LIGNININPOLYMER COMPOSITES
OmarFaruk MohiniSain
Amsterdam • Boston • Heidelberg • London • NewYork • Oxford Paris • SanDiego • SanFrancisco • Singapore • Sydney • Tokyo
WilliamAndrewisanimprintofElsevier
WilliamAndrewisanimprintofElsevier
TheBoulevard,LangfordLane,Kidlington,Oxford,OX51GB,UK 225WymanStreet,Waltham,MA02451,USA
Copyright 2016ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical, includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthe publisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefound atourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmay benotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbe mindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforany injuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseor operationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-35565-0
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ForinformationonallWilliamAndrewpublications visitourwebsiteat http://store.elsevier.com/
Publisher: MatthewDeans
AcquisitionEditor: DavidJackson
EditorialProjectManager: PeterGane
ProductionProjectManager: SusanLi
Designer: MarkRogers
TypesetbyTNQBooksandJournals www.tnq.co.in
PrintedandboundintheUnitedStatesofAmerica
Dedicated to MyBelovedWife “ShailaShumi” and MyBelovedDaughter “OrnelaSuhiya”
OmarFaruk,Canada
UmeshP.Agarwal USDAForestService,Forest ProductsLaboratory,OneGiffordPinchotDrive, Madison,WI,USA
AbdullahAlMamun InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
PriyankaBhattacharya ProtonPower,Inc.,Lenoir City,TN,USA
SabornieChatterjee ChemicalSciencesDivision, OakRidgeNationalLaboratory,OakRidge, TN,USA
HoyongChung DepartmentofChemicaland BiomedicalEngineering,FloridaStateUniversity, FAMU-FSUCollegeofEngineering,Tallahassee, FL,USA
NorbertEisenreich Fraunhofer-ICT,Germany
GunnarEngelmann FraunhoferInstitutefor AppliedPolymerResearchIAP,Potsdam-Golm, Germany
OmarFaruk CentreforBiocompositesand BiomaterialsProcessing,FacultyofForestry, UniversityofToronto,Toronto,ON,Canada
MaikFeldmann InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
JohannesGanster FraunhoferInstituteforApplied PolymerResearchIAP,Potsdam-Golm,Germany
AzadehGoudarzi DepartmentofMaterials Engineering,TheUniversityofBritishColumbia, Vancouver,BC,Canada
ShayestehHaghdan DepartmentofWoodScience, ForestSciencesCentre,TheUniversityofBritish Columbia,Vancouver,BC,Canada
Hans-PeterHeim InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
Contributors
EmiliaReginaInone-Kauffmann Fraunhofer-ICT, Germany
JohnF.Kadla DepartmentofForestBiomaterials, NorthCarolinaStateUniversity,Raleigh,NC,USA
AdelR.Kakroodi CentreforBiocompositesand BiomaterialsProcessing,FacultyofForestry, UniversityofToronto,Toronto,ON,Canada
MuzafferA.Karaaslan DepartmentofMaterials Engineering,TheUniversityofBritishColumbia, Vancouver,BC,Canada
SimonKleinhans InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
FrankK.Ko DepartmentofMaterialsEngineering, TheUniversityofBritishColumbia,Vancouver,BC, Canada
MarkT.Kortschot DepartmentofChemical EngineeringandAppliedChemistry,Advanced MaterialsGroup,UniversityofToronto, Toronto,ON,Canada
YingjieLi DepartmentofMaterialsEngineering, TheUniversityofBritishColumbia,Vancouver,BC, Canada
Li-TingLin DepartmentofMaterialsEngineering, TheUniversityofBritishColumbia,Vancouver,BC, Canada
HelmutNaegele TecnaroGmbH,Germany
MohammadAliNikousaleh InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
NumairaObaid DepartmentofChemical EngineeringandAppliedChemistry,Advanced MaterialsGroup,UniversityofToronto,Toronto, ON,Canada
NikhilD.Patil FacultyofForestry,Universityof Toronto,Toronto,ON,Canada
JuergenPfitzer TecnaroGmbH,Germany
ScottRenneckar DepartmentofWoodScience, ForestSciencesCentre,TheUniversityofBritish Columbia,Vancouver,BC,Canada
AnnetteRu ¨ ppel InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
MohiniSain CentreforBiocompositesand BiomaterialsProcessing,FacultyofForestry, UniversityofToronto,Toronto,ON,Canada;Centre ofAdvancedChemistry,Adjunct,KingAbdulaziz University,Jeddah,SaudiArabia
TomonoriSaito ChemicalSciencesDivision,Oak RidgeNationalLaboratory,OakRidge,TN,USA
ViolaSauer InstituteforMaterialsEngineering, PolymerEngineering,UniversityofKassel,Kassel, Germany
GregoryD.Smith DepartmentofWoodScience, ForestSciencesCentre,TheUniversityofBritish Columbia,Vancouver,BC,Canada
NicoleM.Stark USDAForestService,Forest ProductsLaboratory,OneGiffordPinchotDrive, Madison,WI,USA
NicolasR.Tanguy FacultyofForestry,University ofToronto,Toronto,ON,Canada
JimiTjong CentreforBiocompositesand BiomaterialsProcessing,FacultyofForestry, UniversityofToronto,Toronto,ON,Canada
NewellR.Washburn DepartmentofChemistry, CarnegieMellonUniversity,Pittsburgh,PA, USA;DepartmentofBiomedicalEngineering, CarnegieMellonUniversity,Pittsburgh, PA,USA
NingYan FacultyofForestry,Universityof Toronto,Toronto,ON,Canada;Departmentof ChemicalEngineeringandAppliedChemistry, UniversityofToronto,Toronto,ON,Canada
DanielJ.Yelle USDAForestService,Forest ProductsLaboratory,OneGiffordPinchotDrive, Madison,WI,USA
LarsZiegler TecnaroGmbH,Germany
OmarFaruk
DrOmarFaruk completedhisBSand MSinchemistryat theUniversityofChittagong,Bangladesh. WithaDAAD (GermanAcademic ExchangeService) scholarship,hejoined atUniversityofKassel, Germany.Heachieved hisPhDinmechanical engineeringat2005. Heworkedatthe DepartmentofForestry,MichiganStateUniversity, USAasavisitingresearchassociatefrom2006to 2009.Since2010,hewasworkingattheCentre forBiocompositesandBiomaterialsProcessing, UniversityofToronto,Canada.HejoinedFord MotorCo.Canadaasresearchanddevelopment engineeronJanuary,2015.Hehasmorethan 70publicationstohiscreditswhichhavebeen publishedindifferentinternationaljournalsand conferences.Healsoeditedonebook,titled Biofiber reinforcementincompositesmaterials published fromWoodheadPublishingLtd.Inaddition,he isinvitedreviewerof58internationalreputed journals,governmentresearchproposals,andbook proposals.
MohiniSainis deanandprofessorat FacultyofForestry, UniversityofToronto. Hespecializesinadvancednanocellulose technology,biocomposites,andbio-nanocomposites.Heis cross-appointedtothe DepartmentofChemicalEngineeringandApplied Chemistry.HeisafellowofRoyalSocietyof Chemistry,UK.Besides,heisalsoanadjunctprofessor oftheChemicalEngineeringDepartmentsatthe UniversityofNewBrunswick,Canada;King AbdulazizUniversity,Jeddah,SaudiArabia;UniversityofGuelph,Canada,UniversityofLulea,Sweden, honoraryprofessoratSlovakTechnicalUniversity andInstituteofEnvironmentalScienceattheUniversityofToronto,andcollaborateswithAmericanand Europeanresearchinstitutesanduniversities.Prof. Sainholdsseveralawards;mostrecentoneisthePlastic InnovationAwardandKalevPugiAwardforhisinnovationandcontributiontoindustry.Authorofmore than300papers,6booksandhi-citedresearcherProf. Sainhugelycontributedtothesocietyatlargeby translatingresearchtocommercializationwhichled tothreenewcompaniesmakingproductsranging frompackagingtoautomotivetobuildingconstruction.

MohiniSain
Ligninisacomplexpolymerabundantlyfoundin plantsanditisthefibrouspartoftheplant.Traditionally,ligninisusedinawiderangeoflow-volume, nicheapplications.Industrialligninsarecurrently obtainedascoproductsofthemanufactureofcellulosepulpforpaper,aswellasfromotherbiomassbasedindustriesandtherearevarioustypesoflignin dependingontheirprocessandpurity.Ligninmarket isstilllimitedinitsapplicationinawiderangeoflowvolume,nicheapplications,butlignincanbeusedin awiderangeofapplicationssuchasinthemanufactureofvanillin,animalfeed,dyedispersants, micronutrients,resins,andcleaningchemicals.In addition,lowawarenessaboutligninamongmanufacturersisthekeyrestrainttothismarket.Itisalso foundthatthereisaweaklinkbetweentheindustry andresearchinstituteswhichresultsinthelow exposureofmanufacturerstothedevelopmentsof ligninindifferentapplications.Furthermore,the extractionandmodificationtechniquesandapplicationofligninarestillataprimarystage,which hamperstheligninmarketalso.Anotherobstacleof ligninexpansioninvalue-addedapplicationsis mainlyduetotheirlow-puritystandards,heterogeneity,smellandcolorproblemsoftheexisting commerciallignins.
Currently,environmentalpollutionandincreasing awarenessoflimitedresources,thereisgrowing
Preface
opportunityintheuseofligninasasubstitutefor fossil-basedrawmaterialsanditcouldbeusedin themanufactureofawiderangeofproductssuch asplastics,chemicalproducts,andcarbonfibers. Recentlyextensiveongoingresearchfocusingon lignindrawbacksisincreasingtheapplicationscope oflignininthemarket.
Inrecentyears,therehavebeenanumberof reviewpaperspublishedonlignincoveringlignin chemistry,modification,polymercompositesfrom lignin,oxidativeupgradeoflignin,biocomposites andnanocompositeswithlignin,industriallignin productionandapplications,andcarbonfibers fromlignin.Thisbookfocusesspecificallyon lignin-basedpolymercomposites(thermoplastic, thermoset,andbiopolymer,rubber,nano,carbon), lignin-basedaerogels,lignin-basedfoamingmaterials,aswellassourcesandtypesoflignin,lignin interunitlinkagesandmodelcompounds,extraction oflignin,characterizationandpropertiesoflignin, andapplicationsoflignin.Thebookwillbehelpful toresearchers,engineers,chemists,technologists, andprofessionalswhowouldliketoknowmore aboutthedevelopmentandpotentialofligninand lignin-basedcomposites.
OmarFarukandMohiniSain
ShayestehHaghdan,ScottRenneckarandGregoryD.Smith DepartmentofWoodScience,ForestSciencesCentre,TheUniversityofBritishColumbia,Vancouver,BC,Canada
1.IntroductiontoLignin
Thetermligninisderivedfromthelatinname lignummeaningwood(MccarthyandIslam,1999). Itwasfirstisolatedfromwoodinascientificreport bytheFrenchscientist Payen(1838) andlatergiven itscurrentnamein 1857 bySchulze.Ligninwas initiallydescribedasanincrustantofcellulose,and thispointisinsightfulaslignificationoccursafterthe depositionofthepolysaccharideframework.Inan extremelysimplifiedviewitisanalogoustothe matrixmaterialforafiber-reinforcedcomposite. Ligninhasseveralfunctionswiththecellwallsuchas changingthepermeabilityandthermalstability,butit hastheprimaryfunctiontoserveasastructural materialthataddsstrengthandrigiditytoplant tissue.Inthesenselignindistinguisheslignocellulosicbiomassfromotherpolysaccharide-richmaterials,byreinforcingthepolysaccharidescaffoldingof thecellwall.Itsperformanceissoeffectivethatit allowstreestooutcompeteotherplantsforsunlight formingthelargestorganismsontheplanet.
