Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Encyclopedia of Biomedical Engineering (vol. 1-3) Min Wang
https://ebookmass.com/product/encyclopedia-of-biomedical-engineeringvol-1-3-min-wang/
ebookmass.com
Encyclopedia of Microbiology, vol.1 4th Edition Edition Thomas M. Schmidt
https://ebookmass.com/product/encyclopedia-of-microbiology-vol-1-4thedition-edition-thomas-m-schmidt/
ebookmass.com
Encyclopedia of Materials: Composites: Composites. Volume 1 1st Edition Dermot Brabazon (Editor)
https://ebookmass.com/product/encyclopedia-of-materials-compositescomposites-volume-1-1st-edition-dermot-brabazon-editor/
ebookmass.com
Netter’s Obstetrics and Gynecology-Elsevier (2017) 3rd Edition Roger Smith
https://ebookmass.com/product/netters-obstetrics-and-gynecologyelsevier-2017-3rd-edition-roger-smith/
ebookmass.com
INTERFACIALCHEMISTRY SURFACESCIENCEANDELECTROCHEMISTRY Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UK 50HampshireStreet,5thFloor,Cambridge,MA02139,USA
Copyright 2018ElsevierInc.Allrightsreserved
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuchasthe CopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybenoted herein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding,changesin researchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmayalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation,methods, compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthe safetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjuryand/or damagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN978-0-12-809739-7
Forinformationonallpublicationsvisitourwebsite at http://store.elsevier.com
Publisher:OliverWalter
AcquisitionEditor:RachelGerlis
ContentProjectManager:FionaPattison Designer:MatthewLimbert
PrintedandboundintheUnitedStates
PREFACE ThemotivationforcompilingandpublishingthisencyclopediaonInterfacialChemistrywastopromotethe communicationbetweenchemists,electrochemists,physicochemists,aswellassolid-stateandsurfacephysicists.Thegrowingdiversi ficationandspecializationofscienceandresearchmakesmutualunderstandingmore andmoredifficult,andthereforeinterdisciplinarycommunicationimperative.Chemiststakegreatadvantageof heterogeneouscatalystsoftenwithoutknowingtheirpropertiesandoperationontheatomicscale.This knowledge,however,isnecessaryforarationaldesignandoptimizationofthecatalysts’ activity,selectivity,and stability.Electrochemistsandsurfacephysicistsworkingonasimilarproblemsuchas filmdepositionand growth,justindifferentenvironment,maynotshareknowledgeduetodistinctlydifferent “languages,” suchas “underpotentialdeposition” and “Franck-van-der-Merwegrowth” forasimilarphenomenon.Andthereare knowndifferencesbetweenthemore “practical” approachofchemistsandthemore “formal” approachof physicists,includingtheresultantcommunication “barriers”.Ontheotherhand,allmodernexperimental analyticaltoolsarebasedonphysicalphenomena,suchasinteractionofradiationwithmatterandelectron tunneling,andareimplementedbyphysicists.Interfacialchemistry,asanexcellentexampleofinterdisciplinary research,canonlyprofitfromanunbiasedcommunicationandmutualunderstandingbetweenthedifferent involveddisciplines.
Interfacesarethedividesbetweenphases,e.g.,solid/gas,solid/liquid,solid/solid,liquid/gas,liquid/liquid, andthusaninherentpropertyofheterogeneoussystems. “Heterogeneous” sounds,andactuallyis,more complexthan “homogeneous” andthereforecallsforamultidisciplinaryapproach.Toinvestigateand understand “electrocatalysis,” itiscertainlyhelpfultotakeintoaccounttheexperienceandknowledgeof electrochemists and solid-stateandsurfacephysicists,twocommunitiesthatarenotknownforaclose connection.
