Electronicmaterials:principlesandapplied sciencePoplavko
https://ebookmass.com/product/electronic-materialsprinciples-and-applied-science-poplavko/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Principles of Electronic Materials and Devices 4th Edition
Safa O. Kasap
https://ebookmass.com/product/principles-of-electronic-materials-anddevices-4th-edition-safa-o-kasap/
ebookmass.com
Mesenteric Principles of Gastrointestinal Surgery: Basic and Applied Science Coffey, John Calvin, Sehgal, Rishabh, Walsh, Dara Mesenteric Principles Of Gastrointestinal Surgery: Basic And Applied Science Coffey
https://ebookmass.com/product/mesenteric-principles-ofgastrointestinal-surgery-basic-and-applied-science-coffey-john-calvinsehgal-rishabh-walsh-dara-mesenteric-principles-of-gastrointestinalsurgery-basic-and-applied-scie/ ebookmass.com
Lectures on Digital Design Principles (River Publishers Electronic Materials, Circuits and Devices) 1st Edition
Pinaki Mazumder
https://ebookmass.com/product/lectures-on-digital-design-principlesriver-publishers-electronic-materials-circuits-and-devices-1stedition-pinaki-mazumder/ ebookmass.com
Temel (How the Aliens Were Won Book 2) Honey Phillips
https://ebookmass.com/product/temel-how-the-aliens-were-wonbook-2-honey-phillips/
ebookmass.com
The Deepest Black Randall Silvis https://ebookmass.com/product/the-deepest-black-randall-silvis/
ebookmass.com
The Palgrave Handbook of Imposter Syndrome in Higher Education Michelle Addison
https://ebookmass.com/product/the-palgrave-handbook-of-impostersyndrome-in-higher-education-michelle-addison/
ebookmass.com
Diagnostic Imaging: Gynecology 3rd Edition Akram M. Shaaban
https://ebookmass.com/product/diagnostic-imaging-gynecology-3rdedition-akram-m-shaaban/
ebookmass.com
Victimology: The Essentials 2nd Edition, (Ebook PDF)
https://ebookmass.com/product/victimology-the-essentials-2nd-editionebook-pdf/
ebookmass.com
Sustainable Development and Energy Transition in Europe and Asia, Volume 9 Bernadette Andreosso-O'Callaghan
https://ebookmass.com/product/sustainable-development-and-energytransition-in-europe-and-asia-volume-9-bernadette-andreossoocallaghan/
ebookmass.com
Midlife Stolen Mate: A Fated Mate Shifter Romance (Bear Mates Over Forty Book 9) Aline Ash
https://ebookmass.com/product/midlife-stolen-mate-a-fated-mateshifter-romance-bear-mates-over-forty-book-9-aline-ash/
ebookmass.com
ElectronicMaterials YuriyM.Poplavko
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
# 2019ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandthe CopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyright bythePublisher(otherthanasmaybenotedherein).
Notices Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professional practices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein. Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthesafety ofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproducts liability,negligenceorotherwise,orfromanyuseoroperationofanymethods,products, instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary ISBN:978-0-12-815780-0
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher:MatthewDeans
AcquisitionEditor:KaylaDosSantos
EditorialProjectManager:KatieChan
ProductionProjectManager:SuryaNarayananJayachandran CoverDesigner:VictoriaPearson
TypesetbySPiGlobal,India
Preface TheauthorofthebookisDr.YuriyPoplavko,aprofessorattheMicroelectronics DepartmentoftheKievPolytechnicInstitute,founded120yearsagobyDmitry MendeleyevandnowcalledtheNationalTechnicalUniversityofUkrainenamed afterIgorSikorsky.Theelectronicmaterialsciencesisstudiedandtaughtatthe departmentformanyyears,andDr.YuriyPoplavkopublishedinthisareaseveral booksinRussianandUkrainian;thisbookextendsthisexperience.
Electronicmaterialsscienceispartof“Solid-statephysics”butaccommodatedto theelectronicengineering.Ingeneral,modernmaterialssciencescoveraverywide rangeofissues,butinthisbookmainlythe electricalproperties ofmetals,semiconductors,dielectrics,andmagnetsaredescribed;inparticular,thosethatareofimportanceforspecialistsinelectronics.
Electronicsisascienceandengineeringdisciplinethatconcernsstudyandapplicationofelectricalphenomenainherentinsubstances(mainly,solids).Basedon thesestudies,electronicdevicesarecreated,aswellastheartofelectroniccircuits andsystemsconstructionisdeveloped.Itisalsopossibletodefineelectronicsasa scienceofelectronsinteractionwithelectromagneticfieldsorasascienceandmethodologyofcreatingelectronicmaterials,instruments,anddevices.Theoreticalproblemsofelectronicsconcernwiththestudyofelectronsinteractionwithmacroscopic fieldsinsidetheworkspaceofanelectronicdeviceaswellaswiththestudyofinteractionofelectronswithmicroscopicfieldsofions,atoms,molecules,orcrystallattice.Practicalproblemsofelectronicsboildowntodesignofelectronicdevicesthat performvariousfunctions,suchasconversionandtransmissionofinformation,control,computingaswellastheenergysupplying.
Thus,electronicsmaterialssciencesareimportantforspecialistsinelectronics.It shouldbealsonotedthatatpresentnotonlyeducational,butalsomonographicliterature,cannotkeeppacewithrapiddevelopmentofmaterialsscience,inother words,ofanappliedsolid-statephysics.Thisconcernsmanyareasofknowledge andtechnology—fromthepreparationofmaterialstotheelectronicdevices.Inthis context,nanophysicsandnanotechnologyareparticularlyfast-growingareas,andit isclearthattheyarenevermentionedinthebooksonsolid-statephysicsissued 20–30yearsago.
Thisbookpresentsconsiderablysimplifiedmathematicaltreatmentoftheories, whileemphasisismadeonthebasicconceptsofphysicalphenomenainelectronic materialsandtheirsimpleexplanation.Mostchaptersaredevotedtotheadvanced scientificandtechnologicalproblemsofelectronicmaterials;inaddition,somenew insightsoftheoreticalfactsrelevantfortechnicaldevicesarepresented.Thisapproach ofpresentationisduetothecontemporarytendencyformutualpenetrationandsynthesisfromdifferentfieldsthatatfirstglancebelongtodifferentareasofscience.
FirstVice-RectorofNationalTechnicalUniversityofUkraine “IgorSikorskyKievPolytechnicInstitute” FullMemberoftheAcademyofSciencesofUkraineYuriyYakimenko
Acknowledgments MysincerestthankstomycolleaguesfromIgorSikorskyKievPolytechnicInstitute fortheircontinuedinterestinthiswork,andforanumberoftheirvaluablecomments.Iamalsothankfultomanystudentswhopointedtohard-to-understandplaces andaskedmeforclarification.
IwanttogivespecialthankstoProf.,Dr.YuriyI.Yakimenkoforhiscontinued interestinthisworkaswellastoDr.VictorA.KazmirenkoandDr.YuriyV. Didenkoforgreathelpinthedesignofthebookanditspreparationforpublication. Theresponsibilityforanyremainingerrorsorshortcomingsis,ofcourse,mine.
Finally,IamdeeplygratefultothestaffoftheElsevierPublishingHouse:Kaila DosSantos,KatieChan,andSuryaNarayananJayachandranfortheirpatiencein eliminatingtheshortcomingsofthemanuscript.
Introduction Electronicsprimarilyusecrystals,polycrystals(ceramics),glasses,composites, amorphoussubstances,liquidcrystals,andsubstancesproducedbythecompaction ofnanocrystallinestructures.
Crystals arecharacterizedbywell-ordered(near-perfect)internalstructurethat canbedescribedbythree-dimensional(3D)spatialperiodicpattern.Distinctive propertyofcrystalsistheirtranslationalsymmetry,thatistosay,theelementarycell composedofafewatomscanbe“infinitely”translatedinalldirections,creatinga regularcrystallattice.Fromoutside,crystalsareusuallyseparatedbythefaces,that is,smoothflatsurfacesthatconvergeat strictlydefinedangles.
