Environmentalpollution andenvironmentalanalysis
ChapterOutline
1.1Introduction1
1.2Emergingpollutants2
1.2.1Persistentorganicpollutants2
1.2.2Nanomaterialspollutants7
1.2.3Microplastics8
1.2.4Heavymetalpollutants10
1.2.5Radioactivepollutants14
1.2.6Dyepollutants15
1.3Sourcesandtransportof pollutants18
1.4Riskassessmentandexposureof pollutants20
1.1Introduction
1.4.1Theidentificationofpotential hazard21
1.4.2Theassessmentof dose response21
1.4.3Theassessmentofexposure22
1.4.4Thecharacterizationof potentialrisk22
1.5Roleofenvironmentalanalysisin environmentalpollution23 1.6Conclusions25 References25
Environmentalpollutionisoneofthesignificantinternationalconcerns today.1 5 Variousemergingpollutantsintheenvironmentsuchaspersistence organicpollutants,nanomaterialspollutants,microplastics,radioactivepollutants,andheavymetalsdisplayharmfuleffectsonthehumanbody,animals, andplants.Themaintypesofenvironmentalpollutionthatleadtoharmful effectstodayarewaterpollution,soilpollution,andairpollution.6 12
Waterpollutionhasanumberofcauses,suchasreleaseofcontaminated effluentsfromvariousindustries,sewagecontainingdomesticwastesand pesticidesfromagriculturallands,thereleaseofsuperheatedwater,andthe releaseofwasteandoilfromrefineries.Theindustrialwaterpollutants,such asmercury,cadmium,chromium,andlead,arepoisonous.Theyarealso capableofenteringthefoodchainandcausingdiseasesinthehumanbody. MercuryisknowntocauseadiseasecalledMinimata.Organochlorinepesticides(OCPs),suchasdichlorodiphenyl-trichloroethane(DDT),inagriculturalwastesarenondegradableandcanbereleasedintothefoodchains. Someindustrialeffluentscanalsocausechangesincolor,odor,andtasteof
2 ModernEnvironmentalAnalysisTechniquesforPollutants
thenaturalwater.Thecontaminationofwateralsoleadstothespreadof waterbornediseases,suchascholeraandamoebiasis.13
Soilpollution,alsoknownassoilcontamination,isdefinedasthepresenceofhazardouschemicalssuchasheavymetals,radioactivemetals,nanomaterialpollutants,andtoxicsolventsinsoil.Thesepollutantscaneasily penetratesoilaffectingtheorganismsthatliveinsoil.However,theeffectof thepresenceofpollutantsinsoilorthelithosphereonbothterrestrialanimals andecosystemsismuchmoreconsiderableasthesesubstancesaccumulate infoodchains.Fossilfuelsmayalsoleadtothepollutionofsoilandwater. Somesourcesoffossilfuel basedsoilpollutioninvolvepetrochemical plants,refineries,andmotorvehicles.Theindiscriminateuseofvarious agriculturalchemicals,suchasherbicidesandpesticides,andtheimproper disposalofindustrialwastesareothercausesofsoilpollution.14 16
Airpollutioncanbedefinedasthepresenceofanyliquid,solid,orgas compoundsintheatmosphereatsuchconcentrationvaluesthatcandirectly orindirectlyaffecthumans,animals,and/orplants.Airpollutioniscaused bycertaindomesticandindustrialandactivities.17 19 Forexample,the increasinguseoffossilfuelsinindustry,mining,transportation,andconstructionofbuildingsarecrucialfactors,whichhaveledtoairpollution. Oneofthemajortypesofairpollutantsissuspendedmaterialssuchasdust, smoke,andfumes.Gaspollutantssuchascarbonmonoxide,nitrogenoxides, andsulfurdioxidearetheothertypeofairpollutants.20 Thecarefuland sensitiveanalysisoftheenvironmentalpollutantsisneeded.
Thischapterprovidesacomprehensiveoverviewofenvironmentalpollution.Itstartswiththedescriptionsandpotentialhazardouseffectsofemergingpollutantsintheenvironment,suchaspersistentorganicpollutants (POPs),nanomaterialpollutants,microplastics,heavymetalpollutants,radioactivepollutants,anddyepollutants.Thenthesourcesandtransportofthese environmentalpollutantsaredescribedandexplained.Inthefourthsection thereisanassessmentofthepotentialriskofandexposuretoenvironmental pollutants.Theroleandtheimportanceofenvironmentalanalysisinenvironmentalpollutionarepresentedinthelastsection.
1.2Emergingpollutants
1.2.1Persistentorganicpollutants
POPsareatypeoftoxicchemicalsthatarereleasedintotheenvironment andcannotbeeasilybrokendown.POPsremainintheenvironmentfora longtime(evenseveraldecades).Theseenvironmentalpollutantscanbe intentionallygeneratedandusedinagriculture,diseaseandpestcontrol, manufacturing,orindustry.Theycanalsobeunintentionallygeneratedfrom wasteincineration,cigarettesmoke,vehicleexhausts,andvariousindustrial processes.
TheStockholmConventiononPOPsisaglobalagreementsignedby152 countriesinSwedentoprotectenvironmentandhumanhealthfromthehazardouseffectsofPOPs.21 ThefirstpurposeofthisagreementwastostopimmediatelytheindustrialproductionanduseofPOPs.In2001itoriginallycovered the12POPsofhighconcernwhicharealsocalledthe“dirtydozen.”These POPsaredieldrin,aldrin,dioxins,chlordane,furans,mirex,DDT,endrin,heptachlor,hexachlorobenzene,PCBs,andtoxaphene.Another16newcompounds (α-hexachlorocyclohexane,chlordecone, β-hexachlorocyclohexane,decabromo diphenylether,hexabromobiphenyl,hexabromodiphenylether/heptabromodiphenylether,hexachlorobutadiene,hexabromocyclododecane,lindane,pentachlorobenzene,perfluorooctanesulfonicacid,pentachlorophenolanditssalts andesters,perfluorooctanesulfonylfluoride,polychlorinatednaphthalenes, short-chainchlorinatedparaffins,endosulfananditsrelatedisomers,tetrabromodiphenylether,andpentabromodiphenylether)wereaddedtothisinternationalagreementandacceptedin2017.ThesePOPsexhibithighresistanceto biologicalandchemicaldegradationintheenvironment.Theyalsoshowgreat stability,bioaccumulativefeatures,andpersistenceinthefoodchainandhave potentialhazardouseffectsonhumanhealthandenvironment.22 27
POPsgenerallyenterthehumanbodythroughswallowingcontaminated waterorfood,breathingindoororoutdooraircontaminatedwithPOPs,vehicleexhaustorcigarettesmoke,andtouchingproductsmadewithPOPs,and maycausesignificanthealthproblemssuchasbirthdefects,variouscancers, anddysfunctionalimmunesystems.WhenwateriscontaminatedwithPOPs, thesecontaminantscanpotentiallybeaccumulatedinaquaticorganisms.28
Asmentionedabove,12POPs,theso-called“dirtydozen,”areextremely hazardouscompoundsandthesecontaminantswerestrictlyregulatedor bannedbymanyinternationalorganizationssuchastheUnitedNations EnvironmentProgram,21 theEuropeanUnion,29 andtheUnitedStates EnvironmentalProtectionAgency(USEPA).30 However,thesePOPsarestill usedinsomedevelopingcountriesandarepresentintheenvironment.
