FlowAssurance VolumeII
Editedby
QiweiWang
SaudiAramco,Dhahran,SaudiArabia
GulfProfessionalPublishingisanimprintofElsevier 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,Oxford,OX51GB,UnitedKingdom
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
ISBN:978-0-12-822010-8
ForInformationonallGulfProfessionalPublishingpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: CharlotteCockle
SeniorAcquisitionsEditor: KatieHammon
SeniorEditorialProjectManager: SaraValentino
ProductionProjectManager: SojanP.Pazhayattil
CoverDesigner: MilesHitchen
TypesetbyMPSLimited,Chennai,India
1.1 Introduction....................................................................................2
1.2 Fundamentalsofhydrate................................................................3
1.2.1Definition............................................................................3
1.2.2Structures.............................................................................3
1.2.3Phasebehavior....................................................................6
1.2.4Properties.............................................................................8
1.3 Hydrateformation........................................................................10
1.3.1Hydrateformationscenarios.............................................10
1.3.2Hydrateformationmechanism.........................................10
1.4 Hydratemanagementinproductionsystems...............................11
1.4.1Riskassessment.................................................................12
1.4.2Hydratemodeling..............................................................12
1.5 Temperaturecontrol.....................................................................27
1.5.1Thermalinsulation............................................................27
1.5.2Activeheating...................................................................32
1.6 Chemicalinhibition......................................................................36
1.6.1Thermodynamichydrateinhibitors...................................36
1.6.2Low-dosagehydrateinhibitors.........................................40
1.7 Dehydration..................................................................................64
1.8 Hydrateremediation.....................................................................66
1.8.1Depressurization................................................................66
1.8.2Heating..............................................................................67
1.8.3Chemicaldissociation.......................................................68
1.8.4Modelpredictionsforremediation...................................69
1.9 Casestudies..................................................................................69
1.9.1Hydratemanagementindrytreefacilityfacilities..........69
1.9.2Low-dosagehydrateinhibitorfieldapplication...............72
1.9.3Tommeliten-gammafield.................................................73
1.9.4RemediationofhydratepluginwestAfricadeepwater floatingproductionstorageandoffloading............................75
1.10 Summary.......................................................................................77 Nomenclature...............................................................................77 References....................................................................................78 v
CHAPTER2Paraffinmanagement
.................................................. 85 MikeNewberryandDavidW.Jennings
2.1 Historyofparaffinmanagementdevelopments..........................85
2.2 Crudeoilandparaffinchemistry.................................................88
2.3 Paraffinanalysisandcrudeoilcharacterization..........................91
2.3.1Paraffinanalysismethods.................................................91
2.3.2Crudeoilcharacterization.................................................93
2.4 Paraffindeposition.....................................................................103
2.4.1Paraffindepositionmechanisms.....................................103
2.4.2Paraffindepositionmodeling..........................................106
2.4.3Paraffindepositcharacteristics.......................................111
2.4.4Paraffindepositioncontrol..............................................114
2.5 Pourpoint/crudeoilgellingproblems.......................................153
2.5.1Crudeoilgellingmechanism..........................................153
2.5.2Gelledflowlinecharacteristics.......................................155
2.5.3Pourpointtreatment........................................................156
2.6 Casehistories..............................................................................165
2.6.1Formationdamage..........................................................165
2.6.2Welltubingdeposition....................................................168
2.6.3Flowlinedeposition.........................................................170
2.6.4Tankbottoms...................................................................175
2.7
3.2.1Compositionandstructure..............................................186
3.2.2Solubilityandaggregation..............................................188
3.3 Experimentaltechniquesforasphaltenestabilityprediction.........191
3.3.1Solidsdetectionsystem...................................................191
3.3.2DeBoerplot.....................................................................192
3.3.3Deadoiltests...................................................................192
3.4 Asphaltenestabilitymodeling....................................................194
3.4.1Asphalteneinstabilitytrendmodeling............................194
3.4.2Asphaltenedepositionmodel..........................................195
3.5 Asphalteneinhibitorlabtests.....................................................198
3.5.1Precipitationtests............................................................199
3.5.2Depositiontests...............................................................201
3.5.3Liveoiltests....................................................................207
3.6 Asphaltenecontrolinoilproduction.........................................211
3.6.1Prevention........................................................................211
