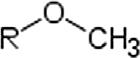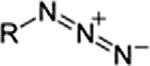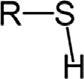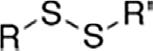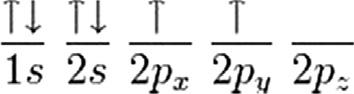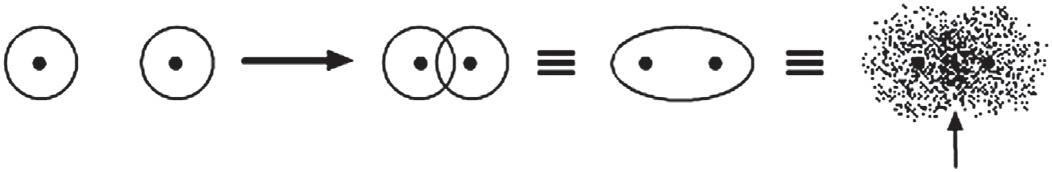AbouttheAuthor
DrJamesG.Speighthasdoctoratedegreesinchemistry,geologicalsciences, andpetroleumengineeringandistheauthorofmorethan80booksinpetroleumscience,petroleumengineering,environmentalsciences,andethics.
Hehasmorethan50yearsofexperienceinareasassociatedwith(1)the properties,recovery,andrefiningofreservoirfluids,conventionalpetroleum, heavyoil,andtarandbitumen;(2)thepropertiesandrefiningofnaturalgas, gaseousfuels,(3)theproductionandpropertiesofpetrochemicals;(4)the propertiesandrefiningofbiomass,biofuels,biogas,andthegenerationof bioenergy;and(5)theenvironmentalandtoxicologicaleffectsoffuels.His workhasalsofocusedonsafetyissues,environmentaleffects,remediation, andreactorsassociatedwiththeproductionanduseoffuelsandbiofuels.
Althoughhehasalwaysworkedinprivateindustrywithemphasison contract-basedwork,DrSpeightwasavisitingprofessorintheCollegeof Science,UniversityofMosul,Iraq,andhasalsobeenavisitingprofessorin chemicalengineeringatthefollowinguniversities:UniversityofMissouriColumbia,theTechnicalUniversityofDenmark,andtheUniversityofTrinidadandTobago.
In1996,DrSpeightwaselectedtotheRussianAcademyofSciencesand awardedtheGoldMedalofHonorthatsameyearforoutstandingcontributions tothefieldofpetroleumsciences.In2001,hereceivedtheScientistsWithout BordersMedalofHonoroftheRussianAcademyofSciencesandwasalso awardedtheEinsteinMedalforoutstandingcontributionsandserviceinthe fieldofGeologicalSciences.In2005,DrSpeightwasawardedtheGold Medal ScientistsWithoutFrontiers,RussianAcademyofSciences,in recognitionofContinuousEncouragementofScientiststoWorkTogether AcrossInternationalBorders.In2007,Dr.SpeightreceivedtheMethanex DistinguishedProfessorawardattheUniversityofTrinidadandTobagoin recognitionofexcellenceinresearch.In2018,hereceivedtheAmerican ExcellenceAwardforExcellenceinClientSolutionsfromtheUnitedStates InstituteofTradeandCommerce,Washington,DC.
Preface
ThesuccessoftheFirstEditionofthistexthasbeentheprimaryfactorinthe decisiontopublishanupdatedSecondEdition.
Thehydrocarbonindustryhaditsmodernoriginsinthelateryearsofthe19th century.Bythetimethatthe19thcenturyhaddawned,itwasknownthat kerosene,afuelforheatingandcooking,wastheprimaryproductofthecrudeoil industryinthe1800s.Rockefellerandotherrefineryownersconsideredgasoline auselessby-productofthedistillationprocess.Butallofthatchangedaround 1900whenelectriclightsbegantoreplacekerosenelampsandautomobilescame onthescene.Newhydrocarbonfuelswerealsoneededtopowertheshipsand airplanesusedinWorldWarI.Afterthewar,anincreasingnumberoffarmers begantooperatetractorsandotherequipmentpoweredbyoil.Thegrowing demandforpetrochemicalsandtheavailabilityofcrudeoilandnaturalgas causedtheindustrytoquicklyexpandinthe1920sand1930s.DuringWorldWar II,vastamountsofcrudeoilwereproducedandconvertedintohydrocarbonfuels andlubricants.
Theterm hydrocarbons (compoundswhichcontaincarbonandhydrogen only)representsalargegroupofchemicalsmanufacturedfromcrudeoiland naturalgasasdistinctfromfuelsandotherproductsthatarealsoderivedfrom crudeoilandnaturalgasbyavarietyofprocessesandusedforavarietyof commercialpurposes.Hydrocarbonsleadtoproductswhichincludesuchitems asplastics,soapsanddetergents,solvents,drugs,fertilizers,pesticides,explosives,syntheticfibersandrubbers,paints,epoxyresins,andflooringandinsulatingmaterials.Hydrocarbonsarealsousedtoproducechemicalsasdiverseas aspirin,luggage,boats,automobiles,aircraft,polyesterclothes,andrecording discsandtapes.Itisthechangesinproductdemandthathavebeenlargely responsiblefortheevolutionofthehydrocarbonindustryfromthedemandfor thehydrocarbonaceousasphaltmasticusedinancienttimestothecurrenthigh demandforgasolineandotherhydrocarbonfuelsaswellasincreasingdemand forawidevarietyofpetrochemicalproducts.
Asaresult,thehydrocarbonindustryisahugefieldthatencompassesmany commercialchemicalsandpolymers.Theorganicchemicalsproducedinlarge volumesaremethanol,ethylene,propylene,butadiene,benzene,toluene,andthe xyleneisomers.Ethylene,propylene,andbutadiene,alongwithbutylenes,are collectivelycalledolefins,whichbelongtoaclassofunsaturatedaliphatichydrocarbons,havingthegeneralformulaCnH2n.Olefinscontainoneormore
doublebonds,whichmakethemchemicallyreactive.Benzene,toluene,andthe xyleneisomers,commonlyreferredtoasaromatics(BTX),areunsaturatedcyclichydrocarbonscontainingoneormorerings.Olefins,aromatics,andmethanolareprecursorstoavarietyofchemicalproductsandaregenerallyreferredto asprimarypetrochemicals.Furthermore,becauseethyleneandpropylenearethe majorbuildingblocksforpetrochemicals,alternativewaysfortheirproduction havealwaysbeensought.Themainrouteforproducingethyleneandpropylene issteamcracking,whichisanenergyextensiveprocess.
Basichydrocarbonchemicalsarethekeybuildingblocksformanufactureof awidevarietyofdurableandnondurableconsumergoods.Consideringtheitems thatareencounteredeveryday clothes,constructionmaterialsusedtobuildour homesandoffices,avarietyofhouseholdappliancesandelectronicequipment, foodandbeveragepackaging,andmanyproductsusedinvariousmodesof transportation hydrocarbonsprovidethefundamentalbuildingblocksthat enablethemanufactureofthevastmajorityofthesegoods.Demandfor chemicalsandplasticsisdrivenbyglobaleconomicconditions,whichare directlylinkedtodemandforconsumergoods.