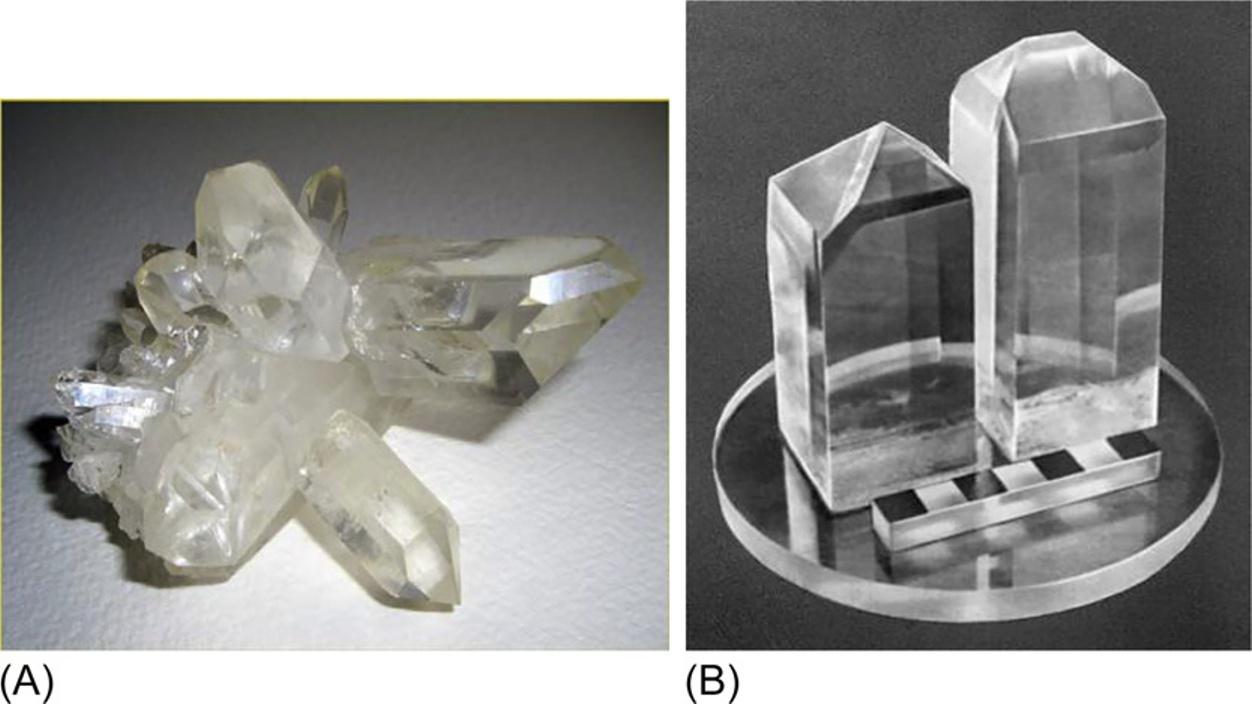
Althoughacrystalmaynotbeshapedlikeapolyhedron,itwillstillmanifestsuch characteristicsthatwillallowdistinguishingstrictlyorderedcrystallinestatefrom anydisorderedglassyoramorphousstate.Crystalsarecharacterizedbyacertain symmetryoftheirphysicalpropertiesthat correspondtosymmetryofinternalstructure.Thissymmetrydeterminesmanyphysicalcharacteristicsofcrystal,especially theanisotropyofitselectrical,thermal,mechanical,andmagneticparameters. Fig.I.1 showsphotosofnaturalquartz(SiO2)andKH2PO4 crystalsartificiallygrown fromaqueoussolution,andthesecrystalsarewidelyusedinelectronics.
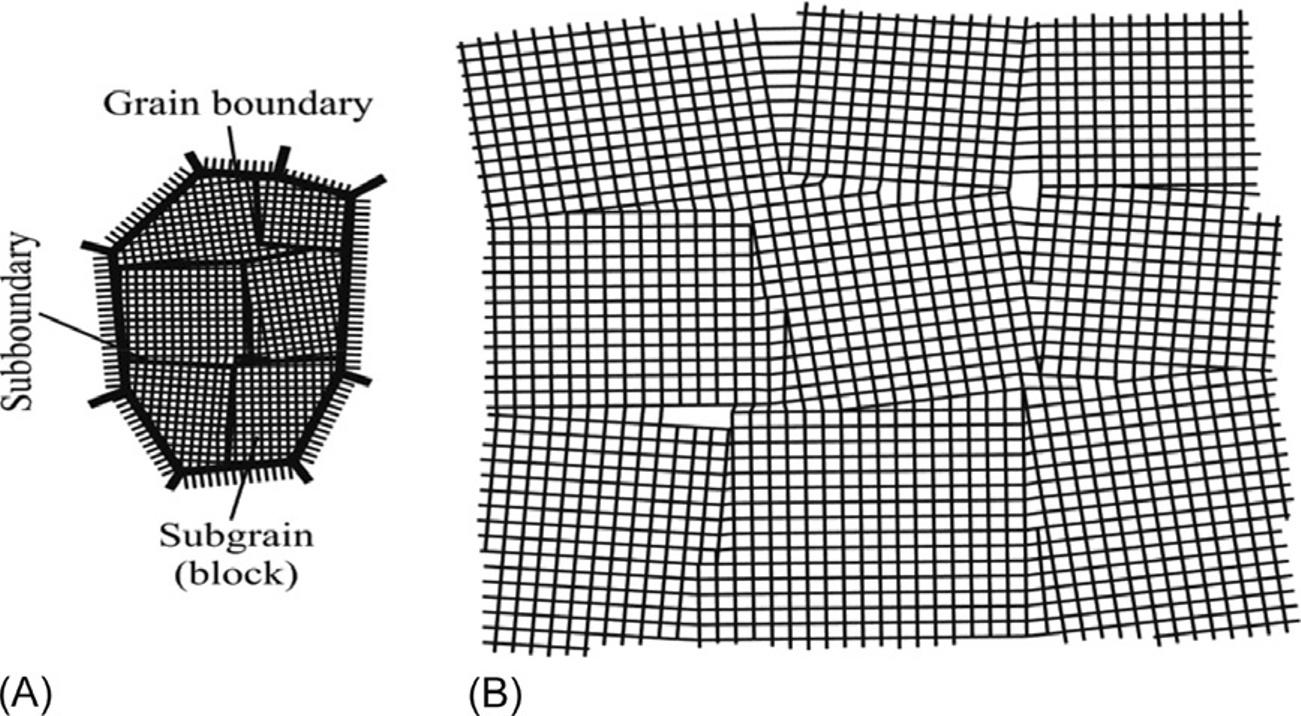
Polycrystals consistofalargenumberofverysmallcrystals(crystallites). Althoughthemacroscopicstructureofpolycrystallinesampleseemsdisordered, itsmicroscopiccomponentparts(i.e.,crystallitesorblocks)arehigh-gradecrystals withperfectmicroscopicstructure,and,therefore,thepolycrystalpracticallyhasthe samepropertiesassinglemonolithiccrystals(Fig.I.2).
The glass-like and amorphous-state solidsaredistinguishedbytheabsenceof anydistant(translational)symmetry.Distributionofatomsinthesebodiesis characterizednotbyalong-rangeordering(asincrystals)butbytheneighborordering.Intherangeofseveralnearbyatoms,thestructureofglassappearsasbeing ordered,thusenablingtodeterminespecificcoordinationnumberforneighbors.
FIG.I.1
Crystalsofquartz(A)andammoniumdihydrophosphate(B).
FIG.I.2
Schemeofcrystallites(grains)withitsborders(A)andblock(mosaic)structureinsidethe crystallites(B).
However,thecrosscorrelationarrangementofremoteatomsinaglassisviolated. Nevertheless,theorderingofglassystateishigherthanthatintheamorphousstate, whichmeansthatthecoordinationnumberismoredefiniteinglassthanintheamorphousstateofasolid.
Other orderedsolids alsomayhavegreatimportanceforpracticaluseinelectronics. Thisappliesprimarilytotwo-dimensional(2D)systems(likefilms).Inthe2D
systems,astrictlyorderedstructureisseenonlyinplane.Ifsuchplanarstructureisregularlyrepeatinginthesemiconductorchip(creatingsuperstructure),itselectronicpropertiescanbecharacterizedbyso-calledquantumwellsthatisatypicalcharacteristicof 2Dnanostructures.
Fig.I.3 showswell-known2Dsystem—graphene,whichhasgreatprospectsin electronics.
FIG.I.3 Crystalstructureofgraphene—2Dhexagonallattice.
FIG.I.4 Modelsofnanotubes.
Accordingly,the linear (wire-like)systemsbelongto1Dnanostructures,where translation-likeorderingisobservedonlyalongonedirection(“quantumthreads”). Theporoussiliconcanbe,inparticular,attributedtothesesystems.However, anotherwell-studiedquasi-1Dstructureisshownin Fig.I.4,carbonnanotubes.
Therearealsosystemswhosedimensionsalongallthreeorthogonaldirections arecommensuratewiththedistancebetweenatoms.Suchzero-dimension(0D)systemscanbeconsideredas“quantumdots,”inwhichonly10–103 atomsarethe orderedsystem. Fig.I.5 demonstratesgermaniumquantumdotgrownonsiliconsubstrate.Ontheareaofonesquaremicron,morethan1,000ofthesequantumdotscan beaccommodated.
FIG.I.5
Three-dimensionalimagesofquantumdotimageusingascanningelectronicmicroscope.
FIG.I.6
Zincoxidenanostructuresusedinsensors:(A)hexagonalnanocrystals;(B)thintubular nanocrystals.
