GLASS NANOCOMPOSITES
Serieseditor
GHENADIIKOROTCENKOV
Editedby
SANJIBBHATTACHARYA
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates ©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-817458-6
ForinformationonallElsevierpublicationsvisitour websiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: KaylaDosSantos
EditorialProjectManager: IsabellaC.Silva
ProductionProjectManager: SojanP.Pazhayattil
CoverDesigner: VickyPearson
TypesetbySPiGlobal,India
Contributors
AmartyaAcharya
CompositeMaterialsResearchLaboratory,SiliguriInstituteofTechnology,Siliguri, Darjeeling,WestBengal,India
ArunKrBar
InstituteofEngineeringandManagement,Kolkata,India
SanjibBhattacharya
EngineeringSciencesandHumanities;CompositeMaterialsResearchLaboratory,Siliguri InstituteofTechnology,Siliguri,Darjeeling,WestBengal,India
SwarupaOjha
Electronics&CommunicationEngineering,OmDayalGroupofInstitutions,Howrah, WestBengal,India
AditiSengupta
ElectronicsandCommunicationEngineering;CompositeMaterialsResearchLaboratory, SiliguriInstituteofTechnology,Siliguri,Darjeeling,WestBengal,India
Prefacetotheseries
Thefieldofsynthesis,study,andapplicationofmetaloxidesisoneofthe mostrapidlyprogressingareasofscienceandtechnology.Metaloxides areoneofthemostubiquitouscompoundgroupsonearth,whichhasalarge varietyofchemicalcompositions,atomicstructures,andcrystallineshapes. Inaddition,metaloxidesareknowntopossessuniquefunctionalitiesthatare absentorinferiorinothersolidmaterials.Inparticular,metaloxidesrepresentanassortedandappealingclassofmaterials,propertiesofwhichexhibita fullspectrumofelectronicproperties—frominsulatingtosemiconducting, metallic,andsuperconducting.Moreover,almostalltheknowneffects includingsuperconductivity,thermoelectriceffects,photoelectricaleffects, luminescence,andmagnetismcanbeobservedinmetaloxides.Therefore, metaloxideshaveemergedasanimportantclassofmultifunctionalmaterials witharichcollectionofproperties,whichhavegreatpotentialfornumerous deviceapplications.Specificpropertiesofthemetaloxides,suchasthewide varietyofmaterialswithdifferentelectrophysical,optical,andchemical characteristics,theirhighthermalandtemporalstability,andtheirability tofunctioninharshenvironments,makemetaloxidesverysuitablematerials fordesigningawidevarietyofitems.Theseincludetransparentelectrodes, high-mobilitytransistors,gassensors,actuators,acousticaltransducers, photovoltaicandphotonicdevices,photo-andheterogeneouscatalysts, solid-statecoolers,high-frequencyandmicromechanicaldevices,energy harvestingandstoragedevices,nonvolatilememories,andmanyothersin theelectronics,energy,andhealthsectors.Inthesedevices,metaloxides canbesuccessfullyusedassensingoractivelayers,substrates,electrodes,promoters,structuremodifiers,membranesandfibers.Metaloxidescanbeused asactiveorpassivecomponents.
Amongotheradvantagesofmetaloxidesarethelowfabricationcostsand robustnessinpracticalapplications.Furthermore,themetaloxidescanbe preparedinvariousforms,suchasceramics,thickfilms,andthinfilm.For thinfilmdeposition,depositiontechniquesthatarecompatiblewithstandard microelectronictechnologycanbeused.Thelastfactorisveryimportantfor large-scaleproductionbecausethemicroelectronicapproachpromoteslow costformassproduction,offersthepossibilityofmanufacturingdevicesona chip,andguaranteesgoodreproducibility.Variousmetaloxidenanostructures,includingnanowires,nanotubes,nanofibers,core-shellstructures
andhollownanostructures,alsocanbesynthesized.Asitisknown,thefield ofmetal-oxidenanostructuredmorphologies(e.g.,nanowires,nanorods, nanotubes,etc.)hasbecomeoneofthemostactiveresearchareaswithin thenanosciencecommunity.
Theabilitytocreateavarietyofmetaloxide-basedcompositesandthe abilitytosynthesizevariousmulticomponentcompoundssignificantly expandtherangeofpropertiesthatmetaloxide-basedmaterialscanhave, makingmetaloxidesbyatrulyversatilemultifunctionalmaterialforwidespreaduse.Asitisknown,smallchangesintheirchemicalcompositionand atomicstructurecanbeaccompaniedbythespectacularvariationinpropertiesandbehaviorofmetaloxides.Evennow,advancesinsynthesizingand characterizingtechniquesarerevealingnumerousnewfunctionsofmetal oxides.
Takingintoaccounttheimportanceofmetaloxidesforprogressin microelectronics,optoelectronics,photonics,energyconversion,sensor andcatalysis,alargenumberofvariousbooksdevotedtothisclassofmaterialshavebeenpublished.However,oneshouldnotethatsomebooksfrom thislistaretoogeneral,somebooksarecollectionsofvariousoriginalworks withoutanygeneralizations,andotheroneswerepublishedmanyyearsago. But,duringthepastdecade,greatprogresshasbeenmadeonthesynthesisas wellasonthestructural,physical,chemicalcharacterization,andapplication ofmetaloxidesinvariousdevices,andalargenumberofpapershavebeen publishedonmetaloxides.Inaddition,untilnowmanyimportanttopics relatedtothestudyandapplicationofmetaloxideshavenotbeendiscussed. Toremedythesituationinthisarea,wedecidedtogeneralizeandsystematizetheresultsofresearchinthisdirectionandtopublishaseriesofbooks devotedtometaloxides.
