1 Cystoscopy and Urinary Bladder Anatomy
Dragos¸ Georgescu, Emanuel Alexandrescu, Ra˘zvan Mult¸escu, Bogdan Geavlete
1.1 GENERALITIES
The ability to approach the urinary tract without requiring surgical incisions has differentiated and continues to differentiate urology from many other surgical specialities. Endourology has permanently evolved over the last decades under the impulse of technological progress that has allowed the approach of the entire urinary tract and the development of modern, advanced endoscopes. Moreover, throughout the history of surgery, urology has had a leading role in the discovery and implementation of minimally invasive techniques.
Cystoscopy is a technique that allows direct visualization of the bladder, and is currently the most frequently performed urological endoscopic procedure, having both diagnostic and therapeutic indications. The endoscopic evaluation of the bladder is frequently accompanied by the concomitant examination of the urethra and, in consequence, can provide a comprehensive evaluation of the lower urinary tract, a procedure known as urethrocystoscopy.
1.2 URINARY BLADDER ANATOMY
1.2.1 Descriptive Anatomy
The urinary bladder is a musculomembranous cavity organ located in the anterior part of the pelvic cavity, superior and posterior to the pubis (Crafts, 1985).
The shape, position, size, and relationships of the bladder vary according to its degree of filling. When empty, the bladder is shaped like a tetrahedron, with an apex, a superior face, two inferolateral faces, a base (the posterior face), and a bladder neck (Carter and Chan, 2007). The bladder apex is oriented superiorly, and is the origin of the urachus, a fibrous structure that is a reminiscence of the allantois from the fetal period. It forms the middle umbilical ligament, which ascends from the bladder apex, on the posterior face of the anterior abdominal wall, between the peritoneum and the transversalis fascia, up to the navel, and fixes the bladder to the abdominal wall (Crafts, 1985).
The superior face of the bladder has a relatively triangular shape, is oriented anterosuperiorly, and is covered by the peritoneum. Anteriorly, the peritoneum is reflected on the anterior abdominal wall (parietal peritoneum). Bladder distension leads to the displacement of the peritoneum away from the abdominal wall and makes it possible to perform suprapubic cystostomy without the risk of intercepting the peritoneal cavity (Carter and Chan, 2007). Through the peritoneum, this portion of the bladder has relationships with the ileal loops and the sigmoid colon in men and with the ileum and uterus in women (Crafts, 1985).
The base of the bladder is oriented posteriorly, and in men, it has relationships with the seminal vesicles, the vas deferens ampoules and the terminal ureter, and inferiorly with the base of the prostate. The part that remains free of these structures is covered by the peritoneum, which is continuous with that located on the anterior rectal wall,
Endoscopic Diagnosis and Treatment in Urinary Bladder Pathology. http://dx.doi.org/10.1016/B978-0-12-802439-3.00001-3
forming the rectovesical pouch. In women, the bladder has posterior relationships with the uterus and upper part of the vagina, structures which separate it from the rectum (Crafts, 1985). The peritoneal reflection on these structures forms the vesicouterine and rectouterine pouches, respectively.
The inferolateral faces of the bladder, as well as the inferior part of the superior face, are separated anteriorly from the pubic bones by the connective tissue and perivesical fat of the retropubic space (the space of Retzius). Posteroinferiorly, the urinary bladder comes into contact with the fascia covering the levator ani and the internal obturator muscles (Crafts, 1985).
The bladder neck is the lowest part of the bladder, located approximately 3–4 cm behind the symphysis pubis, and continues with the prostatic urethra. The degree of bladder filling has very little influence on the position of the bladder neck, which is fixed to the pelvic fascia with the urethra and the prostate (Carter and Chan, 2007).
When distended, the urinary bladder has an oval shape with the maximum diameter oriented anteriorly and superiorly, where it comes into contact with the anterior abdominal wall. Apart from the modifications in shape and position described earlier, the other relationships of the bladder remain unchanged.
