eTextbook 978-0133457100 Strategic Compensation: A Human Resource Management Approach
https://ebookmass.com/product/etextbook-978-0133457100-strategiccompensation-a-human-resource-management-approach/
ebookmass.com
Dirty December (Pine Ridge Universe) S.C. Principale

https://ebookmass.com/product/dirty-december-pine-ridge-universe-s-cprincipale/
ebookmass.com
The Billionaire's Best Friend: A Contemporary Christian Romance (Billionaire Next Door Book 2) Elizabeth Maddrey
https://ebookmass.com/product/the-billionaires-best-friend-acontemporary-christian-romance-billionaire-next-door-book-2-elizabethmaddrey/
ebookmass.com
American Government and Politics Today: The Essentials, Enhanced 19th Edition – Ebook PDF Version
https://ebookmass.com/product/american-government-and-politics-todaythe-essentials-enhanced-19th-edition-ebook-pdf-version/
ebookmass.com
Valuing
Businesses Using Regression Analysis C. Fred Hall
https://ebookmass.com/product/valuing-businesses-using-regressionanalysis-c-fred-hall/
ebookmass.com




William J. Rapaport
https://ebookmass.com/product/philosophy-of-computer-science-anintroduction-to-the-issues-and-the-literature-william-j-rapaport/
ebookmass.com

Dedicated to MyBelovedWife “ShailaShumi” and MyBelovedDaughter “OrnelaSuhiya”
OmarFaruk,Canada
UmeshP.Agarwal USDAForestService,Forest ProductsLaboratory,OneGiffordPinchotDrive, Madison,WI,USA
AbdullahAlMamun InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
PriyankaBhattacharya ProtonPower,Inc.,Lenoir City,TN,USA
SabornieChatterjee ChemicalSciencesDivision, OakRidgeNationalLaboratory,OakRidge, TN,USA
HoyongChung DepartmentofChemicaland BiomedicalEngineering,FloridaStateUniversity, FAMU-FSUCollegeofEngineering,Tallahassee, FL,USA
NorbertEisenreich Fraunhofer-ICT,Germany
GunnarEngelmann FraunhoferInstitutefor AppliedPolymerResearchIAP,Potsdam-Golm, Germany
OmarFaruk CentreforBiocompositesand BiomaterialsProcessing,FacultyofForestry, UniversityofToronto,Toronto,ON,Canada
MaikFeldmann InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
JohannesGanster FraunhoferInstituteforApplied PolymerResearchIAP,Potsdam-Golm,Germany
AzadehGoudarzi DepartmentofMaterials Engineering,TheUniversityofBritishColumbia, Vancouver,BC,Canada
ShayestehHaghdan DepartmentofWoodScience, ForestSciencesCentre,TheUniversityofBritish Columbia,Vancouver,BC,Canada
Hans-PeterHeim InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
Contributors
EmiliaReginaInone-Kauffmann Fraunhofer-ICT, Germany
JohnF.Kadla DepartmentofForestBiomaterials, NorthCarolinaStateUniversity,Raleigh,NC,USA
AdelR.Kakroodi CentreforBiocompositesand BiomaterialsProcessing,FacultyofForestry, UniversityofToronto,Toronto,ON,Canada
MuzafferA.Karaaslan DepartmentofMaterials Engineering,TheUniversityofBritishColumbia, Vancouver,BC,Canada
SimonKleinhans InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
FrankK.Ko DepartmentofMaterialsEngineering, TheUniversityofBritishColumbia,Vancouver,BC, Canada
MarkT.Kortschot DepartmentofChemical EngineeringandAppliedChemistry,Advanced MaterialsGroup,UniversityofToronto, Toronto,ON,Canada
YingjieLi DepartmentofMaterialsEngineering, TheUniversityofBritishColumbia,Vancouver,BC, Canada
Li-TingLin DepartmentofMaterialsEngineering, TheUniversityofBritishColumbia,Vancouver,BC, Canada
HelmutNaegele TecnaroGmbH,Germany
MohammadAliNikousaleh InstituteforMaterials Engineering,PolymerEngineering,Universityof Kassel,Kassel,Germany
NumairaObaid DepartmentofChemical EngineeringandAppliedChemistry,Advanced MaterialsGroup,UniversityofToronto,Toronto, ON,Canada
NikhilD.Patil FacultyofForestry,Universityof Toronto,Toronto,ON,Canada
Ligninisacomplexpolymerabundantlyfoundin plantsanditisthefibrouspartoftheplant.Traditionally,ligninisusedinawiderangeoflow-volume, nicheapplications.Industrialligninsarecurrently obtainedascoproductsofthemanufactureofcellulosepulpforpaper,aswellasfromotherbiomassbasedindustriesandtherearevarioustypesoflignin dependingontheirprocessandpurity.Ligninmarket isstilllimitedinitsapplicationinawiderangeoflowvolume,nicheapplications,butlignincanbeusedin awiderangeofapplicationssuchasinthemanufactureofvanillin,animalfeed,dyedispersants, micronutrients,resins,andcleaningchemicals.In addition,lowawarenessaboutligninamongmanufacturersisthekeyrestrainttothismarket.Itisalso foundthatthereisaweaklinkbetweentheindustry andresearchinstituteswhichresultsinthelow exposureofmanufacturerstothedevelopmentsof ligninindifferentapplications.Furthermore,the extractionandmodificationtechniquesandapplicationofligninarestillataprimarystage,which hamperstheligninmarketalso.Anotherobstacleof ligninexpansioninvalue-addedapplicationsis mainlyduetotheirlow-puritystandards,heterogeneity,smellandcolorproblemsoftheexisting commerciallignins.
Currently,environmentalpollutionandincreasing awarenessoflimitedresources,thereisgrowing
Preface
opportunityintheuseofligninasasubstitutefor fossil-basedrawmaterialsanditcouldbeusedin themanufactureofawiderangeofproductssuch asplastics,chemicalproducts,andcarbonfibers. Recentlyextensiveongoingresearchfocusingon lignindrawbacksisincreasingtheapplicationscope oflignininthemarket.
Inrecentyears,therehavebeenanumberof reviewpaperspublishedonlignincoveringlignin chemistry,modification,polymercompositesfrom lignin,oxidativeupgradeoflignin,biocomposites andnanocompositeswithlignin,industriallignin productionandapplications,andcarbonfibers fromlignin.Thisbookfocusesspecificallyon lignin-basedpolymercomposites(thermoplastic, thermoset,andbiopolymer,rubber,nano,carbon), lignin-basedaerogels,lignin-basedfoamingmaterials,aswellassourcesandtypesoflignin,lignin interunitlinkagesandmodelcompounds,extraction oflignin,characterizationandpropertiesoflignin, andapplicationsoflignin.Thebookwillbehelpful toresearchers,engineers,chemists,technologists, andprofessionalswhowouldliketoknowmore aboutthedevelopmentandpotentialofligninand lignin-basedcomposites.
