S.
Asdescribedpreviously,SECandICmechanismsmayshowdifferentdependenceson temperature.ThedistributioncoefficientsolelydependsonentropicchangesinidealSEC (noenthalpicinteractions)renderingnodependenceontemperature.InLCCC,entropic andenthalpiceffectsarecounterbalanced,hence,anychangeintemperaturewouldrequire adifferentmobilephasecompositiontoretainthecriticalbehavior.Consequently,retention inICdependsbothonenthalpyandentropychanges.Retentionofanypolymeronagiven stationaryphasedependsonthemobilephasecompositionandthetemperature.However, theextentanddirectionofthisdependencevaries.
Thechangesinentropyandenthalpycanbedeterminedfromthevan’tHoffplot,ln K vs 1/T.Variousapproachesthatprimarilydifferinthecalculationofthedistributioncoefficient areusedforthedeterminationofthermodynamicparameters [22,23].
Directproportionalitybetween thedistributioncoefficient K andtheretentionfactor k ¼ ( Ve V0 )/V 0 isoftenappliedinthisregard
whereinterm lnφ correspondstomobileandstationaryphaseratio(generallynotknown), principallyindicatingtheporevolumeandinterstitialvolume.Hence,theslopeand interceptinaplotofln K vs1/T representthethermodynamicparameters Δ H°/R and (Δ S∗/R) ¼ (Δ S°/R) +lnφ.However,thereisnodirectcorrelationbetweenthedistribution coefficient K andtheretentionfactor k asisclearfromequations
10 1.Basicprinciplesofsizeexclusionandliquidinteractionchromatographyofpolymers
Therelationshipbetweenthedistributioncoefficient K andtheretentionfactor k canbe devisedas
Contrarytoatypicalassumption,thereisnodirectproportionalitybetween K and k.The assumptionthat K ¼ k φ isanapproximationandholdsonlyfor K ≫ 1.Theexactrelationcontainsthedistributioncoefficient K;however,thedeterminationofcharacteristicvolumes Vi, Vp,and V0 arerequired.
Itispertinenttomentionherethatthedeterminationofthevalueofvoidvolumeisnot trivial.Numerousarticlesaddresstheissueoftheaccuratedeterminationofthevoidvolume, thedeadvolume,andtheholdupvolume [11,24],havingdifferentdefinitionsrelevanttoparticularsituations [11,25–27].Thevoidvolumeisusuallytakenasthetotalamountofsolvent inthecolumnthatcanbedeterminedgravimetrically.Ontheotherhand,theholdupvolume isconsideredastheelutionvolumeofanunretainedcompoundthatcanbedeterminedby variousmethods [11,24].TheinterstitialvolumecanbedeterminedbyinverseSEC.Itisactuallytheelutionvolumeofacompletelyexcludedpolymerfromtheporesofthestationary phase.However,thesevaluesareverymuchdependentonthemobilephaseandmayassume dissimilarvaluesindifferentmobilephases.
1.4Equipmentandmaterials
Separationsbydifferentmodesofliquidchromatographyofpolymersposeseveralchallengesthatcanbeaddressedbystate-of-the-artHPLCinstrumentation.Typically,aflexible pump(forisocraticandgradientseparations),acolumnoven(forstabletemperature conditions),andareliablesetofdetectors(forquantitativeinformationwithregardtodifferentmolecularparameters)arerequired.
ThemaincomponentsofanyHPLCinstrumentincludeasolventdeliverysystem,asampleinjector,asetofdetectors,andadataacquisitionsystem.Columnsarethecoreofany separationsystemthatcontainstationaryphasesofdifferentnature,whichareselected accordingtothetargetedseparation.
1.4.1Solventdeliveryandinjector
OneofthemostimportantrequirementsofanyHPLCsystemisaconstantandreproduciblesupplyofmobilephase.Severaltypesofpumpsareemployedforareliablemobilephase deliveryinHPLC.Theseincludesyringepumps(workslikeasyringeforpulselessflow)and reciprocatingpumpsthatexistinvariousmodificationsassinglepistons,paralleldualpistons,anddualpistonsinseries.GeneralrequirementsofHPLC/SECwithregardtothepump includeaflowrateprecisionof0.2%,apressureoutputof6000psi,apressurepulsationofless
than1%at1mL/min,aflowraterangeof0.01–10mL/min,chemicalresistancetoawide rangeofsolvents,andsmallholdupvolumesforrapidsolventexchange.Mobilephasesused forHPLCaregenerallydegassedbyinlinedegasserstogetridofdissolvedgases.Analternativeisthedegassingofthemobilephasebyultrasonicationpriortouse.