1.2.1.1Mainsourcesofpersistentorganicpollutants
VegetationfiresandvolcaniceruptionsarethemainnaturalsourcesofvariousPOPs,suchasdibenzofuransanddioxins.ThesePOPsarequitestablein theenvironmentandeasilyentertheatmosphereviamanysources,suchas heatingstations,incineratingplants,andpowerstations.31 Ontheotherhand, otherunintentionalsourcesofPOPsarebushfires,putrefactions,diverse combustions,andincinerations,etc.Inadditiontothesesources,POPscan alsobegeneratedfromvariousactivitiessuchasrecyclingprocesses,buildingdemolition,obsoleteoilusage,pesticidestorage,andbiologicalandmedicalwastes.32
Disposablematerialssuchasplasticinjectors,cups,spoons,forks,and platesareamongthemainproductsofwasteincinerationplants.POPsoccur
4 ModernEnvironmentalAnalysisTechniquesforPollutants
duringtheburningofplasticandpolyvinylchloridematerials.POPsare foundinthefluegasesandashesofcombustionplants.
InastudycarriedoutinPoland33 manysourcesofPOPsinthepastdecadeswerestoppedduetotheirpotentiallytoxicfeatures.Inaddition,alotof wastewaterplantswerebuiltduringthistime.Anothermainreasonobserved forthedecreasingprevalenceofPOPsintheaquaticenvironmentandsedimentsistheecologicalpoliticsofthelocalauthoritiesandthegovernment.
1.2.1.2Categorizationofpersistentorganicpollutants
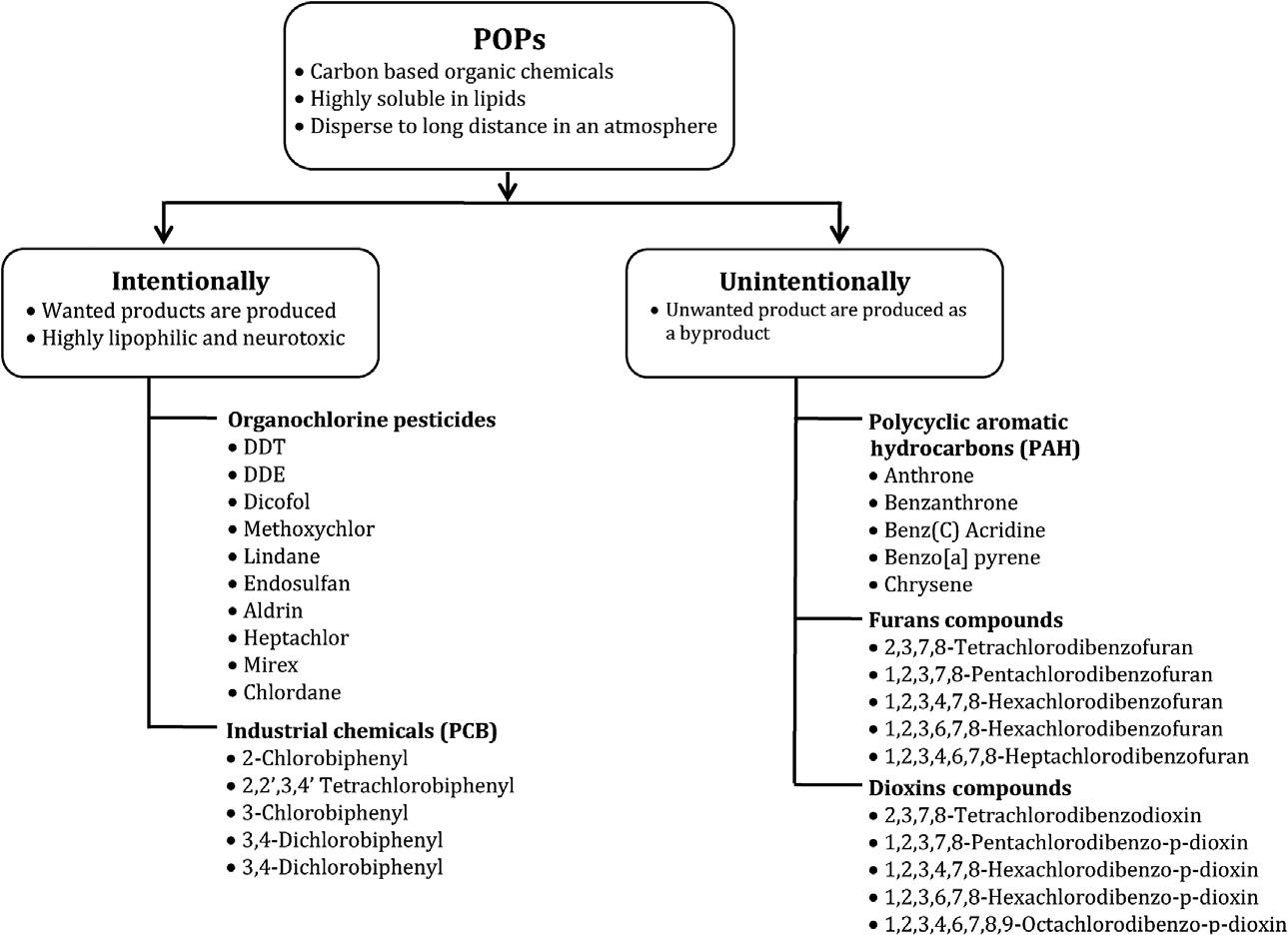
POPscanbecategorizedmainlyintotwocategories.Theseareunintentional POPsandintentionalPOPs,asschematicallyshownin Fig.1.1
OCPsareatypeofintentionalPOPswhichconsistofcarbon,hydrogen, andchlorineatoms.Theywerefirstusedinthe1940sinagriculturalprocessesandtokillinsectsthatdamageplants.OCPsaretoxic,bioaccumulative,degradable,andtheirpotentialfortransportationoverlongdistances wastakenintoaccountintheStockholmConventionduetotheirenvironmentalpotentialaspermanentorganicpollutants.Themostcommonlyused organochlorinepesticideisdichlorodiphenyl-trichloroethane,alsoknownas DDT.35
FIGURE1.1 Theschematicrepresentationofthecategorizationofpersistentorganicpollutants (POPs). ReproducedwithpermissionfromGaur,N.;Narasimhulu,K.;PydiSetty,Y.Recent AdvancesintheBio-RemediationofPersistentOrganicPollutantsandItsEffecton Environment.J.CleanProd.2018,198,1602 1631.34
Althoughpesticidesplayanimportantroleinmodernagriculturalpractices,thepesticideresiduesobservedinfoodproductshavebecomeacrucial problemtobeconsideredforthegeneralpopulation.36 38 Aftertheharmful effectsofOCPsonhumanhealthandtheenvironmentwereprovenbyvariousstudies,theuseofOCPswasrestrictedandbannedbydevelopedcountries.OCPswhicharebannedinmostcountriesarestillusedindeveloping countriessuchassomeAfrican,SouthAsian,andSouthAmericancountries tocombatinsects(mosquitoes,etc.).39
Polycyclicaromatichydrocarbons(PAHs),furans,anddioxinsareunintentionalPOPs.PAHshavetwoormorearomaticringsintheirstructure.PAHs areproducedbythepyrolysisofhydrocarbonsathightemperatures.Coalcombustion,smoke,andindustrialfumesareimportantsourcesofPAHs.PAH compoundsarereadilysolubleinorganicsolventsduetotheirlipophilic/hydrophobicproperties.Thesecompoundsareknowntoincreasethetoxiceffectdue totheincreaseinwatersolubilityasthemolecularweightdecreases.40 PAHs showcarcinogenic,mutagenic,andtoxiceffectsonlivingorganisms.41
Ontheotherhand,furansanddioxinsaretheabbreviatednameofthe polychlorinateddibenzofuransandpolychlorinateddibenzo-p-dioxins, respectively.Combustionandchemicalprocessesarethemainsourcesof dioxinsandfurans.42
1.2.1.3Impactsofpersistentorganicpollutants onenvironmentandhumanhealth
ThePOPscanaffecttheenvironmentthroughabiotic,biotic,andtechnologicalinterference.TheecologicalbalanceisdegradedbythereleasedPOPs intotheenvironmentandthehealthofallorganismsandtheenvironmentis threatened.Forexample,thereleasedPOPscanaccumulateinthebodyof marineanimalsandcausetheirdeath.Thisleadstoachangeinthebalance ofthesea’secology.