Oneshouldnotethattheproposedbookseries“MetalOxides”isthefirst onedevotedtoconsiderationofmetaloxidesonly.Webelievethatcombiningbooksonmetaloxidesinaseriescouldhelpreadersinsearchingrequired informationonthesubject.Inparticular,weplanthatthebooksfromour series,whichhaveaclearspecializationbyitscontent,willprovideinterdisciplinarydiscussionforvariousoxidematerialswithawiderangeoftopics, frommaterialsynthesisanddepositiontocharacterizations,processing,and thentodevicefabricationsandapplications.Thisbookseriesispreparedbya teamofhighlyqualifiedexperts,whichguaranteesitwillbeofahighquality.
Ihopethatourbookswillbeusefulandcomfortableinuse.Iwouldalso liketohopethatreaderswillconsiderthis“MetalOxides”bookserieslikean encyclopediaofmetaloxidesthatenablesreaderstounderstandthepresent
statusofmetaloxides,toestimatetheroleofmultifunctionalmetaloxidesin thedesignofadvanceddevices,andthenbasedonobservedknowledgeto formulatenewgoalsforfurtherresearch.
Theintendedaudienceofthepresentbookseriesisscientistsand researchers,workingorplanningtoworkinthefieldofmaterialsrelated tometaloxides,i.e.,scientistsandresearcherswhoseactivitiesarerelated toelectronics,optoelectronics,energy,catalysis,sensors,electricalengineering,ceramics,biomedicaldesigns,etc.Ibelievethatthis“MetalOxides” bookseriesalsowillbeinterestingforpracticingengineersorprojectmanagersinindustriesandnationallaboratories,whichwouldliketodesign metaloxide-baseddevices,butdon’tknowhowtodoitorhowtoselect optimalmetaloxideforspecificapplications.Withmanyreferencesto thevastresourceofrecentlypublishedliteratureonthesubject,thisbook serieswillserveasasignificantandinsightfulsourceofvaluableinformation, providingscientistsandengineerswithnewinsightsforunderstandingand improvingexistingmetaloxide-baseddevicesandfordesigningnewmetal oxide-basedmaterialswithnewandunexpectedproperties.
Ibelievethatthis“MetalOxides”bookserieswillbeveryhelpfulfor universitystudents,postdocs,andprofessors.Thestructureofthesebooks offersabasisforcoursesinthefieldofmaterialsciences,chemicalengineering,electronics,electricalengineering,optoelectronics,energytechnologies,environmentalcontrol,andmanyothers.Graduatestudentsalso couldfindthebookseriestobeveryusefulintheirresearchandunderstandingfeaturesofmetaloxidessynthesis,study,andapplicationofthismultifunctionalmaterialinvariousdevices.Wearesurethatallofthemwill findinformationusefulfortheiractivity.
Finally,Ithankallcontributingauthorsandbookeditorswhohavebeen involvedinthecreationofthesebooks.Iamthankfulthattheyagreedto participateinthisprojectandfortheireffortsinthepreparationofthese books.Withouttheirparticipation,thisprojectwouldnothavebeenpossible.IalsoexpressmygratitudetoElsevierforgivingustheopportunityto publishthisseries.IespeciallythankalleditorialteamsatElsevierfortheir patienceduringthedevelopmentofthisprojectandforencouragingus duringthevariousstagesofpreparation.
GhenadiiKorotcenkov
Fundamentalsofglasses
SanjibBhattacharya
EngineeringSciencesandHumanities,SiliguriInstituteofTechnology,Siliguri,Darjeeling,WestBengal,India
CompositeMaterialsResearchLaboratory,SiliguriInstituteofTechnology,Siliguri,Darjeeling,WestBengal, India
Abstract
Anincreasinginterestinamorphoussolidshasgrownnotonlyduetotheirvarioustechnologicalapplicationsinelectronic,electrochemical,magneticand,opticaldevices,but alsofromthepointofviewoftheircomplexityinstructure.Glassesareformedasan amorphous(noncrystalline)solidhavingshort-rangeorder,i.e.,thereisnoperiodic arrangementofitsmolecularconstituents.Themostimportantaspectofglasstransition istherelaxationprocessthatoccursasthesupercooledliquidcools.Theconfigurational changescausetherelaxationofthesupercooledliquidandbecomeincreasinglyslow withdecreasingtemperature,untilatagiventemperature(glasstransitiontemperature) thematerialbehavesasasolid.VariousstructuralinvestigationssuchasSEM,TEM,XRD, FT-IR,FESEM,etc.andopticalstudysuchasUV-visiblehavebeencarriedoutondifferent typesofglassnanocompositestoexploretheirvariousproperties.
1.1Introduction:Disorderedsolids-amorphousmaterials
Solidslackinglong-rangepositionalorderarecallednoncrystallinesolids (NCS).Customarily,anetworkisconsideredasasetofvertices(representingcentersofatoms)connectedbystrongshort-range(i.e.,covalent)bonds sothatapathofbondsexistsbetweenanytwovertices.Accordingtothis view,weaklybondedsystemsdonotpossessnetworkstructure.Networks canbecrystalline(havingtranslationalperiodicitywhichisaspecialformof positionallong-rangeorder),quasicrystalline(havinglong-rangepositional orderwithouttranslationalperiodicity)ornoncrystalline(lackinglongrangepositionalorder).Formostsolids,thecrystallinestateisthenatural onesincetheenergyoftheorderedatomicarrangementislowerthanthat ofanirregularpackingofatoms.However,whentheatomsarenotgivenan opportunitytoarrangethemselvesproperly,byinhibitingtheirmobility, amorphousmaterialsmaybeformed;anexampleisamorphouscarbon formedasadecompositionproductatlowtemperatures.Certainpolymers arecomposedofverylargeandirregularmolecules,andinsuchcases,acrystallinepackingisnoteasilyobtained.Inothercases,thesolidstatemay
https://doi.org/10.1016/B978-0-12-817458-6.00001-9
correspondtoasupercooledliquidinwhichthemoleculararrangementof theliquidstateisfrozenin;becauseofrapidcoolingandhighviscosityof liquid,crystalsmaynothavehadenoughtimetogrow,andglassymaterial results.Randomnesscanoccurinseveralforms,ofwhichtopological,spin, substitutional,andvibrationaldisordersarethemostimportant.Disorderis notauniqueproperty;itmustbecomparedtosomestandard,andthatstandardistheperfectcrystal [1,2].
