Leachingpractice
Thechemistryofprocessesaimedatthedissolutionof valuablemetalsfromvariousfeedmaterialswascoveredin Chapter6inVolume1.Inthischapter,thevariousmethods employedtocarryouttheleachingreactionswillbeoutlined Thechoiceofaparticularleachprocessandtheequipmentto beuseddependsontheperformancethatcanbeachievedbythe variousoptions.Themainfactorstobetakenintoaccountin assessingleachperformancearethefollowing.
• Degreeofdissolutionofdesiredspecies.
• Selectivityofleachingprocesswithrespecttothedesired species.
• Leachingtimerequiredtoachievethedesiredextraction.
• Operatingcost(lixiviants,power).
• Capitalcost.
Thereisgenerallyaneconomicoptimumthatdeterminesthe mostappropriatestrategyforleaching.Thus,fortheleachingof goldores,theobjectiveshouldbetomaximizeextractiongiven thatoperatingcostsaregenerallynothighrelativetothevalue oftheproduct.Ontheotherhand,inthecaseoftheleaching oflow-gradecopperores,theoperatingcostsarethemostimportantconsiderationandheapleachingistheonlyviableoption. Eveninthiscase,highacidconsumptioncanruleoutheapleachingforsomeores.
1.1Leachingmethods
Thetechniquesshownnextareappliedintheleachingofores andconcentrates.
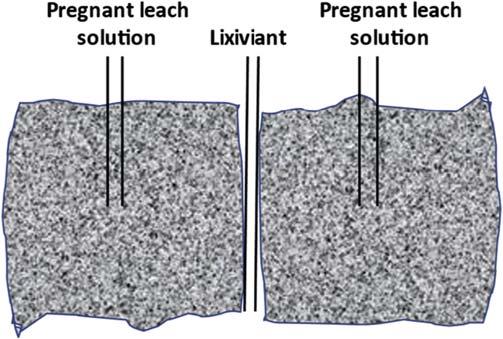
Insituleaching: Lixiviantpumpeddirectlyintofracturedorebodyandpregnantsolutionrecovered.
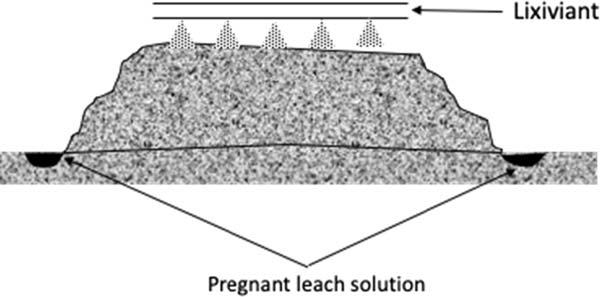
Heap(ordump)leaching: Lixiviantsprinkledoverheapsof mined(crushed)oreinheapsbuiltovertheimperviousbase.
Vatleaching: Crushedore fillsalargevatthatisthen filledwith lixiviantandlefttoleach.Abatchprocessthatisnotvery common.
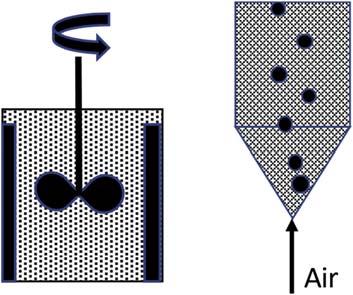
Agitationleaching: Milledorecontactedwithlixiviantin agitatedtanks(mechanicalorairsparging).Batchormultistage continuousreactors.
Pressureleaching: Milledoreorconcentratecontactedwithlixiviantinhigh-pressure,high-temperaturereactors(autoclaves) thataregenerallyoperatedincontinuousmode.
Bacterialleaching: Avariationofheaporagitationleachingin whichbacteriaassistintheleachingreactions.