SemivolatileOCPsarevaporizedatambienttemperaturesandtransported overlongdistancesthroughtheatmosphereduetotheirlowvaporpressures. Thereforetheycanbeobservedeveninplacesfarfromwheretheyare used.43 OCPscanbereleasedintothewaterenvironmentthroughleaksor viatheatmosphere.DuetotheirhydrophobicstructureOCPsaregenerally adsorbedtothesurfaceofthesolidorganicparticlesinthewaterand collapseintothesedimentlayeratthebottom.OCPcompounds,whichtend toaccumulateinthefattytissuesoflivingbeingsduetotheirlipophilic structure,areprogressingupwardsinthefoodchain(biomagnification).Asa resultoflaboratoryinvestigations,itwasfoundthatOCPsexhibitcarcinogeniceffectsonlivingorganisms.Theyhavenegativeeffectsonrespiratory, nervous,andimmunesystems.44
Dioxinsformedduringcombustionarereleasedintotheairandcontaminatesoilandagriculturalproductsandwhileairbornecantravelthrough
longdistances.Dioxinsaccumulateinthefattissuewhentheyenterthe humanbody.Dioxinsaccumulateinthefoodchaininnature.Birdsaresignificantlyaffectedbecausetheyfeedclosetothesoil.Dioxinscanbe absorbedbyalgaeandthenpassedtofishandthenmoveupthefoodchain. Inthelongterm,exposuretolowdosesofdioxinsandfuranscancauseseveraldiseasessuchasdisordersoftheimmunesystem,disordersofthenerve andendocrinesystem,congenitalanomalies,liverfunctiondisorders,breast cancer,andothertypesofcancer.Dioxinsarealsoassociatedwitha decreasedspermcount,behavioralproblems,andincreasedriskofdiabetes. Epidemiologicalstudieshavealsofoundarelationshipbetweendioxinexposureandheartdisease.High-doseexposureofdioxinsmaycauseskinulcers, alsoknownaschloracne.45 Furansaresimilartodioxinsandarealsopotentiallycarcinogeniccompounds.
Therearemanyreportedstudiesintheliteraturethatinvestigatethe impactsofPOPsonenvironmentandhumanhealth.Forexample,the descriptionofthepotentialhazardsofPOPsontheenvironmentwascarried outbyWalker.46 Wongandcolleaguesreviewedtheenvironmentaleffects ofPOPsinNorthAmericaandChina.47
LetcherandcoworkersinvestigatedtheimpactsofPOPsonvarioustrophicanimalspecies,thatis,seabirds,polarfoxes,bears,andsleddogs.48 TheyevaluatedthepotentialhazardsofPOPsintermsofbiochemicalprocesses,suchaspathologicalchangesintissueandtheendocrineandimmune systems.Theyalsohighlightedthephysiologicalandecologicalstressors. Theotherparameters,suchasnutritionchanges,speciesinvasion,temperature,climatechanges,food,weredescribed.Theauthorssuggestedfurther studyandbetterinvestigationoftheimpactsthePOPsonanimals.
InanotherstudyreportedbyNoyesetal.,49 theeffectsofPOPsonthe climatechangehavebeeninvestigated.Theinvestigationwascarriedoutby monitoringthechangesinenvironmentalfactorssuchassalinity,precipitation,andtemperature.
TheimpactsofPOPsontheenvironmentwerealsodiscussedinother reviews. 50 54 Inanimportantstudy 55 Jenssenetal.evaluatedtheeffects ofdisruptingcompoundsonclimatechange.Forthispurpose,dichlorophenyldichloroethylene,polychlori natedbiphenyls,oxychlordane,and hexachlorobenzenewereusedasdisruptingcompoundsandtheireffects onthehormonaldisturbanceofseab irdsandmammalsledtoecological disturbance.Theobtainedresultsindi catedthatcortisol,sexsteroidhormones,andthyroidhormonewereaffectedbythedisruptingcompounds usedinthisstudy.Theseeffectsalsoledtomorphologicalbehavioral changes.
Inanotherinterestingstudy56 amodelassessmentfortheimpactsofland useandclimatechangebecauseofthereleaseofOCPswasapplied. Theimpactsoflandcover,seasonalchangesinthestabilityofair,andsoil temperatureonthereleaseofOCPsintotheenvironmentwereinvestigated.
Theobtainedresultsshowedthattheorganochlorinepesticiderelease occurredbecauseoftemperaturechangesandagriculturalactivities.
1.2.2Nanomaterialspollutants
Nanotechnologyandnanosciencearethescientificresearch,design,preparation,characterization,andapplicationofextremelysmallmaterialsatthe nanoscalebetween1and100nm.Nanotechnologycansuccessfullybe appliedinmanybranchesofscience,suchasengineering,chemistry,physics,biology,materialscience,pharmaceuticalscience,andmedicine.Allof thesebranchesofscienceinvolvethedesign,preparation,characterization, andapplicationofnanomaterials.
