HeterocyclicOrganicCorrosionInhibitors: PrinciplesandApplicationsMumtazA.Quraishi
https://ebookmass.com/product/heterocyclic-organiccorrosion-inhibitors-principles-and-applications-mumtaz-aquraishi/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Organic Corrosion Inhibitors: Synthesis, Characterization, Mechanism, and Applications Chandrabhan Verma
https://ebookmass.com/product/organic-corrosion-inhibitors-synthesischaracterization-mechanism-and-applications-chandrabhan-verma/
ebookmass.com
Eco-Friendly Corrosion Inhibitors : Principles, Designing and Applications Lei Guo
https://ebookmass.com/product/eco-friendly-corrosion-inhibitorsprinciples-designing-and-applications-lei-guo/
ebookmass.com
Eco-Friendly Corrosion Inhibitors : Principles, Designing and Applications Lei Guo
https://ebookmass.com/product/eco-friendly-corrosion-inhibitorsprinciples-designing-and-applications-lei-guo-2/
ebookmass.com
Chemistry in Focus: A Molecular View of Our World 7th Edition
Nivaldo J Tro
https://ebookmass.com/product/chemistry-in-focus-a-molecular-view-ofour-world-7th-edition-nivaldo-j-tro/
ebookmass.com
Lobotomy Nation: The History of Psychosurgery and Psychiatry in Denmark 1st Edition Jesper Vaczy Kragh
https://ebookmass.com/product/lobotomy-nation-the-history-ofpsychosurgery-and-psychiatry-in-denmark-1st-edition-jesper-vaczykragh/
ebookmass.com
Workbook for Textbook of Radiographic Positioning and Related Anatomy John P. Lampignano Med Rt(R)(Ct) & Leslie E. Kendrick Ms Rt(R)(Ct)(Mr)
https://ebookmass.com/product/workbook-for-textbook-of-radiographicpositioning-and-related-anatomy-john-p-lampignano-med-rtrct-leslie-ekendrick-ms-rtrctmr/
ebookmass.com
The Third Option: Covert Action and American Foreign Policy Loch K. Johnson
https://ebookmass.com/product/the-third-option-covert-action-andamerican-foreign-policy-loch-k-johnson/
ebookmass.com
Sexy Book of Sexy Sex Schaal
https://ebookmass.com/product/sexy-book-of-sexy-sex-schaal/
ebookmass.com
Snappy Santa: Welcome to Kissing Springs, Book 8 Grace Grahme & Kissing Springs Book Babes
https://ebookmass.com/product/snappy-santa-welcome-to-kissing-springsbook-8-grace-grahme-kissing-springs-book-babes/
ebookmass.com
Design of Thermal Energy Systems Pradip Majumdar https://ebookmass.com/product/design-of-thermal-energy-systems-pradipmajumdar/
ebookmass.com
HeterocyclicOrganic CorrosionInhibitors PrinciplesandApplications
MumtazA.Quraishi
DheerajS.Chauhan
ViswanathanS.Saji
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright © 2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperiencebroaden ourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingand usinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformation ormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhom theyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeany liabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceor otherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthe materialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-818558-2
ForinformationonallElsevierpublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionEditor: AnitaAKoch
EditorialProjectManager: SaraValentino
ProductionProjectManager: JoyChristelNeumarinHonestThangiah
CoverDesigner: MarkRogers
TypesetbyTNQTechnologies
Listofabbreviationsandsymbols AbbreviationFullform
WLWeightloss
OCPOpencircuitpotential
EISElectrochemicalimpedancespectroscopy
CPEConstantphaseelement
DEISDynamicelectrochemicalimpedancespectroscopy
EFMElectrochemicalfrequencymodulation
CFCausalityfactor
LPRLinearpolarizationresistance
PDPPotentiodynamicpolarization
CVCyclicvoltammogram/voltammetry
SEMScanningelectronmicroscopy
EDXEnergydispersiveX-rayspectroscopy
AFMAtomicforcemicroscopy
FTIRFourier-transforminfraredspectroscopy
NMRNuclearmagneticresonance
XRDX-raydiffraction
XPSX-rayphotoelectronspectroscopy
SECMScanningelectrochemicalmicroscopy
ToF-SIMSTimeof flightsecondaryionmassspectrometry
DFTDensityfunctionaltheory
HSABHardandsoftacidandbaseprinciple
B3LYPBecke3-parameterLee Yang Parr
HOMOHighestoccupiedmolecularorbital
LUMOLowestunoccupiedmolecularorbital
MCMonteCarlo
MDMoleculardynamics
MSMildsteel
CSCarbonsteel
SAMSelf-assembledmonolayer
SymbolMeaning
CR Corrosionrate
q Surfacecoverage
h%Corrosioninhibitionefficiency
RUniversalgasconstant
T Temperature
Ea Energyofactivation
DH Enthalpyofactivation
DS Entropyofactivation
Kads Equilibriumconstantforadsorption
DGoads Standardfreeenergyofadsorption
Qads Heatofadsorption
EOCP Opencircuitpotential
Rct Chargetransferresistance
Rs Solutionresistance
Rp Polarizationresistance
Cdl Doublelayercapacitance
Ecorr Corrosionpotential
icorr Corrosioncurrentdensity
ba AnodicTafelslope
bc CathodicTafelslope
EHOMO Energyofthehighestoccupiedmolecularorbital
ELUMO Energyofthelowestunoccupiedmolecularorbital
DE Energygap
c Electronegativity
m Chemicalpotential
h Globalhardness
u Electrophilicityindex/Angularfrequency
ε Nucleophilicityindex
DN Fractionofelectronstransferred
fk Fukuifunction
Preface Thescienceandtechnologyofcorrosioninhibitorsisoneofthemostimportantareasinthe fieldofcorrosion.Amongthedifferentclassofinhibitors,theheterocyclicorganicinhibitors haveattractedagreatdealofattentionduetotheeaseofsynthesisandhighcorrosioninhibitionattributes.ThroughthelonepairofelectronspresentintheheteroatomssuchasO, N,SandP,theycanbeeffectivelychemisorbedonthemetalsurface.Thisbooksaimsto provideacomprehensiveoverviewofheterocycliccorrosioninhibitorsindifferent applications.
