HANDBOOKOF CHITINAND CHITOSAN PREPARATIONAND
PROPERTIES
VOLUME1
Editedby
SREERAG GOPI
CenterforInnovationsandTechnologies(CIT), ADSONaturalsPrivateLimited,Bangalore,India
SABU THOMAS
MahatmaGandhiUniversity,Kottayam,India
ANITHA PIUS
TheGandhigramRuralInstitute(DeemedUniversity),Dindigul,India
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,includingphotocopying,recording,oranyinformationstorageandretrieval system,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundat ourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusing suchinformationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers, includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproducts liability,negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress ISBN:978-0-12-817970-3
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
EditorialProjectManager: KelseyConnors
ProductionProjectManager: SujathaThirugnanaSambandam
CoverDesigner: ChristianJ.Bilbow
TypesetbyMPSLimited,Chennai,India
Listofcontributorsix
1. Chitinandchitosan:origin,properties,andapplications1
SUNEETAKUMARIANDRUPAKKISHOR
1.1Introduction2
1.2Chitinandchitosan3
1.3Extractionofchitin5
1.4Chitosanpreparationmethods8
1.5Physicochemicalproperties8
1.6Characterizationofchitinandchitosan13
1.7Applicationofchitinandchitosan22 References28
2. Chitinandchitosan:chemistry,solubility,fiberformation, andtheirpotentialapplications35
HAKIMAELKNIDRI,ALILAAJEBANDAHMEDLAHSINI
2.1Introduction36
2.2Chitinandchitosan:chemistryandsolubility36
2.3Chitinandchitosan:fiberformation41
2.4Conclusions50 References50
3. PEGylatedchitinandchitosanderivatives59
ADIBH.CHISTY,RIFATA.MASUD,M.MEHEDIHASAN,M.NURUZZAMANKHAN, ABULK.MALLIKANDMOHAMMEDMIZANURRAHMAN
3.1Introduction60
3.2Chitinandchitosan61
3.3PEGylationandPEGylatedchitin/chitosanderivatives64
3.4FabricationofPEGylatedchitosanderivatives65
3.5CharacterizationofPEGylatedchitosanandchitinderivatives71
3.6ApplicationsofPEGylatedderivativesofchitosan86
3.7Conclusions93 References93
4. Solubility,chaincharacterization,andderivativesofchitin101 MIFENG,XINGMEILU,DANFENGHOUANDSUOJIANGZHANG
4.1Solubilityofchitin102
4.2Chaincharacterizationofchitin107
4.3Derivativesofchitin112
Acknowledgment125 References125
5. Solubility,degreeofacetylation,anddistributionofacetyl groupsinchitosan131
E.I.AKPAN,O.P.GBENEBOR,S.O.ADEOSUNANDODILICLETUS
5.1Introduction132
5.2Chemistryandstructureofchitosan132
5.3Acetylationofchitosan134
5.4Solubilityofchitosan145
5.5Conclusion153 References154
6. Chitinnanomaterials:preparationandsurfacemodifications165
ABULK.MALLIK,MD.NURUSSAKIB,MD.SHAHARUZZAMAN,PAPIAHAQUEAND MOHAMMEDMIZANURRAHMAN
6.1Introduction166
6.2Structureandpropertiesofchitin168
6.3Chitin-basednanomaterials169
6.4Preparationofchitin-basednanomaterials178
6.5Surfacemodificationofchitin185
6.6Conclusions189 References189
7. Importanceofelectrospunchitosan-basednanoscalematerials forseafoodproductssafety195
ZAFERCEYLAN,RACIYEMERAL,FATIHO ¨ ZOGULANDMUSTAFATAHSINYILMAZ
7.1Optimization196
7.2Determinationofelectrospinningparameters202
7.3Characterizationoffabricatednanoscalematerial(s)207
7.4Useofelectrospunnanomaterialsforseafoodproductssafety212
7.5Conclusion216 References217
8. Alternativemethodsforchitinandchitosanpreparation, characterization,andapplication225
GEORGEM.HALL,CLAUDIAH.BARRERAANDKEIKOSHIRAI
8.1Introduction226
8.2Chitinproduction226
8.3Currentchitosanproduction231
8.4Conclusions240 References241
9. Currentresearchontheblendsofchitosanasnew biomaterials247
A.RAJESWARI,SREERAGGOPI,E.JACKCINASTOBELCHRISTY,K.JAYARAJANDANITHAPIUS
9.1Introduction248
9.2Chitosanbiomaterial249
9.3Modificationofchitosan256
9.4Naturalpolymersblendswithchitosan258
9.5Chitosanblendswithsyntheticpolymers264
9.6Chitosan-basedhydrogels274
9.7Conclusions275 Acknowledgment275 References275
10. Chitinandchitosan-basedaerogels285
E.JACKCINASTOBELCHRISTY,A.RAJESWARI,SREERAGGOPIANDANITHAPIUS
10.1Introduction286
10.2Chitinandchitosan-basedaerogels:preparationprocess292
10.3Characterizationofchitinandchitosan-basedaerogels296
10.4Futureaspectsofaerogel324
10.5Conclusions327 Acknowledgments328 References328
11. Chitin,chitosan,marinetomarket335
G.M.OYATOGUN,T.A.ESAN,E.I.AKPAN,S.O.ADEOSUN,A.P.I.POPOOLA,B.I.IMASOGIE, W.O.SOBOYEJO,A.A.AFONJA,S.A.IBITOYE,V.D.ABERE,A.O.OYATOGUN, K.M.OLUWASEGUN,I.E.AKINWOLEANDK.J.AKINLUWADE
11.1Introduction336
11.2Originandsourcesofchitinandchitosan337
11.3Synthesisofchitinandchitosan339
11.4Propertiesofchitinandchitosan348
11.5Potentialapplicationsofchitinandchitosan354
11.6Economicpotentialofchitinandchitosan361
11.7Conclusions364 References364 Furtherreading376
12. Miscibility,properties,andbiodegradabilityofchitinand chitosan377
MUHAMMADARSHAD,MUHAMMADZUBAIRANDAMANULLAH
12.1Introduction378
12.2Physicochemicalpropertiesofchitinandchitosan378
12.3Biologicalpropertiesofchitinandchitosan385
12.4Biodegradabilityofchitinandchitosan389
12.5Concludingremarks392 References392
13. Chitinandchitosan:currentstatusandfutureopportunities401 RUCHIMUTREJA,ABHIJEETTHAKURANDARUNGOYAL
13.1Introduction402