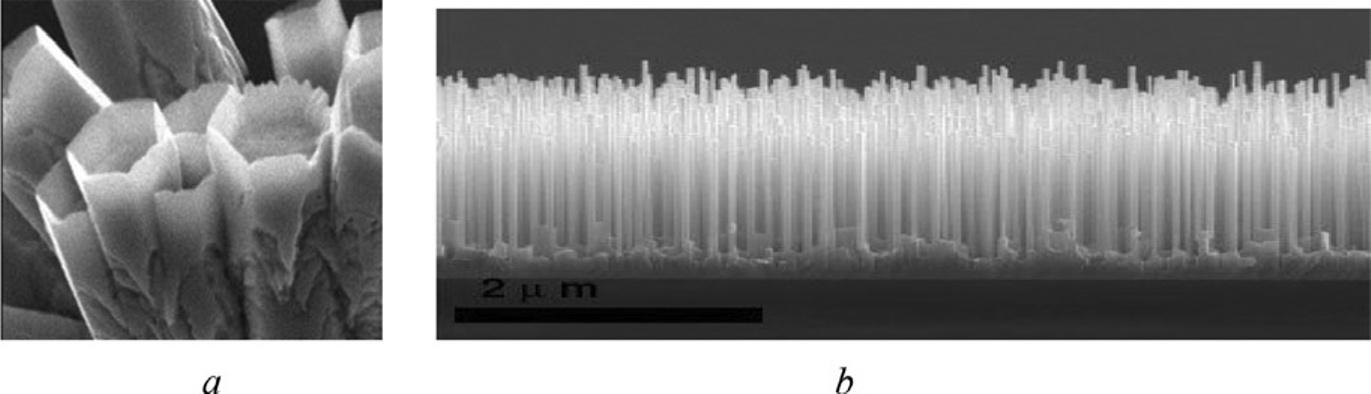
Nanostructuresarecharacterizedbyahugevarietyofshapes. Fig.I.6 givesexamplesofZnOnanostructuresthatcanbeusedinvarioussensors(humidity,gas,and evensmellsensors).
“Scalp-like”finestZnOnanocrystalsshownin Fig.I.6Bactuallyhaveanarrangementthatisthousandsoftimesdenserthanhumanhair.
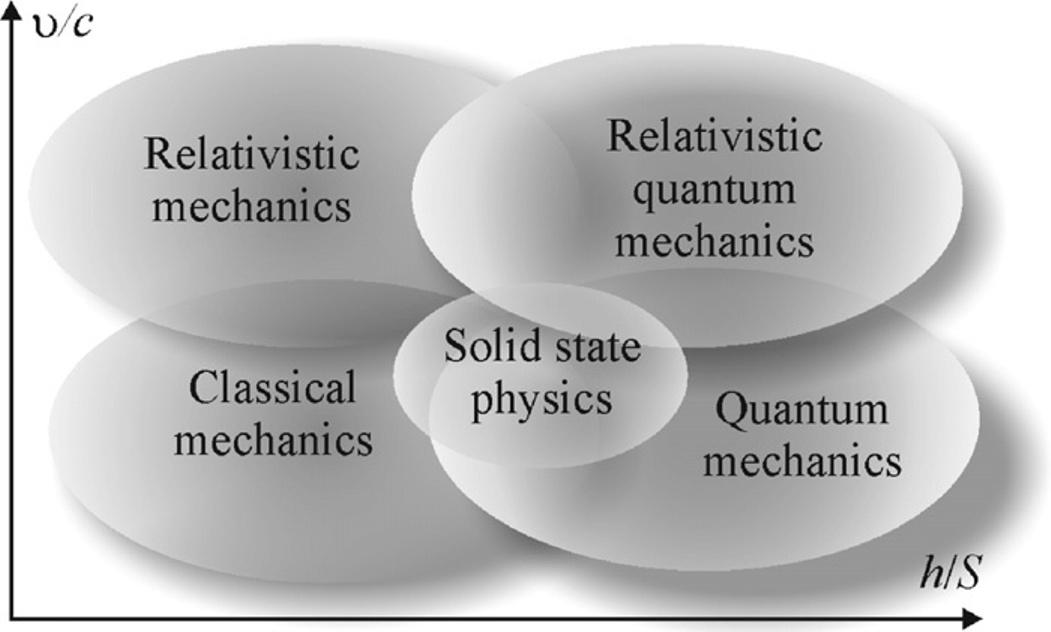
I.1 CONNECTIONBETWEENELECTRONICMATERIALS PHYSICSANDGENERALPHYSICS Technicaluniversitycourseofelectronicmaterialsscience,alsoknownas condensed matterphysics or solid-statephysics,usuallyisstudiedinthefinalpartofaseriesof physicscoursessothatitcanbeknowledgegainedbystudentsinpreviouscoursesof physics.Themappingbetweensolid-statephysics,classicalmechanics,quantum mechanics,andrelativisticmechanicsisillustratedin Fig.I.7.Solids-statephysics islocatedbetweenclassicalandquantummechanics.
Fig.I.7 showsthatclassicalphysicsisafieldofresearchoflowvelocities υ (as comparedwiththespeedoflight c)andacertainratioofPlanckconstant h tothe actionparameter S.Planckconstanthasaphysicaldimensionof“momentum”that istheproductof“energy × time ¼ impulse × length.”Theactionparameter S characterizesmovementofparticlewhenitswayismultipliedbyitsimpulse.Atthat, bothaxesusedin Fig.I.7 aredimensionless,becausethedimensionof S isthesame asthedimensionofPlanck’sconstant h .
Condensedmatterphysics(whichisthegeneralizationofsolid-statephysics)is basednotonlyonclassicalmechanics,butalsoonprincipalmethodsandnotionsof quantummechanics(see Fig.I.7).Einstein’srelativisticmechanicsisnotusedinthe solid-statephysics,butsomeoflawsofrelativisticquantummechanicsareimportant
FIG.I.7
Connectionbetweendifferentareasofphysics.
forunderstandingthenatureofmagnetisminatoms,thinfilms,andnanosizedstructures.Solid-statephysicsexploresthenatureofsolidbodyformationanditsstructure (atomicandenergetic),aswellasitsbasicphysicalproperties—electromagnetic, optical,thermal,mechanical,andothers—thatcontributetowidespreaduseofsolids inelectronics,instrumentation,electrical,andmechanicalengineering.
Electronicmaterialsscienceexaminesrathercomplexspatiallatticescomposed ofmicroscopicparticles—atoms,ions,ormolecules.Theforcesactingbetweenparticleshaveapredominantlyelectrostaticorigin.Althoughatomasaparticleisneutral,itselectricalchargesarenotlocalizedatasinglepoint;theyareslightlyspaced. Therefore,duringtheformationofasolidbody,inwhichatomsarelocatedcloseto eachother,theoppositechargesattracteachother,whilechargeswithequalsign pushoff.Thus,theforcesactingbetweenatomshavebothattractionandrepulsion inthem.Theinfluenceofoneatomonthemotionofelectronsinanotheratomissuch thattheresultantforceisalwaystheforceofattraction.
Themutualattractionofatoms(orions,ormolecules)thatactsatalongdistance isactuallythecauseofformationandexistenceofsolids.However,theattraction dominatesonlyuntilatomscomeneareachothersocloselythattheyalmostcollide. Thentherepulsionbeginstodominate,asthoseforcesareshort-rangeones.Atasufficientlysmalldistance,therepulsiveforcebecomesequaltotheforceofattraction; thenafewatomsorionsformthemolecule,whereasawholesetofatomsconstitutes thesolidbody.
Formaterialsscience,itisimportanttoestablishthenatureofrepulsiveforces.As electronis100,000timessmallerthanatom(soasatomicnucleus),fromthepointof viewofclassicalmechanicstheatomlookslike“emptiness,”asthespaceoccupied byelectronsandnucleusissosmall.However,solid-statephysics(especiallyinits importantsection—crystallography)makesquiteareasonableassumptionthatatom (orion)behavesasslightlydeformablebutsolidball.Forthisreason,awidelyused conceptinmaterialsscienceandsolid-statephysicsistheatomicorionic radius (data foratomsandionsofvariouselementscanbeseeninreferencetables).Onecan assumethatcrystalcanberepresentedinaformofregularlattice,composedofsolid balls(ions,atoms,ormolecules).High“hardness”ofapparentlyemptyatomballsis explainedbyquantummechanics;namely,whenatomsapproacheachother,thepossiblespaceforboundelectronsrapidlydecreases,becauseuncertaintyofrespective coordinatesdiminishes.AccordingtoHeisenberg’suncertaintyprinciple,theposition x andimpulse p ofaparticlecannotbeestablishedsimultaneouslywitharbitrarilyhighprecision: Δx Δp > h /2.Theshorterdistancesbetweenatomsleadto greateruncertaintyinimpulseand,thus,toanincreaseinthemagnitudeofimpulse. Asaresult,thekineticenergyofelectronsincreasesaswellastheirtotalenergy, whichfinallyresultsinrepulsion.Energeticallyfavorablepositionforatomsimposes certaindistancesbetweenthem.Therefore,repulsiveforce,whichprovidesbalance inthesolidstructure,hasquantumnature.
Basicmaterialsofelectronicsaresemiconductors,metals,insulators,magnetics, andnanomaterials.Obviously,mostimportantforelectronicsaretheirelectrical characteristics,and,amongthem,electricalconductivity(σ).Besides,thisparameter
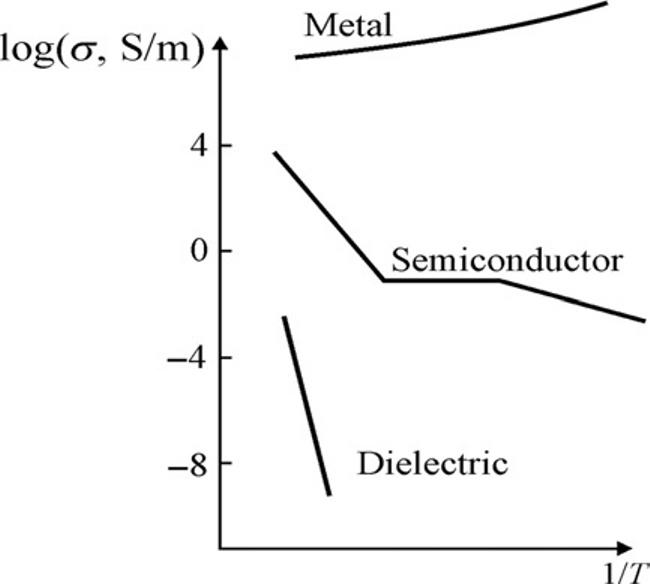
isveryconvenientforthe classification ofsolids.Theunitformeasurementofconductivityis[S/m](Siemenspermeter).Itdeterminescurrentdensity j [A/m2]ina givenmaterial,arisingunderappliedelectricalfield E [V/m]accordingtoOhm’s law: j ¼ σЕ .Itisimportanttonotethattemperaturedependenceofconductivity σ(T)isquitedifferentforvarioustypesofelectronicsmaterials(Fig.I.8).
