https://ebookmass.com/product/encyclopedia-of-infection-and-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Clinical Immunology Nima Rezaei
https://ebookmass.com/product/clinical-immunology-nima-rezaei/
ebookmass.com
Translational Autoimmunity, Volume 1 : Etiology of Autoimmune Diseases Nima Rezaei
https://ebookmass.com/product/translational-autoimmunityvolume-1-etiology-of-autoimmune-diseases-nima-rezaei/
ebookmass.com
Translational Immunology. Volume 2: Treatment of Autoimmune Diseases Nima Rezaei
https://ebookmass.com/product/translational-immunologyvolume-2-treatment-of-autoimmune-diseases-nima-rezaei/
ebookmass.com
The Summer You Found Me (The Summer Series Book 3) Elizabeth O'Roark
https://ebookmass.com/product/the-summer-you-found-me-the-summerseries-book-3-elizabeth-oroark/
ebookmass.com
Decolonial Feminisms, Power and Place: Sentipensando with Rural Women in Colombia Laura Rodríguez Castro
https://ebookmass.com/product/decolonial-feminisms-power-and-placesentipensando-with-rural-women-in-colombia-laura-rodriguez-castro-2/
ebookmass.com
Painting with Light John Alton
https://ebookmass.com/product/painting-with-light-john-alton/
ebookmass.com
Guides to the Evaluation of Permanent Impairment, fourth edition 4th Edition, (Ebook PDF)
https://ebookmass.com/product/guides-to-the-evaluation-of-permanentimpairment-fourth-edition-4th-edition-ebook-pdf/
ebookmass.com
Marginalized, Mobilized, Incorporated. Women and Religious Nationalism in Indian Democracy Rina Verma Williams
https://ebookmass.com/product/marginalized-mobilized-incorporatedwomen-and-religious-nationalism-in-indian-democracy-rina-vermawilliams/
ebookmass.com
Brutal Secrets: (The Brutal Duet Part ■ Book 1) Bj Alpha
https://ebookmass.com/product/brutal-secrets-the-brutal-duetpart-%e2%85%b1-book-1-bj-alpha-2/
ebookmass.com
The Physics of Everyday Phenomena 8th Edition – Ebook PDF Version
https://ebookmass.com/product/the-physics-of-everyday-phenomena-8thedition-ebook-pdf-version/
ebookmass.com
AND IMMUNITY VOLUME \ Editor in Chief Nima Rezaei
ENCYCLOPEDIAOF INFECTIONANDIMMUNITY ENCYCLOPEDIAOF INFECTIONANDIMMUNITY EDITOR-IN-CHIEF NimaRezaei
ResearchCenterforImmunodeficiencies,Children’sMedicalCenter, TehranUniversityofMedicalSciences,Tehran,Iran
DepartmentofImmunology,SchoolofMedicine, TehranUniversityofMedicalSciences,Tehran,Iran
NetworkofImmunityinInfection,MalignancyandAutoimmunity(NIIMA), UniversalScientificEducationandResearchNetwork(USERN),Stockholm,Sweden
VOLUME1 SectionEditors
Section1: TheImmuneSystem ,EditedbyNimaRezaei
Section2: PathogensandImmunity,EditedbyRowenaJenkins
Section3: Bacteria,EditedbyFernandoCobo
Elsevier Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright © 2022ElsevierInc.Allrightsreserved
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,including photocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhow toseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuchasthe CopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite:www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybenoted herein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding,changesin researchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmayalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation,methods, compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthe safetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjuryand/or damagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN978-0-12-818731-9
Forinformationonallpublicationsvisitour websiteat http://store.elsevier.com
Publisher: OliverWalter
AcquisitionEditors: KelseyConnorsandBlerinaOsmanaj
ContentProjectManager: LauraJackson
AssociateContentProjectManager: SajanaDevasiPK Designer: MilesHitchen
EDITOR-IN-CHIEF NimaRezaei gainedhismedicaldegree(MD)fromTehranUniversityof MedicalSciencesandsubsequentlyobtainedanMScinmolecularandgenetic medicineandaPhDinclinicalimmunologyandhumangeneticsfromthe UniversityofSheffield,UK.Healsospentashort-termfellowshipofpediatric clinicalimmunologyandbonemarrowtransplantationintheNewcastleGeneral Hospital.ProfessorRezaeiisnowthefullprofessorofimmunologyandvicedean ofInternationalAffairs,SchoolofMedicine,TehranUniversityofMedicalSciences,andtheco-founderandheadoftheResearchCenterforImmunodeficiencies.HeisalsothefoundingpresidentofUniversalScientificEducationand ResearchNetwork(USERN).Prof.Rezaeihasalreadybeenthedirectorofmore than100researchprojectsandhasdesignedandparticipatedinseveralinternationalcollaborativeprojects.Prof.Rezaeiistheeditor,editorialassistant,or editorialboardmemberofmorethan40internationaljournals.Hehasedited morethan50internationalbooks,haspresentedmorethan500lectures/posters incongresses/meetings,andhaspublishedmorethan1100scientificpapersin theinternationaljournals.
EDITORIALBOARD Editor-in-Chief
NimaRezaei
ResearchCenterforImmunodeficiencies,Children’sMedicalCenter,TehranUniversityofMedicalSciences,Tehran,Iran; DepartmentofImmunology,SchoolofMedicine,TehranUniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity(NIIMA),UniversalScientificEducationandResearchNetwork (USERN),Stockholm,Sweden
SectionEditors Section1.TheImmuneSystem
NimaRezaei
ResearchCenterforImmunodeficiencies,Children’sMedicalCenter,TehranUniversityofMedicalSciences,Tehran,Iran; DepartmentofImmunology,SchoolofMedicine,TehranUniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity(NIIMA),UniversalScientificEducationandResearchNetwork (USERN),Stockholm,Sweden
Section2.PathogensandImmunity
DrRowenaJenkins
SwanseaUniversityMedicalSchool,Swansea,UnitedKingdom
Section3.Bacteria
FernandoCobo
DepartmentofMicrobiologyandInstitutodeInvestigaciónBiosanitariaibs.GRANADA,UniversityHospitalVirgendelas Nieves,Granada,Spain
Section4.Viruses
NimaRezaei
ResearchCenterforImmunodeficiencies,Children’sMedicalCenter,TehranUniversityofMedicalSciences,Tehran,Iran; DepartmentofImmunology,SchoolofMedicine,TehranUniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity(NIIMA),UniversalScientificEducationandResearchNetwork (USERN),Stockholm,Sweden
Section5.Fungi,ProtozoanParasites,andHelminths Fungi
FannyLanternier
HôpitalUniversitaireNecker-Enfantsmalades,AssistancePublique HôpitauxdeParis,Paris,France; Universitéde Paris,InstitutPasteur,UnitédeMycologieMoléculaire,UMR2000,CNRS,CNRdesMycosesInvasivesetantifongiques, Paris,France
ProtozoanParasitesandHelminths
FabrizioBruschi,MD
DepartmentofTranslationalResearch,N.T.M.S.,UniversitàdiPisa,Pisa,Italy
Section6.InsectandMites
Prof.GiovanniBenelli,Ph.D.
DepartmentofAgriculture,FoodandEnvironment,UniversityofPisa,Pisa,Italy
Section7.ClinicalInfectiousDisease
FedericoIovino,PhD.
AssociateProfessorandPrincipalInvestigator,IovinoGroup,LaboratoryofNeuro-InfectionsandNeuro-Inflammation, DepartmentofNeuroscience – KarolinskaInstitutet,Stockholm,Sweden
Section8.ClinicalImmunology
HansD.Ochs
UniversityofWashingtonandSeattleChildren’sResearchInstitute,Seattle,WA,UnitedStates
NimaRezaei
ResearchCenterforImmunodeficiencies,Children’sMedicalCenter,TehranUniversityofMedicalSciences,Tehran,Iran; DepartmentofImmunology,SchoolofMedicine,TehranUniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity(NIIMA),UniversalScientificEducationandResearchNetwork (USERN),Stockholm,Sweden
Section9.LaboratoryTests
NimaRezaei
ResearchCenterforImmunodeficiencies,Children’sMedicalCenter,TehranUniversityofMedicalSciences,Tehran,Iran; DepartmentofImmunology,SchoolofMedicine,TehranUniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity(NIIMA),UniversalScientificEducationandResearchNetwork (USERN),Stockholm,Sweden
Section10.TreatmentProtocols
NimaRezaei
ResearchCenterforImmunodeficiencies,Children’sMedicalCenter,TehranUniversityofMedicalSciences,Tehran,Iran; DepartmentofImmunology,SchoolofMedicine,TehranUniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity(NIIMA),UniversalScientificEducationandResearchNetwork (USERN),Stockholm,Sweden
LISTOFCONTRIBUTORSFORVOLUME1 RaquelAbad
NationalReferenceLaboratoryforNeisserias,National CentreofMicrobiology InstitutodeSaludCarlosIII, Majadahonda,Spain
HassanAbolhassani
DivisionofClinicalImmunology,Departmentof LaboratoryMedicine,KarolinskaInstituteatKarolinska UniversityHospitalHuddinge,Stockholm,Sweden
JeffreyKActor
UniversityofTexasHealthScienceCenter,McGovern MedicalSchool,DepartmentofPathologyandLaboratory Medicine,Houston,TX,UnitedStates
JawadAhmed DepartmentofInternalMedicine,DowUniversityof HealthSciences,Karachi,Pakistan
MahzadAkbarpour
NetworkofImmunityinInfection,Malignancyand Autoimmunity(NIIMA),UniversalScientificEducation andResearchNetwork(USERN),Tehran,Iran; ImmunologyBoardforTransplantationandCell-Based Therapeutics(ImmunoTACT),UniversalScientific EducationandResearchNetwork(USERN),Tehran,Iran; AdvancedCellularTherapeuticsFacility(ACTF), HematopoieticCellularTherapyProgram,Sectionof Hematology&Oncology,DepartmentofMedicine, UniversityofChicagoMedicalCenter,Chicago,United States
LuisAliaga
DepartmentofMedicine,UniversityofGranada,Granada, Spain;DepartmentofInternalMedicine,Hospital UniversitarioSanCecilio,Granada,Spain
JorgeAmich
ManchesterFungalInfectionGroup(MFIG),Division ofInfection,ImmunityandRespiratoryMedicine, SchoolofBiologicalSciences,FacultyofBiology, MedicineandHealth,UniversityofManchester, ManchesterAcademicHealthScienceCentre, Manchester,UnitedKingdom
JorgeArca-Suarez
DepartmentofMicrobiology,ComplexoHospitalario UniversitarioACoruña(CHUAC),Institutode InvestigaciónBiomédicaACoruña(INIBIC),ACoruña, Spain
BeatrizPlataBarril
DepartmentofInfectiousDiseasesandClinical Microbiology,JaénUniversityHospital,Jaén,Spain
JayABerzofsky
VaccineBranch,CenterforCancerResearch,National CancerInstitute,NIH,Bethesda,MD,UnitedStates
JaimeBorrego
HospitalUniversitarioVirgendelasNieves.Granada,Spain
HelenLouiseBrown SchoolofDentistry,CardiffUniversity,Cardiff,United Kingdom
ElizabethCalatrava
DepartmentofMicrobiologyandInstitutodeInvestigación Biosanitaria,ibs.GRANADA,UniversityHospitalVirgen delasNieves,Granada,Spain