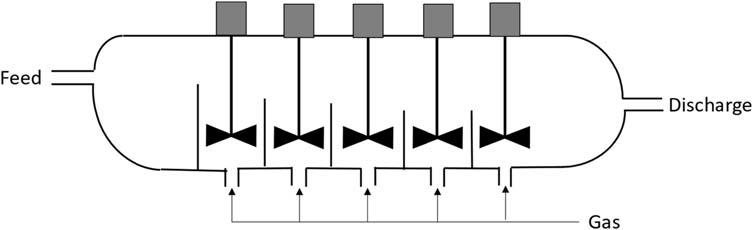
Someofthemoreimportantpracticalmethodsusedto dissolveorleachvaluablecomponentsofanore,concentrate, orotherintermediateproductaresummarizedin Fig.1.1.The actualmethodtobeadopteddependslargelyonthevalueof thematerialtobetreatedwithlow-gradematerialsrequiring methodstotheleftandhighgradematerialstotherightofthe diagram.
1.2Typicalleachingprocesses
Thedissolutionofasolidspeciesisachemicalreactionthat canbeclassi fiedintooneofseveraltypessuchasacidleaching
In-Situ Lix.
Increasing value of material leached
Figure1.1 Selectionofleachingmethodwithgradeoffeedmaterial.
andoxidativeleachingasoutlinedinChapter6inVolume1.The chemistrythatispossibleinthedissolutionofasolidisoftenextensiveandvariedanddependsontheingenuityoftheresearcher. However,practicalconsiderationsdeterminedlargelybythecost ofthelixivianthaverestrictedthechoiceoftheleachingprocess. Table1.1 summarizessomeofthemoreimportantleaching processesthatareinoperationinvariouspartsoftheworld. Thedesignandoperationofleachingprocessesarecritically dependentonanunderstandingandapplicationofthekinetics ofthereactionstakingplaceinleachreactors.Inthefollowing sections,wewillshowhowkineticinformationcanbeusedto describehowwecandesignandoperatealeachingprocess.
1.3Batchleachingkinetics
InChapter6inVolume1,wedealtwiththeexpectedprofiles fortheleachingofparticlesforseveralcasesinwhichthe
Heaps Crush Crush Crush
Grind Grind Tank
Tank Lix. Ore Lix. Lix. Lix.
Table1.1Someleachingprocessesinoperation.
FeedLixiviantOxidantTemp.PressureEquipment
OxidizedCuoreDilH2SO4
NoneAmbientAtmos.Heaps
SulfideCuoreDilH2SO4 Ferric,bacteriaAmbientAtmos.Heaps
Cu,Ni,Co,Zn concentrates, mattes
DilH2SO4 Ferric,oxygen >100 C1 10bar.Tanks, autoclaves
ZinccalcineDilH2SO4
None40 90 CAtmos.Tanks
NimatteDilHClFerric,cupric <100 CAtmos.Tanks
NilateriteoreDilH2SO4 None250 C50bar.Autoclaves
ReducedlateriteoreNH3/CO2 Air,Cu(II)40 80 CAtmos.Tanks
UraniumoreDilH2SO4 Ferric þ MnO2 40 50 CAtmos.Tanks,insitu
OxidizedgoldoreNaCNAirAmbientAtmos.Heaps,tanks
RefractoryAuore, concentrates H2SO4/NaCNFerric/Air150 200 C/Ambient30 40bar/AtmosAutoclaves/ tanks
BauxiteNaOHNone150 200 C20 40barAutoclaves
RoastedVoreWaterNone50 90 CAtmos.Tanks,columns
ScheeliteSodaashNone200 C40barAutoclaves
RoastedLioresDilH2SO4 None <100 CAtmos.Tanks
rate-determiningstepiseithermasstransportorchemicalreaction.Inmanyrealcases(seelater)involvingoresand,toalesser extent,concentrates,thecurveoffractionleachedversustime doesnotconformtoanyofthetheoreticalforms.Underthese conditions,wehavetoresorttoempiricalrateequations.
Considertheleachingofatypicalgoldoreforwhichthe followingrateequationhasoftenbeenfoundtodescribethe rateofdissolutionofgoldfromcyanidepulp,
inwhich[Au]istheconcentration(massgold/unitmassofpulp) atanytime, t,and[Au]f isthecorrespondingconcentrationafter aninfinitetime,thatis,itistheultimateachievablebarrenconcentration(mainly “lockedgold” notaccessibletothelixiviant). kisarateconstant.
