
High-efficiencyphotoelectrochemicalcathodic protectionperformanceoftheTiO2/AgInSe2/In2Se3 multijunctionnanosheetarrayXuhongJiang& MengmengSun&ZhuoyuanChen&JiangpingJing& ChangFeng https://ebookmass.com/product/high-efficiencyphotoelectrochemical-cathodic-protection-performance-of-thetio2-aginse2-in2se3-multijunction-nanosheet-array-xuhongjiang-mengmeng-sun-zhuoyuan-chen-jiangping-jing-chang-feng/

Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
A novel TiO2 nanotube arrays/MgTixOy multiphaseheterojunction film with high efficiency for photoelectrochemical cathodic protection Chang Feng & Zhuoyuan Chen & Jiangping Jing & Mengmeng Sun & Guiying Lu & Jing Tian & Jian Hou
https://ebookmass.com/product/a-novel-tio2-nanotube-arrays-mgtixoymultiphase-heterojunction-film-with-high-efficiency-forphotoelectrochemical-cathodic-protection-chang-feng-zhuoyuan-chenjiangping-jing-mengmeng-sun-guiyi/ ebookmass.com
Elsevier Weekblad - Week 26 - 2022 Gebruiker
https://ebookmass.com/product/elsevier-weekbladweek-26-2022-gebruiker/
ebookmass.com
Jock Seeks Geek: The Holidates Series Book #26 Jill Brashear
https://ebookmass.com/product/jock-seeks-geek-the-holidates-seriesbook-26-jill-brashear/
ebookmass.com
Health Care Ethics: Critical Issues for the 21st Century 4th Edition
https://ebookmass.com/product/health-care-ethics-critical-issues-forthe-21st-century-4th-edition/
ebookmass.com




A Preface to Wilfred Owen 1st Edition John Purkis
https://ebookmass.com/product/a-preface-to-wilfred-owen-1st-editionjohn-purkis/
ebookmass.com
Treblinka: Transit Camp Or Extermination Camp? Carlo Mattogno
https://ebookmass.com/product/treblinka-transit-camp-or-exterminationcamp-carlo-mattogno/
ebookmass.com
Porth’s Pathophysiology: Concepts of Altered Health States 10th Edition, (Ebook PDF)
https://ebookmass.com/product/porths-pathophysiology-concepts-ofaltered-health-states-10th-edition-ebook-pdf/
ebookmass.com
THE WOODS AT DUSK a totally gripping crime thriller full of twists (Detective Chris Bailey Book 2) Kate Watterson
https://ebookmass.com/product/the-woods-at-dusk-a-totally-grippingcrime-thriller-full-of-twists-detective-chris-bailey-book-2-katewatterson/
ebookmass.com
Clear Threat (Disgardium Book #10): LitRPG Series Dan Sugralinov
https://ebookmass.com/product/clear-threat-disgardium-book-10-litrpgseries-dan-sugralinov/
ebookmass.com





https://ebookmass.com/product/stand-by-me-pat-simmons-2/
ebookmass.com


CorrosionScience
journalhomepage: www.elsevier.com/locate/corsci
High-efficiencyphotoelectrochemicalcathodicprotectionperformanceof theTiO2/AgInSe2/In2Se3 multijunctionnanosheetarray
XuhongJianga,b,d,1,MengmengSuna,b,c,1,ZhuoyuanChena,b,c,*,JiangpingJinga,b,c, ChangFenga,b,d
a KeyLaboratoryofMarineEnvironmentalCorrosionandBio-fouling,InstituteofOceanology,ChineseAcademyofSciences,7NanhaiRoad,Qingdao,266071,China
b CenterforOceanMega-Science,ChineseAcademyofSciences,7NanhaiRoad,Qingdao,266071,China
c OpenStudioforMarineCorrosionandProtection,PilotNationalLaboratoryforMarineScienceandTechnology(Qingdao),No.1WenhaiRoad,Qingdao,266237,China
d UniversityofChineseAcademyofSciences,19(Jia)YuquanRoad,Beijing,100049,China
ARTICLEINFO
Keywords:
A.AgInSe2/In2Se3 nanoparticles
A.Multijunctions
A.TiO2 nanosheetarray
C.Photoelectrochemicalconversion performance
C.Photoelectrochemicalcathodicprotection performance
1.Introduction
ABSTRACT


Optimizationofmultijunctionphotoelectricconversionmaterialswithamuchnegativebandpotentialisvery importantforimprovingthephotoelectricconversionandphotoelectrochemicalcathodicprotectionperformancebecausethewell-matchedmultijunctioncanassistthefasttransportofphotogeneratedelectrons.Inthis paper,a “green” AgInSe2/In2Se3 sensitizedTiO2 nanosheetarray(NSA)photoanodewasprepared.Verticallygrowntwo-dimensionalTiO2 NSAwiththemultijunctionofTiO2 NSA/AgInSe2(7)/In2Se3(3)improvestheseparationefficiencyandthetransferofphotoinducedchargecarrierscomparedwithsingleAgInSe2 sensitized TiO2 NSA.TheTiO2 NSA/AgInSe2/In2Se3 photoanodeachieveshighlyefficientphotoelectrochemicalcathodic protectionperformancefor316LSSinNaClsolutionunderAM1.5lightillumination.
Itisvitaltocontrolcorrosionforthebenefitofmankindsince corrosioncancausehugeeconomiclossesandcatastrophicaccidents [1].Amongallofthecorrosionprotectionmethods,thephotoelectrochemicalcathodicprotection(PECCP)hasattractedwideattentions owingtoitsenergy-saving,environmentallyfriendly,andeconomical virtues,whichmainlyutilizesthephotoelectricconversioneffectof semiconductorstoconvertsolarenergyintoelectricalenergyandthen transportsthephotogeneratedelectronstothecoupledmetals,thus achievingthecathodicprotectioneffect[2,3].Thekeyofthistechnologyistochoosesuitablesemiconductormaterialsandtakeadvantageoftheirexcellentphotoelectrochemical(PEC)conversion properties.TiO2 hasbeenprovedtobethemostpromisingsemiconductormaterialsduetoitsappropriateelectronicbandstructure, environmentalfriendliness,highphotostabilityandlowcost,playinga centralrolein fieldsofphotovoltaiccells[4,5]aswellasphotocatalytic pollutantsdegradation[6].However,thebandgapofTiO2 is3.2eV, whichlimitsitslightabsorptiononlytotheultravioletregion[7],and alsothephotogeneratedcarriersareeasytoberecombined.Recent researcheshavebeenfocusingonovercomingtheabovedefects.Forthe
highcarrierrecombinationrate,aneffectivemethodistoconstruct directelectricalpathwaysinordertotransportthephotoinduced electronsrapidlyandreducethegrainboundariessoastoenhancethe photogeneratedcarriercollectionefficiency.Differentmorphologiesof well-organizedTiO2 nanostructures,suchasone-dimensional(1D)nanorods[8,9]andnanotubes[10–14],two-dimensional(2D)nanosheet [15,16]andthree-dimensional(3D)nanotree,nanoforestandnanolawn [17–21]etc.havebeendeveloped,andtheelectron-transportefficiency andPECconversionperformancehavebeenimprovedtodifferentextents.Especially,the2DTiO2 nanosheetarray(NSA)isofsignificance forimprovingtheperformanceofthePECsystem[22–26],whichcan providesufficientnanoparticles(NPs)loadingandlightharvesting area,improvetheelectrontransportandchargeseparationefficiency, thusresultinachievingexcellentPECproperties.
Apartfrommorphologymodification,theconstructionofsuitable semiconductorheterojunctionalsoplaysasubstantialroleonreducing therecombinationrateofthephotogeneratedelectron-holepairsby effectivelyseparatingthem.Andmoreimportantly,couplingTiO2 with visiblelightresponsivenarrowbandgapsemiconductormaterialscan widenthelightresponsetovisiblelightregion,andgreatlyimprovethe PECconversionperformance[27–31].Comparedwithbulk
⁎ Correspondingauthorat:KeyLaboratoryofMarineEnvironmentalCorrosionandBio-fouling,InstituteofOceanology,ChineseAcademyofSciences,7Nanhai Road,Qingdao,266071,China.
E-mailaddress: zychen@qdio.ac.cn (Z.Chen).
1 XuhongJiangandMengmengSunaretheco-firstauthors.
https://doi.org/10.1016/j.corsci.2020.108901
Received28April2020;Receivedinrevisedform15July2020;Accepted26July2020
Availableonline29July2020
0010-938X/©2020ElsevierLtd.Allrightsreserved.
semiconductormaterials,NPsaremoresufficientlyinheterojunction withmatchedenergybandsandenergylevels,andhasbeenextensively investigated[32–35].And,unliketheCdandPbelementswhose toxicityhashinderedtheirfurtherapplications,theI–III–VI2-group(I: Cu/Ag,III:Ga/In,VI:S/Se/Te)ternarychalcogenideshaveattracted moreattentionbecauseoftheirnarrowbandgaps,solarenergy-absorbingability,lightstabilityandlowtoxicity.CuInS2 [36,37],AgInS2 [38],CuInSe2 [39,40],AgInSe2 [41–43]etc.arebecomingalternatives ofthePb-andCd-basedsemiconductorsforPECconversionapplicationslyingintheirhighabsorptioncoefficientandabilitytoincrease photoconversionefficiency[44].Recently,novelnano-photocatalystof TiO2-decoratedAgInSe2 hasbeensynthesizedandwasappliedtodye degradation,whichshowedeffectivedegradationefficiencyandstability[41].ThebandgapofAgInSe2 isaround1.20eV[41],closetothe optimalbandgapforabsorbingthesolarspectrum.Thiscouldbean idealPECmaterialtosubstitutecontaminatedsubstancesintheareasof PECconversionandPECCP.
Besides,forheterojunctionstructure,attheinterfaceofsemiconductorcomposite,unmatchedenergybandarrangementandhigh surfacestatedensitymayexist,whichresultsinachanceofcharge recombinationattheinterface[45].Therefore,interfacialoptimization oftheheterojunctionisalsonecessarytofurtherimprovethePEC conversionefficiency[46].Tosolvethisproblem,amultijunction heterostructurecompositewithwell-matchedbandpotentialscanbe constructed,thenthephotogeneratedelectron-holepairswillbeseparatedandtransferredmoreefficiently[47–49].Changetal.fabricateda seriesofTiO2/CuInS2,TiO2/Cu2S/CuInS2,TiO2/Cu2Se/CuInS2,TiO2/ In2S3/CuInS2,TiO2/In2Se3/CuInS2 configurations,whichuseCu2S, Cu2Se,In2S3,In2Se3 asthebufferlayersofthebulkmaterial,andthe PECconversionefficiencieswere0.58%,1.06%,1.22%,0.89%and 1.35%,respectively.Thisresultandotherreportshavedemonstrated thatamongallthecurrentlystudied “green” co-sensitizers,suchas Cu2S,Cu2Se,In2S3,In2Se3,InP,ZnS,ZnSeandZnTe,In2Se3 hasbeen provedtobethemosteffectiveonetoactasacompositelayerofthe multijunctionowingtoitsbetterPECperformance[50–54].Therefore, thestudiesfordesigningeffective,energybandwell-matchedmultijunctionsystems,especiallycombiningwithassistlayers,arecriticalto furtherpromotethecarriertransmissionefficiencyattheheterointerfaceandthusenhancethePECactivityofphotoanodes.
Inthepresentwork,anAgInSe2/In2Se3 co-sensitizedTiO2 NSA multijunctionphotoanodewasconstructed,andthePECconversionand PECCPperformancewereexploredinsimulatedseawater(3.5wt% NaClsolution)undersimulatedsunlight(AM1.5light)illumination. Thisconditiondoesnotcontainanyaddedman-madehole-scavengers andismorecloselytotherealisticmarineenvironment.ThePECperformanceofthepreparedTiO2 NSA/AgInSe2/In2Se3 wasinvestigated underintermittentAM1.5lightillumination.Besides,theenergyband potentialofthen-typesemiconductorofTiO2/AgInSe2/In2Se3 multijunctioniscomparativelynegative,therefore,itsapplicationinthe PECCPfor316Lstainlesssteel(SS)wasalsoexplored.And,ahighly efficientPECCPperformanceinNaClsolutionunderAM1.5lightilluminationwasobtained.Simultaneously,therelationshipbetweenthe multijunctionstructureandthePECandPECCPperformanceaswellas thecorrespondingmechanismwasanalyzed.Theresultsobtainedin
thisworkwillcontributefordesigningefficientphoto-functional coatingtodeveloptheexpandedPECapplicationsinthePECCP field.
2.Experimental
2.1.PreparationofTiO2 NSAandTiO2 NSA/AgInSe2/In2Se3 photoanodes Allreagentsusedinthisstudywereofanalyticalgradesandwere directlyusedwithoutfurthertreatments.A fluorine-dopedtinoxide (FTO)glass(1×2cm2)wasultrasonicallycleanedwithacetoneand ethanolfor10min,respectively,andthendriedinairbeforegrowing TiO2 NSAonit.The2DTiO2 NSA filmwaspreparedonthecleanedFTO conductiveglassbyasimpleone-stephydrothermalmethod.Inbrief, 15mLconcentratedhydrochloricacid(massfraction36.5–38%)and 15mLdeionizedwaterweremixedtogetherandstirredfor5min.Then, 0.5mLtitaniumbutoxide(TBT)wasaddedtothepreparedHClsolution.Afterstirringforanother5min,0.25gammoniumhexafluorotitanate((NH4)2TiF6)wasaddedandfurtherstirredfor5min. Then,themixturewastransferredtoa50mLTeflon-linedstainlesssteel autoclave.Finally,theFTOsubstratewasplacedatanangleagainstthe walloftheTeflonlinerwiththeconductivesidefacingdown.The hydrothermalsynthesiswasconductedat170,180and200°Cfor12h inanelectricoven,respectively.Afterthat,theautoclavewascooled down,andtheFTOsubstratewastakenoutandrinsedwithdeionized waterthoroughlyanddriedintheair.
TheAgInSe2 NPsweredepositedontotheTiO2 substratebysuccessiveionlayerabsorptionandreaction(SILAR)technique.Typically, theobtainedTiO2 NSA filmsubstratewassuccessivelyimmersedinto threedifferentaqueoussolutionsfor5minforfourtimes, firstlyina 0.02MAgNO3 aqueoussolution(cationprecursor)andthenina0.02M Na2SeSO3 aqueoussolution(anionprecursor),thirdlyina0.02M In2(SO4)3 aqueoussolution(cationprecursor),and finallyina0.02M Na2SeSO3 aqueoussolutionagain.Betweeneachimmersionstep,the sampleswererinsedthoroughlywithdeionizedwatertoremovethe excessweakly-boundedions.TheNa2SeSO3 aqueoussolutionwas synthesizedbydissolvingelementalseleniumpowder(99.99%)inan aqueoussodiumsulfitesolutionat90°C,adjustingpHto12–14with sodiumhydroxide,asadoptedfrompreviousliterature[55,56].The Se2+ solutionpreparationmethodinthepresentpaperismorestable thanthosepreparedbyreducingthealcoholsolutionofseleniumdioxidewithsodiumborohydride[50,57].Such4-timesimmersionprocedureistermedasonecycleofthedepositionprocess,andseveral timesofthisimmersioncyclewererepeateduntilspecifiedamountof AgInSe2 NPswascompounded.Byadjustingthenumberofdeposition cycles,theamountofdepositedAgInSe2 NPscanbecontrolled.
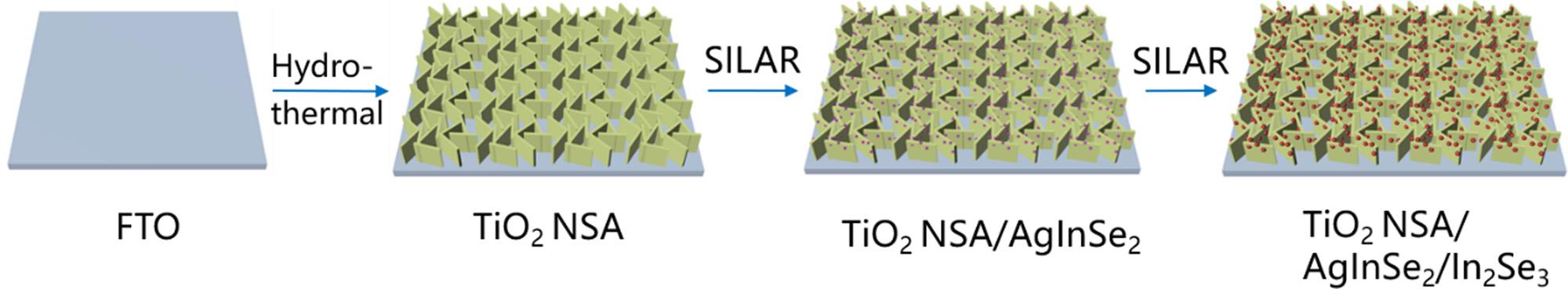
Afterthat,theIn2Se3 layerwasincorporatedontheTiO2 NSA/ AgInSe2 photoanodebyanotherSILARdeposition.TheIn2Se3 depositioncycleissimilartotheAgInSe2 depositionprocedurebutwithout immersingintotheAgNO3 aqueoussolution.Finally,theobtained samplewasdenotedasTiO2 NSA/AgInSe2(m)/In2Se3(n),wheremand nrepresentthenumberofthedepositioncyclesofAgInSe2 andIn2Se3 NPs,respectively.ThepreparationprocedureoftheTiO2/AgInSe2/ In2Se3 multijunctionnanosheetarrayphotoanodeisschematicallyillustratedin Scheme1
The316LSSelectrodewaspreparedbyembeddinga316LSS squareinepoxyresin,exposinganareaof10×10mm2 fortesting. Afterthat,the316LSSelectrodewassuccessivelywetgroundwithSiC paperto2000grits,andthenultrasonicallycleanedinanalyticalgrade ethanolfor5minanddried.