Followedbythefirstchapteronbasicsofheterocycliccompounds,wehaveprovided twochaptersonvariousexperimentalandcomputationalmethodsusedtostudycorrosion inhibition.Chapters4 7highlighttheheterocycliccorrosioninhibitorswithreferencetotheir aqueous/industrialapplications,viz.acidpickling/acidizing,sweet/sourcorrosion,and corrosioninneutralpHandalkalineenvironments.Thelasttwochapters,respectively, explainheterocyclicvaporphaseinhibitorsandgreeninhibitors.
Chapter1providesanoverviewofthefundamentalsofheterocycliccompoundswitha viewtotheirapplicationsincorrosioninhibition.Thechapteralsodescribesthemajorheterocycliccorrosioninhibitorsandtheinfluenceofvarioussubstituentgroupsoncorrosion inhibition.Chapter2entirelyfocusesonvariousexperimentalmethodswhichareusedfor evaluationoftheinhibitorperformance.Themethodsdescribedincludegravimetricweight loss,electrochemicaltechniques,surfacecharacterizationtechniques,andadsorptionisotherms.Chapter3givesaconcisedescriptionofdifferentcomputationalapproachesusedin corrosioninhibitionstudies.Techniquessuchasdensityfunctionaltheory,MonteCarlo,and moleculardynamicssimulationsaredescribedwithemphasisonthedifferentreactivity parametersthatarecalculatedusingthesetechniques.
Chapter4explainsindetailthereportedheterocycliccorrosioninhibitorsforacidic environments.Thesevere/harshcorrosiveenvironmentcreatedbytheuseofconcentrated acidsrequirestheuseofthermallystableefficientcorrosioninhibitorsinapplicationssuchas acidpicklingandacidizationprocesses.Chapter5isspecificforheterocycliccorrosioninhibitorsforsweetandsourcorrosionenvironment.ThecorrosionduetoH2SandCO2 are veryimportantinvariousindustrialsectorssuchasoilandgas.Chapter6providesagood descriptiononheterocycliccorrosioninhibitorsthatarebeingusedinneutralenvironments, whereasChapter7detailstheheterocycliccorrosioninhibitorsforalkalineenvironments. Chapter8givesaconcisedescriptiononheterocyclicvolatilecorrosioninhibitors.Thelast chapter(Chapter9)highlightstheimportantfeaturesofenvironmentallybenignheterocyclic corrosioninhibitors.Thecriteriaforclassificationofcorrosioninhibitorsaccordingto PARCOMguidelines,thedifferentgreenchemistrymetrics,andthetypeofenvironmentally benignheterocyclicinhibitorsareoutlined.Theenvironmentalawarenessandstrictlegislationsrelatedtotheuseoftoxiccorrosioninhibitorsdiscussed.Witheachchapter,several
tablesandschemesareprovidedonthenatureandtypeofinhibitorsandtheirsynthesis approaches.
Wehopethatthebookwillbeahandyreferencetoolforstudentsandresearchers workinginthefieldofcorrosioninhibition.
M.A.Quraishi,D.S.Chauhan,V.S.Saji
Acknowledgment WewouldliketoexpressourgratitudetotheDeanshipofScientificResearch,KingFahd UniversityofPetroleumandMinerals(KFUPM),SaudiArabia,forprovidingfundsonbook writingproject(BW181003).Wewouldalsoliketoexpressourgratitudetoallthosewho grantedusthecopyrightpermissionsforreproducingillustrations.Oursincerethanksforthe Elsevierteaminevolvingthisbookintoitsfinalshape.
Heterocycliccorrosioninhibitors Chapteroutline 1.1Introduction.....................................................................................................................................1
1.1Introduction
Theterm“corrosion”isusuallyreferredtoasthedeteriorationofmetallicmaterialsby itssurrounding.Ingeneral,corrosioncanbedefinedasachemicalorelectrochemical reactionbetweenametalanditsenvironment,whichresultsinitsdeterioration.The seriousconsequencesofcorrosionhavebecomeaproblemwithglobalimplication. Corrosionlosscausesasubstantialeconomicandecologicalimpactonentireglobal infrastructureandconsumes3% 4%ofthegrossdomesticproductofindustrialized countries[1 5].
Selectionandapplicationofsuitablecorrosionpreventionmethodsarehencehighly essentialfortheprotectionandefficientuseofmetallicstructures.Mostoftheindustries includingoilandgas,waterdesalination,andchemicalindustriesaresufferingfrom variouscorrosionissuesresultinginenormouseconomicloss.Thevirtueisthatbythe adoptionofsuitablecorrosionpreventionstrategies,asignificantextentofthelosscan beavoided.Amongthevariouscorrosioncontrolstrategies,theuseofcorrosioninhibitorsisperhapsthesimplest,economic,andeffectiveapproachthatisinroutineuse inindustries.Acorrosioninhibitorcanbedefinedasasubstancethatwhenaddedin suitablequantitytoacorrosiveenvironmentlowersthecorrosionratesignificantly[1,2].