NoeliaCalvoSánchez
ServiciodeMicrobiología,HospitalClínicoUniversitariode Salamanca,Salamanca,Spain;DepartamentodeCiencias BiomédicasydelDiagnóstico.FacultaddeMedicina, UniversidaddeSalamanca,Salamanca,Spain
IsabelCasanovasMoreno-Torres DepartmentofMicrobiology,HospitalSantaAna, Granada,Spain
FernandoCobo
DepartmentofMicrobiologyandInstitutodeInvestigación Biosanitariaibs.GRANADA,UniversityHospitalVirgende lasNieves,Granada,Spain
ClaudioCortes
DepartmentofFoundationalMedicalStudies,Oakland UniversityWilliamBeaumontSchoolofMedicine, Rochester,MI,UnitedStates
ElenaCuadrosMoronta
DepartmentofClinicalMicrobiology,SierrallanaHospital, Torrelavega,Spain
FranciscodeAsísRamirez
PrimaryCareCenter “ElValle",Jaén,Spain;Centrode Salud “ElValle”,Jaén,Spain
AdolfodeSalazarGonzález
DepartmentofMicrobiology,HospitalUniversitarioClínico SanCecilio,Granada,Spain
JoshuaDeSousaCasal
DepartmentofImmunology,UniversityofToronto, Toronto,ON,Canada;SunnybrookResearchInstitute, Toronto,ON,Canada
ZlatkoDembic
MolecularGeneticsLaboratory,DepartmentofOralBiology, FacultyofDentistry,UniversityofOslo,Oslo,Norway
RomanDeniskin
UniversityofKentuckyandKentuckyChildren'sHospital, Lexington,KY,UnitedStates
RebeccaADrummond
InstituteofImmunologyandImmunotherapy,Universityof Birmingham,Birmingham,UnitedKingdom;Instituteof MicrobiologyandInfection,UniversityofBirmingham, Birmingham,UnitedKingdom
NohaMousaadElemam
DepartmentofClinicalSciences,CollegeofMedicine, UniversityofSharjah,Sharjah,UnitedArabEmirates; Immuno-OncologyGroup,SharjahInstituteforMedical Research(SIMR),Sharjah,UnitedArabEmirates
PooyaFarhangnia
DepartmentofImmunology,SchoolofMedicine,Iran UniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity (NIIMA),UniversalScientificEducationandResearch Network(USERN),Tehran,Iran;ImmunologyBoardfor TransplantationandCell-BasedTherapeutics (ImmunoTACT),UniversalScientificEducationand ResearchNetwork(USERN),Tehran,Iran
VivianaPFerreira
DepartmentofMedicalMicrobiologyandImmunology, UniversityofToledoCollegeofMedicineandLifeSciences, Toledo,OH,UnitedStates
CarlaForondaGarcía-Hidalgo HospitalUniversitarioVirgendelasNieves,Granada, Spain
CristinaGómez-Camarasa MicrobiologyDepartment,HospitalUniversitarioClínico SanCecilio,Granada,Spain
EstherGómez-Vicente DepartmentofMicrobiology,HospitalVirgendelasNieves, Granada,Spain
LauraViñuelaGonzález MicrobiologyDepartment,HospitalUniversitarioClínico SanCecilio,Granada,Spain
AGonzález-Martínez ServiciodeMicrobiología,HospitalUniversitarioVirgende lasNieves,Granada,Spain
JuanFranciscoGutiérrezBautista HospitalUniversitarioVirgendelasNieves,Serviciode AnálisisClínicoseInmunología,Granada,Spain
SofiaHain InstituteofImmunologyandImmunotherapy,Universityof Birmingham,Birmingham,UnitedKingdom
LlinosGHarris MicrobiologyandInfectiousDiseases,InstituteofLife Sciences,MedicalSchool,FacultyofMedicine,Healthand LifeScience,SwanseaUniversity,Swansea,United Kingdom
JennyAHerbert UniversityofManchester,Manchester,UnitedKingdom
PaulaHernándezCalvo ServiciodeMicrobiología,HospitalClínico UniversitariodeSalamanca,Salamanca,Spain; DepartamentodeCienciasBiomédicasydelDiagnóstico. FacultaddeMedicina,UniversidaddeSalamanca, Salamanca,Spain
SavannahBHowe VaccineBranch,CenterforCancerResearch,National CancerInstitute,NIH,Bethesda,MD,UnitedStates
ImogenAnneJones FacultyofHealth,SchoolofBiomedicalSciences, UniversityofPlymouth,Plymouth,UnitedKingdom
LovleenTinaJoshi FacultyofHealth,SchoolofBiomedicalSciences, UniversityofPlymouth,Plymouth,UnitedKingdom
MahdisKeshavarz-Fathi CancerImmunologyProject(CIP),UniversalScientific EducationandResearchNetwork(USERN),Tehran,Iran; SchoolofPharmacy,ShahidBeheshtiUniversityofMedical Sciences,Tehran,Iran
MahsaKeshavarz-Fathi SchoolofMedicine,TehranUniversityofMedicalSciences, Tehran,Iran;CancerImmunologyProject(CIP),Universal ScientificEducationandResearchNetwork(USERN), Tehran,Iran
BariaaAKhalil
DepartmentofClinicalSciences,CollegeofMedicine, UniversityofSharjah,Sharjah,UnitedArabEmirates; Immuno-OncologyGroup,SharjahInstituteforMedical Research(SIMR),Sharjah,UnitedArabEmirates
AnaLara-Oya DepartmentofMicrobiology,HospitalUniversitariode Jaén,Jaén,España
CarmenLiébana-Martos FacultativoEspecialistadeÁreaenMicrobiología,Servicio deMicrobiología,HospitalUniversitariodeJáen,Jaén, España
AnaFuentesLópez MicrobiologyDepartment,HospitalUniversitarioClínico SanCecilio,Granada,Spain
AzzamAMaghazachi DepartmentofClinicalSciences,CollegeofMedicine, UniversityofSharjah,Sharjah,UnitedArabEmirates; Immuno-OncologyGroup,SharjahInstitutefor MedicalResearch(SIMR),Sharjah,UnitedArab Emirates
FarheenMalik DepartmentofInternalMedicine,DowUniversityof HealthSciences,Karachi,Pakistan
JosefaMartínez DepartmentofInternalMedicine,HospitalUniversitario SanCecilio,Granada,Spain
HamidRezaMirzaei
DepartmentofMedicalImmunology,SchoolofMedicine, TehranUniversityofMedicalSciences,Tehran,Iran
Hamid-RezaMohammadi-Motlagh
MedicalBiologyResearchCenter,HealthTechnology Institute,KermanshahUniversityofMedicalSciences, Kermanshah,Iran
SaraMomtazmanesh ResearchCenterforImmunodeficiencies,Children's MedicalCenter,TehranUniversityofMedicalSciences, Tehran,Iran;NetworkofImmunityinInfection, MalignancyandAutoimmunity(NIIMA),Universal ScientificEducationandResearchNetwork(USERN), Tehran,Iran
ManuelaMoreno-Higueras DepartmentofInternalMedicine,HospitalUniversitario SanCecilio,Granada,Spain
JuanLuisMuñozBellido ServiciodeMicrobiología,HospitalClínicoUniversitariode Salamanca,Salamanca,Spain;DepartamentodeCiencias BiomédicasydelDiagnóstico.FacultaddeMedicina, UniversidaddeSalamanca,Salamanca,Spain
PavelPNesmiyanov MedicalResearchandEducationCenter,Lomonosov MoscowStateUniversity(MoscowStateUniversity Clinic),Moscow,Russia;EngelhardtInstituteof MolecularBiology,RussianAcademyofSciences,Moscow, Russia
MJOlivares-Duran ServiciodeAnálisisClínicoseInmunología,Hospital UniversitarioVirgendelasNieves,Granada,Spain
PurevdorjBOlkhanud VaccineBranch,CenterforCancerResearch,National CancerInstitute,NIH,Bethesda,MD,UnitedStates
AnastasiaSampedroPadilla HospitalNeurotraumatológicodelHUdeJaén,Jaén,Spain
StavrosPanagiotou UniversityofManchester,Manchester,UnitedKingdom
SamiraRajaei
ImmunologyDepartment,SchoolofMedicine,Tehran UniversityofMedicalSciences,Tehran,Iran
JARegueraMárquez ServiciodeMicrobiología,HospitalUniversitarioVirgende lasNieves,Granada,Spain
NimaRezaei
DepartmentofImmunology,SchoolofMedicine,Tehran UniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity (NIIMA),UniversalScientificEducationandResearch Network(USERN),Tehran,Iran;ResearchCenterfor Immunodeficiencies,Children'sMedicalCenter,Tehran UniversityofMedicalSciences,Tehran,Iran;Cancer ImmunologyProject(CIP),UniversalScientific EducationandResearchNetwork(USERN),Tehran, Iran
JohannaRhodes
MRCCentreforGlobalDiseaseAnalysis,ImperialCollege London,London,UnitedKingdom
LotharRink
InstituteofImmunology,MedicalFaculty,RWTHAachen University,Aachen,Germany
JavierRodríguez-Granger
DepartmentofMicrobiology,HospitalUniversitarioVirgen delasNieves,Granada,Spain
LauraLucíaRojas-García DepartmentofMicrobiology,UniversityHospitalSan Cecilio,Granada,Spain
CarlosRosales
DepartamentodeInmunología,InstitutodeInvestigaciones Biomédicas,UniversidadNacionalAutónomadeMéxico, MexicoCity,Mexico
MonaSadeghalvad
DepartmentofImmunology,SchoolofMedicine,Tehran UniversityofMedicalSciences,Tehran,Iran;Networkof ImmunityinInfection,MalignancyandAutoimmunity (NIIMA),UniversalScientificEducationandResearch Network(USERN),Tehran,Iran;ResearchCenterfor Immunodeficiencies,Children'sMedicalCenter,Tehran UniversityofMedicalSciences,Tehran,Iran
AntonioSampedro MicrobiologyService,VirgendelasNievesUniversity Hospital,Granada,Spain
EstherSerrano-CondeSánchez MicrobiologyDepartment,HospitalUniversitarioClínico SanCecilio,Granada,Spain
LisaForbesSatter
BaylorCollegeofMedicineandTexasChildren'sHospital, Houston,TX,UnitedStates
JenniferScott ManchesterFungalInfectionGroup(MFIG),Division ofInfection,ImmunityandRespiratoryMedicine, SchoolofBiologicalSciences,FacultyofBiology, MedicineandHealth,UniversityofManchester, ManchesterAcademicHealthScienceCentre, Manchester,UnitedKingdom
KatieSilver
FacultyofHealth,SchoolofBiomedicalSciences, UniversityofPlymouth,Plymouth,UnitedKingdom
KeriCSmith
SABAMedicalSchool,SABA,DutchCaribbean
GabrieleSorci Biogéosciences,CNRSUMR6282,Universitéde BourgogneFranche-Comté,Dijon,France
EileenUribe-Querol DivisióndeEstudiosdePosgradoeInvestigación,Facultad deOdontología,UniversidadNacionalAutónomade México,MexicoCity,Mexico
SylviaValdezate ReferenceandResearchLaboratoryforTaxonomy, NationalCentreofMicrobiology,InstitutodeSaludCarlos III,Madrid,Spain
JulioAVázquez
NationalReferenceLaboratoryforNeisserias,National CentreofMicrobiology InstitutodeSaludCarlosIII, Majadahonda,Spain
IngaWessels
InstituteofImmunology,MedicalFaculty,RWTHAachen University,Aachen,Germany
RachaelCWilkinson
SirWilliamDunnSchoolofPathology,Oxford,United Kingdom
ThomasSWilkinson InstituteofLifeScience,MicrobiologyandInfectious Disease,SwanseaUniversityMedicalSchool(SUMS), Swansea,UnitedKingdom
CatrinFWilliams SchoolofBiosciences,CardiffUniversity,Cardiff,Wales, UnitedKingdom
LisaKWilliams DepartmentofAgricultureandAnimalScience,Hartpury University,Gloucester,UnitedKingdom
JuanCarlosZúñiga-Pflücker DepartmentofImmunology,UniversityofToronto, Toronto,ON,Canada;SunnybrookResearchInstitute, Toronto,ON,Canada
FOREWORD Itisagreathonortobeaskedtointroduce,withthisforeword,the EncyclopediaofInfectionandImmunity.This workwillbereadandenjoyedbymanystudents,bothgraduateandpostgraduate,includingclinicians, laboratorypractitioners,andscientists.Manycolleagueshavecontributedtothisimportantwork,withover 230originalchaptersassembledinsectionsandlistedalphabetically.Ioffermywarmcongratulationstothe editorsofthisbook,forsolicitingawidebodyofexpertiseandassemblingitinaformthatgivessomuch pleasureandinformationtothereader.