13.2Propertiesofchitinandchitosan403
13.3Chitin,chitosan,andtheirderivatives405
13.4Applicationsofchitinandchitosan407
13.5Conclusionandfutureperspectives412 References413
14. Fungalchitosan:prospectsandchallenges419
JOSEPHSEBASTIAN,TAREKROUISSIANDSATINDERKAURBRAR
14.1Introduction420
14.2Currentcommercialproductionanditsdisadvantages426 14.3Greensynthesisofchitosan427 14.4Fungalchitosan429
14.5Futureprospects446 14.6Conclusion447 14.7Acknowledgments448 References448 Onlineresource452
15. Preparation,properties,andapplicationof low-molecular-weightchitosan453
NGUYENCONGMINH,NGUYENVANHOAANDTRANGSITRUNG
15.1Introduction454
15.2Preparationoflow-molecular-weightchitosan454
15.3Propertiesoflow-molecular-weightchitosan460
15.4Applicationsoflow-molecular-weightchitosan461
15.5Agriculture463
15.6Conclusions464 References464 Index473
ListofContributors
V.D.Abere DepartmentofMineralProcessing,NationalMetallurgical DevelopmentCentre,Jos,Nigeria
S.O.Adeosun DepartmentofMetallurgicalandMaterialsEngineering, UniversityofLagos,Lagos,Nigeria
A.A.Afonja DepartmentofMaterialsScienceandEngineering,Obafemi AwolowoUniversity,Ile-Ife,Nigeria
K.J.Akinluwade DepartmentofResearchandDevelopment,Prototype EngineeringDevelopmentInstitute(NationalAgencyforScienceand EngineeringInfrastructure,NASENI),Ilesa,Nigeria
I.E.Akinwole DepartmentofMaterialsScienceandEngineering,Obafemi AwolowoUniversity,Ile-Ife,Nigeria
E.I.Akpan InstituteforCompositeMaterials,TechnicalUniversity, Kaiserslautern,Germany
MuhammadArshad DepartmentofAgricultural,FoodandNutritional Science,UniversityofAlberta,Edmonton,AB,Canada
ClaudiaH.Barrera BiotechnologyDepartment,LaboratoryofBiopolymers andPilotPlantofBioprocessingofAgro-IndustrialandFoodBy-Products, AutonomousMetropolitanUniversity,MexicoCity,Mexico
SatinderKaurBrar INRS-ETE,Universite ´ duQue ´ bec,Que ´ bec,QC,Canada; DepartmentofCivilEngineering,LassondeSchoolofEngineering,York University,Toronto,ON,Canada
ZaferCeylan FacultyofFisheries,DepartmentofSeafoodProcessing Technology,VanYuzuncuYilUniversity,Van,Turkey
AdibH.Chisty DepartmentofAppliedChemistryandChemicalEngineering, FacultyofEngineeringandTechnology,UniversityofDhaka,Dhaka, Bangladesh
OdiliCletus MaterialsandMetallurgicalEngineering,UniversityofLagos, Lagos,Nigeria
HakimaElKnidri Catalysis,MaterialsandEnvironmentLaboratory,Higher SchoolofTechnology,SidiMohamedBenAbdellahUniversity,Fez,Morocco
T.A.Esan DepartmentofRestorativeDentistry,ObafemiAwolowoUniversity, Ile-Ife,Nigeria
MiFeng CASKeyLaboratoryofGreenProcessandEngineering,StateKey LaboratoryofMultiphaseComplexSystems,BeijingKeyLaboratoryofIonic LiquidsCleanProcess,InstituteofProcessEngineering,ChineseAcademyof Sciences,Beijing,P.R.China;SchoolofChemicalandEngineering,University ofChineseAcademyofSciences,Beijing,P.R.China
O.P.Gbenebor MaterialsandMetallurgicalEngineering,UniversityofLagos, Lagos,Nigeria
SreeragGopi DepartmentofChemistry,TheGandhigramRuralInstitute— DeemedtobeUniversity,Dindigul,India
ArunGoyal CarbohydrateEnzymeBiotechnologyLaboratory,Departmentof BiosciencesandBioengineering,IndianInstituteofTechnologyGuwahati, Guwahati,India
GeorgeM.Hall CentreforSustainableDevelopment,UniversityofCentral Lancashire,Preston,UnitedKingdom
PapiaHaque DepartmentofAppliedChemistryandChemicalEngineering, FacultyofEngineeringandTechnology,UniversityofDhaka,Dhaka, Bangladesh
M.MehediHasan DepartmentofAppliedChemistryandChemical Engineering,FacultyofEngineering,BangabandhuSheikhMujiburRahman ScienceandTechnologyUniversity,Gopalganj,Bangladesh
DanfengHou CASKeyLaboratoryofGreenProcessandEngineering,State KeyLaboratoryofMultiphaseComplexSystems,BeijingKeyLaboratoryof IonicLiquidsCleanProcess,InstituteofProcessEngineering,Chinese AcademyofSciences,Beijing,P.R.China
S.A.Ibitoye DepartmentofMaterialsScienceandEngineering,Obafemi AwolowoUniversity,Ile-Ife,Nigeria
B.I.Imasogie DepartmentofMaterialsScienceandEngineering,Obafemi AwolowoUniversity,Ile-Ife,Nigeria
E.JackcinaStobelChristy DepartmentofChemistry,TheGandhigramRural Institute—DeemedtobeUniversity,Dindigul,India
K.Jayaraj DepartmentofChemistry,TheGandhigramRuralInstitute— DeemedtobeUniversity,Dindigul,India
M.NuruzzamanKhan DepartmentofAppliedChemistryandChemical Engineering,FacultyofEngineeringandTechnology,UniversityofDhaka, Dhaka,Bangladesh
RupakKishor DepartmentofChemicalEngineering,MANIT,Bhopal,India
SuneetaKumari DepartmentofChemicalEngineering,B.I.T.Sindri,Dhanbad, India
AliLaajeb Catalysis,MaterialsandEnvironmentLaboratory,HigherSchoolof Technology,SidiMohamedBenAbdellahUniversity,Fez,Morocco
AhmedLahsini Catalysis,MaterialsandEnvironmentLaboratory,Higher SchoolofTechnology,SidiMohamedBenAbdellahUniversity,Fez,Morocco
XingmeiLu CASKeyLaboratoryofGreenProcessandEngineering,StateKey LaboratoryofMultiphaseComplexSystems,BeijingKeyLaboratoryofIonic LiquidsCleanProcess,InstituteofProcessEngineering,ChineseAcademyof Sciences,Beijing,P.R.China;SchoolofChemicalandEngineering,University ofChineseAcademyofSciences,Beijing,P.R.China