Amorphousmaterialsbyitselfarenotnew;theiron-richsiliceousglassy materialsrecoveredfromthemoonbytheApollomissionaresomebillions yearsold,andmanhasbeenmanufacturingglassymaterials(principallyfrom silica)forthousandsofyears.Whatisnew,however,isthescientificstudy andtechnologicalapplicationsofamorphousmaterials,andtherehasbeenan explosionofinterestrecentlyasmorenewmaterialsareproducedinan amorphousform.Thisisobviouslyafast-movingfield.Theinterestindisorderedamorphousmaterialsisperhapstwofold.Firstly,thereisthematerialsscienceandengineeringaspect.Awidediversityofmaterialscanbe renderedamorphous;indeed,almostallmaterialscan.Thisisinsharpcontrasttotheknowledgeoftheaveragelayman,forwhomtheword“glass” signifiesonlythattransparentmaterials(madefromsilicawiththeaddition ofafewalkalioxide)whichisplacedinwindows.
Thesecondinterestinamorphousmaterialsisinthefundamentalphysics ofsuchasystem:Whyiswindow“glass”transparentwhentheconventional solid-stateexplanationofbandgapsdependcruciallyontheassumptionof periodicityintheunderlyinglattice,andhence,onthepresenceofBloch electronwavefunctions?Furthermore,amorphousmaterialsexhibitmany propertiesthatareuniquetothemandarenotsharedbycrystallinesolid atall.
Anincreasinginterestinamorphoussolidshasgrownnotonlydueto theirvarioustechnologicalapplicationsinelectronic,electrochemical,magnetic,andopticaldevices [1–6],butalsofromthepointofviewoftheircomplexityinstructure.Thestudyofamorphousmaterialsstartedmuchlater, comparedtocrystallinematerialsandinspiteofalargenumberofinvestigationsalreadymade [1,2,7,8],theglassformationabilityandphysical propertiesofthesematerialsarenotwellunderstood.Thisisbecauseof thecomplicatedtheoriesandmodelsneededtoexplainthenonperiodic potentialoftheelectronsintheamorphousmaterialsinsharpcontrastto thewell-knownbandtheoryforthecrystallinematerials [9]
Fromthetechnologicalpointofview,theadvantagesoftheamorphousmaterialsovertheircrystallinecounterpartsaremanifold.First,large
areahomogeneousthinfilmsofamorphousnatureareeasytoprepare. Currently,Sisemiconductorsareinh ugecommercialuseassolarcells, photo-sensors,flat-screendisplays,etc.Secondly,bulkglassescanbereadilyformedfromthemeltbyslowquenchingandthematerialsremain workable(i.e.,theviscosityisrelativelylow)overarangeoftemperatures. Thisparticularpropertyallowsthematerialstobeeasilyfashionedinto variousshapes,specificallydrawnintolongthinfibers,whichhasbeen thekeyissuefortherecentprogressinopticalcommunication [1,2] Moreover,asaresultoftheirstruc turalhomogeneity,thephysical propertiesoftheamorphousmaterialsareisotropic,unlikecrystalline materialsforwhichtheintrinsicbehaviorofthesinglecrystalmaybeanisotropic,andthepresenceofgrainboundariesinpolycrystallinesamples maydominatetheoverallbehavior.Furthermore,amorphousphasescan beformedinmixed-componentsystemsoverawiderangeofcompositions,whichallowstheirpropertiest ocontinuouslyvarywithcomposition [1,2] .
Thestudyofamorphousmaterialsstartedmuchlater,comparedtocrystallinematerials.Inspiteofalargenumberofinvestigationsalreadymade [8,9],theglassformationabilityandphysicalpropertiesofthesematerials arenotwellunderstood.Thisisbecauseofthecomplicatedtheoriesand modelsneededtoexplainthenonperiodicpotentialtotheelectronsin theamorphousmaterials,insharpcontrasttothewell-knownbandtheory forthecrystallinematerials [9].
1.2Whatisglass?
Oneofthemostusedmaterials,otherthanthemetalsinthehistoryof humancivilization,istheglass.Fromtheoldtothemedievalandtothe modernage,glasshaschangeditsdimensionofusagefrommirrortowindowglasstotechnologicallyadvancedsolidelectrolytes.Thedefinitionof glassgoeslike:
Aglassisanamorphoussolidwhichexhibitsaglasstransition.
S.R.Elliot [1]
Inordernottobeoverlyrestrictive,wearelefttodefineglassasa “solidwithliquid likestructure,”“anon-crystallinesolid,” orsimply “anamorphoussolid … ” Varshnyea [10]
Historicallyandcommonly,glassesaredefinedas [11]: Therigidmetastable solidproducedbyquenchingaliquidformrapidlyenoughtopreventcrystallization.
Althoughthisdefinitionisfrequentlyadopted,glassescanbepreparedat roomtemperaturewithoutmeltingby“sol-gelroute.”
Here,thequestionariseswhetheranoncrystallineoramorphoussolid madebymelt-quenchingorbyothermethodswillbecalledglass?The answerisyes,aslongasthesolidmaintainstheshort-rangeorderofitsmelt. Forexample,silicacanbemadeintoglassbymelt-cooling,bysol-gel,andby vapordepositionmethods.Allthreenoncrystallinesilicashavesimilarproperties(differencesbeingmainlyduetodifferentthermalhistoriesinvolved) andareappropriatelycalledbyasinglename:silicaglass.“Glassy”materials, therefore,neednotbepreparedsolelybyquenchingfromthemelt.Itistobe notedthatglassysolidsareasubsetofamorphousmaterialsor,toexpressitin adifferentway,allglassesareamorphous,butnotallamorphoussolidsare necessarilyglasses.
