Metal-OrganicFrameworksforChemicalReactions: FromOrganicTransformationstoEnergy Applications1stEditionAnishKhan(Editor)
https://ebookmass.com/product/metal-organic-frameworks-forchemical-reactions-from-organic-transformations-to-energyapplications-1st-edition-anish-khan-editor/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Metal-Organic Frameworks (MOFs) for Environmental Applications Sujit K. Ghosh
https://ebookmass.com/product/metal-organic-frameworks-mofs-forenvironmental-applications-sujit-k-ghosh/
ebookmass.com
Metal-Organic Frameworks with Heterogeneous Structures Ali Morsali
https://ebookmass.com/product/metal-organic-frameworks-withheterogeneous-structures-ali-morsali/
ebookmass.com
Metal-free synthetic organic dyes Kruger
https://ebookmass.com/product/metal-free-synthetic-organic-dyeskruger/
ebookmass.com
Eisenhower and American Public Opinion on China Mara Oliva
https://ebookmass.com/product/eisenhower-and-american-public-opinionon-china-mara-oliva/
ebookmass.com
Contemporary Retail Marketing in Emerging Economies: The Case of Ghana’s Supermarket Chains David Eshun Yawson
https://ebookmass.com/product/contemporary-retail-marketing-inemerging-economies-the-case-of-ghanas-supermarket-chains-david-eshunyawson/ ebookmass.com
Kierkegaard'u Nas■l Okumal■y■z? 1st Edition John Caputo
https://ebookmass.com/product/kierkegaardu-nasil-okumaliyiz-1stedition-john-caputo/
ebookmass.com
Jacob Schiff and the Art of Risk 1st ed. Edition Adam Gower
https://ebookmass.com/product/jacob-schiff-and-the-art-of-risk-1st-ededition-adam-gower/
ebookmass.com
The Oxford History of Life-Writing, Volume 2: Early Modern Alan Stewart
https://ebookmass.com/product/the-oxford-history-of-life-writingvolume-2-early-modern-alan-stewart/
ebookmass.com
Oxford Studies in Agency and Responsibility, Volume 5: Themes from the Philosophy of Gary Watson D. Justin Coates (Editor)
https://ebookmass.com/product/oxford-studies-in-agency-andresponsibility-volume-5-themes-from-the-philosophy-of-gary-watson-djustin-coates-editor/
ebookmass.com
Groups Process and Practice 10th Edition Marianne Schneider Corey https://ebookmass.com/product/groups-process-and-practice-10thedition-marianne-schneider-corey/
ebookmass.com
Metal-OrganicFrameworksfor ChemicalReactions FromOrganicTransformationstoEnergyApplications
Metal-Organic Frameworksfor ChemicalReactions FromOrganicTransformationstoEnergy Applications Editedby
AnishKhan
CenterofExcellenceforAdvancedMaterialsResearch, KingAbdulazizUniversity,Jeddah,SaudiArabia
FrancisVerpoort
StateKeyLaboratoryofAdvancedTechnologyforMaterialsSynthesis andProcessing,WuhanUniversityofTechnology,Wuhan,China; GhentUniversity–GlobalCampus,Ywonsu-Gu,Incheon, RepublicofKorea
AbdullahM.Asiri
ChemistryDepartment,FacultyofScience,KingAbdulazizUniversity, Jeddah,SaudiArabia;CenterofExcellenceforAdvancedMaterials Research,KingAbdulazizUniversity,Jeddah,SaudiArabia
MdEnamulHoque
DepartmentofBiomedicalEngineeringattheMilitaryInstituteof ScienceandTechnology(MIST),Dhaka,Bangladesh
AnwarL.Bilgrami
DepartmentofEntomology,RutgersUniversity,NewJersey,United States;DeanshipofScientificResearch,KingAbdulazizUniversity, Jeddah,SaudiArabia
MohammadAzam
AssociateProfessorofChemistry,KingSaudUniversity,Riyadh,Saudi Arabia
K.ChandraBabuNaidu
GITAMSchoolofScience(GSS),GITAMDeemed-to-BeUniversity, Bangalore,India
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2021ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyright LicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professionalpractices,or medicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein. Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthesafety ofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterof productsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress ISBN:978-0-12-822099-3
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: EmilyM.McCloskey
EditorialProjectManager: LenaSparks
ProductionProjectManager: DebasishGhosh
CoverDesigner: VictoriaPearson
TypesetbyMPSLimited,Chennai,India
1.Metal-organicframeworksandtheircomposites
M.RameshandC.Deepa
2.Metal-organicframeworkforbatteriesand
M.Ramesh,N.KuppuswamyandS.Praveen
3.Titanium-basedmetal-organicframeworksfor
A.Ratnamala,G.DeepthiReddy,M.Noorjahaan,H.Manjunatha, S.Janardan,N.SureshKumar,K.ChandraBabuNaidu,AnishKhan andAbdullahM.Asiri 3.1Introduction
4.Electrochemicalaspectsofmetal-organicframeworks
H.Manjunatha,S.Janardan,A.Ratnamala,K.VenkataRatnam, L.VaikuntaRao,S.Ramesh,K.ChandraBabuNaidu, N.SureshKumar,AnishKhanandAbdullahM.Asiri
4.1Introduction
5.Permeablemetal-organicframeworksforfuel(gas) storageapplications
S.Janardan,PC.V.V.EswaraRao,H.Manjunatha,K.VenkataRatnam, A.Ratnamala,K.ChandraBabuNaidu,A.Sivarmakrishna, AnishKhanandAbdullahM.Asiri
5.1Introduction
5.3Permeablemetal-organicframeworksforH2
5.4Permeablemetal-organicframeworksforCH4
5.5Permeablemetal-organicframeworksforC2H2 storage applications
5.6Permeablemetal-organicframeworksforCO2
6.Excessivelyparamagneticmetalorganicframework nanocomposites
B.VenkataShivaReddy,N.SureshKumar,K.ChandraBabuNaidu, K.Srinivas,H.Manjunatha,A.Ratnamala,AnishKhanand AbdullahM.Asiri
6.1Introduction
7.Expandingenergyprospectsofmetal-organic frameworks
K.RamaKrishnaReddy,D.PrakashBabu,N.SureshKumar, G.RanjithKumar,K.ChandraBabuNaiduandAnishKhan
7.1Introduction
7.2Metal-organicframeworksinLi-ionbatteries
7.3Applicationsofmetal-organicframeworksaselectrode materialforlithium-ionbatteries
7.4Applicationsofhighconductivemetal-organicframeworks
7.5Utilizationofmetal-organicframeworksaselectric double-layercapacitors(supercapacitors)
7.6Utilizationoflithium oxygenasseparators
7.7Utilizationofsolid-stateelectrolytes
7.8Applicationsofelectrode electrolytealliances
7.9Fuelcellapplications
7.10Electrocatalyticapplications
7.11Conclusion
8.Metal-organicframework
PrasunBanerjee,AdolfoFrancoJr,K.ChandraBabuNaidu, AnishKhan,AbdullahM.AsiriandSrinivasanNatarajan
8.31D-metal-organicframework
8.42D-metal-organicframework
8.53D-metal-organicframework
9.Applicationsofmetal-organicframeworksin analyticalchemistry
RuthRodr´ıguez-Ramos,A ´ lvaroSantana-Mayor, B ´ arbaraSocasRodr´ıguez,AntonioV.Herrera-Herrera andMiguelA ´ ngelRodr´ıguezDelgado
9.1Introduction
9.2DesirablecharacteristicsofMOFsforanalyticalchemistry
9.3Recentapplications
10.Modifiedmetal-organicframeworksasphotocatalysts 231
WeiNiandAnishKhan
11.Thesensingapplicationsofmetal-organicframeworks andtheirbasicfeaturesaffectingthefateofdetection
TolgaZorlu,LucaGuerriniandRamonA.Alvarez-Puebla
12.Thermomechanicalandanticorrosioncharacteristics ofmetal-organicframeworks
MohammadRamezanzadehandBahramRamezanzadeh
13.Metal-organicframeworks:preparationand applicationinelectrocatalyticCO2
RajasekaranElakkiyaandGovindhanMaduraiveeran
14.Metal-organicframeworksasdiversechemical applications
ShahidPervezAnsari,AhmadHusain,MohdUroojShariq andAnishKhan 14.1Introduction
14.2Electrochemicalapplications
14.3Metal-organicframeworksinsupercapacitorapplications
14.4Wastewatertreatment
14.5Drugdelivery
15.Metal-organicframeworksaschemicalreactionflask
RakeshKumarAmetaandParthMalik
15.1Introductiontometal-organicframeworks
15.4Utilityofmetal-organicframeworkaschemical reactionflask
16.Uniqueattributesofmetal-organicframeworks indrugdelivery 389
ParthMalik,RachnaGuptaandRakeshKumarAmeta 16.1Introduction
16.2Synthesisofmetal-organicframeworks
16.3Aspiringfeaturesformetal-organicframeworks’application indrugdelivery:toxicologicalcompatibility,stability,and biodegradation
16.4Surfacemodificationofmetal-organicframeworks
16.5Synthesisofnanoscalemetal-organicframeworks
16.6Therapeuticefficacyofmetal-organicframeworks
16.7Howmetal-organicframeworkscanadvancethepresent successofdrugdelivery?