Indielectricsandmetals,the σ(T)dependencesareopposite.Whileindielectrics, withtemperaturerise,conductivityincreasesexponentially(becauseatomicthermal motioninamattergeneratesnewchargecarriers),inmetalsconductivitydecreases approximatelyas Т 1 owingtochargecarrierscatteringonthelatticethermalvibrations.Therefore,atlowtemperatures,conductivityinmetalsbecomesverylarge, reachinginfinitywhenatzerotemperature(incaseofsuperconductivity).Indielectrics,incontrast,low-temperaturevalueof σ becomesclosetozero,becausecharge carriersarenotgeneratedintheabsenceofthermalmotion(andanyradiation effects).Evenatroomtemperature(Т 300 К),dielectricshaveverylowconductivity(σ < 10–10 S/m),andforthisreasontheyareoftenarereferredtoas“electrical insulators.”
Withregardto σ(T)dependence,metalsanddielectricsarequalitativelydifferent (see Fig.I.8).Yetinsemiconductorsthe σ(T)dependencelookslikeindielectrics; thedifferenceliesinmuchhigherconductivityofsemiconductor.Iftemperature becomeshigher,thisdifferencebecomeslessnoticeable(indielectricsandsemiconductors,chargecarriersappearthroughthetemperature-drivenactivationprocess).
Variouspropertiesofsolidmaterialsarerelatedtothenatureoftheirchemical bondsandtoenergyspectrumofelectrons.Thestudyofatomicstructureandelectronicenergyspectraofcrystallineandnoncrystallinesolidsisafundamentalproblemofsolid-statephysics,becauseitfacilitatesaconscioussearchformaterialswith predeterminedproperties.
FIG.I.8
Temperaturedependenceofconductivityinsolids:(A)normalscale,(B)logarithmicscalefor σ andinversedtemperature.
Distinguishingcharacteristicofsolidsistheirstructuralorderingthatisdependentonpositionsofneighboringatoms.Thecorrelationoftheirpositionmaymanifestitselfexclusivelyintheshort-rangeorderingofatoms.Intheamorphoussolids, ashort-rangeorderingissignificantlylimited;sometimes,theiratomicregulation mayberestrictedtothemicrocrystallitesthataredisorderedwithrespecttoeach other.However,mostsolidshavethelong-rangeordering,thatis,undistortedcrystal latticethatextendstorelativelylargeareas.Awidevarietyofpossiblegeometric structuresandspatialrelationshipsinlatticesleadstoalargenumberofdifferentphenomenainsolids.
Anycrystalmaymanifestsomedeviationfromidealgeometricstructure.Inaddition,anyphysicalbodyhasafinitesize;thusacrystalisalwayslimited,eitherby externalsurfacesorbyinternalbordersbetweencrystallites.Thisassertionistrivial, butitisessentialformanyphysicalphenomena.Itisnotpossibletoentirelyneglect thedisturbancesinbodyofarealcrystal,causedbothbytheinclusionsofforeign atomsinmainlatticeandbytheimpuritiesandviolationsoflocallatticeperiodicity.
Thethermalmotionofatomsincrystallatticesalsoleadstoadeviationfrom strictperiodicity.Indeed,periodiclatticereflectsnotrealpositionsofatoms,buttheir imaginaryequilibriumpositions,wheretheywouldfindthemselvesattemperature ofabsolutezero;onlythiscasecorrespondstothegroundstateofacrystal.Each deviationfromthegroundstatemeansadisturbance.However,atnormaltemperatures,thesedeviationsaresosmallthatorderedlatticedominantlydeterminespropertiesofacrystal.
Thepropertiesofelectronicsmaterialsare,atfirstapproximation,reducedtotwo complexproblems:
•Determiningthegroundstateofsolidsandreasonsforitsstability(i.e.,clarifying thenatureofforcesthatholdatomsinlattices).
•Describingbehaviorofsolidsunderexternalinfluences(i.e.,justifyingand predictingvariousphysicalpropertiesofsolidssuchaselectrical,thermal, mechanical,etc.).
Thefirstsetofcomplexproblemsarecharacterizedbytermssuchascrystalstructure,natureofchemicalbonds,cohesionforces,andenergyofbonding.However,it isonlyatfirstglancethesolutionoftheseproblemsisindependentofbehaviorof solidsundertheexternalinfluences.Infact,solutiontothefirstproblemcanbe obtainedonlythroughasettlementofthesecondproblem,becauseeachexperiment impliesthedisturbanceofgroundstate.Anyconclusionsaboutpropertiesofsolidsin thegroundstatecanbemadeafterinvestigationonhowitisaffectedbytheapplied electricalfield,temperature,exposuretolight,etc.
Severalexternalimportantimpactswillbediscussedinthisbook:
• Electricalfield.Firstofall,thechargetransfershouldbestudied,thatis,electrical current.Thisinvestigationwouldenablephenomenologicalclassificationof solidsasmetals,semiconductors,anddielectrics.Themechanismofelectrical chargetransferinanelectricalfieldalsomakesitpossibletodetermine
mechanismsofconductivity(electronicorionic).Second,theinvestigationof solidsintheelectricalfieldenablestostudythemechanismofelectrical charge separation,thatis,electricalpolarization,whosenaturemaybeelectronic,ionic, anddipole.
• Magneticfield.Differentresponsesofsolidstotheimpactofmagneticfield dependontheirchemicalcompositionandstructure.Theygiverisetosuch phenomenaasdiamagnetism,paramagnetism,ferromagnetism,and antiferromagnetism(andtheircombinations—ferrimagnetism).Thewidelyused investigationmethodisthemagneticfieldapplicationduringchargetransfer phenomenastudyintheelectricalfield.Theseadditionalparametersgiverisetoa numberofemergingeffectsandallowtoobtainsignificantinformationabout mainpropertiesofsolids.
• Temperaturegradient. Itdeterminesthedirectionofenergyflowfromhotterto colderareasofsolids.Itisnoteworthythatsimultaneouslywithheattransferthe electricalchargetransferisalsopossible.Theenergyandchargetransfercanbe describedbyvariousmechanisms.
• Illuminationbylight.Absorption,reflection,andscatteringoflightprovide importantinformationaboutinteractionofelectromagneticwaveswithvarious solids.
• Irradiationbyelectrons,positrons,neutrons,andothercorpuscularparticles serveasprobetostudyvariouspropertiesofsolids.
• Dosedimplantationofadditives intothecrystallatticeenablestogetimportant informationaboutcrystalproperties.Thismightbedonethroughforeignatom inclusionintocrystalstructure,formationofvacancies,andatomicsubstitutions inalattice,etc.
I.2 SOMECOMMENTSONTHEORETICALAPPROACHES Theoreticaldescriptionofallthelistedphenomenabyusinganyuniversalmodel seemsimpossible.Thereforeitisnecessarytoapplyapproximations.Thereforein specificcasesofinvestigation,thesimplifiedmodelsshouldbeused,whicharesuitableforagivenproblem.Thepurposeofvarioustheoriesofsolidsshouldbeeventual reductionofvarioustheoreticalfacetsofphenomenaintounifiedconcepts. Oneofsuchconceptsisbasedontheideaof quasiparticles, whichareelementary excitationsinthesolids.Infact,evenincaseofveryweakimpactonsolidtheintroducedenergycannotbedeliveredexclusivelytooneelementofcrystalindependentlyofallothers.Thepointisthatthestronginteractionexistsbetweenall particlesofacrystal(atoms,ions,andelectrons),and,therefore,theenergy,even beingappliedtoasingleparticle,shouldberapidlydistributedbetweenotherparticles.Thisprocesscanbemodeledbyemissionandabsorptionofquantaofenergy attributedtoimaginaryparticles.Theconceptofquasiparticles,whichcorrespondsto variousformsofexcitationinsolids,canbeconsideredasthebasicprinciplefor solid-statephysics.
Thustheobjectofstudyisasolidinthe excitedstate.Excitationenergycanbe thermalenergy thatisimposedfromoutsideorappearasexternalviolationofcrystal lattice.Theenergyofthermalexcitationistransferredtodifferentsubsystemsofa solid.Forexample,itcanbetransferredtovalenceelectronortoion,aswellasitcan appearaskineticenergyofioniclatticevibrationsorastheenergyofcoupledspinsin aferromagnetic.