Thesearchforalternativewaystoproducemonomersandchemicalsfrom sourcesotherthancrudeoil.Infact,FisherTropschtechnology,whichproduces lowmolecularweightolefinsinadditiontohydrocarbonfuels,couldenable non crudeoilfeedstocks(suchasextraheavyoil,tarandbitumen,coal,oil shale,andbiomass)tobeusedasfeedstocksforpetrochemicals.
Inaddition,thecontinuedhighdemandforhydrocarbonproducts,suchas liquidfuels(gasolineanddieselfuel)andpetrochemicalfeedstocks(suchas aromaticderivativesandolefinderivatives),isincreasingthroughouttheworld. TraditionalmarketssuchasNorthAmericaandEuropeareexperiencingasteady increaseindemand,whereasemergingAsianmarkets,suchasIndiaandChina, arewitnessingarapidsurgeindemandforhydrocarbons.Thishasresultedina tendencyforexistingrefineriestoseekfreshrefiningapproachestooptimize efficiencyandthroughput.Furthermore,theincreasinguseoftheheavierfeedstocksforrefineriesisforcingtechnologysuppliers/licensorstorevamptheir refiningtechnologiesinanefforttocatertothegrowingcustomerbase.
Theevolutioninproductspecificationscausedbyvariousenvironmental regulationsplaysamajorroleinthedevelopmentofcrudeoilrefiningtechnologies.Inmanycountries,especiallyintheUnitedStatesandEurope,gasoline anddieselfuelspecificationshavechangedradicallyinthepastdecadesandwill continuetodosointhefuture.Currently,reducingthesulfurlevelsofhydrocarbonfuelsisthedominantobjectiveofmanyrefiners.Thereisalsoan increasingdemandforhydrocarbonderivativesforotheruses.Thisispushingthe technologicallimitsofhydrocarbonproductiontothemaximum,andthe continuingissueistheprocessesthatwillincreasehydrocarbonproductionand purity.
Refineriesmust,andindeedareeagerto,adapttochangingcircumstances andareamenabletotryingnewtechnologiesthatareradicallydifferentin
character.Currently,refineriesarealsolookingtoexploitheavy(moreviscous) crudeoilsandtarandbitumen(sometimesreferredtoasextraheavycrudeoil) providedtheyhavetherefinerytechnologycapableofhandlingsuchfeedstocks. Transformingthehigherboilingconstituentsofthesefeedstockcomponentsinto singlehydrocarbonderivativeaswellashydrocarbonfuelsisbecominga necessity.
Thereadermightalsobesurprisedatthenumberofolderreferencesthatare included.Thepurposeofthisistoremindthereaderthatthereismuchvaluable workcitedintheolderliterature.Workthatisstillofvalueand,eventhoughin somecases,therehasbeensimilarworkperformedwithadvancedequipment, theolderworkhasstoodthetestoftime.Manyoftheideasarestillpertinentand shouldnotbeforgottenintermsofthevaluablecontributionstheyhavemadeto crudeoilscienceandtechnology.However,manyoftheolderreferences includedinpreviouseditionsofthisbookhavebeendeleted unavailabilityof thesourceforthegeneralscientificresearcherandthecurrentlackofasubstantiatedsources(otherthanthefilescollectedbytheauthor)havebeentheroot causeofsuchomissions.
Therefore,itisthepurposeofthisbooktoprovidethereaderwithadetailed overviewoftheproductionandpropertiesofhydrocarbonderivativesandhydrocarbonfuelsastheworldevolvesintothe21stcentury.Withthisinmind, manyofthechaptersthatappearedintheFirstEditionhavebeenrewrittento includethelatestdevelopmentsrelatedtohydrocarbonproducts.Updatesonthe evolvingprocessesandnewprocessesaswellasthevariousenvironmental regulationsarepresented.However,thetextstillmaintainsitsinitialpremise, thatis,tointroducethereadertothehydrocarbonscienceandtechnologyaswell astheproductionofawidevarietyofproductsandpetrochemicalintermediates. However,thetextwillalsoproveusefulforthosescientistsandengineersalready engagedinthecrudeoilindustryaswellasinthecatalystmanufacturingindustry whowishtogainageneralovervieworupdateofthescienceofcrudeoil.
Thus,thebookfocusesontheinterfacesbetweenchemicaltechnologyand biotechnologyespeciallywheretheseimpactonhealthandsafetyandthe environment.Also,thebookhasbeenadjusted,polished,andimprovedforthe benefitofnewreadersaswellasforthebenefitofreadersofthefourprevious editions.
Chemistryandchemical technology
1.Introduction
Chemistry(fromtheArabic alkhymia)isthescienceofmatterandisconcernedwiththecomposition,behavior,structure,andpropertiesofmatter,as wellasthechangesmatterundergoduringchemicalreactions.Chemistryisa physicalscienceandisusedfortheinvestigationofatoms,molecules,crystals, andotherassemblagesofmatterwhetherinisolationorcombination,which incorporatestheconceptsofenergyandentropyinrelationtothespontaneity orinitiationofchemicalreactionsorchemicalprocesses.
Disciplineswithinchemistryaretraditionallygroupedbythetypeofmatter beingstudiedorthekindofstudyandincludethefollowing(presented alphabetically):(i)analyticalchemistry,whichistheanalysisofmaterial samplestogainanunderstandingoftheirchemicalcompositionandstructure, (ii)biochemistry,whichisthestudyofsubstancesfoundinbiologicalorganisms,(iii)inorganicchemistry,whichisthestudyofinorganicmatter (inorganicchemicals,suchasminerals),(iv)organicchemistry,whichisthe studyoforganicmatter(inorganicchemicals,suchashydrocarbonderivatives),and(v)physicalchemistry,whichisthestudyoftheenergyrelationsofchemicalsystemsatmacro,molecular,andsubmolecularscales.
Infact,thehistoryofhumanculturecanbeviewedastheprogressive developmentofchemicaltechnologythroughevolutionofthescientificand engineeringdisciplinesinwhichchemistryandchemicalengineeringhave playedmajorrolesinproducingawidevarietyofindustrialchemicals, especiallyindustrialorganicchemicals(Alietal.,2005).Chemicaltechnology,inthecontextofthepresentbook,reliesonchemicalbondsofhydrocarbonderivatives.Naturehasfavoredthestorageofsolarenergyinthe hydrocarbonbondsofplantsandanimals,andtheevolutionofchemical technologyhasexploitedthishydrocarbonenergyprofitably.
Thefocusofthisbookishydrocarbonderivativesandthechemistry associatedwiththeseorganiccompoundswhichwillbeusedtoexplainthe aspectsofhydrocarbonproperties,structure,andmanufacture.Thebookwill provideinformationrelatingtothestructureandpropertiesofhydrocarbon
derivativesandtheirproductionthroughprocesschemistryandchemical technologytotheirconversionintocommercialproducts.