Inrecentyearsscientistshavegivenconsiderableefforttothedesignand preparationofengineerednanomaterialstotakeadvantageoftheirexcellent properties,suchaslighterweight,higherstrength,largesurfacearea,small size,enhancedcontrolofthelightspectrum,andexcellentchemicalreactivity,comparedtotheircounterpartsatthemicroscale.57 Thephysicochemical featuresoftheseuniquematerialsintherangebetween1and100nmare completelydifferentcomparedtothesamematerialsatmicroscale.For example,goldnanoparticlesarearedcolorwhilethebulkgoldparticles exhibitayellowcolorduetotheinteractionbetweenlightandtherestricted electronsofthegoldnanoparticles.Therelativesurfaceareaofamaterial increaseswhenitssizedecreases.Thephysicochemicalfeaturesofthematerialsatthenanoscalecanefficientlybecontrolledbystructuraldesign,the incorporationofappropriatefunctionalgroupsorcompounds,andthemodificationofthesurface.
Despitetheuniquefeaturesandmanyapplicationsofthenanomaterials, therearestillsomequestions,challenges,drawbacks,andconcernsaboutthe impactsofthesenanomaterialsonhumanhealthandtheenvironment.One ofthepotentialconcernsofnanomaterialsistheirtoxicityandsafety.For example,nanoparticleshavethepotentialtoenterthehumanbodyviathe respiratorysystem.Theymayalsocomeintocontactwiththeskinandcause somehealthproblems.Thetoxicityfeaturesofnanoparticlesareaffectedby manyphysicochemicalpropertiessuchaschemicalcomposition,reactivity, andparticlesize.58 Evensmallamountsofthesenanoparticlesmayhaveserioustoxiceffectssincetheyhavealargerelativesurfaceareathatisusually correlatedwithhighreactivitybytheformationoffreeradicals.Whenthey enterthebloodstreamafterpassingthroughtherespiratorysystem,theymay accumulateatthespecificsitesofthevariousorgans.Theymayeven directlypenetratethebrain.59 Thustheeffectsofnanomaterialsonhuman healthshouldbecarefullyinvestigatedanddefined.Recentlymucheffort wasmadeforthedevelopmentofinnovativetechniquesfortheevaluationof thetoxiceffectsofthesenanomaterials.Theseintensiveeffortsledtothe birthofanewfieldoftoxicologywhichiscalled“nanotoxicology.”60
Thematerial’ssafetyandtheriskstohealthandtheenvironmentneedto becarefullyinvestigatedsinceeachnanomaterialisunique.61 Beforethe nanomaterialsareusedforinvivobiologicalapplications,crucialphysiologicalfactors,suchasdistribution,absorption,andtoxicityofnanomaterials, shouldbeconsideredandevaluated.Thepotentialtoxicityofnanomaterials shouldbeinvestigatedaccordingtotheinternationalregulatoryguidelines whicharestillunderdevelopment.62,63
Theresearchersshouldalsobecarefulduringthedisposalstepofthe nanomaterials.Disposalofthenanomaterialsmaygeneratenewformsof environmentalnanowasteswhichareanotherchallengefortherecycling effortsandwastemanagement.
Nanomaterialshavealsopotentialtoxicimpactsontheenvironment.The releaseofthesematerialsintotheenvironmentcausesenvironmentalpollutionandecotoxicity.Forexample,nanoparticlesarerapidlyreleasedintothe environmentasanaturalconsequenceoftherapidincreaseinnanoparticlecontainingconsumerproductsandnanoparticleproductionfacilities.This releaseresultsinthenanoparticlesbeingintroducedintotheair,water,or soilbyvariousprocesses.Thefateofnanoparticlesintheenvironment dependsontheirtype,shape,andphysicochemicalproperties,andthereceptiveenvironmentcharacteristics.Thereleaseofnanoparticlesintotheenvironmentcanoccurduringtheproductionprocess,oronthecompletionof thelifecycleofaproduct.Environmentaloscillationsofnanoparticlesinthe industrialproductionstageareinevitable.Inadditiontoproduction,nanoparticleswhichareseparatedfromtheproductsduringtransport,storage,and usestagesarereleasedintotheenvironmentbydischargesandleaks,are spreadintheecosystemviadiffusion,andcomeintocontactwiththeair, water,andnutrientsandhumansasapartoftheecosystem.Consideringthe applicationareasofnanoparticle-basedproductsduringproductioninindustry,ithasbeenobservedthatitisquiteeasytospreadthenanoparticlesto theenvironmentbothbywaterandair.64
InastudyreportedbyBennandWesterhoff65 itwasfoundthattheAg nanoparticlesoncommerciallyavailablesockswerereleasedintotheaquatic environmentbywashingthemwithwater.Inanotherstudythewastewater ofalaundrywasinvestigatedandAgClnanoparticlesweredetected.66
1.2.3Microplastics
Plasticreferstoaclassoforganicpolymerobtainedfrompetroleumsources suchasnylonpolyvinylchloridepolyethylene,polystyrene,polypropylene, low-densitypolyethylene,andpolyacrylates.67 70 Theindustrialproduction andapplicationofplasticpolymershavegainedsignificantattentionoverthe last50years.Theworldwideproductionofthesematerialswasapproximately335milliontonsin2016.71
Microplasticsaresmallplasticparticles(smallerthan5mmindiameter). Primarymicroplasticsareintentionallyproducedinsmallerparticlesize, suchasresinpellets,microparticlesforpersonalcareproducts,plasticpowdersthatarecommonlyusedformolding,andscrubbersthatareusedforthe preparationofcleaningmaterials.Secondarymicroplasticsoriginatefromthe fragmentationofplasticparticlesthathavelargerdiameter.72,73 Anyofthese typesofmicroplasticshavethepotentialtoendupinenvironmentalsamples suchaswastewater.
Inresponsetosignificantlyincreasingconcernsaboutthemicroplasticsbasedenvironmentalpollution,variouscountriessuchasAustria,the Netherlands,Belgium,Sweden,andLuxembourgagreedonajointstatement totheEuropeanUnionEnvironmentMinistersregardingthebanonmicroplasticsthatareusedintheproductionofpersonalcareproducts.74
Microplastics-basedenvironmentalpollutionisexpectedtoincrease,even thoughthereleaseofmacroplasticstotheenvironmentbecamerelatively undercontrol,duetothedegradationoftheplasticlitterpresentintheenvironment.Becauseofthesmallsizeofmicroplastics,thesemicroparticles mayeasilybeingestedbymanyorganismswhichleadtosignificantharmful effectsonthem.Inaddition,theycanleachadsorbedmetalsandorganic compoundsthatarealsopollutantstotheorganisms,whichfinallyleadsto bioaccumulationthroughthefoodchain.75 77
Microplasticsalsohavethepotentialtocausesignificantharmtohuman healtheitherbyexposuretomicroplastics-contaminatedwaterorseafood.78 Inaddition,fishing,agriculture,andtourismarealsoaffectedbythis microplastics-basedenvironmentalpollution.