Forthoseofuswhoworkinthefieldofinfectionandimmunity,thelasttwoyearshavebeenexceptionally challenging.Atthecurrenttimeweare22monthsintoamajorpandemiccausedbyanovelcoronavirusandits variants,andworldwide,sadly,therehavebeenmanyhospitalizationsanddeaths.Cliniciansandscientistsin ourfieldhavebeenworkingflatout,caringforpatients,generatingorutilizingdiagnostictests,generatingnew knowledgeinimmunitytocoronaviruses,ordiscovering,testing,anddeployingnewvaccines.PublicHealth practitionershaveorganizedourmedico-socialresponsestolimittransmissionanddealwiththeconsequences ofinfection.Behavioralscientistshavetransformedourapproachtoinformingthepublic,gettingthebest possibleoutcomesfromeffectivecommunication.Governmentshavelearnedthedangerofignoringthe expertiseofscientists,andinmanycountries,thepubliclookuponcliniciansandscientistswithgratitude andrespect.Itisatestamenttotheadvancementofinfectionandimmunitysciencethatthegenomeofthevirus wassequencedwithinafewweeksofCOVID-19emerging,andthatwithin12months,thefirsttrialsofnovel vaccineshadbeencompleted.Thesevaccinesincludedsomewithexceptionallynoveltechnologies,including theRNAvaccinesandtheviral-vectoredvaccines.
UnderpinningtheacademicagilityofstudentsandpractitionersinInfectionandImmunityistheuniversal strengthofsoundeducation.Readersofthisbookwillbeseekingtheknowledgetheyneedforacomprehensive understandingofinfectiousdiseaseandimmunology.Weworkinafascinatingdiscipline.Ourfieldisuniquein thatpathogenesisinvolvesatleasttwosetsofgenomes ontheonehandthemicrobe,andontheother,the host.Themicrobialgenomeprogramsitsmicroenvironmenttoenableacquisitionofandresidenceinaniche forsufficienttimetoreplicateandfurtherdisseminatetootherhosts.Thehumangenomehasevolvedto tolerateandcontainawiderangeofsymbiontsandpathobionts,withhighlysophisticatedimmune decision-makingatthecellularlevel,buttorespondtoinvadingspecieswithanelaboratecoordinated repertoireofinnateandacquiredimmunity.Thecomplexconversationbetweenmanydifferentmicrobesand thehumanhostgeneratesawiderangeofdiseasephenotypes.Thismakesthejobofaninfectiousdisease physiciansochallengingbutatthesametimesofulfilling.
Ourfieldisenrichedbythemanyscientistsandclinicianswhoarewillingtosharetheirexperiencewiththeir peersandstudents.Thisbookreflectsthegreatvarietyofknowledgethathasemergedovercenturiesofinquiry andendeavor,andIbelieveyouwillberewardedbyusingitasoneofyourreferencesources.
RobertC.Read Editor-in-Chief,JournalofInfection ProfessorofInfectiousDiseasesandConsultantPhysician UniversityHospitalSouthampton,UnitedKingdom
PREFACE Althoughinfectionshavebeenoneofthemostimportanthealththreatssincecenturiesago,themechanismsof humandefenseagainstinfectionshavebeenunclearuntillastcentury.Thescientificworldhasremarkable progressesinthefieldofimmunologyduringrecentdecades.Indeed,thenoveldiscoveryofgenesrelatedto differentpathogenshasenhancedourknowledgeabouttheimmunesystem.Understandingdifferentpathogensandtheimmunemechanismspromotediagnosticstrategiesanddesignmoreefficienttherapeuticagents.
EncyclopediaofInfectionandImmunity isacomprehensivetext,whichcoverstopicsnotonlyonbasic immunologyandmicrobiologybutalsoonclinicalimmunologyandinfectiousdiseases.Section1focuses ontheimmunesystem,whileSection2describestheinteractionbetweenpathogensandimmunity.Bacteria, viruses,fungi,protozoanparasitesandhelminths,andinsectandmitesareexplainedinSections3–6,respectively.Section7discussestheclinicalinfectiousdiseasechallenges,whereasclinicalimmunologyiscoveredin Section8.LaboratorytestsandtreatmentprotocolsarethemainfocusesofSections9and10,respectively.
EncyclopediaofInfectionandImmunity istheresultofvaluablecontributionofmorethan400scientistsfrom manywell-knownuniversities/institutesworldwide.Iwouldliketoherebyacknowledgetheexpertiseofall contributors,forgenerouslydevotingtheirtimeandconsiderableeffortinpreparingtheirrespectivechapters. IwouldalsoliketoexpressmygratitudetoElsevierforprovidingustheopportunitytopublishthis Encyclopedia.
Finally,Ihopethatthiscomprehensivebookwillbeofspecialvalueforresearchersandclinicianswhowish toextendtheirknowledgeoninfectionandimmunity.
NimaRezaei,MD,PhD
CONTENTSOFVOLUME1 Section1:TheImmuneSystem,EditedbyNimaRezaei
NimaRezaei,MonaSadeghalvad,andHamid-RezaMohammadi-Motlagh
Hamid-RezaMohammadi-Motlagh,MonaSadeghalvad,andNimaRezaei
MonaSadeghalvad,Hamid-RezaMohammadi-Motlagh,andNimaRezaei
JoshuaDeSousaCasalandJuanCarlosZúñiga-Pflücker
JayABerzofsky,SavannahBHowe,andPurevdorjBOlkhanud
MonaSadeghalvad,Hamid-RezaMohammadi-Motlagh,andNimaRezaei
VivianaPFerreiraandClaudioCortes
NohaMousaadElemam,BariaaAKhalil,andAzzamAMaghazachi
PooyaFarhangniaandMahzadAkbarpour
HassanAbolhassani
JeffreyKActorandKeriCSmith
LotharRinkandIngaWessels
MahsaKeshavarz-Fathi,MahdisKeshavarz-Fathi,andNimaRezaei
Section2:PathogensandImmunity,EditedbyRowenaJenkins
LovleenTinaJoshi,ImogenAnneJones,andKatieSilver
RachaelCWilkinson
HelenLouiseBrown
LisaKWilliams
LlinosGHarris
CatrinFWilliams
PrimaryMetabolismofHumanPathogenicFungi,ImportanceforVirulenceandPotentialforDrug
JenniferScottandJorgeAmich
SofiaHainandRebeccaADrummond
JohannaRhodes
GabrieleSorci
Section3:Bacteria,EditedbyFernandoCobo
PaulaHernándezCalvo,NoeliaCalvoSánchez,andJuanLuisMuñozBellido
FernandoCobo
CarlaForondaGarcía-Hidalgo
JawadAhmedandFarheenMalik
ElizabethCalatrava
Genus Neisseria
RaquelAbadandJulioAVázquez
Corynebacteriumdiphtheriae
JaimeBorrego
Listeria and Erysipelothrix
CristinaGómez-Camarasa
CoryneformGram-PositiveBacilli
LauraLucíaRojas-García
Nocardia,Rhodococcus,Streptomyces andOtherAerobicActinomycetes
SylviaValdezate
InfectionsCausedbyAnaerobicMicroorganisms
FernandoCobo
HumanPathogenic Enterobacterales
LauraViñuelaGonzález
Vibrio
AnaFuentesLópez,EstherSerrano-CondeSánchez,LauraViñuelaGonzález,andCristinaGómezCamarasa
Pseudomonas Infections
LuisAliaga,ManuelaMoreno-Higueras,JosefaMartínez,andJavierRodríguez-Granger
Stenotrophomonas, Burkholderia andOtherRelatedMicroorganisms
AnaLara-Oya
Moraxella andOtherNon-FermentativeGram-NegativeBacilli
EstherGómez-Vicente
Francisella, Brucella and Pasteurella 673
BeatrizPlataBarril
Campylobacter and Helicobacter 685
IsabelCasanovasMoreno-Torres
Haemophilus,Bordetella and Bartonella 694
AdolfodeSalazarGonzálezandJorgeArca-Suarez
Mycobacterium infections 703
AGonzález-Martínez,MJOlivares-Duran,JuanFranciscoGutiérrez-Bautista,JARegueraMárquez,LuisAliaga,andJavierRodríguez-Granger
Leptospira,Borrelia and Treponema 719
AntonioSampedroandFranciscodeAsísRamirez
Mycoplasma and Ureaplasma 730
CarmenLiébana-Martos
Legionella,Chlamydia and Chlamydophila 737 ElenaCuadrosMoronta
Ehrlichia,Anaplasma and RelatedBacteria
JuanFranciscoGutiérrezBautista,FranciscodeAsísRamirez,andAnastasiaSampedroPadilla
749
GeneralConceptsofImmunity NimaRezaeia,b,c,MonaSadeghalvada,b,andHamid-RezaMohammadi-Motlaghd, aDepartmentofImmunology,SchoolofMedicine, TehranUniversityofMedicalSciences,Tehran,Iran; bNetworkofImmunityinInfection,MalignancyandAutoimmunity(NIIMA),Universal ScientificEducationandResearchNetwork(USERN),Tehran,Iran; cResearchCenterforImmunodeficiencies,Children’sMedicalCenter, TehranUniversityofMedicalSciences,Tehran,Iran; dMedicalBiologyResearchCenter,HealthTechnologyInstitute,KermanshahUniversityof MedicalSciences,Kermanshah,Iran
©2022ElsevierInc.Allrightsreserved. Introductiontoimmunesystem
Antigenpresentation:AmechanismforTcellsactivationwhichismediatedbyMHCmolecules11
Maintaininghomeostasisinresponsetoenvironmentalalterationsisasignificantchallengeforlivingorganisms.Eliminationof invadingpathogensanddamagedorharmfulcellularcontentsisaninevitablechallengethatallorganismshavetocontendwithit. Thebody’sdefenseagainstthepotentialdangersandre-establishhomeostasisrelyon “immunesystem” whichconsistsofthecells andmoleculesresponsibleforprotectingthehostbodyfromharmfulagents.Inthisway,thecomprehensiveandorganized responseofimmunesystemcomponentsleadstotheformationofan “immuneresponse.” Althoughanidealimmuneresponse woulderadicatetheimminentdangerandrestorehomeostasistoaffectedtissues,itsimproperactivitycanalsobedetrimentaland causetissuedamage.Hence,theregulationofimmuneresponsesiscrucial,allowingtheorganismtomodulatetheintensityand durationoftheresponses(Chaplin,2010).