Onewayofclassificationofinhibitorsis(i)inorganicand(ii)organicinhibitors.When comparedtoconventionalsurfacepassivatinginorganicinhibitors,organicinhibitors (adsorption-type)ingeneralareattractiveduetotheirhighefficiencyandenvironmental friendliness.Organicinhibitorsarewidelyemployedinvariousindustriesforvarious aggressiveenvironments.Theirinhibitionperformanceiscorrelatedwiththeirchemical structureandphysicochemicalpropertiessuchasthenatureoffunctionalgroups, electrondensityatdonoratoms,p-orbitalcharacter,andthemolecularelectronic structure.Theinhibitionismainlyattributedtoadsorptionandformationofaprotective barrierfilm[1,2].Amongthevariousorganicinhibitors,thebestavailableclassis perhapstheheterocycliccompounds.Organiccompoundshavingheteroatomssuchas O,N,andSarefoundtohavehigherbasicityandelectrondensityandthusactasbetter inhibitors.
Thefirstpartofthechapterprovidesaconcisedescriptionofthefundamentalsof heterocycliccompounds(types,structure,andnomenclature)withaviewoftheir applicationininhibitiontechnologythatisfollowedbyanaccountonthebasicsof heterocycliccorrosioninhibitors.Formoredetailsonfundamentalsofcorrosionand corrosioninhibitors,thereaderisreferredtobonafidetextbooksavailable[1 5].
1.2Heterocycliccompounds Anorganiccompoundcontainingthecarbonatomsarrangedintheformofaringis calledacarbocycliccompound.However,ifanatomofadifferentelementreplacesone ofthecarbonatoms,thenthiscompoundisreferredtoasaheterocycliccompound[6,7].
Nitrogen(N),sulfur(S),andoxygen(O)arethemostcommonlyoccurringheteroatoms, althoughheteroaromaticringscontainingotherheteroatomsarealsowellknown.Itis noteworthytomentionthattheheterocycliccompoundsconstitutethebuildingblocks ofmanydrugs.Thephytochemicalsfoundindifferentpartsofaplantsuchastheroot, stem,leaf,flower,seed,fruitetc.,arealsocomposedofcomplexheterocycles.Inaddition,severalessential/nonessentialaminoacids,carbohydrates,proteinsetc.,arealso madeofheterocycles[7 10].
Bydefinition,anyatomotherthancarboncanbedesignatedasaheteroatom,andthe organicringcanbetermedasaheterocycle.Amongtheheterocyclics,thecompounds havingN,S,O,andParethemostcommon(Fig.1.1).
CarbocycleHeterocycle
PyridinePyrroleThiopheneFuran
FIGURE1.1 Examplesofheterocycliccompounds.
Inaheterocycliccorrosioninhibitor,thelonepairofelectronsintheheteroatomsis readilyavailablefordonationtothetargetedmetalandthatineffectcanresultinan effectivechemicaladsorptionoftheinhibitormolecule.Further,theheteroatomswhen presentinacid/alkalinemediamayundergoprotonation/deprotonationresultinginthe developmentofpositive/negativechargeontheatoms.Thiscanpromoteeither acceptanceofelectronsfromthemetalatomsviabackdonationorelectrondonationto themetalsurface[6,7].Thefollowingsectiondescribesanoverviewofthedifferenttypes ofheterocycliccompounds:
(1) Heterocycloalkanes:Inthesecompounds,theringissaturated,forexample, dioxane,thiane,dithiane,piperidine,andpiperazine(Fig.1.2A).
CyclohexaneX = NH (Piperidine) X = NH (Piperazine)
X = S (Thiane) X = S (1,4-dithiane)
X = O (Oxane) X = O (1,4-dioxane)
CyclohexeneX = NHX = NH X = N X = SX = SX = S+
X = OX = OX = O+
BenzeneX = NH (Pyrrole)X = N (Pyridine)X = N (Imidazol)X = N(Pyrimidine)
X = S (Thiophene)X = S (Thiinium ion)
X = O (Furan)X = O (Pyrylium ion)
FIGURE1.2 Saturatedandunsaturatedheterocycliccompounds. ReproducedwithpermissionfromT.Eicher,S. Hauptmann,A.Speicher,TheChemistryofHeterocycles:Structures,Reactions,Synthesis,andApplications,Wiley, NewJersey,2013;Copyright2013 © JohnWileyandSons.
(A)
(B)
(C)
(2) Heterocycloalkenes(partiallyunsaturatedsystems):Inthesecompounds, p bonds arepresentinring(Fig.1.2B).Theheteroatompresentintheringcanalsobethe partofadoublebondinsidetheheterocycle.InthecaseofX ¼ Oþ,thecompoundsactasoxeniumsalts,X ¼ Sþ,sulfeniumsalts,andX ¼ N,imines.
(3) Heteroaromaticsystems:Theseareheterocycliccompoundsthatfollowthe Hu ¨ ckel’sRule,i.e.,theringspossessing(4n þ 2) p-electrons.Prominentexamples arefuran,thiophene,pyrrole,pyridine,imidazole,andpyrimidine(Fig.1.2C).
1.3Importantheterocyclicsystems Inthissection,wehavegivensomeoftheimportanttypesofheterocyclicsystemsthat constitutethemajorcorrosioninhibitors.
1.3.1Azoles
HeterocycliccompoundscontainingoneNatomandatleastoneothernoncarbon atoms(e.g.,N,S,O)arrangedinafive-memberedringareknownasazoles.These compoundsconstituteawiderangeofpharmaceuticalcompounds.Examplesinclude pyrazoles,imidazoles,benzimidazole,benzotriazole,etc.