Theexcitedstatethatliesclosetotheenergyofgroundstatemaybecharacterized byrelativelysmallnumberofthe independentoscillators.Thismethodisusedinthe latticedynamicsofsolidstodescribesmalloscillationsofalatticearounditsequilibriumpositions.Thuscomplicatedcollectivevibrationstateisdecomposedintothe normalindependentvibrations.Thesevibrationscanbequantized,andthecorrespondingquantaarethe phonons, theexampleofelementarydisturbances.Insome sense,theyresembleelementaryexcitationquantaofelectromagneticfield— photons.
Thesecondexampleofformaldescription,simplifyingmultiparticlesystemwith strongcollectiveinteraction,isasfollows.Anideaisintroducedthatthemotionof chargedparticlescanbedescribedasa“gas”ofchargedparticles.Obviously,each particlemustpushoffothersimilarparticlesfromitssurroundings.Formally,this casecanbedescribedbytheassumptionthatthereisnointeractionbetweenparticles,becauseanyobservedparticleisaccompaniedbythe“cloud”ofoppositesign chargesthatpartlycompensatethechargeofgivenparticle.Thus,theinteraction, whichmeansotherparticles’influenceonamovementofgivenparticle,canbe replacedbytheinertialchargedcloudthatthegivenparticleshould“entail”during itsmotion.Insuchaway,thesystemofinteractingparticlesisreplacedbythesystem ofnoninteractingparticles;however,dynamicpropertiesofnewquasiparticlesare changed.
Insolid-statephysics,therearemanyexamplesofelementaryexcitation.Along withphonons,whicharequantaoflatticevibrations,therearecollectiveexcitations ofelectronsinmetals,called plasmons. Inthesameway,magneticspinsystemcan bedescribedbyspinwaveswithcorrespondingquanta—the magnons.Inaddition,in dielectricstheelementaryelectronicexcitationsarethe polarons,whileinthesemiconductorstheymightbethe excitons.Thenatureofvariousquasiparticlesmightbe different.Whendescribingelectrons,itisnecessarytoconsiderthatduringtheir movementthroughacrystaltheyareexposedtodifferentinteractions.Therefore movingelectronscanbedescribedasdifferentquasiparticles,dependingonthe natureofinteraction.Electronscanbehaveasfreeelectrons,orpolarons(escaped electrons),orexcitons(boundbylocalinteractions),orCooperpairs,etc.
Theviolationsofcrystallattice,suchaslocalizedimpurityatoms(orvacancies) inthecrystallattice,canalsobemodeledbyelementaryexcitations.Theseelementaryexcitations,atfirstapproximation,canbeconsideredasnoninteracting.Ina morerefinedapproach,theirinteractionsalsomustbetakenintoconsideration.However,inthiscase,aconceptof“excitation”isalsoapplicable,anditcanbetakeninto considerationbymethodsofthe perturbationtheory.
Evenwhenitispossibletoneglectinteractionofelementaryexcitationsofthe sametype,thequestionofinteractionbetweendifferenttypesofexcitationsremains animportantproblem.Withthisapproach,asignificantvarietyofphenomenain solidscanbeinvestigated.Theprocessofestablishingequilibriuminsolidsalso requiresconsideringtheinteractionofquasiparticles,thatis,energyexchange betweendifferentsystemsofelementaryexcitations.
Theconceptofelementaryexcitationsshouldbeappliedonlyincaseofweak deviationsfromagroundstate.Ifthenumberofcollectiveexcitationsandquasiparticlesisquitelargeandtherelationshipbetweenthemistoostrong,thenthe describedtheoreticalmodelbecomesverycomplicatedduetoalargenumberof details,andtheconceptofquasiparticlesceasestobeeffective.
Structureofelectronic materials 1 CONTENTS 1.1AtomicBondinginMetals,Semiconductors,andDielectrics.................................. 1
1.2SymmetryofCrystals.........................................................................................
1.3BasicStructuresofCrystalsUsedinElectronics.................................................
1.4LatticeDefectsinCrystals.................................................................................
1.5StructureandSymmetryofQuasicrystalsandNanomaterials...............................
1.6StructuresofCompositesandMetamaterials.......................................................
Beforedescribingthemostdiversepropertiesofmaterialsusedinelectronics,itis necessarytoconsiderthefeaturesoftheirstructures,inwhichthesepropertiesare realized.
Theformationofcrystal,amorphous,andothersubstancesfromatomsisaccompaniedbyadecreaseoftheenergyinasystemascomparedtounconnectedatoms. Theminimumenergyinsolidscorrespondstoaregulararrangementofatomsthat agreeswiththespecificdistributionofelectronicdensitybetweenthem.Inaccordancewiththeelectronictheoryofvalence,interatomicbondsareformeddueto theredistributionofelectronsintheirvalenceorbitals,resultinginastableelectronic configurationofnoblegas(octet)duetoformationofionsorofsharedelectronpairs betweenatoms.
1.1 ATOMICBONDINGINMETALS,SEMICONDUCTORS, ANDDIELECTRICS Anyconnectionsofatoms,molecules,orionsareconditionedbyelectricalandmagneticinteractions.Atlongerdistances,electricalforcesof attraction dominate betweenparticleswhereas,atshortdistances, repulsion ofparticlesincreases sharply.Thebalancebetweensuchlong-rangeattractionandshort-rangerepulsion isthecauseofthebasicpropertiesofsubstances.Theatomicconnectionisattributabletotherestructuringofatomicelectronicshells,thuscreating chemicalbonds.In otherwords,chemicalbondsarethephenomenonofatomicinteractionbymeansof overlapoftheirelectronicclouds,andthisisaccompaniedbyadecreaseofthetotal energyofasystem.
https://doi.org/10.1016/B978-0-12-815780-0.00001-3
Chemicalbondingischaracterizedbyboth energy and length. Ameasureofbond strengthistheenergy,expendedincaseofbonddestruction,ortheenergygained duringcompoundformationfromindividualatoms.Consequently,theenergyof chemicalbondsequalstheworkthatmustbeexpendedtoseparateparticlesthat areconstrained,ortoalienatethemfromeachotherontheinfinitedistance [1].
Duringtheformationofchemicalbonds,exactlythoseelectronsthatbelongto the valenceshells playamajorrolebecausetheircontributiontosolidbodyformationismuchgreaterthanthatoftheinnerelectronsofatoms.However,divisioninto ionicresiduesandvalenceelectronsisamatterofconvention.Forexample,inmetals itissufficienttoconsiderthatvalenceelectronsaretransformedintoconduction electronswhereasallotherelectronsbelongtoionicresidues.
Inthe atoms ofametal,theirouterelectronicorbitsarefilledwitharelatively smallnumberofelectronsthathave lowionizationenergy.Whentheseatomscome together(i.e.,whencrystalisformedfromatoms),theorbitsofvalenceelectrons stronglyoverlap.Asaresult,valenceelectronsinmetalsbecomeuniformlydistributedinaspacebetweencations,andtheseelectronshavea commonwavefunction. Thereforevalenceelectronsinmostmetalsarefullycollectivized,andthussuch crystalsconstitutealatticeofpositivelychargedionscrowdedby“electronic gas.”Unlike,forexample,covalentbonds,thecompletedelocalizationofelectrons isadistinctivefeatureofmetallicbonds.
Itisinthiswaythatthespatialdistributionofvalenceelectronsliesattheheartof theclassificationofsolids(dielectrics,semiconductors,andmetals).Thedivisionof crystalsintodifferentclassessuggeststhatsolidsconsistof:
• ionicresidues,thatis,nucleithemselvesandthoseelectronsthataresostrongly associatedwiththeirnucleithattheresiduesformedcannotsignificantlychange theirconfigurationascomparedwiththeatom;
• valenceelectrons,thatis,electrons,thedistributionofwhich,insolids,may differsignificantlyfromtheconfigurationexistinginisolatedatoms.
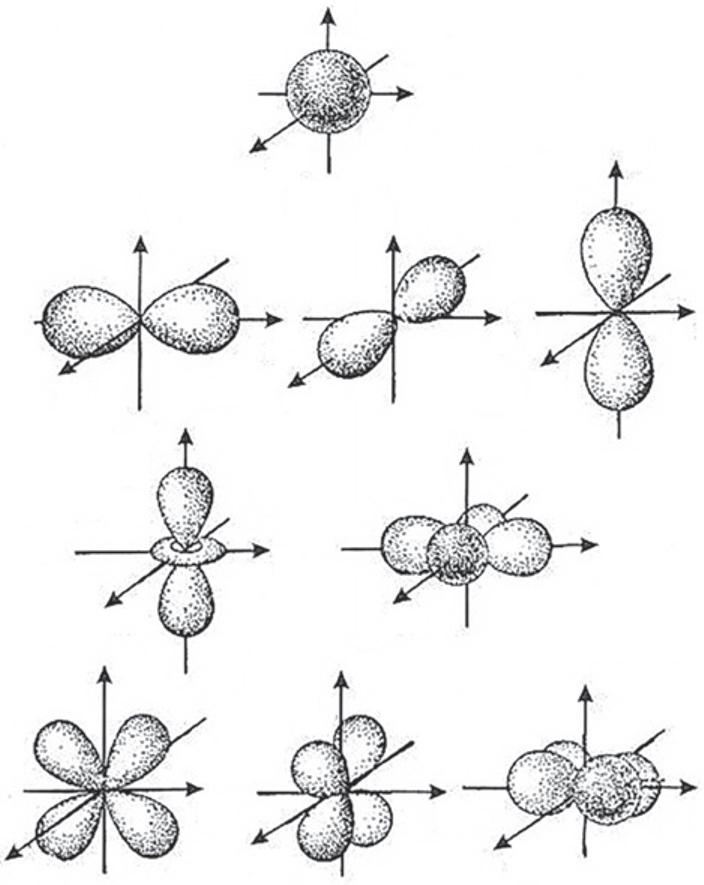
Thespatialdistributionofelectronicorbitalsofcertainatomshasastronginfluenceon thebondstrengthandtheirdirection. Fig.1.1 schematicallyshowshowmajorelectronicorbitalsfor s-,p-,and d-statesofelectronsintheatomsmightlook.Onlythe s-orbitalischaracterizedbysphericalsymmetry.Incontrast,the p-orbitalhasavery specificform,andthisisespeciallytrueforthe d-orbitals:theirformsareconsideredto contributetothespecificpropertiesoftransitionmetals.Rareearthmetalshave f-electrons,andtheymayplayadualrole:asresidueelectronsof“atomiccore”andas“free” electrons(becauseoftheircomplexity, f-orbitalsarenotshownin Fig.1.1).