TheselectionofthepumpforanyHPLCsystemdependsontheintendedapplication.SyringepumpsaresuitableforSECandLCCCduetotheirflowstabilityandminimumevaporationofthesolvent.However,syringepumpsarenotsuitablewherehighpressuremixing isrequired.Reciprocatingpumpsallowlowpressuremixingandarethepreferredchoicefor gradientelution.Recently,reciprocatingpumpsindifferentvariationsarealmostexclusively employedforHPLCbyallthemanufacturers.
Theinjectionofsampleasanarrowbandisimperativetoavoidpeakbroadeningfrominjection.Thisrequiresasharpplugofsamplesolutioninjectedintothemobilephase.Sample injectionistypicallyconductedthroughatwo-position,six-portvalvewhichmaybeoperated manuallyorautomatically.Forprecisemeasurements,thesampleloopshouldbecompletely filled.Thesizeofthesampleloopdependsonthecolumndimensions,thesensitivityofthe detectors,andthenatureofseparation.Largerloops(50–100 μL)withdilutesamplesolutions arerecommendedforSECratherthanusinghigherconcentrationswithsmallerloops.Onthe otherhand,higherconcentrationsarepreferredusingsmallerloops(10–50 μL)forICand LCCC.Moderninstrumentsareequippedwithautosamplingdevicesthatallowanalysis ofmultiplesampleswithoutinterventionofoperator.Mostcommercialautosamplerspermit injectionofanyvolumeintherange0–2000 μLwithaprecisionof 0.5%.Additionally,modernautosamplersarealsoequippedwithsamplefiltration,variablespeedandtemperature mixing,needlewash,etc.
1.4.2Columndimensions
Typesanddimensionsofchromatographiccolumnsdependonthemodeofoperation.In contrasttoothermodesofHPLC,separationinSECispredominantlydeterminedbythetype ofstationaryphasewithminimalinfluenceofthemobilephase.Theseparationtakesplacein theporevolumethatequals30%–40%oftotalelutionvolume.Therefore,forachievingfairly goodseparationefficiency,longercolumns(25–60cm)withlargervolumesofstationary phasearerequired.Typically,amultiple-columnsetisusedforSECanalysis.Innerdiameters ofcommerciallyavailablecolumnsareintherangeof5–8mmforanalyticalSECand 22–25mmforsemipreparativeSEC.Theplateheightdecreaseswithadecreaseinparticlesize resultinginahighernumberofplatesperunitlength.SECcolumnsareclassifiedinto microbore,narrowbore,analytical,semipreparativeandpreparativewithincreaseinparticle size,columndiameter,andlengthinthesameorder.Separationefficiencyofanychromatographicsystemisexpressedintermsofnumberoftheoreticalplates,whereatheoreticalplate referstoasingleequilibriumstep.Highernumbersoftheoreticalplatesrefertobetterseparation.Inthiscontext,plateheight, H,isobtainedbydividingthelengthofcolumnbythe numberoftheoreticalplates.Smallerparticlesarepreferredtoallowhigherpackingdensities henceprovidinghigherplatenumbers;however,thisresultsinanincreasedcolumnback pressure.Thebackpressureshouldnotexceed150barformostofthepackings.Besides thepackingdensityofthestationaryphasethecolumnbackpressurealsodependsonthe
viscosityofthemobilephase.Asaruleofthumb,1mL/minshouldbetheflowrateofacolumnwith8mminnerdiameterwhile0.25mL/minforacolumnwith4mminnerdiameter. Thetotalcolumnlengthshouldbeadjustedaccordingtothebackpressureproducedina givenmobilephaseatoptimumflowrate.Highercolumntemperaturesarepreferredasatool toreducethebackpressurecomparedtolowerflowratesduetothefactthatlowerflowrates resultinarapidincreaseinplateheightandthusdecreaseinseparationefficiency.