1.3.2Indoles
Indoleisabicyclicstructure,inwhichthebenzeneringisfusedtoafive-membered pyrrolering.Thesecompoundsshowawiderangeofbiologicalactivityandconstitute anintegralpartofmanydrugs.
1.3.3Pyridines
Thesearesix-memberedheterocycleswithstructuresimilartothatofbenzenewhere oneCHisreplacedbyNatom.
Pyrazole
Indole
Pyridine
1.3.4Diazines DiazinesareaclassoforganiccompoundshavingmolecularformulaC4H4N2,thatis, eachdiazineringcontainsabenzeneringwhereNatomsreplacetwoCHgroups.Three isomersofdiazinesaregivenbelow:
1.3.5Quinolines TheseareheterocycliccompoundshavingchemicalformulaC9H7N,wherethebenzene ringisfusedwithpyridine.Theisomerofquinolineisisoquinoline.
QuinolineIsoquinoline
Fig.1.3A D showsexamplesofwell-knownheterocyclicorganiccompounds.Foran easyunderstanding,theheterocyclesareshowninredcolorthroughoutthebook.
FIGURE1.3A Examplesofnaturalproductsbasedonheterocycliccompounds.
FIGURE1.3B Examplesofheterocyclicaminoacids.
AdenineNicotineIndole-3-acetic acid
HistidineHistamineTryptophan
Examplesofheterocyclicdrugs.
Examplesofheterocyclicvitamins.
1.4Nomenclatureofheterocycliccompounds Therearethreeimportantsystemsbywhichheterocycliccompoundsarenamed: (i)commonnames,(ii)Hantzsch Widmansystem,and(iii)replacementnomenclature.
1.4.1Commonortrivialnames Thecommonnamesofheterocycliccompoundsarebasedonthefollowingguidelines:
(1) Thenamesarebasedonoccurrence,origin,andspecialproperties.Examples: furan,thiophene,pyrrole,pyridine,indole,quinolone,etc.
(2) Inaheterocyclicring,numberingpreferablycommencesatasaturatedrather thanatanunsaturatedheteroatom(Fig.1.4).
(3) Ifmorethanonetypeoftheheteroatomsispresent,theringisnumberedfrom theheteroatomofthehigherpriority(O > S > N).Thenumberingisdoneinsuch awaysothatheteroatomgetsthesmallestvalue.
FIGURE1.4 Numberingintheheterocyclicrings.
StreptomycinCarbamazepineQuinine
FIGURE1.3C
Riboflavin (Vitamin B2)Niacin (Vitamin B3)Pyridoxine (Vitamin (B6)
FIGURE1.3D
ImidazoleIsoxazole4-bromo-5-methylisoxazole1,2-dihydropyridine
BenzofuranBenzopyrrole
FIGURE1.5A Namingtheheterocyclicringaccordingtotheparentmolecule.
Pyridine1,4dihydropyridine
2H-pyran 2-dihydropyran
4H-pyran 4-dihydropyran
FIGURE1.5B Nomenclatureofpartiallyhydrogenatedsystems.
(4) Nameoftheheterocyclicringischosenastheparentcompound,andthenames ofthefusedringareattachedasaprefix.Theprefixinsuchnameshavethe ending“o,”e.g.,benzo,naptho,andsoon(Fig.1.5A).
(5) Inpartiallyhydrogenatedsystems,thetermsdihydro,trihydro,etc.areused.The numberindicatesthelocationofhydrogenation.Forexample,2-dihydropyran (2H-pyran),4-dihydroydropyran(4H-pyran),1,4-dihydropyridine,2,3dihydropyridine,etc.(Fig.1.5B).
1.4.2Hantzsch Widmannomenclature TheHantzsch Widmannomenclatureisbasedonthetypeofheteroatom,theringsize (n),andthenatureofthering,whetheritissaturatedorunsaturated.Thisnomenclature isbasedonthefollowingguidelines:
(1) Typeofheteroatom:Thetypeofheteroatomisdesignatedbyaprefix,e.g.,“aza” forN,“thia”forS,“oxa”forO,and“phospha”forP.
(2) Ringsize:Theringsizeofthesaturated/unsaturatedsystemsisindicatedby appropriatesuffixesusingLatinnumeralsasgivenin Table1.1.Theending
Table1.1 Theringsizeanddegreeofunsaturationofheterocycles[9].
RingsizeUnsaturatedSaturatedSaturatedwithN 3-irene-irane-iridine 4-ete-etane-etidine 5-ole-olane-olidine 6-ine-inane 7-epine-epane 8-ocine-ocane
8HeterocyclicOrganicCorrosionInhibitors indicatesthedegreeofunsaturationinthering.Forexample,three-membered saturatedandunsaturatedheterocyclesarenamedasiraneandirine,respectively. Suffix“ir”representstheringsize.IfNispresent,thenamewillbeiridine.
(3) Monocyclicsystems:Thecompoundhavingthehighestnumberofnoncumulative doublebondsisconsideredastheparentmolecule.Thenomenclatureisdoneby linkingoneormoreprefixeswithasuffix[7].
Monocyclicsystemswithoneheteroatom:Thenumberingstartsfromheteroatom(Fig.1.6).
Monocyclicsystemswithtwoormoreidenticalheteroatoms:Theprefixesdi-, tri-,tetra-,etc.,areused.Thenumberingisdoneinsuchawaythatheteroatomsgetthesmallestnumber(Fig.1.7A).
