GREENSUSTAINABLE PROCESSFOR CHEMICALAND ENVIRONMENTAL
SonochemicalOrganic Synthesis
Editedby INAMUDDIN
RAJENDERBODDULA
ABDULAHM.ASIRI
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical, includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwriting fromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspolicies andourarrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency, canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanas maybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodsthey shouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessional responsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityfor anyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromany useoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-819540-6
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: KostasMarinakis
EditorialProjectManager: EmeraldLi
ProductionProjectManager: DebasishGhosh
CoverDesigner: ChristianJ.Bilbow
TypesetbySPiGlobal,India
Contributors
HilalAcidereli
SenResearchGroup,DepartmentofBiochemistry,DumlupınarUniversity,K € utahya,Turkey
MortezaMadadiAsl
DepartmentofPolymerScienceandEngineering,UniversityofBonab,Bonab,Iran
ElhamAzadi
OrganicPolymerChemistryResearchLaboratory,DepartmentofChemistry,IsfahanUniversity ofTechnology,Isfahan,Iran
SmritilekhaBera
SchoolofChemicalSciences,CentralUniversityofGujarat,Gandhinagar,India
AmarJyotiBhuyan DepartmentofChemistry,RajivGandhiUniversity,ArunachalPradesh,India
PubanitaBhuyan DepartmentofChemistry,RajivGandhiUniversity,ArunachalPradesh,India
MehmetHarbiCalimli
SenResearchGroup,DepartmentofBiochemistry,DumlupınarUniversity,K € utahya;Tuzluca VocationalHighSchool,IgdirUniversity,Igdir,Turkey
AnshuDandia
DepartmentofChemistry,CentreofAdvancedStudies,UniversityofRajasthan,Jaipur,India
AntonV.Dolzhenko
SchoolofPharmacy,MonashUniversityMalaysia,BandarSunway,Selangor,Malaysia; SchoolofPharmacy,CurtinHealthInnovationResearchInstitute,CurtinUniversity,Perth, WA,Australia
MuhammadFaisal
DepartmentofChemistry,Quaid-i-AzamUniversity,Islamabad,Pakistan
NehaN.Gharat
DepartmentofChemicalEngineering,InstituteofChemicalTechnology,Mumbai,India
ShyamL.Gupta GovernmentPolytechnicCollege,Kalimori,Alwar,India
MehdiHatami
DepartmentofPolymerScienceandEngineering,UniversityofBonab,Bonab,Iran
SaeedKhalili
DepartmentofPolymerScienceandEngineering,UniversityofBonab,Bonab,Iran
AnilKumar
SyntheticOrganicChemistryLab,FacultyofSciences,ShriMataVaishnoDeviUniversity,Katra, Jammu&Kashmir,India
BhagirathLimbani
SchoolofChemicalSciences,CentralUniversityofGujarat,Gandhinagar,India
ManzarMahmoudian
DepartmentofPolymerScienceandEngineering,UniversityofBonab,Bonab,Iran
ShadpourMallakpour
OrganicPolymerChemistryResearchLaboratory,DepartmentofChemistry;Research InstituteforNanotechnologyandAdvancedMaterials,IsfahanUniversityofTechnology, Isfahan,Iran
DhananjoyMondal
SchoolofChemicalSciences,CentralUniversityofGujarat,Gandhinagar,India
MinaNaghdi
DepartmentofChemicalEngineering,InstituteofChemicalTechnology,Mumbai,India x Contributors
OrganicPolymerChemistryResearchLaboratory,DepartmentofChemistry,IsfahanUniversity ofTechnology,Isfahan,Iran
KarunaN.Nagula
DepartmentofPharmaceutics,ParulInstituteofPharmacy&Research,Vadodara,Gujarat,India
MehmetSalihNas
DepartmentofEnvironmentalEngineering,FacultyofEngineering,IgdirUniversity,Igdir, Turkey
HaiTruongNguyen
DepartmentofOrganicChemistry,UniversityofScience,VietnamNationalUniversity,HoChi MinhCity,Vietnam
VijayParewa
DepartmentofChemistry,CentreofAdvancedStudies,UniversityofRajasthan,Jaipur,India
PravinD.Patil
DepartmentofBiotechnology,PriyadarshiniInstituteofEngineeringandTechnology,Nagpur, Maharashtra,India
VirendraK.Rathod
DeeptiRathore
AlankarP.G.GirlsCollege,Jaipur,India
LakhinathSaikia
DepartmentofChemistry,RajivGandhiUniversity,ArunachalPradesh,India
FatihSen
SenResearchGroup,DepartmentofBiochemistry,DumlupınarUniversity,Kutahya,Turkey
MuhammadSyafiqShahari
SchoolofPharmacy,MonashUniversityMalaysia,BandarSunway,Selangor,Malaysia
RashmiSharma
SyntheticOrganicChemistryLab,FacultyofSciences,ShriMataVaishnoDeviUniversity,Katra, Jammu&Kashmir,India
RuchiSharma
DepartmentofChemistry,CentreofAdvancedStudies,UniversityofRajasthan,Jaipur,India
SergeiA.Smolnikov
SchoolofPharmacy,MonashUniversityMalaysia,BandarSunway,Selangor,Malaysia
ManishkumarS.Tiwari
DepartmentofChemicalEngineering,NMIMSMukeshPatelSchoolofTechnology Management&Engineering,Mumbai,Maharashtra,India
PhuongHoangTran
DepartmentofOrganicChemistry,UniversityofScience,VietnamNationalUniversity,HoChi MinhCity,Vietnam
Ultrasound-assistedorganicsynthesis
NehaN.Gharat,VirendraK.Rathod DepartmentofChemicalEngineering,InstituteofChemicalTechnology,Mumbai,India
1.Introduction
Traditionalmethodstocarryoutorganicsynthesisreactionsfacedrawbackssuchaslong reactiontime,non-satisfactoryyields,moresolvent,toxic/costlyreagentrequirements andhightemperaturesandontheotherhand,resultsinuneconomicalproducts.Use ofheterogeneoussystemsgivesrisetomasstransferresistanceissuesdependingonthe numberandtypeofphasespresent.Itmayalsoleadtoagglomerationofparticleswhich lowerssurfaceareaandeventuallyslowsdownthereactionrate.Toovercomeallthese issues,theuseofultrasound(US)isacost-effectivemethodtointensifyvariousreactions suchasaqueousandnonaqueoushomogeneousreactions,heterogeneousreactions, phase-transferreactions,metal-organicframeworks,bio-enzymatic,amongothers.
