Walking
https://ebookmass.com/product/walking-the-edge-sue-ward-drake-drake-2/
ebookmass.com
Contentslistsavailableat ScienceDirect
CorrosionScience
journalhomepage: www.elsevier.com/locate/corsci
FacilefabricationofSnO2 modifiedTiO2 nanorods filmforefficient photocathodicprotectionof304stainlesssteelundersimulatedsolarlight
JuantaoZhanga,c,HualongYangb,YuanWanga,XiaohuCuib,ZhangWenb,YunpengLiua,c,*, LeiFana,*,JiangtaoFengc,*
a NPCTubularGoodsResearchInstitute,StateKeyLaboratoryforPerformanceandStructureSafetyofPetroleumTubularGoodsandEquipmentMaterials,Xi’an,Shaanxi 710077,China
b PetroChinaTraimOilfieldCompany,Korla,841000,China
c DepartmentofEnvironmentalScience&Engineering,Xi’anJiaotongUniversity,Xi’an,710049,China
ARTICLEINFO
Keywords:
TiO2 nanorodsarray SnO2 304stainlesssteel Photocathodicprotection
ABSTRACT
SnO2/TiO2 nanorods(TNRs)composite filmwassuccessfullyfabricated.TheresultsshowedthattheSnO2 nanoparticlesweredepositedontothesurfaceoftheTNRs film,andthiscomposite filmexhibitedexcellentlight absorptionability.Meanwhile,theSnO2/TNRscomposite filmexhibitedthesuperiorphotoelectrochemical performance.Withwhitelightillumination,thiscomposite filmmadethepotentialofthecoupled304stainless steel(304SS)ina0.5mol·L 1 NaClsolutiondropby240mV,exhibitingmoreefficientphotocathodicprotection (PCP)effect.What’smore,itcouldprovidedelayprotectionforthesteelindarknessattributingtothe “electron pool” effectofSnO2
1.Introduction
304SSiswidelyusedasengineeringsteelinindustrialproduction duetoitsoutstandinganti-corrosionproperty.Nevertheless,pittingand crevicecorrosiononthemetalsarebarelytobeavoidedinthepresence ofCl [1–3].Sofar,manyanti-corrosiontechnologieshavebeendeveloped,suchasanti-corrosioncoatings,corrosioninhibitors,electrochemicalprotection,etc[4,5].Thesetraditionalprotectiontechnologiesarelimitedbecauseoftheconsumptionofmaterialandenergy,and theenvironmentalpollution.Onbehalfofgreensteel-protection methods,thePCPiscommonlyconsideredastheoneofthemosteffectivetechnology[6].Whenthephotoanodeisirradiated,thephotoexcitedelectronsareimmigratedtotheconnectedsteel,andthesurface potentialoftheprotectedsteelshiftednegativelybelowitscorrosion potential,resultinginthecathodicprotectioneffectformetals[7].
TiO2 hasattractedmanyattentionsin fieldofphotocatalysis,owing toitslowcost,lowtoxicityandlargespecificsurfaceareaproperties. ComparedwithtraditionalTiO2 particlesandTiO2 nanotubes(TNTs) array,TNRsarrayhasbeenextensivelyexploitedinpollutiondegradation,hydrogenproductionandsolarcellsduetoone-dimensional (1D)electrontransmissionshortcutandexcellentelectronicmobility [8].However,TNRsstillsufferstheinherent flawsofTiO2,namely,the widebandgap(3.1eV)ofrutileTiO2 limitsitsPCPapplicationonlyin
⁎ Correspondingauthors.
theUVregionanddecreasetheutilizationratioofsolarlight[9]. Meanwhile,therapidrecombinationofphotogeneratedelectrone/hole (e /h+)pairswillhappenaftercuttingoff thelight,resultinginthe loseeffectivenessofthecathodicprotectionindarkness[10].Thus,in ordertoenhancethePCPperformance,itisnecessarytosensitizeTNRs asavisiblelightabsorber.Atpresent,thecombinationofTNRsand narrowband-gapsemiconductors(suchasBi2S3,WO3,CdSe,etc.)have beenprovedtobeeffectivemethodstohinderwiththeseproblems [11].
SnO2,akindofstableandnontoxicn-type(3.5eV)semiconductor, hasbeusedtocombinewithTiO2 toformphotoanodeinsomePCP researches[12].ThestaggerbandstructurebetweenSnO2 andTiO2, benefitstheseparationofthee /h+ pairstoenhancephotoelectric performance.Inaddition,sincetheconductionband(CB)ofTiO2 is morepositivethanthatofSnO2,SnO2 canserveasan “electronpool” to storagee inthebinaryphotoanodematerial,toultimatelyprovide delayprotectionforthecoupledsteelindarkness[13].Huetal.have synthesizedSnO2 nanoparticlesmodifiedTiO2 nanotube films,and providedaneffectivecathodicprotectionfor403stainlesssteel[9]. Zhangetal.havefabricatednanoflowerlikeSnO2-TiO2 nanotubes photoanode,andthedelayprotectionfor304SSwasachievedin darkness[13].However,astheexcellentsuccedaneousmaterialsof TNTs film,therehavebeennoreportsonthePCPefficiencyeffectsof https://doi.org/10.1016/j.corsci.2020.108927
E-mailaddresses: liuyunpeng1994@stu.xjtu.edu.cn (Y.Liu), damifan198802@163.com (L.Fan), fjtes@xjtu.edu.cn (J.Feng).
Received7April2020;Receivedinrevisedform12July2020;Accepted2August2020
Availableonline07August2020
0010-938X/©2020ElsevierLtd.Allrightsreserved.
theSnO2/TNRscompositefor304SS.
Therefore,inthiswork,themainobjectiveistosynthesizethe SnO2/TNRs filmviahydrothermaltreatmentandelectrodeposition methodontheconductive fluorine-dopedtinoxide(FTO)substrates,to achieveexcellentPCPefficiencyfor304SS.Meanwhile,theirphotoelectrochemicalpropertiesandPCPmechanismfor304SSwerealso proposed.
2.Materialsandmethods
2.1.Chemicals
Hydrochloricacid(HCl),nitricacid(HNO3),stannicchloride (SnCl2),sodiumhydroxide(NaOH),sodiumchloride(NaCl),sodium sulphide(Na2S),sodiumnitrate(NaNO3)andtitaniumbutoxide (C16H36O4Ti)wereallpurchasedfromSinopharmChemicalRegentCo. Ltd,(Shanghai,China).Allthechemicalswereanalyticalreagent withoutanypurificationanddistilledwater(DW)wasusedinthe preparationofallsolutions.
FTOglass(7 Ω cm 2,2.2mm-thick)wasboughtfromGuluoGlass Co.Ltd,(Luoyang,China).Beforeusingofthetestes,theFTOglasses werecleanedultrasonicallybyDW,followedbyacetoneandalcoholfor 30mineach.Intheend,thosestructureswereair-driedinambient condition.
2.2.SynthesisofSnO2/TNRscomposite films
2.2.1.PreparationofTNRs film
TheTNRsarray filmwasfabricatedonFTOsubstratebythetraditionalhydrothermalreactionfollowingthesesteps.Inthe firstplace,60 mLHClaqueoussolution(6mol·L 1)injectedwith1.0mLC16H36O4Ti, waspouredintoa100mLTeflon-linedstainless-steelautoclave.Thena sheetofFTOstructurewasputatanangleagainstthewalloftheabove autoclavewiththeconductingsidefacingdown,andbeingheatedat 150°Cinanelectricoven(for10h).Finally,the filmwaswashedwith DW,andplacedinamufflefurnaceat500°Cinair(for2h).
2.2.2.PreparationofSnO2/TNRscomposite film
SnO2/TNRscomposite filmswerepreparedinatraditionalthreeelectrodesystem.Theworkelectrode(WE),thereferenceelectrode (RE)andthecounterelectrode(CE)arecorrespondedtotheTNRs film, Ag/AgClelectrodeandPtfoil,respectively.Theelectrodepositionsolutioncomprised25mmol·L 1 SnCl2 and50mmol·L 1 HNO3.The electrodepositionmethodwascarriedoutpotentiostatically(CHI660D, ShanghaiChinstrumentsco.,Ltd,China)at0.5Vunderroomtemperaturecondition.Afterthoroughlyrinsedwithdeionizedwaterand driedinavacuumdryingovenat50°C(for12h),theseas-deposited composite filmswereannealedat450°Cunderairatmosphere(for2h). Fig.1 displaytheschematicillustrationforpreparingthesecomposites. TocomparethePCPperformancesoftheSnO2/TNRscomposite films withdifferentdepositiontime,theSnO2/TNRscomposite filmswith
differentelectrodepositiontime(0.5,1and1.5h)wassynthesized, denotingasSnO2/TNRs-0.5hSnO2/TNRs-1handSnO2/TNRs-1.5h, respectively.
2.3.Characterizations
Alltheas-preparedsampleswerecharacterizedbyscanningelectron microscope(SEMZeissGeminiSEM500,Germany),X-raydiffraction instrument(BrukerD8ADVANCE,Germany),Ramanspectra(LabRAM HREvolutionwithanexcitationof325nmlaserlight,France),X-ray photoelectronspectroscopy(XPS,ThermoScientificEscaLab250Xi., USA),UV–visdiffusereflectanceabsorptionspectra(DRS,PE Lambda950,USA).Thephotoluminescence(PL)spectraweremeasured usinganEdinburghFLS9luminescencespectrofluophotometerwitha Xelamppresentinganexcitationlightof340nm.Thetimeresolved photoluminescence(TRPL)wasalsostudiedonaFLS9seriesof fluorescencespectrometers.
