Contributors
Daniel A. Arber, MD
Ronald F. Dorfman, MBBCH, FRCPATH Professor of Hematopathology
Stanford University Stanford, California
Adam Bagg, MD Professor Department of Pathology and Laboratory Medicine University of Pennsylvania Philadelphia, Pennsylvania
Barbara J. Bain, MB BS, FRACP, FRCPath
Professor of Diagnostic Haematology Department of Haematology
St Mary’s Hospital Campus of Imperial College London London, United Kingdom
Todd S. Barry, MD, PhD Medical Director
Spectrum Pathology Mission Viejo, California
Govind Bhagat, MD
Professor of Pathology and Cell Biology Division of Hematopathology Department of Pathology and Cell Biology Columbia University Medical Center New York, New York
Michael J. Borowitz, MD, PhD Professor of Pathology and Oncology Department of Pathology Johns Hopkins Medical Institutions Baltimore, Maryland
Pierre Brousset, MD, PhD
Senior Pathologist Department of Pathology
Institut Universitaire du Cancer Toulouse Oncopole Toulouse, France
Russell K. Brynes, MD
Professor of Clinical Pathology Chief, Hematopathology Service Department of Pathology
Keck School of Medicine of the University of Southern California Los Angeles, California
Elias Campo, MD Chief, Hematopathology Unit Professor of Anatomic Pathology
Hospital Clinic University of Barcelona Barcelona, Spain
Lorenzo Cerroni, MD Department of Dermatology
Medical University of Graz Graz, Austria
Devon Chabot-Richards, MD
Assistant Professor Department of Pathology University of New Mexico Health Sciences Center Albuquerque, New Mexico
Alexander C. L. Chan, MBBS, FRCPA Consultant Pathologist Department of Pathology
Queen Elizabeth Hospital Kowloon, Hong Kong
John K. C. Chan, MBBS, FRCPath, FRCPA Consultant Pathologist Department of Pathology
Queen Elizabeth Hospital Kowloon, Hong Kong
Karen L. Chang, MD
Physician-in-Charge Department of Anatomic Pathology/Histology and Immunohistochemistry
Kaiser Permanente Southern California Los Angeles, California
Yi-Hua Chen, MD
Associate Professor of Pathology Director, Hematopathology Section Director, Hematopathology Fellowship Program Northwestern University Northwestern Memorial Hospital Chicago, Illinois
Sindhu Cherian, MD Department of Laboratory Medicine University of Washington Medical Center Seattle, Washington
Joseph M. Connors, MD
Clinical Director
BC Cancer Agency Centre for Lymphoid Cancer
Vancouver, British Columbia, Canada
James R. Cook, MD, PhD
Department of Laboratory Medicine
Cleveland Clinic
Associate Professor of Pathology
Cleveland Clinic Lerner College of Medicine Cleveland, Ohio
Fiona E. Craig, MD
Professor of Pathology
Division of Hematopathology
Department of Laboratory Medicine and Pathology
Mayo Clinic Phoenix, Arizona
Magdalena Czader, MD, PhD
Professor
Department of Pathology and Laboratory Medicine
Indiana University School of Medicine Indianapolis, Indiana
Laurence de Leval, MD, PhD
Head and Chief
Institute of Pathology
Professor of Pathology
University Hospital of Lausanne Lausanne, Switzerland
Georges Delsol, MD
Senior Pathologist
Department of Pathology
Institut Universitaire du Cancer Toulouse Oncopole Toulouse, France
Amy S. Duffield, MD, PhD
Assistant Professor
Department of Pathology
Johns Hopkins Medical Institutions Baltimore, Maryland
Kojo S. J. Elenitoba-Johnson, MD
Peter C. Nowell, MD, Professor
Department of Pathology and Laboratory Medicine University of Pennsylvania Philadelphia, Pennsylvania
Fabio Facchetti, MD, PhD
Chief
Department of Pathology
University of Brescia School of Medicine
Spedali Civili Brescia Brescia, Italy
Brunangelo Falini, MD
Institute of Hematology
University of Perugia
Centro di Ricerche Onco-Ematologiche (CREO)
Ospedale S. Maria della Misericordia Perugia, Italy
Andrew L. Feldman, MD
Associate Professor of Laboratory Medicine and Pathology College of Medicine
Mayo Clinic Rochester, Minnesota
Falko Fend, MD
Full Professor and Chair Institute of Pathology
University Hospital Tübingen Tübingen, Germany
Judith A. Ferry, MD
Director of Hematopathology and Pathologist
Department of Pathology
Massachusetts General Hospital Professor of Pathology
Harvard Medical School Boston, Massachusetts
Armando C. Filie, MD
Senior Clinician
Laboratory of Pathology
National Cancer Institute Bethesda, Maryland
Simona Fisogni, MD
Senior Assistant
Department of Pathology
Spedali Civili of Brescia Brescia, Italy
Kathryn Foucar, MD Professor
Department of Pathology
University of New Mexico Health Sciences Center Albuquerque, New Mexico
Randy D. Gascoyne, MD, FRCPC
Hematopathologist
British Columbia Cancer Agency and the Centre for Lymphoid Cancer Vancouver, British Columbia, Canada
Philippe Gaulard, MD
Professor
Department of Pathology
University Hospital Henri Mondor Créteil, France
Tracy I. George, MD
Associate Professor Department of Pathology University of New Mexico Albuquerque, New Mexico
Dita Gratzinger, MD, PhD
Assistant Professor Department of Pathology Stanford University School of Medicine Stanford, California
Nancy Lee Harris, MD Department of Pathology
Massachusetts General Hospital Harvard Medical School Boston, Massachusetts
Robert P. Hasserjian, MD
Associate Professor of Pathology Department of Pathology Director, Hematopathology Fellowship Massachusetts General Hospital Boston, Massachusetts
David R. Head, MD
Professor Department of Pathology, Microbiology, and Immunology
Vanderbilt University School of Medicine Nashville, Tennessee
Hans-Peter Horny, MD Professor
Institute of Pathology University of Munich Munich, Germany
Eric D. Hsi, MD
Professor of Pathology
Cleveland Clinic Lerner College of Medicine
Chairman Department of Laboratory Medicine
Cleveland Clinic Cleveland, Ohio
Robert E. Hutchison, MD
Director of Hematopathology/Clinical Pathology
Department of Pathology
State University of New York Upstate Medical University Syracuse, New York
Elizabeth M. Hyjek, MD, PhD
Associate Professor Department of Pathology Hematopathology Section University of Chicago Chicago, Illinois
Peter G. Isaacson, MB ChB, DM, FRCPath, FRS
Professor
Department of Cellular Pathology
Royal Free Hospital London, United Kingdom
Elaine S. Jaffe, MD
Pathologist Bethesda, Maryland
Ronald Jaffe, MB, BCh
Professor of Pathology University of Pittsburgh School of Medicine Pittsburgh, Pennsylvania
Patty M. Jansen, MD, PhD
Department of Pathology
Leiden University Medical Center Leiden, The Netherlands
Pedro Jares, PhD
Pathology Department Hospital Clinic Barcelona, Spain
Dan Jones, MD, PhD
Professor of Pathology and Vice Chair, Division of Molecular Pathology
Ohio State University College of Medicine
Director of Molecular Pathology
Ohio State University Comprehensive Cancer Center Columbus, Ohio
Marshall E. Kadin, MD
Professor
Department of Dermatology
Roger Williams Medical Center
Providence, Rhode Island Professor
Department of Dermatology
Boston University School of Medicine Boston, Massachusetts
Werner Kempf, MD
Professor and Consultant Physician
Department of Dermatology
University Hospital Zürich Co-director Kempf and Pfaltz Histological Diagnostics Zürich, Switzerland
Philip M. Kluin, MD
Professor of Hematopathology
Department of Pathology and Medical Biology
University Medical Center Groningen University of Groningen Groningen, The Netherlands
Young Hyeh Ko, MD, PhD
Professor
Department of Pathology
Samsung Medical Center