1.3Glasstransition:Thethermodynamics
Sometimes,itissaidthataglassisneitheraliquidnorasolid.Ithasadistinctlydifferentstructurewithpropertiesofbothliquidsandsolids.Itwould beconvenientifonecouldconcludethatglassymaterialschangefrombeing asupercooledliquidtoanamorphoussolidatthe“glasstransition temperature.”Thus,onecandefineglassmoregenerallyas,“Glassesare amorphousmaterials,whichexhibitglass-transitionphenomenon.”Then aquestionarises [11],“Whatistheglasstransitionphenomenon?”
Theglasstransitionisaphenomenoninwhichasolidamorphousphase exhibitsamoreorlessabruptchangeinderivativethermodynamicproperties(suchasspecificheat,thermalexpansioncoefficient,etc.)from“crystallike”to“liquid-like”valueswiththechangeintemperature.Thisdefinition hasanadvantage:thetermglassyisconfinedtothosematerialsthatcanbe obtainedinareproduciblestate,sincethematerialscanbeinastateofinternalequilibriumabovetheglasstransition.Thesechangescanbeobserved readilybymonitoringthevolumeasafunctionoftemperature(usingadilatometer).Atypicalresultisshownin Fig.1.1.Itcanbeobservedthatthe liquid $ crystaltransformationischaracterizedbyanabruptchangeofslope involumeatthemeltingorfreezingtemperature(Tf).
Ontheotherhand,theliquid $ glasstransformationexhibitsagradual breakinslope,andtheregionoverwhichsuchchangeofslopeoccursis termedas“glasstransitiontemperature” Tg.Astheglasstransitiontemperatureisnotwelldefined,anothertemperaturecalledthefictivetemperature,
Fig.1.1 Schematicillustrationofthetwocoolingpathsbywhichliquidmaysolidify. Averyslowcoolingrateleadstoadiscontinuouschangeinvolumetoacrystalstate (curve1).Rapidquenchleadstoacontinuouschangeinvolume(curve2).
whichisobtainedbytheintersectionoftheextrapolatedliquidandglass curves,isdefined.
Thenatureofglasstransitionisverycomplexandisevennowpoorly understood.Manyattemptshavebeenmadetowardsitsunderstanding. Duringglasstransition,boththespecificheatandthermalexpansioncoefficientschangeinanarrowtemperaturerangefromalowvaluecharacteristic ofcrystaltoahighvaluecharacteristicofliquid.Thus,fromthethermodynamicaspectsofglasstransition,thisbehaviorisveryclosetothatexpected forasecondorderphasetransition.Itisworthwhiletomentionthatasecond orderphasetransitioninvolvesadiscontinuityinthespecificheat,heat capacity,etc.,whichisthesecondorderoftheGibbsfunction.However, thesechangesforglassesarenotassharpastheyshouldbeinatruesecond orderphasetransition,butinstead,arediffuseoccurringoverasmalltemperatureintervalratherthanatasharplydefinedtemperature.Thisisalso thecaseforotherthermodynamicalvariablessuchasentropyandenthalpy. Thisimpliesthatat Tg,thereshouldbeadiscontinuityinderivativevariables,suchascoefficientofthermalexpansion
)P,com-
Thekineticaspectsoftheglasstransitionarealsoimportant.Theglass transitiontemperaturedependsoncoolingrate.Thisinfluenceofthecoolingrateonglasstransitionistheclearestproofthattheglasstransitiondiffers fromastrictthermodynamictransition.Thedependenceoftheglass
transitiontemperature,understoodintermsofinterplaybetweenthetime scaleoftheexperimentandthekineticormolecularrecovery,isthemain manifestationofthekineticdimensionoftheglasstransition.However, thereareotheraspects,whichbearmateriallyonthequestionofanunderlyingthermodynamicphasetransition.
Themostimportantaspectofglasstransitionistherelaxationprocessthat occursasthesupercooledliquidcools.Theconfigurationalchangescause therelaxationofthesupercooledliquidandbecomeincreasinglyslowwith decreasingtemperature,untilatagiventemperature(glasstransitiontemperature),thematerialbehavesasasolid.Ifthetimeofobservationislonger thanthestructuralrelaxationtime,thematerialappearsliquid-like,while fortimeofobservationshorterthanstructuralrelaxationtime,thematerial behavesasasolid.Atransitiontakesplaceifthevaluesofliquid-likeparametersdiffersignificantlyfromsolid-likeones.Thusaglasstransitionoccurs, whenthetimeofobservationisequaltothestructuralrelaxationtime.For temperaturesbelowglass-transitiontemperature,thestructuretendsto approachtheequilibriumstateofthesupercooledliquid.Thisprocesscan occurintimesoftheorderofminutesfortemperaturenearglasstransition temperature,butmaytakeyearsfortemperaturefarbelowglasstransition temperature.
Anothertheoryconcernedwithcertainaspectsofglasstransitionthat havemanysimilaritieswithaspectsofrelaxationtheoryisthefreevolume theory.Inthistheory,totalvolumeofaliquidissupposedtobedividedinto twoparts:onepartisoccupiedbytheatomsormoleculesandtheotherpart providesfreespaceforthemtomove.Thelattervolumepermittingdiffusive motionistermedfreevolume.Asthetemperatureofaliquidislowered, boththeoccupiedvolumeandthefreevolumeareexpectedtocontract. Theglasstransitionoccurswhenthefreevolumeofthesupercooledstate decreasesbelowsomecriticalvalue.Theredistributionoffreevolumeno longeroccurs;i.e.,thefreevolumeisfrozeninthelocationswhentheglass isformed.Whenpercolationaspectsaretakenintoconsideration,thefree volumetheorypredictstheglasstransitiontobemorelikelyafirstorder transitionincontrasttothermodynamictheory.