2.2.Characterizations
Thesurfacemorphologyandthemicrostructureoftheprepared photoanodeswereexaminedbya field-emissionscanningelectronmicroscope(SEM)(ULTRA55,ZeissCompany,Germany)andahighresolutiontransmissionelectronmicroscope(HRTEM,TecnaiG20,FEI Company,USA).Thecrystallinestructuresofthepreparedmaterials werecharacterizedusingUltimaIVX-RayDiffractometer(XRD) (RigakuCo.,Tokyo,Japan)withCuKα radiation.Fouriertransform infrared(FTIR)spectraweretestedusingaFouriertransforminfrared spectroscopy(FTIR,Thermo-Nicolet8700,ThermoElectronScientific Inc.,USA)atroomtemperature.Theelementalcompositionsandthe bondinginformationwereidentifiedusingX-rayphotoelectronspectroscopy(XPS)onanX-rayspectrometer(ModelThermoESCALAB25 XI,AlKα,hν =1486.6eV,MonoX-raysource).Theopticalabsorption propertiesweremeasuredonaUV/Visspectrophotometer(SHIMADZU UV-2600,Japan).Thephotoluminescence(PL)emissionintensitiesof thepreparedmaterialswererecordedbya fluorescencespectrometer (FluoroMax-4,HORIBAJobinYvon,France).
2.3.ElectrochemicalandPECperformancemeasurements
ThePECperformanceofthepreparedmaterialswasdeterminedby i-Vcurvesandopencircuitpotential(OCP)variationsaswellasthe photoinducedcurrentdensitiesandpotentiodynamicpolarization curvesunderintermittentsimulatedsolarlight(AM1.5)illumination. Thei-Vcurveswereconductedatthepotentialrangeof 0.8Vto 0.4V.Thepotentiodynamicpolarizationcurvesweremeasuredfrom 250to250mV(vs.OCP).Theelectrochemicalimpedancespectroscopy(EIS)testswereperformedinthedarkatopencircuitpotential overthefrequencyrangebetween105 and10 2 Hz,withanACvoltage magnitudeof5mV.Mott-Schottkyplotswereconductedinthedark withthepotentialrangeof-0.8Vto0.4Vandthefrequencyof1000Hz andtheACvoltagemagnitudeof10mV.Theabovementionedi-V,EIS, andMott-Schottkymeasurementswereallconductedin0.1MNa2SO4, whiletheOCP,photoinducedcurrentdensityandpolarizationcurves weremeasuredin3.5wt%NaClsolution.Allofthemeasurementswere performedusingaCHI660Delectrochemicalworkstation(Shanghai ChenhuaInstrumentCo.,Ltd.),byemployingaPtelectrodeasthe counterelectrodeandanAg/AgCl(saturatedKCl)asthereference electrode,respectively.
2.4.PECCPperformancemeasurements
Thephotoinducedvariationsofthecurrentdensitiesofthegalvanic couplingbetweenthe316LSSelectrodeandthephotoanodesandthe mixedpotentialsofthe316LSSelectrodecoupledwiththephotoanodeswereexaminedtostudythePECCPperformanceoftheprepared samples.Allmeasurementswereperformedin3.5wt%NaClsolution underintermittentsimulatedAM1.5solarlightilluminationusingthe CHI660Delectrochemicalworkstation(ShanghaiChenhuaInstrument Co.,Ltd.).Theexperimentalarrangementisschematicallyillustratedin Fig.1,whichissimilartothoselistedinpreviousreports.Byusingthis experimentalsetup,thecurrentdensityandpotentialcanbemeasured simultaneously[2,58].Thegalvaniccurrentdensitiesbetweenthe photoanodeandthe316LSSelectrodeweremeasuredwithoutany appliedpolarization.A300-WXearclamp(PLS-SXE300,BeijingPerfectLightCo.Ltd.,China)wasusedasthelightsourcetogenerate AM1.5lightwiththepowerenergydensityof100mWcm 2
3.Resultsanddiscussion
3.1.MorphologyandstructureanalysesofthepreparedAgInSe2/In2Se3 decoratedTiO2 NSA
Inordertopreparethephotoanodeswithbestperformance,the influenceofhydrothermaltemperatureonthemorphologiesandperformanceofthepreparedphotoanodeswasperformedandtheresults areshownin Fig.2.Withtheincreaseofhydrothermaltemperature from170°Cto200°C,theshapeofeachnanosheetisbarelychanged, however,thedensity,sizeandthicknessoftheTiO2 NSAincreasewith thehydrothermaltemperature(Fig.2a–c).HigherhydrothermaltemperaturecanpromotethegrowthoftheTiO2 NSA.Meanwhile,the nanosheetswhicharetoosparseortoocrowdedwillnotbenefitthe lightharvestingandtheelectrontransport.Asconfirmedby Fig.2d,the photoinducedcurrentdensityoftheTiO2 NSAsubstratepreparedat 180°Cachievedthehighestvalue,indicatingthattheTiO2 NSApreparedat180°CpossessesthebestPECconversionperformance.As shownin Fig.2b,thepreparedplainTiO2 NSAat180°Cconsistsofa seriesofverticallyarrangedandstaggerednanosheets.Theinsetin Fig.2bshowsthepartialenlargedviewoftheformednanosheets.A well-facetedcrystalstructurecanbeseen,andthejunctionedgeofthe adjacentdifferentcrystallographicplanescanalsobeclearlyseen.The nanosheetsareinterlacedandinterconnectedwitheachother.Theside lengthofthesenanosheetsisapproximately2 μm,andthethicknessofa singlenanosheetisapproximately200nm.Thisorderedstructurewill facilitatethetransportofthephotogeneratedcarriers.Subsequent analyseswerebasedonthephotoanodespreparedundertheoptimal hydrothermaltemperatureof180°C.
XRDpatternswererecordedtoinvestigatethecrystalphasesofthe preparedAgInSe2/In2Se3,AgInSe2,In2Se3 decoratedTiO2 NSAandpure TiO2 NSAsamples,andtheresultsareshownin Fig.3.Thediffraction peakscorrespondingtoanataseTiO2 andFTOglassareclearlyshownin Fig.3.Thediffractionpeaksat2θ =25.3°,37.8°,48.0°,55.1°and62.7° areattributedto(101),(004),(200),(211)and(204)crystalplanesof anataseTiO2 (JPCDSNo.21-1272)[59,60],respectively.Asshownin Fig.3,muchhigherintensityratiosofthediffractionpeakof(004) crystalplanetothoseofotherdiffractionpeaksareobservedincomparisonwiththoseinthestandardpatternofanataseTiO2,demonstratingthatthesynthesizedTiO2 hasahighlyexposed(004)crystal plane.However,nodiffractionpeaksfromAgInSe2 andIn2Se3 aredetectedin Fig.3,indicatingthelowamount,welldispersionand/orlow degreeofcrystallinityofthedepositedAgInSe2 andIn2Se3 inthesynthesizedphotoanodes.SimilarresultsofloadingNPsusingthesame SILARmethodwerealsoreportedinpreviousliterature[38,55,61].
Themorphologiesofthepreparedcompositephotoanodesandthe combinationstatesofAgInSe2 andIn2Se3 ontheTiO2 NSAwereanalyzed,and Fig.4a–eshowtop-viewSEMimagesofthepreparedTiO2 NSA/AgInSe2(7),TiO2 NSA/AgInSe2(7)/In2Se3(3),TiO2 NSA/ AgInSe2(3)/In2Se3(3)andTiO2 NSA/AgInSe2(11)/In2Se3(3)photoanodesandcross-sectional-viewimageofTiO2 NSA/AgInSe2(7)/ In2Se3(3)photoanode,respectively.Asshownin Fig.4a,forTiO2 NSA/ AgInSe2(7),plentyof fineNPsaredistributeduniformlyonthesurface oftheTiO2 NSA.While,withthefurtherdepositionofIn2Se3 onTiO2 NSA/AgInSe2(7),theformedNPsonTiO2 NSA/AgInSe2(7)/In2Se3(3) becomelargerandeasiertobeobserved,asshownin Fig.4b.Thelarger NPsofAgInSe2(7)/In2Se3(3)distributeuniformlyonthesurfaceofTiO2 NSA,andexhibitawellcombinationontothesheets.Thisensuresthe largelight-harvestingofboththenanosheetsandthelargeamountof AgInSe2/In2Se3 NPs.While,in Fig.4c,forTiO2 NSA/AgInSe2(3)/ In2Se3(3),severalscatteredNPsdepositedonthesurfaceoftheTiO2 NSAcanbeseen.ForTiO2 NSA/AgInSe2(11)/In2Se3(3)photoanode (Fig.4d),alotofNPsaredepositedontoTiO2 NSA,someofwhich aggregatetogethertoformlargerparticles.Thiswillhindertheelectron transportprocess.TheamountoftheNPsontheTiO2 NSA/AgInSe2(7)/ In2Se3(3)photoanodeisbetweenthoseoftheTiO2 NSA/AgInSe2(3)/
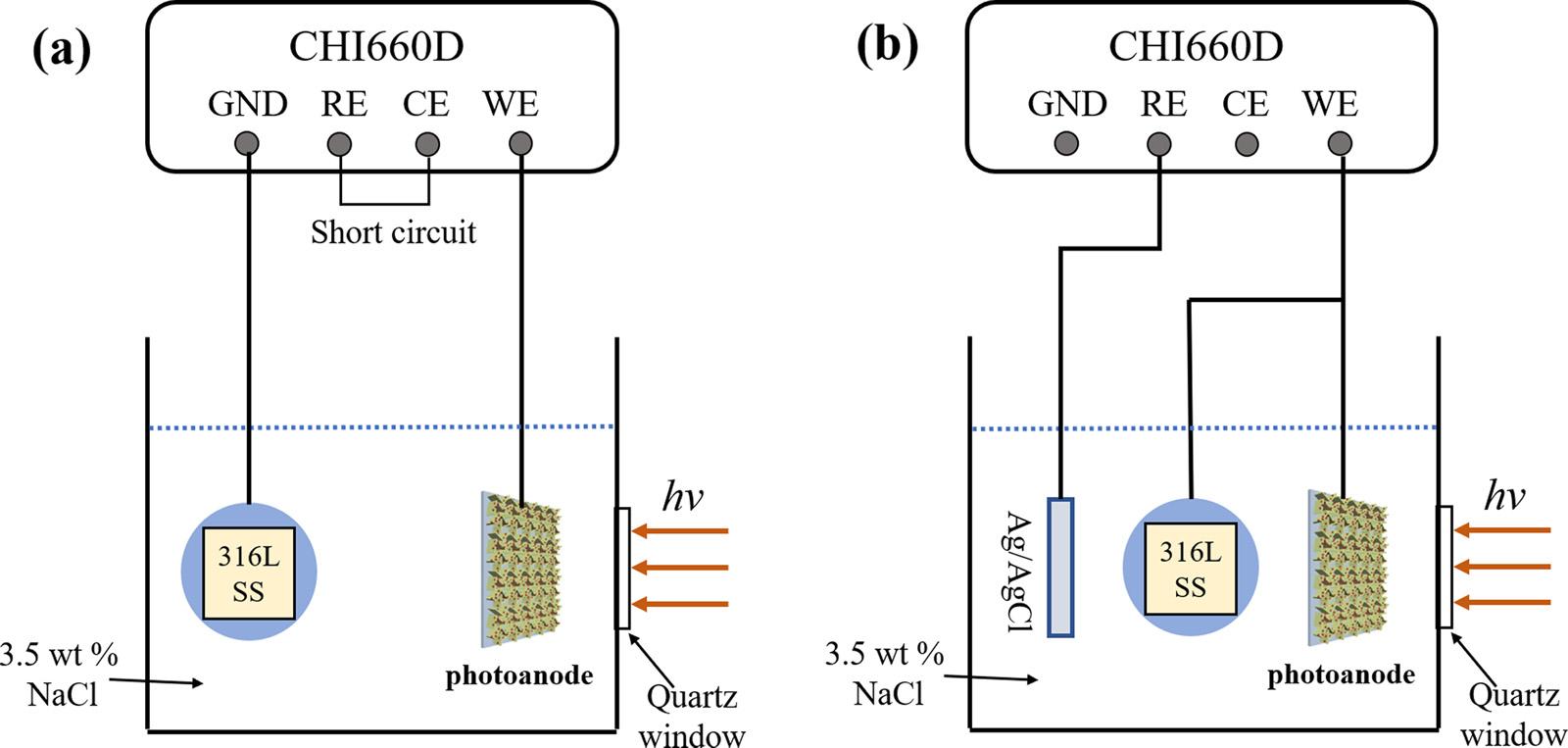
Fig.1. SchematicillustrationsoftheexperimentalsetupforthePECCPmeasurments:(a)thephotoinducedcurrentdensitybetweenthepreparedphotoanodeandthe 316LSSelectrodeand(b)thephotoinducedmixedpotentialsofthecoupled316LSSelectrodeandthepreparedphotoanode.
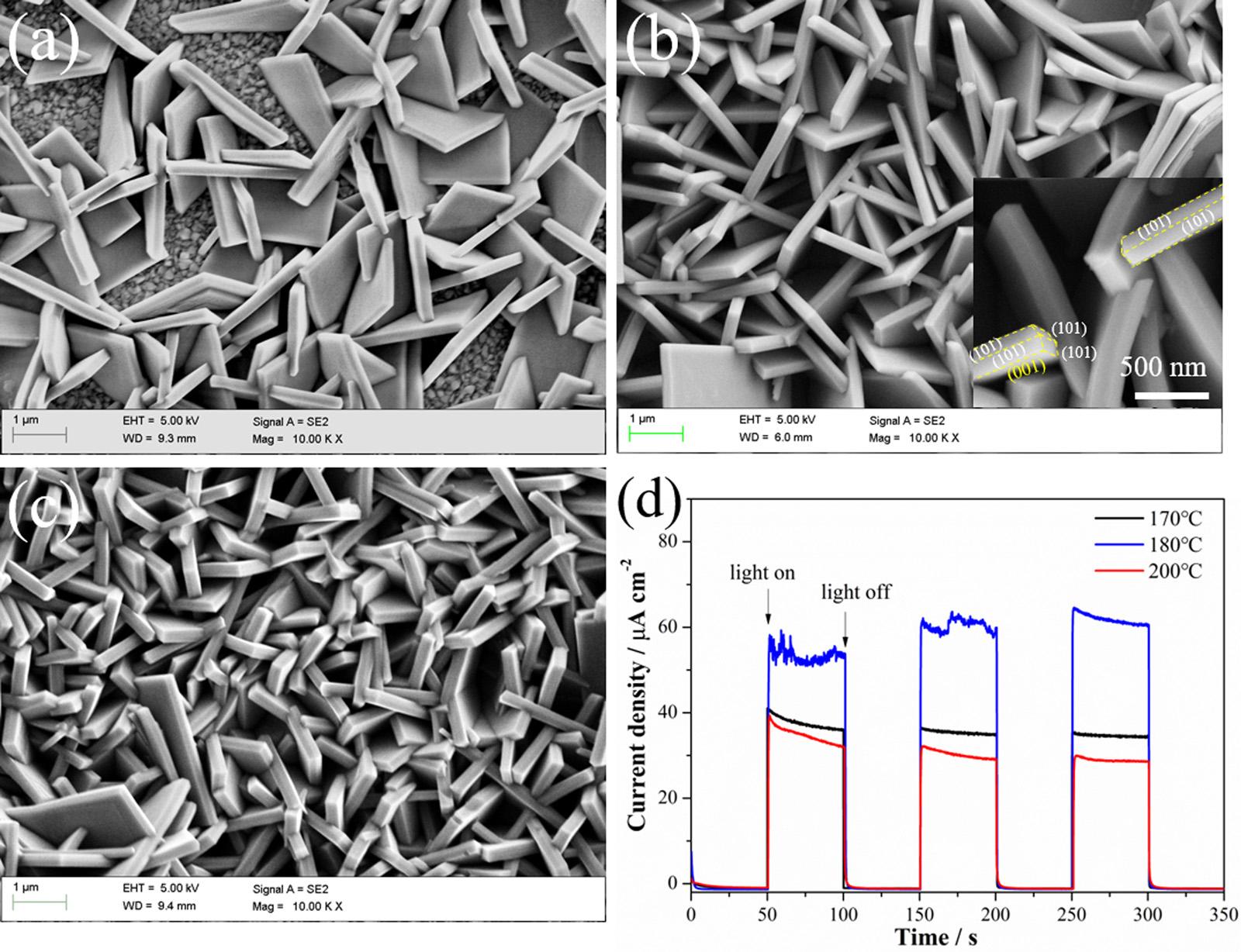
Fig.2. SEMimagesoftheTiO2 NSAsubstratespreparedunderdifferenthydrothermalreactiontemperatures:(a)170°C,(b)180°Cand(c)200°C;and(d)the correspondingphotoinducedcurrentdensitiesoftheTiO2 NSAphotoanodespreparedatdifferenttemperatureunderintermittentAM1.5lightillumination.