2.4.Photoelectrochemicaltests
Inthephotoelectrochemicalexperiments(asshownin Fig.2),the testrigconsistedofacorrosioncell(3.5wt%NaClsolution)anda photoelectrodecell(0.1mol·L 1 Na2S+0.2mol·L 1 NaOHmixedsolution),andthetwocellswereconnectedbyasaltbridge(saturatedKCl inagargel).Inthecorrosioncell,the304SSelectrode,Ag/AgClelectrodeandPtfoilwereconnectedtoWE,REandCEpolesofthe CHI660Delectrochemicalworkstation,respectively,andtheas-
Fig.1. SchematicillustrationoftheconstructionprocessofSnO2/TiO2
Fig.2. Schematicofphotoelectrochemicaltestdevice.
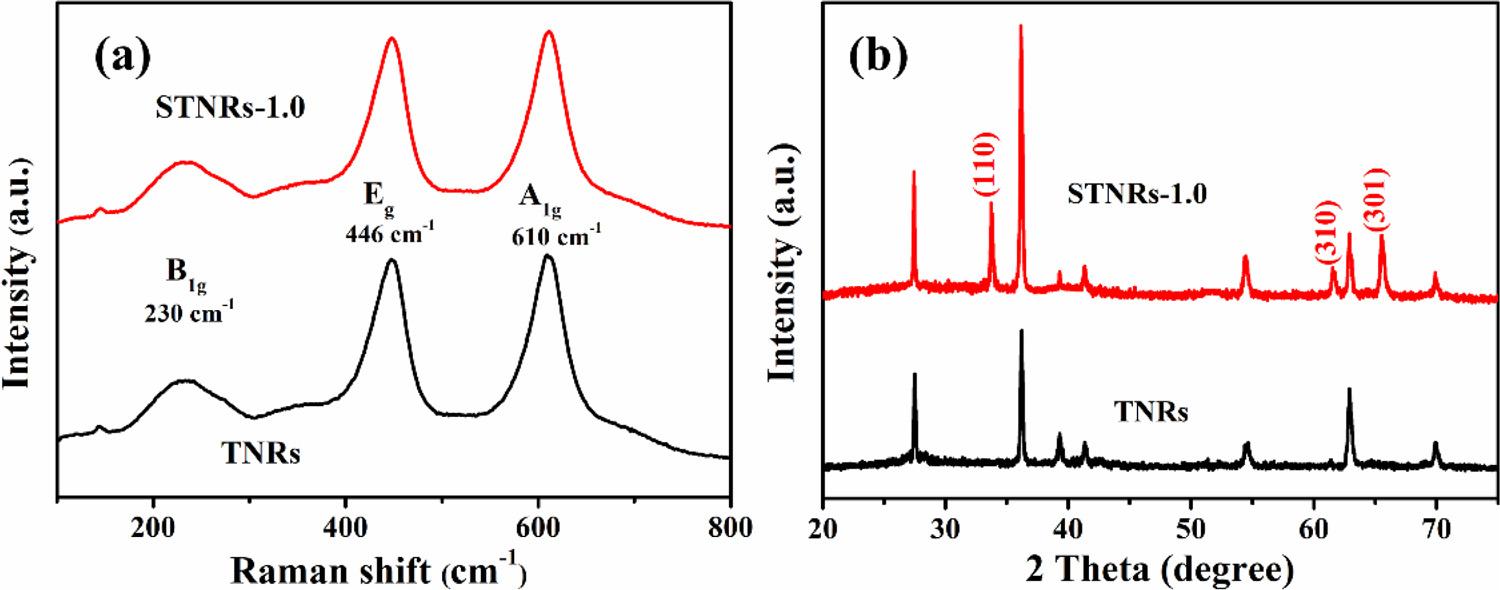
Fig.3. TheRamanspectra(a)andtheXRDpatterns(b)fortheTNRsandSTNRs-1.0.
preparedphotoelectrodeasthephotoanodewasimmersedinphotoelectrochemicalcell,whichwascoupledwiththesteelelectrodeviaa copperwire,asdescribedpreviously[3].Thephotoanodeswithan effectiveirradiationareaof1cm2 wereradiatedbya500WXenonarc lamp(CHF-XM-500W,BeijingChangtuotechnologyCo.,Ltd.Beijing, China)coupledwithanAM1.5G filtercalibratedto100mWcm 2 Thescanningratesofopencircuitpotentials(OCP)andTafelcurves were1mV/s.Meanwhile,nobiasvoltagewasappliedinthetestof photocurrentdensityofthephotoelectrode.TheTafelresultwas fitted tocalculatethecorrosionpotential(Ecorr)andthecorrosioncurrent density(Icorr).Theefficientareaof304SSwas1cm2,whichwasground with2000-meshabrasivepaperandrinsedwithacetone.
3.Resultsanddiscussion
3.1.Structuralcharacteristics
ThestructuralinformationwasprovidedbytheirRamanspectra andXRDpatterns.Asdisplayedin Fig.3a,threestrongcharacteristic Ramanpeaks(thecurveofTNRs)werefoundtolocatedat230,446and 610cm 1,ascribedtotheB1g,Eg,A1g modesofrutileTiO2,respectively [14].Moreover,anythecharacteristicpeaksofanataseTiO2,cannotbe observed,indicatingpurerutilephasewasobtainedafterhydrothermal treatment.InthecurveofSTNRs-1.0,thesecharacteristicpeaksofSnO2 wereoverlappedbytherutileTiO2 signalforthiscomposite film,so thereisnodifferencebetweenTNRsandSTNRs-1.0.Furthermore,to confirmthecompositionoftheSTNRs-1.0 film,theXRDresultsofthe TNRsandSTNRs-1.0weredisplayedin Fig.3b.Thediffractionpeaksof theTNRsat27.5°,36.1°,39.3°,41.3°,54.5°62.9°and69.8°correspond welltothe(110),(101),(200),(111),(211),(002)and(112) planesofrutileTiO2 (JCPDScardno:65-0912),respectively,whichis consistentwiththeaboveRamanresult[15].AftertheelectrodepositionofSnO2 onthesurfaceofTNRs film,theXRDcurveoftheSTNRs1.0exhibitsnewdiffractionpeaksat33.7°,61.6°and65.5°,which matcheswellwiththecharacteristicXRDpeaks((110),(310)and(30 1))ofcassiteriteSnO2 (JCPDScardno:41-1445)[16].TheXRDresult demonstratesthesuccessfuldepositionofSnO2 onthesurfaceofTNRs film.
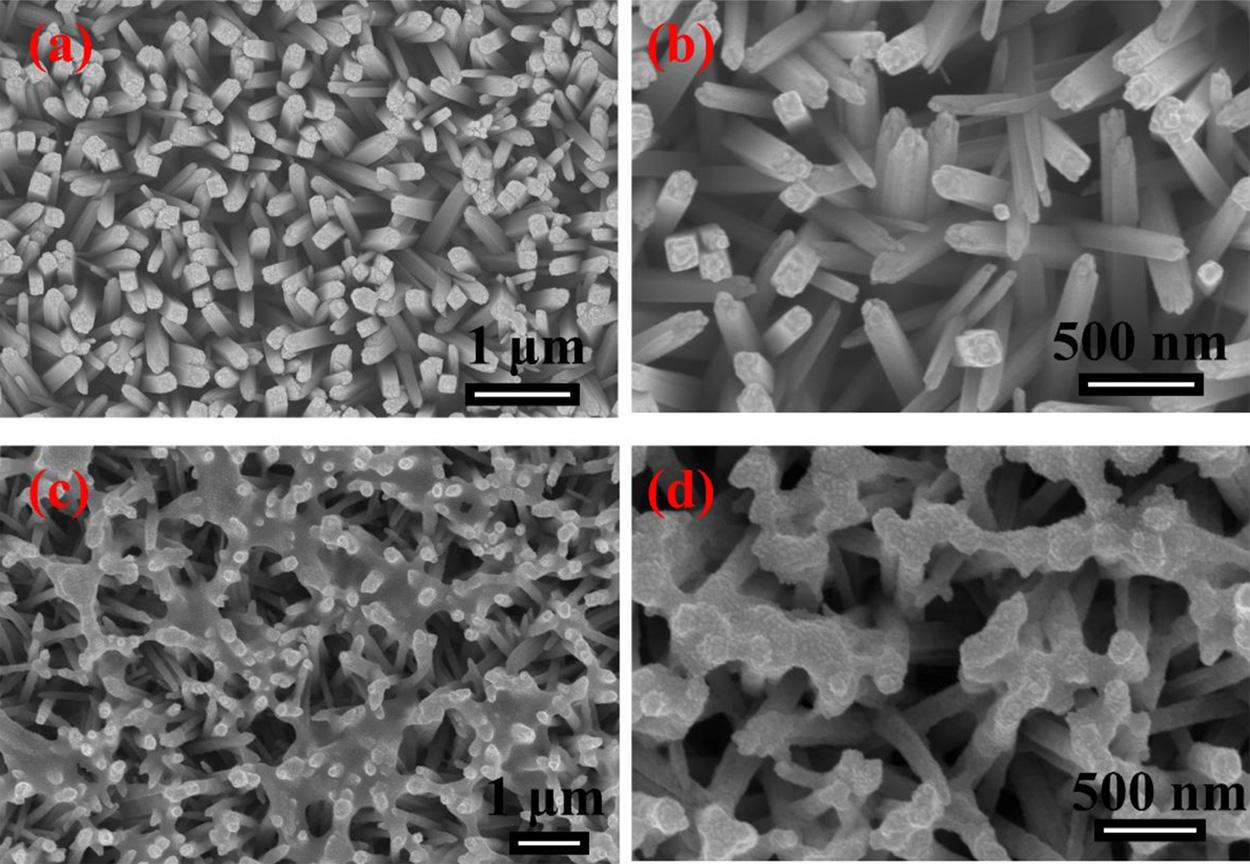
Fig.4 displaystheSEMimageswithtwodifferentmagnificationsof TNRs filmbeforeandafterSnO2 loading.FromtheimagesofTNRs (Fig.4aandb),theTNRsgrewuniformlyanddenselyonthesurfaceof FTOglass,andthemeandiameterofnanorodsisabout150nm.After electrodepositiontreatment,thereareamountofSnO2 nanoparticleson thesurfaceofTNRs filmasshownin Fig.4c.Moreover,therod-like structureofTNRsmaintainsgoodpropertyduringtheelectrodeposition process(Fig.4d).ThisresultalsoindicatesthattheSnO2 nanoparticles aresuccessfullyloadedontheTNRs film.