2.Organicchemistry
Organicchemistry isadisciplinewithinchemistrythatinvolvesstudyofthe structure,properties,composition,reactions,andpreparation(bysynthesisor byothermeans)ofcarbon-basedcompounds(inthiscontext hydrocarbon derivatives).
Ontheotherhand,inorganicchemistryisthebranchofchemistryconcernedwiththepropertiesandbehaviorofinorganiccompounds.Thisfield coversallchemicalcompoundsexceptthemyriadofcarbon-basedcompounds(suchasthehydrocarbonderivatives),whicharethesubjectsof organicchemistry.Thedistinctionbetweenthetwodisciplinesisfarfrom absolute,andthereismuchoverlap,mostimportantlyinthesubdiscipline oforganometallicchemistryinwhichorganiccompoundsandmetalsform distinctandstableproducts.Anexampleistetraethylleadwhichwas formerlyusedingasoline(untilitwasbannedbyvariousnationalenvironmentalagencies)asanoctaneenhancertopreventengineknockingor pingingduringoperation.Otherthanthisclarificationandbriefmentionhere, neitherinorganicchemistrynororganometallicchemistrywillbedescribed furtherinthistext.
2.1Organicchemicals
Organiccompoundsarestructurallydiverse,andtherangeofapplicationof organiccompoundsisenormous.Inaddition,organiccompoundsmaycontain anynumberofotherelements,includingnitrogen,oxygen,sulfur,halogens, phosphorus,andsilicon.Theyformthebasisof,orareimportantconstituents of,manyproducts(suchasplastics,drugs,petrochemicals,food,explosives, andpaints)and,withveryfewexceptions,theyformthebasisofalllife processesandmanyindustrialprocesses.
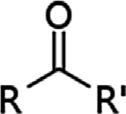
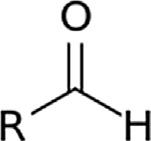
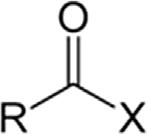
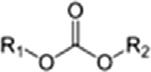
Organiccompounds ofwhichthehydrocarbonderivativesarea subgroup areclassifiedaccordingtothepresenceoffunctionalgroupsin themolecule(Table1.1, Table1.2 , Table1.3 , Table1.4 ).A functionalgroup isamolecularmoietythattypicallydic tatesthebehavior(reactivity)ofthe organiccompoundintheenvironment andthereactivityofthatfunctional groupisassumedtobethesameinavarietyofmolecules,withinsomelimits andifstericeffects(thatarisefromthethree-dimensionalstructureofthe molecule)donotinterfere.Thus,mos torganicfunctionalgroupsfeature heteroatoms(atomsotherthancarbonandhydrogen,suchas:nitrogen,oxygen,andsulfur).Theconceptoffun ctionalgroupsisamajorconceptin organicchemistry,bothtoclassifythe structureoforganiccompoundsandto predictthephysicalandchemicalpropertiesespecially,inthecontextofthis
TABLE1.1
Generalclassesofhydrocarbons.
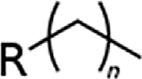
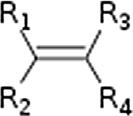
AlkeneAlkenylR2C ¼ CR2
AlkyneAlkynylR1ChCR2
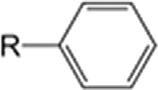
BenzenederivativePhenylRC6H5
book,thosepropertiesthatrelatetobeh aviorandreactivityintechnological processes.
Forexample,whencomparingthepropertiesofethane(CH3CH3)withthe propertiesofpropionicacid(CH3CH2CO2H),whichisachemicalthatis formedduetothereplacementofahydrogenatomintheethanemoleculebya carboxylicacidfunctionalgroup(CO2H)thechangeinpropertiesand behaviorisspectacular.Alternatively,thereplacementofamethylgroup (CH3)intotheethanemoleculebythecarboxylicacidfunctiontoproduce aceticacid(CH3CO2H)(orthereplacementofahydrogeninthemethane molecule(CH4)bythecarboxylicacidfunction)producessignificantchanges inthepropertiesoftheproductvis-a ` -vistheoriginalmolecule.
Thus,organicchemicalsfromverysimplecompoundssuchasmethane (CH 4)toorganicchemicalsthatcontainmorethanonecarbonatom,asmany as10carbonatomstochemicalsthatcontainhundredsormorecarbonatoms thatarelinkedincarbon carbonbonds.Thosethatcontainonlycarbonand hydrogenarecalledhydrocarbons;asimpleexampleisH3C(CH 2)3CH3 (pentane).Organicchemicalscommonlycontainotherelementstoo,suchas oxygen,nitrogen,orsulfur.Anorganometallicchemicalhasacarbonatom bondedtoametalasintetraethyllead.Someorganometallicchemicalsare foundnaturally.
Manyorganicchemicalsaresynthetic,i.e.,producednotbylivingcreatures,butmanufacturedbyhumanbeings.However,thefeedstocksfrom whichthechemicalsaremadecomefromnature.Commonlysyntheticorganic chemicalsaremadefromcrudeoilornaturalgasfeedstocks,whichare
TABLE1.2 Generalclassesofoxygencompounds.
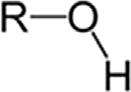
ChemicalclassGroupFormulaStructuralformula AlcoholHydroxylROH
KetoneCarbonylRCOR0
AldehydeAldehydeRCHO
AcylhalideHaloformylRCOX
CarbonateCarbonateesterROCOOR
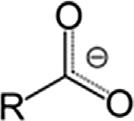
CarboxylateCarboxylateRCOO
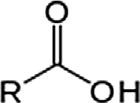
CarboxylicacidCarboxylRCOOH
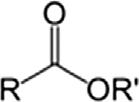
EsterEsterRCOOR0
MethoxyMethoxyROCH3
TABLE1.3
Generalclassesofnitrogencompounds.
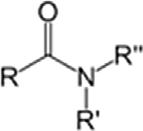
ClassGroupFormulaStructuralformula AmideCarboxamideRCONR2
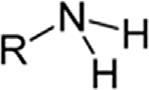
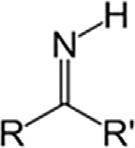
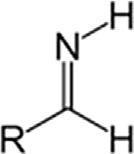
AminesPrimaryamineRNH2
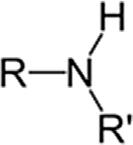
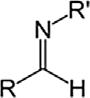
SecondaryamineR2NH
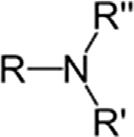
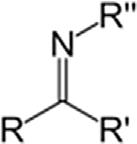
TertiaryamineR3N
4degreesammoniumionR4Nþ
IminePrimaryketimineRC(¼NH)R0
SecondaryketimineRC(¼NR)R0
PrimaryaldimineRC(¼NH)H
TABLE1.3 Generalclassesofnitrogencompounds. cont’d
ClassGroupFormulaStructuralformula
SecondaryaldimineRC(¼NR0 )H
ImideImide(RCO)2NR0
AzideAzideRN3
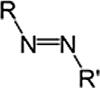
AzocompoundAzo(di-imide)RN2R0
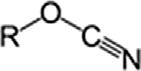
CyanatesCyanateROCN
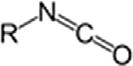
IsocyanateRNCO
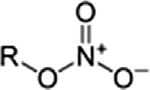
NitrateNitrateRONO2
NitrileNitrileRCN
IsonitrileRNC
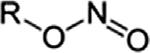
NitriteNitroso-oxyRONO
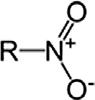
NitrocompoundNitroRNO2
TABLE1.4 Generalclassesofsulfurcompounds.