In2Se3(3)andTiO2 NSA/AgInSe2(11)/In2Se3(3)photoanodes.Onone hand,properamountofNPscanguaranteeenoughlightabsorptionand generatealargestamountofelectrons,ontheotherhand,comparativelysmallsizeofNPscanmakesurethegeneratedelectronstomigraterapidlyattheinterfacesofNPsandnanosheets.Thecross-sectionalviewSEMimageoftheTiO2 NSA/AgInSe2(7)/In2Se3(3)isshown in Fig.4e,thethicknessofthewholeTiO2 NSA filmisapproximately 2 μm.And,theTiO2 NSA filmisnearlyperpendiculartotheFTOsubstrate,whichisconsistentwiththetop-viewSEMimageshownin Fig.4b.
Fig.5 showstheSEMimageandthecorrespondingEDSelemental mappingofTiO2 NSA/AgInSe2(7)/In2Se3(3),fromwhichtheTi,O,Ag,
InandSeelementsareclearlyobserved.ThedistributionofTiandO elementsintheEDSmappingisingoodagreementwiththecorrespondingSEMimage.While,theelementsofAg,InandSeareevenly distributed,indicatingthattheAgInSe2(7)/In2Se3(3)NPsaredispersed evenlyonthesurfaceoftheTiO2 NSA.
ThepreparedTiO2 NSA/AgInSe2(7)andTiO2 NSA/AgInSe2(7)/ In2Se3(3)photocatalystswerefurtherinvestigatedbyTEMandHRTEM toconfirmthedepositedNPs,andtheresultsareshownin Fig.6 Fig.6a andbaretheTEMimagesofTiO2 NSA/AgInSe2(7)andTiO2 NSA/ AgInSe2(7)/In2Se3(3),respectively.BothimagesillustratetheNPsin thesizeof20 50nmonasheet-likestructure.Thedifferencebetween Fig.6aandbisthatsomeclustersareobservedtosurroundtheNPsin
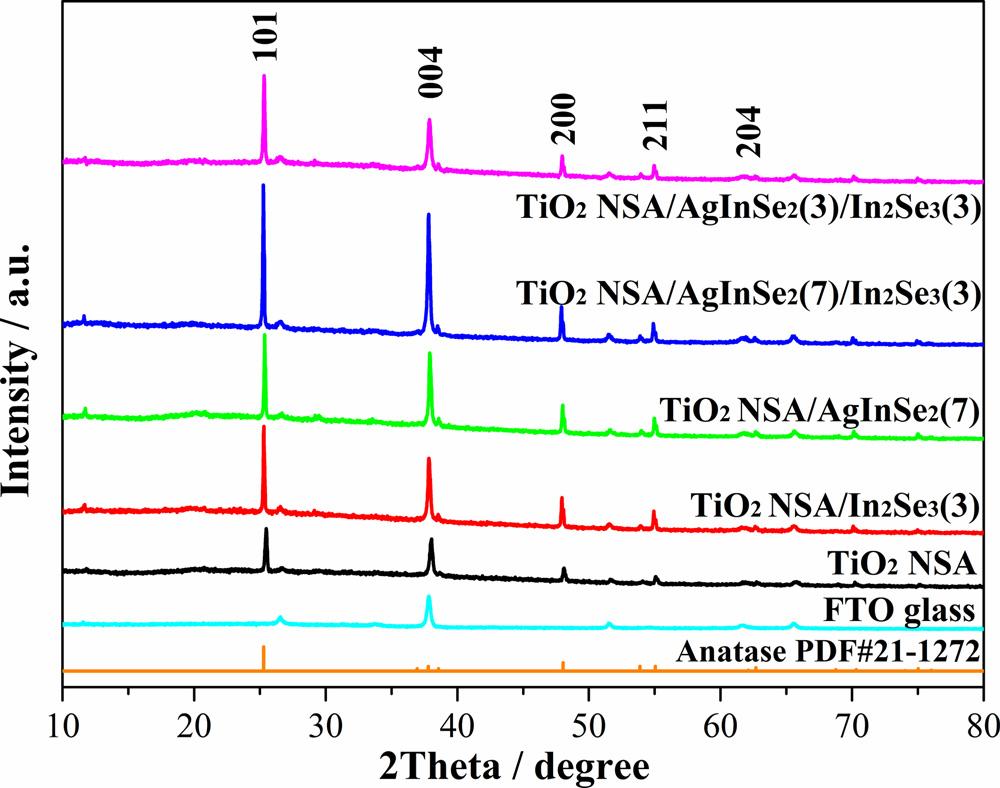
Fig.3. XRDpatternsofthepreparedsamples.
Fig.6b,while,in Fig.6a,theboundariesofnanosheetsandNPsare clearandobvious.FurtherHRTEManalyseshelptodistinguishthe differentlatticesandtheresultsareshownin Fig.6a1,a2,b1andb2. Fig.6a1anda2in Fig.6 arethecorrespondingHRTEMimagesofTiO2
NSA/AgInSe2(7)sampleinthesquareareasof Fig.6a,whileFigures 6b1and6b2in Fig.6 correspondtoHRTEMimagesofTiO2 NSA/ AgInSe2(7)/In2Se3(3)sampleinthesquareareasof Fig.6b.Thelattices spacingof0.352nmand0.189nmcorrespondtothe(101)and(200) planesofanataseTiO2 (JCPDS12-1272)[62,63],implyingtheformationofanatasecrystalofTiO2.Theobservedfringespacingof0.211nm and0.327nmareconsistentwiththe(220)and(111)latticedistances incubicAgInSe2 (JCPDS65-7084)[42,64,65],confirmingtheprepared crystallinestateoftheAgInSe2 NPs.TheHRTEMresultsshownin Fig.6 confirmthesuccessfuldepositionofAgInSe2 NPsontothesurfaceof TiO2 nanosheets.
ApartfromtheclearlyseenAgInSe2 NPs,theweakcrystallinity layeraroundtheAgInSe2 NPsmightbetheIn2Se3 phase,whichisattributedtothelowcrystallinityofIn2Se3 obtainedviatheSILARdepositionprocess[50,61,66].Moreover,theTEMandHRTEManalyses ofthepreparedTiO2 NSA/In2Se3(10)wasconductedtostudythestate ofIn2Se3 depositedonTiO2 NSAbytheSILARdepositionmethod.High concentrationofIn3+ andSe2 precursorsolutions(tentimes)were employedtodepositedsufficientamountofIn2Se3 ontoTiO2 NSA. Fig.7a,candearetheTEMimagesoftheTiO2 NSA/In2Se3 atlow magnifications, Fig.7b,dandfaretheHRTEMimagesinthecorrespondingsquareareasshownin Fig.7a,cande.FromtheTEMimageof TiO2 NSA/In2Se3(10),theTiO2 nanosheetsseemtobecoatedby something.FromalloftheHRTEMimages,anataseTiO2 nanosheetsare detected,eachnanosheetiscoatedwithalayerontheedge,which
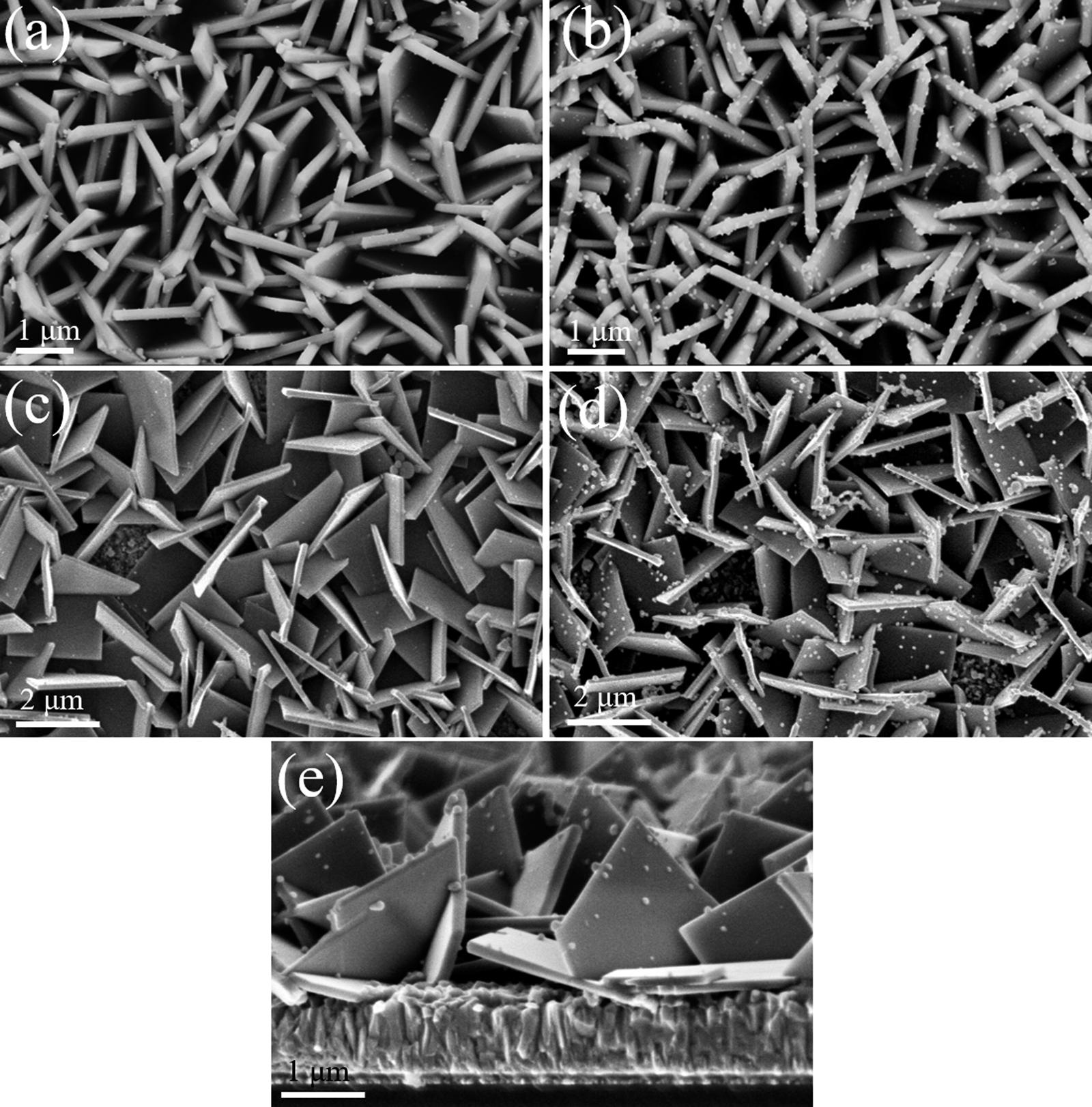
Fig.4. Top-viewSEMimagesof(a)TiO2 NSA/AgInSe2(7),(b)TiO2 NSA/AgInSe2(7)/In2Se3(3),(c)TiO2 NSA/AgInSe2(3)/In2Se3(3)and(d)TiO2 NSA/AgInSe2(11)/ In2Se3(3)photoanodes;Cross-sectional-viewSEMimage(e)ofTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanode.
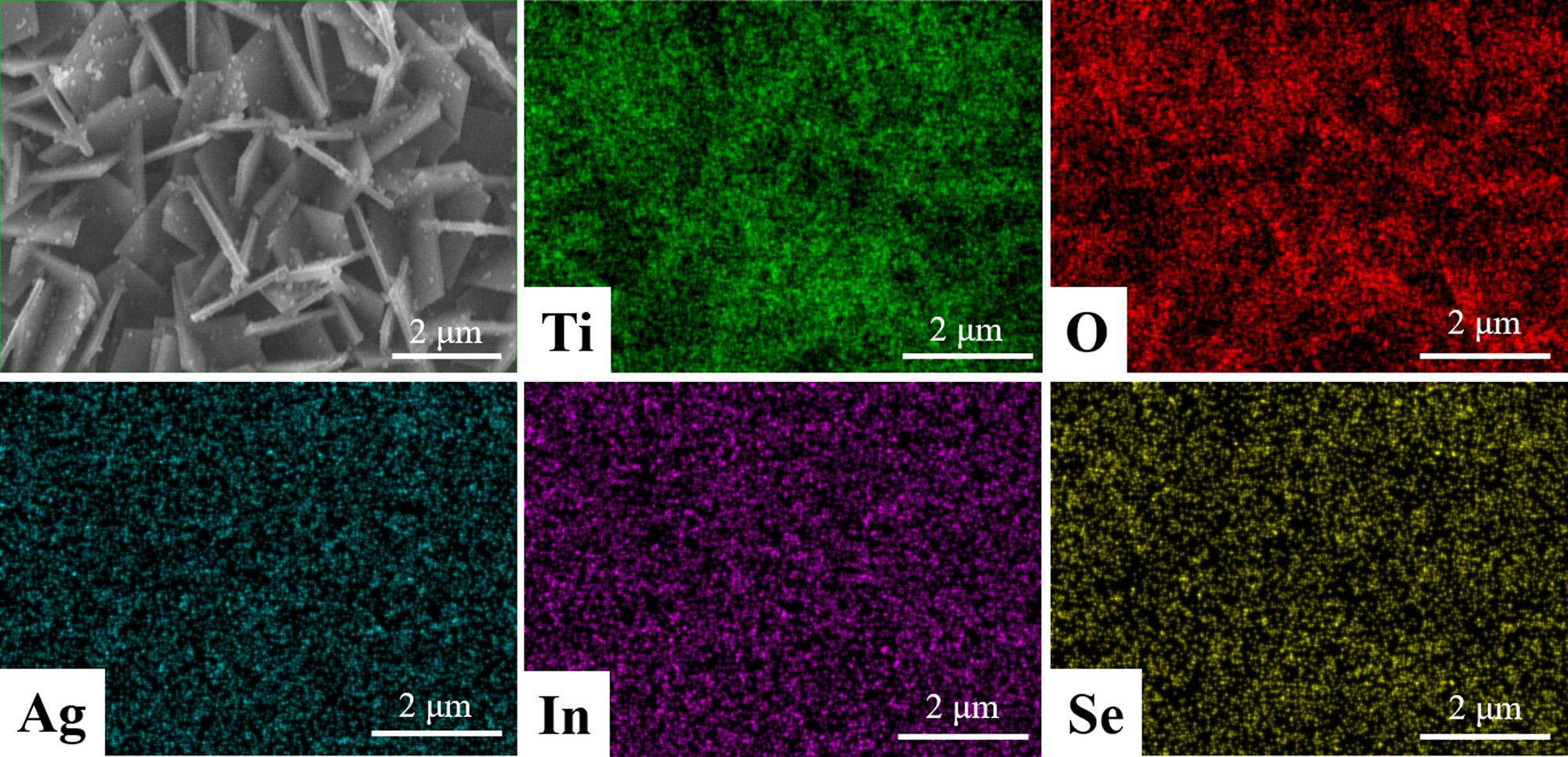
Fig.5. SEMimageandthecorrespondingEDSelementalmappingresultsoftheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanode.