Sungkyunkwan University School of Medicine Seoul, Republic of Korea
Steven H. Kroft, MD
Professor and Excecutive Vice Chair Director of Hematopathology
Department of Pathology
Medical College of Wisconsin Milwaukee, Wisconsin
Laurence Lamant-Rochaix, MD, PhD
Senior Pathologist
Department of Pathology
Institut Universitaire du Cancer Toulouse Oncopole Toulouse, France
Philip E. LeBoit, MD
Professor of Dermatology and Pathology
University of California, San Francisco San Francisco, California
Megan S. Lim, MD, PhD
Professor
Department of Pathology and Laboratory Medicine
University of Pennsylvania Philadelphia, Pennsylvania
Michael A. Linden, MD, PhD
Associate Professor
Director of Hematopathology
Department of Laboratory Medicine and Pathology University of Minnesota Minneapolis, Minnesota
Abner Louissaint, Jr., MD, PhD
Assistant Professor of Pathology
Harvard Medical School Department of Pathology
Massachusetts General Hospital Boston, Massachusetts
Robert W. McKenna, MD
Emeritus Professor
Senior Consultant in Hematopathology
Department of Laboratory Medicine and Pathology University of Minnesota Minneapolis, Minnesota
Manuela Mollejo, MD
Department of Pathology
Complejo Hospitalario de Toledo Toledo, Spain
William G. Morice II, MD, PhD
Professor and Chair Department of Laboratory Medicine and Pathology
Mayo Clinic, Rochester, Minnesota
Krzysztof Mrózek, MD, PhD
Research Scientist
Comprehensive Cancer Center
The Ohio State University Columbus, Ohio
Yasodha Natkunam, MD, PhD
Professor
Department of Pathology
Stanford University School of Medicine Stanford, California
Phuong L. Nguyen, MD
Associate Professor of Laboratory Medicine and Pathology
Division of Hematopathology
Mayo Clinic Rochester, Minnesota
Robert S. Ohgami, MD, PhD
Assistant Professor
Department of Pathology
Stanford University Stanford, California
Attilio Orazi, MD, FRCPath (Engl)
Professor of Pathology
Department of Pathology and Laboratory Medicine
Weill Cornell Medical College
New York, New York
German Ott, MD
Professor of Pathology
Head, Department of Clinical Pathology
Robert-Bosch-Hospital and Dr. Margarete FischerBosch Institute of Clinical Pharmacology Stuttgart, Germany
LoAnn C. Peterson, MD Professor Department of Pathology
Northwestern University Feinberg Medical School Chicago, Illinois
Laura B. Pincus, MD
Assistant Professor of Dermatology and Pathology
University of California, San Francisco San Francisco, California
Miguel A. Piris, MD
Department of Pathology
Hospital Universitario Marqués de Valdecilla Santander, Spain
Stefania Pittaluga, MD, PhD
Senior Research Physician
Hematopathology Section
Laboratory of Pathology, Center for Cancer Research, National Cancer Institute
National Institutes of Health Bethesda, Maryland
Sibrand Poppema, MD, PhD, FRCPC
President of the Board of the University Professor of Pathology Department of Pathology University of Groningen Groningen, The Netherlands
Anna Porwit, MD, PhD
Professor Lund University Faculty of Medicine
Department of Clinical Sciences
Division of Oncology and Pathology
Lund, Sweden
Leticia Quintanilla-Martinez, MD
Professor of Pathology
Institute of Pathology and Neuropathology
University Hospital Tübingen and Comprehensive Cancer Center
Eberhard-Karls-University Tübingen, Germany
Frederick Karl Racke, MD, PhD
Medical Director
Hematopathology and Coagulation
Nichols Institute
Quest Diagnostics
San Juan Capistrano, California
Mark Raffeld, MD
Chief, Molecular Diagnostics Section Laboratory of Pathology
National Institutes of Health, National Cancer Institute Bethesda, Maryland
Sherif A. Rezk, MD
Associate Professor of Clinical Pathology
Chief of Pathology and Laboratory Medicine
University of California Irvine Medical Center Orange, California
Scott J. Rodig, MD, PhD
Department of Pathology
Brigham and Women’s Hospital
Harvard Medical School Boston, Massachusetts
Nancy S. Rosenthal, MD
Clinical Professor of Pathology Department of Pathology
University of Iowa Carver College of Medicine
Iowa City, Iowa
Jonathan W. Said, MD
Professor of Pathology
David Geffen School of Medicine
Chief of Anatomic Pathology
University of California Los Angeles Medical Center
Los Angeles, California
Itziar Salaverria, PhD
Research Scientist
Institut d’Investigacions Biomèdiques August Pi I Sunyer
Hospital Clínic Barcelona, Spain
Bertram Schnitzer, MD
Professor of Pathology Department of Pathology
University of Michigan
Ann Arbor, Michigan
Reiner Siebert, MD
Professor of Human Genetics, Director, Institute of Human Genetics University of Ulm University Hospital of Ulm Ulm, Germany
Aliyah R. Sohani, MD Department of Pathology Massachusetts General Hospital Harvard Medical School Boston, Massachusetts
Karl Sotlar, MD Professor Institute of Pathology University of Munich Munich, Germany
Maryalice Stetler-Stevenson, MD, PhD
Laboratory of Pathology Center for Cancer Research
National Cancer Institute National Institutes of Health Bethesda, Maryland
Steven H. Swerdlow, MD Professor of Pathology Division of Hematopathology Department of Pathology University of Pittsburgh School of Medicine Pittsburgh, Pennsylvania
Naheed Usmani, MD
Associate Professor of Pediatrics University of Massachusetts Medical School Worcester, Massachusetts
Peter Valent, MD Professor Department of Internal Medicine I Division of Hematology and Hemostaseology
Ludwig Boltzmann Cluster Oncology
Medical University of Vienna Vienna, Austria
James W. Vardiman, MD
Professor Emeritus
Department of Pathology University of Chicago Chicago, Illinois
Maria E. Vergara-Lluri, MD
Assistant Professor of Clinical Pathology
Hematopathology Section Department of Pathology University of Southern California Los Angeles, California
Maarten H. Vermeer, MD Department of Dermatology Leiden University Medical Center Leiden, The Netherlands
Edward G. Weir, MD President
Clinical Pathology Associates Austin, Texas
Lawrence M. Weiss, MD
Medical and Laboratory Director
Clarient Diagnostic Services, Inc. Aliso Viejo, California
Rein Willemze, MD
Department of Dermatology
Leiden University Medical Center Leiden, The Netherlands
Carla S. Wilson, MD, PhD Professor Department of Pathology University of New Mexico Health Sciences Center Albuquerque, New Mexico
Bruce A. Woda, MD
Vice Chairman Department of Pathology University of Massachusetts Medical School Worcester, Massachusetts
Tadashi Yoshino, MD, PhD
Professor and Chairman Department of Pathology
Okayama University Okayama, Japan
Constance M. Yuan, MD, PhD
Laboratory
of Pathology
Center for Cancer Research
National Cancer Institute
National Institutes of Health
Bethesda, Maryland
Qian-Yun Zhang, MD, PhD
Professor
Department of Pathology
University of New Mexico Health Sciences Center Albuquerque, New Mexico
Lawrence Zukerberg, MD
Associate Pathologist
Massachusetts General Hospital Boston, Massachusetts
The first edition of Hematopathology was published in 2011, shortly after the introduction of the fourth edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. The timing of the second edition has been coordinated with the release of the revised WHO classification. The reader will find not only the most current terminology, but also a discussion of key changes in the classification of lymphomas and leukemias and of histiocytic disorders. Thus this book will be a valuable resource for the pathologist trying to keep up with this rapidly changing field.