Thisequationcanbeintegratedtogive,
where[Au]o istheinitialconcentration.Thisequationcanbewrittenintheform
inwhichk0 ¼ k([Au]o [Au]f)andX ¼ ([Au] [Au]f)/([Au] o [Au]f)is thefractionofgoldleached.
Theparameters[Au]f andkcanbeobtainedfromasmall-scale batchleachcarriedoutundertypicalenvisagedplantconditions ofpulpdensity,cyanideconcentration,andpH.Forexample, thetimerequiredtoachievea50%dissolutionofgoldfroma batchofpulpforwhich[Au]o ¼ 5g/t,[Au]f ¼ 0.2g/t,and k ¼ 0.05/h,willbegivenby
Inapracticalsituation,thetimerequiredto fillandemptythe batchreactorwillhavetobeaddedtotheactualleachingtime. Thisgenerallyismostefficientwithlargereactors.
Thus,forabatchleachplanttotreat100t/hoftheabovepulp inatankthatcanhold950t(forcomparisonwithalatersection seeAppendix)ofpulp,theleachtimewillbe4.5h,whichwillleave 4.5hforcharginganddischargingthetank,thatis,anaverage pulp flow-rateof420t/hduringtheseoperations.
Noticethatinthecaseofabatchreactor,alloftheoreparticlesareexposedtothelixiviantforthesameleachingperiod.
Batchleachingisseldomusedinpracticeexceptforrelatively small-scaleoperationssuchas,forexample,thedissolutionofa preciousmetalconcentrate(goldand/orplatinumgroupmetals) in,forexample,achlorine/chloridesystem.Therequirementfor accurateaccountingofthemetalinvariousstagesofprocessing isalsoconsiderablysimpli fiedinbatchprocessing.Theformation ofmetastablespeciesinsolution(suchashydratedsilica)may alsobecontrolledmoreappropriatelyinabatchratherthanin acontinuousreactor.
1.4Continuousleaching micro-and macro fluids
Incontinuousleaching,theore,concentrate,orothermaterial containingthemetalormineraltobeleached(generallyasa slurry)andthelixiviantarefedcontinuouslyintoastirredtank reactor(CSTR)andtheleachedslurrydischargedcontinuously generallyintoanotherreactorinseries.
Theanalysisoftheperformanceofthistypeofreactordependsonthenatureofthe fluid. Microfluids consistofsolutions suchthatonecannotdistinguishonemoleculeofasolute,suchas anionorsolventmoleculefromanother.Thus,inaCSTRtreating amicro fluid,allreactantsareattheirexit(i.e.,low)concentrations,reactantsenteringareimmediatelydilutedtoexitconcentrationsbyperfectmixing,andthereactions,therefore, takeplaceatarelativelylowrate,asdictatedbythereactantconcentrations.The flowofamicro fluidisoftencharacterizedas nonsegregated.
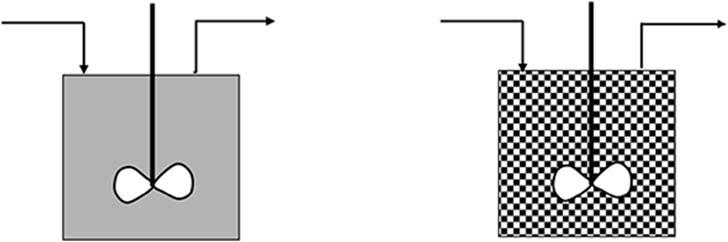
Ontheotherhand,asshownin Fig.1.2, macro fluids consistof suspensionsofparticleseachofwhichisdistinguishableandeach ofwhichisanaggregate(crystalinsomecases)ofalargenumber ofatomsormolecules.Thus,forexample,aparticleoforesuspendedinaslurrywillbehaveinaCSTRinthesamewayasa batchreactor.Thus,thereactantconcentrations(inthesolid phase)donotimmediatelydroptoalowvaluebutdecreaseas theywouldinabatchreactorandtheextentofreactionineach oftheoreparticlesinthereactordependsonlyonthelengthof stay(residencetime)inthereactor.Thisisequallytrueforany particleintheexitstream.Thus,thefractionalconversioninthe exitstreamisdeterminedbysummingtheconversionsofallthe particles(PopulationBalanceMethod).Thisisanexampleof segregated flow.