The bladder wall consists of four layers: serous, muscular, submucosal, and mucosal. The serous layer (tunica serosa) is derived from the peritoneum and is incomplete, only covering the superior face, the upper parts of the lateral faces, and a small area at the base of the bladder, in men (Crafts, 1985).
The muscular tunic (bladder detrusor) consists of three layers of smooth muscle fibers with different orientations: the external and internal layers are longitudinal, while the middle layer has circular fibers. The muscle layers do not follow this orientation throughout the bladder, and have a tendency to overlap and form a network (Crafts, 1985). Thus, in the upper part of the bladder, there is no clear delimitation of muscle layers, whereas as it approaches the trigone and the bladder neck, there is a clear separation between the three types of fibers of the detrusor (Carter and Chan, 2007).
The fibers of the external longitudinal layer have a maximum thickness at the base of the bladder where, on the midline, they are inserted on the apex of the trigone and merge with smooth muscle fibers from the prostate in men and from the vaginal wall in women. This ensures that the trigone has a strong muscular support. Laterally, the fibers of the posterior plane pass anteriorly and loop around the bladder neck, thus being involved in the continence mechanism at this level. Some muscle fibers of the external muscular layer are involved in the formation of the puboprostatic ligament in men and pubourethral ligament in women (Carter and Chan, 2007). The middle muscular layer is poorly represented with an irregular disposition of the fibers, which have an oblique and circular orientation.
The fibers of the internal layer are thin and arranged in a reticular manner, without losing their tendency for longitudinal orientation. Muscle fibers located in the periureteral bladder wall surround the ureteral orifice from the same side, then merge with fibers from the lateral and posterior ureteral wall and descend in order to be inserted in the bladder neck and the prostatic median lobe (Carter and Chan, 2007). These fibers constitute the so-called ureteral muscle, described by Sir C. Bell, which acts during bladder contraction in order to maintain the oblique path of the ureteral orifices and thereby to prevent vesicoureteral reflux (Dyson, 1995).
In the trigone, there are three different muscle layers. The superficial layer includes smooth muscle fibers that originate in the ureters, which descend through the trigone in order to enter the verumontanum. The deep layer stems from Waldeyer’s sheath and enters the bladder neck, while the middle layer consists of urinary bladder fibers (external longitudinal and middle circular) (Carter and Chan, 2007).
The submucous tunic serves to connect the muscular layer with the mucosa, and it intimately adheres to the latter (Crafts, 1985). It consists of a relatively thick layer of fibroelastic connective tissue that allows for considerable bladder distension, and it is crossed by numerous blood vessels (Carter and Chan, 2007).
The internal surface of the bladder is covered by transitional epithelium (urothelium) consisting of six cell layers and a basal membrane. This epithelium continues with the epithelium of the ureters and the renal tubular membrane, as well as that of the proximal urethra (Crafts, 1985).
1.2.2 Endoscopic Anatomy
The normal endoscopic anatomy is a prerequisite for the correct endoscopic assessment of the urinary tract. When properly distended using irrigation fluid, both the bladder and the entire upper urinary tract are accessible to an endoscopic approach.
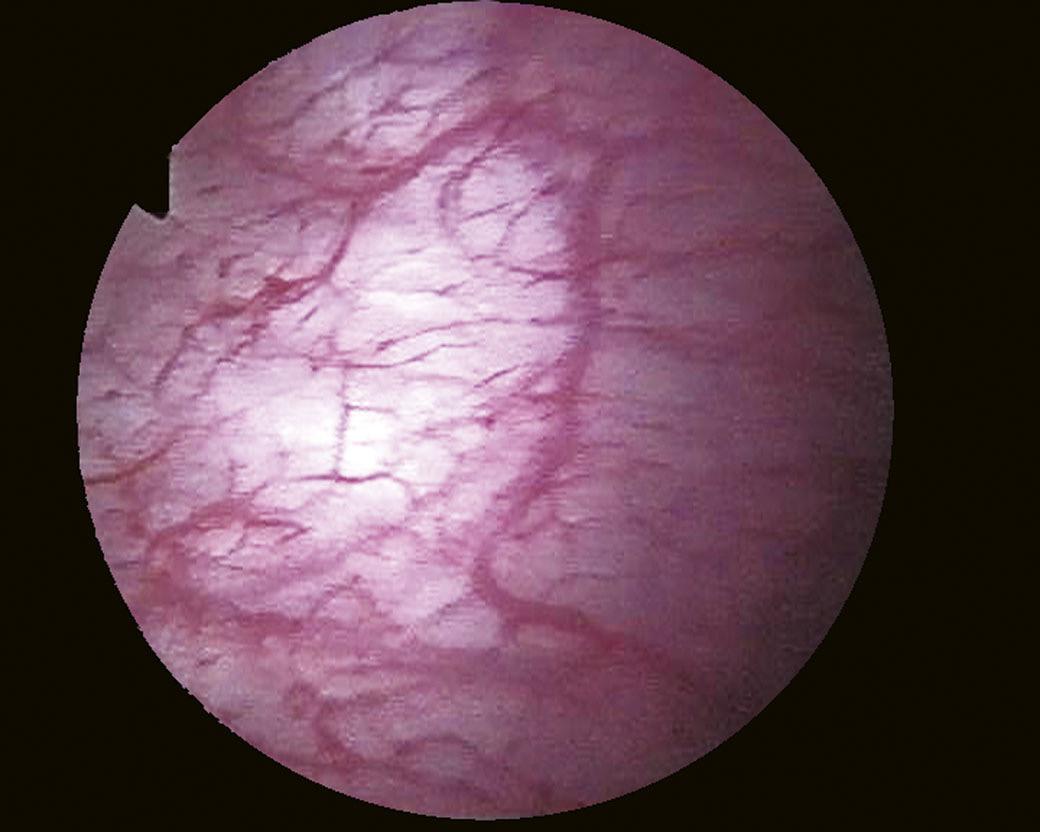
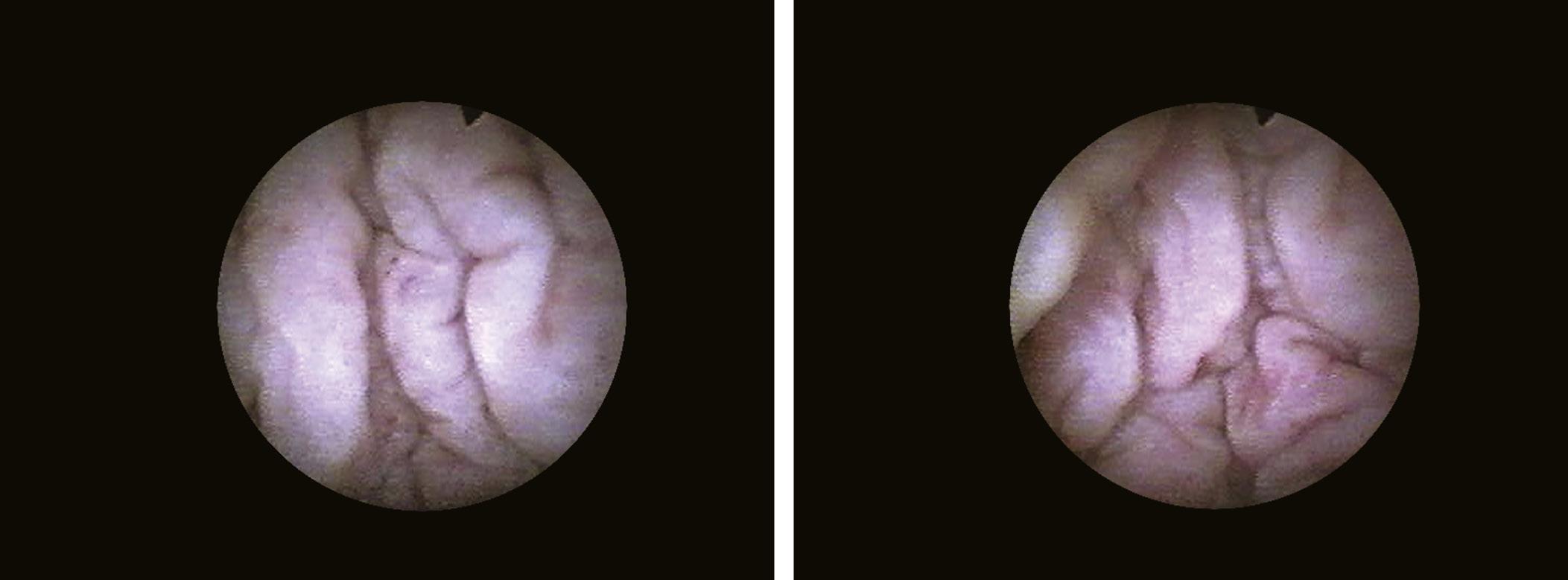
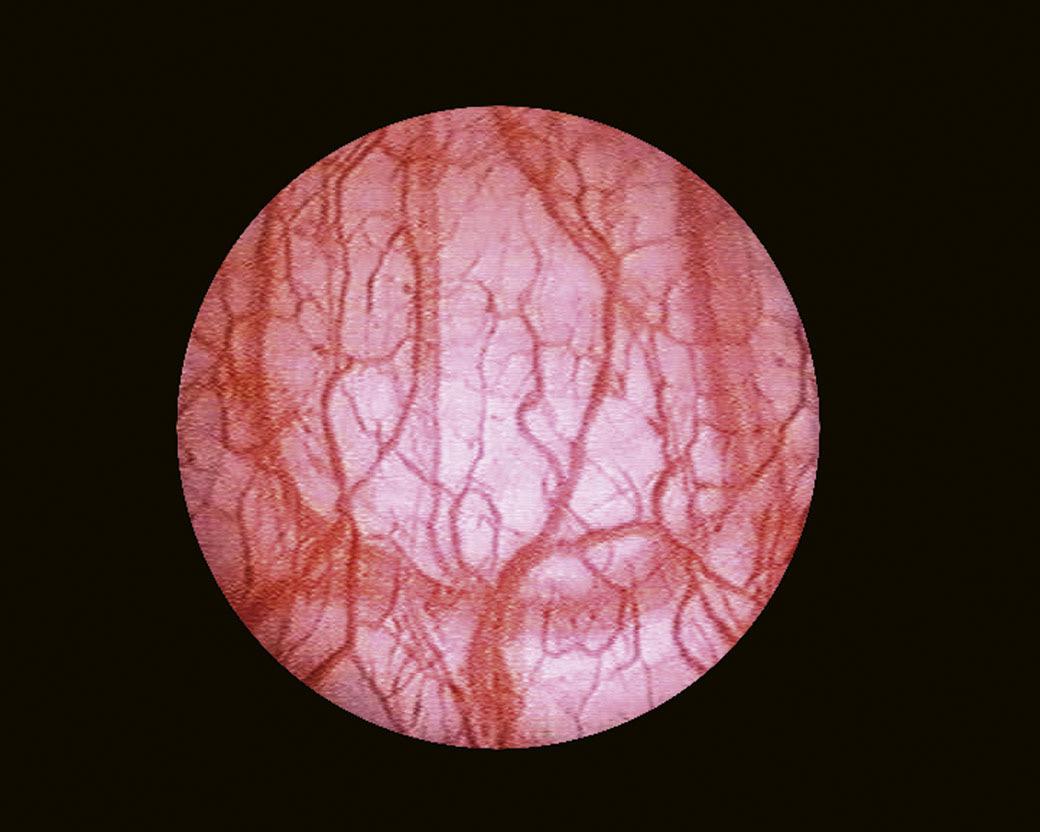
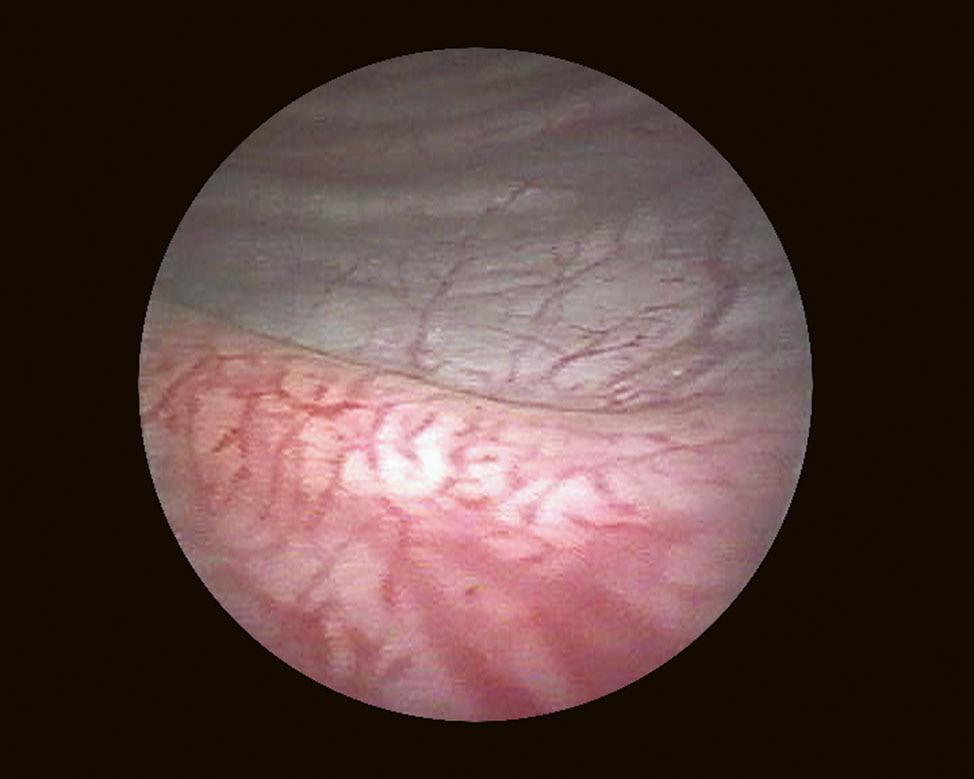
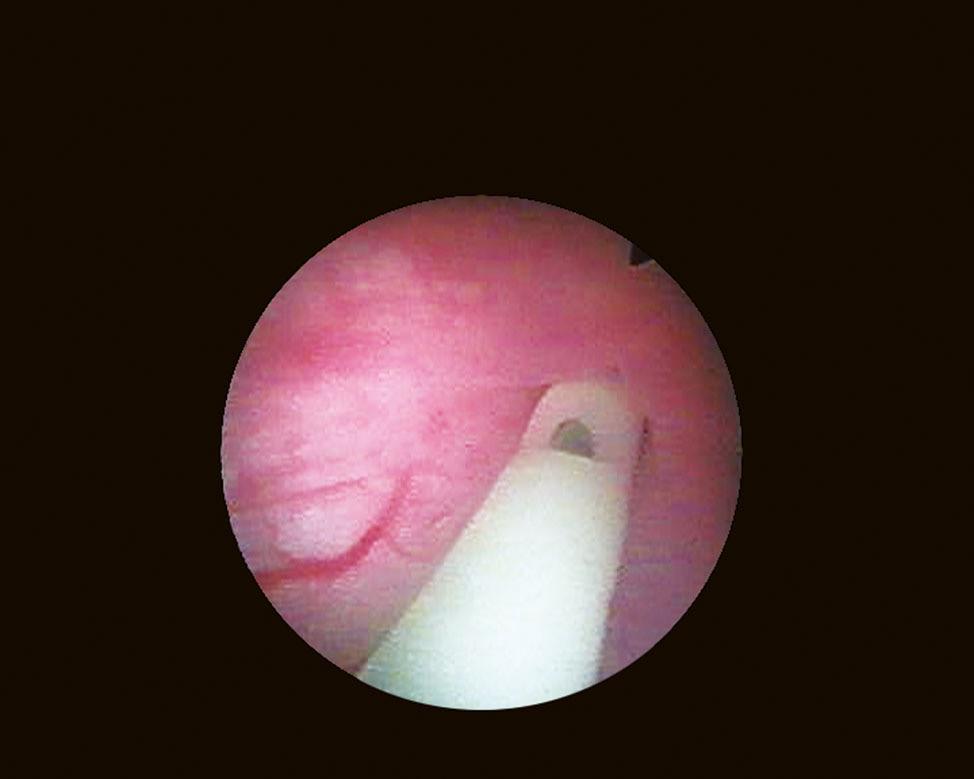
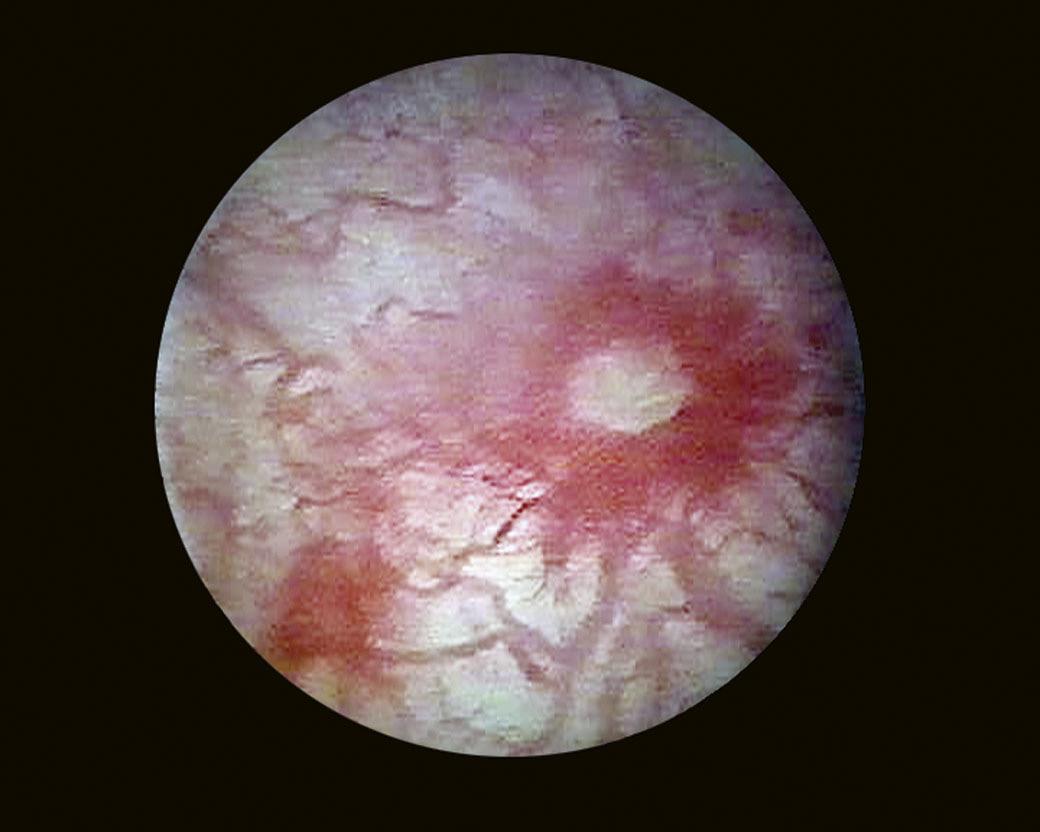
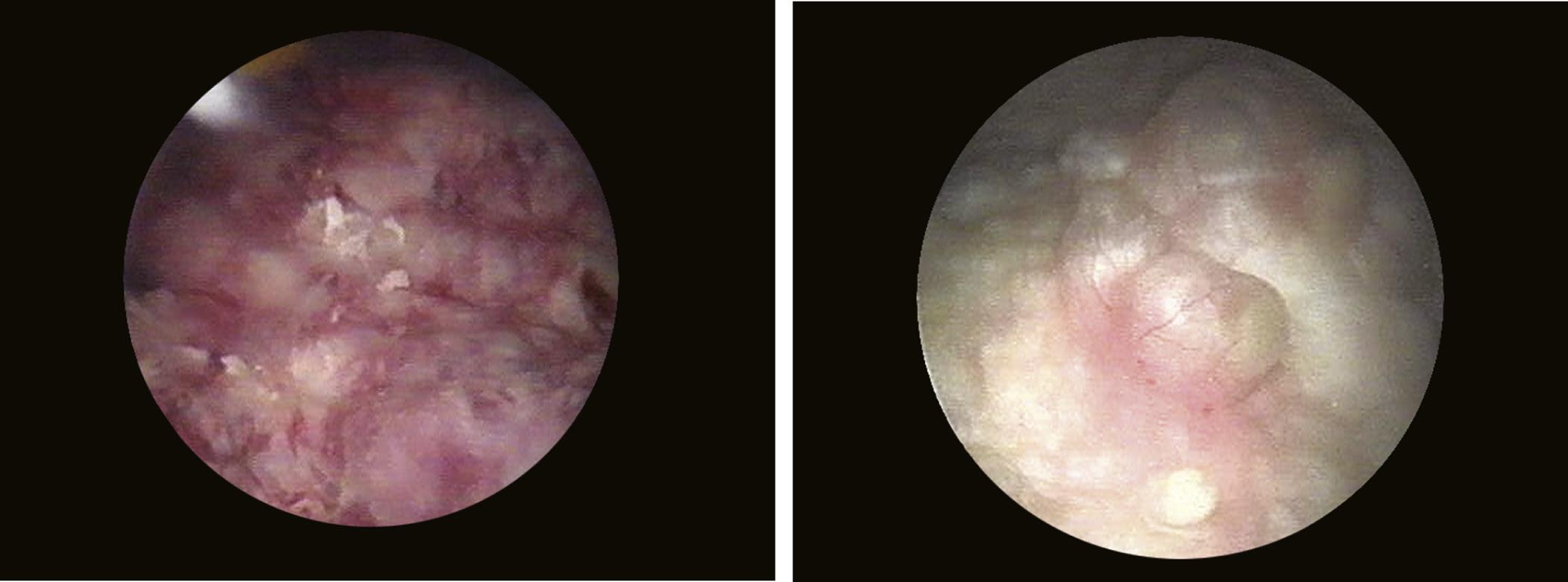
When it is sufficiently distended, the bladder is shaped like a sphere with smooth, regular mucosa (Fig. 1.1); while when partially filled or empty, numerous mucosal folds can be observed at endoscopy (Fig. 1.2). The color of the bladder mucosa is usually yellow or pale pink, while its translucent nature allows endoscopic visualization of the submucosal vascular network (Fig. 1.3) (Cundiff, 2001).
FIGURE 1.1 Distended bladder wall.
FIGURE 1.2 Aspect of depleted bladder wall.
FIGURE 1.3 Endoscopic visualization of the submucosal vascular network.