Hematopathology is a discipline in which traditional methods of clinical and morphologic analysis are interwoven with newer, biologically based studies to achieve an accurate diagnosis. Studies of hematologic malignancies have been at the forefront in applying the principles of basic research to the understanding of human disease. All cancers are increasingly recognized as genetic diseases, with precise genetic alterations often defining entities. Advances in immunologic and molecular genetic technology have rapidly migrated to the clinical laboratory, where they play a role in routine diagnosis, and the introduction of next-generation sequencing is changing the face of molecular diagnostics. The discussion of each disease includes a description of morphologic, immunophenotypic, and clinical features, along with relevant genetic findings. These data inform our understanding of disease pathogenesis and provide valuable and often critical adjuncts to diagnosis. The goal is to provide concise, up-to-date, and practical information that is easily accessed by the reader.
Equally relevant to the diagnostic pathologist is an appreciation of the spectrum of reactive and inflammatory lesions of hematolymphoid tissues occurring in immunocompetent patients and in those with dysimmunity. Thus the reader will find a discussion of reactive lymphadenopathies and primary and iatrogenic immunodeficiency disorders. Additional chapters deal with the bone marrow response to inflammatory, infectious, and metabolic diseases; the findings in a number of inherited and congenital disorders that affect hematopoiesis; and the impact of therapy on bone marrow morphology. Finally, we also include some non-lymphoid or nonhematopoietic lesions that may be encountered in lymph nodes or bone marrow that are important in differential diagnosis.
The use of correct technique is critical in producing a lymph node or bone marrow biopsy specimen that is suitable for accurate diagnosis. Many diagnostic errors stem from poor technique related to fixation, processing, cutting, or staining. The first section of the book deals with technical aspects in the processing of lymph node and bone marrow specimens Although the use of fine needle aspiration for primary diagnosis is controversial, it is critical to be aware of how this diagnostic tool can be used and to understand its limitations. Thus a chapter is devoted to this topic. Finally, several chapters deal with the implementation of techniques used in hematopathologic diagnosis, including immunohistochemistry, flow cytometry, molecular genetic techniques in diagnosis, and both classic and interphase cytogenetics.
Pathologic diagnosis cannot occur in a vacuum, and the pathologist must understand the key clinical characteristics of the diseases being considered in one’s differential. Therefore discussion of each disease includes a description of expected clinical features at the time of diagnosis, including signs, symptoms, and relevant staging procedures. Chapters dealing with neoplastic disorders incorporate a discussion of patterns of spread, relapse, and prognostic factors.
We hope that this book will be of value to hematologists and oncologists, in addition to pathologists. It is increasingly important that clinicians be aware of basic principles of hematopathology diagnosis; hematologists and hematopathologists must work as a team to achieve the correct diagnosis. Just as the pathologist must use clinical data to make an accurate diagnosis, the clinician should have sufficient knowledge of diagnostic principles to appreciate when the pathologic diagnosis just does not quite fit.
The reader will find that most of the chapters deal with a specific disease entity or a group of related diseases. Several key tables have been included in each chapter to facilitate use and access to key facts. These include Major Diagnostic Features, Differential Diagnosis, and Pearls and Pitfalls. The book is generously illustrated, and the consistent use of color photography throughout should make it easy to appreciate key diagnostic features.
The Editors appreciate that the reader needs to have access to key source material and that a richly referenced book provides important information for those who wish to delve further into the topic. The scientific literature is voluminous, and we thought it was important to include older historical references, as well as the most recent scientific data. All the references are accessible on the Expert Consult website, with the benefit of electronic access to the PubMed links instantaneously. However, the authors provide key references in print in each chapter to provide the reader with the most useful sources to examine the topic in greater depth.
We were delighted to add Leticia Quintanilla-Martinez, one of the premier hematopathologists in Europe, to our editorial team for the second edition. In addition, we thank the many authors who both adhered to deadlines and strove to include the latest information on their respective topics. We hope this book will prove to be a constant and valued resource for pathologists and clinicians dealing with hematologic diseases and will ultimately benefit the patients and their families.
