ListofContributors
HusseinT.Abdulrazzaq UniversityofMaine,Orono,ME,UnitedStates
ElhamAryafard DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
AngeloBasile
InstituteonMembraneTechnologyoftheItalianNationalResearchCouncil (CNR-ITM),Rende,Italy
MarcoBasile UniversityofCalabria,Rende,Italy
AbhijeetP.Borole
OakRidgeNationalLaboratory,OakRidge,TN,UnitedStates;TheUniversityof Tennessee,Knoxville,Knoxville,TN,UnitedStates
BerndCermenek
GrazUniversityofTechnology,InstituteofChemicalEngineeringand EnvironmentalTechnology,Graz,Austria
MarcelloContestabile
ImperialCollegeLondon,London,UnitedKingdom
FrancescoDalena UniversityofCalabria,Rende,Italy
MonicaDan NationalInstituteforResearchandDevelopmentofIsotopicandMolecular Technologies,Cluj-Napoca,Romania
LuisaDiPaola UniversityCampusBio-MedicoofRome,Rome,Italy
FaustoGallucci
InorganicMembranesandMembraneReactors,EindhovenUniversityof Technology,Eindhoven,Netherlands
KamranGhasemzadeh
UrmiaUniversityofTechnology,Urmia,Iran
ViktorHacker
GrazUniversityofTechnology,InstituteofChemicalEngineeringand EnvironmentalTechnology,Graz,Austria
RistoIlves
EstonianUniversityofLifeSciences,Tartu,Estonia
CristinaItaliano
InstituteforAdvancedEnergyTechnologies(ITAE)“NicolaGiordano”,National ResearchCouncil(CNR),Messina,Italy
AdolfoIulianelli
InstituteonMembraneTechnologyoftheItalianNationalResearchCouncil (CNR-ITM),Rende,Italy
ElhamJalilnejad
UrmiaUniversityofTechnology,Urmia,Iran
SiminKeshtkari
DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
MarcelKohler
UniversityofKwaZulu-Natal,Durban,SouthAfrica
ArneKu ¨ u ¨ t
EstonianUniversityofLifeSciences,Tartu,Estonia
KeioKuut
EstonianUniversityofLifeSciences,Tartu,Estonia
MihaelaD.Lazar
NationalInstituteforResearchandDevelopmentofIsotopicandMolecular Technologies,Cluj-Napoca,Romania
ZongyuanLiu
BrookhavenNationalLaboratory,Upton,NY,UnitedStates
LieMeng
ArizonaStateUniversity,Tempe,AZ,UnitedStates
MariaMihet
NationalInstituteforResearchandDevelopmentofIsotopicandMolecular Technologies,Cluj-Napoca,Romania
Ju ¨ riOlt
EstonianUniversityofLifeSciences,Tartu,Estonia
AlfredoPachecoTanaka
InorganicMembranesandMembraneReactors,EindhovenUniversityof Technology,Eindhoven,Netherlands
AlessandraPalella
InstituteforAdvancedEnergyTechnologies(ITAE)“NicolaGiordano”,National ResearchCouncil(CNR),Messina,Italy
LidiaPino
InstituteforAdvancedEnergyTechnologies(ITAE)“NicolaGiordano”,National ResearchCouncil(CNR),Messina,Italy
MohammadRezaRahimpour
DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
JohannaRanninger
GrazUniversityofTechnology,InstituteofChemicalEngineeringand EnvironmentalTechnology,Graz,Austria
KaieRitslaid
EstonianUniversityofLifeSciences,Tartu,Estonia
AlirioE.Rodrigues
UniversityofPorto,Porto,Portugal
Jose ´ A.Rodriguez
BrookhavenNationalLaboratory,Upton,NY,UnitedStates
SeyyedMohamadSadatiTilebon
IranUniversityofScience & Technology,Tehran,Iran
ThomasJ.Schwartz
UniversityofMaine,Orono,ME,UnitedStates
SanjayaD.Senanayake
BrookhavenNationalLaboratory,Upton,NY,UnitedStates
AlessandroSenatore
UniversityofCalabria,Rende,Italy
LacrimioaraSenila
INCDO-INOE2000,ResearchInstituteforAnalyticalInstrumentation, Cluj-Napoca,Romania
RamonaO.Stefanescu-Mihaila
SpiruHaretUniversity,Bucharest,Romania
PrakashD.Vaidya
InstituteofChemicalTechnology,Mumbai,India
MartinvanSintAnnaland
InorganicMembranesandMembraneReactors,EindhovenUniversityof Technology,Eindhoven,Netherlands
AntonioVita
InstituteforAdvancedEnergyTechnologies(ITAE)“NicolaGiordano”,National ResearchCouncil(CNR),Messina,Italy
Yi-JiangWu
EastChinaUniversityofScienceandTechnology(ECUST),Shanghai,China
TaherYousefiAmiri UniversityofZanjan,Zanjan,Iran
Preface
Therapidgrowthinglobalenergydemand,greenhousegasemissions,andglobal warming,associatedwiththeuseoffossilfuels,isstimulatingacontinuousresearch foralternativeandrenewablefuelsshowingaverylowenvironmentalimpact.
Inaddition,nowadays,theenergydependenceonfossilfuelscreatesastrong instabilityintheglobalmarketbecausetheworldreservesoffossilfuelsarerunning outwitharelativeinstabilityoffuelprices.
Atpresent,ethanolisconsideredaviablecompetitoroverthosederivedfrom fossilfuels,representingoneofthebestbiofuelsfortransportation.Indeed,itcan beburneddirectlyorblendedwithpetroltoimprovefuelcombustioninvehicles, resultinginlowerCO2 emissiontoreducegreenhousegasesintheatmosphere. Furthermore,ethanolisnotonlyconsideredanexcellentfuelbutalsoitrepresents anextremeversatilechemicalproduct.Itisanimportantrawmaterialforboth foodprocessingandtheproductionofchemicalproducts,anditisalsoparticularly usedinthepharmaceuticalindustry.Itsapplicationsincreasedaybydayandturnout tobeofcrucialimportancefromaworldresearchpointofview.Theaimofthisbook istoprovide,withcontributionscomingfromsomeofthemostrepresentative scientistsinthefield,anoverviewonthestateoftheartaboutthemostrecent researchesonethanolproduction,application,anditseconomicalroleintheglobal marketasdeeplydiscussedinthededicatedfoursectionsofthisbook.
Inthefirstsection(ScienceandProduction),Chapter1(Abdulrazzaqand Schwartz)describesthecatalyticconversionofethanoltocommodityandspecialty chemicalsofindustrialandcommercialinterest.Chapter2(Dalena,Senatore,Iulianelli,DiPaola,M.Basile,andA.Basile)givesanoverviewontheethanolproductionprocessesandtheirfutureperspectivesrelatedtothebiomassexploitation. Chapter3(A.Kuut,Ritslaid,K.Kuut,Ilves,andOlt)dealswithanoverviewabout thestateoftheartontheconventionalprocessesforethanolproduction.Chapter4 (JalilnejadandGhasemzadeh)concludesthefirstsectionofthebookwithbioethanol productionprocessandthecriticismsrelatedtothefoodversusfuelutilization.
Inthesecondsection(ApplicationandInnovation),Chapter5(Liu,Senanayake, andRodriguez)involvesareviewonthemostcommoncatalystsusedintheethanol reformingprocesses.Steamreforming,partialoxidation,andautothermalreforming ofethanolforhydrogenproductioninconventionalreactorsarethetopicsdealtwith inChapter6(Vita,Pino,Italiano,andPalella),inwhichthemostimportantethanol reformingprocessesinthefieldofhydrogenproductionarereviewed.Thesame topicwasdiscussedusingmembranereactorstechnologyinChapter7(Iulianelli, Dalena,andBasile),givingdetailsaboutthebenefitsofthistechnologyovertheconventionalsystems.InChapter8(Ilves,Kuut,andLot),ethanolutilizationasan internalcombustionenginefuelisextensivelydiscussed,whileChapter9(Borole) dealswiththeopportunitiesgivenbytheethanolexploitationforelectricityand hydrogenproductionviabioelectrochemicalsystems.Chapter10(Lazar,Senila, Dana,andMihet)describesthechallengesofusingcrudebioethanolforhydrogen
productionbyethanolsteamreformingreaction,pointingoutthebenefitsanddrawbacksofthisapproach.SectiontwoconcludeswithChapter11(Meng),whichis dedicatedtoethanolutilizationinautomotiveapplications,includingtheenvironmentalimpactsofethanolexploitationintermsofairpollutionandgreenhouse gasemissionsaswell.
