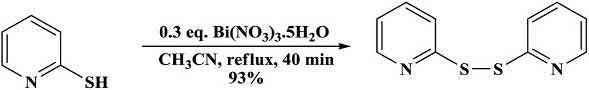Metal- and N,onmetalAssisted Synthesis of Six-Membered Heterocycles
DR. NiA,VJEET MUiR
Department of Qleminry. Bananllall Vidyapith, Banasthali, Rajastnan, IndEa
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyright LicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby thePublisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professionalpractices, ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatter ofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-820282-1
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: AnnekaHess
EditorialProjectManager: LizHeijkoop
ProductionProjectManager: VigneshTamil
CoverDesigner: ChristianBilbow
TypesetbyMPSLimited,Chennai,India
1.Six-membered N-heterocycles1
1.1 Introduction1
1.2 Metal-andnonmetal-assistedsynthesisofsix-membered N-heterocycles1 References48
2.Six-memberedfused N-heterocycles65
2.1 Introduction65
2.2
Metal-andnonmetal-assistedsynthesisofsix-membered N-heterocyclesfusedwithotherheterocycles66 References108
3.Six-memberedfused N-polyheterocycles121
3.1 Introduction121
3.2 Metal-andnonmetal-assistedsynthesisofsix-membered N-polyheterocyclesfusedwithotherheterocycles121 References169
4.Six-membered N,N-heterocycles183
4.1 Introduction183
4.2 Metal-andnonmetal-assistedsynthesisofsix-membered heterocycleswithtwonitrogenatoms183 References229
5.Six-membered N,N-polyheterocycles243
5.1 Introduction243
5.2 Metal-andnonmetal-assistedsynthesisofsix-membered polyheterocycleswithtwonitrogenatoms244 References284
6.Six-membered O-heterocycles295
6.1 Introduction295 v
6.2 Metal-andnonmetal-assistedsynthesisofsix-membered oxygencontainingheterocycles296 References338
7.Six-membered O,O-heterocycles351
7.1 Introduction351
7.2 Metal-andnonmetal-assistedsynthesisofsix-membered oxygen-containingpolyheterocycles352 References398
8.Six-membered O,N-heterocycles413
8.1 Introduction413
8.2 Metal-andnonmetal-assistedsynthesisofsix-membered O,N-heterocycles414 References447
9.Six-membered S-heterocycles459
9.1 Introduction459
9.2 Metal-andnonmetal-assistedsynthesisofsix-membered heterocycleswithsulfurheteroatom460 References491
Index 505
Abouttheauthor

Dr.NavjeetKaur wasborninPunjab,India. ShereceivedherBScfromPunjabUniversity, Chandigarh(Punjab,India)in2008.In2010she completedherMScinchemistryfromBanasthali Vidyapith.ShewasawardedwithherPhDin2014 bythesameuniversity,underthesupervisionof Prof.D.Kishore.Presently,sheisworkingasan assistantprofessorintheDepartmentofChemistry, BanasthaliVidyapithandhasenteredintoaspecializedresearchcareerfocusedonthesynthesisof 1,4-benzodiazepine-basedheterocycliccompounds(OrganicSynthetic andMedicinalChemistry).With9yearsofteachingexperience,shehas publishedover150scientificresearchpapers,reviewarticles,bookchapters,andmonographsinthefieldoforganicsynthesisinnationaland internationalreputedjournals.Shehaspublishedtwobooks, “Palladium AssistedSynthesisofHeterocycles” and “MetalsandNon-metals:Five-Membered N-HeterocycleSynthesis” withCRCPress,Taylor&FrancisGroup.She waspresentedwiththeProf.G.L.TelesaraAwardin2011byIndian CouncilofChemists(Agra,UttarPradesh)atOsmaniaUniversity (Hyderabad),andtheBestPaperPresentationAwardinNational Conferenceon “EmergingTrendsinChemicalandPharmaceutical Sciences” (BanasthaliVidyapith,Rajasthan).Shehasattendedabout 40conferences,workshops,andseminars.Apartfromallthese,shehas beenworkingasNSSProgramOfficersince2016,memberofUBA (UnnatBharatAbhiyan)since2018andhasdeliverednumerousradiotalks.
Dr.Navjeet findsinterestinSikhliteratureandhascompleteda2-year SikhMissionarycoursefromSikhMissionaryCollege(Ludhiana,Punjab).
Dr.NavjeetKaur iscurrentlyguiding5researchscholars-MeenuDevi, YaminiVerma,PoojaGrewal,PranshuBhardwaj,andNehaAhlawat-as theirPhDsupervisor.
Preface
Awidevarietyofbiologicalactivitiesareexhibitedbynitrogen-,oxygen-, andsulfur-containingheterocyclesandrecentlymanyreportshaveappeared forthesynthesisoftheseheterocycles.Thesynthesisofheterocycleswiththe helpofmetalsandnonmetalshasbecomeahighlyrewardingandimportant methodinorganicsynthesis.Newstrategieshavebeendevelopedforthe preparationofheterocyclesinrecentdecades.
Thelargestclassicaldivisionsoforganicchemistryareconstitutedby heterocycles.Heterocyclesareofimmenseimportancebiologically,industrially,andforthefunctioningofhumansociety.Heterocyclesarepresent inmanypharmaceuticallyactivecompoundsandnaturalproducts. Transitionmetal-catalyzedreactionsarethemostattractiveprotocols amonganumberofnewsyntheticmethodologiesbecausemultiplesubstitutedmoleculesareconstructeddirectlyundermildconditionsfromeasily availablestartingsubstrates.Thedevelopmentofnewertransformations forheterocyclesynthesesusingatomicallyeconomicalandefficientpathwaysisapopularresearchareacurrently.Thereisaneedforthedevelopmentofarapid,efficient,andversatilestrategyforthesynthesisof heterocyclicrings.Metal-andnonmetal-involvingmethodshavegained prominencebecausetraditionalconditionshavedisadvantagessuchaslong reactiontimes,harshconditions,andlimitedsubstratechoices.