couldbeIn2Se3 withalowcrystallinityandobscuredmorphology [38,50,55,61,67].ThisresultisalsoagreedwiththeSEMimageofTiO2 NSA/AgInSe2(7)/In2Se3(3)shownin Fig.4e,inwhichtheedgeofthe TiO2 nanosheetsbecomeobscuredcomparedwiththoseofpureTiO2 NSAin Fig.2b.AstheTEMandHRTEMimagesofTiO2 NSA/
AgInSe2(7)/In2Se3(3)shownin Fig.6b,b1,b2,alargeamountofobscuredparticlesareobserved,whichcouldbetheIn2Se3 particlesdepositedonthesurfaceoftheTiO2 NSAbySILARprocess.TheseIn2Se3 layersactasthecompositelayeroftheAgInSe2 NPssensitizedTiO2 NSA,andtheformedmultijunctionstructurecanhelptofurther
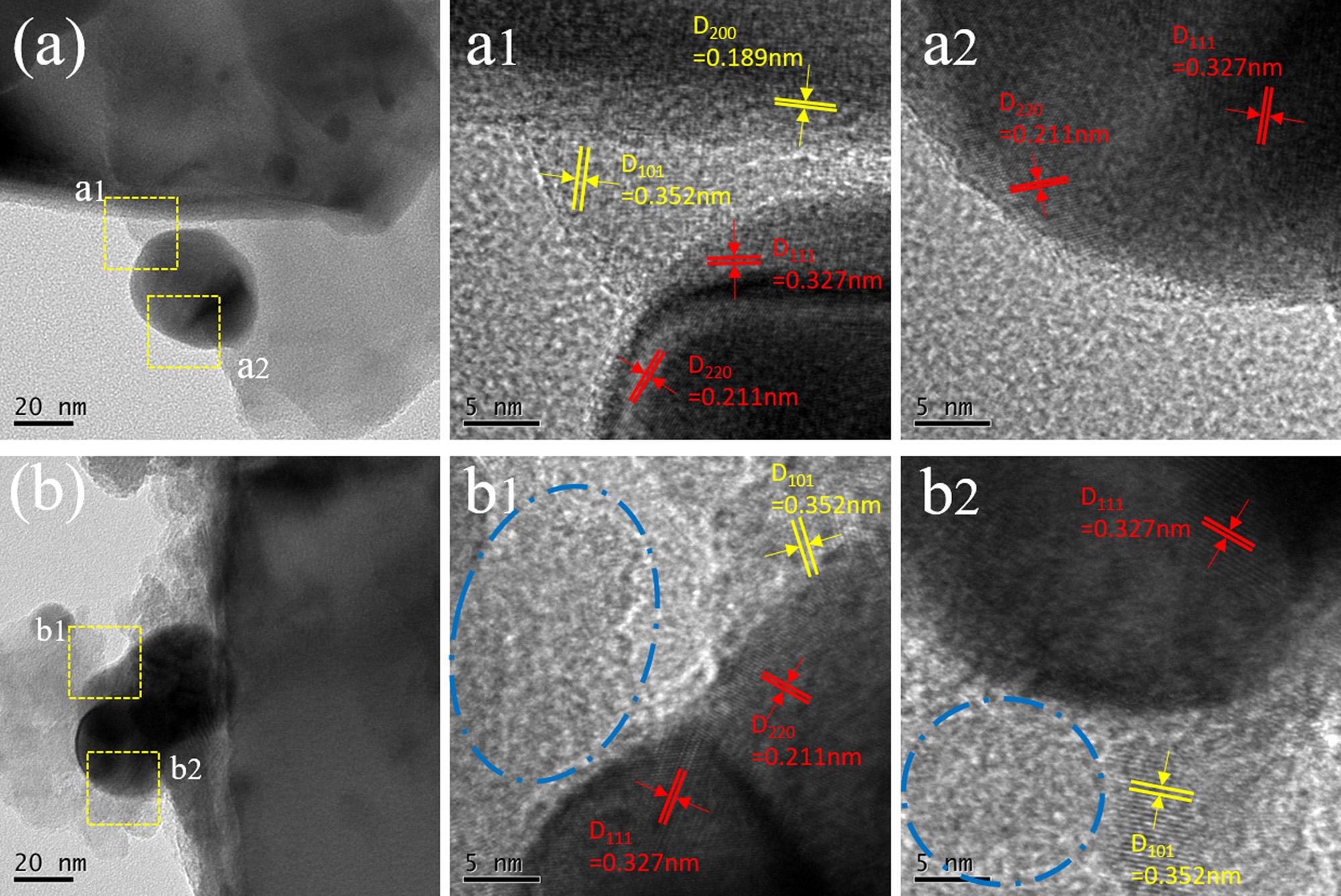
Fig.6. TEMandHRTEMimagesoftheprepared(a)TiO2 NSA/AgInSe2(7)and(b)TiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodes.a1anda2showthecorresponding HRTEMimagesofTiO2 NSA/AgInSe2(7)inthesquareareaof(a);b1andb2showthecorrespondingHRTEMimagesofTiO2 NSA/AgInSe2(7)/In2Se3(3)inthesquare areaof(b).
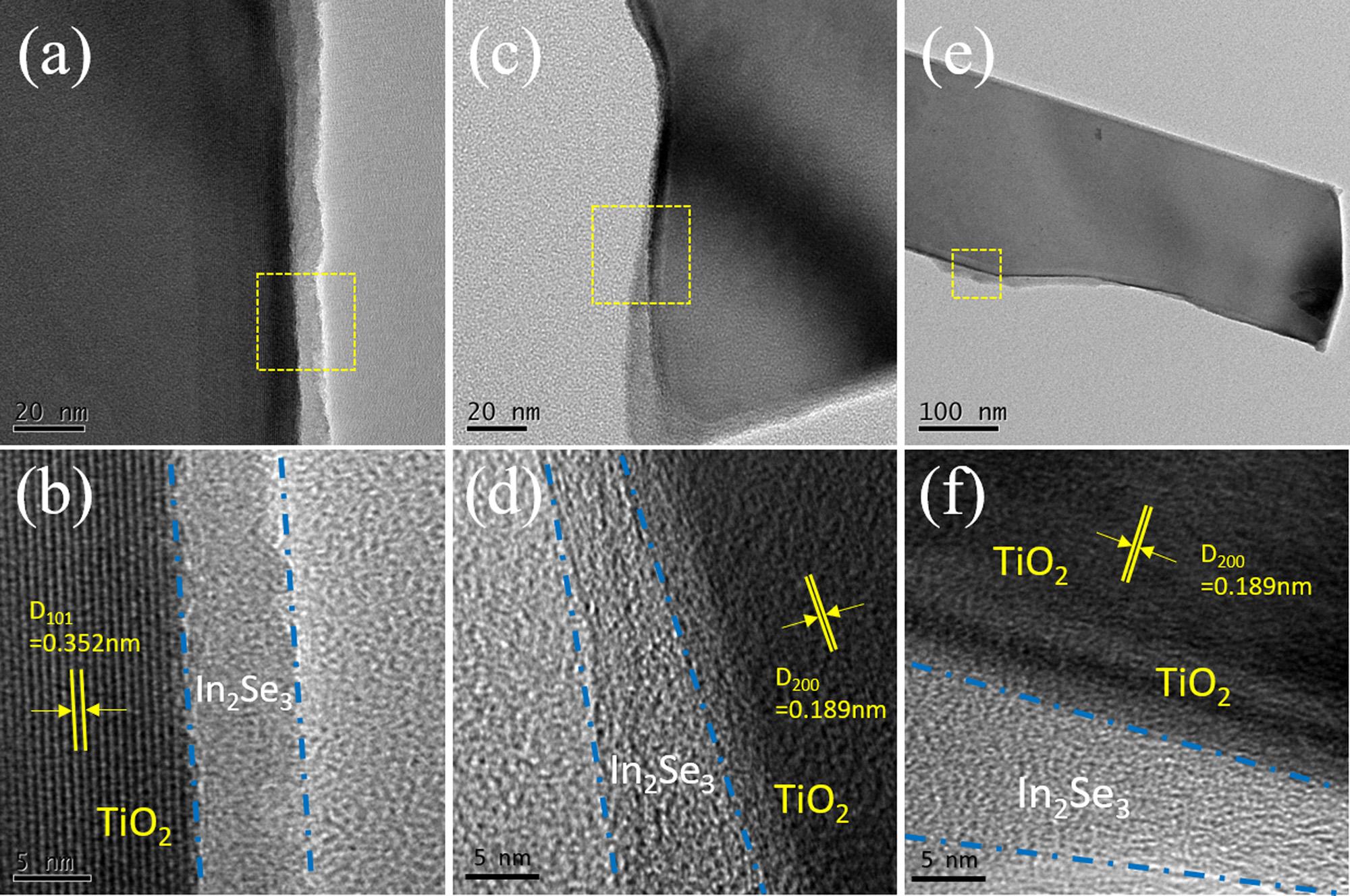
Fig.7. TEMandHRTEMimagesofthepreparedTiO2 NSA/In2Se3 photoanode;a,c,e:TEMimages;b,d,f:thecorrespondingHRTEMimagesinthesquareareas.
separatethephotoinducedelectronsandholesefficiently,andalsothe NSAarchitecturecanpromotethetransferofthephotoinducedelectronsasadirectelectrontransmissionpath.
XPSspectrawereusedtoanalyzethestatesofAg,InandSeinthe preparedTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanode,andtheresults areshownin Fig.8 Fig.8aillustratesthetotalsurveyspectrum,which revealsthepresenceofTi,O,Ag,In,SeaswellasCimpurityfromthe absorptionofCO2 gaseousmoleculesandSifromtheFTOglass.HighresolutionXPSspectraofAg,In,Secoreregionsaregivenin Fig.8b–d. ThebindingenergiesforAg3d5/2 and3d3/2 areobservedat368.3and 374.3eV,respectively,whichareattributedtothemonovalentstateof Ag(Ag+)[68].IntheXPScorelevelspectraofIn3d,twopeaksatthe bindingenergiesof445.3eVand452.9eVareobserved,whichcorrespondtotheIn3d5/2 andIn3d3/2 statesandconfirmthepresenceofthe trivalentnatureofIn(In3+)inpreparedTiO2 NSA/AgInSe2(7)/ In2Se3(3)[41].InSeXPScorelevelspectra,abroadpeakat54.3eV correspondstotheSe3dstateandconfirmstheexistenceofSe2 inthe preparedTiO2 NSA/AgInSe2(7)/In2Se3(3)[69–73].Allofthedetected Ag+,In3+ andSe2 areinagreementwiththepreviousreportsof AgInSe2 andIn2Se3 nanocrystals[69–73],demonstratingthatAgInSe2 andIn2Se3 weresuccessfullypreparedinTiO2 NSA/AgInSe2(7)/ In2Se3(3).
3.2.Analysesoftheopticalpropertiesofthepreparedphotoanodes
TheopticalpropertiesofthepreparedAgInSe2/In2Se3,AgInSe2 and In2Se3 NPssensitizedTiO2 NSAandpureTiO2 NSAnanostructureswere studiedusingUV–visdiffusereflectionspectroscopy,asshownin Fig.9a.Owingtothewidebandgapof3.2eV,theTiO2 NSAshowsits fundamentalabsorptionsharpedgerisingat387nmintheUVlight region.ForIn2Se3 sensitizedTiO2 NSAphotoanode,theadsorptionregionisconsistentwiththatofTiO2 NSA.However,fortheAgInSe2 and
AgInSe2/In2Se3 NPssensitizedTiO2 NSA,theabsorptionpropertiesin thevisible-lightregionof400 800nmareextremelyenhanced,which isduetothesensitizationofAgInSe2 andAgInSe2/In2Se3 NPs.And,the absorptionintensityofTiO2 NSA/AgInSe2(7)/In2Se3(3)ishigherthan thatofTiO2 NSA/AgInSe2(7),whichisrelevanttotheadditionalIn2Se3 compositelayer.DuetothenarrowbandgapofAgInSe2/In2Se3 NPs withvisiblelightresponsecapability[41],thelightabsorptionregion ofTiO2 NSA/AgInSe2(7)/In2Se3(3)isbroadenedtovisiblelightregion, whichovercomestheshortcomingofnarrowphotoresponserangeof TiO2
ThePLanalysesoftheTiO2 NSA/AgInSe2(7)/In2Se3(3),TiO2 NSA/ AgInSe2(7)andTiO2 NSAphotoanodeswerealsoconductedtoreveal theefficiencyofchargecarriertrapping,transferandseparationin semiconductors,andtheirPLemissionspectraareshownin Fig.9b.The lowerPLintensitydemonstratesthelowerrecombinationrateofthe photoinducedelectronsandholes,indicatingahigherPECconversion activity[74].ForTiO2 NSA/AgInSe2(7)sample,thePLemissionpeaks at614,688,712,732,764nmarelowerthanthoseofpureTiO2 NSA photoanode.ForTiO2 NSA/AgInSe2(7)/In2Se3(3),thePLintensitiesare furtherreducedcomparedwiththoseofTiO2 NSA/AgInSe2(7).Therefore,theheterojunctionofTiO2 NSA/AgInSe2(7)willimprovetheseparationofphotogeneratedelectronsandholescomparedwithpure TiO2 NSA.And,themultijunctionofTiO2 NSA/AgInSe2(7)/In2Se3(3) willfurthersignificantlyseparateandtransferthephotoinducedelectronsandholesmoreefficientlycomparedwithTiO2 NSA/AgInSe2(7). ThePLresultsconfirmtheimportanceofthemultijunctionofTiO2 NSA/AgInSe2/In2Se3 inpreventingtherecombinationofthephotoinducedcarriers.
3.3.PECconversionperformanceofthepreparedphotoanodes
Thephotoinducedi-VcurvesoftheTiO2 NSA/AgInSe2(7)/In2Se3(3),
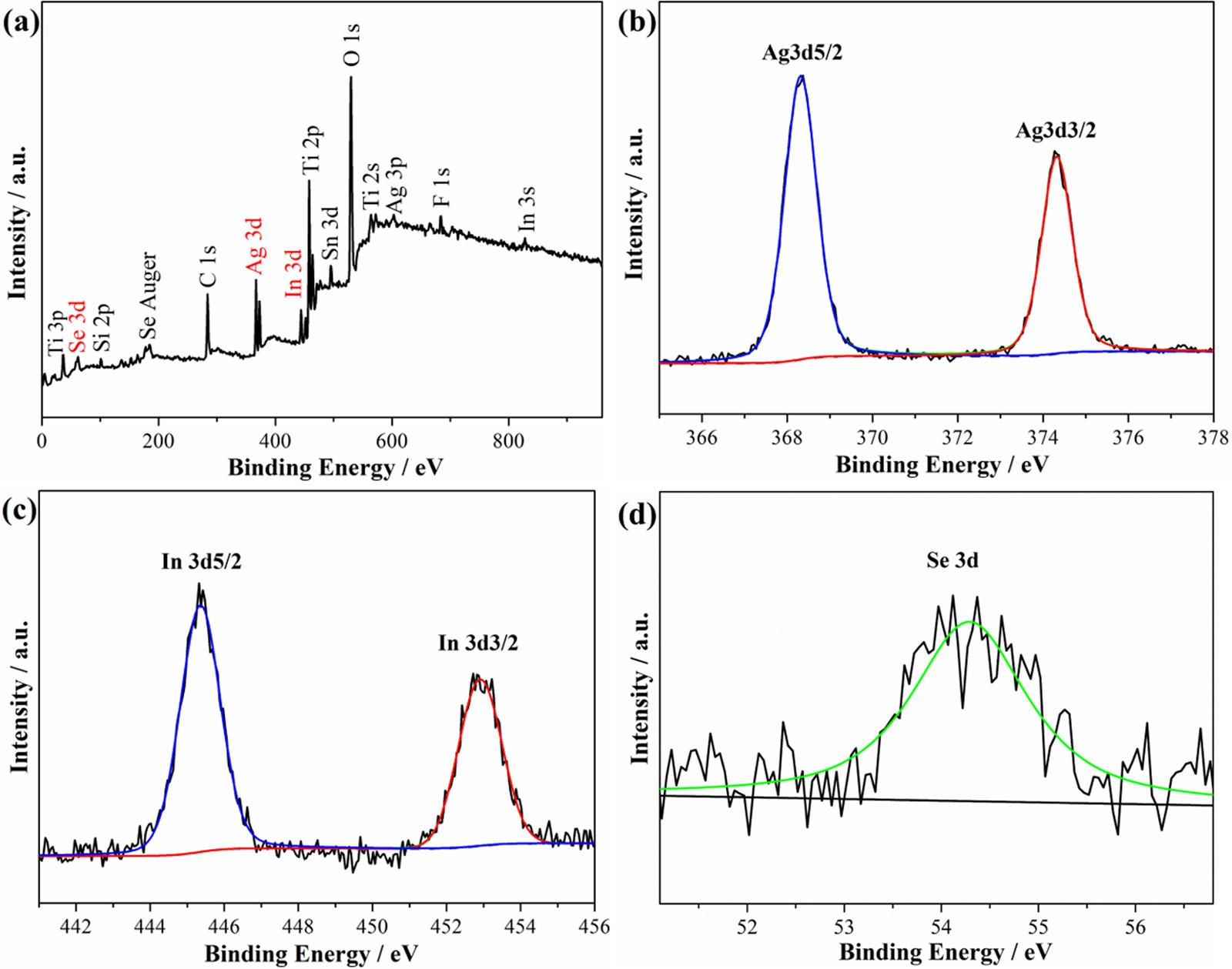
Fig.8. (a)XPSsurveyspectrumandhigh-resolutionXPSspectraof(b)Ag3d,(c)In3dand(d)Se3dofthepreparedTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanode.
