BasicCoordinationChemistry
1.INTRODUCTION
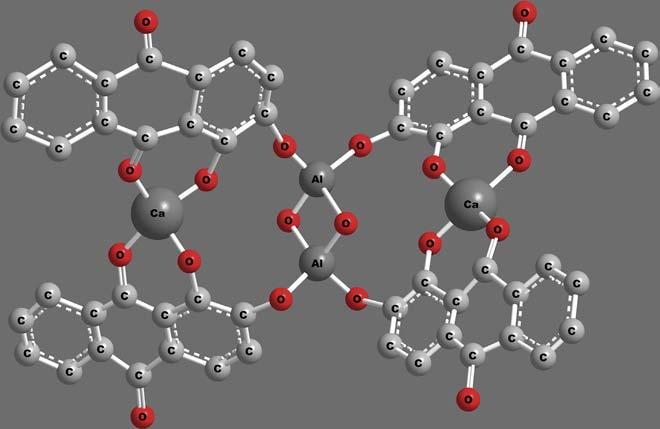
Thecoordinationcompoundsfoundtheirapplicationslongbeforethe establishmentofcoordinationchemistry.Brightredcolouredalizarindyes wereunderapplicationsevenbeforethe fifteenthcentury.Thisbrightred dye,nowcharacterizedasachelatedcomplexofhydroxyanthraquinone withcalciumandaluminiummetalions,isshownin Figure1.
Later,inthesixteenthcentury,theformationofawell-knownmember oftoday’scoordinationchemistryfamily,thetetraamminecupricion
[Cu(NH3)4]þ2,wasrecordeduponcontactbetweenbrassalloyand ammoniumchloride.AdditionofPrussianblueFe4[Fe(CN)6]3$xH2O increasedtheuseofcoordinationcompoundsindyesandpigments.A platinumcomplexK2[PtCl6]offeredanapplicationfortherefinementof platinummetal.Thus,beforethecoordinationchemistrywasstructured, thecoordinationcompounds,complexesandchelatesfoundtheir applications.
Asystematicinvestigationofstructureandbondingincoordination chemistrybeganwiththeinquisitivenessofTassaert(1798),whichwas extendedbydistinguishedchemistslikeWilhelmBlomstrand,Jorgensen andAlfredWerner [1] untiltheendofthenineteenthcentury.Inthe events,Werner’scoordinationtheory(1893)becamethebaseofthe moderncoordinationchemistry.Itisworthnotingthattheelectronwas discoveredsubsequenttoWerner’stheory.
ThebondingincompoundslikeCoCl3 andNH3 wereeasilyunderstoodandexplainedandhencesuchcompoundswereregardedassimple compounds.Forinstance,the þ3formaloxidationofcobaltincobalt chlorideisbalancedbythreeuni-negativechlorideionsandthecoexistence oftheseionicmoietiestoformamoleculeisunderstoodandexplained. Similarly,thevalenceshell(n ¼ 2)ofnitrogen(N ¼ 7)contains five electronsandfourorbitals(2s,2px,2py and2pz).Keepinganelectronpair
Figure1 Structureofalizarindye.
inoneoftheseorbitalswhiletheotherthreeremainshalf filled,anopportunityforthreehydrogenatomstocontributeoneelectroneachforthe formationofacovalentbondwithnitrogen,canalsobeexplained.Thusan ammoniamoleculehasthreeN Hcovalentbondsandonelonepairof electronsoverthenitrogenatom.Itisworthnoticingherethatallthe valenciesofalltheatomsinboththemoleculesarefullysatisfiedandhence thereisnofurtherscopeofbonding.
A ‘complex’ situationariseswhenonecomestoknowthatthemolecule CoCl3 canencompasssixammoniamolecules,resultingintoathirdindependententity.Thissituationwasfullyunderstoodandexplainedby Werner’scoordinationtheory,followedbynamingtheentityas ‘complex’
1.1Definitions
Coordinationcompounds arethecompoundscontainingoneormore coordinatecovalentbonds.