16.8Drugreleasemechanismsofmetal-organicframeworks
16.9Conclusionandfuturedirections
17.Metal-organicframeworksandpermeablenatural polymersforreasonablecarbondioxidefixation
M.Ramesh,M.MuthukrishnanandAnishKhan
18.Nanomaterialsderivedfrommetal-organicframeworks forenergystoragesupercapacitorapplication
LakshmananGurusamy,SambandamAnandanandJerryJ.Wu
Listofcontributors RamonA.Alvarez-Puebla DepartmentofPhysicalandInorganicChemistryand EMaS,UniversitatRoviraIVirgili,Tarragona,Spain;ICREA,Barcelona,Spain
RakeshKumarAmeta SchoolofChemicalSciences,CentralUniversityofGujarat, Gandhinagar,India;DepartmentofChemistry,SriMMPatelInstituteof SciencesandResearch,KadiSarvaVishwavidhyalaya,Gandhinagar,Gujarat, India
SambandamAnandan DepartmentofChemistry,NationalInstituteofTechnology, Trichy,India
ShahidPervezAnsari DepartmentofAppliedChemistry,ZakirHusainCollegeof EngineeringandTechnology,AligarhMuslimUniversity,Aligarh,India
AbdullahM.Asiri ChemistryDepartment,FacultyofScience,KingAbdulaziz University,Jeddah,SaudiArabia;CenterofExcellenceforAdvancedMaterials Research,KingAbdulazizUniversity,Jeddah,SaudiArabia
D.PrakashBabu SchoolofAppliedSciences,REVAUniversity,Bangalore,India
PrasunBanerjee DepartmentofPhysics,GITAM(DeemedtobeUniversity), Bangalore,India;InstitutodeFisica,UniversidadeFederaldeGoias,Goiania, Brazil
K.ChandraBabuNaidu DepartmentofPhysics,GITAM(Deemedtobe University),Bangalore,India
C.Deepa DepartmentofComputerScienceandEngineering,KITKalaignarkarunanidhiInstituteofTechnology,Coimbatore,India
RajasekaranElakkiya MaterialsElectrochemistryLaboratory,Departmentof Chemistry,SRMInstituteofScienceandTechnology,Kattankulathur,Chennai, India
PC.V.V.EswaraRao DepartmentofChemistry,GITAMSchoolofScience, GITAM(DeemedtobeUniversity),Bangalore,India
AdolfoFranco,Jr InstitutodeFisica,UniversidadeFederaldeGoias,Goiania, Brazil
LucaGuerrini DepartmentofPhysicalandInorganicChemistryandEMaS, UniversitatRoviraIVirgili,Tarragona,Spain
RachnaGupta SchoolofChemicalSciences,CentralUniversityofGujarat, Gandhinagar,India;DepartmentofBiotechnology,Visva-Bharati,Santiniketan, Bolpur,India
LakshmananGurusamy DepartmentofEnvironmentalEngineeringandScience, FengChiaUniversity,Taichung,Taiwan
AntonioV.Herrera-Herrera InstitutoUniversitariodeBio-Org ´ anicaAntonio Gonz ´ alez,UniversidaddeLaLaguna(ULL),SanCristo ´ baldeLaLaguna,Espan ˜ a
AhmadHusain DepartmentofAppliedChemistry,ZakirHusainCollegeof EngineeringandTechnology,AligarhMuslimUniversity,Aligarh,India
S.Janardan DepartmentofChemistry,GITAMSchoolofScience,GITAM (DeemedtobeUniversity),Bangalore,India
AnishKhan ChemistryDepartment,FacultyofScience,KingAbdulazizUniversity, Jeddah,SaudiArabia;CenterofExcellenceforAdvancedMaterialsResearch, KingAbdulazizUniversity,Jeddah,SaudiArabia
G.RanjithKumar SchoolofAppliedSciences,REVAUniversity,Bangalore,India
N.SureshKumar DepartmentofPhysics,JNTUA,Anantapuramu,India
N.Kuppuswamy DepartmentofAeronauticalEngineering,KITKalaignarkarunanidhiInstituteofTechnology,Coimbatore,India
GovindhanMaduraiveeran MaterialsElectrochemistryLaboratory,Departmentof Chemistry,SRMInstituteofScienceandTechnology,Kattankulathur,Chennai, India
ParthMalik SchoolofChemicalSciences,CentralUniversityofGujarat, Gandhinagar,India
H.Manjunatha DepartmentofChemistry,GITAMSchoolofScience,GITAM (DeemedtobeUniversity),Bangalore,India
M.Muthukrishnan DepartmentofMechanicalEngineering,KITKalaignarkarunanidhiInstituteofTechnology,Coimbatore,India
SrinivasanNatarajan SolidStateandStructuralChemistryUnit,IndianInstituteof Science,Bangalore,India
WeiNi VanadiumandTitaniumResourceComprehensiveUtilizationKey LaboratoryofSichuanProvince,PanzhihuaUniversity,Panzhihua,P.R.China; InstituteforAdvancedStudy,ChengduUniversity,Chengdu,P.R.China; MaterialCorrosionandProtectionKeyLaboratoryofSichuanProvince,Sichuan UniversityofScienceandEngineering,Zigong,P.R.China
M.Noorjahaan DepartmentofChemistry,PalamuruUniversity,Mahbubnagar,India
S.Praveen DepartmentofMechanicalEngineering,KIT-Kalaignarkarunanidhi InstituteofTechnology,Coimbatore,India
M.Ramesh DepartmentofMechanicalEngineering,KIT-Kalaignarkarunanidhi InstituteofTechnology,Coimbatore,India
S.Ramesh DepartmentofPhysics,GITAM(DeemedtobeUniversity),Bangalore, India
BahramRamezanzadeh DepartmentofSurfaceCoatingsandCorrosion,Institute forColorScienceandTechnology,Tehran,Iran
MohammadRamezanzadeh DepartmentofSurfaceCoatingsandCorrosion, InstituteforColorScienceandTechnology,Tehran,Iran
Listofcontributors xiii
L.VaikuntaRao DepartmentofChemistry,GITAMSchoolofScience,GITAM (DeemedtobeUniversity),Visakhapatnam,India
K.VenkataRatnam DepartmentofChemistry,GITAMSchoolofScience,GITAM (DeemedtobeUniversity),Bangalore,India
