https://ebookmass.com/product/green-sustainable-process-for-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Green Sustainable Process for Chemical and Environmental Engineering and Science: Supercritical Carbon Dioxide as Green Solvent Dr. Inamuddin (Editor)
https://ebookmass.com/product/green-sustainable-process-for-chemicaland-environmental-engineering-and-science-supercritical-carbondioxide-as-green-solvent-dr-inamuddin-editor/ ebookmass.com
Green Sustainable Process for Chemical and Environmental Engineering and Science: Switchable Solvents Dr. Inamuddin
https://ebookmass.com/product/green-sustainable-process-for-chemicaland-environmental-engineering-and-science-switchable-solvents-drinamuddin/
ebookmass.com
Green Sustainable Process for Chemical and Environmental Engineering and Science: Sonochemical Organic Synthesis 1st Edition Dr. Inamuddin (Editor)
https://ebookmass.com/product/green-sustainable-process-for-chemicaland-environmental-engineering-and-science-sonochemical-organicsynthesis-1st-edition-dr-inamuddin-editor/
ebookmass.com
(eTextbook PDF) for Microbiology: A Systems Approach 4th Edition
https://ebookmass.com/product/etextbook-pdf-for-microbiology-asystems-approach-4th-edition/
ebookmass.com
Advanced Fiber Access Networks Cedric F. Lam https://ebookmass.com/product/advanced-fiber-access-networks-cedric-flam/
ebookmass.com
Coming In Hot (Brothers of Fire Book 6) Kathryn Shay
https://ebookmass.com/product/coming-in-hot-brothers-of-firebook-6-kathryn-shay/
ebookmass.com
Holidays on the Ranch Carolyn Brown
https://ebookmass.com/product/holidays-on-the-ranch-carolyn-brown/
ebookmass.com
The War of 1812 Carl Benn
https://ebookmass.com/product/the-war-of-1812-carl-benn/
ebookmass.com
Teaching and Learning for Social Justice and Equity in Higher Education: Foundations 1st ed. Edition Laura Parson
https://ebookmass.com/product/teaching-and-learning-for-socialjustice-and-equity-in-higher-education-foundations-1st-ed-editionlaura-parson/
ebookmass.com
Les Hill
https://ebookmass.com/product/les-terres-dissidentes-tome-2-lesoulevement-nastasia-hill/
ebookmass.com
GREEN SUSTAINABLE PROCESSFOR CHEMICALAND ENVIRONMENTAL ENGINEERING ANDSCIENCE GREEN SUSTAINABLE PROCESSFOR CHEMICALAND ENVIRONMENTAL ENGINEERING ANDSCIENCE CarbonDioxideCapture andUtilization Editedby
Inamuddin
DepartmentofAppliedChemistry,ZakirHusainCollegeofEngineeringandTechnology,Facultyof EngineeringandTechnology,AligarhMuslimUniversity,Aligarh,UttarPradesh,India
TariqAltalhi
DepartmentofChemistry,CollegeofScience,TaifUniversity,Taif,SaudiArabia
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2023ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatment maybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
ISBN:978-0-323-99429-3
ForInformationonallElsevierpublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: AnitaKoch
EditorialProjectManager: FranchezcaA.Cabural
ProductionProjectManager: SwapnaSrinivasan
CoverDesigner: ChristianJ.Bilbow
TypesetbyAptara,NewDelhi,India
Contributorsxi
Chapter1Carbondioxidecaptureandits utilizationtowardsefficientbiofuels production1
AbhinayThakur,andAshishKumar
1.1Introduction1
1.2Utilizationofcapturedcarbondioxide forbiofuelproduction5
1.3Conclusionandfutureperspectives13 References13
Chapter2Deepeutecticliquidsforcarbon capturingandfixation17
ZainabLiaqat,SumiaAkram,HafizMuhammadAthar, andMuhammadMushtaq
2.1Carbondioxideemissions17
2.2Deepeutecticliquids19
2.3Typesofdeepeutecticliquids19
2.4PreparationofDELs20
2.5AuthenticationofDELs21
2.6DELbasedCO2 absorption22
2.7Carboncaptureefficiencyofvarious HBDs24
2.8CO2 absorptioninaqueoussolution ofDELs40
2.9CO2 absorptioninternaryDELs41
2.10Ammonium-BasedDELs42
2.11PhosphoniumbasedDELs44
2.12AzolebasedDELs44
2.13Bio-phenolderivedsuperbasebasedDELs45
2.14HydrophobicDELs45
2.15Non-ionicDELs46
2.16DELsupportedmembranes46
2.17DELswithmultiplesitesinteraction47
2.18Conclusionandfutureprospects48
Acknowledgment49 References49
Chapter3Cookstovesforbiochar productionandcarboncapture53 MashuraShammi,JulienWinter,Md.MahbubulIslam, BeautyAkter,andNazmulHasan
3.1Introduction53
3.2Cookstovesdesignedforbiochar production54
3.3Biocharproductionandclimate-change implications62
3.4Conclusion64 References65
Chapter4Metalsupportinteractionfor electrochemicalvalorizationofCO2 69 AbinayaStalinraja,andKeerthigaGopalram
4.1Introduction69
4.2MetalsupportsforECRofCO2 70 4.3Conclusion80 Acknowledgment80 References81
Chapter5Utilizationofcarbondioxideasa buildingblockinsynthesisofactive pharmaceuticalingredients85 MuhammadFaisal
5.1Introduction85
5.2 N–Nucleophile-triggered CO2 -incorporatedcarboxylation toformC–Nbonds87
5.3 N–Nucleophile-triggered CO2 -incorporatedmethylation toformC–Nbonds95
5.4 O–Nucleophile-triggered CO2 -incorporatedcarboxylation toformC–Obonds96
5.5CO2 -catalyzedoxidationof alcoholstoformC–Obonds97
5.6 C-Nucleophile-triggered CO2 -incorporatedreductivecarboxylation toformC–Cbonds97
5.7 C-nucleophile-triggered CO2 -incorporateddirectC–Hcarboxylation toformC–Cbond99
5.8 C-nucleophile-triggeredCO2 -incorporated organozinc-mediatedcarboxylationtoform C–Cbonds101
5.9 C-nucleophile-triggeredCO2 -incorporated organolithium-mediatedcarboxylation toformaC–Cbond102
5.10 C-Nucleophile-triggeredCO2 -incorporated organomagnesium-mediatedcarboxylation toformaC–Cbond108
5.11Conclusion111
References113
Chapter6ElectrochemicalCarbonDioxide Detection119 S.Aslan,C.I¸sık,andA.E.Mamuk
6.1Introduction119
6.2CapturetechnologiesofCO2 121
6.3Fundamentalsofelectrochemistry126
6.4Directpotentiometricmethods128
6.5Summaryandconclusion139 References141
Chapter7Carbondioxideinjectionfor enhancedoilrecoveryandunderground storagetoreducegreenhousegas149 ShubhamSaraf,andAchintaBera
7.1Introduction149
7.2OilrecoveryusingCO2 156
7.3Undergroundstorageof CO2 inunconventionalreservoirs167
7.4Currentstatus,challengesand futuredirections169
7.5Conclusions170 Acknowledgment172 References172
Chapter8Ionicliquidsaspotential materialsforcarbondioxidecapture andutilization177
MdAbuShahynIslam,MohdArhamKhan,NimraShakeel, MohdImranAhamed,andNaushadAnwar
8.1Introduction178
8.2TypesofILs179
8.3FutureapplicationsofILandGR-basedIL191
8.4Conclusion192 References193 Chapter9Recentadvancesin carbondioxideutilization asrenewableenergy197
MuhammadHussnainSiddique,FareehaMaqbool,TanvirShahzad, MuhammadWaseem,IjazRasul,SumreenHayat,MuhammadAfzal, MuhammadFaisal,andSaimaMuzammil
9.1Introduction197
9.2CO2 utilizationtechnologies198
9.3DevelopmentsinworldwideCO2 utilizationprojects204
9.4Marketscaleandvalue205
9.5Regulationandpolicy205
9.6Conclusionandfutureprospects206 References206
Chapter10MetalOrganicFrameworks asanEfficientMethodforCarbon dioxidecapture211
BhartiKataria,andChristineJeyaseelan
10.1Introduction211
10.2Metalorganicframework(MOF)212
10.3SynthesisofsomeMOFS215
10.4PropertiesofMOFs217