Asmentionedabove,microplasticsarecategorizedas primarymicroplastics and secondarymicroplastics.
Primarymicroplasticsareproducedforthespecificindustrialapplications.Theseapplicationsincludetheproductionoftoothpaste,facialcleansers,cosmeticproductssuchasshowergelsandscrubs,72 deodorants,baby products,haircoloringproducts,andsunscreens.79
Theapplicationofmicroplasticsinthepharmaceuticalindustryhasalso gainedconsiderableinterestfromresearchers.80 Microplastic-basedscrubbers andrelatedproductsarecommonlyusedasfacialscrubsinsteadofconventionallyusedingredientssuchaspumice,groundalmonds,andoatmeal.The applicationsofexfoliatingcleansershavingmicroplasticshaveconsiderably increasedbecauseoftheapplicationsofsomanypatentsformicroplasticbasedscrubbersinthecosmeticindustrysincethe1970s.81 Forinstance, Gregory82 reportedtheuseofpolypropyleneandpolyethylenegranuleparticles(smallerthan5mm)andpolystyrenebeadswhichhaveparticlesizes smallerthan2mminthepreparationofcosmeticformulations.
In2009FendallandSewell81 reportedtheuseofirregularlyshaped microplastics(typicallysmallerthan0.5mmindiameter)forthepreparation ofacosmeticproduct.
Airblastingtechnologyisanotherapplicationareaofprimarymicroplastics.Thistechnologyinvolvestheblastingofpolyesteroracrylic-based microplasticscrubbersandboathullsfortheremovalofpaintandrust.Since thescrubbersareusedmanytimesuntiltheylosetheircuttingperformance andbecomesmaller,thesematerialsarefrequentlycontaminatedbyvarious heavymetalssuchaslead,cadmium,andchromium.
Ontheotherhand,secondarymicroplastics,whicharepresentinsoiland water,aretheresultoflargerparticlesofplasticmaterialsthatbreakdown intothesmallerparticles.Thisdegradationiscarriedoutwhenlargerplastic materialisexposedtoultraviolet(UV)lightforalongtime.Thecombinationofvariousenvironmentalparameters,suchastemperatureandsunlight, andthefeaturesofthepolymer,suchasitsdensityandsize,affectsthedisintegrationofmacroplasticdebris.Thelong-termexposureoflargeplastic materialstoUVlightfromthesunleadstothephotodegradationofplastic materialsthroughthecleavageofthebondsinthepolymericstructure.The photodegradationcontinuesuntiltheparticlesbecomesmaller.Sincethe largeplasticmaterialsdegradeintomicroplastics,theabundanceofthese plasticsaspollutantsintheenvironmentsignificantlyincreases.Sincetheir particlesizebecomessmaller,microplasticscaneasilybeingestedbymany organismsinthemarineenvironment,suchasplankton,fish,andwhale.On theotherhand,toxicchemicalscanbeadsorbedtothesurfaceofmicroplasticpollutantsinthemarineenvironmentwhichcanbereleasedintothefood chain.83
Bothprimaryandsecondarymicroplasticsarefoundinthemarineenvironmentathighconcentrations.Ithasbeenpredictedthatapproximately 245tonsyear 1 ofmicroplasticsendupinthemarineenvironmentwhere themicroplasticsareeasilyingestedandincorporatedintothetissuesand organsoforganisms.84
Theexposureofmarineorganismstomicroplasticpollutantsiscrucial forunderstandingthefateandeffectsofthesepollutantsintheaquaticenvironment.85 Inrecentyearsmanystudieswerecarriedoutinvestigatingthe ingestionofmicroplasticpollutantsinthemarineenvironment.86 89
Whenmicroplasticpollutantsareingestedbyorganismsinthemarine environmenttheycausemechanicalharm(i.e.,cloggingthedigestivesystem, hinderingthemobilityoforganisms)throughtheadsorptionprocess.They alsoleadtochemicalharmsuchashepaticstress,inflammation,and decreasedgrowth.90
1.2.4Heavymetalpollutants
Aconsiderableamountofresearchonenvironmentalpollutionhasfocused ontheinnovativeclean-upprocessesforheavymetals,whicharetoxicand persistentpollutants.Heavymetalsaredefinedasoneofthemostcommon andhazardousenvironmentalpollutants,andtheyhaveaspecificdensityof
morethan5gcm 3.Metalssuchasiron,copper,zinc,andmanganeseare crucialforthelifeprocess.Ontheotherhand,someothermetals,suchas mercury,arsenic,nickel,andcadmium,donothaveanyphysiologicalfunctionbutoftenresultinharmfuleffectsatahigherconcentrations.91
Heavymetalcontaminationisdefinedastheincreasedlevelsoftoxic metalsintheenvironment.Manyanthropogenicactivities,suchasmining, refiningofores,combustionoffossilfuels,fertilizersandpesticides,metallurgy,andmunicipalsolidwastes,resultinmetalcontamination.
Metalsionsareconsideredasprioritypollutantsbecauseoftheirmobility andtoxicfeaturesinecosystems.Themainproblemassociatedwithmetal pollutionisthattheyarehighlypersistentandnotbiodegradableintheenvironment.Thereforetheycanbeaccumulatedinlivingtissueswhichcan causevariousdisordersanddiseases.92 Themostwell-knownexamplesof heavymetalpoisoningareItai-Itaidiseasecausedbythecadmiumpollution ofJinzugawariverbyaJapaneseCompany(MitsuiMining&SmeltingCo.) andMinimatadiseasecausedbymercurytoxicityfromcontaminatedfishin Japan.93 Duetotheirtoxicnaturethemanagementofheavymetalsisofspecialconcern.Thusthesensitiveanalysisandefficientremovalofheavy metalpollutantsfromenvironmentalsamples,suchaswaterandsoil,isvery important.
Heavymetalsarereleasedintotheenvironmentintwodifferentways. Thesearenaturalandanthropogenicsources.Themostimportantnatural resourcesareerosionandvolcaniceruptions.Theothersourcescanbelisted asmining,thermalpowerplants,domesticheatingsystems,motorvehicles, fertilizers,pesticides,iron steel,sugar,cement,petrochemical,andmetal industries.94,95 Themainreasonforthepresenceofheavymetalsintheecologicalsystemanditsdistributionwithinnaturalresourcesisnotnatural cycles.Themainreasonishuman-inducedindustrialeffects.