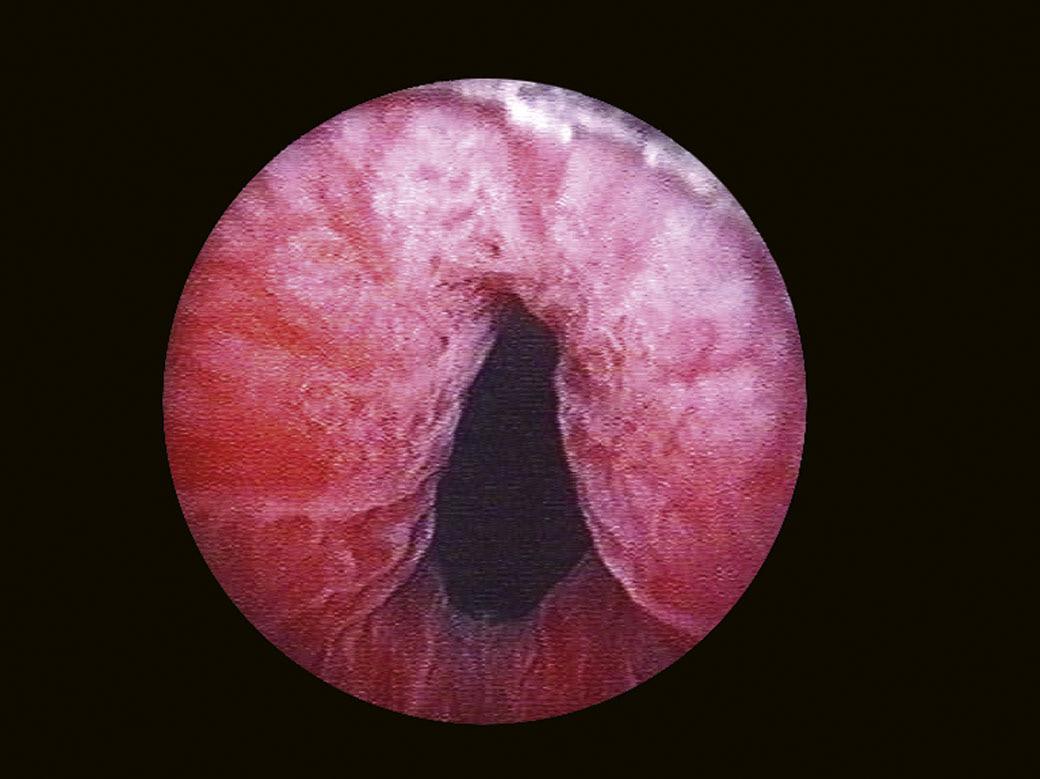
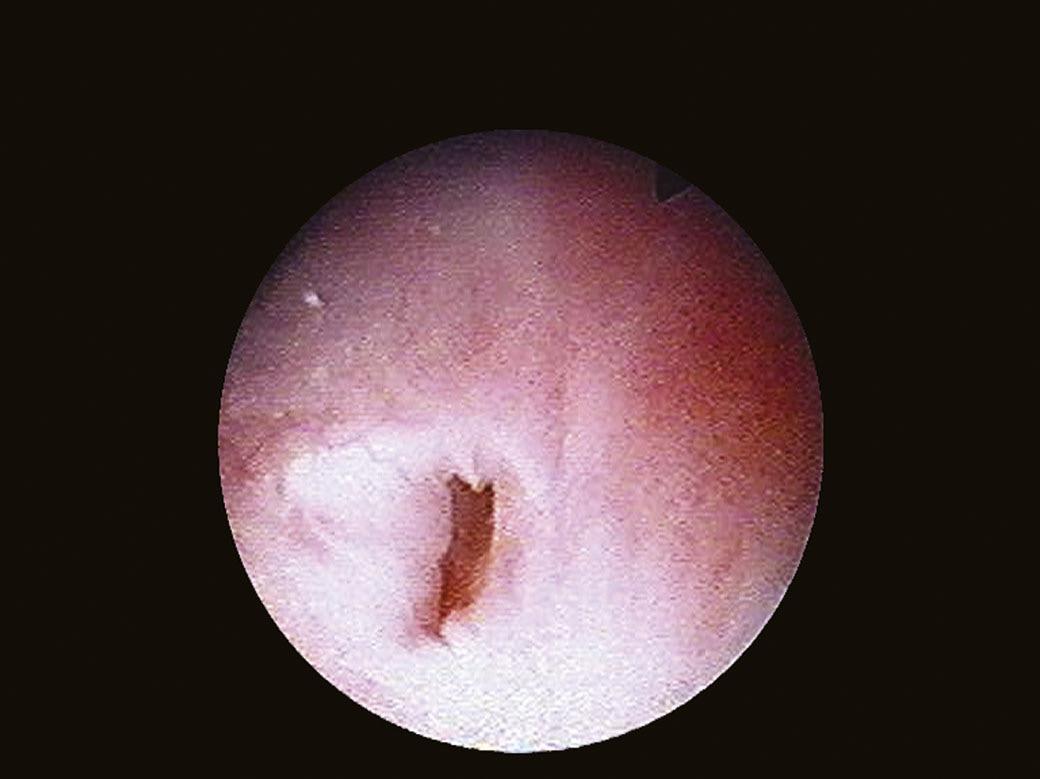
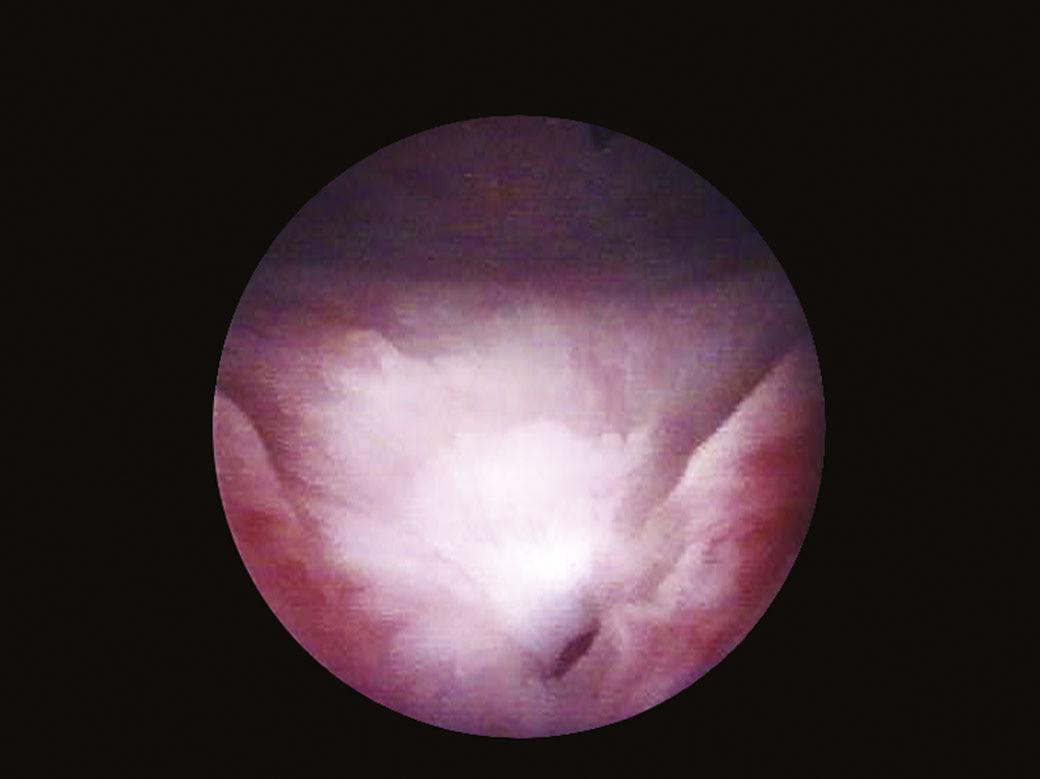
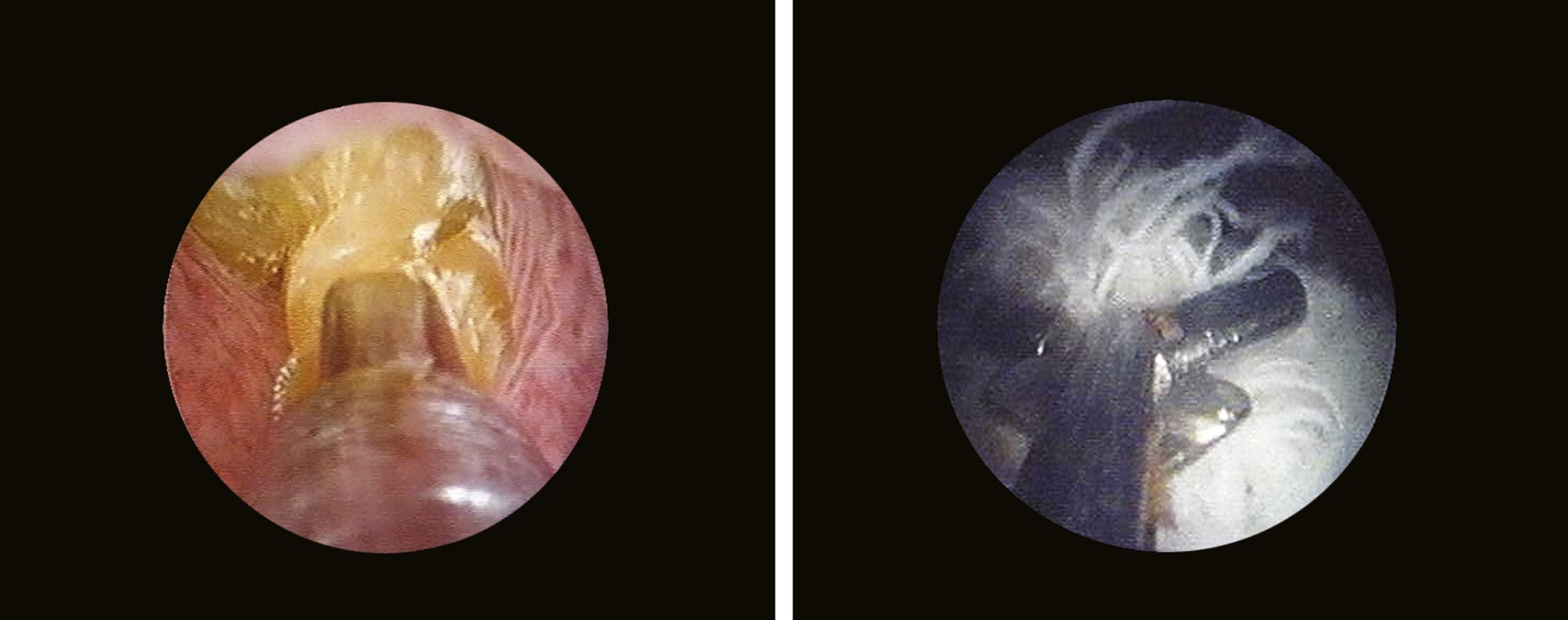
The bladder neck (Fig. 1.4) represents the distal (lower) limit of the bladder and remains the major landmark and point of reference for its endoscopic anatomy. The term bladder neck refers not only to an anatomical region, but at the same time defines a functional entity. At this level, the urethral lumen and the bladder muscles converge. At present, there is a tendency to consider the bladder neck as a separate structure both from the bladder and the urethra, due to its unique functional characteristics.