3.6.2Remediation....................................................................213
3.7 Casestudies................................................................................213
3.7.1Labscreeningmethodsforfieldapplications................213
3.7.2Evaluatingasphalteneinhibitorsforanoffshore alaskanproducer.............................................................214
3.7.3Developmentofmultifunctionalstabilizersof asphaltenes......................................................................215
3.8 Conclusionandpathforward.....................................................215 Acknowledgment.......................................................................217 Nomenclature.............................................................................217 References..................................................................................218
CHAPTER4Naphthenateandcarboxylatesoaptreatment ......... 227 JonathanJ.Wylde
4.1 Introduction................................................................................228
4.1.1Overviewandchapterorientation..................................228
4.1.2Naphthenatesandrecenthistory.....................................228
4.1.3Definitions:acidcrudeoiland“naphthenates”..............231
4.1.4Originofacidiccrude.....................................................234
4.1.5Carboxylateandnaphthenatesoapoperational challenges........................................................................235
4.1.6Thecontinuummodel.....................................................237
4.2 Foulingmechanismsofnaphthenateandcarboxylate soaps...........................................................................................238
4.2.1Analyticaltechniquesforacidicspeciesin crudeoils.........................................................................238
4.2.2Soapemulsions...............................................................244
4.2.3Naphthenatesoapsolids..................................................252
4.2.4Highcalciumincrudecausedbyoil-dispersible naphthenates....................................................................259
4.2.5Refinerychallengesoverview.........................................262
4.3 Chemicalcontrolmethodologiesandlaboratorytesting...........263
4.3.1Introduction.....................................................................263
4.3.2Preventivechemicalstrategies........................................264
4.3.3Remediationandremedialchemicalstrategies..............270
4.3.4Laboratoryandfieldtesting............................................271
4.4 Concludingremarksandremainingchallenges.........................272
GordonMichaelGrahamandDarioMarcelloFrigo
5.1 Introduction................................................................................288
5.1.1Theroleofwater.............................................................289
5.1.2Inorganicmineralscalingintheoilenvironment..........291
5.2 Basicprinciplesofinorganicscaleformation...........................293
5.2.1Typesofinorganicmineralscale...................................293
5.2.2Inorganicmineralscaleformation..................................298
5.2.3Scalenucleationandgrowth...........................................305
5.2.4Thermodynamicsandkinetics........................................309
5.3 Scaleprediction..........................................................................311
5.3.1Scalepredictionasacomponentofscale management....................................................................311
5.3.2Scalepredictionoutputs..................................................312
5.3.3Theoryofscaleprediction..............................................315
5.3.4Importanceofqualityinputdata....................................319
5.3.5Exampleoftheutilityofmodernscale predictionpackages.........................................................321
5.3.6Limitationsofscaleprediction.......................................322
5.4 Scalecontrol...............................................................................323
5.4.1Treatmentoptionsandscalecontrolstrategies..............323
5.4.2Chemicalinhibition.........................................................330
5.4.3Typesofscaleinhibitorscommonlyusedinoil industry............................................................................334
5.4.4Briefhistoryofscaleinhibitordevelopment..................341
5.4.5Chemicaldeployment.....................................................342
5.4.6Chemicalinhibition.........................................................344
5.4.7Factorscontrollingtheperformanceof genericallydifferentinhibitorchemistries.....................348
5.4.8Laboratoryassessmentofscaleinhibitors......................359
5.4.9Chemicalqualification:finalconsiderations..................371
5.5 Scaleinhibitorsqueeze...............................................................372
5.5.1Chemicalsqueezeprocess..............................................372
5.5.2Chemicalretentionmechanisms.....................................381
5.5.3Chemicaltestingofscaleinhibitorsqueeze treatments:reservoirconditioncoreflooding................387
5.5.4Theimportanceofappropriatecorefloodtesting protocols..........................................................................388
5.5.5SIApplicationconsiderations:formationdamage andinhibitorretention/releaseproperties.......................392
5.5.6Importanceofaccurateassayandmonitoring................403
5.5.7Isothermderivationandnear-wellboresimulation.........406
5.6 Scaleremediation.......................................................................412
5.6.1Acidsolublevsacidinsolublescales.............................413
5.6.2MechanicalRemediation/PhysicalMethods...................414
5.6.3Chemicaldissolution.......................................................416
5.6.4Chemicaldeploymentinscaledissolution.....................417
5.6.5AcidsforScaleDissolution............................................419
5.6.6Chelatingagentsforscaledissolution............................422
5.7 Summary.....................................................................................426 Nomenclature.............................................................................426 References..................................................................................428