Elaine S. Jaffe, MD
Daniel A. Arber, MD
Elias Campo, MD
Nancy Lee Harris, MD
Leticia Quintanilla-Martinez, MD
PART I
Technical Aspects
1. Processing of the Lymph Node Biopsy Specimen, 3
Dita Gratzinger and Yasodha Natkunam
2. Fine Needle Aspiration of Lymph Nodes, 15
Magdalena Czader and Armando C. Filie
3. Collection, Processing, and Examination of Bone Marrow Specimens, 29
Phuong L. Nguyen
4. Immunohistochemistry for the Hematopathology Laboratory, 41
Stefania Pittaluga, Todd S. Barry, and Mark Raffeld
5. Flow Cytometry, 53
Maryalice Stetler-Stevenson, Sindhu Cherian, and Constance M. Yuan
6. Molecular Diagnosis in Hematopathology, 69
Kojo S. J. Elenitoba-Johnson, Megan S. Lim, and Adam Bagg
7. Important Chromosomal Aberrations in Hematologic Neoplasms and Key Techniques to Diagnose Them, 105
Itziar Salaverria, Reiner Siebert, and Krzysztof Mrózek
PART II
Normal and Reactive Conditions of Hematopoietic Tissues
8. Normal Lymphoid Organs and Tissues, 131
Elias Campo, Elaine S. Jaffe, and Nancy Lee Harris
9. Reactive Lymphadenopathies, 153
Eric D. Hsi and Bertram Schnitzer
10. Normal Bone Marrow, 179
Barbara J. Bain
11. Evaluation of Anemia, Leukopenia, and Thrombocytopenia, 195
Carla S. Wilson, Maria E. Vergara-Lluri, and Russell K. Brynes
12. Bone Marrow Findings in Inflammatory, Infectious, and Metabolic Disorders, 235
Nancy S. Rosenthal
PART III Lymphoid Neoplasms
13. Principles of Classification of Lymphoid Neoplasms, 253
Elaine S. Jaffe, Nancy Lee Harris, and Elias Campo
SECTION 1 • MATURE B-CELL NEOPLASMS
14. B-Cell Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Monoclonal B-Cell Lymphocytosis, and B-Cell Prolymphocytic Leukemia, 261
Devon Chabot-Richards, Qian-Yun Zhang, and Kathryn Foucar
15. Lymphoplasmacytic Lymphoma and Waldenström Macroglobulinemia, 285
Aliyah R. Sohani, Scott J. Rodig, and Nancy Lee Harris
16. Hairy Cell Leukemia, 299
Robert P. Hasserjian and Brunangelo Falini
17. Splenic Marginal Zone Lymphoma and Other Small B-Cell Neoplasms in the Spleen, 309
Miguel A. Piris and Manuela Mollejo
18. Follicular Lymphoma, 321
Judith A. Ferry, Laurence de Leval, Abner Louissaint, Jr., and Nancy Lee Harris
19. Extranodal Marginal Zone Lymphoma: MALT Lymphoma, 353
James R. Cook and Peter G. Isaacson
20. Primary Cutaneous B-Cell Lymphomas, 369
Rein Willemze, Maarten H. Vermeer, and Patty M. Jansen
21. Nodal Marginal Zone Lymphoma, 383
Elaine S. Jaffe
22. Mantle Cell Lymphoma, 397
Elias Campo and Pedro Jares
23. Diffuse Large B-Cell Lymphoma, 415
Alexander C. L. Chan and John K. C. Chan
24. Burkitt’s Lymphoma and Its Mimics, 447
Randy D. Gascoyne, Reiner Siebert, Joseph M. Connors, and Philip M. Kluin
25. Plasmablastic Neoplasms Other Than Plasma Cell Myeloma, 465
Elias Campo
26. Plasma Cell Neoplasms, 473
Robert W. McKenna, Steven H. Kroft, and Michael A. Linden
27. Nodular Lymphocyte–Predominant Hodgkin’s Lymphoma, 507
Andrew L. Feldman and Sibrand Poppema
28. Classical Hodgkin’s Lymphoma and Related Lesions, 525
Falko Fend
29. Virally Associated B-Cell Lymphoproliferative Disease, 547
Stefania Pittaluga and Jonathan W. Said
SECTION 2 • MATURE T-CELL AND NK-CELL NEOPLASMS
30. Virally Associated T-Cell and NK-Cell Neoplasms, 565
Young Hyeh Ko, John K. C. Chan, and Leticia Quintanilla-Martinez
31. T-Cell and NK-Cell Large Granular Lymphocyte Proliferations, 599
William G. Morice II
32. T-Cell Prolymphocytic Leukemia, 609
Anna Porwit
33. Adult T-Cell Leukemia/Lymphoma, 619
Tadashi Yoshino and Elaine S. Jaffe
34. Hepatosplenic T-Cell Lymphoma, 631
Philippe Gaulard
35. Peripheral T-Cell Lymphoma, Not Otherwise Specified, 641
Laurence de Leval
36. Angioimmunoblastic T-Cell Lymphoma, 659
Leticia Quintanilla-Martinez and German Ott
37. Anaplastic Large Cell Lymphoma, ALK Positive and ALK Negative, 673
Laurence Lamant-Rochaix, Andrew L. Feldman, Georges Delsol, and Pierre Brousset
38. Enteropathy-Associated T-Cell Lymphoma and Other Primary Intestinal T-Cell Lymphomas, 693
Govind Bhagat and Peter G. Isaacson
39. Mycosis Fungoides and Sézary Syndrome, 713
Philip E. LeBoit and Laura B. Pincus
40. Primary Cutaneous CD30-Positive T-Cell Lymphoproliferative Disorders, 731
Marshall E. Kadin and Werner Kempf
41. Primary Cutaneous T-Cell Lymphomas: Rare Subtypes, 747
Lorenzo Cerroni
SECTION 3 • PRECURSOR B- AND T-CELL NEOPLASMS
42. Precursor B- and T-Cell Neoplasms, 761
Amy S. Duffield, Frederick Karl Racke, and Michael J. Borowitz
43. Acute Leukemias of Ambiguous Lineage, 775
Amy S. Duffield, Edward G. Weir, and Michael J. Borowitz
PART IV Myeloid Neoplasms
44. Principles of Classification of Myeloid Neoplasms, 785
Daniel A. Arber
45. Myelodysplastic Syndromes, 793
Robert P. Hasserjian and David R. Head
46. Acute Myeloid Leukemia, 817
Daniel A. Arber
47. Myeloproliferative Neoplasms, 847
James W. Vardiman
48. Myelodysplastic/Myeloproliferative Neoplasms, 883
Elizabeth M. Hyjek and James W. Vardiman
49. Mastocytosis, 911
Tracy I. George, Karl Sotlar, Peter Valent, and Hans-Peter Horny
50. Eosinophilia and Chronic Eosinophilic Leukemia, Including Myeloid/Lymphoid Neoplasms with Eosinophilia and Rearrangement of PDGFRA, PDGFRB, FGFR1, or JAK2, 931
Barbara J. Bain
51. Blastic Plasmacytoid Dendritic Cell Neoplasm, 943
Fabio Facchetti and Simona Fisogni
PART V
Histiocytic Proliferations
52. Non-neoplastic Histiocytic Proliferations of Lymph Nodes and Bone Marrow, 957
Sherif A. Rezk, Naheed Usmani, and Bruce A. Woda
53. Histiocytic and Dendritic Cell Neoplasms Including Langerhans Cell Histiocytosis and Langerhans Cell Sarcoma, 969
Ronald Jaffe, Karen L. Chang, and Lawrence M. Weiss
PART VI
Immunodeficiency Disorders
54. The Pathology of Primary Immunodeficiencies, 999
Stefania Pittaluga
55. Iatrogenic Immunodeficiency-Associated Lymphoproliferative Disorders, 1013
Steven H. Swerdlow and Fiona E. Craig
PART VII
Site-Specific Issues in the Diagnosis of Lymphoma and Leukemia
56. Bone Marrow Evaluation for Lymphoma, 1033
Yi-Hua Chen and LoAnn C. Peterson
57. Evaluation of the Bone Marrow After Therapy, 1065
Robert S. Ohgami and Daniel A. Arber
58. Non-hematopoietic Neoplasms of the Bone Marrow, 1089
Robert E. Hutchison
59. Non-lymphoid Lesions of the Lymph Nodes, 1099
Lawrence Zukerberg and Dan Jones
60. Spleen: Normal Architecture and Neoplastic and Non-neoplastic Lesions, 1113
Attilio Orazi and Daniel A. Arber
61. Diagnosis of Lymphoma in Extranodal Sites Other Than Skin, 1133
Judith A. Ferry
Processing of the Lymph Node Biopsy Specimen
Dita Gratzinger and Yasodha Natkunam
OUTLINE
INSTRUCTIONS FOR THE SURGEON
GROSS PROCESSING OF THE LYMPH NODE
BIOPSY BY THE PATHOLOGIST
Gross Examination
Frozen Sections
Cytologic Preparations
Sectioning

In recent years, technical strides in immunophenotyping and molecular genetic testing have revolutionized the diagnosis of hematolymphoid malignancies. Stained sections prepared from paraffin-embedded fixed tissues remain the foundation of histopathologic diagnosis. The accurate classification of lymphoid tumors and the subsequent clinical management of patients rely on the availability of adequate diagnostic tissue. A multiparameter approach to diagnosis is central to the World Health Organization (WHO) classification schemes of hematolymphoid tumors.1,2 This approach emphasizes the integration of clinical and ancillary data in the formulation of a precise diagnosis. An inadequate lymph node biopsy specimen not only precludes accurate morphologic assessment but also compromises immunophenotypic, cytogenetic, and molecular diagnostic studies. When this first step in making a diagnosis is jeopardized, even the most sophisticated DNA and RNA amplification techniques may not salvage enough information for a definitive diagnosis, and a repeat procedure may be necessary. With the current mandate to provide cost-effective health care and with mounting pressure to make diagnoses based on needle aspirations and cytologic preparations, repeating an open lymph node biopsy procedure is not trivial. Thus it is imperative that the pathologist ensure the optimal procurement and processing of lymph node specimens.