Monocyclicsystemswithtwoormoredifferentheteroatoms:Fordifferent kindsofheteroatoms,prefixesareused,forexample,thepreferenceisasfollows:S > N > O.TheheteroatomhighestinTable2.1isassignedthe1-position inthering,andtheleftoverheteroatomsareallocatedthesmallestpossibleset ofnumberlocants(Fig.1.7B):
Identicalsystemslinkedbyasinglebond:Insuchcases,prefixesbi-,ter-, quater-,etc.,areused(Fig.1.7C)[7].
Theabovestructuresinwheretwoormoreheterocyclicringsareseparatedbysingle bondsareknownasisolatedheterocycliccompounds.
(4) Heterocyclicsystemsfusedwithbenzenerings:Inthiscase,carbocyclicringis designatedasbenzoandtrivialnameisgiventoheterocycle(Fig.1.8).
Numberinginmonocyclicsystemswithsingleheteroatom.
FIGURE1.7 Numberinginmonocyclicheterocycles(A)havingmorethanoneheteroatom,(B)havingmorethan oneheteroatomofdifferenttypes,and(C)identicalsystemsconnectedbyasinglebond.
Pyrrole Pyridine Azepine
FIGURE1.6
(benzo[b]pyrole)
Quinoline (benzo[b]pyridine)
FIGURE1.8 Someofthecommonfusedringheterocycles.
pyrido[2,3-d]pyrimidinepyrido[3,2-d]pyrimidine
FIGURE1.9 Numberinginmorethanoneheterocyclesfusedtogether. Reproducedwithpermissionfrom T.Eicher,S.Hauptmann,A.Speicher,TheChemistryofHeterocycles:Structures,Reactions,Synthesis,and Applications,Wiley,NewJersey,2013;Copyright2013 © JohnWileyandSons.
(5) Heterocyclicsystemsfusedwithotherheterocyclicrings:Thiscategorycontains compoundswhereoneheterocyclicringisfusedtooneormoreheterocyclicrings (Fig.1.9).
Here,firstly,thesystemisdividedintoitscomponents.Thenameofthefused component,byreplacingtheterminal‘‘e’’with‘‘o’’isaddedpriortobasecomponent’s name.Numbersandlettersinsquarebracketsdescribetheatomscommontobothrings, wheretheorderofthenumbersmustagreetothedirectionoftheletteringofthebase component[7].
1.4.3Thereplacementnomenclature Inreplacementnomenclature,theparentcompoundisnamedascarbocycliccompound andtheheteroatomasprefix“aza”,“oxa”,and“thia”forN,O,andSringatom, respectively.Theheterocyclicringsarenumberedsothattheheteroatomhasthelowest possiblenumber.
(1) Monocyclicsystems:Thetypeofheteroatomisindicatedbyaprefix(Table1.1). Thelocationandprefixofheteroatomsarewritteninfrontofthecorresponding hydrocarbonname.Theorderandnumberingoftheheteroatomsfollowsthe guidelinesasdiscussedabove.
Indole
Phenanthrene3,9-diazaphenanthreneBicyclo[2.2.1]heptane7-oxabicyclo[2.2.1]heptane
FIGURE1.10 Replacementnomenclatureofheterocycliccompounds. ReproducedwithpermissionfromT.Eicher, S.Hauptmann,A.Speicher,TheChemistryofHeterocycles:Structures,Reactions,Synthesis,andApplications, Wiley,NewJersey,2013;Copyright2013 © JohnWileyandSons.
(2) Bi-andpolycyclicsystems:Herealsothelocationandprefixareputinfrontofthe hydrocarbonname,withthenumberingretainedassuch(Fig.1.10).
Adetaileddiscussionofthesystematicnomenclatureforpolycyclicsystems,inwhich severalaromaticorheteroaromaticringsarefusedtogether,isbeyondthescopeofthis chapter.Moredetailsaboutstructureandnomenclatureofthesecompoundsare availableinRefs.[7,8].
1.5Heterocyclicsystemsascorrosioninhibitors Therearelargefamiliesofheterocycliccompoundscontaining3,4,5,6,7,andeven largerringsizes.Whencomparedto5or6-memberedheterocycliccompounds,the3-or 4-memberedheterocyclesareconsiderablylessstableduetotheringstrain[7,8]. Therefore,consideringtheapplicabilityincorrosioninhibition,onlythemolecules havingfiveormoreringmemberswillbeconsideredinthisbook.Then,thebenzenefusedringsystemsarecoveredfollowedbythecondensedsystemshavingmorethan twofusedheterocyclesinthesamegroup.
1.5.1Five-memberedheterocycles Amongthefive-memberedheterocycles,t heN-containingcorrosioninhibitors especiallythosehavingtwoormoreNatomsareofthegreatestimportance. Examplesarethederivativesofpyrazoles,imidazoles,triazoles,tetrazoles,etc.Next theinhibitorscontainingOorSatomsin additiontoNconstitutetheimportant types.Examplesarethederivativesofoxazoles,thiazoles,thiadiazoles,etc.Amongthe fusedring basedheterocycles,therearederivativesofindole,benzimidazole, benzotriazole,benzoxazole,etc.Someofthecommonfive-memberedheterocycles withsingleandfusedringsareshownin Figs.1.11and1.12,respectively. Fig.1.13 showsthestructuresofsomeheterocycliccorrosioninhibitorsbasedonfivememberedheterocycles.
1H-pyrazole1H-imidazoleImidazolineImidazolidine
OxazoleIsoxazoleThiazoleIsothiazole
1,2,3-triazole1,2,4-triazole1,3,4-oxadiazole1,3,4-thiadiazole
FIGURE1.11 Five-memberedheterocycles.