1.4Glassnanocomposites
Wheninacompositeoftwoormorephases,atleastonephaseisoftheorder ofnanometer(10 9 m)dimension,andthecompositematerialiscalleda nanocomposite [12].Theprecipitationofmetalsortheformationofclusters
inaglassmatrixgivesbirthtoglassnanocomposites.Thecompositecontainingnanoclustersshowsdifferentphysicalpropertyfromthoseoffreeatoms andbulksolidshavingsimilarchemicalcomposition.Behaviorofnanocompositesissometimesdominatedbyinterphasicinteractionandsometimesby thequantumeffectassociatedwiththenanostructure.Nanostructuredmaterialsaregivenconsiderableattentionbecauseoftheirnovelphysicalpropertiesexhibitedbymatterhavingnano-dimensionalstructure [12].Oxide superionicglasseshavearandomnetworkstructurewithphysicalvoidspaces and,therefore,canbeexploitedasnano-templatesinwhichdistributed nano-structuredparticlescanbegenerated.Thesetypesofoxideglassescontainingthedistributionofnanoparticlesornanoclustersaredesignatedas glassnanocomposites.Theresultantglassnanocompositesexhibitadifferenceintheelectricalconductivityandactivationenergyaswellasmechanicalbehaviorlikemicro-hardnessfromthatofthehostglassmatrix.
1.5Glassfamilies:Proper tiesandcompositions
Theunderstandingofthestructureandthetransportpropertiesofglassand glassnanocompositesrequiretherecognitionofthefollowingaspects:
(a) Physicalstructure,whichdescribesthearrangementofatomswith respecttoeachother.
(b) Mechanicalpropertieslikemicro-hardness,whichisveryimportantin creatinghighlyreliableelectricdevicesoperatinginsevereenvironmentalconditions [13]
(c) Chemicalstructuredescribesthenatureofbonding(covalent,ionic, etc.)betweenthreedifferentspecies(twocationsandoxygenanion).
(d) Bondingenergystructure,whichdescribesthestrengthof variousbonds.
(e) Electricalpropertiesi.e.,conductivity,current-voltagecharacteristics, etc.
1.5.1Molybdateglassesandglassnanocomposites
Thestructureofmolybdateglassesisconstructedfromseveralasymmetric units,mainlyMoO42 tetrahedral,andMo2O72 ions [14].Mostoftheglasses andglassnanocompositescontainingMoO3,exhibitsabsorptionpeaks875, 780,and320cm 1 (ν1, ν2 and ν3 modesofMoO42 tetrahedralions)which areconfirmedfromtheFouriertransforminfrared(FT-IR)study [14,15]. Kawamuraetal. [16] showedthattheprogressivechangeofactivation energyobservedintheAgIdopedmolybdateglassescouldbeattributed
totheorder-disordertransitioninthe α-AgIcrystal.Theionicconductivityoftheseglassesoccurredduetothecooperativeliquid-likemotionof themobileions,andthenetworkstructureofglassesprobablycausedthe non-ArrheniusbehaviorintherapidlyquenchedAgI-Ag 2O-MoO 3 glasses.
Eckertetal. [17] demonstratedthelocalstructuresofmolybdenumspeciesintheglassesofthesystemAgI-Ag2O-MoO3 usingnearinfraredFouriertransform(NIR-FT)Ramanspectroscopy.Theyshowedthatinglasses withtheAg2O/MoO3 ratioofunity,themolybdenumspecieswerepresent onlyastetrahedralmonomericorthomolybdateions,MoO42 .Onthe otherhand,intheglasseswithAg2O/MoO3 molarratioslessthanunity, molybdenumspecieswerepresentastetrahedralorthomolybdateanions, MoO42 .ThepreponderanceofevidencefromNMRandvibrationalspectroscopysuggeststhatthisunitcontainslinkedMoO4 tetrahedraandMoO6 octahedra.Theyalsoshowedthatthestructureoftheseunitswasprobably similartothechainionspresentincrystallineNa2Mo2O7.Minami andTanaka [14] showedthatglasseswithmolarratioAg2O/MoO3 ¼ 1 containednocondensedmacroanions,butonlydiscreteAg+,I and MoO42 .Intheirmodel,onlyapartofthesilverionswerebelievedtoparticipateintheconductionprocess.
Arecentreport [18] onelectricalpropertiesofsemiconductingtellurium molybdateglassesadequatelyexplainedthesmallpolarontheory.Thisreport alsoshowedthattheglass-formingoxidegreatlyaffectedthemagnitudeof theconductivityandtheactivationenergyforhoppingconduction.
IonicallyconductingglassesandglassnanocompositescontainingMoO3 haveattractedmuchattentionbecauseoftheirpotentialapplicationinmany electrochemicaldevices,suchassolidstatebatteries,electro-chromicdisplays,andchemicalsensors [19].Inparticular,silverionconductingglasses areatthefocusofcurrentinterestbecauseoftheirhighstabilityagainst humidityandtheirhighelectricalconductivityintherangeof10 1 S/cm atroomtemperature.GlassesinthesystemAgI-Ag2O-MoO3,firstreported byMinami [14,15],belongtothisgroupofmaterials,andtheirglassformingregions,electricalproperties,glass-transitiontemperatures,and localstructureshavebeenexaminedextensively [14,15,19–22]
Theglass-formingregionofthesuperionicsystemcontainingMoO3 [17] isshownin Fig.1.4.Inparticular,thestructureofglasseswithcompositionsonthetielineAgI-Ag2MoO4 havebeeninvestigatedbymany researchers,usingIR [14,15,23],Raman [24],EXAFS [25],andneutron diffraction [26].Whilemanystudiesagreethatthemolybdenumspecies
existastetrahedralortho-molybdateanionMoO42 intheseglasses [14,15, 24,25],otherreportshaveclaimedoctahedralmolybdenumenvironment [26,27].
