Metal-OrganicFrameworks(MOFs)forEnvironmental ApplicationsSujitK.Ghosh
https://ebookmass.com/product/metal-organic-frameworks-mofsfor-environmental-applications-sujit-k-ghosh/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Metal-Organic Frameworks for Chemical Reactions: From Organic Transformations to Energy Applications 1st Edition
Anish Khan (Editor)
https://ebookmass.com/product/metal-organic-frameworks-for-chemicalreactions-from-organic-transformations-to-energy-applications-1stedition-anish-khan-editor/ ebookmass.com
Metal-Organic Frameworks with Heterogeneous Structures Ali Morsali
https://ebookmass.com/product/metal-organic-frameworks-withheterogeneous-structures-ali-morsali/
ebookmass.com
Novel strategies for the formulation and processing of aluminum metal-organic framework-based sensing systems toward environmental monitoring of metal ions Yongbiao Hua
https://ebookmass.com/product/novel-strategies-for-the-formulationand-processing-of-aluminum-metal-organic-framework-based-sensingsystems-toward-environmental-monitoring-of-metal-ions-yongbiao-hua/ ebookmass.com
Rescued By My High School Crush: A Second Chance Romance (Bearberry Bay Series Book 3) Kassie Kline
https://ebookmass.com/product/rescued-by-my-high-school-crush-asecond-chance-romance-bearberry-bay-series-book-3-kassie-kline/ ebookmass.com
Knot Again: An Omegaverse Romance (Scornedverse Book 2)
Lucy Scott Bryan
https://ebookmass.com/product/knot-again-an-omegaverse-romancescornedverse-book-2-lucy-scott-bryan/
ebookmass.com
Going global – Travel and the 2019 novel coronavirus Alfonso J. Rodríguez-Morales
https://ebookmass.com/product/going-global-travel-and-the-2019-novelcoronavirus-alfonso-j-rodriguez-morales/
ebookmass.com
Pain Medicine: A Case-Based Learning Series: The Spine 1st Edition Steven D. Waldman
https://ebookmass.com/product/pain-medicine-a-case-based-learningseries-the-spine-1st-edition-steven-d-waldman/
ebookmass.com
Meat, Mercy, Morality: Animals and Humanitarianism in Colonial Bengal, 1850-1920 Samiparna Samanta
https://ebookmass.com/product/meat-mercy-morality-animals-andhumanitarianism-in-colonial-bengal-1850-1920-samiparna-samanta/
ebookmass.com
Stop. Think. Invest.: A Behavioral Finance Framework for Optimizing Investment Portfolios Michael Bailey
https://ebookmass.com/product/stop-think-invest-a-behavioral-financeframework-for-optimizing-investment-portfolios-michael-bailey/
ebookmass.com
Facharztprüfung Allgemeinmedizin: in Fällen, Fragen und Antworten - Mit Zugang zur Medizinwelt 5th Edition Detmar Jobst
https://ebookmass.com/product/facharztprufung-allgemeinmedizin-infallen-fragen-und-antworten-mit-zugang-zur-medizinwelt-5th-editiondetmar-jobst/
ebookmass.com
Metal-OrganicFrameworks(MOFs) forEnvironmentalApplications Metal-Organic Frameworks(MOFs)for Environmental Applications Editedby SujitK.Ghosh
DepartmentofChemistry,IndianInstitute ofScienceEducationandResearch(IISER), Pune,India
CentreforEnergyScience,IndianInstitute ofScienceEducationandResearch(IISER), Pune,India
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2019ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationabout thePublisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyright ClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/ permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroaden ourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmay becomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingand usinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformation ormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesfor whomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeany liabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceor otherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthe materialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
LibraryofCongressCataloging-in-PublicationData AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-12-814633-0
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionEditor: EmilyMcCloskey
EditorialProjectManager: MichaelLutz
ProductionProjectManager: PremKumarKaliamoorthi
CoverDesigner: GregHarris
TypesetbyMPSLimited,Chennai,India
ListofContributors MelihBaci DepartmentofChemistry,TexasA&MUniversity,CollegeStation, TX,UnitedStates
MoisesA.Carreon DepartmentofChemicalandBiologicalEngineering, ColoradoSchoolofMines,Golden,CO,UnitedStates
WenmiaoChen DepartmentofChemistry,TexasA&MUniversity,College Station,TX,UnitedStates
KevinDedecker InstitutLavoisierdeVersailles,UMRCNRS8180,Universite ´ de VersaillesSt-Quentin-en-Yvelines,Universite ´ Paris-Saclay,78035Versailles Cedex,France;CentredeRecherchesurlaConservation(CRC),Muse ´ umNational d’HistoireNaturelle,CNRS,Ministe ` redelaCulture,USR3224,36rueGeoffroy SaintHilaire,75005,Paris,France
AamodV.Desai DepartmentofChemistry,IndianInstituteofScienceEducation andResearch(IISER),Pune,India
EddyDumas InstitutLavoisierdeVersailles,UMRCNRS8180,Universite ´ de VersaillesSt-Quentin-en-Yvelines,Universite ´ Paris-Saclay,78035Versailles Cedex,France
SubhajitDutta DepartmentofChemistry,IndianInstituteofScienceEducation andResearch(IISER),Pune,India
PaoloFalcaro InstituteofPhysicalandTheoreticalChemistry,GrazUniversityof Technology,Graz,Austria
YuFang DepartmentofChemistry,TexasA&MUniversity,CollegeStation,TX, UnitedStates
OmarK.Farha DepartmentofChemistry,InternationalInstituteofNanotechnology, NorthwesternUniversity,Evanston,IL,UnitedStates;DepartmentofChemistry, FacultyofScienceKingAbdulaziz,UniversityJeddah,Jeddah,SaudiArabia
MingbaoFeng DepartmentofEnvironmentalandOccupationalHealth,Schoolof PublicHealth,TexasA&MUniversity,CollegeStation,TX,UnitedStates
SujitK.Ghosh DepartmentofChemistry,IndianInstituteofScienceEducation andResearch(IISER),Pune,India;CentreforEnergyScience,IndianInstituteof ScienceEducationandResearch(IISER),Pune,India
AshleeJ.Howarth DepartmentofChemistryandBiochemistry,Concordia University,Montre ´ al,QC,Canada
AngeloA.Kirchon DepartmentofChemistry,TexasA&MUniversity,College Station,TX,UnitedStates
SusumuKitagawa InstituteforIntegratedCell-MaterialScience(iCeMS),Kyoto, Japan
AmritKumar DepartmentofChemicalSciences,BernalInstitute,Universityof Limerick,Limerick,RepublicofIreland
SubhajitLaha MolecularMaterialsLaboratory,ChemistryandPhysicsof MaterialsUnit,SchoolofAdvancedMaterials,JawaharlalNehruCentrefor AdvancedScientificResearch,Bangalore,India
BertrandLave ´ drine CentredeRecherchesurlaConservation(CRC),Muse ´ um Nationald’HistoireNaturelle,CNRS,Ministe ` redelaCulture,USR3224,36rue GeoffroySaintHilaire,75005,Paris,France
ShengqianMa DepartmentofChemistry,UniversityofSouthFlorida,Tampa,FL, UnitedStates
ZacharyLawrenceMagnuson DepartmentofChemistry,UniversityofSouth Florida,Tampa,FL,UnitedStates
MarekB.Majewski DepartmentofChemistryandBiochemistry,Concordia University,Montre ´ al,QC,Canada