FIGURE1.12 Five-memberedheterocycleswithfusedrings.
PyrroleFuranThiophene
Tetrazole
IndoleIsatin
BenzimidazoleBenzothiazoleBenzoxazole
Benzotriazole
2-mercapto-benzimidazoleImidazole derivative4-amino-3-hydrazino-5-thio-1,2,4triazole
3-alkyl-5-mercapto-salicylidene 4-amino-1,2,4-triazole
2-aminobenzothiazole2-salicylideneamino-6-chlorobenzothiazole
2-amino-4-phenylthiazole2-cinnamalideneamino-4phenylthiazole
2,2’-diamino (1,3,4-thidiazol-2-yl) methane
5,5’- (1,2-phenylene) bis(1,3,4-thidiazol-2-amine)
1-furfurylidene-3thiocarbohydrazide
N, N´-bis (isatin)-1,2-diaminoethane
N, N´-bis (isatin) thiocarbohydrazone
N, N´-bis (isatin)-o-phenylene diamine
FIGURE1.13 Structuresofsomeheterocycliccorrosioninhibitorsbasedon five-memberedheterocycles.
1.5.2Six-memberedheterocycles Ringstraininsix-memberedheterocyclesistheleast[7].Therefore,theyconstitute majorclassofcorrosioninhibitors.Thekeycorrosioninhibitorsinthisgroupcomeagain fromtheN-basedheterocycles,i.e.,pyridine,diazines(pyrazine,pyridazineand
Pyridine4H-PyranPiperidinePiperazine
PyridazinePyrimidinePyrazineMorpholine
FIGURE1.14 Six-memberedheterocycles.
CinnolineQuinazolineQuinoxaline
FIGURE1.15 Six-memberedheterocycleswithfusedrings.
pyrimidine)(Fig.1.14),triazines,tetrazines,andtheirderivatives.Amongfusedrings,the mostcommoninhibitorscomefromthecategoryofquinolinederivatives(Fig.1.15).
Structuresofsomeofthecorrosioninhibitorsbasedonsix-memberedheterocyclicrings areshownin Fig.1.16.
1.5.3Macrocycliccompounds Macrocycles(largeringcompounds)arecommonlydescribedasmoleculesandions containing12ormorememberedring.Well-knownexamplesarethecrownethers, calixarenes,porphyrins,andcyclodextrins.Themacrocycliccompoundsdefinelarge, matureareaofchemistry,andsomeofthemembersthathavebeenusedascorrosion inhibitorsareshownin Fig.1.17
1.6Effectofsubstituentsoncorrosioninhibitionef ficiency
Electron-donatingandelectron-withdrawingsubstituentssignificantlyaffecttheinhibitionefficiencyoftheorganicinhibitorsastheyaffectelectrondensityovertheactive sites.Effectofsubstituentsonthesubstitutedaromaticandaliphaticaminescanbe
QuinolineIsoquinoline
14HeterocyclicOrganicCorrosionInhibitors PyridineAcridine & their n-hexadecyl derivatives 2,2’-Biquinoline
2-mercaptopyrimidine1,1’-ethylene-3,3’dimethyl-bispyridinium-iodide
7-nitroso-8-hydroxyquinoline5-acetyl-6-methyl-4-(3-nitro phenyl)-3, 4-dihydro-pyrimidin2(1H)-one
1,10-phenanthroline
Phenothiazine & vinylpyridine polymers
5-(2-hydroxybenzylidene) pyrimidine-2, 4, 6-trione
N-(morpholinomethyl) isatin-3isonicotinoyl hydrazone
N-(2-thiobenzimidazolyl methyl) isatin-3-isonicotinoyl hydrazine
N-(piperadinomethyl) isatin-3isonicotinyl hydrazone
N-(morpholino methyl) isatin-3thiocarbohydrazone
7-amino-5-(4-nitrophenyl)-2, 4dioxo-2, 3, 4, 5-tetrahydro-1Hpyrano [2, 3-d] pyrimidine-6carbonitrile
FIGURE1.16 Structuresofsomeheterocycliccorrosioninhibitorsbasedonsix-memberedheterocycles.
Tetraphenyl-dithiaoctaazacyclotetradecane hexaene
Tetraphenyl-dithiahexaazacyclobidecane hexaene
Tetraphenyl-dioxo-hexaazacyclo bidecane hexaene
1,2,5,10,13-tetraoxo-1,6,9,14tetraazacyclohexadecane
7,8:15,16-dibenzo-2,5,10,13tetraoxo-1,6,9,14-tetraazacyclohexadecane
7,14-dimethyl-5,12-dioxo-1,4,8,11tetaazacyclotetradeca-1,7-diene
2,3:9,10-dibenzo-7,14-dimethyl5,12-dioxo-1,4,8,11tetraazacyclotetradeca-1,7-diene
3,4:11,12-dibenzo 2,5,10,13tetraoxo-1,6,9,14-tetraazacyclohexaxecane
FIGURE1.17 Macrocycliccompoundsreportedascorrosioninhibitors. ModifiedafterM.Quraishi,J.Rawat,Areviewonmacrocyclicsascorrosioninhibitors,CorrosionReviews19(2001)273 299.
describedwiththehelpofHammettsubstituentconstants(s).Thesimplifiedformsof Hammettequationsaregivenbelow[2,12 15]:
where KH and KR denotetheequilibriumconstantsforadsorption desorptionprocessof inhibitormoleculewithoutandwithsubstituent R,respectively. h%, q,and r correspondtopercentageinhibitionefficiency,surfacecoverage,andreactionparameter. Valueofthe r representsthetotalelectroniceffectofthesubstituent(s)attheactivesites
Table1.2 ValuesofsomeHammettsubstituentconstants(s) [12,16].