CHAPTER6Sandcontrolcompletionusingin-situresin
PhilipNguyenandMikeSanders
6.1 Sandcontrol................................................................................443
6.1.1Mechanismsandcausesofsandproduction..................443
6.1.2Problems/issuesofsandproduction...............................444
6.1.3Sandcontrolmethods......................................................444
6.1.4Otherwellborestabilizationmethodsfor sandcontrol.....................................................................447
6.1.5Perforatingtechniquesforcompletionsusing sand-consolidationtreatments.........................................448
6.1.6Chemicalsandconsolidation..........................................451
6.2 FinesMigrationcontrol..............................................................472
6.2.1Mechanismsandcauses..................................................472
6.2.2Previousfinesmigrationcontrolmethods......................474
6.2.3Controllingfinesmigrationintoproppantpack.............475
6.2.4Finesmigrationfieldcasehistories................................478
6.3 Proppantflowbackcontrol.........................................................479
6.3.1Primaryproppantflowbackcontrol................................479
6.3.2Remedialmethodsforproppantflowback.....................490
6.3.3Lessonslearned/recommendations.................................493 Nomenclature.............................................................................494 References..................................................................................494
CHAPTER7Condensateandwaterblockingremoval ................ 503 MashhadFahes
7.1 Introduction................................................................................503
7.2 Backgroundtheory.....................................................................505
7.2.1Fluidphasebehavior.......................................................505
7.2.2Pressureprofiles..............................................................506
7.2.3Two-phaseflowchallenges............................................509
7.3 Fieldexamplesandindustrypractice........................................514
7.3.1Ichthysgas-condensatefieldinAustralia.......................514
7.3.2Cupiaguagas-condensatefieldinColumbia..................515
7.3.3Otherexamples...............................................................516
7.4 Recentadvancesinresearchanddevelopment.........................520
7.4.1Wettabilityalteration......................................................520
7.4.2CO2 huff-n-puff...............................................................528
7.4.3Othernewtechnologies..................................................530
7.5 Finalremarks..............................................................................530 Nomenclature.............................................................................531
CHAPTER8Foam-assistedliquidlift
8.1 Introduction................................................................................542
8.2 Liquidloadinganddeliquification.............................................544
8.2.1Liquidloading.................................................................544
8.2.2Continuousdeliquification..............................................549
8.2.3Intermittentdeliquification.............................................551 8.3 Foam-assistedlift.......................................................................552
8.3.1Foam-assistedliftperformance.......................................552
8.3.2Foam-assistedliftoperatingenvelope............................555
8.3.3Foam-assistedgaslift......................................................556
8.3.4Surfacefoam-assistedlift................................................556
8.4 Foam-assistedliftapplication....................................................556
8.5 Wellperformance.......................................................................557
8.5.1Collectwelldata.............................................................557
8.5.2Diagnoseandforecastliquidloading.............................557
8.5.3Predictfoam-assistedliftoperatingparameters.............559
8.6 Laboratorytesting......................................................................562
8.6.1Foamerperformance.......................................................564
8.6.2Secondaryperformance..................................................566
8.7 Foam-assistedliftfieldtesting...................................................568
8.7.1Batchfoam-assistedlifttrial...........................................569
8.7.2Continuousfoam-assistedlifttrial..................................570
8.8 Foam-assistedliftapplication....................................................571
8.8.1Continuousorintermittentfoam-assistedlift.................571
8.8.2Solidorliquidfoamer.....................................................572
8.8.3Annulusorcapillary........................................................572
8.8.4Capillaryspecifications...................................................576
8.8.5Surfaceinjectionsystem.................................................578
8.9 Foam-assistedliftoperation.......................................................582
8.9.1Optimizingfoam-assistedlift.........................................583
8.9.2Sustainingfoam-assistedlift...........................................587
8.10 Casestudiesofsuccessfulfoamerapplications.........................590
8.10.1Optimizehorizontalwellswithbatchfoamer treatment........................................................................590
8.10.2Continuousfoaminconjunctionwithdry-gas-lift.......593
8.10.3Innovativefoamerassustainabledeliquification solution..........................................................................596
8.10.4Usefoamtoremoveliquidinsubseaflowlines andenhanceproduction................................................598
8.10.5Foam-assistedlifttooptimizematureoilwells...........602
8.11 Remainingchallenges................................................................603 Nomenclature.............................................................................604 References..................................................................................605