TapasKumarMaji MolecularMaterialsLaboratory,ChemistryandPhysicsof MaterialsUnit,SchoolofAdvancedMaterials,JawaharlalNehruCentrefor AdvancedScientificResearch,Bangalore,India
SoumyaMukherjee DepartmentofChemicalSciences,BernalInstitute, UniversityofLimerick,Limerick,RepublicofIreland
RaffaeleRicco InstituteofPhysicalandTheoreticalChemistry,GrazUniversityof Technology,Graz,Austria
SyamantakRoy MolecularMaterialsLaboratory,ChemistryandPhysicsof MaterialsUnit,SchoolofAdvancedMaterials,JawaharlalNehruCentrefor AdvancedScientificResearch,Bangalore,India
ParthaSamanta DepartmentofChemistry,IndianInstituteofScienceEducation andResearch(IISER),Pune,India
ChristianSerre InstitutdesMate ´ riauxporeuxdeParis(IMAP),UMRCNRS 8004,EcoleNormaleSupe ´ rieuredeParis,EcoleSupe ´ rieuredePhysiqueetde ChimieIndustriellesdeParis,PSLUniversity,75005,Paris,France
ShivaniSharma DepartmentofChemistry,IndianInstituteofScienceEducation andResearch(IISER),Pune,India
VirenderK.Sharma DepartmentofEnvironmentalandOccupationalHealth, SchoolofPublicHealth,TexasA&MUniversity,CollegeStation,TX,United States
NathalieSteunou InstitutLavoisierdeVersailles,UMRCNRS8180,Universite ´ deVersaillesSt-Quentin-en-Yvelines,Universite ´ Paris-Saclay,78035Versailles Cedex,France
MarkJ.Styles CSIROManufacturing,ClaytonSouth,VIC,Australia
QiWang DepartmentofChemistry,TexasA&MUniversity,CollegeStation,TX, UnitedStates
ShuaoWang StateKeyLaboratoryofRadiationMedicineandProtection,School forRadiologicalandInterdisciplinarySciences(RAD-X),CollaborativeInnovation CenterofRadiationMedicineofJiangsuHigherEducationInstitutions,Soochow University,Suzhou,P.R.China
ChengliangXiao StateKeyLaboratoryofRadiationMedicineandProtection, SchoolforRadiologicalandInterdisciplinarySciences(RAD-X),Collaborative InnovationCenterofRadiationMedicineofJiangsuHigherEducationInstitutions, SoochowUniversity,Suzhou,P.R.China
LiqiuYang DepartmentofChemicalandBiologicalEngineering,ColoradoSchool ofMines,Golden,CO,UnitedStates
MichaelJ.Zaworotko DepartmentofChemicalSciences,BernalInstitute, UniversityofLimerick,Limerick,RepublicofIreland
PengZhang DepartmentofChemistry,TexasA&MUniversity,CollegeStation, TX,UnitedStates
Hong-CaiZhou DepartmentofChemistry,TexasA&MUniversity,College Station,TX,UnitedStates
Introduction
SujitK.Ghosh1,2,* andSusumuKitagawa3,*
1CentreforEnergyScience,IndianInstituteofScienceEducationandResearch(IISER), Pune,India, 2DepartmentofChemistry,IndianInstituteofScienceEducationand Research(IISER),Pune,India, 3InstituteforIntegratedCell-MaterialScience(iCeMS), Kyoto,Japan
Correspondingauthors:e-mailaddress:sghosh@iiserpune.ac.inandkitagawa-g@icems. kyoto-u.ac.jp
Metal-organicframeworks(MOFs)orporouscoordinationpolymers(PCPs)forma distinguishedsubsetinthedomainofcoordinationchemistry:theyarebroadly regardedascoordinationnetworksbearingpotentialvoids [1].Constructedfrom organicstrutswhichextendinfinitelyinaperiodicmannerthroughmetalnodes, MOFsrepresentanadvancedclassofporousmaterialsthathavebeensubjecttoa remarkableupsurgeoverthelastfewdecades.Themyriadchoiceofbuilding blocksalongwithaccesstocombinatorialchemistryaffordsthesynthesison demandofarchitectureswhosepropertiescanbedirectlylinkedtothestructural characteristics.ThesefeatureshavemadeMOFshighlysought-afterovercongener poroussolidmaterials,forexample,zeolitesandactivatedcarbon.Further,the abilitytomodulatenanospaceproperties,controlporesizeandnature,accessmesoporosity,utilizereticularchemistry,andincorporateactivesitesinporouscompoundsasperdemandhasresultedinMOFscommandingresearchinterestacross disciplines.ThespectrumofpotentialapplicationsusingMOFshasbroadenedwith theevolutionofthisfield,whichprominentlyincludegasstorage/separation, adsorptiveseparationofindustriallychallengingliquidmixtures,ion-conduction, sensingandphotonics,heterogeneouscatalysis,drugdelivery,etc. [2,3].
TheriseofMOFchemistrycanbeprimarilyascribedtothefollowingbroad reasons:growingknowledgeofstructuralcharacteristicsretrievedfromalibrary ofreportedcompounds,andthegreaterprecisiontoobtainread-outandcontrol host guestchemistryindifferentmedia.Inparticulartheriseofthirdgenerationof MOFs(softporouscrystals)hasactuatedthedevelopmentofMOFstowardapplicationsbasedonsuchhost guestchemistry [4].Theabilitytohaveguest-responsive structuraldynamicshasfurtherpropelledtheirabilitytooperateasfunctionalsolids withahighprecisionandselectivityofguestaccommodationandrecognition response.Apartfromsoftness,MOFsbeartheinherentabilitytoincorporate specificactivesiteswhichhavepromotedtheapplicabilityofthesecompounds towardawidearrayofgasorliquidadsorption,whichareeitherenvironmentally noxiousorindustriallyrelevant.Inadditiontothelibertyofdesigningaspecific Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications.DOI: https://doi.org/10.1016/B978-0-12-814633-0.00001-6 © 2019ElsevierInc.Allrightsreserved.
probe/sorbent,MOFsofferthechoicetopicktransductionmechanismsbasedon thespecificfieldofapplication,suchaselectromagneticresponses,photonic responses,andnaked-eyecolorimetry.Otherthanconventionalapplications, recentlyMOFshavestarteddemonstratingaffirmativepotentialtowardtheirutility intherecognition,capture,anddetoxificationofenvironmentaltoxinsthatare presentindifferentmediaincludingair,water,andsoil.
Addressingenvironmentalpollutionhasbecomeoneofthegreatestchallenges forhumancivilizationinthe21stcentury.Thisisclearlyreflectedinthelistof sustainabledevelopmentgoalsbenchmarkedbytheUnitedNations [5].Thedirect correlationinthehigheremissionorreleaseoftoxicspecieswiththechangein ecologicalbalancehaspromptedthepressingneedtocurbthepresenceofpollutants.Forinstance,in2018,areportbyWorldHealthOrganization(WHO)raised thealarm,bysuggestingthatmorethan90%ofthehumanpopulationonEarthis breathingpollutedair [6]
Porousmaterialshaveemergedassuitablereceptorstoentrapenvironmentalpollutantsordetoxifythemintosafesubstancesonaccountofveryhighsurfaceareas, highporosity,andeaseindesignofmolecularscalefunctionalvoids.Duetoseveral advantagesMOFsscoreoverconventionalporousadsorbents,butthemajorbottleneckinpursuitofMOFsrealizingapplicabilityinthisdomainhasbeenthestability underoperatingconditionsorsusceptibilityofMOFcompoundstodecompose uponexposuretomoisture.Recently,therehasbeenaconsciouseffortinthefield toaddressthisissueandencouragingprogressandlong-termpromisehasbeenwitnessed [7].Apartfromstability,anotherissuewhichhasretardedtheinvestigation ofMOFsunderreal-timeconditionshasbeenthelackofstrategiestoformdevices frommicrocrystallineMOFpowders.Hence,compositesbasedonMOFshave receivedgreaterattention,andmoreintensiveresearchinthisregardcanpropelthe realizationofMOF-baseddevices [8].Inadditiontothesemajorroadblocks,issues suchastoxicity,durability,life-cycleassessmentstudies(LCA),bulk-scalesynthesis,andcost-effectivenessareunavoidableandrequirecarefulconsiderationwhile proposingtheutilityofanewlydevelopedmaterialinreal-timescenarios [9,10].