AbulK.Mallik DepartmentofAppliedChemistryandChemicalEngineering, FacultyofEngineeringandTechnology,UniversityofDhaka,Dhaka, Bangladesh
RifatA.Masud DepartmentofAppliedChemistryandChemicalEngineering, FacultyofEngineering,BangabandhuSheikhMujiburRahmanScienceand TechnologyUniversity,Gopalganj,Bangladesh
RaciyeMeral FacultyofEngineering,DepartmentofFoodEngineering,Van YuzuncuYilUniversity,Van,Turkey
NguyenCongMinh InstituteofBiotechnologyandEnvironment,NhaTrang University,NhaTrang,Vietnam
RuchiMutreja DepartmentofBiotechnology,IndianInstituteofTechnology Roorkee,Roorkee,India
K.M.Oluwasegun DepartmentofMaterialsScienceandEngineering,Obafemi AwolowoUniversity,Ile-Ife,Nigeria
A.O.Oyatogun DepartmentofMaterialsScienceandEngineering,Obafemi AwolowoUniversity,Ile-Ife,Nigeria
G.M.Oyatogun DepartmentofMaterialsScienceandEngineering,Obafemi AwolowoUniversity,Ile-Ife,Nigeria
FatihO ¨ zogul FacultyofFisheries,DepartmentofSeafoodProcessing Technology,C¸ukurovaUniversity,Adana,Turkey
AnithaPius DepartmentofChemistry,TheGandhigramRuralInstitute— DeemedtobeUniversity,Dindigul,India
A.P.I.Popoola DeparmentofChemical,MetallurgicalandMaterials Engineering,TshwaneUniversityofTechnology,Pretoria,SouthAfrica
MohammedMizanurRahman DepartmentofAppliedChemistryand ChemicalEngineering,FacultyofEngineeringandTechnology,Universityof Dhaka,Dhaka,Bangladesh
A.Rajeswari DepartmentofChemistry,TheGandhigramRuralInstitute— DeemedtobeUniversity,Dindigul,India
TarekRouissi INRS-ETE,Universite ´ duQue ´ bec,Que ´ bec,QC,Canada
Md.NurusSakib DepartmentofAppliedChemistryandChemical Engineering,FacultyofEngineeringandTechnology,UniversityofDhaka, Dhaka,Bangladesh
JosephSebastian INRS-ETE,Universite ´ duQue ´ bec,Que ´ bec,QC,Canada
Md.Shaharuzzaman DepartmentofAppliedChemistryandChemical Engineering,FacultyofEngineeringandTechnology,UniversityofDhaka, Dhaka,Bangladesh
KeikoShirai BiotechnologyDepartment,LaboratoryofBiopolymersandPilot PlantofBioprocessingofAgro-IndustrialandFoodBy-Products, AutonomousMetropolitanUniversity,MexicoCity,Mexico
W.O.Soboyejo FacultyofEngineering,WisconsinPolytechnicInstitute, Menomonie,WI,UnitedStates
AbhijeetThakur CarbohydrateEnzymeBiotechnologyLaboratory, DepartmentofBiosciencesandBioengineering,IndianInstituteofTechnology Guwahati,Guwahati,India
TrangSiTrung FacultyofFoodTechnology,NhaTrangUniversity,Nha Trang,Vietnam
AmanUllah DepartmentofAgricultural,FoodandNutritionalScience, UniversityofAlberta,Edmonton,AB,Canada
NguyenVanHoa FacultyofFoodTechnology,NhaTrangUniversity,Nha Trang,Vietnam
MustafaTahsinYilmaz FacultyofEngineering,DepartmentofIndustrial Engineering,KingAbdulazizUniversity,Jeddah,SaudiArabia;Chemicaland MetallurgicalEngineeringFaculty,DepartmentofFoodEngineering,Yıldız TechnicalUniversity,Istanbul,Turkey
SuojiangZhang CASKeyLaboratoryofGreenProcessandEngineering,State KeyLaboratoryofMultiphaseComplexSystems,BeijingKeyLaboratoryof IonicLiquidsCleanProcess,InstituteofProcessEngineering,Chinese AcademyofSciences,Beijing,P.R.China;SchoolofChemicaland Engineering,UniversityofChineseAcademyofSciences,Beijing,P.R.China
MuhammadZubair DepartmentofAgricultural,FoodandNutritional Science,UniversityofAlberta,Edmonton,AB,Canada
SuneetaKumari1 andRupakKishor2
1DepartmentofChemicalEngineering,B.I.T.Sindri,Dhanbad,India, 2DepartmentofChemicalEngineering,MANIT,Bhopal,India
1.Chitinandchitosan:origin,properties,andapplications
1.1Introduction
Chitinandchitosanarenaturallyabundantandrenewablepolymers. Theyhaveexcellentpropertiessuchasbiodegradability,biocompatibility, andnontoxicity [1].Chitinisacopolymerof N-acetyl-D-glucosamineand D-glucosamineunitslinkedwith β-(1-4)glycosidicbonds,asshownin Fig.1.1[2],where N-acetyl-D-glucosamineunitsarepredominantinthe polymericchain [3].Thedeacetylatedformofchitinreferstochitosan (Fig.1.1).Chitinandchitosancanbefoundassupportingmaterialsin manyaquatic,terrestrial,andsomemicroorganisms [4],asshownin Fig.1.1A.Almostasmuchchitinisestimatedtobeproducedannuallyas cellulose.Ithasbecomeofgreatinterestnotonlyasanunderutilized resourcebutalsoasanewfunctionalbiomaterialwithhighpotentialin variousfields [5].
Chitinisawhite,hard,inelastic,nitrogenouspolysaccharidefoundin theexoskeletonandintheinternalstructureofinvertebrates.Theproductionofchitosanfromcrustaceanshellsobtainedasafoodindustry wasteiseconomicallyfeasible,especiallyifitincludestherecoveryof carotenoids.Therearemanyapplicationsinwastewatertreatment,such astheremovalofmetalions [6,7] anddyes [8],asamembraneinpurificationprocesses [9],inthefoodindustry(anticholesterolandfatbinding),asapackagingmaterial,asapreservativeandfoodadditive [10], inagriculture(seedandfertilizercoating) [11],forcontrolledagrochemicalrelease [12],inthepulpandpaperindustry(surfacetreatmentadhesivepaper) [2],incosmetics(bodycreamsandInmaculadaAranaz, lotions,etc.),intissueengineering [13],inwoundhealing [14],andas excipientsfordrugdelivery [15] andgenedelivery [16].Additionally,It canbeeasilyprocessedintogels [17],membranes [18],nanofibers [19], beads [10],microparticles,nanoparticles,scaffolds [15],andsponges [20],asshownin Fig.1.1C.