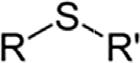
ChemicalclassGroupFormulaStructuralformula ThiolSulfhydrylRSH Sulfide(thioether)SulfideRSR
DisulfideDisulfideRSSR
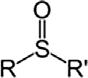
SulfoxideSulfinylRSOR
SulfoneSulfonylRSO2R
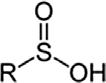
SulfinicacidSulfinoRSO2H
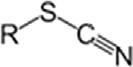
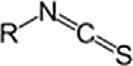
SulfonicacidSulfoRSO3H ThiocyanateThiocyanateRSCN
IsothiocyanateRNCS
ThioneCarbonothioylRCSR
ThialCarbonothioylRCSH
referredtoaspetrochemicals(Speight,2014,2017).Coalorwoodalso sometimesserveasfeedstocksfororganicchemicals.Plasticsaresynthetic organicchemicalsandtheso-calledbioplastics,whichhumansproducefrom plantmaterials,involvesomesyntheticchemistry.Somecommercialorganic chemicalsareproducedtoobyculturesofmoldsorbacteria;suchchemicals mustthenbepurifiedfromtheseculturesbyhumanactions.
Finally,forclarification,abiochemicalisanorganicchemicalsynthesized byalivingcreature.Proteins,fats,andcarbohydratesarebiochemicals.Sucrose (tablesugar)andtheaceticacid(CH3CO2H)invinegarareexamplesofsimple biochemicals.Manybiochemicalscanalsobemadesynthetically,notonly simplechemicalssuchasvinegarorthesugars,sucrose,andxylose,butalso complexones.Ifthestructureofachemicalmadebysyntheticmeansisexactly thesameasthatfoundinnature,itisindeedthesamechemical thebodytreats bothinexactlythesamewaysothereisnobiologicaldifferencebetweenthem. Chemicalssynthesizedbylivingcreaturescanalsobeextensivelymanipulated duringextractionandpurificationandstilllegallybecallednatural.
2.2Thechemicalbond
Themostbasicconceptinallofchemistryisthechemicalbond.Thechemical bondisessentiallythesharingofelectronsbetweentwoatoms,asharing whichholdsorbondstheatomstogether.
Atomshavethreecomponents:protons,neutrons,andelectrons.Protons haveapositivechargeof þ1,neutronshave0charge,andelectronshavea negativechargeof 1.Theprotonsandneutronsoccupythecenteroftheatom asapieceofsolidmattercalledthenucleus.Theelectronsexistin orbitals surroundingthenucleus.Inreality,itisimpossibletotelltheprecisetrajectory ofanelectronandthebestthatcanbeachievedistodescribetheprobabilityof locatingtheelectroninaregionofspace.
Thesimplestcaseiswhenthenucleusissurroundedbyjustoneelectron (forexample,thehydrogenatom).Inthiscase,theprobabilityoffindingan electroninitslowestenergy,ormoststable,stateisitbeingdistributedina sphericallysymmetricwayaroundthenucleus.Theprobabilityoffindingthe electronishighestatthenucleusanddecreasesasthedistancefromthenucleusincreases.
Thesphericallysymmetric 1sorbital isthelowestenergyorbitalthatan electroncanoccupy,butseveralhigherenergyorbitalsaresignificantin organicchemistry.Thenextlowestenergyorbitalthatanelectroncanoccupy isthe 2sorbital,whichlooksmuchlikethe 1sorbital exceptthattheelectron ismorelikelytobefoundfartherfromthenucleus.Thethirdlowestenergy orbitalisthe 2porbital.Themajorandhighlyimportantdifferencebetweena porbital andan sorbital isthatthe porbital isnotsphericallysymmetricand isorientedalongaspecificaxisinspace.Therearethree p orbitals,whichare orientedalongthex,y,andzaxes.
2.3Bondingincarbon-basedsystems
Achemicalbondisessentiallythesharingofelectronsbetweentwoatoms. Sinceelectronsarenegativelychargedandexertanattractiveforceonnuclei, theyservetoholdtheatomstogetheriftheyarelocatedbetweentwonuclei. Whentwoatomsapproacheachother,their atomicorbitals overlap.The overlappedatomicorbitalscanaddtogethertoforma molecularorbital (linear combinationofatomicorbitals,LCAO).Theareaofgreatestoverlapbetween theoriginalatomicorbitalsrepresentsthechemicalbondthatisformedbetweenthem.Sincethesharingofelectronsisthebasisofthechemicalbond, themolecularorbitalsformedrepresentchemicalbonds.
Forexample,inthecaseofhydrogen,thetwo 1s orbitalsgraduallycome closertogetheruntilthereisagooddealofoverlapbetweenthem.Atthis point,theareainspaceofgreatestelectrondensitywillbebetweenthetwo nuclei,whichthemselveswereatthecenteroftheoriginalatomicorbitals. Thiselectrondensity,nowpartofanewmolecularorbital,representsthe chemicalbond.Whentheareaofgreatestoverlapoccursdirectlybetweenthe twonucleionanaxiscontainingthenucleiofbothatoms(internuclearaxis), thebondisa sigmabond (s bond)(Fig.1.1).
Morethanoneatomicorbitalfromasingleatomcanbeusedtoformnew molecularorbitals.Forexample,a 2s orbitalanda 2p orbitalfromoneatom mightaddtogetherandoverlapwithoneormoreorbitalsfromasecondatom toformnewmolecularorbitals.Second,partsoforbitalscanpossessasign (þor-).The s orbitalhasthesamesignthroughout,whileinthe p orbitals,one lobeis þ andtheotherlobeis-.Signsdonotmatterwithrespecttoelectron density,buttheymustbeconsideredwhenorbitalsareaddedorsubtracted.If twoorbitalsofthesamesignareadded,electrondensitywillincrease,whileif twoorbitalsofoppositesigns(charges)areadded,thesharedelectrondensity willcancelout.
Carbonhassixelectrons onlytwoelectronscanoccupyan s orbitalata time.Thefirsttwoelectronsincarbonoccupythe 1s orbitalandthenexttwo occupythehigher-energy,butsimilarlyshaped 2s orbitalwhilethefinaltwo electronsoccupythe 2p orbitals.
electrondensity
FIGURE1.1 Twohydrogen 1s atomicorbitalsoverlaptoformahydrogenmolecularorbital.