Sectionthreeofthebook(ModellingandTechnology)startswithChapter12 (Ghasemzadeh,Jalilnejad,andTilebon),inwhichtheopportunitiesgivenbyethanol exploitationinthefieldofhydrogenproductiontechnologiesarereviewed.Chapter 13(Vaidya,Wu,andRodrigues)analyzesfromakineticpointofviewethanolproductionprocessesforhydrogenproduction.Chapter14(Gallucci,PachecoTanaka, andvanSintAnnaland)illustratesethanolreforminginthermallycoupled,fluidized bed,bubblecolumn,andmembranereactors,reviewingtheadvantagesanddisadvantagesofeachkindofreactortoproducehigh-gradehydrogen.
ThetopicofChapter15(Cermenek,Ranninger,andHacker)isrepresentedby thealkalinedirectethanolfuelcell,whichrepresentsapromisingenvironmentfriendlyenergyconverterfortheproductionofcleanandefficientpower.Chapter 16(Contestabile)contributestothisbookpointingouttheroleofbioethanolutilizationinbatteries,hydrogenfuelcells,andpassengercars,whereasChapter17 (Rahimpour,Keshtkari,andAryafard)pointsoutthebenefitsandunsolvedissues regardingethanolasapotentialsubstitutetocurrentaviationfuel.
Thelastsectionofthisbook(EnvironmentandEconomy)startswithChapter18 (AmiriandGhasemzadeh),inwhichtheauthorsdescribetheimpactofanethanol economyontheenvironmentaswellasondemandandmarketing.Chapter19 (Kohler)undertakesaneconomicassessmentofethanolproductionbasedon thevolatilenatureofinternationalcrudeoilprices,growingglobalconcernsover energysecurity,andGHGemissions.Lastbutnotlessimportant,Chapter20 (Mihaila-S xtefanescu)endsthisbookpresentingatechnicalandeconomiccomparisonofbioethanolexploitationwithinagricultureandindustry.
Inconclusion,theeditorsappreciatethehardworkdonebytheauthorsandwish tothankthemallforthefruitfulcooperationinthepreparationofthisbook.TheauthorsalsothankallthestaffofElsevierfortheirpreciousandirreplaceablehelpin preparing,stepbystep,thisvolume.
AngeloBasile AdolfoIulianelli FrancescoDalena T.NejatVeziroglu
2. PRODUCTSFROMDIRECTETHANOLCONVERSION: COMMODITYCHEMICALS
2.1 ETHYLENE
Ethyleneisoneofthemostsignificantcommoditychemicalinproductiontoday.It istheplatformmoleculeofchoiceforthesynthesisofpolyethylene,polyvinyl chloride,polyethyleneterephthalate,polystyrene,ethyleneglycol,ethylbenzene, andmanyotherchemicalproducts.Ordinarily,ethyleneisproducedindustriallyby crackingnaphthaandgasoils,withapproximately98% 99%ofworldproduction basedonthismethod.Currently,theshalegasrevolutionhasshiftedattentionto theuseofethaneasafeedstock,whichisabundantinmanyshalewells.Theimportanceofsteamcrackingofethanehasincreaseddramaticallyinrecentyears,anditis consideredtobeaproductivealternativerouteforethyleneproduction.Leadingupto thisshift,manytechnologiesandmethodsweredevelopedtousealternativeresources forethyleneproductioninsteadofusingconventionalcrackingprocesses(Drumm, 2006;Sandersetal.,2007),andsometechnologydevelopmentinthisareacontinues tothisday.
IntheyearsfollowingtheSecondWorldWar,thepracticeofethyleneproduction viaethanoldehydrationwasnotuncommon,andintheinterveningyearsthedehydrationreactionhasbeenevaluatedusingmanydifferenttypesofcatalystssuchas metaloxides,alumina,clays,silica,H-ZSM-5andotherzeolites,amorphoussilica alumina,andphosphates,amongothers(Rass-Hansenetal.,2007;ZhangandYu, 2013;Angelicietal.,2013;Morschbacker,2009).
Ethanoldehydrationisanendothermicreactionrequiringhightemperatures, generallyreportedintherangeof473 723K(Angelicietal.,2013).Themostchallengingfactorinethanoldehydrationisthetendencytoproducesideproductssuch asdiethylether.Inparticular,high-temperaturereactionsleadtoethylenewhereas low-temperaturereactionstendtoincreasethepossibilityofformingdiethylether andotherproducts.
Avarietyofcatalystshavebeentestedforthisprocess.Earlyreportsfocusedon phosphoricacid(Morschbacker,2009),whereasmorerecentstudieshaveshown thatalumina-containingcatalystsarethemostpromising.Inparticular,H-ZSM-5 affordedahighyieldofabout95%(Bietal.,2010;Zhangetal.,2008)compared with g-Al2O3, whichgaveayieldofapproximately80%atlowtemperatures (Angelicietal.,2013).Othercatalyticsystemshavealsobeenexamined forlow-temperaturedehydration.Silicoaluminophosphate(SAPO)catalystsdoped withMn2þ andZn2þ showed97.8%yieldofethyleneat613K(Chenetal., 2010). Zhangetal.(2008) probedfourdifferentcatalysts,Al2O3,H-ZSM-5, Silicoaluminophosphate-34(SAPO-34),andNi-dopedSAPO-34(NiSAPO-34),and testedthemunderdifferentconditions.ThemostactivecatalystswereH-ZSM-5
andNiSAPO-34.Bothshowedhighselectivityandgreaterthan90%yieldtoward ethylene,whereastheothersshowedlowselectivity.Theauthorsattributedthelow selectivityovertheothercatalyststoanabundanceofstrongacidsitesintheirstructures.Aluminaisoneofthemostactivecatalystsforthisreaction,withethyleneproductionoccurringinatemperaturerangeof300 500 C,obtaininghighethylene yieldsbetween94%and99%(ZhangandYu,2013).
2.2 PROPYLENE
Inadditiontoethylene,itisalsopossibletoalsoproducehigherolefinsfromethanol. ThesuccessoftheExxonMobil(formerlyMobil)Methanol-to-Olefinsprocesshas driventheuseofH-ZSM-5asacatalystfortheconversionofethanoltopropylene. Inparticular,thegroupofTadahiroFujitanihasbeenquiteprolificinthisarea.As earlyas2009,theirgroupshowedthatH-ZSM-5isactivefortheproductionofpropylenefromethanolusingcommercialzeolitespurchasedfromZeolyst(Songetal., 2009).Thehighestselectivitiesobservedinthatworkwere w30%andobtainedusing aZr-impregnatedH-ZSM-5,whichtheauthorsattributetothepresenceofmoderately acidicactivesitesthatpromotetheformationofC3þ hydrocarbonsfromethanol.Ina follow-upstudy,thisgroupsynthesizedaseriesofzeoliteswithMordeniteFramework Inverted(MFI)topology,andtheyconcludedthatthereactivityisindependentof theacidsitedensity(i.e.,allacidsitesinthesecatalystsareequallyactiveforthisreaction)(Xiaetal.,2010).Theuseofmetal-containingH-ZSM-5zeolitesalsoleadsto w30%selectivityinthereaction.Forexample,thepropyleneyieldusingSr HZSM5was32%atcompleteethanolconversion.Thismaterialshowedsimilarstabilityto theZr-impregnatedzeolite(Gotoetal.,2010).