Thusduringchemicalbondformation,valenceelectronsplayadominantrole because,atcrystalformation,theircontributionismuchgreaterthanthatofelectrons,whichformatomicinternalorbitalsintheresidues.
Aclassificationofthepossiblebondsofparticlesincrystalsisshownin Fig.1.2. Thisdivisionisratherconditional,becauseitcorrespondstosimplifiedmodels. Inmanycases,theactualbondingismorecomplicatedandoftenpresentsasanintermediatecasebetweensimplemodels.
FIG.1.1
Formsof s-,p-,and d-orbitals:angulardependenceofsquarewavefunctions.
FIG.1.2
Variousmodelsofatomicbondsincrystals [2]
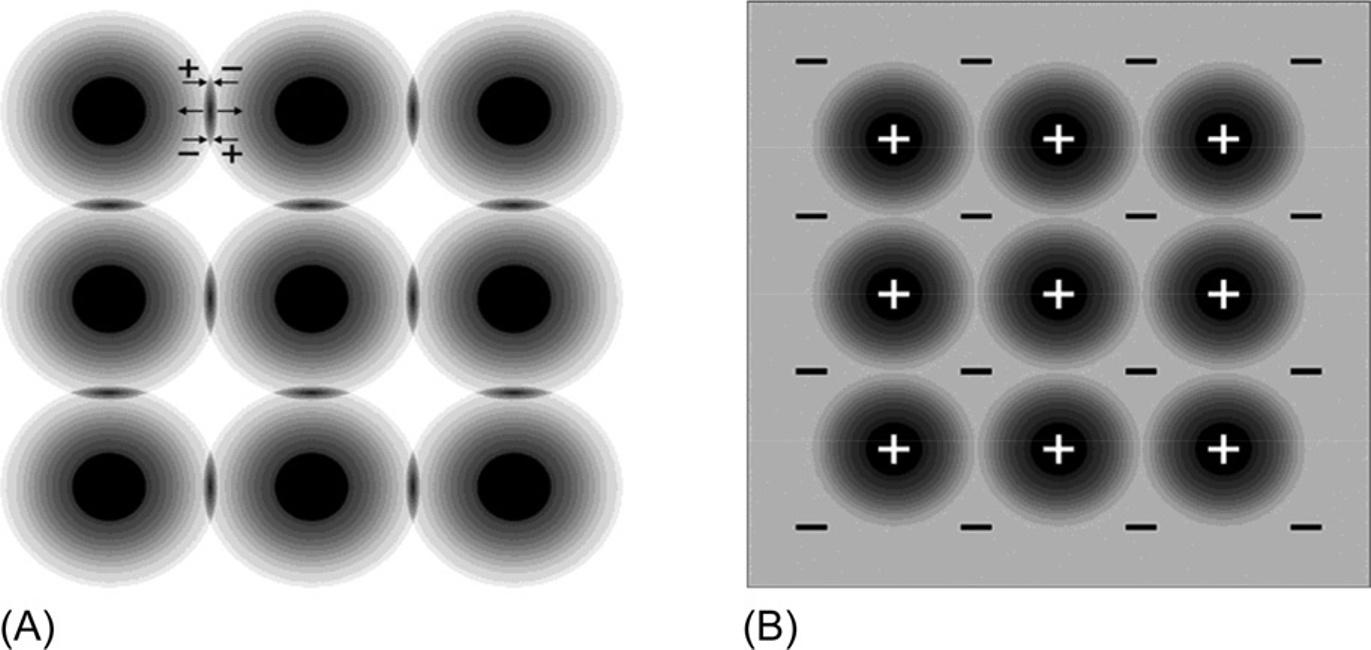
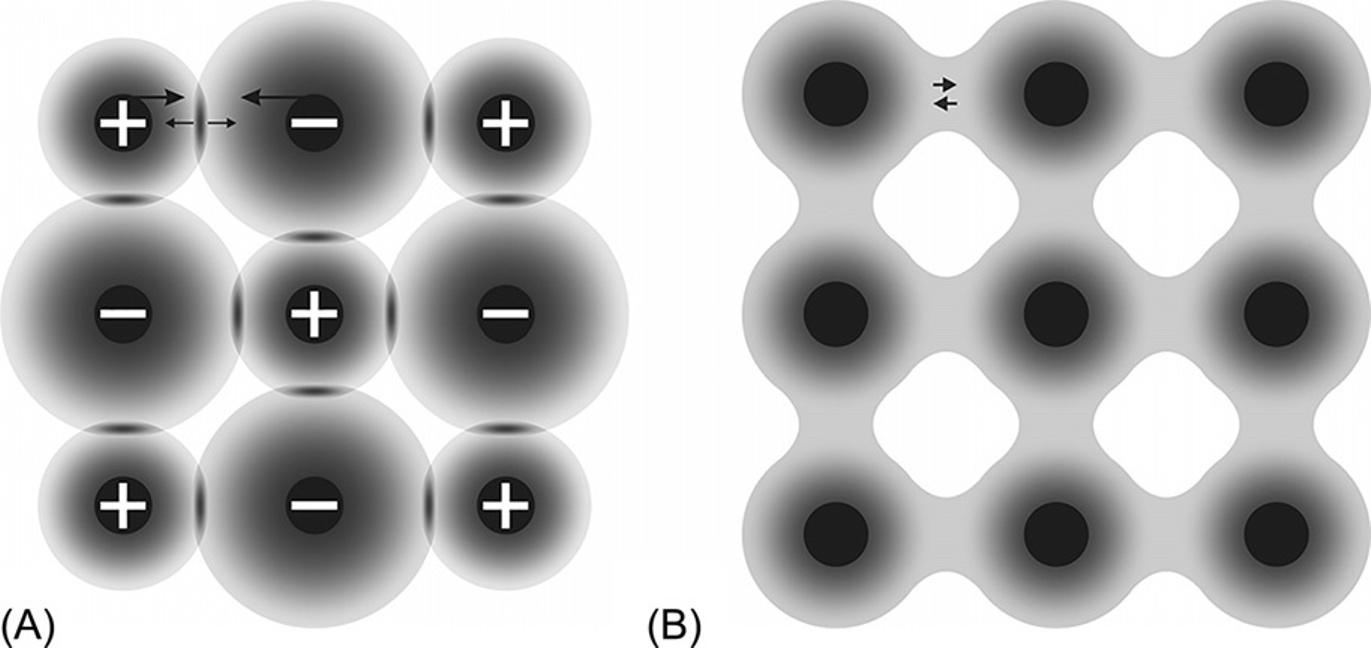
Molecularandmetallicbondsareshownattheoppositesidesofthescheme, becausetheyareabsoluteopposites.Inmolecularcrystals,electronsusuallyare completelylocked intheirmolecules(oratoms; Fig.1.3A).Thenucleiaresurroundedbyspaces(shownasblackballs),wherethedensityoftheelectroniccloud reachessignificantvalues.
Thesimplestexamplesofmolecularbondsareatomiccrystalsofsolidinert gases:neon,argon,krypton,andxenon.Thesehavecompletelyfilledelectronic shells,andsuchastableelectronicconfigurationundergoesonlyminorchangesduringtheformationofsolids.Therefore,theinertgascrystalisanexampleofarigid bodywithstrongelectronbondingexclusively inside atoms,whereastheelectron densitybetweenatomsisrathersmall,becauseallelectronsarewelllocalizednear theirownnuclei.
FIG.1.3
Two-dimensionalimageofelectricalchargedistribution:(A)molecularcrystal,inwhich quadrupoleelectronicfluctuation(+ … +)resultsintheattractionofatoms,whereas partialoverlappingofelectronicshellsleadstorepulsion( !),therebybalancingthis attraction;(B)metalcrystal,blackcirclesrepresentpositivelychargedatomicresidues, immersedinelectronicgas.
Themetalbond. Asalreadynoted,inmetalatomstheirouterelectronicorbitals containarathersmallamountofelectronsthathavelowionizationenergy.When suchatomscomecloser(i.e.,whenametalcrystaloralloyisformed),orbitalsof valenceelectronslargelyoverlapeachother.Asaresult,theseelectronsbecomedistributedalmostuniformlyinthespacebetweenions(Fig.1.3B).Indeed,X-raystudieshavepracticallyindicatedauniformelectronicdensityinthelatticeofmetals.