CHAPTER9Corrosioninhibition .................................................. 609 JeremyMoloney,DharmendrKumar, VenkataMuralidharKandThunyalukPojtanabuntoeng
9.1 Corrosioninhibitors....................................................................610
9.1.1Environmentalconditioners/scavengers.........................611
9.1.2Interfaceinhibitors..........................................................611
9.2 Mechanismofcorrosioninhibition............................................612
9.2.1Environmentalconditioners/scavengers.........................612
9.2.2Interfaceinhibitors..........................................................613
9.3 Measurementofcorrosioninhibition.........................................617
9.4 Oilfieldcorrosioninhibitorchemistryexamples.......................619
9.5 Molecularmodelingofcorrosioninhibitors..............................619
9.5.1Computationofquantumchemicaldescriptors..............625
9.5.2Metalinhibitorinteractionsusingdensityfunctional theory...............................................................................628
9.5.3Moleculardynamicsstudy..............................................630
9.5.4MonteCarlosimulations.................................................632
9.5.5Synergisticeffectstudyusingmolecularmodeling.......633
9.5.6Screeningofcorrosioninhibitorsusingmachine learning............................................................................635
9.6 Corrosioninhibitorperformanceevaluation..............................637
9.6.1Metalsamples.................................................................639
9.6.2Solutionchemistry..........................................................640
9.6.3Testparameters...............................................................641
9.7 Corrosionratemeasurementtechniques....................................654
9.7.1Gravimetry(weightlossmeasurements)........................654
9.9.1Atomicforcemicroscopy................................................666
9.9.2Fouriertransforminfraredspectroscopy........................667
9.9.3X-rayphotoelectronspectroscopy..................................668 9.9.4Others..............................................................................668 9.10 Compatibilitytests......................................................................668
9.10.1Compatibilitywithmetallicmaterials..........................669
9.10.2Compatibilitywithnonmetallicmaterials....................671
9.10.3Foamingandemulsiontendency..................................672
9.10.4Physicalproperties,productstability, andadditionalconsiderations.......................................674
9.11.1Corrosionmonitoring....................................................676
9.11.2Corrosioninhibitorresidualmeasurements..................677
9.11.3Waterchemistryanalysis..............................................679
9.12 Casestudies................................................................................680
9.12.1Fieldapplicationofcorrosioninhibitorsinsweet (carbondioxidecontaining)systems............................680
9.12.2Fieldapplicationofcorrosioninhibitorsinsour (hydrogensulfidecontaining)systems.........................684
9.12.3Learningsfromtheliteraturecasestudies....................688 9.13 Summary.....................................................................................689
CHAPTER10Microbialcontrol
XiangyangZhu
...................................................... 709
10.1 Introduction................................................................................709
10.2 Majormicroorganismsinoilandgasindustry..........................711
10.2.1Sulfate-reducingbacteriaandarchaea..........................712
10.2.2Methanogens.................................................................715
10.2.3Acid-producingbacteria................................................717
10.2.4Iron-andmanganese-oxidizingbacteria......................718
10.3 Biocideclassification.................................................................719
10.3.1Nonoxidizingbiocides..................................................720
10.3.2Oxidizingbiocides........................................................726
10.3.3Preservatives..................................................................728
10.4 Biocideselectionandperformanceevaluation..........................729
10.4.1Considerationsforbiocideselectionand performanceevaluation.................................................729
10.4.2Methodsforbiocideevaluation....................................732
10.5 Biocidetreatmentpractices........................................................743
10.6 Biocideresidualmonitoring.......................................................746
10.7 Microbialmonitoringfortreatmenteffectiveness.....................747
10.8 Alternativemethodsformicrobialcontrol................................749
10.8.1Nitrate............................................................................749
10.8.2Bacteriophage................................................................751
10.8.3Physicalprocesses.........................................................752
10.9 Finalremarks..............................................................................756 Nomenclature.............................................................................757 References..................................................................................758 Index......................................................................................................................775
Thispageintentionallyleftblank
1.2 Fundamentalsofhydrate
1.2.1 Definition
Intheoil/gasindustry,gashydrates,clathratehydrates,andhydratesareoftenused interchangeably.Theyareice-likesolid compoundsthattypicallyformunderhigh pressureandlow-temperatureconditions inpresenceofbothwaterandlighthydrocarbonmolecules [2].Naturalgashydratesarecomposedofapproximately85mol.% water,thereforetheyhavemanyphysicalpropertiessimilartothoseofice.For instance,theappearanceandmechanicalpropertiesofhydratesarecomparableto thoseofice.Thedensitiesofhydratesvarysomewhatduetothenatureoftheguest molecule(s)andtheformationconditionsbutaregenerallycomparabletothatofice.
