C Programming For Dummies 2nd Edition Dan Gookin
https://ebookmass.com/product/c-programming-for-dummies-2nd-editiondan-gookin/
ebookmass.com
The Economics of Optimal Growth Pathways: Evaluating the Health of the Planet’s Natural and Ecological Resources S. Niggol Seo
https://ebookmass.com/product/the-economics-of-optimal-growthpathways-evaluating-the-health-of-the-planets-natural-and-ecologicalresources-s-niggol-seo/
ebookmass.com
Neuroradiology Imaging Case Review Salvatore V Labruzzo
https://ebookmass.com/product/neuroradiology-imaging-case-reviewsalvatore-v-labruzzo/
ebookmass.com
Reluctant Cold Warriors: Economists and National Security
Vladimir Kontorovich
https://ebookmass.com/product/reluctant-cold-warriors-economists-andnational-security-vladimir-kontorovich/
ebookmass.com
Thermofluids:
From
Nature to Engineering Ting
https://ebookmass.com/product/thermofluids-from-nature-to-engineeringting/
ebookmass.com
First Aid for the Basic Sciences. General Principles 3rd Edition Tao Le
https://ebookmass.com/product/first-aid-for-the-basic-sciencesgeneral-principles-3rd-edition-tao-le/
ebookmass.com
Preparations,Properties,Applications andProspects
KazuyukiTakai
SeiyaTsujimura
FeiyuKang
MichioInagaki
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher ’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperiencebroaden ourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationor methodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomthey haveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeany liabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceor otherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthe materialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-819576-5
ForinformationonallElsevierpublicationsvisitour websiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionEditor: GlynJones
EditorialProjectManager: NaomiRobertson
ProductionProjectManager: SruthiSatheesh
CoverDesigner: MarkRogers
TypesetbyTNQTechnologies
Preface
TheNobelPrizeinPhysicsfor2010wasawardedtoProfs.A.GeimandK.Novoselovof theUniversityofManchesterfortheirgroundbreakingexperimentsongraphene.Theterm “graphene”wasproposedin1986inrelationtotheterminologyusedforgraphite intercalationcompounds.AftertheNobelPrize,scientificandtechnologicalinterestin grapheneincreasedrapidly;asaconsequence,atremendousamountofliteraturedeclaring itsresearchtargettobegraphenehasbeenpublished.Unfortunately,thisrapidgrowthin interesthascausedseriousconfusionregardingthedefinitionandterminologyofgraphene eveninscientificjournals.
Twoofthecurrentauthors,M.InagakiandF.Kang,haveauthoredthreebooksinaseries, entitled MaterialsScienceandEngineeringofCarbon:Fundamentals, AdvancedMaterials ScienceandEngineeringofCarbon,and MaterialsScienceandEngineeringofCarbon: Characterization.Itwasanticipatedthatthesebookswouldprovideacomprehensive understandingofawiderangeofcarbonmaterials(graphite,graphitizedcarbons,carbon blacks,activatedcarbons,pyrolyticcarbons,glass-likecarbons,porouscarbons,carbon fibers,etc.,inadditiontodiamond,fullerenes,carbonnanotubes,andgraphene)throughthe detailedexplanationanddiscussionoftheirstructures,nanotextures,andfundamental properties.However,theoccurrenceofmisperceptionsandtheflood-likeincreasein researchpapersongraphene-related(graphene-like)materialsledthesetwoauthorsto believeitwasnecessarytoeditabookfocusingongraphene-relatedmaterials,inaddition tothethreepreviouslymentionedbooks,althoughgrapheneisamembersofthecarbon family.Therefore,theyinvitedtwoauthors,K.TakaiandS.Tsujimura,whoarespecialists inthephysicalchemistryofgraphene,andelectrochemistryapplicationstobiomedicals, respectively,tocoverthewidelyspreadapplicationsofgrapheneinthisbook.
Inthisbook,theauthorsattemptedtoprovidesummariesandreviewsongrapheneandits relatedmaterialsbydifferentiatingmaterialswithahighperfectionofstructure(graphene) fromthosethatarehighlydefective,evenwithvariousfunctionalgroupsattached(reduced grapheneoxide).Inaddition,itisemphasizedthatthenumberoflayersgovernsthe propertiesoftheflakesofgraphene(thecharacteristicsofgraphene)whicharequite differentfromthoseofgraphite(manylayersstacked)andpossibletobeobtainedonthe flakeofonlyafewhighly-crystallinelayersstacked.
Tounderstandgrapheneanditsrelatedmaterials,awiderangeoffundamentalknowledge onvariouscarbonmaterialsisessential:thatis,knowledgesuchascarbonization, graphitization,intercalation,andsoon,inadditiontobasicknowledgeaboutchemistry, physics,biology,andothers.Forthereaders’convenience,itisrecommendedtoconsultthe threebooksmentionedpreviously,whicharepublishedbyTsinghuaUniversityPressand Elsevier.Thesebookswillsupplyfundamentalknowledgeaboutcarbonmaterialsand provideanunderstandingofabroadrangeoftopicsinthecurrentbook.
Itwillbetrulypleasingtoalloftheauthorsifthecontentofthisbookdeliversuseful informationtothereadersandwillleadreaderstothecorrectunderstandingofgraphene andrelatedmaterials.
Acknowledgments
Theauthorswouldliketoexpresstheirsincerethankstothepeoplewhokindlyprovided thedataandfiguresforthisbook.Thenamesandaffiliationsofthecontributingpersonsare mentionedinthecaptionsoffigures.Theauthorsalsothankallofthepeoplewhotookcare ofthisbookatTsinghuaUniversityPressandalsoatElsevier.
Introduction
ChapterOutline
1.1Whatisgraphene?1
1.2Fundamentalsofmaterialsscienceforcarbonmaterials5
1.2.1Classificationofcarbonmaterials5
1.2.2Structureandnanotextureofcarbonmaterials6
1.2.3Carbonizationandgraphitization8
1.2.4Carbonmaterials12
1.2.4.1Highlyorientedgraphitematerials12
1.2.4.2Syntheticgraphitematerials17
1.2.4.3Fibrouscarbonmaterials20
1.2.4.4Nanoporouscarbons23
1.2.4.5Sphericalcarbonmaterials26
1.2.4.6Glass-likecarbons28
1.3Constructionandpurposesofthecurrentbook29 References31
1.1Whatisgraphene?
Theterm“graphene”wasfirstlyproposedin1986 [1] andthenrecommendedbythe InternationalUnionofPureandAppliedChemistry [2] asthenameforasingletwodimensionallayerofcarbonatomsbondingusingsp2 hybridorbitals,whichoccursin graphiteintercalationcompounds.Thesinglecarbonlayeroccurringintheintercalation compoundswasproposedtobecalled“graphene”whichcomesfromthesuffix“-ene”for polycyclicaromatichydrocarbonssuchasnaphthaleneandanthraceneandtheprefix “graph-”fromgraphite.Intheearliestliterature [1],theauthorsnotedthat“itshouldbe adoptedforgraphiteintercalationcompounds.”Inthefirst-stagestructureofthe compounds,atwo-dimensional(2D)carbonlayerissandwichedbytwointercalatelayers andisolatedfromothercarbonlayers,althoughmorethantwocarbonlayersarestackedin parallelwithregularityincompoundswithhigherthanasecondstage,asschematically illustratedin Fig.1.1.