Ontheotherhand,usingsmaller(highefficiency)columnsisatrendinHPLCcomparedto SECwherehighefficiencycanonlybeachievedusinglongercolumns.RetentioninHPLC dependsonthedistributioncoefficientoftheanalytebetweenthemobileandthestationary phase.IncontrasttoSEC,separationinHPLCisgovernedbythecompositionofthemobile phase.Smallercolumnsleadtofasteranalysisandlowersolventconsumption.Thereare, however,limitationsofminiaturizationduetothefactthatsmallerparticlesgivinghigher separationefficiencyresultinanincreasedbackpressure.Furthermore,thelengthsanddiametersofconnectingcapillariesandtheinternalvolumeofthedetectorcellhavetobesmall foranacceptableoverallefficiencyofthesystem.Smallsizedparticlesresultinsmallerplate heightthatinturnincreasesthenumberoftheoreticalplatesperunitvolume.Microbore HPLCrequiresspecializedinjectionsystems,capillaries,anddetectors.Narrowborecolumns canbeoperatedwithnormalHPLCsystems.
1.4.3Stationaryphases
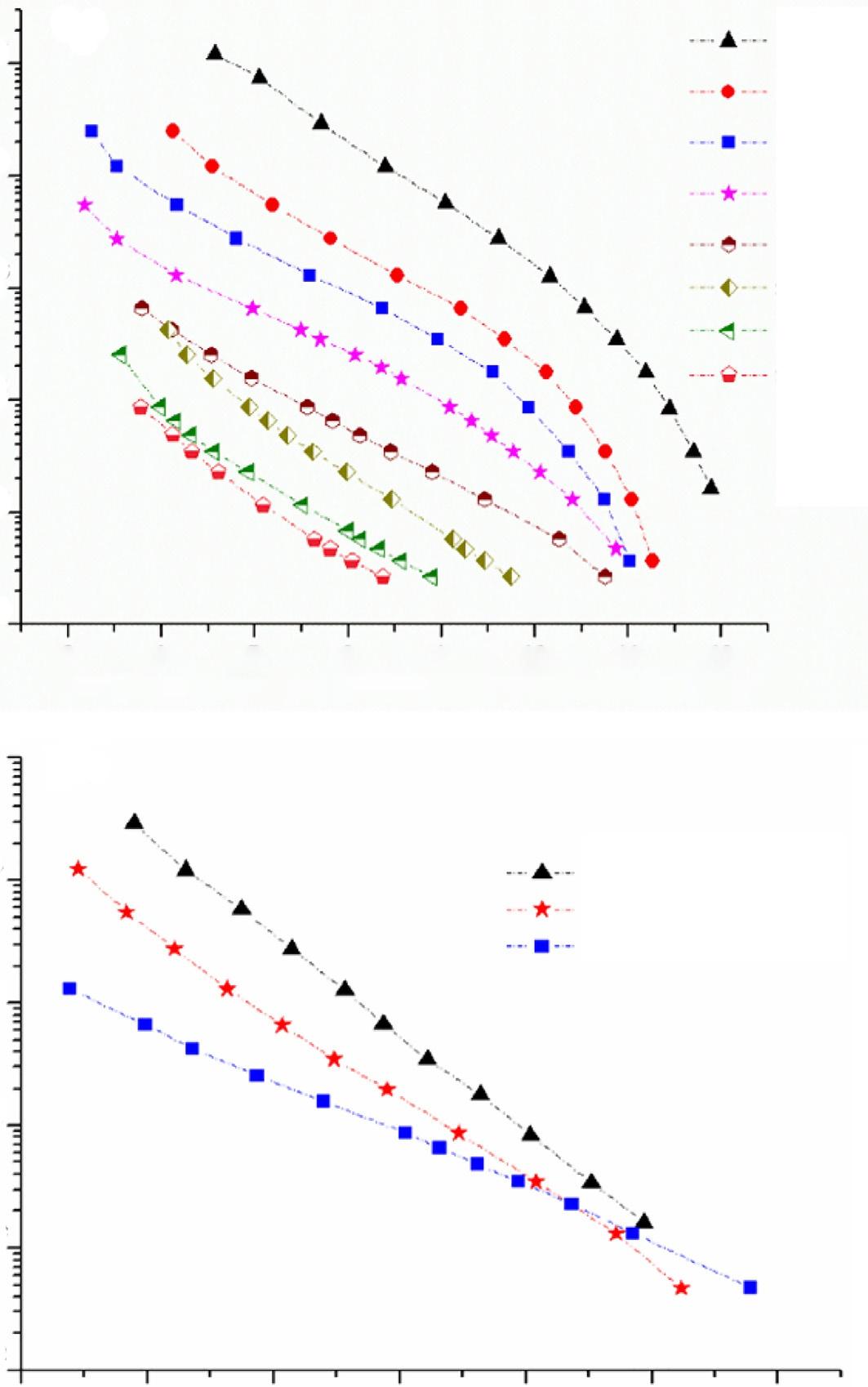
Stationaryphasesareselectedwithrespecttotheintendedseparation.Poroussilicaor cross-linkedorganicgelsarecommonlyusedstationaryphasesforSEC.StyrenedivinylbenzenecopolymeristhemostwidelyemployedstationaryphaseforSECinorganic solvents.Modifiedsilicaorcross-linkedhydrophilicpolymersareacommonchoiceforaqueousSEC.Nonetheless,polymerpackingsareavailablethatcanbeusedforalargevarietyof differentmobilephases.Generally,silica-basedpackingsareratherrobustcomparedtoorganicpolymer-basedpackings.Commerciallyavailablecolumnpackingsfororganicand aqueousSEChavingvarietiesofporesizesandapplicablemolarmassrangeareproduced