A.Ratnamala DepartmentofChemistry,GITAMSchoolofScience,GITAM (DeemedtobeUniversity),Bangalore,India
G.DeepthiReddy DepartmentofChemistry,PalamuruUniversity,Mahbubnagar, India
K.RamaKrishnaReddy SchoolofAppliedSciences,REVAUniversity, Bangalore,India
MiguelA ´ ngelRodr´ıguezDelgado DepartamentodeQu´ımica,Unidad DepartamentaldeQu´ımicaAnal´ıtica,FacultaddeCiencias,UniversidaddeLa Laguna(ULL),SanCristo ´ baldeLaLaguna,Espan ˜ a
RuthRodr´ıguez-Ramos DepartamentodeQu´ımica,UnidadDepartamentalde Qu´ımicaAnal´ıtica,FacultaddeCiencias,UniversidaddeLaLaguna(ULL),San Cristo ´ baldeLaLaguna,Espan ˜ a
A ´ lvaroSantana-Mayor DepartamentodeQu´ımica,UnidadDepartamentalde Qu´ımicaAnal´ıtica,FacultaddeCiencias,UniversidaddeLaLaguna(ULL),San Cristo ´ baldeLaLaguna,Espana
MohdUroojShariq DepartmentofChemistry,AligarhMuslimUniversity,Aligarh, India
A.Sivarmakrishna DepartmentofChemistry,SchoolofAdvancedSciences,VIT University,Vellore,India
B ´ arbaraSocasRodr´ıguez DepartmentofChemistry,CentreforAnalysisand Synthesis,LundUniversity,Lund,Sweden
K.Srinivas DepartmentofPhysics,GITAM(DeemedtobeUniversity),Bangalore, India
N.SureshKumar DepartmentofPhysics,JNTUA,Anantapuramu,India
B.VenkataShivaReddy DepartmentofPhysics,GITAM(Deemedtobe University),Bangalore,India
JerryJ.Wu DepartmentofEnvironmentalEngineeringandScience,FengChia University,Taichung,Taiwan
TolgaZorlu DepartmentofPhysicalandInorganicChemistryandEMaS, UniversitatRoviraIVirgili,Tarragona,Spain
Chapter1 Metal-organicframeworksand theircomposites M.Ramesh1 andC.Deepa2
1DepartmentofMechanicalEngineering,KIT-KalaignarkarunanidhiInstituteofTechnology, Coimbatore,India, 2DepartmentofComputerScienceandEngineering,KITKalaignarkarunanidhiInstituteofTechnology,Coimbatore,India
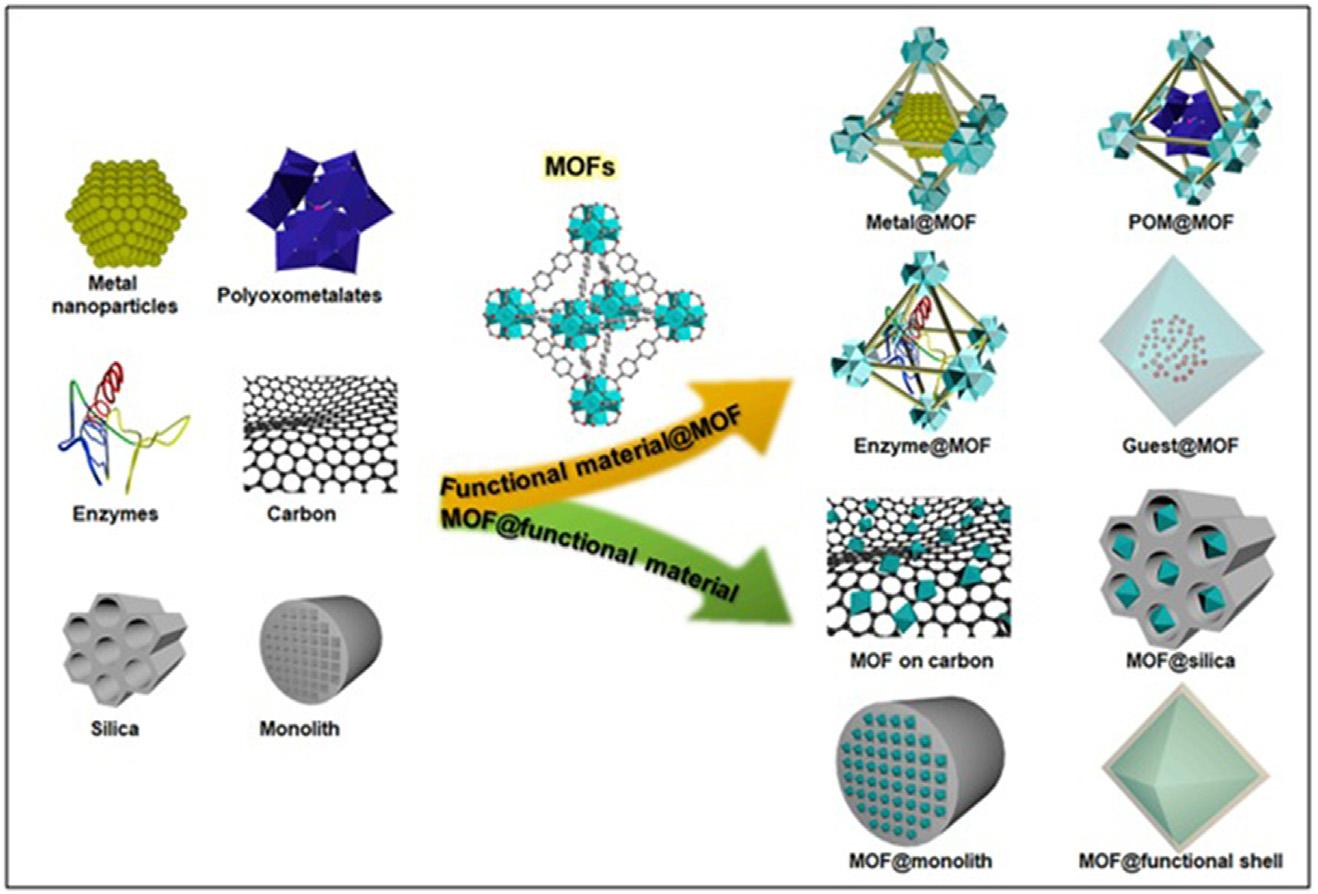
1.1Introduction Metal-organicframeworks(MOFs),alsocalledasporouspolymers,created frominorganicionswithorganicconnectors,haveemergedasapromising classofmaterialswithmanypeculiarproperties,suchashighporosity, diversecomposition,versatileporestructure,andflexiblefunctionality [1 3].Suchareevolvingadsorbentmaterialscomposedofmetalionsor clustersofmetalionsboundbyorganiclinkers [4].TheMOFsattractalot ofinterestbecauseoftheirhighcrystallinity,porosity,andmodularity [5] Thebenefitsofstructuraltuningandotherphysicalorchemicalproperties, obtainedbyastuteselectionandvariationintheshapeoflinkers,theirscale andarrangement,andpre-andpost-syntheticmodification,havedriveneverexpandingresearchintotheuseofMOFsinvariousfieldsandapplications [6].Throughtheadventofmetalnanoparticles,metaloxides,graphene,carbonnanotubes(CNTs),quantumdots(QDs),biomolecules,polymers,polyoxometalates,organicchemicals,proteins,silicaandpolymers,etc.,avariety ofMOFcompositeshavenowbeensuccessfullysynthesized [7 9].The MOFcompositesareconstructedofoneormoreMOFmaterialsshownin Fig.1.1[1].AnanalysisofTangandTanase’ssyntheticapproaches [10] is fortheproductionofMOFsandtheircomposites.Theyobservedthatwhen comparedtotheperformanceofpurepolymermembranes,MOFsembedded inpolymermatricesincreasedmixtureefficiencyandpermeability.
1.2Metal-organicframeworkcomposites MOFsthemselvesarepartofthemajorcompositematerialclass.MOFcompositesarecomparativelyrecentmaterials,usedindiverseapplications.Itis,
https://doi.org/10.1016/B978-0-12-822099-3.00001-0
2 Metal-OrganicFrameworksforChemicalReactions
however,necessarytofindsuitablecomplementarymaterialsandadaptive pathwaystoformcompositesbasedonMOF.Itsflexiblecrystallinearchitecturesandcompactunitsofmetalionsandorganicligandsareidealforthe furtherdevelopmentofcomposites [11,12].Thecompositearchitectureof MOFmaterialswithinthespecialporousstructureoffersthegreatpractical abilitytoconstructcompositematerialsbasedonMOF [13 16].Overthe lastdecade,MOFsformedthroughtheself-assemblyphaseofmetalcations ormetalclustersandsmoothorganicligandsreceivedsignificantattention [17,18].MOF-basedcompositesareprovidedwithhighstrengthandgood catalyticactivitybyintegratingthefunctionalizednanoparticlesintoMOF structuresthatprocesstheadvantagesoversingle-componentMOFs [19].
1.2.1Processingofmetal-organicframeworkcomposites ForthepreparationofMOFcomposites,threeexcellentlydevelopedtechniquesexist,suchasship-in-bottle,bottle-around-ship,andone-potsynthesis [1].Theship-in-bottlemethodentailsapplyingmetalprecursorstoanMOF usingvariousmethodssuchaschemicalvapordeposition,solventimpregnation,firmgrinding,andmicrowaveirradiation,followedbyareductionin metalprecursorstoformnanoparticlesofmetal.Thebottle-around-ship methodologyreferstotheassemblyaroundthemetalofnanoparticlesfrom MOFs.Thetricktoacquiringthecore shellstructureistoprevent
FIGURE1.1 MOFsandotherfunctionalmaterialsincorporatedatMOFcomposites [1]. MOFs,Metal-organicframeworks.
