Table of Contents
Cover image
Title page
Copyright
Contributors
Chapter 1: Switchable solvents for bio-refinery applications
Abstract
1: Introduction
2: Concept of biorefinery
3: Switchable solvents for biorefinery
4: Concluding remarks and future perspectives
References
Chapter 2: Polarity-changing solvents for CO2 capture
Abstract
1: Introduction
2: Switchable solvent for CO2 capture
3: Switchable ionic liquid solvent for CO2 capture
4: Conclusions
References
Chapter 3: Applications of switchable solvents in science and technology
Abstract
1: Introduction
2: Switchable water (SW)
3: Technological and analytical applications of switchable solvents
4: Conclusions
References
Chapter 4: Switchable solvents for CO2 capture
Abstract Acknowledgments
1: Introduction
2: Environmental challenges due to CO2
3: Switchable solvents for the detection of CO2
4: Switchable solvents for CO2 extraction
5: Reversible ionic liquid solvents for the capture of CO2
6: Conclusions
References
Chapter 5: Switchable water
Abstract
1: Introduction
2: Switchable polarity solvents
3: Switchable hydrophilicity solvents
4: Switchable water
5: Conclusion References
Chapter 6: Switchable solvents as alternative solvents for green chemistry
Abstract
1: Introduction
2: Discovery of switchable solvents
3: Types of switchable solvents
4: Chemistry and development of SS
5: Generally desirable properties of SS [76]
6: Acceptability of switchable solvents for green chemistry
7: Applications of switchable solvents as green alternatives [46, 78, 79]
8: Future prospects and conclusions
References
Chapter 7: Nanomaterials synthesis in switchable solvents
Abstract
1: Introduction
2: Ionic liquids as switchable solvents
3: Reverse micelles for nanoparticles synthesis
4: Reverse micro-emulsions for nanoparticle synthesis
5: Reversible capping agents based on amines
6: Conclusion
References
Chapter 8: CO2-triggered switchable polarity solvents and their advancements
Abstract
1: Introduction
2: Chemistry of CO2-triggered SPS
3: CO2-triggered SPS
4: Challenges and future considerations
5: Conclusion References
Chapter 9: Switchable green solvents for lipids extraction from microalgae
Abstract
1: Introduction
2: Green solvents
3: Microalgae lipid and extraction process
4: Perspective and conclusion
References
Further reading
Chapter 10: CO2 triggered switchable and tunable solvents for biocatalysis
Abstract
Acknowledgments
1: Introduction
2: Tunable solvents for biocatalysis
3: Switchable solvents for biocatalysis
4: Conclusions and future perspectives
References
Chapter 11: Basic synthesis and solvatochromic parameters in switchable solvents
Abstract
1: Introduction
2: Chemical synthesis in a switchable solvent
3: Theory on solvatochromism process
4: Solvatochromic parameters
5: Conclusion
References
Chapter 12: Switchable solvents for catalysis
Abstract
1: Introduction
2: Switchable hydrophilic solvents
3: Solvents with switchable polarity
4: Switchable water
5: Surface operation (switchable cationic/anionic surfactants)
6: Materials that respond to stimuli
7: Application of switchable solvents as catalyst
8: Switchable solvents for biocatalysis
9: Conclusions References Index
Copyright
Elsevier
Radarweg 29, PO Box 211, 1000 AE Amsterdam, Netherlands
The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom
50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States
Copyright © 2022 Elsevier Inc. All rights reserved.
No part of this publication may be reproduced or transmi�ed in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions.
This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein).
Notices
Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary.
Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility.
To the fullest extent of the law, neither the Publisher nor the authors, contributors, or editors, assume any liability for any injury and/or damage to persons or property as a ma�er of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions, or ideas contained in the material herein.