Asligninconstitutes15 40%ofdryweightof woodyplants,itisthemostabundantaromatic polymerontheearthandthesecondmostabundant organicpolymeraftercellulose.Basedonyearly biomassgrowthrates,theoverallproductionof ligninisontheorderof5 36 108 tons(dosSantos
etal.,2014).Hence,ligninhasthepotentialtobean importantsourceofaromaticchemicalsforthe chemicalindustry,arisingfromtheconversionof moderneraCO2,anditsefficientutilizationsolves apotentialpuzzleincreatingvaluableby-productsin abiorefineryscheme.Thisreasoningisbecauseif woodisconvertedtothebilliontonscaleforbiofuels andbiochemicals,thentherewillbegreaterthan 300milliontonsofligninpotentiallyavailable.To putthisinperspectiveitisroughlythesizeofthe globalpolymermarket.
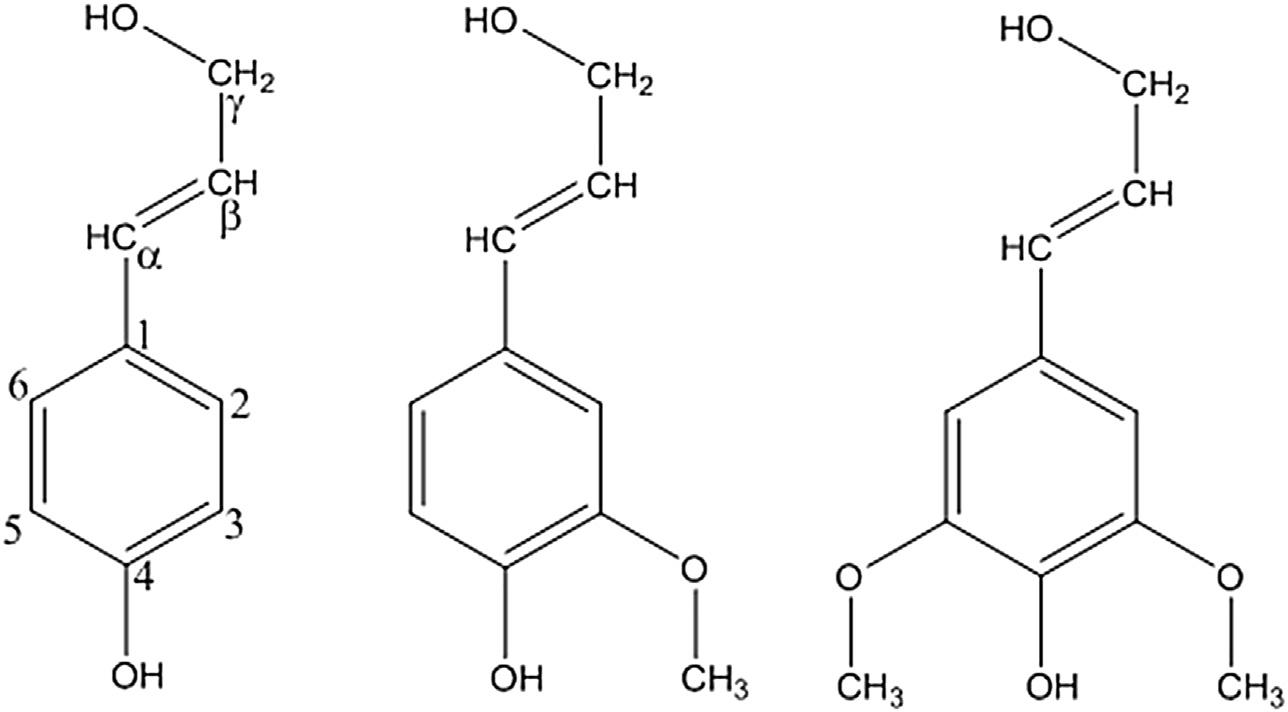
Asmentionedabove,ligninisanaromaticpolymer.Themonomericprecursorshaveaphenolicring withathreecarbonsidechainprovidingabasicnine carbonstructurecommonlyreferredtoasaC9-unit and/orphenylpropaneunitasshownin Figure1.
Thesidechainisterminatedwithaprimary hydroxylgroupontheCg,whiletheCa andCb are connectedtogetherwithanunsaturatedbond.The phenolicringismethoxylated( OCH3)tovarious degrees,dependentuponthespecies; p-coumaryl alcohol,coniferylalcohol,andsinapylalcoholhave none,one,ortwomethoxylgroupsatthe3-and 5-positions,respectively.Basedontheligninmonomericcompositioninvolvedinpolymerization,the resultingligninisclassifiedintothreetypes:(1)lignin thatcontainsmainlyconiferylalcoholiscalled guaiacyl(G)ligninandisfoundpredominantelyin
gymnosperms;(2)ligninformedfromsinapylalcohol iscalledsyringyllignin(S)andmixturesofGandS arefoundinangiospermlignin;and(3)ligninwhich incorporates p-coumarylalcoholis p-hydroxyphenyl (H)lignin,andthethreetypesofmonomersare commonlyfoundingrasses.Overallhardwoodshave aG:Sratiothatapproaches1:2andsoftwoodshave approximately95%Glignin(LinandDence,1992).
Onesimplewayofdeterminingthisratioisbasedon themethoxycontentofthelignin,whileothertechniquescanbeusedtoidentifythespecificC9 structureseitherdirectlyusingnuclearmagnetic spectroscopyordeterminedfromthederivatized thioacidolysisproductsusinggaschromatography.
Themonomericunitsarebuiltintothemacromoleculeligninfromtheoxidativeradicalcoupling ofthesesubstructures(Adametal.,2011;Dinisetal., 2009;DelaCruz,2014;Bowyeretal.,2007).
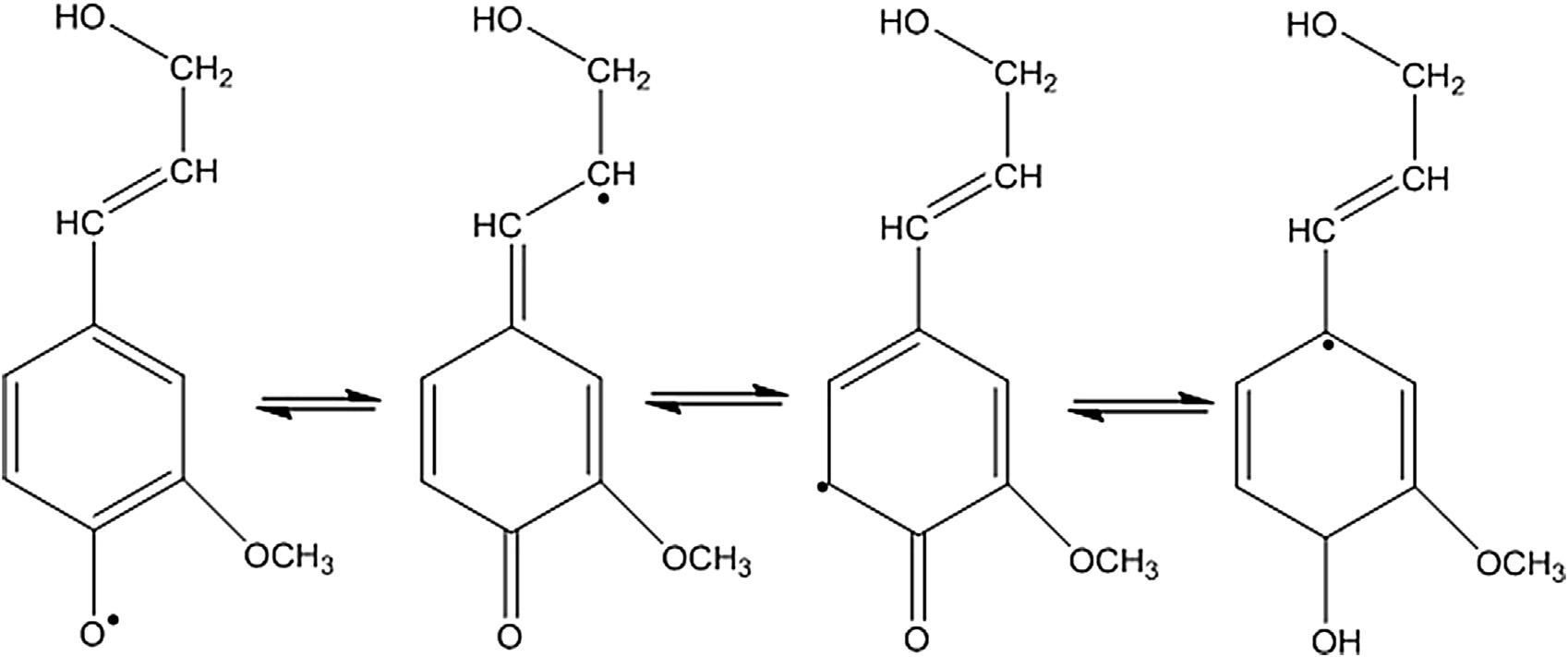
Lignificationisinitiatedwhenaphenolichydroxyl hydrogenatomisabstractedbytheenzymeperoxidasetoformaphenoxyfreeradical,typically referredtoasdehydrogenativepolymerization,as describedby FreudenbergandNeish(1968).This phenoxy-freeradicalwillthendelocalizetoboth aromaticandsidechaincarbonatomsbytheprocess ofresonancestabilization(Figure2).
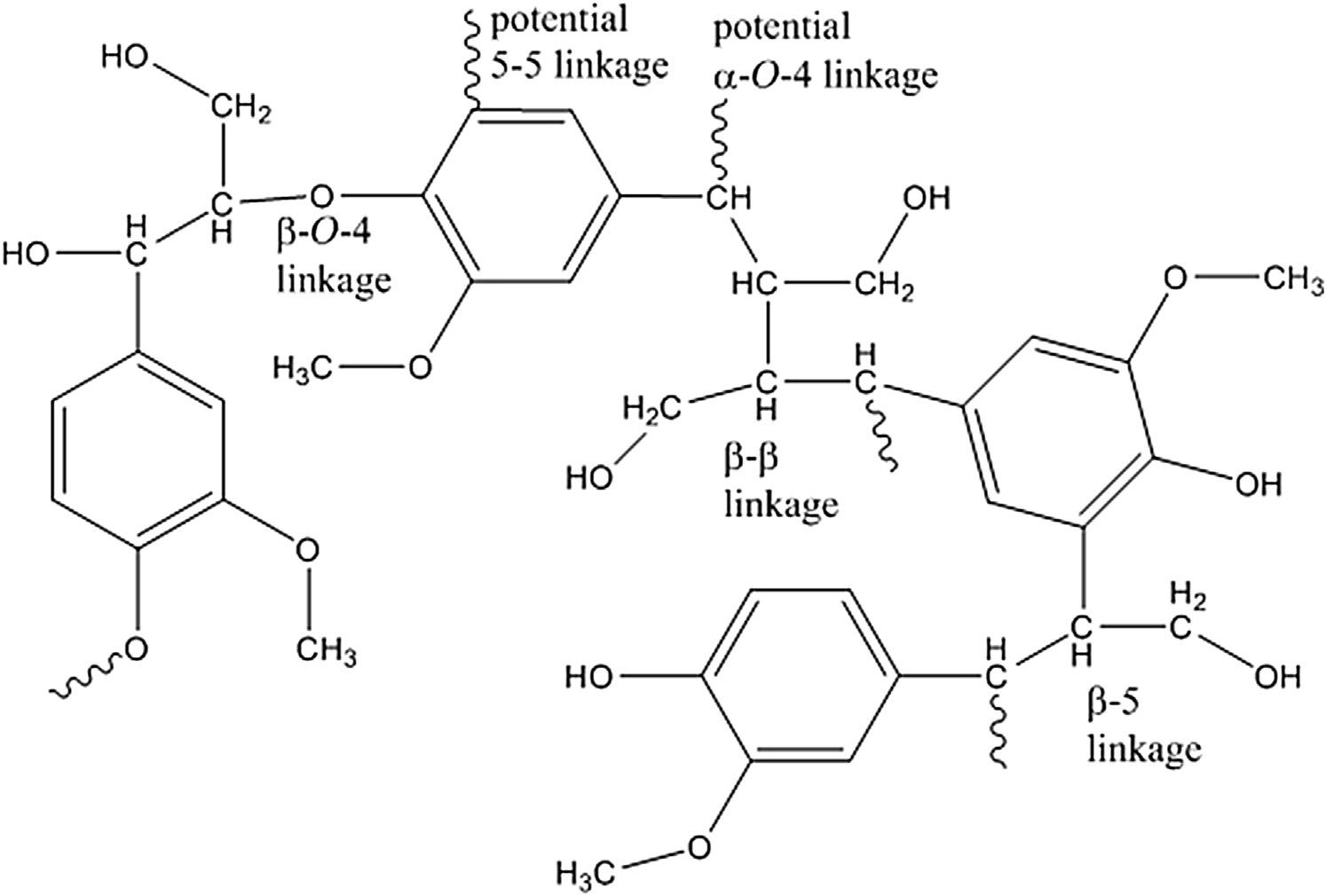
Couplingoftheseradicalsmayformetherlinkages,carbon carbonbonds,andbondsoccasionally tomorethanoneotherphenylpropaneunit.Basedon thestabilityoftheradicalateachlocation,thereis ahigherprobabilitythatcertaincarbonswillhostthe freeradical.This,inturn,willprovidepreferred linkagesbetweenligninunits.Ithasbeendetermined thatapproximately50%ofthesebondsare b-O-4 ethertype(DelaCruz,2014;Bowyeretal.,2007) withotherbondsassociatedsuchas b-5, b-1, b-b,
Figure1 ExamplesofC9 monomers: p-coumarylalcohol,coniferylalcohol,andsyringylalcohol.