Similarresultswereobtainedby Inoueetal.(2010) usinglow Alcontent H-ZSM-5catalysts.ImpregnationofthesecatalystswithLaledtosimilarselectivitiesof w30%.Importantly,deactivationwasobservedforallthecatalystsdescribed thusfar,althoughtheLa-promotedcatalystofInoue’sgroupisqualitativelymore stablethantheZr-promotedmaterialfromFujitanietal.
MotivatedbytheprevioussuccessofphosphorousimpregnationofZSM-5to improvestabilityinotherreactions,Fujitanietal.evaluatedthissamecatalyst forpropyleneformation( Songetal.,2010 ).Similarpropyleneselectivitieswere obtained(w30%maximum),althoughcatalystde activationwaseliminatedduring short(w8h)reactions.Inafollow-upstudy( Takahashietal.,2012),thissame groupshowedthattheP-containingH-ZS M-5catalystdeactivatesonlyslightly overthecourseof100hoftime-on-stream,whereasanunpromotedH-ZSM-5 catalystlosesallofitsactivitywithin20hatthesameconditions.Theynote thattheimprovedstabilityisdependen tonthepresenceofwatervaporinthe reactor.
Directconversionofethanoltopropyleneisnottheonlyprocessavailable. BeforethedevelopmentoftheZSM-5 basedcatalystsdescribedearlier,Braskem undertookdevelopmentofabiomass-derivedethanol-to-propyleneprocess.Intheir route,ethanolisfirstdehydratedtoethylene,whichisoligomerizedtobutene.
UsingtheLummusprocess,thebuteneissubsequentlyconvertedtopropyleneand ethyleneviaolefinmetathesis(BruijnincxandWeckhuysen,2013).Itisworth noting,though,thattheprevalenceofcheapshalegasintheUnitedStatesinthe earlypartofthe21stcenturyhasmadebothbioethanol-to-ethyleneand bioethanol-to-propyleneprocesseseconomicallyunattractive.Notably,asignificant amountofpropaneisrecoveredwithmethaneandethaneinshalegas,andthereis well-establishedtechnology(i.e.,Houdrydehydrogenationforconvertingpropane topropyleneandsteamcrackingforconvertingethanetoethylene)thatmaybe moreeconomicallyattractiveaslongasshalegasliquidsareinexpensiverelative tolignocellulose.
2.3 ACETALDEHYDE
Acetaldehydeisanothersignificantchemicalthatcanbeproducedcatalyticallyfrom ethanol.Itisacommoditychemicalwhichiscommonlyusedasanintermediate inthesynthesisofimportantindustrialchemicals,suchasaceticacid,ethylacetate, crotonaldehyde,butadiene,pentaerythritol,butyleneglycol,andmanyothers (SunandWang,2014).Importantly,becauseethanolisnativelyoxygenated,productionofacetaldehydefromethanolavoidsthehazardsassociatedwithhydrocarbon oxidation.
Historically,acetaldehydehasbeenproducedcommerciallybyhydratingacetylenetovinylalcohol,whichthentautomerizestoacetaldehyde(Wittcoff,1983). Thisprocesswasoftenperformedinthepresenceofsulfuricacidasacatalyst. Asthepriceofethylenedroppedinthe1960s,ethylenesawuseasarawmaterial intheprocessofacetaldehydeproduction.Inthiscase,acetaldehydeisproduced eitherbyhydrationofethylenetoethanolfollowedbydehydrogenationorbypartial oxidationofethanolwithairoverasilvergauzecatalysttogiveacetaldehyde. Currently,themostcommonprocessforacetaldehydesynthesisistheWacker process,whichdirectlyoxidizesethylenetoacetaldehydeoverpalladiumorcopper chlorides.
Acetaldehydecanbeproducedbiorenewablybythedehydrogenationofethanol. Thisprocesshasbeenperformedovermanydifferenttypesofcatalystsandhasbeen testedatawiderangeoftemperatures,from150to350 C.Generally,theprocessis highlyselectiveusingnoblemetalssuchassilverorgold.Copperhasalsobeen observedtobeeffectiveforthedehydrogenationreaction.
GuanandHensen(2009) investigatedtheuseofarangeofsilica-supportedgold nanoparticlecatalystsforthecatalyticoxidationofethanol.Theystudiedtheinfluenceofboththesupportstructureandthegoldnanoparticlesizeinboththepresence andabsenceofoxygen.Acetaldehydeselectivityintheabsenceofoxygenat temperaturesbelow350 Cwasbetween60%and80%,dependingonAuparticle size,atcompleteconversion.At400 Ctheselectivitytoacetaldehydewasabove 90%atcompleteethanolconversionregardlessoftheAuparticlesize.Thebest resultswereobtainedusingcatalystswithmidsizedparticles,suggesting thereisanoptimuminAunanoparticlesizeforacetaldehydeproduction.
Additionally,co-feedingoxygenwithethanolledtoincreasedactivityandselectivitytowardacetaldehyderelativetoreactionscarriedoutunderanaerobic reactions.Thehighestconversionwasobtainedinthepresenceofoxygenusing anMCM-41 supportedAucatalystat250 C,whichresultedin90%selectivity towardacetaldehydeat20%ethanolconversion.Incontrast,Wittcoffpreviously reportedtheoxidationofethanoloversilveroxideorsilvergauzeatmuchhigher temperatures(e.g.,480 C),withconversionsrangingbetween74%and82%and selectivitiestoacetaldehydeofaround80%(Wittcoff,1983).
UsingAu/TiO2 ascatalystleadstoabout65%acetaldehydeselectivityat35% ethanolconversionat90 Cand42%selectivityat95%ethanolconversionat 280 Cinthepresenceofoxygen(Simakovaetal.,2010).However,atelevatedtemperaturesthecatalystdeactivatedandtheselectivitytoacetaldehydewasonly approximately40%. LiuandHensen(2013) havereportedthataternaryspinel MgCuCr2O4-supportedgoldnanoparticlecatalystishighlyactiveandableto achieve90%stableconversionwith85%selectivitytowardacetaldehydeat200 C. Changetal.(2006) havetestedvariousloadingsofcoppersupportedonRice HuskAsh(RHA),whichhasahighcontentofamorphoussilica.At275 Cusing a5.75wt%Cu/RHAcatalyst,thisgroupobtainedover80%ethanolconversion andnearlyquantitativeselectivitytoacetaldehyde.TheCu/RHAwasalsomore activethancoppersupportedonsilicagelpreparedbythesameprocedure(Angelici etal.,2013).TheimprovementusingRHAasasupportwasascribedtochangesin theparticlesizeandsurfaceareaofthecopperparticlesinthecatalyst.Alsousing non preciousmetalcatalysts, Kimetal.(2008) studiedtheconversionofethanolto acetaldehydeusingvanadium tungstenmixedoxidecatalystswithvariousratiosof vanadium-to-tungsten.Acatalystcontaining95%vanadiumshowedthebestperformanceoveradifferentrangeoftemperatures,withthehighestyieldofacetaldehyde ofabout90%at300 C.
2.4 ACETICACIDANDETHYLACETATE
Relatedtoacetaldehydeproduction,ethanolcanbefurtheroxidizedtoacetic acidandethylacetate.Industrially,aceticacidisproducedbyeithertheoxidationofacetaldehydewithdioxygenorby carbonylationofmethanolormethyl acetateandsomeisalsoproducedbydirectoxidationofbutane( Wagner, 2014).Renewableaceticacidcanbeobtain edbyoxidationofethanol-derived acetaldehyde.
Aerobicconversionofethanolleadstomixturesofacetaldehyde,aceticacid,and ethylacetate.Pd-basedcatalystsareparticularlyusefulinthisregard.Forexample, Gasparetal.(2010) showedthatpalladiumoxidesupportedonmonocliniczirconia (PdO/m-ZrO2)canbeusedtoproduceaceticacid,ethylacetate,andacetaldehyde (32%,35%,and25%selectivities,respectively,at32%ethanolconversion)from ethanolat175 Cinthepresenceofdioxygen.Notably,thephaseoftheZrO2 support issignificantforachievinghighselectivity.PhysicalmixturesofthePd-containing catalystwithotherzirconiasleadtoanear-completedeactivationoftheaceticacid
productionpathwayandincreasedproductionofacetaldehydeandethylacetate. Similarly,theseauthorsnotethattheuseofCuO/ZnOsupportedonZrO2 leadsto productionofacetaldehydeandethylacetate,butnotaceticacid(regardlessof thezirconiacrystalstructure).
