https://ebookmass.com/product/high-temperature-oxidationand-corrosion-of-metals-2nd-edition-david-john-young/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
High Temperature Gas-cooled Reactors Takeda https://ebookmass.com/product/high-temperature-gas-cooled-reactorstakeda/
ebookmass.com
Corrosion of Aluminium 2nd Edition Christian Vargel
https://ebookmass.com/product/corrosion-of-aluminium-2nd-editionchristian-vargel/
ebookmass.com
Electrocorrosion and Protection of Metals 2nd Edition Joseph Riskin Alexander Khentov
https://ebookmass.com/product/electrocorrosion-and-protection-ofmetals-2nd-edition-joseph-riskin-alexander-khentov/
ebookmass.com
Urban Ecology: Emerging Patterns and Social-Ecological Systems 1st Edition Pramit Verma (Editor)
https://ebookmass.com/product/urban-ecology-emerging-patterns-andsocial-ecological-systems-1st-edition-pramit-verma-editor/
ebookmass.com
New Challenges for Macroeconomic Policies: Economic Growth, Sustainable Development, Fiscal and Monetary Policies Gilles Dufrénot
https://ebookmass.com/product/new-challenges-for-macroeconomicpolicies-economic-growth-sustainable-development-fiscal-and-monetarypolicies-gilles-dufrenot/ ebookmass.com
Indian Economy Key Concepts | Sixth Edition (English) Karuppiah
https://ebookmass.com/product/indian-economy-key-concepts-sixthedition-english-karuppiah/
ebookmass.com
Focus on Middle East respiratory syndrome coronavirus (MERS-CoV) A. Bleibtreu
https://ebookmass.com/product/focus-on-middle-east-respiratorysyndrome-coronavirus-mers-cov-a-bleibtreu/
ebookmass.com
Three Single Wives Gina Lamanna
https://ebookmass.com/product/three-single-wives-gina-lamanna-4/
ebookmass.com
Chemical Youth: Navigating Uncertainty in Search of the Good Life 1st Edition Anita Hardon
https://ebookmass.com/product/chemical-youth-navigating-uncertaintyin-search-of-the-good-life-1st-edition-anita-hardon/
ebookmass.com
Test-Driven Development in Go Simion https://ebookmass.com/product/test-driven-development-in-go-simion/
ebookmass.com
HighTemperature Oxidationand CorrosionofMetals SecondEdition
DavidJ.Young
SchoolofMaterialsScienceandEngineering,University ofNewSouthWales,Sydney
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UK 50HampshireStreet,5thFloor,Cambridge,MA02139,USA
Copyright 2016,2008ElsevierLtd.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangements withorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency, canbefoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein. Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthesafety ofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproducts liability,negligenceorotherwise,orfromanyuseoroperationofanymethods,products, instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguinginPublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
ISBN:978-0-08-100101-1
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/
Publisher: JohnFedor
AcquisitionEditor: KostasMarinakis
EditorialProjectManager: SarahWatson
ProductionProjectManager: AnithaSivaraj
Designer: MariaInesCruz
TypesetbyTNQBooksandJournals
Coverimage:Burnerrigtestsinbothheatingandcoolingpositions thermalbarriercoatedsuper alloyhot.CourtesyoftheNationalAeronauticsandSpaceAdministration,JohnH.Glenn ResearchCenteratLewisField(NASAIdentifierGRC-C-1999-2487).
Foreword Thedepletionofthefirsteditionprintrunandanenormousincreaseinpublishedresearchonhigh-temperaturecorrosionhavecombinedtomakea secondeditionofthisbookdesirable.Recentworkonmasstransportin aluminaand,moregenerally,onoxidegrainboundarydiffusionhascontributedimprovedclaritytoourunderstandingofhowprotectivealuminaand chromiascalesbehave.Similarly,newinvestigationsintowatervapoureffects onscalingprocesseshaveexpandedandrefinedourknowledge,althougha simple,coherentpictureremainselusive.Thesecontributions,andseveral others,havebeendrawnuponinupdatingtheoriginaltext.
Twonewtopicshavebeenadded,reflectingthelargebodyofpublished researchnowavailableandthetechnologicaldevelopmentswhichdrovethat work.Chapter10treatscorrosionbycarbondioxide,animportantissueifCO2 istobecapturedfromcombustiongasstreams.Inaddition,thethermal propertiesofcarbondioxide,alongwithitspressure-volume-temperature characteristics,makeitattractiveasaheattransferandworkingfluid.Forthese reasons,itisacandidateforuseinnuclearreactorsandconcentratedsolar thermalpowergeneration.Unfortunately,itisalsocorrosivetoavarietyof alloys.
AnewChapter12, CorrosioninComplexEnvironments,isconcernedwith thecorrosionphenomenaarisingfromthepresenceofionicmeltsandvolatilisinghalides.Interestinthesetopicshasarisenoutofthemuchincreased useofbothbiomassandmunicipalwasteasfuelsforthermalpowergeneration.Theresultingfluegasesanddepositscanberemarkablycorrosive,and boileroperatingtemperaturesarestrictlylimitedasaresult.
Asinthefirstedition,Ihavetriedtoacknowledgeimportantcontributions toourunderstandingmadebymanyresearchers,andIapologiseforany omissions.Thesecondeditionhasbenefitedfromcolleaguesaroundtheworld whohaveofferedhospitalityand/orgenerouslygaveexpertcommentary: BrianGleeson(UniversityofPittsburgh),DanielMonceau(INPT-CIRIMAT, Toulouse),BrucePint(OakRidgeNationalLaboratory),JoeQuadakkers (Forschungszentrum,Ju ¨ lich),MichaelSchutze(Dechema,Frankfurt)andJim Smialek(NASA,Lewis).Rectifyinganimportantomissionfromthefirstedition,Ithankmywifeandfamilyfortheirsupportandremarkableforbearance. DavidJ.Young
Preface Almostallmetalsandalloysoftechnologicalinterestoxidiseandcorrodeathigh temperatures.However,thenatureoftheirreactionproductsandtheratesat whichmetalsurfacesaredegradedvarywidely,andacapacityforpredictionis highlydesirable.Thisbookisconcernedwithprovidingafundamentalbasisfor understandingthealloy-gasoxidationandcorrosionreactionsobservedin practiceandinthelaboratory.Itspurposeistoenablethepredictionofreaction morphology,kineticsandrateasafunctionoftemperatureandthecomposition ofbothalloyandgas.
Theterm‘oxidation’isusedinagenericsenseforanychemicalreaction whichincreasesthemetaloxidationstatebyformingacompoundsuchasan oxide,sulphide,carbide,etc.Alloyoxidationreactionscanbeconceivedofas occurringinthreestages.Initially,allreactivecomponentsofanalloyincontact withahotgasarelikelytoreactsimultaneously.Subsequently,morethermodynamicallystablecompoundsreplacelessstableones,andastateofnear equilibriumislocallyapproached.Thereactingsystemcanthenbemodelledasa seriesofspatiallyadjacentlocalequilibriumstateswhichvaryincrementallyin reactantchemicalpotentials.Duringthisstage,thereactionmorphologyand compositiondistributionareinvariantwithtime.Ultimately,this‘steadystate’is lost,andallreactivealloycomponentsareconsumedinafinalbreakdownstage.
