
https://ebookmass.com/product/ion-channels-as-therapeutictargets-part-a-1st-edition-donev/



https://ebookmass.com/product/ion-channels-as-therapeutictargets-part-a-1st-edition-donev/

https://ebookmass.com/product/the-mena-powers-and-the-nile-basininitiative-okoth/
ebookmass.com

AcademicPressisanimprintofElsevier
50HampshireStreet,5thFloor,Cambridge,MA02139,USA 525BStreet,Suite1800,SanDiego,CA92101-4495,USA
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UK 125LondonWall,London,EC2Y5AS,UK
Firstedition2016
Copyright © 2016ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyright LicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby the Publisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professionalpractices, ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyand thesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterof productsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-12-804794-1
ISSN:1876-1623
ForinformationonallAcademicPresspublications visitourwebsiteat http://store.elsevier.com
AnaLu ´ ciaS.Rodrigues
LaboratoryofNeurobiologyofDepression,DepartmentofBiochemistry,Centerof BiologicalSciences,FederalUniversityofSantaCatarina,Floriano ´ polis,SantaCatarina, Brazil
SusanSchenk
SchoolofPsychology,VictoriaUniversity,Wellington,NewZealand
GeraldSeifert
InstituteofCellularNeurosciences,MedicalFaculty,UniversityofBonn,Bonn,Germany
ChristianSteinha ¨ user
InstituteofCellularNeurosciences,MedicalFaculty,UniversityofBonn,Bonn,Germany
StephanieE.Titus
CenterforTranslationalPsychiatry,DepartmentofPsychiatryandBehavioralSciences, MedicalSchool,TheUniversityofTexasHealthScienceCenteratHouston,Houston, Texas,USA
TalitaTuon
LaboratoryofNeurosciences,GraduatePrograminHealthSciences,HealthSciencesUnit, UniversityofSouthernSantaCatarina,Criciuma,SantaCatarina,Brazil
Ya-JuanWang
CenterforProteomicsandBioinformaticsandDepartmentofEpidemiologyand Biostatistics,CaseWesternReserveUniversitySchoolofMedicine,Cleveland,Ohio,USA
MattiWeckstromw DivisionofBiophysics,DepartmentofPhysics,UniversityofOulu,OulunYliopisto, Finland
JohannesWeller
InstituteofCellularNeurosciences,MedicalFaculty,UniversityofBonn,Bonn,Germany
HongmeiWu
CollegeofLifeSciences,ShaanxiNormalUniversity,Xi’an,Shaanxi,PRChina
w MattiWeckstr€ omhasdied.
Ionchannelsarepore-formingmembraneproteinsexpressedinalmostall celltypes.Theseproteinstriggerelectricalsignalingthroughoutthebody bygatingtheflowofionsacrossthecellmembrane.Twocharacteristicfeaturesofionchannelsdistinguishthemfromothertypesofiontransporter proteins.First,thisistheveryhighrateofiontransportthroughthechannel comparedtoothertransporterproteins(often106 ionspersecondorgreater) andsecond,ionspassthroughchannelsdowntheirelectrochemicalgradient withouttheparticipationofmetabolicenergy.
Thesequencingofthehumangenomehasidentifiedmorethan400 putativeionchannels.However,onlyafractionofthesetheoreticallyidentifiedchannelshavebeenclonedandfunctionallycharacterized.Thewidespreadtissuedistributionofionchannels,alongwiththemultiple physiologicalconsequencesoftheiropeningandclosing,makestargeting ofionchannelsverypromisingtargetsfordevelopmentoftherapeutics. Thepotentialvalidationofionchannelsasdrugtargetsprovidesanenormousmarketopportunityfortheirreemergenceaskeytargetsindrugdiscovery.However,torealizethegreatpotentialofthistargetclass,an understandingofthevalidationofthesetargetsaswellasdevelopmentof suitablescreeningtechnologiesthatreflectthecomplexityofionchannel structureandfunctionremainskeydriversforexploitationofthis opportunity.