Thereforevalenceelectronsinmetalsareajointcollectiveinthecrystalasa whole,andmetalrepresentsthelatticeofpositivelychargedionswhereinthe “electronicgas”exists.Thisisareasonforthe delocalization ofmetalbonds;moreover,metalbondsare unsaturated and nondirectional.Metalsare,amongcrystals, characterizedbythehighest coordinationnumber (CN)ofions(usuallyinmetals, thisnumberis12;itisthenumberofthenearestneighborstoagivenparticle). Forcomparison,itshouldbenotedthatinioniccrystalsthisnumberisoften6or 8.Similarly,theCNinthecovalentcrystalsisevensmaller—itis4forsemiconductorswithadiamondstructure.
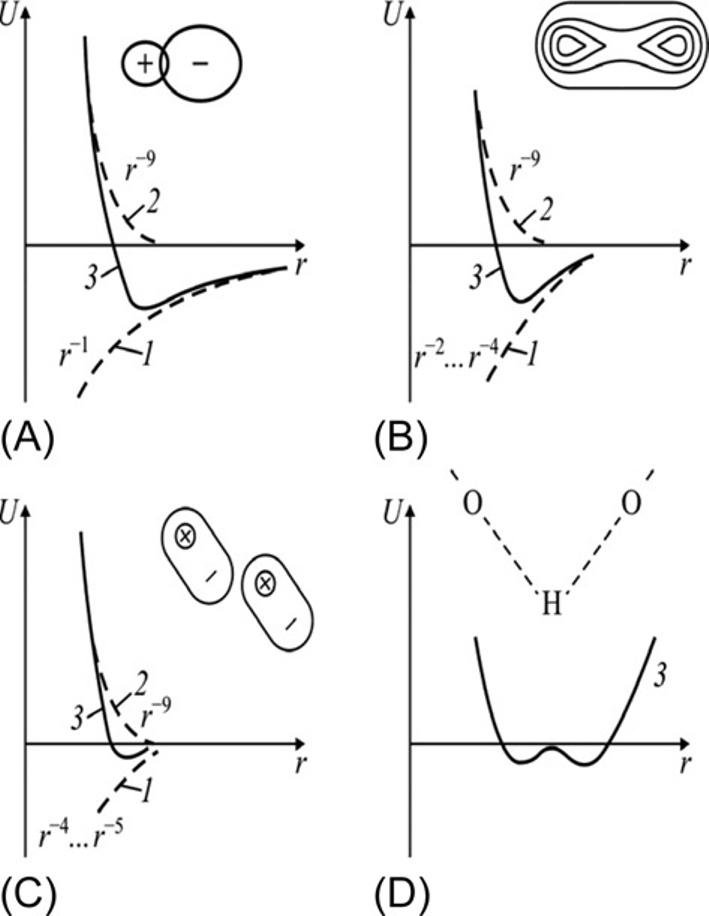
Thebondingin dielectricsandsemiconductors differssignificantlyfrommetal bonds. Fig.1.4 schematicallyshowsthe energydependence onthedistancebetween atomsforbondsinbasictypesofdielectricsandsemiconductors(metalbondsarenot shown).Betweenparticles(atoms,molecules,orions)whencreatingasemiconductorordielectricmaterial,atrelativelylargedistances,theforcesofattractiondominate:thecorrespondingenergyis negative andcharacterizedbythecurve 1.Atshort distances,theforceofrepulsionbecomesmuchmorepowerful;itsenergyis positive
FIG.1.4
Dependenceofattractionenergy(1),repulsionenergy(2),andtotalenergy(3)onthe distancebetweenparticles r :(A)ionicbond,(B)covalentbond;(C)molecular(quadrupole) bond;and(D)hydrogenbond.
andcharacterizedin Fig.1.4 bycurve 2.Thetotalpotentialenergyofinteraction betweenparticlesisshownbycurve 3 thattheminimumenergycorrespondstoa stabledistancebetweentheinteractingparticles(thisis parameteroflattice).
Thestrongrepulsionbetweenapproachingatomsorionscanbemodeledbydrasticenergydependence: Urep r 8 r 12;thisdependencecharacterizesthemutual impenetrability ofelectronicorbitals:electronicshellsofneighboratomsorionscan penetrateeachotheronlyveryslightly.Thisisthereasonthatatoms,ions,ormoleculescanbepresentedbythe“hardspheres”ofcertainradii,thesizeofwhich remainspracticallyunchanged [3]
Theattractionforcesthattieatoms,ions,andmoleculestogetherinsolidsareof anelectricalnature.Itshouldbenotedthatcrystalsareclassifiedjustbythe natureof attractionforces.Asshownin Fig.1.4,themaintypesofchemicalbondsindielectricsandsemiconductorsarethe covalent,ionic,molecular, and hydrogen bonds. Themetalbond(notshownin Fig.1.4)canbeconsideredalimitingcaseofthe covalentbond.
Theionicbond.Ioniccrystals(suchassodiumchloride,Na+ Cl )arechemical compoundsformedfrommetalandnonmetallicelements.Theenergyofionicattractionvarieswithdistanceratherslowly;thereforeionicbondsarethemost long
Two-dimensionalimageofelectronicchargedistributionin:(A)ioniccrystal,whereion attractionisbalancedbypartialoverlappingofelectronicshells;(B)covalentcrystal, black diffusedcircles representatomicresiduessurroundedbyregions,whereelectronicdensity reachessignificantvalues.
ranging incomparisonwithothers.Similartoatomicormolecularcrystals(shownin Fig.1.3A),ioniccrystalscanbecharacterizedbysuchadistributionofelectronic chargethatisalmostcompletely localizednearions.Inthesimplestmodelofthe ioniccrystal(Fig.1.5A),ionsare“nearlyimpenetrablechargedballs.”Thisapproximationisrathersuitableforionsthathavecompletelyfilledelectronicshells.
Typically,cationsandanionsacquireelectronicconfigurationoftheinertgas, andthereforethechargedistributioninthemhasanalmost sphericalsymmetry. Ions withoppositechargesattracteachotherduetolong-rangeCoulombforces;therefore theenergyoftheirattractionvarieswithdistanceveryslowly: Uatt r 1 (Fig.1.4A). Atthesametime,therepulsiveenergyofionsisinverselyproportionaltotheinteratomicdistance: Urep r 8 … r 12 (dependingonthepropertiesofthegivencrystal). Thereforetheioniccrystalcanbeconsideredamolecularcrystalinwhichthelattice isbuilt,notfromatoms,butfromtheions(e.g.,ionsNa+ andCl intherocksalt). Thuschargedistributionintheion,locatedinasolidbody,isonlyslightlydifferent asthoughitwereanisolatedion.Itisimportantthatparticles,whichformioniccrystalsarenotneutralatoms:betweenions, largeelectrostaticforces existthatplaya majorroleanddeterminethemainpropertiesofioniccrystals(thatdiffersignificantlyfromthepropertiesofmolecularcrystals).
Thusinthesimplestmodelofanioniccrystal,allionsarepresentedas “interactingnearlyimpenetrablechargedspheres”;thisapproximationissufficient forionswithentirelyfilledelectronicshells.Whereasintheatomic(ormolecular) crystal,allelectronsremain locked intheirnativeatoms,intheioniccrystal,valence electronsare moved fromcationstoanions.
Thereforetheionicbondoccursbetweenparticlesoftwotypes,oneofwhicheasilyloseselectrons,formingpositivelychargedions(cations)andotheratomsthat
FIG.1.5
readilygetelectronsthenform,respectively,negativelychargedions(anions).Most oftheelectropositivecationsbelongtogroupsIandIIoftheperiodictable,whereas mostanionsbelongtogroupsVIandVII.
Asarule,ionsincrystalsarepackedtightly,aseachofthemissurroundedbythe largestnumber ofoppositelychargedions.Stabilizationoftheionicsolidstructure takesplaceatCNs6,8,and,occasionally,even12.Itshouldbenotedthationicradii varynoticeablywiththevalueoftheCN.Ionicbonds,unlikemetalbonds,are saturated,but,asinmetals,theyare notdirected [4]
Thecovalentbond incrystalsistypicalfor semiconductors.Thedependenceof bindingenergyoninteratomicdistanceisshownin Fig.1.5B;attractiveforcesin caseofcovalentbondsare notsolongranging asinthecaseoftheionicbond: theattractionenergychangeswithdistanceas r 2 r 4
Inprinciple,thenatureofthecovalentbondisveryclosetothatofthemetalbond; however,incovalentcrystals,valenceelectronsaresharedonlybetweenthe nearest neighboringatoms whereas,inmetals,valenceelectronsaresharedwithinthecrystal lattice.Usually,acovalentbond(i.e., homeopolar bond)isformedwithapairof valenceelectronsthathaveoppositespindirections.Duringcovalentchemicalbond formation,thereductionoftotalenergyisachievedbythequantumeffectof exchangeinteraction.ThesimplestexampleofacovalentbondisthehydrogenmoleculeH2,whereinbothelectronsbelongsimultaneouslytobothatoms.