Successfulalloysarethosewhichevidencelengthyperiodsofslow,steadystatereaction.Forthisreason,considerableemphasisisplacedonanalysingthe underlyinglocalequilibriumconditionandtestingitsapplicabilitytoparticular metaloralloy-oxidantsystems.Whenanalloy-gasreactionisatasteadystate, theconstantcompositionprofiledevelopedthroughthereactionzonecanbe mappedontotherelevantsystemphasediagramasa‘diffusionpath’.Frequent useismadeofthesepathsinunderstandingreactionproductdistributionsandin predicting,oratleastrationalising,reactionoutcomes.
Analysisofthealloyoxidationproblemrequiresamultidisciplinary approach.Physicalmetallurgy,materialsscienceandphysicalchemistryprovide thetoolswithwhichtodissectalloyphaseconstitutionsandtheirtransformations,oxidepropertiesandchemicalkinetics.Deliberateemphasisis placedontheuseofchemicalthermodynamicsinpredictingoxidationproducts anddescribingsolidsolutionphases.Equalattentionispaidtothedetailedunderstandingofdefect-baseddiffusionprocessesincrystallinesolids.TheintroductoryChapter1indicateshowthesevariousdisciplinescancontributetothe
analysis.ThelengthyChapter2reviewsthethermodynamic,kineticand mechanicaltheoriesusedinthisbook.Italsocontainstabulateddataandrefers toAppendicesonalloycompositionanddiffusion.
Afterthesepreliminaries,thebookisarrangedinasequenceofchapters reflectingincreasingcomplexity,whichequateswithgreatersystemcomponent multiplicity.Ananalysisofthereactionbetweenpuremetalsandsingleoxidant gasesisfollowedbyadiscussionofmetalreactionswithmixedoxidantgases andthen,inChapters5 7,anexaminationofalloyreactionswithasingle oxidant.MuchofthisdiscussionisbasedontheearlyworkofCarlWagner, whichstillprovidesagoodconceptualframeworkand,inseveralcases,auseful analyticalbasisforquantitativeprediction.However,aswillbeshown, increasingsystemcomplexityisaccompaniedbyaweakeningintheoretical completeness.Theproblemsarisefrommulticomponenteffectsandfrom microstructuralcomplexity.
Considerfirsttheeffectofincreasingthenumberofalloycomponents.A steady-statereactingsystemconsistingofabinaryalloyandasingleoxidantcan bemodelledinatwocoordinatedescriptionofboththermodynamicsand diffusionkinetics,providedthattemperatureandpressureareconstant.Substantialthermodynamicanddiffusiondataisavailableformanysuchsystems, andthisisusedindevelopingdiffusionpathdescriptions.Increasingthenumber ofalloycomponentsleads,however,tochemicalandstructuralinteractions amongthem,renderingtheexperimentalproblemmuchlesstractable,and diagrammaticrepresentationimpossible.Intheabsenceoftherequisiteextensivethermodynamicanddiffusiondata,theWagnertheorycannotbeapplied. Instead,higherorderalloysarediscussedfromthepointofviewofdilute additioneffectsonthebehaviourofbinaries.
Wagner’stheoryisbasedonlatticediffusion.However,thetransport propertiesofslow-growingoxidesarelargelydeterminedbytheirgrain boundariesand,insomecasesperhaps,microporosity.Additionalalloycomponentscanaffectboththeoxidegrainsizeandthediffusionpropertiesofthe grainboundaries.Adescriptionofthesephenomenais,atthisstage,largely empirical.
Thelatterpartofthebookisconcernedwiththeeffectsofothercorrodents andtemperaturevariations.Chapters8and9dealwithsulphurandcarbonbearinggases.Theveryrapiddiffusionratesinvolvedinsulphidationandcarburisationmakesthempotentiallythreateningcorrosionprocessesinanumber ofindustrialtechnologies.Offundamentalinterestarethecomplicationsarising outofthecomplexgas-phasechemistriesandthesometimesslowhomogeneous gas-phasereactions.Itbecomesnecessaryindiscussingthebehaviourofthese gasmixturestoconsidertheroleofcatalysts,includingthealloysinquestionand theircorrosionproducts.Itemergesthatnotonlythegasphase,butalsothegassolidinterfacecanbefarremovedfromlocalequilibrium.Inparticular,analysis ofthecatastrophic‘metaldusting’corrosioncausedbycarbon-supersaturated gasescallsfortheuseofnonequilibriummodels.
TheeffectsofwatervapouronoxidationarediscussedinChapter10.In manyrespectsthisistheleastwellunderstoodaspectofhigh-temperature corrosion.Thereasonforthedifficultyistobefoundinthemultiplewaysin whichwatermoleculescaninteractwithoxides.Preferentialadsorption, hydrogenuptake,latticedefectchanges,grainboundarytransportproperty changes,gasgenerationwithinoxideporesandscaleandscale-alloyinterface mechanicalpropertychangesneedalltobeconsidered.
Finally,theeffectsoftemperaturecyclingonoxidescalegrowthare consideredinChapter11.Acombinationofdiffusionmodellingwitharather empiricalscalespallationdescriptionisfoundtoprovideareasonablysuccessful wayofextrapolatingdataforparticularalloys.However,thereisaneedfor developmentofmorepredictivedescriptionsoftherelationshipbetweenspallationpropensity,alloypropertiesandexposureconditions.
Discussionisfocusedthroughoutondevelopinganunderstandingofthe fundamentalsofhigh-temperatureoxidation.Frequentuseismadeofexperimentalinformationonrealalloysinordertoillustratetheprinciplesinvolved. However,noattemptismadetosurveytheveryextensiveliteraturewhichexists foralloyoxidation.Thusmostexamplesconsideredconcerneitheriron-or nickel-basealloys,whereascobalt-basealloysarelargelyignored.Nickelaluminidesarediscussed,butotherintermetallicsareseldommentioned.Thescope ofthebookisfurtherlimitedbytheexclusionofsomeparticulartopics. Examplesinclude‘pesting’(disintegrationbygrainboundaryattack)ofsilicides, andextensiveoxygendissolutionbymetalssuchastitaniumandzirconium.No bookofmanageableproportionscaneverbecomplete,orevenfullyuptodate.
Itisremarkablethatsincetheearly,verysubstantialprogressmadebyCarl Wagnerandassociatesinunderstandingoxidationphenomena,theresearch efforthasnonethelesscontinuedtoexpand.Thereason,ofcourse,isthe continuingneedtooperateequipmentateverhighertemperaturestoachieve greaterefficienciesandreducedemissions.Theneedtodevelopsuitablematerialscanbeexpectedtodriveevenmoreresearchinyearstocome.
Writingthisbookhasbeenalargetask,anditscontentinevitablyreflectsmy ownexperience,aswellastheideasandresultsofothers.Ihavetriedto acknowledgeimportantcontributionstoourunderstandingmadebymany researchers,andapologiseforanyomissions.Myownresearchinthisareahas benefitedfrominteractionwithmanytalentedstudents,researchfellowsand colleagues,allacknowledgedbydirectreference.Ithasalsobeensustainedin largepartbytheAustralianResearchCouncil,abodytobecommendedforits willingnesstosupportfundamentalresearch.Thisbookhasbenefitedfrom colleaguesfromaroundtheworldwhoofferedhospitalityand/orgenerously gaveexpertcommentaryasIwrote:BrianGleeson(IowaStateUniversity),Jack Kirkaldy(McMasterUniversity),DanielMonceau(CIRIMAT,Toulouse), ToshioNarita(HokkaidoUniversity),JoeQuadakkers(Forschungzentrum, Julich),JimSmialek(NASA,Lewis)andPeterTortorelli(OakRidgeNational Laboratory).
xviii Preface
Finally,Iacknowledgewithgratitudeandaffectiontheinspirationprovided bymymentorsandfriendsatMcMasterUniversity,WaltSmeltzerandJack Kirkaldy.