Asheavymetalsspreadtotheenvironment,theycancauseseveraldiseasesinplants,animals,andhumansdependingontheirconcentrationin theenvironment.Manyoftheheavymetalsandmetal-containingcompounds(metalloids)aretoxic,sotheycancauseundesirableeffectsand problemseveninverysmallconcentrations.96 Heavymetalsinthesoil damagethenumberofmicroorganismsinthesoil,andthusreducingtheir numbersandbiologicalactivitiesovertime.Itisthereforenecessary tocloselymonitortheheavymetalaccumulationinsoil.Inmanyindustrializedcountries,thisfollow-upiscarriedoutusingalargenumberofdifferentmonitoringprograms.Heavymetalsmixedintotheaireventually canreachlandandfromtherepasstoanimalsandhumansthroughplants andthefoodchain.Inaddition,heavymetalsintheair,asaerosolor powder,alsopassfromanimaltoanimalandontohumans.Heavymetals canalsocausewaterpollutionthroughenteringawatersupplyby industrialandconsumerwasteandreleasingheavymetalsintostreams, lakes,riversandgroundwater. Environmentalpollutionandenvironmentalanalysis Chapter|1 11
TABLE1.1 Regulatorylimitsforthemainheavymetals inthesoil.97 HeavymetalRegulatorylimit(mgkg
TheregulatoryguidelinesreleasedbytheUSEPAforsomeofthemain heavymetalspresentinthesoilaresummarizedin Table1.1
1.2.4.1Lead
AccordingtotheAgencyforToxicSubstances,DiseaseRegistry(ATSDR)’s PriorityListofHazardousSubstances,98 lead(Pb)isthesecondmosthazardousheavymetalandithascarcinogenicpotentialforhumans. AnthropogenicandnaturalsourceshavecrucialrolesinreleasingPbinto theenvironment.SomeofthemainsourcesofthecontaminationofPbinthe environmentaresmeltingandmining,metalsweldingwithPbpaint,Pbbasedbatteries,gasolinewithlead,pulpandpaper,andexplosivecompounds havinglead.99 101 OneofthemainsourcesofcontaminationofPbinthe environmentislead-basedpaints.Therearecurrentlymorethan20million houseswherePb-basedpaintshavebeenusedintheUnitedStates,although thesekindofpaintshavebeenbannedbytheUSEPAintheUnitedStates since1978.102 Becauseoftheanthropogenicandnaturalsources,suchas wastedisposal,flaking,chipping,weathering,andscrapping,Pbpresentin exteriorpaintsmayleachoutandformdustparticlescontainingPb.103 Becauseoftheirhandtomouthcontact,childrenyoungerthan6yearsold aremoresusceptibletoPbexposure.104,105
1.2.4.2Mercury
Mercury(Hg)isthethirdmosthazardousheavymetalaccordingtothe ATSDR’sPriorityListofHazardousSubstances.Hgcancausemanyhealth riskstohumans,especiallytochildrenwhoareinthedevelopingstage. Peoplecancomeintocontactwiththreedifferentformsofmercury.These areelementalormetallicmercury,inorganicmercury(HgCl2),andorganic mercury(C2H5HgorCH3Hg).Themostimportantsourceofinorganicmercurycontactinhumansisamalgamtoothfiller.106 Oneoftheotherimportant sourcesisfishlivinginwatercontaminatedwithmercury.
ThetoxicityofHgsignificantlyaffectsthenervoussystem,immunesystem,renalsystem,ocularsystem,anddigestivesystem.Coal-firedpower plantsareoneofthemainsourcesofHgreleaseintotheenvironment.The othersourcesincludeoilpipelines,mining,incinerationofwaste,andresidentialheatingsystems.AfterthereleaseofHgintotheenvironment,the naturaltransformationofelementalHgintoC2H5Hgoccurs,whichleadsto accumulationofC2H5Hginshellfishandfish.Theconsumptionofseafood contaminatedwithC2H5HgandtheinhalationofelementalHgarethemain sourcesofhumanexposuretoHgpollution.107
Organicmercurycompoundsaresolubleinoilandtheycanbeeasily absorbedfromthegastrointestinalsystemduetotheirshorthydrocarbonstructure.Methylmercurycanalsoeasilypassthroughtheplacenta,blood brain barrier,andmilkchannels.Mostofthemethylmercuryexistsininorganic mercuryform.108
1.2.4.3Cadmium
Cadmium(Cd)isanotherheavymetalwhichexhibitscarcinogeniceffects forhumansanditisrankednumberseveninthePriorityListofHazardous SubstancesreleasedbyATSDR.8 Althoughitnaturallyexistsintheenvironmentatverylowconcentrationvalues,thelevelofCdhasbeenconsiderably increasedbyanthropogenicactivities.ZnandPbrefineries,Ni/Cdbatteries disposalofindustrialwastescontaminatedwithCd,electronicproducts,pesticides,andfertilizersaremainsourcesofCdexposure.Duetoitsanticorrosiveproperty,CdisalsocommonlyusedinthepreparationofCd-based coatingsformarinevessels.
Cdhasthehighestsolubilityinwatercomparedtotheotherheavymetals. ThereforetherateofCdpropagationinnatureishighanditisnotanessential elementforhumanlife.Duetoitswater-solubleproperties,Cdistakenintobiologicalsystemsbyplantsandmarineorganisms.Cdexhibitslong-termpersistenceintheenvironmentandeasilyaccumulatesinvegetables,crustaceans,and mollusksovertime.TheremovalofCdisextremelydifficultwhenitentersthe humanbody.109,110 ThetoxicityofCdaffectsthekidneyswhichcancause kidneydysfunction.Itstoxicityalsoaffectsrespiratoryandskeletalsystems.