Applicationofsoundwavesalongwithitschemicaleffectsistermedassonochemistry. TheapplicationofultrasoundwaveswasfirsttriedintheearlyninetiesbyRichardsand Loomis.ThiswasfollowedbySchultesandFrenzelwhostudiedtheaqueoushydrogen peroxideformationat540kHz.Furthermore,in1936,SchultesandGohrobservedthat lightcouldbeproducedbythehigh-intensitysonochemicalreactionofliquidsinthe rangeof190–750nmwavelength.Thisphenomenonwastermedassonoluminescence. Thislongjourneyofsonochemistryhasbeenremarkableafterthe1980s,wherecavitationphenomenawasconsidered.
Ultrasoundhasbeenusedasaprocessintensificationtool,between20kHzand 5MHzfrequencyrange,fortheremovalofbiologicallyactivecompoundsnanoscale operationsandformationofmedicines.Chemicalreactivityincreasesbyultrasound throughtheformationandcollapseofcavitationbubblesinaliquidmedium.Thepropagationofultrasoundwavestakesplaceinaliquidmediuminalternatecompressionas wellasrarefactionandcavitiesareformed.Oncetheattractiveforcesoftheliquidovercomebyrarefactioncycle,thecavitiesgrowtoamaximumsizeandthenburstresulting inenergydissipation(Fig.1) [1–3].Duetoturbulence,correspondingwiththeliquid circulationassociatedwiththecreationandbreakdownofthebubbles,masstransferrates areimproved.Whetherultrasoundcanbeappliedtoacceleratethereactionchemicallyas wellasphysicallydependsonthelocalhotspotsandbyenhancingthemasstransferrates, respectively.Inadditiontoimprovementinmasstransferrates,italsogivesbettercatalyst
Fig.1 Formationofbubble,development,andcollapse. (ReproducedwithpermissionfromP. Chowdhury,T.Viraraghavan,Sonochemicaldegradationofchlorinatedorganiccompounds,phenolic compoundsandorganicdyes areview,Sci.TotalEnviron.407(8)(2009)2474–2492.)
effectiveness.Typically,theeffectsofcavitationinaqueousmediumincorporateelevated temperatures(2000–5000K),pressuresupto1800atmosphere.Ultrasoundisbeneficial toacceleratechemicalreactionsbyimprovingyields,loweringreactiontimesaswellas increasingselectivity.Duetoallthesereasons,ithasbeenpopularizedasanovelapproach fortheproductionoforganiccompoundssincethepastcoupleofdecades [4].Even though,theuseofultrasoundisbeneficialforlaboratory-scaleoperations;foritscommercialimplementationfororganicsynthesis,therearesomeengineeringconcernssuchas missingscale-upprocedures,efficientdesigns [5].
2.Extrinsicvariablesaffectingultrasoundirradiation
Theintensityofcavitationproducedbyultrasoundisdependentonvariousparameters. Theseparametersnotonlyaddinthecostoftheprocessbutalsodecidethescale-upof
thereactor.Thus,itisessentialtooptimizetheseparametersfortheparticularsystemto getmaximumyieldatminimumcost.WhilecarryingoutUS-assistedreactions,itis advisabletostudythefollowingparametersforthedevelopmentofnewanalytical applications.
2.1Influenceofsolvent
Thechangeinsolventalsochangesthephysicochemicalproperties,i.e.,density,vapor pressure,surfacetension,andviscositywhichaffectscavitationintensity,butchemical reactivityofthesolventhasanintenseeffectonultrasonication.Thesecondaryreactions ofsonolysisofwatervaporbetweenOH• andH• explainaqueousultrasonication.Atelevatedtemperature,solventdoesnotshowinertbehavior.Itcanbeovercomebyapplicationoflowvaporpressuresolvent(excepthalocarbons)whichreducestheir concentrationinthevaporphaseofultrasonication.Inordertolowertheviscosityof themedium,thesolventplaysanimportantrolebymakingituniformandmiscible [6].Therefore,theselectionofsolventhasgreatimportanceinultrasoundirradiation.
Thepolarityofthesolvent,alongwithdenaturationconditionsaretheparameters thatneedtobeconsideredwhileselectingthesolventforUS-assistedenzymaticreactions.Inacomparisonofoilwithmethanolasareactant/solventforthesynthesisofbiodiesel,thebubblegetscollapsedatahigherrateinmethanolthanthatofoil.Asthe viscosityofmethanolislowerthanoil,therateofbubblecollapseinmoreinmethanol. Therefore,methanolispreferredasasolventincaseofbiodieselformationduetothe advantagesofenhancedinterfacialareaandrateofreaction [7]
2.2Influenceofpower
Chemicaleffectsonareactioncanbecausedbysupplyingsufficientacousticenergyso thatthecavitationthresholdofthemediumisovercome.“Cavitationalzone”isthe regionwherecavitationoccursaroundtheradiatingsource.Thisenhanceswithincreasingintensityofdissipation.Cavitationalintensitycanbeincreasedbyincreasingultrasonicpower.Also,withanincreaseinpower,enhancementintherateofreactionis observedandduetocontinuoususeofpowerforalongtime,rateofreactiondecreases [8] whichresultsinthegenerationofthecloudofcavitationbubblearoundtheprobe. Collapsingofthosecavitationbubblestakesplacenearhighpowerdissipation [9].With anincreaseinexposuretime,bubbleformation,aswellasimplosion,increases.Also,due toprolongeduseofultrasound,denaturingofcatalystalongwiththetransducersoccurs [10].Thereactorconfigurationanditsspecificapplicationaretheparametersresponsible foroptimumpowerdissipation [11].Acousticenergypresentinreactionmixturegets decreasedduetodecouplinglosses [12]
Overallliteraturehypothesizesthatfrequency,dutycycle,catalystloading,andmolar ratioaretheimportantparameters.Bykeepingthemconstant,theinfluenceofultrasonic
powerinorganicsynthesishasbeenoptimized.Effectofpoweronthereleaseofiodine bysonolysisofaqueousKIwasreported [13].Also,theinitialpowerdissipationwasproportionaltoiodinerelease,till40Wandremainedconstanttill100Wwhichdropped significantlyafter100W.