S.No.Substituent sm sp 1-H0.000.00
2-F þ0.34
3-Cl
4-Br
5-I
0.39
0.35
0.23
0.28
6-NH2 0.16 0.66
7-NO2 þ0.71 þ0.78
8-OH þ0.12 0.37
9-OCH3 þ0.12 0.22
10-SH þ0.25 þ0.15
11-CN þ0.56 þ0.66
12-COOH þ0.36 þ0.43
13-CHO þ0.36 þ0.22
14-CONH2 þ0.28 þ0.36
15-CF3 þ0.43 þ0.54
16-CH3 0.07 0.17
17-CH2CH3 0.07 0.15
18-CH(CH3)2 0.07 0.15
19-C(CH3)3 0.10 0.20
sm and sp denotetheHammettconstantvalueofsubstituentspresentatmeta-andparaposition,respectively.
oftheinhibitormolecule(s). Table1.2 denotesthevaluesofHammettsubstituentconstantforseveralcommonsubstituentspresentatmeta-(m-)andpara-(p-)positionof thearomaticrings.
Generally,theoccurrenceofelectron-releasingsubstituentssuchas OH, OCH3, NH2, NHMe, NMe2,and CH3 augmentstheelectrondensityatthedonorsites, whichresultsintheimprovementoftheinhibitionefficiencyoftheorganicinhibitors, whereaselectron-withdrawingsubstituentssuchas NO2, COOH,and COOC2H5 reducetheelectrondensityatthedonorsites,therebydecreasingtheinhibitionefficiency[12,17 22].Normally,halogensalsoactaselectron-withdrawing(duetonegative inductiveeffect)substituents;therefore,theydecreasetheprotectionefficiencyofthe inhibitors.However,inmanycases,halogenscanenhancetheinhibitionefficiency becauseoftheirresonanceeffect[12,20,22 26].Ingeneral,aninhibitorhavinghydrophobicchain(s)showsbetterprotectionefficiencythantheinhibitorwithoutthehydrophobicchain.Theincreasingcarbonchainlengthalsoenhancesinhibition performancebyincreasinghydrophobicnatureoftheinhibitors[12,27].
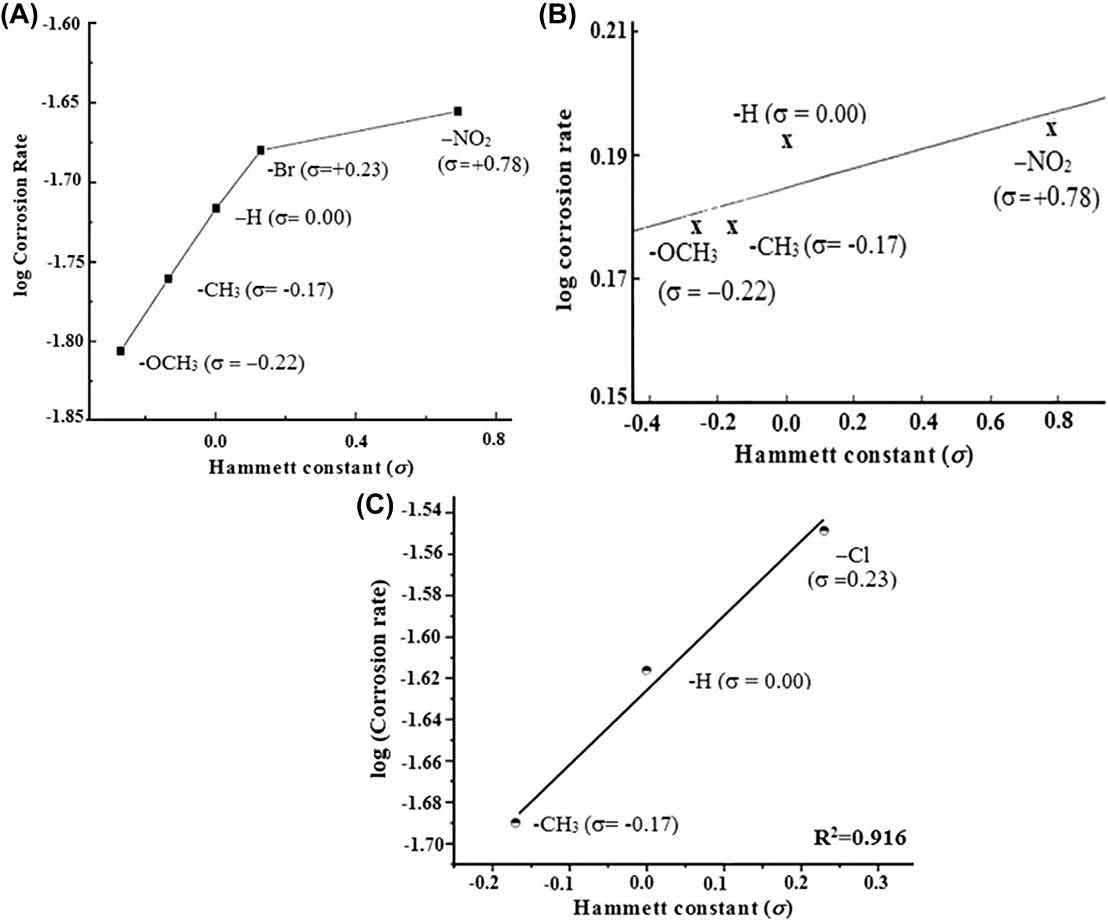
Fig.1.18 showsthevariationofcorrosionrateswith s forseveralorganicinhibitors [12,28 30].Thecorrosioninhibitorhavingthehighestnegativevalueof s displayedthe lowestcorrosionrateandthusthehighestinhibitionefficiencythatcouldbecreditedto thehighestcapabilityofelectrondonation.Thepresenceofelectron-withdrawing
FIGURE1.18 Variationincorrosionratesof(A)5-arylazothiazole,(B)1-methyl-4[4](- X)-styrylpyridiniumiodides, and(C)1-benzoyl-4-phenyl-3-thiosemicarbazidederivativeshavingdifferentsubstituentswiththeirHammettconstant(s)values. ReproducedwithpermissionfromC.Verma,L.Olasunkanmi,E.E.Ebenso,M.Quraishi, Substituentseffectoncorrosioninhibitionperformanceoforganiccompoundsinaggressiveionicsolutions:areview,JournalofMolecularLiquids,251(2018)100 118;Copyright2018 © Elsevier.