Toprovokeanefficientresponse,theimmunesystemmustrecognizeawiderangeofinfectiousagentsnamedpathogenssuchas viruses,bacteria,parasites,andfungianddiscriminatethemfromthehost’sowntissues.Thisprocessisperformedbyavarietyof receptorsexpressedontheimmunecells(MedzhitovandJaneway,1998).Anysubstancesrecognizedbyimmunesystemcomponentsnamed “Antigen” andthespecificpartofanantigenrecognizedbyimmunereceptorsiscalled “Epitope.” Oneoftheunique featuresoftheimmunesystemistheabilitytodistinguishbetween “self” and “non-self” antigens.Topreventinappropriate reactions,theimmunesystemmusthavetoleranceagainsthost’sownmolecules.Thisisanimportanteventwhichstatestheconcept of “immunetolerance” theory.Disruptionofself-toleranceorinappropriateregulationofimmuneresponsescouldleadtoimmune reactiontohostcellularcomponentsresultinginastateknownas “autoimmunity” (Chaplin,2010).
Inoverall,immunesystemcanbecategorizedintotwotypesinclude “innate” and “adaptive” immunity.Theinnateimmune systemisthefirstlineofdefensewhichquicklyactivatedagainstpathogens.Thistypeofimmuneresponsereactsinthesamewayto allforeignantigens,whichiswhyitisnamedasthe “non-specific” immunity.However,anefficientimmuneresponserequiresthe involvementofadaptiveimmunity.Theadaptiveimmunesystemspecificallyrecognizestheantigensandrespondsslowerthan innateimmunesystem.Theadvantageofadaptiveimmunityistheproductionof “ memory ” cellswhichcanreactimmediately againstpathogens.Therefore,whenaknownantigenisencounteredforsecondtime,theadaptiveimmunesystemcanreactfaster (McCulloughandSummerfield,2005).Inthisarticle,weaimedtodescribethebasicconceptsofimmunesystemincludingthe importantcomponentsofinnateimmunityandadaptiveimmunityaswellasthebasiceffectivemechanismsinordertopathogen elimination.
Innateimmunity:Thefirstlineofdefenseagainstpathogens Asanoldevolutionarydefensemechanism,theinnateimmunesystemornativeimmunity,providethefirstlineofdefenseagainst pathogens.Innateimmunityisresponsibleforrespondimmediately(withinminutes)tomicrobialcomponentsandharmful agentscausedbycellularstressortissuedamage.Innateimmunesystemconsistsofthecellularandbiochemicalcomponentsthat arepresentactiveevenbeforeinfection.Althoughsomeofthemincludingcomplementsystemandcellularcompartmentsbecome activeupondangerexposure.Animportantfeatureoftheinnateimmunesystemisitsabilitytoactivatetheadaptiveimmune system(Tosi,2005; Beutler,2004).
Innateimmuneresponseprovidestheimmunityagainstpathogensthroughseveralwaysincluding:
1.Pathogensblockingfromenteringthebody.
2.Pathogenuptakebyphagocytosisorpinocytosis.
3.Pathogenkillingviacytotoxicmediators.
4.Increasedrecruitmentoftheotherimmunecellsbyproductionofinflammatorymediators.
5.Pathogenprocessingandantigenpresentationtoadaptiveimmunecells.
Effectormechanismsofinnateimmuneresponse Recognitionofconservedmolecularpatternsofpathogens
Innateimmuneresponsereliesontherecognitionofmolecularpatternsexistedonthepathogensordamagedassociatedmolecules whichproducedduringinjury,infection,ordiseasecondition.Thesemolecularpatternsareincluding “pathogen-associated molecularpatterns” (PAMP)suchaslipopolysaccharide(LPS)ofGram-negativebacteria,peptidoglycanofbacterialcellwall, andviralRNA;and “damaged-associatedmolecularpatterns” (DAMP)suchasheatshockproteins(HSPs)andhighmobilitygroup box1(HMGB1).PAMPsorDAMPscouldberecognizedbynon-specificreceptorsnamed “patternrecognitionreceptors” orPRRs expressedonimmunesystemcomponents.SeveraltypesofPRRsincludingToll-likereceptors(TLR),nucleotide-bindingoligomerizationdomain(NOD)-LeucineRichRepeats-containingreceptors(NLR),retinoicacid-induciblegene1(RIG-1)-likereceptors (RLR),andtheC-typelectinreceptors(CLRs)havebeenidentifiedwhicharedifferentlydiffusedinsubcellularcompartmentssuch ascytosol,cellmembrane,andendosomalmembrane.Ligand-inducedactivationofPRRsinitiatesinflammatoryresponses resultinginthepromotionofpathogeneliminationaswellastissuehomeostasis(Mogensen,2009).
Phagocytosis:Akillingmechanismbyphagocytes
Phagocytesarethemajorinnateimmunecellsactasthefirstlineofdefenseagainstmicroorganisms.Theimportantphagocytesare monocytesandmacrophages,neutrophils,eosinophils,basophils,mastcells,anddendriticcells.Anatomicallytheyareabundantly foundinthelocationswheretheyencountermostpathogens,suchassubmucosaltissueoftherespiratorysystem,gut,skin,and urogenitaltract(Rabinovitch,1995).
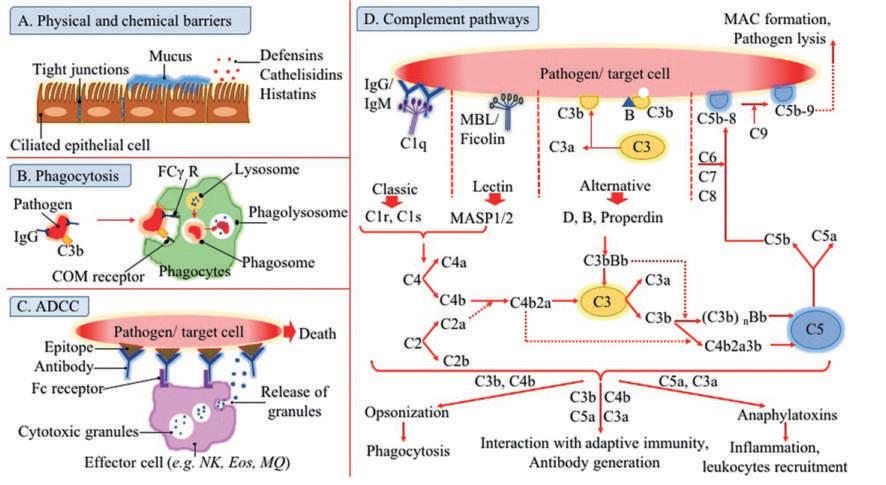
Theinitiationofphagocytosisisdependentontheinteractionofmicrobialsurfacewithcertainreceptorsonthephagocytes. Subsequently,theboundedmicroorganismissurroundedbythecellmembraneofthephagocytesandinternalizedinavesicle named “phagosome.” Then,lysosomeswhichareenrichedinantimicrobialenzymesarefusedintophagosomeleadingtothe formationof “phagolysosome.” Inthiscontext,pathogenkillingwouldoccurthroughtheenzymaticprocessorbytheinfluenceof antimicrobialpeptidespresentinphagolysosomes(Fig.1)(Flannaganetal.,2012).
Severalreceptorshavebeenidentifiedthatstimulatethephagocytosisandintracellularkillingofpathogens. “Dectin-1” is expressedbymacrophages,neutrophils,anddendriticcellswhichrecognizesthepolymersofglucoseespeciallyonthefungalcell wall(Herreetal.,2004; DambuzaandBrown,2015).Furthermore, “Mannosereceptor” (MR)isexpressedbymacrophagesand dendriticcellswhichrecognizesmannoseresiduesinbacteria,virus,andfungi(Ezekowitzetal.,1990).Also, “Scavengerreceptor” (SR)onmacrophagesidentifiesdifferentanionicpolymersandlow-densitylipoproteins(LDL)(Peiseretal.,2000).
Phagocytosiscouldbeenhancedbybindingofsomecomponentsnamed “opsonins” tothepathogen.Immunoglobulinsand complementfragments(e.g.,iC3b)areidentifiedasthekeyopsoninmoleculeswhichinduceefficientphagocytosis(Rosalesand
Fig.1 Theeffectormechanismsofinnateimmunesystem. Ig,Immunoglobulin; ADCC,antibody-dependentcellcytotoxicity; NK,naturalkillercell; Eos,eosinophil; MQ,macrophage; COM,complement.
Uribe-Querol,2017).Therefore,complementreceptors(CRs)andFcreceptors(FCRs)aretheotherreceptorsthatfacilitate phagocytosisandenhancepathogenkillingfunction(Fig.1)(Rosales,2007).
Duringthepathogenkillingbyphagocytosis,sometoxiccompoundsareproducedthatincludingsuperoxide O2 ðÞ,hydroxyl radical,nitricoxide(NO),hypochlorite(HOCl),andhydrogenperoxide.Thisprocessisaccompaniedbyamechanismknownas the “respiratoryburst” whichincludedatransientincreaseinoxygenconsumptionbythephagocytes.Thereisanothermechanism topathogentakeupknownas “macro-pinocytosis. ” Inthisway,largeamountsofextracellularfluidalongwiththemicroorganism areingested(Uribe-QuerolandRosales,2017).
Inflammatoryresponse:Aprocessinducedbypathogenrecognitionandtissuedamage
Recognitionofpathogensordamage-relatedmoleculeselicitsaninflammatoryresponsewithininitialhoursofinfectionthatresults inincreasedinfiltrationofleukocytestotheaffectedtissueaswellaselevatedproductionofacutephaseproteins(APPs).This responseisaccompaniedbyheat,redness,pain,andswellingatthesiteofinfection(KotasandMedzhitov,2015; Nathan,2002). Interestingly,inflammatorysignalinginducesbloodclottinginsmallvesselsatthesiteofinfectionwhichplaysanimportantrolein preventingthedisseminationofinfectionthroughoutthebody(Levietal.,2003).