The lymph node presents certain unique challenges for the pathologist and the histotechnologist because of its innate organizational structure. The lymph node is composed of millions of small cells held together by fine strands of connective tissue surrounded by a fibrous capsule that is relatively impervious to fixation and processing chemicals. Histologic sections of excellent quality can be obtained only if each step in the processing of a lymph node is handled with
Fixation
Contribution of the Histotechnologist
ROUTINE HISTOLOGIC, HISTOCHEMICAL, AND SPECIAL STAINS
CHOICE OF ANCILLARY STUDIES
REPORTING THE LYMPH NODE BIOPSY
care and with knowledge of the underlying factors that result in optimal versus suboptimal preparations. This chapter reviews the essential steps for producing excellent-quality histologic sections of lymph node specimens, discusses the common pitfalls, and suggests how to avoid or correct these errors.
INSTRUCTIONS FOR THE SURGEON
Knowledge of the patient’s clinical history and the suspected diagnosis or differential diagnosis facilitates the search for a lymph node sample that best represents the underlying pathologic process. Despite the obvious appeal of convenient access, minimal discomfort, and procedural simplicity of excising a superficial lymph node, these lymph nodes are not always of diagnostic value. The surgeon should be encouraged to examine the patient thoroughly and sample the largest and most abnormal-appearing lymph node whenever possible (Fig. 1-1). This approach avoids the erroneous sampling of enlarged or inflamed nodes adjacent to a previous biopsy site and enables more representative sampling. Imaging studies may help guide the surgeon to the most abnormal lymph node.
Excisional biopsy of an entire lymph node is preferred to an incisional or needle core biopsy because fragments of lymph nodes preclude a proper assessment of architecture, an important feature in establishing a morphologic differential diagnosis. When an infectious cause is suspected, the surgeon should be advised to submit a portion from one pole of the lymph node for appropriate microbiologic studies directly from the sterile environment of the operating room. In all other circumstances, the intact specimen should be submitted fresh to the pathologist in a specimen container and immersed
Figure 1-1. Selection of a lymph node for biopsy. Diagram of a neck dissection for Hodgkin’s lymphoma showing the distribution of positive (black) and negative (tan) lymph nodes. Many of the most superficial and easily biopsied nodes are either benign or only atypical, whereas the diagnostic nodes are deeper, larger, and less accessible. This experience illustrates the need to remove the largest possible lymph node for diagnosis, because it is most likely to contain diagnostic tissue. (Redrawn by Dr. TuDung Nguyen, Stanford University Medical Center, Stanford, CA, from Slaughter DP, Economou SG, Southwick HW. Surgical management of Hodgkin’s disease. Ann Surg. 1958;148:705-709.)
in saline or culture medium to ensure that the specimen does not dry out during transit. Wrapping the specimen or laying it on gauze, sponges, or towels should be avoided because this leads to desiccation of the lymph node cortex, especially when the specimen is exposed to air. Request for a “lymph node workup” should be clearly indicated on the requisition slip or specimen tag, or both. Ideally, the pathologist should be notified at the time of the biopsy to avoid a delay in the handling of the specimen. When a delay in delivery to the pathologist is anticipated, the specimen should be refrigerated to minimize autolysis. Storage at 4° C for up to 24 hours can yield satisfactory but not optimal morphologic, immunologic, and genetic preservation.1,3-10 When long delays are expected before the pathologist receives the specimen, the surgeon may be instructed to bisect the lymph node and make air-dried imprints, after which the specimen can be sliced thinly and placed in buffered formalin. Portions should also be set aside for special studies.
GROSS PROCESSING OF THE LYMPH NODE BIOPSY BY THE PATHOLOGIST
Gross Examination
The gross appearance of lymph nodes, including their color, consistency, and changes in contour, may provide useful information about the diagnosis and should be recorded during the gross inspection of the fresh specimen (Fig. 1-2). Preservation of the hilus and the presence or absence of
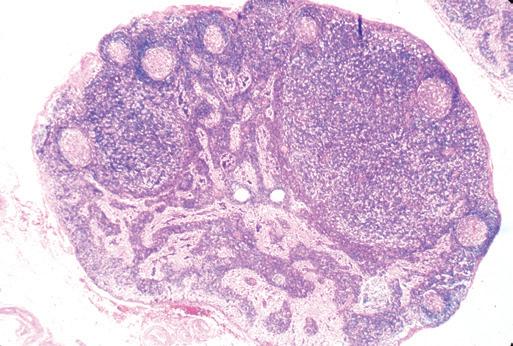
nodularity and fibrosis can offer important diagnostic clues.1,6,7 Preservation of the hilus is rare in lymphomas, and its presence suggests a reactive process (see Fig. 1-2, A and B). Necrosis within the node raises the possibility of an infectious process and may prompt microbiologic studies. Adherence of the node to the surrounding fat may denote extracapsular extension of disease and should be noted in the gross description. Most lymphomas completely efface the nodal architecture, and a nodular appearance or fibrosis can be seen on gross examination (see Fig. 1-2, C to E).
Although the gross findings can be helpful in narrowing the differential diagnosis, an accurate pathologic diagnosis is virtually never possible on the basis of the gross findings alone. Thus these findings must be interpreted in conjunction with microscopic features and immunophenotypic and genetic studies to establish a definitive diagnosis.