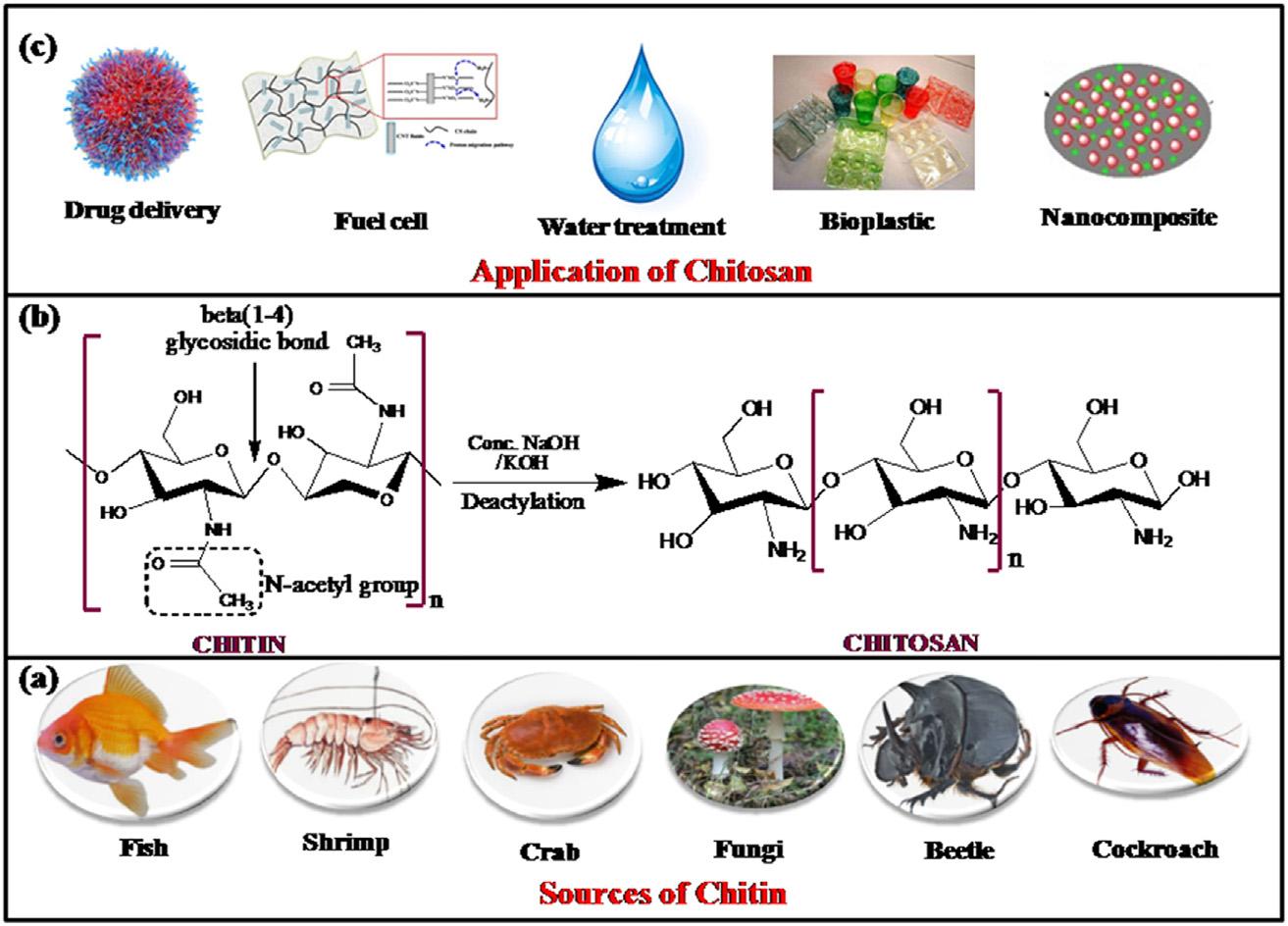
FIGURE1.1 (A)Sourcesofchitin,(B)deacetylationofchitin(synthesisofchitosan), and(C)applicationofchitosan.
Themainobjectiveofthischaptertogiveabriefintroductionabout chitinandchitosananditssynthesis.Thephysicalandchemicalpropertiesofchitosandeterminedbyusingdifferentanalyticaltechniquesare alsodiscussed.Finally,themostrecentapplicationsofchitinandchitosanarediscussed.
1.2Chitinandchitosan
Chitin(C8H13O5N)n,isderivedfromtheGreekword“chiton,”meaning acoatofmail.Itisanaturalpolysaccharideof β-(1-4)-N acetyl-D-glucosaminemonomers,firstidentifiedbythechemistHenriBraconnotin1811 [21].Itsstructureissimilartocellulosebutwith2-acetamido-2-deoxy-β-Dglucose(NAG)monomerunits(Fig.1.1B).Chitinhaslimitedapplications becauseofitsacetylgroups,butthroughthedeacetylationprocesschitinis convertedintochitosan.Duringthedeacetylationprocess,theacetylgroup presentinchitinisconvertedintohydroxyl( OH)andamino( NH2) groupsinthechitosan.Themodificationofthereactivefunctionalgroups presentinchitosanopensthepossibilityofbroadapplicationinmany fields.Chitosan’sstructuralmodificationispossiblebychemicalmethods
4 1.Chitinandchitosan:origin,properties,andapplications
andmostofthenewapplicationsfocusonthepropertiesandmodification ofitscomposites [22].
1.2.1Sourcesofchitin
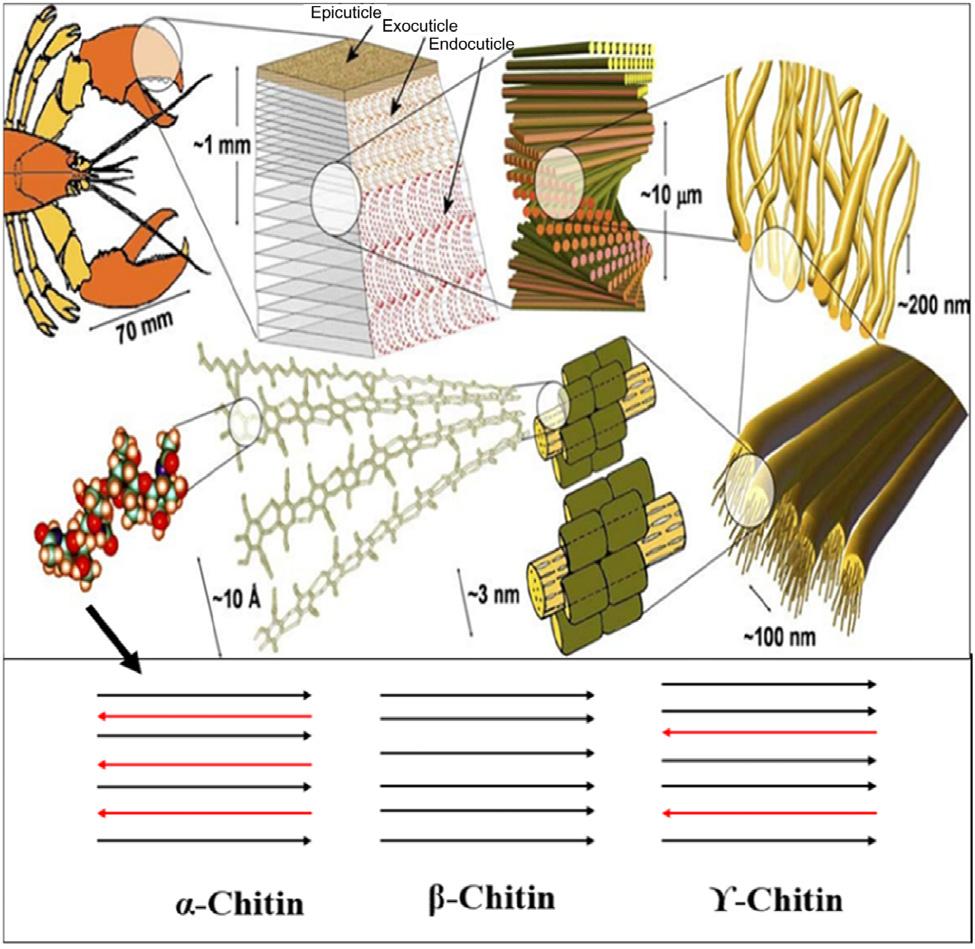
Chitinisusuallyisolatedfromtheexoskeletonsofarthropods’chitin-based tissue(30% 40%protein,30% 50%calciumcarbonate,and20% 30% chitin),suchascrustaceans,mollusks,insects,andcertainfungi [2,23 26].It isabiologicalnanocompositematerialstrictlyhierarchicallyorganizedwhich revealsvariousstructurallevels.Atthe molecularlevelisthepolysaccharide chitinitself(Fig.1.2).Thenextstructurallevelisthearrangementofc.18 25 ofsuchmoleculesintheformofnarrowandlongcrystallineunits,whichare wrappedbyproteins,formingnanofibrilsofabout2 5nmdiameterand about300nmlength.Thenextstepinthescaleconsistsoftheclusteringof someofthesenanofibrilsintolongchitin proteinfibersofabout50 300nm diameter(Fig.1.2).