Thedifferenceinpropertiesoneithersideofthedividehastwoimportantconsequences:(1)interfacesare thelocationsofgradientsthatareadrivingforceforprocessesand(2)interactionsacrosstheinterfacewill obviouslyalsoalterthepropertiesininterface-nearlayersoftheadjacentphases.Ifasolidironsurfaceis exposedtooxygengas,thesurfacewilloxidize;theresultantoxidelayerdiffersfromthepureironunderneath.If twodifferentsolids(orliquids)formacommonphaseboundary,interdiffusionwillchangethecompositionof theinterface-nearregionsonbothsides.Ifaplatinumelectrodeinsulfuricacidsolutionisnegativelypolarized, protons(hydroniumcations)areattractedwiththeconsequencethatthepHnearthePt/electrolyteinterfaceis lowerthaninthebulkofthesolution.Andifacopperelectrodeinhydrochloricacidsolutionispositively polarized,chlorideanionswillbeattracted,adsorb,andrestructurethesurface before surfaceatomsformsoluble copperchloridespecies.
Interestingly,notonlythepresenceofinteractionsacrossaninterfacecausesalterationsoneitherside,but alsothesudden absence ofinteractionsacrossaninterfacehasadecisiveinfluence:Whileanatominthebulkof asolidinequilibriumissurroundedbyandinteractswithatomsonallsides,anatomattheverysurfacein vacuumhasnoneighborstointeractwithonthevacuumside.Thisunbalancedrivesthesystemtowardanew equilibriumandcausesarepositioningofthesurfaceatoms;mostintuitivelythesurfaceatoms “relax” toward thebulkofthesolid.Butthereareevencaseswherethesurfaceatomlayerasawholeassumesatwodimensionallatticestructuredifferentfromthatofaparallelplaneinthebulk,thesurface “reconstructs.” Duetothemissingneighbors,the “unsaturatedbonds” of surface atoms(incontactwithvacuum)addtothe totalenergyofthesolid.Thesurface “relaxation” and “reconstruction” are aresponseofthesystemtolowerthis “excesssurfaceenergy.” Theverysameargumentexplainswhyinequilibriumalsothe surfacecomposition of multicomponentmaterials,e.g.,alloys,solutions,mustbeexpectedtobedifferentfromthebulkcomposition.
Thispageintentionallyleftblank
J-CDong
XiamenUniversity,Xiamen,China
KDuanmu
ChemicalandBiomolecularEngineeringDepartment, UniversityofCalifornia,LosAngeles,CA,UnitedStates
AKEngstfeld
TechnicalUniversityofDenmark,Lyngby,Denmark
AErbe
NorwegianUniversityofScienceandTechnology, Trondheim,Norway
BEren
WeizmannInstituteofScience,Rehovot, Israel
SFearn
ImperialCollegeLondon,London,UnitedKingdom
JMFeliu InstitutodeElectroquímica,UniversidaddeAlicante, Alicante,Spain
SFerrer
AlbaSynchrotronLightSource,Barcelona,Spain
MFilez
UtrechtUniversity,Utrecht,TheNetherlands
AFoelske-Schmitz
ViennaUniversityofTechnology,Vienna, Austria
H-JFreund
FritzHaberInstituteoftheMaxPlanckSociety,Berlin, Germany
TFukuma
KanazawaUniversity,Kanazawa,Japan
KFushimi
HokkaidoUniversity,Sapporo,Japan
HHGirault
ÉcolePolytechniqueFédéraledeLausanne(EPFLValais Wallis),Sion,Switzerland
MJGladys UniversityofNewcastle,Callaghan,NSW,Australia
CGoletti
PhysicsDepartment,UniversityofRomeTorVergata, Roma,Italy
IMNGroot