Figure2 Resonancestabilizationofradicalsthatleadtointerunitlinkagesinlignin.
Figure3 Examplesofinterunitconnectionsinlignin.Note,thediagramistosimplyillustratepossiblelinkages andthisstructureisnotarepresentative“segment”oflignin.
and5-5(Figure3).ItisevidentthattheCg retainsits hydroxylgroupthroughthisprocess,providingthe nativeligninopportunitytoalsoretainacertainlevel ofhydrophilicity.Furthermore,asindicatedin Figure3,thebenzyliccarbon(attheCa)istypically highlyunstableandundergoesreactionswithnucleophiliccompounds.Thesereactionscanleadtothe formationofasecondaryhydroxylgroupattheCa by reactionwithwaterwhichisareactivesiteforlinkagestocarbohydratescreatinglignin carbohydrate complexes.Theselinkagesformedtothepolysaccharidesarebothetherandesterlinkages dependentuponthefunctionalgroupsofthemonosaccharide(FengelandWegener,1983).
Analysisofligninimpactsseveralchangestoits nativestatemakingitdifficulttohaveanexact structureofligninwithinthecellwall.However, thereareisolationmethodsthatcanbeusedtoget abetterideaofthecharacteristicsofalessseverely modifiedlignin.Onesuchmethodinvolvesthe resizingofwoodintoafineflourthroughballmilling, usingcellulose-degradingenzymestoremovethe bulkofthepolysaccharidesandthenanacidolysis reactiontobreakseveralofthelignincarbohydrate linkages(WuandArgyropoulos,2003).Theisolation ofthisenzymaticmildacidolysislignin(EMAL) preservesseveralaspectsaboutligninandisusedas astandardtocompareotherligninisolationmethods.
Whenanalyzedusingquantitativenuclearmagnetic resonance(NMR)spectroscopy,itrevealsthatthe quantityoffunctionalgroupsisbasedonthequantity oflignin(GranataandArgyropoulos,1995).Itisalso revealedthatmostligninshave w4mmolaliphatic hydroxylgroupsattachedtothesidechainpergram ofligninand0.2 1mmolfreephenolicgroupsper gramoflignin(Puetal.,2011).Furthermore,itis possibletoanalyzeligninwithinthewholecellwall withouttheneedforisolation.Thisapproachrequires themillingofwoodintoafinepowderandthenusing specialsolventsforcellwalldissolution.Dissolved woodmaterialisanalyzedusingtwo-dimensional (2D) 13C 1HheteronuclearsinglequantumcoherenceNMRspectroscopy(Mansfieldetal.,2012).The techniquecandeterminetherelativeconcentrations ofinterunitligninstructuresprovidinganinsightinto thestructureoflignin.
Asmaybeinferredfromtheabovedescriptionof isolatingEMAL,therearetwoprocessesthatoccur duringdelignification.Thefirstinvolvesbreakageof keylignin carbohydratelinkages.Thisbond breakagewillallowextractabilityoftheligninas seenduringmildacidolysisofligninwheredilute hydrochloricacidisusedtobreaktheselinkages. Thesecondprocessinvolvescleavageofsomeof theinterunitbondsofligninthatmayreduce themolecularweight.Hence,delignification
technologiesdramaticallyimpactligninfunctionalityandmolecularweight.Asaresult,thepropertiesofligninrelatedtoitssolubilityaremodified. Severalstudieshaveshownthatlignincanbefractionatedbyusingseveralsolventswithlargely differentsolventparameters(Moercketal.,1986).A smallfractionofligninmaybesolubleinanonpolar solventliketoluene,nonhydrogenbondingsolvents likedichloromethane,whileotherfractionsare solubleinmorepolarsolventslikealiphaticalcohols.Thedifferentsolubilityofthefractionsshed lightontheheterogeneousnatureofligninevenifit isisolatedfromasoftwoodligninthatcontains95% guaiacyllignin.Delignificationcanmodifythe lignintoappreciativedegreesbytheadditionofthe reactantssuchassulfuroralcoholtotheligninor increasethemolecularweight(orchangetheinterunitlinkages)byreactionsofligninwithitself duringdelignification.Theseisolationprocesses resultinligninthatcontainsacidicgroups,lose aliphatichydroxylgroups,increasethefreephenolic groupsbybreakageofthe b-O-4linkage,and containmorecarbon carbonbondsbetweenunits (whicharereferredtoascondensedstructures). Becauseofthevarietyofintermolecularlinkages betweentheprecursors,ligninisalsoahighly heterogeneouspolymer(FengelandWegener,1983; Baucheretal.,1996).Thevarietyofbondsresultsin abranchedligninpolymerwithapotentiallycrosslinkedthree-dimensionalstructure(Schmidl,1992). Therecentliteraturehassuggestedthatligninmaybe moreuniformthanpreviousthought,witheither linearchaintopologies(Crestinietal.,2011)oreven beingcappedbyspecificligninmonomers(Sangha etal.,2014).However,itisclearthatligninpolymerizationisleftuptothefatesofthermodynamics asthepolymerizationprocessoccursoutsidethe controlofthecellcytoplasm(Ralphetal.,2004). Hence,thecellularcontrolislimitedtotheproductionandreleaseofthemonomersinvolvedinlignificationandtheenzyme-activateddehydrogenated monomerswillpolymerizewiththegrowinglignin chain.
Anotherimportantaspectofpolymericmaterialsis theirmolecularweight.Absoluteknowledgeabout lignin’smolecularweighthasbeendifficulttoachieveasremovinglignintoanalyzeitautomatically impactstheligninstructure.Additionally,lignin molecularweightmeasurementssufferfromissues surroundingsolubility,aggregation,adsorption,and fluorescencemakingitoneofthemostdifficult
polymerstoanalyze.Earlyexperimentslookinginto themolecularweightoflignosulfonatessuggested thatdelignificationoccurredlikebreakinganetwork polymer(Gorning,1971).However,severaldifferent isolatedligninshavebeenanalyzedwithmodern analyticalequipment,andligninusuallyhas abimodaldistributionwithnumber-averagemolecularweightbetween3000and10,000andweightaveragemolecularweightfrom8000to80,000 dependentuponsolutionconditions,andlignintype (Guerraetal.,2007).Manytechnicallignins describedintheliteratureeitherhavelowermolecularweightsbecauseoffragmentationoflignininto oligomersormuchgreatermolecularweights.When delignificationoccurs,anumberofreactionscan occurbutmanyinvolveradicalformationand subsequentradicalcouplingwhereligninfragments canbecomebonded,increasingthemolecularweight.
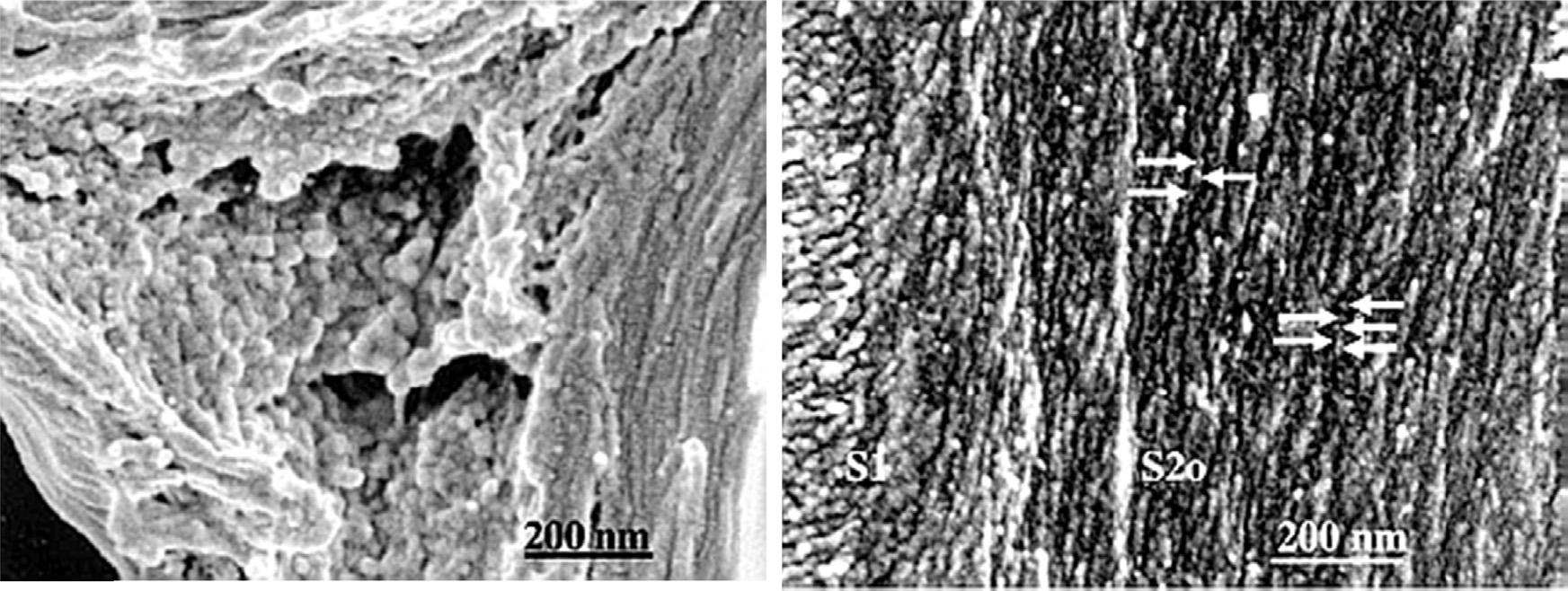
Additionalheterogeneityoflignininvolveshow thestructureofthispolymerdifferswithinagiven cellwalldependentonitslocationinthewall,plant species(hardwoodvssoftwood),andgrowth conditions,especiallyrelatedtoreactionwood formation(CampbellandSederoff,1996;deWild etal.,2010).Thesedifferencesarisefromthe monomericcompositionavailableduringthelignificationreactions.Overall,onecanseethe complexityoftryingtoanalyzealigninthatmaybe differentinvariouslocationsofthecellwall.Terashimahasshownthatligninmicrostructureis dependentuponitslocationinthecellwall.Inthe cellwallcorners,ligninformssphericalclusters, whilelignininthesecondarycellwallisgreatly reducedindimensionssurroundingthemicrofibril lamellarstructureofthecellwalllayersasshownin Figure4 (Terashimaetal.,2004).Workwiththe Ramanspectroscopyhasindicatedthatligninin thesecondarywallhasapreferredorientationalong themicrofibrilstructure(AtallaandAgarwal,1985).