Enzymecatalyzedreactions,preferloweroptimumpowerdissipationbecauseenzymesshowthenegativeeffectathigherpowerdissipation.Severalreportssuchas [14] lipasecatalyzedesterificationreactionofmethylcaffeate(MC)withmethanolalongwith caffeicacidusingNovozym435undertheinfluenceofultrasoundirradiationhavebeen reported.Theimpactofpoweronreactionhasbeenstudiedbytheincreaseinpower underpulsemodeat25kHzfrequencyandtemperatureof5°Cwhichresultedinenhancedyield(99%conversion)at150-Wpowerdissipationunderthetimeintervalof9h.
Inthecaseofheterogeneousreactions,asimilartrendwasrepeated,whichshowed 93%yield(highest)at75Wasoptimumpowerdissipation.Beyond80W,therewasno furtherimprovementobserved.Theinfluenceofultrasonicpowerontheformationof methylbutyratecatalyzedbyamberlyst-15overtherangeof50–145Winputpowerat 22kHzfrequencywasstudied [15],wheretheenhancementinconversion,till100W wasobservedandbeyondthatconversion,wasfoundtodecrease.
Alltheabove-reportedtrendsshowthatwithanincreaseinpowerdissipationuptoa certainoptimumvalue,conversionoftheUS-assistedorganicsynthesisreactionsreaches themaximumvalue.Beyondcertainoptimumpowerdissipation,conversiongets reduced,whichdependsonthespecificclassofreactioncarriedout.Therefore,thereactorconfiguration,i.e.,numberandpositionofthetransducersandthetypeofreactionare thefactorsonwhichoptimumpowerdissipationwouldbedependent.Laboratory-scale investigationsinreactorswhicharealmostsameasthedesignofthecommercial-scale unitcangiverisetotheactualvalueofpowerdissipation.
2.3Influenceoffrequency
Ultrasonochemicalwavesproducefrequenciesintherangeof16–100kHzwhich enhancethecavitationeffect.Frequencyactsastheoriginofthedramaticimpactof powerdissipationonchemicalreactivity.Inthecaseofradicalformationmechanism, higherfrequenciesarepreferredastheyfacilitateandaccelerateagivenreaction.
Thebubbleformationisproceededbypassingultrasonicwavesviaamediumwhich beginswithgrowthanddisruptionofthebubble [16].Theclassificationofultrasonicfrequencieshasbeendonebasedonitslarge-scaleoperationinthreegroupssuchaspower ultrasonication(16–100kHz),high-frequencyultrasonication(100kHz–1MHz),and diagnosticultrasonication(1–10MHz) [17].Whennegativepressureoftherarefaction exceedstheattractiveforcesbetweenthemoleculesoftheliquid,voidsgetformed. Asthefrequencyincreases,cavitationgoesondecreasing,duetolesspressureproduced
byrarefactioncyclewhichisrequiredtoattaincavitationandsometimestherateofthe compressionexceedsthedurationwhichisrequiredforthecollapsingofbubbles [18]. Thereforethemainphenomenaofacousticstreamingarisewherecavitationproduced byultrasonicwaveshavingfrequency16–100kHzresultsintheformationoflargercavitationbubbleswithelevatedtemperatureandpressureconditions [19].Inthecaseof homogeneousliquids,frequencyplaysanimportantroletoattainoptimumcavitation becausehomogeneousultrasonicationiscarriedoutbeyondoptimum(offset)value. Toovercomethiscounteractingbehavior,thereisaneedtospecifyanoptimumrange offrequencies.
Inthesonochemicaldegradationofcarbontetrachloride,theauthorshaveclaimed thatat500kHz,thehigherdegradationrateisobservedascomparedto20kHz.Similarly, decompositionofchlorobenzeneandremovalof2-chlorophenolwasreported [20,21] whichdemonstratesthatforradicals,higherfrequencyispreferableasdominantchemical effectsoccurthatparticularlyintensifytheoxidationreactionswhereamajorcontrolling factoristhehydroxylradicals.Forheterogeneousreactions,theoppositetrendshave beenobservedintheliterature.
Also,thesynthesisof4-methylcinnamoylglycerolusingenzymeoverthefrequency of20–40kHzin48hhasbeenreported.Asfrequencyincreasesfrom20to35kHz,conversionincreasesandbeyond35kHz,itdecreasesbecauseatthisfrequencydeactivation oftheenzymeoccurs,duetophysicaleffectsandhighshearforces [22]
Overviewingtheseliteraturetrends,itcanbesaidthatforenzymaticreactionsorheterogeneousreactions,frequenciesintherangeof20–50kHzarepreferred.Physical effectsarerequiredinordertoeliminatemasstransferresistancesandtoretainthecatalyst activity.Similarly,inthecaseofhomogeneousreactionsorheterogeneousreactions, whichinvolveradicalmechanisms,higherfrequencies(200–500kHz)resultinenhanced reactionrates [23].
2.4Influenceofdutycycle
Theexistentexposuretimeofultrasonicationinonecycleisknownasthedutycycle. TheON/OFFtimeofultrasonicationcanbeadjustedtochangethedutycycle [24].FormationofthebubbletakesplaceinONtimewhereasitsenlargementiscarriedoutin restingtime(OFFtime).InUSsonochemistry,theconversionofaproductincreasesas thedutycycleincreases.Atthesametime,itmightaffectthetransducers,whichleadto wearandteareffects.Toavoidthiseffect,anothertypeoftransducerssuchaspulsemode isreported [25,26].Inordertoenhancethetransducerslife,thereactionshouldcarryout atminimumdutycycle.
Also,keepingtheultrasound“on”foralongtimecandamagethetransducers,thus usingrecentinstrumentswithbetterqualitytransducersshouldbepreferred [27,28]
Similarly,reportshavebeenvalidated [25] thatwithanincreaseintheultrasonicduty cycle,enzymeactivitygetsreducedasaresultofthephysicaleffectsofshearandturbulence.Thesynthesisofisoamylbutyratehasbeenstudied [29],where83%asanoptimum dutycyclehasbeenreported.Thesameresultofanoptimumdutycycleinheterogeneouslycatalyzedformationofmethylbutyratehasbeenreported [15] andinvestigated thatwithanincreaseinthedutycycleovertherangeof25%–75%,conversionincreases from72%to91.64%andasobserved,beyond75%dutycycle,conversiongetsreduced. Typically,itcanbeestablishedthatdependingontheconfigurationofthereactoralong withthetypeofreactionsysteminthepresenceofacatalyst,theoptimumdutycycle wouldbeintensifiedtoobtainbenefitsofultrasoundoperations.