Duringinflammatoryresponse,immunecellsproduceavarietyofinflammatorymediatorsincludingtheproteinsorglycoproteinsnamed “cytokines” and “chemokines.” Interleukin-1(IL-1),IL-6,andtumornecrosisfactor-a (TNF-a)arethemostimportant pro-inflammatorycytokinesproducedbyinnateimmunecells.Thesemediatorsincreasethevascularpermeabilityandthe recruitmentoftheotherinnateoradaptiveimmunecellsleadingtothepromotionofimmuneresponseagainstpathogenorinjury (Gilroyetal.,2004; HeadlandandNorling,2015).Inthiscontext,inflammatoryresponseinducestheexpressionofcell-adhesion moleculesontheendothelialcellsleadingtothepromotionofleukocytesbindingandtheirmigrationfrombloodstreamto affectedtissue,amechanismknownas “extravasation.” Meanwhile,Neutrophilsarethefirstleukocytesattractedtotheinfectionsite (Mitroulisetal.,2015).
Surfacemoleculesofmicroorganismsalsoactivatecomplementsystemleadingtoproductionofcomplement-associated moleculessuchasC5aorC3awhichactas “anaphylatoxins” andincreasetherecruitmentofimmunecellstotheinfectionsite (Merleetal.,2015).
Complementsystem
Complementsystemasapivotaleffectormechanismofinnateimmunesystemiscapableofdestructionofavarietyofmicroorganismsincludingbacteria,viruses,andparasites.Thissystemconsistsofmorethan30solubleandcellsurfaceproteins(Fig.1) (Merleetal.,2015).Uponstimulation,theseproteinsbeingactivatedandinitiateacascadewhichresultsin:
1.Pathogendestructionbyformingporesonthecellmembraneofmicroorganism
2.Increasedrecruitmentofinflammatoryimmunecellsbyproductionof “anaphylatoxins” 3.Enhancedphagocytosisbyproductionofopsonins
Thecomplementsystemcouldbeactivatedbythreepathwaysincluding:
1.Classicalpathway:activatedbytheimmunecomplexesofantigen-antibody
2.Alternatingpathway:activatedbythespontaneoushydrolysisofC3component
3.Lectinpathway:activatedbytherecognitionofcertaincarbohydratesonthemicrobialsurface
Animmunecomplexwhichconsistsofantibodies(IgG1orIgM)attachedtothecellsurfaceofpathogensiscapableofactivatingthe classicalpathway.Inthismanner,C1complex(C1q,C1r,C1s)bindstotheFcregionofantibodies.Uponactivation,C1sactsasa serineproteaseandcleavesC4andC2intoC4a,C4bandC2a,C2b,respectively.Subsequently,C4balongwithC2aformC4bC2a complexwhichinteractswithpathogensurface.Thiscomplex(C4bC2a)actsasanenzymenamed “C3convertase” whichcleavesC3 intoC3aandC3bfragments.Thisstepisthepointinwhichallcomplementpathwaysconverge.C3bfragmentcovalentlybindsto themicrobialsurfaceanditsassociationwithC4bC2amakesacomplex(C4bC2AC3b)namedC5convertasewhichinturnconverts C5intoC5aandC5b.BindingofC5btothemicrobialsurfaceleadingtoactivationofothercomponentsincludingC6,C7,C8,and C9.Ultimately,polymerizationofC9onthepathogensurfaceresultsintheformationofseveralporesnamed “membraneattack complex” orMACwhichcandirectlylysetargetedpathogen.Thelectinpathwayissimilartoclassicalpathway,butitsactivationis antibody-independent.Inthispathway,patternrecognitionreceptors(PRRs),suchasmannose-bindinglectin(MBL)andficolins recognizethecarbohydratesonthemicrobialsurface.MBLiscomplexedwithMBL-associatedserineproteases(MASPs)-1,-2,and-3 whichtheiractivationresultsincleavageofC2andC4,andsubsequentlythegenerationoftheC3convertase(C4bC2)ofboth classicalandlectinpathways.ThealternativepathwayisactivatedwiththespontaneoushydrolysisofC3intoC3aandC3b.Inthe presenceofmicrobialinfection,C3bbindstothemicrobialsurface.Subsequently,theassociationofBandDfactorswithC3bleads toadditionalC3cleavageandformationofalternativepathwayC3convertase(C3bBb)andC5convertase(C3bBbC3b).Aprotein named “properdin” stabilizesalternativepathwayconvertasesandfacilitatesalternativeactivation.AnaphylatoxinsincludingC4a/ C3a/C5aarepotentinflammatorymoleculeswhichpossessseveralfunctionssuchasincreasesinvascularpermeability,leukocyte infiltration,smoothmusclecontraction,phagocytosisandproductionofinflammatorymediatorssuchaspro-inflammatory cytokines(Merleetal.,2015).
Antibody-dependentcellcytotoxicity(ADCC):Anextracellularkillingmechanism ADCCcouldbeconsideredasanextracellularkillingmechanismresultinginpathogenelimination.Thisprocessrequires: (1)immunecellsexpressingFcreceptorontheirsurface,and(2)targetantigencoatedbyantibody(Fig.1).Immunoglobulin G(IgG)anditsreceptornamedFcgRIII(CD16)playanimportantroleinADCCactivation.NKcellswhichexpresshighlevelsof CD16areregardedasthekeyplayersinthisprocess.However,inadditiontoNKcells,macrophages,neutrophils,andeosinophils areabletomediatingADCC(Hubertetal.,2011; Sidersetal.,2010; Valeriusetal.,1990).
IgGpossessabifunctionalstructurewhichisrelatedtothefragmentantigen-binding(Fab)andFcportionsofantibody.ADCCis initiatedbytheengagementofFabandFcregionsofantibodieswiththepathogenandFcgRoneffectorcells,respectively. Subsequently,degranulationofeffectorcells(mainlyNKcell)leadingtopathogenlysis(Nigroetal.,2019).
Thecomponentsofinnateimmunesystem Physicalandchemicalbarriers
Theinnateimmunesystemincludesanatomicalandchemicalbarriersthatimpedemicrobesfromenteringthebody.Epithelialcells whichcomprisetheskinandtheliningoftherespiratory,gastrointestinal,andgenitourinarytractsactasthefirstlineofdefense againstpathogens.Inadditiontoformingthetightjunctions,epithelialcellsproducealargeamountof “ mucus ” enriched glycoproteinsknownas “mucins” whichtrapsmicroorganismsandpreventtheirdissemination(Fig.1).Epithelialcellsalsoprovide achemicalbarrierbyproducingvariouschemicalsubstanceswithantimicrobialproperties.DigestiveenzymesandacidicpHofthe stomach,lysozyme,phospholipaseA2,defensins,cathelicidins,andhistatinsareidentifiedastheimportantchemicalbarriersto infection(Schleimeretal.,2007).
“Lysozyme” isaglycosidaseenzymeintearsandsalivawhichhastheabilitytodisruptionofpeptidoglycanpresentinthe bacterialcellwallespeciallyinGram-positivebacteria.Panethcells,atypeofepithelialcellsinthesmallintestineproduceseveral microbiocidalproteinssuchaslysozymeandphospholipaseA2responsibletokillingthepathogensinthegutlumen(Sukhithasri etal.,2013).
“Defensins” asthecationicpeptidesformaporeinthecellmembraneofpathogenslikebacteriaandfungiaswellastheviruses’ envelope.Twodistincttypesofdefensinswithdifferentactivitiesandstructureareimplicatedinhumansincluding a-and b-defensins.Neutrophilsandpanethcellsproduce a-defensins.Theproductionof b-defensinsisinducedbyinfectionconsiderably inskin,airwayandurogenitaltracts(GanzandLehrer,1997; OuelletteandSelsted,1996).
“Cathelicidins” areantimicrobialpeptidesproducedbymacrophages,neutrophils,mastcells,NK,T,B,sebocytes,andepithelial cellsinresponsetoinfection(RobyandDiNardo,2013).
“Histatins” astheimportantanti-fungalpeptideshaveakeyroleinhostdefenseintheoralcavity.Histatinsareprimarily producedinresponsetopathogenicfungisuchas Cryptococcusneoformans and Candidaalbicans (EdgertonandKoshlukova,2000).
Innateimmunecells
Themostcellsoftheinnateimmunesystemarederivedfrommyeloidlineageincludingneutrophils,eosinophils,basophils,mast cells,monocytes,macrophages,anddendriticcells.Whileothers,includingNKcells,naturalkillerT(NKT)cells,andinnate lymphoidcells(ILCs)areoriginatedfromlymphoidlineage.Thesecellswithdistinctphenotypescontributetopathogenkilling throughespecialfunctions. Table1 liststheinnateimmunecellsandtheireffectormechanismsinhostdefense.
Monocyteandmacrophage
Monocytesandmacrophagesaretheimportantcomponentsofinnateimmunesystemwhichpossessvariousinflammatoryand regulatorycapabilities.Thesecellsareknownasprofessionalphagocytesandplaythekeyrolesincludingcytokineproduction, antibody-dependentcellcytotoxicity(ADCC),antigenpresentation,Tcellproliferationandactivation,tissuerepairandregeneration,resultinginpathogeneliminationandtissuehomeostasis.
Monocytesaredevelopedinbonemarrowandthencirculateinthebloodstream.Thesecellsemigratetodifferenttissues throughoutthebodywheretheydifferentiatetomacrophagesandmyeloid-deriveddendriticcells.Threesubpopulationsof monocyteswithdistinctphenotypeshavebeenfoundinhumanswhichexhibitdifferentfunctions.Theseareincludingclassical (CD14++ CD16 ),intermediate(CD14++ CD16+),andnon-classical(CD14+ CD16++)monocytes(Ziegler-Heitbrocketal.,2010). Classicaltypecomprisesthelargestpopulationofmonocytesthatarehighlyskilledinphagocytosisandantibody-dependentcell cytotoxicity(ADCC).Thesecellsareimportantininitiatingtheinflammatoryresponses.Non-classicaltypesareknownas anti-inflammatorymonocytesthatareinvolvedinmaintainingvascularhomeostasis.Intermediatemonocyteshaveseveral
Table1 Themajorcomponentsandimportantfunctionsofinnateandadaptiveimmunesystems.