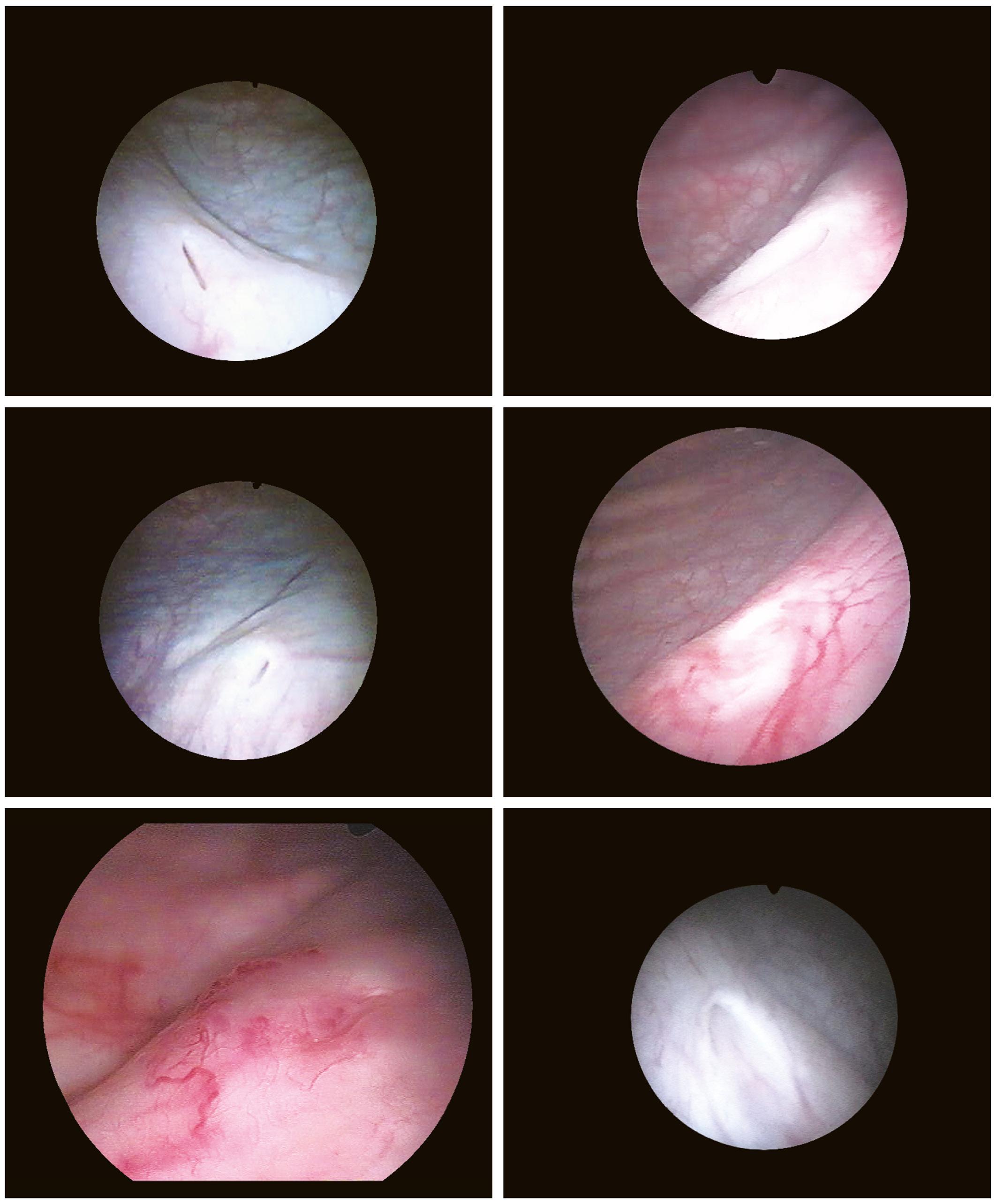
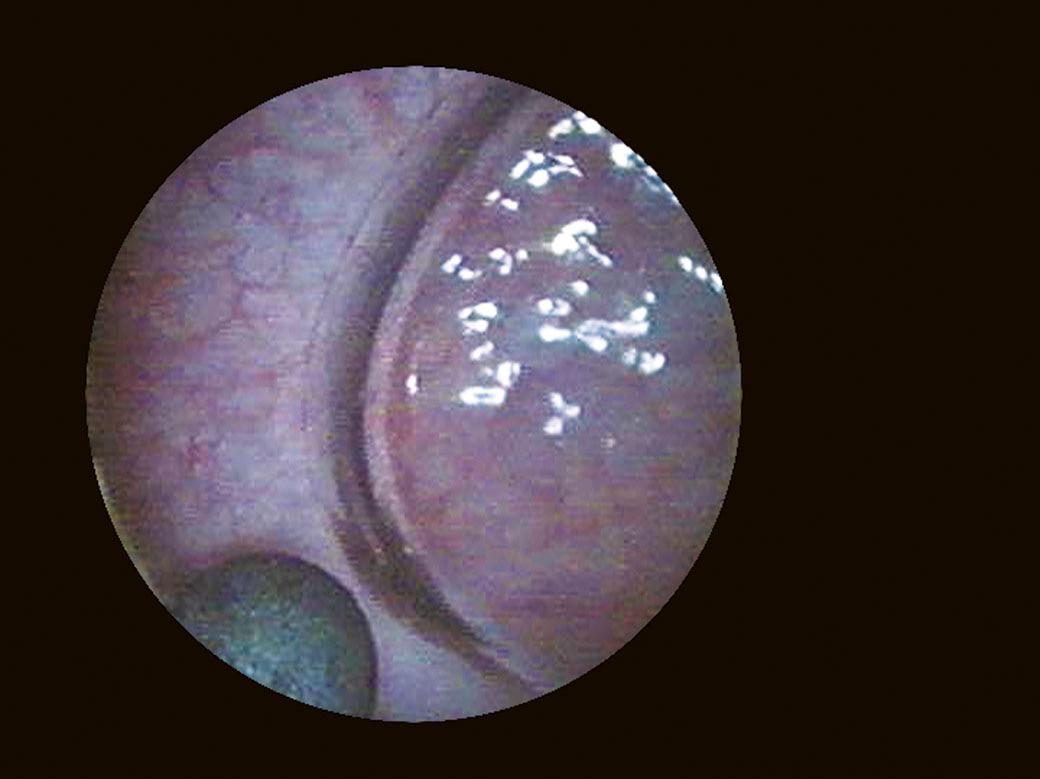
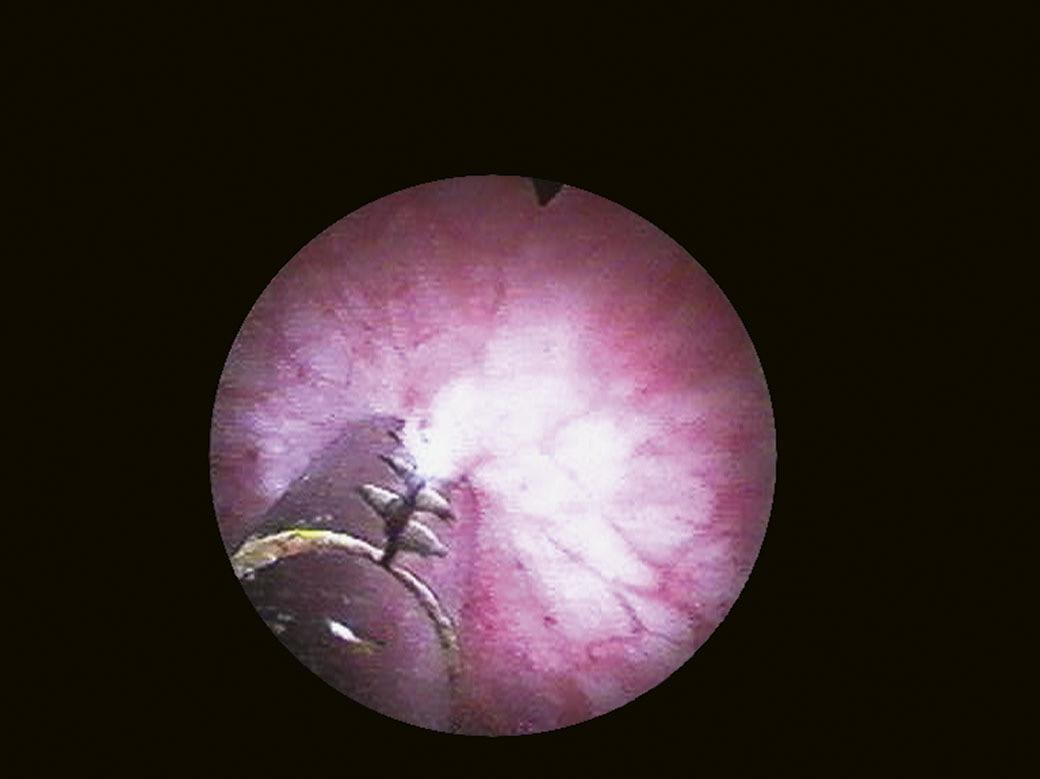
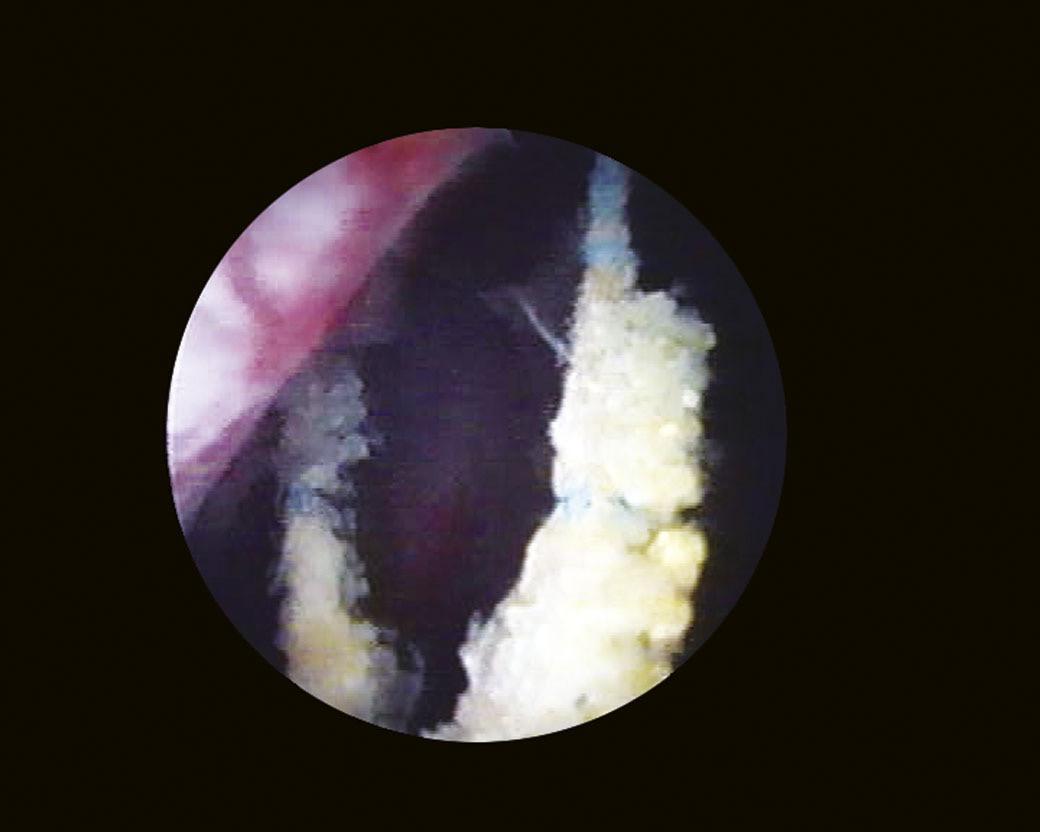
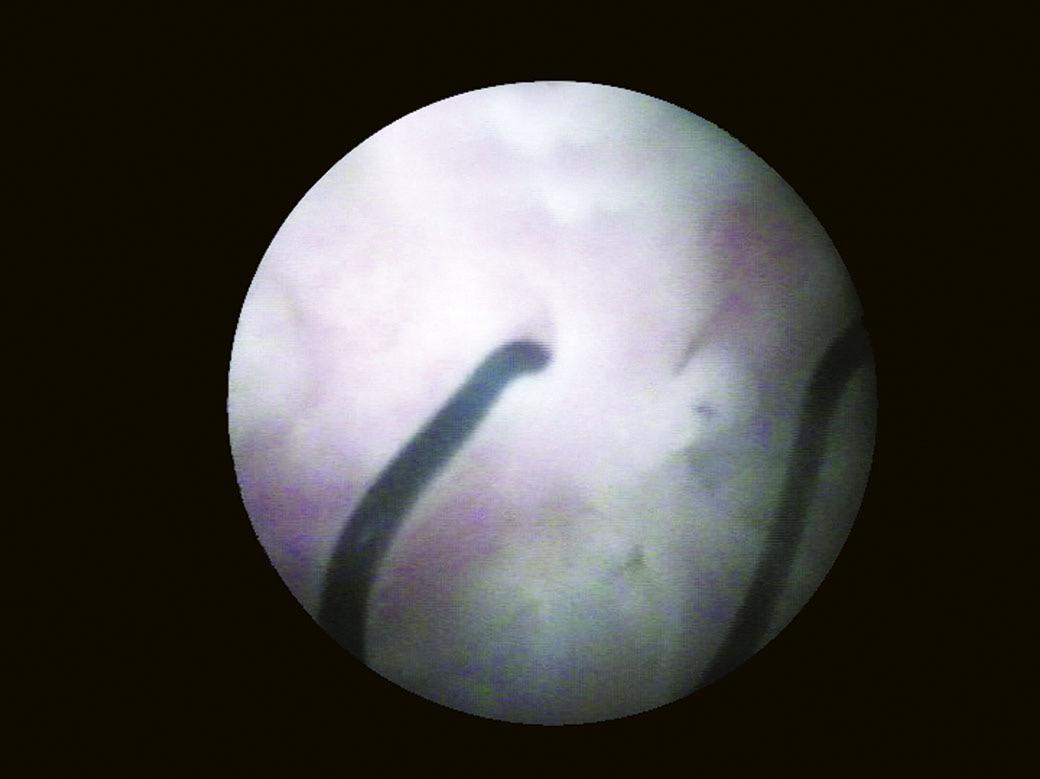
Another landmark of endoscopic anatomy is represented by the bladder trigone. It defines a portion located on the posterior wall of the bladder, delimited by the two ureteral orifices and the bladder neck. The name derives from the relatively triangular shape of this area, where the ureteral orifices delimit the base, while the bladder neck marks the tip of the trigone. The interureteral bar (Fig. 1.5) extends between the two ureteral orifices and appears endoscopically as an elevated part of the mucosa. The identification of the interureteral bar during cystoscopy helps locate the ureteral orifices. The ureteral orifices may have a variety of endoscopic aspects (Fig. 1.6) but, in general, they appear as small circular or oval openings situated on top of a mucosal fold. The intramural part of the ureters can often be visualized endoscopically as a bump in the bladder mucosa, which descends obliquely from the sidewalls toward the trigone and the ureteral orifices (Cundiff, 2001). With the peristaltic outflow of urine, the ureteral orifices open and the mucosa that delimits them retracts toward the intramural part of the ureters (Fig. 1.7).