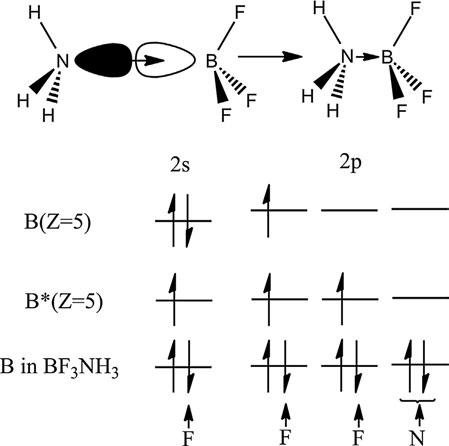
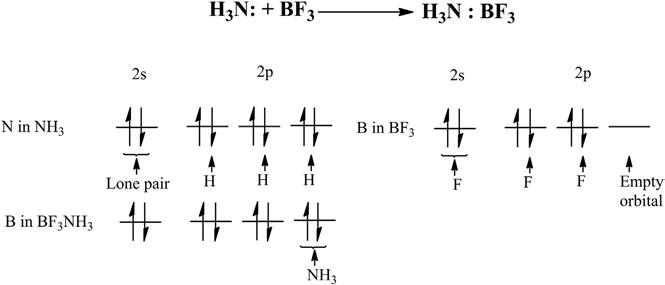
Coordinatecovalentbonds arethecovalentbondsinwhichboththe bondingelectronsarecontributedbyoneofthebondpartners. Figure2 distinguishesthecovalentbondsfromthecoordinatecovalentbondin NH3BF3.WhilethethreeB Fcovalentbondsareformedduetothe sharingofelectronpairsresultingfromcontributionsofbothboronand fluorineatoms,anN Bbondisformedduetothedonationofalonepair
Figure2 BondinginNH3BF3.
ofelectronsfromnitrogenintotheemptyorbitalsofboron.Thecoordinate covalentbondisshownbyanarrowwithitsheadpointingtowardsthe directionofthedonationofanelectronpair,asshownin Figure2
A complex isamolecule/ioncontainingacentralmetalatom/ion surroundedbyadefinitenumberofligandsheldbysecondaryvalencesor coordinatecovalentbonds.
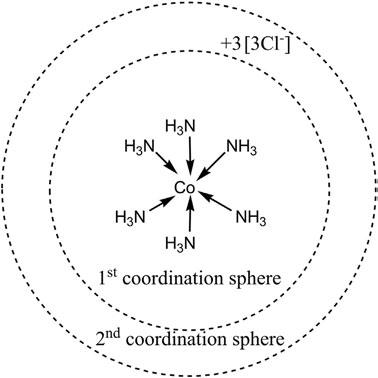
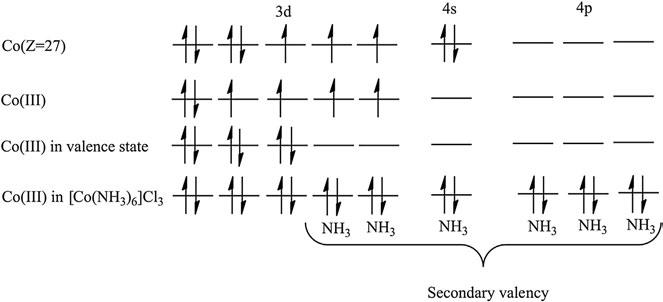
Primaryvalency referstothechargeoverthemetalione.g.Co(III) has þ3charge,whichcanbebalancedby 3charge-formingcompounds likeCoCl3.Theprimaryvalencyisionicandissatisfiedinthesecond coordinationsphere,asshownin Figure3.
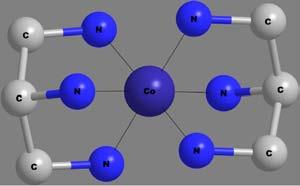
Secondaryvalency isthenumberofemptyvalenceorbitals,asillustratedfor[Co(NH3)6]Cl3 inthe figure.TheCo(III)ionhassixempty valenceorbitals.Henceitssecondaryvalencyissix.Secondaryvalencyisa coordinatecovalentvalency,anditissatisfiedinthe firstcoordination sphereofthemetalion,asshownin Figure4.
Coordinationnumber isapropertyofthemetalionrepresentingthe totalnumberofdonoratomsdirectlyattachedtothecentralatom.Inthe abovecase,thecoordinationnumberofCo(III)issix,assixnitrogendonor atomsaredirectlyconnectedtothecentralmetalion(cobalt(III)).
Ligand isanyatom,ionorneutralmoleculecapableofdonatingan electronpairandbondedtothecentralmetalionoratomthroughsecondaryvalency.
Figure3 Firstandsecondcoordinationspheresin[Co(NH3)6]Cl3.
Dentatecharacter isapropertyofaligandrepresentinganumberof coordinatingatoms.
Inthecaseof[Co(NH3)6]Cl3,ammonia,NH3 theligandcontainsone donoratom(N).Henceitsdentatecharacterisoneandisclassifiedasa monodentateligand.Similarly,chloro(Cl )isananionic,monoatomicand monodentateligand,whilehydroxo(OH )isadiatomic,monodentateand anionicligand.Aquo(OH2)representsaneutraltriatomicmonodentate ligand.Afewpopularligandsandtheircharacteristicsareshownin Figure5.