DavidJ.Young
August2007
AbbreviationsandAcronyms APT Atomprobetomography
CTGA Continuousthermogravimetricanalysis
CVD Chemicalvapourdeposition
EBSD Electronbackscattereddiffraction
EDAX EnergydispersiveanalysisofX-rays
EELS Electronenergylossspectroscopy
EPMA Electronprobemicroanalysis
FIB Focusedionbeam
IGCC Integratedgasificationcombinedcycle
ppm Partspermillion(unitofrelativeconcentration)
ppma Partspermillionbyatoms
ppmm Partspermillionbymass
PVD Physicalvapourdeposition
SAD Selectedareadiffraction
SCC SupercriticalCO2
SEM Secondaryelectronmicroscope
SIMS Secondaryionmassspectrometry
TBC Thermalbarriercoating
TEM Transmissionelectronmicroscope
TGA Thermogravimetricanalysis
TGO Thermallygrownoxide
XPS X-rayphotoelectronspectroscopy
XRD X-raydiffraction
YSZ Yttria-stabilizedzirconia
Symbols GREEKSYMBOLS a Coefficientofthermalexpansion
a Enrichmentfactorformetalininternaloxidationzone
d Thicknessofgasphaseboundarylayer
d Deviationfromstoichiometryinoxide
hi Electrochemicalpotentialofcomponent i
hg Viscosityofgas
g Surfacetension,freeenergyperunitsurfacearea
gi Activitycoefficientofcomponent i
l Interplanardistance,jumpdistance
l x =t 1 2 ,forparametricsolutionstoFick’sequation
mi Chemicalpotentialofcomponent i
n Stoichiometriccoefficientinchemicalreactionorcompound
vg Kinematicviscosityofgas
niv Kineticfrequencyterm
vP Poisson’sratio
j Electrostaticpotential
r Density
s Mechanicalstress
q Fractionofsurfacesites
x Extentofreaction
x Molefractionofoxide BO insolidsolution A1 x Bx O
εc Criticalstrainformechanicalfailureofscaleorscale-alloyinterface
εik Wagnerinteractioncoefficientsforsolutecompounds i and k
εOX Mechanicalstraininoxide
SYMBOLS
A Surfaceareaofoxidisingmetal
ai Chemicalactivityofcomponent i
a0 o ; a00 o Boundaryvaluesofoxygenactivityatmetal-scaleandscale-gas interfaces
Bi Mobilityofspecies i
Ci Concentrationofcomponent i
C 0 , C 00 Boundaryvaluesofconcentrationatmetal-scaleandscale-gasinterfaces.
D Diffusioncoefficient
d Grainboundarywidth
DA Intrinsicdiffusioncoefficientforspecies A
DA* Tracerorself-diffusioncoefficientofspecies A
Dij Diffusioncoefficientrelatingfluxofcomponent i toconcentration gradientincomponent j
D Chemical(orinter)diffusioncoefficient
Do Diffusioncoefficientforsoluteoxygeninalloy
Do,i Diffusioncoefficientforoxygenalonganinterface
E Electricfield
EOX Elasticmodulusofoxide
EA Activationenergy
e0 Freeelectron
F TheFaraday(96,500C)
f Fraction
fv Volumefraction
G TotalormolarGibbsfreeenergy
GOX Shearmodulusofoxide
Gv Freeenergyperunitvolume
gBO Volumefractionofinternallyprecipitatedoxide,BO
H Totalormolarenthalpy
hl Positivehole
ijS Species i adsorbed(bound)tosurfacesite iozInternaloxidationzone
Ji Fluxofcomponent i
K Chemicalequilibriumconstant
k Rateconstant
k Boltzmann’sconstant
kc Parabolicrateconstantformetalconsumption,corrosionrateconstant
kl Linearrateconstantforscalethickening
km Gaseousmasstransfercoefficient
ks Surfaceareafractionofoxidespalled
k ði Þ p Parabolicrateconstantforinternaloxidation
kp Parabolicrateconstantforscalethickening
kw Parabolicrateconstantforscalingweightgain
kv Vaporisationrate
Kp Equilibriumconstantatfixedpressure
Ksp Solubilityproduct
KIC Fracturetoughness,criticalstressintensityfactor
Lij Generalmobilitycoefficient,Onsagerphenomenologicalcoefficient
L Lengthofmaterialoverwhichgasflows
l Halfthicknessofalloysheet
MWMolecularweight
mi Molarconcentrationofcomponent i
ml , m0 Numberofchargeunitsonlatticepointdefectspecies
n Numberofmoles
Ni Molefractionofcomponent i
NAV Avogadro’snumber
NM,i MolefractionofcomponentMatscale-alloyinterface
NM,min MinimummolefractionofcomponentMrequiredtosupportgrowthof externalMOscale
N ðoÞ M MolefractionofcomponentMoriginallypresentinalloy
N ðsÞ O Molefractionofdissolvedoxygenatalloysurface
P Pressure
pDA/DB,ratioofmetalself-diffusioncoefficientsinternaryoxide
pi Partialpressureofcomponent i
PT Totalpressureofgasmixture
Q Activationenergy
q Charge
R Generalgasconstant
ri Rateconstantforindicatedgas-solidreaction
S Totalormolarentropy
S Spacingofperiodicmicrostructure
S Surfacesite
S X M Species S locatedoncrystallatticesiteM,witheffectivecharge X
T Temperature
t Time
t *Timeattemperatureincyclicexposureconditions
U Totalormolarinternalenergy
Ui Buildingunitincrystallinecompound
V Volume
v Velocity
Vi Molarvolumeofphase i
W Weight
X Scalethickness
x Positioncoordinate
XM Metalsurfacerecession
Xss Steady-statescalethicknesswhengrowthbalancedbyevaporation
X(i) Depthofinternaloxidationzone
y Positioncoordinateforscale-alloyinterfacerelativetotheoriginal, unreactedsurfacelocation
yz/zs (or x/X),positionwithinscalenormalisedtoitsthickness
Z Effectivecharge,valence
z Positioncoordinateinreferenceframewithoriginatscale alloy interface
TheNatureofHigh TemperatureOxidation Athightemperatures,mostmetalswillinevitablyoxidiseoverawiderangeof conditions.Thepracticalissuesofmateriallifetimesandcorrosionprotection methodsthereforecentrearoundtherateofoxidation,andhowtocontrol reactionmorphology.Answerstothesecondquestionturnouttoberather interestingandinvolvetheneedforafundamentalunderstandingoftheprocessesinvolvedandwaystomodifythem.Thegeneralnatureoftheproblem canbeappreciatedfromaconsiderationofsomepracticalexamples.