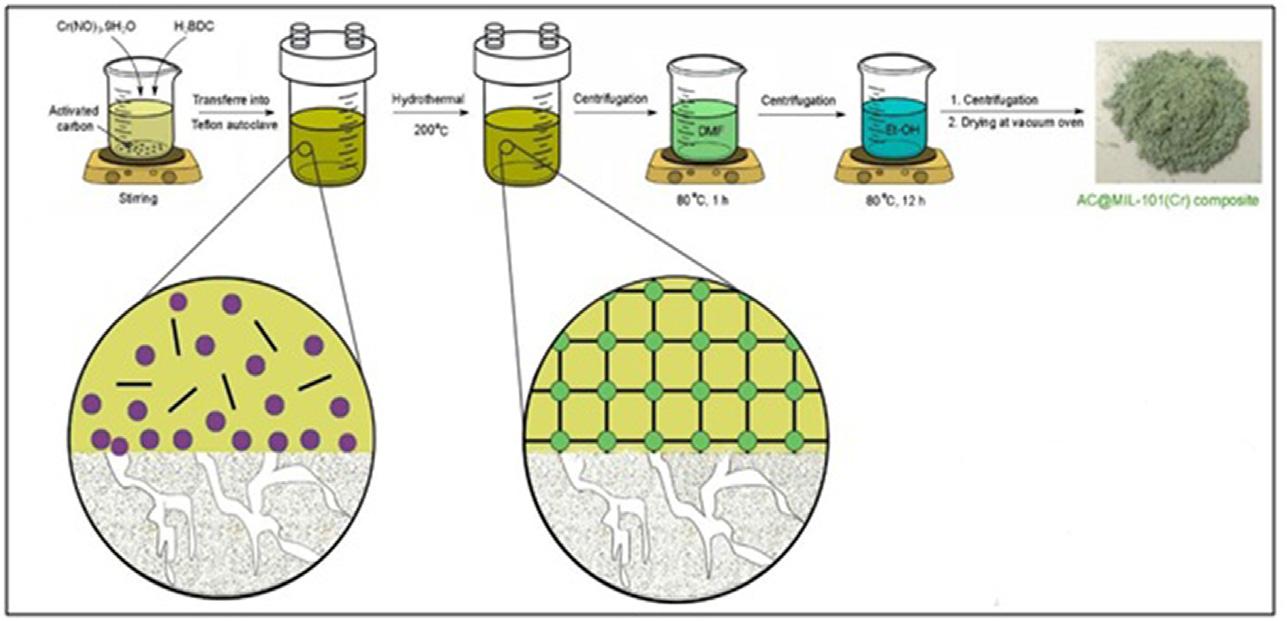
aggregatingmetalnanoparticlesandself-nucleatingMOFshells.Dueto reducedproductioncosts,shorterprocessingtimes,andeasyscaling,the one-potmethod,throughthedirectmixingofthemetalprecursorsandMOF precursorsintoonepot,hasrecentlyattractedmuchinterest.Activatedcarbon@MIL-101(Cr)nanocompositewaspreparedbyMIL-101(Cr)insitu synthesisataconversionrateofabout96%.TheprecursorMOFwastreated with25mgactivatedcarbonandautoclavedfor12hoursat473K.The resultingstockwascentrifugedandsoakedat353Kfor60minutes,toeliminateimpurities.Thepowder,obtainedthroughcentrifugation,wasdissolved inethanolandheatedat353Kfor12hours.Finally,thesynthesizedgreen powderwascentrifugedanddriedat373Kinavacuumovenfor12hours. Aschematicrepresentationofthesynthesisisshownin Fig.1.2[19].
1.2.2Typesofmetal-organicframeworkcomposites 1.2.2.1Metal-organicframework polymercomposites
Polymersareexceptionalintheirrangeofpropertiesthatincludethermal, chemical,andsoftnessstability.AsynthesisofMOFsandpolymerswillgenerateinnovativeandversatilematerialsthatshowjointpropertiesfor framestabilityandactionenhancement [21].Roweetal. [22] preparedthe gadolinium(Gd)MOFcompositesbasedonmulti-functionalpolymer.Poly (N-isopropylacrylamide)-co-poly(N-acryloxysuccinimide)-co-poly(fluorescein O-methacrylate)copolymerswereconstructedthroughreversibleadditional fragmentationchaintransfer(RAFT)polymerization.Tobindatherapeutic agentsuchasmethotrexateandatargetingligandsuchas H-glycine-arginine-glycine-aspartate-serine-NH(2)peptide,succinimide’sfunctionalitywas usedasascaffold.TheuseofatrithiocarbonateRAFTagentallowedthe reductionofpolymerendgroupstothiolatesandprovidedameansofcopolymerattachmentonthesurfaceofGdMOFparticlesthroughvacantorbitals ontheGd(3 1 )ions.Theseversatile,nanoscalescaffoldshavebeendemonstratedtobebiocompatibleandarecapableofkillingcancercells,biomedicalimaging,andtreatingdiseases.Thisrevolutionaryapproachoffereda simplebutversatilepathfortheproductionofpolymernanoparticlesthe agnosticmaterialswithanunparalleleddegreeofflexibilityindesign,theoreticallyenablingcustomizableloadingcapacitiesandspatialloadingoftargetingortreatmentagents,therebycombiningbimodalimagingcapabilities viabothmagneticresonanceandfluorescencemicroscopy.
1.2.2.2Metal-organicframework quantumdotcomposites
Thecombinationofhighsurfacearea,microporosity,andflexibleMOFcompositionswithQDsenablesthepreparationofcompositematerialswith improvedpropertiesformanyapplicationssuchasphotocatalysis,energy storage,andgasstorageandsensing [23].Despitetheirunusualelectronic
4 Metal-OrganicFrameworksforChemicalReactions
Synthesisprocedureofactivatedcarbon basedMOFcomposites [20] MOF, Metal-organicframework.
andopticalpropertiesdependingondimension,QDswithasizerangeof 2 10nmhavereceivedconsiderableattention.Theencapsulationof QDswithinMOFswillimprovetheirstabilityandmodulateratesof electron holepartrecombination.DifferentformsofQDssuchasnitride-, oxide-,carbon-,andchalcogenide-basedcompoundshavebeenintegrated intoMOFs,andtheresultantcompositematerialshaveenhancedtheir propertiesandapplications [7]
1.2.2.3Metal-organicframework metalnanoparticle composites
Metalnanoparticleshaveacquiredalotofinterestbecauseoftheirhigh chemicalprocessesandspecificities.Nevertheless,theseparticleshavea highsurface-to-volumeratioandhighsurfaceenergy,andhencetendtocollectandignite.Forexample,arrangingnanoparticlesofmetalintoporous materialssuchasmetaloxides,zeolites,mesoporoussilicates,andcarbon willeffectivelylimittheaccumulationofmetalnanoparticlesinrestricted cavities.Asanewclassofporousmaterials [1,24 26],MOFswithlargesurfacesandporosityaresuitableassupportsformetalnanoparticles.MIL-100 (Fe)MOFcompositesandmagneticnanoparticleshavealsobeenshownto bequicklyandeasilyabsorbentforextractingaciddyes [27,28].Shustova etal. [29] observedfluorescenceinanotherwisenonemissivezinc-MOF sample.Theseresearchersincorporatedtetraphenylethylenecoresintothe MOF,andtheresultingstructurewasobservedtoobtainfluorescence becauseofthematrixcoordination’sinducedemissioneffect.
ThesemiconductingbehaviorofstrontiumMOF(Sr-MOF)hasbeen demonstratedexperimentallyandbytheoreticalcalculations [30]. Temperature-dependentcurrent voltagetestsfoundtheMOFhadan
FIGURE1.2
electricalconductivityvalueontheorderon106Scm 1.Achangeinthe temperatureattheannealingcausedtheMOFtoexponentiallyincreaseits conductivity.Insteadofthethermallymediatedcarriersandvariablehopping,theArrheniusconductivityplotshowedSr-MOF’ssemiconducting transportactions.Forthedevelopmentofadirectwhitelight-emittingdiode forsolid-statelighting,anSr-MOFcompositeformedwithasemiconductive organicligand(1,4,5,8-naphthalenetetracarboxylicacidhydrate)wasdocumented [31].Thephotoluminescencespectraoftheaboveelectroluminescent Sr-MOFconfirmedtheexistenceofuniqueemissionpeaksleadingtointermetallicelectronictransitionsinstrontium,transitionsbetweenmetallic energystates,andmetal-to-ligandconversionofcharges.