2.5Influenceoftemperature
Thoughtemperatureandthechoiceofsolventareinterrelatedvariables,bothareessential.Withariseinsolventvaporpressure,themaximumbubblecollapsetemperatureand pressuredecreases.Therefore,inareactionwheresonochemicalactivationtakesplace throughcavitationalcollapseandalow-boilingsolventispresent,lowtemperatureis beneficial.Conversely,highboilingsolventsarepreferredwherereactionrequireselevatedtemperatures.Inthecaseofheterogeneousreactions,anexternalsurfaceofacatalystoraninorganicsolidreagentgetsaffectedbysonicationwhereabalanceshouldbe maintainedsothatpropercavitationtakesplaceaswellasthethermodynamicsofthe reactiondoesnotgetaffected.Also,theenergyrequiredtoinitiatecavitationisenhanced byapplyingexternalpressuretoareactionsystem,whichultimatelyenhancesthehydrostaticpressureoftheliquid.Thehighesttemperaturesandpressurescanbefacedatthe timeofbubblecollapsewhenthresholdenergyexceedstheavailableirradiationsource andcorrespondinghydrostaticpressureincreasesthesonochemicaleffect.
InUS-assistedreactions,theeffectofbulkliquidtemperatureisalsodependentonthe temperatureofthecounteractingreagent [9].Withariseinthereactiontemperature, chemicalkineticsgetenhancedasinthecaseofconventionalreactionsystems,but thecavitationalintensitydecreases.Aninverseeffectoftemperatureoncavitationintensitytakesplaceduetoformationofvaporouscavities,whichreducesbubblecollapse [30].
Novozyme435-assistedhydrolysisofsoybeanwiththehelpofmethanol(1:6)using 40kHzfrequencyhasbeenreported [31].Thereactionwascarriedoutovertherangeof 30–70°C,andthehighestactivitywasobservedat40°Candreducedbeyond40°C, whichmightbeduetodecreaseinviscosityathighertemperatureswhichimproves emulsification.Infinitesimalincreaseintemperaturecausesthebettercollisionsof enzymealongwiththesubstrateswhichresultinenzyme-substratecomplexwith increasedenzymeactivityat40°C [32].
Fromtheaboveexamples,itcanbeeffectivelyconcludedthatthemaximumrateof reactioncanbeobtainedatanoptimumtemperature.InUS-assistedorganic
homogeneousorheterogeneouschemicalsynthesis,duetoreducedcavitationalactivity, theconversiongetsreducedaswellasinUS-assistedenzymaticroute,alowertemperatureispreferredasanoptimumtemperature.Beyondthisvalue,thereducedrateof reactionisseenduetodenaturationofenzymesathighertemperatures.
3.Originsofthechemicaleffectsinsonochemistry
Theearlierassumptionaboutultrasoundwasthatitcanbecommonlyusedtoassistthe reactionsassociatedwithsolidreagentsonly;thisisnotparticularlycorrect.Alargenumberofgroupsareseekingtoachieveknowledgeofsonochemistry,whichcanhelpthem toanticipatethetypeofreactionaffectedbysonication.Luchehasclassifiedsonochemical reactionsintothreecategoriesbaseduponthecavitationalchemicaleffects.Otherthan chemicaleffects,therearesomemechanicaleffectsrelatedtocavitationbubblecollapse whicharetreatedasphysicaleffectsandevaluatedtobenegativesonochemistry.Those negativeeffectsarealsosignificant,whichhavebeenusedintheanalysisandincludedin thebelowthreereactioncategoriesaffectingsonochemicalenrichment.
1. Homogeneousreactionsinvolvingradicalmechanismarealteredbyultrasoundirradiation.However,sonicationdoesnotinfluenceionicmechanisms.Inthehomogeneousliquidsystem,thecollapsingofthecavitationbubbleleadstoimmediateinflow ofliquidtocoverthevoidspaceformedthroughthecollapsingcavity.Therupturing ofbondsincompoundstakesplaceduetoshearforcesproducedbythisinflowinthe surroundingbulkliquid.Thesimilareffecthasbeenobservedinthereactionofpolymericmaterialdecomposedinthefluid [33] (Fig.2).
In the cavity extreme conditions on collapse 5000°C and 2000 atmospheres
In the bulk media intense shear forces
Fig.2 Cavitationinhomogeneousphase. (ReproducedwithpermissionfromS.V.Sancheti,P.R.Gogate, Areviewofengineeringaspectsofintensificationofchemicalsynthesisusingultrasound,Ultrason. Sonochem.36(2017)527–543.)
2. Heterogeneousreactionprogressesviaionicintermediates.Theyareaffectedbya decreaseintheparticlesizeaswellasenhancementinmasstransferrate,whichare thephysicalimpactsofcavitation.Insuchreactions,theselectionofoperatingparametersiscrucialduetolessimportanceofthechemicaleffectsofsonication.
Inphase-transferheterogeneousreactionsduetothecollapseofacavitationalbubble,layerdisruptionalongwiththerapidblendingproducesultrafinemixtures(Fig. 3).Formaintainingstability,surfactantsareemployedinmakingtheemulsion. Enhancementinthesurfaceareaoccursasaresultofthegenerationoffineemulsions, givingthedesiredintensification.Duetocavitationaleffects,phase-transferheterogeneousreactionsrequirelesscatalyst.
Fig.3 Cavitationindual-phasicliquidsystem. (ReproducedwithpermissionfromS.V.Sancheti,P.R. Gogate,Areviewofengineeringaspectsofintensificationofchemicalsynthesisusingultrasound, Ultrason.Sonochem.36(2017)527–543.)
3. Thereactionsthatinvolvearadicalmechanismorradical,aswellasionicmechanism, getinfluencedbysonication.Radicalreactionsareaffectedbythechemicaleffectsof ultrasoundirradiation,whereasionicreactionsareinfluencedbyenhancedmasstransferrates.
Forheterogeneoussystemsinthepresenceofsolids,thebubblecollapsemaybe symmetricorasymmetric.Thesizeandtypeofthematerialinshockwavesraisethe motionofsolidparticlesinsolution.Thebreakdownofthebubbleduetosucha motionofsolidparticleresultsintheavailabilityofincreasedareaforreactionand activationofthecatalystcanalsobedonebyphysicaleffects.Thatresultsinthecleaningofthesurfacesandalsoenhancestransportratesthatdependontheinterruptionof theboundarylayers(Fig.4).