ShayestehHaghdan,ScottRenneckarandGregoryD.Smith DepartmentofWoodScience,ForestSciencesCentre,TheUniversityofBritishColumbia,Vancouver,BC,Canada
1.IntroductiontoLignin
Thetermligninisderivedfromthelatinname lignummeaningwood(MccarthyandIslam,1999). Itwasfirstisolatedfromwoodinascientificreport bytheFrenchscientist Payen(1838) andlatergiven itscurrentnamein 1857 bySchulze.Ligninwas initiallydescribedasanincrustantofcellulose,and thispointisinsightfulaslignificationoccursafterthe depositionofthepolysaccharideframework.Inan extremelysimplifiedviewitisanalogoustothe matrixmaterialforafiber-reinforcedcomposite. Ligninhasseveralfunctionswiththecellwallsuchas changingthepermeabilityandthermalstability,butit hastheprimaryfunctiontoserveasastructural materialthataddsstrengthandrigiditytoplant tissue.Inthesenselignindistinguisheslignocellulosicbiomassfromotherpolysaccharide-richmaterials,byreinforcingthepolysaccharidescaffoldingof thecellwall.Itsperformanceissoeffectivethatit allowstreestooutcompeteotherplantsforsunlight formingthelargestorganismsontheplanet.
Asligninconstitutes15 40%ofdryweightof woodyplants,itisthemostabundantaromatic polymerontheearthandthesecondmostabundant organicpolymeraftercellulose.Basedonyearly biomassgrowthrates,theoverallproductionof ligninisontheorderof5 36 108 tons(dosSantos
etal.,2014).Hence,ligninhasthepotentialtobean importantsourceofaromaticchemicalsforthe chemicalindustry,arisingfromtheconversionof moderneraCO2,anditsefficientutilizationsolves apotentialpuzzleincreatingvaluableby-productsin abiorefineryscheme.Thisreasoningisbecauseif woodisconvertedtothebilliontonscaleforbiofuels andbiochemicals,thentherewillbegreaterthan 300milliontonsofligninpotentiallyavailable.To putthisinperspectiveitisroughlythesizeofthe globalpolymermarket.
Asmentionedabove,ligninisanaromaticpolymer.Themonomericprecursorshaveaphenolicring withathreecarbonsidechainprovidingabasicnine carbonstructurecommonlyreferredtoasaC9-unit and/orphenylpropaneunitasshownin Figure1.
Thesidechainisterminatedwithaprimary hydroxylgroupontheCg,whiletheCa andCb are connectedtogetherwithanunsaturatedbond.The phenolicringismethoxylated( OCH3)tovarious degrees,dependentuponthespecies; p-coumaryl alcohol,coniferylalcohol,andsinapylalcoholhave none,one,ortwomethoxylgroupsatthe3-and 5-positions,respectively.Basedontheligninmonomericcompositioninvolvedinpolymerization,the resultingligninisclassifiedintothreetypes:(1)lignin thatcontainsmainlyconiferylalcoholiscalled guaiacyl(G)ligninandisfoundpredominantelyin
technologiesdramaticallyimpactligninfunctionalityandmolecularweight.Asaresult,thepropertiesofligninrelatedtoitssolubilityaremodified. Severalstudieshaveshownthatlignincanbefractionatedbyusingseveralsolventswithlargely differentsolventparameters(Moercketal.,1986).A smallfractionofligninmaybesolubleinanonpolar solventliketoluene,nonhydrogenbondingsolvents likedichloromethane,whileotherfractionsare solubleinmorepolarsolventslikealiphaticalcohols.Thedifferentsolubilityofthefractionsshed lightontheheterogeneousnatureofligninevenifit isisolatedfromasoftwoodligninthatcontains95% guaiacyllignin.Delignificationcanmodifythe lignintoappreciativedegreesbytheadditionofthe reactantssuchassulfuroralcoholtotheligninor increasethemolecularweight(orchangetheinterunitlinkages)byreactionsofligninwithitself duringdelignification.Theseisolationprocesses resultinligninthatcontainsacidicgroups,lose aliphatichydroxylgroups,increasethefreephenolic groupsbybreakageofthe b-O-4linkage,and containmorecarbon carbonbondsbetweenunits (whicharereferredtoascondensedstructures). Becauseofthevarietyofintermolecularlinkages betweentheprecursors,ligninisalsoahighly heterogeneouspolymer(FengelandWegener,1983; Baucheretal.,1996).Thevarietyofbondsresultsin abranchedligninpolymerwithapotentiallycrosslinkedthree-dimensionalstructure(Schmidl,1992). Therecentliteraturehassuggestedthatligninmaybe moreuniformthanpreviousthought,witheither linearchaintopologies(Crestinietal.,2011)oreven beingcappedbyspecificligninmonomers(Sangha etal.,2014).However,itisclearthatligninpolymerizationisleftuptothefatesofthermodynamics asthepolymerizationprocessoccursoutsidethe controlofthecellcytoplasm(Ralphetal.,2004). Hence,thecellularcontrolislimitedtotheproductionandreleaseofthemonomersinvolvedinlignificationandtheenzyme-activateddehydrogenated monomerswillpolymerizewiththegrowinglignin chain.
Anotherimportantaspectofpolymericmaterialsis theirmolecularweight.Absoluteknowledgeabout lignin’smolecularweighthasbeendifficulttoachieveasremovinglignintoanalyzeitautomatically impactstheligninstructure.Additionally,lignin molecularweightmeasurementssufferfromissues surroundingsolubility,aggregation,adsorption,and fluorescencemakingitoneofthemostdifficult
polymerstoanalyze.Earlyexperimentslookinginto themolecularweightoflignosulfonatessuggested thatdelignificationoccurredlikebreakinganetwork polymer(Gorning,1971).However,severaldifferent isolatedligninshavebeenanalyzedwithmodern analyticalequipment,andligninusuallyhas abimodaldistributionwithnumber-averagemolecularweightbetween3000and10,000andweightaveragemolecularweightfrom8000to80,000 dependentuponsolutionconditions,andlignintype (Guerraetal.,2007).Manytechnicallignins describedintheliteratureeitherhavelowermolecularweightsbecauseoffragmentationoflignininto oligomersormuchgreatermolecularweights.When delignificationoccurs,anumberofreactionscan occurbutmanyinvolveradicalformationand subsequentradicalcouplingwhereligninfragments canbecomebonded,increasingthemolecularweight.