Chitinismainlyoccursinthreedifferentpolymeric α, β-,and γ-forms. Thechainsarearrangedinstacksorsheetsin α-chitinandadjacentsheets alongthec-axishavethesamedirectioninaparallelarrangement. The α-chitinoccursintheexoskeletonsofcrustaceans(e.g.,crabs, lobsters,andprawns).Inthecaseof β-chitin,theadjacentsheetsalong
FIGURE1.2 Hierarchyofthemainstructurallevelsandmicrostructureelementsofthe exoskeletonmaterial [25].
thec-axispresentinoppositedirectionsinanantiparallelarrangement anditcanbefoundinsquidpen,certaindiatoms,andvestimentiferans (aclassofdeep-seaanimal) [27].Howevereverythirdsheetisinthe oppositedirectiontotheprecedingsheetsin γ-chitin.Itmainlyexistsin fungiandyeast [28].
1.3Extractionofchitin
Cuticlesofvariouscrustaceanslikecrabs,shrimps,andlobsterand fishscalesarethemajorsourcesofchitin.Crustaceanscarryanexoskeletoncomposedofproteins,chitin,andcalciumcarbonatewhich bindtogethertoformanexternalshell [26].Proteinandchitosanbind togetherandasmallpartoftheproteinisavailableinthepolymercomplex.Hence,chitin’sseparationfromtheshellrequirestheelimination oftwomajorconstituents,proteinandminerals.Proteinisremovedby adeproteinizationprocessandmineralsareremovedbyademineralizationprocess.Insomecasesanadditionalprocessofdecolorizationis carriedouttoremovepigments.Avarietyofmethodshavebeen adoptedtoproducechitin.Amongallmethodschemicalandbiological processesareprimaryfortheproductionofchitin.Moreover,among thetwomethodbiologicalmethodsthesequenceoftheprocessoffers someadvantagesspecificallyintimeconsumptionandqualityofchitin produced.Thereforeextractionalwaysbeginsbychoosingcrustacean shells.Foranyparticularseparation,shellsofidenticalsizeandspecies arepreferred.Inthecaseofshrimpshell,theseparationiseasierasthe shellwallisthinnerbuttheyieldislessincomparisonwithcraband lobster.However,crabandlobsterallowabetterqualityofchitintobe recovered.Thustheavailabilityofcrustaceansremainsakeyfactorfor theextractionofchitin [29].
1.3.1Chemicalextraction
1.3.1.1Demineralizationprocess
Demineralizationofshellsisbasedonacidictreatmenttoremoveminerals likecalciumcarbonateandcalciumphosphate.Themostcommonreagents areHCl,HNO3,H2SO4,CH3COOH,andHCOOH.Thenthedemineralized shellisfilteredundervacuumandwashedwithdistilledwaterfor30min untilthepHbecomeneutral.Thenthedemineralizedshellsaredriedinan ovenataround60 Cfor24h. ð1:1Þ
Thisreactionuseshydrochloricacidtodecomposecalciumcarbonate intocalciumchloridewiththereleaseofwaterandcarbondioxide [30].
Similarlymineralsalsoreactwiththeacidandproducesolublesalts. Thesaltsareremovedbyfiltration.Chitinisrecoveredbywashingwith distilledwateranddrying [31].Thedemineralizationprocessdiffers withdifferenttypesofshells,timeofextraction,temperature,shellsize, concentrationofacid,andsolute/solventratio.Hence,thesolute/solventratioisbasedonstoichiometry(2molofacidfordecomposed 1molofcalciumcarbonate).Thedemineralizationprocessgenerally occursathightemperatureasthisallowsthesolventtodiffuseintothe chitinmatrixmoreeasilyanddiffusiondependsontheparticlesize. Moreover,ahighconcentrationofacid,hightemperature,andprolongedprocesstimeadverselyaffectthepropertiesofchitin [32].
1.3.1.2Deproteinizationprocess
Deproteinizationofdemineralizedshellsiscarriedoutbyusingan alkalitreatmentwithcommonreagentssuchasNaOH,Na2CO3, NaHCO3,KOH,K2CO3,Ca(OH)2,Na2SO3,NaHSO3,CaHSO3,Na3PO4, andNa2S.Theproteinisremovedfromdemineralizedshellsusing KOH/NaOHwithconstantstirringfor2hataround90 Candfiltered undervacuumwithdistilledwateruntilpHneutral.Thenthedeproteinizedshellisdriedintheovenataround60 Cfor24h.Duringthe deproteinizationprocesscleavageofchemicalbondsoccursbetween proteinandchitin.KOH/NaOHhasthetendencytodepolymerizeand degradethechitin.Thedeproteinizationprocessreactionconditionis dependentonthesourceofthecrustaceans.Hence,alkalitreatment leadstothepartialdeacetylationofchitinwithlowmolecularweight (Mw) [33].
1.3.1.3Decolorization
Decolorizationofchitinisthefinalstageofchitinpreparation. Acetoneoranotherorganicsolventmixtureisusedtoremovethepigments.Decolorizingisdonewithacetonefor10 20minandthendrying for2hatambienttemperature.
1.3.2Biologicalextraction
Thechemicaltreatmentprocessofchitinhasmanydrawbacks.Chemical chitinpurificationisextremelyhazardous,energyconsuming,andthreateningtotheenvironment,duetothehighconcentrationofmineralsandcaustic employed.Biologicalextractionisanalternativemethodtoextractchitin fromcrustaceans’shells.Itovercomes theenvironmentalproblemsassociated withacidicandalkalitreatment.The advantagesofbiologicalmethods
includetheproductionofchitinwithhigherreproducibility.Moreover,the solubilityofchitinislimitedandthebiologicalapproachislimited.
1.3.2.1Enzymaticdemineralization
Mineralandproteinpresentinthecrustaceanshellsaredissolvedby organicacidwithmicroorganismssuchaslacticacid-producingbacteria. Thisenzymaticdemineralizationreactioninvolvesorganicacidand microorganismreactingwithcalciumcarbonatepresentintherawshells andcalciumsaltsprecipitatetheorganicacid.Theprecipitatedsaltsare removedbytheculturemediumwithspecialcare.Theprecipitated organicsaltsarealsoremovedbywashingandareusedaspreservative andantiicingagents [34].