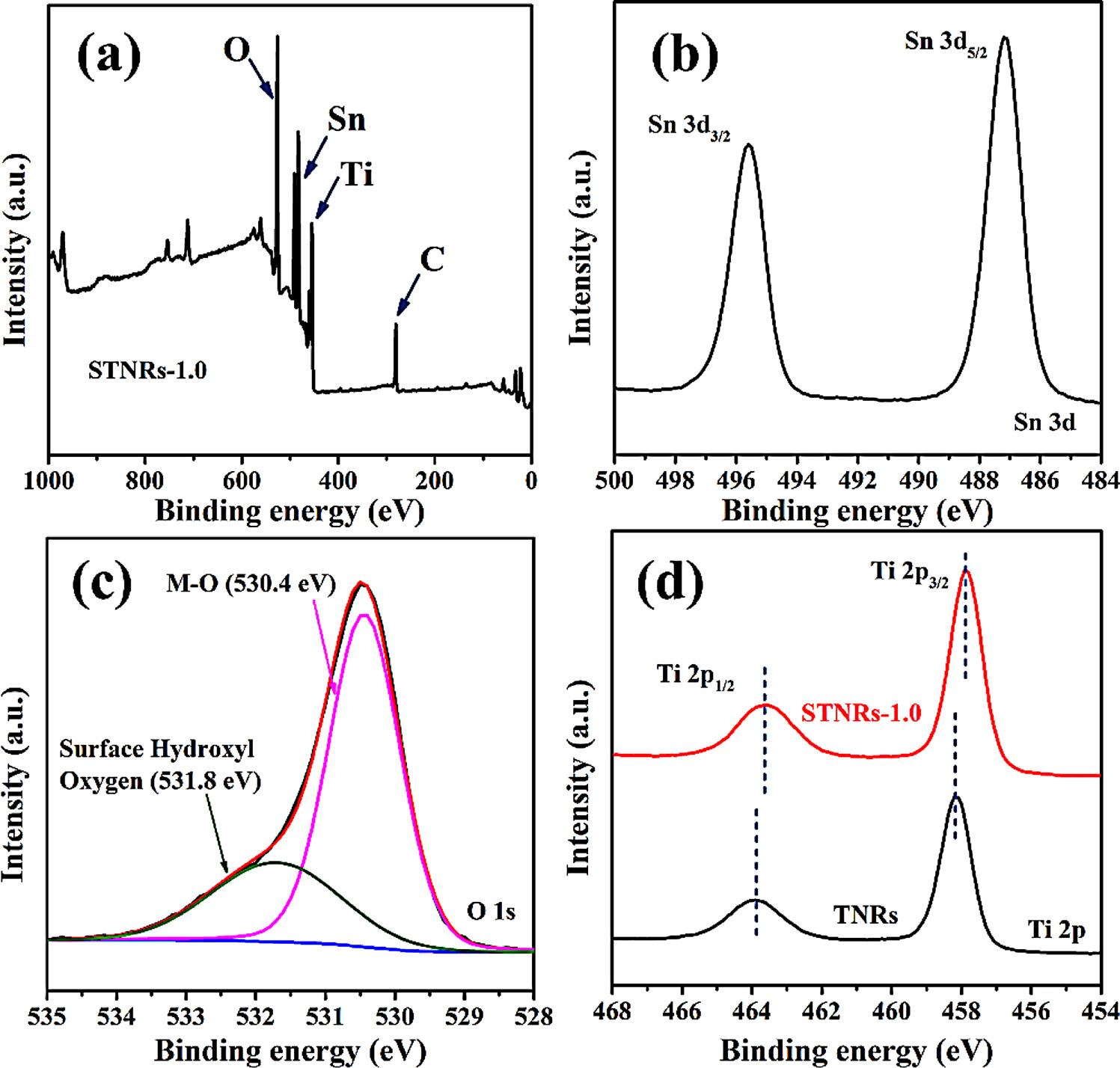
Tofurtherconfirmthechemicalcompositionsandelementalchemicalstatusesofthecomposite film,theas-synthesizedSTNRs-1.0 film
wascharacterizedbyXPS,andtheresultisdisplayedin Fig.5a-d.From thesurveyspectrashownin Fig.5a,theTi,SnandOwerepresentinthe sample,andtheC1speakinthe figureisduetothesurfacepollutionof C.HighresolutionXPSspectraforSn3d,O1sandTi2paredisplayedin Fig.5b,candd,respectively.Thetwopeaksat495.6and487.2eVare ascribedtoSn3d3/2 andSn3d5/2,respectively(Fig.5b),implyingthat themainvalencestateofSninthissampleis+4[17].Asshownin Fig.5c,bindingenergiesat530.4eVand531.8eV,arecorresponding tothecontributionoflatticeoxygeninTiO2 andSnO2 crystallattice, andsurficialhydroxylgroups(O H),respectively[18,19].Asshownin theXPScomparisoncurvesofTi2pofTNRsandSTNRs-1.0(Fig.5d), thebindingenergiesat463.9eVand458.2eVofTNRsareascribedto Ti2p1/2 andTi2p3/2,respectively[20].Comparedwiththeprevious reports[21],bothoftheTi2ppeaksoftheTNRsslightlyshiftedtothe lowerbandingenergy,whichisascribedtothedestroyofTi-O-TilinkagesoftheTiO6 octahedrainstrongacidsolutions,generatingasmall amountofoxygenvacancies(OVs)inthesynthesisprocessofnanorods [22].However,thecontentofOVsisnotsufficienttoimpactthepure rutilephaseofTNRs,accordingtotheresultsofRamanandXRD spectra.AfterelectrodepositionofSnO2,alltheTi2ppeaksofthe STNRs-1.0 filmsshiftednegatively,indicatingtheinteractionbetween TiO2 andSnO2.Withtheformationofheterojunction,theredistribution ofvalenceelectronsbetweenTiO2 andSnO2 occurs[23],andthepositiveshiftofTi2pindicatesthatthetransfertendencyfromSnO2 to TiO2.
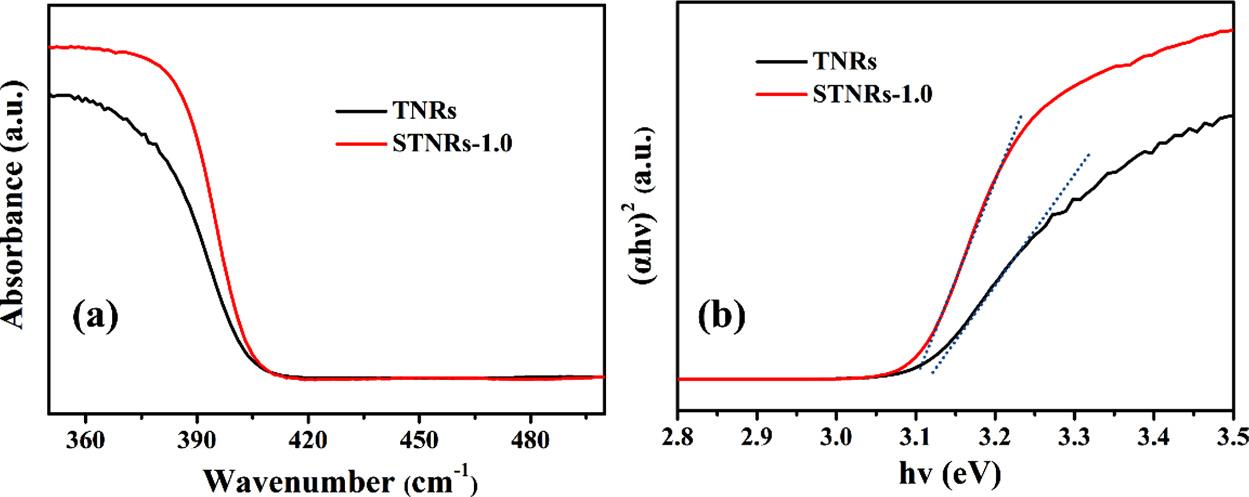
UV–visDRSwasemployedtostudytheopticalpropertiesofTNRs andSnO2/TNRs films.Asdisplayedin Fig.6,theTNRsshowsatypical absorptionband-edgeatabout390nm,whichagreeswellwiththe bandgapofrutileTiO2 (Eg =3.10eV)[3,24].AfterdepositionofSnO2, theas-preparedSTNRs-1.0compositeshowsmuchstrongerabsorption abilitytotheUVlight,andslightred-shiftoftheabsorptionedgetothe longerwavenumberregioncomparedwiththepureTNRs.Themeasuredband-gapvalueofSTNRs-1.0compositeisnarrowedtobeabout 3.06eV.ThisresultdemonstratesthatthecombinationofTNRsand SnO2 notonlyimprovethesolar-energyutilizationefficiencyofthis photoanode,butalsoefficientlysuppressthebulkrecombinationand surfacerecombinationofthee /h+ pairs,and finallypromoteitsPCP propertiesfor304SS.Meanwhile,SnO2 nanoparticlescannotabsorb visiblelightduetoitsrelativelywidebandgap[25],resultinginthe veryslightopticalabsorptionred-shiftofSTNRs-1.0composites.