Thus,inaCSTRtreatingaslurry,thesolutionphasewould behaveasamicrofluidandthesolidphaseasamacrofluid. Thus,inanidealCSTRinwhichallparticlesoftheabovegold orehavethesameresidencetimetR (¼ Vol.ofreactor/Volumetric flowrateofslurry),theperformancewouldbeidenticaltothatof thebatchreactordiscussedearlier.
Figure1.2 Schematictodistinguishbetweenmicrofluid(left)andmacrofluid(right).
1.4.1ResidencetimedistributioninaCSTR
Inanidealcompletelymixedreactorvessel,anentering fluid elementisinstantaneouslybrokenupintotinyfragmentsthatare uniformlydistributedthroughoutthevolumeofthevessel.Some ofthese fluidfragmentsareimmediatelydrawnintotheeffluent streamwhileotherscirculatewithinthevesselforvariouslengths oftimebefore findingtheirwayout.Thus,atanyinstant,the reactoreffluentiscomposedof fluidparticlesthathavespent variouslengthsoftimeinthevessel.Incontrast,every fluid elemententeringa plug flow vesselfollowstheelementthat enteredbeforeitwithoutanyintermixingandexitsthereactor inexactlythesameorder.Atanyinstantthen,theexitstreamis madeupof fluidelements,allofwhichhavebeenresidentin thereactorforexactlythesamelengthoftime.Thetimespent inthereactorbya fluidelementiscalledits exitage.Thedistributionofexitagesofall fluidfragmentsinthereactoreffluentis calledthe residencetimedistribution (RTD)andisindicativeof themixingand flowdistributionpatternswithinthereactor.
Intheory,ina “perfectlymixedtank”,theresidencetimes coverthewholerangefromzerotoinfinity,althoughtheaverage ormeanresidencetime(tR)isthesameasforbatchtreatment, namelythemassofpulpinthetankdividedbythemass flowrate. Mixingtheoryshowsthatthewaytoovercomethe “shortcircuiting” thatoccursinasingletankistodividethesametotal volumeormassamonganumberoftanksinseries themore tanksthereareinseries,thehigherwillbetheproportionof pulpthatwillhavearesidencetimeclosetothemeanvalue.
Forthemeanresidencetime,tR ¼ V/Q,inwhichVisthevolumeof fluidinthetankandQthevolumetric flowrate,theactual residencetime/meanresidencetime ¼ t/tR ¼ q.
Theresidencetimedistributionfunction,E(q),istherelative proportionofthedischargepulphavingaresidencetimebetween q and q þ dq.Thatis,E(q).dq isthefractionoftheexit streamofagebetween q and qþdq.
Foranidealplug flowreactor,theexpectedRTDfunction,E(q) isshownastheverticalarrowin Fig.1.3.
Ontheotherhand,foracompletelymixed(CSTR)reactor, E ðqÞ¼ 1 tR expð qÞ (1.4)
AgraphicalrepresentationoftheexpectedRTDfunctionfora completelymixedvesselisshownin Fig.1.3.
Consideranumberofimixedtanksinseriesthatareassumed tobecompletelymixedandtoeachhavethesamevolume.Thus,
ResidencetimedistributionforasingleCSTR.
ifthetotalvesselvolumeisV,eachtankhasvolumeVi ¼ (V/N). TheRTDfunctionforthissituationcanbederivedandisgivenby
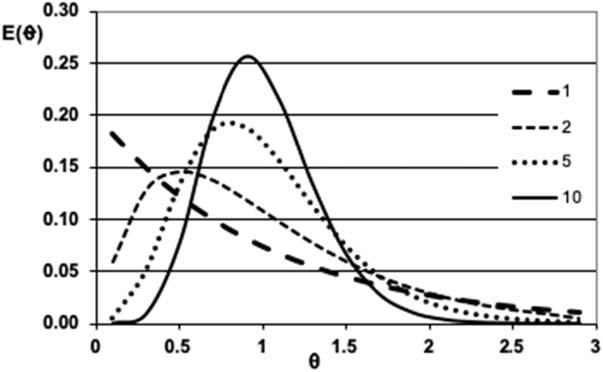
AfamilyofRTDcurvesforanumberoftanksinseriesis shownin Fig.1.4.