2.LigninFunctions
Ligninispartofthestructuralframeworkin plants,formingpartoftheprimaryload-bearing elementofthecellwall.Fromanevolutionary pointofview,ligninhasbeencreditedastheterrestrialadaptionthatpermitssignificantverticalgrowth. Asanessentialpartofthecell,ligninsupportsthe plantbyimpartingrigiditytothecellwall.Theplant withstandsnaturalenvironmentalstressesbecauseof
Figure4 Cellcornerregion.Largeglobularmodulusformaggregatesatrandom,leftandligninmodules(arrows) aregrowingalongcellulosemicrofibrils(CMFs)atanalmostregulardistance,right.
thiscellwallbuildingblock(Lopesetal.,2011; SarkanenandLudwig,1971).Ligninnotonly providesstiffnesstotheplantbutinconjunctionwith theheteropolysaccharides,itprovidesflexibility whichisfundamentalforanappropriateresponseto dynamicloadsfromwindandsnow.Inadditionto mechanicalsupport,ligninmodifiesthepolysaccharidenetworktomakeitresistanttooutside organisms.Ligninhelpstoprotectwoodytissuefrom themicrobialandfungalattackencasingthecarbohydratestructure,providingreducedaccessibilityof enzymesforhydrolysis.Limitedsolubilityand complexityoftheligninmakeitresistanttodegradationbymostmicroorganisms(Prasongsuketal., 2009;Bholayetal.,2012;BergandMeentemeyer, 2002;Crowetal.,2009;VermaandDwivedi,2014). Onecanimagineifligninonlycontainsasingle linkagethatwouldbecomethecellwall’sAchilles’ Heal.However,withthevarietyoflinkageswooddestroyingorganismsrequirethebreakageofboth arylcarbonbondsandaryletherbondsneedingto expandthecostofproductionofspecificenzymesor developnonspecificpathwaysfordelignification.In addition,ligninislesshydrophilicthanthepolysaccharideshelpingtochangethepermeabilityofthe cellwallbysealingitandenablingwatertransport throughthevasculartissue(TenandVermerris, 2013).Finally,thearomaticityofligninlendsitselfto enhancethethermalstabilityofwoodprovidingchar layer.Thishasbeenexploitedwithisolatedtechnical lignin,turningitintocarbonfibermaterialby controlledhigh-temperatureheating.Whileligninis seenasmorethermallystablebecauseofit,thenative structureofligninisgreatlyimpactedbythethermal modificationandresearchersshouldbecautioned
thatligninstructurecanchangeduringprocessingat temperaturesusedintheproductionorsomethermoplasticmaterials.Suchmodificationincludes depolymerization,lossoftheCg hydroxyl,and formationofnewacidicgroups.
3.SourcesofLignin
Thesourcefromwhichligninisobtained,the extractionmethodsandthesecondarytreatments applied,havestrongimpactsonitsphysicaland mechanicalproperties(Garciaetal.,2011;Khanam etal.,2006).Lignincanbederivedfromvarious sourcessuchaswood,pulpandpaper,sugarcane bagasse,andcerealstrawsusingavarietyofpulping methods.Intermsofweight,thelignincontentin woodyplantsfromgymnospermsandangiospermsis thehighestwiththeorderof30 40%whileother sourcesonlycontainaround3 25%(Smolarski, 2012).Thepulpandpapersectorproducesalarge amountoflignin(Jungmeier,2010;Dohertyetal., 2011)withevengreaterpotentialfromfuturelignocellulosicbiorefineries.Minimalligninisrecovered fromgrass,branches,leaves,andsolidwasteinurban andruralareaswheretheirlignincontentisestimated tobelessthan15%(Wangetal.,2011;Philippidis andHatzis,1997).
3.1Wood
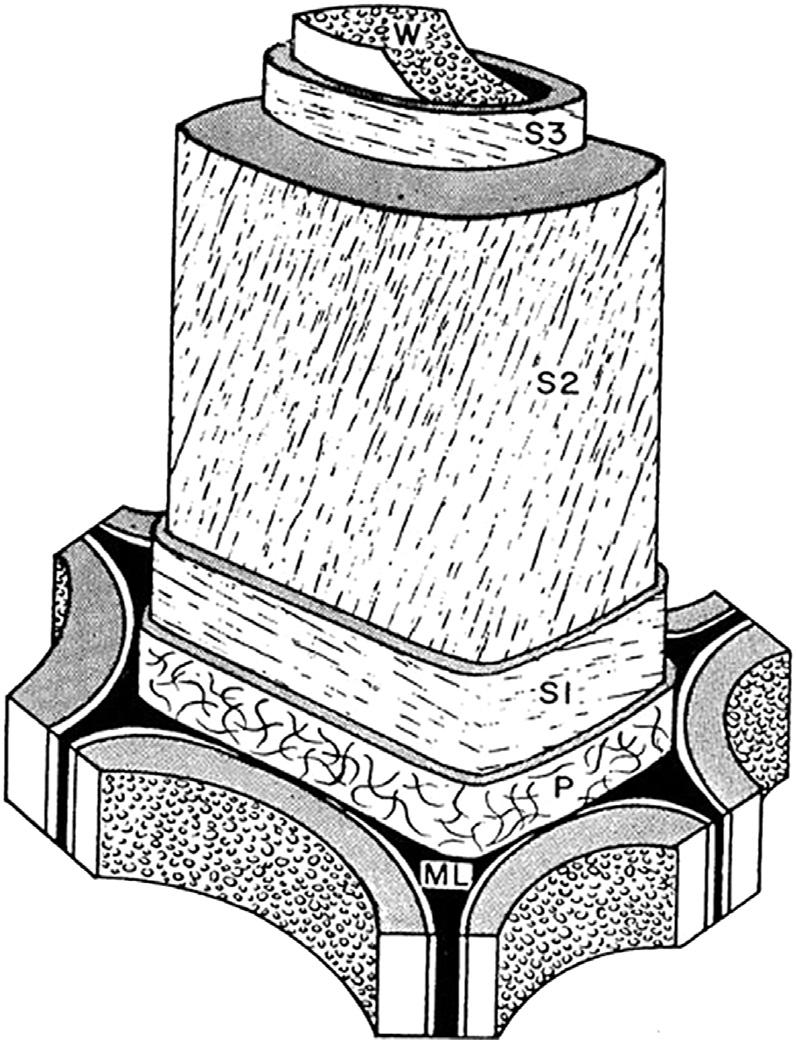
Ligninhasnonuniformthicknessinthemiddle lamella,andintheprimaryandsecondarycellwalls dependingontheplantspeciesandcelltype (Figure5).Approximately70%ofthetotallignin contentofthecellwallisconcentratedinthe
thickestlayerofthesecondarycellwall.Lignin quantityandqualityvarynaturallyamongwood specieswith19 28%inhardwoods(angiosperms) and24 33%insoftwoods(gymnosperms)(Fengel andWegener,1983).Infact,hardwoods,ingeneral, containmorehemicelluloseandlessligninthan softwoods(Bjornsson,2014).Notonlytheamount ofligninvariesbetweenhardwoodsandsoftwoods, therelativeconcentrationalsodiffersinthelocation withinatree.Thejuvenilewoodhasahigherlignin contentthanlatewood(DelaCruz,2014;Bowyer etal.,2007).Lignincontentisalsovariablewithin populationsofplantsofthesamegenus.For example,theaveragecontentofligninrangesfrom 25%in Pinusmonticola to30%in Pinuspalustris withinthegenus Pinus.Reactionwoodforms mechanicalstressandhasdifferentlignincontentin comparisonwiththenormalwooddependingonthe conditionsandspecies(Tiimonen,2007).
Lignininhardwoodsissyringyl guaiacyltype andinsoftwoodsistypicallyguaiacylwithlimited p-hydroxyphenyllignininbothtypes.Bothofthese structureshavedifferencesarisingfromtheinterunit linkages.Guaiacyllignincanundergocoupling reactionsatthe5-positionofthephenylpropaneunit, andthisprovidesasignificantplaceforbranchingand cross-linkingreactions,especiallyoccurringduring
Figure5 Woodcellwallincludingmiddlelamella(ML), primarycellwall(P),layersofthesecondarycellwall (S1,S2,S3),andwartylayer(W)(Sjostrom,1993).
delignificationprocess.Thepresenceofsyringylunits makeshardwoodligninmorereadilyremovedduring thepulpingprocessbylimitingligninforming condensedstructuresattheopenmethoxyposition.In thecaseofcompression-woodlignin,itismore difficulttohydrolyzebecauseitcontainsahigher proportionofcondensed p-hydroxyphenylunits (CampbellandSederoff,1996;Novaesetal.,2010).
3.2PulpandPaperIndustry
Softwoodandhardwoodspecies,andcertaintypes ofannualplants,havecommercialinterestasasource ofcellulosefibersfortheproductionofpaperand boardproducts.Ligninisnoteasytoisolateinthe nativeformfromplantmaterial(Leisolaetal.,2012). Thepulpandpaperindustryistheprimarily commercialsourceoflignin,however,thedelignificationprocessmodifieslignintovariousdegrees.In technicalfiberliberationprocesses,suchasalkaline orsulfitepulping,hugequantitiesofligninaredissolvedasalkaliligninandlignosulfonates,respectively.Largeamountsofligninaremadeavailable annuallyfromthepulpandpaperindustryasbyproductsofthedelignificationprocess.Thesesulfite orsulfateligninshavevaryinglevelsofcovalently bondedsulfurresultinginthepolymerwithdifferent characteristicsthantheoriginallignin(Sainsbury, 2013;Khanametal.,2006;LoraandGlasser,2002). Typically,ligninimpuritiesincludelow-molecularweightsugarsandresinacidsthatareremovedduring thepurificationprocess.Forexample,kraftligninis usuallypurifiedfromkraftblackliquorswhichare complexmixturesoffibrousmaterialsanddissolved organicssuchaslignins,hemicelluloses,sugars,acids, andresinsandalsoinorganicsaltssuchasash(Wallbergetal.,2003).Therecenttechnologyinenhancing ligninrecoveryfromblackliquorbyCO2 acidification hasbeentransferredtotheindustryandhascreated areadilyavailabledryligninpowderstream(Ohman etal.,2013;Kouisnietal.,2012).Asshownin Table1, inallcommercialpulpingprocessesthelignin extractionmethodswillaffectthevalueoftheproducts thatcanbederivedfromitandwillalterthestructureof theligninincomparisonwiththenativeone(Smolarski,2012).
3.3SugarcaneBagasse
Sugarcanebagasseisthefiberthatremainsafterthe sugarshavebeenextracted.Asanagro-industrial residue,sugarcanebagasse(Saccharumofficinarum)
Table1 LigninPuritywithItsAssociatedSources(Holladayetal.,2007)
LigninTypeLigninPuritya
LignosulfonatesMedium(somereducingsugar (upto20wt%)andsulfur)
PotentialProducts
Dispersants,agriculturalchemicals,emulsion stabilizer,industrialbinders,carbonblack,ink andpigments,andconcreteadditives
KraftMedium(someashandsulfur)Dispersants,carbonfibers,emulsifiers, activatedcharcoal,andbinders
OrganosolvHigh(sulfurfree)Aromaticpolyols,newdiacids,activatedcarbon, phenolicresins,carbonfibers,vanillin,phenol derivatives,andantioxidants
aPresenceofresidualcarbohydrates,ash,andproteinsdependsonfeedstockandprocess.
isanothersourceofligninrawmaterial.Itisabyproductofthesugarcaneindustrywithapproximately32 34%cellulose,19 24%hemicellulose, 25 32%lignin,6 12%extractives,and2 6%ash (SakdaronnarongandJonglertjunya,2012;Rezende etal.,2011;Pandeyetal.,2000).Chemicalcompositionofsugarcanebagasseissimilartotheotherplant cellwalls.Eachclassofplants,grasses,softwoods, andhardwoodsproducesaligninrichinonetypeof thephenylpropanerepeatunit.Sugarcanebagasse ligninhasahigherproportionofH-typelignin, phydroxyphenyl,andhencealowermethoxycontent thansoftwoodandhardwoodlignins(Dohertyetal., 2007).Approximately250 280kgofbagasseis generatedfromprocessingeachtonofsugarcane whichroughlyyielded54milliontonsofbagasse annually(Canilhaetal.,2012).Currently,alarge amountofbagasseisburntasalow-gradefuelfor energyrecovery,andonlyalimitedquantityhasbeen usedtomakepulps,boardmaterials,andcomposites. Itisestimatedthat200milliontonsofligninis producedannuallyfrombagasse(Singhetal.,2005).