LeidenUniversity,Leiden,TheNetherlands
H-LHan
LawrenceBerkeleyNationalLaboratory,Berkeley,CA, UnitedStates
ARHead
LawrenceBerkeleyNationalLaboratory,Berkeley,CA, UnitedStates
YHorowitz
LawrenceBerkeleyNationalLaboratory,Berkeley,CA, UnitedStates;andUniversityofCalifornia,Berkeley, CA,UnitedStates
KHubkowska
UniversityofWarsaw,Warsaw,Poland
TJones
Fritz-Haber-InstitutderMax-Planck-Gesellschaft, Berlin,Germany
MJurczyszyn UniversityofWrocł aw,Wrocł aw,Poland
MMKappes
KarlsruheInstituteofTechnology(KIT),Karlsruhe, Germany
MKeddam
SorbonneUniversités,Paris,France
AKlyushin
Fritz-Haber-InstitutderMax-Planck-Gesellschaft, Berlin,Germany;andHelmholtz-ZentrumBerlin fürMaterialienundEnergieGmbH,Berlin, Germany
AKnop-Gericke
Fritz-Haber-InstitutderMax-Planck-Gesellschaft, Berlin,Germany
JKnudsen
MAXIVLaboratory&LundUniversity,Lund,Sweden
FLaMantia
UniversitätBremen,Bremen,Germany
ALasia
UniversitédeSherbrooke,Sherbrooke,QC,Canada
ALesch
ÉcolePolytechniqueFédéraledeLausanne(EPFLValais Wallis),Sion,Switzerland
J-FLi
XiamenUniversity,Xiamen,China
T-ELin
ÉcolePolytechniqueFédéraledeLausanne(EPFLValais Wallis),Sion,Switzerland
M q ukaszewski UniversityofWarsaw,Warsaw,Poland
IMatsuda
TheInstituteforSolidStatePhysics,TheUniversityof Tokyo,Chiba,Japan
CONTENTSOFVOLUME1 VOLUME1.1:EXPERIMENTALMETHODS AReviewonInSituSumFrequencyGenerationVibrationalSpectroscopyStudies ofLiquid SolidInterfacesinElectrochemicalSystems1 H-LHan,YHorowitz,andGASomorjai
AmbientPressureX-RayPhotoelectronSpectroscopy13 ARHeadandHBluhm
Angle-ResolvedPhotoelectronSpectroscopyatSurfacesWithHigh-OrderHarmonicGeneration28 C-TChiang
AtomicScaleSTMImagingofAlloySurfacesWithChemicalResolution39 AKEngstfeld
CyclicVoltammetry
48 VClimentandJMFeliu
DifferentialCapacitanceMeasurementsonPassiveFilms75 FDiQuarto,FDiFranco,MSantamaria,andFLaMantia
EISTechniqueinPassivityStudies:DeterminationoftheDielectricPropertiesofPassiveFilms93 BTribollet,VVivier,andMEOrazem
ElectrochemicalScanningTunnelingMicroscopy108 MNowickiandKWandelt
ElectronParamagneticResonanceSpectroscopyatSurfaces129 PMClawin,NFRichter,WRiedel,HRonneburg,andTRisse
EllipsometryinPassiveFilms 143 TOhtsukaandKFushimi
ExperimentalMethodsinInterfacialandSurfaceChemistry157 KWandelt
FieldIonandFieldDesorptionMicroscopy:SurfaceChemistryApplications162 YSuchorski
High-SpeedElectrochemicalSTM180 MJRost
HowtoProbeStructure,Kinetics,andDynamicsatComplexInterfacesInSituand OperandobyOpticalSpectroscopy199 AErbe,SNayak,Y-HChen,FNiu,MPander,STecklenburg,andCToparli
ImagingChemicalReactionsOneMoleculeataTime220 ZNovotny,ZZhang,andZDohnálek
ImpedanceSpectroscopyAppliedtotheStudyofElectrocatalyticProcesses241 ALasia
InSituPhotoelectronSpectroscopy264 ABraun
InSituProbingofAdsorbatesatElectrochemicalInterfacesWithVibrational SumFrequencySpectroscopy 280 YTongandRKCampen
InSituReal-TimeLow-EnergyElectronMicroscopy287 MBCasu
IonConductanceProbeMicroscopy MolecularResolution295 YZhou,TFukuma,andYTakahashi