Duetoahigherdentatecharacterofligands,avarietyofcomplexes knownaschelateisalsoformedsometimes. Chelate isacompoundformed whenapolydentateligandusesmorethanoneofitscoordinatingatomsto formaclosed-ringstructure,whichincludesthecentralmetalion.Fiveandsix-memberedringsareknowntoprovideextrastabilitytothechelates. Theprocessofchelateformationisknownaschelation.Apolydentate ligandinvolvedinchelateformationisalsoknownasachelatingligand. Chelatesgenerallyexhibithigherstabilitythananalogouscomplexes.
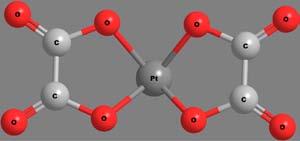
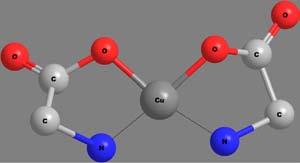
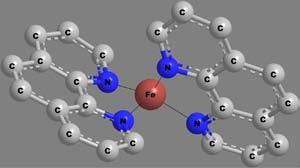
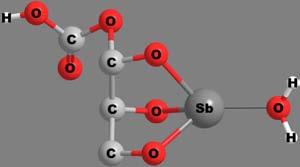
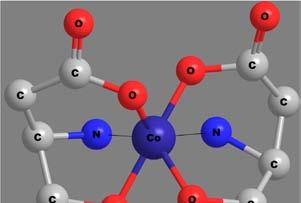
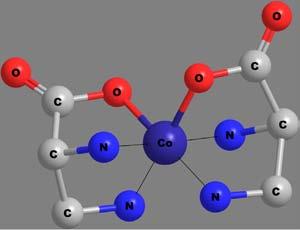
Apolydentateligandmaybeattachedtothecentralmetalionthrough morethanonekindoffunctionalgroup.Thenumberandkindoflinkages bywhichthemetalionisattachedwiththeligandscanthusbecomea criterionfortheclassificationofchelates.Thecovalentbondsareformedby thereplacementofoneormoreH-atoms,whilecoordinatecovalentbonds areformedbythedonationofanelectronpairfromtheligands.Someof thechelatesinvolvingavarietyofpolydentateligandsandlinkagesare shownin Figure6.Thecoordinatecovalentlinkagesareshownbythin, thread-likebonds.
Figure4 SecondaryvalencyofCo(III)in[Co(NH3)6]Cl3.
etatnedib,lartuen,)ne(enimaidenelyhtE
)caca(enotecaelytec A , neutral, bidentate
sanwonkoslaedyhedlazneblyxordyh-2
salicylaldehyde (sal), anionic, bidentate
,)gmd(emix oylglyhtemi D anionic, bidentate
2,2′-bipyridine, neutral, bidentate
Figure5 Structuresandcharacteristicsofafewimportantligands.
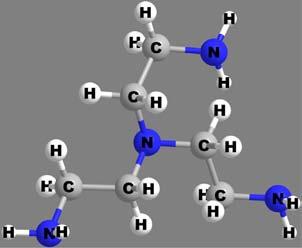
Diethelenetrimine (dien), neutral, terdentate
Nitrilotriacetic (nta), anionic, quadridentate
2,2ʹ,2ʹʹ-triaminotriethylamine (tren), neutral, quadridentate
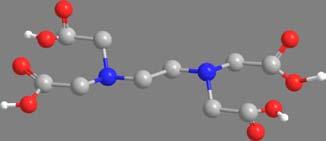
Ethylenediaminetetraaceticacid (edta), anionic, sexadentate
Cont'd
Figure5
EXAMPLE
CHARACTERISTICS
Oxalic acid
Anionic
Bidentate
Two covalent bonds
Two five-membered rings in the chelate
Glycine
Anionic Bidentate
One covalent and one coordinate covalent bond
Two five-membered rings in the chelate
1,10-phenanthroline
Neutral
Bidentate
Two coordinate covalent bonds
Two five-membered rings in the chelate
1,2,3-trihydroxypropyl hydrogen carbonate
Anionic
Tridentate
Three covalent bonds
Two five-membered rings and one six-membered ring in the chelate
2-aminosuccinic acid also known as aspartic acid
Anionic
Tridentate
Two covalent and one coordinate covalent bond
Two five-membered rings, two six-membered rings and two seven memebered rings in the chelate
Figure6 Structuresandcharacteristicsofafewchelates.