1.1METALLOSSDUETOTHESCALINGOFSTEEL Carbonsteelisproducedinprodigiousquantities(about1.7 109 tworldwide in2014).Almostallofitiscastintolargepiecessuchasslabs,whichare subsequentlyreheatedtoaround1000 1200 Ctobeformedintomoreuseful shapes(Fig.1.1).Thereheatingoperationiscarriedoutindirectfiredfurnaces wheresteelworksgases,orsometimesnaturalgas,arecombustedwithexcess air.Thecombinationofhightemperature,heatingtimesofaroundtwohours, andoxidisinggasesleadstothegrowthofathickironoxidescaleonthesteel. Theamountofsteelconsumedinthiswayisabout1 2%ofthetotal. Obviously,withsteellossesof17 34Mtin2014,plustheaddedcostof removingthescaleandrecyclingit,thereisconsiderableeconomicmotivation tocontrolorslowthisprocess.However,therearedifficulties.
Asdiscussedlater,andasisintuitivelyreasonable,thesteelscalingrate dependsonthreevariables:steelchemistry,temperatureandthegas
atmosphere.Thefirstcannotbechanged,becauseitiscriticaltothefinalsteel properties.Temperatureisdeterminedbysteelchemistryandisthereforealso fixed.Changesingascompositionshould,however,bepossible.Thereactions producingthefurnaceatmospherescanbedescribedas
where x representsthesurplusofoxygenabovestoichiometricrequirements forcompletecombustion.Innormalpractice,excessair(x > 0)isusedto ensurecompletecombustion.However,itwasrecognisedlongago [1] thatfor x < 0,theatmospherewouldbemuchlessoxidisingandtheextentofscaling mighttherebybelessened.
Inanalysingthissuggestion,werecognisethatitisnecessarytocalculatethe furnacegaspartialpressureofoxygen, pO2 ,asafunctionof x andtemperature, thatthepossibleoxidesofironmustbeidentified,andthattherangesof pO2 valuesatwhichtheyexistneedtobeestablished.Thenecessary pO2 valuescanbe calculatedfromtheequilibriumof reactions[1.1]and[1.2] andthoseoftheiron oxideformationreactions,usingthetechniquesofchemicalthermodynamics describedinChapter2.Suchananalysisshowsthatitisnotpossibletolower pO2 belowthevalueatwhichironoxidisesandstillhavesufficientcombustiontoheat thesteel.Giventhatsteelscalingcannotbeprevented,itisimportanttoknowhow therateofscalegrowth(andsteelconsumption)varieswith pO2 andtemperature.
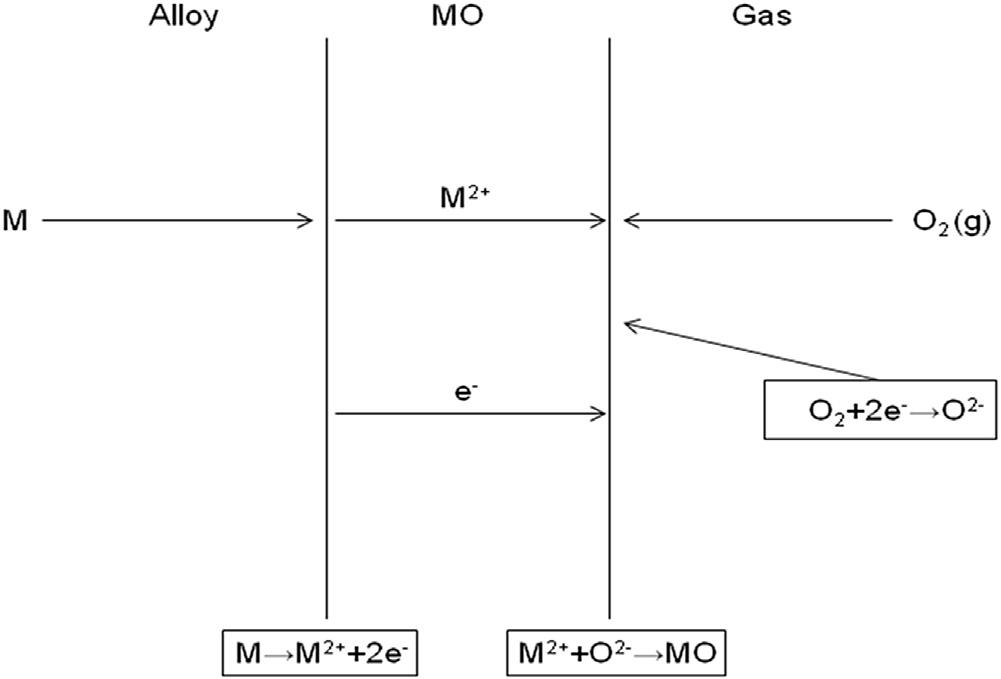
Aschematiccross-sectionalviewofagrowingoxidescaleisshownin Fig.1.2.Theoveralloxidationprocesscanbesubdividedintoseveralsteps:
1. Deliveryofoxidanttothescale gasinterfaceviamasstransferinthegas phase.
2. Incorporationofoxygenintotheoxidescale.
3. Deliveryofreactingmetalfromthealloytothealloy scaleinterface.
4. Incorporationofmetalintotheoxidescale.
5. Transportofmetaland/oroxygenthroughthescale.
Evaluationoftheratesatwhichthesestepsoccurinvolvescalculationof thegasphasemasstransfer,solid-statemasstransferordiffusionintheoxide andalloyandconsiderationoftheinterfacialredoxreactions
wheree representsanelectron.Theredoxreactionsarerapidanddonot usuallycontributetoratecontrol.Otherscale gasinteractionscanbedealt
FIGURE1.2 Reactionsandtransportprocessesinvolvedinthegrowthofanoxidescale.
withusingthemethodsofsurfacechemistry.Gasphasemasstransferratescan becalculatedfromthemethodsoffluiddynamics,whilstmasstransferinthe solidoxideandalloyisdescribedusingdiffusiontheory.
Theprincipalconstituentofanironoxidescaleat T > 570 Ciswu ¨ stite, FeO,inwhichtheFe2þ speciesdiffusesrapidlyathightemperatures.Athigh valuesof pO2 ,diffusioninFeOcontrolstherateatwhichthisoxideaccumulates [2].However,inacombustiongas,where pO2 canbequitelow,reactionwiththe oxidantspeciesCO2 and/orH2Oisslowerthanwustitediffusion,andcontrols thescalingrate [3].Thusitappearspossiblethatsteelscalingcanbeslowedby operatingreheatfurnacesundersubstoichiometriccombustionconditions.Of course,theeconomicfeasibilityofthisprocessalterationwouldhavetobe establishedthroughquantificationoftheactualbenefittobeexpected(aswellas thecosts).Suchanexerciserequirestheabilitytopredictscalingratesasa numericalfunctionofprocessvariables,aprincipalconcernofthisbook.