Inspiteofsomeimportantdrugstargetingionchannelswhicharetoday inclinicaluse,asaclass,ionchannelsremainunderexploitedindrugdiscovery.Furthermore,manyexistingdrugsarepoorlyselectivewithsignificant toxicitiesorsuboptimalefficacy.Thisthematicvolumeofthe Advancesin ProteinChemistryandStructuralBiology isdedicatedtoionchannelsastherapeutictargetsandmorespecificallyaspromisingtreatmenttargetsinneurologicalandpsychiatricdisorders.Chapter1inthisvolumesummarizes currentadvancesabouttheproteinbiogenesisprocessoftheCys-loop receptors.Operatingonindividualbiogenesisstepsinfluencesthereceptor cellsurfacelevel;thus,manipulatingtheproteostasisnetworkcomponents canregulatethefunctionofthereceptors,representinganemergingtherapeuticstrategyforcorrespondingchannelopathies.Chapter2proposesfor thefirsttimeanovelconceptualframeworkbindingtogethertransient receptorpotential(TRP)channels,voltage-gatedsodiumchannels(Nav),
technologyandbiomedicalknowledgesuggestthattheseproteinsarepromisingtargetsforfuturetherapeuticdevelopment.Therefore,theaimofthis volumeistopromotefurtherresearchinthestructure,function,andregulationofdifferentfamiliesofionchannelswhichwouldresultindesigning newefficienttargeteddrugswithsignificantlyfeweradverseeffects.
DR.ROSSEN DONEV BiomedConsultLtd
Yan-LinFu*,Ya-JuanWang†,Ting-WeiMu*,1
*DepartmentofPhysiologyandBiophysics,CaseWesternReserveUniversitySchoolofMedicine,Cleveland, Ohio,USA
†CenterforProteomicsandBioinformaticsandDepartmentofEpidemiologyandBiostatistics,CaseWestern ReserveUniversitySchoolofMedicine,Cleveland,Ohio,USA
1Correspondingauthor:e-mailaddress:tingwei.mu@case.edu
Contents
1. Introduction2
2. Folding,Assembly,andDegradationofCys-LoopReceptorsintheER5
2.1 FoldingandAssemblyofCys-LoopReceptors5
2.2 ERADoftheCys-LoopReceptors
3. TraffickingofCys-LoopReceptorsfromERtoGolgiandtoPlasmaMembrane10
4. ProteinQualityControlofCys-LoopReceptorsonthePlasmaMembrane11
TheCys-loopreceptorsplayprominentrolesinthenervoussystem.Theyinclude γaminobutyricacidtypeAreceptors,nicotinicacetylcholinereceptors,5-hydroxytryptaminetype-3receptors,andglycinereceptors.Proteostasisrepresentsanoptimalstateof thecellularproteomeinnormalphysiology.Theproteostasisnetworkregulatesthe folding,assembly,degradation,andtraffickingoftheCys-loopreceptors,ensuringtheir efficientfunctionalcellsurfaceexpressions.Here,wesummarizecurrentadvancesabout theproteinbiogenesisprocessoftheCys-loopreceptors.Becauseoperatingonindividualbiogenesisstepsinfluencesthereceptorcellsurfacelevel,manipulatingthe proteostasisnetworkcomponentscanregulatethefunctionofthereceptors,representinganemergingtherapeuticstrategyforcorrespondingchannelopathies.