Additionalheterogeneityoflignininvolveshow thestructureofthispolymerdifferswithinagiven cellwalldependentonitslocationinthewall,plant species(hardwoodvssoftwood),andgrowth conditions,especiallyrelatedtoreactionwood formation(CampbellandSederoff,1996;deWild etal.,2010).Thesedifferencesarisefromthe monomericcompositionavailableduringthelignificationreactions.Overall,onecanseethe complexityoftryingtoanalyzealigninthatmaybe differentinvariouslocationsofthecellwall.Terashimahasshownthatligninmicrostructureis dependentuponitslocationinthecellwall.Inthe cellwallcorners,ligninformssphericalclusters, whilelignininthesecondarycellwallisgreatly reducedindimensionssurroundingthemicrofibril lamellarstructureofthecellwalllayersasshownin Figure4 (Terashimaetal.,2004).Workwiththe Ramanspectroscopyhasindicatedthatligninin thesecondarywallhasapreferredorientationalong themicrofibrilstructure(AtallaandAgarwal,1985).
2.LigninFunctions
Ligninispartofthestructuralframeworkin plants,formingpartoftheprimaryload-bearing elementofthecellwall.Fromanevolutionary pointofview,ligninhasbeencreditedastheterrestrialadaptionthatpermitssignificantverticalgrowth. Asanessentialpartofthecell,ligninsupportsthe plantbyimpartingrigiditytothecellwall.Theplant withstandsnaturalenvironmentalstressesbecauseof
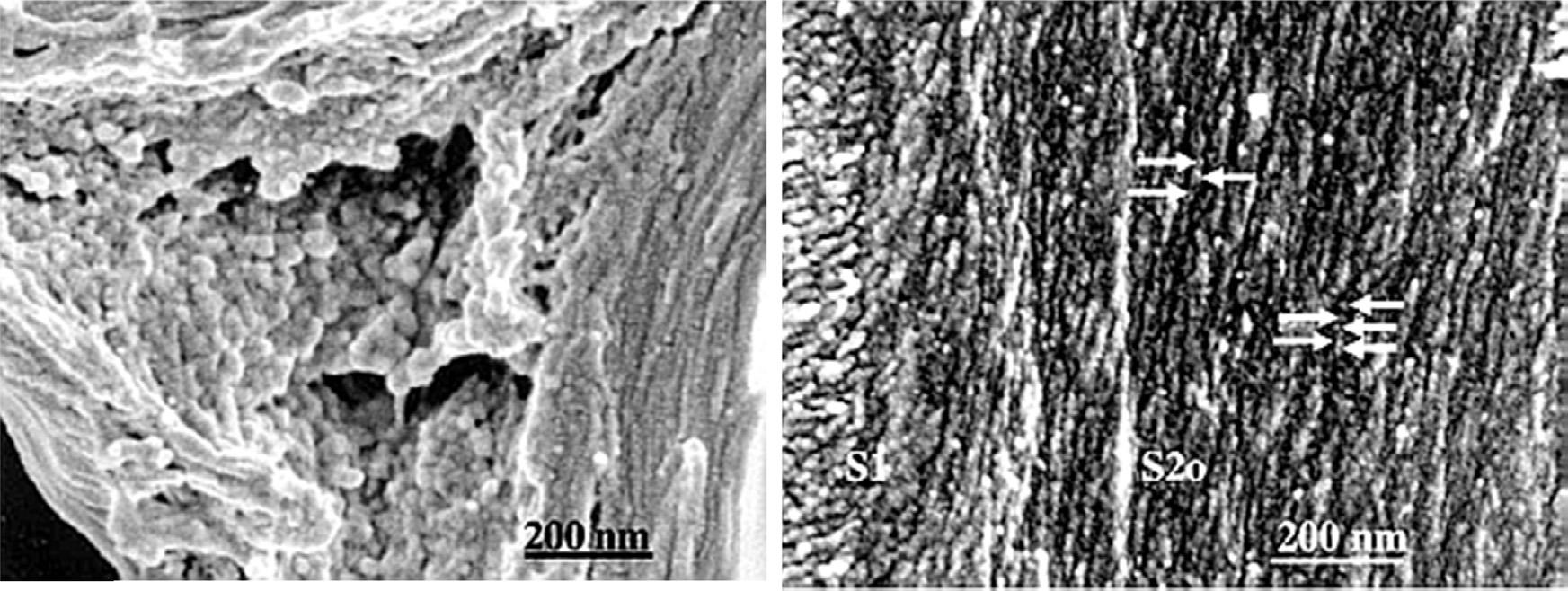
Figure4 Cellcornerregion.Largeglobularmodulusformaggregatesatrandom,leftandligninmodules(arrows) aregrowingalongcellulosemicrofibrils(CMFs)atanalmostregulardistance,right.
thiscellwallbuildingblock(Lopesetal.,2011; SarkanenandLudwig,1971).Ligninnotonly providesstiffnesstotheplantbutinconjunctionwith theheteropolysaccharides,itprovidesflexibility whichisfundamentalforanappropriateresponseto dynamicloadsfromwindandsnow.Inadditionto mechanicalsupport,ligninmodifiesthepolysaccharidenetworktomakeitresistanttooutside organisms.Ligninhelpstoprotectwoodytissuefrom themicrobialandfungalattackencasingthecarbohydratestructure,providingreducedaccessibilityof enzymesforhydrolysis.Limitedsolubilityand complexityoftheligninmakeitresistanttodegradationbymostmicroorganisms(Prasongsuketal., 2009;Bholayetal.,2012;BergandMeentemeyer, 2002;Crowetal.,2009;VermaandDwivedi,2014). Onecanimagineifligninonlycontainsasingle linkagethatwouldbecomethecellwall’sAchilles’ Heal.However,withthevarietyoflinkageswooddestroyingorganismsrequirethebreakageofboth arylcarbonbondsandaryletherbondsneedingto expandthecostofproductionofspecificenzymesor developnonspecificpathwaysfordelignification.In addition,ligninislesshydrophilicthanthepolysaccharideshelpingtochangethepermeabilityofthe cellwallbysealingitandenablingwatertransport throughthevasculartissue(TenandVermerris, 2013).Finally,thearomaticityofligninlendsitselfto enhancethethermalstabilityofwoodprovidingchar layer.Thishasbeenexploitedwithisolatedtechnical lignin,turningitintocarbonfibermaterialby controlledhigh-temperatureheating.Whileligninis seenasmorethermallystablebecauseofit,thenative structureofligninisgreatlyimpactedbythethermal modificationandresearchersshouldbecautioned
thatligninstructurecanchangeduringprocessingat temperaturesusedintheproductionorsomethermoplasticmaterials.Suchmodificationincludes depolymerization,lossoftheCg hydroxyl,and formationofnewacidicgroups.