10.5CO2 captureusingMOF217
10.6Adsorptionofcarbondioxidein metalorganicframeworks219
10.7MethodstoenhanceCO2 adsorption219
10.8MethodstoenhanceMOF stability222
10.9Conclusion227 References227
Chapter11Industrialcarbondioxide captureandutilization231
UzmaHira,AhmedKamal,andJaveriaTahir
11.1Introduction231
11.2CO2 collectionsystemsbasedonliquid233
11.3CO2 capturingwithionicliquidsolvents242
11.4Applications,implementation andchallenges244
11.5SolidCO2 adsorbentsforlow-temperature applications245
11.6Carbonadsorbents246
11.7Zeoliteadsorbents248
11.8AdsorbentsoftheMOF(metal–organic framework)type250
11.9Adsorbentspredicatedon carbonate-basedalkalis252
11.10Layereddoublehydroxides (LDHs)-basedadsorbents254
11.11Adsorbentsmadeofmagnesium oxide(MgO)255
11.12SolidCO2 sorbentsfor high-temperatureapplications257
11.13Pre-combustionapplications, implementationandproblems260
11.14TheutilisationofCO2 in industrialprocesses262
11.15Conclusionsandprospects268 References270
Chapter12Ionicliquidsforcarbon capturingandstorage279
FaizanWaseemButt,HafizMuhammadAthar,SumiaAkram, ZainabLiaqat,andMuhammadMushtaq
12.1Introduction279
12.2CO2 capturetechnologies280
12.3Ionicliquids(ILs)281
12.4FeaturesofILs281
12.5ILasabsorbentsforCO2 capture283
12.6ILhybridsasadsorbentsfor CO2 capture289
12.7ILhybridswithmembranesfor CO2 capture289
12.8Ionicliquidsupportedmembrane290
12.9PolyILsmembrane290
12.10Compositemembranes290
12.11Conclusionandfutureinsights291 References291
Chapter13Advancesin utilizationofcarbon-dioxideforfood preservationandstorage297
AdeshinaFadeyibi
13.1Introduction297
13.2Utilizationofcarbon-dioxidein foodpreservation298
13.3Utilizationofcarbon-dioxidein foodstorage302
13.4Prospectsandconclusion305 References305
Chapter14Aninsightintotherecent developmentsinmembrane-basedcarbon dioxidecaptureandutilization311
PritamDey,PritamSingh,andMitaliSaha
14.1Introduction311
14.2Carbondioxidecapturetechnologies312
14.3Abriefaboutmembranetechnology314
14.4CO2 separationusingmembranes316 14.5CO2 utilizationusingmembranes321 14.6Conclusions322 References323
Chapter15Carbondioxidetofuelusing solarenergy327
SrijitaBasumallick
15.1Introduction327
15.2CO2 reductionontosemiconductor surface327
15.3MajorbottleneckforCO2 reduction328
15.4Differenttypesofphotocatalyst329
15.5ReductionofCO2 tomethanol usingCu2 Oasphotocatalyst330
15.6ReductionofCO2 tomethanolusing Cu2 Oaselectrocatalyst330
15.7BenefitsofusingRGOinthe compositecatalyst331
15.8Conclusions333
Acknowledgment333 References333
Chapter16Adsorbentsforcarbon capture337
VijayVaishampayan,MukeshKumar,MuthamilselviPonnuchamy, andAshishKapoor
16.1Introduction337
16.2Carboncaptureprocesses338
16.3AdsorbentsforCO2 capture338
16.4Futureperspectiveandconclusion342 References342
Chapter17Carbondioxidecaptureand utilizationinionicliquids345
GuocaiTian
17.1Introduction345
17.2CaptureofCO2 inILs349
17.3ElectroreductionofCO2 inILs391
17.4Conclusions406 Acknowledgments407 References407
Chapter18Hydrothermalcarbonizationof sewagesludgeforcarbonnegativeenergy production427
MilanMalhotra,AnushaSathyanadh,andKhanh-QuangTran
18.1Introduction427
18.2Sludgeasapotentialsourceof alternateenergy431
18.3Hydrothermal(HT)treatmentsforthe productionoffuel432
18.4Hydrothermalcarbonization+ gasification+ccs437
18.5Conclusion437 Acknowledgement438 References438
Chapter19Utilizationofsupercritical CO2 fordryingandproduction ofstarchandcelluloseaerogels441
JeieliWendelGasparLima,ClaraPrestesFerreira,
JhonatasRodriguesBarbosa,andRaulNunesdeCarvalhoJunior
19.1Introduction441
19.2CO2 application–Supercriticaldrying442
19.3StarchaerogelandCO2 utilization445
19.4CelluloseaerogelsandCO2 utilization447
19.5Conclusions448 Authorcontributions449 Ethicalapproval449 Declarationofcompetinginterest449 Acknowledgment449 References449
Chapter20Advancesincarbon bio-sequestration451
NigelTwi-Yeboah,DacostaOsei,andMichaelK.Danquah
20.1Introduction451
20.2Carbonsequestrationmethods452
20.3Limitationsofcarbonsequestration methods453
20.4Overviewofbiologicalsequestration (Cycle/Mechanism)454
20.5Bioresourcesforcarbon bio-sequestration455
20.6Cyanobacteria456
20.7Microalgae457
20.8Plants457
20.9Bacteria458
20.10Nanomaterialsincarbonsequestration458
20.11Futureperspectives459
20.12Conclusion459 References460
Chapter21Photosyntheticcellfactories, anewparadigmforcarbondioxide (CO2 )valorization463
BijayaNag,AbdalahMakaranga,MukulSureshKareya, AshaArumugamNesamma,andPannagaPavanJutur
21.1Introduction463
21.2Carboncapture,utilizationand storagemechanism465
21.3Biologicalmechanismofcarboncapture469
21.4ProductsfromCCU470
21.5Challengesandopportunities472
21.6Futureperspectivesandconclusions475 Fundinginformation476 References476
Chapter22Carbondioxidecapture andsequestrationtechnologies–currentperspective,challenges andprospects481
IfeanyiMichaelSmarteAnekwe,EmmanuelKweinorTetteh, StephenAkpasi,SamailaJoelAtuman, EdwardKwakuArmah,andYusufMakarfiIsa
22.1Introduction481
22.2Carboncaptureandsequestration(CCS) technologies484
22.3CO2 transportation,storage andopportunities/applicationsfor CCStechnologies493
22.4Currentperspectiveandpoliciesof CSStechnologiesinvariouscountries throughouttheworld497
22.5Challengesandsocio-economic implicationsofCCStechnologies501
22.6Applicationsandopportunities forCCStechniques504
22.7Prospectsandfuturework considerationsforCCSapproaches507
22.8Conclusion508 References509
Chapter23Microbialcarbon dioxidefixationfortheproduction ofbiopolymers517
TubaSaleem,IjazRasul,MuhammadAsif,andHabibullahNadeem
23.1Introduction517
23.2SourcesofCO2 emission519
23.3SequestrationmethodsofCO2 519
23.4Carbonconcentratingmechanisms520
23.5Advancementsincarboncaptureand storage&carboncaptureutilization521
23.6Carbondioxidefixationpathways521
23.7Factorsaffectingthecarbondioxide biofixation527
23.8Productionofbiopolymers/bioplastics527
23.9Conclusion529 References529
Chapter24Carbondioxidecapture anditsenhancedutilization usingmicroalgae531
PinkuChandraNath,BiswanathBhunia, andTarunKantiBandyopadhyay
24.1Introduction531
24.2PhotosynthesisandCO2 fixationusing microalgae532
24.3Cultivationsystemsforcarbondioxide capturebymicroalgae533
24.4CO2 captureimprovementstrategies541
24.5Conclusion541 References541
Chapter25Supportedsingle-atom catalystsincarbondioxide electrochemicalactivation andreduction547
AmosAfugu,CarolineR.Kwawu,ElliotMenkah,andEvansAdei
25.1Introduction547
25.2CO2 ERRproducts549
25.3Single-Atomcatalystsefficiency descriptors549
25.4Single-Atomcatalystsupports551
25.5MechanismsforCO2 ERRon single-atomcatalysts554
25.6Conclusion556 References557
Chapter26Organicmatterand mineralogicalacumensinCO2 sequestration561
SantanuGhosh,TusharAdsul,andAtulKumarVarma
26.1Overview562
26.2Introduction562
26.3Geo-sequestration563
26.4Bio-sequestration563
x Contents
26.5Mechanismsofcarboncapture564
26.6Transportofcarbondioxide566