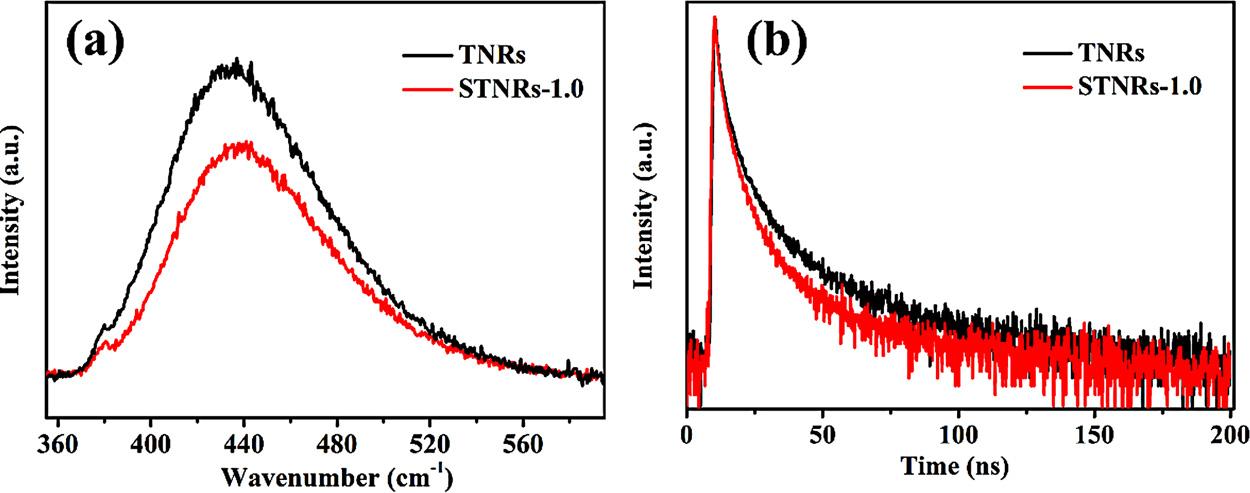
InordertostudytheinfluenceoftheSnO2 nanoparticles,PLemissionspectrumandTRPLspectrummeasurementswereappliedtoreveal themigration,transferandrecombinationprocessesofthee /h+ pairs intheinterfacebetweenSnO2 andTNRs.Asshownin Fig.7a,themain emissionpeakislocatedat435nmforthepureTNRs,whichcanbe contributedtotheband-to-bandrecombinationprocessofthee /h+ pairs[26].ThehigherPLintensity,thefasterrecombinationrateof photocarriers.ThePLpeakintensityoftheSTNRs-1.0wasmuchlower forcomparisonwiththatofthepureTNRs,implyingthattheseparation
Fig.4. SEMimagesoftheTNRs(aandb)andSTNRs-1.0(candd).
Fig.5. FullscansurveyXPSspectrum(a)andhigh-resolutionspectraof(b)Sn3dand(c)O1sofSTNRs-1.0composite film,andtheXPScomparisonspectrumofTi 2p(d)ofTNRsandSTNRs-1.0 films.
efficiencyofthee-/h+ pairsinthecomposite filmhasbeenenhanced becauseofthedepositionofSnO2.Formoreinsightsintothephotogeneratedcharges,TRPLanalysiswasdisplayedin Fig.7b,andthe lifetimeofSTNRs-1.0(8.30ns)ishigherthanthatofpureTNRs(5.34 ns),indicatingrapidchargeimmigrationbetweenSnO2 andTNRs. Theseresultsdemonstratedthattheformationofbuilt-inelectric field intheinterfacecanefficientlyimprovethetransferofphoto-induced electronsandsimultaneouslyhinderedtherecombinationofthee /h+ pairs.
UV–visabsorptionspectra(a)andtheplotsof(αhν)2 versus hν (b)fortheTNRsandSTNRs-1.0 films.
3.2.PhotoelectrochemicalpropertiesofTNRsandSnO2/TNRs film
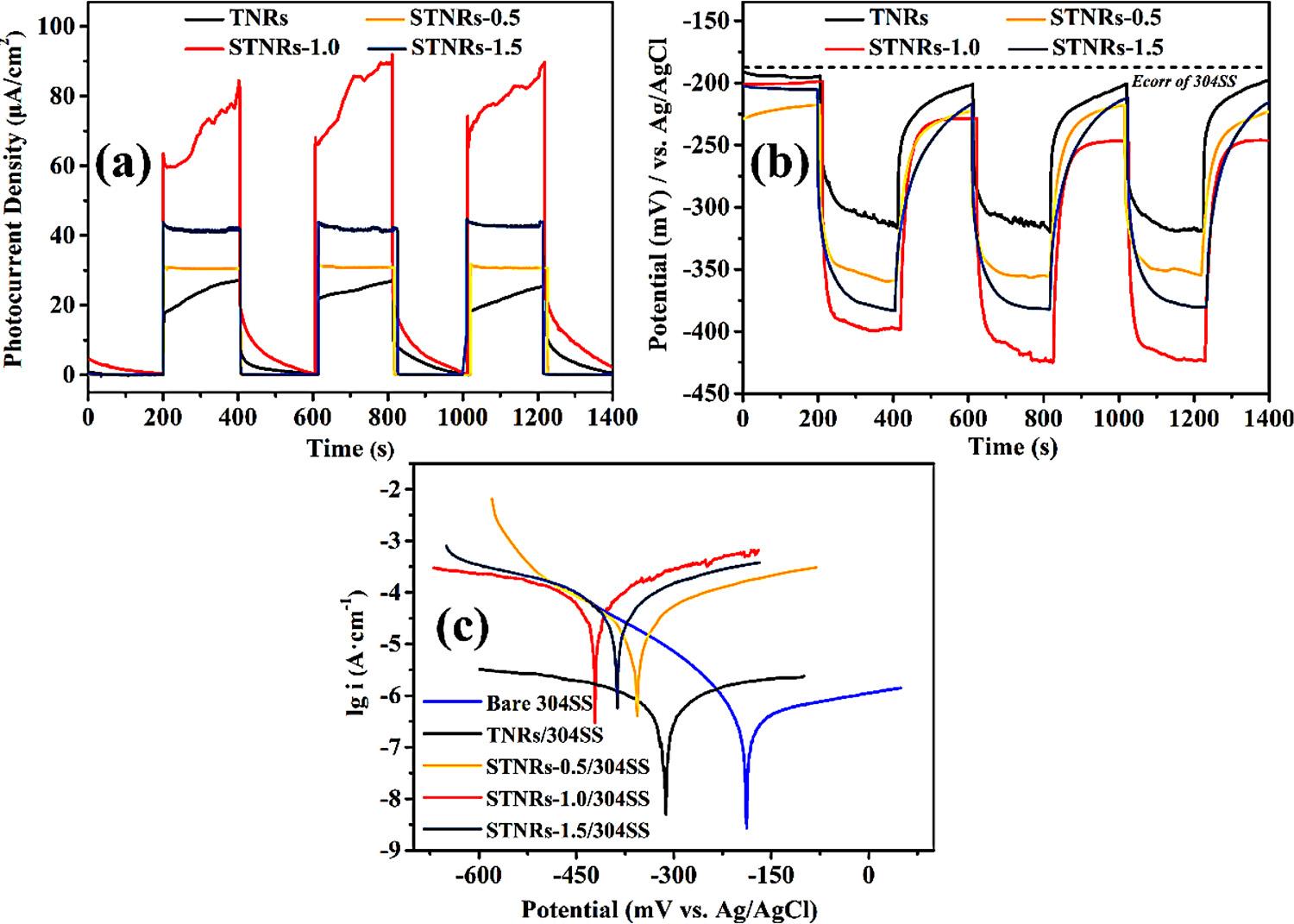
Thetransferofphotoinducedelectronsplayanimportantrolein PCPprocess,soit’sessentialforassessingthePCPefficiencyofaphotoanodetoinvestigatethephotocurrentdensity[27]. Fig.8ashowsthe photocurrentdensityoftheTNRs,andSTNRs-0.5,STNRs-1.0and STNRs-1.5 filmsconnectedwithmetalwithintermittentwhitelight irradiation.Beforeillumination,thephotocurrentvaluesofthefour sampleswerealmostzero.Afterthelightsourcewasturnedon,the photoelectricresponseofSnO2/TNRscomposite(31–84 μAcm 2)were highercomparedwiththeunmodifiedTNRs film(27 μAcm 2),indicatingthatphoton-to-currentconversionefficiencyofSnO2/TNRs filmwasoutstandinglyimproved.Namely,moreelectronscanbe photoexcitedinthiscomposite filmunderwhitelightillumination, suggestingthattheSnO2/TNRs filmmightprovidemoreeffective cathodicprotectionfor304SSthantheTNRs film.From Fig.8a,the valueorderofphotocurrentdensityisasfollows:STNR-1.0>STNR1.5>STNR-0.5,suggestingthattheSTNR-1.0mightexhibitsuperior PCPperformanceon304SS.Inaddition,theSnO2/TNRsphotoanode hasastableandreproduciblephotoelectrochemicalpropertyduring threeon-off cyclesoftheillumination.Therefore,theSnO2/TNRs film hasanadvantageforanexcellentphotocatalyticperformance.