3.SourcesofLignin
Thesourcefromwhichligninisobtained,the extractionmethodsandthesecondarytreatments applied,havestrongimpactsonitsphysicaland mechanicalproperties(Garciaetal.,2011;Khanam etal.,2006).Lignincanbederivedfromvarious sourcessuchaswood,pulpandpaper,sugarcane bagasse,andcerealstrawsusingavarietyofpulping methods.Intermsofweight,thelignincontentin woodyplantsfromgymnospermsandangiospermsis thehighestwiththeorderof30 40%whileother sourcesonlycontainaround3 25%(Smolarski, 2012).Thepulpandpapersectorproducesalarge amountoflignin(Jungmeier,2010;Dohertyetal., 2011)withevengreaterpotentialfromfuturelignocellulosicbiorefineries.Minimalligninisrecovered fromgrass,branches,leaves,andsolidwasteinurban andruralareaswheretheirlignincontentisestimated tobelessthan15%(Wangetal.,2011;Philippidis andHatzis,1997).
3.1Wood
Ligninhasnonuniformthicknessinthemiddle lamella,andintheprimaryandsecondarycellwalls dependingontheplantspeciesandcelltype (Figure5).Approximately70%ofthetotallignin contentofthecellwallisconcentratedinthe
thickestlayerofthesecondarycellwall.Lignin quantityandqualityvarynaturallyamongwood specieswith19 28%inhardwoods(angiosperms) and24 33%insoftwoods(gymnosperms)(Fengel andWegener,1983).Infact,hardwoods,ingeneral, containmorehemicelluloseandlessligninthan softwoods(Bjornsson,2014).Notonlytheamount ofligninvariesbetweenhardwoodsandsoftwoods, therelativeconcentrationalsodiffersinthelocation withinatree.Thejuvenilewoodhasahigherlignin contentthanlatewood(DelaCruz,2014;Bowyer etal.,2007).Lignincontentisalsovariablewithin populationsofplantsofthesamegenus.For example,theaveragecontentofligninrangesfrom 25%in Pinusmonticola to30%in Pinuspalustris withinthegenus Pinus.Reactionwoodforms mechanicalstressandhasdifferentlignincontentin comparisonwiththenormalwooddependingonthe conditionsandspecies(Tiimonen,2007).
Lignininhardwoodsissyringyl guaiacyltype andinsoftwoodsistypicallyguaiacylwithlimited p-hydroxyphenyllignininbothtypes.Bothofthese structureshavedifferencesarisingfromtheinterunit linkages.Guaiacyllignincanundergocoupling reactionsatthe5-positionofthephenylpropaneunit, andthisprovidesasignificantplaceforbranchingand cross-linkingreactions,especiallyoccurringduring

Figure5 Woodcellwallincludingmiddlelamella(ML), primarycellwall(P),layersofthesecondarycellwall (S1,S2,S3),andwartylayer(W)(Sjostrom,1993).
delignificationprocess.Thepresenceofsyringylunits makeshardwoodligninmorereadilyremovedduring thepulpingprocessbylimitingligninforming condensedstructuresattheopenmethoxyposition.In thecaseofcompression-woodlignin,itismore difficulttohydrolyzebecauseitcontainsahigher proportionofcondensed p-hydroxyphenylunits (CampbellandSederoff,1996;Novaesetal.,2010).
3.2PulpandPaperIndustry
Softwoodandhardwoodspecies,andcertaintypes ofannualplants,havecommercialinterestasasource ofcellulosefibersfortheproductionofpaperand boardproducts.Ligninisnoteasytoisolateinthe nativeformfromplantmaterial(Leisolaetal.,2012). Thepulpandpaperindustryistheprimarily commercialsourceoflignin,however,thedelignificationprocessmodifieslignintovariousdegrees.In technicalfiberliberationprocesses,suchasalkaline orsulfitepulping,hugequantitiesofligninaredissolvedasalkaliligninandlignosulfonates,respectively.Largeamountsofligninaremadeavailable annuallyfromthepulpandpaperindustryasbyproductsofthedelignificationprocess.Thesesulfite orsulfateligninshavevaryinglevelsofcovalently bondedsulfurresultinginthepolymerwithdifferent characteristicsthantheoriginallignin(Sainsbury, 2013;Khanametal.,2006;LoraandGlasser,2002). Typically,ligninimpuritiesincludelow-molecularweightsugarsandresinacidsthatareremovedduring thepurificationprocess.Forexample,kraftligninis usuallypurifiedfromkraftblackliquorswhichare complexmixturesoffibrousmaterialsanddissolved organicssuchaslignins,hemicelluloses,sugars,acids, andresinsandalsoinorganicsaltssuchasash(Wallbergetal.,2003).Therecenttechnologyinenhancing ligninrecoveryfromblackliquorbyCO2 acidification hasbeentransferredtotheindustryandhascreated areadilyavailabledryligninpowderstream(Ohman etal.,2013;Kouisnietal.,2012).Asshownin Table1, inallcommercialpulpingprocessesthelignin extractionmethodswillaffectthevalueoftheproducts thatcanbederivedfromitandwillalterthestructureof theligninincomparisonwiththenativeone(Smolarski,2012).
3.3SugarcaneBagasse
Sugarcanebagasseisthefiberthatremainsafterthe sugarshavebeenextracted.Asanagro-industrial residue,sugarcanebagasse(Saccharumofficinarum)
Table1 LigninPuritywithItsAssociatedSources(Holladayetal.,2007)
LigninTypeLigninPuritya
LignosulfonatesMedium(somereducingsugar (upto20wt%)andsulfur)
PotentialProducts
Dispersants,agriculturalchemicals,emulsion stabilizer,industrialbinders,carbonblack,ink andpigments,andconcreteadditives
KraftMedium(someashandsulfur)Dispersants,carbonfibers,emulsifiers, activatedcharcoal,andbinders
OrganosolvHigh(sulfurfree)Aromaticpolyols,newdiacids,activatedcarbon, phenolicresins,carbonfibers,vanillin,phenol derivatives,andantioxidants
aPresenceofresidualcarbohydrates,ash,andproteinsdependsonfeedstockandprocess.
isanothersourceofligninrawmaterial.Itisabyproductofthesugarcaneindustrywithapproximately32 34%cellulose,19 24%hemicellulose, 25 32%lignin,6 12%extractives,and2 6%ash (SakdaronnarongandJonglertjunya,2012;Rezende etal.,2011;Pandeyetal.,2000).Chemicalcompositionofsugarcanebagasseissimilartotheotherplant cellwalls.Eachclassofplants,grasses,softwoods, andhardwoodsproducesaligninrichinonetypeof thephenylpropanerepeatunit.Sugarcanebagasse ligninhasahigherproportionofH-typelignin, phydroxyphenyl,andhencealowermethoxycontent thansoftwoodandhardwoodlignins(Dohertyetal., 2007).Approximately250 280kgofbagasseis generatedfromprocessingeachtonofsugarcane whichroughlyyielded54milliontonsofbagasse annually(Canilhaetal.,2012).Currently,alarge amountofbagasseisburntasalow-gradefuelfor energyrecovery,andonlyalimitedquantityhasbeen usedtomakepulps,boardmaterials,andcomposites. Itisestimatedthat200milliontonsofligninis producedannuallyfrombagasse(Singhetal.,2005).