26.7Mechanismofcarbonaccommodation566
26.8Carbondioxidesequestration inorganicmatter566
26.9Mineralogicalacumenofcarbon sequestration573
26.10AnoteonCO2 disposalinbasalt formations587
26.11Summary587 References588 Index595
Contributors EvansAdei DepartmentofChemistry,Kwame NkrumahUniversityofScienceandTechnology,Kumasi,Ghana
TusharAdsul CoalGeologyandOrganicPetrologyLaboratory,DepartmentofAppliedGeology,IndianInstituteofTechnology(Indian SchoolofMines)Dhanbad,Jharkhand,India
AmosAfugu DepartmentofChemistry,Kwame NkrumahUniversityofScienceandTechnology,Kumasi,Ghana
MuhammadAfzal DepartmentofBioinformaticsandBiotechnology,GovernmentCollege UniversityFaisalabad,Faisalabad,Pakistan
MohdImranAhamed DepartmentofChemistry,FacultyofScience,AligarhMuslimUniversity,Aligarh,UP,India
StephenAkpasi GreenEngineeringResearch Group,DepartmentofChemicalEngineering, FacultyofEngineeringandtheBuiltEnvironment,DurbanUniversityofTechnology,Durban,SouthAfrica
SumiaAkram DivisionofScienceandTechnology,UniversityofEducationLahore,Pakistan
BeautyAkter DepartmentofEnvironmental Sciences,JahangirnagarUniversity,Dhaka, Bangladesh
IfeanyiMichaelSmarteAnekwe Schoolof ChemicalandMetallurgicalEngineering, UniversityoftheWitwatersrand,Johannesburg, SouthAfrica
NaushadAnwar DepartmentofChemistry,FacultyofScience,AligarhMuslimUniversity, Aligarh,UP,India
EdwardKwakuArmah SchoolofChemicaland BiochemicalSciences,DepartmentofApplied Chemistry,C.K.TedamUniversityofTechnologyandAppliedSciences,Navrongo,Upper EastRegion,Ghana
MuhammadAsif DepartmentofBioinformatics andBiotechnology,GovernmentCollegeUniversityFaisalabad,Faisalabad,Pakistan
S.Aslan DepartmentofChemistry,Facultyof Science,MuglaSitkiKocmanUniversity,Mugla, Turkey
HafizMuhammadAthar DepartmentofChemistry,GovernmentCollegeUniversity,Lahore, Pakistan
SamailaJoelAtuman SchoolofChemicaland MetallurgicalEngineering,Universityofthe Witwatersrand,Johannesburg,SouthAfrica; DepartmentofChemicalEngineering,Faculty ofEngineering,AbubakarTafawaBalewaUniversityBauchi,Nigeria
TarunKantiBandyopadhyay Departmentof ChemicalEngineering,NationalInstituteof TechnologyAgartala,Jirania,Tripura,India
JhonatasRodriguesBarbosa InstituteofTechnology(ITEC),FacultyofFoodEngineering (FEA),FederalUniversityofPara(UFPA),Rua AugustoCorrêaS/N,Guamá,Belém,PA,Brazil
SrijitaBasumallick AsutoshCollege,University ofCalcutta,Kolkata,India
AchintaBera DepartmentofPetroleumEngineering,SchoolofEnergyTechnology,PanditDeendayalEnergyUniversity,Gandhinagar, Gujarat,India
BiswanathBhunia DepartmentofBioEngineering,NationalInstituteofTechnologyAgartala, Jirania,Tripura,India
FaizanWaseemButt DepartmentofChemistry, GovernmentCollegeUniversity,Lahore,Pakistan
MichaelK.Danquah DepartmentofChemical Engineering,UniversityofTennessee,ChattanoogaTN,UnitedStatesofAmerica
PritamDey DepartmentofChemistry,National InstituteofTechnologyAgartala,Tripura,India
AdeshinaFadeyibi DepartmentofFoodand AgriculturalEngineering,FacultyofEngineeringandTechnology,KwaraStateUniversity, Ilorin,KwaraState,Nigeria
MuhammadFaisal CreativeResearchCenterfor BrainScience,BrainScienceInstitute(BSI),KoreaInstituteofScienceandTechnology(KIST), Seoul,RepublicofKorea;DivisionofBioMedicalScience&Technology,KISTSchool, KoreaUniversityofScienceandTechnology (UST),Seoul,RepublicofKorea;Department ofChemistry,Quaid-i-AzamUniversity,Islamabad,Pakistan;InstituteofPlantBreedingand Biotechnology,MNS-UniversityofAgriculture, Multan,Pakistan
ClaraPrestesFerreira InstituteofTechnology (ITEC),FacultyofFoodEngineering(FEA), FederalUniversityofPara(UFPA),Rua AugustoCorrêaS/N,Guamá,Belém,PA, Brazil
SantanuGhosh CoalGeologyandOrganic PetrologyLaboratory,DepartmentofApplied Geology,IndianInstituteofTechnology(Indian SchoolofMines)Dhanbad,Jharkhand,India; OrganicGeochemistryLaboratory,Department ofEarthSciences,IndianInstituteofTechnology Bombay,Mumbai,Maharashtra,India;DepartmentofGeology,MizoramUniversity,Aizwal, Mizoram,India
KeerthigaGopalram DepartmentofChemical Engineering,SRMInstituteofScience&Technology,Kancheepuram,TamilNadu,India
SumreenHayat DepartmantofMicrobiology, GovernmentCollegeUniversityFaisalabad, Faisalabad,Pakistan
NazmulHasan TheUnitedGraduateSchoolof AgriculturalSciences,KagoshimaUniversity, Kagoshima,Japan;FruitScienceLaboratory, SagaUniversity,Saga,Japan
UzmaHira SchoolofPhysicalSciences(SPS), UniversityofthePunjab,Lahore,Pakistan
YusufMakarfiIsa SchoolofChemicalandMetallurgicalEngineering,UniversityoftheWitwatersrand,Johannesburg,SouthAfrica
Md.MahbubulIslam BangladeshBiocharInitiative,Dhaka,Bangladesh
MdAbuShahynIslam InterdisciplinaryNanotechnologyCentre,ZHCET,AligarhMuslim University,Aligarh,UP,India
C.I¸sık DepartmentofChemistry,FacultyofScience,MuglaSitkiKocmanUniversity,Mugla, Turkey
ChristineJeyaseelan DepartmentofChemistry, AmityInstituteofAppliedSciences,AmityUniversity,Noida,UttarPradesh,India
RaulNunesdeCarvalhoJunior Instituteof Technology(ITEC),FacultyofFoodEngineering(FEA),FederalUniversityofPara(UFPA), RuaAugustoCorrêaS/N,Guamá,Belém,PA, Brazil
PannagaPavanJutur OmicsofAlgaeGroup,IndustrialBiotechnology,InternationalCentrefor GeneticEngineeringandBiotechnology,New Delhi,India
AhmedKamal SchoolofPhysicalSciences(SPS), UniversityofthePunjab,Lahore,Pakistan
AshishKapoor DepartmentofChemicalEngineering,HarcourtButlerTechnicalUniversity, Kanpur,UttarPradesh,India
MukulSureshKareya OmicsofAlgaeGroup, IndustrialBiotechnology,InternationalCentre forGeneticEngineeringandBiotechnology, NewDelhi,India
BhartiKataria DepartmentofChemistry,Amity InstituteofAppliedSciences,AmityUniversity, Noida,UttarPradesh,India
MohdArhamKhan InterdisciplinaryNanotechnologyCentre,ZHCET,AligarhMuslim University,Aligarh,UP,India
AshishKumar NCE,DepartmentofScienceand Technology,GovernmentofBihar,India
MukeshKumar DisciplineofChemistry,Indian InstituteofTechnology,Gandhinagar,Gujarat, India
CarolineR.Kwawu DepartmentofChemistry, KwameNkrumahUniversityofScienceand Technology,Kumasi,Ghana
ZainabLiaqat DepartmentofChemistry,GovernmentCollegeUniversity,Lahore,Pakistan
JeieliWendelGasparLima InstituteofTechnology(ITEC),FacultyofFoodEngineering
(FEA),FederalUniversityofPara(UFPA),Rua AugustoCorrêaS/N,Guamá,Belém,PA,Brazil
AbdalahMakaranga OmicsofAlgaeGroup,IndustrialBiotechnology,InternationalCentrefor GeneticEngineeringandBiotechnology,New Delhi,India
MilanMalhotra DepartmentofEnergyandProcessEngineering,NorwegianUniversityofScienceandTechnology,Trondheim,Norway
A.E.Mamuk DepartmentofPhysics,Facultyof Science,MuglaSitkiKocmanUniversity,Mugla, Turkey
FareehaMaqbool DepartmentofBioinformatics andBiotechnology,GovernmentCollegeUniversityFaisalabad,Faisalabad,Pakistan
ElliotMenkah DepartmentofChemistry, KwameNkrumahUniversityofScienceand Technology,Kumasi,Ghana
MuhammadMushtaq DepartmentofChemistry,GovernmentCollegeUniversity,Lahore, Pakistan