1.2.2.4Metal-organicframework grapheneoxidecomposites TheproductsbettersuitedforMOFcompositesynthesisaregrapheneoxide (GO).Owingtoitssuperiorpropertiessuchaswidesurfacearea,mechanical stability,robustelectrical,andopticalproperties,GO,afunctionaloxygencontaininggraphenewithchemicalgroups,recentlyattractedresurgentinterests [32].Theflexible,freestanding,andthree-dimensionalcobalt-based MOFs/reducedGO(CoMOF/rGO)compositewaspreparedwithasimple electrochemicaldepositionofCoMOFonthesurfaceoftherGOelectrode [33].Musyokaetal. [34] preparedacompositeusinginsituapproachusing zirconium-basedMOFandrGO.Thiscompositewasusedinstorageapplicationsandshowedgreaterhydrogenstorageefficiencycomparedwith ZrMOF.Zhangetal. [35] synthesizedGOnano-sheetswithtwo-stepcobasedMOFusinganinsitugrowthandcalcinationprocess.Thismaterial wasusedasamediumforelectromagneticabsorptionanddemonstratedelectromagneticdissipationathighefficiency.Fangetal. [36] preparedandconstructeduniform,highperformance,andflexiblenanofiltrationmembrane MOFcompositesbasedonZr.Twophaseswereusedtobuildthesubstratum:(1)dopingGOsheetsintoapolyacrylonitrile(PAN)membranecasting solutionandforming2D 3Dbindingporesbyphaseimmersionprocessand (2)immersingoftheGO@PANsubstratumintoadopaminesolutionfor self-polymerizationintomacromolecularchainsobtainingahighlystableand flexiblesubstratum.Linetal. [37] showedthattheinclusionofrGOonZrbasedMOFincreasesbothadsorptiveandphotocatalyticefficiencybyeliminatingacidcolors.
1.2.2.5Metal-organicframework polyoxometalatecomposites
Polyoxometalatesareaclassofanionicmetal oxygenclusterswithawide rangeofadditives,flexibleshapesandproportions,solubility,redoxpotential,andhighacidity.Suchpropertiesprovidegreatopportunitiesinavariety ofcatalytictransformations,particularlyinacidandoxidationreactions. However,theirimplementationisconstrainedbyitslowspecificareaand
lowstability.TheimmobilizationofpolyoxometalatesintoMOFsisapromisingapproachforthestabilizationandrefiningofpolyoxometalatestoboost theircatalyticproperties.Becauseoftheircompositionalstabilityandstructuralstrength,polyoxometalatescanbeusedasversatilebuildingblocks (nodesorbases,orprototypesinsidethecages)fortheconstructionof polyoxometalate-basedMOF.Inaddition,polyoxometalatescanbeencapsulatedinMOFporesbyhost guestinteractionstoformMOFpolyoxometalatecomposites [38,39].
1.2.2.6Metal-organicframework
enzymecomposites Enzymesareaclassofextremelyefficientbiocatalystswithhighactivity andmildchemo-,enantio-,andareaselectivity,whichareveryeffectivein catalyzingvariousreactions.However,theirextensivecatalyticusesare greatlyhinderedbythedelicateexistenceofenzymes,suchaspoorthermal stability,limitedoptimumpHranges,andlowresistancetoorganicsolvents anddenaturants.Inaddition,lengthypurificationandisolationstepsare requiredtocontaminatetheenzymesinthetargetproducts.MOFshave provedtobeeffectiveenzymeimmobilizationmechanismstoshieldthem fromdeactivatingreactionconditions,improvingtheirrecyclability,and reducingproductdegradation.Preciseregulationofporedepth,form,and compositionofMOFsenablesenzymeconfinementwithmatchedthickness, therebyreducingself-aggregationandenzymeleaching.Inaddition,MOF’s inorganicnodesandfunctionallinkerswillcreatethoseenzymeinteractions bycoordination,covalentbonding,hydrogenbonding,andvanderWaalsto stabilizeleachingenzymes [40 44].Theencapsulationofrhodaminewas obtainedinabio-MOFbasedonadenine [45].Theresultingcompositeprovidedhighquantumefficiencyincolortuningtoshowthepossibleapplicabilityoflight-emittingdevicesandvisiblelightcommunication.Several scholarshavestudiedthedifferentMOFcompositeswithdifferingrhodamineratiosrelativetospecificationssuchascolortenability,emissionefficiencies,andlifetimeofemissions.
1.2.2.7Metal-organicframework cellulosecomposites Cellulosehasgreatpotentialassubstratesbecauseofitshighstrength,lightweight,lowcost,waterresilience,flexibility,nontoxicity,andexcellentprocessability [46 49].MOFdispersiononthecellulosesurfacesisbeing successfullypreparedtoproducecompositeMOF cellulosefabricswith newpracticalefficiency [50].TheMOFandcelluloseaerogelcomposite materialswereprocessedusingtheinsitugrowthtechniqueatroomtemperature.Suchmaterialshavebeendescribedbyastudyofscanningelectron microscopy(SEM),X-raydiffractionanalysis(XRD),atomicabsorption spectrometer,andthermogravimetry.Theamountofadsorbedmetalionsis equivalenttothenumberofMOFsandcelluloseaerogels,indicatingthatthe
MOFsarenotblockedandthereforeadsorbentafterthedevelopmentofcelluloseaerogels.CompositeplasticMOF celluloseaerogelshavebeenshown toberecyclableinwatertoadsorbPb21 andCu21 afterquickwashing.This resultshowsthatthesecompositematerialscouldadsorbheavymetalionsin waterbypreventingsecondarycontaminationanddemonstrategreatpotential inwatertreatment [51].ThecompositeMOF celluloseaerogelswerepreparedatroomtemperaturebysimpleinsituproduction.Inthemetalionprecursor,thepre-syntheticaerogelofcelluloseissoaked,andthentheorganic ligandisaddedtoallowtheMOFstobuildonthecelluloseaerogel.The effectivenessofadsorbingheavymetalionsinwaterbycelluloseaerogelhas beeninvestigated.TheMOFswerestillworking,andthechannelswerenot obstructedbycomparingtheadsorptionequilibriumofheavymetalionswith celluloseaerogel,MOFs,andcompositeMOF celluloseaerogel.
1.2.2.8Metal-organicframework silicacomposites
Silicaparticlesandnanostructureshaveefficientmechanismsthathave drawnsignificantinterestincatalyticapplicationsforperformingvarious nanoscalefunctionssuchasporosity,stabilization,andhydrophilicity.The synthesisofsilicawithMOFsincorporatestheessentialpropertiesofall materialsandcontributestoinnovativeapplications.Thereareactuallytwo maintypesofMOF silicacomposites:SiO2@MOFsandMOFs@SiO2.The formerincludestheinjectionofdispersedsilicaparticlesintoMOFpores/ channelsorthegrowthofanMOFshellintoapreformedsilicasphere,while thelatterusessilicaasacoatinglayerproducedontheMOFsurfaceorasan aidtothegrowthofMOFparticles [52 54].
1.2.2.9Metal-organicframework activatedcarboncomposites
MOFfibers,weresynthesizedformethanerecoveryandcarbondioxidecapturebyKayaletal. [55].Nevertheless,theeffectivenessofMOF activated carboncompositesinaqueousconditionswasnotinvestigatedforthe removalofchemicalspecies.Thesecompositesweresynthesizedbyasimple hydrothermalcycleandusedastheadsorbentorganicdye.Throughtesting theadsorptionkineticsundervariousconditions,suchasadsorbentthickness, contacttime,anddyeconcentration,theadsorptionpotentialofthecompositeparticlesisshowntobesuperiortobothactivatedcarbonandMOF.The surfacereactiontechniquedependingonthecorecompositeconceptwas usedtodeterminethemosteffectiveconditionsfortherapidandproductive removalofdye.Apotentialorganicdyestructureandadsorptionmechanism isgivenoncarbonnanocompositeenabledwithMIL-101(Cr).Mahmoodi etal. [56] synthesizedthegreenMOFnanocompositewith2,5,and10wt.% activatedcarbonratiosbasedoncucumberpeelactivatedcarbon and chromium-basedMOF[MIL-101(Cr)].Characterizationofcompoundswas performedusingTGA,Brunauer Emmet Teller(BET),XRD,SEM,and
Fourier-transforminfraredspectroscopy(FTIR).Thefindingsrevealedoctahedralcrystalform,andcompositematerialsexhibitedthesamemorphology asMIL-101(Cr)crystalsonanactivatedcarbonsurface.Thefindings revealedthatthemethodsofthesurfacereactionmodelestimatedthereal datawithhighprecision.
Hasanzadehetal. [20] preparedthecompositesoftheactivatedcarbon / chromium-basedMOF[MIL-101(Cr)]withhighlysolubleadsorbents.The polymerhasahighsurfaceareaandagrossvolumeofaround1.3cm3 g 1 inpore.Toillustratetheefficacyofthecompositeasanadsorbent,the removalkineticsofanionicdyesfromaqueoussolutionsareexhibitedbased onthevolumeofcomposite,adsorptiontime,dyeconcentration,andpH. MIL-101(Cr)-activatedcarboncompositekineticswasshowntobequicker thanMIL-101(Cr)undernear-neutralpHconditions.Halftheprocessing timeisroughly3minutes,andafter5minutes85%ofthecolorislost.