3.4AgriculturalResidues
Theadvantagesofusingagriculturalresiduesare threefolds:economic,environmental,andtechnological.Unlikewoodpulps,agriculturalpulpscanbe producedusingmoreenvironmentallybenignprocessingandbleachingmethods(Clancy-Hepturn, 1998).Inthewoodpulpingprocess,mostofthepulp isbleachedusingchlorineorchlorine-basedchemicalswhilestrawcanbetreatedwithminimaladditionsofchlorine-freechemicalswhichresultsinno productionoftoxicchemicals.Moreover,agriculturalresidueshavegenerallyamoreporousstructure andalowerlignincontentthanwoodyplantswhich facilitatetheirpulpingprocess.Examplesofthe
agriculturalresiduesarericeandwheatstrawswhich arediscussedinthissection.Ricestrawalsocalledas cerealstrawisanothersourceforligninproduction. Totalworldproductionofricestraw, Oryzasativa,is 525milliontonsperyear.
About90%ofthericestrawisproducedinAsia. Chinahasthegreatestcapacityforpulpingricestraw. Ithasapproximately30 35%cellulose,25 30% hemicellulose,15 28%lignin,and4 7%ash (Marquesetal.,2010).Thehighashcontentarises fromthesilicainthecellwalllimitingtheabilityto burnthismaterialwithoutcausingasignificantenvironmentalnuisance.Highsilicacontentinricestraw, ashighas18%incleanricestraw,makesthepulping processmorecostlyduetoincreasedchemical recoverydifficultiesandcosts.Inadditiontorice straw,wheatstraw, Triticumaestivum,isannually generatedinabundanceof529milliontonsperyear. Whenfarmedintensively,wheatstrawcanbe producedinalargerscale.Theamountofligninof wheatstrawvariesbetween5%and17%depending onthegeographicallocationoftheplantswhichis comparabletohardwoods(BuranovandMazza, 2008).Wheatstrawhasoneofthehighestcellulose contentsofalloftheagriculturalfibers.Strawpapers areknowntopossessgoodprintingqualitiesandare madefrompulprequiringlowenergyrelativetothat requiredtoprocesswoodpulp.Researchersfoundthat wheatstrawmustbepulpedunderconditionsofless energyandfewerchemicalsincomparisonwiththe woodpulpstomaximizepulpyields(Clancy-Hepturn, 1998).Tomakethestrongestproduct,papermakers willlikelycombinesomestrongerhardwood,kenaf, orhemppulpwithstrawpulp.Despitetheadvantages ofusingagriculturalresidues,thehighcollection, transportation,andhandlingcostsassociatedwith theseresources,aswellastheirseasonalavailability, limittheirapplicationsinpaperproduction.
4.LigninPotential
Currentlyaround50milliontonsofligninis producedperyearbythepulpandpapersectorbutonly 2%ofthatisusedforapplicationsotherthancombustionandenergyproduction(deWildetal.,2014;Fengel andWegener,1983;Schmidl,1992;Thielemansetal., 2002).Therearelimitationsinutilizationduetothe ligninstructure,heterogeneity,andtheindustrialprocessingcostsfordelignification(Holladayetal.,2007; Vanholmeetal.,2010;Neutelings,2011;ElHageetal., 2009;Chiang,2005).Toimproveupontheselimitations,differenttypesofmodificationsaredoneto increaseitschemicalreactivityanduniformity,reduce thebrittlenessoflignin-derivedpolymers,increaseits solubilityinorganicsolvents,andimprovetheeaseof processingthelignin(Argyropoulos,2012).Lignin potentialscanalsobeconsideredintheproductionof additives,resins,andcoatingmaterials(Jungmeier, 2010;VishtalandKraslawski,2011).Thisrenewable aromaticpolymercanequallybereplacedwith syntheticpolymersandaromaticchemicals(Zakzeski etal.,2012).Highantioxidantcapacityofligninandits applicationsinpolymerenhancementhavebeen experimentallyproved(Piazzaetal.,2014).Thefindingsofresearchonlignindepolymerizationshowed thatduetotheligninmacromolecularstructureoflignin withmanyphenolicunits,lignindepolymerizationcan beusedasalarge-scalesourceforproductionofvaluablechemicals(Kleinertetal.,2009;Meieretal.,1992; Hora ´ ceketal.,2012).Ligninalsohaspotentialtobe usedinthefabricationofcompositematerials. ElMansourietal.(2007) showedthatexterior-grade particleboardsmadewithlignin-basedadhesives successfullymettherequirementsofinternational standards.Thepotentialoflignininpolymeric compositeswillbediscussedinlaterchapters.
References
Adam,M.,Ocone,R.,Briens,C.,Berruti,F.,2011. ModellingthePyrolysisofLignin. Argyropoulos,D.,2012.HighValueLigninDerivatives,Polymers,andCopolymers,andUseThereof inThermoplastic,Thermoset,andComposite Applications.USPatentApplication.
Atalla,R.H.,Agarwal,U.P.,1985.Ramanmicroprobe evidenceforligninorientationinthecellwallsof nativewoodytissue.ComptesRendusBiologies 227(4687),636 638.
Baucher,M.,Chabbert,B.,Pilate,G.,Van Doorsselaere,J.,Tollier,M.T.,Petit-Conil,M., 1996.Redxylemandhigherligninextractability bydown-regulatingacinnamylalcoholdehydrogenaseinpoplar.PlantPhysiology112, 1479 1490.
Berg,B.,Meentemeyer,V.,2002.Litterqualityin anorthEuropeantransectversuscarbonstorage potential.PlantandSoil242(1980),83 92.
Bholay,A.,Bhavna,B.,Jadhav,P.,Palekar,K., Dhalkari,M.,Nalawade,P.,2012.Bacteriallignin peroxidase:atoolforbiobleachingandbiodegradationofindustrialeffluents.UniversalJournalof EnvironmentalResearchandTechnology2(1), 58 64.
Bjornsson,S.,2014.AdvancedControlMethodology forBiomassCombustion.UniversityofWashington. Bowyer,J.,Shmulsky,R.,Haygreen,J.,2007.Forest ProductsandWoodScience:AnIntroduction, fifthed.BlackwellPublishing,Ames,IA.