ThebookattemptstofocusontheprogressofMOFsandunderstandthelongtermtrendswhichhavebeenstudiedandproposedbytheleadingnamesacrossthe globe.Chapter8,Metal-organicframeworksfordetectionanddesensitizationof environmentallyhazardousnitro-explosivesandrelatedhighenergymaterials,by GhoshandcoworkersemphasizestheutilityofMOFstowardsensinganddesensitizationofnitroaromaticcompounds(NACs)andrelatedexplosivecompounds.
Chapter2,Metal-organicframework-basedcarboncaptureandpurificationtechnologiesforcleanenvironment,byZaworotkoandcoworkersrevisitsthemajormilestonesinthedomainofMOFsforthestorageandseparationofgreenhousegas carbondioxide(CO2)andexplainstheroadmapgoingahead.CarreonandcoworkershavedescribedtheroleofMOFsasheterogeneouscatalystsforthegreener waysofgeneratingfuelsinChapter9,Greendeoxygenationoffattyacidsto transportfuelsovermetal-organicframeworksascatalystsandcatalyticsupports. Thesubsequenttwochapters,Chapter3,Sensingandsequestrationofinorganiccationicpollutantsbymetal-organicframeworks(Maandcoworkers)andChapter4, 2Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
Metal-organicframeworksforrecognitionandsequestrationoftoxicanionicpollutants(Ghoshandcoworkers),dealwiththevariousapproacheswhereinMOFshave beenabletosenseortrapionicpollutants.Chapter5,Metal-organicframeworksfor thecaptureofvolatileorganiccompoundsandtoxicchemicals,bySerreandcoworkersdiscussesthestate-of-artforutilizingMOFsforair-pollutioncontrol. Chapter6,Metal-organicframeworksforcaptureanddetoxificationofnerve agents,byFarhaandcoworkersextendsthediscussioninpreviouschapterand highlightstheprogressofMOFstowardthecaptureanddetoxificationofchemical warfareagents.Zhouandcoworkers(Chapter7:Metal-organicframeworksforcaptureanddegradationoforganicpollutants)andMajiandcoworkers(Chapter10: Potentialofhydrophobicmetal-organicframework-basedmaterialsforenvironmentalapplications)provideaflavoroftheresearchcarriedouttowardremediationof waterpollutionwiththefocusesondegradationoforganicpollutantsandseparation ofoil watermixtures,respectively.Thegrowingdependenceonnuclearpowerhas resultedintheemissionofhighquantumofnuclearwasteinaqueousstreams. Wangandcoworkers(Chapter11:Radionuclidesequestrationbymetal-organicframeworks)describetheprogressofMOFstowardthecaptureofsuchradioactive species.FinallythechapterbyRiccoandcoworkers(Chapter12:Metal-organic framework-baseddevicesfordetectionandremovalofenvironmentalpollutants) givesthereaderaflavoroftheresearchinthecommunitydevotedtowardthe developmentofMOFsinworkableformsorascomponentsofportabledevices.
Allthecontributingauthorsarethankedfortheirinsightfulcontributions,and theElsevierteamarethankedforallthebackroomsupport.Thebookendeavorsto providethereader,eitherinacademiaorindustry,withanessenceoftheresearch inthedomainofMOFsdevotedtowardpressingpracticalconcerns,andtheeditor hopesthatthewidearrayoftopicscoveredinthisbookcanaccomplishthat objective.
References [1] S.R.Batten,N.R.Champness,X.-M.Chen,J.Garcia-Martinez,S.Kitagawa,L. Ohrstrom,etal.,Terminologyofmetal organicframeworksandcoordinationpolymers (IUPACRecommendations2013),PureAppl.Chem.85(2013)1715 1724.
[2] H.Furukawa,K.E.Cordova,M.O’Keeffe,O.M.Yaghi,Thechemistryandapplications ofmetal-organicframeworks,Science341(2013)1230444.
[3]S.Yuan,L.Feng,K.Wang,J.Pang,M.Bosch,C.Lollar,etal.,Stablemetal organic frameworks:design,synthesis,andapplications,Adv.Mater.(2018).Availablefrom: https://doi.org/10.1002/adma.201704303.
[4] S.Horike,S.Shimomura,S.Kitagawa,Softporouscrystals,Nat.Chem.1(2009) 695 704.
[5] www.un.org/sustainabledevelopment/sustainable-development-goals/ [6] http://www.who.int/airpollution/data/cities
[7] A.J.Howarth,Y.Liu,P.Li,Z.Li,T.C.Wang,J.T.Hupp,etal.,Chemical,thermaland mechanicalstabilitiesofmetal organicframeworks,Nat.Rev.Mater.1(2016)15018.
4Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
[8] P.Falcaro,R.Ricco,C.M.Doherty,K.Liang,A.J.Hill,M.J.Styles,MOFpositioning technologyanddevicefabrication,Chem.Soc.Rev.43(2014)5513 5560.
[9] P.A.Julien,C.Mottillo,T.Friscic,Metal organicframeworksmeetscalableand sustainablesynthesis,GreenChem.19(2017)2729 2747.
[10] C.A.Grande,R.Blom,A.Spjelkavik,V.Moreau,J.Payet,Life-cycleassessmentasa toolforeco-designofmetal-organicframeworks(MOFs),Sust.Mater.Technol.14 (2017)11 18.
Metal-organicframeworkbased carboncaptureandpurification technologiesforclean environment SoumyaMukherjee,AmritKumarandMichaelJ.Zaworotko* DepartmentofChemicalSciences,BernalInstitute,UniversityofLimerick,Limerick,
RepublicofIreland
Correspondingauthor.emailaddress:xtal@ul.ie
2.1Introduction—thesocietalrelevanceofcarbon captureandsequestration Thehumanraceisnowinthe“ageofgas,”whichmeansthatgasesandvapors havelargelyreplacedliquidswithrespecttofuelsforsocietyandfeedstocksforthe chemicalindustry [1].Theenergyfootprintassociatedwithgasandvaporpurificationisenormous;currently40%oftheenergyconsumedinthechemicalindustryis usedjustforcommodityseparationsandpurification.Thisequatestoroughly15% ofglobalenergyproductionandithasbeenestimatedthatthedemandforcommoditiescouldtripleby2050 [2].WhereasEUmemberstatesarecommittedtoa40% reductioningreenhousegasemissionsaspartofthe2030climateandenergy framework [3],whichalsocallsfor27%improvementinenergyefficiency,these ambitioussocietalgoalswilllikelybecomemootifthehighenergyfootprintassociatedwithcommoditypurificationremainsunaddressed.