SaimaMuzammil DepartmantofMicrobiology, GovernmentCollegeUniversityFaisalabad, Faisalabad,Pakistan
HabibullahNadeem DepartmentofBioinformaticsandBiotechnology,GovernmentCollege UniversityFaisalabad,Faisalabad,Pakistan
BijayaNag OmicsofAlgaeGroup,Industrial Biotechnology,InternationalCentreforGenetic EngineeringandBiotechnology,NewDelhi, India
PinkuChandraNath DepartmentofBioEngineering,NationalInstituteofTechnologyAgartala,Jirania,Tripura,India
AshaArumugamNesamma OmicsofAlgae Group,IndustrialBiotechnology,International CentreforGeneticEngineeringandBiotechnology,NewDelhi,India
DacostaOsei ChemicalandPetroleumEngineeringDepartment,UniversityofKansas,KS, UnitedStatesofAmerica
MuthamilselviPonnuchamy Departmentof ChemicalEngineering,CollegeofEngineering andTechnology,SRMInstituteofScienceand
Technology,Potheri,Kattankulathur,Tamil Nadu,India
IjazRasul DepartmentofBioinformaticsand Biotechnology,GovernmentCollegeUniversity Faisalabad,Faisalabad,Pakistan
MitaliSaha DepartmentofChemistry,National InstituteofTechnologyAgartala,Tripura,India
TubaSaleem DepartmentofBioinformaticsand Biotechnology,GovernmentCollegeUniversity Faisalabad,Faisalabad,Pakistan
ShubhamSaraf DepartmentofPetroleumEngineering,SchoolofEnergyTechnology,PanditDeendayalEnergyUniversity,Gandhinagar, Gujarat,India
AnushaSathyanadh DepartmentofEnergyand ProcessEngineering,NorwegianUniversity ofScienceandTechnology,Trondheim, Norway
NimraShakeel DepartmentofChemistry,FacultyofScience,AligarhMuslimUniversity,Aligarh,UP,India
MashuraShammi Hydrobiogeochemistryand PollutionControlLaboratory,Departmentof EnvironmentalSciences,JahangirnagarUniversity,Dhaka,Bangladesh
TanvirShahzad DepartmantofEnvironmental SciencesandEngineering,GovernmentCollege UniversityFaisalabad,Faisalabad,Pakistan
MuhammadHussnainSiddique Department ofBioinformaticsandBiotechnology, GovernmentCollegeUniversityFaisalabad, Faisalabad,Pakistan
PritamSingh DepartmentofChemistry,NationalInstituteofTechnologyAgartala,Tripura, India
AbinayaStalinraja DepartmentofChemicalEngineering,SRMInstituteofScience&Technology,Kancheepuram,TamilNadu,India
JaveriaTahir SchoolofPhysicalSciences(SPS), UniversityofthePunjab,Lahore,Pakistan
EmmanuelKweinorTetteh GreenEngineering ResearchGroup,DepartmentofChemicalEngineering,FacultyofEngineeringandtheBuilt Environment,DurbanUniversityofTechnology,Durban,SouthAfrica
Contributors
AbhinayThakur DepartmentofChemistry,FacultyofTechnologyandScience,LovelyProfessionalUniversity,Phagwara,Punjab,India
GuocaiTian StateKeyLaboratoryofComplex NonferrousMetalResourcesCleanUtilization, FacultyofMetallurgicalandEnergyEngineering,KunmingUniversityofScienceandTechnology,Kunming,YunnanProvince,China
Khanh-QuangTran DepartmentofEnergyand ProcessEngineering,NorwegianUniversityof ScienceandTechnology,Trondheim,Norway
NigelTwi-Yeboah OperationsDepartment, GhanaNationalGasCompany,WesternRegion, Ghana
VijayVaishampayan DepartmentofChemical Engineering,IndianInstituteofTechnology, Ropar,Punjab,India
AtulKumarVarma CoalGeologyandOrganic PetrologyLaboratory,DepartmentofApplied Geology,IndianInstituteofTechnology(IndianSchoolofMines)Dhanbad,Jharkhand, India
MuhammadWaseem DepartmantofMicrobiology,GovernmentCollegeUniversityFaisalabad,Faisalabad,Pakistan
JulienWinter Privateconsultant,Cobourg,ON, Canada
1 Carbondioxidecaptureandits utilizationtowardsefficient biofuelsproduction AbhinayThakur a andAshishKumar b a DepartmentofChemistry,FacultyofTechnologyandScience,LovelyProfessional University,Phagwara,Punjab,India b NCE,DepartmentofScienceandTechnology, GovernmentofBihar,India
1.1Introduction Whenreleasedintotheatmosphere,carbondioxide(CO2 ),aforemostgreenhousegas, retainsheatbyreflectinginfraredlightbacktotheEarth’ssurface.Asaresult,increasedCO2 emissionsareaworldwideproblembecausetheyareoneoftheprimarycausesofclimate disruption [1–4].Furthermore,worldwideCO2 emissionpatternsindicateanannuallyrise, whichisaccompaniedbyanannuallyriseinoverallwarming.TheriseinCO2 concentration was2.460.26ppm y 1 inOctober2021,aspercurrentstatistics,butthetemperaturehas enhancedatanannualizedlevelof0.08Cperdecadesince1980,asperNationalOceanicand AtmosphericAdministration(NOAA)2020annualclimatedisclose.Theresultisprogressive warmingandwitheringoftheenvironment,which,amongotherthings,iscreatingenormous anddisastrouswildfiresaroundtheworld,whichinturnemitenormousvolumesofCO2 intotheenvironment,makingcarbonreleasesevenmoreofaproblem [5–7].Inactuality, risingCO2 levelsintheenvironmenthaveavarietyofotherenvironmentalconsequences, includingadjustmentsinthehydrogeologicalprocess,theenhancedincidenceofnumerous severeclimateoccurrences,sea-levelrise,speciationmigratory,harvestlosses,andenhanced eventsthatoccurredofinfectiousdiseases,andsoon.From2010to2040,consumptionfor fossilfuelsisexpectedtoincreaseby40percent.Asaresult,alternateenergysourceshavebeen andcontinuetobeinvestigatedinordertomeetourenergyrequirements.Renewableenergy resourcesincludesunlight,air,andbiomass.Inthelastseveralyears,biomass,whichisformed throughaphysiologicalorigin,hasbeenexploitedtogeneratebiofuelsandbio-products. Thereare4phasesofbiofuels,basedonthesortofbiomass.Biodiesel,bioethanol,bioethanol,
1.Carbondioxidecaptureanditsutilizationtowardsefficientbiofuelsproduction biohydrogen,andbioethersareexamplesofbiofuels.Bioethanolandbiodiesel,whichboth constitutethefirstgenerationofbiofueltechnologies,arethemostcommonbiofuels,as pertheDepartmentofEnergy.TheUnitedStatesofAmerica,Australia,andtheEuropean Unionhaveallfinancedbiofuelexperiments.TheUnitedStatesprovidedfinancingtoNew Mexico(2009),Arizona(2008),Florida(2013),andMassachusetts(2011)whiletheEuropean Unionprovidedfundsforfourexperimentalinitiatives,threeoftheseoperatedduring2011to 2015/16andtheremainingbetween2012and2017.Biomassisconsideredasdiametrically opposedtotheusageoffossilfuels,andhencepreventsthereleaseoffreshCO2 intothe environment.Utilizingbiomass(andbiofuelsproducedfromit)isregardedanoperationthat doesnotaddCO2 totheenvironmentfromfossilfuels.Althoughthoughitisn’tpreciselyso, burningbiomassorbiofuelsisregardedazero-emissionsolutionforpowergenerationand consumption.Despitecontrast,comprehensivelifecycleanalysisinvestigationsdemonstrate that,intheexistingproduction–utilization–accountingframework,theusageofbiofuelsisa carbontransmissionthroughsubsurfacesubsoiltotheenvironment,equivalenttotheusage offossilfuels,albeitconsiderablylessintensively.Intruth,biomassismadeupofCO2 from theenvironment,andwhenburnt,itisthoughttorestoretheequivalentquantityofCO2 tothe environment,asiftheprocesswereacomponentofthebiologicalprocess.Inreality,unlike inreality,thecycleisnottrulyended.Inessence,incontrasttothecarbondioxideproduced bycombustionprocesses,onemustalsoevaluatethequantityofCO2 releasedbynumerous humanactionsthatprecedebiomassgenerationandprocessing,aswellasthesoilcarbon depletioninducedbyagriculturalmethods[8–15].