3.4AgriculturalResidues
Theadvantagesofusingagriculturalresiduesare threefolds:economic,environmental,andtechnological.Unlikewoodpulps,agriculturalpulpscanbe producedusingmoreenvironmentallybenignprocessingandbleachingmethods(Clancy-Hepturn, 1998).Inthewoodpulpingprocess,mostofthepulp isbleachedusingchlorineorchlorine-basedchemicalswhilestrawcanbetreatedwithminimaladditionsofchlorine-freechemicalswhichresultsinno productionoftoxicchemicals.Moreover,agriculturalresidueshavegenerallyamoreporousstructure andalowerlignincontentthanwoodyplantswhich facilitatetheirpulpingprocess.Examplesofthe
agriculturalresiduesarericeandwheatstrawswhich arediscussedinthissection.Ricestrawalsocalledas cerealstrawisanothersourceforligninproduction. Totalworldproductionofricestraw, Oryzasativa,is 525milliontonsperyear.
About90%ofthericestrawisproducedinAsia. Chinahasthegreatestcapacityforpulpingricestraw. Ithasapproximately30 35%cellulose,25 30% hemicellulose,15 28%lignin,and4 7%ash (Marquesetal.,2010).Thehighashcontentarises fromthesilicainthecellwalllimitingtheabilityto burnthismaterialwithoutcausingasignificantenvironmentalnuisance.Highsilicacontentinricestraw, ashighas18%incleanricestraw,makesthepulping processmorecostlyduetoincreasedchemical recoverydifficultiesandcosts.Inadditiontorice straw,wheatstraw, Triticumaestivum,isannually generatedinabundanceof529milliontonsperyear. Whenfarmedintensively,wheatstrawcanbe producedinalargerscale.Theamountofligninof wheatstrawvariesbetween5%and17%depending onthegeographicallocationoftheplantswhichis comparabletohardwoods(BuranovandMazza, 2008).Wheatstrawhasoneofthehighestcellulose contentsofalloftheagriculturalfibers.Strawpapers areknowntopossessgoodprintingqualitiesandare madefrompulprequiringlowenergyrelativetothat requiredtoprocesswoodpulp.Researchersfoundthat wheatstrawmustbepulpedunderconditionsofless energyandfewerchemicalsincomparisonwiththe woodpulpstomaximizepulpyields(Clancy-Hepturn, 1998).Tomakethestrongestproduct,papermakers willlikelycombinesomestrongerhardwood,kenaf, orhemppulpwithstrawpulp.Despitetheadvantages ofusingagriculturalresidues,thehighcollection, transportation,andhandlingcostsassociatedwith theseresources,aswellastheirseasonalavailability, limittheirapplicationsinpaperproduction.
4.LigninPotential
Currentlyaround50milliontonsofligninis producedperyearbythepulpandpapersectorbutonly 2%ofthatisusedforapplicationsotherthancombustionandenergyproduction(deWildetal.,2014;Fengel andWegener,1983;Schmidl,1992;Thielemansetal., 2002).Therearelimitationsinutilizationduetothe ligninstructure,heterogeneity,andtheindustrialprocessingcostsfordelignification(Holladayetal.,2007; Vanholmeetal.,2010;Neutelings,2011;ElHageetal., 2009;Chiang,2005).Toimproveupontheselimitations,differenttypesofmodificationsaredoneto increaseitschemicalreactivityanduniformity,reduce thebrittlenessoflignin-derivedpolymers,increaseits solubilityinorganicsolvents,andimprovetheeaseof processingthelignin(Argyropoulos,2012).Lignin potentialscanalsobeconsideredintheproductionof additives,resins,andcoatingmaterials(Jungmeier, 2010;VishtalandKraslawski,2011).Thisrenewable aromaticpolymercanequallybereplacedwith syntheticpolymersandaromaticchemicals(Zakzeski etal.,2012).Highantioxidantcapacityofligninandits applicationsinpolymerenhancementhavebeen experimentallyproved(Piazzaetal.,2014).Thefindingsofresearchonlignindepolymerizationshowed thatduetotheligninmacromolecularstructureoflignin withmanyphenolicunits,lignindepolymerizationcan beusedasalarge-scalesourceforproductionofvaluablechemicals(Kleinertetal.,2009;Meieretal.,1992; Hora ´ ceketal.,2012).Ligninalsohaspotentialtobe usedinthefabricationofcompositematerials. ElMansourietal.(2007) showedthatexterior-grade particleboardsmadewithlignin-basedadhesives successfullymettherequirementsofinternational standards.Thepotentialoflignininpolymeric compositeswillbediscussedinlaterchapters.
References
Adam,M.,Ocone,R.,Briens,C.,Berruti,F.,2011. ModellingthePyrolysisofLignin. Argyropoulos,D.,2012.HighValueLigninDerivatives,Polymers,andCopolymers,andUseThereof inThermoplastic,Thermoset,andComposite Applications.USPatentApplication.
Atalla,R.H.,Agarwal,U.P.,1985.Ramanmicroprobe evidenceforligninorientationinthecellwallsof nativewoodytissue.ComptesRendusBiologies 227(4687),636 638.
Baucher,M.,Chabbert,B.,Pilate,G.,Van Doorsselaere,J.,Tollier,M.T.,Petit-Conil,M., 1996.Redxylemandhigherligninextractability bydown-regulatingacinnamylalcoholdehydrogenaseinpoplar.PlantPhysiology112, 1479 1490.
Berg,B.,Meentemeyer,V.,2002.Litterqualityin anorthEuropeantransectversuscarbonstorage potential.PlantandSoil242(1980),83 92.
Bholay,A.,Bhavna,B.,Jadhav,P.,Palekar,K., Dhalkari,M.,Nalawade,P.,2012.Bacteriallignin peroxidase:atoolforbiobleachingandbiodegradationofindustrialeffluents.UniversalJournalof EnvironmentalResearchandTechnology2(1), 58 64.
Bjornsson,S.,2014.AdvancedControlMethodology forBiomassCombustion.UniversityofWashington. Bowyer,J.,Shmulsky,R.,Haygreen,J.,2007.Forest ProductsandWoodScience:AnIntroduction, fifthed.BlackwellPublishing,Ames,IA.
Buranov,A.U.,Mazza,G.,2008.Lignininstraw ofherbaceouscrops.IndustrialCropsand Products28(3),237 259. http://dx.doi.org/10. 1016/j.indcrop.2008.03.008.
Campbell,M.M.,Sederoff,R.R.,1996.Variationin lignincontentandcomposition.PlantPhysiology 110,3 13.
Canilha,L.,KumarChandel,A.,dosSantos Milessi,T.S.,FernandesAntunes,F.A.,daCosta Freitas,W.L.,dasGrac¸asAlmeidaFelipe,M.,da Silva,S.S.,2012.Bioconversionofsugarcane biomassintoethanol:anoverviewaboutcomposition,pretreatmentmethods,detoxificationof hydrolysates,enzymaticsaccharification,and ethanolfermentation.JournalofBiomedicine& Biotechnology1 16. http://dx.doi.org/10.1155/ 2012/989572.
Chiang,V.L.,2005.Understandinggenefunctionand controlinligninformationinwood.Agricultural Biotechnology17,139 144.
Clancy-Hepturn,M.,1998.Agriculturalresidues: apromisingalternativetovirginwoodfiber.JournalofReinforcedPlasticsandComposites29. Washington,DC,USA.IssueinResources Conservation,BriefingSeriesNo.1Resources ConservationAlliance.
Crestini,C.,Melone,F.,Sette,M.,Saladino,R., 2011.Milledwoodlignin:alinearoligomer. Biomacromolecules12(11),3928 3935. http:// dx.doi.org/10.1021/bm200948r.
Crow,S.E.,Lajtha,K.,Filley,T.R.,Swanston,C.W., Bowden,R.D.,Caldwell,B.A.,2009.Sourcesof plant-derivedcarbonandstabilityoforganicmatter insoil:implicationsforglobalchange.Global ChangeBiology15(8),2003 2019. http://dx.doi. org/10.1111/j.1365-2486.2009.01850.x.
DelaCruz,F.,2014.FateandReactivityofLignin inMunicipalSolidWaste(MSW)Landfill, p.192.
Dinis,M.,Bezerra,R.,Nunes,F.,Dias,A.,Guedes,C., Ferreira,L.,Rodriques,M.,2009.Modificationof wheatstrawligninbysolidstatefermentationwith white-rotfungi.BioresourceTechnology100(20), 4829 4835.
Doherty,W.,Halley,P.,Edye,L.,Rogers,D., Cardona,F.,Park,Y.,Woo,T.,2007.Studieson polymersandcompositesfromligninandfiber derivedfromsugarcane.PolymersforAdvanced Technologies18(March),673 678. http://dx. doi.org/10.1002/pat.
Doherty,W.,Mousavioun,P.,Fellows,C.,2011. Value-addingtocellulosicethanol.Ligninpolymers.IndustrialCropsandProducts33(2), 259 276.
ElHage,R.,Brosse,N.,Chrusciel,L.,Sanchez,C., Sannigrahi,P.,Ragauskas,A.,2009.Characterizationofmilledwoodligninandethanolorganosolv ligninfrom Miscanthus.PolymerDegradationand Stability94(10),1632 1638. http://dx.doi.org/10. 1016/j.polymdegradstab.2009.07.007.
ElMansouri,N.-E.,Pizzi,A.,Salvado,J.,2007. Lignin-basedpolycondensationresinsforwood adhesives.JournalofAppliedPolymerScience 103,1690 1699. http://dx.doi.org/10.1002/app. Fengel,D.,Wegener,G.,1983.Wood:Chemistry, Ultrastructure,Reactions.WalterdeGruyter, Berlin,p.613.
Freudenberg,K.,Neish,A.C.,1968.Constitutionand BiosynthesisofLignin.Springer-Verlag,Berlin, p.129.
Garcia,A.,Amendola,D.,Gonzalez,M.,Spigno,G., Labidi,J.,2011.Ligninasnaturalradicalscavenger.Studyoftheantioxidantcapacityofapple treepruningligninobtainedbydifferentmethods. ChemicalEngineeringTransactions24,925 930. Gorning,D.A.I.,1971.Polymerpropertiesoflignin andligninderivatives.In:Sarkanen,K.V.(Ed.), Lignins,3055.
Granata,A.,Argyropoulos,D.S.,1995.2-Chloro-4, 4,5,5-tetramethyl-1,3,2-dioxaphospholane,areagent
fortheaccuratedeterminationoftheuncondensed andcondensedphenolicmoietiesinlignins. JournalofAgriculturalandFoodChemistry43, 1538 1544.
Guerra,A.,Gaspar,A.R.,Contreras,S.,Lucia,L.A., Crestini,C.,Argyropoulos,D.S.,2007.Onthe propensityoflignintoassociate:asizeexclusion chromatographystudywithligninderivativesisolatedfromdifferentplantspecies.Phytochemistry 68(20),2570 2583. http://dx.doi.org/10.1016/j. phytochem.2007.05.026.
Holladay,J.E.,White,J.F.,Bozell,J.J.,Johnson,D., 2007.TopValue-AddedChemicalsfromBiomass. Springfield,VA,pp.1 87.
Hora ´ cek,J.,Homola,F.,Kubickova ´ ,I.,Kubicka,D., 2012.Lignintoliquidsoversulfidedcatalysts. CatalysisToday179(1),191 198. http://dx.doi.org/ 10.1016/j.cattod.2011.06.031.