TiO2 NSA/AgInSe2(7),TiO2 NSA/In2Se3(3)andTiO2 NSAphotoanodes, togetherwiththeTiO2 NSA/AgInSe2/In2Se3 photoanodeswithdifferent AgInSe2 depositioncyclesaredepictedin Fig.10aandb.Amongallof thesamplesinvestigated,theTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodeexhibitsthehighestphotoinducedcurrentdensity.ThisisattributedtothedepositedIn2Se3 layeraroundAgInSe2 NPsthatfurther improvesthephoto-to-currentconversionthroughthewell-matched energybandandacceleratesthetransferofphotogeneratedelectronsas wellastheseparationofphotoinducedelectron-holesinthesystem. Themultijunctionelectric fieldattheinterfaceofTiO2 NSA/AgInSe2/ In2Se3 facilitatestheseparationofthephotogeneratedcarriersand henceimprovesthePECperformanceofTiO2 NSA/AgInSe2/In2Se3 Fig.10cpresentsthephotoinducedOCPvariationsoftheTiO2 NSA, TiO2 NSA/In2Se3(3),TiO2 NSA/AgInSe2(7),TiO2 NSA/AgInSe2(7)/ In2Se3(3)photoanodesconductedin3.5wt%NaClsolutioninthedark andunderAM1.5lightillumination.Assoonasswitchingonthelight,
theOCPsofthesephotoanodesshiftnegativelyasaresultofthegenerationandaccumulationofthephotoinducedelectronsontheelectrodesurface.Afterswitchingoff thelight,theOCPsofthesephotoanodesshowpositiveshifts.TheTiO2 NSA/AgInSe2(7)/In2Se3(3) photoanodeexhibitsthemaximumphotoinducedpotentialdropof approximately280mV,indicatingthattheTiO2/AgInSe2/In2Se3 multijunctiongreatlyenhancesthePECperformanceunderAM1.5light illumination.ThemorenegativethephotoinducedOCPis,themore negativethequasi-Fermilevelofthephotoanodeis,andthebetter PECCPperformanceis.
ThepotentiodynamicpolarizationcurvesoftheTiO2 NSA,TiO2 NSA/AgInSe2(7),TiO2 NSA/In2Se3(3)andTiO2 NSA/AgInSe2(7)/ In2Se3(3)photoanodesweremeasuredin3.5wt%NaClsolutioninthe absenceandpresenceofAM1.5lightillumination,andtheresultsare shownin Fig.10d.Underlightillumination,theOCPsofthesephotoanodesshifttomorenegativevalues,whichareagreedwiththose
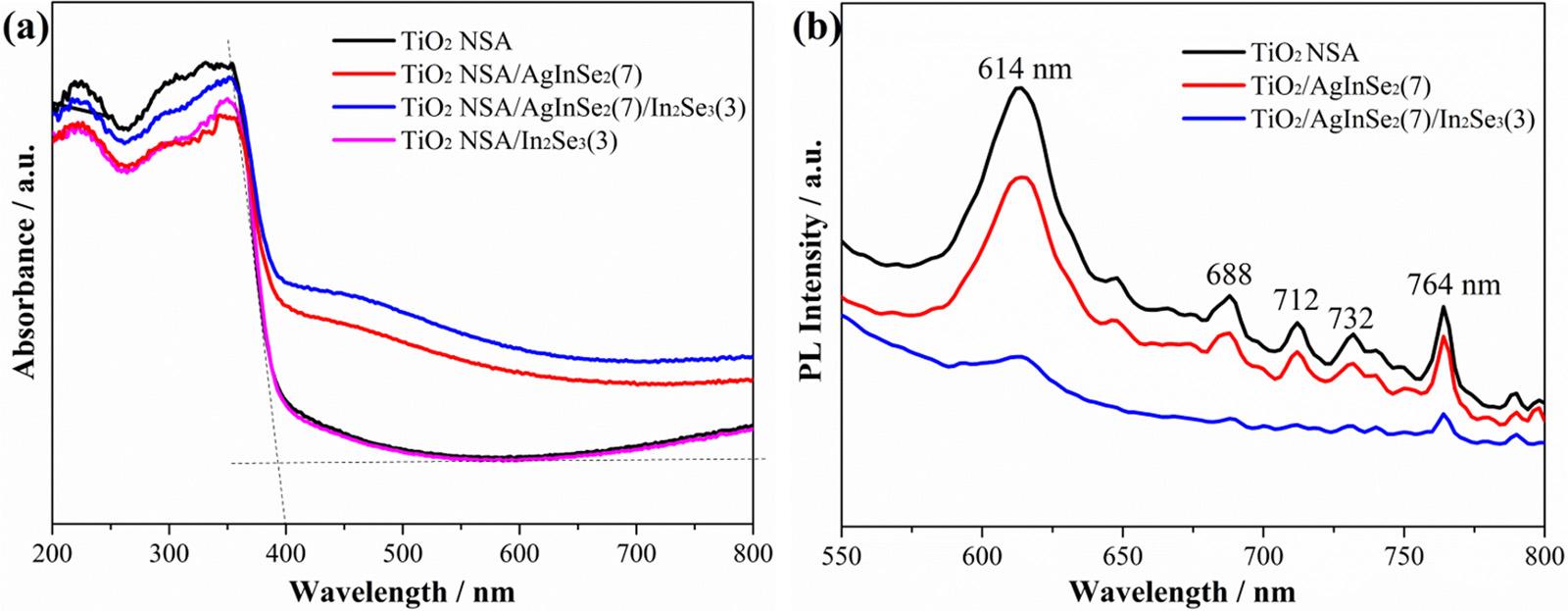
Fig.9. (a)UV/Visdiffusereflectancespectraofthepreparedsamples,(b)PhotoluminescencespectraofpureTiO2 NSA,AgInSe2 andAgInSe2/In2Se3 NPssensitized TiO2 NSA.
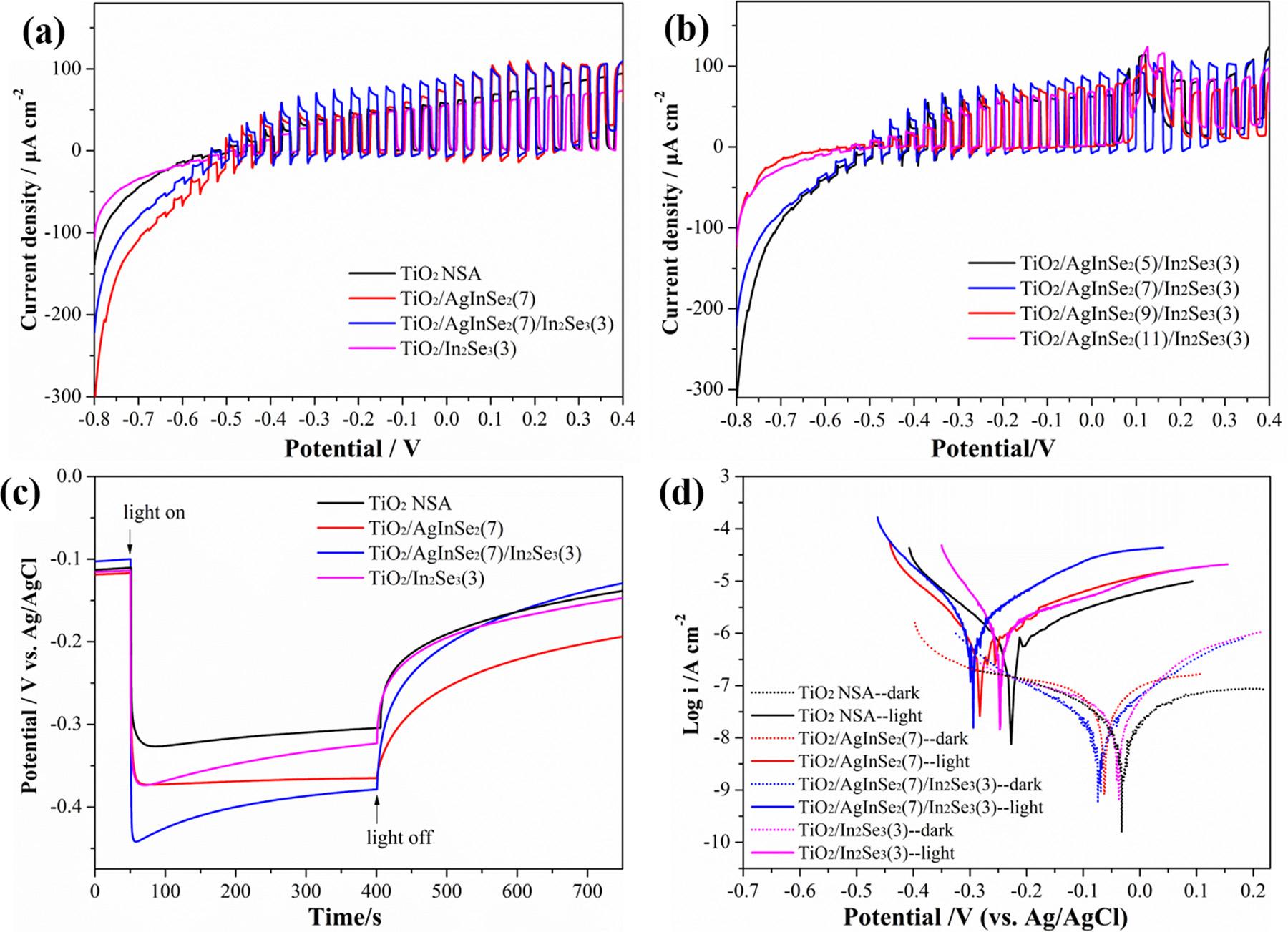
Fig.10. Thephotoinducedvariationsofthei-Vcurvesof(a)theAgInSe2/In2Se3,AgInSe2,In2Se3 NPssensitizedTiO2 NSAphotoanodesandpureTiO2 NSA photoanodeand(b)TiO2 NSA/AgInSe2/In2SephotoanodeswithdifferentAgInSe2 depositioncyclesunderswitchingonandoff theAM1.5lightin0.1MNa2SO4 solution;(c)ThephotoinducedvariationsoftheOCPsoftheAgInSe2/In2Se3,AgInSe2,In2Se3 NPssensitizedTiO2 NSAphotoanodesandpureTiO2 NSAphotoanodein 3.5%NaClsolutionunderAM1.5lightillumination;(d)ThepotentiodynamicpolarizationcurvesoftheTiO2 NSA,TiO2 NSA/AgInSe2(7),TiO2 NSA/In2Se3(3)and TiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodesmeasuredin3.5wt%NaClsolutionintheabsenceandpresenceofAM1.5lightillumination.
observedin Fig.10candareduetothegenerationandaccumulationof photoinducedelectronsonthephotoanodes.Boththeanodicand cathodicpolarizationcurrentdensitiesofthesephotoanodesmeasured underlightilluminationshowsignificantincreasescomparedwith thoseobtainedinthedark,whicharecausedbytheparticipationofthe photogeneratedholesandelectronsintheanodicandcathodicreactionsoftheelectrochemicalpolarizationprocess.Amongwhich,the TiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodepossessesthehighest anodicpolarizationcurrentdensityandthemostnegativeOCPamong allthepreparedphotoanodes.ThisresultindicatesthatthephotoinducedelectronsandholesgeneratedbytheTiO2 NSA/AgInSe2(7)/ In2Se3(3)photoanodeareseparatedtothegreatestextent,whichmakes ithavethemostphotogeneratedholestoparticipateintheanodicwater oxidationreactionsoftheelectrochemicalpolarizationprocessandthus makesitsanodicpolarizationcurrentdensitybeinggreatlyenhanced. Meanwhile,thelargestnumberofthephotoinducedelectronsaccumulateontheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodeduetothe maximumseparationofthephotoinducedcarriersgeneratedbyit.This resultsintheobservationofthemostnegativeOCPofthisphotoanode underlightillumination.Theelectrochemicalpolarizationresults shownin Fig.10dfurtherdemonstratethattheTiO2 NSA/AgInSe2(7)/ In2Se3(3)photoanodepossessesthelargestseparationefficiencyofthe photogeneratedelectronsandholesamongallofthepreparedphotoanodes,whichmakesithavethebestphotoelectrochemicalcathodic protectionperformance.Moreover,forthecoupledphotoanodes/316L SSelectrodes,therearemajorissuesforthepotentiodynamicpolarizationcurvesmeasuredonthembecausetheyaregalvaniccorrosion systems.Whencouplingthephotoanodewiththe316SSelectrode,it becomesimpossibletosortoutwhichelectrodethecurrentiscoming
from.Therefore,thepotentiodynamicpolarizationcurvesofthecoupledphotoanodes/316SSelectrodewerenotperformedinthepresent work.
3.4.PECCPperformanceofthepreparedphotoanodes
InordertocharacterizethePECCPperformanceoftheprepared photoanodes,thephotoinducedvariationsofthecurrentdensitiesof thegalvaniccouplingbetweenthe316LSSelectrodeandthephotoanodeswithoutanyappliedbiaspotentialandthephotoinducedvariationsofthepotentialsofthe316LSSelectrodecoupledwiththe photoanodesunderintermittentsimulatedsunlight(AM1.5)illuminationweremeasured.Theresultsareshownin Fig.11.Duringthe measurements,boththephotoanodeandthe316LSSelectrodewere immersedin3.5wt%NaClsolution. Fig.11ashowsthevariationsin thecurrentdensitiesbetweenthe316LSSelectrodeandtheprepared photoanodes.Positiveexcitationcurrentdensitiesareobtainedunder lightillumination,signifyingthatthephotoinducedelectronsgenerated bythesemiconductortransferfromthephotoanodetothecoupled 316LSSelectrodeandthusprovidecathodicprotectionforit.The variationsofthecurrentdensitiesintheabsenceandpresenceoflight illuminationarethephotoinducedcurrentdensities.Inthiswork,the maximumphotogeneratedcurrentdensityofapproximately7 μAcm 2 belongstotheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanode,whichis muchhigherthanthoseoftheTiO2 NSA/AgInSe2(7)andTiO2 NSA/ In2Se3(3).
Asshownin Fig.11b,thevariationsofthepotentialsofthe316LSS electrodecoupledwiththephotoanodesexhibitnegativeshiftswhen thelightisswitchedon.Withswitchingoff thelight,thepotentials
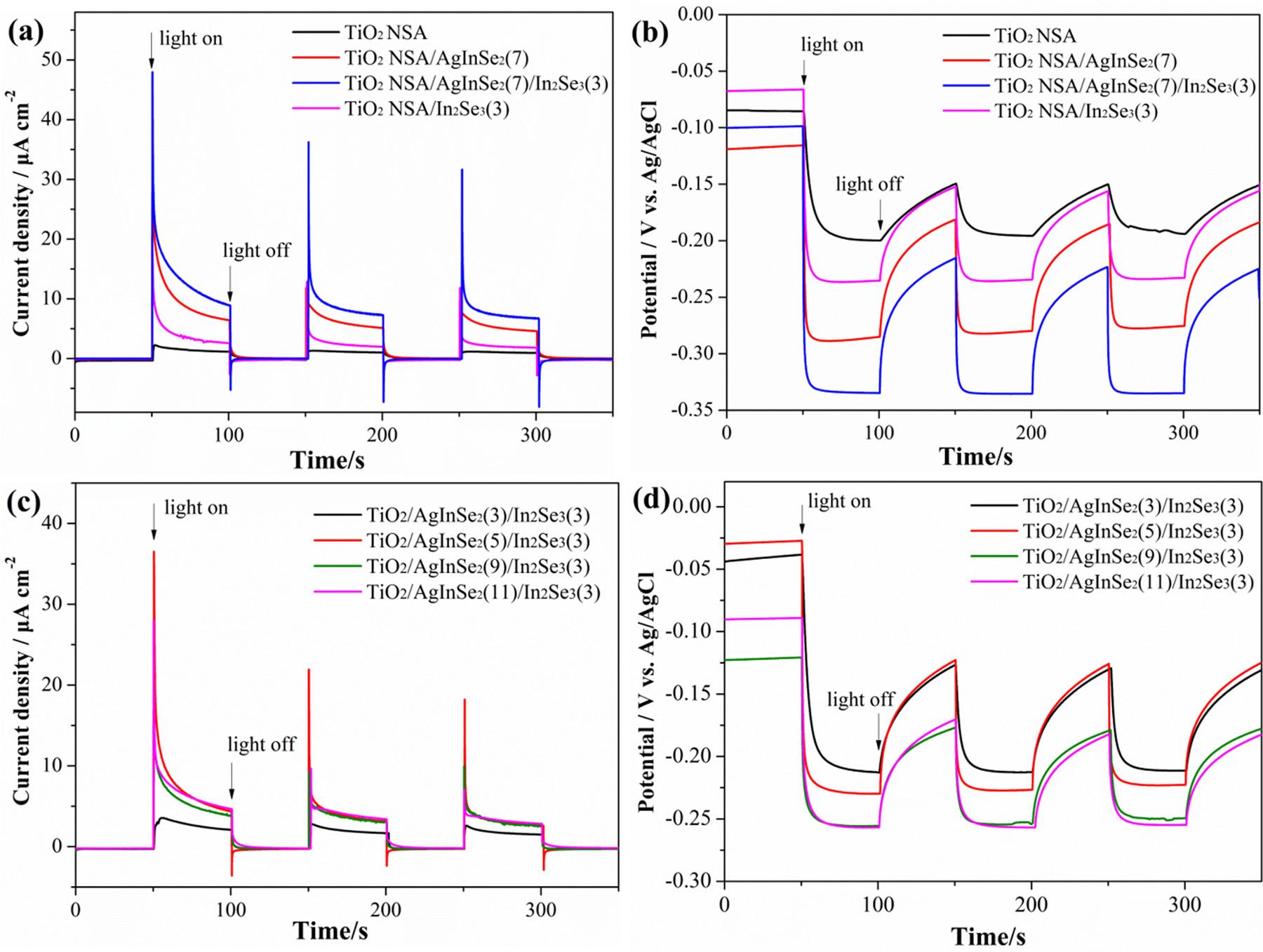
Fig.11. (a)Thephotoinducedvariationsofthecurrentdensitiesbetweenthepreparedphotoanodesandthe316LSSelectrode,(b)thephotoinducedvariationsof themixedpotentialsofthe316LSSelectrodecoupledwiththephotoanodes,(c)thephotoinducedvariationsofthecurrentdensitiesbetweenthe316LSSelectrode andtheAgInSe2/In2Se3 NPssensitizedTiO2 NSAphotoanodespreparedbydepositingdifferentamountofAgInSe2 NPs,and(d)thephotoinducedvariationsofthe mixedpotentialsofthe316LSSelectrodecoupledwiththeAgInSe2/In2Se3 NPssensitizedTiO2 NSAphotoanodespreparedbydepositingdifferentamountofAgInSe2 NPsunderintermittentAM1.5lightilluminationin3.5wt%NaClsolution.