Library of Congress Cataloging-in-Publication Data
A catalog record for this book is available from the Library of Congress
British Library Cataloguing-in-Publication Data
A catalogue record for this book is available from the British Library
ISBN: 978-0-12-819850-6
For information on all Elsevier publications visit our website at h�ps://www.elsevier.com/books-and-journals
Publisher: Susan Dennis
Acquisitions Editor: Anita Koch
Editorial Project Manager: Emerald Li
Production Project Manager: Kumar Anbazhagan
Cover Designer: Christian J. Bilbow
Typeset by STRAIVE, India
Contributors
Meenu Aggarwal Department of Chemistry, Aggarwal College, Faridabad, Haryana, India
Mohd Imran Ahamed Department of Chemistry, Aligarh Muslim University, Aligarh, U�ar Pradesh, India
Mohammad Faraz Ahmer Department of Electrical and Electronics Engineering, Mewat Engineering College, Nuh, Haryana, India
Naushad Anwar Department of Chemistry, Aligarh Muslim University, Aligarh, U�ar Pradesh, India
K.K. Athira Department of Chemistry, Indian Institute of Technology Madras, Chennai, India
Shokufeh Bagheri Department of Chemical Engineering, Shiraz University, Shiraz, Iran
Pinki Chakraborty School of Basic & Applied Sciences, Galgotias University, Greater Noida, India
Dr. Elsa Cherian Department of Food Technology, SAINTGITS College of Engineering, Ko�ayam, Kerala, India
Santanu Dasgupta Reliance Industries Limited, Jamnagar, Gujarat, India
Ramesh L. Gardas Department of Chemistry, Indian Institute of Technology Madras, Chennai, India
Anjali Gupta Department of Chemistry, School of Basic and Applied Sciences, Galgotias University, Greater Noida, India
Saurabh Jain Department of Biotechnology, MGIMT, Banthra, Lucknow, India
Wriju Kargupta Monash University, Melbourne, VIC, Australia
Mohd. Farhan Khan
Nano Solver Lab, Department of Mechanical Engineering, Z. H. College of Engineering & Technology, Aligarh Muslim University Department of Science, Gagan College of Management and Technology, Aligarh, India
Satish Kumar Department of Chemistry, St. Stephen's College, University Enclave, Delhi, India
Mohammad Luqman Chemical Engineering Department, College of Engineering, Taibah University, Yanbu Al-Bahr, Kingdom of Saudi Arabia
Anuradha Mishra Department of Applied Chemistry, SoVSAS, Gautam Buddha University, Gautam Budh Nagar, India
M.A. Quraishi Center of Research Excellence in Corrosion, Research Institute, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia
Mohammad Reza Rahimpour Department of Chemical Engineering, Shiraz University, Shiraz, Iran
Zeynab Rezaeiyan Department of Chemical Engineering, Shiraz University, Shiraz, Iran
Karthikay Sankhydhar School of Basic & Applied Sciences, Galgotias University, Greater Noida, India
Debanjan Sanyal Reliance Industries Limited, Jamnagar, Gujarat, India
Ajit Sapre Reliance Industries Limited, Navi Mumbai, Maharashtra, India
Nishant Saxena Reliance Industries Limited, Jamnagar, Gujarat, India
Mohammad Amin Sedghamiz Department of Chemical Engineering, Shiraz University, Shiraz, Iran
Nimra Shakeel Department of Chemistry, Aligarh Muslim University, Aligarh, U�ar Pradesh, India
Anupama Sharma School of Basic & Applied Sciences, Galgotias University, Greater Noida, India
Shashank Sharma Department of Chemistry, SBAS, Galgotias University, Greater Noida, India
G. Venkata Subhash Reliance Industries Limited, Navi Mumbai, Maharashtra, India
Divya Bajpai Tripathy Department of Chemistry, School of Basic and Applied Sciences, Galgotias University, Greater Noida, India
Aman Ullah Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, AB, Canada
Qasim Ullah Physical Sciences Section, School of Sciences, Maulana Azad National Urdu University, Hyderabad, Telengana, India
Muhammad Zubair Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, AB, Canada
Chapter 1: Switchable solvents for bio-refinery applications
Muhammad Zubair; Aman Ullah⁎ Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, AB, Canada
⁎ Corresponding author. ullah2@ualberta.ca
Abstract
Switchable solvents have excellent advantages over traditional solvents including low volatility and flammability, reuse or recycling, and benign to the environment. These a�ributes make them an excellent candidate to be used in biorefinery for fractionation, separation, and development of different products with multiple functions. This chapter focuses primarily on the use of different switchable solvents such as switchable polarity solvents, switchable hydrophilicity solvents, and switchable water in the biorefinery processes. This chapter also discusses the concept of biorefinery briefly and ends with the future perspective for utilization of switchable solvents in the biorefinery.