Powerful disruption of phase boundary
Inrush of liquid from one side of collapsing bubble forces powerful jet onto solid surface
Fig.4 Cavitationinheterogeneoussystems. (ReproducedwithpermissionfromS.V.Sancheti,P.R. Gogate,Areviewofengineeringaspectsofintensificationofchemicalsynthesisusingultrasound, Ultrason.Sonochem.36(2017)527–543.)
4.Reactordesignandconfiguration
Therearemanydevicesavailabletocarryoutultrasonicirradiation.Theyareknownas sonochemicalreactors.Theclassificationofsonochemicalreactorsdependsonthenature ofirradiationlikedirectandindirect.Directmodereferstothedirectcontactofultrasonictransducerwiththereactionmedium.Inindirectmode,itreferstoeitheracontact betweenseparatereactorhavingreactants,whichissuspendedintoultrasonicvesselfilled withacouplingliquidorsonicationvesselitselfcontainingliquidmixture.Sonochemical reactorsaregenerallydividedintothreedesigns.
1. Ultrasoniccleaningbath.
2. “Cup-horn”sonicator.
3. Directimmersionultrasonichorn.
Inallcases,theoriginalsourceoftheultrasoundisapiezoelectricmaterial,usuallyalead zirconatetitanateceramic(PZT),whichissubjectedtoahighACvoltagewithanultrasonicfrequency(typically15–50kHz).Thepiezoelectricsourceexpandsandcontractsin thiselectricfieldandisattachedtothewallofacleaningbathortoanamplifyinghorn.
4.1Ultrasoniccleaningbath
Fortheindirectirradiation,asonicationbathisaveryfrequentlyusedsonochemicalreactor.Itisaninexpensiveinstrumentinwhichtheultrasoundisappliedwiththehelpof transducers(Fig.5).ItisusedprimarilyinlaboratoryultrasoundoperationsofUS-assisted heterogeneoussystems.Averylessamountofenergyissuppliedtotheactualreaction mixturewithinthemediumascomparedtoultrasonichorn(whichareusedindirect irradiationmode).Dependingonthesizeofthevesselandthenumberoftransducers, distributionofthecavitationalactivitytakesplaceinthecaseofultrasoundbath.
Solid surface Diffusion layer
Fig.5 Ultrasoniccleaningbath. (ReproducedwithpermissionfromE.M.Hussein,K.S.Khairou, Sonochemistry:synthesisofbioactiveheterocycles,Synth.Commun.44(15)(2014)2155–2191.)
Alongwiththebenefitsofultrasoundbath,thereareseveralpotentialdrawbackslike variableacousticintensity.Itvariesfrombathtobathandonemanufacturertoanother. Therefore,theissueofreplicabilityfromonebathtoanothermayarise.Also,theplacementofthereactionflask,temperature,theheightofboththebathliquidandofthesolutionwithinthereactionvesselarecriticalparameters [34,35].Coolantsareusedfor thermostating,astheyarepassedthroughcoppercoilswithoutcontactingwalls.In thecaseofhomogeneousliquids,marginalacousticintensitiesarepresentforthegenerationofcavitation.Also,incaseofsolids,thetensilestrengthoftheliquidattheinterface causesthecavitationwellbelowthresholdsascomparedtosimplesolutions.However, forheterogeneousultrasoundirradiation,thesonicationbathcanbeconsideredwithlimitedpotential.
4.2 “Cup-horn ” sonicator
Togethighcavitationalintensityatlowoperatingvolumes,ultrasonichornsaremost commonlyusedforlaboratory-scaleoperations.Bykeepingthefrequencyofirradiation constant,thepowerdissipatedwithinthereactionmixturecanbevariedwithanintroductionoftheacousticenergydirectlyintotheliquid.Thediameteroftheprobeandthe heightoftheliquidinthereactorarethecontrollingfactorsintermsofcavitationalactivityandapplicationpointofview.Thereisacertainlimitationinthecaseofthehorn
systemwherecontaminationshouldbestrictlyprohibited.Duetoextensiveuseofhigher levelsofpowerdissipation,erosionofmetaltakesplaceintoliquidalongwiththepitting ofthetip [36].Also,theshapeofthehorncontrolsthecavitationalactivityalongwiththe transferofenergyconsideringtheminimumeffectonthesurfaceerosion.Cuphornsarea knownmodificationwhereasmallsonicationbathisdrivenbyahornextendingthrough itsbase(Fig.6).Initially,itwasusedincelldisruption,butnowadaysithasfoundapplicationinsonochemistryinindirectirradiation,butmorepowerdissipationoccursin comparisonwiththetypicalsonicationbath.Anultrasonichornismuchbetterwith highercapabilityforprocessedvolume.Inthecaseofultrasonicprobeinstandardpiezoelectrictransducers,itismadeupofTitaniumcrucible.Incomparisonwithultrasonic cleaningbath,itisadvantageousintermsofacousticintensities,frequencycontrol,aswell asthermostatingcapacity.Asthereisnocontactbetweenradiatingsurfaceandreaction solution,theresultingintensitiesaresmallerthanthatofdirectimmersionultrasonic horn.Therefore,homogeneousultrasoundirradiationisdisadvantageousintermsof activeness.Butitisalsoadvantageousasitisfreefromcontaminationagainsttheerosion ofthetitaniumhorn.

Fig.6 UltrasonicprobeCuphorn. (ReproducedwithpermissionfromP.Chowdhury,T.Viraraghavan, Sonochemicaldegradationofchlorinatedorganiccompounds,phenoliccompoundsandorganic dyes areview,Sci.TotalEnviron.407(8)(2009)2474–2492.)