Buranov,A.U.,Mazza,G.,2008.Lignininstraw ofherbaceouscrops.IndustrialCropsand Products28(3),237 259. http://dx.doi.org/10. 1016/j.indcrop.2008.03.008.
Campbell,M.M.,Sederoff,R.R.,1996.Variationin lignincontentandcomposition.PlantPhysiology 110,3 13.
Canilha,L.,KumarChandel,A.,dosSantos Milessi,T.S.,FernandesAntunes,F.A.,daCosta Freitas,W.L.,dasGrac¸asAlmeidaFelipe,M.,da Silva,S.S.,2012.Bioconversionofsugarcane biomassintoethanol:anoverviewaboutcomposition,pretreatmentmethods,detoxificationof hydrolysates,enzymaticsaccharification,and ethanolfermentation.JournalofBiomedicine& Biotechnology1 16. http://dx.doi.org/10.1155/ 2012/989572.
Chiang,V.L.,2005.Understandinggenefunctionand controlinligninformationinwood.Agricultural Biotechnology17,139 144.
Clancy-Hepturn,M.,1998.Agriculturalresidues: apromisingalternativetovirginwoodfiber.JournalofReinforcedPlasticsandComposites29. Washington,DC,USA.IssueinResources Conservation,BriefingSeriesNo.1Resources ConservationAlliance.
Crestini,C.,Melone,F.,Sette,M.,Saladino,R., 2011.Milledwoodlignin:alinearoligomer. Biomacromolecules12(11),3928 3935. http:// dx.doi.org/10.1021/bm200948r.
Schmidl,G.W.,1992.MolecularWeightCharacterizationandRheologyofLigninsforCarbonFibers. UniversityofFlorida.
Schulze,H.,1857.Neuenburg.Einegeschichtlich staatsrechtlicheSkizzenebsteinerBeleuchtung derneuestenschweizerischenDenkschriftvom7. Heinicke,p.48.
Singh,R.,Singh,S.,Trimukhe,K.D.,Pandare,K.V., Bastawade,K.B.,Gokhale,D.V.,Varma,A.J.,2005. Lignin-carbohydratecomplexesfromsugarcane bagasse:preparation,purification,andcharacterization.CarbohydratePolymers62(1),57 66. http://dx.doi.org/10.1016/j.carbpol.2005.07.011.
Sjostrom,E.,1993.WoodChemistry:Fundamentals andApplications.Nature,p.293.
Smolarski,N.,2012.High-ValueOpportunitiesfor Lignin:UnlockingItsPotentialLigninPotential. Frost&Sullivan,pp.1 15.Retrievedfrom: http:// www.greenmaterials.fr/wp-content/uploads/2013/ 01/Highvalue-
Ten,E.,Vermerris,W.,2013.Functionalizedpolymersfromlignocellulosicbiomass:stateofthe art.Polymers5(2),600 642. http://dx.doi.org/ 10.3390/polym5020600.
Terashima,N.,Awano,T.,Takabe,K.,Yoshida,M., 2004.Formationofmacromolecularligninin ginkgoxylemcellwallsasobservedbyfield emissionscanningelectronmicroscopy.Comptes RendusBiologies327(9 10),903 910. http://dx. doi.org/10.1016/j.crvi.2004.08.001.
Thielemans,W.,Can,E.,Morye,S.S.,Wool,R.P.,2002. Novelapplicationsofligninincompositematerials. JournalofAppliedPolymerScience83(2), 323 331. http://dx.doi.org/10.1002/app.2247.
Tiimonen,H.,2007.LigninCharacteristicsand EcologicalInteractionsofPtCOMT-modified SilverBirch.UniversityofOulu.
Vanholme,R.,Demedts,B.,Morreel,K.,Ralph,J., Boerjan,W.,2010.Ligninbiosynthesisand
structure.PlantPhysiology153(3),895 905. http://dx.doi.org/10.1104/pp.110.155119.
Verma,S.R.,Dwivedi,U.N.,2014.Ligningenetic engineeringforimprovementofwoodquality: applicationsinpaperandtextileindustries,fodder andbioenergyproduction.SouthAfricanJournal ofBotany91,107 125. http://dx.doi.org/10.1016/ j.sajb.2014.01.002.
Vishtal,A.,Kraslawski,A.,2011.Challengesin industrialapplicationsoftechnicallignins.Bioresources6(3),3547 3568.
Wallberg,O.,Jonsson,A.-S.,Wimmerstedt,R.,2003. Fractionationandconcentrationofkraftblack liquorligninwithultrafiltration.Desalination154 (2),187 199. http://dx.doi.org/10.1016/S00119164(03)80019-X.
Wang,M.,Wang,J.,Tan,J.X.,2011.Lignocellulosic bioethanol:statusandprospects.EnergySources, PartA:Recovery,Utilization,andEnvironmental Effects33(7),612 619. http://dx.doi.org/10.1080/ 15567030903226249.
deWild,P.J.,Huijgen,W.J.J.,Gosselink,R.A.,2014. Ligninpyrolysisforprofitablelignocellulosicbiorefineries.Biofuels,BioproductsandBiorefining8 (5),645 657. http://dx.doi.org/10.1002/bbb.
deWild,P.J.,vanderLaan,R.R.,Wilberink,R.,2010. Thermolysisofligninforvalue-addedproducts.In: XVMeetingoftheInternationalHumicSubstances Society.EnergyResearchCentreoftheNetherlands,Tenerife,CanaryIslands,Spain,pp.1 27. Wu,S.,Argyropoulos,D.S.,2003.Animproved methodforisolatinglignininhighyieldand purity.JournalofPulpandPaperScience29(7), 235 240.
Zakzeski,J.,Jongerius,A.L.,Bruijnincx,P.C.A., Weckhuysen,B.M.,2012.Catalyticligninvalorizationprocessfortheproductionofaromaticchemicals andhydrogen.ChemSusChem5(8),1602 1609. http://dx.doi.org/10.1002/cssc.201100699.