Keywords
Biorefinery; Biomass; Switchable hydrophilicity solvents; Switchable polarity solvents; Switchable water
1: Introduction
In the present century, human beings are facing numerous challenges such as huge demand of supplies due to the ever-growing world population, climatic changes as a result of rapid industrialization, exhaustion of fossil fuel feedstocks, and, most importantly, geopolitical apprehensions effecting supply of raw materials across the globe. Moreover, concerns regarding the environment pollution have led to an increase in the demand of shift of global economy towards low cost, efficient, renewable, and sustainable feedstock [1, 2]. It has been predicted that the industry in coming decades will be heavily based on state of the art routes derived from renewable raw materials and providing chemicals with at least same or more innovative features than their petroleum-based counterparts [3, 4].
However, the production of bio-based products from sustainable resources poses certain challenges for an eco-based economy. The most suitable approach is the use of renewable biomass feedstock to provide a continuous supply chain that is not only the viable sustainable option to substitute for fossil fuel resources but also a source of organic compounds with a short span of life and infinite supply. These a�ributes make them an a�ractive candidate for research and development of biomass-based materials in a sustainable way [5]. The concept of biorefinery is considered an excellent opportunity to replace the petro-based materials as biorefinery utilizes biomass as a cheap feedstock to produce materials in both chemical as well as biological industries.
There is a growing research interest in the value of bio-based materials derived from residual biomass. It is essential to focus on the extraction, recovery, and/or synthesis of bio-based products for industrial applications to implement sustainable approach in a future bio-derived economy. The efficient and effective use of biomass such as cellulose, hemicellulose, lignin, proteins, lipids has become a�ractive starting materials for the synthesis of various chemicals and materials to replace the fossil fuel-derived materials [6, 7].
These large-scale applications are evolving from innovative approaches that are being designed to advance the biorefineries for particular bio-derived feedstocks [5]. The focus of the biorefineries is the extraction of valuable chemicals from the biomass to develop food additives, pharmaceuticals, fragrances, biofuels, dyes, coating, nutraceuticals, and other commodities [8–14]. In the synthesis and extraction process, solvents are ubiquitous auxiliary substances and play the most vital role in terms of their efficiency, cost, availability, and mostly importantly environmental impact.
For the last two decades, research efforts of scientists have shifted towards study of the harmful impacts of solvents utilized in various industrial processes to produce diverse materials. Specifically, the introduction of green chemistry 12 principles signified the importance to diminish the hazards associated with human health, environment, and safety due to the tremendous use of traditional chemical processes [15, 16]. Similarly, in biorefinery various solvents/reagent have been used during the extraction of valuable components and production of materials [17, 18]. Thus, the progress in the utilization of environmentally benign solvents is presently a favorable choice in this rapid technological era, especially in biorefineries. Green solvents are being used in biorefineries as they have less harmful impacts on the health, safety, and environment as well as short life cycle. Ionic liquids derived from bio-based materials or natural eutectic solvents, supramolecular solvents, supercritical substances, and switchable solvents are few examples of greener solvents [19–22].
Amongst green solvents, switchable solvents, also called smart solvents, are one of the suitable choices to replace the traditional solvent systems. They have excellent ability to alter both their physical and chemical properties reversibly under the influence of external stimulus [23, 24]. For example, addition or removal of CO2 or a change in temperature results in the formation of switchable solvent. The solvent's behavior can be switched by exposing them to triggers such as light, gas, and heat and ultimately used them for decontamination of solute in a separation process. They also
facilitate their reuse and thus reduce the generation of waste [22]. Each of the trigger has advantages, but their most important characteristics are being environmentally benign and economically feasible with a straightforward reversible mechanism [25–28]. So, this chapter presents the existing switchable solvents used in the industrial biomass processing applications within a biorefinery context and highlights the concept of biorefinery.