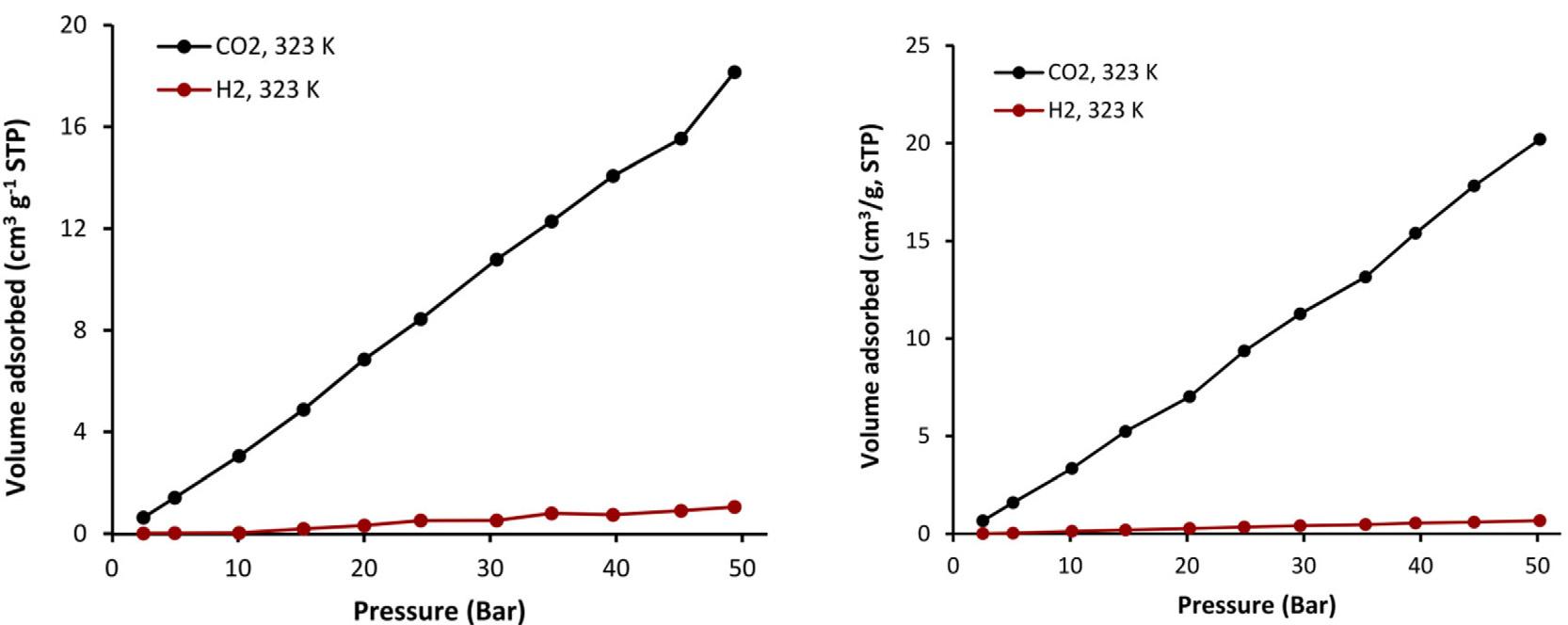
Bycombiningtelmisartanandasuitabletin(IV)chloride,Hadietal. [16] wasableto createunique,permeable,extremelyaromaticorganotin(IV)structures.Thesurfaceareaof theproducedmesoporousorganotin(IV)complexeswas32.3–130.4m 2g 1 ,theporecapacity was0.046–0.162cm 3g 1 ,andtheporediameterwasroughly2.4nm,accordingtoBrunauer–Emmett–Teller(BET)calculations.Tincomplexeswithabutylgroupwerefoundtobemore effectiveascarbondioxidestoragedevicesthanthosewithaphenylgroup.Ataregulatedtemperature(323K)andcompression,thedibutyltin(IV)moleculeofferthegreatestBETinterface region(128.871m 2g 1 ),thehighestquantity(0.162cm 3g 1 ),andtermedtobeeffective forCO2 retention(8.3wtpercent)(50bars).Thesorptionofcompoundswasinvestigated underaspecifiedtemperature(323K)andstrain.TheH2 andCO2 adsorptionisothermsin thepresenceofcompoundsareshownin Fig.1.1.ComplexesabsorbedalotofCO2 ,which mightownbecauseofintensevanderWaalscontactamongthemandCO2 .Forcomplexes, theamountofabsorbedCO2 was17.9,21.2,15.7,and34.9cm 3g 1 .Evidently,thesestructures havethemaximumCo2 absorptionaperture(6.9wtpercent)oftheorganotin(IV)compound, thatcouldbeduetothefactthattheyhavethebiggestBETinterfaceregion(128.871m 2g 1 ). Furthermore,withintheorganotin(IV)complexes,significantdipole-quadrupoleencounters inCO2 orH2 bondingandheteroatomsmayoccur.Whencomparedtoothergaseslikenitrogen andmethane,highlypermeableorganicpolymershavingnitrogen,oxygenorsulphuratoms areefficientatpreferentiallyabsorbingCO2 .Furthermore,insimilarcircumstancesasthose employedforCO2 absorption,complexesdisplayverylittleH2 adsorption(0.5–1.1cm 3g 1 ). It’spossiblethatthisbehaviorisowingtominimalcontact.
Similarly,Nasiretal. [17] usedthepartialpressuresofmethaneandCO2 ,aswellas theproportionsofseveralmembranematerials(polymer,amine,andfiller),tolinkthree optimalresultsinaunifiedmodel:CO2 permeance,CH4 permeance,andCO2 /CH4 selectivity.
FIGURE1.1 CO2 andH2 adsorptionisothermsforcomplex(AdaptedfromRef. [16])MDPI2019.Publishedin accordancewithCreativeCommonattributionLicenseCCBY4.0.
Thesevariablesaidedinforecastingmembraneefficiencyandinfluencingsecondaryvariables includingmembranelife,effectiveness,andproductquality.ForCO2 permeability,CH4 permeability,andCO2 /CH4 selectivity,themodelfindingsaccordwithexperimentaldata havinganrelativedeviationof5.9percent,3.8percent,and4.1percent,approximately.The findingssuggestthatthemodelcouldforecastvaluesunderavarietyofmembraneformation configurations.
Scholesetal. [18] investigatedthecapacityofacovalentlylinkedpolyether-polyamideblock copolymer(PEBAX2533)andpolyethyleneglycoldiacrylatetoextractcarbondioxidethrough N2 andCH4 inabasicandintegratedgascircumstances,aswellaswhen500ppmH2 Swas involved.TheLennardJoneswelldepthwasfoundtobeastrongerpredictorofgassolubility withinthesepolymersthanessentialtemperature.DuetocompetingsorptionfromCH4 or N2 ,CO2 penetrationwasdecreasedindrymixedgascircumstancesrelativetosinglegas measurements.Bothpolymers,though,maintainedCO2 selectivity.Waterinthefeedcaused thePEGmembranetoexpand,leadinginaconsiderableimprovementinCO2 penetrationas comparedtothegas(dry)environment.Interestingly,thesensitivitywasmaintainedeven whenthesupplygaswasmoist.TheinclusionofH2 SreducesCO2 penetrationviaboth membranesmerelyslightly.
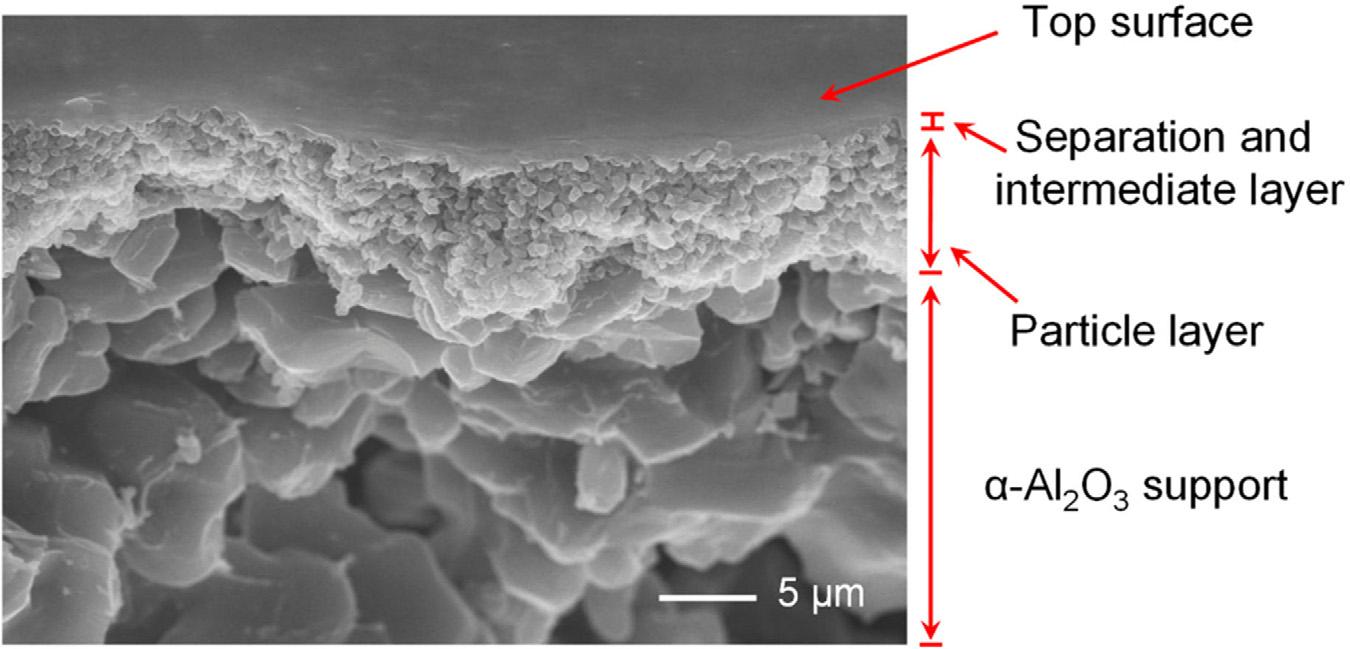
Jiangetal. [19] conductedextensiveresearchontheeffectsofcalcinationdegreesuponthe systemarchitecturesoforganosilicafilms.TheprecursorBis(triethoxysilyl)acetylene(BTESA) waschosenformembranemanufacturingusingthesol-gelmethod.Calcinationdegrees influencedfilmporouswidthandsilanoldensity,asindicatedbyTG,FT-IR,N2 adsorption, andmoleculetunablegaspermeationmeasurements.Thedisintegratedacetylenebridges resultedinaloosearchitectureintheBTESAmembrane,whichhadanextremehighCO2 permeationof15,531GPUbutalimitedCO2 /N2 sensitivityof4.1.BTESAmembranesshowed remarkablepotentialforCO2 extractionapplicationswhentheywerecalcinedat100°C,with aCO2 permeabilityof3434GPUandaN2 /CO2 sensitivityof21.FE-SEMwasusedtoanalyse BTESAcompositemembranesthathadbeencalcinedat100°Cinordertolearnmoreabout theirchemistry,asshownin Fig.1.2.
FIGURE1.2 SEMimagesoftheBTESA-100porousfilm.(AdaptedfromRef. [19])MDPI2022.Publishedin accordancewithCreativeCommonattributionLicenseCCBY4.0.