groups(havingapositivevalueof s)resultedinariseinthecorrosionrateanda diminutionintheefficiency.Theseobservationssupporttheabovementioneddiscussionontherelationbetween s and h%.
DetaileddescriptionsonvariousheterocycliccompoundscanbefoundinChapters 4 7basedontheirapplicationsinacidic,sweetandsour,neutral,andalkalinemedia. VaporphaseandgreencorrosioninhibitorsareprovidedinChapters8and9,respectively.
Suggestedreading
M.G.Fontana,CorrosionEngineering,TataMcGraw-HillEducation,2005. V.S.Sastri,Corrosioninhibitors:Principlesandapplications,Wiley,1998.
18HeterocyclicOrganicCorrosionInhibitors T.Eicher,S.Hauptmann,A.Speicher,TheChemistryofHeterocycles:Structures, Reactions,Synthesis,andApplications,JohnWileyandSons,2013.
J.A.Joule,K.Mills,HeterocyclicChemistryataGlance,JohnWileyandSons,2012. J.Alvarez-Builla,J.J.Vaquero,J.Barluenga,ModernHeterocyclicChemistry,John WileyandSons,2011.
D.T.Davies,AromaticHeterocyclicChemistry,OxfordUniversityPress,1992.
References [1]V.S.Sastri,CorrosionInhibitors:PrinciplesandApplications,Wiley,NewYork,1998.
[2]V.S.Sastri,GreenCorrosionInhibitors:TheoryandPractice,Wiley,NewJersey,2012.
[3]M.G.Fontana,CorrosionEngineering,TataMcGraw-HillEducation,NewDelhi,2005.
[4]R.W.Revie,Uhlig’sCorrosionHandbook,Wiley,NewJersey,2011.
[5]R.Cottis,M.Graham,R.Lindsay,S.Lyon,T.Richardson,D.Scantlebury,H.Stott,Shreir’s Corrosion,Elsevier,Amsterdam,2010.
[6]D.T.Davies,AromaticHeterocyclicChemistry,OxfordUniversityPress,NewYork,1992.
[7]T.Eicher,S.Hauptmann,A.Speicher,TheChemistryofHeterocycles:Structures,Reactions, Synthesis,andApplications,Wiley,NewJersey,2013.
[8]J.A.Joule,K.Mills,HeterocyclicChemistryataGlance,Wiley,NewJersey,2012.
[9]S.Derese,NomenclatureofHeterocyclicCompounds,LectureNotes,UniversityofNairobi,Kenya.
[10]S.Kirchhecker,M.Antonietti,D.Esposito,Hydrothermaldecarboxylationofaminoacidderived imidazoliumzwitterions:asustainableapproachtowardsionicliquids,GreenChemistry16(2014) 3705 3709.
[11]M.Quraishi,J.Rawat,Areviewonmacrocyclicsascorrosioninhibitors,CorrosionReviews19(2001) 273 299.
[12]C.Verma,L.Olasunkanmi,E.E.Ebenso,M.Quraishi,Substituentseffectoncorrosioninhibition performanceoforganiccompoundsinaggressiveionicsolutions:areview,JournalofMolecular Liquids251(2018)100 118.
[13]J.Leffler,E.Grunwald,RatesandEquilibriaofOrganicReactions,Wiley,NewYork,1963.
[14]F.M.Donahue,K.Nobe,Theoryoforganiccorrosioninhibitorsadsorptionandlinearfreeenergy relationships,JournaloftheElectrochemicalSociety112(1965)886 891.
[15]Z.Szklarska-Smialowska,M.Kaminski,Effectofvarioussubstituentsinthiopheneontheinhibitor efficiency,CorrosionScience13(1973)1 10.
[16]E.McCafferty,IntroductiontoCorrosionScience,SpringerScience&BusinessMedia,NewYork, 2010.
[17]S.K.Saha,A.Dutta,P.Ghosh,D.Sukul,P.Banerjee,NovelSchiff-basemoleculesasefficient corrosioninhibitorsformildsteelsurfacein1MHClmedium:experimentalandtheoretical approach,PhysicalChemistryChemicalPhysics18(2016)17898 17911.
[18]S.Deng,X.Li,H.Fu,Twopyrazinederivativesasinhibitorsofthecoldrolledsteelcorrosionin hydrochloricacidsolution,CorrosionScience53(2011)822 828.
[19]M.Yadav,L.Gope,N.Kumari,P.Yadav,CorrosioninhibitionperformanceofpyranopyrazolederivativesformildsteelinHClsolution:gravimetric,electrochemicalandDFTstudies,Journalof MolecularLiquids216(2016)78 86.