ToassessingthePCPperformancesofas-preparedphotoelectrodes onthesteel,thematerials(TNRs,STNRs-0.5,STNRs-1.0andSTNRs-1.5 films)serveasphotoanodeinthephoto-inducedOCPtestsviamonitoringthechangeofsteelsurfacepotentialunderwhitelightillumination(asdisplayedin Fig.8b).Theresultshowsthecorrosionpotentialofbaresteelisabout 180mVvs.Ag/AgCl,meanwhile,the OCPvalueof304SScoupledtoTNRs filmshiftsslightlytonegative values( 320mVvs.Ag/AgCl).Nevertheless,forSnO2/TNRs films,the muchmorenegativeOCPvalue( 400mVvs.Ag/AgCl)ofthesteelcan beobservedwhenthisphotoanodeisirradiatedbywhitelight firstly. Afterthen,thesurfacepotentialofthesteelconnectedtoSnO2/TNRs filmsgoondroppingslightly,andsustain finallyanegativevalue ( 350to 420mVvs.Ag/AgCl).Asexplainedabove,theformationof n-nheterojunctionbetweenSnO2 andTiO2 canimprovetheseparation efficiencyofe /h+ pairs,andthenmorephotoelectronsare
immigratedtotheconnectedsteeltodecreaseitssurfacepotential, resultinginsuperiorPCPefficiency.Additionally,aftercuttingoff the light,theOCPvalueof304SSconnectedtoTNRs filmreturnstothe initiallevelbeforeillumination(about190mVvs.Ag/AgCl),indicating thatitcan’tprovidevaliddelayprotectionfor304SS.Nevertheless,itis obviousthatallthesurfacepotentialofthesteelcoupledwithSnO2/ TNRscomposite filmsaremorenegativethanthecorrosionpotentialof 304SSindarkness( 210to 230mVvs.Ag/AgCl),whichcanbe ascribedtotheeffectof “electronpool”.Meanwhile,thepotentialof 304SScoupledwithSTNRs-1.0issignificantlylowerthanthatwhenit iscoupledwithpureSTNRs-0.5andSTNRs-1.5withlightillumination, implyingthatSTNRs-1.0isoptimalforanti-corrosionof304SS.This resultcouldbeattributedtotheappropriatedepositioncontentofSnO2 nanoparticlescoordinatedwiththesustainabilityofthe1Delectron transmissionshortcutoftheTNRs,thereforeenhancingthephoton-toelectronconversionefficiencyandinsuringabundantgenerationand rapidimmigrationofphotoelectrons.ForSTNRs-0.5,becauseofthelow depositioncontentofSnO2 nanoparticles,itisunsatisfactorytogeneratesufficientphotoelectronstothemaximumextent.ForSTNRs-1.5, owingtotheexcessivecoverageofSnO2 nanoparticlesontothesurface ofTNRs film,thelight-absorptionabilityisreduced,andsectionalSnO2 nanoparticlesmaybetherecombinationcenterofthee /h+ pairs. Moreinterestingly,theOCPvalueof304SScoupledtotheSTNRs-1.0 compositekeepsgoingdowntoa fixedstate(approximately 250mV vs.Ag/AgCl)aftercuttingoff thelightsourceforthesecondandthird time.WhentheSTNRs-1.0 filmisirradiatedforthe firsttime,extra electronswastemporarilystoredinSnO2 andthenreleasedtoachieve dark-stateprotection.Theseunconsumedelectronsindarknesswillbe continuedtotransferredtothecoupledsteelwhenthelightisprovided again,resultinginmorenegativesurfacepotentialof304SS.Afterthe secondon-off cycle,thedark-stateOCPshiftmorenegativelytoa fixed valueduetononeedofmoreelectronstobestored.Therefore,the STNRs-1.0 filmcanserveasapotentialphotoelectrodeinthePCPapplicationsfor304SS.
TheTafelpolarizationcurvesof304SSinthe0.5mol·L 1 NaCl solutionwerealsoemployedtoevaluatethePCPefficiencyoftheaspreparedsamplesunderwhitelightillumination(Fig.8c).Itisobvious
Fig.7. PLemissionspectra(a)andTRPLspectra(b)ofTNRsandSTNRs-1.0composite films.
Fig.6.
thattheEcorr valueof304SSconnectedtotheas-preparedsampleis morenegativethanthatofbare304SS( 187mVvs.Ag/AgCl),andthe Ecorr of304SScoupledwithSTNRs-1.0 filmexhibitsthemostnegative value(-421mVvs.Ag/AgCl),whichisinagreementwiththeOCPresults.ThisresultindicatethatSTNRs-1.0 filmpossessthehigherphotoelectricconversionefficiencythanpureTNRs film,andmoreelectrons areimmigratedtothecoupledsteelunderwhitelightirradiation.Itis wellknownthatthehigherIcorr value,themoreeffectiveanti-corrosion propertywillbe.TheIcorr ofSTNRs-1.0ismarkedlyhigherthanthoseof theothersamples/304SSandbare304SS,sincesuperiorsolarlight utilizationcapacityandseparationefficiencyofthee-/h+ pairs.Accordingtotheaboveanalysis,theSTNRs-1.0compositecouldbesupposedtodisplayremarkablePCPefficiencyfor304SS.
3.3.ThestabilityevaluationofSTNRs-1.0 filminPCPsystemof304SS
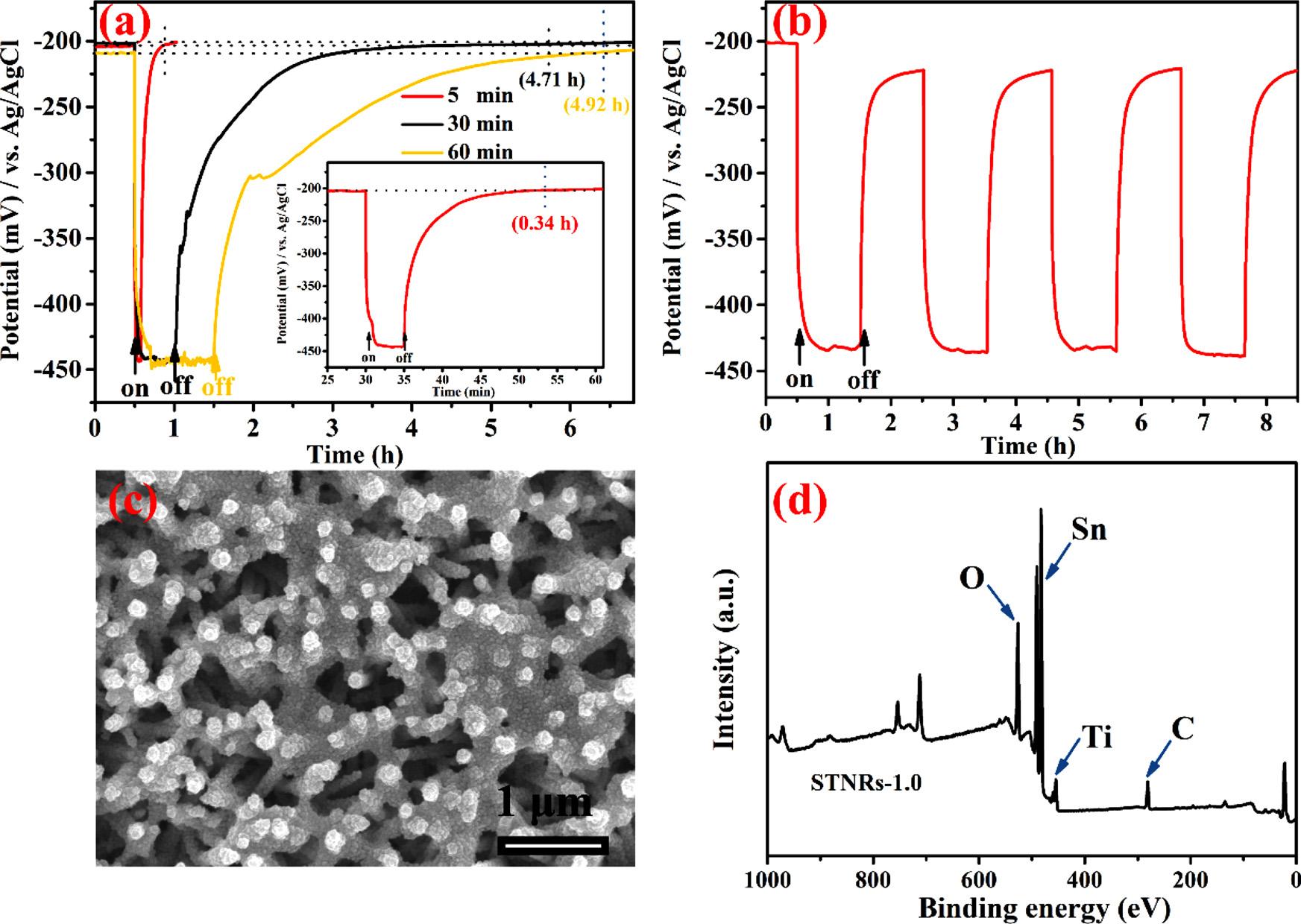
Asshownin Fig.9a,therelationshipbetweentheilluminationtime ofwhitelightandthedark-statedelayPCPperformanceof304SS coupledwithSTNRs-1.0isinvestigated.AftercoupledwiththeSTNRs1.0composite,the304SSpotentialnegativelyshiftstoaround 200 mVvs.Ag/AgClbeforeilluminationbecauseofthegalvaniceffect. Whenprovidedwithwhitelightirradiation,allthepotentialvalues rapidlydroppedto 440mVvs.Ag/AgCl,whichareconsistentwith theaboveresults.WhentheSTNRs-1.0 filmwasonlyirradiatedwith whitelightfor5min,theOCPvalueofthe304SSrecoveredtoitsthe initialpotential( 200mVvs.Ag/AgCl)after20min(about0.34h). Afterextendingtheilluminationtimeto30min,thedurationofthe delayprotectioncouldbeeffectivelyincreasedto4.71h.However, whentheilluminationtimewasextendedto60minfurther,thedurationofthedelayprotection(about4.92h)isalmostidenticaltothatof theaboveresult(illuminationfor30min).Theseresultsdemonstrate thattheincreaseofilluminationtimeisinfavorofthedark-stateprotectionof304SS,nevertheless,theenhancementextentofdelayprotectionislimitedandtheSTNRs-1.0 filmcanprovidecertaindegreeof delayprotectionevenwhenthelightsourceisturnedoff duringthis period.