immediatelyshifttowardspositivedirectionandthengoslowlybackto theirinitialpotentials.Thephotoinducedpotentialdropisthedifferenceofthepotentialsintheabsenceandpresenceoflightillumination. Thenegativelyshiftofthepotentialsdemonstratesthatthephotoinducedelectronsgeneratedbythephotoanodearetransferredtothe coupled316LSSelectrode,therebyprovidingcathodicprotectionfor 316LSS.Theseresultsareconsistentwiththoseofthephotoinduced currentdensitiesshownin Fig.11a.Thephotoinducedpotentialdrops oftheTiO2 NSA/AgInSe2(7)/In2Se3(3)-316LSS(approximately 236mV)arealsomuchhigherthanthoseofotherphotoanodes.The multijunctionTiO2 NSA/AgInSe2/In2Se3 furtherenhancestheseparationefficiencyofthephotoinducedelectronsandholesbythemultijunctioneffect,andthusimprovesthePECCPperformanceunder AM1.5lightillumination.BecauseofthedifferenceintheFermilevels andenergybandstructuresofAgInSe2,In2Se3 andTiO2,numerousinternalmultijunctionelectrostatic fieldscanbebuiltattheinterfacesof thepreparedTiO2 NSA/AgInSe2(7)/In2Se3(3),thustheseparationof thephotoinducedelectronsandholeswillbegreatlypromotedunder theexcitationofthesimulatedsunlight.Asaconsequence,theTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodeexhibitsthehighestPECCP performance.
Moreover,theeffectofthedepositionamountofAgInSe2 NPsonthe PECCPperformanceofthepreparedTiO2 NSA/AgInSe2/In2Se3 photoanodefor316LSSunderintermittentAM1.5lightilluminationhasalso beenstudiedbychangingthedepositioncyclesofAgInSe2,andthe resultsareshownin Fig.11candd.ForTiO2 NSA/AgInSe2(3)/In2Se3(3) photoanode,thephotogeneratedcathodicprotectioncurrentdensity andthephotoinducedpotentialdropare2 μAcm 2 and160mV,
respectively,demonstratingalowPECCPefficiencyduetotheinsufficientloadingamountofAgInSe2 NPs.Withtheincreaseofthe loadingamountofAgInSe2 NPs,thePECCPperformanceisenhanced, andtheTiO2 NSA/AgInSe 2(7)/In2Se3(3)photoanodeexhibitsthelargestphotoinducedcathodicprotectioncurrentdensityof7 μAcm 2 in NaClsolutionandthephotoinducedpotentialdropof236mV.Withthe furtherincreaseoftheloadingamountofAgInSe2 NPs,thedecreased PECCPperformanceisobserved.TheexcessivedepositionofAgInSe2 NPswillleadtotheagglomerationofthedepositedNPs.Thisreduces theeffectiveheterojunctionarea,therebyreducingthemultijunction effectamongAgInSe2,In2Se3 andTiO2 NSA,andcausingtherecombinationofthephotoinducedelectronsandholes.Theexcessive depositionofAgInSe2 NPsresultsintheagglomerationofAgInSe2 NPs canalsobeprovedbytheSEMimages(Fig.3d),fromwhichtheNPs loadedonTiO2 NSAbecomemoreandthenclustertogetherwiththe increasedloadingamountofAgInSe2 NPs.
Inordertocharacterizethestabilityofpreparedphotoanodes,the SEMimageoftheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodeafterthe PECCPtestsandtheXRDpatternsandtheFTIRspectraoftheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodebeforeandafterthePECCP testswererecorded,andtheresultsareshownin Fig.12.Asshownin Fig.12a,themicromorphologyofTiO2 NSA/AgInSe2(7)/In2Se3(3) photoanodeafterPECCPdoesnotchangesignificantly,maintainingthe samemorphologyofthatbeforethePECCPtests(Fig.4b).Besides,the XRDpatternafterPECCPtestsshownin Fig.12bwasbasicallyconsistentwiththatbeforethePECCPtests.Furthermore,theFTIRspectrumoftheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodeafterPECCP testsarehighlyconsistentwiththosebeforePECCPtests,asshownin
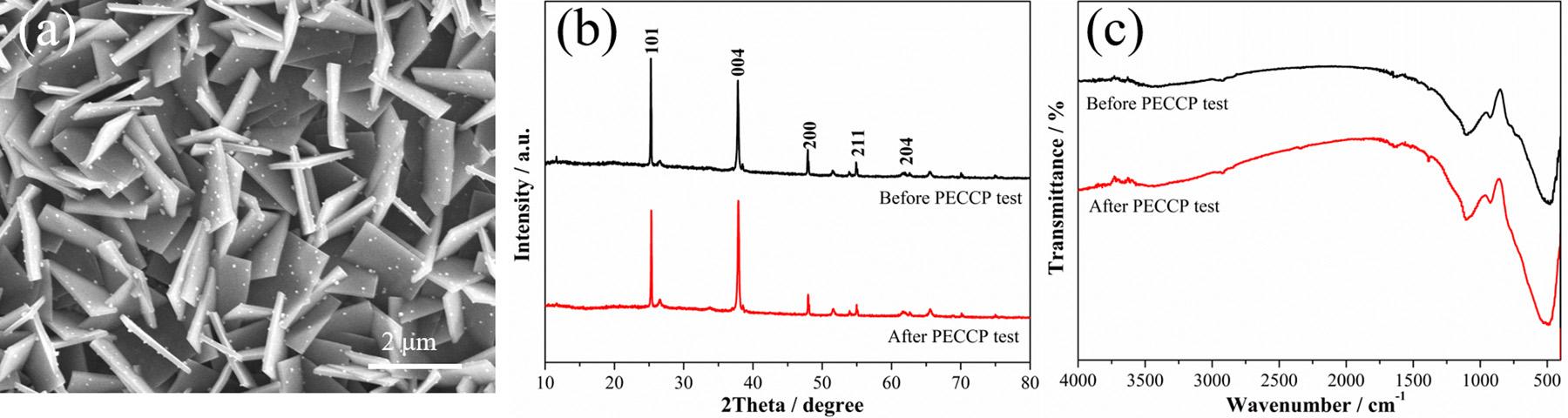
Fig.12. (a)SEMimageoftheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodeafterPECCPtests,(b)XRDpatternsand(c)FTIRspectraoftheTiO2 NSA/AgInSe2(7)/ In2Se3(3)photoanodebeforeandafterPECCPtests.
Fig.12c.Theresultsshownin Fig.12 indicatethattheTiO2 NSA/ AgInSe2(7)/In2Se3(3)photoanodehasgoodPECCPstability.
3.5.Chargetransferpropertyand flatbandpotentialoftheprepared photoanodes
EISanalyseswereperformedtostudythechargetransferproperty ofthepreparedphotoanodes,andtheresultsareshownin Fig.13a.In general,smallerdiameterofthesemicirclearcoftheNyquistplotindicatesfasterinterfacialchargetransfercapability.Asshownin Fig.13a,thediametersofthesemicirclearcdecreaseintheorderof TiO2 NSA>TiO2/In2Se3(3)>TiO2/AgInSe2(7)>TiO2/AgInSe2(7)/ In2Se3(3),indicatingthehighestinterfacechargetransferefficiencyof TiO2/AgInSe2(7)/In2Se3(3).TheNyquistplotswere fittedusingthe equivalentcircuitshownintheinsetin Fig.13a.Inthisequivalent electricalcircuit,Rs representsthesolutionresistance;Qmeansthe constantphaseangleelement,whoseimpedanceisequalto (Y0(jω)n) 1,and ω istheac-voltageangularfrequency(rads 1), Y0 and narethefrequency-independentparameters.Rf andQc representthe resistanceandcapacitanceofthesurface film,respectively.Rct andQdl representtheresistanceandcapacitanceofthedoublelayer,respectively,and W representstheWarburgresistance.In Fig.13a,themeasureddataarethedotswithdifferentsymbols,andthe fittedresultsare thesolidlines.Themeasureddataare fittedverywell.The fitted parametersarelistedin Table1.Asshownin Table1,Rf ofTiO2/ AgInSe2(7)/In2Se3(3)andTiO2/AgInSe2(7)aresmallerthantheother twophotoanodes,suggestingthattheresistancesoftheTiO2/ AgInSe2(7)/In2Se3(3)andTiO2/AgInSe2(7)aredecreasedwiththedepositionofAgInSe2 NPs.AndtheTiO2 NSA/AgInSe2(7)/In2Se3(3) photoanodeshowsthesmallest Rf value,revealingthatTiO2 NSA/ AgInSe2(7)/In2Se3(3)photoanodepossessesthesmallestresistanceand
thehighestelectrontransmissionperformance.Thisalsoindicatesthat theelectrontransferisfurtheracceleratedandtheinterfacialcharge transferbarrierisfurtherreducedafterdepositingtheIn2Se3 layer, leadingtothepromotionofthePECandPECCPperformanceofthe TiO2 NSA/AgInSe2(7)/In2Se3(3)photoanode.
Mott-Schottkyplotreportsontherelationbetweenthecapacitance ofthespacechargeregionandtheappliedpotentialwiththespecific formulaforann-typesemiconductorlistedasfollows[3]:
(1) where Csc isthecapacitanceofthespacechargeregioninthesemiconductor, ε istherelativepermittivityofthesemiconductor, ε0 isthe vacuumpermittivity(8.854×10 14 Fcm 1), e istheelectroncharge (1.602189×10 19 C), ND isthechargecarrierdensity, E istheapplied potential, Efb isthe flatbandpotential, k istheBoltzmannconstant (1.38066×10 23 JK 1)and T istheabsolutetemperature(298K). Mott-Schottkyplotsofthepreparedphotoanodesareshownin Fig.13b. TheslopesoftheMott-Schottkyplotsofthepreparedphotoanodesare positive,indicatingthen-typesemiconductorcharacteristicsofthe preparedsamples.AccordingtoEq. (1),the Efb ofthesemiconductors canbeobtainedfromthehorizontalintercept.Asshownin Fig.13b,the Efb ofTiO2 NSAisapproximately-0.46V(vs.Ag/AgCl),whichequalsto -0.26V(vs.SHE).Consideringthen-typesemiconductorcharacteristics ofthepreparedmaterials,itcanbeconcludedthattheCBpotentialof pureTiO2 locatesatapproximately-0.26V(vs.SHE),whichiscloseto thereported-0.29V(vs.SHE)oftheCBofTiO2 [17].Afterdecorated withAgInSe2 NPs,the Efb negativelyshiftsto-0.57V(vs.Ag/AgCl), revealingthattheFermilevel(Ef)ofTiO2 waspulledtoamorenegative valueandformamorenegative Eco-f duetothedepositedAgInSe2 NPs withmorenegativeenergyband.Furthermore,afterIn2Se3 decoration,
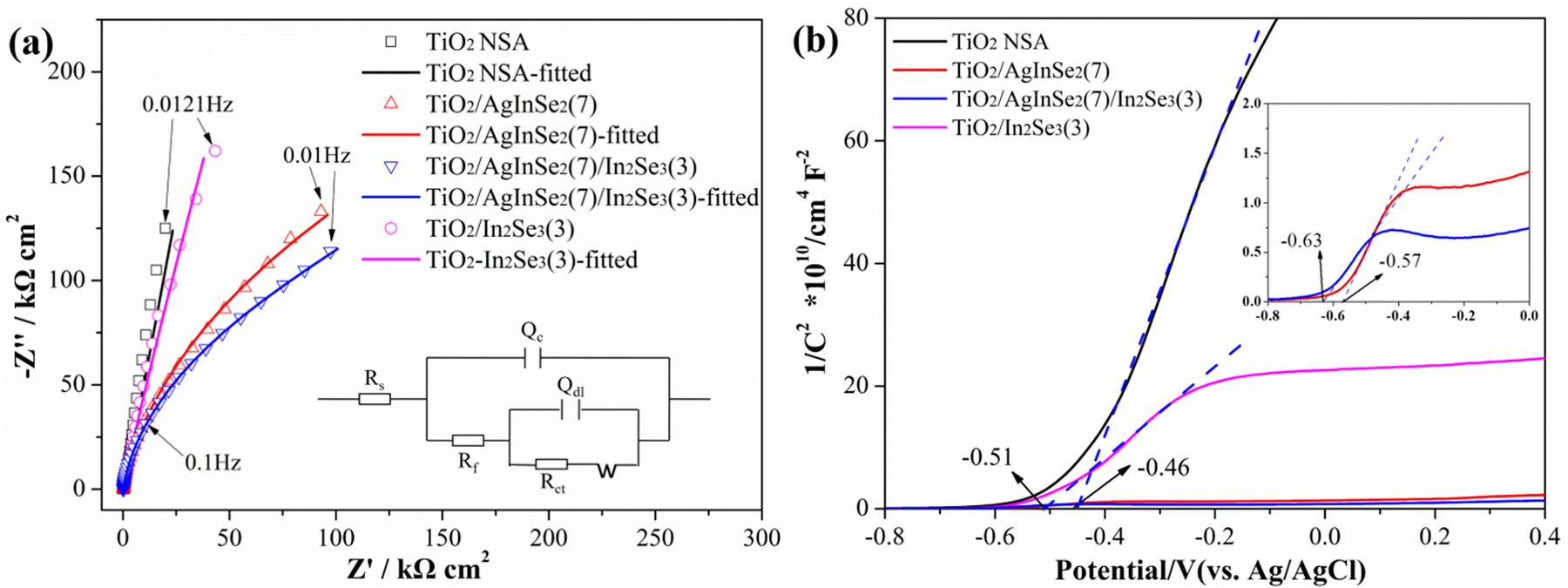
Fig.13. (a)EISplotsoftheAgInSe2/In2Se3,AgInSe2,In2Se3 NPssensitizedTiO2 NSAphotoanodes,andpureTiO2 NSAphotoanodein0.1MNa2SO4 solutioninthe dark.(b)Mott-SchottkyplotsoftheAgInSe2/In2Se3,AgInSe2,In2Se3 NPssensitizedTiO2 NSAphotoanodes,andpureTiO2 NSAphotoanodein0.1MNa2SO4 solution inthedark.
Table1
FittedparametersoftheEISequivalentcircuitdatashownin Fig.13a.
theTiO2/AgInSe2(7)/In2Se3(3)photoanodeshowsthemostnegative Efb of-0.63V(vs.Ag/AgCl).ThecomparativelynegativeEfb denoteshigher activityandutilizationrateofthephotogeneratedelectronsforreductionreactions,suchasthephotocatalytichydrogenevolutionfrom watersplitting,aswellasthePECCPformetallicmaterials.
Furthermore,the ND ofthesemiconductorsisinverselyproportional totheslopeoftheMott-Schottkyplots.Thesmallerslopecontributesto thelarger ND anddenotesthehigherconcentrationofchargecarriers [3].From Fig.13b,theobtainedthechargecarrierdensitycanbe rankedas ND (TiO2 NSA/AgInSe2(7)/In2Se3(3))> ND (TiO2 NSA/ AgInSe2(7))> ND (TiO2 NSA/In2Se3(3))> ND (TiO2 NSA).TheTiO2 NSA/AgInSe2(7)/In2Se3(3)hasthesmallestslope,correspondingtothe largest ND andthehighestchargecarrierdensity.Thiswillbenefitthe generationandtransferofphotoinducedelectronsinann-typesemiconductorphotoanode.Therefore,theaforementionedresultsreveal thattheTiO2 NSA/AgInSe2(7)/In2Se3(3)withcomparativelynegative Efb andhighchargecarrierdensitypossessessuperiorPECconversion capabilityintheutilizationofelectrons.