Jungmeier,G.,2010.Classificationandassessment ofbiorefineryconcepts.In:IEABioenergyTask 42Biorefineries.ICPSConference,Leipzig, Germany.
Khanam,L.A.M.,Talukder,D.,Hye,M.A.,2006. Toxicandrepellentactionofsugarcanebagassebasedligninagainstsomestoredgraininsect pests.UniversityJournalofZoology,Rajshahi University25,27 30.
Kleinert,M.,Gasson,J.R.,Barth,T.,2009.Optimizingsolvolysisconditionsforintegrateddepolymerisationandhydrodeoxygenationofligninto produceliquidbiofuel.JournalofAnalyticaland AppliedPyrolysis85(1 2),108 117. http://dx. doi.org/10.1016/j.jaap.2008.09.019.
Kouisni,L.,Holt-Hindle,P.,Maki,K.,Paleologou,M., 2012.Thelignoforcesystem:anewprocessforthe productionofhigh-qualityligninfromblackliquor. JournalofScience&TechnologyforForestProducts andProcesses2(4),6 10.
Leisola,M.,Pastinen,O.,Axe,D.D.,2012.LigninDesignedRandomness.BIO-Complexity(3),1 11. Lin,S.,Dence,C.,1992.MethodsinLigninChemistry.Springer,Berlin.
Lopes,F.J.F.,Silve ´ rio,F.O.,Baffa,D.C.F., Loureiro,M.E.,Barbosa,M.H.P.,2011.DeterminationofsugarcanebagasseligninS/G/Hratioby pyrolysisGC/MS.JournalofWoodChemistryand Technology31(4),309 323. http://dx.doi.org/ 10.1080/02773813.2010.550379.
Lora,J.H.,Glasser,W.G.,2002.Recentindustrial applicationsoflignin:asustainablealternativeto
nonrenewablematerials.JournalofPolymersand theEnvironment10(1 2),1 10.
Mansfield,S.D.,Kim,H.,Lu,F.,Ralph,J.,2012.Whole plantcellwallcharacterizationusingsolution-state 2DNMR.NatureProtocols7(9),1579 1589. http://dx.doi.org/10.1038/nprot.2012.064.
Marques,G.,Rencoret,J.,Gutie ´ rrez,A.,Rı´o,J.C., 2010.Evaluationofthechemicalcomposition ofdifferentnon-woodyplantfibersusedforpulp andpapermanufacturing.TheOpenAgricultural Journal4,93 101.
Mccarthy,J.L.,Islam,A.,1999.Ligninchemistry, technology,andutilization:abriefhistory.In:Lignin: Historical,Biological,andMaterialsPerspectives. AmericanChemicalSociety,pp.2 99.
Meier,D.,Ante,R.,Faix,O.,1992.Catalytichydropyrolysisoflignin:influenceofreactionconditions ontheformationandcompositionofliquidproducts.BioresourceTechnology40(2),171 177. http://dx.doi.org/10.1016/0960-8524(92)90205-C.
Moerck,R.,Yoshida,H.,Kringstad,K.P., Hatakeyama,H.,1986.Fractionationofkraftlignin bysuccessiveextractionwithorganicsolvents.1. Functionalgroups(13)CNMRspectraandmolecularweightdistributionsAGRIS:International InformationSystemfortheAgriculturalScience andTechnology.Holzforschung40,51 60.
Neutelings,G.,2011.Ligninvariabilityinplantcell walls:contributionofnewmodels.PlantScience 181(4),379 386. http://dx.doi.org/10.1016/j. plantsci.2011.06.012.
Novaes,E.,Kirst,M.,Chiang,V.,Winter-Sederoff,H., Sederoff,R.,2010.Ligninandbiomass:anegative correlationforwoodformationandlignincontent intrees.PlantPhysiology154(2),555 561. http:// dx.doi.org/10.1104/pp.110.161281.
Ohman,F.,Theliander,H.,Tomani,P.,Axegard,P., 2013.MethodforSeparatingLigninfromBlack Liquor.
Pandey,A.,Soccol,C.R.,Nigam,P.,Soccol,V.T., 2000.Biotechnologicalpotentialofagro-industrial residues.I:sugarcanebagasse.BioresourceTechnology74(1),69 80. http://dx.doi.org/10.1016/ S0960-8524(99)00142-X.
Payen,A.,1838.Me ´ moiresurlaconge ´ lationdes pommesdeterre.Huzard,pp.1795 1871. Philippidis,G.P.,Hatzis,C.,1997.Biochemical engineeringanalysisofcriticalprocessfactorsin thebiomass-to-ethanoltechnology.Biotechnology Progress13(3),222 231.
Piazza,G.J.,Lora,J.H.,Garcia,R.A.,2014.Flocculationofhighpuritywheatstrawsodalignin.BioresourceTechnology152,548 551. http://dx.doi. org/10.1016/j.biortech.2013.11.040.
Prasongsuk,S.,Lotrakul,P.,Imai,T.,Punnapayak,H., 2009.Decolourizationofpulpmillwastewaterusing thermotolerantwhiterotfungi.ScienceAsia35, 37 41. http://dx.doi.org/10.2306/scienceasia15131874.2009.35.037.
Pu,Y.,Cao,S.,Ragauskas,A.J.,2011.Application ofquantitative31PNMRinbiomassligninand biofuelprecursorscharacterization.Energy& EnvironmentalScience4(9),3154 3166. http:// dx.doi.org/10.1039/c1ee01201k.
Ralph,J.,Lundquist,K.,Brunow,G.,Lu,F., Kim,H.,Schatz,P.F.,Boerjan,W.,2004.Lignins: naturalpolymersfromoxidativecouplingof 4-hydroxyphenyl-propanoids.Phytochemistry Reviews3(1 2),29 60. http://dx.doi.org/10. 1023/B:PHYT.0000047809.65444.a4.
Rezende,C.A.,deLima,M.A.,Maziero,P., Deazevedo,E.R.,Garcia,W.,Polikarpov,I.,2011. Chemicalandmorphologicalcharacterizationof sugarcanebagassesubmittedtoadelignification processforenhancedenzymaticdigestibility. BiotechnologyforBiofuels4(54),1 18. http:// dx.doi.org/10.1186/1754-6834-4-54.
Sainsbury,P.,2013.BiocatalyticValorisationofLignin viaGeneticorChemicalInterventionofBacterial AromaticDegradationPathways.Universityof Warwick.
Sakdaronnarong,C.,Jonglertjunya,W.,2012.Rice strawandsugarcanebagassedegradation mimickinglignocellulosedecayinnature:an alternativeapproachtobiorefinery.ScienceAsia 38(4),364 372. http://dx.doi.org/10.2306/ scienceasia1513-1874.2012.38.364.
Sangha,A.K.,Davison,B.H.,Standaert,R.F., Davis,M.F.,Smith,J.C.,Parks,J.M.,2014. Chemicalfactorsthatcontrolligninpolymerization.JournalofPhysicalChemistryB118(1), 164 170. http://dx.doi.org/10.1021/jp411998t.
dosSantos,P.S.B.,Erdocia,X.,Gatto,D.A.,Labidi,J., 2014.Characterisationofkraftligninseparatedby gradientacidprecipitation.IndustrialCropsand Products55,149 154. http://dx.doi.org/10.1016/j. indcrop.2014.01.023.
Sarkanen,K.,Ludwig,C.(Eds.),1971.Lignins: Occurrence,Formation,StructureandReactions. JohnWiley&Sons,Inc.,p.360.
Schmidl,G.W.,1992.MolecularWeightCharacterizationandRheologyofLigninsforCarbonFibers. UniversityofFlorida.
Schulze,H.,1857.Neuenburg.Einegeschichtlich staatsrechtlicheSkizzenebsteinerBeleuchtung derneuestenschweizerischenDenkschriftvom7. Heinicke,p.48.
Singh,R.,Singh,S.,Trimukhe,K.D.,Pandare,K.V., Bastawade,K.B.,Gokhale,D.V.,Varma,A.J.,2005. Lignin-carbohydratecomplexesfromsugarcane bagasse:preparation,purification,andcharacterization.CarbohydratePolymers62(1),57 66. http://dx.doi.org/10.1016/j.carbpol.2005.07.011.
Sjostrom,E.,1993.WoodChemistry:Fundamentals andApplications.Nature,p.293.
Smolarski,N.,2012.High-ValueOpportunitiesfor Lignin:UnlockingItsPotentialLigninPotential. Frost&Sullivan,pp.1 15.Retrievedfrom: http:// www.greenmaterials.fr/wp-content/uploads/2013/ 01/Highvalue-
Ten,E.,Vermerris,W.,2013.Functionalizedpolymersfromlignocellulosicbiomass:stateofthe art.Polymers5(2),600 642. http://dx.doi.org/ 10.3390/polym5020600.
Terashima,N.,Awano,T.,Takabe,K.,Yoshida,M., 2004.Formationofmacromolecularligninin ginkgoxylemcellwallsasobservedbyfield emissionscanningelectronmicroscopy.Comptes RendusBiologies327(9 10),903 910. http://dx. doi.org/10.1016/j.crvi.2004.08.001.
Thielemans,W.,Can,E.,Morye,S.S.,Wool,R.P.,2002. Novelapplicationsofligninincompositematerials. JournalofAppliedPolymerScience83(2), 323 331. http://dx.doi.org/10.1002/app.2247.
Tiimonen,H.,2007.LigninCharacteristicsand EcologicalInteractionsofPtCOMT-modified SilverBirch.UniversityofOulu.
Vanholme,R.,Demedts,B.,Morreel,K.,Ralph,J., Boerjan,W.,2010.Ligninbiosynthesisand
structure.PlantPhysiology153(3),895 905. http://dx.doi.org/10.1104/pp.110.155119.
Verma,S.R.,Dwivedi,U.N.,2014.Ligningenetic engineeringforimprovementofwoodquality: applicationsinpaperandtextileindustries,fodder andbioenergyproduction.SouthAfricanJournal ofBotany91,107 125. http://dx.doi.org/10.1016/ j.sajb.2014.01.002.
Vishtal,A.,Kraslawski,A.,2011.Challengesin industrialapplicationsoftechnicallignins.Bioresources6(3),3547 3568.
Wallberg,O.,Jonsson,A.-S.,Wimmerstedt,R.,2003. Fractionationandconcentrationofkraftblack liquorligninwithultrafiltration.Desalination154 (2),187 199. http://dx.doi.org/10.1016/S00119164(03)80019-X.
Wang,M.,Wang,J.,Tan,J.X.,2011.Lignocellulosic bioethanol:statusandprospects.EnergySources, PartA:Recovery,Utilization,andEnvironmental Effects33(7),612 619. http://dx.doi.org/10.1080/ 15567030903226249.
deWild,P.J.,Huijgen,W.J.J.,Gosselink,R.A.,2014. Ligninpyrolysisforprofitablelignocellulosicbiorefineries.Biofuels,BioproductsandBiorefining8 (5),645 657. http://dx.doi.org/10.1002/bbb.
deWild,P.J.,vanderLaan,R.R.,Wilberink,R.,2010. Thermolysisofligninforvalue-addedproducts.In: XVMeetingoftheInternationalHumicSubstances Society.EnergyResearchCentreoftheNetherlands,Tenerife,CanaryIslands,Spain,pp.1 27. Wu,S.,Argyropoulos,D.S.,2003.Animproved methodforisolatinglignininhighyieldand purity.JournalofPulpandPaperScience29(7), 235 240.
Zakzeski,J.,Jongerius,A.L.,Bruijnincx,P.C.A., Weckhuysen,B.M.,2012.Catalyticligninvalorizationprocessfortheproductionofaromaticchemicals andhydrogen.ChemSusChem5(8),1602 1609. http://dx.doi.org/10.1002/cssc.201100699.