1.3.2.2Enzymaticdeproteinization
Intheenzymaticdeproteinizationprocess,proteases(alcalase,pepsin, papain,pancreatin,devolvase,andtrypsin)frombacteriacaneliminate proteins.Commonly,proteolyticenzymesareobtainedfromplants, microbes,andanimalsources.Theyremoveproteinsandreducethe stepsinpreliminaryprocesses.Alcalaseisgenerallypreferredforthe productionofchitin,proteinhydrolyzate,andastaxanthinrecovery.Its hydrophobicaminogroupcontrolshydrolysis.Alcalaseisaserineendopeptidaseobtainedfrom Bacilluslicheniformis.Itisselectedduetoits specificityforterminalhydrophobicaminoacids.Itgenerallyleadsto theproductionofnonbitterhydrolyzateandallowsaneasycontrolof thedegreeofhydrolysis.RawshellsaredemineralizedafterdeproteinizationusingHCltreatmentandtheresidualproteincontentis higherinthechitinisolatedwiththeenzymaticdeproteinizationthan thatobtainedwithalkalitreatment [35].
1.3.2.3Fermentation
Theenzymeprocesshasahighcostduetotheenzymes.Thecostof usingenzymescanbedecreasedbyperformingdeproteinizationusinga fermentationprocess [36].Fermentationmethodsareseparatedintotwo majorcategories:lacticacidfermentationandnonlacticacidfermentation. Fermentationofcrustaceanwasteresultsinasolidfractioncontaining crudechitinandtheproductionofliquorrichinnaturalprotein,mineral, andpigments.Moreover,theactionofthelacticacid-producingbacteriais twofold [37].Theyproduceaspectrumofproteasesthatdetachprotein fromthesolidchitin CaCO3 complexbypartialhydrolysis.Thusextractionbybiologicalactivityisgaininginimportanceoverchemicaltreatment.Itisaneco-friendlyprocessandtheby-productscanberecovered andrecused [38].
Chitosanispreparedbyadegreeofacetylation.Acetylgroupsare removedduringthedeacetylationprocessandMwchangesduetothedepolymerizationreaction.Therearetwoprocesses,thatis,theenzymaticprocessandchemicalprocess,andchitosanisproducedbychemicalprocess. Itispreferableforlarge-scaleproduction.
1.4.1Chemicalandbiologicaldeacetylationofchitin
Glycosidicbondsareattractedtowardacidsandalkalis.Chitinisprocessedhomogeneouslyorheterogeneously.Inthehomogeneousmethod, chitinisdiffusedinconcentratedalkaliat25 Cfor3handallowedtodisperseincompressediceataround0 C [39].Intheheterogeneousprocess thechitinistreatedwithhothigh-concentrationalkaliandthenwashed withdistilledwateruntilthepHisneutral.Itisdifficulttoproducehigher deacetylatedchitosan.Theaddition ofthiophenolasacatalystduringthe processwouldminimizethedegradationbytrappingoxygenandenhance theeffectivedeacetylation.Theeffectivedeacetylationprocessofchitin achievesthepreparationofchitosanifthealkaliconcentrationisfourtimes greaterthanthetotalaminogroupinthepolysaccharideatatemperature around100 Cforthedurationof1h.Itisrecommendedtouselowconcentrationalkaliandashortcontacttimebetweenalkaliandpolymer [40]
Chemicaldeacetylationhasmanydisadvantageslikehighenergyconsumptionandenvironmentalpollutionproblems [41].Analternative methodofenzymedeacetylationhasbeendevelopedtoovercomethese drawbacks.Chitindeacetylationenzymeactsasacatalysistohydrolyze Nacetamidebonds [42].Thisenzymeisextractedfromthefungi Mucorrouxii, Absidiacoerulea, Aspergillushidulans,andtwostrainsof Celletotrichumlindemuthianum.Thisenzymeisthermallystableandhasabindingaffinity toward β-(1,4)-linked N acetyl-D-glucosominepolymers [43].Mostofthe timetheenzymeprocessiscarriedoutinbothbatchandcontinuousculture. InthebatchprocesstheMwofchitosanislowerwithrespecttotime. Moreover,chitosanofhigherMwisobtainedinaspecificcultureeven thoughtheyieldiscomparativelylow [44].
1.5Physicochemicalproperties
Chitinisacolorless,crystallineoramorphouspowderthatisinsoluble inwater,organicsolvents,diluteacids,andalkalis.Itdissolvesinconcentratedmineralacidswithsimultaneousdegradationofthepolymer [45] Althoughchitosanisinsolubleinwater,itdoesdissolveinaqueous
organicacids,forexample,aceticandformicacids,aswellasinorganic acids.Chitinandchitosanpreparationcanvaryduetothesource,with compositionaldifferences.Similarly,thephysicochemicalcharacteristics ofchitinandchitosandifferbetweencrustaceanspeciesandpreparation methods [46].Severalstudieshaveclearlydemonstratedthespecific characteristicsoftheseproducts.TheMwanddegreeofdeacetylation (DD)varywithprocessconditions [46 48].Thephysicochemicalcharacteristicsofchitinandchitosaninfluencetheirfunctionalproperties [24,49].MwandDDvarywithprocessconditionsordifferentextraction methods [24].Chitosan’sapplicationdependsonphysical,biological, andchemicalpropertiesandchitosandependsontwoparameters, suchasDDandMw [50].