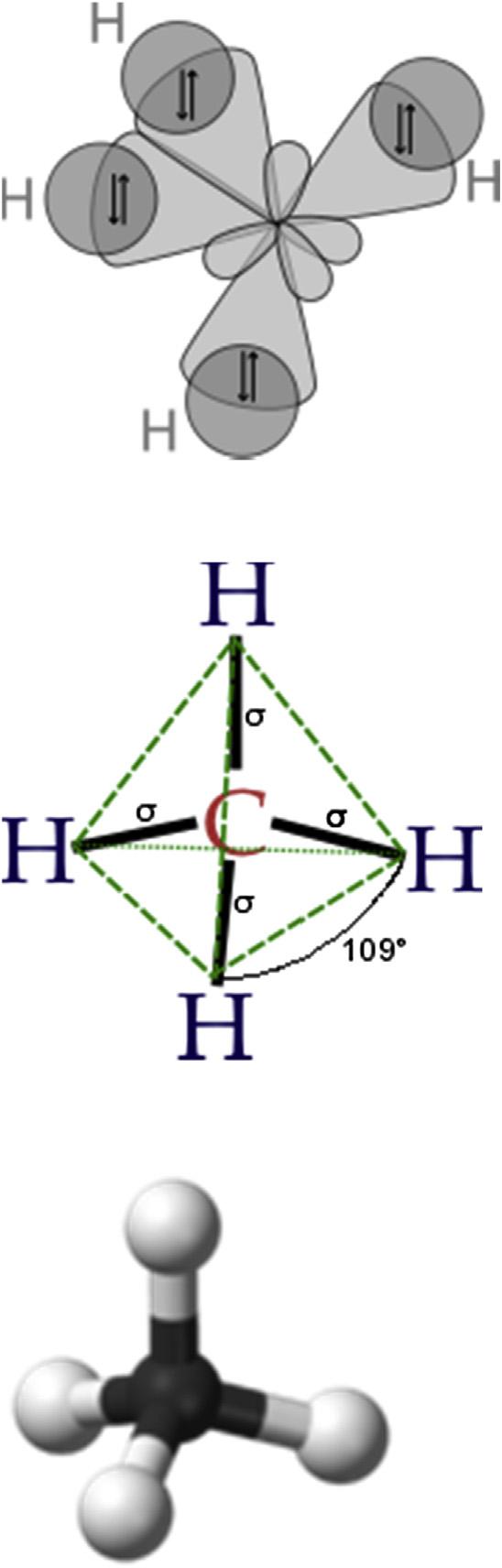
Incarbon,theelectronsinthe 1s orbitalaretoolowinenergytoformbonds. Thus,electronsusedtoformbondsmustcomefromthe 2s and 2p orbitals. Carbonveryoftenmakesfourbondsbyredistributionofthe 2p electrons: Whenitdoesso,thesebondsarearrangedsothattheyareasfarawayfrom eachotheraspossible.Thisarrangementisreferredtoasa tetrahedralbond (Fig.1.2).
Theindividual 2s orbitalandthe 2porbitalcannotformbondsinthis arrangementduetotheirgeometry.The 2s orbitaliscompletelysymmetric, whilethe 2p orbitalsarealignedalongspecificaxes.Noneoftheseorbitalsis well-equippedtoformbondsinthetetrahedralgeometryalone.
Sinceachemicalbonddoesnothavetobeformedfromindividualatomic orbitals,butcanbeformedfromacombinationofseveralatomicorbitalsfrom thesameatom,eachbondthatismadeinthetetrahedralgeometry,apartof the 2s andapartofeachofthe 2p orbitalswillcontribute,resultingina tetrahedralarrangementandthereisa109.5degreesanglebetweeneachofthe bonds(Fig.1.2).Toachievethisgeometry,boththe 2s andallthreeofthe 2p orbitals(2px, 2py,and 2pz)mustcontribute.Thenewbondsthatareformedare called sp 3 bonds,since1 s orbitaland3 p orbitalswereusedtoformthebonds.
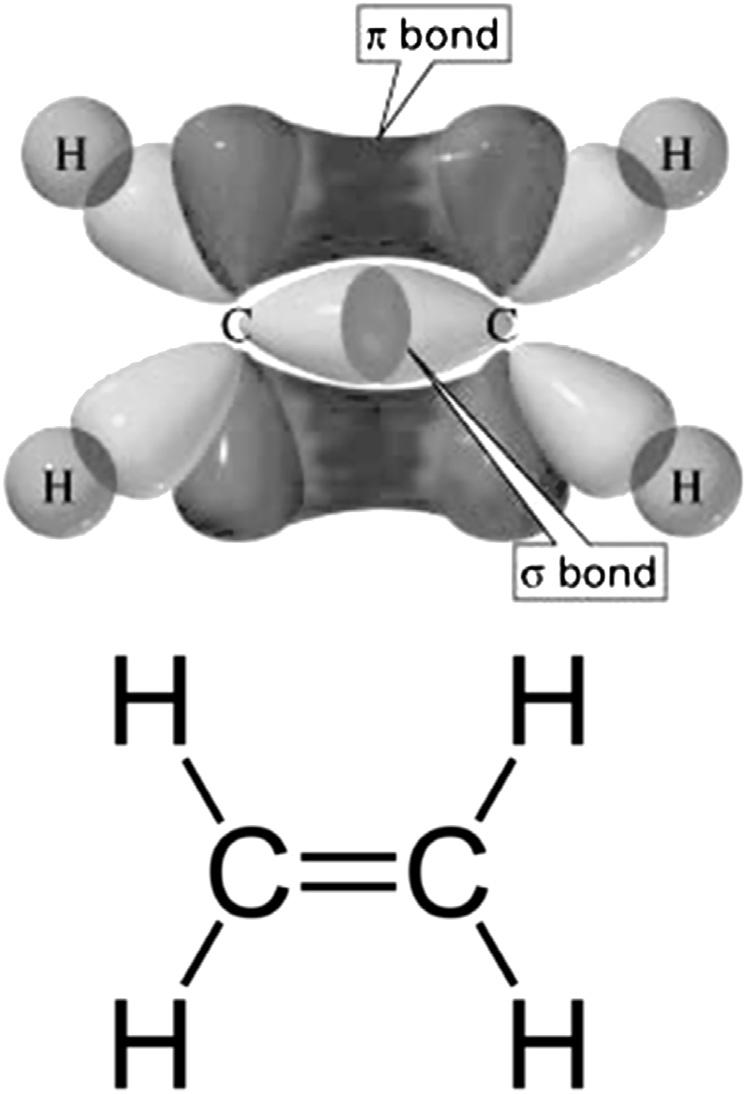
Carbonsometimesmakesthreebondsinsteadoffour.Inthiscase,notallof the 2p orbitalscombinewiththe 2s orbitaltoformbonds.Instead,acombinationofthe2sorbitalandtwoofthe 2p orbitalsmakethree sp 2 bonds,while theother p orbitaldoesnotparticipateinthiscombinationandcanmakea fourthbondonitsown.