Frozen Sections
The diagnosis of lymphoid malignancies can be challenging even on permanent sections. Because of the numerous artifacts generated during the preparation of a frozen section, a diagnosis of lymphoma based on frozen tissue is perilous and best avoided.1,6-9 Although certain lymphomas can be distinguished on frozen sections, clinical colleagues should be advised of the unreliability of frozen sections for the accurate diagnosis and classification of lymphoma. In the rare event that a rapid interpretation is necessary for patient care, touch imprints or scrape preparations should be examined in conjunction with frozen sections. Imprints yield cytologic details that may not be appreciated on frozen tissue sections; for example, Reed-Sternberg cells may be more readily apparent on imprints than on frozen tissue sections. Even if diagnostic cells are identified on imprints or frozen sections, caution is necessary in the diagnosis of classical Hodgkin’s lymphoma because atypical cells with Reed-Sternberg cell–like morphology may be present in infectious mononucleosis, angioimmunoblastic T-cell lymphoma posttransplant lymphoproliferative disorders, diffuse large B-cell or anaplastic large cell lymphoma, poorly differentiated carcinoma, sarcoma, melanoma, and fat necrosis.1,11
The appropriate use of frozen sections of lymph node biopsy specimens is to estimate the adequacy of the tissue for diagnosis and to assess for morphologically evident nonhematolymphoid processes such as metastatic carcinoma. Frozen sections also offer the pathologist the opportunity to allocate tissue for ancillary studies based on the preliminary differential diagnosis.1,7-9,12 The frozen portion of the node should always be retained frozen for future immunophenotypic or molecular studies. In addition, microbiologic, cytogenetic, or flow cytometry studies can be initiated rapidly, with optimal preservation of cell viability. If the changes seen on frozen sections suggest a reactive process in a patient in whom there is a strong clinical suspicion of lymphoma, the surgeon can be advised to explore the patient further to find a more abnormal lymph node.
Cytologic Preparations
The utility of imprints in the evaluation of lymphoid lesions should not be underestimated. Cytologic imprint preparations complement tissue diagnosis and are useful both at the time
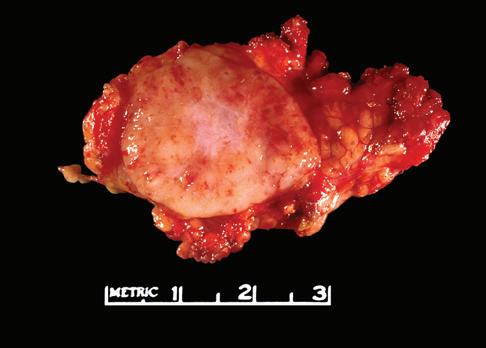
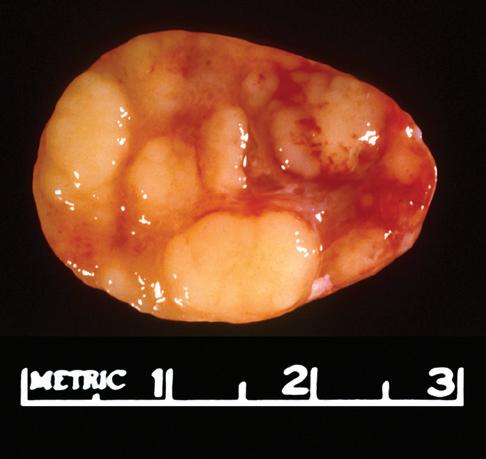
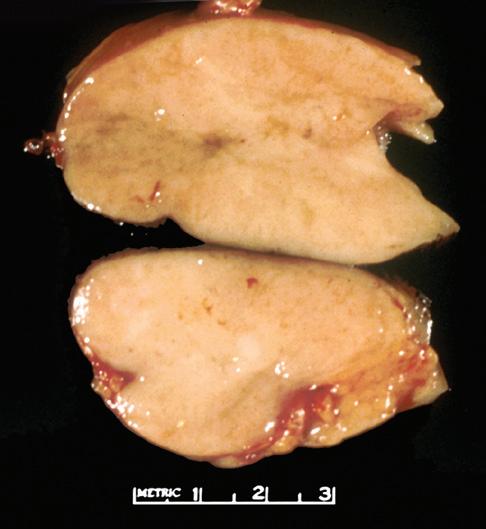
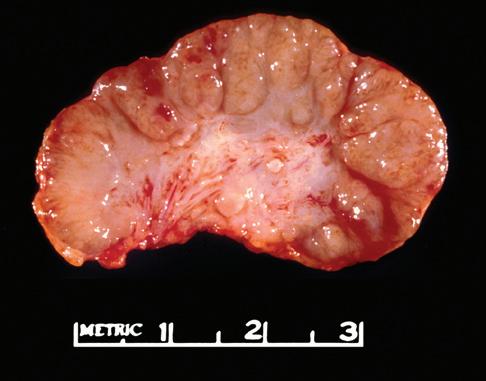
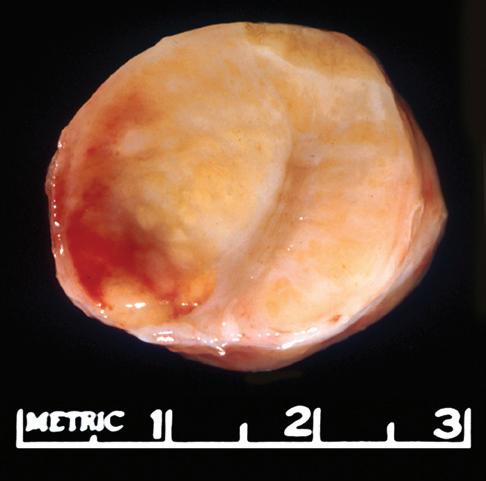
Figure 1-2. Gross appearance of lymph nodes involved by a variety of processes. A, Intraparotid lymph node with reactive hyperplasia shows preservation of the hilus (gray structure in the center). B, Lymph node with dermatopathic lymphadenitis has a brownish color to the cut surface, possibly reflecting melanin deposition. The hilus is preserved in this lymph node as well, suggesting a reactive process. C, Lymph node with both progressively transformed germinal centers and nodular lymphocyte-predominant Hodgkin’s lymphoma has an obviously nodular architecture on cut section. D, Lymph node containing nodular sclerosis Hodgkin’s lymphoma has fibrous bands traversing the cut surface. E, Lymph node involved by follicular lymphoma has a homogeneous, fleshy cut surface with obliteration of the hilus, which is typical of lymphomatous involvement.
of frozen section and when examining permanent tissue sections. Touch and scrape imprints are encouraged for all intraoperative consultations for lymphoid lesions and should be examined in conjunction with the frozen tissue sections. Most important, imprints can be stored at 4° C for days to weeks or frozen at 70° C indefinitely and used for selected immunophenotypic studies or fluorescence in situ hybridization (FISH) analysis.6,9,12 Imprints can also facilitate the intraoperative assessment of hematolymphoid lesions of bone when frozen sections cannot be obtained.
When cytologic imprints are prepared from lymph node specimens, it is best to prepare and label six to eight slides ahead of time. For touch imprints, the cut surface of the lymph node should be positioned on a flat surface such as a towel. While the slide is held firmly at one end, the slide is gently lowered and brought into contact with the cut surface of the node, avoiding smearing or sideways movement. This process can be repeated three to five times, creating a series of touch imprint slides. The imprint slide should immediately be placed in a Coplin jar with 95% alcohol. Buffered formalin or formaldehyde can also be used as a fixative. A few imprint slides may be air dried. For scrape preparations, the fresh-cut surface of the lymph node is gently scraped with the edge of a slide or the blunt edge of a scalpel and immediately smeared onto a previously labeled slide. Alcohol-dried and air-dried slides can be generated as for touch imprints. Although there is almost always enough material available to make touch imprints, scrape preparations are best avoided when dealing with very small samples to prevent inadvertent crushing or distortion of the tissue.