Thefractionof fluidthathasaresidencetimebetween q1 and q2 isgivenbythatareaunderthecurvebetween q1 and q2 relativetothetotalareaunderthecurve.Thesevaluesareshown in Table1.2 forseveralintervals.
Figure1.4 Residencetimedistributionsfortanksinseries.
Figure1.3
Table1.2Fractionof fluidwithvariousresidencetimes.
Reactors,N
0.10 0.20.18140.05960.02480.00460.0001
0.30.2 0.40.14850.12860.09670.04990.0094
0.50.4 0.60.12160.14570.14800.13140.0773
0.70.6 0.80.09960.13750.16040.18530.1979
0.90.8 1.00.08150.11890.14630.18760.2563
1.11.0 1.20.06670.09760.12040.15520.2148
1.31.2 1.40.05460.07740.09260.11220.1334 1.51.4 1.60.04470.05990.06780.07360.0667 1.71.6 1.80.03660.04560.04790.04490.0283 1.91.8 2.00.03000.03420.03290.02590.0106
2.12.0 2.20.02460.02530.02210.01420.0036 2.32.2 2.40.02010.01860.01450.00760.0011 2.52.4 2.60.01650.01360.00940.00390.0003 2.72.6 2.80.01350.00980.00600.00200.0001
2.92.8 3.00.01100.00540.00120.00030.0000 >3.00.04900.02240.00890.00150.0000
Itisapparentthatasthenumberoftanksincreases,thedistributionofresidencetimesbecomessharperwithalargerfraction ofthepulphavingaresidencetimeclosetotR.
Forareactioninthetankthatisa first-orderprocess,itcan beshownthatnoerrorisintroducedbyassumingthatallparticleshavethesame(mean)residencetime. Thisisnottrueforany otherreactionorder(seeAppendixfordetails).
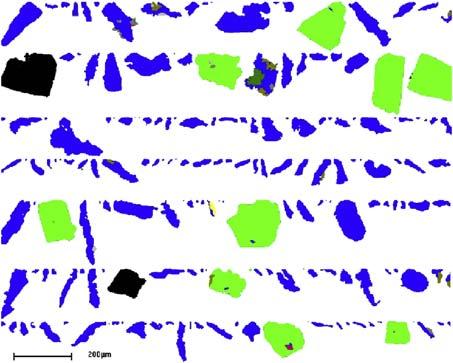
Thesituationwithreactionsinvolvingsolidparticlesisfurther complicatedbythefactthatapulpinvariablycontainssolidswith anonuniformparticlesizedistribution,probabledifferent shapes,andevenadistributionofreactivities.Thus, Fig.1.5 showsamineralliberationanalyzerpictureofthegoldparticles (blue)inatypicalgoldconcentrateproducedbygravity separation.
Thus,onecouldexpectto findadistributionofresidence times,particlesizes,shapes,andreactivitysuchasshownin Fig.1.6.
Itisnotdif ficulttovisualizethatthelargerparticleswillprobablyhavealongerresidencetimeinthetankandmay,iflarge
Figure1.5 Distributionofparticlesizesandshapesinagold(blue(darkgrayinprint))concentrate.Thegreen particles(lightgrayinprint)arepyrite.
Fraction Within Each Class
Particle size,
shape,reactivity
Figure1.6 Schematicdistributionofvariouscharacteristicsofore/concentrateparticles.
enough,settleinthebottomofthetankwhilethe fineparticles willprobablyhaveashortermeanresidencetime.Thiseffect will,inpart,acttocompensateforthenormalRTDinthatwe wantthelargerparticlestohavealongerresidencetime.For thisreason,thecomplicationscausedbytheRTDeffectareoften ignored.