Likethe sp 3 bonds,the sp 2 bondsareorientedsuchthattheyareasfar awayfromeachotheraspossible(trigonalplanargeometry).Eachofthe bondspointstooneoftheverticesofatriangle,butallthreebondsarelocated inthesameplane.Theother 2p orbital,theonewhichdidnotaddtomake sp 2 bondsexistsperpendiculartotheplaneinwhichthe sp 2 bondsform.Ittoois abletoformbonds,anditdoessoindependentlyofthe sp 2 bonds.
Whentwocarbonatomswith sp 2 orbitalsformabondtoeachotherusing their sp 2 orbitals,a s bondisformedbetweenthen.Moreover,theextra p orbitals,whichexistaboveandbeloweachcarbonatom,alsooverlapwith eachother.Thisoverlapbetween p orbitalsleadstotheformationofasecond bondinadditiontothe s bondformedbetweenthe sp 2 orbitals.Thissecond bondwhichdoesnotoccurdirectlybetweenthenucleiontheinternuclearaxis butaboveandbelowtheinternuclearaxisisa p bond(pibond).Whena s
FIGURE1.2 Tetrahedralgeometryasexhibitedby thecarbonatomsurroundedbyfourhydrogenatoms (methane).
bondanda p bondformtogetherbetweentwoatoms,itleadstotheformation ofadoublebond(Fig.1.3).
Briefly,manyinorganiccompoundsareioniccompounds,consistingof cationsandanionsjoinedbyionicbonding.Examplesofsalts(whichareionic compounds)aremagnesiumchloride(MgCl2)whichconsistsofmagnesium cations(Mg2þ)andchlorideanions(Cl ).Inanysalt,theproportionsofthe
FIGURE1.3 Themoleculeethylene isformedfromtwocarbonatomsand fourhydrogenatoms a s bondis formedfromtwo sp 2 orbitalsanda p bondisformedfromtwo 2p orbitalsto compriseadoublebond. 12 HandbookofIndustrialHydrocarbonProcesses
ionsaresuchthattheelectricchargescancelout,sothatthebulkcompoundis electricallyneutral.Importantclassesofinorganiccompoundsaretheoxides, thecarbonates,thesulfates,andthehalides.Manyinorganiccompoundsare characterizedbyhighmeltingpoints,easeofcrystallization,andsolubility wheresomesalts(suchassodiumchloride,NaCl)arehighlysolubleinwater, others(suchassilica,siliconoxide,SiO2)areinsolubleinwater.
3.Chemicalengineering
Chemicalengineering isthebranchofengineeringthatdealswiththeapplicationofphysicalscience(suchaschemistry)totheprocessofconvertingraw materials(forexample,crudeoil)orchemicalsintomoreusefulorvaluable forms.
Chemicalengineeringlargelyinvolvesthedesign,improvement,andmaintenanceofprocessesinvolvingchemicaltransformationsforlarge-scalemanufacture.Chemicalengineers(processengineers)ensuretheprocessesare operatedsafely,sustainably,andeconomically.Chemicalengineeringisapplied inthemanufactureofawidevarietyofproducts.Thechemicalindustrymanufacturesinorganicandorganicindustrialchemicals,ceramics,fuelsand
petrochemicals,agrochemicals(fertilizers,insecticides,herbicides),plasticsand elastomers,oleo-chemicals,explosives,detergentsanddetergentproducts(soap, shampoo,cleaningfluids),fragrancesandflavors,additives,dietarysupplements, andpharmaceuticals.Closelyalliedoroverlappingdisciplinesincludewood processing,foodprocessing,environmentaltechnology,andtheengineeringof crudeoil,glass,paints,andothercoatings,inks,sealants,andadhesives.
Chemicalengineersdesignprocessestoensurethemosteconomical operationinwhichtheentireproductionchainmustbeplannedandcontrolled forcosts.Achemicalengineercanbothsimplifyandcomplicateshowcase reactionsforaneconomicadvantage.Usingahigherpressureortemperature makesseveralreactionseasier;ammonia,forexample,issimplyproduced fromitscomponentelementsinahigh-pressurereactor.Ontheotherhand, reactionswithalowyieldcanberecycledcontinuously(recycledtoextinction inwhichnofurtherproductismade),whichwouldbecomplex,arduouswork ifdonebyhandinthelaboratory.Itisnotunusualtobuild6-step,oreven12stepevaporatorstoreusethevaporizationenergyforaneconomicadvantage. Incontrast,laboratorychemistsevaporatesamplesinasinglestep.
Theindividualprocessesusedbychemicalengineers(e.g.,distillationor filtration)arecalledunitoperationsandconsistofchemicalreactions,masstransferoperations,andheattransferoperations.Unitoperationsaregrouped togetherinvariousconfigurationsforthepurposeofchemicalsynthesisand/or chemicalseparation.Someprocessesareacombinationofintertwinedtransportandseparationunitoperations,suchasreactivedistillationinwhichthe productisformedasthestilltemperatureisraisedandtheproductdistillsfrom thereactionmixture.
Threebasicphysicallawsthatunderliechemicalengineeringdesignare (i)conservationofmass,(ii)conservationofenergy,and(iii)conservationof momentum.
3.1Conservationofmass
Thelawofconservationofmass(principleofmass/matterconservation) statesthatthemassofaclosedsystem(inthesenseofacompletelyisolated system)remainsconstantovertime.Themassofanisolatedsystemcannot bechangedasaresultofprocessesact inginsidethesystembutwhilemass cannotbecreatedordestroyed,itmayberearrangedinspace,andchanged intodifferenttypesofparticles.Putsimply,thelawstatesthatmattercannot becreatedordestroyedinachemicalreaction.Thisimpliesthatforany chemicalprocessinaclosedsystem,themassofthereactantsmustequalthe massoftheproducts.
Thelawimpliesthatmasscanneitherbecreatednordestroyed,althoughit mayberearrangedinspace,ortheentitiesassociatedwithitmaybechanged inform.Thisisillustratedinchemicalreactionsexampleinwhichthemass ofthechemicalcomponentsbeforethereactionisequaltothemassofthe
componentsafterthereaction.Thus,duringanychemicalreactionandlowenergythermodynamicprocessesinanisolatedsystem,thetotalmassofthe reactants(thestartingmaterials)willbeequaltothemassofthereaction products.Forexample,usingthemolecularproportionsastheweightsofthe reactantsandtheproducts:
þ 2O2 / CO2 þ 2H2O 16 þ (2 32) / 44 þ (2 18)
Massofthereactants ¼ massoftheproducts
Thechangeinmassofcertainkindsofopensystemswhereatomsor massiveparticlesarenotallowedtoescape,butothertypesofenergy(suchas lightorheat)wereallowedtoenterorescape,wentunnoticedduringthe19th century,becausethemass-changeassociatedwithadditionorlossofthe fractionalamountsofheatandlightassociatedwithchemicalreactions,was verysmall.Massisalsonotgenerallyconservedinopensystems(evenifonly opentoheatandwork),whenvariousformsofenergyareallowedinto,orout of,thesystem.Massconservationforclosedsystemscontinuestobetrue exactly.Themass-energyequivalencetheoremstatesthatmassconservationis equivalenttoenergyconservation,whichisthefirstlawofthermodynamics. Themass-energyequivalenceformularequiresclosedsystems,sinceifenergy isallowedtoescapeasystem,masswillescapealso.