1.2.2.10Metal-organicframework aluminumcomposites
Aluminumtris(8-hydroxyquinoline)(Alq3)waswidelyusedasasolventin metalchelates.Alq3 thinfilmsaselectroluminescentmaterialshavebeen reportedtodemonstratehigheffectivenessandstability.Theiruseincommerciallight-emittingsystemshasalsodrawninterestinthinfilmfeatures. MOFshavebeenproposedasahostmatrixformodulatingtheluminescence propertiesofAlq3 molecules.TheincorporationofAlq3 moleculesintoMOF poresornanochannelsisveryeffectiveinavoidingtheiraggregation,which inturnhelpstoeffectivelyincreasethelifespanandtheyieldofthechromophore.FortheMOF Alq3 composite,therewasanincreaseinthelifetime ofexcitedstateemissionsofasmuchas65%relativetoAlq3 alone.Inadditiontothis,aconstantblueemissionshiftcouldbeachievedbyincreasing theAlq3 loadinthecomposite.Thisactivitywasexplainedbecauseof molecularinteractionswiththeMOFinAlq3.Assuch,thelatterstudycan beconsideredoneofthemostimportantstudiesforunderstandingthetuning ofemissionpropertiesofchromophoresfollowingtheirencapsulationin MOFs [57 59].
1.2.2.11Metal-organicframework molecularspecies composites
Astoolsforstudyingandimitatingthefunctionalitiesofbiologicalstructures,homogeneousmolecularspeciessuchasdyemolecules,salinecomplexes,porphyrins,andmetalloporphyrinswereextensivelyresearched. However,thelifetimeofthesemoleculesisshortenedduetointeractions betweentheactivesitesandoxidativeself-degradationduetoselfaggregation.TheimmobilizationofmolecularspeciesinMOFs,wherethe activesitescanbeseparatedandprotected,isaviablealternativetotheheterogenizationofhomogeneousmolecularcatalysts,combiningthebenefitsof
allcatalystgroupsandreducingthedrawbacks.Molecularspeciescanbe incorporatedinanMOFbackboneviacovalentbondingorencapsulatedvia noncovalentinteractionwithinMOFpores.Directencapsulationdoesnot disturbthesphereofinteractionbetweenmolecularspeciesandthusretains thestabilityandpropertiesofthemolecularspecies.Furthermore,direct encapsulationcanmaintainthemobilityofmolecularmoleculestosome degree,enablingthemtocooperatewiththeMOFbackbone.ThreedimensionalMOFswithwidecavitiesinterconnectedbysmallwindowsare especiallyidealforstabilizingmolecularspecies,giventhatthewidecavities canhandlemolecularspecies,andthesmallporouswindowsavoidleaching andaggregationofmolecularspecies [60 64].
1.2.2.12Metal-organicframework hybridcomposites Monamaetal. [65] producedanovelhybridcompositedependingon4tetranitrocopper(II)phthalocyanin(TNCuPc)producedfromMOFasanoble, hydrogen-freecatalyst.Thecomposition,surfacearea,andmorphologyof thecompositebareMOF,TNCuPc,andTNCuPc/MOFarecharacterizedby XRD,FTIRspectroscopy,ultraviolet visiblespectroscopy,BET,SEM, transmissionelectronmicroscopy(TEM),andsimultaneousthermalanalysis. Thecompositedevelopedexhibitedhighbehavioragainsthydrogenreaction evolution,strongthermalstability,andexcellentresistance.Thusthenonnobleelectro-catalystTNCuPc/MOFmaybeapromisingelectrochemical catalystfortheproductionofelectrochemicalhydrogentoreplaceplatinumbasedcatalysts.ThespectroscopicstudyindicatedastrongcompositesynthesisofTNCuPc.Themorphologicalfindingsshowedthatrod-likeTNCuPc structureswerebeingformedontheMOFsheet.Thegreensolvothermal processforthepreparationofthecompositeMOF(Cu-BTC)andMOF/graphenehybridistobeusedasanefficientadsorbentoftheproductfuels.The formulatedadsorbentsaredistinguishedbytheapplicationofvariousanalyticaltechniquessuchasXRD,FTIR,BET,andTEM.Laboratoryadsorption resultsrevealedthat,underoptimalexperimentalconditions,theMOF/Gr compositecontentexhibitsexceptionallystrongadsorptionofdibenzothiopheneatanadsorptionrateof46.2mgSg 1 [66].
1.3Characterizationofmetal-organicframeworkcomposites ToadvancethecharacterizationofMOFparticlesforreal-worldapplications, thewiderangeofmaterialssuchasactivatedcarbons,rGO/GO,multiwalled CNTs,biomaterials,andnano-fibrousmembraneshavebeenregardedassupportbedsforstabilizingandcreatingbetterandbetterMOFnanocrystals.To date,MOFcompositeshavebeensynthesizedasthinfilmsusingaseeding andgrowthprocessorlayer-by-layergrowthofMOFsondifferentsubstrates,includingporousoxidesupports,graphite,CNT,andactivatedcarbon
[67 72].ThischapteraddressedthemostimportantcharacterizationtechniquesusedbytheseMOFcomposites,suchasXRD,X-rayphotoelectron spectroscopy(XPS),FTIR,andSEManalytics.
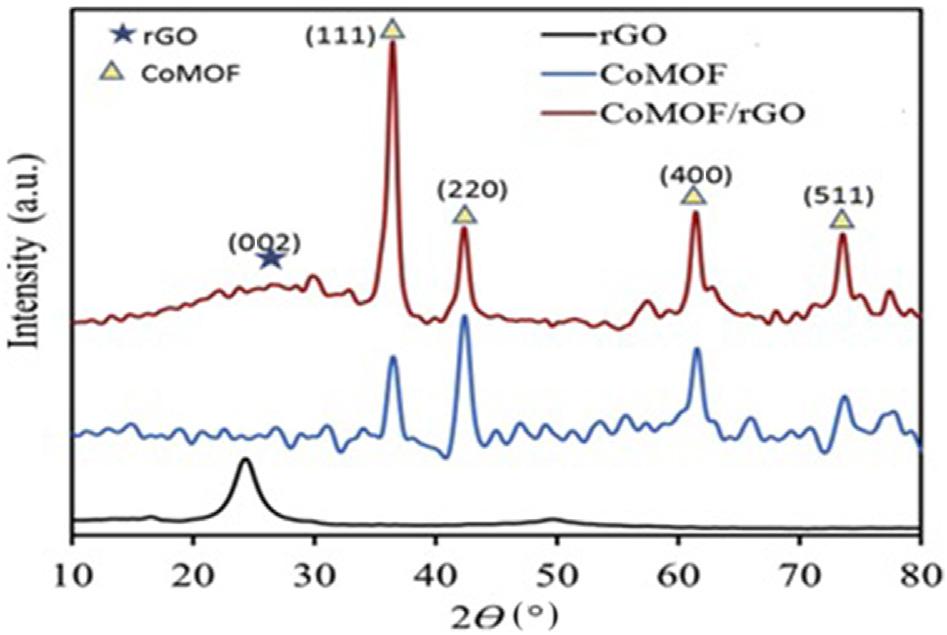
1.3.1X-raydiffractionanalysis UsingtheXRDprocess,therGO,CoMOF/rGOcomposites,andCoMOF powdercrystalstructuresweredetermined(Fig.1.3).IntherGOpaper,XRD spectrumthepeakat25.4degreeswasobserved,referringtothetypicalcrystaldiffractionofthegraphenestructure(002).InCoMOF,XRDresults fromthepeaksatroughly35.4,42.5,61.7,and73.4degreescorrespondto the(111),(220),(400),and(511),respectively,Coform.TheXRDpatternoftheCoMOF/rGOpapershowsdiffractionpeaksofbothrGOand CoMOFcomposition,suggestingthegoodpreparationoftheCoMOF/rGO composites [33].