Au-containingcatalystsarealsoact ivefortheproductionofaceticacid underthecorrectconditions. Takeietal.(2011) showedthatZnO-supported Aunanoparticlesareparticularlysele ctiveforaceticacid,obtaining46%yield toaceticacidand44%yieldtoacetald ehydeatcompleteconversionat220 Cin thevaporphase.Muchoftheirstudy,though,focusedonacetaldehydeproduction, andtheyobtainedhighyieldstoacetaldehyde(someinexcessof90%atcomplete ethanolconversion)usingavarietyofbasicsupports. Tembeetal.(2009) made similarobservationsintheconversionofethanolusingAu/ZnOandAu/TiO2 catalystsintheliquidphase.Byusingaslightexcessofoxygenatthestartofthebatch reaction,theyobtainednearlyquantitativeyieldofaceticacidfromethanolusing Au/ZnOat150 C.Similarly, Christensenetal.(2006) obtainedhighyields(greater than90%)toaceticacidduringliqu id-phaseoxidationusingaAu/MgAl2O4 catalyst.
Thechemistryrequiredforproducingethylacetateiscloselyrelatedtothatfor producingaceticacid,andmanyoftheworksdescribedintheearlierparagraphsalso detailtheproductionofethylacetate.Indeed,theconversionofaceticacidtoethyl acetaterequiresonlyaFischeresterification,suggestingthatethylacetateproductionmaybecatalyzedbyaceticacidattherelevantreactionconditions.Au-based catalystscanbeusedinthisregard. ZhengandStucky(2006) observed20%selectivitytoethylacetateat45%ethanolconversionusingacarefullysynthesizedAu/ SiO2 catalyst,withtheremainderoftheselectivitygoingtoacetaldehyde.Better selectivitycanbeobtainedwhenanadequateoxidationcatalystispairedwithasupportcontainingacidicorbasicsitesactiveforFisheresterification.Theuse,for example,ofaPdcatalystsupportedonacidicY-zeoliteleadstooxidationofethanol toaceticacidfollowedbyacid-catalyzedesterification(YanandChang,2000). Theseauthorsobtained49.6%selectivitytoethylacetateat83.3%ethanol conversion.
Itispossibletoobtainethylacetatefro methanolwithoutpassingthroughacetic acidasanintermediate.Interestingly, Freitasetal.(2016 )obtainedselectivities near80%toethylacetateusingCu Ag/ZrO2 catalystsintheabsenceofadded oxygen.Theynotethattheselectivitytoet hylacetatedecreaseswithincreasing Agloading,attributedtoasilver-inducedchangeinthecopperoxidationstate. Similarresultswereobtainedby Satoetal.(2013) usingmonometallicCu/ZrO 2 catalysts,andtheseauthorsnotethatthe zirconiaphaseplaysanimportantrole inobtaininghighselectivitytoethylacetate,obtaining80.5%selectivityat 48.9%ethanolconversion.ACu Zn Zr Al Ocatalystwasreportedby Inuietal.(2004) tobesimilarlyactiveforacetaldehydeproduction.Othercatalysts arealsoactiveforproducingethylacetatealongwithacetaldehydeandaceticacid, and Galloetal.(2014) provideanexcellentreviewofwhichmaterialsareactivefor whichreaction.
3. PRODUCTSFROMETHANOLDIMERIZATIONAND OLIGOMERIZATION:HIGH-VALUECOMMODITIESAND SPECIALTYCHEMICALS
3.1 BUTADIENE
Oneofthemorepromisingchemicalsthatcanbeproducedfromethanolisbutadiene.Butadieneisanimportantbuildingblockfortheproductionofpolymers suchasStyrene ButadieneRubber,polybutadiene,andstyrenebutadienelatex. Approximately95%ofbutadieneiscurrentlyobtainedasaby-productofthe naphthacrackingprocessusedtoproduceethylene.However,therearedownsides tothisrouteasaresultofthecomplex,expensiveseparationprocessthatrequires severalextractivedistillationsteps.Moreover,asdiscussedearlier,inthefirsttwo decadesofthe21stcentury,therehasbeenashifttowardlighterfeedstocksfor ethyleneproductionintheUnitedStates,whichhasledtoadecreasedoutputof butadieneandputpressureonthatmarket.Consequently,attentionhasshifted towardtheuseofalternativerenewableresourcesfor“on-purpose”butadiene synthesis.
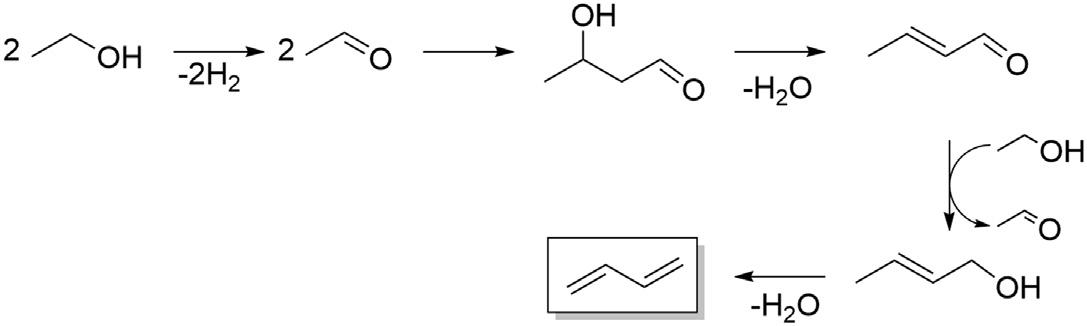
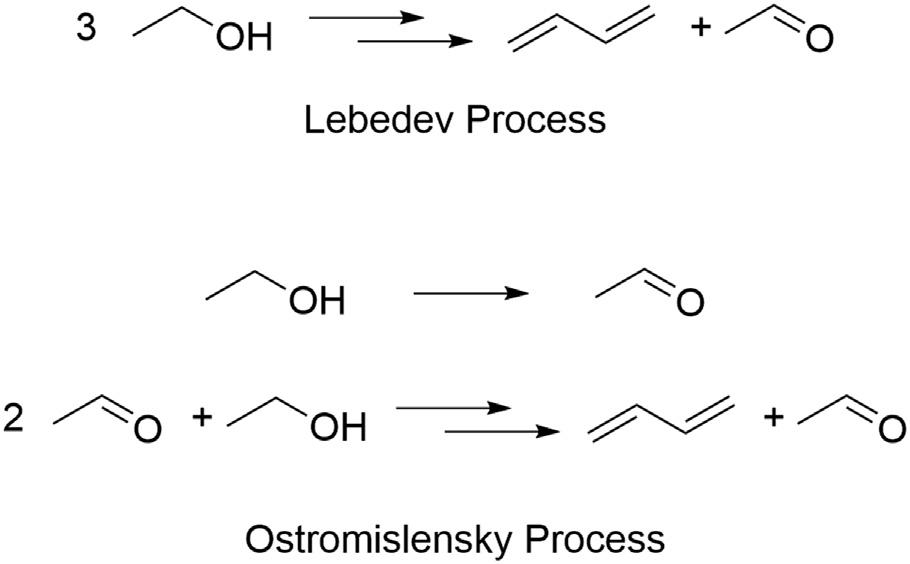
Theone-stepprocessforbutadieneproductionfromethanolwasfirstdeveloped inRussiaintheearly20thcenturyby Lebedev(1933).TheLebedevprocessforthe directconversionofethanolintobutadieneinvolvesfirstdehydrogenatingethanolto acetaldehyde,followedbyaldolcondensationofacetaldehydetogive 3-hydroxybutanalthatissubsequentlydehydratedtogivecrotonaldehyde.Crotonaldehydeisreducedtogivecrotylalcohol,whichisfinallydehydratedtoproduce butadiene,asshownin Fig.1.1.Thewholeprocessoccursoverasinglecatalyst inasinglereactor.TheOstromislenskyprocesswasdevelopedatUnionCarbide inparalleltotheLebedevprocessandisreferredtoasthe“two-stepprocess” (Waclaw,1944;Corsonetal.,1950)whereinethanolisdehydrogenatedtoacetaldehydeinonereactorfollowedbycondensationofthisacetaldehydewiththe remainingethanoltoproducebutadieneinasecondreactor(see Fig.1.2).