“Graphene”iscommonlydefinedasanisolatedsinglelayerofcarbonhexagonsconsisting ofsp2 hybridizedC Cbondingwith p-electronclouds.Fromanengineeringpointof view,thinflakesconsistingofafewlayersofcarbonatoms,includingsingle-layer
Graphene. https://doi.org/10.1016/B978-0-12-819576-5.00001-3 Copyright © 2020ElsevierInc.Allrightsreserved. 1
Stagestructureofgraphiteintercalationcompounds.
graphene,couldbeimportantbecauseoftheirinterestingstructural,chemical,andphysical characteristics.Thepromisingpotentialapplicationsofgrapheneanditsrelatedmaterials havebeenpointedoutintechnologicalfields.
Numerousreportshavebeenpublished,particularlyaftertheNobelPrizeinPhysicswas awardedin2010toProfs.A.GeimandK.NovoselovoftheUniversityofManchesterfor theirgroundbreakingexperimentsingraphene [3].Suddenscientificandtechnological interestingrapheneanditsrelatedmaterialshascausedsomeconfusionaboutthe definitionandterminologyofgraphene-relatedmaterials,eveninscientificjournals.A proposalregardingthenomenclaturefortwo-dimensionalcarbonmaterialswasbeen presentedinthejournal Carbon [4].Inmuchoftheliterature,however,theterm “graphene”hasnotbeenusedaccordingtoitsstrictdefinition,i.e.,asinglelayerofcarbon atomsconsistingofsp2 hybridizedbonds.Someauthorsdonotpayenoughattentionto howmanylayersarestackedintheirmaterials,althoughtheyhavecalledthem“graphene” Therefore,here,theterms“single-layergraphene”(ormonolayergraphene),“doublelayeredgraphene”(orbilayeredgraphene),and“multilayeredgraphene”areusedonlyfor productsthathaveconfirmednumbersofstackedlayers.
Numerousreviewshavebeenpublishedfromvariousviewpoints:providinganoverviewof graphenebypointingoutwhatisfascinatingaboutit [5 13],focusingonitsproductionin relationtoitsstructure [14 23],applicationsinelectronics [24 30],energystorage applications [31 48],biologicalapplicationsincludingsensors [49 68],functionalization includingtheformationofcomposites [69 80],mechanicalapplications [81,82],thermal properties [83,84],dopingtoimproveitsproperties [85,86],anditstoxicity [87,88]
Figure1.1
Beforesummarizingresearchongraphene-relatedmaterialsanddiscussingtheresults,it isworthwhiletounderstandtheflakesand/orfilmsthatdeterminehowmanynumbersof layerscanexhibittheuniquefunctionalitiescharacteristicofgraphene,todifferentiateit fromgraphite. Fig.1.2 comparesRamanspectraareforgraphiteandgrapheneflakes withdifferentnumbersofstackedlayers [89].Asshownin Fig.1.2A ,highlyoriented pyrolyticgraphite(HOPG),forexample,showsasharpandstrongG-bandandG0 -band (2Dband).Thelatterispresentedasdecomposingintotwobands,G0 1 andG 0 2,with heightsofroughly¼and½oftheG-band,respectively.Ontheotherhand,single-layer grapheneexhibitsasharpandsingleprofileforbothG-andG0 -bandsandtheG0 -band ismuchstrongerthantheG-bandbyalmostfourtimes.Asshownin Fig.1.2B,the G 0 -bandgraduallyshiftstoahigherposition,changingitsprofiletounsymmetricaland decreasesitsintensityrelativetotheG-bandwithanincreasingnumberofstacked layers.
Amarkedfieldeffectonelectricalconductionisacharacteristicofgraphene.Pioneering workswerereportedonfew-layeredgrapheneflakes [90] Fig.1.3A showsresistivity changewithgatevoltage Vg onfew-layeredgrapheneflakesasafunctionoftemperature, revealingamarkedfieldeffect,apeakofseveralkU withrapiddecaytoabout100 U at high Vg,andastrongdependenceontemperature.Thisfieldeffectisshownbyplotting therelativecarrierconcentration n/n0 (n0 istheconcentrationofcarriersat4K)against temperatureforthreeflakes,few-layeredflakes,few-layeredbutmuchthickerflakes,and multilayeredflakesin Fig.1.3B.Theresultsclearlyrevealthatthefieldeffectdependson thenumberofstackedlayers:thesmallerthenumberoflayers,thestrongerthefield effect.
Figure1.2
Ramanspectrameasuredbyalaserwith514-nmwavelengthforgraphitetosingle-layergraphene [89]:(A)comparisonofgraphiteandsingle-layergraphene,(B)ChangeinG0 -bandwith increasingnumberofstackedlayers. 2D,two-dimensional;a.u.,arbitraryunits.
Fieldeffectforfew-layeredgrapheneflakes [90]
Largest data
Averaged value
Theoretical prediction I
Theoretical prediction II
Figure1.4
Changeinthermalconductivityatroomtemperaturewithnumberoflayersstacked [91]
Extremelyhighthermalconductivity,morethan5000W/m K,isexpectedforsingle-layer graphene [91].Itdependsonthenumberoflayersstackedintheflake.In Fig.1.4,thermal conductivitiesexperimentallydeterminedfromthetemperaturedependenceoftheposition ofthemicro-RamanG-bandtogetherwiththeoreticalpredictionsareplottedagainstthe numberofstackedlayers [91].Amarkeddependenceofroomtemperaturethermal conductivityonthenumberoflayersisclearlyshown.Single-layergraphenehasextremely highconductivity,butthatofaflakecomposedoffourlayersiscomparabletobulkgraphite withhighcrystallinity,suchasHOPG,althoughitishighcomparedwithmetals.
Figure1.3
Thesethreeexperimentalresultssuggestthatweneedaflakeand/orfilmcomposedofless thanfourlayerstoobtaincharacteristicsintrinsictographene.
1.2Fundamentalsofmaterialsscienceforcarbonmaterials
Grapheneisamemberofcarbonmaterials,whichincludegraphite,carbonblacks,carbon nanotubes,carbonfibers,activatedcarbons,porouscarbons,diamond,andfullerenes.To preparegrapheneflakesandfilms,graphitematerialssuchasnaturalgraphite,HOPG,and kishgraphitehavebeenemployedasstartingmaterials.Inadditiontographene,other carbonmaterialssuchasactivatedcarbonsandporouscarbonsincludingcarbonfoamsand carbonnanotubeshavebeencitedinreferencesand/orcompetitivematerials.
Beforediscussinggraphene,thefundamentalsofcarbonmaterialssciencewillbebriefly explained,emphasizinghighlyorientedgraphitematerialsthathavebeenusedasraw materialsofgraphene.Foradetailedexplanationanddiscussionofcarbonmaterials, readersofthisbookaresuggestedtorefertofundamentalbookswrittenbythecurrent authors(M.I.andF.K.) [92,93].
1.2.1Classificationofcarbonmaterials
Manykindsofcarbonmaterialshavebeenmanufactured,synthesized,andwidelyusedin variousindustries.Thesecarbonmaterialsareproposedtobeclassifiedonthebasisof theirchemicalbondsofconstituentcarbonatomsusingsp3,sp2,andsphybridorbitals. Thesp2 hybridbondingofcarbonatomsresultsintwostructures:flatlayerscomposedof six-memberedcarbonrings,whichhadbeenrepresentedbygraphitebutnowinclude graphene;andcurvedlayers,whichintroducefive-memberedcarbonringsintosixmemberedrings,asoccursinfullerenes.Layerscomposedofsp2 orbitals,bothflatand curved,areintrinsicallyanisotropicandhave p-electroncloudsatbothsidesofthelayer, whichcreatesbroaddiversityinthestructureandpropertiesofthematerials.Carbon nanotubescanbeplacedbetweenfullereneandgraphene,becausethetipsofthetube includefive-memberedrings(fullerene-like)anditswalliscomposedofsix-membered ringsalthoughitisrolledup(graphene-like). Fig.1.5 showstheclassificationofcarbon materialsbasedonhybridbonds,togetherwiththediversityofeachmaterial.