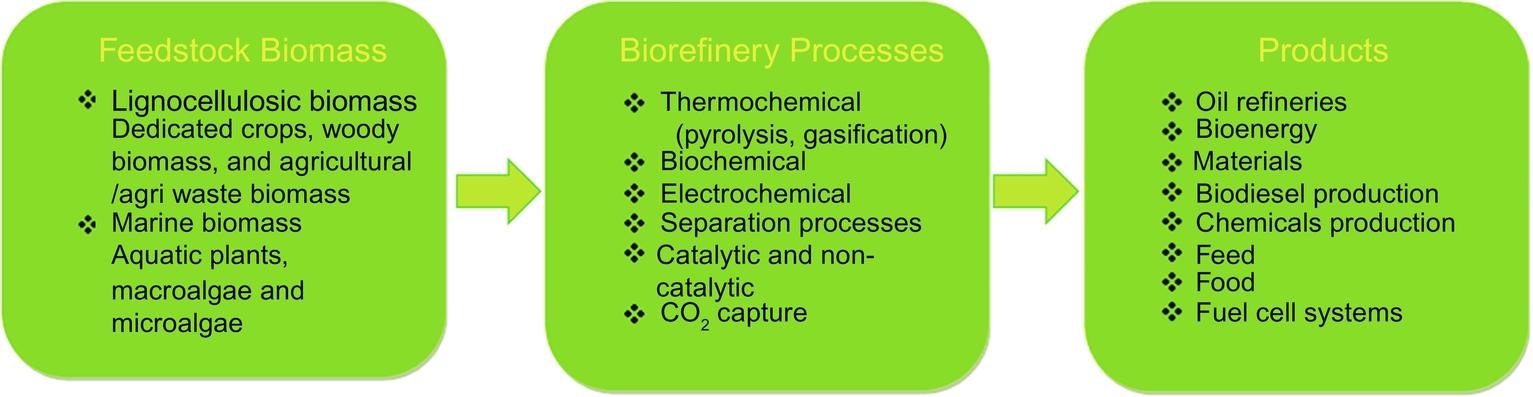
2: Concept of biorefinery
International Energy Agency Bioenergy Task 42 defines biorefinery and its most accepted definition in the scientific community. It states biorefinery as a “sustainable processing of biomass into a spectrum of marketable products (food, feed, materials, chemicals) and energy (fuels, power, heat)” [29]. In other words, biorefinery concept is to develop wide range of chemicals, materials, fuels at least similar or superior in properties than petroleum-based materials at industrial scale from biomass in a sustainable manner [30] as summarized in Fig. 1. The main goal of the future biorefineries is to extract valuable components that are present in the biomass and transform them into the bio-derived materials, bioenergy, and other commercially viable commodities. Most importantly, biorefineries only be feasible if the extraction of chemicals from biomass and conversion efficiency into other materials is exploited maximum using proper production techniques [31].
FIG. 1 Concept of biorefinery.
Biorefinery is basically a conversion process of biomass feedstock into commercial products such as fuels, chemicals, and materials similar to petroleum resources [32]. In biorefinery processes, proper utilization of feedstock is essential to produce low-cost products while maintaining the concept of sustainable economy [33]. Biomass has the inherent property of diverse and complex molecular structures that makes them ideal candidates to transform into different chemicals and materials with excellent properties.
Corn, sugar cane, corn stover, switch grass, plam oils, and microalgae are the major sources of biomass feedstocks which consist of mainly lignocellulosic and lipids components. These components are being extracted and converted into a variety of materials with multiple functions properties based on their composition. These biomass-derived materials at industrial scale can be categorized into energy, molecules, and materials [34]. The energy products can be synthesized using direct combustion or thermochemical conversion processes [35]. These conversions methods utilize the whole biomass fractions for the synthesis of biocrude or biogas. Similar to petroleum refineries, produced biocrude can be further fractionated into different hydrocarbons and syngas [36, 37]. In terms of molecules, extracted portion can be used either as chemical solvents or as basic unit to produce other chemicals [34]. Furthermore, the extracted components can be utilized to synthesize polymers or fibers with inherent properties of strength and biodegradability.
The biomass can be processed to obtain chemicals or materials through different techniques including mechanical or physicochemical, fractionation, and conversion reactions, however these methods require large amount of energy. In addition, these processing steps involve solvents such as hexane, methanol, and dimethyl sulfoxide. Most of the organic solvents are inherently hazardous due to high toxicity and inflammability. In addition, at the end of the process, solvent separation from final product involves energy and ultimately more cost for the product production [38]. Hence, the be�er processing methods, especially the ones related to the use of suitable solvents, are essential to determine
economic viability, cost and environmental, health, and safety impacts of the biorefineries [29].
3: Switchable solvents for biorefinery
The unique properties of switchable solvents such as easy conversion into hydrophilic or hydrophobic phase, less energy for separations as well as recovery/recycling of solvent and catalysts, make them suitable for biorefinery operations [39]. There are numerous data available in the literature where switchable solvents are studied for the pretreatment, extraction, and fractionation of valuable components from lignocellulosic biomass such as pretreatment with butadiene sulfone [40], phenols extraction [41], and spruce wood fractionation with ionic liquids [42].