Heterocycleshavebeensynthesizedunderconventionalandtraditional conditionsforuseinindustry,becausethesereactionsarerobust,reliable, andeconomicallyeffective.However,thesereactionsalsocreatewaste by-products.Manyefficientstrategieshavebeendevelopedforthesynthesisofheterocycles,howeverthesearchforimprovedmethodshas continuedunabated.Oneofthemajorresearchendeavorsinsynthetic organicchemistryisthedevelopmentofnewsyntheticprotocolstoward heterocycles,aimingatachievingbetterfunctionalgroupcompatibilities andgreaterlevelsofmolecularcomplexityinatomicallyeconomicaland convergentwaysundermildreactionconditionsusingeasilyavailable startingsubstrates.
Thesynthesisofheterocycliccompoundsinthepresenceofmetaland nonmetalcomplexeshasbecomeincreasinglycommonbecauseametalcatalyzedreactioncanformcomplicatedmoleculesdirectlyfromeasily
x Preface
availablestartingmaterialsundermildconditions.Inmetal-andnonmetalassistedtools,smallunreactivemolecules,suchasCO2,CO,orethylene, havebeenefficientlyutilizedforthesynthesisofheterocycles.Despitethesignificantprogressthathasbeenmadewithmetalandnonmetalchemistryof heterocycles,thereremainsahighdemandforefficient,general,andsustainablestrategiesfortheconstructionofthesemolecules.Inthisbook,the authorhasfocusedontheutilizationofmetalsandnonmetalsforthesynthesis ofseveralsix-memberedheterocycliccompounds.
Six-membered N-heterocycles
1.1Introduction
Heterocyclesandimplicitnitrogen-containingheterocyclesarebecoming veryimportantinallaspectsofpureandappliedchemistry [1a,b]. Developmentofnewsyntheticmethodology,isolationfromnatural sources,findingmodernapplicationsofvariousheterocyclesinthepharmaceuticalfield,inindustry,chemistry,ormedicinearethesubjects intensivelystudiedbychemists,biologists,andresearchers [2a,b]. Heterocyclesarenotonlyimportantduetotheirabundanceinorganic chemistrybuttheyarealsoveryimportantfortheirchemical,biological, andtechnologicalapplications [3].Formanydecades, N-heterocycles havebeenusedasmedicinalcompounds,andformthebasisforvarious drugslikemorphine(analgesic),captopril(hypertension),andvincristine (cancerchemotherapy) [4a i,5a h]
Thesix-memberedheterocycliccompoundsarepharmaceutical actives.Six-memberedheterocycleslikesubstitutedpyridinespossessa widerangeofpharmacologicalactivity.Theyareusedtomodulateanginapectoris,antidiabetic,hypertension,antitumor,actasCa2 1 channel blockers,andheptaprotectiveproperties.Inaddition,pyridinederivatives arealsousedasorganicbasesandorganocatalystsinorganicsynthesis.The fusedquinolinefunctionalityisalsopresentinanumberofnaturally occurringandbiologicallyactivecompounds [6a h,7a g,8a,b,9a,b,10a, b,11a,b,12a,b,13a d,14a e,15a,b,16].
1.2Metal-andnonmetal-assistedsynthesisofsixmembered N-heterocycles
1.2.1Aluminum-assistedsynthesis
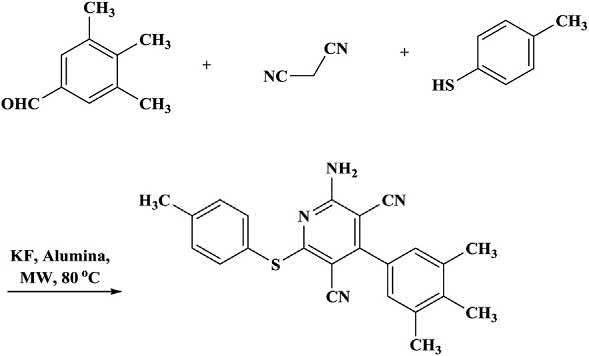
Amulticomponentreactionofmalononitrile,aromaticaldehydes,and thiophenolswasusedfortheone-potsynthesisofhighlyfunctionalized pyridines(Scheme1.1) [17 21].Thereactionwascatalyzedwithpotassiumfluorideimpregnatedalumina.Goodyieldswereobtainedinthe
Metal-andNonmetal-AssistedSynthesisofSix-MemberedHeterocycles DOI: https://doi.org/10.1016/B978-0-12-820282-1.00001-4
2 Metal-andNonmetal-AssistedSynthesisofSix-MemberedHeterocycles
caseofbenzaldehydes,astheyhavebothelectron-withdrawingand electron-donatingsubstituents.Itwasreportedthatheterocyclicoraliphaticaldehydesdidnotundergoareactionwithsatisfactoryyields.For comparison,thereactionswerealsoperformedinethanolusinganoil bathunderconventionallyheatedrefluxconditions.Themicrowaveassistedreactionsconsistentlyprovidedbetterresults(62% 93%yields) whencomparedtothereactionsunderconventionalrefluxconditionsin ethanol(56% 82%yields) [22].
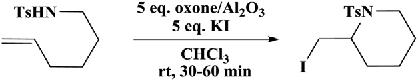
The N-tosyliodopiperidineswereobtainedingoodyieldsbyiodocyclizationofunsaturatedtosylamideswithoxoneoxidationinpotassium iodide.Asimple,newmethodwasdevelopedforthetransformationof alcoholstotosylamides(Scheme1.2) [23].
Afterthecyclizationofsubstratesbearingaprenylenefunctionality, thefateofanalogoussubstratespossessingacrotylmoietywasinvestigated undersimilarreactionconditions.However,thisreactionprovidedamixtureofproducts,eachinlowyields,whichindicatedthatthesubstrate wasnotgoodforthisreaction(Scheme1.3) [24,25].