Becauseoftheanisotropicnatureandthepresenceof p-electroncloudsinthecarbon materialscomposedmainlyofsp2 hybridorbitals,thenumberoflayersstackedinparallel hasastronginfluenceontheirproperties.Theimportanceofthenumberofstackedlayers hasbeenpointedoutforcarbonnanotubesandfullerenes,andnowforgraphene,as mentionedintheprevioussection.Largenumbersoflayersstackedwithregularityhave beencalledgraphite,andvariousgraphite-relatedmaterialshavebeenproducedin industriesandusedasimportantindustrialmaterials,someofwhicharelistedin Fig.1.5
sp3
Diamond
Crystalline Cubic Hexagonal
Non-crystalline Diamond-like carbon (DLC)
Crystalline Hexagonal Rhombohedral
P1=1.00
d002=0.3354 nm
Oriented
Planar orientation
Highly-oriented graphite
Planar Graphene
Single-layer to Multi-layered
Graphite
Synthetic (artificial) graphite
Intercalation compounds
Pyrolytic carbons
C 2s22p2
sp2 + π
Carbon nanotubes
Single wall
Chirality
Multi-walled
Graphitization degree
Nanotexture
Axial orientationPoint orientation
Carbon fibers C/C Composites
Figure1.5
Carbon blacks
Curved Fullerenes sp+ 2π
Single wall C60, C70, ··· Multi-walled
Carbyne
Chain length, etc.
Non-crystalline Turbostratic
P1=0.00
d002>0.344 nm
Not-oriented
Random orientation
Activated carbons
Glass-like carbons
Classificationsanddiversityofcarbonmaterials.
1.2.2Structureandnanotextureofcarbonmaterials
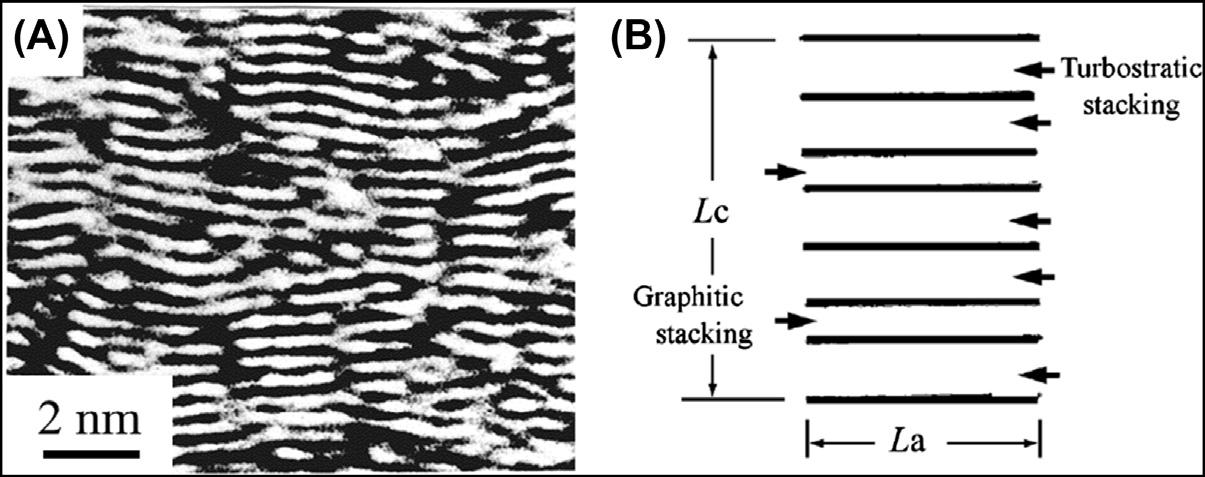
Mostcarbonmaterialscomposedofflatlayersusingsp2 hybridorbitalsconsistofsmall unitsoflayersstackedinparallel,asshownbythetransmissionelectronmicroscopy (TEM)latticefringeimageandschematicillustrationin Fig.1.6.Theseunitsarecalled basicstructuralunits(BSU)orcrystallites.Inaunit,twokindsoflayerscoexistwith stackingregularity:randomandregularstacking.Theformeriscalledturbostraticstacking andthelatterisgraphiticstacking(usuallywrittenasABstacking).TheseBSUsare stronglyanisotropicinthenatureoftheirbond,withstrongcovalentbondingusingsp2 hybridorbitalalongthelayerandweakvanderWaalsbondingacrossthelayers.
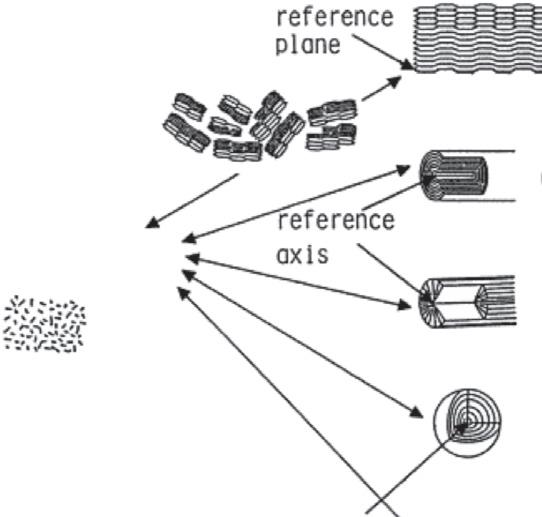
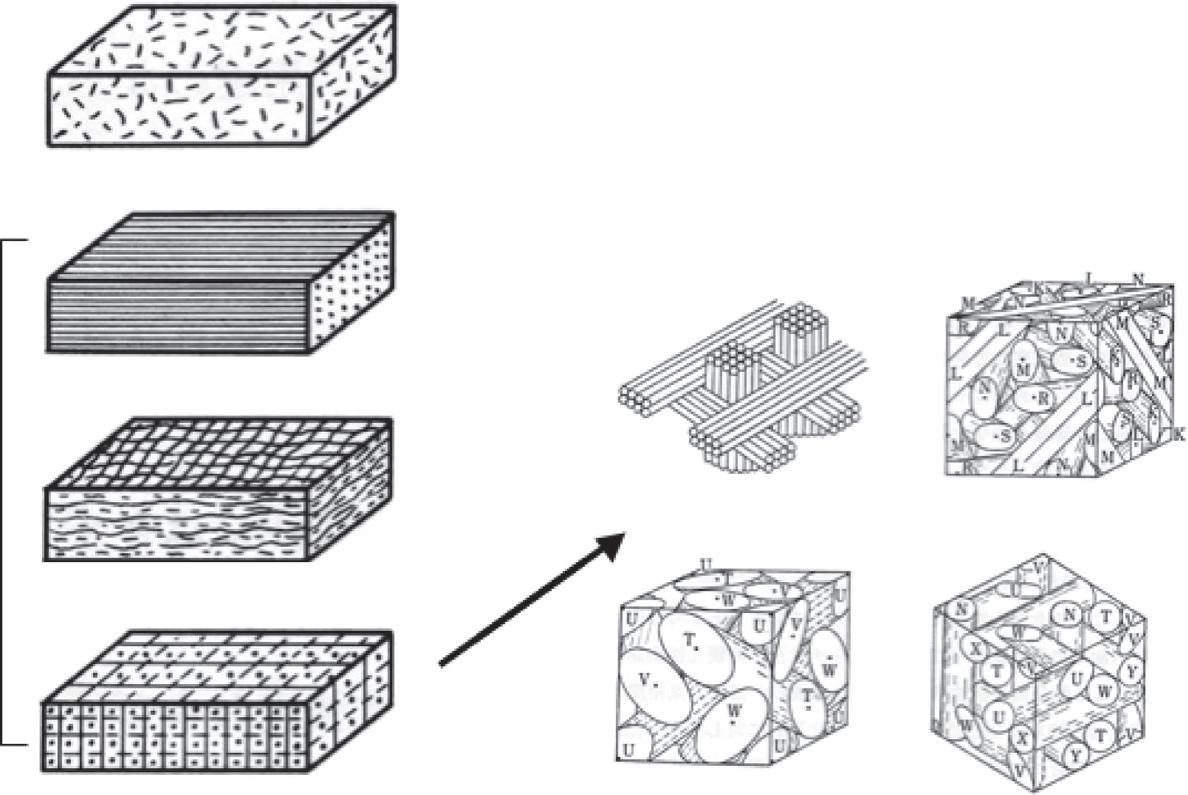
TheaggregationoftheseanisotropicnanosizedBSUsintheparticlesresultsindifferent texturesowingtothedifferentschemaofpreferredorientationsofanisotropicBSUs(i.e., planar,axial,point,andrandomorientations),asillustratedwithsomerepresentative carbonmaterialsin Fig.1.7.TheaggregationofBSUsindifferentschemaiscalled nanotextures.Bytheplanarorientationscheme,filmsandplateletsofhighlyoriented
Figure1.6
Basicstructuralunitofcarbonmaterials:(A)latticefringeimageand(B)schematicillustration ofaunit.