3.2Conservationofenergy
Thelawofconservationofenergystatesthatthetotalamountofenergyinan isolatedsystemremainsconstantovertime.Aconsequenceofthislawisthat energycanneitherbecreatednordestroyed;itcanonlybetransformedfrom onestatetoanother.Theonlythingthatcanhappentoenergyinaclosed systemisthatitcanchangeform,suchasatransformationofchemicalenergy tokineticenergy.
Conservationofenergyreferstotheconservationofthetotalsystemenergyovertime.Thisenergyincludestheenergyassociatedwiththemassof thereactantsaswellasallotherformsofenergyinthesystem.Inanisolated system,althoughmassandenergy(heatandlight)canbeconvertedtoone another,boththetotalamountofenergyandthetotalamountofmassofsuch systemsremainconstantovertime.Ifenergyinanyformisallowedtoescape suchsystems,themassofthesystemwilldecreaseincorrespondencewiththe loss.Theconservationofenergyisafundamentalconceptofphysicsalong withtheconservationofmassandtheconservationofmomentum.Within someproblemdomain,theamountofenergyremainsconstantandenergyis neithercreatednordestroyed.Energycanbeconvertedfromoneformto another(potentialenergycanbeconvertedtokineticenergy)butthetotal energywithinthedomainremainsfixed.
CH4
Thus,energyconservationistheeffortmadetoreducetheconsumptionof energybyusinglessofanenergyservice.Thiscanbeachievedeitherbyusing energymoreefficiently(usinglessenergyforaconstantservice)orby reducingtheamountofserviceused(forexample,bydrivingless).Energycan beconservedbyreducingwastageandlosses,improvingefficiencythrough technologicalupgrades,andimprovingoperationandmaintenance.However, energycanonlybetransformedfromoneformtoanother.
3.3Conservationofmomentum
Momentumistheproductofthemassandthevelocityofanobject.The conservationofmomentumisafundamentallawofphysicswhichstatesthat themomentumofasystemisconstantiftherearenoexternalforcesactingon thesystem.Momentumisaconservedquantityinsofarasthetotalmomentum ofanyclosedsystem(asystemnotaffectedbyexternalforces)cannotchange. Oneoftheconsequencesofthelawisthatthecenterofmassofanysystemof objectswillalwayscontinuewiththesamevelocityunlessactedonbyaforce fromoutsidethesystem.Inanisolatedsystem(onewhereexternalforcesare absent)thetotalmomentumwillbeconstant,whichdictatesthattheforces actingbetweensystemsareequalinmagnitude,butoppositeinsign,isdueto theconservationofmomentum.
Thus,fortwoobjects(AandB)collidinginanisolatedsystem,thetotal momentumbeforeandafterthecollisionisequal themomentumlostbyone objectisequaltothemomentumgainedbytheother.ByNewton’sthirdlawof thermodynamics,foreveryactionthereisanequalbutoppositereaction. Hence,theforceexertedbyobjectAonobjectBisequalbutoppositetothe forcethatobjectBexertsonobjectA.ThenbyNewton’ssecondlawof thermodynamics,thisforceisequaltotheproductofthemassandtheaccelerationoftheobjects.Hence,theproductofthemassandaccelerationof objectAisequalbutoppositetotheproductbetweenthemassandaccelerationofobjectB.inotherwords,momentumisconserved.
4.Chemicaltechnology
Thedefinitionsoftechnologyvaryfromonedisciplinetoanother.However,in thecurrentcontext,chemicaltechnologyisthepracticalapplicationof chemistryandchemicalengineeringtotheneedsofcommerceorindustryand isamulticomponentdisciplinewhich,inthiscontext,dealswiththeapplicationofchemicalknowledgetothesolutionofpracticalproblems(Badger andBaker,1941;Henglein,1969;JessandWasserscheid,2013).Chemical technologyisalsoahumanactionthatinvolvesthegenerationofknowledge and(usuallyinnovative)processestodevelopsystemsthatsolveproblemsand extendhumancapabilities.
Inchemicaltechnology,whenareactionnotstudiedbeforeisplanned,itis oftheutmostimportancetoknowandtocalculatetheequilibrium,thatis,the equilibriumconstantsatvarioustemperatures,beforeexpensiveequipmentfor experimentalstudiesisinstalled.Itistheaimofchemicalthermodynamicsto answertechnicallyimportantquestions,suchasthefinalstateofchemical transformations,reactionmechanisms(passingintermediatestages),andreactionrates.
4.1Historicalaspects
Historically,theword technology isamoderntermandrosetoprominence duringthe industrialrevolution whenitbecameassociatedwithscienceand engineering.Theword technology canalsobeusedtorefertoacollectionof techniques,whichreferstothecurrentstateofhumanity’sknowledgeofhow tocombineresourcestoproducedesiredproducts,tosolveproblems,fulfill needs,orsatisfywants;itincludestechnicalmethods,skills,processes,techniques,tools,andrawmaterials.Thedistinctionbetweenscience,engineering, andtechnologyisnotalwaysclear.However,technologiesarenotusually exclusivelyproductsofsciencebecausetheyhavetosatisfyrequirements, suchasutility.
Inthecontextoftechnologyasatechnicalendeavor,engineering technologyistheprocessofdesigningandmakingtoolsandsystemsto exploitnaturalphenomenaforprac ticalhumanmeans,often(butnotalways)usingresultsandtechniquesf romchemistryandothersciences. Thus,thedevelopmentoftechnologymaydrawuponmanyfieldsofknowledge fromthescientificandengineeringdisciplinesinordertoachieveapractical result.
Tosome,technologyisoftenaconsequenceofscienceandengineering in thissense,scientistsandengineersmaybothbeconsideredtechnologists;the threefieldsareoftenconsideredasoneforthepurposesofresearchand reference.
Chemicaltechnologyisthestudyoftechnologyrelatedtochemistry.Tobe morespecific,chemicaltechnologytakeschemistrybeyondthelaboratoryand intotheindustrialworldwhereproductsaremadethroughknowledgeof chemistry.Thus,chemicaltechnologyalsoinvolvesvariousaspectsofchemical engineeringsuchasreactordesignandperformance.Thisdiffersfromchemistryitselfbecausethefocusisalsoonthemeansbywhichchemistrycanbe employedtomakeusefulproducts.Chemicaltechnologistsaremorelikelythan technicianstoparticipateintheactualdesignofexperiments,andmaybe involvedintheinterpretationofexperimentaldata.Theymayalsoberesponsiblefortheoperationofchemicalprocessesinlargeplants,andmayeven assistchemicalengineersinthedesignofthesame.