InordertoestimatethephotoresponsestabilityofSiO2/TNRs film inthePCPsystem,theOCPvaluechangesofthe304SScoupledwith STNRs-1.0 filmtointermittentlywhitelightirradiationweremeasured
Fig.8. (a)Thephotoinducedcurrentdensity betweentheas-preparedsamplesand304SS underintermittentlightillumination.(b)The potentialchangeofthe304SSelectrodescoupledwiththeas-prepared films.Polarization curves(c)ofbare304SS,and304SScoupled withTNRs,andSnO2/TNRs filmselectrodes underillumination.
(asshownin Fig.9b).ThisresultdisplaysthattheSTNRs-1.0 filmcan preventcontinuouslyandeffectively304SSfromcorrosion,andthe OCPvalue(around 220mVvs.Ag/AgCl)of304SSisstilllowerfor comparisonwithitsinitialvalue(around 200mVvs.Ag/AgCl)and corrosionpotentialunder8h’ intermittentlightirradiation,displaying distinguishedstabilityofthiscomposite films.TheSEMimageof STNRs-1.0 filmmeasuredintheabovestabilityevolutionexperiment, areexhibitedin Fig.9c.ItcanbeobviouslyseenthatamassofSnO2 are stillsteadilyanchoredonthesurfaceofTNRs film,andthereisn'tmuch massSnO2 tostripfromthesubstrate.Therefore,suchcomposite film hasgoodpotentialinPCPapplication.
InthePCPsystem,theS2 oftheelectrolyteofthephotoelectrochemicalcellplaysanextremelykeyroletoeliminateh+ (Eq. (2)), aimingtopromotetheseparationofphotocarriersandfurtherenhance thePCPefficiency[28].However,theaccompanyingelementalsulfur (S)maydepositontothesurfaceofthecomposite filmtohinderthe immigrationoffreecarrier.TheXPSsurveyspectrumoftheSTNRs-1.0 filmafterPCPexperimentisshownin Fig.9d,andthereisnopeaksfor elementalS,whichsuggeststhatthereaction-generatedelementalS formsdissolvablepolysulfideionsinthesolution(Eq. (3) and (4))[29]. Therefore,theelementalSoriginatedfromtheoxidationreactionof S2 doesnotexistontothesurfaceoftheelectrode,andhavelittle influenceonthePCPperformance.
3.4.Mechanism
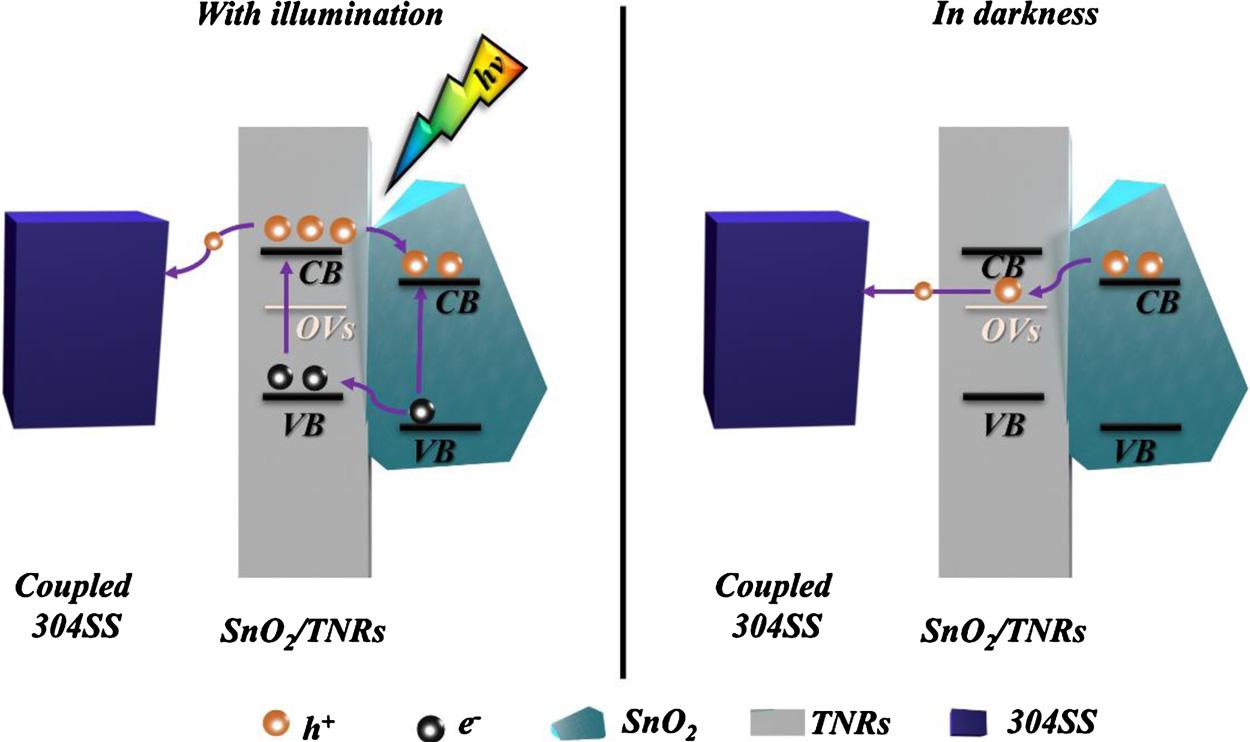
AfterevaluatingthePCPperformancesoftheTNRsandSnO2/TNRs films,thee /h+ separationandimmigrationmechanismforthisSnO2/ TNRscompositeelectrodewithandwithoutwhitelightirradiationin thePCPapplicationfor304SSweredescribedin Fig.10.Thebandgap ofSnO2 iswiderthanthatofrutileTiO2,andtheCBandvalanceband (VB)ofSnO2 canmatchwellwiththoseofTiO2 [14].Whenthewhite lightilluminedthiscomposite film,thephotogeneratedelectronsinthe VBofTiO2 andSnO2 werephotoexcitedandimmigratedtotheirCB,
Fig.9. (a)Thepotentialchangeofthe304SSelectrodescoupledwiththeSTNRs-1.0 filmunderdifferent-timeintermittentvisiblelightillumination.(b)Thepotential changeofthe304SSelectrodescoupledwiththeSTNRs-1.0 filmunder4lighton/off cycles.TheSEMimages(c)andthefullscansurveyXPSspectrum(d)ofthe STNRs film,whichwasusedintheabovestabilityevaluationexperimentsfor8hunderintermittentvisiblelightillumination.
respectively.Meanwhile,amajorityofphotoelectronswererapidly transferredtothe304SSthroughFTOsubstratebecauseofalower potential(304SS)[30],resultinginthattheprotectedsteelwasinthe stateofthermodynamicstability.ComparedwiththepureTNRsphotoanode,bothofthephotoexcitedelectronsoftwokindsmaterials couldimmigratedtothecoupledsteel,leadingtoabetterPCPperformance
Atthesametime,sectionalelectronsinTiO2 CBcouldbeimmigratedtoSnO2 CBunderwhitelightillumination,becausetheCBof thelatterismorenegativethanthatoftheformer.AndthephotogeneratedholesassembledinTiO2 VBintheoppositedirectionare vanishedbyS2 atthephotoanode/electrolyteinterface.Theordered
band-gapstructureisfavouroftheseparationofe-/h+ pairs,resulting inthatmoreelectronsofSnO2 andTiO2 areimmigratedtothesteel surface.Meanwhile,sectionalelectronscouldbeservedinSnO2 CB temporarily,duetoitsinherentpropertyviaareductionreactionas follows[13,31–33]:
++⇄== −+ SnOxexMMSnOMNaHx(or,1or2) x 22 withwhitelightillumination,theelectronswillbesavedinSnO2 temporarily.Aftercuttingoff thelight,abovereactionreversestodischargethephotoelectrons.Meanwhile,thereareasmallamountofOVs intheTNRs,andtheenergylevelsofOVshavebeenreportedtobe about1.18eVbelowtheCBoftherutileTiO2 [34].Therefore,the
Fig.10. ThesketchoftheproposedchargephotogenerationandtransfermechanismintheSnO2/TNRs filmforthePCPapplicationwithandwithoutsolarlight illumination.
electrontransitionsfromtheCBofSnO2 totheOVslevelsofTiO2 contributedtotheelectrontransferfromSnO2 toTiO2 alongthe1D electronshortcut.Andthisistheprincipalfactortoachievingthedelay protectionfor304SSinthedark.