Theforemostanthropogenicgascarbondioxide,CO2,liesattheheartofachievingenergysustainabilityforseveralreasons.Sustainedburningoffossilfuelssince thebeginningoftheindustrialrevolutionhasincreasedatmosphericCO2 concentrationto . 400ppmfrom280ppm [4].Today’spowerplantswillemitafurther300 billiontonsofCO2 inthefuture,enoughtocontributeanadditional20ppmofCO2 totheatmosphere.AtmosphericCO2 isnotjustrelevanttoclimatechange,it becomesamajorhealthissueasconcentrationsofaslittleas600ppmcanleadto respiratoryillnesses [5].OccupationalhealthandsafetyregulationsdictatethatCO2 concentrationsinconfinedspacesshouldbelimitedto , 0.5%CO2 [6].Inthiscontext,airpurificationdevicesareemployedinspacecraftandsubmarinestocontrol CO2 concentrations [7].Industrially,traceremovalofCO2 isimportantinpurificationoffluegasandnaturalgas(NG)processing.SpecificationsrequireCO2 concentrationsinNGliquefaction(LNG)of , 50ppmtoavoidcorrosionandCO2
6Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
freezing [8].TheseapplicationsencompassadiverserangeofCO2 concentrations, conditions,andgasmixtures,therebymakingitunlikelythatthereisasinglesolutionforC-capture.Rather,bespokeapproachesandmaterialswithoptimalpropertiesareneededtoaddressthespecificrequirementsofagivencarboncapture application.
Sincefossilfuelswillremaintheprimaryenergysourceforgenerationsand gaseswithCO2 impuritiesareimportantfeedstocksforthechemicalindustry,new approachestocost-effectiveandenergy-efficientcarboncaptureandsequestration (CCS)areurgentlyneeded.Thebroadroll-outofCCShasthusfarbeenhampered byhighcosts(50 100US$pertonofCO2 capturedatbest),thetechno-economic uncertaintiesofCCStechnologies,andtodate,thelackofsuitablematerialalternativestoliquidamine-basedCCStechnologies [9].LiquidamineCCStechnologies arethecurrentstate-of-the-artforcarboncaptureandtheyhavebeenutilizedinthis contextforoverhalfacentury.Thisisdespitethefactthatliquidaminesrelyupon chemicalreactionsthatareenergy-intensive,theyarenoteconomicallyviablefor broaddeployment,andtheyofferlittleroomforinnovation.Theambitioustargets forCO2 emissionsreductionsby2030thereforerequireinnovativeapproachesto CCStechnologiesthatimproveboththeenergyfootprintandcost-effectivenessof CCS.Inthischapter,wefocusuponthepotentialofferedbynewclassesof advancedphysisorbentsforcarboncapture,C-capture,whichinprinciplearecapableofreducingtheenergyfootprintofC-captureprocessesbyupto90% [2] Table2.1 liststhevariousapproachestoC-captureandpresentstheiradvantages/ disadvantagesalongwithanassessmentoftheirupsidepotentials.Thestate-of-theartwithrespecttobulk-scaleC-capturecanbesubdividedintotwomainclassesas definedbythemodeofcapture.1
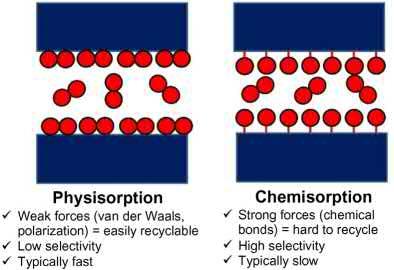
1. Chemisorption functionsthroughchemicalreactionbetweenthecapturematerial(sorbent) andCO2 (Fig.2.1,left).Theprimaryadvantageofchemisorbentsisthattheyaretypically highlyselectivebut,becauserecyclingofthesorbentrequiresthebreakageofchemical bonds,theytendtoexhibitahighenergyfootprint.Inaddition,thekineticsofchemisorptionprocessesislikelytoberelativelyslowifCO2 concentrationsarelow.Examplesof chemisorbentsincludethefollowing:
a. Thestate-of-the-artforbulk-scaleC-captureatindustrialscaleinvolves liquidamines aschemisorbents.Theseprocesses,initiallydevelopedinthe1930s,arebuiltaround liquidaminesolutionsthatchemicallyreactwithCO2 Drawbacks:Whereasliquid amine-based“wetscrubbers”areeffectiveatC-capturetheysufferfromhighregenerationenergy( . 140 C),largeequipmentsize,aminedegradation,andequipmentcorrosion [10].Thesefactorscollectivelyamounttoa30% 40%reductionintheoverall efficiencyofapowerplantwhenliquidamineCCSisemployedforpostcombustion C-captureandtheassociatedcostsareintherangeof h70 100perton.Further,there isverylimitedpotentialtosignificantlyimproveperformance.
b. Solidchemisorbentssuchas sodalime havealsobeenusedforC-capture,especiallyin closedcircuitbreathingenvironments. Drawback:reactionsaretooslowforlargescaleC-captureapplications.
c. LiquidchemisorbentsdevelopedforwetairC-capturehavealsousedthe soda-lime process. Drawback:CO2 abatementcostsareprohibitivelyhigh,the American PhysicalSociety estimatingcostsofUS$600pertonofCO2 [11]
Table2.1 ComparisonofthestrengthsandweaknessesofselectedC-capturemethods.
Aminegrafted inorganics Liquid amines Aminegrafted MOFs IonicliquidsSodalimeZeolitesMOFsHUMsMolecular sieves
SelectivityHighHighHighHighHighLowLowVeryhighInfinite StabilityHighLowMediumHighHighHighLowMediumLow HumidityeffectLowLowMediumLowLowHighHighMediumLow MaterialcostMediumLowHighLowLowLowMedium/highLowHigh ProcesscostMediumLowHighMediumLowLowMediumLowHigh RecyclingcostMediumHighMediumMedium/highVeryhighHighHighLowN/A WorkingcapacityMediumMediumMediumLowMediumMediumHighMediumN/A KineticsMediumFastMediumFastFastMediumMediumFastN/A UpsidepotentialMediumLowMediumMediumLowLowHighHighHigh
8Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
Figure2.1 Typesofsorptionbasedseparation/purification:chemisorptionversusphysisorption.
d. Amine-modifiedporousmaterials:theintroductionor“grafting”ofaminefunctional groupsontoporousmaterials(typicallyMOFs,mesoporoussilicas,andzeolites)can significantlyincreasetheaffinityofaporousmaterialtowardsCO2. Drawbacks:high regenerationenergy( . 90 C),thematerialscanbetypicallyexpensiveand/ordifficult toproduce,andtheycansufferfromchemicalandthermalinstability [12,13].
e. Ionicliquids(ILs) whichcontainaminegroupshavebeentoutedasapotentiallongtermreplacementforliquidaminesinCCStechnologiesasILshavebeenfoundto exhibitsignificantlybetterchemicalandthermalstabilitythanaqueousamines. Drawbacks:ILsexhibitrelativelylowuptakeandworkingcapacityandIL CO2 interactionsarebaseduponthesamechemisorptionprocessasliquidaminessotheyalso requireelevatedtemperaturesduringtheILregenerationcycle [14]
2. Physisorption representsafundamentallydifferentapproachtoC-capturesinceitrelies uponthephysicalpropertiesofCO2 suchasitsabilitytoengageinnoncovalentinteractionsanditssize(Fig.2.1,right).Inprinciple,asindicatedby Table2.1,advancedphysisorbentsthatselectivelycaptureCO2 fromgasmixturesofferthegreatestupsidepotential forarevolutioninC-capturetechnologysincetheywouldrequiremuchlessenergyfor recycling,canexhibithighworkingcapacity,and,ifdesignedproperly,willbeunaffected bymoisture.However,therearestillobstaclesthatmustbeovercomebeforedeployment becomesfeasible.