Immune system ComponentsFunctions
Innate immunity
Adaptive immunity
PhysicalandchemicalbarriersSuchasskin,mucusmembrane,chemicalsecretions
Preventpathogensfromenteringthebody,pathogenkillingviacytotoxicmediators
MonocytesandmacrophagesPhagocytosis,actasprofessionalAPC,ADCC,Tcellproliferationandactivation,tissuerepairandregeneration
DendriticcellsInitiatingandpolarizationoftheimmuneresponses phagocytosisormacropinocytosis actasprofessionalAPC,ADCC,Tcellproliferationandactivation
NeutrophilsMostnumerousinnateimmunecells,phagocytosis,ADCC, promotionofbothinnateandadaptiveimmuneresponses formationofNETs
Eosinophils,Basophils,Mast cells defenseagainstparasiticinfectionsandallergicreactions,ADCC
Innatelymphoidcellspromotiontheadaptiveimmuneresponses,respondingtopathogenictissuedamage,tissuehomeostasis, repairandregeneration
Naturalkillercellsstrongcytotoxicfunctionimportantlyagainstvirus-infectedcellsandcancerouscells,ADCC
NaturalkillerTcellsrecognizethelipidderivativesofpathogenspresentedbyCD1molecule,activationofothercellsininnateand adaptiveimmunesystem
BcellsB1cellsProductionofnaturalantibodieswithoutthepresenceofanyinfections
FollicularBcellsMostfrequentBcellsinthecirculation,productionofthemajorityofantibodiesduringaninfection
Marginalzone
Bcells Defenseagainstblood-bornpathogens
TcellsCD4+ ThelperTh1:Defenseagainstintracellularpathogens,cytokineproduction,enhancephagocytosis,inducingBcellsto makeIgG1andIgG3antibodies
Th2:Defenseagainstextracellularpathogens,cytokineproduction,inducingBcellstomakeIgG4andIgE antibodies
CD8+ TcytotoxicDefenseagainstintracellularpathogensandcancerouscells,enhancephagocytosis
AntibodiesIgGThemajorantibodyinvolvedinopsonizationandneutralization
IgGhavesoursubclasses:IgG1,IgG2,IgG3,IgG4
IgAHighlevelsinmucosaltissues,saliva,tear,breastmilk Haveneutralizationactivityandmoderateopsonizationcapacity,complementactivation
IgMThefirstproducedantibodyuponinfection ActsasmembraneboundreceptorforBcell Excellentcomplementactivation Noopsonization,moderateneutralization
IgDMembraneboundreceptorforBcell Noopsonizationandneutralizationactivities
IgEThemajorantibodyinvolvedinparasiticinfectionsandinflammatoryconditionsincludingallergyandasthma Noopsonization,moderateneutralization
APC,Antigenpresentingcell; ADCC,antibody-dependentcellcytotoxicity; TH,Thelper; Ig,immunoglobulin; NETs,neutrophilextracellulartraps.
functionsincludingtheproductionofreactiveoxygenspecies(ROS),Tcellactivationbycytokineproductionandantigen presentation(Sampathetal.,2018).
Macrophagesarefoundapproximatelyinalltissuesinthebody.Thesecellsarecapabletorecognitionofthepathogensor damagedcellsbyseveralsurfacereceptorssuchasSR,TLR,andNLR(Tayloretal.,2005).
Macrophagescouldbeclassifiedintotwoimportantsubtypes,including “M1orclassicallyactivatedmacrophages” and “M2or alternativelyactivatedmacrophages” thataredifferentintheirsurfacemarkers,producedcytokines,andfunctions(Yaoetal.,2019).
M1macrophagesexertpro-inflammatory,anti-microbialandanti-tumoralactivities,whileM2macrophagesexhibit anti-inflammatoryandimmunomodulatoryproperties,tissue-repairing,pro-tumoralandpro-angiogenicfunctions,andpossess effectivephagocyticactivity.
Macrophagepolarizationishighlydependentonthesignalsinthemicroenvironment.Forexample,LPSandcytokinesofTh1 suchasinterferongamma(IFN-g)andTNF-a areidentifiedasthemainstimulatorofM1polarization.Ontheotherhand,M2 macrophagesarethoughttobeassociatedwithTh2cytokinessuchasIL-4,IL-13,IL-10,IL-33,andTGF-b (Yaoetal.,2019).
M1macrophagesareproducedinresponsetointracellularbacterialike Mycobacteriumtuberculosis, Listeriamonocytogenes, Salmonellatyphi, Salmonellatyphimurium (Benoitetal.,2008).ThesecellsarecharacterizedbytheexpressionofCD80(B7.1), CD86(B7.2),MHCII,TLR2,andTLR4.Duringinflammatoryresponses,M1macrophagesproducepro-inflammatorycytokinesand chemokinessuchasIL-1,IL-6,TNF-a,IL-12,CCL15,CCL20,CXCL9,andCXCL10uponligandrecognitionandpromotethe Th1-relatedresponses(Yaoetal.,2019).
M2macrophagesaremostlyrelatedtochronicinfectiousdiseaseslikechronicbrucellosis,chronicmycobacterialinfections (Benoitetal.,2008).M2macrophagessecreteanti-inflammatorycytokinessuchasIL-10,TGF-b andtheycanstimulateTh2 responses.Thesecellsexpressthemarkersincludingmannosereceptor(CD206),CD163,CD209(DC-SIGN)(Yaoetal.,2019).
Dendriticcell
Dendriticcellsaretissue-residentorcirculatingleukocyteswhichknownastheimportantcellsinmakingalinkagebetweeninnate andadaptiveimmuneresponses.Thesecellsareimportantininitiatingandpolarizationoftheimmuneresponses.Severalfunctions ofDCsareincluding:
1.Pathogenuptakethroughphagocytosisorpinocytosis
2.AntigenprocessinganditspresentationtoTcellsresultingintriggeringtheadaptiveimmuneresponses;thesecellsareknownas theprofessionalantigenpresentingcells(APCs)
3.Cytokineproduction
Functionally,DCsaredividedintotwotypesincludingimmatureandmatureDCs(Hawigeretal.,2001; Steinmanetal.,2003). ImmatureDCsareskilledinantigen(Worbsetal.,2017)phagocytosisorpinocytosis,whilematureDCshavetheabilitytoactivate Tcellresponses.DCmaturationisinitiatedbytherecognitionofpathogensordamage-associatedmoleculesbymediatingseveral receptorssuchasTLRs(HemmiandAkira,2005; Cerbonietal.,2013).Subsequently,theymigratefromperipheraltissuesto secondarylymphoidorgans(e.g.,lymphnode)wheretheycanactivateTcellresponsesbyactingasanAPC.MatureDCexpresshigh levelsofMHCII,CCR7,andco-stimulatorymoleculeswhichareessentialforTcellactivation.Besides,increasedlevelsof pro-inflammatorycytokineshaveshowntobeproducedbymatureDCscomparedwithimmatureDCs.Theinteractionbetween DCsandTlymphocytesleadingtodifferentiationofTcellstodifferentsubtypesincludingTh1,Th2,Th17,orregulatoryTcells (Treg)whichcanbeinfluencedbycytokineprofileinthetissuemicroenvironmentorthetypeofrecognizedpathogen.Forexample, highlevelsofIL-12andIL-4inmicroenvironmenthaveshowntobeassociatedwithTh1andTh2differentiation,respectively (Patenteetal.,2019).
HeterogeneouspopulationanddifferentsubtypesofDCshavebeenidentifiedwithdifferentorigin,phenotypes,andbiological functions.TwomainsubtypesofDCsareincludingconventional(cDCs)andplasmacytoiddendriticcells(pDCs).cDCs(CD11c+ CD123 )areregardedashavinghighabilityforantigenuptakingandpresentationtonaïveCD4+ Tcellsandshowntoproduce pro-inflammatorycytokines(Haniffaetal.,2013).pDCs(CD11c CD123+)arewell-knownfortheproductionoftypeIinterferon (IFN-I)duringviralinfection,althoughtheyalsohaveotheractivitiessuchasstimulationofTcellsandNKcellsandtheproduction ofpro-inflammatorycytokines(SwieckiandColonna,2015).IncontrasttoconventionalDCs,thesecellsareabundantlyfoundin bloodstream.
Inaddition,monocytescouldbedifferentiatedtodendriticcellsknownasmonocytes-derivedDCs,duringinfectionand inflammation(Schlitzeretal.,2015).
Neutrophil
Neutrophilsareregardedasthemostnumerousinnateimmunecellswhichactasthefirstlineofdefenseagainstinfection. Inresponsetopathogens,neutrophilsrapidlymigratefromthebloodstreamtothedamagedtissue.CXCL8asachemotacticfactor playstheimportantroleintheirrecruitment.Thesecellsplaytheimportantroleininitiationoftheinflammatoryresponse importantlybyphagocytosisandreleasethecytotoxicgranules(Rosalesetal.,2016).Somepathogenssuchas Staphylococcusaureus isshowntoevadephagocytosisandthereforehavethepotentialtosuppressdefensebyneutrophils(KumarandSharma,2010). Neutrophilsdisplaytheimportantfunctionsresultinginthepromotionofbothinnateandadaptiveimmuneresponses. Eliminationtheextracellularpathogensbyphagocytosisisthemostimportantfunctionofneutrophils(Rosalesetal.,2017). UponstimulationwithvariousstimulisuchasmicrobialcomponentsorincreasedlevelofintracellularCa2+,neutrophilsrelease
theirgranulesbyaprocessknownas “exocytosis” resultinginpathogenkilling.Theirgranulesenrichedwithvariousantimicrobial moleculessuchascationicpeptides,proteases,lactoferrin,myeloperoxidase,gelatinase,cathepsinG,elastase,proteinase3,and azurocidin(KumarandSharma,2010).
Deficiencyinneutrophil-associatedgranulesleadstotheincreasedsusceptibilitytoGram-positiveorGram-negativebacterial infections.Forexample,cathepsinG-deficientandelastase-deficientmicearemoresusceptibletothebacterialinfectionsby Staphylococcusaureus and Escherichiacoli,respectively(KumarandSharma,2010).
Inadditiontophagocytosis,neutrophilscanfightagainstpathogensbyformingneutrophilextracellulartraps(NETs).FormationofNETsisimportanttolocalizingtheearlyinfection.NETsareconsistingofneutrophilnuclearmaterial(e.g.,chromatin), cytoplasmicandgranularproteins.Extracellularreleaseofthesecomponentsleadstobacterialdegradation.NETsplayanimportant roleindefenseagainstinfectionsincluding Shigellaflexneri, Mycobacteriumtuberculosis, Streptococcuspyogenes, S.aureus, Candida albicans,fungus,andparasitessuchas Leishmaniaamazonensis (KumarandSharma,2010).
Eosinophil
Eosinophilsaretheimportantcellsindefenseagainstparasiticinfectionsandallergicreactionssuchasasthma,aswellasintissue homeostasisincludingtissuerepairandremodeling.Uponstimulationbyseveralstimulisuchascytokines,complementsystem proteins,andimmunoglobulins,eosinophilsareinfiltratedfromthebloodstreamtotheinflamedtissue(Kita,1996).Th2immune responseandtheircytokinessuchasIL-4,IL-5,andIL-13havethesignificantroleineosinophilrecruitmentandactivation(Sher etal.,1990; Horieetal.,1997).