Althoughhydrateswerefirstdiscoveredover200yearsago,nopractical implicationwasrealizeduntil1934whenitwasdiscoveredthatitwasgas hydrates,notice,thatpluggednaturalgaspipelines.Thisbroughtarenewedburst ofresearchinterestsongashydrates,especiallyfocusingondeterminingthermodynamicandstructuralpropertiesandpreventinghydrateplugs.
NaturallyoccurringnaturalgashydrateswerepredictedandfoundbyRussian researchersin1960s.Thisbroughtanothersurgeofresearchinterestthatconsidered gashydratesasapotentialenergyresourceandasanimportantfactoraffecting globalclimatechanges.Thecumulativeefforts,beginningwithHumphreyDavyin 1810,providedtremendousamountsofknowledgeaboutthethermodynamic,physical,andstructurepropertiesofgashydratesandarichcollectionofhydrateformers,includingnitrogen,carbondioxide,hydrogensulfide,methane,ethane, propane, iso-butane,n-butane,andsomebranchedorcyclicC5 C8hydrocarbons.
1.2.2 Structures
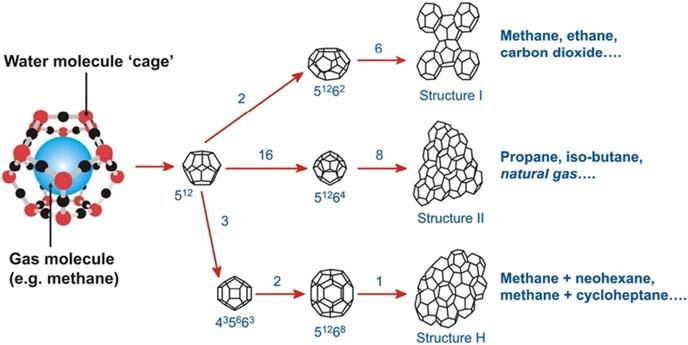
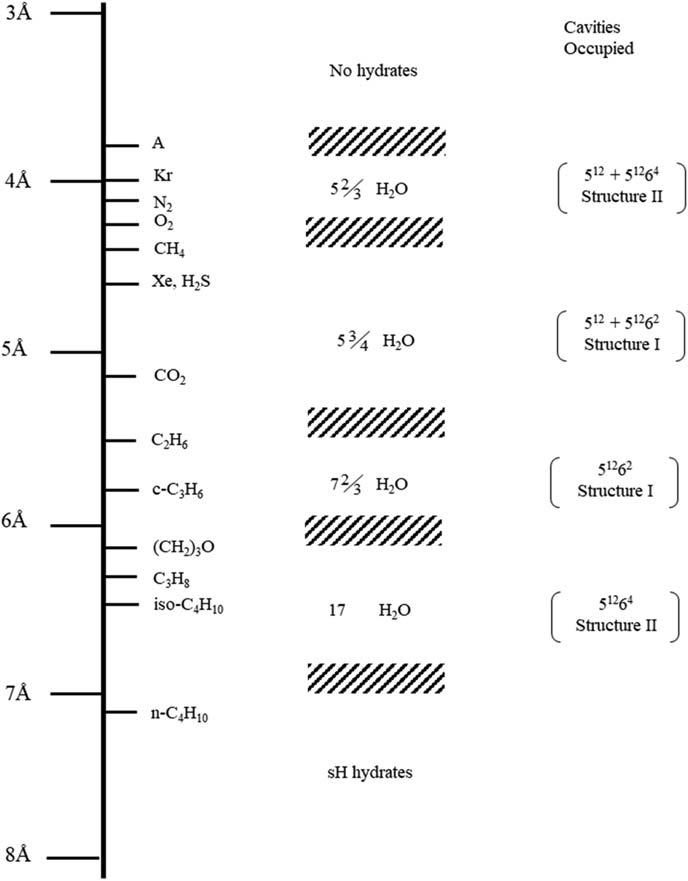
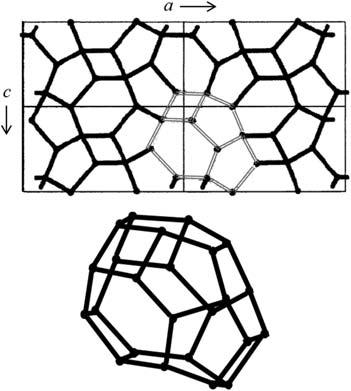
Inhydratestructures,watermoleculesarehydrogenbonded,whilegaseousmoleculesarebondedtothoseonlyviavanderWaalsforces.Thoughtheenergy requiredtodissociateonehydrogenbondisabout5kcal/mol,only0.3kcal/molis neededtobreakonevanderWallsbond,suggestingthatgaseousmoleculesare onlyconsideredphysicallybutnotchemicallyentrappedintocrystallinewater cages [2].Dependingonthesizesoftheguestmoleculesincludedinthegas hydrates,threehydratestructuresaretraditionallyfound,whicharestructureI,II, andH(Table1.1, Figs.1.1and1.2).ThebasicrepeatingunitinstructureIisa primitivecubiclatticeconsistingoftwopentagonaldodecahedra(512)(5isthe numberofedgesinafaceand12isthetotalnumberofthistypeoffacesina cage)andsixtetra-decahedra(51262)clathratecageswithatotalnumberof48 