Micro-SpectroscopytoInterrogateSolidCatalystsatWork304 MFilez,ZRistanovic,andBMWeckhuysen
OnlineChromatographicDetection321 AVRudnev
On-LineInductivelyCoupledPlasmaSpectrometryinElectrochemistry:BasicPrinciples andApplications 326 SCherevkoandKJJMayrhofer
OperandoScanningProbeMicroscopy,SurfaceX-RayDiffraction,andOptical MicroscopyforCatalysisStudies336 IMNGroot
PerspectivesofAdvancedIonBeamAnalysisofElectrochemicallyActiveSurfaces354 SFearnandERuiz-Trejo
PhotocurrentSpectroscopyinPassivityStudies361 FDiQuarto,FDiFranco,AZaffora,andMSantamaria
PhotoelectronDiffraction 372 DPWoodruff
PhotoemissionTomography:ValenceBandPhotoemissionasaQuantitativeMethod forInvestigatingMolecularFilms380 PPuschnigandMGRamsey
PorousElectrodesinBioelectrochemistry392 TVidakovic-KochandKSundmacher
QuartzCrystalNanobalanceMeasurementsinElectrocatalysis402 KHubkowska,M Łukaszewski,andACzerwinski
ReflectanceAnisotropySpectroscopy413 CGoletti
RotatingDiskandRing DiskElectrodes421 SVesztergom
ScanningElectrochemicalMicroscopyforBioimaging445 T-ELin,ABondarenko,ALesch,andHHGirault
ScanningElectrochemicalMicroscopyintheAC-Mode453 MKeddam,CMSánchez-Sánchez,andVVivier
PERMISSIONACKNOWLEDGMENTS ThefollowingmaterialisreproducedwithkindpermissionofAmericanAssociationfortheAdvancementof Science
Figure12CatalysisforEnergyConversion
Figure3(left)BiophotovoltaicSystems
Figure1bConductanceandLightEmissionFromOn-surfaceSynthesizedMolecularWires
Figure2bConductanceandLightEmissionFromOn-surfaceSynthesizedMolecularWires
Figure12Silicene
Figure7AtomicScaleSTMImagingofAlloySurfacesWithChemicalResolution http://www.aaas.org/
ThefollowingmaterialisreproducedwithkindpermissionofNaturePublishingGroup
Figure8SubstrateMediatedInteractions
Figure3(middle)BiophotovoltaicSystems
Figure4BiophotovoltaicSystems
Figure12A-CBiophotovoltaicSystems
Figure14G-HBiophotovoltaicSystems
Figure3Lanthanide-Based2DCoordinationNetworks
Figure2TemperatureControlofReactionPathways:Intramolecularvs.Intermolecular
Figure3TemperatureControlofReactionPathways:Intramolecularvs.Intermolecular
Figure5TemperatureControlofReactionPathways:Intramolecularvs.Intermolecular
Figure6TemperatureControlofReactionPathways:Intramolecularvs.Intermolecular
Figure1aConductanceandLightEmissionFromOn-surfaceSynthesizedMolecularWires
Figure1dConductanceandLightEmissionFromOn-surfaceSynthesizedMolecularWires
Figure2dConductanceandLightEmissionFromOn-surfaceSynthesizedMolecularWires
Figure1On-surfaceSynthesisofGrapheneNanoribbons
Figure2bOn-surfaceSynthesisofGrapheneNanoribbons
Figure2cOn-surfaceSynthesisofGrapheneNanoribbons
Figure2dOn-surfaceSynthesisofGrapheneNanoribbons
Figure3aOn-surfaceSynthesisofGrapheneNanoribbons
Figure3bOn-surfaceSynthesisofGrapheneNanoribbons
Figure3cOn-surfaceSynthesisofGrapheneNanoribbons
Figure3dOn-surfaceSynthesisofGrapheneNanoribbons
Figure6aOn-surfaceSynthesisofGrapheneNanoribbons
Figure8aOn-surfaceSynthesisofGrapheneNanoribbons