byseveralcompaniessuchasAgilent,Macherey-Nagel,TosohHaas,PolymerLaboratories, Merck,Phenomenex,PolymerStandardsService,Shodex,Malvern,andWaters.Commerciallyavailablecolumnscanbesubdividedintotwomajorcategories,namelysingleporecolumnsandmixedcolumns(alsotermedaslinear). Fig.1.3Ademonstratestheelutionvolume ofnarrowmolarmassPSstandardsasafunctionofmolarmassonSDVcolumnsofvarious poresizes.Ascanbenoticed,thecalibrationcurveonsingleporesizedcolumnsisnotlinear. Approximatelytwodecadesofmolarmasses(e.g.,103–105 g/mol)arecoveredbytraditional singleporesizecolumns.Recently,theuseofmixedbedcolumns,madeofmixingparticlesof differentporesizes,isinfashion.Linearormixedcolumnsallowtocoverawidermolarmass rangeandtheobtainedplotofelutionvolumeasafunctionofmolarmassislinear, Fig.1.3B.
TraditionalHPLCcolumnsthatareusedfortheseparationoflowmolarmassorganiccompoundsareequallyapplicabletointeractivemodesofliquidchromatographyofpolymers.As ageneralrule,sphericalparticlesaresuperiortoirregularparticles.Theefficiencyofacolumn increaseswithdecreasingparticlesizeofthestationaryphasethat,ontheotherhand,leadsto anincreasedbackpressure.SECissolelybasedonthelimitedaccessibilityofthepolymer
FIG.1.3 ElutionvolumeasafunctionofmolarmassofPSstandardsonSDVcolumnsinTHF(A)singleporesize columns,(B)linearormixedcolumns.DataprovidedbyPolymerStandardsService [28]
moleculestotheporesofthestationaryphase,whileseparationinICisbasedontheavailable surfaceareaofthestationaryphase.InLCCC,bothadsorption/interactionandexclusion effectsarecompensated,henceporesizeisveryimportant. Thenatureoftherequiredstationaryphaseisbasicallydeterminedbytheseparation problem.StationaryphasesforHPLCaremostlybasedonsilicaorcross-linkedpolymers.