watermoleculesandadimensionof1.2nm.Theaveragecavityradiusofeach typeofcageis3.95and4.33A ˚ ,respectively.Methane,ethane,CO2 andXenon aretypicalstructureIhydrateformers.WhilemethaneandXenonoccupyboth small(512)andlarge(51262)cages,CO2 andethaneareonlysmallenoughto dwellinlargecages.
Table1.1 Characteristicsofhydratecagesofdifferentstructures.
StructureI(sI)StructureII(sII)StructureH(sH) SmallLargeSmallLargeSmallMediumLarge Cages512 51262 512 51264 512 435663 51268
No.ofcagesperunitcell26168321
No.ofwatersperunitcell4813634
Averagecageradius(Å)3.954.333.914.733.914.055.71
Coordinationnumber20242028202036
FIGURE1.1
DifferenttypesofcavitiesfoundinhydratesandrespectiveunitstructuresofsI,sIIandsH structures [3]
ReproducedwithpermissionfromE.D.Sloan,C.A.Koh,ClathrateHydratesofNaturalGases,thirded.,CRC Press,NewYork,2008.
TherepeatingunitofstructureIIhydratealsocontainstwotypesofcavities16 pentagonaldodecahedra512 (3.91A ˚ )and8hexadecahedra51262 (4.37A ˚ )composed of136watermolecules.Itslatticetypeisface-centeredcubicanditsunitdimension is1.7nm.Mosthydratesintheoil/gasindustryareexpectedtobestructureII hydrates.InstructureH,alayerof512 (3.91A ˚ )cavitiesconnectsalayerof51268 (4.06A ˚ )and435663 (5.71A ˚ )cavities.Initshexagonalunitcell(a 5 1.21nm, c 5 1.01nm),34watermoleculesformthree512,two51268,andone435663.One uniquefeatureofstructureHisthatbothsmallandlargesizesofmoleculesare requiredtostabilizethestructure.Forexample,neohexeneandcycloheptane,which cannotformhydratesalone,formstructureHhydrateswiththehelpofmethane. Thesethreehydratestructuresareimportantfortheoil/gasindustrybecausethetypes ofhydrocarbonsencounteredinthefieldcanformallthesethreetypesofhydrates.
Comparisonofguestmoleculesizesandcavitiesoccupiedassimplehydrates.