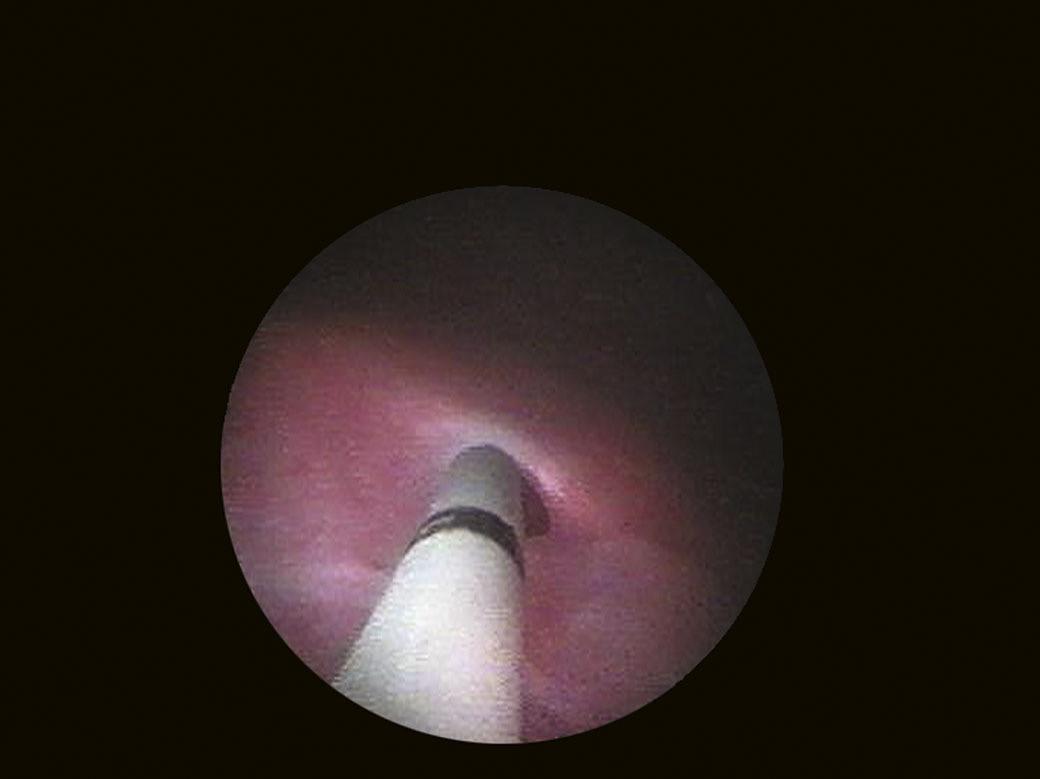
Visualization of the trigone is achieved by aligning the tip of the cystoscope inferiorly (caudally), immediately after passing the bladder neck, while the ureteral orifices are identified by directing the instrument laterally and posteriorly. The bladder base is located behind the trigone, while the sidewalls extend superiorly, toward the dome. The bladder dome (anterior wall) is identified by locating the air bubble that enters the bladder during cystoscopy (Fig. 1.8).
1.3 HISTORY
The concept of exploring the inside of the human body and its various organs date back to antiquity. The Hippocratic Corpus describes probably the first rudimentary attempts at endoscopy using a rectal speculum. Most historians attribute the first attempt of an endoscopic maneuver to Philipp Bozzini (1773–1809) (Fig. 1.9), a German army surgeon, who attempted to study the cavity organs of the human body. In 1806, he designed the Lichtleiter device that consists of a system of angled mirrors, positioned to project the image of different internal structures of the body toward the examiner’s eye. Bozzini’s device consists of two aluminum tubes that were inserted into the body orifice that was to be examined, and used a candle as a light source (Natalin and Landman, 2009).
This device is considered to be the first endoscope of modern medicine (Verger-Kuhnke et al., 2007).
FIGURE 1.4 Endoscopic aspect of the bladder neck in men.
FIGURE 1.5 Endoscopic aspect of the interureteral bar.
FIGURE 1.6 Various aspects of ureteral orifices.
In 1826, Pierre Salomon Segalas (1792–1875), a French urologist, presented an improved variant of the Lichtleiter device by adding an additional light source (candle) and a cannulated catheter that was meant to evacuate the bladder content. The new device was made of an elastic material similar to rubber, with the purpose of improving the safety and comfort of the procedure (Natalin and Landman, 2009).
However, the term endoscope is attributed to the French urologist Antonin Jean Desormeaux (1815–1894) and was introduced in 1853 in order to describe an extensively modified version of Bozzini’s Lichtleiter device. He replaced the light produced by the Lichtleiter’s candle with the flame obtained by burning a solution of 96% alcohol and turpentine in a lamp. Also, he reconfigured the mirror angles so that the light may be focused more accurately, leading to a superior visualization of the region that was examined (Verger-Kuhnke et al., 2007). Desormeaux performed the first endoscopic surgical intervention, reporting the excision of a urethral polyp and the incision of a urethral stricture; these procedures being made possible due to new technology (Léger, 2004). The German urology professor Maximilian Carl Friedrich Nitze (1848–1906) (Fig. 1.10) noted two major shortcomings of the endoscopes used at that time: a poor visual field and poor illumination. Based on these observations, Nitze created an innovative optical system using prisms and lenses that provide a much improved visual field, and introduced the first intracorporeal
FIGURE 1.7 Open ureteral orifice, evacuating urine.
FIGURE 1.8 The gas bubble that marks the bladder dome.
FIGURE 1.9 Philipp Bozzini.
light source, using a platinum filament electric lamp (1878) (Herr, 2006). These changes led to the creation of what most historians consider to be the first successful modern cystoscope. The device with prisms and lenses allowed the transurethral visualization of the urinary bladder, while the light source was incorporated into the tip of the endoscope. The shortcomings of the instrument were related to the lamp’s complicated water cooling system, the difficulties in transurethral insertion, and the fact that the displayed image was inverted (Natalin and Landman, 2009). Nitze’s discovery revolutionized endoscopy and paved the way for minimally invasive surgery (Verger-Kuhnke et al., 2007).
In 1910, Christian Jacobaeus used Nitze’s cystoscope to perform the first endoscopically guided laparoscopy (Herr, 2006).
In 1879, the development of cystoscopes was influenced by the appearance of incandescent electric lamps (light bulbs) that consisted of a carbon filament incorporated into a vacuumed glass envelope. In 1883, David Newman adapted this type of lamp so that it could be used as a light source incorporated into the cystoscope. This greatly simplified the production of cystoscopes, reduced the production cost, and led to the widespread use of these instruments (Natalin and Landman, 2009).
Another significant advance in the field of endourology was achieved with the creation of the first resectoscope by Maximilian Stern (1877–1946) in 1926. Subsequently, in 1931, the addition of a mobile resection loop made this instrument appropriate for transurethral resections, procedures that were to become standard interventions for most cases of benign prostatic hyperplasia and noninvasive bladder tumors (Reuter and Reuter, 1999).
Subsequently, the physicist Harold Hopkins (Fig. 1.11) attempted, in turn, to improve the optical system of cystoscopes. Hopkins discovered optical fibers with a thickness of 0.1 mm, which he merged into coaxial fascicles, creating a new means of transmitting light and images. In 1960, this technology led to the creation of the first flexible endoscope (Gow, 1998).
Furthermore, Hopkins developed a telescope consisting of cylindrical lenses separated by sections of air; this modification allows light to be transmitted more than 80 times faster at the same diameter (1959) (Shah, 2002).
FIGURE 1.10 Maximilian Carl Friedrich Nitze.
The Hopkins telescope with cylindrical lenses and the use of optical fibers are the foundation for the design of current cystoscopes.
The development of optical fiber technology at the beginning of the 1970s led to the appearance of flexible cystoscopes. In 1973, Tsuchida and Sagawara described the first bladder exploration using a cystoscope that, although requiring a rigid sheath for insertion, had a mobile distal extremity (Quayle et al., 2005). Subsequently, continuous technological progress turned flexible cystoscopy into an integral part of modern urological practice. Major disadvantages of optical fibers were excessive fragility and poor image resolution. This was solved by the use of cystoscopes equipped with distal sensors (distal chip technology) consisting of millions of photodiodes. The sensor is capable of converting light particles (photons) into electrical impulses and subsequently into a digital image (Natalin and Landman, 2009). This technology significantly improved the reliability of flexible cystoscopes and provided an excellent image resolution and the possibility of storing these images (Quayle et al., 2005). The development of fluorescence cystoscopy also represented an important progress in the diagnosis of noninvasive bladder tumors using agents with an affinity for cancer cells, which can be visualized using this technique.
Endocystoscopy is a recent technique, currently under assessment, that uses an imaging device similar to a catheter, which can be inserted through the working channel of most endoscopes (Anandasabapathy, 2008). This device achieves a 450- to 1125-fold amplification of the image, while histological level resolutions are generated with the concomitant use of tissue coloring agents, allowing the in vivo differentiation between benign and malignant superficial lesions of the bladder mucosa (Ohigashi et al., 2006; Sasajima et al., 2006). The images allow the observation of the cellular components, similar to conventional histological samples, and in most cases, the correct assessment of tumor grade. However, there are plenty of controversial aspects to be overcome before this technology can be effectively used for the in vivo clinical diagnosis of bladder tumors (Ohigashi et al., 2006).
Virtual endoscopy uses computer-assisted three dimensional reconstruction of anatomic information provided by computerized tomography and magnetic resonance imaging in order to generate images that provide a detailed endoscopic perspective of the studied organ. This type of reconstruction was applied to most cavity organs of the human body, including the bladder (Allan and Tolley, 2001), and may represent an inexpensive and noninvasive method for evaluating the urinary tract. However, virtual cystoscopy does not yet provide sufficient sensitivity for it to replace standard cystoscopy in diagnosis of bladder tumors and detection of recurrences after transurethral resection (Babjuk et al., 2008). Currently, virtual cystoscopy is a feasible method for the diagnosis of bladder tumors larger than 5 mm. Moreover, it allows for a 360° evaluation of the bladder in multiple planes, including areas that are difficult to approach by standard cystoscopy. Presently, this method has a major application only in patients in whom standard cystoscopy cannot be performed or is contraindicated (Kivrak et al., 2009).
Wireless capsule endoscopy (WCE) represents the most recent technique in the field of lower urinary tract endourology. This method uses a miniaturized video camera and a wireless radio transmitter integrated into a small capsule, which is inserted into the urinary tract. The system is also composed of an external device that receives the
FIGURE 1.11 Harold Hopkins.
signal, a data storage unit, and a computer for data interpretation. The capsule has two main components: the optical system and the guidance system. The optical system consists of a short focus lens, a narrow external slot to increase the depth of the field, and a dual light-emitting diode illumination system. The guidance system is based on a set of magnets that enable manipulation of the capsule inside the examined organ (Natalin and Landman, 2009).
WCE was initially used in 2001 and its main indication is the exploration of the digestive tract when classic endoscopy is unsuccessful (occult bleeding, reflux disease, Crohn’s disease, etc.) (Saurin, 2007). In 2009, at the American Urology Association Congress in Chicago, the first use of such a perfected device was reported – Cellvizio, for the examination of the bladder. The study, which included 27 patients, was the first in vivo microscopic evaluation of the bladder and allowed real-time differentiation between cancer cells and normal bladder mucosa.
However, there are some potential obstacles to be considered for the clinical application of this method. Unlike the gastrointestinal tract, the introduction and mobilization of the WCE inside the bladder is performed in a nonphysiological manner, requiring additional handling capabilities. The bladder has a relatively small capacity compared with the gastrointestinal tract and is easily accessible to conventional endoscopic exploration. Moreover, tissue sampling is much more difficult when using the WCE. Finally, the size of the WCE raises issues regarding placement, mobility, and recovery of the device from the urinary tract (Gomella, 2009).
1.4 INDICATIONS
The primary indication of cystoscopy is represented by the diagnosis of lower urinary tract disorders. Urethrocystoscopy achieves a direct and complete visualization of the urethra, bladder neck, and bladder. The main indications of cystoscopy are imposed by clinical manifestations that require detailed information regarding the structural anatomy and macroscopic pathology of the bladder. Moreover, cystoscopy enables tissue sampling for the histological (Fig. 1.12) or cytological examination, as well as endoscopic access to the upper urinary tract (Fig. 1.13).
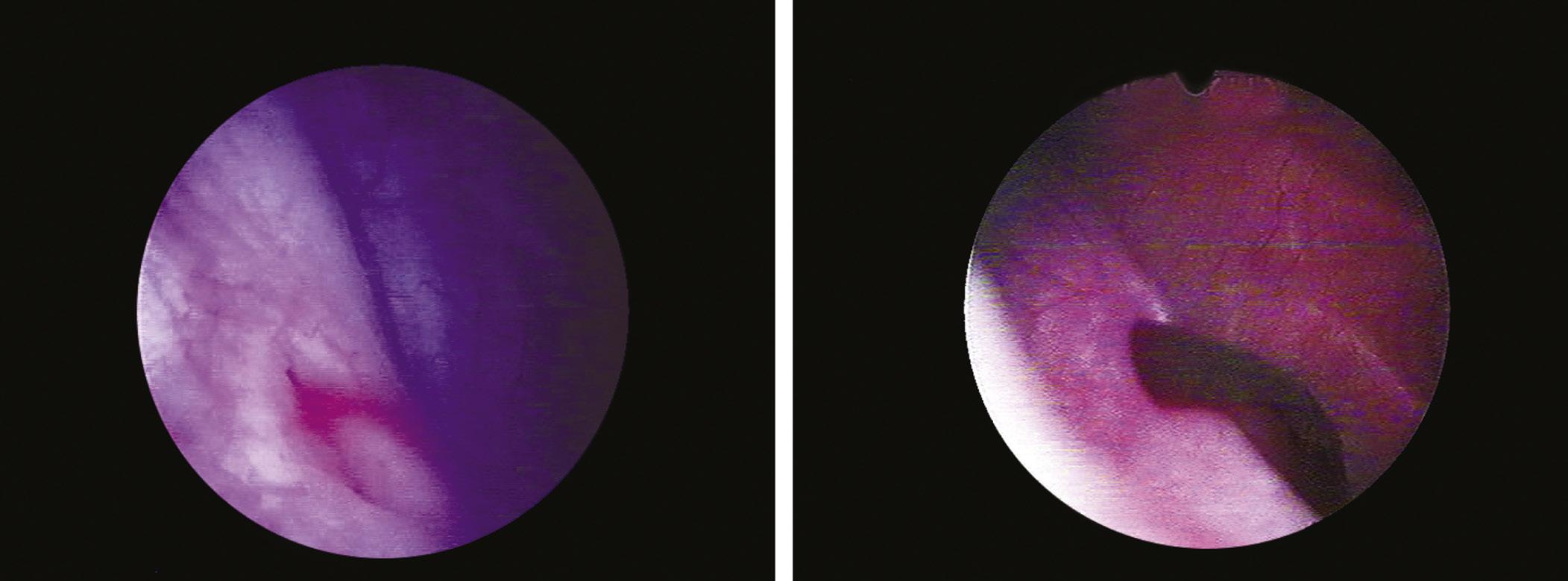
A major indication of cystoscopy is represented by the diagnosis of microscopic or macroscopic hematuria. In the protocol for evaluating hematuria, the endoscopic exploration is preceded by other investigations (radiological, ultrasound), but cystoscopy remains the only direct method of diagnosing bladder lesions (Manyak and Scherr, 1996). Moreover, in case of ongoing total hematuria that presents as the only symptom, cystoscopy becomes the first method of investigation (Proca, 1978). Thus, the causes of hematuria can be directly diagnosed as originating in the bladder or upper urinary tract (Fig. 1.14).