HoyongChung 1 andNewellR.Washburn 2, 3
Ligninisthesecondmostabundantterrestrial biopolymeraftercelluloseandisthelargestrenewablesourceofaromaticgroupsinnature.Ligninis foundinplantcellwallsandisanimportantstructuralcomponentofwoodyplants.Themainfunctions oflignininplantsaretoprovidephysicalstrength, toformwater-conductingvascularnetworksusing hydrophobicinteractionsandtoprotectplantsfrom microorganismsandinsects.Chemically,ligninis composedofarandomnetworkofphenylpropane groups.Thethreebasicstructuralmonomerunitsare coumarylalcohol,coniferylalcohol,andsinapyl alcohol,asshownin Figure1.Innature,these monomerunitsoxidizetophenoxyradicalsby peroxidaseandthenundergopolymerizationthrough multiplereactivesitestoformacomplexthreedimensionalpolymer.Thisbiosynthesisisreferred toasdehydrationpolymerization,andtheresulting monomerunitswithinligninarereferredtoas p-hydroxyphenyl(H),guaiacyl(G),andsyringyl (S).Differencesinligninaccordingtovariousplant sourcescanbemanifestedindifferencesinmonomerconcentrations(Vanholmeetal.,2010),with gymnosperms,suchasthevarioustypesofpines,
comprisingprimarilyofG-unitswithsmallconcentrationsofH-units.Incontrast,angiospermdicots, includingmanyhardwoods,haveamixtureofGand S-units,whichreducebranchingconcentrationsand canimproveligninprocessibility.
Asaresultoftherandomradicalpolymerizationof phenylpropanemonomers,ligninadoptscomplex three-dimensionalstructureswithvarioustypesof functionality.Asshownin Figure2,importantC O linkagesare b-O-4, a-O-4,and4-O-5;andC Clinks are b-5,5-5, b-1,and b b linkages(Koch,2008; Calvo-FloresandDobado,2010).Also,common functionalgroupsinligninincludemethoxyl,phenolic hydroxyl,aliphatichydroxyl,andothercarbonyl groups(ChakarandRagauskas,2004).
Figure1 Monomericligninbuildingblocks: p-coumaryl alcohol,coniferylalcohol,andsinapylalcohol.
Figure2 Schematicmodeloftheligninstructurewithimportantlinkages(Koch,2008;Calvo-FloresandDobado, 2010; LaurichesseandAverous,2014).
Chemicalcharacterizationoflignin,ligninderivatives,andotherlignin-basedmaterialsisnota simpletaskduetothethree-dimensionalarchitecture, diversityofchemicallinkagesandfunctionalgroups, difficultisolation,andpoorsolubilityinmany organicsolvents(Figure2).Thisisfurthercomplicatedbymanydifferenttypesoflignindependingon theplantsourceandprocessingmethod.Numerous analyticalapproacheshavebeenemployedtocharacterizethestructureofligninandotherlignin-based materials.
Nuclearmagneticresonancespectroscopyisoneof themostusefultechniquestodeterminethechemical structureoflignin(Ralphetal.,1999;Ralphand Landucci,2010).Lignincontainsahighfractionof methoxygroupsandaromatichydrogens,allowing thesegroupstobereadilydeterminedby 1HNMR. However, 1HNMRisnotsuitableforcharacterizing themostabundantreactivegroup,thehydroxylgroup. Hydroxylgroupcontentisbestdeterminedafter acetylationofhydroxylgroupsbyaceticanhydride inthepresenceofpyridine(BonnerandMcnamara, 1968; ChungandWashburn,2012).Theacetoxy groupsproducedinthisreactioncorrespondto
hydroxylgroupsofligninandappearatchemical shiftsofapproximately2ppm(Lundquist,1992; ChungandWashburn,2012).Afteracetylation,the phenolichydroxylgroupcanbedeterminedselectivelybyrelativelyfastandselectivedeacetylation ofphenolicacetylgroups(Ma ˚ nsson,1983).Alkylationofthehydroxylgroupwasperformedtodeterminehydroxylgroupsin 1HNMRspectra(Adler etal.,1987).While 1HNMRprovidesquantitative information,certainpeaks,suchasthosedueto methoxygroups,canappearbroadandfeatureless becauseofthediversityofchemicalenvironments (Liitiaetal.,2003).Two-dimensional 1H-13CNMR spectroscopycanprovidedetailedinformationon connectivity.Modellignincomplexesprovided detailedinformationnecessaryforpeakassignments (EdeandRalph,1996),andnumerouspulsesequences havebeendevelopedtoelucidatetheconnectivityof functionalgroupsandidentifyspecificlinkages (EdeandBrunow,1992;Kilpelainenetal.,1994). Forexample,thesyringyl-to-guaiacyl(S/G)ratio wasfollowedduringkraftpulpingusingheteronuclearsinglequantumcorrelationNMR,which providedinformationonchangesduetooxidation
duringprocessing(Ibarraetal.,2007).Adifferent approachthatprovidesinformationonalcohol functionalityutilizes 31PNMRinwhichhydroxyl groupswereconvertedtophosphitylgroupsby 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane todo 31PNMRanalysis(GranataandArgyropoulos, 1995;Froassetal.,1998).For 31PNMRanalysis, phosphitylationwascarriedoutbyanotheragent, 1,3,2-dioxaphospholanylchloride(Argyropoulos, 1994;Froassetal.,1996).Mostoftheligninsfrom theprocessingmethodsdescribedherehavebeen characterizedbyNMRspectroscopy.
Size-exclusionchromatography(SEC)analysisof ligninisanimportantanalysistodeterminemolecular weightandmolecularweightdistribution,whichare criticaldataformaterialsdevelopment(Himmeletal., 1995;Hortlingetal.,1995).However,thereliability ofSECanalysiscanbelimitedduetothemany functionalgroupsinlignin,suchashydroxylgroups andcarboxylicacids,andtheligninanalytecanhave stronginteractionswiththecolumnsupportmaterials orbetweenotherligninspecies(Sarkanenetal.,1981, 1982,1984;Iversen,1985).Therefore,thehydroxyl groupsareacetylatedandcarboxylicacidgroups aremethylatedpriortotheSECanalysistoexclude possiblenoncovalentinteractions,suchashydrogen bondingandelectrostaticaggregation,fromthelignin (Himmeletal.,1995;Kimetal.,2007).Ligninisalso characterizedbyvariousmorphologicalmethodssuch
asthermalanalysisandRaman/IRanalysis(Agarwal andAtalla,1999,2010;GlasserWolfgang,1999).
Thechemistryandmaterialpropertiesoflignin havebeenreviewedextensively(Goldsteinetal., 1983;Lewisetal.,1999;ChakarandRagauskas, 2004;Calvo-FloresandDobado,2010; Hatakeyama andHatakeyama,2010; DuvalandLawoko,2014)as wellaschemicalmethodsformodifyingandblending it(Wangetal.,1992;Meister,2002;Liitiaetal., 2003;Mohantyetal.,2009;Dohertyetal.,2011; WashburnandChung,2012).Inthischapter,we presentextractionmethodsandtypesofcommercialgradeligninfromarawbiomass.Abetterunderstandingoftypesandproductionmethodsofligninis animportantstartingpointtostudyligninasnew materials,biofuel,andrenewablesourcesoffine aromaticchemicals.