Scheme1.1
Scheme1.2
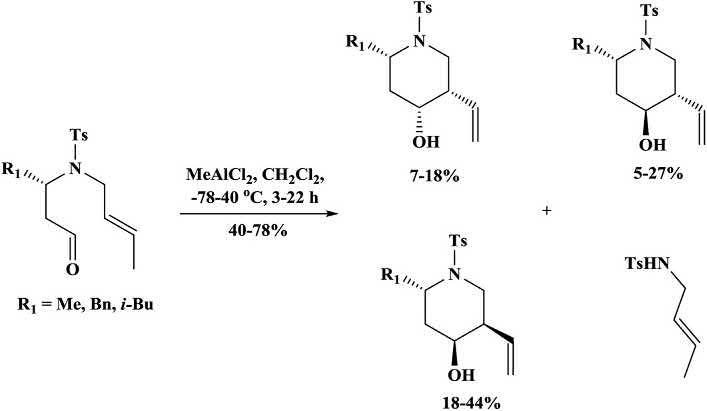
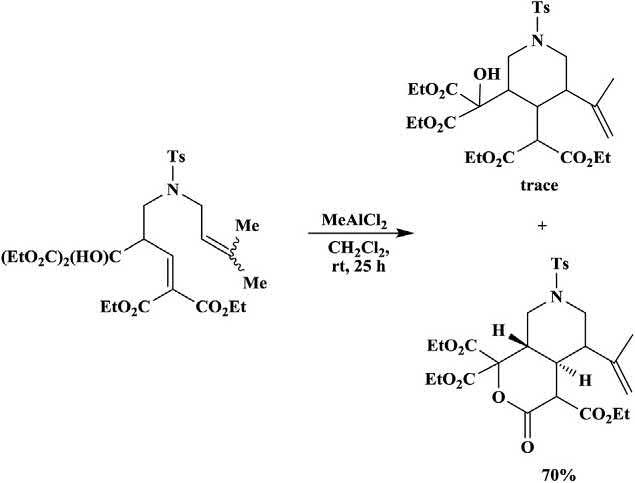
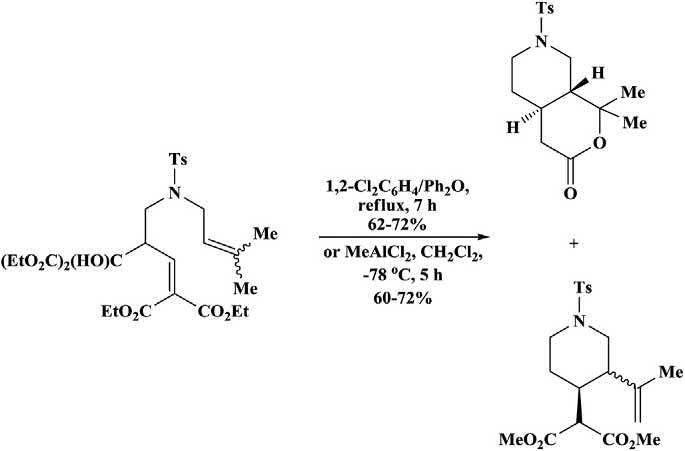
Various4-aza-1,7-dienesbearingactivatedenophileunderwentthermal orLewisacid-catalyzedenecyclizationtoformtheenecyclizationproduct, substitutedpiperidines,alongwithbicycliclactones,formedviaacompeting hetero-Diels Alderreaction(Schemes1.4and1.5).Athermalenecyclizationwasfacilitateduponactivationoftheenophilewithasingleester,but thereactionwasnotamenabletoLewisacidcatalysis.Itwasreportedthat theLewisacid-catalyzedreactionwasfacilewithotheractivatinggroupson theenophile,althoughtherewasafinebalancebetweenthecompetinghetero-Diels Alderreactionandthedesiredenecyclization,withtheproduct distributionbeinginfluencedbythenatureoftheenecomponent,theactivatinggrouponenophile,andtheLewisacid [25,26]
Scheme1.3
Scheme1.4
4 Metal-andNonmetal-AssistedSynthesisofSix-MemberedHeterocycles
Scheme1.5
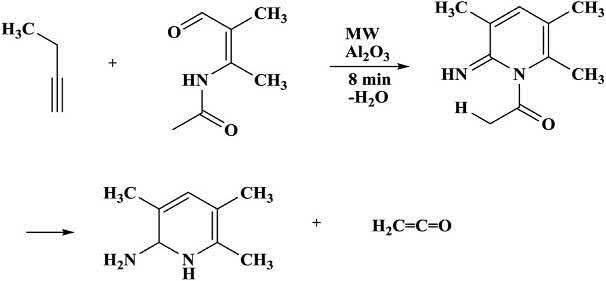
Thepyridinederivativewasformedfromacylaminoketoneandnitrile bymicrowave-assistedreaction.Goodyieldsof3,5,6-trisubstituted 2-aminopyridinederivativewereobtainedaftertheeliminationofacyl residue.Thehigh-energy-moleculeketenewasformedasasideproduct producedbyanelectrocyclicreaction(Scheme1.6) [27,28].
Scheme1.6
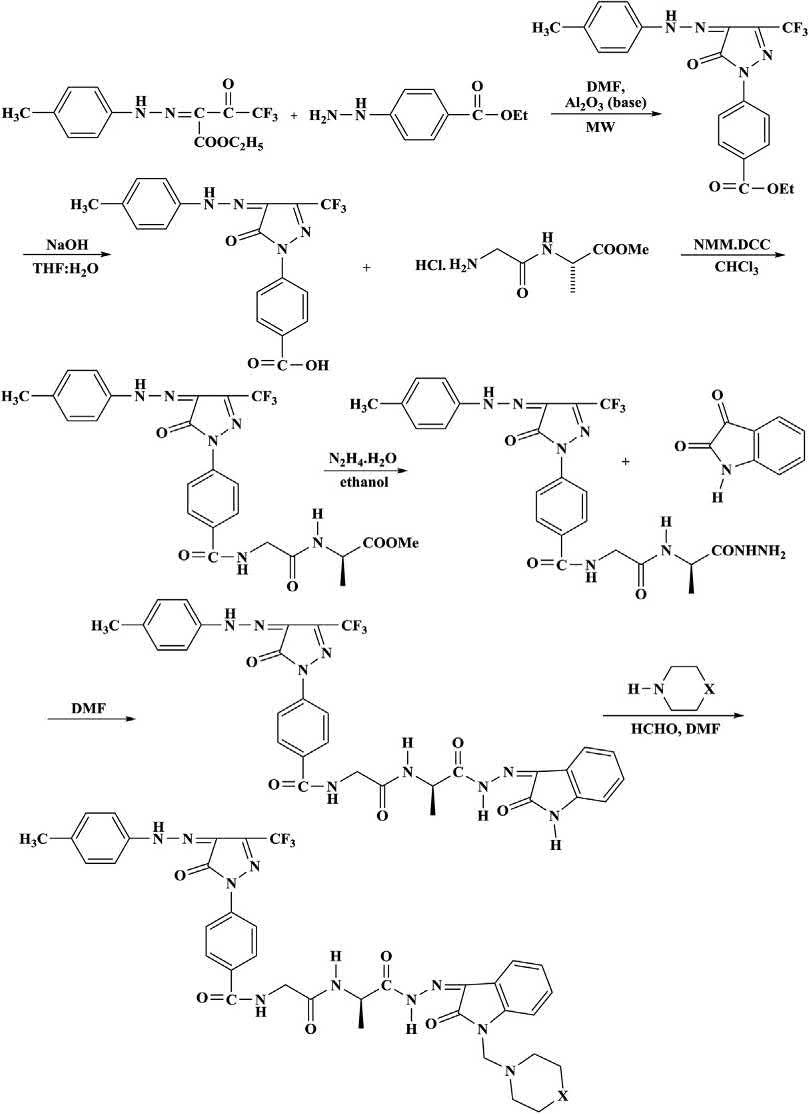
NovelMannichbases N-(2-(R)-1-(Z)-2-(1-(4-methylpiperazin-1-yl) methyl)-2-oxoindolin-3-ylidene)hydrazinyl)-1-oxopropan-2-ylamino)-2oxoethyl)-4-(5-oxo-4-(2-phenylhydrazono)-3-(trifluoromethyl)-4,5-dihydro1 H -pyrazol-1-yl)benzamidewereprepared.The N -(2-oxo-2-( R )-1oxo-1-(Z)-2-(2-oxoindolin-3-ylidene)hydrazinyl)propan-2-ylamino)ethyl)4-(5-oxo-4-(2-phenylhydrazono)-3-(trifluoromethyl)-4,5-dihydro-1H-pyrazol-yl)benzamideweresynthesizedbycondensationof( R )- N -(2-(1hydrazinyl-1-oxopropan-2-ylamino)-2-oxoethyl)-4-(5-oxo-4-(2-phenylhydrazono)-3-(trifluoromethyl)-4,5-dihydro-1H-pyrazol-1-yl)benzamide withisatin.The N-(2-oxo-2-(R)-1-oxo-1-(Z)-2-(2-oxoindolin-3-ylidene) hydrazinyl)propan-2-ylamino)ethyl)-4-(5-oxo-4-(2-phenylhydrazono)3-(trifluoromethyl)-4,5-dihydro-1H-pyrazol-yl)benzamidewassubjectedto
Scheme1.7