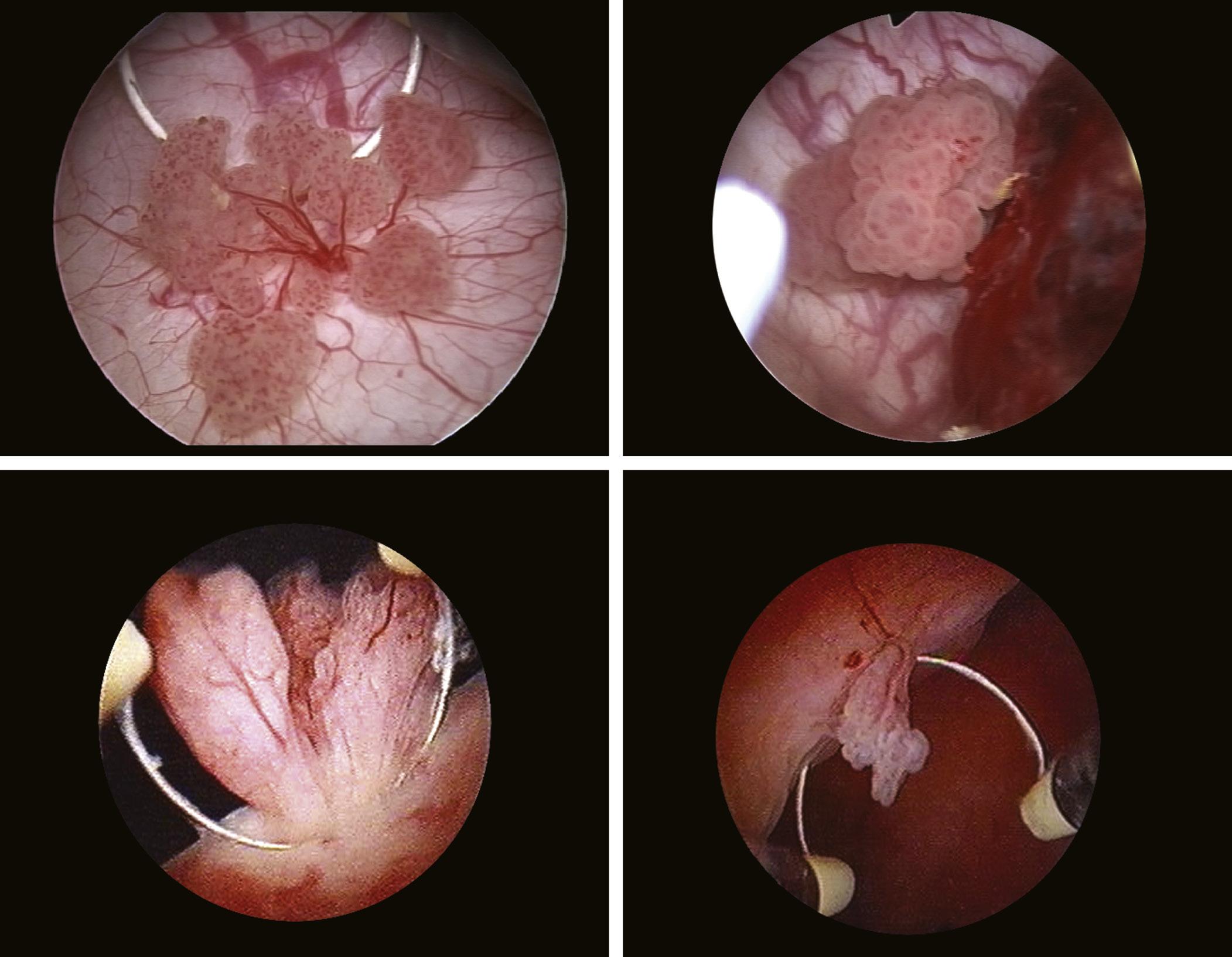
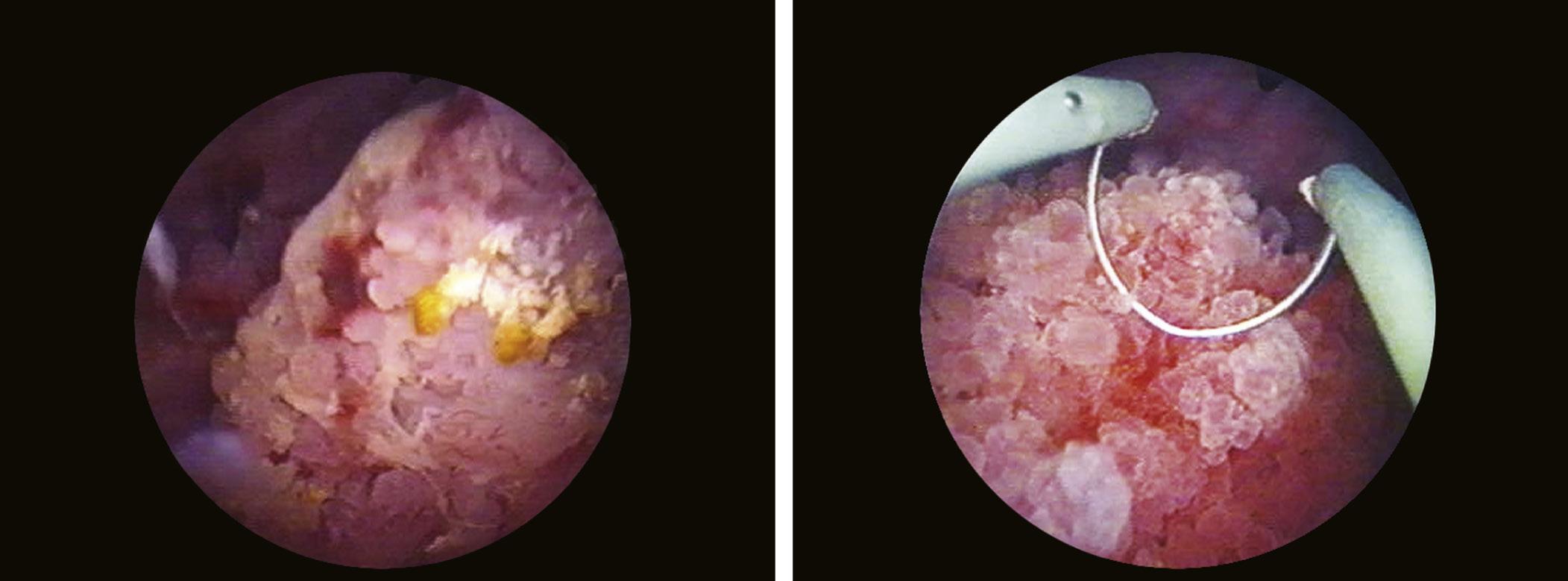
Bladder tumors clinically manifest through hematuria in 80% of cases, while diagnosis through imaging investigations (radiological) is possible in approximately 60% of these. Accordingly, cystoscopy represents the primary diagnostic method, allowing to assess the location, number, and size of bladder tumors, as well as to take tissue samples for histological examination (Manyak and Scherr, 1996). Any bladder tumor, papillary (Fig. 1.15) or sessile
FIGURE 1.12 Bladder biopsy.
FIGURE 1.13 Insertion of a ureteral catheter in order to perform retrograde pyelography.
FIGURE 1.14 Bleeding through the ureteral orifice.
FIGURE 1.15 Different aspects of papillary bladder tumors.
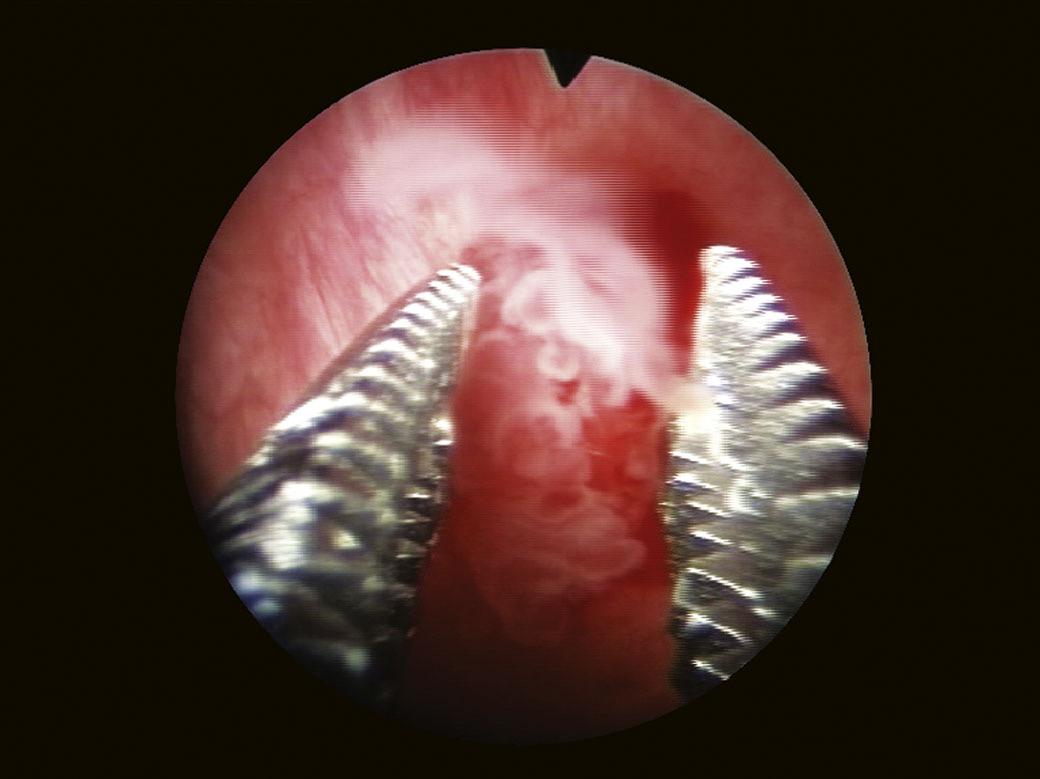
(Fig. 1.16), requires a biopsy (Fig. 1.17). Biopsy samples must include samples of the muscle layer for a correct tumor staging (Fig. 1.18).
Another indication of cystoscopy is the assessment of irritative or obstructive micturition disorders, in order to establish the etiology: inflammatory (Fig. 1.19), neurological, tumoral, or other.
The approach of the upper urinary tract by cystoscopy allows to examine the entire upper urinary tract using a contrast medium (retrograde pyelography), to obtain samples for cytological or histological examination, as well as to perform therapeutic maneuvers (placement of ureteral catheters) (Fig. 1.20) (Carter and Chan, 2007).