ThesuggestedsafeconcentrationlimitofCdbytheWorldHealth Organization(WHO)islowerthan200mgCdkg 1.Ithasbeenreported thatthetoxicityofCdnotonlyimpactsthebonesandkidneysbutalsosignificantlyaffectstheotherorgansofhumansandothermammals.111
1.2.4.4Chromium
Chromium(Cr)isametalwhichisnaturallyfoundintheenvironment(i.e., soils,rocks,andvolcanicdusts)intwostates:trivalentCr(Cr(III))andhexavalentCr(Cr(VI)).112,113 Cr(VI)compoundshavealmost100timesmore toxicitythanCr(III))compounds.114 AlthoughthereleaseofCroccurs
ModernEnvironmentalAnalysisTechniquesforPollutants
throughnaturalsources,themainsourcesoftheCrpollutionintheenvironmentisfromindustrialprocesses.Crcompoundsarecommonlyusedin manyindustrialapplicationssuchastheproductionofdyesinthetextile industry(i.e.,potassiumchromate,ammoniumdichromate),theproduction ofinks,Cr-basedpaints,plasticmaterials(i.e.,zincchromate,sodiumchromate,leadchromate,andbariumchromate),andanticorrosivematerials(i.e., strontiumchromate,zincchromate,andcalciumchromate).115
Cralsoexistsinanimalbodiesandplantsasatraceelementthatmeans thesmallamountsofCrareessentialforthegrowthoflivingorganisms. However,thehighlevelofCrconcentrationinthelivingorganismsmaybe highlyhazardoustothem.116
Cr(VI)hasahightoxicityandisanindustrialpollutantthatcanbedangeroustohumans,leadingtoCr-induceddiseases,suchasliverandkidney damage,respiratoryproblems,andimmunesystemproblems.Ingeneral,the suggestedsafeconcentrationlimitofCrinsoilanddrinkingwaterisinthe rangebetween1and1000mgkg 1 and0.1mgL 1,respectively.117
1.2.4.5Arsenic
Arsenic(As)isatypeofmetalloidthatispresentinalmostallkindsofenvironmentalsamples.Itisoneofthemostwidelyavailableenvironmentalcontaminantsinwaterbodiesacrosstheworld.Arsenicisthemosthazardous heavymetalaccordingtotheATSDR’sPriorityListofHazardousSubstances. Itisawell-knownheavymetalwhichcancausecancertohumans.Soluble inorganicAsdisplaysacutetoxicity.Arsenicexistsinfourvalencestates:elementalAs(As0),arsenite(As(III)),arsenate(As(V)),andarsine(AsH3)gas.118
ThepresenceofAspredominantlyleadstoanincreaseinthetoxicity levelsofdrinkingwater.ThepermissiblelimitofAsindrinkingwaterand insoilsis0.05mgL 1 and1 50mgkg 1,respectively.Theeffluents releasedfromindustrieshavelargeamountsofarsenicandthedirectdischargeintotheenvironmentleadstoenvironmentalpollution.Arsenicisan extremelytoxicheavymetalthatcanaffecthumansthroughtheinhalationof pollutedair,water,andcontaminatedfood.Ingeneral,arsenicisineither organicorinorganicformswhichcanaffectboththeperipheralandcentral nervoussystemsbecauseofitsneurotoxictoxicity.Neurotoxicitystartswith sensorychangesandtendernessfollowedbygradualweaknessfromthe proximaltodistalmusclegroups.119 ThusAsisconsideredasanonessential elementtohumansandtheenvironmentwhichmaybereleasedintotheecosystemthroughanthropogenicornaturalactivities.
1.2.5Radioactivepollutants
Radioactivecontaminationisaninvoluntaryreleaseofradioactivesubstances.Theelectronsemittedbyradioactivesubstancescancause
Environmentalpollutionandenvironmentalanalysis Chapter|1 15
irreversibledamagetoair,water,andnature.Withincreasingtechnology, theneedforenergyandtheinabilityofexistingresourcestomeetthisneed haveincreasedtheimportanceofnuclearenergy.However,nuclearpower plantsarethemainsourcesofradioactivepollution.120 Inadditiontothe powerplants,nuclearweaponsfactoriesandwastesofradioactivematerials playanimportantroleinradioactivepollution.Inthelast60years,nuclear testshavebeenconductedformilitarypurposesandenergyproductioninthe world.121 Thesenucleartrialshavecausedaconsiderableamountofradioactivecontaminationtotheenvironment.Radioactivesubstancesarenotonly activeinthetestareabutalsoaffecttheairandwater.Theworld’sworst nucleardisastersoccurredin1986inChernobyl,Ukraineandin2011in Fukushima,Japan.Thousandsofpeoplediedduetotheradiationreleased fromthenuclearplants.122,123
Thecontaminationcausedbytheradioactivepollutantsintheenvironmentisacrucialissuethataffectsthequalityofpublichealthandtheenvironment.Ingeneral,anthropogenic(artificial)andnaturalradionuclidesare themainpollutantradionuclidesintheenvironment.Radionuclidessuchas 238U, 232Th, 222Ra,and 87Rbcanexistinvariousenvironmentalmatrices suchasrocks,soil,sediments,air,andwater.Ontheotherhand,themain sourcesofanthropogenicradionuclides(i.e., 137Cs, 239Pu, 241Am, 90Sr,and 91Y)arenuclearweaponsandnuclearpowerplants.
However,variousradionuclidessuchasradiocarbon(14C)andtritium (3H)havebothartificialandnaturalsources(releasedbybothnuclearexplosionsandcosmicradiation.
Thestrategiesandapproachesforthecarefulcontrolofradioactivepollutantsreleasedintotheenvironmentshouldbethesameforanthropogenic andnaturalradionuclidesaccordingtotheguidelinesoftheInternational CommissiononRadiologicalProtection.124
Somenaturalandartificialradioactivepollutantsandtheirhealtheffects aregivenin Table1.2.
Themainriskofthenuclearhazardisthetransmissionofradioactiveisotopesintothewater.Ontheotherhand,radioactiverainisoneofthemost importantfactorsforradioactivityinthesurroundingwater.Themostimportantissueinradiationaccidentsistheurgentandsensitivedetectionofradioactivepollutantsinthesurroundingwaters.Theradiationtransmittedtothe waterpassesdirectlytotheplantswhichleadstothecontinuousreleaseof theradiation.Thewateroftheradiation-contaminatedareaisextremelydangerousnotonlyfordrinking,butalsoforagriculturalproducts.125
1.2.6Dyepollutants
Dyecompoundsarewidelyusedinmanyfieldssuchasthepaper,food, cosmetic,andtextileindustries126,127 andthesecompoundshavethepotential togeneratelargevolumesofcontaminatedeffluents.Thereleaseof
TABLE1.2 Somenaturalandartificialradioactivepollutantsandtheir healtheffects.
RadioactivepollutantSourceHealtheffect
222RnNaturalCarcinogen
226RaNaturalCarcinogen
228RaNaturalCarcinogen
238UNaturalCarcinogen,toxictokidneys
234ThNaturalCarcinogen
152EuAnthropogenicCarcinogen
241AmAnthropogenicCarcinogen
239PuAnthropogenicCarcinogen
91YAnthropogenicCarcinogen
contaminatedeffluentsintotheenvironmentisthemainsourceofenvironmentalpollutionwhichisoneoftheseriousenvironmentalproblemsthat needstobesolvedallovertheworld.Thesecontaminantsmustefficiently beanalyzedandremovedfromthewastewhichisacrucialandchallenging process.Thussimple,effective,cheap,andenvironment-friendlytechniques arerequiredforthesensitiveanalysisofthesedyecontaminantsinenvironmentalsamples.
Todaymorethan100,000differenttextiledyesandpigmentsarecommerciallyavailableandextensivelyusedinindustryandtheglobalannual productionofsyntheticdyesismorethan7 3 105 tons.128 130 Thechemical industryandpublichealtharenegativelyaffectedbythedyecompounds releasedfromthetextileindustry.