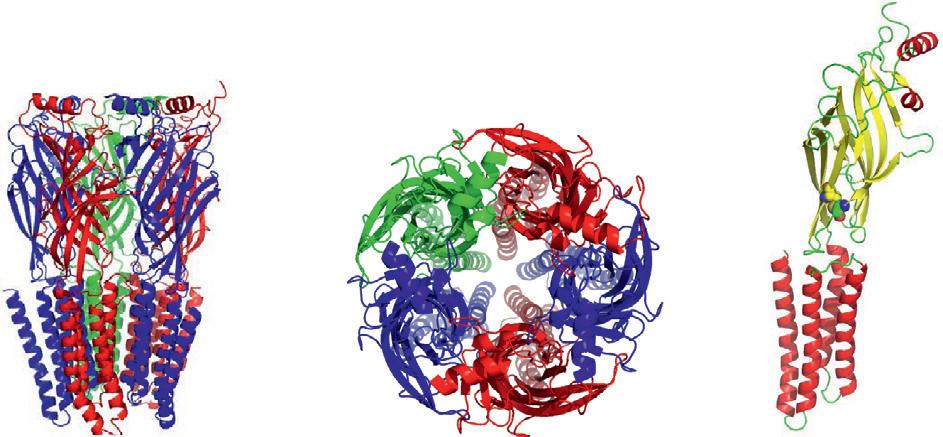
Figure1 StructuralcharacteristicsoftheCys-loopreceptors.(A)TheCys-loopreceptors arepentameric,formingacentralionpore.(B)EachsubunithasalargeERlumen domain,fourtransmembranehelices,andalargeintracellularloopdomain(ICD) betweenTM3andTM4.Thetwocysteinesthatformthesignaturedisulfidebondare showninspheremodel.ThecartoonsarebuiltfromthecrystalstructuresofGABAA receptors(4COF).
M3andM4isimportantformodulatingthetraffickingofthereceptorsand subunitclusteringoncellmembrane.Italsoaffectsthechannelconductance byinfluencingtheaccessibilityofthechannelporetoions(Thompson, Lester,&Lummis,2010).TheTMdomainsplayanimportantroleinchannelfolding,assembly,andgating.
ProteostasismaintenanceofCys-loopreceptorsensurestheirnormal functional(Balch,Morimoto,Dillin,&Kelly,2008).Theproteostasisnetworkregulatestheirfunctionalcellsurfaceexpressionlevelsbyoperatingon theirfolding,assembly,trafficking,anddegradationalongproteinbiogenesis pathways(Fig.2).Tofunction,individualsubunitsofCys-loopreceptors needtofoldintotheirnativestructuresandassemblecorrectlywithother subunitsintheendoplasmicreticulum(ER).Properlyassembledreceptors willbeabletobetransportedfromtheERthroughGolgitocellsurface. UnassembledsubunitsormisfoldedsubunitswillundergotheER-associated degradation(ERAD)pathway,beingretrotranslocatedintothecytosoland degradedbytheproteasome(Guerriero&Brodsky,2012;Olzmann, Kopito,&Christianson,2013;Smith,Ploegh,&Weissman,2011;Wang, Tayo,etal.,2014).Problemsinanystepduringthebiogenesisofthe Cys-loopreceptorsaffectthenormalsurfaceexpressionlevelofthereceptors,thuscausingdiseases.Forexample,manymutationsofhuman GABAARsleadtoepilepsybyabolishingthefolding,assembly,andtraffickingofthemutantreceptors(Macdonald,Kang,&Gallagher,2010).Also, thereceptorsonthecellsurfaceundergocontinuousendocytosisand
toreducedagonist-inducedcurrentsandamyotrophiclateralsclerosis (Richardsetal.,2011).TheS143L,C128S,andR147Lmutationslocated at N-terminalextracellulardomainof ε subunitsfornAChRsinfluencethe subunitassemblyandarelinkedtocongenitalmyasthenicsyndromes(Engel, Ohno, &Sine,1999).