Mannichreactionwithcyclicsecondaryaminelikemorpholine/piperidine/ N-methylpiperidineinDMFinthepresenceofformaldehydetoaffordthe excellentyieldsofMannichbase N-(2-(R)-1-(Z)-2-(1-(4-methylpiperazin1-yl)methyl)-2-oxoindolin-3-ylidene)hydrazinyl)-1-oxopropan-2-ylamino) -2-oxoethyl)-4-(5-oxo-4-(2-phenylhyd razono)-3-(trifluoromethyl)-4,5dihydro-1H-pyrazol-1-yl)benzamide.Theyieldwasimprovedto90%under microwaveirradiation.Furtherstepsinvolvedsimplereactionconditionsand goodyieldprocedure(Scheme1.7) [29]
1.2.2Antimony-assistedsynthesis
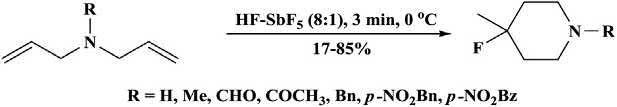
Thibaudeauandcoworkers [30] reportedarapidtransformationofseveral amidesand N,N-diallylicaminestofluorinatedpiperidinesinsuperacid, HFeSbF5,byanovelcyclization-fluorinationprocess(Scheme1.8) [25].
Scheme1.8
1.2.3Bismuth-assistedsynthesis
ThiolsunderwentoxidativecouplinginthepresenceofBi(NO3)3 5H2O toaffordthedisulfides.Theheteroaromaticthiol2-pyridinethiolwas reactedinthismethodtosynthesizethe2,20 -dipyridinedisulfidein93% yield(Scheme1.9) [31,32].
Scheme1.9
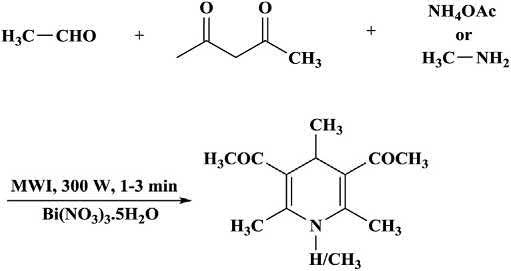
TheclassicalHantzschreactionisoneofthemosteconomicaland simplestmethodsforthepreparationofpharmacologicallyusefulandbiologicallyimportant1,4-dihydropyridinederivatives.BismuthnitratepentahydrateactedasaveryefficientcatalystunderMWIforaone-pot three-componentpreparationof1,4-dihydropyridines(1,4-DHPs)in excellentyieldsfromdiversealdehydes,amines/ammoniumacetate,and 1,3-dicarbonylcompoundsundersolvent-freeconditionswithin 1 3min.Theextremerapidityandexcellentyieldofreactionwasdueto aconcurrenteffectofMWIandcatalyst.Anextremelyfastandeasy methodwasreportedforthesynthesisof1,4-DHPsincatalyticamounts ofbismuthnitrateundersolvent-freeconditionsandMWI.Thisideawas extendedtothereactiononcarbonylcompounds(both1,3 dicarbonyl compoundsandaldehydes)withasuitableNH3 sourceundersolvent-free conditionsinthepresenceofcatalyticamountsofbismuthnitrate.Many bismuthsaltssuchasbismuthtriflate,bismuthchloride,bismuthbromide,
bismuthsubnitrate,bismuthnitrate,andbismuthiodidepentahydrate werescreenedusingethylacetoacetate,benzaldehyde,andammonium acetateasamodelreactionunderautomatedCEMMWIconditions (300W,1min,50°C).Thereactionoccurredin52%yieldwithinamin withoutanycatalyst(onlyMWI).Aseriesof1,4-DHPswasprepared usingdiverse1,3 diketocompounds,aldehydes,andammoniumacetate/ aminesunderMWIinthepresenceofbismuthnitratepentahydrate (5mol%)ascatalyst(Scheme1.10) [33].