1,2,3-triaminopropane
Neutral
Tridentate
Three coordinate covalent bonds
Four five-membered rings and two six-membered rings in the chelate
2,3-diaminopropanoicacid
Anionic
Tridentate
One covalent and two coordinate covalent bonds
Four five-membered rings and two six-membered rings in the chelate
Polynuclearcomplex isacomplexwithmorethanonemetalatom/ ion.Thesemetalionsaresometimesbridgedthroughappropriateligands, resultingintotheformationofabridgedpolynuclearcomplex.
2.NOMENCLATURE
Asystematicnomenclatureofcoordinationcompoundsrequiresacareful considerationofthefollowingrules [2].Thelearnershould firstlearnallof theserulesbyheartandthendosuf ficientpracticetomastertheprocedure.
2.1ForWritingtheCoordinationFormula
1. Placethesymbolofthecentralatom firstfollowedbythesymbolofthe ligandinthefollowingorder:anionic,neutralandcationic.Enclosethe complexinasquarebracket.
2. Iftheformulaofachargedcomplexiswrittenwithoutanycounter-ion, thechargeistobeindicatedoutsidethesquarebracketasarightsuperscriptwiththenumberprecedingthesign,asin[PtCl6]2 or [Cr(OH2)6]3þ;theoxidationnumberofacentralatom/ionmaybe optionallyrepresentedbyaRomannumeralplacedasarightsuperscript ontheelementsymbol,asin[CrIIICl3(OH2)3]and[FeII(CO)4]2 .
Figure6 Cont'd
3. Theanionicligandsarecited firstandinalphabeticalorder,accordingto the firstsymbolsoftheirformulae.
4. Theneutralandcationicligandsarethenlistedinthefollowingorder: H2O,NH3,otherinorganicligandsandorganicligandsinalphabetical order.
5. Thestructuralinformationmaybegivenbyprefixessuchas cis-, trans-, fac-, mer-etc.
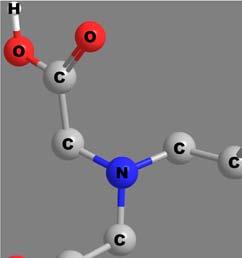
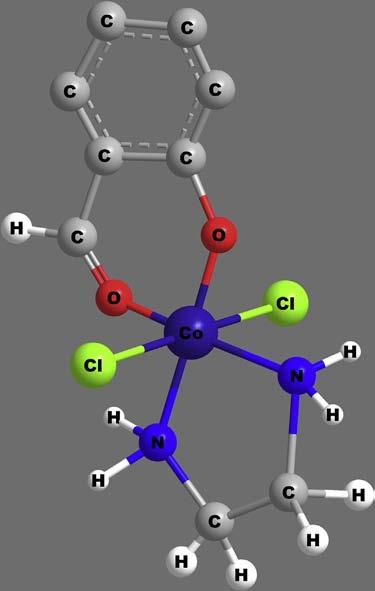
Toillustratetheaboverules,considerthewritingofacoordination formulaforthecompoundshownin Figure7 as trans-[CoIIICl2(sal)(en)].
N.B.:Theligandabbreviationsandtheformulaeofthepolyatomic ligandsaregenerallyplacedinparentheses.
2.2ForWritingtheNames
1. Thecationisnamed first,followedbythenameoftheanionirrespectiveofwhetherthecationortheanionisthecomplexspecies.
Figure7 trans-[CoIIICl2(sal)(en)].
2. Inacomplex(cationic,anionicorneutral),thenamesoftheligandare citedalphabeticallyirrespectiveoftheirchargewithoutseparation,and thenameofthecentralmetalatomisthelast.
3. Thenamesoftheanioniccomplexeshavethespecificending ‘-ate’ or ‘-ic’ (ifnamedasacid),whilethereisnosuchspecificendingofcationic orneutralcomplexes.
4. TheoxidationstateofthecentralatomisindicatedbyaRoman numeralintheparenthesisattheendofthenameofthecomplex.
5. Nameoftheligands:thespecificending ‘ -o ’ isgiventoorganic/ inorganicanionicligands.Inthecaseofligandnamesendingin ‘-ide’ , ‘-ite’ or ‘-ate’ , ‘ e ’ isreplacedby ‘ o ’,giving ‘-ido’ , ‘-ito’ or ‘-ato’ respectively,asin-azide ðN3 Þ becomesazido,-nitrite ðNO3 Þ becomesnitritoand-sulphate ðSO4 2 Þ becomessulfato.
However,certainanionicligandsareexceptionstotheaboverule andarenamedasshownin Table1.
Thereisnochangeinthenameofneutralligands.Theyarenamed asamoleculeasinNH2CH2CH2NH2 (en)asethylenediamineand NH2CH2CH2NHCH2CH2NH2 (dien)asdiethylenetriamine.Here also,theexceptionsareH2O(namedasaquo),NH3 (asammine), NO(asnitrosyl)andCO(ascarbonyl)etc.