1.2HEATINGELEMENTS Theuseofmetalsaselectricalresistanceheatingelementsiscommonplacein smalldomesticappliancesandlaboratoryfurnaces.Ofcoursethemetalsused mustresistoxidationinair.Twogroupsofalloysarewidelyusedforthispurpose:nickelalloyscontainingaround20w/o(weightpercent)chromiumand ironalloyscontainingabout20w/oCrand5w/oAl.Aspuremetals,eachofFe, Ni,CrandAloxidisesinair,butatvastlydifferentrates.Oxidationratemeasurementsarediscussedlaterinthischapter,butforthemoment,itissufficientto useacomparisonofdifferentoxidescalethicknessesgrowninaparticulartime. Datafor100hreactionat800 CinpureO2 at1atmareshownin Table1.1. Itisclearthatpureironwouldbequiteunacceptableasaheatingelement, andthataluminiumandchromiumappearmuchmoreattractive.However,
4
TABLE1.1 MetalOxideScaleThicknesses
thesearenotpracticalchoices:aluminiummeltsat660 Candpurechromium isbrittleandcannotbeformedatroomtemperature.Nickelhasneitherof thesedeficienciesandmighthaveanacceptablescalingrateforsomeapplications.However,likemostmetalsinthepurestate,nickelhasquitepoorhigh temperaturestrengthandcannotbeused.Ontheotherhand,appropriate alloyingcanprovidebothstrengthandoxidationresistance.
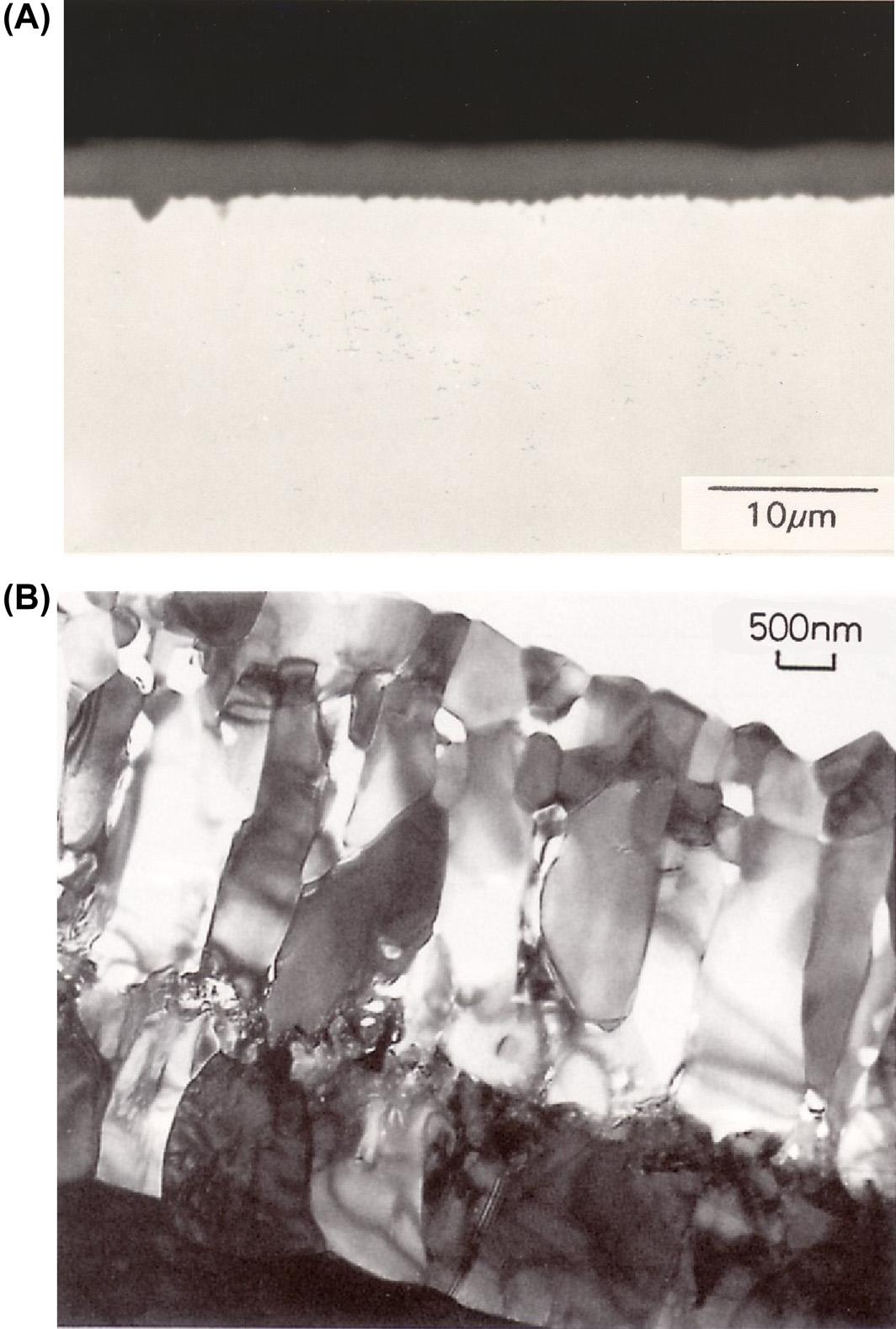
Cross-sectionalviewsofoxidisedsurfacesofNi-28CrandFe-20.1Cr5.6Al-0.08Laalloys(allcompositionsinweight%)areshownin Fig.1.3. Single-phaseoxides,Cr2O3 andAl2O3,respectively,growasalmostuniform scales,providingsatisfactorilyslowalloyconsumptionrates.Itwouldbe usefultobeabletopredictwhatconcentrationsofchromiumandaluminium arerequiredtoachievetheirpreferentialoxidationandtherebyavoidreaction ofthenickeloriron.Todealwiththissituation,itwillbenecessarytoconsider thethermodynamicsofcompetitiveoxidationprocessessuchas
þ 3NiO ¼ Cr2 O3
whereunderliningindicatesthemetalispresentasanalloysolute.Anadditionalfactorcanbeexpectedtocomplicatethisprediction.Selectiveoxidation ofametalimpliesitsremovalfromthealloyandaloweringofitsconcentrationatthealloysurface.Thusitwillalsobenecessarytoconsiderthe diffusionprocessesinbothalloyandoxide.
1.3PROTECTINGTURBINEENGINECOMPONENTS Thegasturbineenginesusedtopropelaircraftandtogenerateelectricpower havebeendevelopedtoaremarkableextentsincetheirinventioninthemid20thcentury.Asshownin Fig.1.4,fueliscombustedwithinaturbineto producealargevolumeofhotgas.Thisgasimpingesonangledbladesinthe hot(turbine)stageoftheengine,causingittorotateanddrivethecompressor stage,whichdrawsinairtosupportcombustion.Powerisobtainedfromthe engineeitherasrotationalenergyviaadriveshaft,orasthrust,generatedby thejetofhotexhaustgas.
2Cr
þ 3Ni[1.5]
FIGURE1.3 Cross-sectionofslow-growingprotectivescales:(A)OpticalmicrographofCr2O3 onNi-28Crafter24hat900 Cand(B)Brightfieldtransmissionelectronmicroscopyviewof Al2O3 onFe-20Cr-6Al-0.08Laafter400hat1150 C [4] PublishedwithpermissionofScience Reviews.
Theefficiencyoftheengine,whichistheproportionofthethermalenergy convertedtomechanicalpower,isrelatedtothetheoreticalmaximumwork available,givenby
max ¼ T To T q [1.6] where q istheheatexchanged, To istheambienttemperatureand T theoperating temperature.Itisclearthatthehighertheturbineoperationtemperature,the
greateristheefficiencypotentiallyavailable.Sincehigherefficiencyisthe equivalentoflowercostandlessgreenhousegasproductionperunitofoutput, itsdesirabilityhasdrivenasteadyincreaseinturbinegastemperatures.However,becausethistemperatureislimitedtowhateverthematerialsofthefirsthot stagecomponentscanwithstand,anincreaseinmaterialscapabilityhasalso beennecessary.