The diamond mightbeaclassicexampleofacovalentcrystal(Fig.1.5B),where carbonatomsarelocatedinaratherroomyconfiguration:theirCNisonly4.Therefore,diamond(aswithsemiconductorsofsimilarstructure—germaniumandsilicon) ischaracterizedbyacomparativelyhigh-densityelectroniccloudintheatomicinterstitials:electronsareconcentratedmainlynearthelinesconnectingeachcarbon atom withitsfournearestneighbors.Althoughdiamondisdielectric,thehighchargedensityinareasbetweenatomsisacharacteristicfeatureofsemiconductors.
Covalentbonds,unlikemetalbonds,are stronglydirected;moreover,theyare saturated.Thesaturationofacovalentbondistheabilityofatomstoforma limited number ofcovalentbonds.Thenumberofbondsformedbytheatomisdetermined byitsouterelectronicorbital.Thedirectivityofcovalentbondsiscausedbytheir peculiarelectronicstructureandgeometricalshapeofelectronicorbitals(theangles betweentwobondsarethe valenceangles).
Sometimes,covalentbondingmighthavepronounced polarity andincreased polarizability thatdeterminesmanychemicalandphysicalpropertiesofcorrespondentcompounds.Thepolarityofacovalentbondisduetotheunevendistributionof electronicdensityaccruabletoadifferenceintheelectronegativityofatoms(thereforecovalentbondsaredividedintononpolarandpolarbonds).Thepolarizabilityof bondscanbeexpressedbythenonsymmetricspontaneousdisplacementofbinding electrons [5].
Thefollowingpolarconnectionsaredistinguished [4]:
•The nonpolar (simple)covalentbondarisesfromthefactthateachatomprovides oneofitsunpairedelectrons,buttheformalchargeofatomsremainsunchanged, becauseatomsthatformthebondequallyhaveasocializedelectronpair.
•The polar covalentbond,whereinatomsaredifferent,thedegreeofoverlapofthe socializedpairofelectronsisdeterminedbythedifferenceinthe electronegativityofatoms.Theatomwithagreaterelectronegativitymore stronglyattractselectrons;thereforeitsrealchargebecomesmorenegative.With theelectronegativechargeanatomacquires,thereisadditionalpositivechargeof thesamemagnitude.
•The donor-acceptor bondariseswhenbothconnectingelectronsareprovided byoneoftheatoms(calledthe donor)whereasthesecondatom,involvedin theformationofabond,isthe acceptor.Whencreatingthispair,formal chargeofthedonorisincreasedbyoneandtheformalchargeofthe acceptorisreducedbyone.Theelectronpairofoneatom(donor)goesinto acommonuse,whereasanotheratom(acceptor)providesitsfreeorbital, becausethedonoratomsusuallyserveatomsthathavemorethanfour valenceelectrons.
•The σ-bond and π-bond areapproximatedescriptionsofsometypesofcovalent bondsindifferentcompounds.Thereforethe σ-bondischaracterizedbythe maximumelectronicclouddensityalongtheaxisjoiningthenucleiofatoms.The formationofthe π-bondischaracterizedbythelateraloverlapofelectronic clouds“above”and“below”theplaneofthe σ-bond.
Unlikemetalliccoupling,theemergenceofacovalentbondisaccompaniedbysuch redistributionofelectronicdensitythatitsmaximumlocalizes between theinteractingatoms.Asinmetals,incaseofacovalentbond,the collectivization oftheouter valenceelectronsisseen,butthenatureofelectronicallocationisdifferentfromthat inmetals.Inthegroundstateofcovalentcrystals,thatis,at T ¼ 0K,therearenopartiallyfilledelectronicenergybands.
Inotherwords,thecovalentcrystalcannotbedescribedbyuniformdistribution ofelectronicdensitybetweenatoms,asistypicalforsimplemetals.Conversely,in covalentcrystals,theelectronicdensityisincreasedalongthe“bestdestinations,” leadingto chemicalbonds.Thestrongerthecovalentbond,thegreatertheoverlap ofelectroniccloudsofinteractingatoms.Ifthisbondisformedbetweensimilar atoms,thecovalentbondisthe homeopolar and,whenatomsaredifferent,itis the heteropolar.
Incaseswheretwointeractingatomsshareoneelectronpair,a single connection isformed;whentherearetwoelectronpairs,the double bondiscreated,andwhen therearethreeelectronpairs,a triple bondiscreated.Thedistancebetweenbound nucleiisdefinedasthe length ofthecovalentbond.Bondlengthdecreaseswhenthe orderofthebondincreases.Forexample,thelengthofa“carbon-to-carbon”bond dependsonmultiplicity:foraC Cbond,itslengthis1.54 10 1 nm;incaseofa C]Cbond,thelengthis1.34 10 1 nm,whereasforC^C,itisonly 1.20 10 1 nm [3].Withanincreaseofthebondorder,itsenergyincreases.
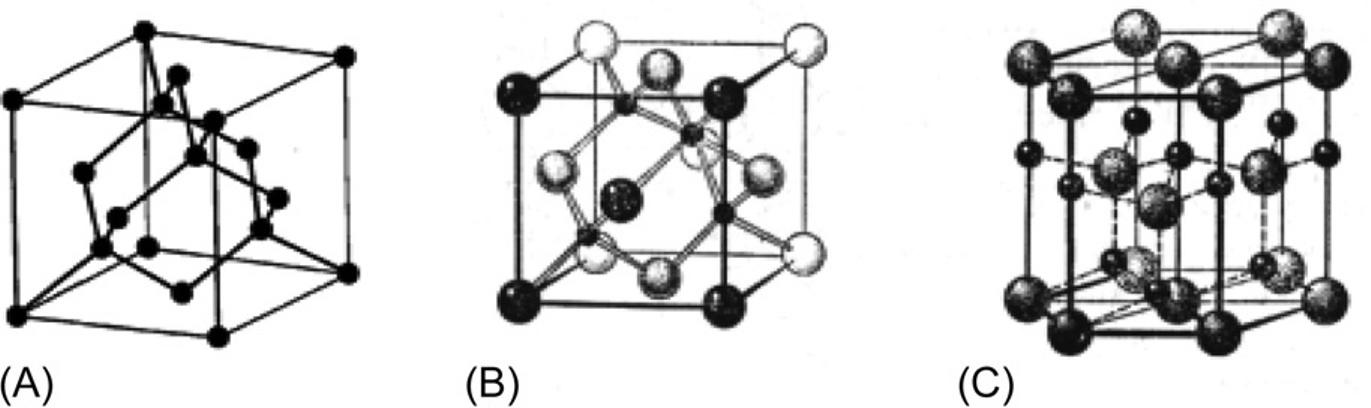
The directivity ofcovalentbondscharacterizesthefeaturesofelectronicdensity distributioninatoms.Forinstance,ingermaniumandsiliconcrystals(thathavea diamondstructure),eachatomislocatedinthecenterofatetrahedron,formedby
FIG.1.6
Structureofmainsemiconductors:diamond(A),sphalerite(B),andwurtzite(C) [6]
fouratoms,theirclosestneighbors(Fig.1.6A).Inthiscase,tetrahedralbondsare formedwheneachatomhasonlyfournearestneighboringatoms.
Mostcovalentbondsarecreatedbytwovalence(hybridized)electrons—one fromeachinteractingatom.Incaseofsuchaconnection,theelectronsarelocalized inthespacebetweenthetwoatoms;thusthespinsoftheseelectronsareantiparallel. Asshownin Fig.1.5B,theplaneschemecangiveonlyanapproximaterepresentationoftheactuallocationofatoms.Infact,therelativepositionoftheseatomsinreal crystalscanbequitecomplex,asshownin Fig.1.6BandC.Thestructureofthemineral sphalerite (zincsulfide,ZnS)istypicalofAIIIBV semiconductors,suchasgalliumarsenide.The wurtzite structure(calciumselenide,CaSe)istypicalofAIIBVI semiconductors.
Simplifiedschemesofelectronicdensitydistributionincovalentandioniccrystalsareshownin Fig.1.7AandB. However,sphaleriteandwurtzitebelongtopolar crystalsthathaveahybridbonding.
Thehybridionic-covalentbond. Aswiththemodelofa“purelycovalent”structure,amodelofa“purelyionic”crystalisidealized.Inrealcrystals(especially,in someAIIIBV andAIIBIV typesofsemiconductorsandinactivedielectrics),theintermediatecasebetweenionicandcovalentbondsexists.Inthecovalentsiliconcrystal (Fig.1.7A),electronsareequallydistributedaroundatoms;therefore,theelectronic densitybetweenatomsisratherlarge.Inanioniccrystal,theattractionofcationand anioniscompensatedbytherepulsionofpartiallyoverlappingelectronicshells.
Theconceptofintermediatetypebondsagreeswiththetheoryofiondeformation bytheirpolarization.Thismayoccur,forexample,bythedistortionofananion’s electronicorbital,mainlybythedifferentelectronegativitiesofadjacentions.Therefore,theelectronicdensitybetweenionicresiduesincreases,thatis,themixed covalent-ionicbondwithagreaterdegreeofchargeseparationbecomesthe polar bond.Theexactpresenceofsuchbondsdeterminesthenoncentrosymmetricstructureofsomecrystals.Thehybridionic-covalentbondingisthemaincauseofpyroelectric,ferroelectric,andpiezoelectricproperties.Mostsuch active(functional) dielectrics belongtocrystalsortootherorderedpolarsystems(liquidcrystals,electrets,polarpolymers,etc.).Thusthephysicalhypothesis,relevanttothenatureofthe