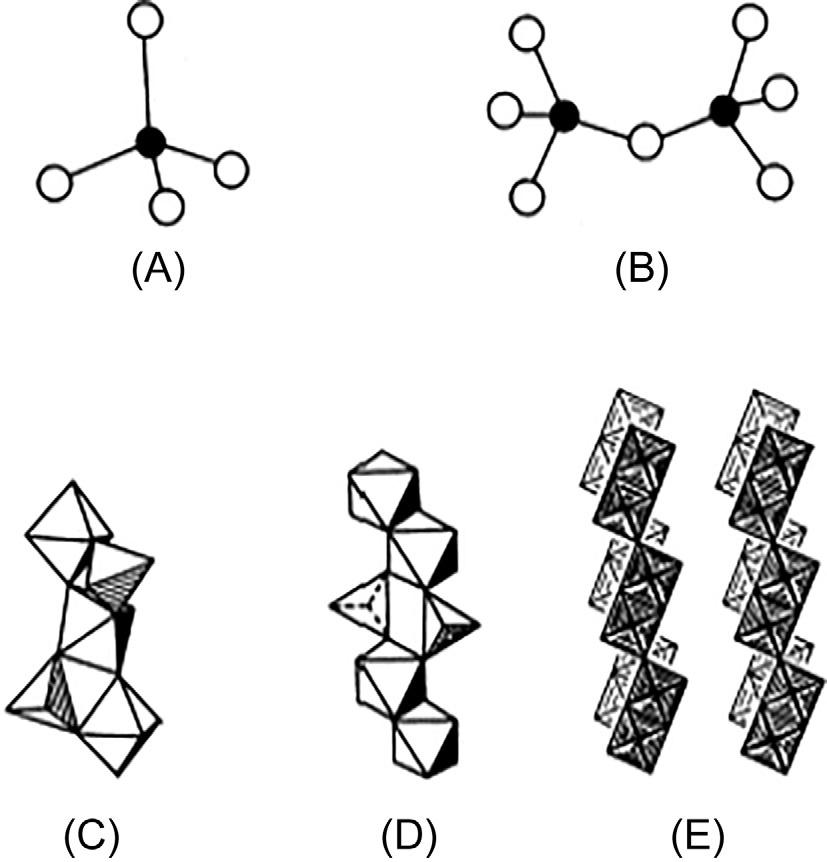
Fig.1.2 summarizestypicalstructuralfeaturesincrystalswiththedimolybdatestoichiometry.Comparativelyfewstudieshavebeencarriedouton glasseswithcompositionratioAg2O/MoO3 < 1.Intheseglasses,twoadditionalinfraredabsorptionbandshavebeenobservedat600and450cm 1 [14,15].ThestructureofcrystallineNa2Mo2O7 [31] isbasedoninfinite chainsformedbyMoO4 tetrahedraandMoO6 octahedra [29] asshown in Fig.1.3.AninterpretationoftheIRspectrahasbeengivenbyCaillet etal. [32].Inspectionofthecrystalstructuresofothercrystallinemolybdates basedonMo2O72 anionicunitsrevealsasubstantialstructuralvariety.InfinitechainsofinterlinkedMoO4 andMoO6 unitsalsoexistinthecompoundsK2Mo2O7 [30] and(NH4)2Mo2O7 [33].Discretedimeric bitetrahedralMo2O72 anions(Fig.1.3B)areknowntobepresentin (n-Bu4N)2Mo2O7 [34],(PPN)2Mo2O7 (PPN][Ph3P]N]PPh3]+) [35], MgMo2O7 [36],K2Mo2O7-KBrdoublesalt [37],K2Mo2O7 melt [38], and(n-Bu4N)2Mo2O7–CH3CNsolution [34].Thecrystalstructureof Ag2Mo2O7 consistsofinfinitechainsformedbyblocksoffouredge-shared MoO6 octahedrajoinedbyedge-sharing [28] (Fig.1.3E).Inprinciple,allof thesearrangementsprovidepossibleexplanationsfortheextraIRbands observedinglasseswithAg2O/MoO3 ratios <1.
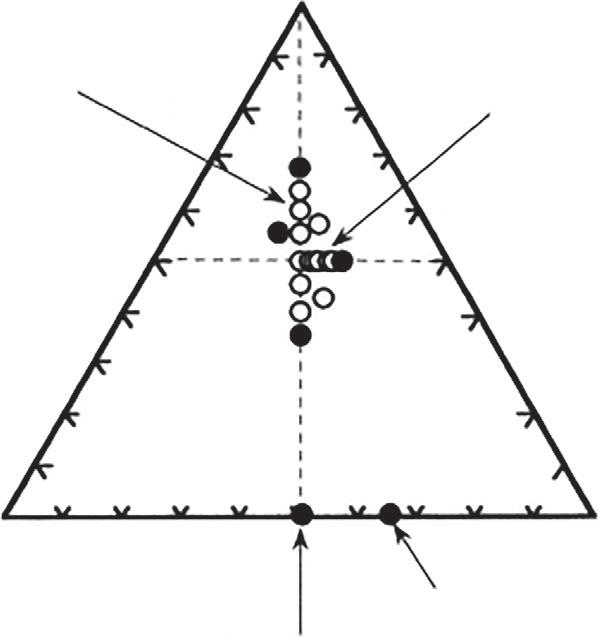
Fig.1.2 Glass-formingregionandsamplesinvestigatedinthesystemAgI-Ag2O-MoO3; ( )glassy(•)crystallinesamples.