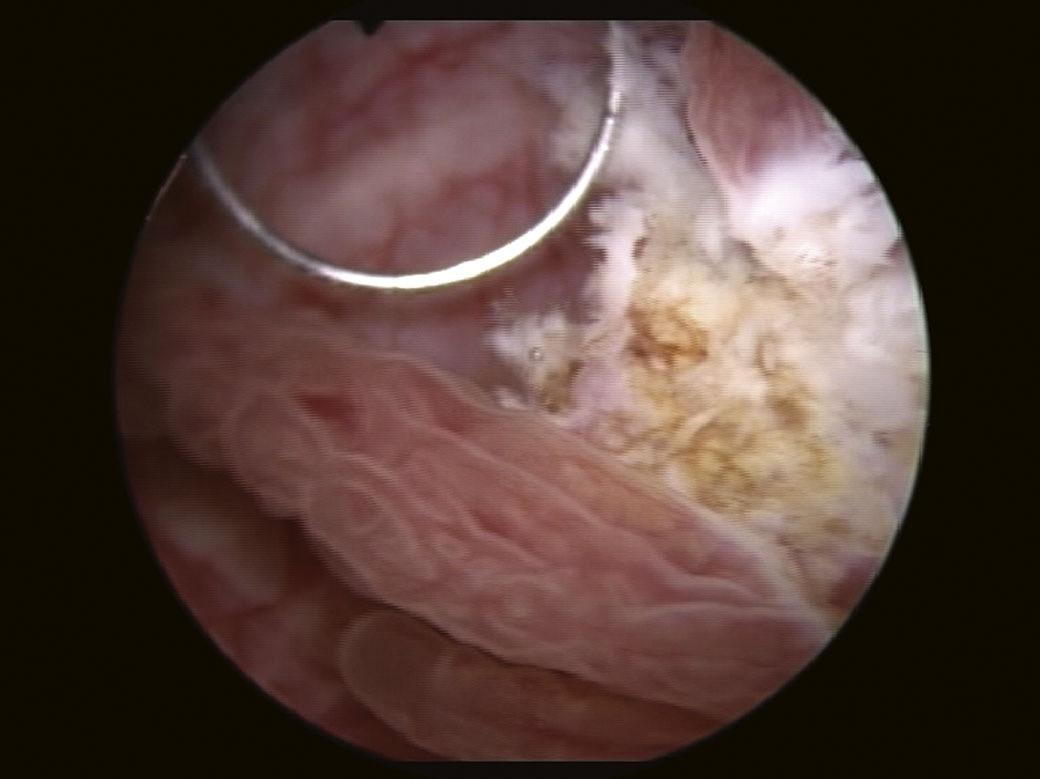
In cases of recurrent or persistent urinary tract infections, cystoscopy may identify the presence of predisposing factors such as bladder outlet obstruction, cancers, fistulas (Fig. 1.21), malformations, etc. (Boos, 2001).
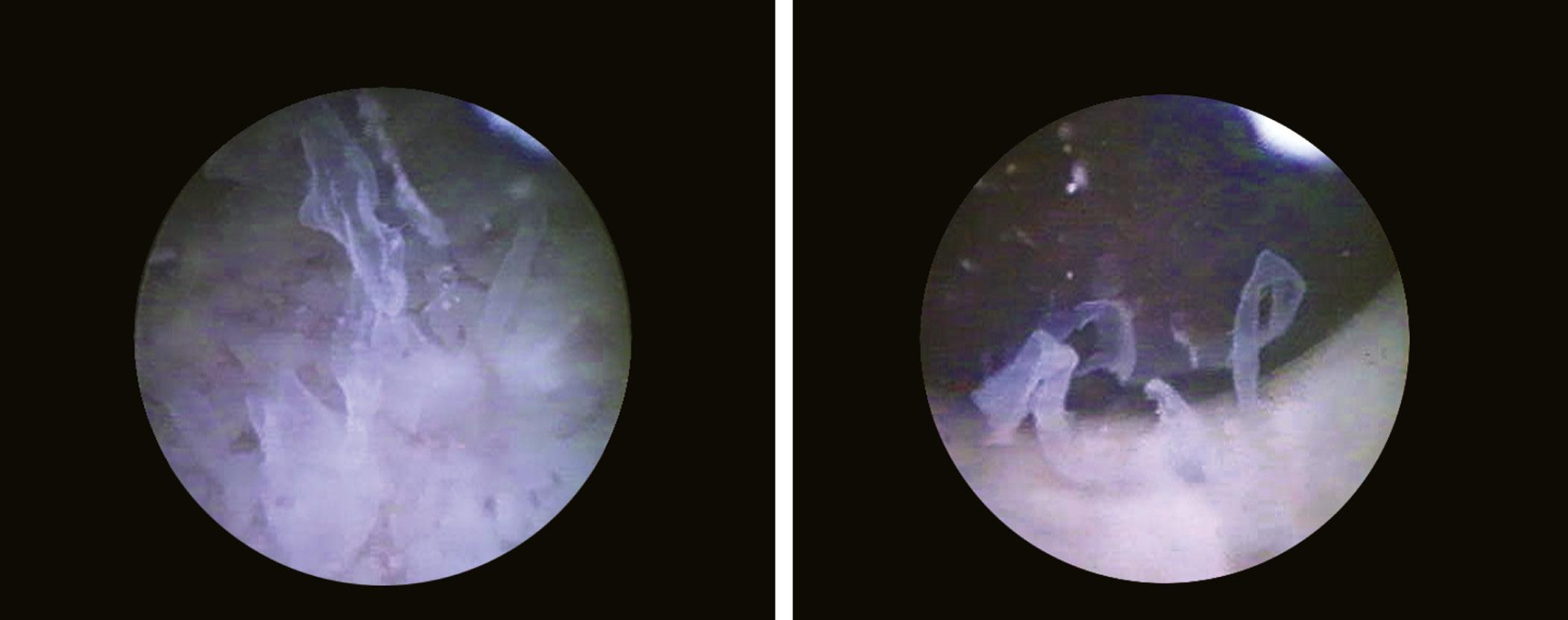
The diagnosis of interstitial cystitis is based on clinical and endoscopic criteria. Modifications specific to this condition (Fig. 1.22) are revealed during cystoscopy in over 90% of cases (Porru et al., 2003; Hanno et al., 1999).
Traumatic injuries of the lower urinary tract are the result of different types of accidents involving the pelvis, or they may be iatrogenic. Both the bladder and the ureters may be injured during abdominal or pelvic surgical procedures. Statistically, gynecological interventions are responsible for over 75% of these injuries. Urethrocystoscopy has an important role in the diagnosis and minimally invasive treatment of these conditions (Fig. 1.23) (Hurt, 1999).
Cystoscopy may be performed as a diagnostic or a therapeutic procedure.
FIGURE 1.16 Solid, infiltrative bladder tumors.
FIGURE 1.17 Bladder tumor biopsy using forceps.
FIGURE 1.18 Tumor biopsy that includes the muscle layer.
FIGURE 1.19 Pseudotumoral edema of the bladder mucosa.
FIGURE 1.20 Insertion of an autostatic ureteral catheter under cystoscopic control.
FIGURE 1.22 Characteristic aspect of interstitial cystitis (Hunner’s ulcer).
FIGURE 1.23 Transfixiant intravesical thread extracted during cystoscopy.
FIGURE 1.21 Vesico-vaginal fistula observed at cystoscopy.
1.4.1 Diagnostic Indications
The diagnostic indications of cystoscopy include (Leyh and Paul, 2005):
• to establish the etiology of macroscopic or microscopic hematuria
• the diagnosis and follow-up of bladder tumors (primary, metastases, or by local invasion) (Fig. 1.24)
• the assessment of patients with recurrent or persistent urinary infections, interstitial cystitis
• to establish the etiology and therapeutic protocol in patients with urinary incontinence, neurogenic bladder, etc.
• the diagnosis of stones (Fig. 1.25) and intravesical foreign bodies
• the assessment of patients with urinary fistulas (vesicovaginal fistula, vesicoenteral fistula, etc.)
• the diagnosis of ureteral orifice conditions (vesicoureteral reflux, ureterocele, etc.)
• the assessment of patients with lower urinary tract symptoms, with etiology that could not be determined by other diagnostic methods
• endoscopic access to the upper urinary tract
• the following results of surgical interventions performed on the lower urinary tract
FIGURE 1.24 Invasion of the bladder trigone by extension of a cervical cancer.
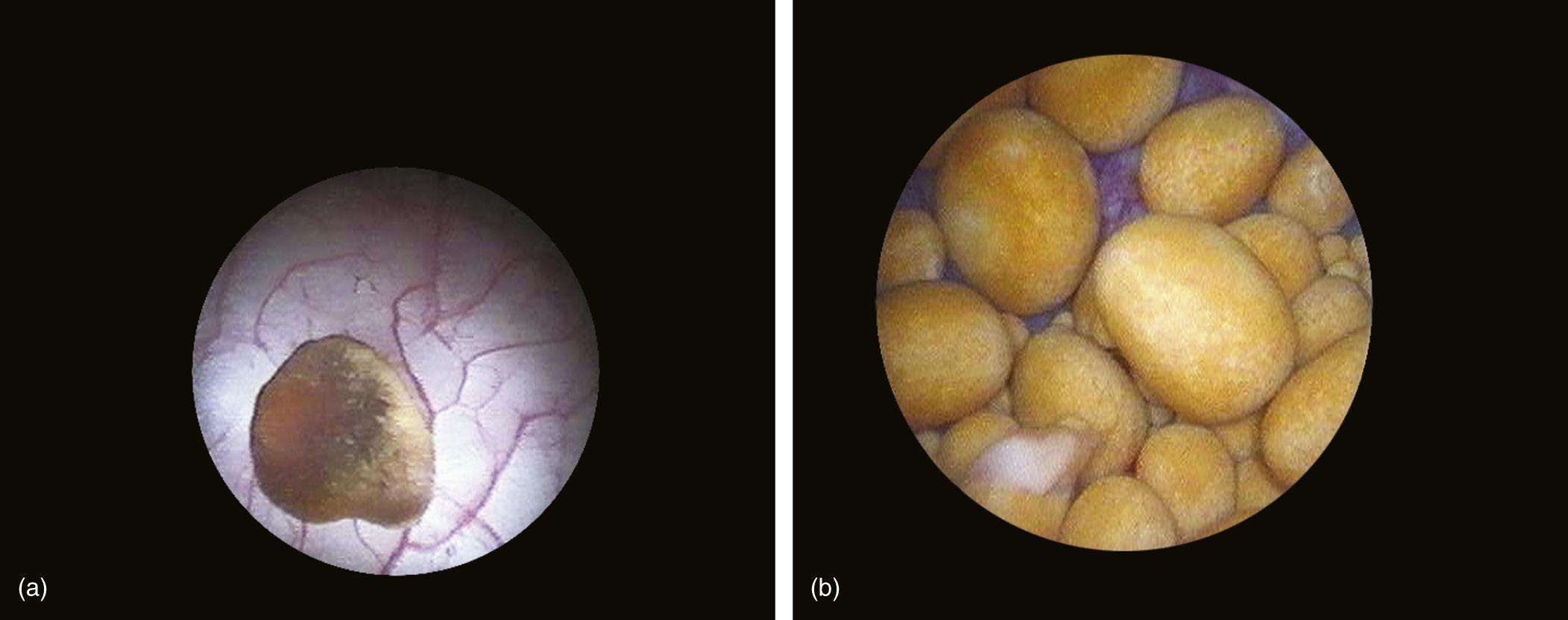
FIGURE 1.25 Solitary (a) and multiple (b) bladder stones.
1.4.2
Therapeutic Indications
The therapeutic indications of cystoscopy include (Mantorani et al., 1993):
• extraction of intravesical foreign bodies (Fig. 1.26)
• treatment of bladder stones (litholapaxy)
• insertion or extraction of ureteral catheters
• bladder biopsy
• intravesical injection of therapeutic agents (anti-incontinence, overactive bladder)
1.5 CONTRAINDICATIONS
The contraindications for the endoscopic evaluation of the lower urinary tract are represented by:
• acute urethritis
• acute prostatitis
• acute epididymitis
• fever caused by urinary tract infections
• severe coagulopathies
• urethral strictures
• urethral or bladder ruptures (abdominal or pelvic trauma) (Carter, 1992)
1.6 TECHNIQUE OF CYSTOSCOPY
1.6.1 Instruments
The endoscopic assessment of the urinary bladder is performed using cystoscopes. Endoscopic equipment is measured according to the external diameter, expressed in French units (1 F = 0.33 mm) (Carter and Chan, 2007).
Currently used cystoscopes are of two types:
• rigid
• flexible
FIGURE 1.26 The endoscopic extraction of intravesical foreign bodies.
1.6.1.1 Rigid Cystoscopes
Rigid cystoscopes (Fig. 1.27) were the first endoscopic instruments used in urological practice, and for a long time, the endoscopic exploration and treatment of lower urinary tract disorders was based exclusively on this type of instruments. The diameter of rigid cystoscopes varies between 6 F and 27 F; the most commonly used in adults having a diameter ranging between 15 F and 25 F (Akornor et al., 2005).
The size of the cystoscope is chosen according to the indication of the endoscopic procedure, the general rule being to use the instrument with the smallest diameter that is suitable for the intervention that needs to be performed (Manyak and Scherr, 1996).
Existing rigid cystoscopes, regardless of size, are composed of three main parts (Fig. 1.28):
• external sheath with obturator
• intermediate piece (bridge)
• telescope (optical system)
The external sheath has the role of protecting the telescope and at the same time, of introducing the telescope and the irrigation fluid into the bladder. The diameter of the sheaths varies between 6 F and 27 F; the ones with 21–22 F diameters being the most frequently used for standard diagnostic cystoscopy in adults. When a therapeutic maneuver is expected, 24–27 F sheaths are preferred because they allow for an easy insertion of various instruments through the working channel (Carter and Chan, 2007). The sheath is fitted at its proximal end with two connectors (ports), flow and return, for the introduction and the evacuation of the irrigation fluid, respectively.
The obturator is connected to the sheath and facilitates the atraumatic urethral passage of the sheath when choosing to introduce it in a “blind” manner, without the help of a video camera.
The intermediate piece serves to connect the sheath to the telescope and is fitted with one or two ports that enable the introduction of various instruments into the bladder (Cundiff, 2001). Thus, depending on the diameter of the sheath’s working channel, several types of instruments may be used: biopsy forceps, guidewires, laser fibers, lithotripsy probes, injection needles, etc. There are variants of cystoscopes in which the sheath and the telescope are connected directly, without the intermediate piece. The Albarran sheath is an intermediate piece variant that is fitted with a distal deflecting system, which facilitates the handling of instruments that are introduced through the working channel in areas with difficult access. The use of the Albarran sheath is avoided by some urologists because of the traumatic risk (mucosal lesions or perforations).