Inarealagitatedtankreactor,however,thereisoftenadegree ofshort-circuitingofthepulpduetoinef ficientblendingofthe incomingpulpwiththecontentsofthetankcoupledtoinappropriatepositioningofthefeedandexitpoints.Thesettlingof largerparticlescanalsooftenresultinasignificantfractionof
thetankvolumebeingunavailableforreaction.Thisis,asexpected,moreprevalentinthe firsttankinaseriesandtheperiodicuseoftracerteststoestablishtheactivevolumeofthe tankwillenablethisproblemtobehighlighted.
Itisapparentthatafulltreatmentofthekineticsofleaching ofarealoreorconcentratewillrequireinformationontheparticlesizedistribution.Givenallthepreviouslymentionedcomplicationswhendealingwithrealleachsystems,theadded complexityofthepopulationbalancemodelscannotgenerally bejustified.TheAppendixoutlinestheapplicationofconventionalCSTRtheoryforthetreatmentofleachingreactions bearinginmindthepreviouslymentionedproblemsinparticulatesystems.
1.5Counter-currentleaching
Thepreviouslymentionedleachingprocessesinvolvecocurrent flowoftheoreorconcentrateandthelixiviant.Whilethis isoftenaconvenientmethodofoperating,greaterefficiencies intermsofoverallleachingrecoveryandmaximumutilization ofthelixiviantcanbeaccomplishedbycontactingthelixiviant withtheoreinacounter-currentfashion.Thus,
• Maximum extraction isachievedbycontactingaleachresidue withfresh,concentratedlixiviant.
• Maximum utilization ofthelixiviantcanbeachievedbycontactingfresh,reactiveoreorconcentratewithlixiviantthat hasalreadybeenusedinapriorstage.
Thus,considertherealexampleshownin Fig.1.7 ofathreestageleachingcircuitforazinccalcinethatisdesignedto maximizezincrecoveryandutilizeallthelixiviantwhilestillminimizingthedissolutionofironbylimitingthepHtovaluesabove3 inthe firststageleachandprovidingasolutionofthedesired compositionforpurificationbeforeelectrowinning.
Asthisisthe firstexampleofatypicalhydrometallurgical flowsheet,itisworthspendingsometimeonthedetails.Ignoreinthe firstinstancetheoperationsshownindashedlines theseare usedtobleedironfromthecircuitandarenotessentialinterms ofcounter-currentleachingandweshallreturntothissection later.Intermsofoverallleachinginonestage,oneissimply reactingthecalcinewithacidinthespentelectrolytefromelectrowinningthatcontainsexcessacidgeneratedattheanodeduringelectrowinning.However,inthiscase,inthe firstleachstage, thecalcineiscontactedwith(a)solutionfromthesecondstage leachthatcontainsexcessacidandzincdissolvedinthe first
Spent Electrolyte
1st Stage Leach, 50o C, pH 4
LLL S
2ndStage Leach, 80o C, 10 g/L acid LLL L S
3rdStage Leach, 80oC, 50 g/L acid
Calcine, NH 4 +
LLS L LLL S Residues
Figure1.7 Athree-stagecounter-currentleachingprocessforzinccalcine.
stageand(b)smallamountsofspentelectrolytetocontrolthepH ofthesolutionleavingthe firststage.Thepulpleavingthe first stageis filtered(orsettled)toseparatethesolutionfromthe leachresidue.Thesolutionnowcontainsupto200g/Lzinc andissenttothepurificationstageoftheplantandthereafter toelectrowinning.
Thesolidresiduefromthe firststagestillcontainszincandis subjectedtoleachingundermoreextremeconditionstodissolve mostoftheresidualzinc.Thesolutionusedtodissolvethiszincis madeupofasolutionfromthethirdstageleachandsomespent electrolyte.Thepulpfromthisstageissubjectedtosolid/liquid separationandthesolutionisroutedtothe firststageandasmall amountissentforironremoval.Thesolidfromthissecondstage isthefeedtothethirdstageinwhichanyresidualrefractoryzinc isdissolvedathightemperatureandacidityusingthespentelectrolyte.Aftersolid/liquidseparation,thesolutionphaseisusedin thesecondstageleach,andthesolidiswashedandreportedas the finalresidue.