RANDOM NANOTEXTURE
Glass-like carbons
Random orientation
ORIENTED NANOTEXTURE
Planar orientation
Axial orientation
Figure1.7
Highly-oriented graphites
Cokes Carbon nanotubes
Carbon fibers
Point orientation
Fullerenes
Carbon blacks
Nanotexturesincarbonmaterialsonthebasesofpreferredorientationsofbasicstructuralunits.
graphiteareproducedandsomecokeparticlesareprincipallycomposedofplanar orientationofBSUs.Bytheaxialorientationscheme,fibrouscarbonmaterialsare producedfromdifferentprecursorssuchascarbonnanotubesandvapor-growncarbon fiberswithacoaxialmodeoforientationandsomemesophasepitch-basedcarbonfibers witharadialmode.Bythepointorientationscheme,variouscarbonspheresareproduced, aswellasvarioussizedfullereneparticlesanddifferentnanosizedcarbonblackswith concentricmodeandmesophasesphereswitharadialmode.Theparticlescomposedof theseorientednanotexturesarestillanisotropic.Inaddition,randomaggregationofsmall BSUsoccursinso-calledglass-likecarbon(glassycarbon),theparticlesofwhichare isotropicinnature.
1.2.3Carbonizationandgraphitization
Mostcarbonmaterialsusedinindustryareproducedfromorganicprecursorssuchas pitches,biomasses,andorganicpolymersviaheattreatmentathightemperaturesinan inertatmosphereduringcarbonizationandgraphitizationprocesses. Fig.1.8 summarizes changesinchemicalandelectronicbandstructures.
Carbonizationisperformedafterpyrolysisoftheprecursorsfrom800to2000 C,inwhich theBSUsareformedandtheirbasicaggregationscheme(nanotexture)isestablished, accompaniedbytheemissionofforeignatoms,oxygen,hydrogen,andnitrogenasgases andthepolycondensationofsix-memberedcarbonrings.Becausethenanotextureofmost carbonmaterialsisestablished,thisprocessismostimportantintheproductionofvarious carbonandgraphitematerials.Thenanotexturegovernsthedevelopmentofcrystalline structuresincarbonmaterialsduringgraphitization.Duringthisprocess,alargeamountof shrinkageandtherapidemissionofgasspeciesoccurthatareassociatedwithcrackingof carbonparticlesthatoccurinmanycases.Therefore,theprocessofcarbonizationis appliedseparatelyfromthatofgraphitization.
Above2000 C,achangeincrystallinestructure(thedevelopmentofagraphitestructure) mainlyoccurs.Thedevelopmentofagraphitestructuremaybeevaluatedbydifferent techniques,includingx-raydiffraction(XRD),electromagneticpropertymeasurements, Ramanspectroscopy,andhigh-resolutionTEM.InBSUsformedduringcarbonization,
Schematicillustrationofchemical,crystallographic,andelectronicbandstructuresincarbon materialswithaplanarorientationnanotexture. MW,molecularweight.
Figure1.9
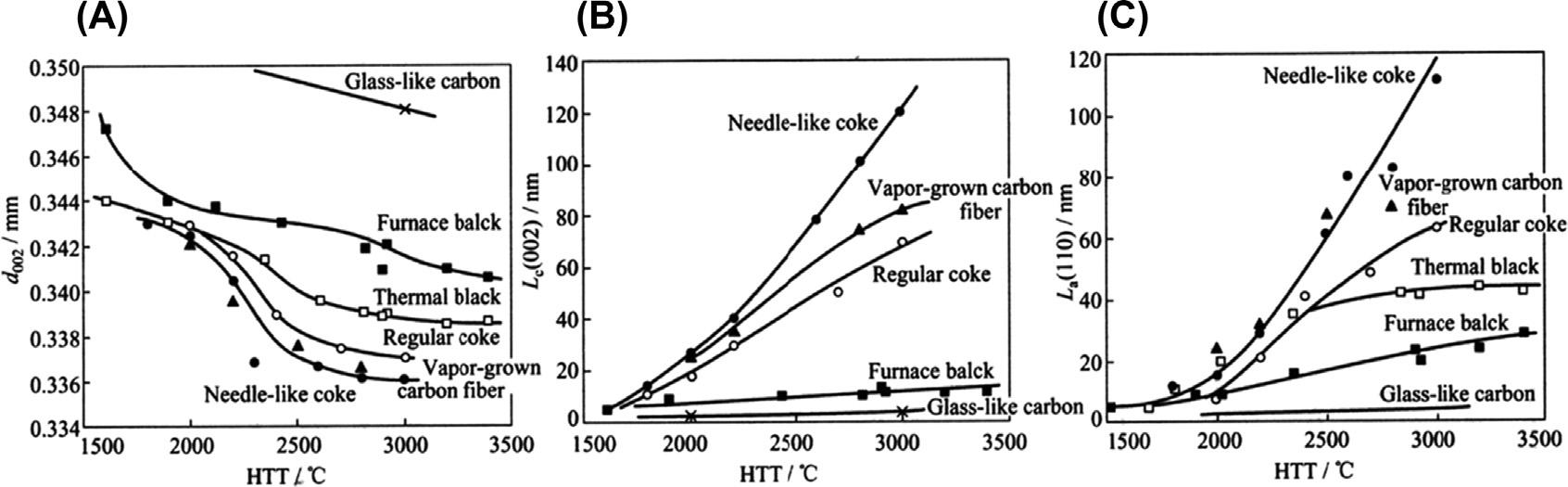
Changesinx-raydiffractionparameterswithheattreatmenttemperatures(HTT)forvarious carbonmaterials:(A) d002,(B) Lc measuredfroma 002 diffractionpeak,and(C) La froma 110 peak.