Although itisessentialfortheCys-loopreceptorstoacquiretheircorrect foldingandassemblystatus,theseprocessesaredifficultbecauseeachreceptor,beingapentamer,hasalarge-molecularweight,whichisabout 250kDa,andeachsubunithasmultitransmembranedomains.Asaresult, theassemblyprocessisgenerallyinefficientandslow.Only25%ofnewly synthesizedGABAARsareassembledintoheteromericreceptors,and 30%ofthetranslated α subunitsofnAChRsareassembled(Gorrieetal., 1997; Wanamaker,Christianson,&Green,2003).Thehalf-lifeofthe nAChR assemblyismorethan90min,muchlongerthan7–10min,the half-lifeofinfluenzahemagglutinintoformhomotrimers(Wanamakeret al., 2003).TheGreengrouphasdeterminedtheassemblymodelsofnAChR by usingpulsechaseandcoimmunoprecipitationassayswithsubunits sequence-specificantibodies(Wanamakeretal.,2003).However,nofolding andassemblymodelsofotherCys-loopreceptorareavailableyet. TheassemblyofCys-loopreceptorsdependsontheN-terminalsignal. TheN-terminalextensionandputative α-helixinthe α1, β2,and γ2subunitsofGABAARsarerequiredfortheintersubunitassemblyandthuscan affectthecellsurfaceexpressionlevelofthereceptors(Wong,Tae,& Cromer, 2015).Also,N-terminalextensionand α-helix of ρ1GABAC receptors,whichalsobelongtoCys-loopreceptorfamily,arealsorequired forthenormalassembly,trafficking,andcellsurfaceexpressionofthereceptors(Wong,Tae,&Cromer,2014).Previousstudiesdeterminedthespecific amino acidslocatedattheN-terminusthatareimportantforthesubunit assemblyforGABAARs,nAChRs(Kreienkamp,Maeda,Sine,&Taylor, 1995; Sumikawa,1992;Sumikawa&Nishizaki,1994;Tsetlin,Kuzmin, &Kasheverov,2011),andGlyRs(Kuhse,Laube,Magalei,&Betz,1993; Tsetlin etal.,2011).However,theassemblyof5-HT3Rs(Connolly& Wafford, 2004),nAChRs(Avramopoulou,Mamalaki,&Tzartos,2004), GlyRs(Kuhseetal.,1993),butnotGABAARs(Buller,Hastings,Kirkness, & Fraser,1994),dependsonN-glycosylationstatusasallcys-loopchannels are glycoproteins.Inaddition,recentstudyshowedthatC-terminal motifsinnAChRsmayalsobeimportantforsubunitassembly(Lo, Botzolakis, Tang,&Macdonald,2008).Ahighlyconservedaspartateresidue at theboundaryoftheM3–M4loopandtheM4domainisrequiredfor
Phosphorylationof α4-nAChRsubunitsataproteinkinaseA(PKA)consensussequenceenhancestheinteractionof14-3-3proteinstothe α4subunitsintheERandpromotestheassemblyofcomplete α4β2-nAChRs (Bermudez&Moroni,2006).
Golgi-specificDHHC(Asp-His-His-Cys)zincfingerprotein (GODZ),whichbelongstoDHHCfamilypalmitoylacyltransferase,specificallypalmitoylatesthe γ2subunitsofGABAARs.Thepalmitoylationis requiredfortargetingthereceptorstoinhibitorysynapses.Knockdownof GODZcausesthelossofGABAARs,thusleadingtoreducedGABAARmedicatedminiatureinhibitorysynapticcurrentamplitudeandfrequency (Fangetal.,2006;Kelleretal.,2004;Luscheretal.,2011).
The brefeldinA-inhibitedGDP/GTPexchangefactor2(BIG2)interactswiththeICDof β subunitsofGABAARs.Itenhancesthetraffickingof β3-containingGABAARsbypromotingthemembranebuddingofvesicles fromGolgiapparatus(Shin,Morinaga,Noda,&Nakayama,2004).