Whilethisapproachisefficientintermsofleaching,theintroductionofasolid/liquidstepbetweeneachcounter-currentstage introducesanadditionalunitoperationthatcanbebothinef ficientandcostlyforpulpsthataredifficultto filterorsettle.The
Calcine
introductionofadditionalwaterforwashingthe filtercakes furthercomplicatestheprocess.Forthisreason,countercurrentleachingisnotappliedaswidelyasmaybeanticipated.
1.6Bacterialoxidationandleaching
Bioleaching istheextractionofametalfromsulfideoresor concentratesusingmicroorganismsthatcatalyzetheoxidation ofsulfideminerals.Anassociatedprocessis biooxidation inwhich sul fidemineralsassociatedwithbutnotnecessarilypartofthe mineralofinterestisoxidizedordissolved.Inbiooxidationofrefractorygoldores,bacteriaareusedtosolubilizeanironsulfidein whichthegoldparticlesarelocatedandthusmakethegoldavailableforcyanideleaching.Likewise,incoaldesulfurization,bacteriaareusedtooxidizethepyritecontaminantinthecoalthus makingthesulfursolubleasferricsulfate.
Bioleachingisusedtodayincommercialoperationstoprocess oresofcopper,nickel,cobalt,zinc,anduranium;whereas,biooxidationisusedingoldprocessingandcoaldesulfurization. Sincebioleachingisanaturalprocess,anundesirableeffectis thecreationofso-calledaciddrainagefromtheslowoxidation ofsulfidemineraloutcropsandfromabandonedtailingsdumps. Bioleachprocessingdiffersdependingonthetypeofresource tobeprocessed.
Dumpleaching wasterock,low-gradeore,orconcentrator tailings(lowgrade,oxides,andsecondarysulfides)areleached fromwastedumps.
Heapleaching newlyminedrun-of-the-mine(ROM)material (intermediategrade,oxides,andsecondarysul fides)isplacedasa heaponanimperviousnaturalsurfaceorapadandleached.ROM maybeleachedasminedormaybepartiallycrushedandmixed withacidbeforedepositingontheheap.
Agitatedleaching concentratesareleachedinatankusing mechanicalagitation.
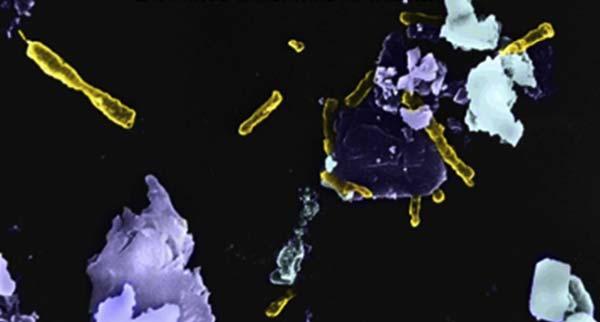
Wastedumpleachinguses mesophilic (ambienttemperature, 35 45 C)microorganisms,thatis,bacteria.Heapleachingof oremayinvolve mesophiles ormoderate thermophiles (hightemperature,50 60 C)microorganisms.Leachingofchalcopyrite andotherprimarysulfideconcentratesrequiresextreme thermophiles (>70 C).Aphotographofthiobacillusferrooxidansbacteria attachedtosulfidemineralsisshownin Fig.1.8.
Insummary,bioleachinginvolves
• Oxidativedissolutionofsul fideswithferricions.
• Reoxidationofferrousbydissolvedoxygencatalyzedbyspecificbacteria.
Figure1.8 Bacteria(yellow(lightgrayinprint))associatedwithsulfideminerals.
• Useofmicroorganismsofwhichthiobacillusferrooxidansand sulphooxidansaremostcommon.
• SourceofCO2,pHabout1.5 2,temperature35 45 C,nutrients(N,P,K),dissolvedO2 greaterthan1ppm.