4.Conclusion
Inthisresearch,SnO2/TNRscomposite filmwassuccessfullysynthesizedwithenhancedPCPperformancesandelectronsstorageability viahydrothermaltreatmentcombinedwithelectrodepositionmethod. ComparedwiththepureTNRs film,theSnO2/TNRscomposite film possessessuperiorlightresponseabilityandphotoelectrochemicalefficiency.Thephotocurrentdensityofthiscomposite filmisalmostthree timeshigherthanthatofthepureTNRsbasedonthedepositionofSnO2 nanoparticles.Withwhitelightirradiation,theSnO2/TNRs filmcould exhibitsuperiorcathodicprotectionperformanceforthe304SSina0.5 mol·L 1 NaClsolutioncomparedwiththepristineTNRs film.More importantly,theSnO2/TNRs filmcouldachievethedelayprotection owingtotheelectronsstoragepropertyofSnO2 material,evenafter cuttingoff thelight.Inaword,SnO2/TNRscompositecanberegarded asapotentialphotoelectrodeinthePCPapplicationsfor304SS.
CRediTauthorshipcontributionstatement
JuantaoZhang: Writing-originaldraft,Methodology. Hualong Yang: Projectadministration. YuanWang: Formalanalysis. Xiaohu Cui: Datacuration. ZhangWen: Conceptualization. YunpengLiu: Supervision,Writing-review&editing. LeiFan: Fundingacquisition. JiangtaoFeng: Investigation,Methodology,Resources,Software.
DeclarationofCompetingInterest
Wedeclarethatwehaveno financialandpersonalrelationships withotherpeopleororganizationsthatcaninappropriatelyinfluence ourwork,thereisnoprofessionalorotherpersonalinterestofany natureorkindinanyproduct,serviceand/orcompanythatcouldbe construedasinfluencingthepositionpresentedin,orthereviewof,the manuscriptentitled.
Acknowledgements
TheauthorsgratefullyacknowledgetheBasicResearchand StrategicReserveTechnologyResearchFundofChinaNational PetroleumCorporationProject(GrantNo.2018Z-01),ShaanxiKeyresearchanddevelopmentprojects,China(GrantNo.2017SF-386)and theFundamentalResearchFundsfortheCentralUniversitiesofChina.
References
[1] J.Zhang,R.G.Du,Z.Q.Lin,Y.F.Zhu,Y.Guo,H.Q.Qi,L.Xu,C.J.Lin,Highly efficientCdSe/CdSco-sensitizedTiO2 nanotube filmsforphotocathodicprotection ofstainlesssteel,Electrochim.Acta83(2012)59–64
[2] H.Li,X.Wang,L.Zhang,B.Hou,PreparationandphotocathodicprotectionperformanceofCdSe/reducedgrapheneoxide/TiO2 composite,Corros.Sci.94(2015) 342–349
[3] Y.Liu,C.Zhao,X.Wang,H.Xu,H.Wang,X.Zhao,J.Feng,W.Yan,Z.Ren, PreparationofPPy/TiO2 core-shellnanorods filmanditsphotocathodicprotection for304stainlesssteelundervisiblelight,Mater.Res.Bull.124(2020) 110751–110756
[4] H.Xu,W.Liu,L.Cao,G.Su,R.Duan,PreparationofporousTiO2/ZnOcomposite filmanditsphotocathodicprotectionpropertiesfor304stainlesssteel,Appl.Surf. Sci.301(2014)508–514
[5] L.Xu,Y.F.Zhu,J.Hu,J.Zhang,Y.Z.Shao,R.G.Du,C.J.Lin,TiO2 nanotube films preparedbyanodizationinglycerolsolutionsforphotocathodicprotectionof stainlesssteel,J.Electrochem.Soc.161(2014)C231–C235
[6] Y.-F.Zhu,L.Xu,J.Hu,J.Zhang,R.G.Du,C.J.Lin,Fabricationofheterostructured SrTiO3/TiO2 nanotubearray filmsandtheiruseinphotocathodicprotectionof stainlesssteel,Electrochim.Acta121(2014)361–368
[7] S.Cui,X.Yin,Q.Yu,Y.Liu,D.Wang,F.Zhou,Polypyrrolenanowire/TiO2 nanotube nanocompositesasphotoanodesforphotocathodicprotectionofTisubstrateand 304stainlesssteelundervisiblelight,Corros.Sci.98(2015)471–477
[8] S.S.Ge,Q.X.Zhang,X.T.Wang,H.Li,L.Zhang,Q.Y.Wei,Photocathodicprotection of304stainlesssteelbyMnS/TiO2 nanotube filmsundersimulatedsolarlight,Surf. Coat.Technol.283(2015)172–176
[9] J.Hu,Q.Liu,H.Zhang,C.D.Chen,Y.Liang,R.G.Du,C.J.Lin,Facileultrasonic depositionofSnO2 nanoparticlesonTiO2 nanotube filmsforenhancedphotoelectrochemicalperformances,J.Mater.Chem.A3(2015)22605–22613
[10] J.Hu,Y.F.Zhu,Q.Liu,Y.B.Gao,R.G.Du,C.J.Lin,SnO2 nanoparticle filmspreparedbypulsecurrentdepositionforphotocathodicprotectionofstainlesssteel,J. Electrochem.Soc.162(2015)C161–C166
[11] H.Li,X.Wang,L.Zhang,B.Hou,CdTeandgrapheneco-sensitizedTiO2 nanotube arrayphotoanodesforprotectionof304SSundervisiblelight,Nanotechnology26 (2015)155704–155714
[12] W.Liu,K.Yin,F.He,Q.Ru,S.Zuo,C.Yao,Ahighlyefficientreducedgraphene oxide/SnO2/TiO2 compositeasphotoanodeforphotocathodicprotectionof304 stainlesssteel,Mater.Res.Bull.113(2019)6–13
[13] J.Zhang,Z.UrRahman,Y.Zheng,C.Zhu,M.Tian,D.Wang,NanoflowerlikeSnO2TiO2 nanotubescompositephotoelectrodeforefficientphotocathodicprotectionof 304stainlesssteel,Appl.Surf.Sci.457(2018)516–521.
[14] T.H.Huy,D.P.Bui,F.Kang,Y.F.Wang,S.H.Liu,C.M.Thi,S.J.You,G.M.Chang, V.V.Pham,SnO2/TiO2 nanotubeheterojunction:the firstinvestigationofNOdegradationbyvisiblelight-drivenphotocatalysis,Chemosphere215(2019)323–332 [15] Y.Zhang,Q.Lin,N.Tong,Z.Zhang,H.Zhuang,X.Zhang,W.Ying,H.Zhang, X.Wang,SimplefabricationofSnO2 quantum-dot-modifiedTiO2 nanorodarrays withhighphotoelectrocatalyticactivityforoverallwatersplitting,Chemphyschem 19(2018)2717–2723
[16] B.Sun,Y.Chen,L.Tao,H.Zhao,G.Zhou,Y.Xia,H.Wang,Y.Zhao,Nanorodsarray ofSnO2 quantumdotsinterspersedmultiphaseTiO2 heterojunctionswithhighly photocatalyticwatersplittingandself-rechargeablebattery-likeapplications,ACS Appl.Mater.Interfaces11(2018)2071–2081
[17] Y.Li,Q.Zhang,L.Niu,J.Liu,X.Zhou,TiO2 nanorodarraysmodifiedwithSnO2Sb2O3 nanoparticlesandapplicationinperovskitesolarcell,ThinSolidFilms621 (2017)6–11
[18] S.V.Mohite,V.V.Ganbavle,K.Y.Rajpure,Photoelectrochemicalperformanceand photoelectrocatalyticdegradationoforganiccompoundsusingGa:WO3 thin films, J.Photochem.Photobiol.A344(2017)56–63
[19] Y.Liu,W.Zhang,C.Zhao,H.Wang,J.Chen,L.Yang,J.Feng,W.Yan,Studyonthe synthesisofpoly(pyrrolemethane)swiththehydroxylindifferentsubstituentpositionandtheirselectiveadsorptionforPb2+,Chem.Eng.J.361(2019)528–537
[20] P.Zhang,S.Zhu,Z.He,K.Wang,H.Fan,Y.Zhong,L.Chang,H.Shao,J.Wang, J.Zhang,C.N.Cao,PhotochemicalsynthesisofSnO2/TiO2 compositenanotube arrayswithenhancedlithiumstorageperformance,J.Alloys.Compd.674 (2016)1–8.
[21] H.Zhu,J.Tao,X.Dong,PreparationandphotoelectrochemicalactivityofCr-Doped TiO2 nanorodswithnanocavities,J.Phys.Chem.C114(2010)2873–2879
[22] P.K.Giri,B.Santara,K.Imakita,M.Fujii,Microscopicoriginoflatticecontraction andexpansioninundopedrutileTiO2 nanostructures,J.Phys.D-Appl.Phys.47 (2014)215302–215315
[23] W.Cui,J.He,H.Wang,J.Hu,L.Liu,Y.Liang,Polyanilinehybridizationpromotes photo-electro-catalyticremovaloforganiccontaminantsover3Dnetworkstructure ofrGH-PANI/TiO2 hydrogel,Appl.Catal.B232(2018)232–245
[24] Q.Shi,Z.Li,L.Chen,X.Zhang,W.Han,M.Xie,J.Yang,L.Jing,SynthesisofSPR Au/BiVO4 quantumdot/rutile-TiO2 nanorodarraycompositesasefficientvisiblelightphotocatalyststoconvertCO2 andmechanisminsight,Appl.Catal.B244 (2019)641–649
[25] M.K.Singh,M.C.Mathpal,A.Agarwal,OpticalpropertiesofSnO2 quantumdots synthesizedbylaserablationinliquid,Chem.Phys.Lett.536(2012)87–91
[26] L.Zhang,Y.Li,Q.Zhang,H.Wang,Well-dispersedPtnanocrystalsontheheterostructuredTiO2/SnO2 nanofibersandtheenhancedphotocatalyticproperties,Appl. Surf.Sci.319(2014)21–28
[27] Y.Yang,Y.F.Cheng,FactorsaffectingtheperformanceandapplicabilityofSrTiO3 photoelectrodesforphotoinducedcathodicprotection,J.Electrochem.Soc.164 (2017)C1067–C1075
[28] J.Ren,B.Qian,J.Li,Z.Song,L.Hao,J.Shi,Highlyefficientpolypyrrolesensitized TiO2 nanotube filmsforphotocathodicprotectionofQ235carbonsteel,Corros.Sci. 111(2016)596–601.