ManyresearchgroupsinboththeUnitedStatesandRussiahavetesteddifferent typesofcatalystsbasedonthesecommonlyacceptedpathwaysforproducingbutadiene.Ultimately,theone-stepprocessprovedtobelessexpensiveandmore
FIGURE1.1
GenerallyacceptedreactionnetworkfortheLebedevprocess.
FIGURE1.2
ComparisonoftheLebedevprocesswiththeOstromislenskyprocess.
acceptableintermsofintermediatestepsforproducingbutadiene.TheLebedevprocessinvolvesacomplicatednetworkofreactions,althoughthenetworkshownin Fig.1.1 remainstobedefinitivelyproved.Recently,newattentionhasbeenpaid totheLebedevprocesswiththeaimofdemonstratingtheconditionsandcatalyticallyactivesitesthatarerequired.Itisgenerallyacceptedthatthereactionrequires asubtlebalanceofvariousactivesites(acid,basic,andredox)togethertoachieve effectivecatalysis.
TheearlycatalystsusedforthisreactionwereAl Znmixedoxides. Quattlebaumetal.(1947) firstproposedthereactionnetworkshownin Fig.1.1 Theyshowedthatbutadieneproductionwasmorefacilewhenusingafeedmixture ofethanolandcrotonaldehydethanwhenusingafeedofacetaldehyde.Followingup onthiswork,Bhattacharyyaetal.carriedoutthereactioninfluidizedbedreactor overanAl2O3 ZnOcatalyst(Al2O3:ZnO ¼ 60:40)andco-fedvariousexpected intermediatecomponentswithethanol,includingacetaldehyde,crotonaldehyde, anddihydrogen(Bhattacharyya,1967).Reactionswithapureethanolfeedwere consistentwiththemechanismofLebedev,whereasthosehavingethanolwith acetaldehydeinvariousratiosalsoledtothesamebutadieneproductivity.Feeding crotonaldehydewitheitherhydrogenorethanolbothledtobutadieneproductionas well.Thus,Bhattacharyyaetal.concludedthatthealdolcondensationofacetaldehydeisnottherate-controllingreactionintheLebedevpathway.
MgO SiO2 mixedoxideshavealsobeenshowntobeactiveforbutadieneproduction. Kvisleetal.(1988) testedaMgO SiO2 catalystproducedbywetkneading, andtheyobtained30%selectivitytobutadieneand55%selectivitytoethyleneat 55%ethanolconversionatsteadystate.Thisgrouphypothesizedthataddingoxygen oracetaldehydetotheethanolfeedwouldincreasetheactivitytowardbutadiene. Theyproposedamechanismparalleltothatproposedby Quattlebaumetal. (1947),buttheirproposedrate-controllingreactionoccursbeforethehydrogen transferstep,whichwouldleadtotheaccumulationonthesurfaceofaC2 species
andnotC4-oxygenatedintermediates.Importantly,theseauthorsindicatethatcatalystmorphologyisessentialforcontrollingtheselectivitytobutadiene,implying thatcarefulcatalystpreparationiscriticalforproducingbutadiene. Makshina etal.(2012) alsoconcludethatcatalystpreparationandmorphologyarecritical forbutadieneproductionwhenstudyingasimilarMgO SiO2 system.
RecentworkbyWeckhuysenandBruijnincxhasfocusedmoredetailonthe MgO SiO2 system,usingCuasapromoter.Theyconcurwiththeobservations of Kvisleetal.(1988) thatthemethodusedtopreparethemixedoxideiscritical forobtainingbutadiene,andtheyobservethatpromotionusingCuOimprovesbutadieneyieldssignificantly(Angelicietal.,2014).Theyexplainthattheinfluenceof morphologyonbutadieneyieldiscausedbyvariationsinthenumberandstrengthof acidicandbasicsites,withasmallnumberofstronglybasicsitescombinedwith moderatelyacidicandbasicsitesleadingtothehighestbutadieneyield(Angelici etal.,2015b).Usingaseriesofinsituand operando experiments,theyelucidate thattheCuOaddedtothecatalysttoimproveperformanceformsasolidsolution intheoxidesupportandisreducedtometalliccopperduringthereaction(Angelici etal.,2015a).Thisobservation,combinedwiththeimprovedperformanceofthe Cu-containingmaterials,suggeststhatethanoldehydrogenationmayplayamore importantroleinbutadieneformationthansuggestedbypreviouswork.
Sushkevichetal.(2014) haveevaluatedMgO,ZrO2,Nb2 O5,TiO2 ,andAl2 O3, allsupportedonsilicaandpromotedwith variousmetals,includingAg,Cu,and Ni.Theethanolconversionoverthesecatalystsrangedfrom2%to90%,andthe bestperformancewasobtainedwithAg/ZrO 2 SiO2 catalystswithselectivity morethan74%towardbutadiene.ThisgroupagreedwiththemechanismofQuattlebaumandothergroups,andtheypropos edthatthealdolcondensationstepis ratecontrollingbecauseace taldehydewasthemostabundantintermediate observedduringtheirreactions.InagreementwiththegroupofKvisle,thisgroup alsosuggeststhatthecatalyststructure iscriticalfora chievingthehighestactivity andselectivitytowardbutadiene.Amongthemetalpromotersstudiedbythis group,metallicNishowedthebeststability,whichisrelatedtotheavoidanceof cokeformationinthepresenceofNi.
Gruveretal.(1995) havehypothesizedanewpathwayforconversionofethanol intobutadieneoveraluminatedsepiolitecatalystsat280 C.Incontrasttoprevious studies,thismechanisminvolvesaPrinsreactionbetweenethyleneandacetaldehydefollowedbydehydrationtogeneratebutadiene.Theyproposedthismechanism basedontrendsintheethyleneandbutadieneselectivity,whichincreasesproportionaltotheoverallfractionalconversion.Thisgroupalsoreportedthatethanol dehydrogenationandethanoldehydrationoccuratLewisacidsites,whichare requiredforthePrinsmechanism.
Niiyamaetal.(1972) havestudiedethanolconversionintobutadieneusing varioussilica magnesiacatalysts.Theytriedtoexplainthereactionmechanism byevaluatingdifferentratiosofcatalystcomponentsandobservedthatsilica magnesiawith85%MgOshowedthebestactivitytowardbutadiene.Theyalso co-feddifferentreactants(i.e.,ethanol,acetaldehyde,crotonaldehyde).Thisgroup
concludedthataddingeitheracetaldehydeorcrotonaldehydetoanethanolfeed increasedtheactivitytowardbutadiene,whichsuggeststhatbothaldehydesare abundantintermediatesinthereaction.Theyalsonoticedthatco-feedingacetaldehydewithethanolsignificantlyimprovedtherateofreaction,suggestingthat acetaldehydeformationistherate-determiningstep.Thus,accordingtothisgroup, controllingandbalancingbasicandacidicsitesisthemostefficientwaytomaintain catalystsactivityandselectivity.Notably,thesegroupseachsuggestdifferent reactionnetworks,indicatingthatthereactionmechanismisnotyetresolved. Furtherworkisneededinthisareatoelucidatehowbutadieneisformedfrom ethanol.
3.2 BUTANOL,HIGHERALCOHOLS,ANDAROMATICS
Incloselyrelatedchemistry, n-butanolcanbeproducedbythedimerizationof ethanol.Butanolisanimportantcommoditychemicalthatisnormallyusedas solventoranadditivetogasolineandcouldbeanothersourcefortheproduction ofethersandesters.Butanolisalsoa“higheralcohol,”whichisimmiscibleinwater, isnoncorrosive,andhasahigherenergydensitythanethanol.Thus,butanolis consideredagoodpotentialbiofuel.