4.3Directimmersionultrasonichorn
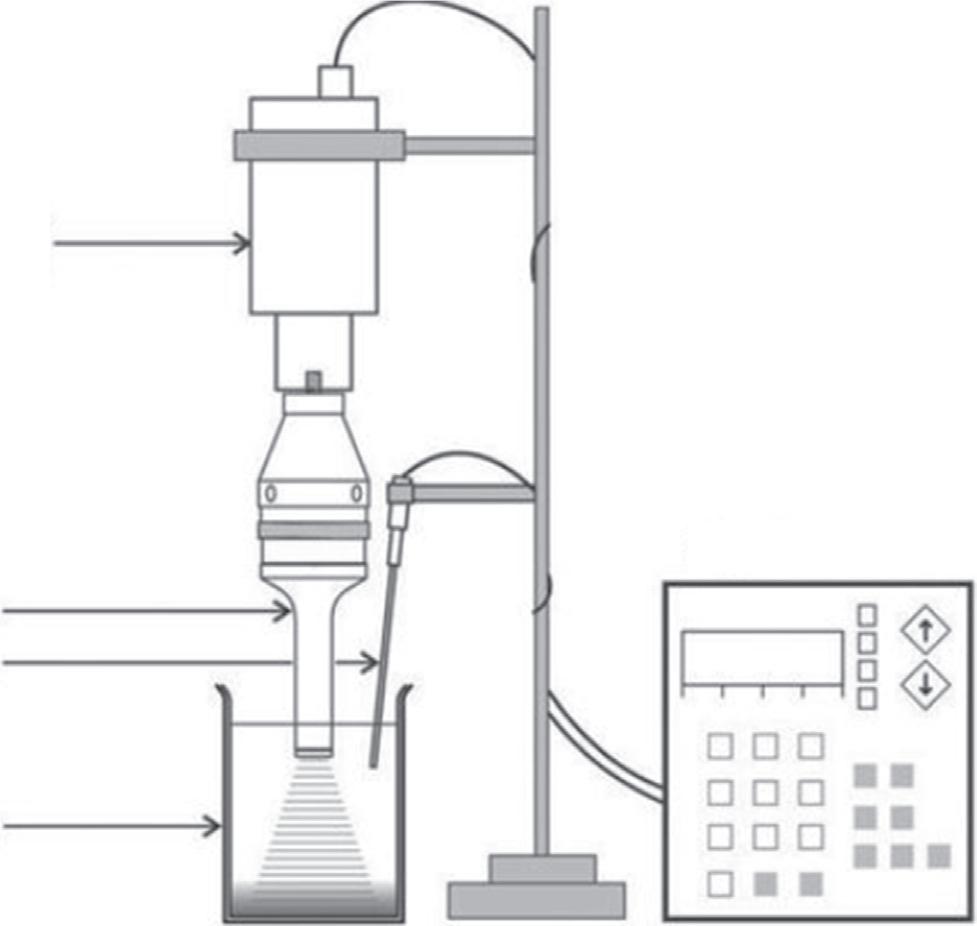
Itisaverypowerfulultrasonicreactorspeciallyusedinachemicallaboratoryinaninert atmosphere(Fig.7).Likeacuphorn,directimmersionultrasonichornisalsodesignedby biochemistsforcelldisruption.Itisreadilyavailableatareasonablecost.Evenvariable shapesoftitaniumhornsandsizesofpowersupplyareprovided.
Forlarge-scaleoperations,theuseofdirectimmersionultrasonichornprovidesthe flexibilityofoperatingatmulti-litercapacity.Thishorngiveshighandvariableintensities atafrequencyof20kHz.Duetomorepowerdissipation,thenecessityoftemperature controllertocooldownthereactionariseswhichdependsonthetypeofconfiguration. Also,itshowsthedrawbackoferosionofthetitaniumtipwithoutchemicaleffects, whichshowsenhancedtensilestrengthwithlessreactivityofTimetal.Thecommercial applicationofthesehornsinvolvescelldisruptiononlargescale,degassingofliquids,dispersionofsolidsintoliquids,andemulsificationofimmiscibleliquids.Thedirectimmersionultrasonichornismostwidelyfoundinindustrialultrasonicreactionsthanatthe laboratoryscale.
Directimmersionultrasonichorns. (ReproducedwithpermissionfromE.M.Hussein,K.S.Khairou, Sonochemistry:synthesisofbioactiveheterocycles,Synth.Commun.44(15)(2014)2155–2191.)
Digital disruptor (piezoelectric ceramic)
Titanium horn
Temperature probe
Heavy duty beaker
Digital control unit
Fig.7
5.ClassificationofUS-assistedorganicsynthesis
Applicationofultrasoundforintensifyingdifferentprocessesresultsinvariousorganic synthesisreactions.Theuseofhighlyintensivesonochemistryinaqueousornonaqueous mediumgivesbenefitsofbenignreactionconditions,lesstimewithenhancedyield, whichmostlydependsonphysicaleffectsthattakeplaceinultrasonication.Here,we areclassifyingreactionsintohomogeneousandheterogeneoussystems.
5.1Homogeneous
Sonochemicaleffectsinhomogeneousliquidsystems,usuallyoccuratdifferentsites. Thosesitesaregivenbelow.
1. extremeconditionsinsidethecollapsingbubble;
2. attheinterfaceinbetweenthecavityandpresentbulkliquid;and
3. inthebulkliquidwheremechanicaleffectsprevail.
Further,wewillproceedwiththeclassificationofUS-assistedhomogeneousorganicsynthesisintoaqueousandnonaqueoushomogeneoussystems.Theydepictthedifferent typeofcavitationeventineachcase.
5.1.1Aqueous
Aqueousirradiationoforganiccompoundsfrequentlygivesrisetoextremelyoxidizedor degradedproductsinmultipleamounts.Inthecaseofaqueousmedia,thereisanoverall deficiencyofspecificityforultrasonicirradiationbecausewhilecarryingoutsonolysisof water,wetendtogetintenselyreactiveintermediatesatrespectablerates.
In1982,aqueoussonochemistrywasreported,inwhichstereoisomerizationofmaleic acidtosynthesizefumaricacidbyultrasoundirradiationusingBr2 wascarriedout.Inthis reaction,thefrequencyusedoveraverywiderangeandBr0 isanefficientcatalystforthe isomerizationobtainedfromsonolysis,whichmakesthisreactionuniqueinsonocatalysis, asgivenbelow.
Therearemanystudies,whichreportedtheeffectsofultrasoundthroughvarioussolvolysisreactions.Oneofthemappliedinbothultrasoundirradiationandcatalyticwet peroxideoxidationforabreakdownof4-hydroxybenzoicacidwherethedetermination ofenhancedrateforthehydrolysisofacetatesandsaponificationoffatshasbeen studied [37]
In2013,thesynthesisofNovelHomoallylicAlcoholDerivativesusingwaterasa mediumwasestablishedbycomparingbothconventionalandUS-Assistedapproach [38].Thereactionwascarriedoutinaqueousmedia,withdifferentcarbonylcompounds
Br'2 2Br' +
withallylicbromidesthroughUS-assistedBarbierreaction.The α-bromoketones reactedwithsodiumbenzenesulfinateinthepresenceofCuI/2,6-lutidine.Simply,sulfonationof α-bromoketoneswascarriedoutwhichproduced β-keto-sulfoneswithsatisfactoryyields.Therefore,thisaqueousmediasulfonationreactionwasbeneficialin termsofexcellentyields,lesserreactiontime,andmoresimpleincomparisonwith thetraditionalone(Scheme1).