Scheme1.10
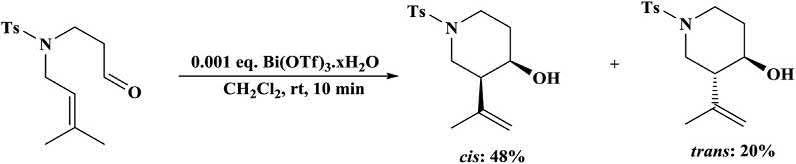
Andersonandcoworkers [34] developedanintramolecularcyclicene reactionofparentaldehydeinthepresenceofBi(OTf)3 xH2Ocatalyst forthesynthesisoftwo3,4-disubstitutedpiperidines(Scheme1.11) [32]
Scheme1.11
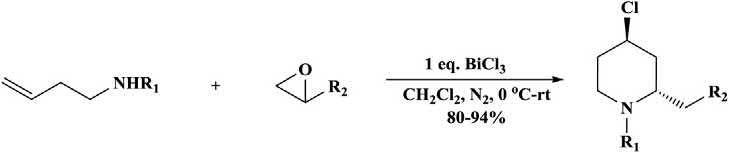
Theaza-Prins-typecyclizationofepoxidesand N-protectedhomoallyl aminesinthepresenceofbismuthchloride(Lewisacid)providedvarious trans-4-chloro-2-substitutedpiperidinesundermildreactionconditions (Scheme1.12) [32,35].
Scheme1.12
8 Metal-andNonmetal-AssistedSynthesisofSix-MemberedHeterocycles
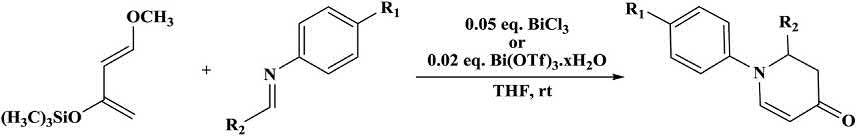
TheBi(OTf)3 xH2Oorbismuthchloride-catalyzedaza-Diels Alder reactionofimineswithDanishefsky’sdienesynthesizedvarious dihydropyridin-4-onesinhighyields(Scheme1.13) [32,36].
1.2.4Cerium-assistedsynthesis
Nairandcoworkers [37] synthesizedfunctionalizedpiperidinesbystereoselectiveintramolecularcyclizationofepoxypropylcinnamylamineswith cericammoniumnitrate(Scheme1.14).Theepoxideringunderwenta single-electrontransferoxidationbycericammoniumnitratetoprovidea radicalcationandthecerium(III)wasoxidizedtocerium(IV) [25].
Scheme1.14
Athree-componentone-stepreact ionofprimaryamine,1,3-dicarbonylcompound,and α,β-unsaturatedaldehydewasreportedby Menéndezetal. [38].ThisprotocolwasestablishedusingCANasa Lewisacidcatalyst.Thismethodhasexcellentsubstratetoleranceand furnished1,4-dihydropyridinesatr oomtemperaturewithmoderate-togoodyields.The β-ketothioesterswereefficientlyusedasdicarbonyl componenttoprovidethe1,4-dihydropyridinesbearingareactive thioestergroup(Scheme1.15 ) [39] .
Scheme1.13
Scheme1.15
1.2.5Cesium-assistedsynthesis
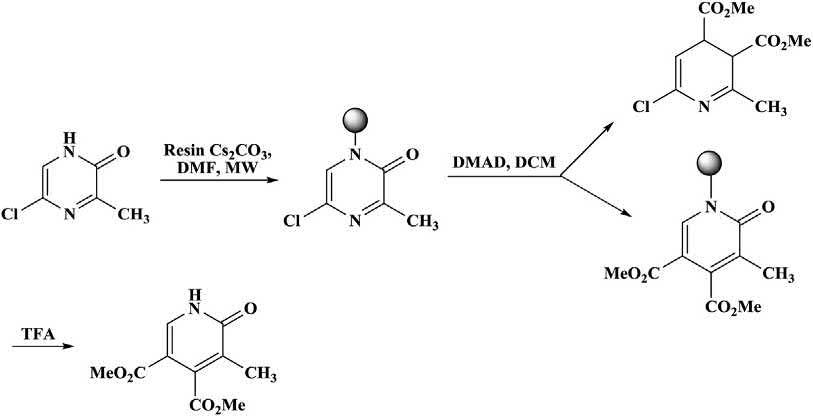
Cycloadditionswereevaluatedonacid-labilepolystyrenesupportssuchas HMPB AMresin,Wangresin,andsyringaldehyde-basedresin.Allsteps (i.e.,linking,cycloaddition,andcleavage)ofsolidphasewereperformed underbothcontrolledMWIandthermalconditions.Generally,thereactionrateincreasedsignificantlyandreactiontimesdecreasedfromhours ordaystominuteswhenconductedunderhigh-temperatureMWconditions(Scheme1.16) [40 44]
Scheme1.16
1.2.6Indium-assistedsynthesis
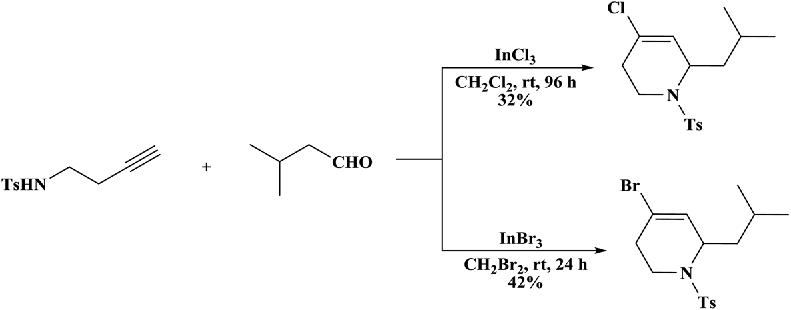
Toexaminethebenefitofironascomparedtoothertypicalmetal halidesusedintheaza-Prinscyclization,afewrunswerecarriedout usingindiumhalidesascatalysts( Scheme1.17 ) [45 49].Itwasreported thatbothindiumbromideandin diumchloridealsoinducedthe
cyclizationofaldehydesandhomopropargyltosylaminetoprovidethe tetrahydropyridines.However,the yieldswerelowerandthereaction rateswereslower.