The GABAAR-associatedprotein(GABARAP),whichbelongstoa ubiquitin-likefamilyproteininmammalsandisenrichedinGolgiand othersomatodendriticmembranecompartments,facilitatesthetrafficking ofGABAARsinhippocampusneuronontoplasmamembranethrough connectingthe γ subunitswithmicrotubules(Nymann-Andersenetal., 2002; Wang,Bedford,Brandon,Moss,&Olsen,1999).ThisGABARAP effect alsodependsontheinteractionofphospholipidstoGABARAP (Chen,Chang,Leil,&Olsen,2007).
Phospholipase C-relatedcatalyticallyinactiveprotein(PRIP)isinositol1,4,5-trisphosphate-bindingproteins.Itmayserveasabridgeprotein whichconnects γ2-containingGABAARswithGABARAPandpromotes thetraffickingofthereceptors.InterruptingtheinteractionofPRIP with γ2subunitsofGABAARsdecreasesthesurfaceexpressionlevelof thereceptorsinbothculturedcelllinesandneurons(Mizokamietal., 2007).
VILIP-1, aneuronalprotein,enhancesthesurfaceexpressionof α4β2nAChRsinhippocampalneuronsbypromotingtheirexitfromthetransGolginetwork.ThiseffectisactivatedbyincreasingintracellularCa2+ Asaresult,itisanimportantfactorthatmediatestheneuronactivityinducedsurfaceexpressionlevelchangeofthereceptors(Zhaoetal.,2009).
ProteinUnc-50,whichisfoundinnematode C.elegans butevolutionarilyconserved,isneededforthetransportofspecifictypesofnAChRsonto thecellsurfacewithunknownmechanism(Eimeretal.,2007).
RestrictionofCys-loopreceptorstodesignatedsitesonthepostsynaptic plasmamembraneisalsotightlyregulated.Thisprocessisimportantfor shapingthepostsynapticsitestypesandregulatingthereceptors-mediated inhibitoryorexcitatoryeffect.
GephyrinregulatestheclusteringofGlyRsandGABAARs.Gephyrinis ascaffoldproteinthatmainlyaccumulatesininhibitoryGABAergicand glycinergicsynapsesinvariousbrainregions.Glycinereceptorswerethefirst tobefounddependingongephyrintoclusteratpostsynapticsites.Glycerine β loopinteractswithEdomainofgephyrin.Gephyrinisalsoinvolvedinthe intracellulartraffickingandlateralmovementofglycinereceptors(Fritschy, Harvey, &Schwarz,2008).Gephyrin-inducedclusteringofGABAARsis subunit-specific.Gephyrinknockoutinmicediminishesthenumberof α2, α3, β2/3,and γ2subunits-containingsynapticsites,butnotthe α1-, α5-containingsynapticsiteswithoutaffectingthenumberoftotalinhibitory synapticsites(Jacob,Moss,&Jurd,2008).Thiscouldbeduetothefactthat there areonlycertaintypesofGABAARsubunitsthatcanassociatewith gephyrin.GephyrinEdomainassociateswitha10-aminoacidhydrophobic motifwithintheintracellulardomainoftheGABAAR α2, α3,andgephyrin alsointeractsweaklywith γ2,and β3subunits(Kneusseletal.,2001;Tretter et al.,2008).Gepyrinisalsoimportantinregulatingtheneuronactivityplasticity. Long-terminhibitorypotentiationofneuronsinvisualcortex increasesGABAAR-mediatedinhibitorypostsynapticcurrentsbyinducing theCaMKIIphosphorylationoftheGABAAR β3S383 residueandenhances gephyrinclusteringof β3-containingGABAARs.PhosphorylationdependentinteractionofPin,apeptidyl-prolylisomerase,withgephyrin modulatesgephyrininteractionwithglycinereceptorsandthustheirclustering(Fritschyetal.,2008).Collybistin,aguanidineexchangefactoractivating cdc-42, formsabindingcomplexwithgephyrin.Knockoutofcollybistinin micedoesnotaffectglycinergicsynaptictransmissionbutdecreases GABAergicsynaptictransmission.Collybistinisnotrequiredfor gephyrin-mediatedGlyRclusteringbutnecessaryforgephyrin-mediated
(Chen&Olsen,2007).Membranesphingolipidsandotherlipidspromote the surfaceexpressionlevelofmuscle-typenAChRsbyaffectingthebiosynthesisprocessinER(Baier&Barrantes,2007).Decreasingthemembrane cholesterol promotestheendocytosisofnAChRsanddecreasestheircell expressionlevel(Borronietal.,2007).Theunderlyingmechanismisthat membrane lipidservesaslipidrafts,whichisrequiredforthetrafficking andmembranestabilizationofthereceptors.