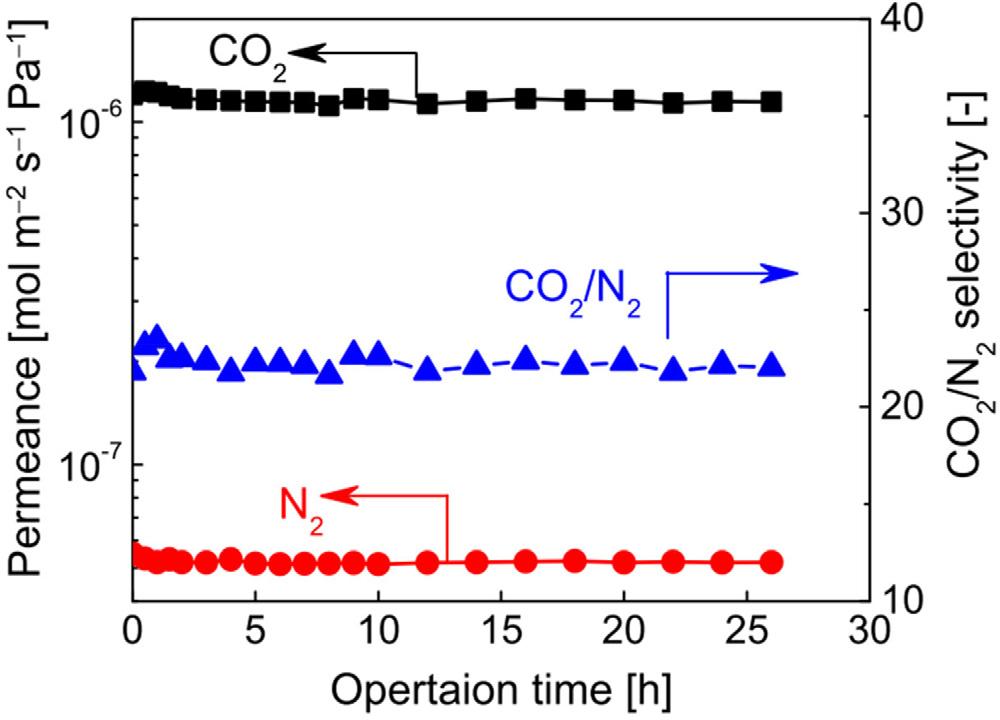
TheBTESA-100membranewasusedtoseparateCO2 /N2 mixturesforanalyticalpurposes. Astudyforthelong-termoperatingdurabilityofbinaryCO2 /N2 (14/79)segregationconductedat323Ktodemonstrateitsdurabilityofitsextractionefficiency,andthefindings arepresentedinFig.3.Inaconstantoperationlastingupto26h,negligiblediscernible lossinCO2 /N2 extractionefficiencyforCO2 /N2 sensitivityandCO2 permeability.During alongextractionexperiment,theBTESA-100membranewasproventobedependable,and ithasalotofpossibilitiesinCO2 collectionapplications.However,owingtothepresence ofmoistureintheoperationalCO2 /N2 separationprocedure,themembranestabilityin humidifiedconditionsmustalsobeevaluated.
Furthermore,Suetal. [20] investigatedtheeffectofpollutantsinthefluegaseous,likeH2 O vapor,O2 andSO2 forthesorptionofCO2 /N2 integrationincarboxyldopedCNTmatrixand carbonnanotubes(CNTs)usingalargecanonicalMonteCarlosimulation.Themosteffective inhibitorofCO2 adsorptionwhenasolitaryuncleangasSO2 wasintroduced,whilewater onlyhadasignificantimpactatlowpressures(0.1psi),whena1DlatticeofH2 -bonded monomersformed.Furthermore,O2 wasdiscoveredtohavenoeffectonCO2 purificationand segregation.Withthreecontaminantsinfluegas,SO2 performedakeyfunctioninsuppressing CO2 adsorptionbydrasticallyloweringtheadsorptionquantity.Thiswasduetothefactthat SO2 exhibitedagreateraffinitywithcarbonwallsthanCO2 .Becauseofcorrelationsamong distinctentities,theinclusionofthreecontaminantsinfluegasincreasedtheadsorption intricacy.TheCNTmatrix’externaladsorptionregionwasheavilydominatedbyH2 O,which hydrophiliccarboxylgroupsmodified,andSO2 effectivelyadsorbsCO2 insidetheduct.These twoimpactsrestrictedCO2 adsorptionwhileincreasingCO2 /N2 selectivity,andthecontest amongthemcontrolledtheCO2 adsorptionpatternwithinandwithoutthetube.Furthermore, itwasdiscoveredthatintheexistenceofimpuritygas,carbonnanotubeconsistentlyretained theoptimumCO2 /N2 sorptionandsegregationefficiency,inbothsingleCNTandCNTarray situations.
Aconsiderableamountofwatermoleculesareabsorbedandaccumulatedamongtubesto createchainformations,asperthemolecularimageofwatermoleculesdepositedin(7,7)
CNTarrayinFig.4,althoughwatermoleculeadsorptionintubesisscarcelydetected.Atthe sametime,astuberadiusincreases,theadsorptionrateofwatermoleculesreduces.Measuring theweightfractionofinterferingcarboxylrevealsthatcarboxylconcentrationhasasignificant impactonwatermoleculeadsorptioncapability.Themasspercentageofcarboxylgroupdrops asthewidthofthetubeincreases,resultinginareductionintheadsorptioncapabilityof watermolecules.TheabsorptionofSO2 insmall-diameternanotubearrayswasaidedbythe existenceofwatermolecules.
1.2Utilizationofcapturedcarbondioxideforbiofuelproduction Worldwideclimatewarmingandrisinggreen-housegasemissions,andtheexhaustionof traditionalfuelsources,havebecomeanincreasingsourceofconcerninrecentdecades.Coal, oil,andnaturalgasburningreleaseupwardsof6billiontonnesofCO2 intotheenvironment eachyear[5,21–29].Inthiscontext,physiologicalCO2 reductionisincreasinginterestsinceit resultsintheproductionofenergythroughbiomassgeneratedbyCO2 fixationviaphotosynthesis.Becauseitisenergyeconomical,durable,andecologicallyfriendly,photosyntheticCO2 fixationisregardedtobeaviabletechnique.GreenplantsmaycaptureCO2 viaphotosynthesis, whichisanaturalprocess.Furthermore,duetothemitigatedratesofgrowthoftraditional landplants,CO2 collectionthroughsustainablenaturalsourcespredictedtobejust4–7percent offossilfueloutputs[12,30–33].Microalgae,oneitherhand,couldpresentapossibilitybecause toitsquantityandrapiddevelopmentproportion.Rapidlymaturingsinglecelledmicrobes calledmicroalgaehavea10–50percentgreatercapacitytoabsorbphotovoltaicradiation overbryophytessimultaneouslyfixingCO2 .Carbonicanhydrase(CA),anextracellularzinc metalloenzyme,aidsintheabsorptionofCO2 fromtheenvironmentbymicroalgalcells. CAcatalyzesthetransformationofCO2 tobicarbonates,thatareabsorbedbymicroalgal cellsviatransporter.TheCO2 collectedbymicroalgaeisretainedascarbohydrates,lipids,or proteins,basedonthegenus.ItmaybepossibletoextractCO2 frommicroalgaelipidstores anduseitasabiofuel.OneoftheleaststudiedmethodsforcapturingCO2 isthebiological pathwayviamicroalgae,inwhichCO2 isinstantlyconvertedtobiomassviasinglesource dischargesinspeciallydesignedplatformslikephotobioreactors.Phototrophicalgae’scarbon fixationhastheabilitytoreduceCO2 emissionsintotheenvironment,hencereducingglobal warming.MicroalgalCO2 biofixationinphotobioreactorsisapotentialmethodforproducing morebiomassandethanol.TheusageofphotobioreactorsforCO2 capturebymicroalgaehas severalbenefits,includingincreasedmicroalgalproductionowingtoregulatedatmospheric factorsandenhancedareaorvolumetricutilization,resultinginmoreeffectiveutilizationof expensiveland.Microalgaemightthusserveadualpurposebyloweringgreenhousegases throughCO2 sequestrationandsupplyingcleanerenergytomeettheexpandingneedfor energy.
1.2.1Photosynthesisandphotooxidationofwater
Photosynthesisisknowntobeabiologicalactivitythatisperformedoutbybacteria, algae,andelevatedplants.Itrelatestotheprocessthroughwhichspeciesturnlightenergy tothechemicalenergythroughgatheringlightandusingitforfuelbyCO2 adsorption.
1.Carbondioxidecaptureanditsutilizationtowardsefficientbiofuelsproduction
FIGURE1.3 At50°Celsius,aextended durabilityexperimentofCO2 /N2 (14/79) mixturesegregationfortheBTESA-100 porousfilmwasperformed.(Adapted fromRef. [19])MDPI2022.Published inaccordancewithCreativeCommon attributionLicenseCCBY4.0.
(A)(B) FIGURE1.4 At1.0bar,300K,amolecularimageofthe(7,7)CNTarrayincross-sections(A)andaxialaxis(B). (AdaptedfromRef. [20])MDPI2022.PublishedinaccordancewithCreativeCommonattributionLicenseCCBY4.0.