Theimportanteffectorfunctionsofeosinophilsareincludingcytokineproduction,releasingcytotoxicgranules,antibody-and complement-mediatedcytotoxicity,phagocytosis,andeosinophilextracellularDNAtraps.Eosinophilsproduceseveralcytokines andlipidmediatorsinresponsetopathogensresultinginthevascularpermeability,constrictionofsmoothmuscle,andincreased secretionofmucus.TheseareincludingIL-6,IL-2,IL-8,TNF-a,INF-g,TGF-a/b,GM-CSF,platelet-activatingfactor(PAF),leukotriene C4(LTC4),andmatrixmetalloproteinases(MMPs).Uponactivation,eosinophilsreleasetheirgranulescontainingdifferent tissue-destructivemediatorswhichcontributetopromotetheinflammatoryresponseandkillingparasiticpathogens.Theseinclude eosinophilcationicprotein(ECP),eosinophilperoxidase(EPO),majorbasicprotein(MBP),andneurotoxinderivedfrom eosinophils(EDN)(Shamrietal.,2011).
IgG1andIgG3immunoglobulinswerefoundtobeeffectiveinpromotingeosinophil-mediatedkilling.Theseantibodiesby interactingwithimmunoglobulinreceptornamedFcgRIIexpressedoneosinophils,inducedegranulationofeosinophils.This processiswell-knownasADCCwhichregardedasamainextracellularkillingmechanism(Kanekoetal.,1995).
Inaddition,eosinophilsareshowntoexhibittheantibacterialrolebyreleasingthematerialsconsistofmitochondrialDNAas wellascytotoxicgranule-derivedcomponentswhichactsastrapsinordertopathogenkilling.LPSofGram-negativebacteria considerablyinduceeosinophil-derivedextracellulartraps(Yousefietal.,2008).
Basophil
Basophilsarethemajorimmunepopulationsinvolvedinallergyresponseandhypersensitivityreactionsincludingasthma, anaphylaxis,andallergicrhinitis.Besides,theycontributetoimmunityagainsthelminthesandbacterialrespiratoryinfection (Chirumboloetal.,2018).Basophil-mediatedresponsescouldalsobeevokedbyseveralbacterialinfectionssuchas S.aureus, non-hemolyticstreptococci, Haemophilusinfluenzae, S.enteritidis and E.coli (AbrahamandArock,1998).
ThesecellsarecapabletoactivateadaptiveimmuneresponsesimportantlythestimulationofTh2immuneresponses.Basophils produceIL-4andIL-13cytokineswhichpromoteTh2responsesresultinginhypersensitivityreactions.Uponactivationbyseveral stimulisuchasimmunoglobulinE(IgE)binding,basophilsreleasetheirgranulescontaininghistamine,heparin,andserine proteases.Inaddition,theycansynthesizeandreleaseothermediatorsincludingleukotrienesandprostaglandins(Chirumbolo etal.,2018).
Mastcell Mastcellsareprimarilyinvolvedinpathophysiologyofallergicreactionsandtheirfunctionalmechanismsaresimilartobasophils. Incontrasttobasophilswhicharefoundincirculatorysystem,mastcellsaremostlylocatedintheskin,mucosalmembranesand aroundbloodvessels.Mastcellspossesscentralfunctionsinresponseagainstbacterialinfectionsandcertainparasiticinfections. Theycandirectly(opsonin-dependent)orindirectly(opsonin-independent)bindtothebacteriaorparasites.Intheindirect recognition(oropsonin-dependent),mastcellsrecognizetheopsonizedpathogenswithcomplementfragments(e.g.,iC3b)or immunoglobulins. Salmonellatyphimurium andthehelminth, Schistosomamansoni havebeenreportedtorecognizebymastcellsvia thismechanism.Indirectinteraction,mastcellspossessastrikingcapacitytorecognizeandbindtopathogenthroughtheir receptorswithoutmediatingopsonins.Forexample, E.coli and K.pneumonia and Leishmania parasiteshavebeenrecognizedto interactdirectlywithmastcells(AbrahamandArock,1998).
Similartobasophils,mastcellsarehighlyskilledforthesynthesisandproductionofseveralactivemediatorsincluding histamine,heparin,serineproteases,leukotrienes,prostaglandins,cytokines,andchemokinesinresponsetocertainmicroorganisms(Gallietal.,1991; Galli,1993).TheyproducevariouscytokinesincludingIL-1,IL-6,TNF,IL-4,IL-5,IL-13,andchemokines MCP-1,MIP-1,RANTES.Thesemediatorsplaycentralrolesinthepromotionofinflammatoryresponses,pathogenkilling, enhancedphagocytosis,anddevelopmentofadaptiveimmuneresponses.Besides,granuleexocytosiscontributeinpathogenesis ofseveralallergicdisorderscausinghypersensitivitysymptoms(AbrahamandArock,1998).
Innatelymphoidcells(ILCs)
ILCsareafamilyofinnateimmunecellswhichprimarilyconsideredastheinnatecounterpartsofTcells.Theyaretissueresident cellsandabundantlyfoundinmucosalsurfacesespeciallyinintestineandlung,andcontributeinmucosalimmunityandtissue homeostasis.ILCsareinvolvedinseveralfunctionsincludingpromotiontheadaptiveimmuneresponses,respondingtopathogenic tissuedamage,tissuehomeostasis,repairandregeneration(MjösbergandSpits,2016; Ebboetal.,2017).
ILCsaredividedintothreemaingroupsbasedontheirphenotype,cytokinepattern,andtheirdevelopmentalpathways, includingILC1,ILC2,andILC3(Vivieretal.,2018).ILC1saresignificantlycontributedtothepromotionofmacrophagesand DCsactivitiesandTh1responses.Inthiscontext,theyproduceIFN-g inresponsetoup-regulationofIL-15andIL-12during infectionortissueinjury.IFN-g enhancestheeliminationofintracellularbacteriaaswellasantigenpresentationbymacrophages andDCs(Fuchsetal.,2013).
ILC2sareinvolvedintheimmuneresponseagainstparasitesinfectionandrepairthedamagecausedbyparasitisorviral infections.TheysecretecytokinessuchasIL-4,IL-5,IL-9,IL-13,andamphiregulininresponsetoIL-25,IL-33,andTSLPwhich promoteTh2responsesandinnatedefenseagainstparasites(PandaandColonna,2019).
ILC3splayanimportantroleinresponsetoextracellularbacteriaandfungi.TheyproduceIL-22andIL-17inresponsetoIL-23 andIL-1b andinduceTh17responsesadneutrophilrecruitment.ThesecellsbytheproductionofIL-22contributeintheup regulationofantimicrobialpeptidesandenhancementofbarrierintegrityespeciallyintheintestine(Aujlaetal.,2008; Sugimoto etal.,2008; Zhengetal.,2008).
Naturalkillercell(NK)
NKcellsareasubsetofinnatelymphocytecellsthatcharacterizebythestrongcytotoxicfunctionimportantlyagainstvirus-infected cellsandcancerouscells.ThedominantpopulationofNKcellsexpressCD16andCD56molecules(Lanieretal.,1986,1989). InrespecttotheNKcellphenotypes,twomajorsubsetsofNKcellsareidentifiedinhumansinaccordingtotheexpressionofCD16 andCD56markersasfollows(Stabileetal.,2017):
1.CD16HighCD56lowNKcellspossesshighlevelsofperforinmediatingcellularcytotoxicityleadingtotheenhancementof pathogenkilling
2.CD16+/-CD56HighNKcellsarecharacterizedbythelowlevelsofperforinandtheyarehighlyskilledincytokineproduction NKcellshavemultipleactivating(e.g.,KIR2DL,KIR3DL,NKG2A,CD85,andCD244)andinhibitoryreceptors(e.g.,NKG2C, NKG2D,KIR2DS,KIR3DS,NKP30,NKP44,andNKP46)allowthemtorecognizethetargetcells.Uponactivation,NKcellsproduce severalcytokinesandchemokinesincludingIFN-g,TNF-a,granulocytemacrophagecolony-stimulatingfactor(GM-CSF), CCL1-CCL5,andCXCL8leadingtoregulationofthefunctionofotherinnateandadaptiveimmunecells(PaulandLal,2017).
MHCclassImoleculewhichexpressnormallyonhealthycellsisconsideredasaligandforNK-inhibitoryreceptorandtheir interactionresultsinNKcelltoleranceandinactivation.Ontheotherhand,decreasedlevelofMHCIisexpressedonthe virus-infectedcellsortumorcellsleadingtoengagementoftheactivatingreceptorsandpropagatescytotoxicactivityofNKcells (Lanier,2008).
NKcellcytotoxicresponseisdependentonforminganimmunologicalsynapsebetweenNKcellandtargetcell.Uponactivation, NKcellsreleasetheircytotoxicgranulescontaining “perforin” and “ granzyme. ” Releasedperforinbypolymerizationonthecell membraneoftargetcellsformstheporeswhichfacilitatestheentryofgranzymesintothetargetcells.Granzymeasaserineprotease activatestheenzymesknownas “ caspase ” responsibletoinductionofcelldeathor “apoptosis” oftargetcells(TophamandHewitt, 2009).NKcell-mediatedapoptosisoftargetcellscouldalsoperformbyengagementthereceptorsnamed “deathreceptor.” These receptorssuchasTNFreceptorandFasreceptorbindtocognateligandsontargetcellsandinduceapoptosisoftargetcells(Sonar andLal,2015; Thorburn,2004).
OneoftheimportantNKcellreceptors,CD16orFcgRIII,mediatesanextracellulardeathnamedasantibody-dependent cell-mediatedcytotoxicity(ADCC).ThisreceptorbybindingtoFcregionofantibodiesespeciallyIgG,inducesthedegranulation ofNKcellsandpromotekillingthetargetcells(Mandelboimetal.,1999).
NaturalkillerTcell(NKT) NKTcellsareasubgroupofinnateimmunecellswithcytotoxicfunctionswhichexpressNKcellmarkersandatypeofTlymphocyte receptornamed ab Tcellreceptoror ab TCR.ThesecellscanearlyactivateinresponsetoIL-12andIL-18producedduringmicrobial infectionsandisresponsibletorecognizethelipidderivativesofpathogenspresentedbyCD1molecule,anon-polymorphicMHC classImoleculeonAPCs.Uponactivation,NKTcellssecreteIFNg andIL-12resultingintheactivationofothercellsininnateand adaptiveimmunesystemincludingTcells,NKcells,andDCs(TerabeandBerzofsky,2008).Gramnegative-derivedLPSisimplied astheimportantstimulusforactivatingNKTcells.However,LPS-negativebacteriaalsohavetheabilitytoinduceNKTresponses (VanKaerandJoyce,2005).NKTcellsarealsocontributedtoresponseagainstparasiticinfectionslike Leishmania andviral infections(TerabeandBerzofsky,2008).
Adaptiveimmunity Adaptiveimmunity,alsoknownasacquiredimmunityorspecificimmunityisthesecondlineofdefensiveresponseagainst invadingpathogens.Thistypeofimmuneresponseoccurstoattacknon-selfpathogenshoweversometimesmaymistakenlyattack
self-tissueswhichresultsinautoimmunediseasessuchaslupus,rheumatoidarthritis,andetc.Thedifferencebetweenadaptive immunityandinnateimmunityisthattheadaptiveimmuneresponseisnotimmediatelystartedasinnateimmuneresponse occurs.However,theeffectoftheadaptiveimmuneresponseislong-lastingandhighlyspecific.