Withtheadvancementofexperimentaltechnologiesandcontinuousresearch effortsonclathratehydrates,somenewtypesofhydratestructuresathighpressures havebeenidentified,mostlynotrelevanttooil/gasindustry.Itwasdiscoveredthat underapressureof0.8GPa,tetrahydrofuran(THF)anddeuteriumoxide(D2O)
FIGURE1.2
FIGURE1.3
Packingandschematicviewofthespace-fillingpolyhedron. ReproducedwithpermissionfromA.Kurnosov,V.Komarov,V.Voronin,etal.,Newclathratehydratestructure: high-pressuretetrahydrofuranhydratewithonetypeofcavity,AngewandteChemieInternationalEdition,43 (2004):2922 2924.
formanorthorhombicstructure,inwhichwatermoleculesform14-hedracages withfourtetra-,fourpenta,andsixhexagonalfaces(445466)thatareabletopack three-dimensionallywithouttheneedforothertypesofpolyhedrons [4].Thestoichiometriccompositionofaunitcellcanbepresentedas4T3 24D2O,whereT3 isa 445466 cage.Projectionofthestructurealongthebaxisispresentedin Fig.1.3
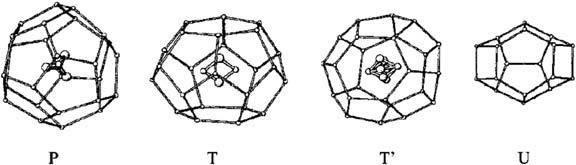
Whileinvestigatingdimethylether(DME)hydrateusingX-raydiffraction, Udachinetal. [5] identifiedanothernewhydratestructureTthatisdenseand highlycomplex(Fig.1.4).Itdoesnothave512 polyhedraandcancontain 51263(P),51262(T),4151063(T’)and425861(U)cages.Thishydratestructureistrigonal,spacegroupP321,a 5 34.995A ˚ ,c 5 12.368A ˚ ,andstoichiometrycanbe describedas12P 12T 24T 12U 348H2O.TheDMEmoleculesareaccommodated inallthreetypesoflargecages(P,T,T’)(Fig.1.5),givinganoverallcompositionofDME 7.25H2O.
1.2.3 Phasebehavior
Theconditionsrequiredforhydrateformationandtheresultinghydratephasediagramvarybasedonthetypesofhydrateformers.Thetypicalfourpillarsofhydrate formationarepresenceoffreewater,hydrateformer(s),highpressure,andlowtemperaturewithonlyafewexceptions,e.g.,THFandethyleneoxidecanformhydrate withwaterunderambientpressureat B4.5 Cand B11 C,respectively.
FIGURE1.4
GeneralviewofthestructureThydrateasdeterminedbysinglecrystal.
ReproducedwithpermissionfromK.Udachin,C.Ratcliffe,J.Ripmeester,Adenseandefficientclathrate hydrate,AngewandteChemieInternationalEdition,40(2001):1303 1305.
FIGURE1.5
Viewofthecagesinthestr.Thydrate.
ReproducedwithpermissionfromK.Udachin,C.Ratcliffe,J.Ripmeester,Adenseandefficientclathrate hydrate,AngewandteChemieInternationalEdition,40(2001):1303 1305.
Mosthydrocarbonsfoundintheoil/gasindustryrequirehighpressuretoform hydrate.Atthesametemperature,lighterhydrocarbonhydrateformerstypically requirehigherpressuretoformhydratethanheavierhydrocarbonhydrateformers. Forexample,at10 C(50 F)withfreshwater,methanehydrate’sequilibriumpressureisabout1000psiawhileitwouldonlyrequireabout250psiatoformethane hydrate.Inaddition,thesalinityofwaterplaysakeyfactorinthehydratephase diagram.Fromamolecularlevel,thepresenceofionsinthewatercancausedisruptionintheformationprocessofhydrogenbondingstructureofclathratecages. Thehigherthesalinity,thehigherthepressurerequiredtoformhydrate.Sincethe hydratestructureitselfissaltfree,thehydrateformationprocessinsaltwaterwould extractwaterinthesolutionandconvertthemtohydratewhilecausingthesalinity oftheremainingwatertoincrease.Forthisreason,thehydrateformationinsalty
FIGURE1.6
Typicalhydratephasediagramofablackoilsystem.