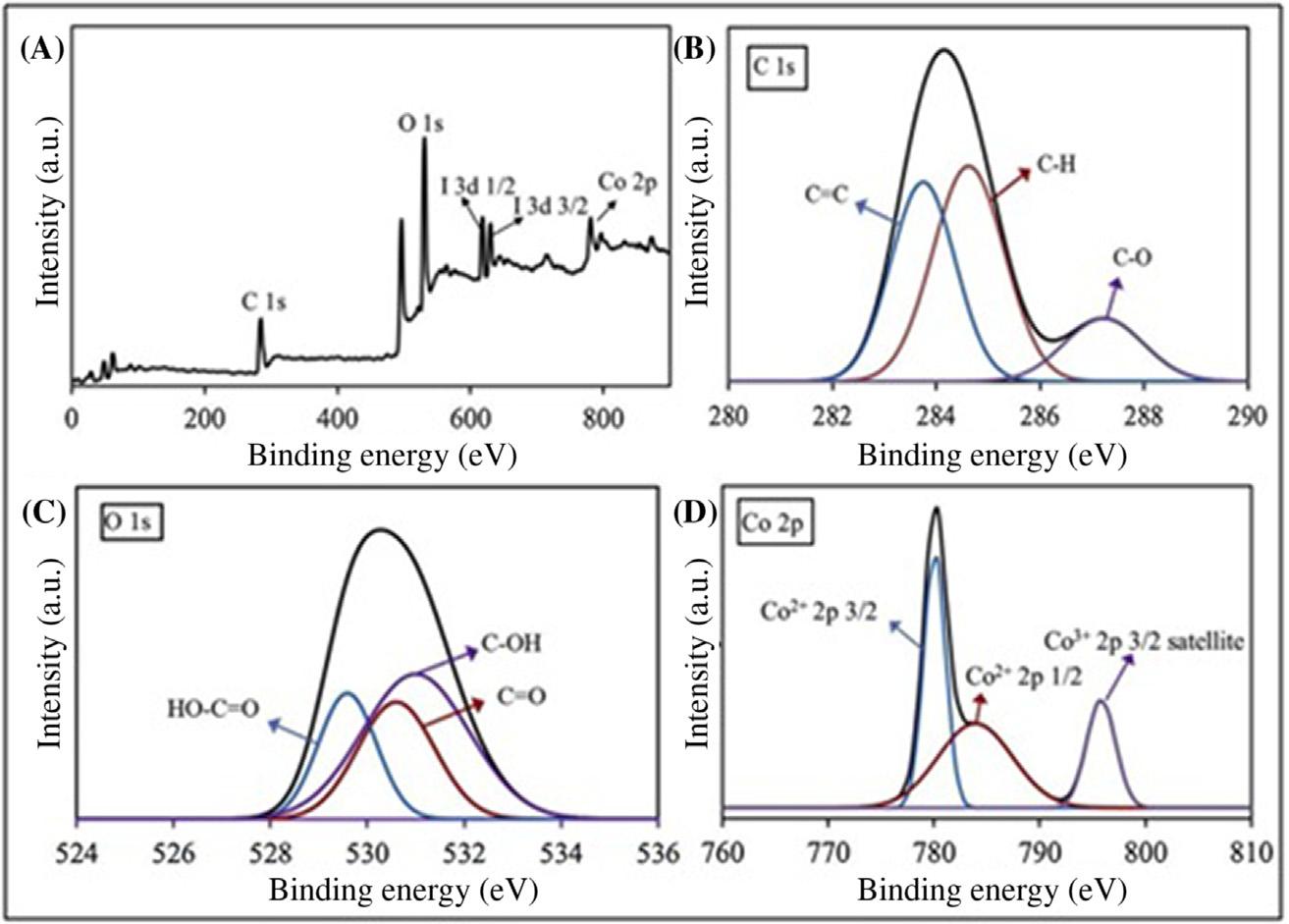
1.3.2X-rayphotoelectronspectroscopy TheXPSexperimentswereconductedonastandardsourcespectrometer withAlX-ray.TheCoMOF/rGOchemicalcompositeelectrodestructurewas calculatedusingXPStechnique,asshownin Fig.1.4.CoMOF/rGO’scompositeelectrodeconsistsof46.432%C,46.445%O,4.721%Co,and2.402% I.TheCoMOF/rGOcompositespectrumofC1s(Fig.1.4B)canbedeconvertedtothreemajorpeaksof283.7,285.5,and287.6eVbindingenergies, respectively.In Fig.1.4C,O1srangecanbegroupedintothreepeaks, respectively,at530.4,531.4,and532.2eV.Thepeakscorrespondtovarious oxidationstructuresofCo,asshownin Fig.1.4D,areCo21 2p3/2(780eV), Co21 2p1/2(784eV),andCo31 2p3/2satellite(796eV).ThehighforIis duetoHIusedduringthechemicalreductionprocesswhenpreparingthe rGOfilmelectrode.SincetheCoMOFisformedonthesurfaceoftherGO electrodeusingtheelectrochemicaloxidationcycle,therGOXPSspectrum showedrelativelygreateroxygencontent.Inevaluatingtheseresults,itwas establishedthattheCoMOF/rGOcompositeelectrodewassuccessfullyprepared [33]
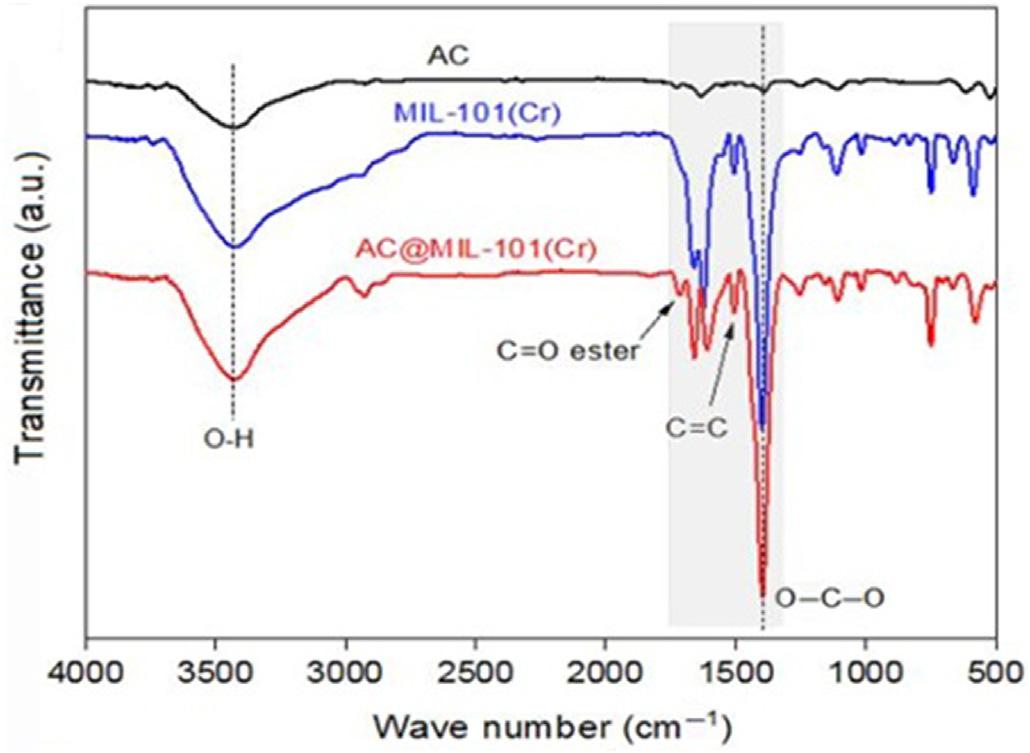
1.3.3Fourier-transforminfraredspectroscopy FTIRspectrumofactivatedcarbonstrengthenedMOFcompositesisseenin Fig.1.5[19].Thebroadbandaround3430cm 1 isrelatedtotheO H stretchingvibrationfromwateradsorbedbytheair.Thebandappearedat 1400cm 1 isduetotheO C Osymmetricvibrationofthecarboxylicacid groupthatindicatesthepresenceof1,4-benzenedicarboxylicacid(H2BDC) ligandwithinthematerialstructure.Thelowpointat1506cm 1 isdueto C 5 Cofthebenzenechain.Theothermotionsat1108,1017,888,and
FIGURE1.3 XRDpatternsofrGO,CoMOF/rGO,andCoMOFcomposites [33]. XRD,X-ray diffractionanalysis.
FIGURE1.4 XPSspectraofCoMOF/rGOcomposite:(A)survey,(B)C1s,(C)O1s,and(D) Co2p [33]. XPS,X-rayphotoelectronspectroscopy.
749cm 1 areduetoC Hdeformationofthebenzenering [55].Reduction ofthebandstrengthat1610cm 1 (carboxylicacidC Ogroup)incombinationwiththeformationofanew1716cm 1 absorptionband(estercarbonyl group)foractivatedcarbon MOFcompositeenablesthepotentialreaction ofH2BDCligandtoactivatedcarbon.Tomappotentialreactionsbetween
FIGURE1.5 FTIRspectrumofactivatedcarbon reinforcedMOFcomposites [20]. FTIR, Fourier-transforminfraredspectroscopy, MOF,metal-organicframework.