1b:R=PhSO2 4:R=F
5a:R=PhSO2 b:R=F 6a-e 5and6R aPhSO2 bF cBr dCH3 eH
Scheme1 Sulfonationof α-bromoketonesinpresenceofultrasonication.
In2017,theformationofnaphthoquinonecombinedoxazinederivativesunderan aqueousmediumbyusingsonicationhasbeeninvestigated [39].Developmentofenergy efficient,aswellastheenvironmentallysafeprocedure,hasbeencarriedoutthroughthe reactionof2-hydroxy-1,4-naphthoquinone,aromaticamineandformaldehydeinasingleapproachusingwaterasanaqueousmediaunderultrasoundirradiation(Scheme2).
Scheme2 Formationofnaphthoquinonecombinedoxazinederivativesusingultrasonicirradiation.
Laterin2017,synthesisofBis(Indolyl)Methanesand3,3-Bis(Indolyl)oxindolesin thecatalyst-freemediumusingAqueousEthylLactatehasbeeninvestigated [40].Inthe reportedapproach,indolesreactedwithaldehydesorisatinsinwaterandethyllactate undertheinfluenceofultrasonication.Inadditiontobenefitsofaqueousmediaalong withtheultrasonication,thisapproachisfreefromthecatalyst,havingabroadsubstrate scopeandapplicableforindustrialsynthesis(Schemes3–5).
Scheme3 Ultrasound-assistedformationofBis(Indolyl)Methanes.
Scheme4 FormationofBis(Indolyl)Methanesinwaterandethyllactateusingultrasonication.
Scheme5 Formationof3,3-Bis(Indolyl)oxindolesinwaterandethyllactateusingultrasonic irradiation.
Also,in2017,thelatestachievementsintheultrasonicallyirradiatedorganicsynthesis usingaqueousmediumhavebeenreported [41].Thereareseveralreportsavailableonthe applicationofanaqueousmediainUS-assistedorganicreactions.Basedontheknowledgeofgreenchemistry,waterisconsideredasthesafestsolventascomparedtoothers. Therefore,itisasustainableapproachthatholdsanactiveassociationbetweenhighly intensiveultrasonicationandaqueousmedium.Also,theapplicationofultrasoundirradiationalongwiththewaterleadstoaneco-friendlyprotocol.
Recentlyin2019,theformationofcrucialheterocyclessuchasQuinoxaline, 1,4-oxazine,1,4-thiazine,and1,4-dioxinderivativesusingcatalyst-freemediuminwater undertheinfluenceofultrasonicationhasbeenstudied [42].Therewerevariousmethods reportedforthesynthesisofthesame.However,thesemethodshavecertaindrawbacks. Therefore,forthesynthesisoftheseheterocycliccompounds,easyandenergy-efficient methodsarerequired(Scheme6).
Scheme6 Thereactionofninhydrinando-phenylenediamineinpresenceofultrasonication.
Thereforetheapplicationofwaterasasolventthanothervolatileorganicsolventsis appreciableingreenchemistry [43–46].However,aqueousmediumgivesincreased reactionrateasaresultofstronghydrogenbonding.Also,theultrasoundusingwater offersbenefitsofnontoxicity,recyclabilitywiththepurificationofproducts [47–52]
Nowadays,catalyst-freeaqueousmediumhasgainedimportanceinlaboratoryscaleas wellasindustrial-scaleduetopropertieslikerapid,energy-efficient,cheap,improved selectivity,environmentallybenign,andeasyisolationofproduct [53,54].
5.1.2Nonaqueous
Homogeneousnonaqueoussonicationwasnotreportedfrequentlyuntilthepastfewyears. In1937,PorterstudiedthesevenfoldaccelerationduetoCurtiusrearrangementof C6H5CON3 toC6H5NCOandN0 2 [32].Laterin1965,WeisslerhasreportedsomesolventswhicharetooslowindegradationlikeCH3CNandCC14 [34].In1967,theinitiationofexplosionsoftetranitromethaneandnitroglycerinewasinvestigated.Also,in1974, thedepolymerizationofhighmolecularweightpolymerswasobserved [55].Principally, aqueoussolutionswithvolatileorganicsolventshavenotbeenreportedforsonochemistry.
Thisledtoageneralbeliefthatwatercanonlyactasahightensilestrengthliquidtosupport intensecavitationalcollapse.Duetohighvaporpressures,alargegroupoforganicliquids reducesthestrengthofcavitationalcollapse.Butithasnowbeenestablishedthatcavitation willbeaccomplishedbyorganicliquidsviafreeradicalsatlowtemperaturethroughbond homolysis.In1983,thesonolysisofalkaneshasbeenstudied,whichisthesameprocessas extremelyhigh-temperaturepyrolysis.Also,in1979,thesonolysisandpyrolysisofbiacetyl, whichproducesacetone,hasbeenreportedandcomparedwiththesonolysisandradiolysis ofmenthonein1980.Thechemistryinvolvedinnonaqueoussonochemistrycanbe tedious,asseeninthetarrypolymerizationofvarioussubstitutedbenzenesreportedbyseveralauthors.Theresultsobtainedfromtheanalysisofaqueoussonochemicalreactionsare challengingtounderstandbecauseofthecomplicationofthesecondaryreactions.Therefore,inthecaseofnonaqueousliquids,theratesofdegradationcanbeadjustedtoslow downquitebelowthanwaterbyproperselectionofsolventaswellasbymaintaining lowvolatility.Highlystableliquidscanbeobtainedatlowertemperatureslikedecane at 10°C.Itisadvantageousbecauseinsteadofthesecondaryreactionsoccurringwithsolventfragments;theprimaryultrasoundirradiationofdecomposedsubstratescanbeinvestigated.Infutureaspectsofsonochemicalstudies,wecanlookforwardtoincreasetheuseof nonaqueoussonochemistrywiththeapplicationoflow-volatilityorganicliquids.
5.2Heterogeneous
Thehasteningofheterogeneousreactionusingultrasoundisanemergingtechnique.Itis accompaniedbyphysicaleffectssuchasformationofemulsionsatliquid-liquidinterfaces, cavitationalerosionandfinally,purificationtakesplaceatliquid-solidinterfaces.Asthe formationofshockwavedamagesaswellasdeformssolidsurfaces,thesurfaceareaof friablesolidsgetsincreased.