turbostraticstackingwithaninterlayerspacingofabout0.342nmisrandomlychangedto graphiticregularstackingwithaspacingof0.3354nm(thespacinginthegraphitecrystal) withanincreasingheattreatmenttemperature(HTT)ofabove2000 C,whichismeasured asadecreaseintheaveragedinterlayerspacing, d002,byXRD,associatedwiththegrowth ofBSUsizes(crystallitesize)alongthea-andc-axes, La and Lc.Thechangein d002 with HTTdependsonthematerialsaftercarbonization(carbonmaterials). Fig.1.9 showsthe changesintheseparameterswithHTTforvariouscarbonmaterials.Inneedle-likecoke withaplanarorientationscheme, d002 decreasesquickly,approachingthevalueofa graphitecrystal(0.3354nm),and Lc and La growrapidly.Inglass-likecarbonwitha randomorientationscheme,incontrast,thereisalmostnodecreasein d002 andno appreciablegrowthin Lc and La evenafter3000 Ctreatment(i.e.,nodevelopmentofa graphitestructure).Carbonblackswithapointorientationschemehaveintermediate behaviors:thelarge-sizedthermalblackshowsmoreimprovementinstructurethandoes thesmall-sizedfurnaceblack.
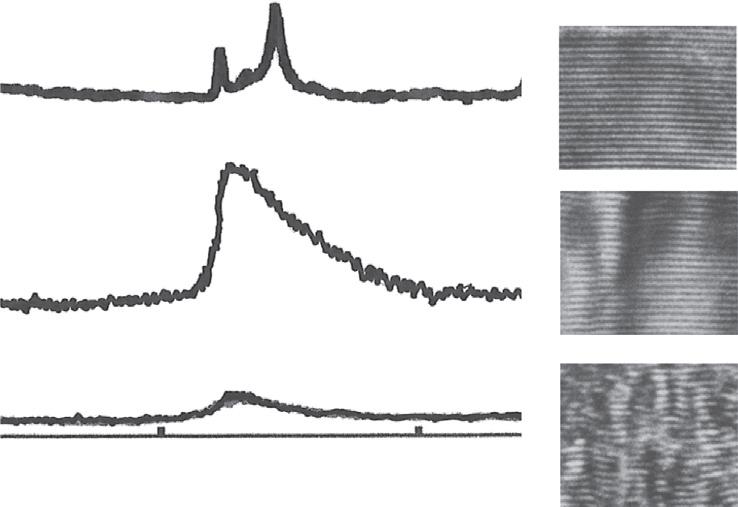
DiffractionpeaksofXRDforcarbonmaterialsareclassifiedintothreegroups: 00l, hk0, and hkl,mainlyowingtothestronganisotropyofBSUsandthecoexistenceoftwo interlayerspacings,asshownin Fig.1.6B.Diffractionpeakswithindicesof 00l give averagedinterlayerspacing,whichdecreasesgraduallyfrommorethan0.344nmfor highlydefectivelayersto0.3354nmforgraphiticstackingandabout0.342nmfor turbostraticstackingwithincreasingHTT:inotherwords,withimprovingcrystallinity ofcarbon,asshownin Fig.1.9A.Inturbostraticstackingoflayers,thereisno3D regularityinstacking(i.e.,no l indexisdefined),andsothediffractionpeakswith hk0 indicesforagraphiticstructureareexpressedas hk diffractionpeaksforcarbon materialsmainlyconsistingoftheturbostraticstackingoflayers.Regularandrandom stackings,graphiticandturbostratic,areclearlydemonstratedindiffractionprofilesof
Figure1.10
d002=0.3345 nm Regular stacking (graphite structure)
d002=0.342 nm Random stacking (turbostratic structure)
d002>0.344 nm Minute BSUs
(A)Correspondencebetweenx-raydiffraction(XRD)profileof hk lineand(B)transmission electronmicroscopy(TEM)image. BSU,basicstructuralunits.
hk0 and hk peaks,asshownfor 101 and 10 peaksin Fig.1.10 .Theprofileofthe 10 peakforaturbostraticstructureshowsacharacteristicunsymmetricalprofile,andthat ofa 100 peakforagraphiticstructureissharpandsymmetricalandisassociatedwith a 101 peakowingtotheformationof3Dstackingregularity.WithincreasingHTT,an unsymmetrical 10 peakismodulatedbytheappearanceofa 101 peak,togetherwithits sharpenedandimprovingsymmetry.Peakswithindicesof hkl arecausedbythe graphitestructure,andso 112 peakisoftenselectedastoindicatetheformationofa graphitestructure,becausethe 112 peakdoesnotoverlapwithotherpeaksalthoughthe 101 peakoverlapswiththe 100 peak,ascanbeseenin Fig.1.10.
Becausemostcarbonparticlesaremoreorlessanisotropic,exceptforglass-likecarbon witharandomorientationscheme,theiraggregationintoablockgivestextureonanlarger scale,whichmaybecalledmicrotextureandmacrotexture.Thetechniqueforevaluating thesemicro-andmacrotextureshasnotyetbeenestablished. Figs.1.11and1.12 demonstrateexamplesofthesetextures,whichmustbecontrolledforpractical applications.In Fig.1.11,scanningelectronmicroscopy(SEM)imagesofcross-sections ofcommerciallyavailableisotropichigh-densitygraphiteareshown,demonstrating differentporestructures [94].Imageprocessingofthesemicrographssuggestsarelation betweenporestructureparameters(forexample,theporearea)andthemechanical propertiesofthesegraphiteblocks.Topreparecarbonfiber reinforcedplasticsandcarbon fiber carboncomposites,differentmacrotexturesbasedontheorientationofcarbonfibers areemployedtoobtainhighstrengthandahighmodulusofthecomposites,asshownin Fig.1.12.
TEM (002 lattice fringe image)
Figure1.11
SEMimagesofthecross-sectionsofisotropichigh-densitygraphiteblocks [94].
Chopped fibers
Random
One-directional
Long fibers
Three-dimensional
Two-directional
Three-directional
Figure1.12
Differentschemeforreinforcingcarbonfibersincomposites.
1.2.4.1Highlyorientedgraphitematerials
Theblocksorplatelets,whicharecomposedofbigcarbonlayersstackedinlargeamount withgraphiticregularity,arecalledhighlyorientedgraphite;theextremecaseofthisisa singlecrystalofgraphite.Inpractice,however,itisdifficulttoobtainsinglelargecrystals andalmostimpossibletogetthosethataremorethanafewsquaremillimeters.Thereare onlytwowaystofindsinglecrystalsofgraphite:innaturalgraphiteoresandinso-called kishgraphite.Resourcesofhigh-qualitynaturalgraphitearelimitedonearthandexistin SriLanka,Madagascar,andChina.Evenintheseores,thepossibilityoffindingsingle crystalsofacertainsizeisslim.Kishgraphiteisformedbytheprecipitationof supersaturatedcarbonfrommoltenironandcannotbelargeinsize,butsomehavevery highcrystallinityandcanbecalledsinglecrystals.Alternativestosingle-crystalgraphite areHOPGandgraphitefilmsderivedfromsomeorganicpolymerprecursorssuchas polyimidesviahigh-temperaturetreatment.