a. PorousphysisorbentsofferpotentialtoimprovetheenergyefficiencyofC-capture becauseoftheirrelativelylowenergyfootprintforrecycling.Thecurrentbenchmark physisorbentsare zeolites and metal-organicframeworks, MOFs,specifically,zeolite 13XandMg-MOF-74,respectively. Drawbacks:thesetop-performingphysisorbents arehandicappedbyrelativelypoorselectivityforCO2 overtheothercomponentsof industrialgasmixtures(principallyCH4,N2,O2,and,especially,H2O),whichlimits theirusefortraceC-capture.Therelativelyhighcostand/orpoorstabilityofMOFs arealsoofconcern [15].
b. Sizeexclusion couldbeusedby molecularsieves toseparateCO2 fromlargergas molecules [16]. Drawbacks:requiresamaterialwithaveryspecificporesize,fabricationintoamembraneisdifficult,andwouldnotrestrictwatervapor,whichexhibitsa smallerkineticdiameter(2.65A ˚ )thanthatofCO2
c. Hybridultramicroporousmaterials(HUMs).HUMs [17,18] arearecentlyintroduced classofphysisorbentsthatexhibitnewbenchmarksforCO2 selectivityversusimportantgasessuchasN2 andCH4.HUMsthereforehavethepotentialtoaddressthefull
Figure2.2 RelationshipbetweenthecostsofC-capture,CO2 selectivity,andworking capacityforsolidphysisorbents [23].
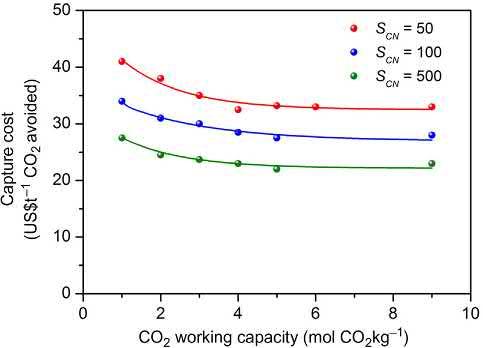
rangeofC-captureapplications,includingtracecapture.HUMscombine , 0.7nm poreswithstrongelectrostaticsthatenhancetheenergeticsofsorbent sorbateinteractions [19 22].HUMsarethereforehighlyselectiveandcantargeteventhemostchallengingseparations/purifications,suchasremovaloftraceimpuritiesofCO2 fromgas mixturesandC-capturefromcomplexwetgasmixtures.Theselectivityperformance ofHUMs(SCN . 1000)issuchthattheycanbeprojectedtosignificantlyimprovethe processcostofcarboncapture(Fig.2.2) [23].
Inthischapterwefocusonthepotentialforadvancedphysisorbentsbasedupon porouscoordinationnetworkssuchasMOFs [24],porouscoordinationpolymers, PCPs [25],andHUMs [17,18] toenableinnovationwithrespecttotheirdeployment inCCStechnologies.Weemphasizehowthemolecularlevelcontrolofporesizeand porechemistrythatcanbeachievedinsuchphysisorbentsandtheircompositional diversitycanbeusedtotailorperformancewithrespecttoseparationofgasmixtures inamannerthatisnotsoreadilyachievedinotherclassesofsorbents.However,we alsoaddressthehurdlesthatremaintobeovercomebeforeimplementationcanoccur.
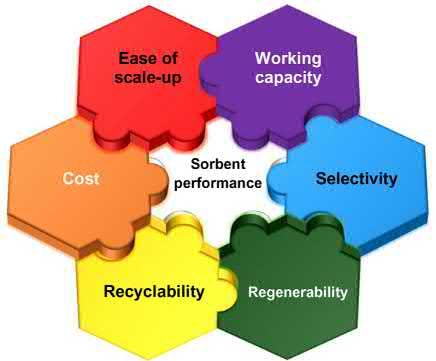
2.2Spectrumofperformanceparametersandcriteria forevaluationofsorbents Beforewediscussspecificclassesofsorbents,itwouldbeappropriatetoaddress relevantperformanceparametersandcriteria.Aspectrumofperformanceparametersmustbeaddressedandevaluatedbeforeasorbentisselectedasaleadcandidate.Somearemorerelevanttoearlystagediscoverywhereasothersmatteratlater stagesofdevelopment.Thesecriteriaarepresentedin Fig.2.3 andarediscussed individuallyinthissection.
10Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
Figure2.3 Thespectrumofperformanceparametersthatmustbemetbyasorbentbeing consideredfordevelopmentwithrespecttostorageand/orpurificationtechnologies.
2.2.1Workingcapacity Workingcapacityisakeycriterionforevaluatingsorbentperformancesinceit influenceshowmuchsorbentisrequiredforaparticularprocess.Thesaturation uptakeofasorbentprovidesinformationonthemaximumamountofCO2 thatcan beadsorbedbyagivensorbentandcanbereadilydeterminedusingpuregas adsorptionexperimentsthatprovidegravimetricorvolumetricmeasuresofuptake. GravimetricsaturationuptakecapacityistheamountofCO2 adsorbedperunit masssorbent(gramCO2 g 1 sorbent,orcm3 CO2 g 1 sorbent)at195K. VolumetricsaturationuptakecapacityistheCO2 uptakepervolumeofsorbent (gCO2 cm 3 sorbent,orcm3 CO2 cm 3 sorbent)at195K.Thesesaturationuptake valuesareusefulasafirstassessmentofthepotentialutilityofasorbent.However, theuptakeofCO2 adsorbedattheloadingconditionsthatarerelevanttotheprocess inquestionisamorerelevantparameter.Workingcapacity(ΔNCO2 ),thedifference betweentheuptakeofCO2 attheadsorption(loading)anddesorption(release)conditionsoftemperatureandpressureoftheprocessinquestion,isthemostrelevant measureofuptakeperformance.
WhereasCO2 uptakevaluesat1bararerelativelyeasytomeasureandfrequentlyreported,theyareunlikelytobereliableindicatorsofsorbentperformance atthepartialpressuresofCO2 typicallypresentinacomplexgasmixture [26].CO2 uptakeattheprocessrelevantpartialpressure(s)servesasamoreappropriate parameterandcanalsobedeterminedfrompuregasisotherms(Fig.2.4). Fig.2.4 alsohighlightshowimportantthetypeofisothermcanbewithrespecttoworking capacity.Mostrigidsorbentsexhibittype-IorLangmuirisotherms(Fig.2.4,left)
ΔNCO2 5 Nads Ndes
Figure2.4 Schematicillustratinghowtheworkingcapacitycandifferbetweenadsorbents withthesameuptakebutwithtwodistinctisothermtypes.Left:atype-Iisothermas exhibitedbymostrigidmicroporoussorbents;right:atypeF-IVisothermasfoundin flexiblesorbentsthatcanswitchbetweenclosed(nonporous)andopen(porous)phases [27].
whereasflexiblesorbentscanexhibitsteppedortypeF-IV [27] isotherms(Fig.2.4, right).Thelatterismuchlesscommonbutcanbehighlyadvantageoussinceworkingcapacitycanbe100%ofuptakecapacityinflexiblematerials.