PhosphorylationaffectstheCys-loopreceptorchannelproperties(Swope, Moss, Raymond,&Huganir,1999)andmodulatestheefficacyofreceptor-mediated effectbyinfluencingtheirtrafficking,endocytosis,and recyclingprocess.Neuronalactivitiesthatleadtothechangeintheintracellularcalciumsignalregulatetheactivityofkinasesandphosphatases, resultinginthealteredthebiogenesisprocessandthusthesurfaceexpression levelofthereceptors.Forexample,enhancedexcitatorysynapticactivities activatephosphatasecalcineurinthroughCa2+/calmodulinpathway followedbyanincreaseinintracellularCa2+ concentration.ActivatedcalcineurindephosphorylatesSer327intheGABAAR γ2subunit,whichleads totheenhancedlateralmobilityofthereceptors,decreasedclustersizeof GABAARs,andreducedGABAergicmIPSC(Bannaietal.,2009).Calcineurin isalsoinvolvedindownregulationofthe α2-containingGABAAR membraneexpressionlevelinprolongedseizuresactivitylinkedtobenzodiazepinepharmacoresistance(Eckel,Szulc,Walker,&Kittler,2015). PRIP, asmentionedabove,modulatestheGABAARsurfaceexpression levelbyaffectingthephosphorylationofthereceptors.PRIPinactivates theproteinphosphatase1α (PP1α),whichdephosphorylatestheGABAARs phosphorylatedbyPKA.Asaresult,PRIPpositivelyregulatesthereceptor surfaceexpressionandreceptor-mediatedinhibitioneffectinhippocampal neuron(Kittler&Moss,2003;Terunumaetal.,2004;Yoshimuraetal., 2001).
Many neurosteroidsorneurotrophicfactorsregulatethesurfaceexpressionlevelofreceptorbyaffectingthetrafficking,endocytosis,andrecycling process.Forexample,neurosteroidspromotethePKCphosphorylationof α4subunitSer443site,whichenhancestheinsertionofthe α4subunitcontainingGABAARsandleadstoincreasedtonicinhibition(Abramian et al.,2010).However,thesameneurosteroiddoesnothaveanyeffecton the α1-and α5-containingGABAARs,whichmediatethephasicinhibition
Bannai,H.,Levi,S.,Schweizer,C.,Inoue,T.,Launey,T.,Racine,V.,etal.(2009).Activity-dependenttuningofinhibitoryneurotransmissionbasedonGABAARdiffusion dynamics. Neuron, 62,670–682.
Bedford,F.K.,Kittler,J.T.,Muller,E.,Thomas,P.,Uren,J.M.,Merlo,D.,etal.(2001). GABA(A)receptorcellsurfacenumberandsubunitstabilityareregulatedbythe ubiquitin-likeproteinPlic-1. NatureNeuroscience, 4,908–916.
Bermudez,I.,&Moroni,M.(2006).Phosphorylationandfunctionofalpha4beta2receptor. JournalofMolecularNeuroscience, 30,97–98.
Bocquet,N.,Nury,H.,Baaden,M.,LePoupon,C.,Changeux,J.P.,Delarue,M., etal.(2009).X-raystructureofapentamericligand-gatedionchannelinanapparently openconformation. Nature, 457,111–114.