Theadaptiveimmunesystemcanlearnandremembersspecificpathogens,thusitcanprovidelong-lastingdefenseand protectionagainstrecurrentinfections.Whiletheadaptiveimmunesystemisencounteredtoanewthreat,someantigensare memorizedtopreventfromgettingthediseaseagain.Oneexampleofimmunologicalmemoryisvaccinations.Bothattenuated wholevirus/bacteriaandtheirparticlescannotactuallycauseanactiveinfectionbutcauseanimmuneresponse.Inotherwords,fail intheimmunologicalmemorysystemcanresultinautoimmunediseases.Molecularmimicryofaselfantigenbyaninfectious foreignpathogen,includingvirusesandbacteria,cantriggerautoimmunediseasebecauseofacross-reactiveimmuneresponse againsttheinfection. Streptococcus infectionisagoodexampleinwhichaforeignorganismusesmolecularmimicrytohidefrom immunologicalresponses(Chaplin,2010).Furthermore,theactivityoftheadaptiveimmunesystemreliesontheclonalexpansion ofTandBlymphocytesandalsoimmuneresponsemediators.
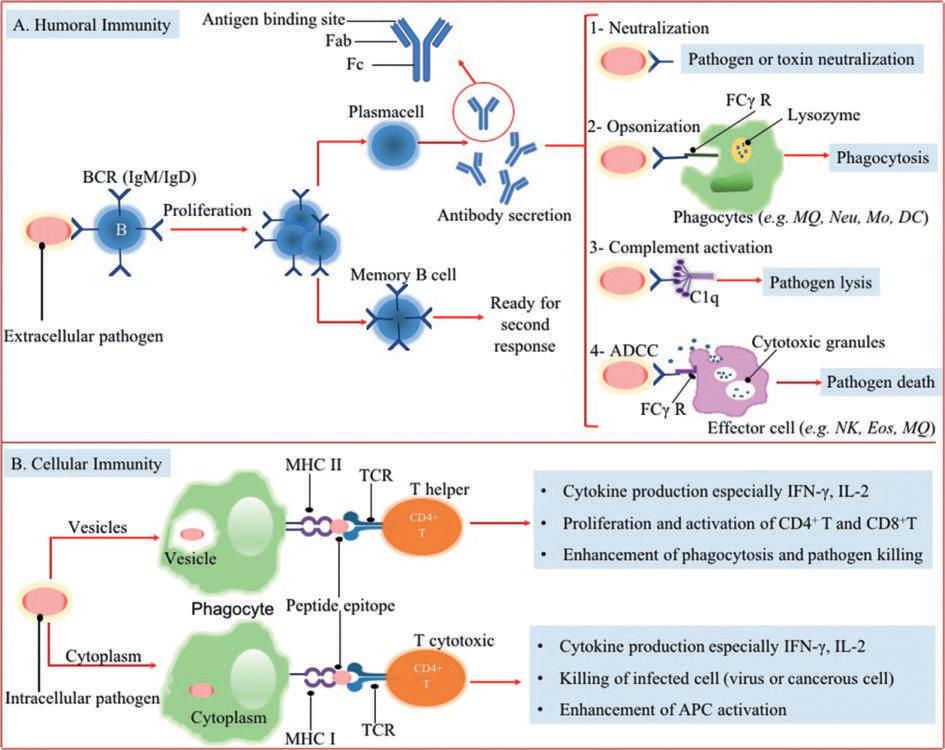
Inoverall,therearetwotypesoftheadaptiveimmunesystem(Fig.2):
1.Cell-mediatedimmunity(orcellularimmunity)thatoccursagainstcellsinfectedwithviruses,intracellularbacteria,and cancerouscells,andmediatedbyhelperandcytotoxicTlymphocytes.
2.Thehumoralimmunity(orantibody-mediatedimmunity)isanefficientimmunityagainstextracellularorcirculatingantigens. ThistypeofimmunityinvolvesBcellsdifferentiationintoplasmaBcellswithaidofType2helperTcells(TH 2)that subsequentlycanproduceimmunoglobulinscalledantibodies.
Inthissection,wediscussinmoredetailaboutthecomponentsofadaptiveimmunesystemandtheirmechanismsofactioninthe specificimmuneresponseagainstpathogens.
Fig.2 Theeffectormechanismsofadaptiveimmunesystemandtherelationbetweeninnateimmunityandadaptiveimmunity. BCR,Bcellreceptor; TCR,Tcell receptor; Fab,fragmentantigen-bindingregion; Fc,fragmentcrystallizableregion; FCg R,Fcgammareceptor(receptorforIgG); MQ,macrophage; Neu,neutrophil; MO,monocyte; ADCC,antibody-dependentcellcytotoxicity; MHC,majorhistocompatibilitycomplex; IFN-g,interferongamma; IL-2,interleukin-2; APC,antigen presentingcell.
Adaptiveimmunecells
TheadaptiveimmunesystemdependsonBandTcellsresponses.Thesecellsarelymphocytesderivedfromspecifictypesofbone marrowstemcells,knownasmultipotenthematopoieticstemcells.Uponformationinthebonemarrow,thesecellsneedtomature andactivate.Inthisregard,eachtypeofcellfollowsdifferentdevelopmentalstagestobecomemature.Tcellsmigratetothethymus inwheretheycompletetheirmaturationintofunctionalTcells.While,thedevelopmentofanimportantpartofBcellstakesplacein thefetalliverandbonemarrowandthentheimmatureBcellsmigratetothespleenfortheirfinalstagesofmaturation (Huston,1997).
BandTcellspossessantigen-specificreceptorswhichenablethemtorecognizespecificforeignantigens.Inthisrespect,both Bcellreceptor(BCR)andTcellreceptor(TCR)usetheprocessknownas “generearrangement” toprovidethenecessarydiversityof receptorspecificitiesduringdevelopmentalprocessofTandBcellsinthymusandbonemarrow,respectively(Gellert,2002).
Blymphocytes
ThematureBcellsornaïveBcellshavedistinctivesurfaceantigen-specificreceptors(BCR)calledmembrane-boundantibodies. ThesesurfaceantibodiesareprimarilyIgMandIgDwhichcanrecognizeepitopesassociatedwithdifferenttypesofantigenic structuresincludingproteins,polysaccharides,andlipopolysaccharides.Bcellreceptorsplayimportantrolesinantigenbinding, internalizationandprocessingoftheantigen,andinducingsignalingpathwayssuchasthecytokineproductionpathwayinorderto communicatewithothercellsoftheimmunesystem.
Bcellactivationoccursuponantigenrecognitioninthesecondarylymphoidorganssuchaslymphnodesorspleen.After bindinganantigentoBCR,BcellsquicklydividetoeitheramemoryBcelloraneffectorBcell(orplasmacell).MemoryBcells expressthemembrane-boundantibody,whileplasmaBcellscansecreteantibodies.Secretedantibodiesidentifyandneutralizethe freepathogenscirculatingthroughoutthebody(Fig.2)(Pieperetal.,2013).
Twosub-classesofBcellshavebeenidentifiedwithdistinctphenotypeandfunctionincludingB1andB2cells.B1cellsarefound intheperipheralorgansandtheylesscommonlypresentintheblood.Theyarehighlyskilledintheproductionofnatural antibodieswithoutthepresenceofanyinfections.B2cellsaredividedintotwotypesincludingBfollicular(BFO)andBmarginal zone(BMZ).BFOsarethemostfrequentBcellsinthecirculation.Theyproducethemajorityofantibodiesduringaninfection. BMZsarefoundprimarilyinthemarginalzoneofthespleenandtheyareinvolvedindefenseagainstblood-bornepathogens (Pieperetal.,2013).
Tlymphocytes
Unlikeantibodies,Tcellreceptorscan’tbindtoantigensdirectly.Theyrecognizetheantigensboundtothemembranesurface moleculesonAPCs,knownasMajorHistocompatibilityComplexclass1(MHCI)andclass2(MHCII).Duringarecombination processintheirgenes,Tcellreceptorsundergoagenerearrangementthatallowsthemfordiversebinding.However,inordertoan appropriateimmuneresponseandavoidmistakenlyattackagainstselfantigens,matureTcellsshouldrecognizeonlypathogens’ antigensboundtotheMHCmolecules.Therefore,Tcellsundergotwo “selectionprocesses” duringtheirdevelopmentalstagesin thethymus;PositiveselectioninwhichTcellsshouldbindself-MHC:peptidecomplexesandthusdistinguishbetweenselfand nonselfpeptidesandproteins.TcellsthatbindmoderatelytoMHCcomplexesonthethymiccellsreceivesurvivalsignals.Ifthese cellsbindtoostronglytotheseMHCcomplexes,theyfailthepositiveselectionandshouldbeeliminatedthroughapoptosis mechanismintheprocessof “negativeselection.” Negativeselectionguaranteesself-tolerancebytestingthespecificallybinding abilityofTcellstoself-MHC:peptidecomplexes.Thecriticalimportanceofthesetwoselectionprocessesistheprotectionofown cellsandtissuesagainstimmuneresponse,andotherwiseautoimmunediseaseswouldoccurmuchmorecommon(Chaplin,2010).
Effectormechanismsofadaptiveimmuneresponse
Inanadaptiveimmuneresponse,theeffectormechanismsbywhichpathogensareeliminatedareessentiallysimilartothoseof innateimmunity.Ontheotherhand,specificrecognitionbyclonallydistributedreceptorsduringanadaptiveimmuneresponse seemstobeevolvedasalateadditionalmechanismtoexistinginnatemechanismsofaction.
Antibodies:Theeffectormechanismofhumoralimmunity
Antibodies,astheproductsofplasmaBcellsinadaptiveimmunesystem,arefoundinthebodyfluidssuchaswholeblood,serum orplasma,andalsoextracellularfluids.Thus,immuneresponsemediatedbyantibodiesisknownashumoralimmuneresponse.
Asshownin Fig.2,anantibodymoleculehasY-shapedstructurewhoseidenticalarmsfunctionastwoantigen-bindingsites knownas “antigenbindingfragment” orFab.However,thesesitesarehighlyvariablefromanantibodymoleculetoanotherwhich resultsindiversespecificantigenrecognition.ThestemoftheYstructurewhichreferredas “fragmentcrystallizableregion” orFcisa constantregionwhichdeterminestheclassofthe antibody anditsfunctionalproperties.FiveclassesofantibodiesincludingIgG, IgM,IgD,IgE,andIgAhavebeenidentifiedthateachclassusesdiversedistinctsetofeffectormechanismsforrecognitionand eliminationofantigens(Table1).Throughamechanismknownas “neutralization,” antibodiesneutralizepathogensortheirtoxic productsbybindingtothemandthuspreventingthemtoentercellsandsubsequenttheirinfection.Thismechanismiscriticalfor protectionagainstbacterialtoxinsandalsopathogenssuchasviruses.Anothermechanismbywhichantibodiescanresponseto pathogensisknownas “opsonization. ” Byopsonization,antibodiesenablephagocytesforingestinganddestroyingtheextracellular