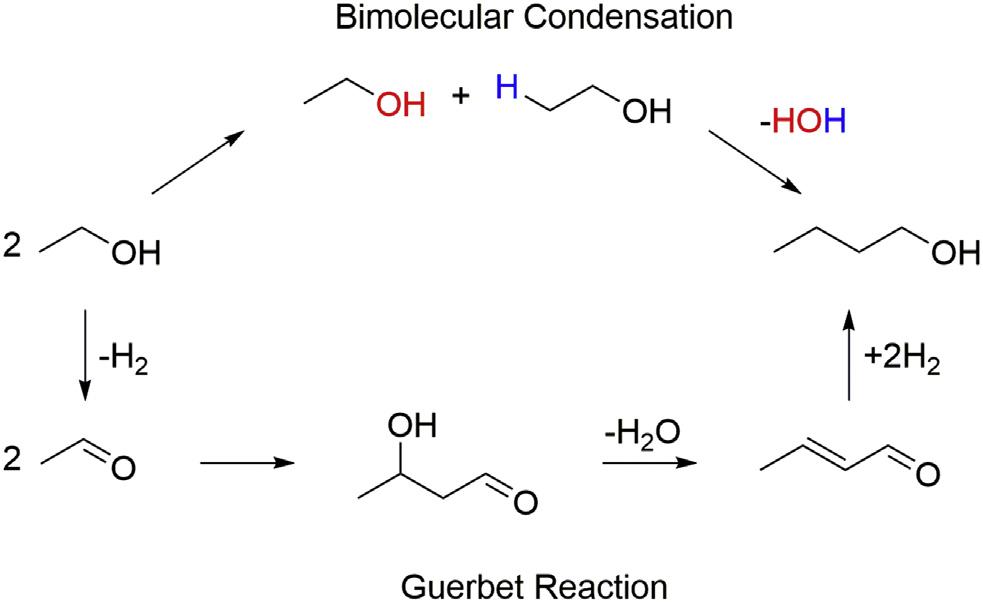
Currently,butanoliscommerciallyproducedfromfossilresourcesbypropylene hydroformylationtogeneratebutyraldehydefollowedhydrogenationtobutanol (Ojimaetal.,2004).Theuseofrenewablefeedstocksispossibleforbutanolproduction,though,eitherbydirectfermentationtoyieldbutanol(e.g.,theacetonebutanol-ethanolprocesses)orethanolcouplingusingtheGuerbetreaction. NumerousrecentstudieshavefocusedontheGuerbetreaction,andseveraltypes ofcatalystswithdifferentpropertieshavebeendevelopedthatleadtobutanol production.ThereactionnetworkispostulatedtobesimilartothatfortheLebedev reaction(see Fig.1.1):thealcoholisdehydrogenatedtoformthecorresponding aldehyde,whichsubsequentlyundergoesaldolcondensation.Theresultingunsaturatedaldehydeisthenreducedtotherequiredalcoholviaoxide-mediatedH-transfer reactions.Achievinghighselectivitytobutanolcanbeachallengeusingthis pathway(Furukawaetal.,1959).
Riittonenetal.(2012) haveprobedawideselectionofmetalssupportedon alumina(e.g.,Ni,Pd,Au,Rh,Ru,andAg)forthedirectconversionofethanol intobutanolat250 C.Inthiswork,Nimetalsupportedonaluminawasreported toshowthebestperformancecomparedwiththeothermetalsintermsofselectivity,achievingmorethan80%selectivitytobutanolat25%ethanolconversion. Incontrast, Dowsonetal.(2013) reportedacollectionofruthenium-based catalystsofwhichthebestachieved94%selectivitytobutanolatethanolconversionsover20%.Thesecatalystsarenotablebecauseoftheirhighselectivity comparedwithothersystems.Theseauthorsalsosuggestthatthebase-catalyzed aldolcondensationofacetaldehydeis mostsignificantforachievinghigh selectivitybecauseimprovingtherateofaldolcondensationledtoincreased butanolselectivity.
Inaddition,therearemanystudiesthathavebeenperformedusingoxide-based catalystsystems.Forexample,YangandMenghavereportedtheuseoflithium-and potassium-exchanged13Xzeolitespreparedbycationexchange(YangandMeng, 1993).Therubidium-containingcatalystachievedthehighestactivityandselectivity towardbutanolamongthezeolitestheytested,withoptimalactivityobservedat 420 C.Thisgroupconsideredthatbutanolmightbeformedbytwodifferentmechanisms:abimolecularcondensationrouteortheclassicalGuerbetreaction (see Fig.1.3).Themechanismwasexploredbyco-feedingacetaldehydeorcrotonaldehydealongwithethanol.Afterfeedingethanolwithcrotonaldehyde,theauthors didnotobserveanyincreaseinbutanolproduction;indeed,theyobservedadecrease inbutanolproductivity.Co-feedingacetaldehydeledonlytotheproductionof crotonaldehyde.Basedontheseexperiments,theauthorsconcludethatbutanolis formedoverbasiczeolitesviabimolecularethanolcondensationincludingtwo ethanolmoleculesbeingconverteddirectlytobutanol(Angelicietal.,2013).
ThegroupofIoan-Cezarreportedconversionofethanoltobutanolover M Mg AlwithM ¼ Pd,Ag,Mn,Fe,Cu,Sm,andYb(Marcuetal.,2013). Theyinvestigatedtheperformanceofthecatalystwiththesedifferentmetalsand studiedtheinfluenceofeachmetalonthedistributionofacidicandbasicsites. Allthecatalystswereselectivetowardbutanol,whereastheCuandPdcatalysts wereparticularlyactive.Pd MgAlOachieved72.7%selectivitytowardbutanol at3.8%ethanolconversion,whichwasthebestamongthecatalystsstudiedin thiswork.Cu MgAlOachieved40.3%selectivitytobutanolat4.1%ethanol conversion,butitwasalsoactivefortheproductionof1-1-diethoxyethane.Additionally,Sm-MgAlOwasalsohighlyselectivetowardbutanol(66.3%selectivity at1.3%ethanolconversion)becauseitpossessesasimilardistributionofbasicsites asPd MgAlO,althoughthedistributionofsitestrengthsisdifferentfortheSm
FIGURE1.3
Twopotentialroutesforconversionethanolintobutanolaccordingto Yangand Meng(1993)
catalystthanforthePdcatalyst.Thisgroupconcludedthattheoptimalcatalystrequiresabalanceofbasicandacidicactivesites,anditisunfavorabletohavealarge acidsitedensity.
Tsuchidaetal.(2006) investigatedtheuseofnonstoichiometrichydroxyapatite (HAP),acalciumphosphate,forbutanolproductionfromethanol.Theyfoundthat butanolcanbeformedfromethanolwithaselectivityof76.3%at14.7%ethanol conversionat300 Cwith1.64ratioofCa/PintheHAP.Theseauthorsobserve 68.8%selectivitytobutanolat26.1%ethanolconversionat350 C,44.8%selectivityat57.4%conversionat400 C,and6%selectivityat95.3%conversionat 450 C.Itisalsopossibletoobtainevenhigheralcohols,suchasC6,andC8 alcohols, withthetotalselectivitybeingmorethan85%usingHAPcatalysts.Theseauthors concludethatthemechanismforbutanolproductionoverHAPcatalystsfollowsthe Guerbetpathway.Insimilarwork, Ogoetal.(2011) alsoreportedtheuseofthe substitutedHAPsSr10(PO4)6(OH)2,Ca10(VO4)6(OH)2,Sr10(VO4)6(OH)2,and Ca10(PO4)6(OH)2 at300 C.Thebutanolselectivitiesoverthesematerialswereas follows:81.2%forSr P,74.5%forCa P,21.9%forCa V,and8.1%forSr V atconversionsrangingbetween1%and24%.Butanolwasthemainproductover allthesecatalysts,butSr PandCa PalsoproducedC6 andC8 alcohols.The Sr PHAPcatalystachievedthehighestselectivityamongallthecatalyststested bythisgroup,anditshowedgoodselectivitytowardcrotonaldehydeandother Guerbetpathwayintermediatesaswell,suggestingthattheGuerbetreactionisthe mechanismusingHAPcatalysts.DetailedreactionkineticsstudiesbytheDavis groupindicatethattheGuerbetmechanismisindeedoperativeoverthesecatalysts (Hanspaletal.,2015;Hilletal.,2015;Youngetal.,2016).Similarworkwas performedby Carvalhoetal.(2012) usingMg Almixedoxides.Basedontheir results,theyhypothesizedthatcatalystcompositionandmorphologyhaveadominanteffectoncatalystperformanceforthisreaction.Theyevaluateddifferentratios ofMg:Alandobservedthebestperformanceusingtheratioof3:1.Othercatalysts havealsobeenstudiedforthisreaction,andKozlowskiandDavishavepublishedan excellentreviewofthecatalystsandreactionmechanismsthathavebeenproposed (KozlowskiandDavis,2013).