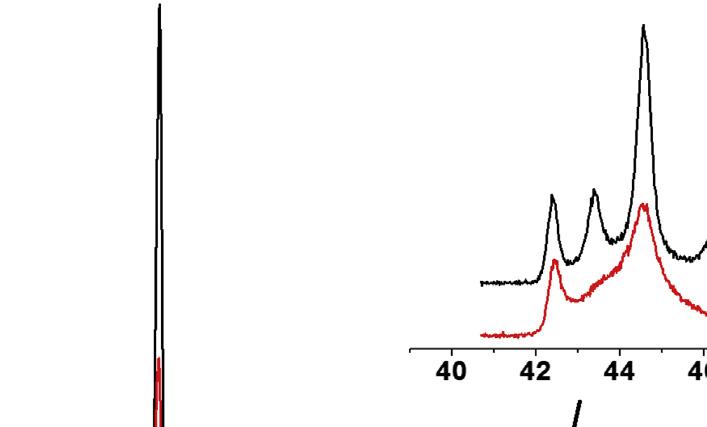
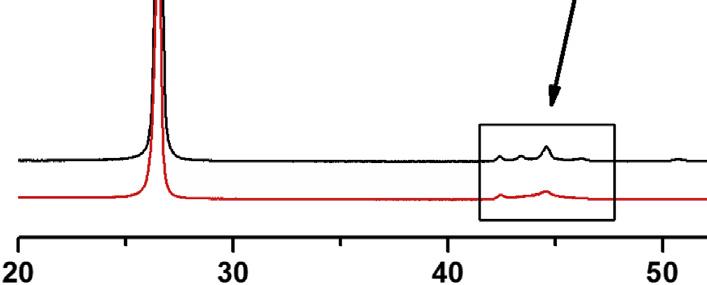
Naturalgraphite:Naturalgraphiteisusuallyrecoveredasapowderfromnaturalores throughmillingandpurificationprocesses.Afterthefinalpurificationprocess,thepowder hasusuallyapurityofmorethan99wt%.Someofitconsistsofflakyparticleswitha highlycrystallinestructureandhighlyorientednanotexture(flakygraphite),butsome consistsofaggregatesofsmallcrystals(calledamorphousgraphite) [95].Thesetwokinds ofnaturalgraphitecanbedifferentiatedbyXRD,asshownin Fig.1.13,whichshows
002
003
2θ / degree
Figure1.13
graphite
X-raydiffractionpatternsofflakyandmicrocrystallinenaturalgraphite [95]
flakygraphitewithsharp 100 and 101 peaksbutamicrocrystallinegraphitebroad 101 peak.Someflakygraphitecontainsmetastablerhombohedralmodificationofthegraphite structure,possiblyasaresultofshearstressduringmilling.
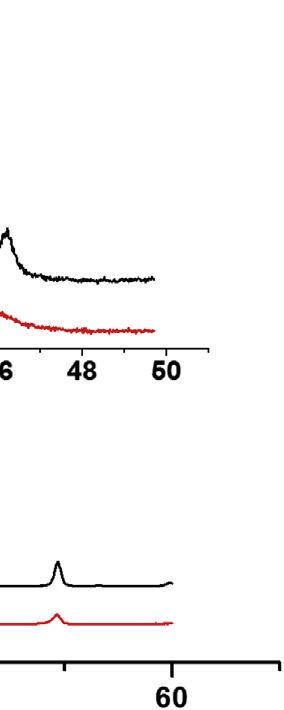
Kishgraphite:Apartofcarbonsdissolvedintomoltenironathightemperaturesis incorporatedintothecrystallatticeofirontoformalloys(differentsteels)andanotherpart segregatesasgraphite.Graphiteflakessegregatedfrommoltenironarecalledkish graphite [96 98].Relativelylargeamountsofkishgraphiteflakesareobtainedasabyproductduringsteelproduction;allofthemdonotalwayshavehighcrystallinitybecause itdependsonthesegregationconditions.Whentheyareproducedatthetemperatureat whichironevaporates,kishgraphiteflakeshaveasinglecrystalnature.Asshownin Fig.1.14,flakesarethinwithanirregularshape.Regularstackingoflayersisobservedby SEMandtheirsinglecrystalnatureisconfirmedbyawell-organizedelectronchanneling pattern.Thehighcrystallinityofkishgraphiteflakeswasalsoconfirmedbymeasurements oftheresistivityratio, r300K/r4.2K,maximumtransversemagnetoresistance,(Dr/r0)max, measuredat77K,andShubnikov-de-Haasoscillationinmagnetoresistance.
Graphitesinglecrystalshavebeensynthesizedfrommoltenironbycontrollingthe segregationprocess [99,100] andfromAl4C3 bytransportingdecompositiongases [101,102].
Highlyorientedpyrolyticgraphite:Carbondepositedonasubstrateby chemicalvapor deposition (CVD)ofhydrocarbongasessuchasmethaneandpropaneathightemperatures canhaveawell-orientedtextureandiscalledpyrolyticcarbon.Pyrolyticcarbonshave extensivelybeenstudiedtocontroltheirstructureandtexturebyapplyingdifferent depositionconditions,suchasprecursorhydrocarbon,theconcentrationandflowrate,the temperatureofdeposition,andthegeometryofthefurnace [103].Byhot-pressingathigh temperaturesunderhighpressure,thepreferredorientationofgraphitecrystallitesandtheir crystallinitycanbemarkedlyimproved.Typicalconditionsareshownin Fig.1.15[104].
Figure1.14
Kishgraphite:(A)opticalmicrograph,(B)scanningelectronmicroscopyimageoftheedge surface,and(C)electronchannelingpatternonthebasalplane. CourtesyofProf.Y.Hishiyamaof TokyoCityUniversity.
Hot pressing
2800-3000 °C, 30-50 MPa
Oriented pyrolytic graphite (OPG)
Annealing under pressure
3400-3600 °C, 10 kg/cm2
Pyrolytic carbon (PC) deposited at 2100-2500 °C mosaic spread of 40-50° mosaic spread of less than 0.5 °C density of 2.226 g/cm3 mosaic spread < 0.4°
Highly-oriented pyrolytic graphite (HOPG)
Figure1.15
Procedureoftheproductionofhighlyorientedpyrolyticgraphite.
TheproductsofthisprocessarecalledHOPG.Toachievehighcrystallinityandhigh orientation,thestartingpyrolyticcarbonsneedselected,aswellasthehot-pressing conditions.
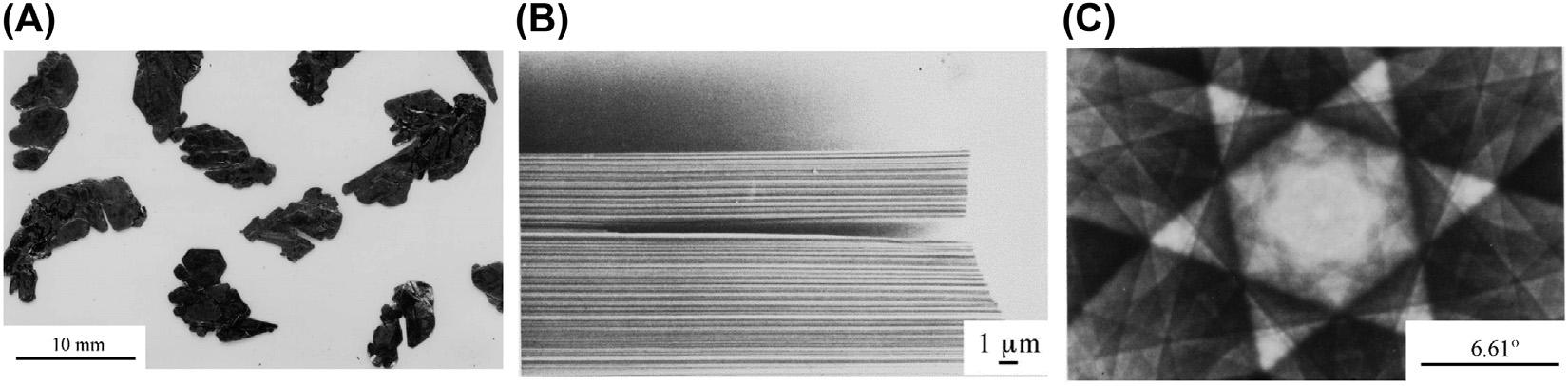
Asshownin Fig.1.16A,HOPGhasahighlyorientednanotexturealongitsplate,but electronchannelinganalysissuggeststhatHOPGconsistsofrandomlyorienteda-axesof crystallinedomains,althoughtheirc-axesarealmostperfectlyorientedperpendicularto theplate.Thechannelingpatternonthesurfaceoftheplate(Fig.1.16B)issomewhat distortedcomparedwiththatofkishgraphite(referto Fig.1.14C)andthechanneling contrastshowstherandomorientationofa-axesofthedomains(Fig.1.16C).