3.6.PromotionmechanismofthePECandPECCPperformanceofTiO2 NSA/AgInSe2/In2Se3 photoanode
Fig.14 schematicallydescribesthemechanismfortheimproved PECconversionandPECCPperformanceofTiO2 NSA/AgInSe2(7)/ In2Se3(3)underAM1.5lightinNaClsolution.InthepreparedTiO2 NSA/AgInSe2/In2Se3 multijunction,AgInSe2 andIn2Se3 arevisiblelight-responsivesemiconductors.Thebandgap(Eg)ofAgInSe2 is 1.57eVwiththeCBandVBpotentialsof-1.64Vand-0.075V(vs.SHE), respectively[41].Besides,the Eg ofIn2Se3 is1.35eV,whoseCBandVB potentialslocateat-0.83Vand0.95V(vs.SHE),respectively[70,75].
The Eg,CBandVBofTiO2 are3.2eV,-0.29Vand2.91V(vs.SHE), respectively[17].TheCBofIn2Se3 islocatedbetweenTiO2 and
AgInSe2,makingitbemoreefficientintransferringphotoinduced electronsbetweentheAgInSe2/TiO2 heterojunction.OncetheTiO2 NSA/AgInSe2(7)/In2Se3(3)isexcitedbysimulatedsunlight,theelectronsintheVBsofAgInSe2,In2Se3 andTiO2 areexcitedtotheirCBsto producethephotogeneratedelectrons.Duetothedifferenceinenergy bandpotentialsofAgInSe2,In2Se3 andTiO2,whoseCBpotentialsarein theorderof ECB(AgInSe2)< ECB(In2Se3)< ECB(TiO2),thephotoinduced electronsgeneratedontheCBofAgInSe2 willtransfertotheCBof In2Se3 andthenfurthertotheCBofTiO2 todecreasetheenergyofthe system.Simultaneously,withtheVBpotentialsintheorderof EVB (AgInSe2)< EVB(In2Se3)< EVB(TiO2),thephotoinducedholesgeneratedontheVBofTiO2 canbetransferredtotheVBofIn2Se3 andfurther totheVBofAgInSe2 and finallyparticipateintheoxidationreaction withtheambientNaClsolution.Therefore,constructinganIn2Se3 compositelayeraroundAgInSe2 helpstheformationofTiO2 NSA/ AgInSe2/In2Se3 multijunctionwithwell-matchedenergybandstructure.TheTiO2 NSA/AgInSe2/In2Se3 multijunctionwillfurtherfacilitate thetransferofthephotogeneratedelectronsandholesbetweenTiO2 andAgInSe2,asdescribedin Fig.14.Besides,theNSAstructurecan offeralargelight-harvestingareaandfastelectrontransmission channel,andthusincreaselightabsorptionareaandpromotethe transferofphotogeneratedelectronstowardssubstrate.Therefore,the TiO2 NSA/AgInSe2/In2Se3 photoanodeexhibitsthehighestphoto-tocurrentconversionefficiency.
SincetheFermilevelsofAgInSe2 andIn2Se3 arerelativelynegative, theTiO2 NSA/AgInSe2/In2Se3 multijunctionwillpulltheFermilevelof TiO2 NSAtoanegativedirection,asillustratedintheMott-Schottky plots.Whenann-typesemiconductorisexposedtosimulatedsunlight, thephotoinducedelectronswillaccumulateonitsCB,inducinganegativeshiftofthequasi-Fermilevelofthephotogeneratedelectrons. Then,thepotentialofthesystemwillnegativelyshift.Hence,forthe TiO2 NSA/AgInSe2(7)/In2Se3(3),alargeamountofthephotoinduced
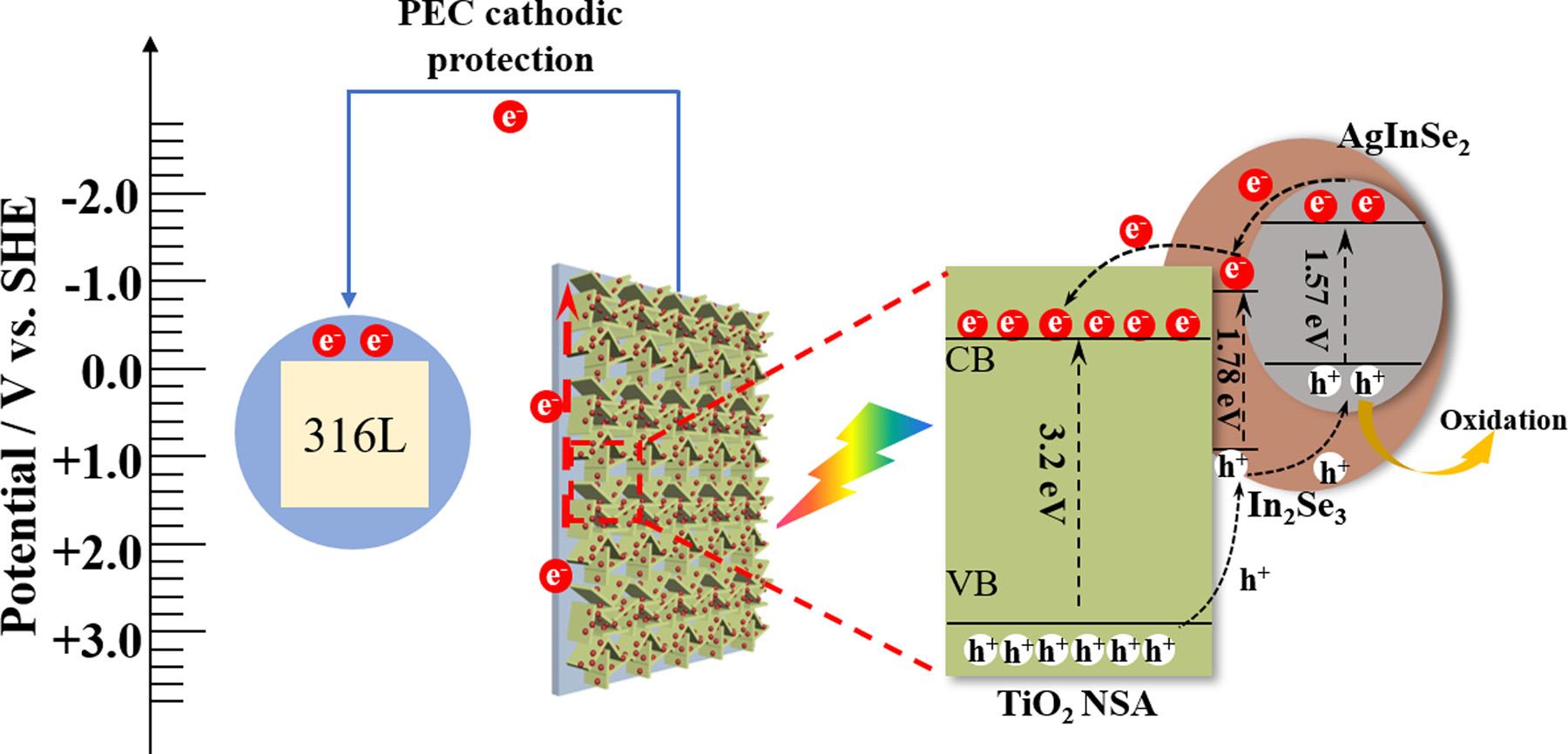
Fig.14. ProposedmechanismsfortheenhancedPECaswellastheenhancedPECCPperformanceofTiO2
electronswillpushthequasi-Fermilevelofthephotogeneratedelectronstoamorenegativelevelthanthosegeneratedbyotherphotoanodes.WhencouplingtheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodewiththe316LSSelectrode,thephotogeneratedelectronswillbe transferredtothecoupled316LSSelectrodeandachievethePECCP effectforit.Therefore,theTiO2 NSA/AgInSe2(7)/In2Se3(3)exhibitsan excellentPECCPpropertyfor316LSSundersimulatedsunlightilluminationandshowsgreatapplicationpotentialsinthe fieldofthe PECCPformetals.Theoptimizationofmultijunctionphotoanodewitha muchnegativebandpotentialcanbebeneficialforthePECCPapplicationofmetalsinmarineenvironment.
4.Conclusions
Theenvironment-friendlyAgInSe2/In2Se3 NPsdecoratedTiO2 NSA photoanodewithamultijunctionstructurewasfabricatedinthispaper. TheTiO2 NSAwithnumerousvertically-growntwo-dimensionalnanosheetscanofferalargelightharvestarea,andthedirectelectron transferpathways,whichbenefitstheseparationofthephotogenerated electronsandholes.Meanwhile,theconstructedTiO2 NSA/AgInSe2/ In2Se3 multijunctionavoidstheunmatchedenergybandarrangement betweenAgInSe2 andTiO2 comparedwithsingleAgInSe2 NPsdecoratedTiO2 NSA,andcanleadtothefurtherpromotionofthecharge generation/separationefficiencyundersimulatedsunlightillumination. FortheTiO2 NSA/AgInSe2(7)/In2Se3(3)photoanode,AgInSe2 and In2Se3 arepreparedatoptimalquantity,anddistributedevenlyonthe surfaceofTiO2 NSA,formingcloseinterfacialadhesiontoTiO2 NSA. Thelightresponserangeisbroadenedduetothevisiblelightresponse ofnarrowbandgapofAgInSe2/In2Se3 NPs.ThePLresultsreflectthat theTiO2 NSA/AgInSe2/In2Se3 multijunctionphotoanodeleadstothe swiftseparationofthephotogeneratedelectronsandholescompared withthebi-junctionofTiO2 NSA/AgInSe2.Moreover,fortheTiO2 NSA/ AgInSe2(7)/In2Se3(3)photoanode,EISandMott-Schottkyanalysesindicatethatthereducedchargetransferbarrier,themorenegativeEfb as wellasthehighestchargecarrierdensitybenefittothetransmissionof thephotogeneratedelectrons.Undersimulatedsunlightillumination, themultijunctionsystemexhibitsenhancedthephoton-to-current conversionactivity.ThisstudyconfirmsthatthecomplexofAgInSe2 sensitizerwithIn2Se3 assistlayercanoptimizetheinterfacialmicrostructureofthephotoanode,therebyeffectivelyimprovingthePEC activity.Whencouplingthemultijunctionphotoanodewith316LSS, thephotogeneratedelectronsonthephotoanodecanbetransportedto themetallicmaterialandprovidecathodicprotectionforit.Finally,the TiO2 NSA/AgInSe2(7)/In2Se3(3)photoanodewithamuchmorenegativebandpotentialexhibitsafurtherenhancedPECCPperformancefor 316LSSbyprovidingthephotogeneratedcathodicprotectioncurrent densityof7 μAcm 2 andthephotoinducedpotentialdropof236mV undersimulatedsunlightilluminationinNaClsolution.
Dataavailability
Theraw/processeddatarequiredtoreproducethe findingscannot besharedatthistimeasthedataalsoformspartofanongoingstudy.
CRediTauthorshipcontributionstatement
XuhongJiang: Investigation,Datacuration,Formalanalysis, Validation,Visualization,Methodology,Writing-originaldraft, Writing-review&editing. MengmengSun: Fundingacquisition, Resources,Investigation,Datacuration,Formalanalysis,Validation, Visualization,Methodology,Writing-originaldraft,Writing-review& editing. ZhuoyuanChen: Fundingacquisition,Resources,Projectadministration,Supervision,Methodology,Validation,Conceptualization, Datacuration,Writing-originaldraft,Writing-review&editing. JiangpingJing: Fundingacquisition,Investigation,Datacuration, Formalanalysis,Validation,Visualization,Methodology. ChangFeng:
Investigation,Datacuration,Formalanalysis,Validation,Visualization, Methodology.
DeclarationofCompetingInterest
Theauthorsdeclarethattheyhavenoknowncompeting financial interestsorpersonalrelationshipsthatcouldhaveappearedtoinfluencetheworkreportedinthispaper.
Acknowledgements
Thisworkwas financiallysupportedbytheNationalNaturalScience FoundationofChina(GrantNos.41676069,41976036,41906034), KeyResearchandDevelopmentProgramofShandongProvince(Grant No.2019GHY112085,2019GHY112066),andQingdaoAppliedBasic ResearchPlanProgram(GrantNo.19-6-2-79-cg).
References
[1] B.Hou,X.Li,X.Ma,C.Du,D.Zhang,Z.Meng,W.Xu,D.Lu,F.Ma,Thecostof corrosioninChina,NPJMater.Degrad.1(2017)4
[2] M.M.Sun,Z.Y.Chen,J.Q.Yu,Highlyefficientvisiblelightinducedphotoelectrochemicalanticorrosionfor304SSbyNi-dopedTiO2,Electrochim.Acta109 (2013)13–19
[3] J.P.Jing,Z.Y.Chen,Y.Y.Bu,M.M.Sun,W.Q.Zheng,W.B.Li,Significantlyenhancedphotoelectrochemicalcathodicprotectionperformanceofhydrogentreated Cr-dopedSrTiO3 byCr6+ reductionandoxygenvacancymodification,Electrochim. Acta304(2019)386–395
[4] G.K.Mor,O.K.Varghese,M.Paulose,K.Shankar,C.A.Grimes,Areviewonhighly ordered,verticallyorientedTiO2 nanotubearrays:fabrication,materialproperties, andsolarenergyapplications,Sol.EnergyMater.Sol.Cells90(2006)2011–2075
[5] K.Zhu,N.R.Neale,A.Miedaner,A.J.Frank,Enhancedcharge-collectionefficiencies andlightscatteringindye-sensitizedsolarcellsusingorientedTiO2 nanotubesarrays,NanoLett.7(2007)69–74
[6] J.Su,L.Zhu,P.Geng,G.Chen,Self-assemblygraphiticcarbonnitridequantumdots anchoredonTiO2 nanotubearrays:anefficientheterojunctionforpollutantsdegradationundersolarlight,J.Hazard.Mater.316(2016)159–168
[7] B.O’Regan,M.Grätzel,Alow-cost,high-efficiencysolarcellbasedondye-sensitizedcolloidalTiO2 films,Nature353(1991)737–740
[8] B.Liu,S.Eray,Growthoforientedsingle-crystallinerutileTiO2 nanorodson transparentconductingsubstratesfordye-sensitizedsolarcells,J.Am.Chem.Soc. 131(2009)3985–3990
[9] X.Zheng,X.Liu,W.Wang,X.Wang,C.Liu,X.Qian,Z.Li,Z.Zhang,Enhanced photoelectrochemicalandphotocatalyticperformanceofTiO2 nanorodarrays/CdS quantumdotsbycoatingTiO2 throughatomiclayerdeposition,NanoEnergy11 (2015)400–408
[10] D.Spanu,S.Recchia,S.Mohajernia,O.Tomanec,S.Kment,R.Zbořil,P.Schmuki, M.Altomare,Templateddewetting-alloyingofNiCubilayersonTiO2 nanotubes enablesefficientnoblemetal-freephotocatalyticH2 evolution,ACSCatal.8(2018) 5298–5305.
[11] M.M.Momeni,S.H.Khansari-Zadeh,H.Farrokhpour,Fabricationoftungsten-irondopedTiO2 nanotubesviaanodization:newphotoelectrodesforphotoelectrochemicalcathodicprotectionundervisiblelight,SNAppl.Sci.1(2019)1160
[12] M.M.Momeni,M.Mahvari,Y.Ghayeb,PhotoelectrochemicalpropertiesofironcobaltWTiO2 nanotubephotoanodesforwatersplittingandphotocathodicprotectionofstainlesssteel,J.Electroanal.Chem.(Lausanne)832(2019)7–23
[13] M.M.Momeni,Y.Ghayeb,N.Moosavi,PreparationofNi-Pt/Fe-TiO2 nanotube films forphotoelectrochemicalcathodicprotectionof304stainlesssteel,Nanotechnology 29(2018)425701
[14] M.M.Momeni,M.Taghinejad,Y.Ghayeb,R.Bagheri,Z.Song,Preparationof variousboron-dopedTiO2 nanostructuresbyinsituanodizingmethodandinvestigationoftheirphotoelectrochemicalandphotocathodicprotectionproperties, J.Iran.Chem.Soc.16(2019)1839–1851
[15] L.Yang,W.H.Wang,H.Y.Jiang,Q.H.Zhang,H.H.Shan,M.Zhang,K.R.Zhu, J.G.Lv,G.He,Z.Q.Sun,ImprovedSERSperformanceofsingle-crystallineTiO2 nanosheetarrayswithcoexposed{001}and{101}facetsdecoratedwithAgnanoparticles,Sens.ActuatorsBChem.242(2017)932–939
[16] S.Feng,J.Yang,H.Zhu,M.Liu,J.Zhang,J.Wu,J.Wan,Synthesisofsingle crystallineanataseTiO2 (001)tetragonalnanosheet-array filmson fluorine-doped tinoxidesubstrate,J.Am.Ceram.Soc.94(2011)310–315
[17] X.Jiang,M.Sun,Z.Chen,J.Jing,C.Feng,AnultrafinehyperbranchedCdS/TiO2 nanolawnphotoanodewithhighlyefficientphotoelectrochemicalperformance,J. Alloys.Compd.(2019)152533
[18] F.Shao,J.Sun,L.Gao,S.W.Yang,J.Q.Luo,Forest-likeTiO2 hierarchicalstructures forefficientdye-sensitizedsolarcells,J.Mater.Chem.22(2012)6824–6830.