Carbongetstransferredfromtheenvironmentintobiomassinthismanner.Thewatersplittingprocess,whichresultsinthecreationofoxygen,isabonuselementofalgae’s photosynthesis.Thephotosynthesisreactionoccurredinchloroplasts,whicharespecialized organelles.Thephysicochemicalandbiologicalprocessesarethetwoseriesofstepsthat makeupphotosynthesis.Thebiophysicalprocessestakeoccurinthechloroplasts’thylakoid discs [34–39].Theabsorbingoflightphotonsbyessentialpigmentssuchaslikexanthophylls andcarotenesisreferredtoasphotonabsorption.Thewaterisoxidized,releasingoxygen (Fig.1.3).Thereductionofnicotinamideadeninedinucleotidephosphateandproductionof adenosinetriphosphate(ATP)arebothaidedbytheelectronsreleasedfromwatermolecules
FIGURE1.5 Photosynthesisandphotolysispathwaysofphotoautotrophicbacteriaaredepictedschematically. (AdaptedfromRef. [40])Springer2017.PublishedinaccordancewithCreativeCommonattributionLicense CCBY4.0.
(NADPH).TheenergyproducedwhileNADPHandATPareintheiractivestatesisusedin thedarkprocessestobindCO2 .Thestromaiswherethemetabolicresponseoccurs,andthe endmetabolitesareprimarilysugarmoleculesandafewotherchemicalcompoundsrequired formetabolicactivityandcellfunction.
1.2.2Bio-sequestrationofCO2
CO2 fromtheenvironmentisabsorbedduringthephotosyntheticcycle,thatiscarriedout bymicroalgaetoproducefeed.TheC3andC4routesarethetwoprocessesthroughwhich greenplantsassimilateCO2 fromtheenvironment.Around250,000speciesofC3plantsand 7500kindsofC4plantshavebeenidentified.ForCO2 fixation,manyalgaeutilizetheC3 pathway(CalvinCycle).CO2 ismixedusinga5-carbonmoleculetoproducedual3-carbon chemicalsinthisprocess.Ribulose-1,5-bisphosphatecarboxylase/oxygenase(RuBisCo)is referredtobeanenzymewhichcatalyzesthisprocess.Mostalgaearephotoautotrophs,which meanstheycanobtainalloftheirenergyfromphotosynthesisandmostoftheircarbonthrough carbondioxideabsorption.DiatomsarecategorisedasC4plantsbecauseitcouldabsorbCO2 inadifferentwaythanterrestrialagriculturalplantslikecorn,cotton,andwheat.C4plants combineCO2 usingatri-carbonmoleculetocreateatetra-carbonmoleculeinsteadusing
1.Carbondioxidecaptureanditsutilizationtowardsefficientbiofuelsproduction
RuBisCotocreatedualthree-carbonmolecules,limitingphotorespirationlossandimproving theefficiencyofCO2 fixation.C4plantsarebelievedtopossessdoublethephotosynthetic rateofC3plants,thoughthatthisadvantagehasbecomefewernoticeablewhenCO2 levels aresufficient.TheabsorbedCO2 isretainedascarbohydratesandlipidsinthealgalcells. TheHatchSlackphaseisusedbyC4plantsincomplementtotheBensonCalvinprocess. Inthisadditionalcycle,thephosphoenolpyruvatecarboxylase(PEPcase)enzymeachievesa pre-acquisitionofcarbondioxideintheformofatetra-carbonmolecule.Thebyproductsof thisprocessareemployedtoincreasethelevelofCO2 atthelocationwhereinRuBisCO(the carboxylationenzymeoftheBensonCalvincycle)isactive,preventingphotorespiration.The extracellularcarbonicanhydrase(CA)enzymeaidsintheabsorptionofCO2 bymicroalgal cells.It’sthoughttobethecarbonconcentratingmechanism’slikelymainenzyme.Theenzyme isinvolvedinabroadvarietyofmacroandmicroalgalorganisms.ItaidsinCO2 absorption bycatalysingtheinteractionbetweenHCO3 andCO2 .Ithasbeendiscoveredthatintracellular CAcanhappenintheidenticalcell.ThegenescodefortheCAisoformsarecontrolledbythe inorganiccarboninthemedia.Asaresult,theactionofCArisesastheamountofinorganic carboninthemediadecreases.InordertoconvertCO2 intoHCO3 inthecytosolduringC4 photosynthesisandfurnishsubstratesforPEPcarboxylase,CAisrequired.Theinformation gatheredfromtheresearchconductedwithCAinhibitorshasprovedthepresenceofCA. AlthoughCAactivityhasbeenstudiedinavarietyofmicroandmacroalgae,investigationsof thestandardgreenalgaChlamydomonasreinhardtiihaveprovidedthemajorityofthepresent knowledgeofthefunctionofCAinalgae.
Enhancingpassiveandactivecarbonabsorptionfromtheatmosphere. ReducingCO2 escapefromhigh-CO2 -concentrationareaswithinthecell.
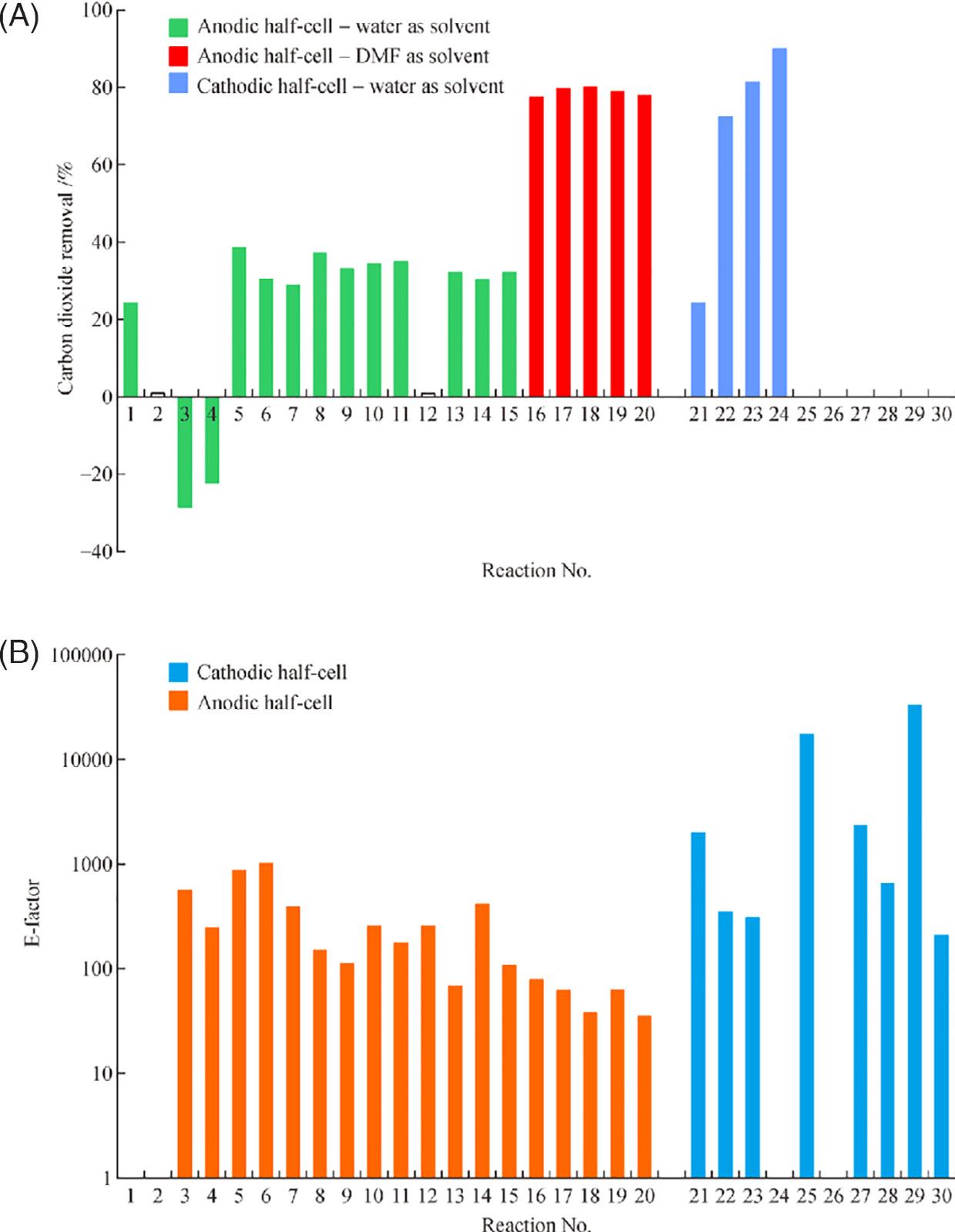
Montazersadghetal. [41] decidedtogenerateanovelelectrochemicalsystemforproducing low-carbone-biofuelsusingmultipurposeelectrosynthesisandintegratingCO2 covalorizationofbiomassresources.Drop-infuelswereproducedbyreducingCO2 nearthecathode, whereasvalue-enhancedchemicalswereproducedneartheanode.Inthisstudy,amathematicalanalysisofacontinuous-flowarchitecturewasestablishedtoevaluatethemosttechnoeconomicallyviablecombinationsbasedonenergyeffectiveness,environmentaleffect,and economicalideals.Afterthen,thereactorarchitecturewastweakedusingparametricstudy. Aconstantelectrolyticcellwasdesignedandconfirmedanalytically.Thealgorithmwasthen utilizedincombinationwithmultiplecellkineticstoestimatetheoptimalcellarchitecture fordistinctescenarios.Aselectionoforganiccompoundswithatleastoneinteractionfrom eachgroupwereusedtogeneratethekinetics.Themostcurrentdevelopmentsinbiomass oxidationforbiofuelgenerationandCO2 electroreductiondynamicsandarealsocoveredin thisresearch.Theoverallperformanceofthecellisimprovedbyusinganon-watersolvent becauseHERwasnotpredominantatthecathode.Whereastheenergycontentoftheprimary commodityprimarilydeterminestheenergyeffectivenessofthecell,properlychoosingthe reactorkineticscouldsignificantlyincreasetheefficiencyofCO2 conversion.Whencontrasted tocertainothermanufacturingapplications,thecumulativeenvironmentalimpact(E-factor) isconsiderable.Thisisowingtothesolvent’shugequantityincomparisontotheoutput, anditcouldbeminimizedbycyclingthesolventthroughtheprocess.Thebyproductsat theanodeforthespecifiedreactionmechanisminfluencethecell’seconomicallyadditional valueoneofthemost.BecausebothcompoundspossessarelativelyhighGibbsenergyoutput,
FIGURE1.6 Forseveralhalf-cellreactions,(A)CO2 captureratioand(B)E-factor.(AdaptedfromRef. [41]) Springer2021.PublishedinaccordancewithCreativeCommonattributionLicenseCCBY4.0.