AssuggestedbytheworkofOgoandcoworkers,videsupra,theGuerbetchemistrycanbealsoextendedtooligomerizeethanoltoyieldmixturesofhigheralcohols containing3 5carbons.Thesemixturesarenormallyburntandusedasfuels (Gabrie ¨ lsetal.,2015).Therehasbeenincreasedinterestinthesehigheralcohols, though,becauseoftheirpotentialtobeusedbothasbiofuelsandasrawmaterials forobtainingheavieroxygenates.
Thepreviouslydescribedmechanismforcouplingalcohols(i.e.,theGuerbet reaction)hasrecentlybeenthesubjectofdebate,andtheproductdistributions, reactionsbetweenintermediates,andotherfactorssuchascatalystmorphology andactivityofvariousreactivesitesarestillnotwellexplainedforallcatalyst materials,especiallyforalcoholproductslargerthanC4.Inpursuitofsuchanexplanation,TakahikoandFlahertyhaveproposedamechanismforethanolcouplingover Ca-andSr-HAPcatalysts(MotekiandFlaherty,2016).Theyhypothesizethat
ethanolmoleculesarefirstdehydrogenatedtogiveacetaldehyde,andthenthefirst carbon carbonbondisgeneratedbyaldolcondensationofacetaldehyde.TheunsaturatedproductishydrogenatedduringaMeerwein Pondorff Verleyreaction, whichyieldsthehigheralcoholproduct.Notably,thecarbondistributioninthe productsofethanolcouplingareconsistentwithexpectationsforastep-growthpolymerizationmodel,andtheratesofbothself-andcross-couplingreactionsbetween C2 andC4 alcoholsareofcomparablemagnitudeforeachalcoholstudied.Importantly,theseauthorsshowthat,whentargetingmoleculeslargerthanC4,thenetwork ofcouplingreactionsleadsinherentlytoabroadproductdistribution.
Generally,smallamountsofaromaticspeciesareobservedintheGuerbetreactionwithethanol(Tsuchidaetal.,2006;Ogoetal.,2011;Hanspaletal.,2015), whichFlahertyandcoworkershaverecentlyshowncanbeexploitedtoproduce aromaticspeciesdirectlyfromethanol(Motekietal.,2016).Theyusethesame Ca-containinghydroxyapatites(Ca-HAP)traditionallyusedtoproducehigheralcoholsbytheGuerbetreactiontoinsteadproduce2-and4-methylbenzaldehydesfrom mixturesofacetaldehydeandethanolat275 C.Thereactionproceeds,asshownin Fig.1.4,byseveralsequentialaldolcondensationsofacetaldehyde,generatedinsitu bydehydrationofethanol,withthearomaticringultimatelyformedbydehydrocyclizationoftheC8 unsaturatedaldehyde(i.e.,2,4,6-octenetrienal).Althoughthis pathwayadequatelyexplainstheformationof2-methylbenzaldehyde(i.e.,the ortho product),dehydrodecyclizationof2,4,6-octenetrienalcannotexplaintheformation of4-methylbenzaldehyde(i.e.,the para product).Indeed,theproductformationrate of4-methylbenzaldehydedoesnotdependontheacetaldehydepartialpressureand isgreatestatlowconversionsofacetaldehyde,suggestingthatthe para productis formedbyself-couplingof2-butenalratherthansequentialacetaldehydecoupling (Motekietal.,2017).
Importantly,theseauthorssuggestthatitispossibletotunetheselectivityto aromaticsversushigheralcoholsbyadjustingreactionconditionsandcatalyst parameters.Theformationof,forexample,2-methylbenzaldehydedependsonthe sequentialaldolcondensationofacetaldehyde.Thus,highselectivitiescanbe obtainedbyusinganoxidecatalystthatishighlyefficientforaldolcondensation ratherthanhydridetransfer(e.g.,viatheMeerwein Ponndorf Verley Oppenauer reaction).Conversely,includingasecondcatalystthatishighlyefficientforethanol dehydrogenationtogeneratelargepartialpressuresofacetaldehydeinthereactor willleadtosimilarlyhighselectivities,andthisgroupshowedthattheuseofa Cu ZnOdehydrogenationcatalystupstreamofaCa-HAPbedleadstoreasonably
FIGURE1.4
Formationof2-methylbenzaldehydefromethanol-derivedacetaldehydeoverCa-HAP catalysts.
highselectivitytoC8 aromatics.StackingseveralbedsofCu ZnOandCa-HAP circumventsequilibriumlimitationsduringethanoldehydrogenationandleadsto atotalyieldofC4þ productsof58%(Motekietal.,2016).
3.3 LUBRICANTS
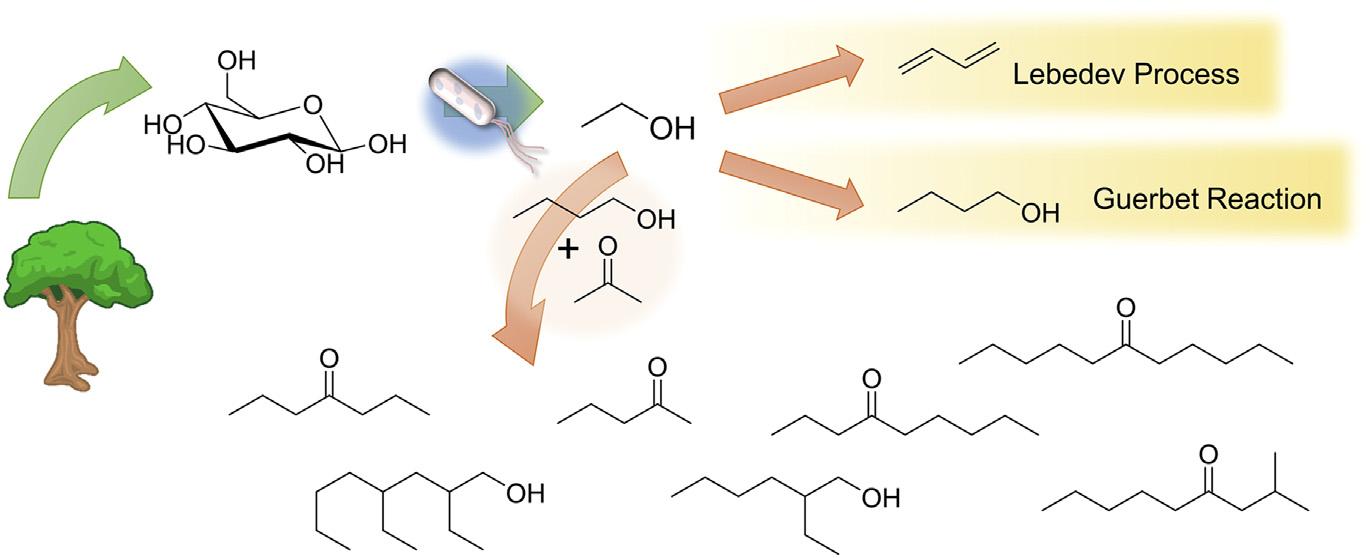
Withtheresurgenceofinterestinbiorenewablefuelsinthefirsttwodecadesofthe 21stcentury,therehasbeenacorrespondinginterestincarbon carbonbond formingreactions.Althoughcarbon carbonbondformationisnotnewinethanol processing(seethediscussionmentionedearlierontheLebedevandGuerbet reactions),someprocesseshavebeendevelopedthatincorporateethanolwithother biomass-derivedsmallmolecules.Notableamongtheseprocessesistheworkofthe TosteandBellgroups,whohavedevelopedroutestonewbiobasedlubricantsand long-chainhydrocarbonfuelsusingthemixtureofacetone,butanol,andethanol producedduringacetone butanol ethanolfermentation(GoulasandToste,2016).