Thegeneratedwastewaterfromtheproductionprocessinthetextile industryisapproximately40 65Lkg 1 products.131 Themajorityofwastewater,whichisimportantintermsofboththeamountandthepollutants involvedinthetextileindustry,isduetothedyeingprocess.Oneofthe mostcharacteristicpollutantparametersofthewastewatergeneratedasa resultofthedyeingprocessisthecolor.Themainsourceofthecolorthat canbedissolvedandcolloidalinsuchwastewatersisthedyeswhichare usedduringthedyeingprocesses.
Dyescontaindifferentchemicalstructuresandtheyareclassifiedaccordingtotheirstructureandapplication.Dyesaregenerallycomposedoftwo compounds,namelythechromophoreandfunctiongroups.Thechromophore isanimportantpartofthedyeprovidingthecolor,andcontainsoneormore bonds.Thesebondsarevariableandabsorbthelight,providingabright
colorappearanceofthedye.Themostcommonlyusedchromophoregroup indyesistheazogroup.Thefunctionalgroupallowsthedyetobebonded tothecottonorwoolyarn.Differenttypesoffunctionalgroupsareusedfor dyeingdifferenttypesoftextilematerials.
Azodyescompriseapproximatelymorethan60%ofthedyesusedin textileindustry.132 Thesedyesaretypicallycharacterizedas NQN and theyhavecrucialstructuraldiversity.Thefeaturesofazodyeshavebeen improvedtoprovideagreatdegreeofbiological,chemical,andphotostabilityandcanresistbreakdownovertime,exposuretomicroorganisms,and sunlight.Thustheyarestronglyresistancetobiologicalandchemicaldegradation.133 Moreover,thereductivecleavageofazobondsofthistypeofdyes isthemainsourceofthegenerationofaminecompoundsthatarehighly toxicandhavecarcinogenicpotential.134
Indigodyesarealsoanimportantpartofthetextileindustrythatprocessescottonfabrics.Indigodyeshaveacomplexapplicationprocedure becausetheyareinsolubleinaqueoussolutions.Nowadays,theuseofthis typeofdyehasdecreased.Indyeingprocessesthereductionofindigodyes iscarriedoutusingstrongreducingagentssuchassodiumdithionite (Na2S2O4).Inthiscasethedyeingbathscauseseriouscontaminationproblemsinoutletwaterandwashingwater.Thereducingagentsareultimately oxidizedtononrecyclablespecies,suchassulfite,sulfate,thiosulfate,and toxicsulfide,whichpollutethewastewaterfromthedyeingunits. Furthermore,dithionitemaybepresentinthewastewatertonegativelyaffect theaerobictreatmentprocessasaresultoftheexcessiveuseofreducing agentsusedtostabilizethedyeingbathsthataresensitivetooxidation reactions.135
Aciddyesareclassifiedasanionicdyes.Aciddyesareorganosulfonic acidsandcommerciallyusedspeciesaresodiumsaltswhichhavehighsolubilityinwater.Theyareusuallyusedfordyeingpolyamidefibers,wool, silk,andmodifiedacrylics.136 Ontheotherhand,basicdyesarecationic dyesandareusedforthedyeingofmaterialswhichhaveanionicgroups.In generally,aquaternaryammoniumgroupispresentinthestructureofthis typeofdyes.
Duetothecolorofthewastewatercontaminatedwithdyecompounds, thecoloredwastewaterleadstoadecreaseinthelightpermeabilityinthe waterenvironmentandnegativelyaffectsthephotosyntheticactivity.Inaddition,theaccumulationofdyesinsomeaquaticorganismscausestheformationoftoxicandcarcinogenicproducts.137 Thereforetheremovalofcolor fromwastewatercontainingdyecompoundsinthetextileindustryisgaining importance.However,dependingonthecomplexchemicalstructureandsyntheticorigin,theremovalofdyesisaverydifficultprocess.
Chemicalandbiologicaltechniquesareusedfortheremovalofindustrial dyesfromcontaminatedeffluents.Inbiologicalprocessesmicroorganisms suchasfungi,bacteria,andalgaehavebeenused.138 140
Ozonationisanefficientapproachforthedegradationofdyesinthe effluentsofthetextileindustry.141 Theconcentrationofdyecompoundsand pHarecrucialparametersthataffectthedecompositionrateofozone.AtpH valueshigherthan7.0,rapiddecompositionofozoneoccurstogenerate
OHradicalsandotherradicalgroupsinthesolution.Ozonebehavesasan elecrophileatthelowerpHvalues(pH , 7.0)anddirectlyattacksorganic compoundsinthesolution.Ozoneisextensivelyappliedfortheremovalof dyecompoundsfromthecontaminatedeffluentsintextileindustry.Itreacts withtheconjugateddoublebondspresentonthedyemoleculethatareassociatedwiththecolorofthedye.
Ontheotherhand,conventionalmaterialssuchasactivatedcarbonare widelyappliedfortheremovalofdyecompoundsfromenvironmentalsamplesduetotheirhighadsorptioncapacity.142 145 However,activatedcarbon exhibitssomedrawbackssuchasitshigh-costandregenerationproblems.
Someothermaterials,suchasmolecularlyimprintedpolymers(MIPs),146 membranes,147,148 andMIP-membranecomposites,149 werealsoappliedfor theremovalofdyepollutantsfromtheenvironmentalsamples.
1.3Sourcesandtransportofpollutants
Themaintypesofenvironmentalpollutantsthathavepotentialtoxiceffects inhumans,animals,andplantsaresoilpollutants,waterpollutants,andair pollutants.
Soilpollutantsorsoilcontaminantscaneasilypenetratesoilwhichaffects theorganismsthatliveinsoil.However,theeffectofpollutantsinsoilon bothanimalsandecosystemsismuchmoreconsiderableasthesesubstances accumulateinfoodchains.Fossilfuelsmayalsoleadtothecontamination ofsoil.Varioussourcesoffossilfuel basedsoilpollutioninvolve petrochemicalplants,refineries,andmotorvehicles.Ontheotherhand, indiscriminateuseofvariousagriculturalchemicalssuchasherbicidesand pesticidesandimproperdisposalofindustrialwastesareothercausesofsoil pollution.150
Thereleaseofsoilpollutantsintotheairorwatercanoccurbasically throughthechangesinthestatusofwatersaturationinthesoil,changesin thecompositionandchemistryofthegasandwaterphase,andthechanges inthefeaturesoftheparticle’ssurface.Therateofthepollutantreleasecan berelativelyrapid(fromminutestohours)orveryslow(years)depending onthetypeofpollutantinthesoil.151
Transportofthesoilpollutantscanalsooccurbetweenbioticandabiotic environments.152 Furthermore,transportprocessintheabioticenvironment havestrongeffectsonthedispersionofthepollutantsinsoilandincludethe migrationofpollutantswhicharedissolvedinthesolutionofsoilandwater. Windisanotherrouteforthetransportofthesoilpollutants.Thetransportoftheadsorbedpollutantsinsoilcanoccurinaerosolsoraswindblown 18 ModernEnvironmentalAnalysisTechniquesforPollutants