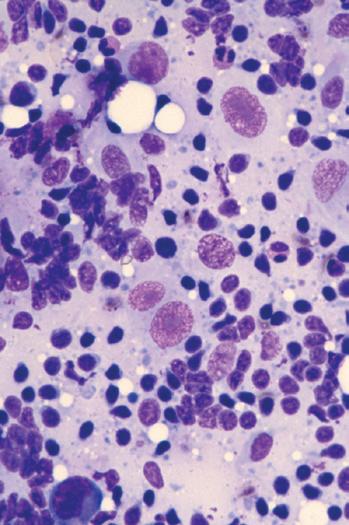
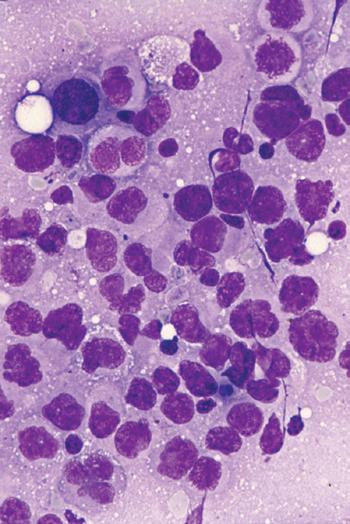
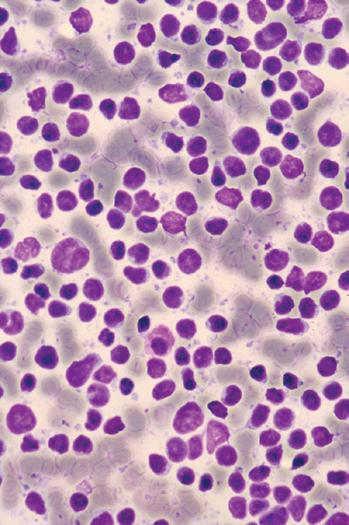
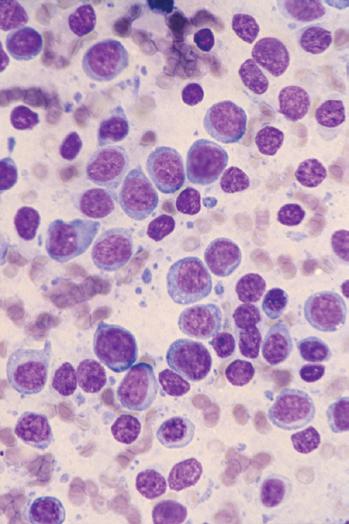
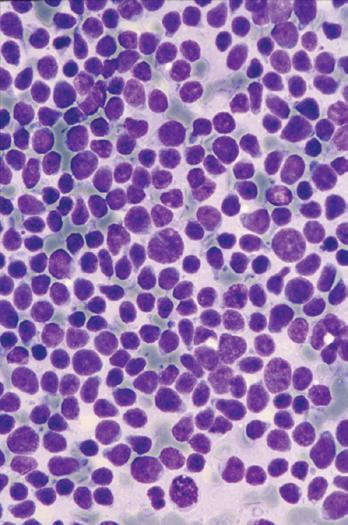
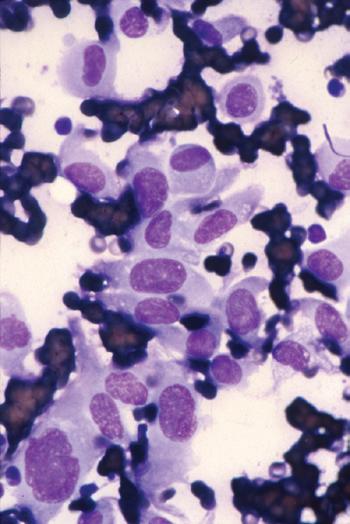
A Wright-Giemsa or Diff-Quik stain is best for identifying and characterizing cells of the hematopoietic system and tumors derived from them, but the Papanicolaou stain is useful for assessing nuclear details such as membrane irregularity, chromatin configuration, and nucleoli. When necrosis and inflammatory cells are present, a Gram stain can be helpful to highlight bacterial organisms. In general, aspirations of lymph nodes are highly cellular and are characterized by a dispersed cell pattern and lymphoglandular bodies (detached cytoplasmic fragments of lymphoid cells). Indolent lymphomas composed of predominantly small cells or a mixed cellular milieu are much more difficult to diagnose on cytologic preparations than are aggressive lymphomas (Fig. 1-3, A).11 Reactive follicular hyperplasia can be nearly impossible to distinguish from follicular lymphoma on cytologic imprints, although the presence of a limited range of maturation together with the absence of tingible body macrophages favors a malignant diagnosis. In aggressive lymphomas, the presence of monotonous sheets of medium to large cells, especially when associated with karyorrhexis and apoptosis, suggests the differential diagnosis of lymphoblastic, Burkitt’s, or large cell lymphoma (see Fig. 1-3, B). Similarly, imprints can be helpful in highlighting Reed-Sternberg cells (see Fig. 1-3, C) or immunoblastic features in diffuse large B-cell lymphoma (see Fig. 1-3, D).1,11 Cytologic preparations can also be useful in the diagnosis of metastatic melanoma and carcinoma (see Fig. 1-3, E and F) and of non-neoplastic lesions in the lymph node such as granulomatous lymphadenitis and Kikuchi’s lymphadenitis. Lesions associated with significant sclerosis seldom yield sufficient material for cytologic preparations.1,9,11
Sectioning
The two most important initial steps in the processing of a lymph node specimen are sectioning (blocking) and fixation. These steps are entirely the responsibility of the pathologist. Blocking should be performed promptly and should precede fixation because an intact lymph node capsule is impervious to fixation. In addition, touch and scrape imprints are best obtained in the fresh state. The objective of good sectioning of a lymph node is to provide an undisrupted section that maintains the overall architecture of the tissue intact and is thin enough to yield significant cytologic detail. Sections should also preserve the relationship between the capsule and the remainder of the lymphoid compartments (Fig. 1-4). The best cross-section of a lymph node results from sectioning perpendicular to the long axis of the node with a sharp knife in one continuous sweep. This technique facilitates excellent preservation of the nodal architecture. For lymph nodes less than 1 cm in diameter, a single cut along the long axis is recommended; such small specimens may be crushed when attempting to perform cross-sections perpendicular to the long axis. The entire specimen should be sectioned in 2- to 3-mm slices and then placed promptly in fixative. Portions of lymph nodes should never be left unfixed or fixed without slicing. Because the fibrous tissue in the capsule may contract when exposed to fixatives, scoring of the capsule by introducing small cuts with a sharp scalpel blade may prevent distortion during processing (see Fig. 1-4, A). When lymph node specimens are fixed whole or when the central portion of the section is too thick, uneven fixation results (Fig. 1-5). This may lead to autolysis of the central areas or retraction of the tissue, causing erosion or cracking of the sections upon cutting with a microtome blade.1,7-9,13-16
Thin slices of 2 to 3 mm should be placed in shallowprofile plastic cassettes (used in most modern surgical pathology laboratories) to allow adequate penetration by fixation and processing reagents. Thorough—if not complete— sampling of the lymph node specimen is essential. This practice prevents sampling errors in disorders that may only partially involve the lymph node, such as nodular lymphocyte–predominant Hodgkin’s lymphoma in patients with progressive transformation of germinal centers and in cases of variations in grade or focal progression of a lowgrade lymphoma such as follicular lymphoma. Under most circumstances, once portions of the lymph node specimen have been removed for ancillary studies, the specimen is small enough to be submitted entirely in a few cassettes. When multiple lymph nodes are submitted or when a lymph node is so large that 10 or more cassettes are required to submit the entire specimen, knowledge of the clinical differential diagnosis and good gross examination skills are helpful. Multiple sections at 2- to 3-mm intervals should be made throughout the specimen, and sections from various portions should be submitted. It is always preferable to err on the side of submitting too much adequately fixed tissue rather than not having enough to establish a definitive diagnosis or to perform ancillary studies. In any lymph node biopsy in which microscopic examination of the initially submitted sections does not yield a definitive diagnosis, all the remaining tissue should be promptly submitted for microscopic examination.