UsingreducedtransitionmetalsandK3PO4 asabase,itispossibletocouple acetone,butanol,andethanolviaalkylation,whichleadstoamixtureofmethyl ketonesthataresubsequentlydoublyalkylated,leadingtomixedC7 C11 ketones asshownin Fig.1.5 (Anbarasanetal.,2012;Sreekumaretal.,2015a).These products,whicharegenerallylinearforreactionscarriedoutatmoderatespace velocities,aresuitableforsubsequenthydrodeoxygenationandconversionto biobasedalkanessuitableforfuelpurposes.Foreaseofprocessing,itis preferabletouseasolidbase,ratherthanK3PO4,andithasbeenshownthat hydrotalcite-supportedPdandCucatalystscanperformthesamereaction,andthe
FIGURE1.5
Routesforcarbon carboncouplingofethanol,includingtheLebedevprocess,the Guerbetreaction,andcross couplingwiththeproductsofacetone butanol ethanol fermentation.
ReprintedfromSchwartz,T.J.,Shanks,B.H.,Dumesic,J.A.,2016.Couplingchemicalandbiologicalcatalysis:a flexibleparadigmforproducingbiobasedchemicals.CurrentOpinioninBiotechnology38,54 62with permissionfromElsevier.
useof i-propanolratherthanbutanolleadstoimprovedproductyields(Sreekumar etal.,2014).TheuseofPd Cubimetallicnanoparticlessupportedonhydrotalcite avoidsthedecarbonylationreactionsthatleadtodecreasedyieldsovermonometallic Pdcatalysts(Goulasetal.,2016).Lifecycleanalysisoftheprocesssuggeststhatit wouldresultinanontrivial(w50% 80%)reductioningreenhousegasemissions whentunedfordieselproduction(Sreekumaretal.,2015b).Modificationofthefeedstockbyincludingadditionalmethylketonesobtainedfromfuransleadstoadditional couplingreactionsandhighermolecularweightproducts(uptoC45)thataresuitable foruseaslubricants(Balakrishnanetal.,2015).
4. CONCLUSIONANDFUTURETRENDS
Throughoutthe20thcenturyandthefirstportionofthe21stcentury,therehavebeen numerousdevelopmentsintheconversionofethanoltobothcommodityand specialtychemicals.Therelativeeaseofethanolproduction,coupledwithitsmonofunctionality,makeitparticularlyattractiveasaplatformchemical(Schwartzetal., 2016).Asignificantamountofdevelopmenthasoccurredalready,inparticularwith regardtocommoditychemicalproduction(e.g.,ethylene,propylene,acetaldehyde, ethylacetate).However,numerouschallengesremain.Althoughethanolitselfisa fairlystablemoleculeatrelevantreactionconditions,manyoftheseproducts (e.g.,ethylene)orreactionintermediates(e.g.,acetaldehyde)arehighlyreactive, andachievinghighselectivityathighethanolconversioninmanyreactionsisnot straightforward.Inmostcases,thereactionmechanismiswelldocumented,and theactivesiterequirementsareunderstood.However,thereisstillroomtorationally applytheseobservationstodesigncatalyststhatcanachievehighcarbonyieldsfor certainreactions.Incaseswherethereactionproductishighlyreactiveandproneto degradation,suchasethyleneproduction,itmaynotbecatalystdesignthatisneeded but,rather,cleverprocessdesign.However,inthecasewherereactionintermediates areunstable,itmaybepossibletoapplycatalystdesignprinciplestoimproveproductyields.Recentdevelopmentsintheproductionofethylacetate,forexample, highlightthepossibilitiesforsuchimprovements.Insuchcases,materialsynthesis willplayanimportantroleinthedevelopmentofsuitablecatalysts.
Targetingtheconversionofethanoltosmall(i.e.,C2 orC3)productsmayface economicchallengesintheimmediatefuture.Theprevalenceofcheapnaturalgas andnaturalgasliquidsfromtheshalegasboomintheUnitedStateswillkeepprices lowforsuchproducts.Thissituationhasdrivenresearchintocarbon carbonbond formingreactionsthatyieldeitherC4 speciesthataredifficulttoobtainfromnewly lightenedpetroleumfeedstocks(e.g.,butadieneor n-butanol),orhighercarbonnumberoxygenatesthatareinherentlyvaluable.Inmanycases,therehavebeenonlya smallnumberofcatalyststhusfardescribedforthechemistry,andthemechanisms arenotuniversallyagreedupon.
Additionalfundamentalstudieswillhe lpshedlightonsuchprocessesandcan guidetherationaldesignofimprovedcatalysts.Oneexamplethatwouldbenefit
fromsuchastudyistheproductionofbutadieneviatheLebedevprocess.Although qualitativestudieshavesuggestedthepresentlyagreed-uponm echanism,asdiscussedearliertherehavebeenrecentstudiesthatcastsomedoubtonthevalidity ofthismechanism,andthereactionhasnotbeenstudiedinasmuchdetailas therelatedconversionofethanolto n -butanolviatheGuerbetreaction.Similar reactionkinetics,stea dy-stateisotopictransient,in frared,andmicrocalorimetric experimentswouldbehelpfulinelucidatingthechemistryassociatedwiththis pathway.
Finally,eventhoughethanolconversionhasbeenstudiedformorethan 100years,itisstillpossibletodevelopnewchemistryviaethanololigomerization reactions,asshownbyrecentworkfromthegroupsofToste,Bell,andFlaherty. Thearomaticsandlong-chainfuelsandlubricantsrecentlydevelopedareonlythree examplesofthetypeofproductsthatcouldbeobtainedfromethanol.Thelistof knowncarbon carbonbondformingreactionsisextensive,onlyafewofwhich havebeenappliedtoethanol-derivedmolecules.Carefulapplicationofsuch chemistrycouldleadtonewbiobasedalternativestopetroleumoreventonovelmoleculesthatcanprovideforimprovedmaterialpropertiesnotcurrentlyavailablefrom petroleumsources.
LISTOFABBREVIATION
Ca-HAPCalcium-ContainingHydroxyapatite
HAPHydroxyapatite
MFIMordeniteFrameworkInverted
NiSAPO-34Ni-containingSAPO-34
RHARiceHuskAsh
SAPOSilicoaluminophosphate
SAPO-34Silicoaluminophosphate-34
REFERENCES
Alonso,D.M.,Bond,J.Q.,Dumesic,J.A.,2010.Catalyticconversionofbiomasstobiofuels. GreenChemistry12(9),1493 1513. Anbarasan,P.,etal.,2012.Integrationofchemicalcatalysiswithextractivefermentationto producefuels.Nature491(7423),235 239.Availableat: http://www.nature.com/ doifinder/10.1038/nature11594.
Angelici,C.,Weckhuysen,B.M.,Bruijnincx,P.C.A.,2013.Chemocatalyticconversionof ethanolintobutadieneandotherbulkchemicals.ChemSusChem6,1595 1614. Angelici,C.,etal.,2014.EffectofpreparationmethodandCuOpromotionintheconversion ofethanolinto1,3-butadieneoverSiO2 MgOcatalysts.ChemSusChem7,2505 2515. Angelici,C.,Meirer,F.,etal.,2015a.Exsituandoperandostudiesontheroleofcopperin Cu-PromotedSiO2 MgOcatalystsfortheLebedevethanol-to-butadieneprocess.ACS Catalysis5(10),6005 6015.Availableat: http://pubs.acs.org/doi/10.1021/acscatal. 5b00755