HoyongChung 1 andNewellR.Washburn 2, 3
Ligninisthesecondmostabundantterrestrial biopolymeraftercelluloseandisthelargestrenewablesourceofaromaticgroupsinnature.Ligninis foundinplantcellwallsandisanimportantstructuralcomponentofwoodyplants.Themainfunctions oflignininplantsaretoprovidephysicalstrength, toformwater-conductingvascularnetworksusing hydrophobicinteractionsandtoprotectplantsfrom microorganismsandinsects.Chemically,ligninis composedofarandomnetworkofphenylpropane groups.Thethreebasicstructuralmonomerunitsare coumarylalcohol,coniferylalcohol,andsinapyl alcohol,asshownin Figure1.Innature,these monomerunitsoxidizetophenoxyradicalsby peroxidaseandthenundergopolymerizationthrough multiplereactivesitestoformacomplexthreedimensionalpolymer.Thisbiosynthesisisreferred toasdehydrationpolymerization,andtheresulting monomerunitswithinligninarereferredtoas p-hydroxyphenyl(H),guaiacyl(G),andsyringyl (S).Differencesinligninaccordingtovariousplant sourcescanbemanifestedindifferencesinmonomerconcentrations(Vanholmeetal.,2010),with gymnosperms,suchasthevarioustypesofpines,
comprisingprimarilyofG-unitswithsmallconcentrationsofH-units.Incontrast,angiospermdicots, includingmanyhardwoods,haveamixtureofGand S-units,whichreducebranchingconcentrationsand canimproveligninprocessibility.
Asaresultoftherandomradicalpolymerizationof phenylpropanemonomers,ligninadoptscomplex three-dimensionalstructureswithvarioustypesof functionality.Asshownin Figure2,importantC O linkagesare b-O-4, a-O-4,and4-O-5;andC Clinks are b-5,5-5, b-1,and b b linkages(Koch,2008; Calvo-FloresandDobado,2010).Also,common functionalgroupsinligninincludemethoxyl,phenolic hydroxyl,aliphatichydroxyl,andothercarbonyl groups(ChakarandRagauskas,2004).
Figure1 Monomericligninbuildingblocks: p-coumaryl alcohol,coniferylalcohol,andsinapylalcohol.
Figure2 Schematicmodeloftheligninstructurewithimportantlinkages(Koch,2008;Calvo-FloresandDobado, 2010; LaurichesseandAverous,2014).
Chemicalcharacterizationoflignin,ligninderivatives,andotherlignin-basedmaterialsisnota simpletaskduetothethree-dimensionalarchitecture, diversityofchemicallinkagesandfunctionalgroups, difficultisolation,andpoorsolubilityinmany organicsolvents(Figure2).Thisisfurthercomplicatedbymanydifferenttypesoflignindependingon theplantsourceandprocessingmethod.Numerous analyticalapproacheshavebeenemployedtocharacterizethestructureofligninandotherlignin-based materials.
Nuclearmagneticresonancespectroscopyisoneof themostusefultechniquestodeterminethechemical structureoflignin(Ralphetal.,1999;Ralphand Landucci,2010).Lignincontainsahighfractionof methoxygroupsandaromatichydrogens,allowing thesegroupstobereadilydeterminedby 1HNMR. However, 1HNMRisnotsuitableforcharacterizing themostabundantreactivegroup,thehydroxylgroup. Hydroxylgroupcontentisbestdeterminedafter acetylationofhydroxylgroupsbyaceticanhydride inthepresenceofpyridine(BonnerandMcnamara, 1968; ChungandWashburn,2012).Theacetoxy groupsproducedinthisreactioncorrespondto
hydroxylgroupsofligninandappearatchemical shiftsofapproximately2ppm(Lundquist,1992; ChungandWashburn,2012).Afteracetylation,the phenolichydroxylgroupcanbedeterminedselectivelybyrelativelyfastandselectivedeacetylation ofphenolicacetylgroups(Ma ˚ nsson,1983).Alkylationofthehydroxylgroupwasperformedtodeterminehydroxylgroupsin 1HNMRspectra(Adler etal.,1987).While 1HNMRprovidesquantitative information,certainpeaks,suchasthosedueto methoxygroups,canappearbroadandfeatureless becauseofthediversityofchemicalenvironments (Liitiaetal.,2003).Two-dimensional 1H-13CNMR spectroscopycanprovidedetailedinformationon connectivity.Modellignincomplexesprovided detailedinformationnecessaryforpeakassignments (EdeandRalph,1996),andnumerouspulsesequences havebeendevelopedtoelucidatetheconnectivityof functionalgroupsandidentifyspecificlinkages (EdeandBrunow,1992;Kilpelainenetal.,1994). Forexample,thesyringyl-to-guaiacyl(S/G)ratio wasfollowedduringkraftpulpingusingheteronuclearsinglequantumcorrelationNMR,which providedinformationonchangesduetooxidation
duringprocessing(Ibarraetal.,2007).Adifferent approachthatprovidesinformationonalcohol functionalityutilizes 31PNMRinwhichhydroxyl groupswereconvertedtophosphitylgroupsby 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane todo 31PNMRanalysis(GranataandArgyropoulos, 1995;Froassetal.,1998).For 31PNMRanalysis, phosphitylationwascarriedoutbyanotheragent, 1,3,2-dioxaphospholanylchloride(Argyropoulos, 1994;Froassetal.,1996).Mostoftheligninsfrom theprocessingmethodsdescribedherehavebeen characterizedbyNMRspectroscopy.
Size-exclusionchromatography(SEC)analysisof ligninisanimportantanalysistodeterminemolecular weightandmolecularweightdistribution,whichare criticaldataformaterialsdevelopment(Himmeletal., 1995;Hortlingetal.,1995).However,thereliability ofSECanalysiscanbelimitedduetothemany functionalgroupsinlignin,suchashydroxylgroups andcarboxylicacids,andtheligninanalytecanhave stronginteractionswiththecolumnsupportmaterials orbetweenotherligninspecies(Sarkanenetal.,1981, 1982,1984;Iversen,1985).Therefore,thehydroxyl groupsareacetylatedandcarboxylicacidgroups aremethylatedpriortotheSECanalysistoexclude possiblenoncovalentinteractions,suchashydrogen bondingandelectrostaticaggregation,fromthelignin (Himmeletal.,1995;Kimetal.,2007).Ligninisalso characterizedbyvariousmorphologicalmethodssuch
asthermalanalysisandRaman/IRanalysis(Agarwal andAtalla,1999,2010;GlasserWolfgang,1999).
Thechemistryandmaterialpropertiesoflignin havebeenreviewedextensively(Goldsteinetal., 1983;Lewisetal.,1999;ChakarandRagauskas, 2004;Calvo-FloresandDobado,2010; Hatakeyama andHatakeyama,2010; DuvalandLawoko,2014)as wellaschemicalmethodsformodifyingandblending it(Wangetal.,1992;Meister,2002;Liitiaetal., 2003;Mohantyetal.,2009;Dohertyetal.,2011; WashburnandChung,2012).Inthischapter,we presentextractionmethodsandtypesofcommercialgradeligninfromarawbiomass.Abetterunderstandingoftypesandproductionmethodsofligninis animportantstartingpointtostudyligninasnew materials,biofuel,andrenewablesourcesoffine aromaticchemicals.
2.Commercial-GradeLignins
Ligninisbiosynthesizedfrommonomericsubstitutephenylpropyleneunits,coumarylalcohol,coniferylalcohol,andsinapylalcohol,aslignocelluloses aswellasnonconjugatedlignin.Becausethelignocellulosesandligninareintimatelyincorporatedin plantstructures,avarietyofprocessingmethodshave beendevelopedtoisolatelignin. Table1 demonstratesdiversesourcesofligninfromrawbiomass
andplant-basedmaterialssuchascottonand bagasse.Rawwoodspecies,hardwood,andsoftwoodcontainahighpercentageoflignin,between 25%and40%.Ligninandotherpolymericcomponentsofeachwoodspeciesdifferasshownin Table2.Glucomannanandglucuronoxylanaretypical typesofhemicelluloseineachwoodyspecies.Over theallwoodyspecies,theportionoftheligninisat least20%.Therefore,itisnecessarytoisolatetwo importantplant-basedpolymers,ligninandcellulose, fromrawbiomassresourcesthroughanefficient, industrial-scalemethod.Eachcommercialisolation methodhastrade-offsintermsofreagentsandenergy requiredthataffectthemolecularweightandpurity ofthefinalproduct,aswellastheextenttowhich newchemicalfunctionalityisintroducedasaresult ofprocessing.Thepulpandpaperindustryisthe majorproducerofcommercial-gradeligninasabyproductofcellulose-richpulp/papermanufacturing butcellulosicethanolfacilitiesarecurrentlycoming online.Itiscriticalforresearcherstounderstandhow ligninprocessinghaschangedthechemistryand propertiesofthematerial.Sixcommoncommercialgradelignin-producingmethodsarediscussedhere.
2.1KraftPulping
Thekraftpulpingprocessisthemostcommonly usedchemicalpulpingmethodstoproducecellulose fibersfromrawwoodandplantsources(Sixtaetal., 2008).Theglobalproductionusingkraftpulpingis thelargestamongallproductionmethodswith 131.2Mtonin2000(Sixtaetal.,2008),makingkraft ligninthemostcommonform(Smook,2002).Kraft pulpingisamethodofchemicalpulpingthatuses chemicalreactionstodegradeanddissolvethelignin inordertoseparatepurecellulosefromthewood (Sixtaetal.,2008).Animportantstepofkraftpulping isatreatmentofbiomasswithsodiumhydroxideand sodiumsulfidetocleaveetherlinkagebysulfideand bisulfideions(Sjostrom,1993).Inthisprocess, phenolicgroupsareconvertedtoquiononemethide groupsbysodiumhydroxideandthenhydrogen sulfideionattacks a-carbonatomsofetherlinkages formingabenzylthiolateanion.Next,the b-phenolate anionisreleasedfromthebenzylthiolateanionto producefreephenolicgroups.Thefreephenolic groupsyieldquiononemethideagain,andthesame processisrepeatedtocleaveetherbondscontinuously andproducelowermolecularweight,solublefragments.Lignincarbon carbonbondscanbeformedat
thelaststageoftheprocess;thisgenerallyinvolves acondensationreactionandyieldsaveryrobust lignincomplex(SaakeandLehnen,2000;Sixtaetal., 2008)(Schemes1and2).Kraftligninishydrophobic atneutralpHandcontainsapproximately1%sulfur (measuredinatomic%)intheformofaliphaticthiol groups(ElMansouriandSalvado,2007).Alsoother importantfunctionalgroupsinkraftlignin(expressed inw/w%)aremethoxygroup(14%),aliphatic hydroxylgroup(10%),phenolichydroxylgroup (2 5%),andcarboxylicacidgroupwithcompositionsthatcanvarydependingontheplantsourceand processingconditions(4 7%)(ElMansouriand Salvado,2007;Dohertyetal.,2011).However,as aby-productinpaperproduction,mostkraftligninis stillburnedasafueltoproducepowerinthepulpmill andtorecoverinorganiccomponents(Saakeand Lehnen,2000;Mohantyetal.,2009;Dohertyetal., 2011).
2.2SulfitePulping
Theprocessofsulfitepulpingisthereactionof woodorbiomasswithsulfurdioxide(SO2),which reactswithwatertoformsulfonicacid,andbasessuch ascalcium,sodium,magnesium,orammoniumat hightemperatures,125 150 C,for3 7h.This processisacid-catalyzedtocleave a-etherlinkage and b-etherlinkagesduringthepulpingprocess (Shimizuetal.,1998).Thelignincanbedissolvedand separatedinaqueoussolutionduetosulfonicacids whichintroducedto a-carbonofetherlinkage(Saake andLehnen,2000).Theresultingligninhasfunctionalgroupsoflignosulfonicacid,lignosulfonate, carboxylicgrouptogetherwithphenolic/aliphatic hydroxylgroups(Vishtaletal.,2011).Duetothehigh contentofsulfonate,theproducedligniniswater soluble.Ligninproducedbythesulfiteprocesshas broadmolecularweightdistributionandrelatively highashcontent.Thecarboxylicacidcontentis approximately4%,whilethesulfonatecontentcanbe 13%(ElMansouriandSalvado,2007).Thus,the materialsapplicationsarelimitedtotheareaof animalfeeds,particleboards,surfactants,adhesives, cementadditives,andstabilizer/dispersingagents (Vishtaletal.,2011;LaurichesseandAverous,2014). Duetothehighcontentofsulfur,approximately 5 6%,mostoftheobtainedligninbythesulfite pulpingprocessisusedasafuelforthepulpmilland torecoverinorganiccomponents(SaakeandLehnen, 2000;Dohertyetal.,2011)(Scheme3).