The telescope is designed to transmit the light inside the bladder, and at the same time, to transmit the image to the examiner. Modern rigid cystoscopes are based on the lens system created by Harold Hopkins, and have various viewing angles that enable a full examination of the bladder (Cundiff, 2001). Thus, cystoscopes with 0° optical angle (forward view) are used particularly for urethroscopy; whereas, those with 12–30° angles (forward and oblique view) are used especially for the examination of the base, posterior wall, and sidewalls of the bladder; and those with 70° (side view) enable the examiner to view the anterolateral walls and the bladder dome. Cystoscopes with 120° angles (retrograde view) are used to inspect the anterior area of the bladder neck (Carter and Chan, 2007). Telescopes with different viewing angles are fitted with a mark at the periphery of the visual field, with the role of facilitating
FIGURE 1.27 Rigid urethrocystoscope.
FIGURE 1.28 Components of the rigid cystoscope.
orientation during cystoscopy. This marker is usually located directly opposite to the viewing angle. In common practice, the 30° telescope is the most widely used, allowing both diagnostic cystoscopy as well as handling of various intravesical instruments, while the 70° telescope is preferred for a complete bladder inspection.
The advantages of rigid cystoscopes include (Leyh and Paul, 2005):
• large visual field, superior telescopes
• wide working channel for auxiliary instruments
• irrigation channel with a large caliber and hence, better visualization
• the possibility to evacuate blood clots or other bladder debris
• easy handling and orientation during the procedure
1.6.1.2
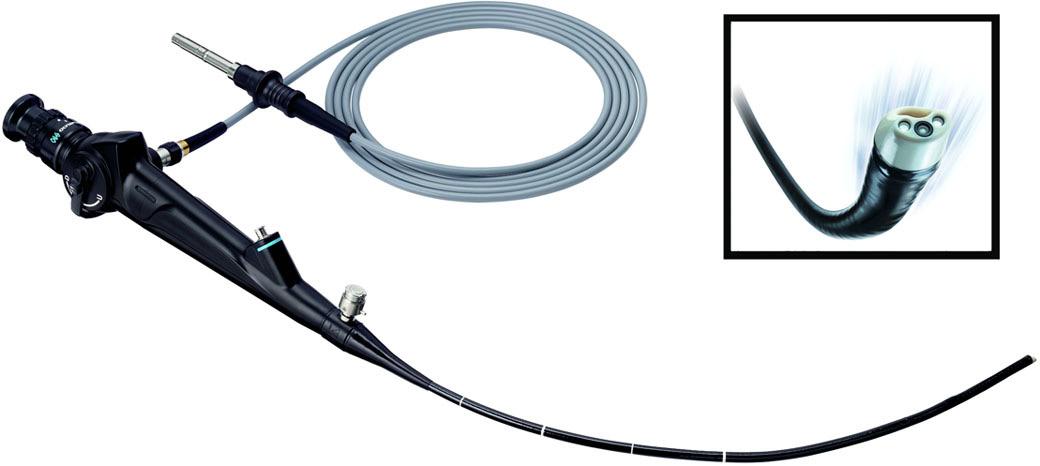
Flexible Cystoscopes
Flexible cystoscopes (Fig. 1.29), unlike the rigid ones, combine both the optical system and the working/irrigation channel into a single unit. The optical system is composed of a bundle of optical fibers that are responsible for the transmission of images and two similar fascicles responsible for transmitting light. The optical fibers are isolated, parallel, can transmit light (the image) even if bent, and are connected to a lens system that serves to magnify and focus the image. Because of the way the optical fibers are arranged, a small space remains between them, causing the granular, “honeycomb” aspect of the image (Cundiff, 2001). However, this drawback has been corrected in the last-generation cystoscopes. Similar to rigid cystoscopes, the optical system features a mark in the visual field (often located at 12 o’clock) that is meant to facilitate orientation during cystoscopy.
The properties of the optical fibers and their parallel layout have enabled to develop a distal deflecting mechanism that is controlled by a button located near the viewfinder. The degree of distal deflection in a single plane varies between 220° and 350°, while the maximum deflection in a single direction ranges from 180° to 220° ( Table 1.1 ).
The distal diameter of flexible cystoscopes ranges between 14 F and 16.2 F, and the usable length between 37 cm and 40 cm, which represents approximately half of the instrument’s total length (Akornor et al., 2005).
In the case of flexible cystoscopes, the working channel is designed to allow the passage of various instruments and also to ensure the flow of irrigation fluid. The irrigation flow is about four times lower in flexible cystoscopes compared to rigid ones with the same diameter. The use of various instruments through the working channel leads to a reduction in the degree of deflection and also a significant reduction in the irrigation flow. Under these circumstances, maintaining a sufficient flow for optimal viewing requires either increasing the irrigation pressure or using small caliber accessory instruments.
FIGURE 1.29 Flexible urethrocystoscope.
TABLE 1.1 technical Characteristics of different models of Conventional flexible Cystoscopes
Instruments, which can be used through the working channel of flexible cystoscopes include biopsy forceps, basket catheters, guidewires, flexible lithotripsy catheters, laser fibers, flexible needles, etc. Knowing the position of the working channel in relation to the visual field is important for a proper handling of these instruments (Akornor et al., 2005).
The advantages of flexible cystoscopes are as follows(Leyh and Paul, 2005):
• increased patient compliance, requiring only local anesthesia
• the complete and detailed evaluation of the bladder with a single instrument
• represents an elective indication for endoscopy of urinary diversions (neobladder) (Fig. 1.30)
• they have a particular indication in patients with joint disorders or neurological conditions, who cannot be placed in the classic lithotomy position for rigid cystoscopy
• similarly, flexible cystoscopy is indicated in patients with semirigid penile prosthesis or “frozen pelvis”
Connecting the video camera to the cystoscope’s telescope allows viewing the exploration on a TV monitor (videocystoscopy). This modality of performing cystoscopy has been adopted virtually by all urologists. Video-cystoscopy has multiple advantages (Jones, 2006; Leyh and Paul, 2005):
• increased comfort for the physician
• binocular vision
• reduced infectious contamination (the physician avoiding contact with the patient’s urine)
• documentation of procedure results by recording the video images and, implicitly, monitoring bladder changes over time (follow-up)
• useful in the training of resident doctors
• patient education
The video-cystoscopy unit (Fig. 1.31) consists of a cystoscope, a video camera, a monitor, a light source, and an image recording/storage device (videorecorder, DVD recorder, etc.).
1.6.2 Distending Medium
The distension of urethra and bladder during urethrocystoscopy is an important element for an optimal examination. The distending medium used can be an electrically conductive liquid (saline 0.9% or 0.45%, lactated Ringer’s solution), a nonconductive fluid (sterile water, 3% sorbitol, 5% mannitol, 1.5% glycine), or gas (carbon dioxide). Carbon dioxide was used in the 1970s and has the advantage that if bleeding occurs during a procedure, the blood drops ooze down the bladder wall and are not dispersed in the distending medium, which ensures enhanced visibility. Currently, liquid distending media are used almost exclusively. The choice of the irrigation fluid depends on its risk of being absorbed during the procedure. For diagnostic cystoscopy, any irrigation fluid may be used (sterile water or saline are most frequently used). Saline solution has a reduced irritative effect on the bladder mucosa and is preferred when the absorption of irrigation fluid is anticipated.
FIGURE 1.30 Nonabsorbed suture thread inside a neobladder, observed during flexible cystoscopy.
Systemic absorption of water can lead to electrolyte imbalances and may cause intravascular hemolysis. Nonconductive liquids such as sterile water or glycine are used when maneuvers involving the use of the monopolar electrocautery are expected (Bagley et al., 1985).
The most commonly used distending fluid instillation system consists of 1- or 2-L bags, suspended 60–80 cm above the patient’s level and connected to the irrigation port of the cystoscope. In this manner, fluid is instilled by gravity. The temperature of the bladder distending fluid should range between room temperature and 37°C (Kok and Dwyer, 2007).
1.6.3 Preliminary Measures
In patients with symptoms or a recent history of urinary tract infection, urine cultures should be performed prior to cystoscopy in order to document urinary tract sterility. Endoscopic exploration in patients with untreated urinary infections can cause exacerbation of their symptoms or even sepsis. Cystoscopy is considered a “clean” procedure that does not require routine prophylactic antibiotherapy; studies showing an incidence of symptomatic urinary infections after the procedure of approximately 5%, while the rate of asymptomatic bacteriuria is 10–35%. Therefore, as a general rule, standard diagnostic cystoscopy does not require prophylactic antibiotics in patients with sterile urine cultures.
A high-risk category is represented by immunosuppressed patients, those with prosthetic heart valves or a history of urosepsis. In these situations, broad-spectrum antibiotics are necessary perioperatively.
Prophylactic antibiotherapy is administered 24 h before surgery in high-risk patients.
1.6.4
Anesthesia
The type of anesthesia is chosen according to several considerations:
• the purpose of the endoscopic exploration (diagnostic or therapeutic)
• the type of cystoscope to be used (rigid or flexible)
• patient gender
FIGURE 1.31 Video-cystoscopy unit.
In current urological practice, rigid or flexible standard diagnostic cystoscopy is performed under local anesthesia that is well tolerated both by women and most of the men.
In male patients, due to the length and anatomy of the urethra, as well as to the increased incidence of subvesical pathology, rigid cystoscopy requires, in some cases, a superior form of anesthesia (intravenous (i.v.) sedation, regional anesthesia).
Regional anesthesia or i.v. sedation is also required in situations where the endoscopic exploration is expected to be difficult, lengthy, or when transurethral therapeutic procedures are necessary. There are also particular cases in which cystoscopy is performed under general anesthesia.
1.6.5 Rigid Cystoscopy Technique
The patient, with an empty bladder, is placed in the lithotomy position (rigid cystoscopy) or in a supine position (flexible cystoscopy). The external genital organs are carefully disinfected and the operative field is delimited.
The necessary instruments are prepared and checked. The cystoscope is selected depending on the purpose of the exploration. In case of diagnostic cystoscopy, small caliber endoscopes are used, while when it is necessary to use various auxiliary instruments, cystoscopes with a higher caliber and larger working channel are preferred.
Sterile water and saline are the most frequently used irrigation fluids.
The cystoscopy begins with the assembly of the endoscope and checking and connecting the light source and the video camera. When necessary, the color (white balance) and image resolution are adjusted. The cystoscope is thoroughly lubricated with anesthetic gel. The urethral meatus is examined before inserting the cystoscope. If a urethral meatal stenosis is observed, it is either dilated or meatotomy is performed using Ottis urethrotome, or a smaller caliber cystoscope is used (Manyak and Scherr, 1996).
In male patients, the cystoscope is initially inserted into the anterior urethra (the navicular fossa), the left hand of the surgeon holding the penis stretched vertically (at a right angle to the abdominal wall). The urethral passage of the cystoscope can be performed in a “blind” manner with the obturator fixed to the sheath, or “under visual control” using the video camera to view the images on the monitor. In the first case, the cystoscope advances without difficulty in a vertical position, up to the bulbar urethra. Passing the bulbar urethra and the sphincter area is achieved by combining an advancing motion with the progressive horizontal tilting of the cystoscope (Carter and Chan, 2007). The presence of a bulky prostate adenoma with a median lobe or bladder neck sclerosis may require additional tilting of the cystoscope, below the horizontal plane, to allow passage into the urinary bladder. If it is too difficult to pass these obstacles, the cystoscope will be inserted under visual control, forcing the urethral passage or brutal maneuvers being contraindicated because of the increased risk of injuries.
In male patients, most urologists prefer to insert the rigid cystoscope under direct visualization on the monitor. The telescope, with the light source and video camera attached, is connected to the sheath, and the irrigation fluid circuit is opened. The cystoscope is inserted with ease by following the urethral lumen, allowing a simultaneous examination of the urethra. Thus, changes to the urethral caliber (strictures, diverticula) or mucosa lesions (inflammatory, tumoral) can be assessed. The external sphincter is observed in the membranous urethra and appears as a circular narrowing of the lumen with mucosal folds arranged in a radial manner. Passing this area requires the physician to apply a slight pressure on the cystoscope. After the sphincter area is passed, the verumontanum is visualized, the length of the prostatic urethra is inspected and assessed, together with the dimensions of the lateral lobes. The bladder is entered by slightly ascending the tip of the cystoscope to pass the bladder neck (Fig. 1.32) (Carter and Chan, 2007).
In women, due to the short urethra, it is preferred to insert the cystoscope with the obturator fixed to the sheath, the maneuver being easier to perform and less traumatic compared with male patients. The visualization of the female urethra is performed in an anterograde manner, when the cystoscope is withdrawn from the bladder. Although in most anatomical texts, the female urethra is described as having a straight trajectory without folds; in many cases, a slight superiorly oriented curvature may be observed, located before the bladder neck. For this reason, the insertion of the cystoscope is easier and less traumatic if the tip is oriented slightly oblique, superiorly (Fig. 1.33) (Jones, 2006).
Once the cystoscope is inserted into the bladder, the obturator is detached (in case of a “blind” insertion) and the telescope is attached. Prior to the actual cystoscopy, urine samples may be taken for bacteriological or cytological examinations, after which the bladder is distended with approximately 200–300 mL of irrigation fluid or until the patient experiences the sensation of micturition. After this volume is reached, the irrigation system can be closed, except for cases that require improved visualization by periodical maneuvers of partial evacuation and refilling of the bladder (hematuria, pyuria, etc.).