1.2.7Iodine-assistedsynthesis
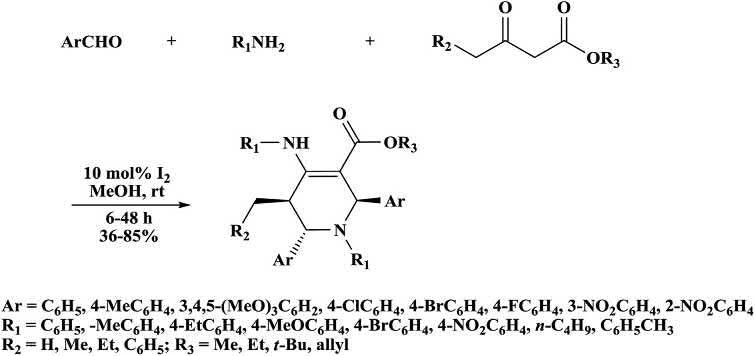
Khanetal. [50] preparedhighlyfunctionalizedpiperidinederivativesby aniodine-catalyzedone-potfive-componentreaction(Scheme1.18).The β-ketoesterswerereactedwithaminesforinsitugenerationofenamine thatunderwentMannich-typereactionwithiodine-activatedSchiff ’sbase toaffordanintermediate.Theformedintermediatereactedwithaldehydestoproducethecompoundthattautomerizedinthepresenceof iodine.TheformedcompoundunderwentintramolecularMannich-type reactiontoaffordthecompoundthattautomerizedtoprovidethecorrespondingproducts [51]
Scheme1.18
Scheme1.17
Six-membered N-heterocycles
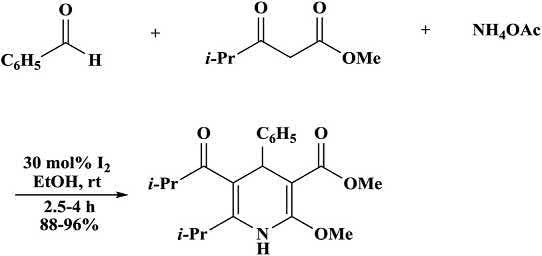
Akbariandcoworkers [52] synthesized1,4-DHPsbymulticomponent reactionsof1,3-dicarbonylcompounds,aldehydes,andammoniumacetateinthepresenceofiodine(30mol%)(Scheme1.19).Thecyclizedproductswereobtainedfrom1,3-dicarbonylcompounds [51]
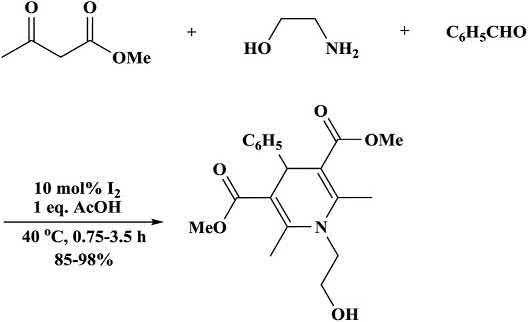
Zolfigolandcoworkers [53] developedareactionforthepreparation ofHantzsch N-hydroxyethyl-1,4-DHPsundermildreactionconditions (Scheme1.20) [51]
RenandCai [54] reportedaone-potHantzschreactionunder solvent-freeconditionsforthesynthesisof2,4,6-triarylpyridines (Scheme1.21).Enolattackedtheiodine-activatedaldehydestoaffordthe β-hydroxyketointermediatethatreactedwithNH3.Theimino-keto intermediatewasproducedbynucleophilicattackofanothermoleculeof enol.The2,4,6-triarylpyridineswereobtainedwhenimino-ketointermediateunderwentintramolecularcyclizationfollowedbylossofwaterand subsequentoxidation [51].
Scheme1.19
Scheme1.20
12 Metal-andNonmetal-AssistedSynthesisofSix-MemberedHeterocycles
Scheme1.21
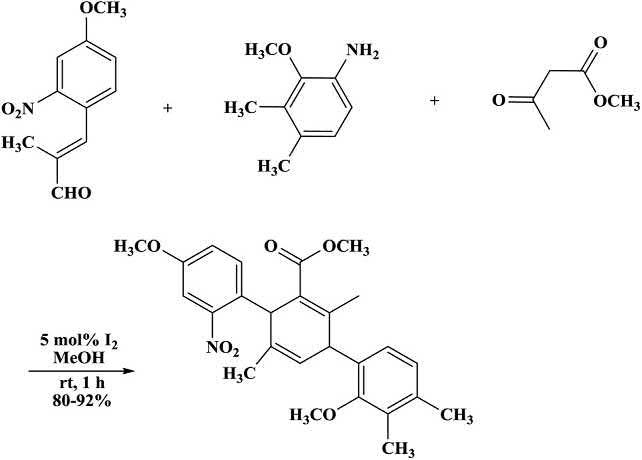
Kumarandcoworkers [55] reportedaniodine-catalyzedthree-componentreactionofsubstitutedanilines,cinnamaldehydes,and2-ketoesters inmethanoltoaffordthe N-aryl-1,4-DHPs(Scheme1.22).Antioxidant andantidyslipidemicactivitiesof1,4-DHPswereevaluatedinvitroand invivo.Anilinesandcinnamaldehydeswerereactedtosynthesizethe Schiff ’sbasethatunderwent1,4-additionwithenoltoproduceanintermediate.The N-aryl-1,4-DHPswereformedbyeliminationofH2Ofollowedbyintramolecularcyclizationandsubsequentlossofproton [51]
Scheme1.22
The(diacetoxyiodo)benzene,[bis(trifluoroacetoxy)iodo]benzene (BTI),and[hydroxyl(tosyloxy)iodo]benzeneareextensivelyusedinmany cationiccyclizationsthatareimportantfortheconstructionof heterocycliccompounds [56 77].Tellituandcoworkers [78 81] reportedaseriesofBTI-promotedintramolecularamidationreactions (Scheme1.23)toprovideseveralfive-,six-,andseven-memberedheterocycliccompounds.Theionicmechanismwasproposedonthebasis ofexperimentalevidences;thismechanisminvolvedtheformationof Nacylnitreniumintermediatesbyinitialreactionoftheamidewithhypervalentiodinereagent [82] .