Withintechnologyfallstheconceptofinnovation,whichisthechangein thethoughtprocessforperformingascientificorengineeringtaskthatwilllead
Chemistryandchemicaltechnology Chapter|1 17
to(i)anewprocess,(ii)anewproduct,or(iii)anewuseforanoldproduct.In fact,innovationmayrefertoincrementalorradicalchangesinproductsand/or processesandthegoalofinnovationisapositivechangeinaproductorprocess.Innovationisconsideredtobeamajordriveroftheeconomy,especially whenitleadstonewproductcategoriesorincreasingproductivity.
Forexample,usingthecrudeoilindustryasanexample,innovativeuseof crudeoilanditsderivatives(particularlyasanasphaltmastic)started6000 yearsago;currentinnovationscanbeconsideredtohavecommencedinthe 1860sandcontinuetothisday(Table1.5)tothepointwhereheavyoil(once consideredadifficult-to-refinefeedstock)isnowrefinedonaveryregular basis(AncheytaandSpeight,2007;Speight,2014).
4.2Technologyandhumanculture
Theuseoftechnologyintheformofthedevelopmentoftoolsandharnessing theenergyoffirehasoftenbeenregardedasthedefiningcharacteristicof Homosapiens, andisameansofdefiningthespecies.Furthermore,thehistory ofhumanculturecanbeviewedastheprogressivedevelopmentofnewenergy sourcesandtheirassociatedconversiontechnologies(Halletal.,2003).Most oftheseenergytechnologiesrelyontheproperties(i.e.,thechemicalbonds)of hydrocarbonderivatives.
Technology,thesystematicapplicationofscientificandengineeringknowledgeindevelopingandapplyingtechnology,hasgrownimmensely.Technologicalknowledgeprovidesameansofestimatingwhatthebehaviorofthingswillbe evenbeforetheyaremadeorobservedinservice.Moreover,technologyoften suggestsnewkindsofbehaviorthathadnotevenbeenimaginedbefore,andso leadstostrategiesofdesign, tosolvepracticalproblems.
Althoughthedevelopmentof huntingweaponscanbeconsideredakey eventintheevolutionofhumanculture,harnessingtheenergyoffirewas probablythemostseminaleventofhumanhistory.This,morethananyother event,assistedhumansintheirexploitationofcolder,morenortherly ecosystems.
Theprincipalenergysourcesofantiquitywereallderiveddirectlyfromthe sun:humanandanimalmusclepower,wood,flowingwater,andwind.Inthe mid-to-late18thcenturytheindustrialrevolutionbeganwithstationarywindpoweredandwater-poweredtechnologies,whichwereessentiallyreplacedby fossilhydrocarbonderivatives:coalinthe19thcentury,oilsincethe20th century,andnow,increasingly,naturalgas(Speight,2014,2017).Furthermore,hydrocarbon-basedenergyhasastrongconnectionwitheconomicactivityforindustrializedanddevelopingeconomies(Halletal.,2001;Tharakan etal.,2001).
Technologyprovidesthe raisond’eˆtre ofscienceandengineering.Technologyisessentialtoscienceandengineeringforpurposesofmeasurement, datacollection,treatmentofsamples,computation,transportationtoresearch
TABLE1.5 Simplehydrocarbonsandthevariations.
NumberofCatoms
1Methane
2EthaneEthylene(ethene)Acetylene(ethyne)
3PropanePropylene(propene)PropyneCyclopropanePropadiene
4ButaneButylene(butene)ButyneCyclobutaneButadiene
5PentanePentenePentyneCyclopentanePentadiene
6HexaneHexeneHexyneCyclohexaneHexadiene
7HeptaneHepteneHeptyneCycloheptaneHeptadiene
8OctaneOcteneOctyneCyclooctaneOctadiene
9NonaneNoneneNonyneCyclononaneNonadiene
sites,samplecollection,protectionfromhazardousmaterials,andcommunication.Moreandmorenewinstrumentsandtechniquesarebeingdeveloped throughtechnologythatmakesitpossibletoadvancevariouslinesofscientific research.
However,technologydoesnotjustprovidetoolsforscience;italsomay providemotivationanddirectionfortheoryandresearch.Scientistsandengineersseepatternsinphenomenatomaketheworldasunderstandableas possibleandbeingabletobemanipulated.Technologyalsopushesscientists andengineerstoshowthattheoriesfitthedataandtoshowlogicalproofof abstractconnectionsaswellasdemonstrabledesignsthatwork.
Technologyaffectsthesocialsystemandculturewithimmediateimplicationsforthesuccessorfailureofhumanenterprisesandforpersonalbenefit andharm.Technologicaldecisions,whetherindesigninganirrigationsystem oracrudeoilrecoveryproject,inevitablyinvolvesocialandpersonalvaluesas wellasscientificandengineeringjudgments.Thisleadstotheissuesregarding thesupplyofhydrocarbonderivatives(intheformofcrudeoilandnaturalgas) andthefutureofthesevaluablechemicals(Speight,2014,2017).
Therumorsofthedeathofthehydrocarbonculturearegreatlyexaggerated (toparaphraseMarkTwainwhoobserved,“Therumorsofmydeathare greatlyexaggerated”).Theworldisnotabouttorunoutofhydrocarbonderivatives,andperhapsitisnotgoingtorunoutofcrudeoilornaturalgasfrom unconventionalsourcesanytimesoon.However,cheapcrudeoilwillbe difficulttoobtainbecausethereservesthatremainarenotonlydifficultto recoverbutthecrudeoilisalow-gradeandwillbemoredifficult(costly)to refinetoproducethedesiredhydrocarbonfuels(Speight,2010,2014).
Asconventionaloilbecomeslessimportant,itisimportanttoinvestina differentsourceofenergy,onefreeingusforthefirsttimefromourdependenceonhydrocarbonderivatives(Speight,2008).However,renewableenergy technologiesrequirefurtherdevelopmentbutsomedoshowadvantagesover hydrocarbonderivativesintermsofeconomicreliability,accessibility,and environmentalbenefits.Withproperattentiontoenvironmentalconcerns, biomass-basedenergygenerationiscompetitiveinsomecasesrelativeto conventionalhydrocarbon-basedenergygeneration.Bycontrast,liquid-fuel productionfromgrainandsolarthermalpowerhasarelativelyloweconomicreturnoninvestment.Butisdoesdependontheinvestmentrequiredto keepafleetonalertoffshoreofvariousoilproducingcountriesaswellasthe willingnessofthepopulationtopayanadditionalpergallonofgasolineorper gallonoffueloilamountforahighermeasureofenergyindependence.
Governmentintervention,inconcertwithongoingprivateinvestment,will speeduptheprocessofsortingthewheatfromthechaffintheportfolioof feasiblerenewableenergytechnologies.Itistimetothinkaboutpossibilities otherthanthenextcheapesthydrocarbonderivatives.Iffornootherreason thantoprotecttheenvironment,alloftheavailabletechnologiesshouldbe broughttobearonthistask.