1.Basicprinciplesofsizeexclusionandliquidinteractionchromatographyofpolymers
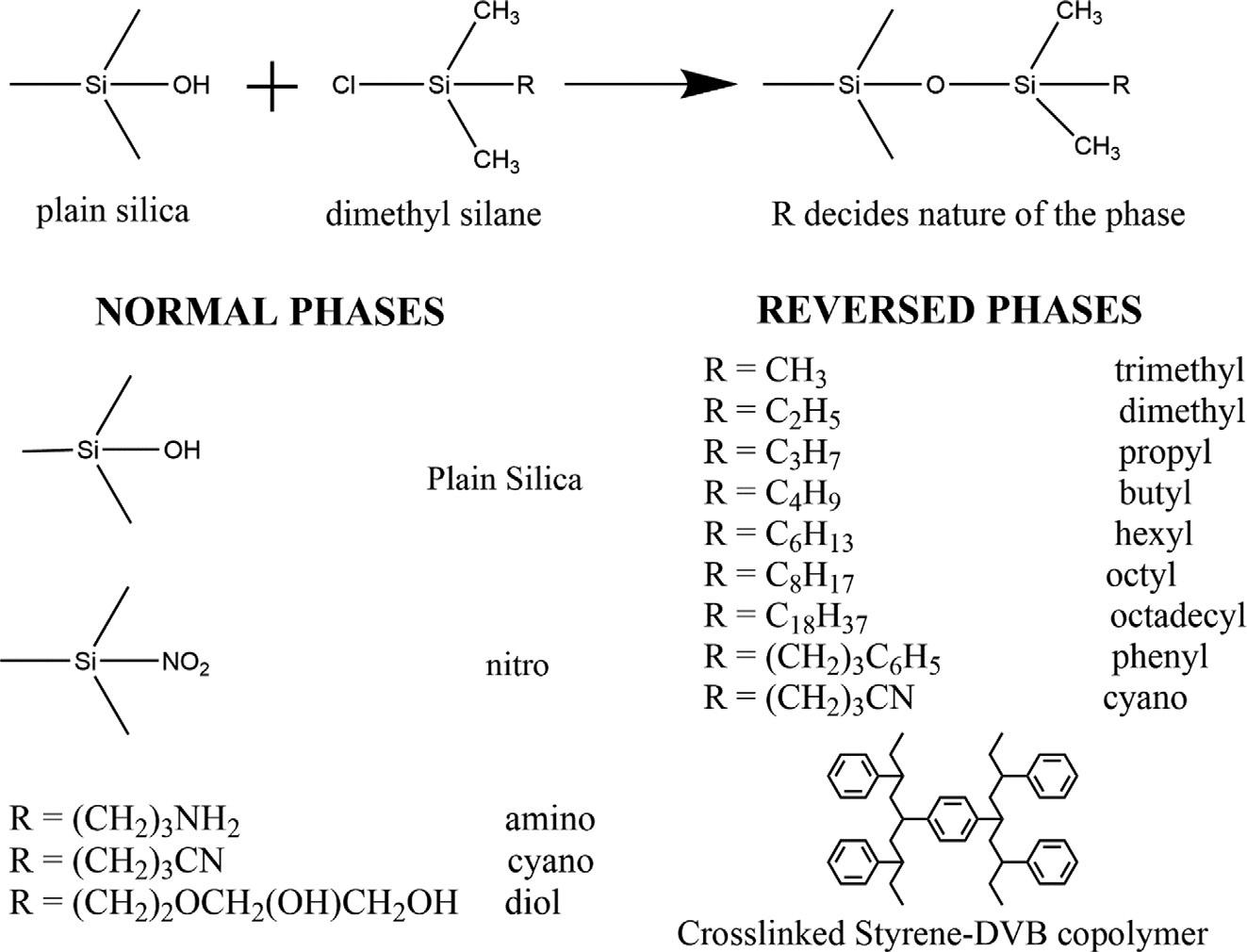
Ageneralclassificationisbasedonthepolarityofstationaryandmobilephases.Thestationaryphaseispolarcomparedtothemobilephaseinnormalphasechromatography(NP).On thecontrary,themobilephaseispolarcomparedtothestationaryphaseinreversedphase chromatography(RP).Typically,plainsilicahavingsilanolgroupsismodifiedwithdimethyl silanehavingavarietyofRgroups.TheRgroupdeterminesthenatureofthestationary phase.Sometypicalstationaryphasesforbothnormalandreversedphasechromatography areshownin Fig.1.4.StationaryphasesbasedonmodifiedsilicaaretypicallyusedinRPchromatography.Theseareobtainedbyreactingsilicawithsilanes.Thereactionofsilicawith silanesisseldomcompleteresultinginresidualsilanolgroupsinthestationaryphase.These residualsilanolgroupsmayaffecttheseparationespeciallyintheanalysisofbasiccompoundssuchasamines.Acurrenttrendtoovercomethisproblemistheapplicationofpackingswithahighcarbonloadandahighdegreeofend-capping.Nonetheless,polymer-based packingssuchascross-linkedstyrene-divinylbenzenecopolymersmaybethebetterchoiceto overcomethislimitation.Itisworthmentioningherethatnitrile-modifiedphasescanbeused eitherasnormalorreversedphasesdependingonthepolarityofthemobilephase.