Graphitefilmsderivedfromorganicfilms:Highlycrystallizedgraphitefilmswereprepared fromfilmsoflimitednumbersoforganicprecursors,suchaspoly(p-phenylenevinylene) (PPV) [105],poly(p-phenylene-1,3,4-oxadiazole)(POD) [106,107],benzimidazobenzophenanthrolineladderpolymerBBL [107 110],andthecommerciallyavailablearomatic
Figure1.16
Highlyorientedpyrolyticgraphite:(A)scanningelectronmicroscopyimageofthecross-section, (B)electronchannelingpattern,and(C)electronchannelingcontrast. CourtesyofProf.A.Yoshida ofTokyoCityUniversity.
Molecularrepeatingunitsofpolymersgivinggraphitefilms. PPV,poly(p-phenylenevinylene); POD,poly(p-phenylene-1,3,4-oxadiazole); BBL,benzimidazobenzo-phenanthrolineladderpolymer; Kapton; PPT,commerciallyavailablearomaticpolyimidefilms.
polyimidesKaptonandPPT [111 113],therepeatingunitsofwhichareshownin Fig.1.17.HeattreatmentofPPV-derivedcarbonfilmsupto3000 Cgaveagraphitefilm; asshowninitsRamanspectrumin Fig.1.18A,noD-bandwasdetected [105].Thefilmof polyoxadiazoleheat-treatedat2800 Cgavesharpandstrong 00l diffractionprofilesand no hk0 diffractionlinesbyreflectionmodeandwell-resolved hk0 and hkl linesandno 00l linesbytransmissionmode,asshownin Fig.1.18B;thisrevealedtheformationofa highlyoriented,well-crystallizedgraphitefilm [106].
BBLpolymerfilmwascastontoaglasssubstratefromitssolutionof trifluoromethanesulfonicacid(TFMSA)andthenheat-treatedathightemperaturesto 3200 Cbysandwichingitbetweentwoartificialgraphiteplates [110].Theresultant ultrathinfilms,whichwerelessthan100nmthick,showedanelectronchanneling patternsimilartothatofHOPGandanorientationofgraphitebasalplanesparalleltothe filmsurface,asshownin Fig.1.19A .Byusingmethanesulfonicacidassolventforthe BBLpolymer,filmwithanorientationofbasalplanesperpendiculartothefilmsurface wasobtained,asshownin Fig.1.19B[109].
Twofilmsderivedfrompolyimidepolytrimethyleneterephthalate(PTT)andKaptonby heattreatmentat3200 Cunderasimplemechanicalconstraint(sandwichingbetweentwo
Figure1.17
Figure1.18
(A)ChangeinRamanspectrumwithheattreatmenttemperatureforpoly(p-phenylenevinylene) film [105] and(B)x-raydiffractionpatternsofpolyoxadiazolefilmheat-treatedat2800 C [106]. a.u.,arbitraryunits; cps,countspersecond.
Figure1.19
002 latticefringeimagesofthecross-sectionofgraphitefilmspreparedfromabasicstructural unitspolymerusingtrifluoromethanesulfonicacid(A)andmethanesulfonicacid(B)assolvents andheat-treatedat2800 C [109]
graphiteplates)gavehighcrystallinitycomparabletokishgraphiteandHOPG; rRT/r4.2K (oneparameterstoevaluatecrystallinityofgraphite)was4.90and4.79forPTT-and Kapton-derivedfilm,respectively,althoughitwas4.7 5.5forkishgraphiteand HOPG [112].
Exfoliatedgraphiteandflexiblegraphitesheets:Becauselargeflakesofnaturalgraphite arenotcommonlyavailableandnaturalgraphiteflakescannotbeformeddirectlyintoa sheet,atechniqueforpreparinggraphitesheetswithnobindingmaterialswasdeveloped byforminggraphiteoxidesandviatheirthermalexfoliationandreduction,followedby compressionintosheets.Theyarecalledflexiblegraphitesheetsandhavepromotedthe applicationofgraphite [114].Graphitesheetshavecharacteristicadvantagessuchas flexibility,resilience,andtheabilitytobeformedintovariousshapeseasily;inaddition, graphitehasintrinsicpropertiessuchaslubricity,chemicalandthermalstability,andhigh electricalandthermalconductivity.
Naturalgraphiteflakesarechemicallyorelectrochemicallyintercalatedtoformacovalent intercalationcompoundofgraphite,graphiteoxide,throughtheuseofamixtureof concentratedsulfuricandnitricacids.Afterrinsinganddrying,residuecompoundsare obtainedinwhichtheregularstagestructureislostbutsomesulfuricacidderivatives remaininthegraphitegallery.Theseresiduecompoundsarethenrapidlyheatedto 900 1200 C,inwhichintercalatesremaininginthegraphitegalleryaredecomposedinto gaseousproducts,yieldingmarkedexfoliationofthepristinegraphite.Thisexfoliated graphiteconsistsofworm-likeparticles,asshownin Fig.1.20.Theyareformedby preferentialexfoliationperpendiculartotheplanesurfaceofthepristineflakes.Worm-like particlesofexfoliatedgraphiteareeithermoldedorrolledintoasheetwithnoadhesives orbinders.Thesearecalledflexiblegraphitesheetsandarewidelyusedasseals,gaskets, andpackings.
Afterrinsinganddryingofintercalationcompounds,theresiduecompoundsare commerciallyavailableasexpandablegraphiteandareusedasthestartingmaterialfor thinflakesofgrapheneoxideinsomeliterature.
1.2.4.2Syntheticgraphitematerials
So-calledgraphitematerialshavebeenmanufacturedindustriallyintovariouslysized blocks,rods,andplatesfordifferentapplications,suchaslargedegreeelectrodesfor electricarcfurnaces,variousjigsforprocessingmetals,cruciblesandheatingelements togrowsemiconductorcrystals,brushesforelectricmotors,andneutronmoderatorsfor nuclearreactors.Graphitematerialsfortheseapplicationshavemostlybeenproduced usingcokeparticlesasfillerswithpitchesasthebinderthroughcarbonizationand graphitizationtreatmentathightemperatures.Theproductionprocessesaresummarized asablockdiagramin Fig.1.21.Briefly,pulverizedcokeparticlesaremixedand
Figure1.20
(A)Scanningelectronmicroscopyimagesofworm-likeparticlesofexfoliatedgraphite.(B) Appearanceoftheparticles.(C)Cross-sectionofaparticle.(D)Distributionofporeareaon cross-section.