Borroni,V.,Baier,C.J.,Lang,T.,Bonini,I.,White,M.M.,Garbus,I.,etal.(2007).Cholesteroldepletionactivatesrapidinternalizationofsubmicron-sizedacetylcholinereceptordomainsatthecellmembrane. MolecularMembraneBiology, 24,1–15. Boyd,G.W.,Doward,A.I.,Kirkness,E.F.,Millar,N.S.,&Connolly,C.N.(2003). Cellsurfaceexpressionof5-hydroxytryptaminetype3receptorsiscontrolledbyan endoplasmicreticulumretentionsignal. TheJournalofBiologicalChemistry, 278, 27681–27687.
Bruneau,E.G.,&Akaaboune,M.(2006).Thedynamicsofrecycledacetylcholinereceptors attheneuromuscularjunctioninvivo. Development, 133,4485–4493.
Buller,A.L.,Hastings,G.A.,Kirkness,E.F.,&Fraser,C.M.(1994).Site-directedmutagenesisofN-linkedglycosylationsitesonthegamma-aminobutyricacidtypeAreceptor alpha1subunit. MolecularPharmacology, 46,858–865.
Castillo,M.,Mulet,J.,Gutierrez,L.M.,Ortiz,J.A.,Castelan,F.,Gerber,S.,etal.(2005). DualroleoftheRIC-3proteinintraffickingofserotoninandnicotinicacetylcholine receptors. TheJournalofBiologicalChemistry, 280,27062–27068.
Castillo,M.,Mulet,J.,Gutierrez,L.M.,Ortiz,J.A.,Castelan,F.,Gerber,S.,etal.(2006). RoleoftheRIC-3proteinintraffickingofserotoninandnicotinicacetylcholinereceptors. JournalofMolecularNeuroscience, 30,153–156.
Chaumont,S.,Andre,C.,Perrais,D.,Boue-Grabot,E.,Taly,A.,&Garret,M.(2013).Agonist-dependentendocytosisofgamma-aminobutyricacidtypeA(GABAA)receptors revealedbyagamma2(R43Q)epilepsymutation. TheJournalofBiologicalChemistry, 288,28254–28265.
Chen,Z.W.,Chang,C.S.,Leil,T.A.,&Olsen,R.W.(2007).C-terminalmodificationis requiredforGABARAP-mediatedGABA(A)receptortrafficking. TheJournalofNeuroscience, 27,6655–6663.
Chen, Z.W.,&Olsen,R.W.(2007).GABAAreceptorassociatedproteins:Akeyfactor regulatingGABAAreceptorfunction. JournalofNeurochemistry, 100,279–294.
Chiou,T.T.,Bonhomme,B.,Jin,H.,Miralles,C.P.,Xiao,H.,Fu,Z.,etal.(2011).Differentialregulationofthepostsynapticclusteringofgamma-aminobutyricacidtypeA (GABAA)receptorsbycollybistinisoforms. TheJournalofBiologicalChemistry, 286, 22456–22468.
Cho,C.H.,Song,W.,Leitzell,K.,Teo,E.,Meleth,A.D.,Quick,M.W.,etal.(2005). Rapidupregulationofalpha7nicotinicacetylcholinereceptorsbytyrosinedephosphorylation. TheJournalofNeuroscience, 25,3712–3723. Christianson,J.C.,&Green,W.N.(2004).Regulationofnicotinicreceptorexpressionby theubiquitin-proteasomesystem. TheEMBOJournal, 23,4156–4165. Comenencia-Ortiz,E.,Moss,S.J.,&Davies,P.A.(2014).PhosphorylationofGABAA receptorsinfluencesreceptortraffickingandneurosteroidactions. Psychopharmacology, 231,3453–3465.
Connolly,C.N.(2008).Traffickingof5-HT(3)andGABA(A)receptors(Review). MolecularMembraneBiology, 25,293–301.