waterwillbecomeself-inhibitingatacertainpointoftheconversionprocess.The additionofwater-solublecompoundssuchasmethanol(MeOH)orglycolintothe waterwillrequirehigherpressuretoformahydrate,asimilareffectisobserved withhighersalinity.Thisisthefoundationtomanagehydrateriskwiththermodynamicinhibitors(mostlyMeOH,glycol,andtoalesserextentethanol). Fig.1.6 is atypicalhydratephasediagramofablackoilsystemwithdifferentsalinities.The hydratephasediagramsfornaturalgasaresimilarbutwithouttheinflectionpoints alongthecurvewheretheblackoilsystemtypicallygoesthroughthebubblepoint. Obtainingaccuratehydratephasediagramsisakeystepinproperhydraterisk assessmentandriskmanagementandwillbediscussedinmoredetailslater.
1.2.4 Properties
Allthehydratesimplicationsandapplicationsarerootedintheiruniqueproperties undervariousconditionsoftemperatureandpressure.Thefollowingareafew propertiesthatareparticularlyimportanttoenergyindustry.
1.2.4.1 Mechanicalproperties
Experimentaldeterminationofthemechanical propertiesofclathratehydratesisdifficultduetothechallengeofmakingpurenonporoushydratesamples.Inaddition,the presenceofresidualwater/iceandfreegasinthesystemduetoincompletehydrate
formationprocesscanalsocontributetothemeasurementreliability.Thereforethere areconsiderableuncertaintiesassociatedwithallthehydratemechanicalpropertymeasurements.Thefollowingareafewgeneralconclusionsaboutthemechanicalpropertiesofgashydratesbasedonexperimental/theoreticalstudiesandfieldobservations.
•Generallyspeaking,thehydratemechanicalpropertiesaresimilartothatofice. Onceformed,theycanbehardtoremovemechanicallyandcannotbescrapped offbysendingdownapiglikeremovingwaxdeposit.Doingsowillonlymake mattersworse.Thereforethemainhydratemanagementstrategyisfocusedon hydratepreventioninthefirstplaceandhydratedissociationonceformed.
•Theelasticpropertiesofgashydratedependontemperature,pressure,and hydratecomposition,includingstructure,guestmolecule,andcageoccupancy. Lowertemperatureandhigherpressurebothcontributetoanincreasedbulk modulusandconsequentlymakethehydrateharder.
•Whenwellwithinthestabilityzone,thecompressivestrengthofhydratesis higherthanthatoficehowever,thestrengthsbecomecloserinvaluewhen hydrateislesssupercooled.Thisdifferenceisattributedtothespecialhydrate latticestructureandthehost,guestandhost guestinteractions.
1.2.4.2 Self-preservationduringdissociation
Hydratedissociationisanendothermicprocesssimilartoicemelting,butitsheatof dissociation(B54kJ/molformethanehydrate)ismuchhigherthanthatofice (6kJ/mole).Duringdissociationprocess,hydratesseparateintowaterandguest moleculesbybreakinguphydrogenbondingnetworksofwatermoleculesandthe vanderWaalsinteractionforcesbetweenguestandhostwatermolecules.Thisprocesstakesupasignificantamountofheatfromtheenvironmentandcausesthetemperaturetodrop,whichcontributestothestabilityoftheremaininghydrate,thatis, aself-preserving/limitingphenomenon.Withoutactivesupplyofheatfromtheenvironment,ahydrateplugcantakeuptoayeartodissociatebyitself.Thereforecontinuoussupplyofheatiskeytomodulatetherateofhydratedissociation.
1.2.4.3
Largegas-to-hydratevolumeratio
Alargevolumeofhydrateformergascanbereleaseduponhydratedissociation. Forexample,onecubicfootofmethanehydratereleasesabout160cubicfeetof gasunderambientcondition.Thishastwokeyimplicationsforoil/gasindustry applications:
•Lowgas-to-oilratio(GOR)fluidcanbecomegas-starvedduringthehydrate formationprocessandbecomeself-limiting.Consequently,thehydrate pluggingriskduringshut-ininsuchsystemcanbemuchlowerthanahigh GORfluid.
•Gasreleaseduringhydratedissociationcanquicklybuildpressureupin confinedvolume,whichcaneithercausepressurecontainmentruptureor rapidaccelerationofdislodgedhydrateplugs.Thiscanposeassetintegrity risksandpersonnelsafetyrisks.