Thehistoryofdevelopmentsinturbinebladematerialsandthetemperaturesatwhichtheyhaveoperatedaresummarisedin Fig.1.1.Inadditionto alloycompositionalchanges,thedevelopmentofthesematerialshasseenan evolutioninproductiontechnologyfromwroughtthroughconventionallycast anddirectionallysolidifiedtosinglecrystalproduction.Currenthotstage materialsarenickel-basedsuperalloys,whichpossessexcellenthightemperaturestrength.Thisisnecessarytowithstandtheenormouscentrifugalforces generatedbythehighrotationalspeeds,around10,000rpminthecaseofjet engines.Themetallurgicaldesignwhichprovidesthestrengthofthesesuperalloysissuchthattheyoxidiseatunacceptablyrapidratesatoperating temperature.Thisproblemhasbeensolvedbyprovidingacoatingofoxidation resistantalloyonthecomponentsurfaces.
Turbinetemperaturesarenowexceedingthecapabilitiesofsuperalloy components,andithasbecomenecessarytocoolthem.Thisisdoneby pumpingairorsteamthroughcoolingchannelsrunningthroughthecomponent interiorsandprovidingthermalinsulation(athermalbarriercoatingorTBC)on topoftheoxidation-resistantcoating.Thewholeassemblyisshownschematicallyin Fig.1.6.TheTBCistypicallyaceramicmadeofY2O3-stabilisedZrO2; theoxidationresistantcoating,knownasabondcoat,isanaluminium-rich material(severaldesignsarepossible);andthesuperalloysarecomplex, nickel-basedalloyscontainingchromium,aluminiumandnumerousotherelements.Someexamplesofsuperalloyandbondcoatcompositionsaregivenin Table1.2.AdditionalsuperalloycompositionsareshowninAppendixA.
FIGURE1.4 Schematicdiagramofgasturbineengine.
S.C(NIMS)
FIGURE1.5 Progressiveincreasesintemperaturecapabilitiesofsuperalloysforturbineengine blades. ReproducedwithpermissionoftheNationalInstituteofMaterials(NIMS),Japan.
FIGURE1.6 Cross-sectionalviewofTBCsystemforgasturbineblade.
Manufactureofthesesophisticatedcomponentsiscomplex.Thesuperalloy itselfiscast,usingadirectionalsolidificationprocess,oftenasasinglecrystal [5].Thebondcoatcanbeappliedinvariousways [6].Chemicalvapour deposition(CVD),inwhichaluminiumfromavapourphasespeciesdiffuses intothealloysurface,formsanaluminidediffusioncoating.Thesecoatingscan bemodifiedbytheincorporationofplatinumandthecodepositionofadditional
metalsfromthevapourphase.MorecomplexcoatingchemistriescanbeachievedbyphysicalcodepositionofvariousMCrAlYcompositionsinwhichM indicatesFe,NiorCo,oramixturethereof.Thesecoatingsaredepositedby sputtering,plasmasprayingorphysicalvapourdeposition,usingahighvoltage electronbeamtovapourisethesourcematerial.Theoutersurfaceofthebond coatisoxidisedtoformathermallygrownoxide(TGO)whichisthesurfaceto whichtheTBCadheres.Thethermalbarriercoatingisdepositedbyeither electronbeamphysicalvapourdepositionorplasmaspraying [7]
Athightemperatures,variousinteractionsbetweenthesematerialscanbe expected.Interdiffusionbetweenthesuperalloyanditsaluminium-richcoating canproducenewphasesaswellasdrainingthecoatingofitsessential aluminium.SomebondcoatconstituentsandmetalsdiffusingfromthesuperalloythroughthebondcoatcandissolveintheTBCtoformmixedoxides. Understandingandpredictingtheseinteractionsrequiresknowledgeofthe phaseequilibriarelevanttoeachparticularsystem.Finally,becausetheTBCis porous,oxygenfromthehotcombustiongaspenetratestothebondcoat surface,causingoxidescalegrowth.Ahighdegreeofresistancetothis oxidationprocessisanessentialfunctionofthebondcoat.Alloftheseprocessesareaccompaniedbyvolumechanges,whichhavethepotentialto mechanicallydisruptthejunctionbetweentheTBCandtheunderlyingoxide scale.ThisinturncanleadtopartialorevencompletelossoftheTBC, subsequentoverheatingofthesubstratemetalandcomponentfailure.Inorder topredictandtherebymanagetheseconsequences,itisnecessarytounderstandthedetailedmechanicsofstressdevelopmentwithinthesuperalloy substrate-bondcoat-TGO-TBCsystemandthewaysinwhichthatstressis accommodatedbydeformationorfractureofoneormoreofthesystem components.
1.4HYDROCARBONCRACKINGFURNACES Manychemicalandpetrochemicalprocessesareoperatedathightemperatures inordertoachievereasonableproductionratesor,asincrackingfurnaces,to promoteendothermicreactions.Cracking(orpyrolysis)furnacesareusedto produceolefinessuchasethyleneandpropylene,whicharesubsequentlyused tomakethecommoditymaterialspolyethyleneandpolypropylene.The crackingreactioncanbewritten
andisaccompaniedbycarbonformation:
Toslowthelatterreaction,steamisaddedtothehydrocarbonfeedstock. Thehydrocarbon-streammixtureisheatedbypassingitthroughatube whichissuspendedwithinafirebox.Asseenin Fig.1.7,tubeunits(orcoils)
arelarge.Thetubesarearound100mmdiameter,with10mmwallthickness andabout10mlong.Thesetubesareexpectedtosurviveforfiveyearsor morewhilstoperatingatwalltemperaturesranginguptoabout1100 C.They mustthereforepossessadequateresistancetocreepdeformation(undertheir ownweight),tooxidationoftheirexternalsurfacebycombustiongasandto attackbybothcarbonandoxygenontheirinnersurface.
Thematerialsusedforpyrolysisfurnacetubesarecentrifugallycastheat resistingsteelsornickelbasealloys,allausteniticalloyscontaininghigh chromiumlevels.Processeconomicsareenhancedbyhigheroperatingtemperatures,creatingademandforimprovedheat-resistantalloys.Thisdemand hasdrivenashiftinmaterialselectionforthecentrifugallycasttubesfromHK grade(25%chromium,20%nickel)toHPgrade(25%chromium,35%nickel) steel,andmorerecentlytoalloyscontaining45or60%nickelandaround25% chromium.Thesehighernickellevelsareintendedtoachievehighercreep
FIGURE1.7 Pyrolysistubeunitbeinginstalledinsteamcrackerfurnace.