Fig.1.3 Idealizeddrawingofsomemolybdatespeciesexistinginvariousdimolybdate crystals;(A)tetrahedralmonomericortho-molybdateionMoO42 ,(B)tetrahedral dimericanionMo2O72 (in(n-Bu4N)2 Mo2O7),(C)infinitechainstructurein Na2Mo2O7,(D)K2Mo2O7 and(NH4)2Mo2O7,and(E)Ag2Mo2O7 [28–30]
AgIdopedsilvermolybdateglassesandtheirnanocompositesareparticularlyinterestingbecauseofthegrowingevidenceofanomalousinthestructureaswellastheintensivepropertieswhencomparedwithsilverborateand silverphosphateglasses [28,39].Experimentally,considerableeffortshave beenmadetoestablisharelationbetweenthemicroscopicstructureandfast ionconductivityinglassesandglassnanocomposites.
1.5.2Seleniteglassesandglassnanocomposites
TheideaofsynthesizingseleniteglassesbelongstoRawson [40] andStanworth [41] whoobtainedglassesintheK2O-SeO2 andSeO2-TeO2-PbO systems.Dimitrievetal. [42] obtainedstablehomogeneousglasseswithhigh contentofSeO2 incombinationwithothernontraditionalnetworkformers: V2O5,TeO2,Bi2O3.FromtheIRspectra,theyshowedthattheindependentSeO3 pyramids ν s ¼ 860–810cm 1 and ν d ¼ 720–710cm 1 participatedinthenetworkwhentheSeO2 concentrationwaslow.Asthe SeO2 contentwasincreased,SeO3 groupsbecameassociatedintochainsthat containisolatedSe]Obondswithavibrationfrequencyat900–880cm 1
Satyanarayanaetal. [43] studiedthedifferentialscanningcalorimetry (DSC)fortheAgI-Ag2O-SeO2-V2O5 glasses.Theyobservedadecrease in Tg withtheincreaseinAgIcontent.Theysuggestedthatalargernumber ofbondsweredestroyedwithintheglassynetworkinordertoallowitsrearrangementtoformamoreopentypethermodynamicallystablephase.
Venkateswarluetal. [44] showedthatthedcconductivityofAgI-Ag2OSeO2-V2O5 systemincreaseswiththeAgIcontentandexhibitedhighest conductivity(σ ¼ 2.63 10 2 Ω 1 cm 1)forthe66.67%AgI-23.07% Ag2O-10.26%(0.8SeO2 +0.2V2O5)glasssystem;withafurtherincrease ofAgIcontent,theconductivitywasfoundtodecrease.Thistypeofdecrementofconductivitywasexplainedfromtheirstructuralbehavior.
1.6Characterizationtechniquesforglassmicrostructure 1.6.1X-raydiffraction
Toensurethenatureofthepreparedsamples,X-raydiffraction(XRD)was carriedoutonthepowderedglasssamplesusingaRich-SeifertX-raydiffractometer(model3000P)forrecordingthediffractiontraces(2θ vsintensity)ofthepowderedsamples.Inthisinstrument,NifilteredCuKα radiation operatingat35kVand25mAinastepscanmodewasused.Thestepsize wastakentobeof0.02° in2θ andaholdtimeof2secondperstep.The hardwareofXRD3000systemsiscomprisedofthegeneratorID3000, themonitor,andaccessorycontrollerC3000andthetimer/counterhardware.Thediffractiontraceswererecordedatroomtemperature.Fromthe diffractionpeaksoftheXRDpattern,theaverageparticlesizeofdifferent nanoparticleswasdeterminedusingtheDebye-Schererformula [1]
where t denotestheaveragegrainsizeoftheparticles, λ standsfortheX-ray wavelength(1.54A ˚ ), θ fortheBraggdiffractionangleand β forthepeak widthinradiansathalf-height.
CrystallitesizeandthelatticestrainofthecrystalcanbeevaluatedseparatelyfromXRDstudybyHall’sequation [2],
where β hkl isthefullwidthhalfmaximumofagiven(hkl)diffractionpeak, λ isthewavelengthoftheX-ray, D isthecrystallitesize, η isthemeasureofthe heterogeneouslatticestrain, θ istheBraggangle,and K isaconstantof0.9. Fromtheplotsbetween β hkl cos θ /λ andsin θ /λ forthemajor(hkl)
diffractionpeaksofdifferentnanocrystalsinthesamples,thevaluesof β hkl weredeterminedfromtheGaussianfunctionwhichwasfittedtothemajor diffractionpeaks.Thevaluesof η,themeasureoflatticestrain,wasobtained fromtheslopeoftheplots.
1.6.2Fieldemissionscanningelectronmicroscopy(FE-SEM) andenergy-dispersiveX-rayspectroscopy
Toexplorethemicrostructureandsurfacemorphologyoftheprepared glassesandglassnanocomposites,fieldemissionscanningelectronmicrographs(FE-SEM)ofthepolishedsurfacesofthesamplesweretakeninafield emissionscanningelectronmicroscope(JEOLJSM-6700F).Athinplatinumcoating( 150A ˚ )wasdepositedonthepolishedsurfacesofthesamples byvacuumevaporationtechniqueforaconductinglayer.Thequantitative investigationofthefinalcompositionshasbeendonefromenergy-dispersive X-rayspectroscopy(EDS)studyofthecorrespondingFE-SEMimage.
1.6.3Transmissionelectronmicroscopy(TEM)
Transmissionelectronmicrocopy(TEM)(JEOLJEM2010)isapowerful anduniquetechniqueforcharacterizationofthemicrostructureofthepreparedglassnanocomposites.Byformingananometersizeelectronprobe, TEMisuniqueinidentifyingandquantifyingthechemicalandelectronic structuresofindividualnanocrystals.Thepowdersamplewassonicatedfor 15mininacetonemediumtoformveryfinesuspendedparticlesinacetone medium.Bypouringaverysmalldropletofthatcolloidalsolutionhaving fineparticlesontocarboncoatedCu-grids(400mesh),grid-preparation wasmadefortransmissionelectronmicroscopy.Thefocusedbeamofelectronisallowedtopassthroughthesamplesintheveryhighvacuumchamber.Thefinaldatawererecordedusingachargecoupleddevice(CCD). Brightanddarkfieldimagesandselectedareaelectrondiffraction(SAED) patternofthesamplesweretaken.Theinterplannerspacing(dhkl)wasfound fromtheSAEDpatternsandalsofromthehighresolutiontransmissionelectronmicroscopy(HR-TEM).