1.4.4Mobilephases
SinglesolventsaretypicallyusedasmobilephasesforSEC.ThemobilephaseforSEC shouldbeathermodynamicallygoodsolventforthepolymertobeanalyzed.Additionally, itshouldbeabletosolvatethestationaryphase,shouldbechemicallyinert,havealow
FIG.1.4 Typicalpolar(normalphase)andnonpolar(reversedphase)stationaryphasesforliquid chromatography.
viscosity,beUVtransparent,andpossessanappropriaterefractiveindexandlowtoxicity. Themobilephaseisselectedwithrespecttothenatureofthepolymerandthestationary phase.FrequentlyemployedorganicsolventsforSECanalysisaretetrahydrofuran,chloroform,toluene,esters,ketones,dimethylformamide,etc.Lowmolarmasselectrolytescan beaddedtominimizenonexclusioneffectsintheanalysisofpolarpolymerssuchaspolyelectrolytes.Polyolefinsarenotsolubleinanysolventatroomtemperatureand,hence,require separationathightemperaturesinhighboilingsolvents,trichlorobenzenebeingthemost widelyusedmobilephaseforpolyolefins(seeChapter5fordetaileddiscussion).
TheprimarycriterionfortheselectionofamobilephaseforinteractivemodesofHPLC includessamplesolubilityandcomplexinteractionsbetweenthesample,thestationary phase,andthesolvent.Solventsareclassifiedwithregardtotheirchemicalnatureandpolarity.ThemostimportantcriterionfortheselectionofamobilephaseinICispolarity.Solventsareclassifiedintermsof“eluotropicseries”withregardtopolarity.Althoughvarying valuesforsolventpolarityarereportedbydifferentsources,theorderremainsthesame.Itis worthmentioningherethatsolventpolaritymayvarywithinchemicallysimilarclassesof solvents.TypicalHPLCsolventswiththeirpolarityindexandUVcutoffarelistedin Table1.1.Anotherimportantconcernwhenselectingasolventforanyseparationsystem isitsmodeofdetection.Thespectroscopicbehaviorofamobilephaseneedstobeconsidered whenspectrometricdetectorssuchasUV,FTIR,orNMRareused.
1.4.5Detectors
Theseparationofthesampleinthecolumnhastobemonitoredbyoneormoredetectors whosesignalmustrepresenttheconcentrationofthepolymer.TypicalHPLCdetectorsfor lowmolarmassanalytesareequallyapplicabletoSEC/HPLCofpolymers.However,there arespecificrequirementsandapproachesthatareassociatedwiththepeculiarnatureoflarge molecules.DetectorsusedinHPLCofpolymerscanbebroadlyclassifiedintotwomajorcategories,concentrationsensitivedetectorsandmolarmassdetectors.Additionally,thereare spectroscopicdetectorsthatcanprovidedirectchemicalcompositioninformation.
1.4.5.1Concentrationsensitivedetectors
Theconcentrationofthesoluteinthesolventisdirectlyrelatedtothedetectorsignalin concentrationsensitivedetectors.Thesedetectorscanbeclassifiedintotwomajorgroups, namely,selectivedetectorsthatmeasureapropertyofasolute,anduniversaldetectorsthat measureabulkpropertyofthemobilephase.Combinationsofdetectorsmayberequiredfor theanalysisofcopolymers.
Selectivedetectors
SeveralselectivedetectorsareavailableinHPLC;however,notallofthemareapplicableto polymers.TheUVdetectoristhemostwidelyemployedselectivedetectorinpolymeranalysis.IRdetectorscanbeusefulbutarelimitedtomobilephasesthatdonotabsorbradiationat thedetectionwavelength.TheintroductionofanevaporativeinterfaceprovidesagoodalternativeforofflinecouplingtoFTIR [29,30].TheeluateissprayedonaGermaniumdiskthatis rotatedataparticularspeed.ThediskisthentransferredtotheFTIRspectrometertoyield
1.Basicprinciplesofsizeexclusionandliquidinteractionchromatographyofpolymers
TABLE1.1 Typicalsolventsusedinliquidchromatographyofpolymers.