2.2.2CO2 selectivity, Sads(CO2) CO2 selectivityforasorbentdefinesthesorptionuptakeratioofCO2 overanother gasunderagivensetofconditions.Selectivityversusnitrogen(SCN)isrelevant withrespecttopostcombustionC-capturewhereasselectivityversusmethane(SCM) isparticularlyrelevantforNGpurification.Asrevealedby Fig.2.2,afivefold increasein SCN,forexample,from100to500,wouldbeexpectedtosignificantly reduceC-captureexpensepertonne( . 45to B30h t 1 CO2) [23].Selectivityalso impactsthepurityofadsorbedgasandlevelsintheoutlet(effluent)gasstream.For abinarymixture,the adsorptionselectivity (Sads or αij)isdefinedasfollows
Sads 5 qi =qj pi =pj
where qi,j and pi,j denotetheworkingcapacitiesandpartialpressuresforcomponents i and j,respectively.Thisisalsoreferredtoas Idealselectivity(IS) or molarselectivity. Analternativemethodistocalculate Henry’slawselectivity,whereintheCO2 selectivityoverN2 iscalculatedbasedontheHenry’sconstants(KH)ofthegases:
5 KH ðCO2 Þ KH ðN2 Þ
TheratiooftheHenry’slawconstants,thatis,Henry’slawselectivityreflects therealmixtureselectivityonlyatverylowpressureandlowloadings [28]
SCN
12Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
IAST.AcommonlyusedmethodtoestimateselectivityisbaseduponIdeal AdsorbedSolutionTheory(IAST),whichusesthesingle-componentadsorptionisothermsofCO2 andcompetinggas(es) [29].IASTcalculations,whichcanbeperformedusingamodifiedversionoftheprogramIAST [29],pyIAST [30],are limitedbythreeassumptions:
1. Thepurecomponentsformanidealmixture(nochangeinareaorenthalpyuponmixing ofpurecomponents),areasonableapproximationformostsystems.
2. Theareaaccessibletobothadsorbatesisequal(i.e.,thesorbentisnotamolecularsieve).
3. Thethermodynamicpropertiesofthesorbentdonotchangerelativetothethermodynamic propertiesofthesorbate(i.e.,thereisnosorbate-inducedphasetransition).
IASTusesthedatapointsoftheexperimentalsingle-componentisothermsunder aparticulartemperaturefordifferentgasesinterpolatedvianumericalquadrature:
Amodelofeachisothermisformulatedwherethespreadingpressure, A=RT πi Px i ,fromabsolutevacuumtothefirstdatapointisassumedtofollow Henry’slaw:
where nx i isuptake,and P ispressure,andtherefore:
Betweenthefirsttothelastexperimentalisothermdatapoints,thefunction nx i P ðÞ isapproximatedvialinearinterpolation:
where mj istheslopeand bj istheinterceptofthelinethatpassesthroughthe points Pj ; nx i Pj and Pj11 ; nx i Pj11 .Theindex k definestherange
Pk # Px i # Pk11 .Thereforetheintegral Ð Px i Pk nx i P ðÞ P dP actsinthesamewayas Eq.(2.4) butaccountsforthelinethatpassesthroughinterpolatedpoints.Thismeansthat thespreadingpressureismostsensitivetothelow-coverageregimeofthesinglecomponentadsorptionisotherm [30].ItisimportanttonotethatLangmuir,BET, Langmuir Freundlich,dual-siteLangmuir Freundlichandquadraticequationscan beemployedinsteadofHenry’slawtofit/modelthepurecomponentisotherms,as applicable [31].
ThepracticaladvantageofselectivityvaluesderivedfromIASTandHenry’s Lawisthattheyenableestimationoftheseparationperformancesolelyfrom single-componentsorptionisothermswithouttheneedforcomplicatedandcostly mixed-gasequipment.However,breakthroughmeasurementsinafixed-bedreactor withmodeli.e.,simulatedgasmixturesaremuchclosertotheactualpracticeof gascapture/separation,andsorptionselectivitypredictedfromIASTcalculations candrasticallydifferfromrealmixed-gasseparationsbecausethelatterisconductedunderkineticflowconditions,oftenundernonequilibriumconditions [32]. Moreover,regenerabilityandrecyclability(particularly,moisture-mediated)cannot beaccuratelyevaluatedusingadsorptioninstruments,whereastheseparameterscan beaddressedbymixedgasbreakthroughmeasurements.Further,afewprocess engineeringaspects,suchasparticlesizeinfluenceonbackpressureofthecolumn orheatmanagementissues,canbemodeledthatarebeyondthereachofpuregas adsorptionmeasurements [33].Therefore,dynamicbreakthroughexperimentsusing dryandwetgasmixturesareingeneralamuchbetterindicatorofreal-world performance.
Breakthroughexperiments aretypicallyconductedatroomtemperaturewith B0.5gofpre-activatedsampleplacedinaquartztubingofpredetermineduniform diametertoforma“fixedbed.”Thesorbentbedispurgedunderasteadyflowof Hebeforethebreakthroughexperimentisconducted.Uponcoolingto25 C,amulticomponentgasmixtureofdesiredcomposition,typicallywithHeasthecarrier gas,isintroduced.ForC-capture/commoditypurificationtesting,thisfeedisgenerallybinaryinnature,thatis,CO2/N2,CO2/CH4,CO2/C2H2,H2/CO2 (each,either dryormoisture-mediated).Whereastheinletgascompositionispreciselycontrolledandkeptunalteredduringexperiment(s),theoutletgascompositioniscontinuouslymonitoredbymassspectrometry(MS)orgaschromatography(GC)until completebreakthroughisachieved.Experimentsinthepresenceofcontrolledrelativehumidity(%RH)areperformedbypassingthegasstreamthroughawater vaporsaturatorat25 C.Aftereachdryandwetbreakthroughexperiment,the packedcolumnbedisregeneratedusingtemperatureorpressureswingoracombinationofboth,underconstantHeflowforextendedtimetoensurecompletesample regeneration.Theamountofadsorbedgas i (qi)iscalculatedfromthebreakthrough curveasfollows:
Here, VT isthetotalflowrateofgas(cm3 min 1), Pi isthepartialpressureof gas i (atm), ΔT isthetimeforinitialbreakthroughofgas i tooccur(minutes),and m isthemassofthesorbent(g).Theseparationfactor(α)forthebreakthrough experiment,thatis,breakthroughderivedselectivity,isdeterminedasfollows:
α 5 q1 y2 q2 y1
where yi isthepartialpressureofgas i inthegasmixture.Inthecasewhereone gascomponenthasnegligibleadsorption,theamountofgasadsorbedistreatedas # 1cm3 forcalculationpurposes.TocomparetherelativeC-capture/purification efficiencyofdifferentadsorbents,itisimportanttoconsiderthreeparameters:separationfactor(α),workingcapacity(ascalculatedfromthebreakthroughprofiles), andbreakthroughtime.StrongCO2 sorbentinteractionsandfastkineticswill improvetheseperformanceparameters.