1.5.1Molecularweight
Chitosananditsderivativeshavebeenusedinawidevarietyof applicationsbuttheeffectivenessofthesematerialshasbeenfoundto bedependentupontheirDD,crystallinity,andMw [51].DDandMwof chitosanaregreatlyaffectedbyreactionconditionsliketemperature, reagentsconcentrations,repetitionofalkalinesteps,time,andatmosphericconditionsofthedeacetylation [52].Chitosanispreparedby chemicalandenzymaticprocesses [1].Thechemicalprocesshasseveral drawbackssuchaslowproductyieldsandpoorstructuralorder(glucosering).Additionally,acidicandalkalitreatmentsduringthechemicalprocesscouldbeasourceofenvironmentalproblems [24].The enzymaticprocessisanalternativewaytosynthesizechitosanwhichis moreenvironment-friendly [53].Duringtheenzymaticprocesslactic acid-producingbacteriaareusedforthedemineralizationofcrustacean shellsinsteadofacidictreatment.Theobtainedlacticacidreactswith calciumcarbonateyieldingcalciumlactate,whichcanbeprecipitated andremoved.Duringthedeproteinationofcrustaceanshells,proteases frombacteria(Pseudomonasaeruginosa K-187, Serratiamarcescens,FS-3, and Bacillussubtilis)areused. Serratia sp.and Bacillus sp.arebacteria thatalsoproducechitindeacetylaseandcanbeusedtogeneratechitosan [54].Theeffectivedeacetylationisattainedbyintermittentlywashingtheintermediateproductwithwaterduringthealkalitreatment [55].TheaverageMwofchitosanis B500kDawith100%ofDDA.The DDAincreasesrapidlytoabout68%duringthefirsthourofalkalitreatment(50%NaOH)at100 Candfurtherslowlyincreaseswithtime [48]. ThereareseveralmethodsusedforthecalculationofMw,suchaslight scattering,gelpermeationchromatography(GPC),andcapillaryviscometry.Capillaryviscometryisthesimplestandmostwidelyusedmethod todeterminetheMwofchitosan.Duringanalysis,anUbbelohde-type
capillaryviscometerisusedtomeasurethepassagetimeofsolutions flowingthroughthecapillaryat25 C.Differentviscositysolutionsofchitosanareusedatvariousconcentrationsrangingfrom0.00125%to0.15%in 0.1Maceticacid 0.2MNaClsolutions.Thecapillaryviscometerisfilled withthesampleandisequilibratedinawaterbathat25 C.Thesolution andsolventflowtimesaremeasuredtocalculaterelativeviscosity.The Mark Houwink Sakuradaequationgivenbelowprovidestherelation betweenintrinsicviscosity(η)andMw [51]:
BasedonMw,chitosanisclassifiedintothreedifferenttypes,namely low-molecular-weightchitosan(LMWC; , 50kDa),medium-molecularweightchitosan(MMWC;50 250kDa),andhigh-molecular-weightchitosan (HMWC)( . 250kDa).SeveralauthorshavereportedthatLMWChas enhancedproperties,suchasantibacterialandantifungal [56],antitumor [57],lipidmetabolism,intestinaldisaccharidase [58],andmucoadhesive properties [59].ApartfromthistheMwalsoplaysanimportantrolein thebiopolymer’srheologicalproperties.Itdirectlyimpactsthedevelopment ofchitosan-basedbiomaterials [60].LMWCisusedasananticreasingagent toproduceafinishingagentandthenappliedintheanticreasingtreatment ofcottonfabrics.HMWCisusedinquaternizedchitosanfilmswithpropertiesofwatersolubilityandfreeradicalscavenging,andHMWCisalsoused forhigh-performancecellsanditseffectsonpolymeraggregationand phaseseparation.
1.5.2Viscosity
Chitosanisthebestknowndeacetylatedderivativeofchitin,asone ofitsuniquepropertiesisitspolycationicnaturewhendissolvedin acidicsolution(thevalueofpKa 5 6.0).Hencethisbiopolymerisfavorableforabroadvarietyofindustrialandbiomedicalapplications.When chitosanisdissolvedinacidicsolution,itgivesaviscoussolution.The viscosityofthesolutionisrelatedtotheMw,DD,concentration,pH, andtemperatureofchitosansolution.
Theviscosityofchitosansolution,atthemolecularlevel,isadirect measureofthevolumeofthepolymermolecules,whichinturnisgovernedbythemolecularsizeorchainlength.Theviscosityofthepolymersolutioniswidelymeasuredbyacapillaryviscometeranditsvalue isusedtodeterminetheaverageMwofpolymerbyusingthe Mark Houwinkequation(Eq.(1.1)):
Where Mv istheaverageMwofthepolymerand α and k areconstants (α 5 0.83and k 5 1.4 3 10 4 for0.25Maceticacidand0.25Msodiumacetatesolventsystem).Theintrinsicviscosity[η]canbedeterminedfrom thefollowingequation(Eq.(1.2)):
where η (dL/g)isthesolutionviscosityand ηs isthereferencesolvent viscosityand C isthesolutionconcentration.Asindicatedin(1.2),when (η ηs)/ηsC,thatis,reducedviscosity(ηred)isplottedagainstconcentration(C),theinterceptcorrespondsto[η].The[η]valueisdirectly usedindeterminingtheMwofdifferentgradesofchitosan.Someofthe typicalchitosanviscositieswithdifferentMwsaresummarizedin Table1.1[61]
Flowactivationenergy(Ef)ofviscouschitosansampleisanalyzedby theArrheniusequation(Eq.1.3):
where A and R arethepreexponentialfactoranduniversalgasconstant absolutetemperature(inKelvin).Thevalueof Ef oftwodifferentgrade ofchitosan91%DOAand70%DOAwas25and15kJ/mol,respectively,asreportedbyWeiWang [62].Thismeansthattheentanglement ofchainsincreaseswiththeincreasingDDofchitosanbecauseofthe natureofthepolymer.
Theviscosityandflowpropertiesoftheconcentratedsolutionsofchitosan withdifferentdegreesofdeacetylationaredifferent.Theviscositiesandthe non-Newtonianflowpropertiesofthesolutionsincreasewiththeincreasing DDofchitosan.Ontheotherhand,additionalsaltdecreasestheviscosities andthenon-Newtonianflowpropertiesofthesolutionsofchitosan.
TABLE1.1 Intrinsicviscosityandviscosityaveragemolecularweightofdifferent gradesofchitosan.
ChitosanIntrinsicviscosity[η],dL/gMolecularweight, MV
CHT-19.40654,127
CHT-24.72285,231
CHT-32.55135,839
CHT-41.5071,676
CHT-50.53520,698
1.5.3Solubility
Thethree-dimensionalchitincrystallinematrixconsistsofacombination ofstronghydrogenbondsandcohesiveforcesofacetyl(OQC),hydroxyl ( OH)andamine( NH)functionalgroups.Thesefunctiongroupslink with NH OQCand OH OQCbyhydrogenbondsinthethreedimensionalmatrix.Purechitincontainsaround90% N-acetylgroupsinits backboneandsomedeacetylationreactionstakeplaceduetotheextraction processofchitinfromthenaturalsources.
ThefirststudyonthesolubilityofchitinwasperformedbyAustin,who testedthesolubilityofchitinindifferentorganicsolvents [63].Itwasa well-organizedevaluationofchitin’ssolubilityindifferenttypesofsolvents,suchasdichloroacetic(DCA)andtrichloroacetic(TCA)acidsinthe presenceorabsenceofalcohol,etc.Lateron,manystudieswereconducted withthesameintentionsbymanyotherresearchersandchitin’ssolubility wasverifiedinmanysolventssuchasdimethylacetamide(DMA)/LiCl mixture,CaBr2 H2Osaturatedmethanol,hexafluoroisopropylalcohol andhexafluoraceton,lithiumthiocyanate,phosphoricacidand N-methyl2-pyrrolidone,etc. [64].Althoughthedissolutionofchitinispossibleby thesesolvents,manyofthemaretoxic,scarcelydegradable,corrosive,or mutagenic.Thereforethechoiceofanappropriatesolventforchitinand chitosansolubilizationisimportantandaprimaryissueforlab-scale researchandscalingupforindustrialpractices [65].