ClassSolventPolarityindexUVcutoff
AlkanesHexane,heptane0.0200
Cyclohexane0.2200
AromaticsBenzene2.7280
Toluene2.4285
Xylene2.5290
EtherDiisopropylether2.2220
Methyl-tert-butylether2.5210
Tetrahydrofuran4.0215
Dioxane4.8215
AlkylhalidesTetrachloromethane1.6263
Dichloromethane2.5235
Dichloroethane4.0225
Trichloromethane4.8245
EstersButylacetate4.0254
Ethylacetate4.4260
KetonesMethylethylketone4.7329
Acetone5.1330
Alcohols n-Butanol3.9215 i-Propanol3.9210 n-Propanol4.0210
Methanol5.1205
NitrilesAcetonitrile5.8190
AmidesDimethylformamide6.4268
CarboxylicacidsAceticacid6.2230
Water9.0200
ReproducedfromH.Pasch,B.Trathnigg,MultidimensionalHPLCofPolymers,Springer,Berlin-Heidelberg-NewYork, 2013,withpermissionfromSpringerNature.Copyright2013.
spectralinformationasafunctionofelutiontimeofthechromatogram.Thissetupmust, however,becombinedwithanadditionalconcentrationsensitivedetectorforaccuratequantification.FluorescenceandelectrochemicaldetectorsareotherselectivedetectorsforHPLC thatarenotapplicabletopolymers.
TheUVdetectoristhemostfamiliarsolutepropertydetector.Itiscommerciallyavailable indifferentmodifications.TheUVdetectorisbasedontheprincipleofabsorptionoflightofa
selectedwavelengthbythechromophore-containinganalyte.Thetypicalwavelengthrange inthiscontextis180–350nm.UVdetectorscanonlybeappliedtosolventswithlowUVcutoff. TherearebasicallythreetypesofUVdetectors,namely,fixedwavelengthdetectors,variable wavelengthdetectors,anddiode-arraydetectors(DAD).Fixedwavelengthdetectorsare mostlyequippedwithalampemittinglightat254nm.Variablewavelengthdetectorsallow forselectionofaparticularwavelengthbymeansofaholographicgrating.DADallowsfor simultaneousmeasurementofthewholeUVspectrumovertheentirechromatogram.
Universaldetectors
Universaldetectorsmeasurethechangeinabulkpropertyofthemobilephase.Important universaldetectorsarerefractiveindex,conductivity,density,andevaporativedetectors. Universaldetectorsarelesssensitivecomparedtoselectivedetectors;however,theyarecommonlyappliedinanalysisofpolymers.Themostwidelyemployeddetectorinthisregardis theRIdetectorthatisavailableinmanymodifications.Applicationsoftheconductivitydetectortopolymersarenotcommon.Densitydetectorworksonthemechanicaloscillatorprincipleandisaneffectivedetectorinpolymeranalysisespeciallyincombinationwithother detectors.Evaporativedetectorsarebasedonthevaporizationofthevolatilecomponent oftheeluate(typicallythesolvent)andthedetectionofthenonvolatilecomponentsbyscatteringofthetransversallightbeam.
RIdetector
ThreetypesofRIdetectorsareavailable,namelydeflectionrefractometers,Fresnelrefractometers,andinterferometricrefractometers.Deflectionrefractometeristhemostcommonly employeddetectorinthisregard.Deflectionrefractometershavealargecellbutabetterlinear rangecomparedtoFresnelrefractometers.Sensitivityofinterferometricrefractometersis higherbyoneorderofmagnitudecomparedtootherRIdetectors.Theresponsefactorof RIdetectorsisdependentuponmolarmassaswellasonchemicalcomposition.Hence,an additionalconcentrationdetectorisrequiredforanalysisofcopolymersandpolymerblends. Moreover,preferentialsolvationofonecomponentofcopolymermayaffectthedetector signal [1].
Densitydetector
DensitydetectorincombinationwithUVorRIdetectorsrevealsadditionalinformation inisocraticelutionmode.Itworksonthemechanicaloscillatorprinciple [31,32].Atypical densitydetectorconsistsofanoscillatingU-shapedcapillarywhoseperioddependsupon thedensityofthecontent.However,therearenorecentapplicationsofdensitydetector foranalysisofpolymers.
Evaporativelightscatteringdetector
TheELSDcanberegardedasuniversaldetectorsinceitdetectsanynonvolatilecomponentsoftheeluate [33,34].OnlyfewcompaniesofferELSDscomparedtoawiderangeof availableUVdetectors.Insuchinstrument,theeluateisnebulizedandthesolventfrom thedropletisevaporated.Particlesareformedbythenonvolatilecomponentsofeachdroplet thatscattertheincidentlightbeaminaphotodiodecell.