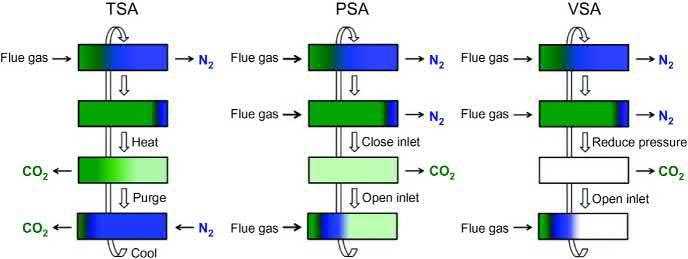
2.2.3Regenerability Minimizingtheenergyinputforregenerationviafine-tuningofthethermodynamics ofinteractionbetweenthesorbentandCO2 isoneofthemostcriticalconsiderationstoimprovetheenergyefficiencyofC-capture [26].Thisisdependentupon twofactors:enthalpyofadsorptionandadsorption/desorptionkinetics.Pressure swing(PSA),vacuumswing(VSA),andtemperatureswing(TSA)arethemethods mostcommonlyusedtoregeneratesorbents.ForPSA(Fig.2.5,middle)orVSA (Fig.2.5,right)recycling,thecolumnpressureisreducedafteradsorptioninorder todesorbthecapturedgas,thatis, Pdes , Pads.ForPSA,theinletgasispressurized viacompressionandflowedthroughthecolumnuntilitreachessaturation.Onclosingtheinletvalve,thecolumnpressuredeclinestowardambientpressure.During thisprocess,significantamountsofsorbatedesorbandelutefromthecolumn.VSA reducescolumnpressuretosubatmosphericpressureafteradsorptionatahigher pressuretoremovethesorbate.Forgasreleasenearambientpressure(e.g.,postcombustion),compressingorevacuatingsuchalargevolumeofgasisdifficult,and TSA(Tdes . Tads)islikelytobemoreviable(Fig.2.5,left) [34].Thatprecombustioninvolvesaninherentlypressurizedgasstream(aftertheconversionreactions)
Figure2.5 Schematicdiagramsoftemperatureswingadsorption(TSA),pressureswing adsorption(PSA),andvacuumswingadsorption(VSA)employedforsorbentregenerationin afixed-bedcolumn.
Source:ReprintedwithpermissionfromK.Sumida,D.L.Rogow,J.A.Mason,T.M. McDonald,E.D.Bloch,Z.R.Herm,etal.,Carbondioxidecaptureinmetal-organic frameworks,Chem.Rev.112(2012)724 781.Copyright(2016)AmericanChemical Society. 14Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
meansthataPSAcycleismostlikelytobepreferred [35].Theefficiencyofsorbentregenerationisaffectedbytwoprimaryfactors,theenthalpyassociatedwith adsorptionandthekineticsofadsorption/desorption.
2.2.3.1Enthalpyofadsorption(Qst) Isostericenthalpyofadsorption, Qst,isameasureoftheheatreleasedduring adsorptionandthereforeprovidesaguidetotheenergyrequiredtoregeneratethe sorbent.Theamountofheatneededtoregenerateasorbentisaparameterthatsignificantlyinfluencesregenerationcost. Qst(CO2)forasorbentcanbecalculated fromitslow-pressureCO2 adsorptionisothermscollectedatdifferenttemperatures (oftenthetemperaturesstudiedare273K,283K,and298K).Ingeneral,virial-type equationsareusedtofitatleasttenlow-pressurepointsintheadsorptiondataat multipletemperatures(Eq.2.5) [36]
Qst isthencalculatedfromthevirialmodelusing Eq.(2.6).
Qst isthecoverage-dependententhalpyofadsorption,and R istheuniversalgas constant.Generally,higherrelativeenthalpiesofadsorptionresultinhigherselectivityandwouldrenderasorbentmoresuitedforremovaloftraceamountsof adsorbatefromindustrialgasstreams.However,ahighenthalpyofadsorption wouldalsobeexpectedtoresultinahighenergyfootprintforrecyclingthesorbent. Thisisanareawherephysisorbentswouldbeexpectedtooffersignificantadvantagesoverchemisorbentsbecauseevenahigh Qst valueforaphysisorbentislikely tobelessthanthatofachemisorbent.Forexample,somephysisorbentsthathave beensubjectedtotemperatureswingregenerationrequirearegenerationtemperatureof , 55 C(e.g.,HUMslikeSIFSIX-2-Cu-i [19],TIFSIX-2-Cu-i [37],SIFSIX14-Cu-i [20]),whereas B75 C 150 Ctreatmentisrequiredforchemisorbents studiedforC-capturesuchasPEI(Polyethyleneimine)modifiedMCM-41 [38] and TEPA(tetraethylenepentamine)modifiedSBA-15 [39].
2.2.3.2Sorptionkinetics Adsorption desorptioncycletimeisgreatlydependentupontheCO2 adsorption desorptionkineticsofagivenmaterial.Kineticscanbemeasuredviadynamic breakthroughandtemperature-programmeddesorptionexperiments.Thisaspectof sorptionperformanceremainsthusfarunderstudiedinMOFsandPCPs.Sorbents thatadsorbanddesorbCO2 inashortertimearepreferredasthesereducecycle
timeaswellastheamountofsorbentrequired,andultimatelycurtailcost.Thisis anotherperformanceparameterwherephysisorbentsmightbeexpectedtooffersignificantadvantagesoverchemisorbents,especiallyfortracecaptureofCO2
2.2.4Recyclability(hydrolytic,mechanical,andthermalstability) Asorbentmustdemonstratestabilityundertheconditionsofaprocessandduring manycyclesofadsorption desorption.Strongthermal,mechanical,andhydrolytic stabilityundertheconditionsofaprocessareessentialforrecyclingofsorbents. Unfortunately,poormechanicalstabilitytopressurechangesand/orpoorhydrolytic stabilitythatresultinpoorrecyclabilityplaguemostMOFs.Sorbentswhichneed heatingduringregeneration,eitherTSAorPTSA(pressure-temperatureswing) mustbethermallyrobustsincerepeatedheatingcycleswillotherwisecausesorbent degradation.
Thethermalstabilityofsorbentscanbeevaluatedusingtwocomplementary methods.High-resolutionthermogravimetricanalysiscoupledwitheitherMSor FT-IRcanbeusedtodeterminetheidentityandquantityofcomponentweightloss versustemperature.Variable-temperaturePXRDcanprobeifelevatedtemperatures resultinreversibleorirreversiblestructuralchanges.Thesetestscanbeusedto determinetheoptimalactivationproceduresforasorbentduringgassorption experimentsandtemperatureswingrecycling.Hydrolyticstabilitycanbeaddressed byusingstandardstabilityteststhatweredesignedtomeasureshelf-lifeofdrug substancesanddrugproducts.Inparticular,acceleratedstabilitytestsconductedat 40 Cand75%RHover1,7,and14days [40] candeterminethelikelyshelf-lifeof asorbent.UnlikemostMOFs,anumberofHUMswerefoundtoexhibithydrolytic stabilitycomparabletoinorganicmaterialssuchassilicasandzeolites [15,41].