Filler
Petroleum coke Pitch coke
Binder Pitch
Pulverization
Coase grains
Sieving
Fine grains
Re-calcination
Mixing & kneading
Pitch, Resin
Extrusion
Molding
Forming
Isostatic pressing
Impregnation
Halogen gases
Purification
Graphitization
Machining
Calcination (Carbonization)
Figure1.21
Productionprocessofsyntheticgraphitematerials.




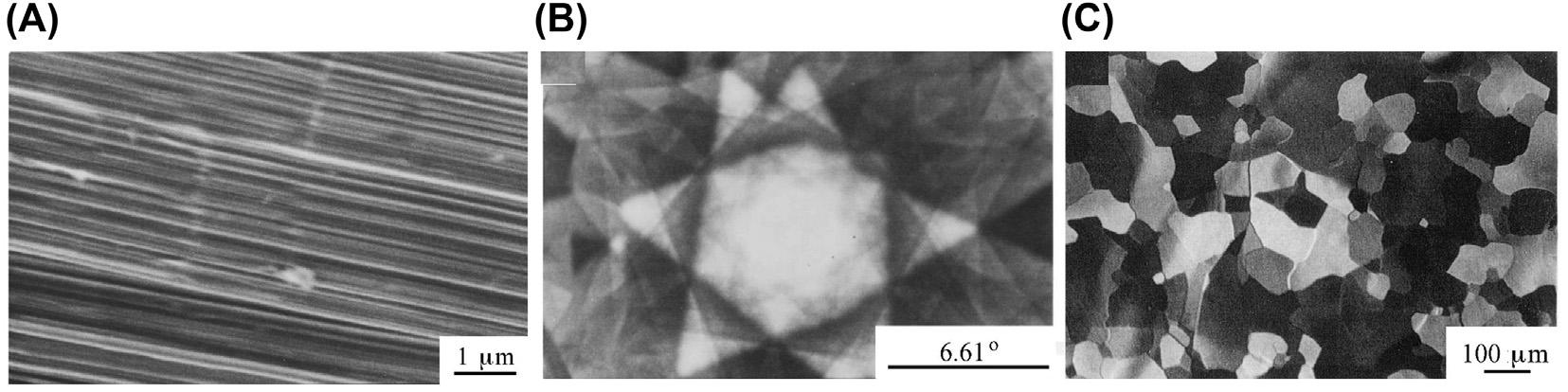
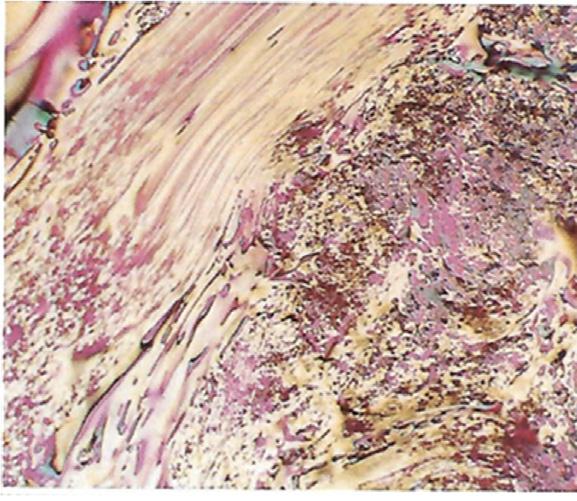
kneadedwithbinderpitchatatemperatureslightlyhigherthanthesofteningpointof thepitch,inwhichthenanotexture(eitherneedle-likeormosaic)andparticlesizeof thefillercokeandthemixingratioofthefillertothebinderhavetobecontrolled accordingtotherequirementsoftheapplications.Afterthemixtureisformedby extrusion,molding,orisostaticcompression,itisheat-treatedat1400 1800 C (calcinationorcarbonization),followedbyheattreatmentatahighertemperatureupto 2800 3000 C(graphitization)(see Fig.1.8).Thefinalproductsofthismanufacturing processarecalledsynthetic(artificial)graphitematerials.Allsyntheticgraphitesare polycrystallinesolids;ofthese,BSUs(crystallites)aremuchsmallerthanthoseinthe highlyorientedgraphitesdescribedpreviously.Toproducegraphitematerialsforusein anelectricarcfurnaceasanelectrode,coarse-grainedcokeparticleswithaneedle-like textureareusuallyemployedtoachievehighthermal-shockresistance.Theyareformed byextrusionormolding,andtheslightorie ntationofcokeparticlescannotbeavoided. Toproduceisotropichigh-densitygraphiteblocks,however,fine-grainedcokeparticles havetobeusedtoachieveahighdensity,andisostaticpressingmustbeemployedfor anisotropicnatureinthefinalblock.Microtexturesoftwographitematerials,electrode gradeandisotropicgrade,arecomparedin Fig.1.22.
Mostsyntheticgraphitematerialsconsistoffillercokeandacarbonizedbinder(binder coke).Theformerisabletoattainahigherdegreeofgraphitestructuredevelopment (graphitizationdegree)comparedwiththelatter.Thedegreeofgraphitizationachieved afterfinalgraphitizationdependsonthenanotextureofthestartingcokeparticles,as shownin Fig.1.23A.Needle-likecokehasanextendedflowpatternowingtothepreferred orientationofcrystallites,andsothereisahigherdegreeofgraphitizationafterhightemperaturetreatmentcomparedwithmosaiccoke,whichhasashortrangeoforientation andislessordered(Fig.1.23B).However,thedegreeofgraphitizationreachedbyneedlelikecokeisfarlowerthanthatofhighlyorientedgraphitematerials.
Figure1.22
Polarizedlightmicrographsofcross-sectionsofsyntheticgraphites:(A)electrodegradeand (B)isotropicgrade.
Polarizedlightmicrographsofcross-sectionofparticles:(A)needle-likeand(B)mosaiccoke.
1.2.4.3Fibrouscarbonmaterials
Avarietyoffibrouscarbonmaterialshavebeenpreparedbydifferenttechniques: electricarc-discharge,catalyticCVD,template-assistedCVD,melt-spinning,and electrospinning. Table1.1 classifiesthesefibrouscarbonsaccordingtowhetherthe 002 latticefringesofcarbonlayersareshortorlongandwhethertheirdiametersareonthe micrometerornanometerscale.Carbonlayersconstitutingthewallofcarbonnanotubes (CNTs)arelongandorientedexactlyparalleltothetube’saxis,butlayersofcarbon nanofibersareshortandorientedindifferentmodes,calledtubular,herringbone,and platelettypes,dependingonthemethodsandconditionsofsynthesis.Carbonfiberswith adiameterofabout7 m mareproducedindustriallybymelt-spinningfromdifferent precursors,polyacrylonitrile(PAN),isotropicandanisotropic(mesophase)pitches, cellulose,andphenolasfilamentyarns,clothes,chops,webs,etc.Vapor-growncarbon fibersonthemarketareproducedbycatalyticCVDandhavetubularnanotextures.By applyingelectrospinning,variousorganicpolymerscanbeusedasprecursorstofabricate carbonnanofibers [115].
Table1.1: Classificationoffibrouscarbonmaterials.
DiameteroffiberLongcarbonlayersShortcarbonlayers
NanometersizeCarbon nanotubes Single-walled Double-walled Multiwalled Carbon nanofibers
Tubulartype
Herringbonetype Platelettype
MicrometersizeGraphitewhiskerCarbonfibersPolyacrylonitrile-based Pitch-based,etc.
Vapor-growncarbon fibers
Figure1.23