2.Commercial-GradeLignins
Ligninisbiosynthesizedfrommonomericsubstitutephenylpropyleneunits,coumarylalcohol,coniferylalcohol,andsinapylalcohol,aslignocelluloses aswellasnonconjugatedlignin.Becausethelignocellulosesandligninareintimatelyincorporatedin plantstructures,avarietyofprocessingmethodshave beendevelopedtoisolatelignin. Table1 demonstratesdiversesourcesofligninfromrawbiomass
Table2 PolymericComponentsofVariousSoftwoodandHardwoodSpecies(Sjostrom,1993;Koch,2008)
SpeciesCommonNamesExtractivesLigninCelluloseGlucomannanGlucuronoxylanPolysaccharides Softwoods
Abiesbalsamea
Pseudotsuga menziesii
Tsuga canadensis
Juniperus communis Commonjuniper3.232.133.016.410.73.2
Pinusradiate Montereypine1.827.237.420.48.54.3
Pinussylvestris Scotspine3.527.740.016.08.93.6
Piceaabies Norwayspruce1.727.441.716.38.63.4
Piceaglauca Whitespruce2.127.539.517.210.43.0
Larixsibirica Siberianlarch1.826.841.414.16.88.7
Hardwoods
Acerrubrum Redmaple3.225.442.03.122.13.7
Acersaccharum Sugarmaple2.525.240.73.723.63.5
Fagussylvatica Commonbeech1.224.839.41.327.84.2
Betulaverrucosa Silverbirch3.222.041.02.327.52.6
Betulapapyrifera Paperbirch2.621.439.41.429.73.4
Alnusincana Grayalder4.624.838.32.825.82.3
Eucalyptus globulus Bluegum1.321.951.31.419.93.9
Acaciamollissima Blackwattle1.820.842.92.628.22.8
forlowerdigestiontemperatures,oftenbelow140 C insteadof170 Corgreaterwithoutit.Thekineticsof theAcetosolvprocesshavebeenstudiedforbothhardwood(eucalyptus)andsoftwood(pine)(Vazquezetal., 1997).Theproposedmechanismisbasedonasequence offirst-orderreactionsinvolvingformationofsoluble ligninspeciesthroughlignin hydrolysis,lignincondensationinsolution,andsubsequentreprecipitation. Effectivedelignificationwasobservedat180 Cand 0.1%HCl,whereasincreasingtheHClconcentrationto 0.2%allowedforatemperaturereductionto140 C,but longerreactiontimescanactuallyreduceyielddueto ligninrecombination.
TheMilox(orFormacell)processtakesitsname fromtheMilieuPureOxidativeprocessdeveloped bytheFinnishPulpandPaperResearchInstitute (KCL)(Sundquist,1986).Itisbasedonuseofperoxyformicacidtoperformtandemdelignification andbleaching.Peroxyacidsreactwithnucleophiles having p-electronsviaoxygentransfer,andin acidicmediathestrongelectrophile þOHisformed. Adiversityoffunctionalgroupsresultfromthis oxidationreaction,includingquinones,carboxylic acids,andalkenes,andformationofrecombination by-productsisminimizedthroughefficientsolubilizationinthewater/formicacidmedium.
Generationofperoxyformicacidisaccomplished byreactingformicacidwithhydrogenperoxide accordingtothereaction: whichhasaforwardrateconstantat22 Cthatis50 timesgreaterthantheanalogousreactionofaceticacid andhydrogenperoxide(SuchyandArgyropoulos, 2002).Peroxyformicacidcanbereadilyseparated fromthereactionmixturebydistillation,andhasbeen appliedtothereactorasaconcentratedsolution. However,theseconcentratedsolutionscanbeexplosive,andmorerecentapproachesrelyongeneratingit insitu.Seistoetal.(SeistoandPoppiusLevlin,1997) establishedmultistageprocessesbasedonimpregnationofbiomasswithformicacidandintroductionof hydrogenperoxidefollowingbyadditionofwaterand cookingatupto120 Cforperiodsrangingfrom60to 180min.Thiswasappliedtothehybridgrass Miscanthus giganteus,andacomparisonwithnative ligninshowedthattheweightandnumberaverage molecularweights(Mw andMn)haddecreasedfrom
31742gmol 1 and2681gmol 1 to7636gmol 1 and 1417gmol 1,respectively,asaresultoftheMilox processing.Inaddition,theconcentrationofcarboxylicacidsitesincreasedfrom0.18mmolg 1 to 0.35mmolg 1 andtotalphenolicgroupsincreasedfrom 0.84mmolg 1 to1.31mmolg 1,whiletheconcentrationofaliphatichydroxylgroupswasreduced from5.54mmolg 1 to1.66mmolg 1.Clearlyeven organosolvprocessingcan havesignificantimpacton thechemicalfunctionalityoflignins.
Alkalineconditionshavealsobeenexploredin organosolvprocesses.Forexample,theOrganocell processfirstimpregnatesbiomasswithamixture ofwaterandmethanol,orsometimesethanol,at temperaturesof110 140 C.Thenalkali,suchas sodiumhydroxide,andanthraquinoneareaddedin thedigesterwherethepulpingliquor,containingup to30%methanolbyvolume,isheatedto170 Cat pressuresoforder10barfor2h.Itisthoughtthatthe methanolmaydirectlyparticipateinthedelignificationbyformingmethylethergroupsatcarbocation siteswherelignincondensationcouldoccur.This wouldmaintainalow-molecularweightofthelignin fragmentscomparedtothoseformedbykraftor sulfitepulping.
2.5SteamExplosionLignin (HydrothermalProcess)
Thesteamexplosionprocessrequireshighly compressedwater,200 2000psi,andhightemperature,180 230 C,duringtheshortprocessingtime of1 20mininthepresenceofacatalyst(Wayman andLora,1978,1979,1980;Dataretal.,2007;Zhang etal.,2007;Ruizetal.,2008).Hydrolysis,arylether cleavageandhomolyticcleavageofC Cbond, demethoxylation,alkylation,andcondensationreactionsoccurduringthesteamprocess(Wangetal., 2009;Kangetal.,2011).Whilethearomaticgroups intheligninarenotdegradedbysteamexplosion process, b-O-4etherlinkagesandCa Cb bondsare beingcleavedduringtheprocess.Theresultinglignin hasasimilarcharacterasorganosolvlignin,low molecularweight,andgoodsolubilityinorganic solvents(WaymanandLora,1978;Shimizuetal., 1998).Steamexplosionhasgreatpotentialbecause thisprocessisthefirststeptoproducebiofuelpriorto fermentationofpolysaccharide(GlasserandWright, 1998).Afewexamplesofligninsubunitsthatcanbe