Scheme1.23
1.2.8Iridium-assistedsynthesis
Thesulfoxoniumylideswereusedasacarbenesourceinthepresenceof simpleandcommerciallyavailableiridiumcatalystformanyinter-and intramolecularX Hbondinsertionssuchasapracticalring-expansion protocolforlactams.Thesulfoxoniumylideswererecommendedaspreferablesurrogatestotraditionaldiazoestersandketonesduetostability andsafety(Scheme1.24) [83]
Scheme1.24
Fujitaandcoworkers [84] used[Cp IrCl2]2 for N-heterocyclization, anditwasemployedforthereactionofanilineandbenzylaminewithseveraldiolstoaffordfive-,six-,andseven-memberedcyclicamines.The borrowinghydrogenprotocolwasusedforthetransformationofprimary aminesintonitrogen-containingheterocyclesviaadoublealkylationwith suitablediols(Scheme1.25) [85,86].
Scheme1.25
1.2.9Iron-assistedsynthesis
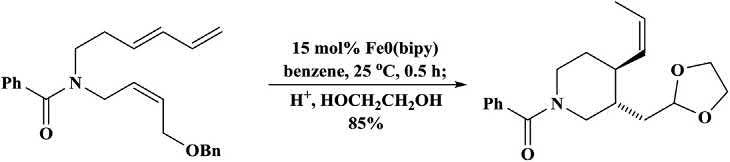
Aniron-catalyzedthermodynamicequilibrationof2-alkenyl6-substituted piperidineswasakeystepintheecofriendlyandhighlydiastereoselective preparationofsubstituted cis-2,6-piperidinesthatallowedtheisolationof enrichedmixturesofthemoststable cis-isomers(Scheme1.26) [87].
Scheme1.26
Theamidederivativesofpiperidinesweresynthesizedincomplete stereoselectivityandgoodyieldsbyinterestingapplicationofthisreaction (Scheme1.27) [88,89]
Scheme1.27
Amongdifferentexaminedsolvents(acetonitrile,tetrahydrofuran,chloroform,ethylacetate,nitromethane,carbontetrachloride,1,2-dichloroethane, anddichloromethane)thebestconditionswereusing1,2-dichloroethane anddichloromethane.Asimilarsolventeffecttotheoxa-alkynePrins cyclizationwasreported.Thechlorovinylderivativewasformedina mixturewiththebromovinylcompoundwhenthereactionwasperformedwithdichloromethaneassol ventandferricbromideascatalyst ( Scheme1.28 ) [49,90]
Scheme1.28
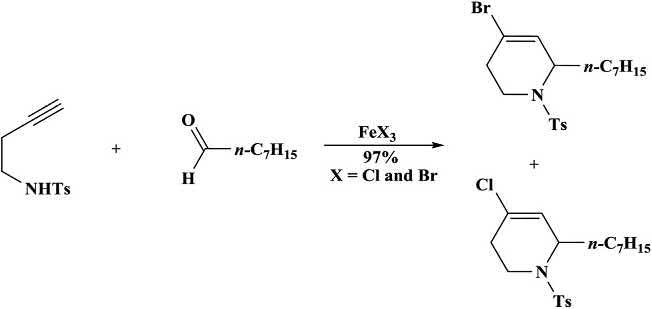
The N-sulfonyliminiumionwasproducedwhenhomopropargyl tosylaminewasreactedwithanaldehydeinthepresenceofferrichalide. Theformedintermediateaffordedtetrahydropyridine(Scheme1.29) [49].
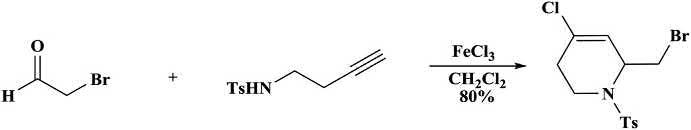
Thedimer2-alkyl-4-chloro-1-tosyl-1,2,5,6-tetrahydropyridinewas synthesizedfromnoncommercialandmoreelaboratedaldehyde (Scheme1.30) [49,91,92].
Althoughthecycloadditionreactionneededlowcatalystloading,the alkynecyclotrimerizationproductsandpyridineproductwereformed.An ironpentamethyl(cyclopentadienyl)acetonitrilesandwichcomplexwassynthesizedbyFerréandcoworkers [93] forthesuccessfulsynthesisofpyridineproductin73%yield.However,thiscycloadditionreactionwas limitedtooneactivatedalkyneandneededtheiron-complexinstoichiometricamounts(Scheme1.31) [94].
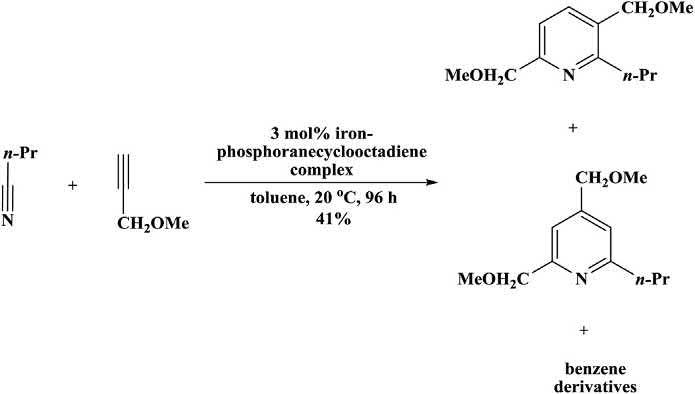
Inthepastironwasusedforthesynthesisofpyridine.SirWilliam Ramsay [95] reportedthisveryfirstexamplein1876.Hepreparedpyridineintracesuponpassingacetyleneandhydrocyanicacidthrougha redhotirontube [96].Knochandcoworkers [97] reportedacycloadditionofnitrilesandalkynesusinganiron-phosphoranecyclooctadiene complexforthepreparationofpyridinederivatives.Althoughthecycloadditionreactionneededlowcatalystl oading,significantcyclotrimerizationofalkynewasalsoreportedtogetherwiththedesiredpyridine products( Scheme1.32 ).