Thecationicligandshavethespecificending ‘ium’,asin NH2 NH3 þ ,namedashydrazinium,andH2 N CH2 CH2 NH3 þ , namedas2-aminoethylammonium.
6. Thenumberofeachkindofsimpleligandisindicatedbyprefixessuchas mono-foroneligand,whichisusuallyomitted,di-fortwoligandsandtriforthreeligandswithoutanyspace.Inthecaseofcomplicatedligands,the prefixesbis-fortwoligands,tris-forthreeligandsandtetrakis-forfourligands(withthenameoftheligandenclosedinparenthesis)isused.
7. Thecoordinatingatomofaligandtothecentralatomisindicatedby placingthesymbol k (Kappa)followedbytheelementalsymbolafter
Table1 NamesusedforanionicligandsaccordingtoInternationalUnionofPure andAppliedChemistry(IUPAC)nomenclature
LigandNameLigandNameLigandName
F FluoroOH HydroxoO2 2 Peroxo
Cl ChloroCN CyanoCH3O Methoxo
Br BromoHS ThioloC5 H5 Cyclopentadienyl
I IodoS2 ThioC6 H5 Phenyl
H HydridoO2 SuperoxoO2 Oxo
thenameoftheligand,asinM-SCN-,itiswrittenasthiocyanato-kS andinM-NCS-,itiswrittenasiosthiocynato-kN.
8. Aligandthatbridgestwocentralatomsisdesignatedbythesymbol m beforeitsname.
HereareafewexamplesofusingIUPACnomenclatureforwriting theformulaeandnamesofcoordinationcomplexes.
Formula Name
[Fe(CN)6]4
Hexacyanoferrate(II)ion [CoCl3(NH3)4]
Triamminetrichlorocobalt(III) [CuCl2(CH3NH2)2]Dichlorobis(methylamine)copper(II) Cis-[PtCl2(NH3)2] Cis-diamminedichloroplatinum(II) [PtCl(en)NH2NH3]þ2
Chloroethylenediaminehydraziniumplatinum(II) ion
[Fe(CN)(CNCH2C6H5)5]þ Pentakis(benzylisocynide)cyanoiron(III)ion [Al(OH)(H2O)5]þ2
Pentaaquohydroxoaluminum(III)ion [Co(CN)(CO)2NO]
[PtClNO3(NH3)2(en)]SO4
K[SbCl5(C6H5)]
Dicarbonylcyanonitrosylcobaltate(0)ion
Diammine(ethylenediamine) chloronitroplatinum(IV)sulphate
Potassiumpentachloro(phenyl)antimonite(V) H2[PtCl6]
Na2[Fe(CN)5NO]
Hexachloroplatinic(IV)acid
Sodiumpentacyanonitrosylferrate(III) [PtPy4][PtCl4]
Tetrakis(pyridine)platinum(II) tetrachloroplatinate(II) [Pt(NH3)4Br2]Br2
Tetramminedibromoplatinum(IV)bromide
3.THEORIESOFBONDINGINCOORDINATION COMPOUNDS
Thetheoriesofbondingincoordinationcompounds [3] haveevolvedsubsequenttoWerner’scoordinationtheory(1893).Wernerintroducedthe conceptofprimaryandsecondaryvalency,explainingtheformationofthe coordinationcompounds.The18-electronrule,statingthatthestablecomplexeswithlowformaloxidationstatesofmetalionsshouldhave18bonding electronsaroundthemetalion,becameanimportantbeginningpointtoward thestudyofthestabilityofthecomplexes.The18-electronruleissignificant inmoderncoordinationchemistryasitisalsosupportedbythemolecular orbitaltheory.However,asmallernumberofcomplexeswithmetalsinlow oxidationstatesrestrictitswideapplicability.Animportantadvanceinthe theoriesofbondingincoordinationcompoundswastheintroductionof
CovalentandcoordinatecovalentbondinginBF3NH3.
valencebondtheory(VBT),whichisactuallyanextensionofLewistheory (1902)andHeitler–Londontheory(1927).Fromtheabovetwotheories,the sharingofelectronpairsbyatomsandtheformationofbondswasunderstood andexplained.TheVBTdevelopedbyLinusPaulingintroducedtwokey conceptsinthetheoriesofbonding,viz,thehybridizationoforbitalsand resonance,theabsenceofwhichwerethedrawbacksoftheprevioustheories.