[29] A.A.Anani,Electrochemicalproductionofhydrogenandsulfurbylow-temperature decompositionofhydrogensulfideinanaqueousalkalinesolution,J.Electrochem. Soc.137(1990)2703–2709
[30] Q.Wei,X.Wang,X.Ning,X.Li,J.Shao,H.Li,W.Wang,Y.Huang,B.Hou, CharacteristicsandanticorrosionperformanceofWSe2/TiO2 nanocompositematerialsfor304stainlesssteel,Surf.Coat.Technol.352(2018)26–32
[31] R.Subasri,T.Shinohara,K.Mori,TiO2-basedphotoanodesforcathodicprotection ofcopper,J.Electrochem.Soc.152(2005)B105–B110
[32] RaghavanSubasri,TadashiShinohara,KazuhikoMori,ModifiedTiO2 coatingsfor cathodicprotectionapplications,Sci.Technol.Adv.Mater.6(2005)507-507
[33] H.Li,X.Wang,Y.Liu,B.Hou,AgandSnO2 co-sensitizedTiO2 photoanodesfor protectionof304SSundervisiblelight,Corros.Sci.82(2014)145–153
[34] N.Wei,Y.Liu,M.Feng,Z.Li,S.Chen,Y.Zheng,D.Wang,ControllableTiO2 coreshellphaseheterojunctionforefficientphotoelectrochemicalwatersplittingunder solarlight,Appl.Catal.B244(2019)519–528
MAPS.
Map 1. Showing the deaths from cholera in Broad Street, Golden Square, and the neighbourhood, from 19th August to 30th September 1854. A black mark or bar for each death is placed in the situation of the house in which the fatal attack took place. The situation of the Broad Street Pump is also indicated, as well as that of all the surrounding Pumps to which the public had access.
Map 2. Showing the boundaries of the Registrar-General’s districts on the south side of the Thames in London, and also the water supply of those districts.
ON THE MODE OF COMMUNICATION OF CHOLERA.
The existence of Asiatic Cholera cannot be distinctly traced back further than the year 1769. Previous to that time the greater part of India was unknown to European medical men; and this is probably the reason why the history of cholera does not extend to a more remote period. It has been proved by various documents, quoted by Mr. Scot,[1] that cholera was prevalent at Madras in the year above mentioned, and that it carried off many thousands of persons in the peninsula of India from that time to 1790. From this period we have very little account of the disease till 1814, although, of course, it might exist in many parts of Asia without coming under the notice of Europeans.
In June 1814, the cholera appeared with great severity in the 1st bat. 9th regt. N.I., on its march from Jaulnah to Trichinopoly; while another battalion, which accompanied it, did not suffer, although it had been exposed to exactly the same circumstances, with one exception. Mr. Cruikshanks, who attended the cases, made a report, which will be alluded to further on.
ON THE MODE OF COMMUNICATI ON OF CHOLERA.
In 1817, the cholera prevailed with unusual virulence at several places in the Delta of the Ganges; and, as it had not been previously seen by the medical men practising in that part of India, it was thought by them to be a new disease. At this time the cholera began to spread to an extent not before known; and, in the course of seven years, it reached, eastward, to China and the Philippine Islands; southward, to the Mauritius and Bourbon; and to the north-west, as far as Persia and Turkey. Its approach towards our own country, after it entered Europe, was watched with more intense anxiety than
its progress in other directions. It would occupy a long time to give an account of the progress of cholera over different parts of the world, with the devastation it has caused in some places, whilst it has passed lightly over others, or left them untouched; and unless this account could be accompanied with a description of the physical condition of the places, and the habits of the people, which I am unable to give, it would be of little use.
There are certain circumstances, however, connected with the progress of cholera, which may be stated in a general way. It travels along the great tracks of human intercourse, never going faster than people travel, and generally much more slowly. In extending to a fresh island or continent, it always appears first at a sea-port. It never attacks the crews of ships going from a country free from cholera, to one where the disease is prevailing, till they have entered a port, or had intercourse with the shore. Its exact progress from town to town cannot always be traced; but it has never appeared except where there has been ample opportunity for it to be conveyed by human intercourse.
There are also innumerable instances which prove the communication of cholera, by individual cases of the disease, in the most convincing manner. Instances such as the following seem free from every source of fallacy.
I called lately to inquire respecting the death of Mrs. Gore, the wife of a labourer, from cholera, at New Leigham Road, Streatham. I found that a son of the deceased had been living and working at Chelsea. He came home ill with a bowel complaint, of which he died in a day or two. His death took place on August 18th. His mother, who attended on him, was taken ill on the next day, and died the day following (August 20th). There were no other deaths from cholera registered in any of the metropolitan districts, down to the 26th August, within two or three miles of the above place; the nearest being at Brixton, Norwood, or Lower Tooting.
The first case of decided Asiatic cholera in London, in the autumn of 1848, was that of a seaman named John Harnold, who had newly arrived by the Elbe steamer from Hamburgh, where the disease was prevailing. He left the vessel, and went to live at No. 8, New Lane, Gainsford Street, Horsleydown. He was seized with cholera on the 22nd of September, and died in a few hours. Dr. Parkes, who made
an inquiry into the early cases of cholera, on behalf of the then Board of Health, considered this as the first undoubted case of cholera.
Now the next case of cholera, in London, occurred in the very room in which the above patient died. A man named Blenkinsopp came to lodge in the same room. He was attacked with cholera on the 30th September, and was attended by Mr. Russell of Thornton Street, Horsleydown, who had attended John Harnold. Mr. Russell informed me that, in the case of Blenkinsopp, there were rice-water evacuations; and, amongst other decided symptoms of cholera, complete suppression of urine from Saturday till Tuesday morning; and after this the patient had consecutive fever. Mr. Russell had seen a great deal of cholera in 1832, and considered this a genuine case of the disease; and the history of it leaves no room for doubt.
The following instances are quoted from an interesting work by Dr. Simpson of York, entitled “Observations on Asiatic Cholera”:—“The first cases in the series occurred at Moor Monkton, a healthy agricultural village, situated to the north-west of York, and distant six miles from that place. At the time when the first case occurred, the malady was not known to be prevailing anywhere in the neighbourhood, nor, indeed, at any place within a distance of thirty miles.
“John Barnes, aged 39, an agricultural labourer, became severely indisposed on the 28th of December 1832; he had been suffering from diarrhœa and cramps for two days previously. He was visited by Mr. George Hopps, a respectable surgeon at Redhouse, who, finding him sinking into collapse, requested an interview with his brother, Mr. J. Hopps, of York. This experienced practitioner at once recognised the case as one of Asiatic cholera; and, having bestowed considerable attention on the investigation of that disease, immediately enquired for some probable source of contagion, but in vain: no such source could be discovered. When he repeated his visit on the day following, the patient was dead; but Mrs. Barnes (the wife), Matthew Metcalfe, and Benjamin Muscroft, two persons who had visited Barnes on the preceding day, were all labouring under the disease, but recovered. John Foster, Ann Dunn, and widow Creyke, all of whom had communicated with the patients above named, were attacked by premonitory indisposition, which was however arrested. Whilst the surgeons were vainly endeavouring to
discover whence the disease could possibly have arisen, the mystery was all at once, and most unexpectedly, unravelled by the arrival in the village of the son of the deceased John Barnes. This young man was apprentice to his uncle, a shoemaker, living at Leeds. He informed the surgeons that his uncle’s wife (his father’s sister) had died of cholera a fortnight before that time, and that, as she had no children, her wearing apparel had been sent to Monkton by a common carrier. The clothes had not been washed; Barnes had opened the box in the evening; on the next day he had fallen sick of the disease.
“During the illness of Mrs. Barnes, her mother, who was living at Tockwith, a healthy village five miles distant from Moor Monkton, was requested to attend her. She went to Monkton accordingly, remained with her daughter for two days, washed hey daughter’s linen, and set out on her return home, apparently in good health. Whilst in the act of walking home she was seized with the malady, and fell down in collapse on the road. She was conveyed home to her cottage, and placed by the side of her bedridden husband. He, and also the daughter who resided with them, took the malady. All the three died within two days. Only one other case occurred in the village of Tockwith, and it was not a fatal case.” (p. 136.)
“A man came from Hull (where cholera was prevailing), by trade a painter; his name and age are unknown. He lodged at the house of Samuel Wride, at Pocklington; was attacked on his arrival on the 8th of September, and died on the 9th. Samuel Wride himself was attacked on the 11th of September, and died shortly afterwards. These comprise the first cases.
“The next was that of a person named Kneeshaw, who had been at Wride’s house. But as this forms one of a series connected with the former, furnished by Dr. Laycock, who has very obligingly taken the trouble to verify the dates and facts of the latter part of the series, it will be best to give the notes of these cases in that gentleman’s own words.
“‘My dear Dr. Simpson,—Mrs. Kneeshaw was attacked with cholera on Monday, September 9th, and her son William on the 10th. He died on Saturday the 15th; she lived three weeks; they lived at Pocklington. On Sunday, September 16th, Mr. and Mrs. Flint, and Mr. and Mrs. Giles Kneeshaw, and two children, went to Pocklington