TheheterogeneoussonochemicalreactionisaveryknowntypeofUS-assistedreaction,inorganicororganometallicchemistry.Further,US-assistedreactionscanbe appliedtotheleachingprocesstointensifytheireffectonleaching.Heterogeneous US-assistedreactionsarefurtherdividedintothreecategories,whicharestatedbelow.
5.2.1Metal-organicframeworks
Materialscientistshavenewlydevelopedaporousmaterialknownasmetal-organicframeworks(MOFs).InMOF’s,metalionstogetherwithorganicmultidentateligands,are boundedthroughcoordinatebonds.Theseorganic-inorganichybridcompoundsarecrystallineinnature.Theyprovidehighsurfaceareaswithbetterfunctionality,poresize,selectivityrangeofshape/size,andimprovementinactivityascomparedtobasemetaloxides. However,theyhaveapplicationasahostinavarietyofguestmolecules [56–58],further usedinadsorption,catalysis,magnetism,sensing,anddrugdelivery [59–61].Natureofthe metal-organicframeworkdecidesthephysicalparametersofMOFslikemagneticsusceptibility,conductivity,andopticalcharacteristics [62].MOF’sactasabridgebetweenzeolites(havingsmallporesize)andsilicates(possesshugesize).MOF’sexhibitreduced chemical,thermal,andhydrothermalstabilityincomparisonwithotheroxides [63]
ForthemanufacturingofMOF’s,transitionmetals,alkalineearthmetals,p-blockelements,actinidesaswellasmixedmetalsareusedsincethepasttwodecades.Itcanbe preparedbylowvapordiffusionandsolvothermaltechniques [64] butneedtobemaintainedatelevatedtemperatureandpressure.Therefore,nowadays,nanoparticleswith particularmorphologiesaresynthesizedbytheUS-assistedmethod [65,66].
In2016,themostsimpleandcommercialmethodfortheformationofnanoMOFs usingultrasonicsonochemistryhasbeenreported [67].Recentlyin2018,Ultrasoundassistedamidefunctionalizedmetal-organicframeworkusedfornitroaromaticsensing. Whilesynthesizingnanoplatesofzinc(II)-basedMOF’sthroughtheultrasonicmethod, surfactantswerenotinvolvedatambienttemperatureandatmosphericpressure. Therefore,FieldEmissionScanningElectronMicroscopy(FE-SEM),powderX-ray diffraction,thermogravimetricanalysis(TGA),elementalanalysis,andFTIRspectroscopyarethemethodsusedtoanalyzeincreasedcontrolofparticlesizeandmorphology. Similarly,byusingdifferentprecursorconcentration,amide-functionalized nanometal-organicframework,[Zn2(oba)2(bpfb)] (DMF)5,TMU-23,(H2oba ¼ 4,40oxybis(benzoicacid);bpfb ¼ N,N0 -bis-(4-pyridylformamide)-1,4-benzenediamine, DMF ¼ N,N-dimethylformamide),wasreportedbyapplicationofultrasound sonochemistry.Also,thedecreaseinflorescenceintensityandanincreaseinselectivity ofsensingcapacityofnitroaromaticcompoundslikenitrophenol,nitroaniline,and nitrobenzeneinacetonitrilesolutionisinvestigated [68] (Scheme7).
5.2.2Phase-transfercatalysis
Mutuallyinsolublecompoundsreactwitheachotherthroughphase-transfercatalysis (PTC).Sinceitisusedinthesynthesisofpharmaceuticals,agriculturalchemicals,flavorings,dyes,perfumes,andenvironmentalprocesses;itwasattemptedtoimprovethe
Scheme7 SynthesisofTMU-23viabothwayshydrothermalaswellasultrasonication.
phase-transfercatalystsefficiency.“Multisite”phase-transfercatalyst(MPTC)isthe newlydevelopedPTCmethod,wherecatalyticefficiencyhasbeenincreased,dueto multiplemoleculesbeingabletobetakenintotheorganicphaseinonecycle [69–73].Asultrasoundsonochemistryreportedadvantageslikehighreactionrate,yield, andselectivity;therefore,itsapplicationsinorganicsynthesishasbeenpredominant.The combinationofPTC,alongwithultrasonicationhasbeenformedtobeaneffective methodfororganicconversion [74–78].Also,PTCassistedbysonicationgivesbenefits ofhighmasstransferrate.In2000,Cannizzaroreactioncarriedoutbyphase-transfer catalysiswithsonochemistryhasbeenestimatedthatthereactionrateincreasedby 20-kHzultrasonicwave [79].Inthecaseofepoxidationanddichlorocyclopropanation of1,7-octadieneandethoxylationofp-chloronitrobenzenebyphase-transfercatalyzed ultrasonication,increasedrateofreactionwasobserved [80–82].Phase-transfercatalysts carriedbyhighlyintenseultrasonicwavesalsopromotesWilliamsonethersynthesis.Due toitspotentialandversatiletechnology,ultrasoundhasearnedimportanceinpolymerizationindustries [83],homopolymers,blockcopolymers [84],hydrogels [85],and polymer-inorganiccomposites,etc. [86].Liquid-liquidphase-transfercatalysis(LLPTC) combinedwithultrasoundirradiationalsoenhancestherateofreaction,butfroman applicationpointofviewisrarelyseenintheliterature.
However,theapplicationofPTCalongwithultrasoundmethod,hasachieved exceptionalattentioninboththecommercialaswellasacademicpointofview,due toitsstrongandflexiblenature.Italsoprovidesmultiplebenefitsthatfavoritsuseindifferentpolymerizationtechniquestosynthesizepolymers.Tothebestofourknowledge, thecombinedapproachofPTCandultrasoundislimited.
In2017,thenovelprocessesinphase-transfercatalyticreactionsundertheinfluenceof ultrasonicationhavebeenstudied [87].Theenhancedyieldatreducedorganicsolventand lessreactiontimewasreported.Theethoxylationreaction,inwhich4-chloronitrobenzene reactswithpotassiumethoxidehasbeenevaluatedinthepresenceofPTCusingultrasound irradiationconditions(28kHz,200W),thatresultedinethoxy-4-nitrobenzene(Scheme8)
Ultrasoundirradiation
Scheme8 Ultrasound-assistedreactionof4-chloronitrobenzenewithpotassiumethoxideinpresence ofPTCtoformethoxy-4-nitrobenzene.