Accordingtothistheory,themetalionsareregardedasLewisacids characterizedbytheavailabilityoflowlying,emptyorbitalssuitablefor accommodatingtheacceptedelectrons.TheligandsaretheLewisbases, characterizedbytheavailabilityofalonepairofelectronsthatcanbe readilydonated,resultingintoaformationofacoordinatecovalentbond. Thus,theacid–basecharacteristicsofmetalandligandcomplementeach othertogiverisetoacoordinatecovalentbond,asshownin Figure8
Hybridizationofatomicorbitals,amathematicaltoolformixingthe atomicorbitalstogiveanequalnumberofhybridorbitalswithsuperior directionalproperties,becameaveryimportantfeatureofthistheoryin explainingvariousgeometriesobservedincoordinationcompounds.
4.GEOMETRIESOFCOMPLEXESWITHDIFFERENT COORDINATIONNUMBERS
1.Coordinationnumber2: Thisisarelativelyuncommoncoordination numberandisrestrictedtoafewcases,suchasCu(I),Ag(I),Au(I),and Hg(II)ionshavingd10 configuration.[CuCl2] and[Ag(NH3)2]þ are therepresentativecomplexesofthiscoordinationnumber.
ThepossiblegeometriesforthecomplexeswithCN ¼ 2arelinear andangular.Lineargeometryiscommonlyobservedinsuchcomplexes,
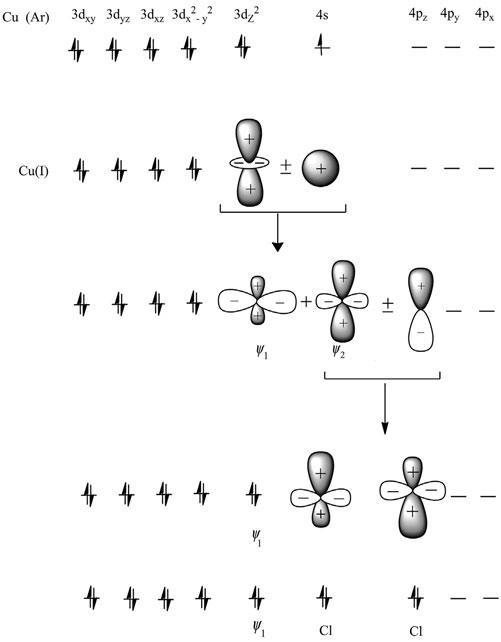
Figure8
asitinvolvesminimumligand–ligandrepulsion.Thesecomplexes involvesd-hybridizationbetweenthecentralmetalatomorbitals,as shownin Figure9
Orgelhassuggestedthat(n 1)dorbitalshavenearlythesameenergy asthatofnsandnporbitals.Thedz2 orbitalscanenterintothishybridizationtoremoveelectrondensityawayfromtheregionofligandsand stabilizethecomplex.Initially ‘4s’ and ‘3dz2 ’ orbitalshybridizetogive twosd-hybridorbitals,namely j1 and j2.The j2 sd-orbitalshavetheir positivelobesalongthe z axis,whilethe j1 isinthe XY plane.The j2 orbitals,furtherhybridizedwithpz orbitals,givethehybridorbitals concentratedalongthe z-direction.Theelectronpairfromthedz2 orbitalsnowoccupythesdorbital XY plane,whilehybridorbitals concentratedalongthe z-directionareavailableforastrongerbond alongthe z axis,resultingintoacomplexwithlineargeometry.
Figure9 Hybridizationexplainingthebondingin[CuCl2] .
Thechelatesofthiscoordinationnumberarelessstablethananalogouscomplexes.Considerthecomplexes[Ag(en)]þ and[(Ag(NH3)2]þ . Inthesecomplexes,thefavouredgeometryislinear.In[Ag(en)]þ chelate,a five-memberedringisformed,andthe ‘ en ’ moleculecannot occupylinearcoordinationpositionsaroundAgþ withoutintroducing straininthechelatering,whichdestabilizesthecomplex.Hence [Ag(en)]þ islessstablethan[(Ag(NH3)2]þ.TheAg(I)chelateswith six-,seven-oreight-memberedringsaremorestable;asinlargersizedchelaterings,thestrainisless,hencestabilityisgreater.