Figure8bOn-surfaceSynthesisofGrapheneNanoribbons
Figure8dOn-surfaceSynthesisofGrapheneNanoribbons
Figure8eOn-surfaceSynthesisofGrapheneNanoribbons
Figure9bOn-surfaceSynthesisofGrapheneNanoribbons
Figure9cOn-surfaceSynthesisofGrapheneNanoribbons
Figure9dOn-surfaceSynthesisofGrapheneNanoribbons
Figure11aOn-surfaceSynthesisofGrapheneNanoribbons
Figure11bOn-surfaceSynthesisofGrapheneNanoribbons
Figure11cOn-surfaceSynthesisofGrapheneNanoribbons
Figure11dOn-surfaceSynthesisofGrapheneNanoribbons
Figure11abSilicene
Figure14Silicene
Figure2OxideThinFilmsforMemristiveDevices
Figure2SteeringMolecularAssembliesandReactionsonSurface
Figure5SteeringMolecularAssembliesandReactionsonSurface
Figure10SteeringMolecularAssembliesandReactionsonSurface
Figure3UllmannCouplingofPorphyrins
Figure4UllmannCouplingofPorphyrins
http://www.nature.com
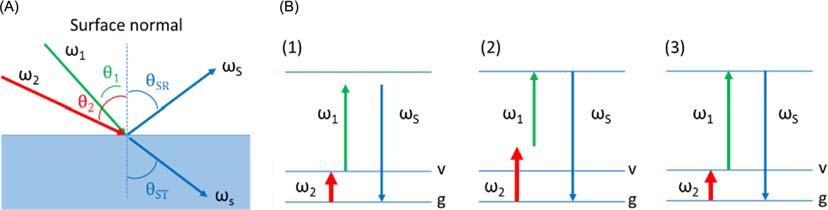
Fig.1 (A)AschematicofaSFGprocessataninterface.(B)EnergydiagramsanddescriptionofresonantSFGVSwith(1) u2 inresonancewith avibrationaltransition,(2) uS inresonancewithanelectronictransition,and(3)both u2 and uS areinresonances.
conditionsandphase-matchingrequirements, k1jj þ k2jj ¼ ksjj .Eq. (1) describestheemittedSFGdirectionbasedonthewavelength oftheinputbeamsandthegeometryofthesetup:
where n istherefractiveindexofthemediuminwhichtheSFGispresent.
Fig.1B showstheenergylevelsfortheexcitationsinaSFGprocess.IntheSFGVS, u1 istypicallyinthevisiblespectralregionand u2 isintheinfrared(IR)spectralregion.Whenevereither u1 or u2 or/and uS areinresonancewiththeelectronicorvibrational modeattheinterface,theSFGintensityincreasesseveralordersofmagnitudes.5 ByscanningtheIRfrequencyandmonitoring theSFGintensity,weobtainthevibrationalspectrumofsurfacemoleculesandthestructuralinformationfromthemolecules presentattheinterface.TheoutputintensityofthereflectedSFGisgivenbythesquareofthesumofthesecond-ordernonlinear susceptibilityandtheintensitiesoftheinputbeams,asshowninEq. (2)
denotetheFresnelfactorsfortheoutputandinputbeams.InSFGVS,inordertocharacterizethe vibrationalmodesofadsorbates,onewouldusetheexpressioninEq. (3) to fittheobservedspectrum.
where Aq ! , uq, Gq aretheamplitude,frequency,anddampingcoefficientofthe qthvibrationmode,respectively, cNR (2) isthesecondordernonlinearsusceptibilityfromthenonresonantbackground. Lu ! istheFresnelfactorat u,andtheselocal fieldfactorscanbe calculatedbasedonthephysicalandgeometricalpropertiesoftheSFGapparatusandexperiment:thebeamangles,therefractive indexofthemedium,etc.TheSFGdatahastobeprocessedsothatonecandeducethesurfacenonlinearsusceptibility, x ! 2 ðÞ s j from theSFGsignalamplitude.Usually,thisiscarriedoutbyremovingthegeometricfactorsandFresnelcoefficientsandsoweareleft withthesignal’samplitudethatinturngives x ! 2 ðÞ s j.Thenonlinearsusceptibilityholdstheinformationoftheadsorbate’ s molecularorientationandtheadsorbatelayerorderingdegree,6 ofwhichwegiveexampleslaterinthissection.