1.6.4Differentialscanningcalorimetry
Todeterminethethermodynamicproperties,suchasglasstransitiontemperature(Tg)andcrystallizationtemperature(Tc),DSCofthepoweredglass samplesisperformedinaPerkin-Elmerdifferentialscanningcalorimeter (DSC7).Itoperatesinthetemperaturerangefrom 150°Cto500°Cin
nitrogenatmosphere.Inthisprocess,finepowersofthesamplearetakenin analuminumpan.PureAl2O3 inothercrucibleisusedasareference.The glasstransitiontemperatures(Tg)andthecrystallizationtemperatures(Tc) weredeterminedfromtheendothermicshoulderandexothermicpeak.
1.6.5FT-IRspectroscopy
TheFT-IRspectraofthepowderedsamplesinKBrmatricesintransmission modewererecordedinaNicolateFT-IRspectrophotometer(Magna IR-750,SeriesII)inthewavenumberrangeof400–4000cm 1 atatemperature25°Candhumidityat50%–60%.Eachbondinginthesamplehasa characteristicfrequencyofvibration.WhenIRistransmittingthroughthe sample,thefrequencyofthebondingofthesampleisexactlymatchedwith theparticularfrequencyoftheIRregion;thensomepeaksarefounddueto resonanceoffrequency.
1.6.6Densityandmolarvolume
Thedensitiesofthepreparedglassandglassnanocompositesampleswere measuredbyArchimedes’sprincipleusingacetoneasanimmersionliquid. Molarvolumeofasubstanceisthevolumeofonemoleofthatsubstance. Molarvolumeofasubstanceisdefinedastheratioofitsmolecularoratomic weight,whicheverissuitabletoitsdensity.Therelationshipbetweendensityandcompositionofanoxideglasssystemcanbeexpressedintermsofan apparentmolarvolumeofoxygen(VM)fortheglasssystem,whichcanbe obtainedusingtheformula
where xi isthemolarfractionand Mi isthemolecularweightofthe ith component.
1.6.7Magneticsusceptibilityofsemiconductingsamples
ThemagneticsusceptibilityofthesemiconductingsamplesatroomtemperaturewasdeterminedusingaEG&GParc(Princeton,NJ)vibratingsample magnetometer(model155).Theelectronicconcentrationwasobtained fromthefollowingrelation
where χ istheparamagneticsusceptibility, Ig istheintensityofmagnetization, Hg istheappliedmagneticfield, M isthemolecularweightofthe
sampleunderconsideration, N istheelectronicconcentration, μBpeff is theeffectivemagneticmomentinBMunit,and R istheuniversalgas constant.
1.7Correlationbetweenstructureandproperties
1.7.1XRDstudy
1.7.1.1AgI-Ag2O-CuOglassnanocomposites
TheXRDpatternsofsamplesI,J,andKarepresentedin Fig.1.4.Itis observedthat(002),(110),and(112)peaksareobserveddueto α-AgInanocrystals [21].Itisalsonotedin Fig.1.3 thatatlowerangles(111)peaksappear duetounstableAgInanoparticles [21,22] insamplesIandJrespectively.But thesepeaksareabsentinsamplesK.Newpeak(202)duetoCuOnanoparticles [21] appearsatahigherangleonlyforsampleKasshownin Fig.1.3 Theinterplanerspacingsbetweentwosuccessiveplanes(d-values)havebeen computedfromXRDanalysis.These d-valuesarealsoinagreementwith thosereceivedfromtheASTMdatasheet [21,22].Thecrystallitesizesof AgInanoparticles, α-AgInanocrystals,andCuOnanoparticlesdispersed inas-preparedglassnanocompositeshavebeendeterminedfromDebyeSchererformula [1] t ¼ 0.89λ/(β cos θ ),where t denotestheaveragegrain sizeoftheparticles, λ standsfortheX-raywavelength(1.54A ˚ ), θ forthe Braggdiffractionangle,and β forthepeakwidthinradiansathalf-height. Thecrystallitesizesofthemarepresentedin Table1.1
1.7.2Transmissionelectronmicroscopy(TEM)study
1.7.2.1Copper-molybdateglassnanocomposites
Thetransmissionelectronmicrographs(TEM)foraparticularsamplewith x ¼ 0.3displaysthedistributionoffrozenCuMoO4 nanoparticlesatroom temperature.TheCuMoO4 nanoparticleshavinggrainsize 18nm dependinguponcompositionisdispersedintheglassmatrix.Itisalso observedthatatlowerCuIcontent,thedensityofdistributionofthe CuMoO4 nanoparticlesisfoundtoincrease.AstheCuIcontentisincreased, thedensityofdistributionofCuMoO4 nanoparticlesisfoundtodecrease. Thecalculated d valuescorrespondingtoCuMoO4 nanoparticlesarein agreementwiththosevaluesobtainedfromASTMdatasheet [16].The decreaseofdistributionofthesizeofCuMoO4 nanoparticlesintheregion 0.1 x 0.5mayberelatedtothesolubilitylimitofglassformationandis yettobeunderstood.
Fig.1.4 X-raydiffractogramsof(A)0.4AgI-0.3Ag2O-0.3CuO(sampleI),(B)0.5AgI0.25Ag2O-0.25CuO(sampleJ)and(C)0.5AgI-0.25Ag2O-0.175CuO-0.075MoO3 (sampleK)glassnanocomposites.
Table1.1 CrystallitesizecalculatedfromXRDforsamplesI,J,andK,respectively.
Sample
Crystallitesize(nm) (α-AgInanocrystals)
Crystallitesize(nm) (AgI-nanocrystal)
Crystallitesize(nm) (CuOnanoparticles)