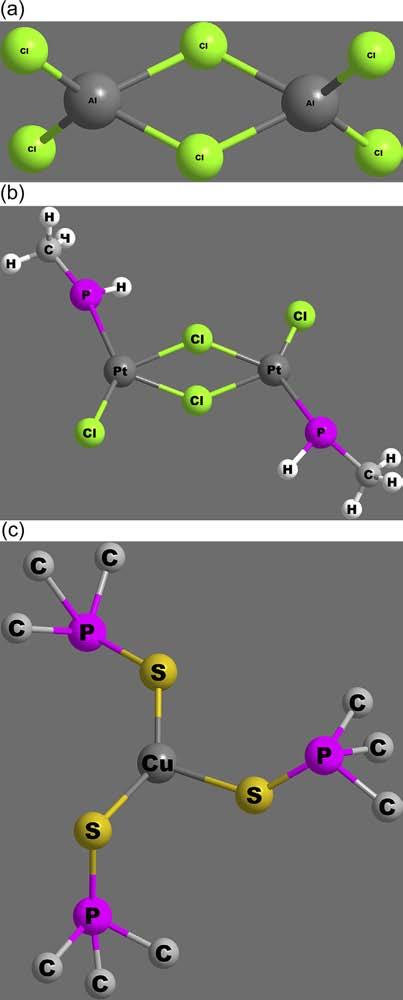
2.Coordinationnumber3: Thisisveryrarecoordinationnumber.In mostcases,detailedstudieshaveshownahighercoordinationnumber attainedbythecentralmetalatomthroughdimerizationandbridging, asinthecasesofAlCl3 andPtCl2(PR3),shownin Figure10.
However,CN ¼ 3isalsoillustratedbyafewtruecomplexessuchas HgI3 and[Cu(SPMe3)]ions.Thesecomplexesexhibittrigonalplanar geometry,asshowninthe figure.
3.Coordinationnumber4: Thisisoneofthemostcommonlyobserved coordinationnumbers.Therearetwopossiblegeometriesassociated withCN ¼ 4,viz,tetrahedralandsquareplanar.
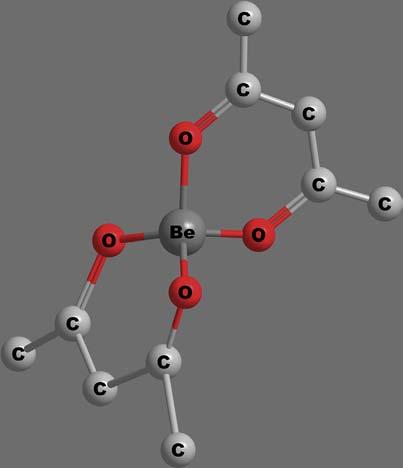
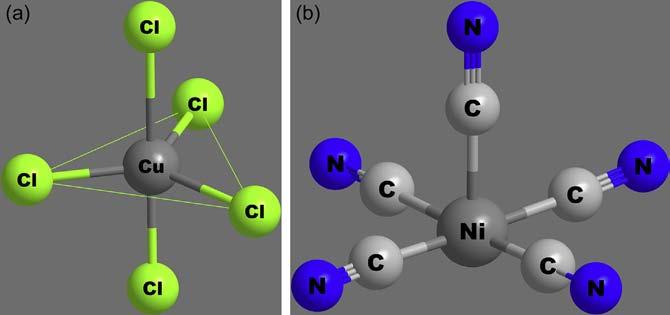
a.Tetrahedralcomplexes: Tetrahedralgeometryisfairlycommon infour-coordinatecomplexesofnontransitionmetalions.Thisgeometryinvolvessp3 hybridizationoforbitalsofthecentralatom. ThisgeometryisfavouredbylargeligandslikeCl ,Br ,I and smallmetalionslikeBeþ2 andAlþ3.Complexeslike[BeF4]2 , [ZnCl4]2 andBe(acac)2,shownin Figure11,illustratethetetrahedralcomplexeswithCN ¼ 4.
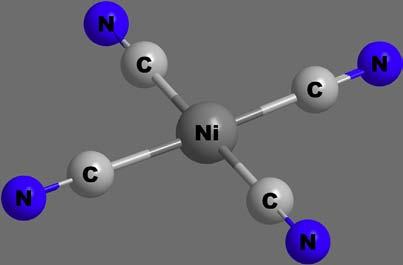
b.Squareplanarcomplexes: Squareplanarcoordinationislessfavouredstructurally(duetostrongerligand–ligandrepulsion)than tetrahedralcoordination,especiallybylargeligands.Iftheligands aresmall,octahedralcoordinationcanbeachieved.Fewmetal ionsareknowntoformsquareplanarcomplexes;itismostly restrictedtod8 tod9 ionslikePt(II),Pd(II),Ni(II),Cu(II)and Au(II),asillustratedin[Pt(NH3)4]2þ,[PtCl2(NH3)2]and [Ni(CN)4]2 shownin Figure12.
4.Coordinationnumber5: Thetwopossiblegeometriesforthiscoordinationnumberaretrigonalbipyramidal(TBP)andsquarepyramidal (SqPy).Inmostcases,thestructureisfoundtobedistorted.Theconversionbetweenthesetwogeometriescaneasilyoccurbyslight
Dimerizationandbridgingin(a)AlCl3 (b)PtCl2P(CH3)and(c)[Cu(SPMe3)].