Whenstudyingelectrifiedinterfaces,theeffectfromanelectric fieldorso-calledelectricdoublelayer(EDL)ontheSFGsignal needstobeconsideredduetotheadditionalcontributionfromthesecond-orderbulknonlinearsusceptibilityinsidetheEDL. Thispotential-inducedcontributionoriginatesfromtheelectric-dipole-allowedthird-orderbulknonlinearsusceptibilityandthe fieldintheEDL.7–9 Theintensityofapotential-dependentreflectedSFGbeaminthepresenceofanEDLcanbeexpressedas7:
ISFG uVIS þ uIR ; f ðÞfI uIR ðÞI uVIS ðÞ c 2 ðÞ eff uIR ; f ðÞþ Z N 0 dzc 3 ðÞ uIR ; z; f ðÞEDC z ðÞ 2 (4)
where f ispotentialand EDC(z)isthestaticelectric fieldfromtheelectrodethatisadecayingfunctionof z.Thethird-order susceptibility, c(3)(uIR, z, f),varieswiththedistance z fromtheelectrodesurfacestartingat z ¼ 0.Ifweassumethatthe field EDC(z)isconstantinthedoublelayerandequalstozerointhebulkelectrolyte,theequationcanbeexpressedasfollows:
ISFG uVIS þ uIR ; f ðÞfI uIR ðÞI uVIS ðÞ c 2 ðÞ eff uIR ; f ðÞ 2 þ 2Re c 2 ðÞ eff uIR ; f ðÞc 3 ðÞ DL uIR ; f ðÞeid hi þ c 3 ðÞ DL uIR ; f ðÞ 2 ðÞ f2 (5) where cDL (3)(uIR, f)isthethird-orderpotential-dependentsusceptibilityofthedoublelayer.Thethird-ordersusceptibilityinthe doublelayeristhesumofthethird-orderhyperpolarizabilitiesofallthemoleculesinthedoublelayer.10 d isaphasefactorthatis
where fq isaphaseanglewithrespecttothenonresonantsignal cNR 2 .Whileobtaining[c2]2 iswellestablishedandisbasedon astraightforwarddesignoftheSFGapparatus,thereisafundamentalshortcomingofthistechnique,wherebyallinformationon thecomplexnatureof c 2 islost.Forexample,foragivenvibrationassociatedwithaspeci ficbond,wecandeducetheangle distributionofthedipolemomentbycalculatingtheintensityratiobetweenSSPandSPS.However,justfromthe c2 2 ssp c2 2 sps ratio,wecannotpredictifthedipolemomentispointingawayfromthesurfaceorpointingtothesurface.Probingthecomplex representationof c2,speci ficallyitsimaginarycomponent, Im[c2]readilyrevealsthedipoleorientation.Nevertheless,itrequires eitheraphase-modulationoraheterodyneSFGsetuptogetherwithsolidmathematicalmodeling.
Inordertosimplifytheinterpretationofphase-sensitiveSFGVS,thatis, Im[c2],MoritaandHynes,begunbymodelingtheSFG spectraaccordingtotheenergyrepresentationofthenonlinearsusceptibility, c2,inmoleculardynamics(MD)calculations.20 They havepioneeredthecomputationalanalysisofSFGspectroscopyingeneralandsuggestedanalternativeMDmodelbasedonthe timecorrelationfunction.21 Recently,Moritaetal.haveincorporatedthechargeresponsekernel(CRK)modelintheirworkbringing thecomputationalmodeltobetterinterprettheexperimentalSFGspectra.22,23 TheCRKwassuccessfullyimplementedininvestigatingthevapor –liquidinterfacestructureofDMCandpropylenecarbonate(PC),bothcommonsolventsinLi-ionelectrolytesolutions.22 ThestudyprobedtheC]Ostretchanditsorientationattheliquid –gasinterface.In Fig.4,wecanseethatthesimulatedMD SFG(A,C)andexperimentalSFG(B,D)spectraofPCandDMCatthesolid –gasinterfacecorrespondwell.However,theimportanceoftheMDsimulationwasdemonstratedbyrevealingthatthereasonfortheC]ObipolarbandslieswithDMChavingan almostisotropicorientationwhilePChasanorderedstructureduetoPCdimersasdiscussedinRef.[23].