Scheme1.29
Scheme1.30
Scheme1.31
Scheme1.32
1.2.10Lanthanum-assistedsynthesis
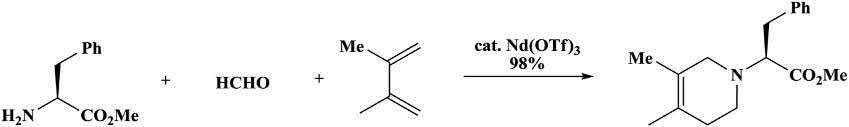
Theoriginalmethodforaza-Diels Alderreactionswaslimitedtoeither activatedaldehydeslikeglyoxylatesorthesimplestaldehyde,formaldehyde [98 103].TheLn(OTf)3-promotedaza-Diels Alderreactionsin waterwerecompatiblewithsubstratesthatweredifficulttoemployunder standardconditions.Forinstance,propanal,hexanal,andphenylethanal reactedinthepresenceofLn(OTf)3toaffordthegoodyields.Lessreactivedienessuchas2-methyl-1,3-butadiene,2,3-dimethyl-1,3-butadiene, and1,3-cyclohexadienedidnotreactwithhigheraldehydesusingthis method;however,theyreactedsmoothlywith L-phenylalaninoesterand formaldehyde(Scheme1.33) [104].Aza-sugarsweresynthesizedusingthis protocol [105].UnlikecarbonDiels Alderreactions,mostoftheavailableprotocolswereauxiliary-basedandenantioselectiveaza-Diels Alder reactionswerefarlessstudied [106 108].
Scheme1.33
Molanderandcoworkers [109] reportedthatthediastereoselective intramolecularhydroaminationofaminoalkenewasusednotonlyforthe synthesisof( )-pinidiolbutalsoforthesynthesisofits(1)-and( )-isomers(Scheme1.34).Thelanthanocenecatalystdisplayedgoodcatalytic
activitybutlowdiastereoselectivity,whichwasimprovedwhenthereactionwasperformedinthepresenceofathreefoldexcessof n-propylamine relativetotheaminoalkenesubstrate.Also,stericallymoreopen ansalanthanocenesshowedhigherdiastereoselectivities.Thesyntheticvalidity ofthisreactionwasdemonstratedforthepreparationofpinidinolwith excellent cis/trans diastereoselectivity [110].
Scheme1.34
1.2.11Lithium-assistedsynthesis
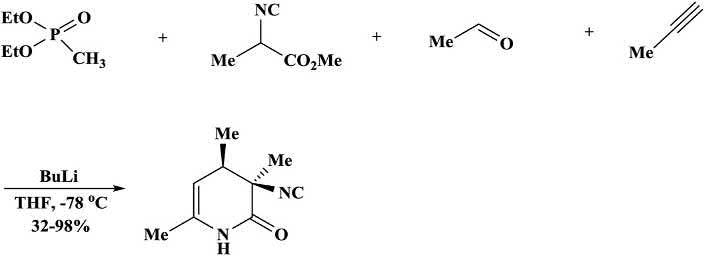
Paravidinoandcoworkers [111] reportedanewdiastereoselectivefourcomponentreactionwherenitriles,phosphonate,isocyanoacetates,and aldehydeswerecombinedtosynthesizethefunctionalized cis-3-isocyano3,4-dihydro-2-pyridones.Heteroaromatic,aromatic,and α,β-unsaturated aldehydesandnitrilesaffordedreasonabletoexcellentyieldsofdesired products cis-3-isocyano-3,4-dihydro-2-pyridones.However,primaryaliphaticnitrileswereavoidedbecausetheywerelessefficientinthesynthesisofazadiene(Scheme1.35) [112].
Scheme1.35
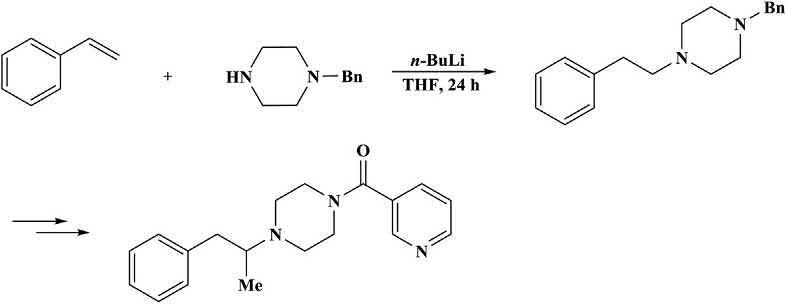
Thebase-catalyzedhydroaminationreactionofstyrenesandmonobenzylatedpiperazinewasreportedbyBelleretal. [113] asakeystepforthe preparationof N-(heteroarylcarbonyl)-N0 -(arylalkyl)piperazines,whichare CNS(centralnervoussystem)activecompounds.Thusstyreneswere reactedwith N-benzylpiperazineat65°C 120°Cinthepresenceof
n-BuLi(0.1 0.2eq.)toaffordthe N-benzyl-N0 -(2-arylethyl)piperazines ingoodyields.Thereactionalsooccurredinsimilarorevenbetteryields atroomtemperaturethanathighertemperatures,whencarriedoutina 2:1olefin/amineratio(Scheme1.36).Avariantinvolvedpriorisomerizationofallylbenzenetomethylstyrene [110]
1.2.12Magnesium-assistedsynthesis
Kadouri-Puchotandcoworkers [114] developedamuchimprovedvariationofene-iminiumcyclizationusingachiralpoolstartingcompound. Enantiomericallypure( S)-phenylglycinolwasreactedwithbutyraldehydetoaffordtheoxazolidine( Scheme1.37 ).The β-aminoalcoholwas obtainedwithhighdiastereoselecti vitywhenoxazolidinewasreacted withorganolithiumspecies.Oxazolidineexistsinequilibriumwiththe ring-openediminetautomerwith E-geometry.Ahighlyordered,chelatedtransitionstatewasformedi nthepresenceoforganolithium reagent.The β-aminoalcoholwasreactedwithglyoxaltoformanimine thatwascyclizedtogivethehemiacetal.Theene-iminiumcyclization occurredwithcompletefacialdiscrim inationasthephenylsubstituent hinderedthe Re faceandthereforetheconcertedprocessproceededto formthebicyclicintermediate.Completionofthesynthesisinvolved oxidativecleavageofterminalalkene,followedbySwernoxidationof hemiacetaltoaffordthelactone.TheketonewasreducedwithKselectridediastereoselectivelyto installthe4-hydroxysubstituent. Finally, trans-6-substituted-4-hydroxyp ipecolicacidwasformedin almostquantitativeyieldbyhydrogenolysis.A27%overallyieldwas achievedinsevensteps.
Scheme1.36