Table2 PolymericComponentsofVariousSoftwoodandHardwoodSpecies(Sjostrom,1993;Koch,2008)
SpeciesCommonNamesExtractivesLigninCelluloseGlucomannanGlucuronoxylanPolysaccharides Softwoods
Abiesbalsamea
Pseudotsuga menziesii
Tsuga canadensis
Juniperus communis Commonjuniper3.232.133.016.410.73.2
Pinusradiate Montereypine1.827.237.420.48.54.3
Pinussylvestris Scotspine3.527.740.016.08.93.6
Piceaabies Norwayspruce1.727.441.716.38.63.4
Piceaglauca Whitespruce2.127.539.517.210.43.0
Larixsibirica Siberianlarch1.826.841.414.16.88.7
Hardwoods
Acerrubrum Redmaple3.225.442.03.122.13.7
Acersaccharum Sugarmaple2.525.240.73.723.63.5
Fagussylvatica Commonbeech1.224.839.41.327.84.2
Betulaverrucosa Silverbirch3.222.041.02.327.52.6
Betulapapyrifera Paperbirch2.621.439.41.429.73.4
Alnusincana Grayalder4.624.838.32.825.82.3
Eucalyptus globulus Bluegum1.321.951.31.419.93.9
Acaciamollissima Blackwattle1.820.842.92.628.22.8
Scheme1 Reactionof b-aryletherlinkagesinnonphenolicphenylpropaneunits.
Scheme2 Reactionofsulfidewithphenolicphenylpropaneunits.Styrenicproductsarecapableoffurtherreactionstoformacomplexpolymermixture.
Scheme3 Mechanismofligninisolationduringthesulfitepulpingprocess.
2.3SodaProcess
Thesodaprocessistheoldestpulpingmethod thatusessodiumhydroxidesasthechemical reagentsofthepulpingprocess,andisnowbeing usedtoprocessnonwoodmaterial,whichincludes mainlyannualplantsandagriculturalwaste,suchas wheatstraw,sisal,kenaf,hemp,andbagasse.Inthis process,biomassreactswithconcentratedsodium hydroxide(ca. 1M)underhightemperature(ca. 170 C)andhighpressure(ca. 10psi)(Gierer,1980).
Asshownin Scheme4,an a-etherbondcanbe hydrolyticallycleavedbythismethod,resultingin lowermolecularweightfragmentswithincreased solubility,makingitaneffectiveprocessfor delignification.
Anthraquinone(AQ)isoftenaddedtothereaction, whichoxidizescarbohydratesandformsanthrahydroquinine(AHQ)(Lundquistetal.,1981).TheAHQ dianioncancleavethe b-O-4etherlinkageeasily duringtheprocessbyreactingwithquininemethides formedfromsodareactionsvianucleophilicattack,
Scheme4 Cleavageofthe a-etherbondinlignin duringthesodaprocess.
thusregeneratingtheAQ.Thisreactionisshownin Scheme5
AdditionofAQcansignificantlyincreasethe delignificationrates;Lunquistetal.reporteda reductionincookingtimefrom250minforsodato 90minforsoda-anthraquinoneinstudiesonspruce wood(Lundquistetal.,1981).Furthermore,soda ligninhasbeenusedasaprecursorforrawmaterials thatisconsideredasbeingclosertonaturalligninsin abroadrangeofapplicationsbecauseoftheabsence ofsulfurfunctionality(Giereretal.,1979;Saakeand Lehnen,2000).
2.4OrganosolvLignin
Organosolvprocessesrepresentalargefamilyof methodsusedtodelignifybiomass,andthereare severalexcellentreviewsonthese(McDonough, 1993; YoungandAkhtar,1998).Commonfeatures includemixturesofwaterandorganicsolventsto removeligninfromthewoodorotherbiomassaswell astheuseofacidorbasecatalysts(PyeandLora, 1991;Stockburger,1993; AkhtarandYoung,1997; Zhangetal.,2007).Ethanol,methanol,formicacid, oraceticacidhavebeenused,anditisknownthatthe organosolvprocessismoreeffectiveintreating annualplantsandhardwoods(PyeandLora,1991; Loraetal.,1993;Creameretal.,1997).Organosolv ligninisfreeofsulfur,andthemolecularweightis lowcomparedtootherligninproductionmethods, generallyaround5kDa,althoughthiswilldependon theorganicsolventused,withcarboxylicacidsoften
beinglesseffectiveatpreventingrecombination thanalcohols.However,theuseoforganicsolvents increasesthecostofprocessingandcanhavea greaterenvironmentalimpact,althoughthelack ofodorofthisprocesscomparedtothekraftprocess anditscompatibilitywithagriculturalresidues providesomebenefitsinthisregard(PyeandLora, 1991;Stockburger,1993;AkhtarandYoung,1997; Zhangetal.,2007).Manyorganosolvprocesses havebeeninvestigatedextensivelyandtestedinpilotscaleplants,includingAlcell,Acetosolv,andMilox. Mostinvolveanacidcatalystorareperformedinan acidicsolvent,whichallowsuseoflowertemperaturesthantraditionalpulpingmethods,andthey generallyavoiduseofsulfur-andchlorine-based chemicals.
TheAlcellmethodisbasedonethanolprocessing usingasulfuricacidcatalyst,whichisbeingactively studiedforthepretreatmentoflignocellulosicsfor ethanolproduction.Ethanolmoreefficientlysolubilizeslignincleavageproductsformedasaresultof catalystactionthanwater.Whileoriginallydevelopedforhardwoods,recentstudieshavefocusedon adaptingthisforsoftwoods,suchaspine(Panetal., 2005),aswellasagriculturalresidues(Cybulska etal.,2012).Optimizedconditionsforsoftwood werefoundtobe170 C,60min,1.1%H2SO4,and 65%ethanol/35%waterfromwhich79%ofthe ligninwasrecovered(Panetal.,2007).Asdeterminedusing 13Cand 31PNMRmeasurements,the majorreactionproductsfromprocessingpinewere formedthroughcleavageofthe b-O-4linkagesas wellasesterbonds(Sannigrahietal.,2010).Characteristicmolecularweightsfortheseligninsare Mw w2000gmol 1 withapolydispersityof w2.0. Interestingly,ethanolorganosolvproductstendtobe richerinphenolichydroxylgroups,makingthem highlyeffectiveatradicalscavenging(Panetal., 2006).
TheAcetosolvprocessusesacookingmedium of93%aceticacidand <1%hydrochloricacidas acatalyst.Theadditionofamineralacidcatalystallows
Scheme5 Cleavageofthe b-O-4etherlinkageinthepresenceofanthrahydroquinineinthesodaprocess.
forlowerdigestiontemperatures,oftenbelow140 C insteadof170 Corgreaterwithoutit.Thekineticsof theAcetosolvprocesshavebeenstudiedforbothhardwood(eucalyptus)andsoftwood(pine)(Vazquezetal., 1997).Theproposedmechanismisbasedonasequence offirst-orderreactionsinvolvingformationofsoluble ligninspeciesthroughlignin hydrolysis,lignincondensationinsolution,andsubsequentreprecipitation. Effectivedelignificationwasobservedat180 Cand 0.1%HCl,whereasincreasingtheHClconcentrationto 0.2%allowedforatemperaturereductionto140 C,but longerreactiontimescanactuallyreduceyielddueto ligninrecombination.
TheMilox(orFormacell)processtakesitsname fromtheMilieuPureOxidativeprocessdeveloped bytheFinnishPulpandPaperResearchInstitute (KCL)(Sundquist,1986).Itisbasedonuseofperoxyformicacidtoperformtandemdelignification andbleaching.Peroxyacidsreactwithnucleophiles having p-electronsviaoxygentransfer,andin acidicmediathestrongelectrophile þOHisformed. Adiversityoffunctionalgroupsresultfromthis oxidationreaction,includingquinones,carboxylic acids,andalkenes,andformationofrecombination by-productsisminimizedthroughefficientsolubilizationinthewater/formicacidmedium.
Generationofperoxyformicacidisaccomplished byreactingformicacidwithhydrogenperoxide accordingtothereaction: whichhasaforwardrateconstantat22 Cthatis50 timesgreaterthantheanalogousreactionofaceticacid andhydrogenperoxide(SuchyandArgyropoulos, 2002).Peroxyformicacidcanbereadilyseparated fromthereactionmixturebydistillation,andhasbeen appliedtothereactorasaconcentratedsolution. However,theseconcentratedsolutionscanbeexplosive,andmorerecentapproachesrelyongeneratingit insitu.Seistoetal.(SeistoandPoppiusLevlin,1997) establishedmultistageprocessesbasedonimpregnationofbiomasswithformicacidandintroductionof hydrogenperoxidefollowingbyadditionofwaterand cookingatupto120 Cforperiodsrangingfrom60to 180min.Thiswasappliedtothehybridgrass Miscanthus giganteus,andacomparisonwithnative ligninshowedthattheweightandnumberaverage molecularweights(Mw andMn)haddecreasedfrom
31742gmol 1 and2681gmol 1 to7636gmol 1 and 1417gmol 1,respectively,asaresultoftheMilox processing.Inaddition,theconcentrationofcarboxylicacidsitesincreasedfrom0.18mmolg 1 to 0.35mmolg 1 andtotalphenolicgroupsincreasedfrom 0.84mmolg 1 to1.31mmolg 1,whiletheconcentrationofaliphatichydroxylgroupswasreduced from5.54mmolg 1 to1.66mmolg 1.Clearlyeven organosolvprocessingcan havesignificantimpacton thechemicalfunctionalityoflignins.
Alkalineconditionshavealsobeenexploredin organosolvprocesses.Forexample,theOrganocell processfirstimpregnatesbiomasswithamixture ofwaterandmethanol,orsometimesethanol,at temperaturesof110 140 C.Thenalkali,suchas sodiumhydroxide,andanthraquinoneareaddedin thedigesterwherethepulpingliquor,containingup to30%methanolbyvolume,isheatedto170 Cat pressuresoforder10barfor2h.Itisthoughtthatthe methanolmaydirectlyparticipateinthedelignificationbyformingmethylethergroupsatcarbocation siteswherelignincondensationcouldoccur.This wouldmaintainalow-molecularweightofthelignin fragmentscomparedtothoseformedbykraftor sulfitepulping.
2.5SteamExplosionLignin (HydrothermalProcess)
Thesteamexplosionprocessrequireshighly compressedwater,200 2000psi,andhightemperature,180 230 C,duringtheshortprocessingtime of1 20mininthepresenceofacatalyst(Wayman andLora,1978,1979,1980;Dataretal.,2007;Zhang etal.,2007;Ruizetal.,2008).Hydrolysis,arylether cleavageandhomolyticcleavageofC Cbond, demethoxylation,alkylation,andcondensationreactionsoccurduringthesteamprocess(Wangetal., 2009;Kangetal.,2011).Whilethearomaticgroups intheligninarenotdegradedbysteamexplosion process, b-O-4etherlinkagesandCa Cb bondsare beingcleavedduringtheprocess.Theresultinglignin hasasimilarcharacterasorganosolvlignin,low molecularweight,andgoodsolubilityinorganic solvents(WaymanandLora,1978;Shimizuetal., 1998).Steamexplosionhasgreatpotentialbecause thisprocessisthefirststeptoproducebiofuelpriorto fermentationofpolysaccharide(GlasserandWright, 1998).Afewexamplesofligninsubunitsthatcanbe