TheadvantagesofSFGvibrationalspectroscopyasasurfacespeci ficspectroscopyaredemonstratedinthestudyofthesolid (electrode) –liquid(electrolyte)interfaceandevenmoresoinexaminingthesolidelectrolyteinterphase(SEI).24 TheSEI25,26 is atermandconceptthathasbeenwidelyacceptedforthereductionproductsformedonananodeoftheliquidhydrocarbonelectrolytemoleculesinteractingwithLi-ionsduringthechargingprocess.TheSEIservesasaphysicalbarrierandachemicallyinert coatingthathindersfurtherelectrolytereductiononthesurfaceoftheanode.Meanwhile,itallowslithiumionsdiffusion,at areasonablerate,throughittothelithiumionhost(anode)uponchargingandbacktotheelectrolytesolutiononcethedischarge oftheLi-ionbatterytakesplace.
OneofthekeystepsforamajorimprovementoftheLi-ionbatteryisunderstandingtheSEI’sstructure-relateddiffusioninenergy storagematerials.TheSEIformationisacrucialstepaschargeanddischargeratesofLi-ionbatteriesarediffusion-limited.The fundamentalunderstandingoftheiontransportanddiffusionmechanismwillultimatelyleadtofastercharging,longerlasting, andsaferLi-ionbatteries.Severalgroupshavesettoexplorethecomposition,structure,andformation/degradationmechanisms ofthesolidelectrolyteinterfaceonthesurfacesofelectrodesatOCPandduringcharge/dischargecyclesbyapplyingadvanced insituSFGvibrationalspectroscopy.
TheSEI’sbehaviorwasalsoinvestigatedunderappliedpotentials.Dlottetal.carefullychoseanelectrochemicalsystemto produceasingleSEIproduct.24 Theycycledlithiumperchlorate(LiClO4)saltdissolvedinanECdilutedwithtetrahydrofuran (THF)incontactwitheithergoldorcopperanodesbetween2.0and0.2V(vs.Li/Liþ).Thesinglereductionproductwaslithium ethylenedicarbonatewithatraceamountofethylenehavingtwoC]Odistinctvibrationalfrequenciesat1782and1800cm 1 , referto Fig.5.Theyhadreportedtwomain findings.The firstisthattheSEI “breaths” asafunctionofappliedpotentialonly whenreducedonacopperelectrode.ThisincreaseanddecreaseoftheSEIwidthisnotapparentwhenagoldelectrodeisused eventhoughthesameelectrolyteandthesameelectrochemicalconditionsasintheCucasearemaintained.Thesecond,presented in Fig.6,wasthattheappliedexternalpotentialaffectstheSFGprofile27 andsooneshouldcarefullyexaminetheSFGpro file obtainedunderpotentiodynamicconditions.
Fig.4 ThesimulatedMDSFG(A,C)andexperimentalSFG(B,D)spectraofpropylenecarbonate(PC)anddimethylcarbonate(DMC)solid–gas interface.Left TheSSP-polarizedSFGspectraareshownin red andSPSspectrain blue.Right TheSSPpolarizedimaginarycomponent,Im[c2], calculatedSFGspectraofPC(up)andDMC(bottom)solid–gasinterface.TheMDsimulationsshowthattheSFGbipolarbandoriginatesfrom differentmolecularinteractions,dimerizationforPC(up)andrandomforDMC(bottom).ReprintedwithpermissionfromWang,L.;Peng,Q.L.;Ye, S.;Morita,A.SurfaceStructureofOrganicCarbonateLiquidsInvestigatedbyMolecularDynamicsSimulationandSumFrequencyGenerationSpectroscopy. J.Phys.Chem.C 2016, 120,15185–15197;Ishiyama,T.;Morita,A.ComputationalAnalysisofVibrationalSumFrequencyGenerationSpectroscopy. Annu.Rev.Phys.Chem. 2017, 68,355–377.Copyright(2017)AmericanChemicalSociety.