Figure10
deformation.However,[CuCl5] 3 and[NiCN5] 3 exhibitTBPand squarepyramidalgeometryrespectively,asshownin Figure13.
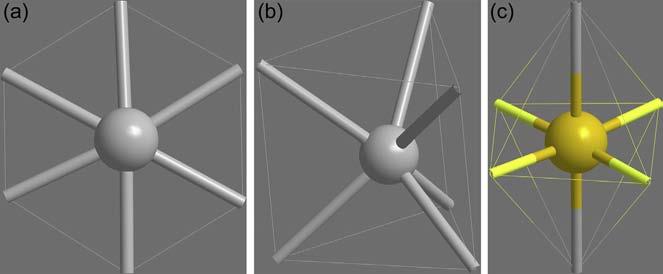
5.Coordinationnumber6: Thisisthemostcommoncoordination number.SomemetalslikeCr(III)andCo(III)almostexclusivelyform six-coordinatecomplexes.Therearethreepopulararrangementsof sixligandsaroundthecentralmetalion,viz,hexagonalplanar,trigonal prismaticandoctahedral,asshownin Figure14
Figure11 TetrahedralBe(acac)2 chelate.
Figure12 Squareplanar[Ni(CN)4]2 complexion.
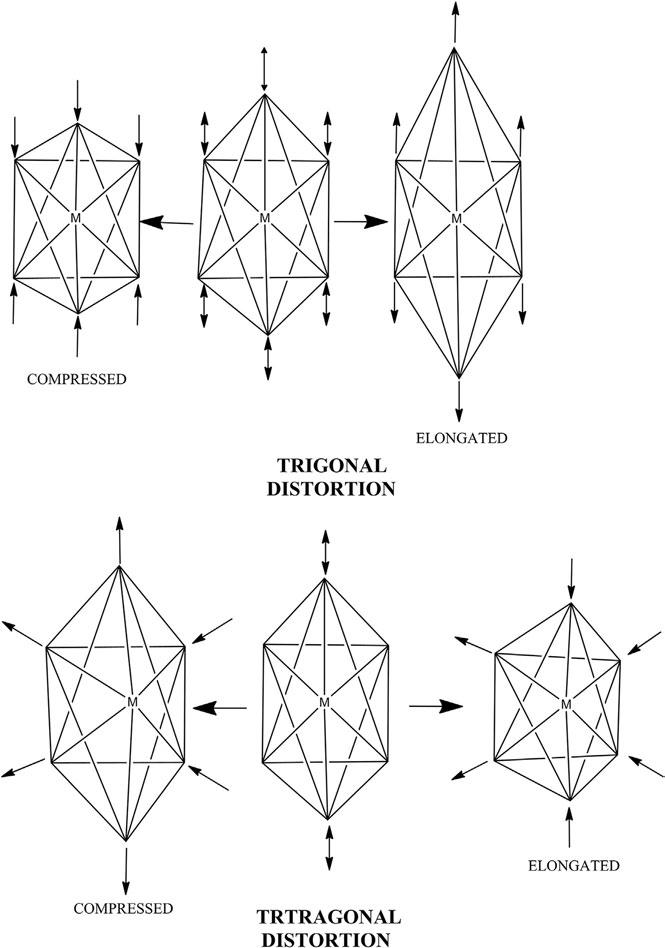
AnextensivestructuralstudyofthecomplexeswithCN ¼ 6hasshown thatthearrangementofsixligandsinasix-coordinationcompoundisalwaysoctahedral.Inoctahedralcomplexes,d2sp3 orsp3d2 hybridizationof orbitalsisobserved.Perfectoctahedralsymmetrycanbeobservedonly whenallofthesixligandsareidentical,otherwisethegeometrygetsdistorted.Itmayshoweithertetragonalortrigonaldistortion,asshownin Figure15.
Complexeswithhighercoordinationnumbershavealsobeenreported, butconsideringthescopeofthebook,thediscussiononsuchcomplexesis knowinglyomitted.
ThoughVBThasremainedanecessarytoolforthestudyofmetal complexes,itisnotsufficienttoexplainthecolourandcharacteristicsofthe absorptionspectraofthecomplexes.Thetheorycouldnotdemystifythe
Figure13 (a)Trigonalbipyramidal[CuCl5] 3 and(b)Squarepyramidal[NiCN5] 3 ions.
Figure14 (a)Hexagonalplanar,(b)trigonalprismaticand(c)octahedralgeometries.
Figure15 Distortionsinoctahedra.