FIGURE1.6 SEMimagesof(A)rGO,(B)CoMOF/rGOcomposite,and(CandD)lowand highmagnificationsofCoMOFnanorods [33] SEM,Scanningelectronmicroscopy.
theactivatedcarbonandH2BDCligand,theFTIRspectrumofactivatedcarbonfunctionalizedwithH2BDCmoleculeswasmeasured.Thepeaksaround 3430and1685cm 1 correspondtotheactivatedcarbonstretchinghydroxyl
FIGURE1.7 SEMimagesoftheMOFcomposites(AandB)MIL-101(Cr)and(CandD)activatedcarbon/MIL-101(Cr) [56] MOF,Metal-organicframework; SEM,scanningelectron microscopy.
vibration,respectively,andtheH2BDCcarbonylgroup.Accordingtothe reactionbetweentheactivatedcarbonandH2BDC,itwasexpectedthatan estercarbonylgroupwillformaround1716cm 1.FTIRspectroscopyhas notshownsuchapeakthatmaybeattributedtothehighH2BDCcarbonyl grouppeakpresentinthislevel.
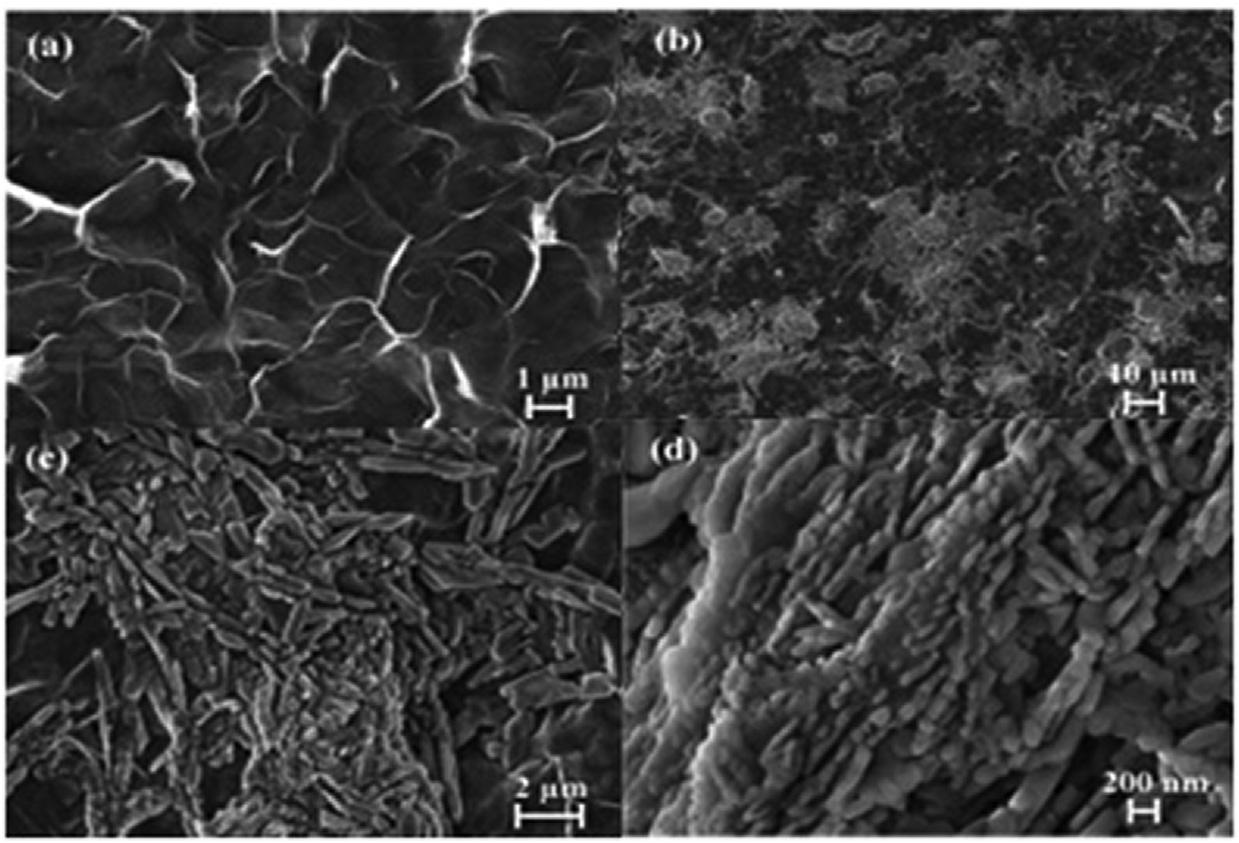
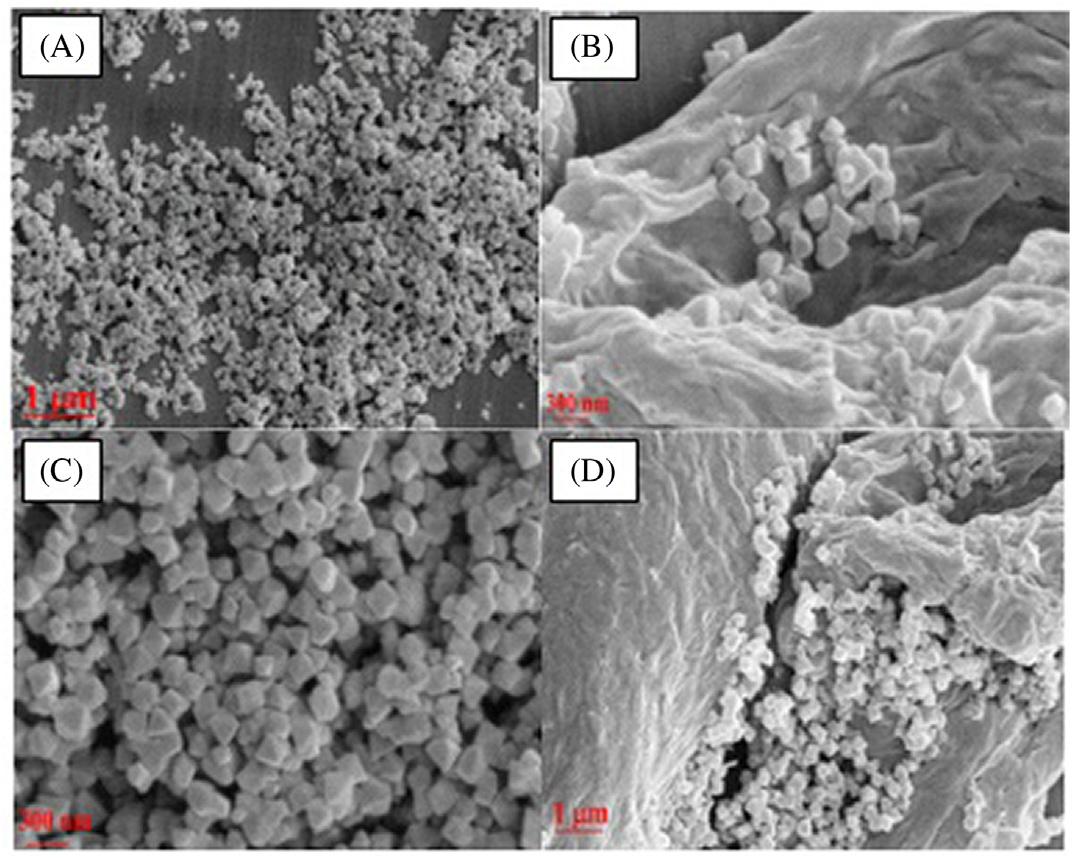
1.3.4Scanningelectronmicroscopyanalysis SEMphotographsweregatheredtoanalyzethemorphologyofthematerials. ThemorphologyofCoMOF/rGOcompositeswasstudiedbySEM. Fig.1.6 displaystheSEMimagesoffreestandingandrobustcompositesrGOand CoMOF/rGOformorphologicalcharacterization.Thecharacteristicwrinkled appearanceofthegraphenesystem’smetallicgraysurfacewasobservedon theSEMpictureoftherGO(Fig.1.6A). Fig.1.6B revealsthatfinger-like CoMOFnanorodsshapedontherGOsurfaceandthatthesefinger-like CoMOFstructurescoatedtheelectrodesurfacehomogeneously(Fig.1.6C andD).A3Dsurfacewasformed,aswellasthesurface’sactivesurface areaisincreasedbysynthesizedfinger-likeCoMOFstructuresontherGO. Such3DCoMOFstructuresarepredictedtoexhibithighefficiencyonthe rGOsurfaceinelectro-catalyticstudies [33].
Fig.1.7 revealedtheSEMimagesofAC/MIL-101(Cr)2%andAC/MIL101(Cr)10%.Asfor Fig.1.7AandB,MIL-101(Cr)SEMphotosrevealeda