2.2.5Sorbentcostbaseduponsubstrates Sorbentsthatexhibitexcellentsorptionperformancemustalsobejudgedbased uponthecostofsubstratesusedtopreparethesorbent.Thepracticalityofusinga MOFsorbentinaC-captureprocesswillnotjustrelyonitsphysicochemicalpropertiesbutalsouponthecostofitsingredients.Thatmetalsaltsaregenerallyinexpensivemeansthatlinkerligandcostistypicallythemainfactorthatwillaffectthe costofasorbent.ManyMOFsorbentsarebaseduponpolycarboxylateligands, someofwhicharepreparedusingcross-couplingreactions.Nevertheless,thereare anumberofcommerciallyavailable,inexpensiveligands.Forexample,4,40 -bipyridine, bipy,isthemostcommonligandintherecentlypublishedMOFsubsetofthe CambridgeStructuralDatabase,CSDwithover4000entries [42].Amongcarboxylates,1,4-benzenedicarboxylate(BDC)basedstructuresarethemostcommonly studiedwith B3100entries.Theseligandswouldbehighlyappropriatestarting pointsforsorbentdesign,synthesis,andmanufacturegiventhescaleofsorbentproductionthatwouldbeneededformostC-captureapplications. 16Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
2.2.6Feasibilityofscale-up Scale-upcanrepresentanobstacletothedevelopmentofasorbent [43].Uponidentifyingasorbentcandidatewithdesirablepropertiesandperformance,itmustbe determinedifitcanbemanufacturedinsufficientquantitytoenablelaterstage developmentincludingformulationandpilot-scaletesting.Syntheticmethodsthat areamenabletoscale-uparegenerallyunderstudiedinthecontextofMOFs,yet suchstudiesareneededinordertomoveasorbenttohighertechnologicalreadiness levels(TRLs).Pilot-scaleMOFproductionthroughdirectmixingorsolvent-free solid-stategrinding,thatis,mechanochemicalmethods(e.g.,ballmilling,highpressurecompression,singleortwin-screwextrusion(TSE),solvent-freegrindingwith heating),isdesirablebutnotalwaysviable.Nevertheless,TSEhasbeenimplementedforkgperhour-scaleMOFproductionandofferstheadvantagesofcontinuous synthesiswithreducedornosolventrequirements [44].Giventhatmechanochemicalsynthesisisasubjectofgrowingacademicinterest [45],theamenabilityofa sorbenttomechanochemicalsynthesisislikelytobecomeapartofsorbentselectionprotocols.
2.3Overallevaluationofsorbentperformance:sorbent selectionparameters Theidealsorbentwouldbeexpectedtooptimallyaddressalloftheparameters discussedin Section2.2 .Intheearlystagesofsorbent development,selectivity, workingcapacity,andadsorp tionenthalpyareofparticul arrelevancetoidentify potentialleadcandidatesforfurtherdevelopment.However,itislikelythat therewillbeatrade-offbetweenthesefactorssince,forexample,highsurface areamaterialsarelesslikelytoofferhighselectivity.Evaluatingaseriesof candidatesorbentsisanontrivialtaskb ecauseoftrade-offs.Afewselection metricshaverecentlyemergedtosimplif ytheprocessofidentifyingleadcandidatesorbents.
● The adsorbentperformanceindicator(API) wasrecentlyproposedbyLlewellynetal. [46].APIconsidersthevolumetricworkingcapacity(WC),adsorptionenthalpies(Δadsh) andselectivityvalues(α1/2),asindicatedbelow:
A,B,andCaremultiplesusedtoweightherelativeimportanceofeachtermthatis usedtocalculatetheAPIforasorbentforgas1over2.Selectivityofcomponent1over component2(α1/2)canbecalculatedfromIASTselectivityormolarselectivity [29].The APIaffordsaparameterthatcanbeusedtocomparesorbentsinordertofacilitateselectionofaleadcandidateforagivenprocess.However,itdoesnotaddresssomeofthe laterstageparametersdiscussedabove.
● The separationpotential (ΔQ)hasbeenputforwardbyKrishnaasakeysorbentselection criteria,whichcombines Sads(CO2)andworkingcapacity(ΔNCO2 )inamannerwhich mimicsaspectsofafixedbedadsorptionprocess [47]
ΔQ 5 Nads;CO2 3 ðpi =pCO2 Þ Nads;i ; pi and pCO2 denotethepartialpressuresforcomponent i (N2 orCH4,ingeneral)andCO2,respectively.
● Keskinetal.proposedanewapproachofconsidering percentregenerability (R%)to comparativelyassessMOFsbecausehighCO2 selectivitiesinsorbentsoftensufferfrom verylow R%( , 75%) [48].
R% 5 ΔNCO2 Nads;CO2 3 100%
● The adsorbentperformancescore(APS) wasintroducedin2018 [49] andistheproduct ofselectivityandworkingcapacity.Efficientadsorbentsthatexhibithighselectivityand highworkingcapacitywouldofferhighAPSvalues.
APS 5 SadsðCO2 Þ=i 3 ΔNCO2 ;ingeneral, i:N2 orCH4 inthebinarymixture.
2.4CurrentCO2 capturetechnologies 2.4.1Point-sourceCO2 capture
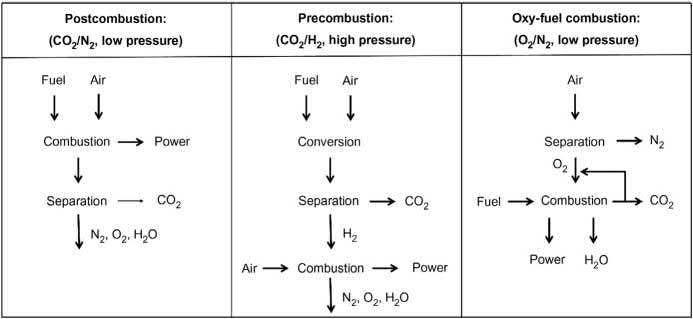
ReductionofCO2 emissionsfrompointsourcessuchaspowerplantscanbe addressedusingthreemainapproaches:(1)postcombustion;(2)precombustion;and (3)oxy-fuelcombustion(Fig.2.6).Allthreeapproachessufferanenergypenaltyof c.10% 14%onaccountoftheenergyfootprintassociatedwithgasfeedseparation,capture,conditioning,andcompression/liquefactionofthecapturedCO2 [50]
Figure2.6 Thethreetypesofpoint-sourceCO2 capture.
Source:AdaptedfromK.Sumida,D.L.Rogow,J.A.Mason,T.M.McDonald,E.D.Bloch,Z. R.Herm,etal.,Carbondioxidecaptureinmetal-organicframeworks,Chem.Rev.112 (2012)724 781. 18Metal-OrganicFrameworks(MOFs)forEnvironmentalApplications
Thisreducespowerplantefficiencyandsomewhatovershadowstheupsidepotentialsofallthesetechnologies [51].
2.4.1.1Postcombustion ProcessesthatinvolveC-captureaftercombustionmusttakeintoaccountfluegas cleaningprocessessuchasremovalofdust,nitrogen,andsulfurcompoundsandare collectivelytermed“Postcombustion”processes [52].Inpostcombustioncapture, CO2 isremovedfromthefluegasthatresultsaftercombustionofthefuelinair (compositionasdescribedin Table2.2).ThisispredominantlyaCO2/N2 gasseparationowingtohighN2 contentintheambientairusedforcombustionandhas beenthemostexploredstrategythusfarsinceapostcombustionC-capturesystem couldberetrofittedtoexistingpowerplants. Liquidamineabsorption or amine scrubbing currentlyrepresentthestate-of-the-artforbulkCCSandCCUbutliquid aminessufferfromcostandcorrosionchallenges.
LiquidamineprocesseshavebeentestedandappliedforindustrialCO2 removal fromprocessgasstreamslargelybecausetheyarebaseduponchemisorptionand theycanexhibithighseparation/purificationperformance [38,53].Themostcommonlyusedaminesarealkanolamines,suchasmonoethanolamine(MEA),diethanolamine(DEA),andmethyldiethanolamine(MDEA),dissolvedinwater.A schematicillustrationofaliquidamineabsorptionplantforCO2 postcombustion captureispresentedin Fig.2.7A.Liquidamineabsorptionisanenergy-intensive technologyanditsmostextensiveuseisinthecontextofNGsweetening.
Table2.2 TypicalconditionsforthethreemajorC-captureprocesses:postcombustion, precombustion,andnaturalgassweeteningprocesses.
Gascomposition(bymole)