Therearetwomonomerunitspresentinthechitinbackboneindifferent fractionsnamely2-acetamino-2-deoxy-D-glucopyronase(N-acetyl-D-glucosamine)and2-amino-2-deoxy-D-glucopyronase(N-amino-D-glucosamine) [66].Thefirstonegroup,2-acetamino-2-deoxy-D-glucopyronase,displays insolubilityduetothestronghydrogenbondsbetweentheacetylgroupsof thesameoradjacentchitinchains.Intheotherunit N-amino-D-glucosamine ishydrophilicinnatureandpositivelychargedinacidicsolution.Thedominationofthehydrophiliccharacterwithahighamountof N-amino-D-glucosamineunitsinthechitinbackbone makeitsolubleinthespecificacidic solution.Thepercentageof N-amino-D-glucosamineunitinthechitinbackbonecanbedeterminedbyDDA.Itistherationof N-amino-D-glucosamine to N-acetyl-D-glucosamine,whilethedegreeofacetylationrepresentsthe detectionfrom100(100-DDA).WhenDDAisbetween60%and90%,anew chemicalentity“chitosan”isformedanditissolubleinorganicacidssuch asaceticacid.Chitosancanbedissolvedinaqueousdilutedacidsasapolycationat B50%DDAormoreduetothepresenceof N-amino-D-glucosamineunits [64].Theotherparameters,suchastemperature,timeof deacetylation,alkalicontraction,andpriortreatment,appliedtochitinisolationandDDalsoaffectthesolubilityofchitosan.Thereforethefractionof N-amino-D-glucosamineunitshasalargeinfluenceonthesolubilityandthe solution’sproperties. 12 1.Chitinandchitosan:origin,properties,andapplications
1.6Characterizationofchitinandchitosan
1.5.4Water-bindingcapacityandfat-bindingcapacity
Water-bindingcapacity(WBC)andfat-bindingcapacity(FBC)of chitinandchitosanismeasuredbyamodifiedmethodofWangand Kinsella [67].Inatypicalprocess,waterorfatabsorptionisinitiallycarriedoutbyweighingacentrifugetubecontaining0.5gofsampleand adding10mLofwaterorsoybeanoilandmixingwithavortexmixer for1mintodispersethesample.Thecontentsareleftatambienttemperaturefor30minwithshakingfor5severy10min,beforebeingcentrifugedat B3200rpmfor25min.Afterthesupernatantisdecanted, thetubeisweighedagain.WBCandFBCarecalculatedasfollows [24].
WBCdifferedwithproductsrangingfrom381%to673%forchitins andfrom458%to805%forchitosans [24].ChitosanhasahigherWBC thanchitin.TheWBCvaluesaredifferentforeachsource(chitin)and chitosan.ThedifferencesinWBCbetweenchitinandchitosanarepossiblyduetodissimilaritiesincrystallinity,thenumberofsalt-forming groups,andtheresidualproteincontentoftheproducts [68].
FBCforproductsofchitinswereintherangeof316% 320%,whereas chitosanshowsdissimilarbindingcapacitiesrangingfrom314% 535%. ThisapparentlysuggeststhatchitincanorcannothavehigherFBCthan chitosandependingontheproducts [24].
1.6Characterizationofchitinandchitosan
Afterseveraldecadesofintenseresearchconcerningthesynthesis, characterization,andapplicationofchitinandchitosan,acompleteand detaileddescriptionofthepolymeranditscharacteristicscannotbe deducedyet;evenfromthosestudiesperformedbyusingadvanceexperimentaltechniquessuchasX-raydiffractionorhigh-resolutionscanning electronmicroscopy(HRSEM).Asaconsequence,suitableatomistic modelshavebeendevelopedtotrytodescribetheiratomicstructurein ordertounderstandtheirdifferentpropertiesandtohelpintailoring themforspecificpurposes.
Adetaileddescriptionofchitinandchitosanmustinclude(1)characteristics oftherawchitinandtheproducedchitosan,bymeansofsurfacefunctional group;(2)thermalanalyses;(3)structuralanalysis;(4)Mw;(5)viscosity;and (6)themorphologyoftheproduct.
Abriefdescriptionofdifferentcharacterizationtechniquesisdiscussed below.
1.6.1Fourier-transforminfraredspectroscopy(FT-IR)analysis
FT-IRanalyticaltechniqueisusedtounderstandthefunctionalgroups presentinthechitinandchitosan.Thechitins(α-, β-,and γ-chitin)canbe distinguishedbyFT-IRspectraandtheseareshownin Fig.1.3.Inthecase of α-chitin,twoseparatepeaksareobservedat B1662and B1630cm 1 . Theyrepresenttheamide-Ibandpresentinthe α-chitin.Itisassociated withtheoccurrenceoftheintermolecularhydrogenband CO...HNand CO HOCH2 [71].Inthecaseof β-chitin,asinglebandisobservedat 1656cm 1 duetothehydrogenbondpresentbetweentheamidegroup ( CQO)oftheneighboringintrasheetchain [27].The NHstretching bandat3264and3107cm 1 isclearlyobservedinboth α-and β-chitin.The bandsat703and750cm 1 representthebendingvibrationof OHgroups andNH groupspresentinthe α-chitin,respectively.Whileinthecaseof β-chitin,theyareshiftedto682and710cm 1 [71].However, β-chitinshows asinglebandatapproximately1656cm 1 whichisassociatedwiththe intermolecularhydrogenbondofCO...HN [69].The β-chitinhavethe weakerinter-andintramolecularhydrogenbondingandamoreloosely orderedstructurecomparedwith α-chitin. γ-Chitinshowsthetwosharp peaksat1660and1620cm 1 fortheamide-Iband,whichisalsoavailable inthe α-chitin.
Apartfromtheexpecteddecreaseinthebandintensityat1665and 1550cm 1 forchitin(amide-I),theyappearedatnearly1604,1598,and 1592cm 1 forchitosan.Thisdemonstratestheeffectivedeacetylationof chitin.Moreover,thebandsassignedtothestretchingvibrationsofthe glycosidicbondofchitosanpolysaccharidestructureat1151,1098,and 1021cm 1 weakeneddistinctlyafterthedeacetylationprocess.The bandsnearlydisappearedat3260and3107cm 1,originatingfrom N Hstretchingafterdeacetylationindicatingthedisturbedthe regularhydrogenbondofN Hinthechitin.
Inthe α-chitinspectra:twoseparatepeaksareobservedat1662and 1630cm 1.Theyareassociatedwiththeoccurrenceoftheintermolecular hydrogenband CO...HNand CO.....HOCH2.Moreover,asinglebandis observedat1656cm 1 incaseofthe β-chitin.Itiscommonlyrelatedtothe stretchingofthe COgrouphydrogenbondtoamidegroupoftheneighboringintrasheetchain [27].Thestrongbandat1430cm 1 isnotedfor β-chitin, whileasharpbandat1416cm 1 presentinthecaseof α-chitin [28]. TheNH stretchingbandat3264and3107cm 1 isclearlyobservedinthe α-chitinbutinthecaseof β-chitin,itisnoteasilyseen(Fig.1.3A).Thebandat 703cm 1 duetothe OHoutofplaneand750cm 1 duetoNH outof planeisshowsinthe α-chitin.Whileinthecaseof β-chitin,itisshiftedto