Despite the fact that switchable solvents have promising future in various biomass applications, optimization of parameters are extremely necessary such as solvent-biomass ratios, solvent recovery, and purity of product [43]. The utility of switchable solvents is mainly dependent on their properties. For example, switchable hydrophilicity solvents should have specific basic values as they tend to behave hydrophilic/hydrophobic at different values and can be efficiently triggered with CO2. In this section, different switchable solvents are discussed for their use with focus in the biorefinery.
3.1: Switchable polarity solvents
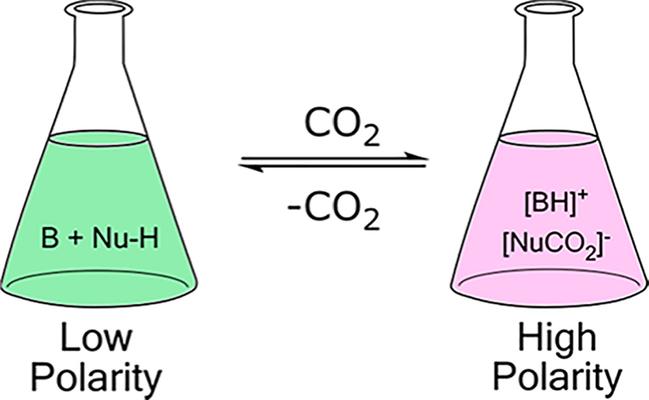
Switchable polarity solvents are of low polarity that is changed to high polarity resulting in the dramatic change in the solubility of many solutes. The low polarity molecular liquid is switched to a higher polarity ionic liquid as CO2 is bubbled from a switchable polarity solvents as shown in Fig. 2 [23].
FIG. 2 Exposure of CO2 results in polarity change [28].
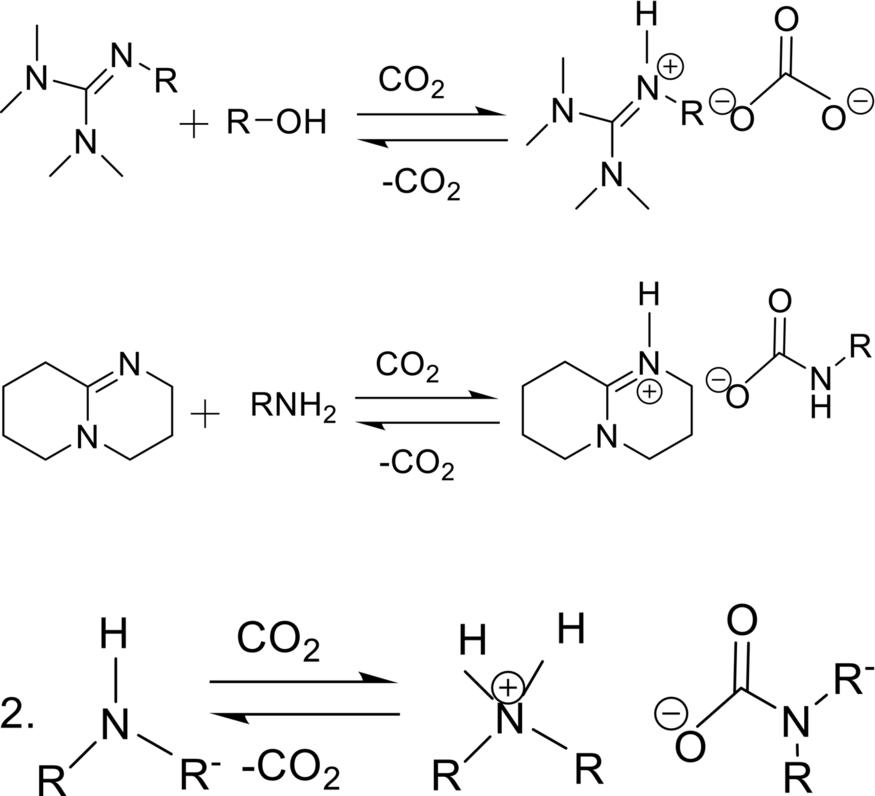
The molecular liquids often have equimolar mixtures of amines and alcohols as a nucleophilic solvents and guanidines, amines, or amidines, as an organic base [44, 45]. When CO2 binds to the nucleophilic moieties, it chemically results in the formation of ammonium carbonate and carbamate salts. In some cases, same molecule act as a nucleophile and a base in one-component switchable solvent system. In some cases, same molecule act as a nucleophile and a base in one-component switchable solvent system [46, 47] as shown in Fig. 3.