strength.Considerationoftheprocessgascompositionrevealsthattheoxygen partialpressureiscontrolledbytheequilibrium
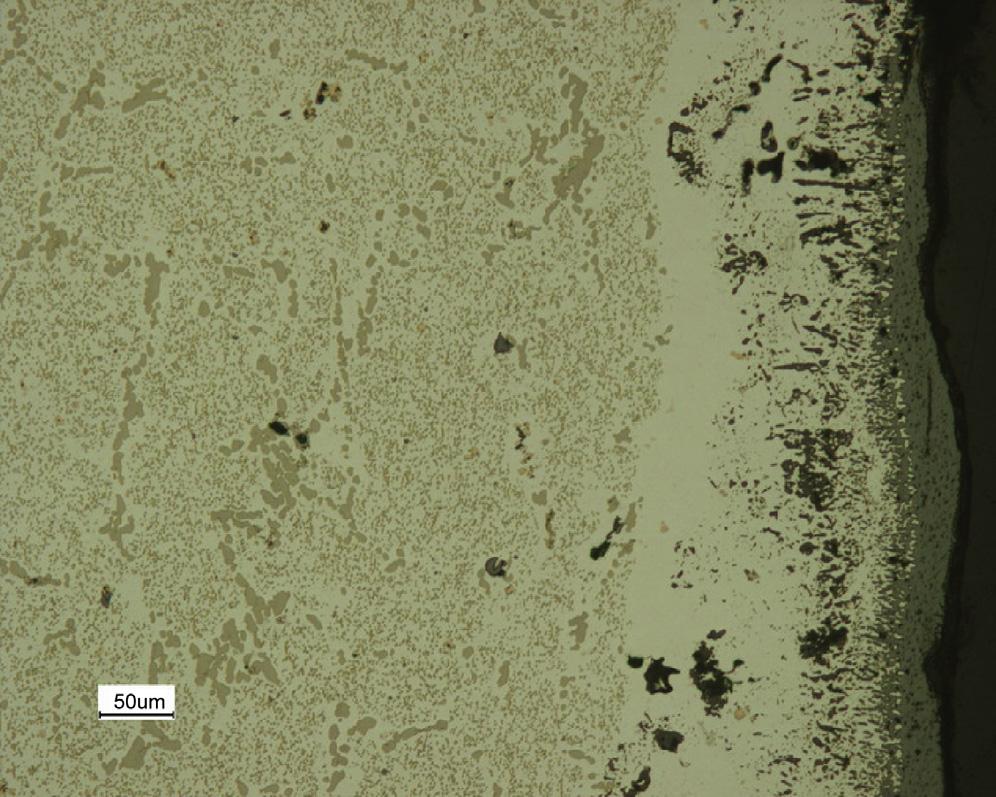
and pO2 z10 24 atmat1000 C.Thecarbonactivityiscontrolledby reaction [1.8] ,and ac ¼ 1.Undertheseconditions,thema inalloyconstituentwhichis reactiveischromium,andallofthecompoundsCr2O 3,Cr7C3 andCr23 C6 are possibleproducts.Thepracticalfindingsarethatanexternalchromiumrichoxidescalegrowsearlyinthelifeofthetube,butthatchromium carbidesprecipitatewithinthealloy,be neathitssurface,la teron.Theresults ofalaboratorysimulationoftheprocessareshownin Fig.1.8.Questions arisingfromtheseobservationsonwhathappenstothealloymightinclude thefollowing.Whydothealloyconstit uentsotherthanchromiumapparently notreact?Whyarethecarbidesformedasdispersedprecipitatesandnotas scalelayers?Whyarecarbidesformedbeneaththeoxideandnotviceversa? Howdoescarbonpenetratetheoxidelayertoreachthealloyinteriors?Why istherealayerofapparentlyunreactedalloyimmediatelybeneaththescale? Inaddition,andasalways,wewishtoknowtheratesatwhichscalegrowth andinternalcarbideprecipitationoccur,andhowtheserateswillvarywith changesintemperature,alloycompositionandgasconditions.
Toanswerthesequestions,itisnecessarytoconsiderfirstthechemical thermodynamicsgoverningreactionsbetweenametalandtwodifferentoxidants.Secondly,adescriptionoftheratesofmasstransferofchromium,
FIGURE1.8 Cross-sectionofcastheat-resistingsteel(HPModgrade)afterlaboratoryexposure tosteam-hydrocarbonmixtureat1100 Cfor500cyclesofonehoureach.
oxygenandcarbonwithinthesolidphasesisrequired.Finally,aknowledgeof theprocesseswherebyprecipitatesnucleateandgrowwithinmetalsisneeded, alongwithanabilitytopredictwhichprecipitatephasescancoexistwith whichalloycompositions.
1.5PREDICTIONANDMEASUREMENT Emergingfromaconsiderationoftheexamplesaboveistheneedtopredict whichreactionproductsresultfromhightemperatureoxidation(orcarburisation,sulfidation,etc.),whetherthoseproductsareformedasexternalscale layersorinternalprecipitates,howfasttheyformandwhattheirmechanical stabilitywillbe,allasfunctionsofalloycomposition,temperatureandgas conditions.Thetheoreticalbasisfortherequisitepredictivemethodologiesare reviewedinChapter2.Thenecessarythermodynamic,kineticandmechanical dataarenotalwaysavailableforcomplex,multicomponentsystems,and furtherexperimentalinvestigationwillbenecessary.Nonetheless,theoretical predictionisstilluseful,asitprovidesqualitativeindicationsoftheexpected effectofexperimentalvariables.Evenifthesearenomorethanhypotheses, theyprovidearationalframeworkforexperimentaldesign,therebyenabling efficientplanningoflaboratoryinvestigations.
Atthesametime,itisadvisabletobeawareofthepossibilitiesaffordedby modernexperimentaltechniques.Usefultheoriesprovidepredictionswhich canbetested,andthemorethoroughlywecantestatheory,themoreconfidencewearelikelytohaveinit.Theoreticaltreatmentsshouldthereforebe exploredwiththeaimnotonlyofachievingthedesiredperformancepredictions,butalsooffindingotherimpliedoutcomeswhichcanbemeasured. Thepointhereisthat‘performance’intermsofcomponentlifetimemightbe tensorevenhundredsofthousandsofhours.Otherpredictedresults,suchas compositional,microstructuralorphaseconstitutionalchangeinalloyorreactionproduct,willbeevidentmuchmorerapidly.Theirverificationtherefore providesanearlyindicationoftheprobabilityofoxidationlifetimebeing achieved.
1.5.1OxidationRates Thecourseofanoxidationreaction
followsakineticratelaw
where x isameasureoftheextentofreactionattime, t.Thus
where n isthenumberofmoles.Itisnecessarytodeterminethequantitative formofthefunction f(t).
Inprinciple,areactioncanbefollowedbymeasuringconsumptionofmetal oroxygenorbyobservingoxideaccumulation,asafunctionoftime.Iftheoxide isagas,thenmetalconsumptioncanbefollowedcontinuouslybyattachingthe metalsampletoabalanceofappropriatesensitivity,heatingitinthereactiongas andmeasuringtheweightloss.Anapparatussuitableforthisexperimentis shownin Fig.1.9.Inthemorecommoncase,theoxideissolid,andmetal consumptioncannotbedirectlyobservedinthisway.Instead,ametalsample couldbereactedforatime,andtheamountofmetalremainingaftersubsequent removaloftheoxidemeasured.Aseriesofsamplesreactedfordifferenttimes wouldthenyieldakineticplot.Difficultiesinremovingallofthescalewithout damagingtheunderlyingmetalrenderweightchangemeasurementsofthissort inaccurate.Analternativetechniqueistomeasurethedifferenceinmetalsection thicknessbeforeandafterreaction.Giventhatthedifferenceswillbesmall, perhapsoforder10 mm,comparedtotheusualspecimenthicknessofsome millimetres,measurementerrorscanbelarge.However,thistechniquehasbeen successfullyappliedtotheoxidationofthinfoils [8]
Theconsumptionofoxidant dnO2 canbefollowedbyobserving DpO2 at constantvolume,orthevolumechangerequiredtomaintain pO2 constant. Giventhevastlydifferentdensitiesofsolidsandgases,itisclearthatthis techniqueisrestrictedtocasesofsmall dx,unlesstheoxidantcanbe replenished.Similarreservationsapplytotheuseofthistechniquewhenthe reactiongasisamixture:as dx increases,thegaschangescomposition.