Fixation
Fixation is the point of no return in the processing of a lymph node specimen. Although subsequent steps, including infiltration, clearing, and dehydration, can be repeated if necessary, inadequate fixation cannot be reversed. Poor fixation is the leading cause of uninterpretable lymph node sections.1,7-9,13-15 Both histotechnologists and pathologists may waste valuable time attempting to reprocess poorly fixed specimens, obtaining special or ancillary studies that may not
be necessary, and seeking expert consultation to establish or confirm a diagnosis.
Excellent-quality slides can be prepared from lymph node specimens using a number of different fixatives, as long as the proper volume and strength of fixative are used and, most important, adequate time is allowed for fixation. The advantages and disadvantages of the most commonly used fixatives for lymph node specimens are outlined in Table 1-1. Many laboratories use a combination of neutral buffered formalin and a metal-based fixative; one or two slices are fixed in a
Figure 1-3. Cytologic preparations of low-grade B-cell lymphoma (A), lymphoblastic lymphoma (B), Hodgkin’s lymphoma (C), diffuse large B-cell lymphoma with prominent immunoblastic features (D), metastatic melanoma (E), and metastatic poorly differentiated carcinoma of unknown primary site (F).
2-mm cross-section
Score capsule to prevent curling in fixative
Figure 1-4. Lymph node sectioning. Lymph nodes should be sectioned to provide a complete cross-section that allows an appreciation of architecture. A, Schematic diagram shows that the lymph node is cut perpendicular to the long axis of the node (best for specimens >1 cm in diameter). The lymph node capsule can be scored, with several small cuts, before placing the section in fixative; this prevents curling as the capsule retracts on exposure to fixative. B, Low-power photomicrograph of a properly oriented section of lymph node showing the capsule, cortex, paracortex, and medulla.
metal-based fixative for speed of fixation and optimal morphology, and the remainder are fixed in formalin for preservation of DNA and long-term storage. Although pathologists’ preferences for metal fixatives vary, B5 neutral Zenker’s solution and zinc sulfate formalin are the most commonly used. Although B5 renders excellent nuclear detail (Fig. 1-6), several factors make its routine use problematic. These include the relatively high cost, the time-sensitive nature of fixation (2 to 4 hours), and the need to remove mercuric chloride crystals from the sections and dispose of the mercury, an environmental hazard. Zinc sulfate (available commercially as B+ from Buffers And Biochemicals Corp., Loveland, Ohio) is an alternative to B5; it offers good nuclear detail, is less costly, and requires no special procedures for handling and disposal because it contains no mercuric chloride. Fixatives that are highly acidic, such as Zenker’s, B5, Bouin’s, and Carnoy’s, are unsuitable for molecular diagnostic studies because they compromise the efficiency of polymerase chain reaction (PCR) amplification by decreasing the ability of the DNA within tissue to function as a template for the amplification of DNA fragments of desirable length. The best fixatives for molecular diagnostic studies are ethanol, acetone, and Omnifix (FR Chemical Inc., Albany, N.Y.), although formalin fixation also works well in most instances. Alcohol-based fixatives enhance the preservation of not only DNA and RNA but also certain antigens targeted for immunohistologic studies. Alcohol preserves intermediate filaments better than other fixatives but
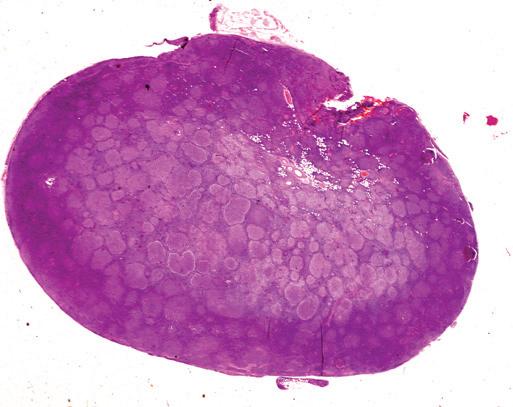
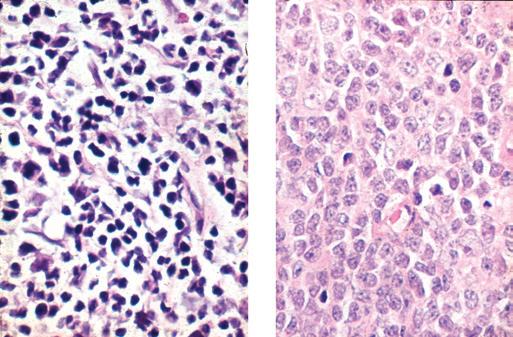
Figure 1-5. Lymph node fixation. This lymph node was placed in fixative without first cutting thin sections. A, Only the outer 1.0 mm of this paraffin section stained with hematoxylin-eosin is well fixed and stained; the center shows fainter staining and evidence of cell retraction. B, At high magnification, the center of the node (left) is autolyzed, with suboptimal cellular detail; the periphery (right) shows good cellular detail.
does not preserve some lymphoid antigens. Alcohol fixation, however, may yield suboptimal morphologic preparations, especially in small biopsies. Several technical modifications are also available to preserve and augment the immunoreactivity of selected antigens. In addition, plastic embedding may be helpful in enhancing cytologic detail.
We find that 10% neutral buffered formalin offers the best overall results by furnishing excellent morphologic preparations with good preservation of immunoreactivity and suitability for molecular diagnostic studies (Table 1-2). In addition, neutral buffered formalin provides the best method for long-term storage of fixed tissue, a particularly important consideration in storing archival material for research purposes. For good morphology, though, fixation in formalin requires at least 12 hours, with a maximum of about 48 hours for optimal morphology and tissue preservation for immunohistochemistry.17
Thus when there is sufficient tissue for more than one fixative, a few slices may be fixed in a metal-based fixative, and the remainder in formalin for overnight fixation before additional processing.