FIG. 3 Guanidine, amidine, and secondary amine (from top to bottom).
So far, many switchable polarity solvents have been reported including a low-polarity liquid mixture of an alcohol [23], binary liquid mixtures such as glycerol-amidine [48] or amidine-primary amine mixtures [45, 49–51] and single component switchable polarity solvents, i.e., diamines [52], secondary amines, primary amines, etc. [53].
A study reported on the use of N,N-dimethylcyclohexylaminethe for the lipids extraction and recovery, directly from wet samples having about 80% water contents. They used microalgal strains and reported lipids extraction without any cell disruption from dilute cultivation media and avoided the use of volatile organic solvents [54].
Anugwom et al. reported switchable ionic liquids prepared from hexanol or butanol and CO2 along with amidine (1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU)) to investigate the effect of solvent on dissolution as well as fractionation of native spruce and preextracted spruce woody material for 5 days at 55°C. The study concluded that after the 5-day treatment, 38 wt% less hemicelluloses was observed in the undissolved fraction in comparison with native spruce. Less energy was required for this process in comparison with existing wood treatments technologies since dissolution/fractionation occurred at 55°C. Moreover, the ionic liquid gave more chances of recycling or reuse due to the possibility of return to their molecular components [42].
It is noteworthy that switchable polarity solvents, particularly alcohols, incline to be water sensitive because it is not easy for solid bicarbonate products to return into their neutral form. Furthermore, bicarbonate formation is thermodynamically more favorable than the carbamate salts formation. Thus, dry conditions are required to obtain be�er separations or chemical reactions while using alcohol containing switchable polarity solvents. The condition is critical particularly in organic reactions where water was produced as a byproduct. Then, it is necessary to be dried before switching the solvent. Samori et al. reported the method to overcome this issue. They did extraction of lipids from microalgae using 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) and an alcohol. Dried and watersuspended samples of the microalga were used for the study. The nonionic nature of the DBU/alcohol SPS for nonpolar compounds was used to extract hydrocarbons from algae. While the DBU-alkyl carbonate form, which is in ionic character and obtained by the addition of CO2, was used for the recovery of hydrocarbons from the switchable-polarity solvents. The comparative study was performed for DBU using alternatively octanol or ethanol. The results indicated that extraction efficiency using octanol showed the maximum yields of extracted hydrocarbons from both freeze-dried and liquid algal samples [55]. SPS having amine nucleophiles are more stable in the presence of water since formation of carbamate is faster than
bicarbonate formation. Organic reactions such as Michael and Heck have been performed in the switchable polarity solvents [56].
Functional groups present in the switchable solvents are not always chemically inert. Therefore, selection of appropriate reactants or reagents is necessary to avoid this problem. Equimolar quantities of alcohol are not required in one component systems still they have potential reactive amine moieties. In addition, in one component systems 2 equivalent of amine are needed for 1 equivalent of CO2 for anion-cation pair. Therefore, in one component systems, large quantity of switchable polarity solvents may involve contingent to the chemical process. The switching of polarity exhibited by the switchable polarity solvent is very critical for extractions/separations of the components predominantly in the complex materials. A transformation of polarity from high to low or low to high, resulted in phase separation or precipitation of a dissolved solute. Hence, switchable polarity solvents have been utilized in numerous applications, for example, oil extraction from soybeans [57], heavy metals recovery [58], pretreatment of lignocellulosic biomass, etc. [59, 60].
Phan and co-workers extracted soy oil from soybean flaked by an amidine as a switchable solvent to overcome the issue related to hexane harmful environmental impacts and cost. Later, the solvent was removed from the soy oil using carbonated water. They evaluated the oil separation ability of different solvents, a secondary amine, an amidine/alcohol, a combination of an amidine and excess water, and use of dioxane. A combination of amidine and excess water showed higher separation of solvent/oil, appropriate extraction of oil, and insensitive to water. Further, this method exhibited advantage of easy switching with CO2 application or removal.
There are studies reported on the use of switchable ionic liquids for the extraction of lignin from the lignocellulosic materials as they are water insensitive and easy to handle [59, 60]. Monoethanol amine (MEA), an amidine, DBU were used as switchable ionic liquids along with CO2 and SO2 as triggers for the dissolution of one/two major