1.gas bottle
2.catch bottle
3.condenser + flask
4.water bath for flask
5.water pump
6.water bath condenser
7.furnace
8.microbalance
9.specimen
10.amplifier
11.computer
FIGURE1.9 Schematicviewofthermogravimetricapparatusformeasuringweightuptake duringhightemperaturereactioninacontrolledgasatmosphere.
Byfarthemostcommonmethodofmeasuringoxidationratesisthe observationofoxideaccumulationwithtime.Gravimetricmeasurementscan beperformedcontinuouslywithamicrobalanceordiscontinuouslyby weighingaseriesofsamplessubjectedtodifferentreactiontimes.Continuous measurementsyieldamoreaccuratedefinitionof Eq.[1.11],butthetimelapse exposureapproachcanbeusedtosimultaneouslyreactalargenumberof differentalloys.Moreoverthemultiplesamplesobtainedforeachalloycanbe usefulincharacterisingthereactionproducts.When dx/dt isverysmall,the measurementprecisionprovidedbyahighqualitymicrobalanceisdesirable, althoughitcanbedifficulttoachieve.
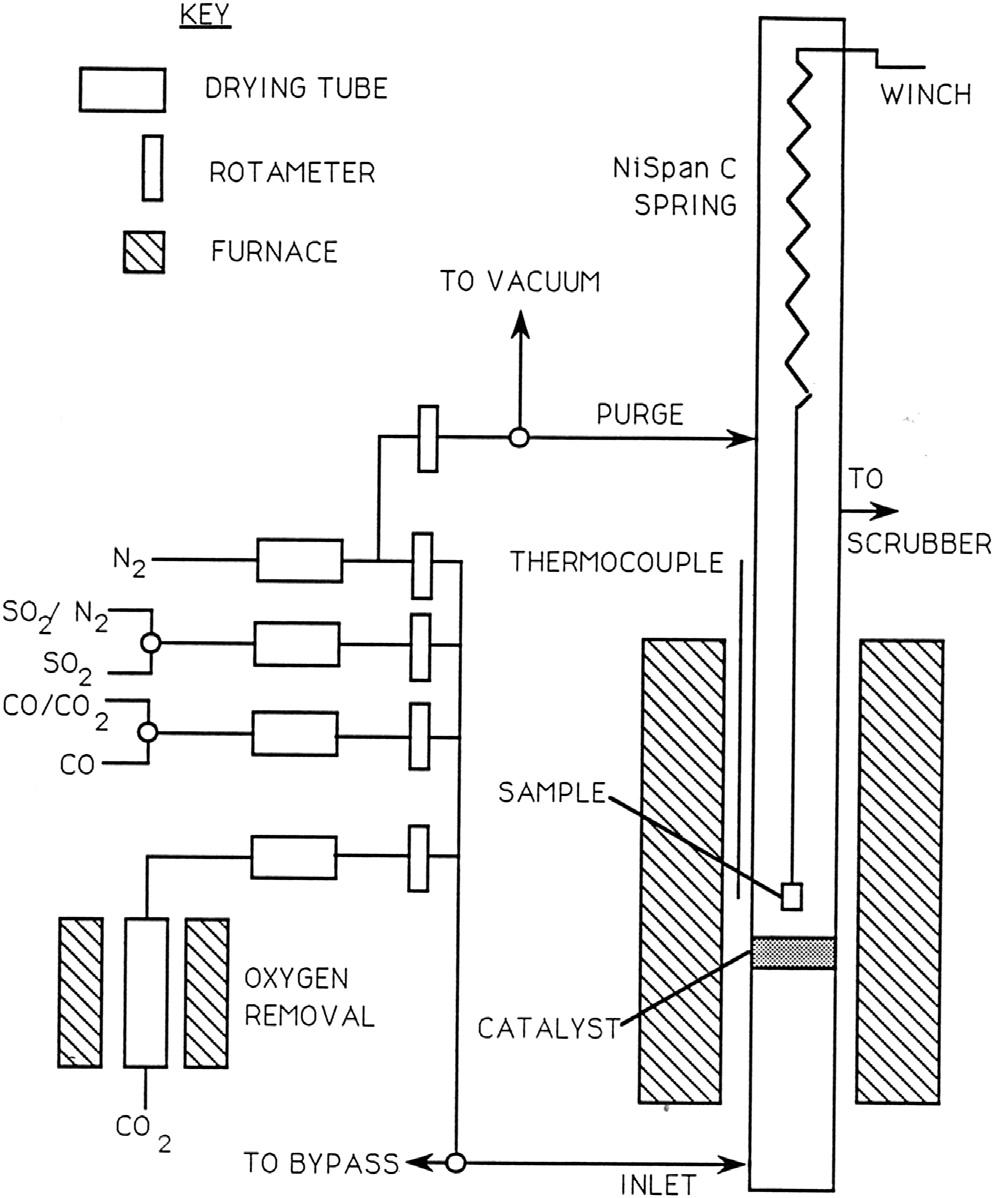
Microbalancesareexpensive.Theymustbeprotectedagainstcorrosionby thereactiongasbypassingacounterflowofunreactivegasthroughthebalance chamber,asshownin Fig.1.9.Inthecaseofparticularlycorrosivespecies suchasSO2 orH2S,itisadvisabletouseacheapspringbalancesuchasthat shownin Fig.1.10.Theelongationofahelicalspringisobservedasasample
FIGURE1.10 Schematicviewofspringbalanceassemblyforobservinghightemperature oxidationkinetics.
suspendedfromitreactsandbecomesheavier.Thespringisusuallymade fromsilicafibreorNiSpanCwire,thelatterbeinganalloywithanelastic modulusinsensitivetotemperature.
Theobservedweightchange, D W,varieswithspecimensurfacearea, A, andthemeasuredquantityisreportedas D W/A .Ifnometalvolatilisation occurs,theweightchangecorrespondstooxidantuptake,anditfollowsfrom Eq.[1.12] that
Thelossofmetalcanthenbeexpressedintermsofweightperunitsurface area, DWM/A,usingtheatomicweight,AWM,
Thislossisequivalenttoadecreaseinvolumegivenby
where rM isthemetaldensity.Recognisingthatuniformremovalofmetal fromaflatsurfaceresultsinarecessionofthesurfacebyadepth
itisseenthat
Similarly,thethickness X ofauniform,single-phaseoxidescalegrownona flatsurfacecanbecalculatedas
whereMWOX isthemolecularweightand rOX thedensityoftheoxide. Oxidescalethicknessescanbemeasureddirectly,byexaminingmicroscopicimagesofcross-sectionssuchasthoseshownin Figs.1.3and1.8.This technique,whichisdescribedbelow,isrelativelysimpleandeconomical.For thisreason,andalsobecausediffusionequationsareexpressedintermsof positioncoordinates,itispreferably torephrasethegeneraloxidationrate Eq.[1.11] as