theincreasedcellularenergyperformancewasenhancedto340percent.CO2 transformation frequencyof69.3percent,presentefficacyof56.7percentwithE-factorof704aresome oftheothercelleffectivenessparameters.TheCO2 conversionrateisalsoanothercrucial productivitycomponentthatcouldbeimproved(see Fig.1.6).BecausetheHERwasnot presentatthecathode,DMFwaspresumedtobethesolvent.
Similarly,Zdebetal. [42] discussedtheempiricalfindingsofincorporatingcarbondioxide asareagentinthevalorizationprocessincoalgasification.Threebasicsetupsfeaturingvaried modeledwasteheatusesituationsweretestedonabatchprocessmovingbedgasifier.CO2 , O2 ,andacombinationof30percentCO2 inO2 wereutilizedasgasificationreagentsat
FIGURE1.7 Configurationonalabscalewitharollingbedreactorandagasificationreagentpre-heatingsystem: (A)aperspectiveand(B)agraphicillustration.(AdaptedwithRef. [42])MDPI2019.Publishedinaccordancewith CreativeCommonattributionLicenseCCBY4.0.
temperaturesof700,800,and900°Celsius.Thecumulativeinfluenceofprocessingparameters oncoaltreatmentefficiencyofgasproductivity,content,andcalorificrangewasinvestigated, andtheempiricalvaluewasanalyzedutilizingPrincipalComponentAssessment.
Inthecontrolledsituationsused,thetrialsconfirmedthepossibilityofproducinggaswith acaloriecontentof4–6MJ/m3 bypyrolysiswithacarbondioxide-containinggasifyingagent. Eventhoughencouraginginthedevelopmentofenergy-efficientandlow-carbonfootprint processes,theconceptofcarbondioxidevalorizationandwasteheatutilizationincoal gasificationrequiresmuchfurtherbreakthroughsinrelationtoworkingassimilationaswell ascost-competitivenessmetricsuntilitcanberegardedforwidespreadapplication.
Similarly,Ahmadetal. [43] createdasystemofdata-basedsoftsensorsthatusesan ensembletechniquecalledboostingtoforecastthecontent,amount,andgradeoffattyacid methylesters(FAME)inthebiofuelsynthesisprocedureusingtheoilofseveralvegetables. Thenon-intrusivepolynomialchaosexpansion(PCE)techniquewasaddedintothesensitive detectorsdesigntoevaluatehowambiguityaffectedtheresults.Ineachoftheelements,flow rateandcetane,auniquemodel(softsensor)wascreated.TheanticipatedresultsofMethyl-Li, -O,-M,-P,-S,FAMEtransmissionrate,andcetanefrequencywere0.27479,0.32227,2.41208, 0.1651,0.82135,0.96546,and0.97013with1percentvariationinallsupplyparametersofthe sensitivedetectorswere0.27479,0.32227,2.41208,0.1651,Thesensorsareextremelypreciseat predictingandquantifyingambiguity,makingthemidealforpracticaluses.
Zhangetal. [44] focusedonthetechnicalandeconomicalconfigurationofsolid-oxideelectrolysisforthemanufactureofgreenmethanolbyHydrogenationofcarbondioxide.System unification,technicalandeconomicalanalysis,andmulti-objectivemanagementarecarried outsuccessfullyforaresearchproject.Theresultsshowatrade-offbetweenenergyefficiency andthecostofgeneratingCH3OH.Theassessedexample’sannualmethanolproduction was100kton,withaqualityof98.6percentweightandacarbondioxideusageof150kton,
offeringitanannualretentioncapacityof800GWhsustainableenergy.Methanolproduction costsapproach560$/tonhavinganelectriccostof74.26$/MWh,makingitcommercially unworkablewithanusablelifeofover13years,despitetheperformancebeingabout70 percentandvaryingwithinasmallrange.Whenthepriceofenergyisreducedto47dollars permegawatthourandsubsequentlyto24dollarspermegawatthour,thecostofproducing methanolfallsto365and172dollarsperton,respectively,witha4.6and2.8-yeareconomic success.Thecostofpowerhasaconsiderableinfluenceonprojectexecution.Thecostofpower variesbycountry,resultinginvariedpaybacktimesinvariousplaces.
Estevesetal. [45] examinedattheeffectsofdifferentlightfrequenciesonbiomassproduction,carbondioxidereduction,andnutrientremovalthroughasyntheticdischarge inTetradesmusobliquus,ChlorellavulgarisandNeochlorisoleoabundans.Light-emitting diodes(LEDs)havingvariedwavelengthswereusedintheexperimentations:620–750nm (red),380–750nm(white)and450–495nm(blue).N.oleoabundanswithwhiteLEDshadthe highestspecificgrowthrate(0.2640.005 d 1 ),whileC.vulgarishadthehighestbiomassoutput (144mgCO2 L 1 d 1 )andCO2 fixationrates(12.5mgCO2 L 1 d 1 ).Thethreemicroalgae investigatedhadthegreatestnitrogenandphosphorusextractionefficiencywhenexposedto whitelight.
Molinoetal. [46] developedScenedesmusalmeriensisintoagreenmicroalgaonabenchscaletotrapCO2 andproducelutein.Inaverticallyhydrodynamiccavitationphoto-bioreactor withasteadystreamofamixtureofgasesofN2 ,O2 andCO2 withtheformerhaving aconcentrationof0.0–3.0percentv/v,heterotrophicgrowthofS.almeriensiswascarried successfully.Batchingwasusedintheliquidphase.ThedevelopmentofS.almeriensiswasoptimized.Furthermore,luteinseparationwasconductedoutat59°Cand9MPautilizingrapid solventseparationusingC2 H5 OHtobeaGenerallyRecognizedasSafe(GRAS)substrate. Utilizingacarbondioxideconcentrationof2.9percentv/v,thehighestbiofuelproductivityof 129.24mgL 1 d 1 wasattainedduringinthedevelopment,allowingforaluteinconcentration of8.54mgg 1 ,thatwas5.6-foldgreaterthanthesimilarprocedureperformedwithoutCO2 . TheionchemistryanalysisofthegrowingmediumrevealedthatrisingCO2 concentrationprogressivelyboostednutrientintakethroughoutthegrowthstage.Becauseitfocusesonpigment creationfromanaturaloriginwhilealsocapturingCO2 ,thisresearchcouldbeofrelevancefor luteinharvestingatanindustriallevel. Fig.1.8 showstheinfluenceofCO2 concentrationon nutrientabsorptionasassessedattheconclusionofS.almeriensis’development.Theresults revealedafullphosphateionconsumption,thatwouldimpedecellularproliferation.The efficiencyofnutrientintakeimprovedasCO2 levelraised.Theextendedcultureperiods(i.e., 20daysforCO2 = 0.5percentv/v;16daysforCO2 = 1.5percentv/v;13daysforCO2 = 3.0percentv/v)did,though,helptoincreasenutritionalintake.Duringinthedevelopment period,nitrateandphosphatewerethemostheavilyabsorbednutrients.Aproposedreason forthisphenomenaisthatproteinproductionrequiresanitrogensupply,andluteinoccurs inmicroalgaeasanitrogenousmacromolecule.ThecurrentinvestigationshowedthatNO3 andPO4 3 ionsarethemostessentialnutrientforcellgrowthinmicroalgaedevelopment. Furthermore,withCO2 levelsof0.0,0.5,1.5,and3.0percentv/v,theabsorptionofNO3 ionswas5.0,59.88,77.26,and87.22percent,correspondingly,throughoutdevelopment.This findingcouldbeexplainedbyarestrictedcarbonsource,thatcausesstraininmicroalgae growthcells,resultinginreducedbiologicalnutrientabsorption.Incontrasttotheintakeof othernutrients,therewasareducedintakeofbothNa+ andClionsattheconclusionofthe