Forexample,thefollowingreactionsoccurduringthebiooxidationofpyrite, FeS2 ðsÞþ 14Fe3þ D 8H2 O ¼ 15Fe2þ D 2H2 SO4 D 12Hþ (1.6) 14Fe2 D þ 7=2O2 þ 14Hþ ¼ 14Fe3þ D 7H2 O(1.7) thatis,overall,
2FeS2 ðsÞþ 15=2O2 D H2 O ¼ Fe2 ðSO4 Þ3 þ H2 SO4 (1.8)
N.B.Inthecaseofpyrite,acidisproducedandmustbe neutralised.
Inthecaseofpyrrotite,
2FeSðsÞþ 9=2O2 þ H2 SO4 ¼ Fe2 ðSO4 Þ3 þ H2 O(1.9) acidisconsumedandmustbeprovidedtokeepthepHintheoptimumregion.
Someofthemostimportantadvantagesofbiooxidationor leachingprocessesare
• Rapidoxidationofiron(II)toiron(III)
• Bacterialoxidationofelementalsulfurlayers
• Lowercapitalcostsforsmalltomediumsizeplants
• Relativelysimple,low-techprocess
• Environmentallyacceptable
Ontheotherhand,therearesomedisadvantagessuchas
• Slowkinetics(severaldaysunderfavorableconditions)
• Sensitivitytoprocessvariations(temperature,lossofaeration, poisonssuchascyanideandsalinity)
• Limitedsolidscontent(<20%)
• Producessolubleiron(III)thatrequiresremovalanddisposal.
• Bioleachingdoesnotrecoverthepreciousmetalsintheore
1.6.1Processparametersforbiologicaloxidation
Theplantsizeisdeterminedbytheoreorconcentrate throughputandtherateofoxidationofsulfidesulfur.Therelative proportionsofeachmineralpresentdeterminetheprocessacid consumption/production,oxygendemand,andcoolingrequirementsasshownin Table1.3.
Majordesignrequirementsofreactorsare:
• Agitationtosuspendsolidsand,moreimportantly,todisperse largevolumesofairoroxygen.
• Coolingcoilstodissipateheatgeneratedbyexothermicreactionsandagitationthatisnotlostbyevaporation,heatingof air,andfeedpulp.
Table1.3Reagentandpowerrequirements.
• Residencetime
• Corrosionresistanceofthematerialsofconstructiongiven acidicconditions.
1.6.2Biooxidationreactorkineticsanddesign
Therateofbiologicaloxidationofasulfidemineralcanoften beexpressedintermsofthe “logisticrateequation”
wherevistherateofoxidation, Xisfractionoxidized, Xm isthemax.fractionthatcanbeoxidized,and k isabacterialgrowthrateconstant
Considerasingle-stageCSTRreactorcontainingavolumeVof pulpthatis flowingatarateQ(volume/unittime)throughthe reactor.
Fromthemassbalanceatsteady-state,weobtaintheCSTR equation,
and,substitutingthepreviousrateequation,
inwhichtR ¼ V/Qisthemeanresidencetime.
Fork ¼ 1/tR,X ¼ 0,andthisisreferredtoasthebacterialcell “wash-out” conditionthatis,theoperatingpointatwhichthe dilutionrate,1/tR,isequaltothemaximumrateofgrowthof thebacterialcells.Thus,
(a) Forthreeequalreactorsinseries:
Cellwash-outwilloccurfortR ¼ 1/k andthesystemresidence timewillbe3tR
(b) Foraprimaryreactorthatistwicethesizeofthesecondary reactorscellwash-outwilloccurforatotalresidence time ¼ 2/k ¼ 2tR wheretR istheresidencetimeintheprimary reactor.
Thisisthebasisforthecommondesignoftwoprimaryreactorsinparallelfeedingsecondaryreactorsofthesamesizeinseries.Thisensuresthatwash-outofthebacteriawillnotoccurat thedesign flowratesforasinglereactor.
Fig.1.9 illustratesthisphenomenonforabioleachreactorin whicharefractorygoldconcentrateisoxidized.Thegoldrecovery shownisthatobtainedbycyanidationoftheresiduefrombiologicaloxidation.Youshouldattempttointerprettheoperating