[19] J.S.Yang,W.P.Liao,J.J.Wu,Morphologyandinterfacialenergeticscontrolsfor hierarchicalanatase/rutileTiO2 nanostructuredarrayforefficientphotoelectrochemicalwatersplitting,ACSAppl.Mater.Interfaces5(2013)7425–7431
[20] F.Zhu,H.Dong,Y.Wang,D.Wu,J.Li,J.Pan,Q.Li,X.Ai,J.Zhang,D.Xu,Dualfunctionalhetero-structuredTiO2 nanotreescomposedofrutiletrunksandanatase
branchesforimprovedperformanceofquantumdot-sensitizedsolarcells,Phys. Chem.Chem.Phys.15(2013)17798–17803
[21] Y.Q.Wang,W.H.Han,B.Zhao,L.L.Chen,F.Teng,X.D.Li,C.T.Gao,J.Y.Zhou, E.Q.Xie,Performanceoptimizationofself-poweredultravioletdetectorsbasedon photoelectrochemicalreactionbyutilizingdendriformtitaniumdioxidenanowires asphotoanode,Sol.EnergyMater.Sol.Cells140(2015)376–381
[22] H.Yao,W.Fu,H.Yang,J.Ma,M.Sun,Y.Chen,W.Zhang,W.Di,P.Lv,M.Li, Verticalgrowthoftwo-dimensionalTiO2 nanosheetsarray filmsandenhanced photoelectrochemicalpropertiessensitizedbyCdSquantumdots,Electrochim.Acta 125(2014)258–265
[23] G.D.Zhou,T.Zhao,R.F.Qian,X.Xia,S.Y.Dai,A.Alsaedi,T.Hayat,J.H.Pan, Decorating(001)dominantanataseTiO2 nanoflakesarraywithuniformWO3 clustersforenhancedphotoelectrochemicalwaterdecontamination,Catal.Today 335(2019)365–371
[24] H.G.Yang,C.H.Sun,S.Z.Qiao,J.Zou,G.Liu,S.C.Smith,H.M.Cheng,G.Q.Lu, AnataseTiO2 singlecrystalswithalargepercentageofreactivefacets,Nature453 (2008)638
[25] L.Jiang,L.Sun,D.Yang,J.Zhang,Y.J.Li,K.Zou,W.Q.Deng,Niobium-doped (001)-DominatedanataseTiO2 nanosheetsasphotoelectrodeforefficientdye-sensitizedsolarcells,ACSAppl.Mater.Interfaces9(2017)9576–9583.
[26] T.Liu,J.Wang,L.Liu,S.Feng,P.Su,H.Yang,W.Fu,Enhancedphotoelectric performanceofCdS/CdSeco-sensitizedTiO2nanosheetsarray films,Sustain. EnergyFuels2(2018)1262–1268
[27] I.Robel,V.Subramanian,M.Kuno,P.V.Kamat,Quantumdotsolarcells.Harvesting lightenergywithCdSenanocrystalsmolecularlylinkedtomesoscopicTiO2 films,J. Am.Chem.Soc.7(2006)2385–2393
[28] O.Niitsoo,S.K.Sarkar,C.Pejoux,S.Rühle,D.Cahen,G.Hodes,Chemicalbath depositedCdS/CdSe-sensitizedporousTiO2 solarcells,J.Photochem.Photobiol.A: Chem.181(2006)306–313
[29] Y.Xie,G.Ali,S.H.Yoo,S.O.Cho,Sonication-assistedsynthesisofCdSquantum-dotsensitizedTiO2nanotubearrayswithenhancedphotoelectrochemicalandphotocatalyticactivity,ACSAppl.Mater.Interfaces2(2010)2910–2914
[30] F.Shen,W.Que,Y.Liao,X.Yin,PhotocatalyticactivityofTiO2 nanoparticles sensitizedbyCuInS2 quantumdots,Ind.Eng.Chem.Res.50(2011)9131–9137
[31] P.P.Khlyabich,B.Burkhart,B.C.Thompson,Efficientternaryblendbulkheterojunctionsolarcellswithtunableopen-circuitvoltage,J.Am.Chem.Soc.133(2011) 14534–14537
[32] Y.L.Lee,Y.S.Lo,Highlyefficientquantum-dot-sensitizedsolarcellbasedoncosensitizationofCdS/CdSe,Adv.Funct.Mater.19(2009)604–609
[33] J.Jean,S.Chang,P.R.Brown,J.J.Cheng,P.H.Rekemeyer,M.G.Bawendi, S.Gradecak,V.Bulovic,ZnOnanowirearraysforenhancedphotocurrentinPbS quantumdotsolarcells,Adv.Mater.25(2013)2790–2796
[34] C.H.Chuang,P.R.Brown,V.Bulovic,M.G.Bawendi,Improvedperformanceand stabilityinquantumdotsolarcellsthroughbandalignmentengineering,Nat. Mater.13(2014)796–801.
[35] J.Zhang,J.Gao,C.P.Church,E.M.Miller,J.M.Luther,V.I.Klimov,M.C.Beard, PbSequantumdotsolarcellswithmorethan6%efficiencyfabricatedinambient atmosphere,NanoLett.14(2014)6010–6015
[36] L.Li,T.J.Daou,I.Texier,T.K.C.Tran,Q.L.Nguyen,P.Reiss,Highlyluminescent CuInS2/ZnScore/shellnanocrystals:cadmium-freequantumdotsforinvivoimaging,Chem.Mater.21(2009)2422–2429
[37] L.X.Yi,Y.Y.Liu,N.L.Yang,Z.Y.Tang,H.J.Zhao,G.H.Ma,Z.G.Su,D.Wang,One dimensionalCuInS2-ZnSheterostructurednanomaterialsaslow-costandhigh-performancecounterelectrodesofdye-sensitizedsolarcells,EnergyEnviron.Sci.6 (2013)835–840
[38] M.Sun,Z.Chen,J.Li,H.Jian,F.Xu,L.Xu,R.Zeng,Enhancedvisiblelight-driven activityofTiO2 nanotubearrayphotoanodeco-sensitizedby “green” AgInS2 photosensitizerandIn2S3 bufferlayer,Electrochim.Acta269(2018)429–440
[39] J.J.Wang,Y.Q.Wang,F.F.Cao,Y.G.Guo,L.J.Wan,Synthesisofmonodispersed wurtzitestructureCuInSe2 nanocrystalsandtheirapplicationinhigh-performance organic-inorganichybridphotodetectors,J.Am.Chem.Soc.132(2010) 12218–12221
[40] J.Xu,C.S.Lee,Y.B.Tang,X.Chen,Z.H.Chen,W.J.Zhang,S.T.Lee,W.Zhang, Z.Yang,Large-scalesynthesisandphasetransformationofCuSe,CuInSe2,and CuInSe2/CuInS2 core/shellnanowirebundles,ACSNano4(2010)1845–1850
[41] A.S.Kshirsagar,P.K.Khanna,Titaniumdioxide(TiO2)-decoratedsilverindium diselenide(AgInSe2):novelnano-photocatalystforoxidativedyedegradation, Inorg.Chem.Front.5(2018)2242–2256.
[42] P.N.Li,A.V.Ghule,J.Y.Chang,DirectaqueoussynthesisofquantumdotsforhighperformanceAgInSe2 quantum-dot-sensitizedsolarcell,J.PowerSources354 (2017)100–107
[43] H.Mustafa,D.Hunter,A.K.Pradhan,U.N.Roy,Y.Cui,A.Burger,Synthesisand characterizationofAgInSe2 forapplicationinthin filmsolarcells,ThinSolidFilms 515(2007)7001–7004
[44] T.Bai,C.Li,F.Li,L.Zhao,Z.Wang,H.Huang,C.Chen,Y.Han,Z.Shi,S.Feng,A simplesolution-phaseapproachtosynthesizehighqualityternaryAgInSe2 and bandgaptunablequaternaryAgIn(S1-xSex)2 nanocrystals,Nanoscale6(2014) 6782–6789
[45] G.S.Wang,H.Y.Wei,J.J.Shi,Y.Z.Xu,H.J.Wu,Y.H.Luo,D.M.Li,Q.B.Meng, SignificantlyenhancedenergyconversionefficiencyofCuInS2 quantumdotsensitizedsolarcellsbycontrollingsurfacedefects,NanoEnergy35(2017)17–25
[46] M.A.Mahadik,P.S.Shinde,M.Cho,J.S.Jang,FabricationofaternaryCdS/ ZnIn2S4/TiO2 heterojunctionforenhancingphotoelectrochemicalperformance: effectofcascadingelectron-holetransfer,J.Mater.Chem.A3(2015) 23597–23606
[47] Z.Jiang,D.Jiang,Z.Yan,L.Dong,K.Qian,J.Xie,Anewvisiblelightactive
multifunctionalternarycompositebasedonTiO2-In2O3 nanocrystalsheterojunction decoratedporousgraphiticcarbonnitrideforphotocatalytictreatmentofhazardous pollutantandH2 evolution,Appl.Catal.B170–171(2015)195–205
[48] S.Obregón,Y.Zhang,G.Colón,Cascadechargeseparationmechanismbyternary heterostructuredBiPO4/TiO2/g-C3N4 photocatalyst,Appl.Catal.B184(2016) 96–103
[49] J.Ke,L.Jie,H.Sun,H.Zhang,X.Duan,L.Ping,X.Li,M.O.Tade,S.Liu,S.Wang, FacileassemblyofBi2O3/Bi2S3/MoS2 n-pHeterojunctionwithlayeredn-Bi2O3 and p-MoS2 forenhancedphotocatalyticwateroxidationandpollutantdegradation, Appl.Catal.B200(2017)47–55
[50] J.Y.Chang,J.M.Lin,L.F.Su,C.F.Chang,ImprovedperformanceofCuInS2 quantum dot-sensitizedsolarcellsbasedonamultilayeredarchitecture,ACSAppl.Mater. Interfaces5(2013)8740–8752
[51] J.L.Blackburn,D.C.Selmarten,R.J.Ellingson,M.Jones,O.Micic,A.J.Nozik, Electronandholetransferfromindiumphosphidequantumdots,J.Phys.Chem.B 109(2005)2625–2631
[52] M.Valdes,M.Vazquez,A.Goossens,ElectrodepositionofCuInSe2 andIn2Se3 on flat andnanoporousTiO2 substrates,Electrochim.Acta54(2008)524–529
[53] J.Y.Chang,L.F.Su,C.H.Li,C.C.Chang,J.M.Lin,Efficient “green” quantumdotsensitizedsolarcellsbasedonCu2S–CuInS2–ZnSearchitecture,Chem.Commun.48 (2012)4848–4850.
[54] S.Jiao,Q.Shen,I.Mora-Sero,J.Wang,Z.Pan,K.Zhao,Y.Kuga,X.Zhong, J.Bisquert,Bandengineeringincore/shellZnTe/CdSeforphotovoltageandefficiencyenhancementinexciplexquantumdotsensitizedsolarcells,ACSNano9 (2015)908–915
[55] J.X.Yang,Z.G.Jin,T.J.Liu,C.J.Li,Y.Shi,Aninvestigationintoeffectofcationic precursorsolutionsonformationofCuInSe2 thin filmsbySILARmethod,Sol. EnergyMater.Sol.Cells92(2008)621–627
[56] Z.Li,L.Yu,Y.Liu,S.Sun,CdS/CdSequantumdotsco-sensitizedTiO2 nanowire/ nanotubesolarcellswithenhancedefficiency,Electrochim.Acta129(2014) 379–388
[57] H.Lee,M.Wang,P.Chen,D.R.Gamelin,S.M.Zakeeruddin,M.Gratzel, M.K.Nazeeruddin,EfficientCdSequantumdot-sensitizedsolarcellspreparedbyan improvedsuccessiveioniclayeradsorptionandreactionprocess,NanoLett.9 (2009)4221–4227
[58] Y.Bu,Z.Chen,J.Yu,W.Li,Anovelapplicationofg-C3N4 thin filminphotoelectrochemicalanticorrosion,Electrochim.Acta88(2013)294–300
[59] Y.Wang,J.Liu,M.Wang,C.Pei,B.Liu,Y.Yuan,S.Liu,H.Yang,Enhancingthe sensingpropertiesofTiO2 nanosheetswithexposed{001}facetsbyahydrogenationandsensingmechanism,Inorg.Chem.56(2017)1504–1510
[60] Y.L.Sui,L.Wu,S.K.Zhong,Q.X.Liu,Carbonquantumdots/TiO2 nanosheetswith dominant(001)facetsforenhancedphotocatalytichydrogenevolution,Appl.Surf. Sci.480(2019)810–816
[61] Y.Shi,Z.Jin,C.Li,H.An,J.Qiu,Effectsofpost-heattreatmentonthecharacteristicsofchalcopyriteCuInSe2 filmdepositedbysuccessiveioniclayerabsorptionandreactionmethod,ThinSolidFilms515(2007)3339–3343
[62] X.Cui,H.Gu,Y.Guan,G.Ren,Z.Ma,Y.Yin,J.Liu,X.Cui,L.Yao,Y.Yin, FabricationofAgInS2 nanoparticlessensitizedTiO2 nanotubearraysandtheir photoelectrochemicalproperties,Sol.EnergyMater.Sol.Cells137(2015)101–106
[63] W.T.Sun,Y.Yu,H.Y.Pan,X.F.Gao,Q.Chen,L.M.Peng,CdSquantumdotssensitizedTiO2 nanotube-arrayphotoelectrodes,J.Am.Chem.Soc.130(2008) 1124–1125
[64] D.Che,X.Zhu,H.Wang,Y.Duan,Q.Zhang,Y.Li,Aqueoussynthesisofhighbright andtunablenear-infraredAgInSe2-ZnSequantumdotsforbioimaging,J.Colloid InterfaceSci.463(2016)1–7
[65] M.A.Abate,J.Y.Chang,BoostingtheefficiencyofAgInSe2 quantumdotsensitized solarcellsviacore/shell/shellarchitecture,Sol.EnergyMater.Sol.Cells182(2018) 37–44
[66] X.Hu,Q.X.Zhang,X.M.Huang,D.M.Li,Y.H.Luo,Q.B.Meng,Aqueouscolloidal CuInS2 forquantumdotsensitizedsolarcells,J.Mater.Chem.21(2011) 15903–15905
[67] T.Koida,Y.Ueno,J.Nishinaga,H.Higuchi,S.Niki,Cu(In,Ga)Se2 solarcellswith amorphousIn2O3-basedfrontcontactlayers,ACSAppl.Mater.Interfaces9(2017) 29677–29686
[68] P.M.Allen,M.G.Bawendi,TernaryI III VIquantumdotsluminescentinthered tonear-infrared,J.Am.Chem.Soc.130(2008)9240–9241
[69] Y.Jin,K.B.Tang,C.H.An,L.Y.Huang,Hydrothermalsynthesisandcharacterization ofAgInSe2 nanorods,J.Cryst.Growth253(2003)429–434.
[70] X.Wei,H.Feng,L.Li,J.Gong,K.Jiang,S.Xue,P.K.Chu,Synthesisoftetragonal prismatic γ-In2Se3 nanostructureswithpredominantly{110}facetsandphotocatalyticdegradationoftetracycline,Appl.Catal.B260(2020)118218
[71] T.Meng,C.B.Ng,J.J.VittalBoothroyd,One-potsynthesisofnew-phaseAgInSe2 nanorods,J.Am.Chem.Soc.128(2006)7118
[72] C.L.Hsin,W.F.Lee,C.T.Huang,C.W.Huang,W.W.Wu,L.J.Chen,Growthof CuInSe2 andIn2Se3/CuInSe2 nano-heterostructuresthroughsolidstatereactions, NanoLett.11(2011)4348–4351
[73] Y.Jiang,Q.Wang,L.Han,X.Y.Zhang,L.X.Jiang,Z.Z.Wu,Y.Q.Lai,D.Z.Wang, F.Y.Liu,ConstructionofIn2Se3/MoS2 heterojunctionasphotoanodetowardefficientphotoelectrochemicalwatersplitting,Chem.Eng.J.358(2019)752–758
[74] Y.F.Chen,W.X.Huang,D.L.He,S.T.Yue,H.Huang,Constructionofheterostructuredg-C3N4/Ag/TiO2 microsphereswithenhancedphotocatalysisperformanceundervisible-lightirradiation,ACSAppl.Mater.Interfaces6(2014) 14405–14414
[75] S.Chen,X.Liu,X.Qiao,X.Wan,K.Shehzad,X.Zhang,Y.Xu,X.Fan,Facile synthesisof γ-In2Se3 nanoflowerstowardhighperformanceself-poweredbroadband γ-In2Se3/Siheterojunctionphotodiode,Small13(2017)1604033
